Introduction
Pancreatic cancer is the fourth leading cause of
cancer death and is expected to become the second major cause of
cancer mortality in Western countries by 2020 (1). Most cases are diagnosed in the late
stage due to the absence of early symptoms and the lack of
validated screening tests for early diagnosis, so the overall
survival of the patients with pancreatic cancer are limited
(2). Current therapeutic
strategies, such as surgical resection, radiation and chemotherapy,
are unsatisfactory (3–5). Thus, identification and development
of more effective treatment strategies is urgently needed.
Tumor cells including pancreatic cancer cells
reprogram their metabolism to meet their bioenergetic and
biosynthetic requirements, and increased glycolysis is a primary
biochemical hallmark of tumors (6). This phenomenon is known as the
Warburg effect and is one of the most predominant metabolic
alterations that occur during malignant transformation (7). In contrast with normal cells which
transform glucose into carbonic anhydride under aerobic conditions,
the tumor cells mostly produce lactate, even in the presence of
sufficient levels of oxygen (8).
It has been documented that tumor glycolysis provides energy for
rapid growth and promotes cancer metastasis (9). Thus, tumor growth inhibition of
pancreatic cells through targeting and blocking of glycolysis could
be an attractive antitumor strategy (4).
V-ets erythroblastosis virus E26 oncogene homolog-1
(ETS-1) belongs to the ETS transcription factor family
characterized as a conserved DNA-binding domain (transcription
activation domain) (10–12). Our previous study (10) and other studies proved the
importance of the ETS family members in the regulation of cell
development, differentiation, proliferation, apoptosis,
angiogenesis, tissue remodeling, migration, invasion, malignant
transformation and metastasis (10,13–17).
ETS-1 is an important transcription factor regulating MMP genes
(18) such as MMP1, MMP3 and MMP9
(18–21). Additionally, it can mediate
extracellular matrix degradation, cell migration, angiogenesis and
drug resistance (22,23). Considerable attention has been paid
to ETS-1 due to its important roles in regulating the energy
metabolism in cancer cells (11,24).
It has been stated that the overexpression of ETS-1 is responsible
for the poor prognosis of breast cancer (10,25–31),
ovarian carcinoma (32–34), and cervical carcinoma (24,35).
Therefore, ETS-1 may be a promising molecular target for the new
therapeutic strategy of pancreatic cancer (36).
Increased uptake of extracellular glucose is
facilitated by the glucose transporter-1 (GLUT-1) which is commonly
upregulated in a wide range of solid tumors and contribute to the
Warburg effect (37,38). It has been shown that GLUT-1 is
associated with the development, poor prognosis of cancers and
inhibition of glycolysis which can effectively block the
proliferation and invasion of the cancer cells. Thus, key
transporters that participate in glycolytic metabolism such as
GLUT-1 could become vulnerable for pancreatic cancer therapy
(39,40).
AMP-activated protein kinase (AMPK) is a
heterotrimer comprised of one catalytic subunit α and two
regulatory subunits β and γ and AMPKα phosphorylation site on
threonine 172 (pT172) that is essential for full activation of AMPK
(41). As a cellular energy
sensor, AMPK is activated when the uptaking of glucose is reduced
and AMP/ATP ratio is increased (42). AMPK plays a pivotal role in
'sensing' energetic stress. It can promote the catabolism to
generate more ATP and inhibit the anabolism of cancer cells to keep
a sustained energy supply (43).
Furthermore, AMPK has been reported to stimulate the process of
autophagy, a cellular process in which components of the cell are
degraded to ensure sufficient metabolites in response to nutrient
limitation (44,45).
In this study, we sought to detect whether silencing
ETS-1 could be a selective anticancer strategy by reducing the
expression of GLUT-1 to inhibit glycolysis and then induce
autophagy to decrease viability of pancreatic cancer cells.
Materials and methods
Cell culture and reagents
The two human pancreatic cancer cell lines, Panc-1,
PaTu-8988 were cultured in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% heat inactivated fetal bovine serum
(FBS), 100 U/ml penicillin and 100 µg/ml streptomycin
(Invitrogen, Carlsbad, CA, USA) in a humidified incubator in 5%
CO2 at 37°C. Cells were in log phase prior to the
following experiments.
Establishing of stable ETS-1 knockdown
cell lines
Panc-1 cells and PaTu-8988 cells were seeded in
6-well plate (5×105 cells/well) separately followed by
transfection with 500 ng/ml of either psi-ETS-1 (PIEE102075355) or
control plasmids (constructed and analyzed sequence by GeneChem
Company, Shanghai, China) conjugated with Lipofectamine 2000
(11668-019; Invitrogen). After transfection, cells were cultured in
Opti-MEM medium (31985-062; Invitrogen) according to the
manufacturer's instructions.
Cell viability assay
Cell viability was assessed by MTT cell
proliferation and cytotoxicity assay kit (C0009; Beyotime
Biotechnology). Panc-1, PaTu-8988 cells were seeded in 96-well
plates at a density of ~10,000 cells/well. Thiazolyl blue
tetrazolium bromide (MTT) solution was prepared following the
instructions, and incubated with the growing cultures at a final
concentration of 0.5 mg/Ml for 4 h at 37°C. Absorbance at 570 nm
was read on a multiwell scanning spectrophotometer
(Infinite®; Tecan, Shanghai China), and the results were
expressed as a percentage (%) of the control.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from untreated and stably
transfected cells using TRIzol reagent (15596-026; Invitrogen).
First-Strand cDNA was synthesized according to the manufacturer's
instructions (DRR036A; Takara, Tokyo, Japan). RT-PCR analysis was
carried out using 2X Power Taq PCR Master Mix (PR1700; BioTeke,
Beijing, China) under the following conditions: 95°C for 5 min to
denature cDNA, followed by 30 cycles of 95°C for 30 sec, 55°C for
15 sec, and 72°C for 30 sec, followed by a terminal extension for 7
min at 72°C. PCR products were electrophoresed on a 2% agarose gel
containing ethidium bromide (16550-100; Invitrogen) at 90 V for 45
min, and the bands were visualized using the UltraPower™ Gel
Imaging system (EP2018; BioTeke). The primer sequences are listed
in Table I. The primers were
obtained from Invitrogen.
 | Table ISequences of the oligonucleotide
primers. |
Table I
Sequences of the oligonucleotide
primers.
| Gene | Primer sequences
(5′-3′) | Product (bp) |
|---|
| ETS-1 | F:
5′-GTCGTGGTAAACTCGG-3′ | 246 |
| R:
5′-CAGCAGGAATGACAGG-3′ | |
| GLUT-1 | F:
5′-CGGGCCAAGAGTGTGCTAAA-3′ | 283 |
| R:
5′-TGACGATACCGGAGCCAATG-3′ | |
| ATG5 | F:
5′-AAGCAACTCTGGATGGGATT-3′ | 239 |
| R:
5′-GCAGCCACAGGACGAAAC-3′ | |
| ATG7 | F:
5′-ACCCAGAAGAAGCTGAACGA-3′ | 267 |
| R:
5′-AGACAGAGGGCAGGATAGCA-3′ | |
| GAPDH | F:
5′-CCACCCATGGCAAATTCCATGGCA-3′ | 251 |
| R:
5′-TCTAGACGGCAGGTCAGGTCCACC-3′ | |
Glucose uptake and lactate release
assay
The culture contains 4.5 g/l glucose which will be
uptaken by cancer cells to supply energy and produce lactate. The
supernatants from untreated and stably transfected cells of Panc-1,
PaTu-8988 cultures were collected respectively at various time
points. Glucose uptake rate in culture supernatants was determined
using a glucose assay kit (F006; Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) according to the manufacturer's
instructions, and quantified by absorption at 450 nm. Lactate
generation was measured using a lactate assay kit according to the
manufacturer's instruction (A019-2; Nanjing Jiancheng
Bioengineering Institute).
ATP detection by colorimetric
measurements
Intracellular ATP was measured by a
luciferin-luciferase method using ATP assay kit (A095; Nanjing
Jiancheng Bioengineering Institute). Briefly, untreated and stably
transfected cells of Panc-1, PaTu-8988 were collected after washing
with PBS. Boiling distilled water was added to the collected cells
and then the suspension was placed in hot bath to homogenize the
cells. After that the suspension was heated in boiling water bath
for 10 min and eddied for 1 min. The supernatants were collected
after centrifugation (12,000 × g, 4°C) for 10 min (Eppendorf
Centrifuge 5424R), followed by mixing with the ATP detection buffer
for analyzing by luminescence spectrometry. The final ATP content
of each sample was normalized to protein concentration measured by
BCA Protein assay kit (A045-2; Nanjing Jiancheng Bioengineering
Institute).
Western blot analyses
Total proteins of cultured cells were extracted
using RIPA buffer containing phenylmethanesulfonyl fluoride (PMSF).
BCA protein assay kit (G2026; Wuhan Guge Bioengineering Institute,
Wuhan, China) was used to determine the protein concentrations.
Samples were resolved by 12.5% SDS-PAGE (Invitrogen) after mixing
with SDS-PAGE loading buffer and boiling for 5 min. Proteins were
transferred to a PVDF membranes and diluted in blocking buffer (5%
dry milk) for 1 h, and then incubated separately with diluted
(1:300) primary antibodies [polyclonal rabbit anti-ETS-1, GLUT-1,
autophagy-related gene 5 (ATG5), ATG7, AMPK, light chain 3 (LC3);
Wuhan Guge Bioengineering Institute] overnight. Then, secondary
HRP-conjugated antibody (1:3,000, GB23301; Wuhan Guge
Bioengineering Institute) was added for 1 h. Membrane was exposed
using the chemiluminescence detection kit (G2019; Wuhan Guge
Bioengineering Institute). Anti-β-actin antibody (GB13001; Wuhan
Guge Bioengineering Institute) was used as control.
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, San Diego,
CA, USA) was used for all statistical analysis. The mean ± SD was
determined for each group in the individual experiments. The
Student's t-test was used to determine the significance of
differences between different groups. P-values <0.05 were
statistically significant.
Results
Transcriptional silencing of ETS-1
attenuated cell viability in Panc-1 and PaTu-8988 cells
To evaluate the function of ETS-1 gene in the
regulation of GLUT-1 expression in pancreatic cancer cells, two
stable ETS-1 silencing cell lines, Panc-1-siETS-1 and
PaTu-8988-siETS-1, were established using a green fluorescent
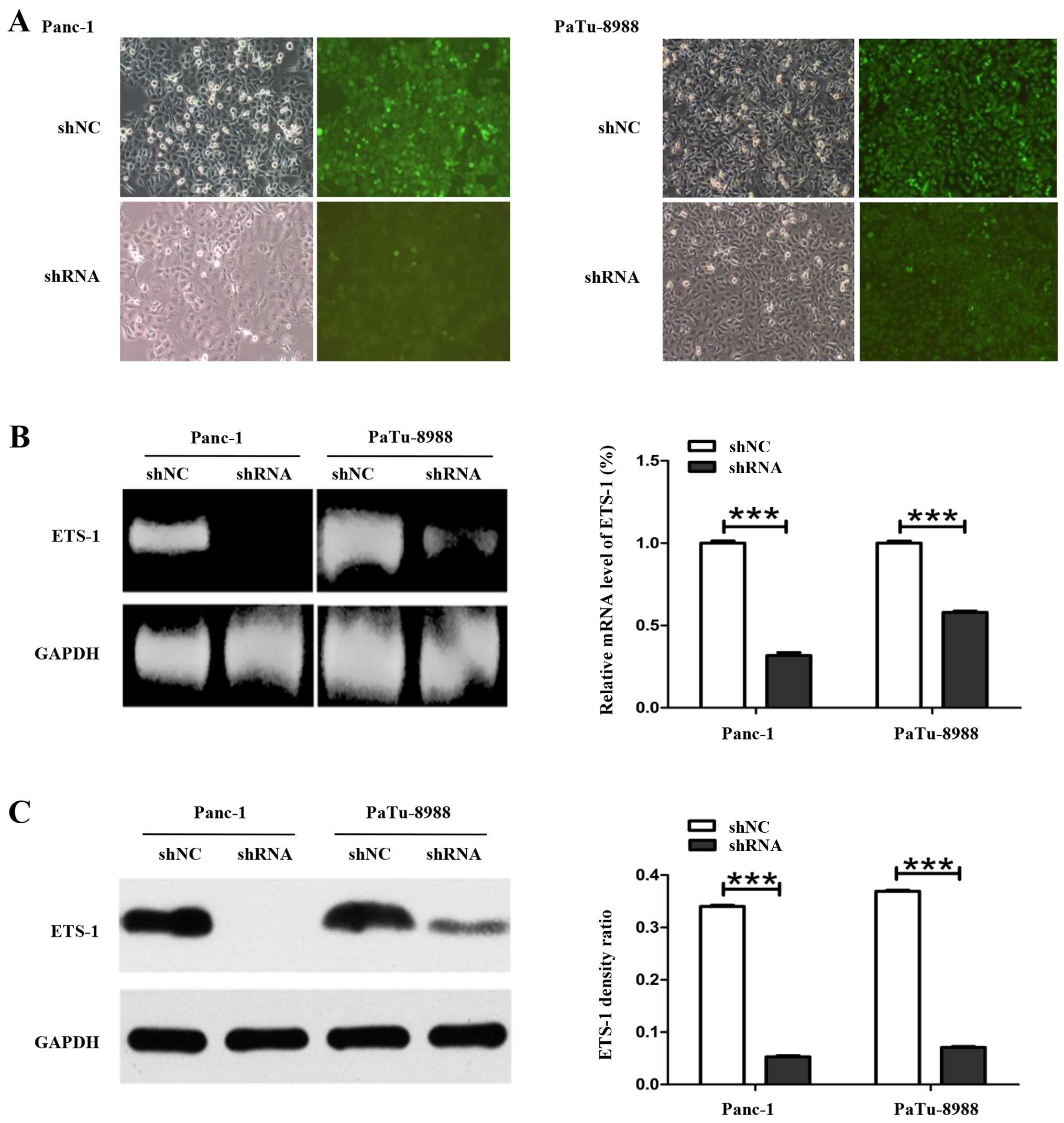
protein (GFP)-expressing adenoviral vector carrying an shRNA
targeting the ETS-1 gene. The success of the plasmid transfection
was monitored by brightfield and fluorescence images
(magnification, ×100). Our results showed the plasmid was
successfully transfected in Panc-1 and PaTu-8988 cells (Fig. 1A). The silencing efficiency of the
ETS-1 shRNA was determined using qRT-PCR and western blot analysis.
qRT-PCR results showed that mRNA expression level of ETS-1 in
Panc-1-siETS-1 and PaTu-8988-siETS-1 transfected cells was reduced
with a percentage of 68 and 52%, respectively compared with control
cells (Fig. 1B). Additionally,
western blot analysis indicated the level of ETS-1 protein was also
significantly decreased in the ETS-1-silenced cells with a
percentage of 88.50 and 80.44% for Panc-1-siETS-1 and
PaTu-8988-siETS-1 cells, respectively (Fig. 1C).
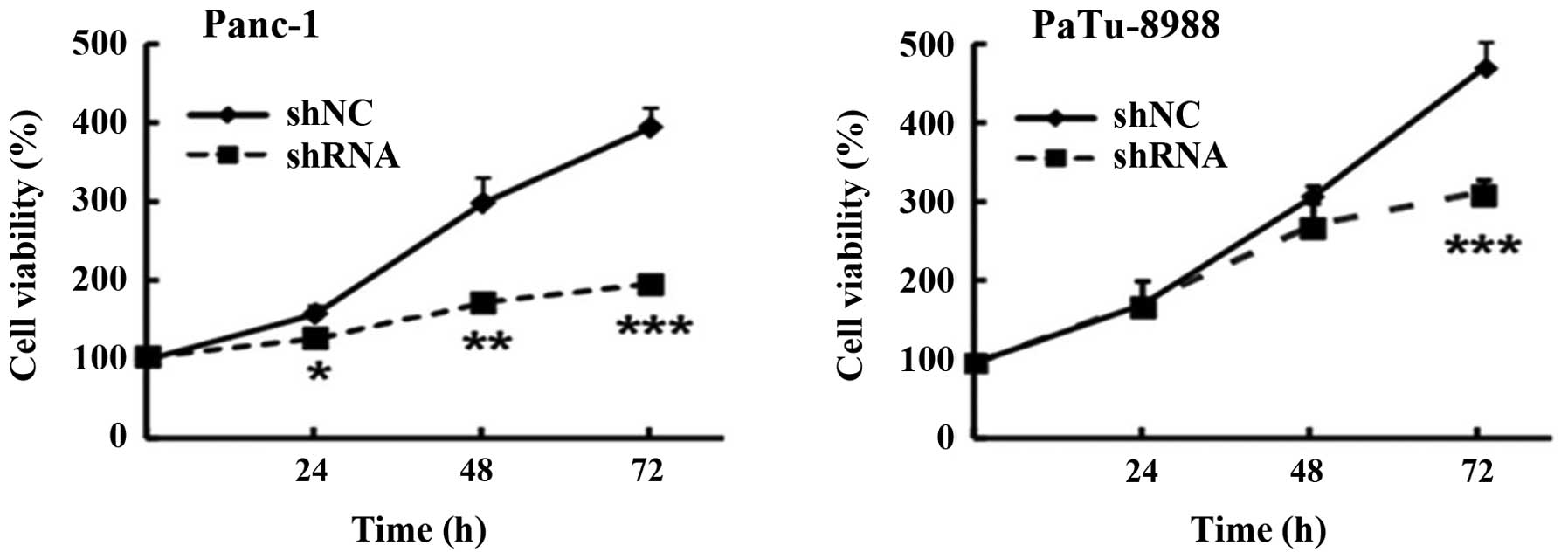
In order to assess the cell viability of pancreatic
cancer cells, MTT assay was performed, the result demonstrated that
the cell viability was significantly reduced in Panc-1 and
PaTu-8988 cells with a percentage of 200 and 180% after
transfection for 72 h, respectively (Fig. 2). Collectively these results
indicated that silencing ETS-1 by transfecting adenoviral vectors
carrying shRNA targeting the ETS-1 gene in pancreatic cancer cells
could reduce cell viability.
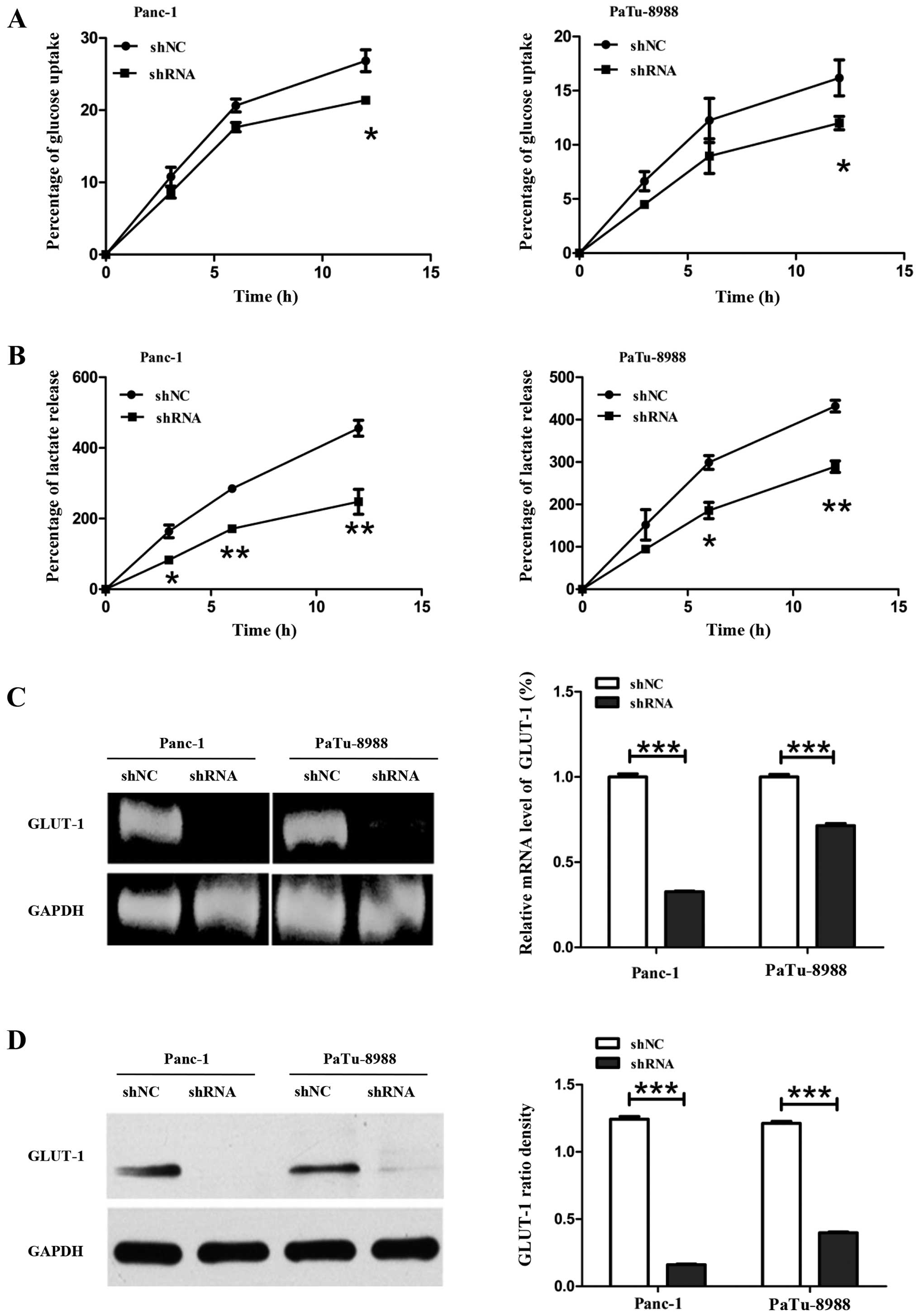
ETS-1 knockdown inhibits glucose
metabolism and activated AMPK
Pancreatic cancer cells predominantly metabolize
glucose transported by GLUT-1 to lactate to meet energy demand. In
order to detect the mechanism of inhibition the glucose metabolism
after silencing ETS-1 in pancreatic cancer cells, we measured
glucose uptake rate, lactate release rate by standard colorimetric
assay kits in Panc-1, PaTu-8988, Panc-1-siETS-1 and
PaTu-8988-siETS-1 silenced cells. We found that glucose uptake rate
was reduced in Panc-1-siETS-1 and PaTu-8988-siETS-1 cells with 5.36
and 4.17%, respectively compared with control group (Fig. 3A). Similarly, lactate releasing
rate was also reduced in Panc-1-siETS-1 and PaTu-8988-siETS-1 cells
with 131.7 and 142.69%, respectively compared with control group
(Fig. 3B).
GLUT-1 is a predominant glucose transporter in human
cells. Previous studies have documented that upregulation of GLUT-1
plays an essential role in cancer cells proliferation (46). To measure the GLUT-1 expression
level in ETS-1 silenced cells, qRT-PCR and western blot analysis
were performed. The results revealed that silencing ETS-1 inhibited
the mRNA level of glycolysis-related GLUT-1 expression with a ratio
of 67% in Panc-1-siETS-1 cells and 29% in PaTu-8988-siETS-1 cells
compared with control group (Fig.
3C). Interestingly, the GLUT-1 protein level was also reduced
with 93.65% in Panc-1-siETS-1 cells and 66.20% in PaTu-8988-siETS-1
cells compared with control cells (Fig. 3D).
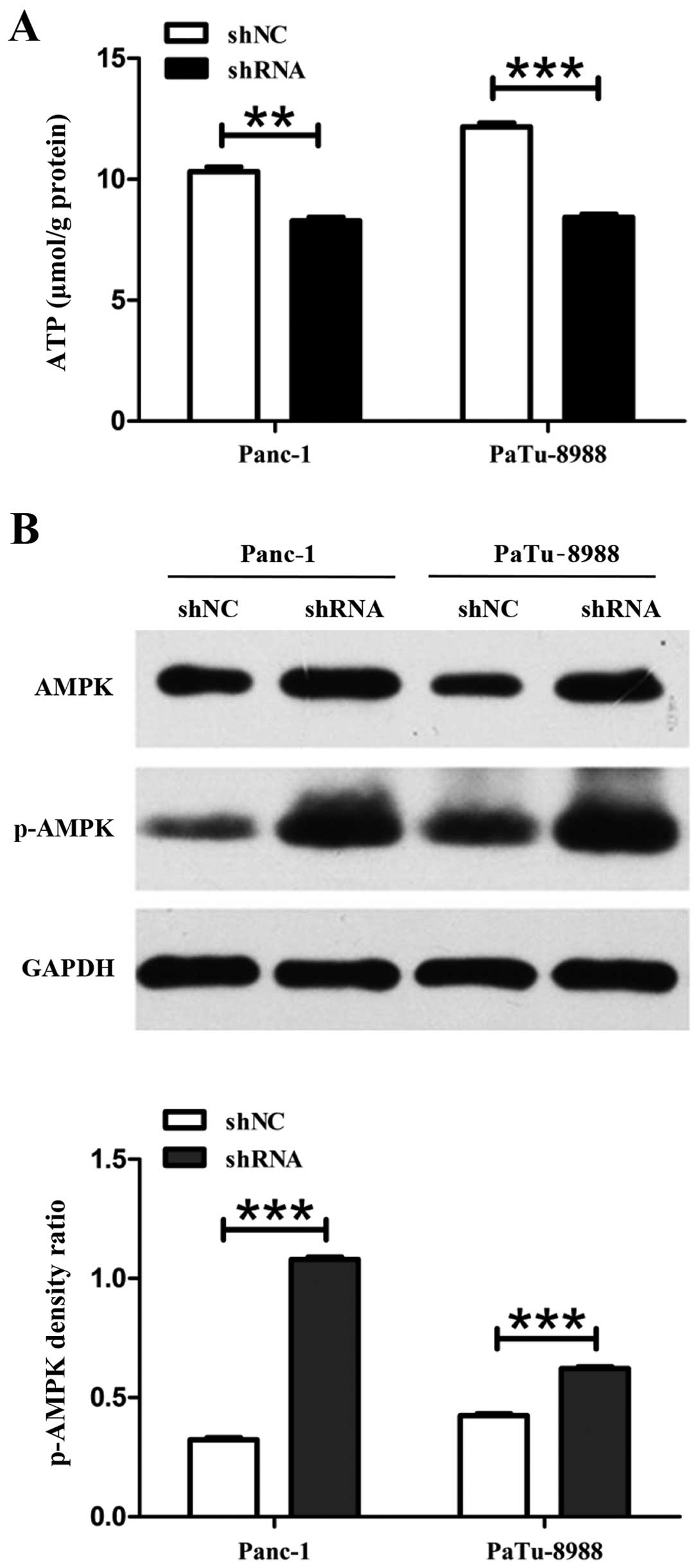
Cancer cells could utilize glucose that was mainly
transported by GLUT-1 through glycolysis to produce lactate and
yield energy in the form of ATP. The decreased production of ATP
could stimulate the process of AMPK phosphorylation. To measure
cellular ATP level, standard colorimetric assay kits were performed
in pancreatic cancer cells. The results showed that cellular ATP
formation was decreased from 10.32 µmol/g protein to 8.28
µmol/g protein and from 12.16 µmol/g protein to 8.43
µmol/g protein in Panc-1-siETS-1 and PaTu-8988-siETS-1
cells, respectively, compared to control cells (Fig. 4A). Besides, the AMPK
phosphorylation was increased 2.36- and 1.29-fold in Panc-1-siETS-1
and PaTu-8988-siETS-1 cells compared with control cells (Fig. 4B).
These findings indicated that glucose metabolism was
inhibited through decreasing the level of GLUT-1 expression after
ETS-1 knockdown followed by AMPK activation.
Transcriptional silencing of ETS-1
induces autophagy
Autophagy is an intracellular lysosomal degradation
processs (47) by which cells
capture intracellular proteins, lipids and organelles, and deliver
them to the lysosomal compartment for degradation. It has been
documented that autophagy promotes cell survival upon nutrient
depletion such as glucose starvation. Among different kinds of
autophagy-related (Atg) proteins, ATG5 and ATG7 proteins play an
essential role in the formation of preautophagosome and isolation
membrane (48,49).
In the previous sections, we demonstrated that ETS-1
interference can attenuate the cell viability and inhibit
glycolysis. In order to investigate the mechanism of the
attenuation on cell viability after silencing ETS-1, qRT-PCR and
western blot analysis were used to analyze the expression level of
autophagy-related genes in Panc-1-siETS-1, PaTu-8988-siETS-1,
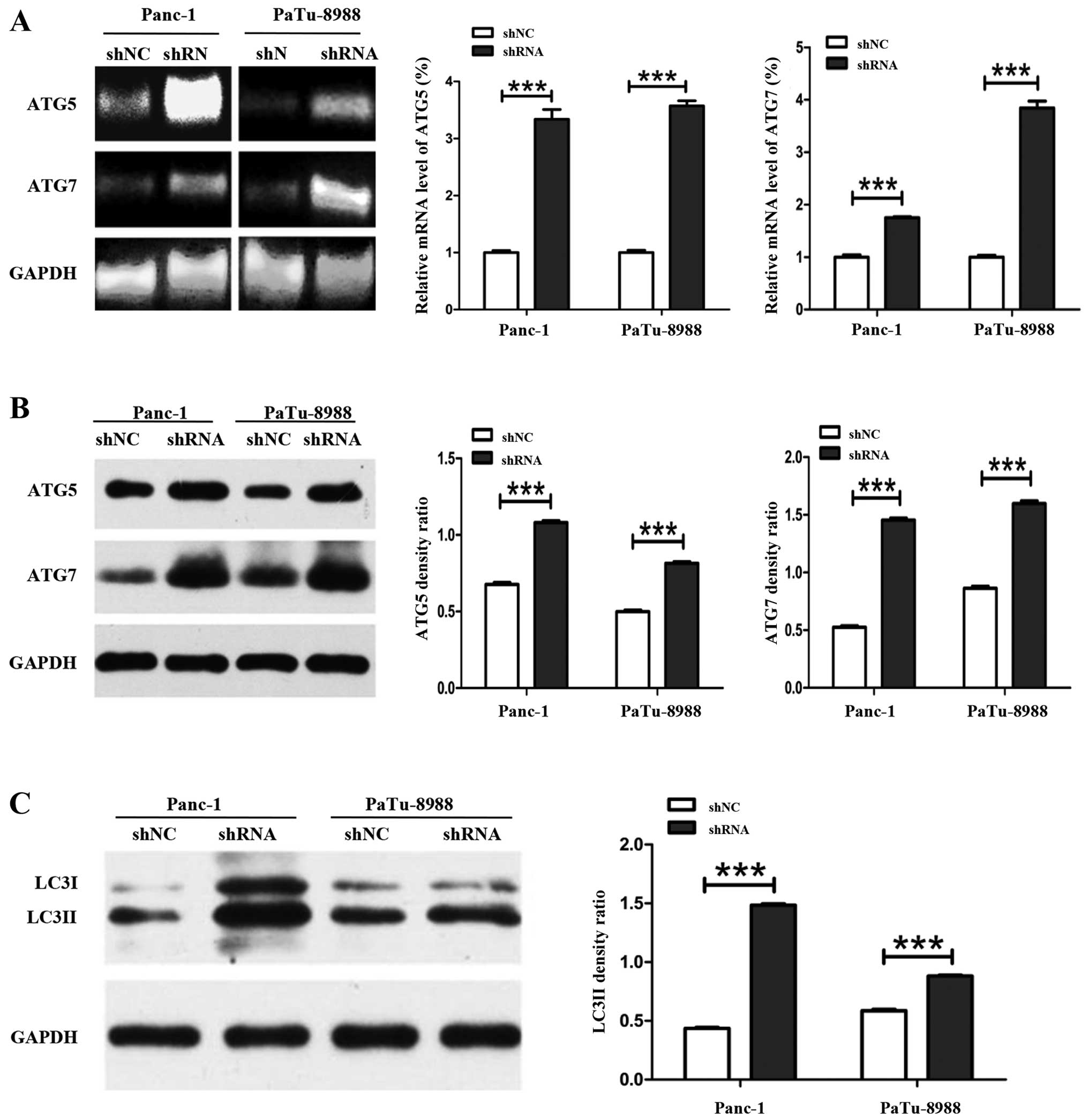
Panc-1 and PaTu-8988 cells. qRT-PCR data showed that the mRNA level
of ATG5 was increased 3.34- and 3.57-fold in Panc-1-siETS-1 and
PaTu-8988-siETS-1 cells, respectively, compared with control cells.
Similarly, the mRNA level of ATG7 was also increased 1.75- and
3.84-fold in Panc-1-siETS-1 cells and PaTu-8988-siETS-1 cells,
respectively, compared with control group (Fig. 5A). Furthermore, the ATG5 protein
expression was increased 1.58- and 1.64-fold in Panc-1-siETS-1 and
PaTu-8988T-siETS-1 cells, respectively, compared with control
cells. The protein expression of ATG7 was also increased 2.67-fold
in Panc-1-siETS-1 cells and 1.89-fold in PaTu-8988-siETS-1 cells
compared with control cells (Fig.
5B).
Microtubule-associated protein LC3 is a mammalian
homolog gene of ATG8 in yeast. LC3I combined with PE to form LC3II,
which is a marker located on the membrane of the autophagosome
during the induction of autophagy (48).
In order to verify whether the attenuation of cell
viability was caused by autophagy, we further analyzed the protein
expression of LC3II by western blot assay. The results showed that
the protein level of LC3II was increased 3.37- and 1.54-fold,
respectively, compared with internal marker in Panc-1-siETS-1 and
PaTu-8988T-siETS-1 cells (Fig.
5C).
Collectively, these results indicated that silencing
of ETS-1 can attenuate the cell viability of pancreatic cancer by
decreasing the expression of GLUT-1. The silencing of ETS-1 can
obviously decrease the expression of GLUT-1 and subsequently
interfere with glycolysis and induce autophagy.
Discussion
Pancreatic cancer remains one of the most lethal
malignancies due to its aggressiveness and its intrinsic resistance
to standard chemotherapy. This is possibly due to a low vascular
density and a prominent fibrotic stromal compartment impairing
successful drug delivery to cancer cells. Therefore, new effective
treatments are urgently needed. Previous studies have noted that
pancreatic cancer cells, even under non-hypoxic conditions,
predominantly employ cytosolic glycolysis and lactate fermentation
rather than mitochondrial oxidative phosphorylation of pyruvate for
their energy production. This metabolic pathway is called 'Warburg
effect', and was first described in the 1920s by Otto Warburg
(37). Thus, we concluded that
targeting the metabolic reprogramming in cancers could be a
promising strategy for pancreatic cancer treatment.
ETS-1, a member of ETS transcription factor family,
playing a vital role in regulating differentiation, proliferation,
apoptosis, angiogenesis, migration, metastasis and metabolism of
cancer cells. Our previous studies have demonstrated that high
expression of transcription factor ETS-1 is associated with
migration, invasion and metastasis of cancer cells, thus ETS-1
could be used as a prognostic biomarker in pancreatic cancer
(10), we also found that
overexpression of ETS-1 enhanced the dependence on glycolysis for
cellular energy production in pancreatic cancer cells. Metabolic
alterations including the increasing of glycolysis dependence are
considered as common characteristic of cancer cells to rapidly
generate energy, support the rapid cell division, uncontrolled
proliferation, and distant metastasis of tumor cells (50). In this study, we sought to identify
the potential role for ETS-1 in the regulation of glucose
metabolism and evaluate whether it could be a novel target for
metabolic therapy in pancreatic cancer. First and foremost, our
results showed that transcriptional silencing of ETS-1 attenuated
cell viability in Panc-1 and PaTu-8988 cells. This finding is
consistent with previous studies that have demonstrated blocking
major pathways such as glycolysis may be successful in reducing
cell growth or tumorigenicity. Therefore, it could be concluded
that inhibiting the reprogramming metabolism pathways could provide
a new chance for tumor treatment.
GLUT-1 is involved in aerobic glycolysis (Warburg
effect) via facilitating glucose transport to generate energy
(51,52). In this study, our results showed
that si-RNA mediated knockdown of ETS-1 obviously downregulating
the level of GLUT-1. We also found that inhibition of GLUT-1
expression significantly reduced the level of glucose uptake,
lactate secretion and intracellular ATP production. The decrease in
ATP generation level is associated with the activation of AMPK via
phosphorylation relying on liver kinase B1 (LKB1) processing
(41). AMPK is a hetero-trimeric
protein complex that plays an essential role in the regulation of
metabolic and energy homeostasis in cancer cells. Under
nutrient-abundant condition, AMPK is suppressed and mTOR is
activated, by which promotes generation of biomass that leads to
cell growth and proliferation. While under glucose-limiting
condition, AMPK, by activating p38 and by inhibiting mTOR,
regulates expression of PGC1-α, which controls mitochondrial
biogenesis in cancer cells, thereby allowing oxidative metabolism
of non-glucose carbon sources, such as glutamine, lactate and fatty
acids, to generate ATP. It is important to mention that autophagy
is promoted by AMPK via suppressing mTORC1 and activating ULK1.
Autophagy, an alternative metabolic pathway, is an important
catabolic program to recycle the energy and nutrients to sustain
the survival of cancer cells under nutrient and metabolic stress
(53). The autophagy is utilized
as a cytoprotective mechanism to rescue cells in energy-deprived
condition, while this kind of insufficient activation of autophagy
may also improve the sensation of chemotherapeutics and ionizing
radiation in tumor cells (48). In
addition, autophagy has been reported as a major mechanism to
induce cancer cell death called type II programmed cell death in
the absence of apoptosis which is blocked or defective in certain
tumor cells (54). In this study
we found that LC3II, the marker of autophagy, increased in si-ETS-1
cells compared with control groups. Importantly we found that
autophagy-related proteins ATG5/7, was obviously increased at mRNA
and protein levels. These results indicated that ETS-1 can reduce
the expression of GLUT-1 to interfere with the energy production of
cancer cells and activate AMPK to prompt autophagy.
These findings suggest that the silence of ETS-1 in
pancreatic cancer cells disturb the expression of GLUT-1 to reduce
the absorption of glucose and production of lactate to impair the
glycolysis through alteration of 'Warburg effect', resulting in
AMPK activation, autophagy induction and reduction of cell
viability.
An important implication of these findings is that
ETS-1 plays functionally significant roles in attenuating
pancreatic cancer cell viability by decreasing the expression of
GLUT-1 to interfere glycolysis and induce autophagy. In our future
research, we aim to combine the strategy of inhibiting glycolysis
with different modulators of autophagy and detect their efficacy in
clinical application.
Abbreviations:
|
ETS-1
|
v-ets erythroblastosis virus E26
oncogene homolog-1
|
|
GLUT-1
|
glucose transporter-1
|
|
AMPK
|
AMP-activated protein kinase
|
|
ATG5
|
autophagy-related gene 5
|
|
ATG7
|
autophagy-related gene 7
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
Acknowledgments
This study was supported financially by the National
Nature Science Foundation of People's Republic of China
(81271699).
References
|
1
|
Perera RM and Bardeesy N: Pancreatic
cancer metabolism: Breaking it down to build it back up. Cancer
Discov. 5:1247–1261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xie D and Xie K: Pancreatic cancer stromal
biology and therapy. Genes Dis. 2:133–143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cid-Arregui A and Juarez V: Perspectives
in the treatment of pancreatic adenocarcinoma. World J
Gastroenterol. 21:9297–9316. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guillaumond F, Iovanna JL and Vasseur S:
Pancreatic tumor cell metabolism: Focus on glycolysis and its
connected metabolic pathways. Arch Biochem Biophys. 545:69–73.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paniccia A, Merkow J, Edil BH and Zhu Y:
Immunotherapy for pancreatic ductal adenocarcinoma: An overview of
clinical trials. Chin J Cancer Res. 27:376–391. 2015.PubMed/NCBI
|
|
6
|
Tennant DA, Durán RV and Gottlieb E:
Targeting metabolic transformation for cancer therapy. Nat Rev
Cancer. 10:267–277. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee M and Yoon JH: Metabolic interplay
between glycolysis and mitochondrial oxidation: The reverse Warburg
effect and its therapeutic implication. World J Biol Chem.
6:148–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou W, Capello M, Fredolini C, Piemonti
L, Liotta LA, Novelli F and Petricoin EF: Proteomic analysis of
pancreatic ductal adenocarcinoma cells reveals metabolic
alterations. J Proteome Res. 10:1944–1952. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lunt SY and Vander Heiden MG: Aerobic
glycolysis: Meeting the metabolic requirements of cell
proliferation. Annu Rev Cell Dev Biol. 27:441–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li C, Wang Z, Chen Y, Zhou M, Zhang H,
Chen R, Shi F, Wang C and Rui Z: Transcriptional silencing of ETS-1
abrogates epithelial-mesenchymal transition resulting in reduced
motility of pancreatic cancer cells. Oncol Rep. 33:559–565.
2015.
|
|
11
|
Verschoor ML, Verschoor CP and Singh G:
Ets-1 global gene expression profile reveals associations with
metabolism and oxidative stress in ovarian and breast cancers.
Cancer Metab. 1:172013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu M, Li M, Zhang F, Feng F, Chen W, Yang
Y, Cui J, Zhang D and Linghu E: FBI-1 enhances ETS-1 signaling
activity and promotes proliferation of human colorectal carcinoma
cells. PLoS One. 9:e980412014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shaikhibrahim Z and Wernert N: ETS
transcription factors and prostate cancer: The role of the family
prototype ETS-1 (Review). Int J Oncol. 40:1748–1754.
2012.PubMed/NCBI
|
|
14
|
Lelièvre E, Lionneton F, Soncin F and
Vandenbunder B: The Ets family contains transcriptional activators
and repressors involved in angiogenesis. Int J Biochem Cell Biol.
33:391–407. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oikawa T: ETS transcription factors:
Possible targets for cancer therapy. Cancer Sci. 95:626–633. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hahne JC, Okuducu AF, Kaminski A, Florin
A, Soncin F and Wernert N: Ets-1 expression promotes epithelial
cell transformation by inducing migration, invasion and
anchorage-independent growth. Oncogene. 24:5384–5388. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shaikhibrahim Z, Langer B, Lindstrot A,
Florin A, Bosserhoff A, Buettner R and Wernert N: Ets-1 is
implicated in the regulation of androgen co-regulator FHL2 and
reveals specificity for migration, but not invasion, of PC3
prostate cancer cells. Oncol Rep. 25:1125–1129. 2011.PubMed/NCBI
|
|
18
|
Gao H, Peng C, Liang B, Shahbaz M, Liu S,
Wang B, Sun Q, Niu Z, Niu W, Liu E, et al: β6 integrin induces the
expression of metalloproteinase-3 and metalloproteinase-9 in colon
cancer cells via ERK-ETS1 pathway. Cancer Lett. 354:427–437. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bu S, Yamanaka M, Pei H, Bielawska A,
Bielawski J, Hannun YA, Obeid L and Trojanowska M:
Dihydrosphingosine 1-phosphate stimulates MMP1 gene expression via
activation of ERK1/2-Ets1 pathway in human fibroblasts. FASEB J.
20:184–186. 2006.
|
|
20
|
Li T and Jiang S: Effect of bFGF on
invasion of ovarian cancer cells through the regulation of Ets-1
and urokinase-type plasminogen activator. Pharm Biol. 48:161–165.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang Y, Xu W, Lu J, He F and Yang X:
Invasiveness of hepatocellular carcinoma cell lines: Contribution
of hepatocyte growth factor, c-met, and transcription factor Ets-1.
Biochem Biophys Res Commun. 286:1123–1130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tomita N, Morishita R, Taniyama Y, Koike
H, Aoki M, Shimizu H, Matsumoto K, Nakamura T, Kaneda Y and Ogihara
T: Angiogenic property of hepatocyte growth factor is dependent on
upregulation of essential transcription factor for angiogenesis,
ets-1. Circulation. 107:1411–1417. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kato T, Fujita Y, Nakane K, Kojima T,
Nozawa Y, Deguchi T and Ito M: ETS1 promotes chemoresistance and
invasion of paclitaxel-resistant, hormone-refractory PC3 prostate
cancer cells by upregulating MDR1 and MMP9 expression. Biochem
Biophys Res Commun. 417:966–971. 2012. View Article : Google Scholar
|
|
24
|
Verschoor ML, Wilson LA, Verschoor CP and
Singh G: Ets-1 regulates energy metabolism in cancer cells. PLoS
One. 5:e135652010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sato T and Miwa A: Ets-1 and integrin
beta3 for lung metastasis from colorectal cancer. APMIS.
110:347–353. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sasaki H, Yukiue H, Moiriyama S, Kobayashi
Y, Nakashima Y, Kaji M, Kiriyama M, Fukai I, Yamakawa Y and Fujii
Y: Clinical significance of matrix metalloproteinase-7 and Ets-1
gene expression in patients with lung cancer. J Surg Res.
101:242–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verschoor ML and Singh G: Ets-1 regulates
intracellular glutathione levels: Key target for resistant ovarian
cancer. Mol Cancer. 12:1382013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li W, Zang W, Liu P, Wang Y, Du Y, Chen X,
Deng M, Sun W, Wang L, Zhao G, et al: MicroRNA-124 inhibits
cellular proliferation and invasion by targeting Ets-1 in breast
cancer. Tumour Biol. 35:10897–10904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang N, Wang X, Huang T, Wu Y and Chen Y:
Role of Ets-1 in fibronectin-derived heparin-binding domain
polypeptides alleviating melanoma cell invasiveness and
chemoresistance. Exp Dermatol. 23:512–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Span PN, Manders P, Heuvel JJ, Thomas CM,
Bosch RR, Beex LV and Sweep CG: Expression of the transcription
factor Ets-1 is an independent prognostic marker for relapse-free
survival in breast cancer. Oncogene. 21:8506–8509. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Puzovic V, Brcic I, Ranogajec I and
Jakic-Razumovic J: Prognostic values of ETS-1, MMP-2 and MMP-9
expression and co-expression in breast cancer patients. Neoplasma.
61:439–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Davidson B, Reich R, Goldberg I, Gotlieb
WH, Kopolovic J, Berner A, Ben-Baruch G, Bryne M and Nesland JM:
Ets-1 messenger RNA expression is a novel marker of poor survival
in ovarian carcinoma. Clin Cancer Res. 7:551–557. 2001.PubMed/NCBI
|
|
33
|
Davidson B, Risberg B, Goldberg I, Nesland
JM, Berner A, Tropé CG, Kristensen GB, Bryne M and Reich R: Ets-1
mRNA expression in effusions of serous ovarian carcinoma patients
is a marker of poor outcome. Am J Surg Pathol. 25:1493–1500. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takai N, Miyazaki T, Nishida M, Nasu K and
Miyakawa I: c-Ets1 is a promising marker in epithelial ovarian
cancer. Int J Mol Med. 9:287–292. 2002.PubMed/NCBI
|
|
35
|
Fujimoto J, Aoki I, Toyoki H, Khatun S and
Tamaya T: Clinical implications of expression of ETS-1 related to
angiogenesis in uterine cervical cancers. Ann Oncol. 13:1598–1604.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kimmelman AC: Metabolic dependencies in
RAS-driven cancers. Clin Cancer Res. 21:1828–1834. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cairns RA: Drivers of the Warburg
phenotype. Cancer J. 21:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mogi A, Koga K, Aoki M, Hamasaki M, Uesugi
N, Iwasaki A, Shirakusa T, Tamura K and Nabeshima K: Expression and
role of GLUT-1, MCT-1, and MCT-4 in malignant pleural mesothelioma.
Virchows Arch. 462:83–93. 2013. View Article : Google Scholar
|
|
39
|
Blum R and Kloog Y: Metabolism addiction
in pancreatic cancer. Cell Death Dis. 5:e10652014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Granja S, Pinheiro C, Reis RM, Martinho O
and Baltazar F: Glucose addiction in cancer therapy: Advances and
drawbacks. Curr Drug Metab. 16:221–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li N, Huang D, Lu N and Luo L: Role of the
LKB1/AMPK pathway in tumor invasion and metastasis of cancer cells
(Review). Oncol Rep. 34:2821–2826. 2015.PubMed/NCBI
|
|
42
|
Kim N, Jeong S, Jing K, Shin S, Kim S, Heo
JY, Kweon GR, Park SK, Wu T, Park JI, et al: Docosahexaenoic acid
induces cell death in human non-small cell lung cancer cells by
repressing mTOR via AMPK activation and PI3K/Akt inhibition. BioMed
Res Int. 2015:2397642015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saito Y, Chapple RH, Lin A, Kitano A and
Nakada D: AMPK protects leukemia-initiating cells in myeloid
leukemias from metabolic stress in the bone marrow. Cell Stem Cell.
17:585–596. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Abdou AG, Eldien MM and Elsakka D: GLUT-1
Expression in cutaneous basal and squamous cell carcinomas. Int J
Surg Pathol. 23:447–453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vuppalapati KK, Bouderlique T, Newton PT,
Kaminskyy VO, Wehtje H, Ohlsson C, Zhivotovsky B and Chagin AS:
Targeted deletion of autophagy genes Atg5 or Atg7 in the
chondrocytes promotes caspase-dependent cell death and leads to
mild growth retardation. J Bone Miner Res. 30:2249–2261. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lao Y and Xu N: Autophagy in cancer
chemoprevention: Identification of novel autophagy modulators with
anticancer potential. Methods Mol Biol. 1379:151–163. 2016.
View Article : Google Scholar
|
|
49
|
Shimizu S, Arakawa S and Nishida Y:
Autophagy takes an alternative pathway. Autophagy. 6:290–291. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang TB, Zhao Y, Tong ZX and Guan YF:
Inhibition of glucose-transporter 1 (GLUT-1) expression reversed
Warburg effect in gastric cancer cell MKN45. Int J Clin Exp Med.
8:2423–2428. 2015.PubMed/NCBI
|
|
52
|
Yoon SO, Jeon TJ, Park JS, Ryu YH, Lee JH,
Yoo JS, Kim JK, Yoon DS and Oh EJ: Analysis of the roles of glucose
transporter 1 and hexokinase 2 in the metabolism of glucose by
extrahepatic bile duct cancer cells. Clin Nucl Med. 40:e178–e182.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kim SE, Park HJ, Jeong HK, Kim MJ, Kim M,
Bae ON and Baek SH: Autophagy sustains the survival of human
pancreatic cancer PANC-1 cells under extreme nutrient deprivation
conditions. Biochem Biophys Res Commun. 463:205–210. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu B, Cheng Y, Liu Q, Bao JK and Yang JM:
Autophagic pathways as new targets for cancer drug development.
Acta Pharmacol Sin. 31:1154–1164. 2010. View Article : Google Scholar : PubMed/NCBI
|