Introduction
The programmed-death 1 receptor (PD-1,
CD279)/programmed-death ligand 1 (PD-L1, B7-H1, CD274) pathway has
emerged as a critical inhibitory pathway that regulates T cell
responses and maintains immune suppression (1–4).
Oral squamous cell carcinoma (OSCC) is a malignant tumour found
most often in the head and neck region (5,6) that
is highly immunosuppressive in general, and PD-L1 expression has
been proposed as a potential mechanism facilitating this phenotype
(7–9). PD-L1 is expressed in many tumours in
a constitutive and interferon-γ (IFN-γ)-inducible manner. PD-L1
binds to an inhibitory receptor (PD-1), which is a member of the B7
family of receptors (4), and to
the costimulatory molecule CD80 (B7-1) (10). PD-1 is expressed on activated T
cells, and ligation of PD-1 by tumour-associated PD-L1 induces
apoptosis or downregulation of effector cytotoxic T lymphocytes
(CTL), resulting in an escape from T-cell mediated immune
surveillance (11). Blockade of
the PD-L1/PD-1 pathway efficiently reduces tumour growth and
improves survival (7,12–15).
Durable tumour regression with blockade of the PD-1/PD-L1
checkpoint has been demonstrated in recent clinical studies
(1,2), leading to the recent registration of
an anti-PD-1 antibody for advanced melanoma and non-small cell lung
cancer. In addition, the clinical efficacy of an anti-PD-1
monoclonal antibody in OSCC has been observed in phase I studies
(16). In general, the response to
PD-1 blockade has been commonly correlated with PD-L1 expression in
treated tumours (1).
Although anti-PD-1 treatment can produce durable
responses, it appears to benefit only a subset of patients. Chen
et al recently demonstrated a molecular link between the
epithelial-mesenchymal transition (EMT) and intratumoural
CD8+ T cell suppression through PD-L1 regulation, in
both animal models and human cell lines (17). EMT is a key process that drives
cancer metastasis and drug resistance, and has been associated with
poor prognosis in multiple cancers, including OSCC (18–20).
For instance, positive staining for the mesenchymal marker vimentin
occurs in specimens from non-small cell lung cancer (NSCLC)
patients who develop resistance to epidermal growth factor receptor
(EGFR) inhibitors, suggesting that EMT has been triggered in such
tumours (21–23). Previously, we found that loss of
EGFR expression in OSCC was associated with EMT and might have
functional implications in the resistance to cetuximab treatment
(24). However, the impact of EMT
on reprogramming the tumour immune microenvironment is largely
unknown.
To predict the efficacy and optimize anti-PD-1
therapy, alone or in combination, it is important to understand the
mechanisms controlling PD-L1 expression. In this study, we focused
on the regulation of PD-L1 expression in OSCC, and the mechanism of
regulation of PD-L1 expression in the tumour microenvironment.
Materials and methods
Cell culture
Three human OSCC cell lines established from tumour
biopsies with different grades of invasive abilities were used,
including OSC-20 cells (low-grade invasive cells), OSC-19 cells
(low-grade invasive cells), and TSU cells (high-grade invasive
cells). The OSC-20 cell line was derived from a 58-year-old female
with tongue cancer metastatic to the cervical lymph nodes (25). OSC-19 was derived from a
61-year-old male with tongue cancer metastatic to the cervical
lymph nodes (26). The TSU cell
line was established from a patient with gingival squamous cell
carcinoma who had developed marked leukocytosis (27). In addition, normal human gingival
fibroblasts (HGFs; ATCC no. CRL-2014) obtained from the American
Type Culture Collection (Manassas, VA, USA) served as a control.
Macrophages and dendritic cells (DCs) were generated from human
peripheral blood mononuclear cells (PBMCs), as described previously
(28,29). PBMCs were obtained by venepuncture
into an 8-ml Vacutainer CPT Cell-Preparation Tube (BD Vacutainer
Systems, Franklin Lakes, NJ, USA). Briefly, monocyte-derived
macrophages were generated by incubating monocytes
(1×106/ml) in RPMI-1640 medium containing 10% fetal
bovine serum (FBS), 2 mM glutamine, 25 mM HEPES, 100 U/ml
penicillin, 100 μg/ml streptomycin, and 50 ng/ml GM-CSF
(PeproTech, Rocky Hill, NJ, USA) at 37°C in a CO2
incubator for 7 days. Induced macrophages were examined using an
anti-macrophage antibody (MAC387; Abcam, Tokyo, Japan).
Monocyte-derived DCs were generated by incubating monocytes at
1×106 cells/ml in G4 medium (G4 Dendritic Cell
Generation kit; Humanzyme, IL, USA) at 37°C in a CO2
(5%) incubator for 7 days. Induced DCs were examined using an
anti-DC antibody (CD83; Abcam). Carcinoma-associated fibroblasts
(CAFs) were generated by the addition of recombinant TGF-β1 to
confluent growth-arrested cell monolayers, as previously described
(30,31). Recombinant TGF-β1 (R&D Systems
Europe Ltd., Abingdon, UK; 50 ng/ml) was prepared in serum-free
culture medium. CAFs were examined using anti-αSMA (R&D Systems
Europe Ltd.) antibodies. Culture inserts (1-μm pore size)
were purchased from Corning Japan (Tokyo, Japan). MyD88
homodimerization-inhibitor peptide and toll-like receptor 4
(TLR4)-inhibitory peptide were purchased from Imgenex (San Diego,
CA, USA) and Novus Biologicals (Littleton, CO, USA), respectively,
and used in the cell-blocking study.
Tissue samples
Experiments using human tissues were approved by the
ethics committee of the Kanazawa University Graduate School of
Medical Science (IRB no. 2014-004, 352-2), and written informed
consent was obtained from patients providing tissue specimens. The
subjects included 24 patients with primary OSCC who underwent
surgical resection at the Department of Oral and Maxillofacial
Surgery at Kanazawa University Hospital between 2000 and 2008. The
patients with carcinoma were aged from 43 to 89 years (64.4±11.2
years, mean ± SD). The grade of tumour differentiation was
determined according to the criteria proposed by the World Health
Organization (32). The mode of
tumour invasion was assessed according to the classification by
Yamamoto et al (33).
RNA extraction, cDNA synthesis, and
quantitative real-time PCR (qPCR)
The mRNA expression levels of PD-L1, PD-L2,
E-cadherin, N-cadherin, Vimentin, and Snail1 were analysed using a
Rotor-Gene Q 2plex System (Qiagen, Hilden, Germany) with
FAM/ZEN/IBFQ probes (Integrated DNA Technologies, Inc., Coralville,
IA, USA; DNA sequences not opened). Total RNA was extracted using
the RNeasy Protect Mini kit (Qiagen), and cDNA was obtained using
the PrimeScript first-strand cDNA Synthesis kit (Takara, Tokyo,
Japan). All reactions were performed according to the
manufacturer's instructions. We amplified 18S rRNA as an internal
standard using HEX/ZEN/IBFQ probes (Integrated DNA Technologies,
Inc.; DNA sequences not opened). Relative expression levels were
calculated using the ΔΔCt method for qPCR (34), which presents the data as
fold-differences in expression level relative to a calibrator
sample; in this case, the mean expression of 3 experimental
measurements of 18S rRNA in control cells or vehicle-treated
cells.
Western blot analysis
The cultured cells were lysed with Pierce RIPA
buffer (Thermo Scientific, Waltham, MA, USA). Lysates mixed with
sample buffer were electrophoretically separated and transferred
onto membranes. Membranes were blocked with Blocking One (Nacalai
Tesque, Kyoto, Japan), followed by incubations with an anti-PD-L1,
E-cadherin, N-cadherin, Vimentin, or Snail1 antibody (Abcam) and an
anti-human β-actin antibody (Cell Signaling Technology, Tokyo,
Japan). After washing with Tris-buffered saline (TBS) with 0.05%
Tween, membranes were incubated with a horseradish
peroxidase-conjugated anti-mouse IgG. After washing with TBS-0.05%
Tween, membranes were incubated with the ECL Prime Western Blotting
Detection reagent (GE Healthcare, Little Chalfont, UK). Signals
were detected and analysed using C-DiGit (M&S TechnoSystems,
Tokyo, Japan).
EMT induction
OSC-20 cells were seeded at 70% confluence and
cultured for 48 and 72 h in Dulbecco's modified Eagle's medium
(Sigma-Aldrich Japan, Tokyo, Japan) with 0.5% FBS (Hyclone, Logan,
UT, USA) to induce EMT. Recombinant human TGF-β1 (R&D Systems,
Minneapolis, MN, USA) was added to a final concentration of 5
ng/ml.
Immunohistochemistry
Each specimen was fixed in 10% buffered formalin and
then embedded in paraffin to prepare serial sections.
Immunohistochemical staining was performed using antibodies against
PD-L1, Snail, Vimentin, CD83, macrophages (MAC387), αSMA, and
fibroblast activation protein (FAP) (Abcam). Primary antibodies
were detected using EnVision Single reagents (Dako Japan, Tokyo,
Japan), Alexa Fluor 488-labeled goat anti-mouse IgG (Cell Signaling
Technology), and Alexa Fluor 546-labeled goat anti-rabbit IgG
(Thermo Fisher Scientific, Rockford, IL, USA). Staining specificity
was confirmed using the Universal Negative Control for IS-Series
Rabbit Primary Antibodies reagent (IS600; Dako Japan) as a negative
control, instead of the matching primary antibody. After mounting
with ProLong Gold Antifade reagent (Thermo Fisher Scientific,
Tokyo, Japan), the tissues were subjected to fluorescence
microscopy. Three regions were image-captured under a light
microscope at ×100 magnification, and the images were evaluated
based on number of stained tumour cells, following the method
described by Kimura et al (24). PD-L1 expression was evaluated based
on the extent of immunolabelling in tumour cell membranes and was
classified on a four-point scale: 0 (no labelling, or labelling in
<10% of tumour cells); 1 (weak labelling, homogeneous or patchy,
in >10% of tumour cells); 2 (moderate labelling, homogeneous or
patchy, in >10% of tumour cells); 3 (intense labelling,
homogeneous or patchy, in >10% of tumour cells). These scores
were subsequently grouped into 2 categories: low expression (0 or
1) and high expression (2 or 3).
Statistical analysis
Statistical analyses were conducted using JMP 12.0
software (SAS Institute, Inc., Cary, NC, USA). The qPCR data are
presented as the mean ± standard error of the mean (SEM).
Differences between groups were tested for statistical significance
using the 2-tailed Mann-Whitney U test. Differences were considered
significant at P<0.05.
Results
PD-L1 and PD-L2 expression in 3 different
grades of invasive OSCC cell lines
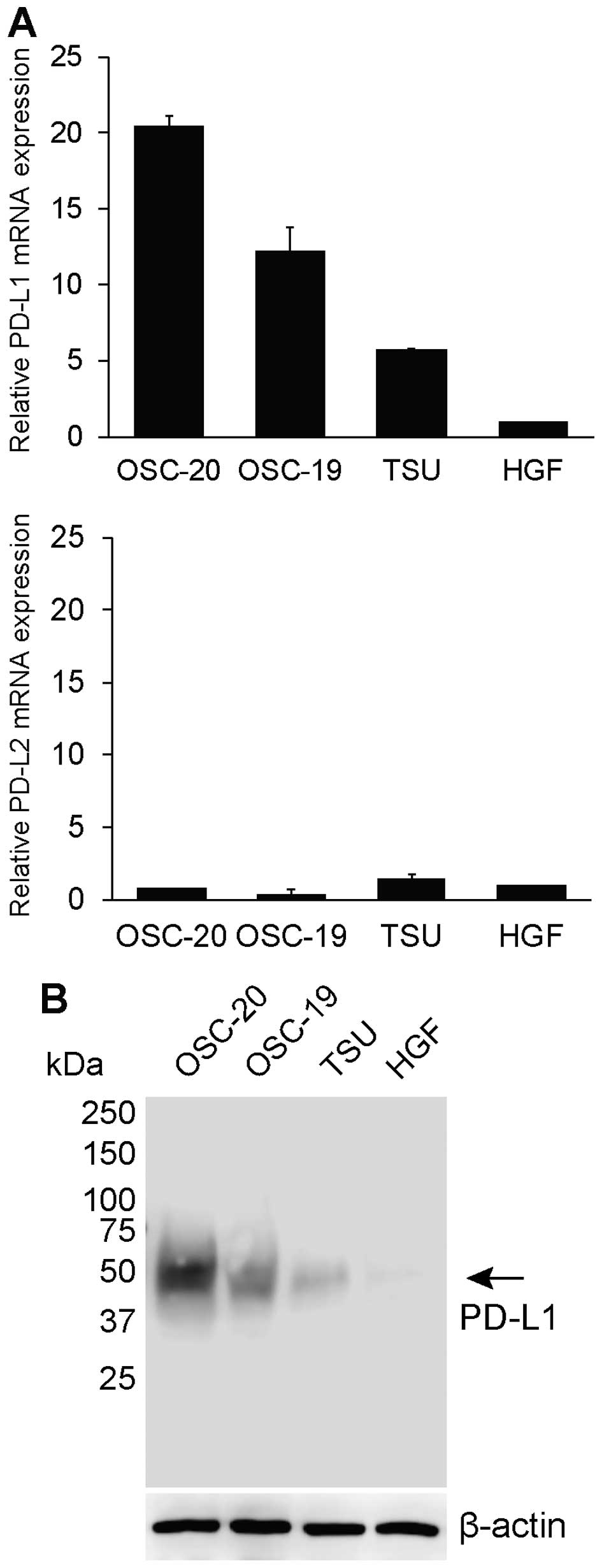
PD-L1 and PD-L2 mRNA expression on 3 human OSCC
lines with different grades of invasiveness (OSC-19, OSC-20, and
TSU) were analysed. The qPCR data are presented as fold-differences
relative to the mean values from 3 experiments with HGF cells
(Fig. 1A). Among the 3 OSCC cell
lines, the PD-L1 mRNA expression level ranged from 5.73 to
20.48-fold higher than in HGF cells (Fig. 1A), while PD-L2 mRNA expression
ranged from 0.38 to 1.48-fold higher than in HGF cells (Fig. 1A). In particular, the expression of
PD-L1 mRNA in low-grade invasive cells (OSC-20 cells) was highest
among the 3 OSCC cell lines. Upregulation of PD-L1 mRNA in
high-grade invasive cells (TSU cells) was not as remarkable
compared to that in the other 2 low-grade invasive cells (OSC-20
and OSC-19 cells). PD-L2 expression on the 3 different invasive
human OSCC lines was much lower, and differences were negligible
compared to that of PD-L1. We further analysed PD-L1 expression at
the protein level. Both low-grade invasive cells (OSC-20 and
OSC-19) had a PD-L1 protein expression level higher than that of
TSU cells. HGF cells exhibited negligible PD-L1 protein expression
(Fig. 1B).
The relationship between EMT and PD-L1
expression in human OSCC cell lines
To evaluate whether PD-L1 expression is associated
with EMT features, we analysed the mRNA- and protein-expression
levels of E-cadherin, N-cadherin, vimentin, and Snail1 in the human
OSC-19, OSC-20, and TSU lines with different degrees of
invasiveness. The mRNA expression levels of these EMT-associated
genes in OSC-19 and TSU cells are presented as fold-differences
relative to those in OSC-20 cells. TSU cells displayed a
mesenchymal phenotype manifested by the loss of E-cadherin and
acquisition of N-cadherin, vimentin, and Snail compared to the
corresponding expression levels in OSC-20 and OSC-19 cells
(Fig. 2A and B). OSC-20 cells
(epithelial phenotype) were exposed to TGF-β1 to determine whether
such exposure could change the mRNA- and protein-expression levels
of EMT-associated genes and PD-L1 (Fig. 2C and D). The mRNA-expression levels
are presented as fold-differences relative to that of vehicle
control-treated cells. Serial examination of EMT markers (loss of
E-cadherin and upregulation of N-cadherin, vimentin, and Snail) at
48 and 72 h showed that TGF-β1 treatment resulted in EMT in OSC-20
cells (Fig. 2C). We further
confirmed E-cadherin downregulation and N-cadherin, vimentin, and
Snail upregulation at the protein level at 72 h after TGF-β1
treatment (Fig. 2D). Strikingly,
the total PD-L1 mRNA and protein levels were reduced in OSC-20
cells after EMT induction by TGF-β1 treatment.
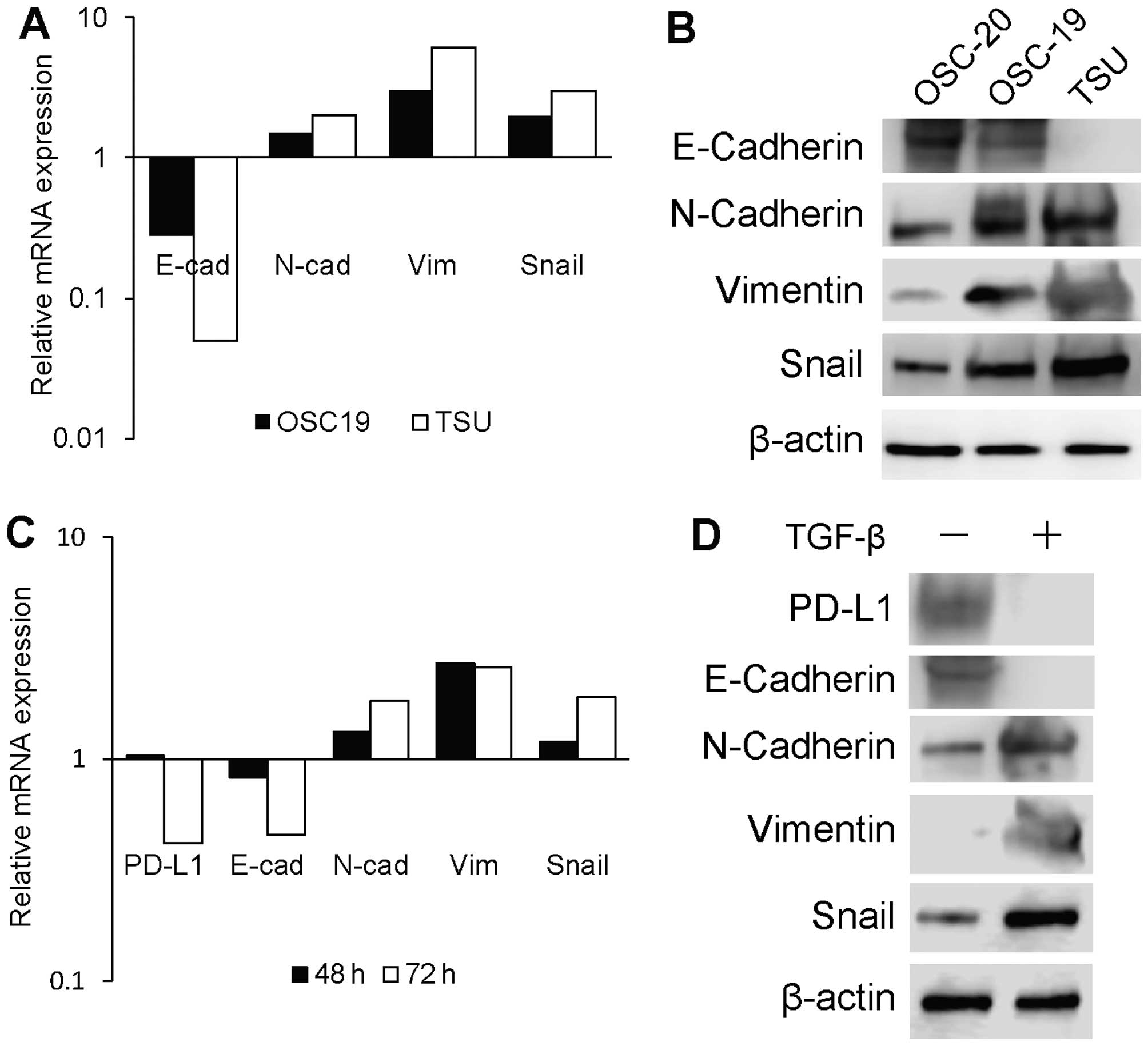 | Figure 2Relationship between EMT and PD-L1
expression on human OSCC cell lines. (A) Relative mRNA expression
levels of the EMT-associated genes E-cadherin, N-cadherin,
vimentin, and Snail, in OSC-20, OSC-19, and TSU cell lines.
Expression differences in OSC-19 and TSU cells are displayed as
fold-changes relative to the expression levels in OSC-20 cells. (B)
Expression of the EMT-associated protein in OSC-20, OSC-19, and TSU
cells was assessed by immunoblot analysis. β-actin was detected as
a loading control. (C) Relative mRNA expression levels of PD-L1 and
the EMT-associated genes E-cadherin, N-cadherin, vimentin, and
Snail in TGF-β-treated OSC-20 cells. OSC-20 cells were treated with
human TGF-β or a vehicle control for 48 and 72 h. Expression
differences are displayed as fold-changes relative to the control
vehicle at 48 and 72 h. (D) Expression of the EMT-associated
protein in TGF-β-treated OSC-20, OSC-19, and TSU cells was assessed
by immunoblot analysis. β-actin was detected as a loading
control. |
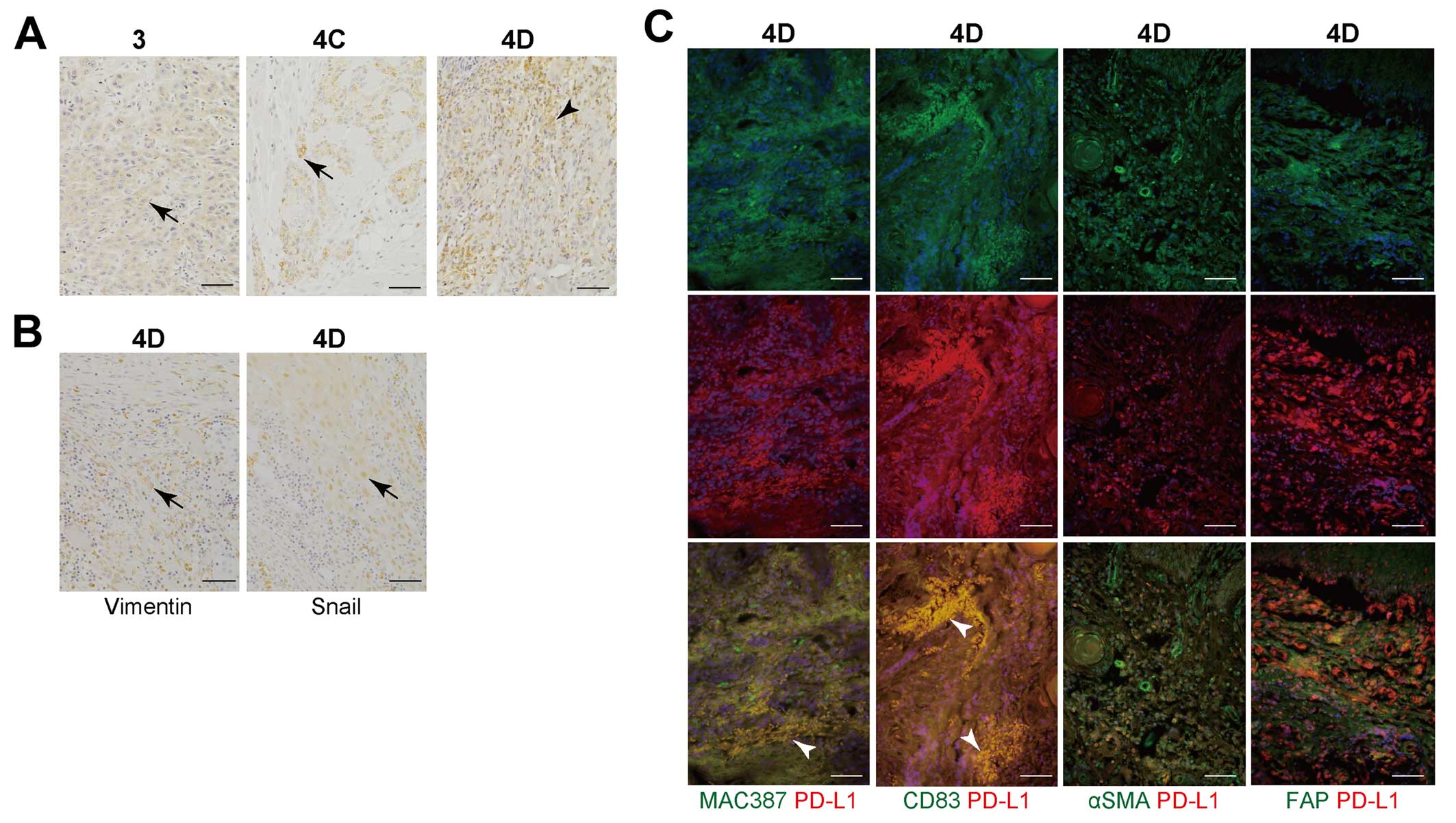
Immunohistochemical analysis of human
OSCC tissues
To confirm downregulation of PD-L1 on high-grade
invasive cells compared to low-grade invasive cells, we further
studied immunohistochemical analysis using 3-, 4C-, and 4D-grade
invasive OSCC tissues. Previously, we demonstrated that OSC-20 and
OSC-19 cells implanted into nude mice successfully reproduced the
same types of diffuse invasive tumours (grades 3 and 4C in terms of
the mode of invasion, respectively) (35), and repeated inoculation with TSU
cells successfully reproduced the formation of grade 4D OSCC
tumours in nude mice (36). With
the low-grade invasive OSCC lines (grades 3 and 4C, mode of
invasion), immunohistochemical analysis demonstrated that PD-L1 was
detected in cancer cells, but not in stromal cells (Fig. 3A). However, PD-L1 signals were
detected in stromal cells of high-grade invasive OSCC (grade 4D,
mode of invasion), whereas PD-L1 was not detected in the cancer
cells (Fig. 3A). Table I displays the correlations between
PD-L1 expression and the clinicopathological factors. PD-L1
expression in high-grade invasive OSCCs (4D, mode of invasion) was
significantly downregulated compared to low-grade invasive OSCCs (3
and 4C, mode of invasion) (P=0.0392). In contrast, PD-L1 expression
did not correlate with tumour size (P=0.873), lymph node metastasis
(P=0.542), local recurrence (P=0.083), or histological
differentiation (P=0.145). Immunohistochemical analysis
demonstrated that signals of EMT-related protein (Vimentin and
Snail1) were detected in cancer cells in human OSCC tissues
classified as grade 4D in terms of the mode of invasion (Fig. 3B). To analyse which cells expressed
PD-L1 in the cancer stroma, we investigated cells surrounding the
cancer cells by studying several biomarkers. Immunofluorescence
analysis demonstrated that most CD83 (DC marker)-positive cells
expressed PD-L1 and a fraction of MAC387 (macrophage
marker)-positive cells expressed the PD-L1 protein in high-grade
invasive OSCC tissues (grade 4D, mode of invasion; Fig. 3C). However, αSMA or FAP (CAF
marker)-positive cells did not co-localize with PD-L1 expressing
cells (Fig. 3C).
 | Table ICorrelation between PD-L1 expression
and the OSCC clinicopathological factors of the patients. |
Table I
Correlation between PD-L1 expression
and the OSCC clinicopathological factors of the patients.
| Factor | PD-L1 expression
| P-value |
|---|
| High (n=13) | Low (n=11) |
|---|
| T category | | | |
| T1 | 4 | 3 | 0.873 |
| T2 | 7 | 5 | |
| T3 | 0 | 1 | |
| T4 | 2 | 2 | |
| N category | | | |
| Negative | 12 | 7 | 0.542 |
| Positive | 1 | 4 | |
| Local
reccurence | | | |
| Negative | 10 | 5 | 0.083 |
| Positive | 3 | 6 | |
|
Differentiation | | | |
| Well | 9 | 7 | 0.145 |
| Moderately | 3 | 1 | |
| Poorly | 1 | 3 | |
| Mode of
invasion | | | |
| Grade 3 | 6 | 2 | 0.0392a |
| Grade 4C | 5 | 3 | |
| Grade 4D | 2 | 6 | |
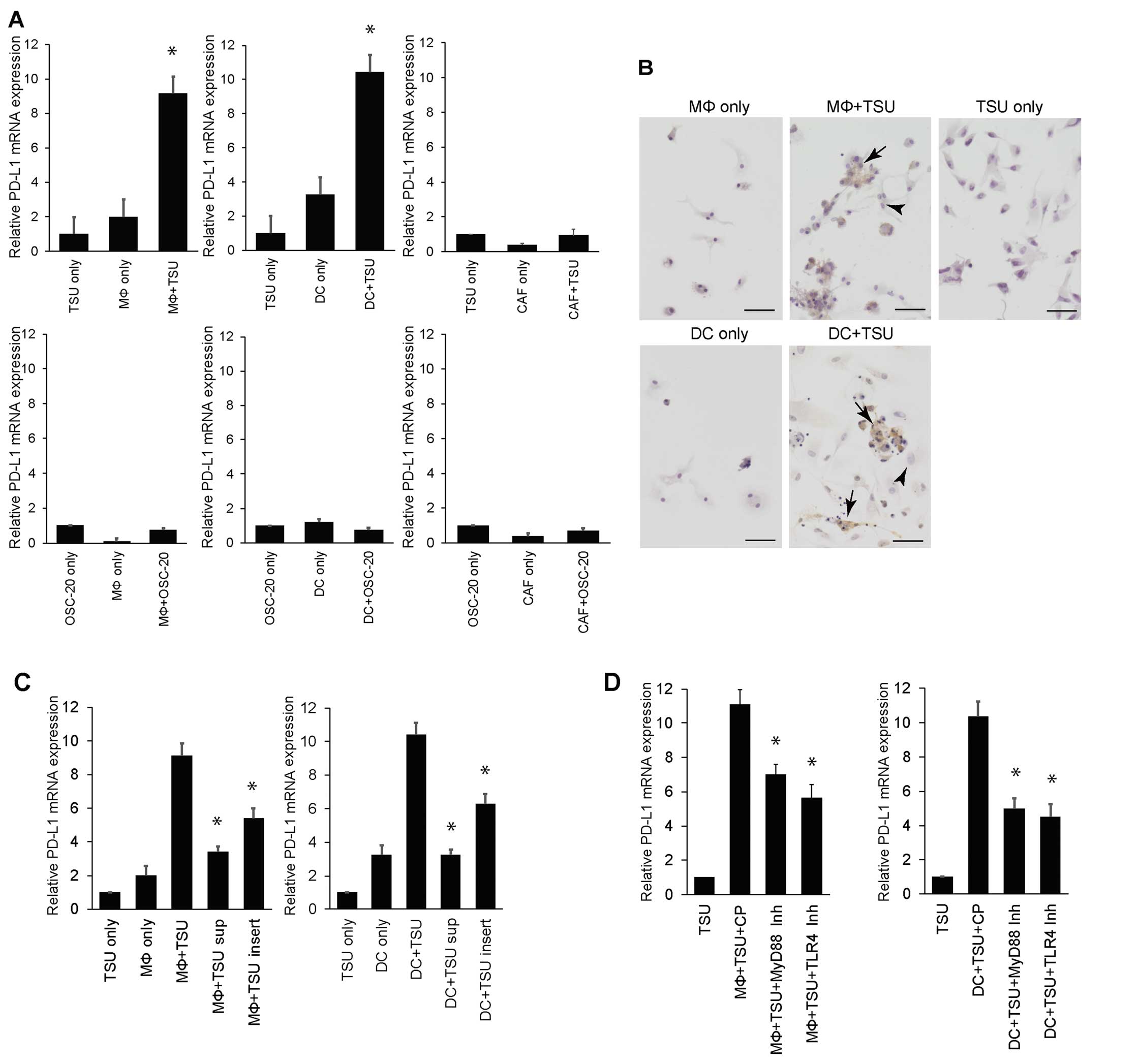
PD-L1 expression in macrophages or DCs
co-cultured with high-grade, invasive OSCC cells
The above immunohistochemical analysis demonstrated
that macrophages and DCs expressed PD-L1 in stromal cells in
high-grade invasive OSCC tissues. Thus, we performed further in
vitro studies to confirm PD-L1 expression in macrophages and
DCs in vitro. We found that PD-L1 expression increased
significantly (~10-fold) in macrophages or DCs co-cultured with TSU
cells, relative to that observed in TSU cells, macrophages or DCs
cultured separately. Differences in PD-L1 expression were
negligible between TSU cells co-cultured with CAFs and TSU cells or
CAFs cultured separately (Fig.
4A). In addition, PD-L1 expression in macrophages or DCs
co-cultured with OSC-20 cells, as well as in separate cultures of
OSC-20 cells and macrophages or DCs, did not change (Fig. 4A). Differences in PD-L1 expression
were negligible between OSC-20 cells co-cultured with CAFs and
OSC-20 cells or CAFs cultured separately (Fig. 4A).
Next, macrophages or DCs co-cultured with TSU cells
were immunostained using an anti-PD-L1 antibody to determine which
cell type expressed PD-L1. PD-L1 signals were detected in
macrophages or DCs, but not TSU cells (Fig. 4B). In particular, macrophages or
DCs attached to TSU cells that expressed PD-L1. PD-L1 was not
detected in the separate cultures of TSU cells, macrophages, or DCs
(Fig. 4B). PD-L1 upregulation was
not observed after adding the supernatant from a TSU cell culture
to macrophages or DCs (Fig. 4C).
PD-L1 upregulation was inhibited after adding TSU cells in culture
inserts to macrophages or DCs (Fig.
4C). Further, we used a blocking peptide to study the mechanism
of toll-like receptors in PD-L1 induction in macrophages or DCs
co-cultured with TSU cells. PD-L1 upregulation in macrophages or
DCs co-cultured with TSU cells was significantly suppressed by
treatment with the MyD88 homodimerization-inhibitor peptide or
TLR4-inhibitory peptide (Fig.
4D).
Discussion
PD-L1 is a ligand of the inhibitory receptor PD-1,
and the PD-L1/PD-1 pathway is involved in the induction and
maintenance of peripheral immunotolerance in cancer tissues
(11,37). Previous data demonstrated that
constitutive expression of PD-L1 occurs in various human cancers,
including squamous cell carcinomas of the lung, esophagus, and head
and neck; other types of carcinomas of the colon, ovaries, bladder,
and breast; melanoma; and glioma (7,12,13,37–45).
Tumour-associated PD-L1 is closely correlated with a poor prognosis
and/or higher malignancy grade. In general, PD-1 blockade has been
commonly correlated with PD-L1 expression in treated tumours
(1). Here, we demonstrated that
PD-L1 expression in 3 OSCC cell lines was significantly upregulated
compared to that in normal cells; thus, PD-L1 may play a greater
role in immunotolerance in the cancer microenvironment than PD-L2
does. However, it was surprising that PD-L1 expression in
high-grade invasive OSCC cell lines was lower than that in a
low-grade invasive OSCC line because these data contradicted
previous findings with other tumour types (7,12,13,37–45).
While the molecular mechanisms that account for this difference
remain to be determined, we suggest that they may be confined to
cancer subtypes with a unique molecular profile. Clearly, further
investigation will be required to test this hypothesis.
Previously, we demonstrated that high-grade invasive
OSCC cells have a mesenchymal phenotype (24). In this study, we confirmed the
EMT-associated genes were upregulated in high-grade invasive OSCC
tumour cells (TSU). Interestingly, PD-L1 expression in TSU OSCC
cells (with a mesenchymal phenotype) was downregulated compared to
that in OSC-19 and OSC-20 cells (with an epithelial phenotype). We
also demonstrated that TGF-β1 caused OSC-20 cells (with an
epithelial phenotype) to undergo an EMT-associated gene switch,
resulting in lost PD-L1 expression. These results indicated that a
close correlation existed between PD-L1 expression and EMT, and
that the net effect of TGF-β1 signalling was PD-L1 downregulation
with a concomitant EMT-associated gene switch in tumour cells.
However, further studies are needed to explain why OSCC maintains
high-grade invasive ability even after PD-L1 downregulation in
cancer cells. Our above data were developed in cell line models,
which do not reflect contributions of the tumour microenvironment
and immune responses, which play important roles in therapeutic
responses. Further studies of the precise molecular mechanisms
underlying the association between EMT and the regulation of PD-L1
expression in a human tumour microenvironment should be
conducted.
By analysing the immunomarkers PD-L1, vimentin, and
snail1, we confirmed the loss of PD-L1 expression and the
mesenchymal phenotype of cancer cells in high-grade invasive human
OSCC tissues (grade 4D). Interestingly, strong PD-L1 signals were
detected in stromal cells of high-grade invasive human OSCC tissues
(grade 4D), rather than in cancer cells. In low-grade invasive
human OSCC tissues (grade 3 and 4C), PD-L1 expression was seen only
in cancer cells, but not in stromal cells. These data suggested
that invasiveness and the EMT signature were inversely correlated
with PD-L1 expression in cancer cells and directly correlated with
PD-L1 expression in stromal cells. Potentially, some interaction
occurred between EMT-induced OSCC cells and stromal cells. A
previous report provided evidence that PD-L1 is not only expressed
by tumour cells, but also by tumour-associated macrophages, DCs,
and CAFs (46,47). In this study, we detected a strong
presence of PD-L1 in macrophages or DCs in cancer stroma tissues
via immunofluorescence analysis. While the expression levels of
PD-L1 in tumour cells, tumour-infiltrating immune cells have
recently been shown to correlate with clinical responses to
anti-PD-1 therapy (48–50). Only a subset of patients with
PD-L1-expressing tumours had clinical responses and other patients
(without PD-L1 staining) demonstrated clinical benefits, indicating
that additional factors in the tumour microenvironment (such as
stroma cell PD-L1 expression) may exist that define the subgroup of
patients who derive benefit.
Here, we showed that macrophages or DCs co-localized
with PD-L1 protein expression in cancer stromal tissues by
immunofluorescence staining. We performed further in vitro
analysis of the effects of direct interactions between cancer cells
and macrophages or DCs on PD-L1 expression. We showed here that
PD-L1 expression was upregulated in macrophages or DCs co-cultured
with TSU cells, but upregulation was not observed in CAFs
co-cultured with TSU cells. Immunostaining of co-cultured
macrophages or DCs and TSU cells revealed that PD-L1 upregulation
was observed only in macrophages or DCs, but not in co-cultured TSU
cells. Although the association between PD-L1 expression and
macrophages or DCs reported here is suggestive of an adaptive
resistance mechanism, it is still necessary to explain why PD-L1
was predominantly expressed by macrophages or DCs, rather than
cancer cells. If cancer cell-derived IFN-γ is indeed a major
mediator of PD-L1 expression in oral cancer, as described in other
settings (51), then oral cancer
cells may be less IFN-γ-responsive than macrophages or DCs. Another
possibility is that IFN-γ is expressed at lower levels in TSU
cells, as we showed here that the supernatant of cultured TSU cells
or TSU cells in culture insert did not induce PD-L1 expression in
macrophages or DCs. Alternatively, perhaps while cleaning up tumour
debris, macrophages and DCs begin to present high levels of tumour
antigens, making them prime targets for T cell recognition. In
fact, in this study, a TLR4-inhibitory peptide successfully
suppressed PD-L1 upregulation in macrophages or DCs co-cultured
with TSU cells. In this scenario, PD-L1 expression may provide a
means for protecting macrophages or DCs against cell death, and
tumour antigen could be induced by EMT, because PD-L1 upregulation
was not observed in macrophages or DCs co-cultured with OSC-20
cells with an epithelial phenotype (Fig. 4A).
Abbreviations:
|
CAF
|
carcinoma-associated fibroblast
|
|
EGFR
|
epidermal growth factor receptor
|
|
EMT
|
epithelial-mesenchymal transition
|
|
HGF
|
human gingival fibroblast
|
|
IFN-γ
|
interferon-γ
|
|
NSCLC
|
non-small cell lung cancer
|
|
OSCC
|
oral squamous cell carcinoma
|
|
PD-1
|
programmed-death 1 receptor
|
|
PD-L1
|
programmed-death ligand
|
|
qPCR
|
quantitative real-time PCR
|
|
TLR4
|
toll-like receptor 4
|
Acknowledgments
This study was supported by grants-in-aid for
Scientific Research from the Ministry of Education, Science, Sports
and Culture, Japan (nos. 15K20510 and 20390504 to H.K., no.
26463038 to K.K., no. 25462882 to H.N., no. 15H05042 to S.K.). We
are grateful to the members of the Department of Oral and
Maxillofacial Surgery of Kanazawa University for their helpful
suggestions and assistance. We thank Elsevier Premium Language
Editing Services for assistance with language editing.
References
|
1
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Topalian SL, Drake CG and Pardoll DM:
Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor
immunity. Curr Opin Immunol. 24:207–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oliveira-Neto HH, Gleber-Netto FO, de
Sousa SF, França CM, Aguiar MC, Silva TA and Batista AC: A
comparative study of microvessel density in squamous cell carcinoma
of the oral cavity and lip. Oral Surg Oral Med Oral Pathol Oral
Radiol. 113:391–398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang X, Zhou H, Liu X, He Y, Tang Y, Zhu
G, Zheng M and Yang J: Effect of local hyperthermia on
lymphangiogenic factors VEGF-C and -D in a nude mouse xenograft
model of tongue squamous cell carcinoma. Oral Oncol. 46:111–115.
2010. View Article : Google Scholar
|
|
7
|
Strome SE, Dong H, Tamura H, Voss SG,
Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, et al:
B7-H1 blockade augments adoptive T-cell immunotherapy for squamous
cell carcinoma. Cancer Res. 63:6501–6505. 2003.PubMed/NCBI
|
|
8
|
Lyford-Pike S, Peng S, Young GD, Taube JM,
Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA, et
al: Evidence for a role of the PD-1:PD-L1 pathway in immune
resistance of HPV-associated head and neck squamous cell carcinoma.
Cancer Res. 73:1733–1741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zandberg DP and Strome SE: The role of the
PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck.
Oral Oncol. 50:627–632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Butte MJ, Keir ME, Phamduy TB, Sharpe AH
and Freeman GJ: Programmed death-1 ligand 1 interacts specifically
with the B7-1 costimulatory molecule to inhibit T cell responses.
Immunity. 27:111–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsushima F, Tanaka K, Otsuki N, Youngnak
P, Iwai H, Omura K and Azuma M: Predominant expression of B7-H1 and
its immunoregulatory roles in oral squamous cell carcinoma. Oral
Oncol. 42:268–274. 2006. View Article : Google Scholar
|
|
14
|
Iwai Y, Terawaki S and Honjo T: PD-1
blockade inhibits hematogenous spread of poorly immunogenic tumor
cells by enhanced recruitment of effector T cells. Int Immunol.
17:133–144. 2005. View Article : Google Scholar
|
|
15
|
Hirano F, Kaneko K, Tamura H, Dong H, Wang
S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, et al: Blockade of
B7-H1 and PD-1 by monoclonal antibodies potentiates cancer
therapeutic immunity. Cancer Res. 65:1089–1096. 2005.PubMed/NCBI
|
|
16
|
Ibrahim R, Stewart R and Shalabi A: PD-L1
blockade for cancer treatment: MEDI4736. Semin Oncol. 42:474–483.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen L, Gibbons DL, Goswami S, Cortez MA,
Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, et al: Metastasis
is regulated via microRNA-200/ZEB1 axis control of tumour cell
PD-L1 expression and intratumoral immunosuppression. Nat Commun.
5:52412014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Prudkin L, Liu DD, Ozburn NC, Sun M,
Behrens C, Tang X, Brown KC, Bekele BN, Moran C and Wistuba II:
Epithelial-to-mesenchymal transition in the development and
progression of adenocarcinoma and squamous cell carcinoma of the
lung. Mod Pathol. 22:668–678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zavadil J, Haley J, Kalluri R, Muthuswamy
SK and Thompson E: Epithelial-mesenchymal transition. Cancer Res.
68:9574–9577. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uramoto H, Iwata T, Onitsuka T, Shimokawa
H, Hanagiri T and Oyama T: Epithelial-mesenchymal transition in
EGFR-TKI acquired resistant lung adenocarcinoma. Anticancer Res.
30:2513–2517. 2010.PubMed/NCBI
|
|
22
|
Chung JH, Rho JK, Xu X, Lee JS, Yoon HI,
Lee CT, Choi YJ, Kim HR, Kim CH and Lee JC: Clinical and molecular
evidences of epithelial to mesenchymal transition in acquired
resistance to EGFR-TKIs. Lung Cancer. 73:176–182. 2011. View Article : Google Scholar
|
|
23
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kimura I, Kitahara H, Ooi K, Kato K,
Noguchi N, Yoshizawa K, Nakamura H and Kawashiri S: Loss of
epidermal growth factor receptor expression in oral squamous cell
carcinoma is associated with invasiveness and
epithelial-mesenchymal transition. Oncol Lett. 11:201–207.
2016.PubMed/NCBI
|
|
25
|
Yokoi T, Hirata S, Nishimura F, Miyakawa
A, Odajima T, Kohama G and Mochizuki Y: Some properties of a newly
established human cell line derived from an oral squamous
carcinoma. Tumor Res. 25:93–103. 1990.
|
|
26
|
Yokoi T, Homma H and Odajima T:
Establishment and characterization of OSC-19 cell line in serum and
protein free culture. Tumor Res. 24:1–17. 1988.
|
|
27
|
Hayashi E, Rikimaru K and Nagayama M:
Simultaneous production of G- and M-CSF by an oral cancer cell line
and the synergistic effects on associated leucocytosis. Eur J
Cancer B Oral Oncol. 31B:323–327. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arrighi JF, Hauser C, Chapuis B, Zubler RH
and Kindler V: Long-term culture of human CD34(+) progenitors with
FLT3-ligand, thrombopoietin, and stem cell factor induces extensive
amplification of a CD34(−)CD14(−) and a CD34(−)CD14(+) dendritic
cell precursor. Blood. 93:2244–2252. 1999.PubMed/NCBI
|
|
29
|
Yang D, Chen Q, Le Y, Wang JM and
Oppenheim JJ: Differential regulation of formyl peptide
receptor-like 1 expression during the differentiation of monocytes
to dendritic cells and macrophages. J Immunol. 166:4092–4098. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Evans RA, Tian YC, Steadman R and Phillips
AO: TGF-beta1-mediated fibroblast-myofibroblast terminal
differentiation - the role of Smad proteins. Exp Cell Res.
282:90–100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adam L, Zhong M, Choi W, Qi W, Nicoloso M,
Arora A, Calin G, Wang H, Siefker-Radtke A, McConkey D, et al:
miR-200 expression regulates epithelial-to-mesenchymal transition
in bladder cancer cells and reverses resistance to epidermal growth
factor receptor therapy. Clin Cancer Res. 15:5060–5072. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thompson L: World Health Organization
classification of tumours: Pathology and genetics of head and neck
tumours. Ear Nose Throat J. 85:742006.PubMed/NCBI
|
|
33
|
Yamamoto E, Kohama G, Sunakawa H, Iwai M
and Hiratsuka H: Mode of invasion, bleomycin sensitivity, and
clinical course in squamous cell carcinoma of the oral cavity.
Cancer. 51:2175–2180. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
35
|
Kawashiri S, Kumagai S, Kojima K, Harada H
and Yamamoto E: Development of a new invasion and metastasis model
of human oral squamous cell carcinomas. Eur J Cancer B Oral Oncol.
31B:216–221. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moriyama M: Development of diffuse
invasive (grade 4D) human oral squamous cell carcinoma model in
severe combined immunodeficiency mice: Microangioarchitectural
analysis and immunohistochemical study. Oral Oncol. 35:395–400.
1999. View Article : Google Scholar
|
|
37
|
Zou W and Chen L: Inhibitory B7-family
molecules in the tumour microenvironment. Nat Rev Immunol.
8:467–477. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wintterle S, Schreiner B, Mitsdoerffer M,
Schneider D, Chen L, Meyermann R, Weller M and Wiendl H: Expression
of the B7-related molecule B7-H1 by glioma cells: A potential
mechanism of immune paralysis. Cancer Res. 63:7462–7467.
2003.PubMed/NCBI
|
|
39
|
Konishi J, Yamazaki K, Azuma M, Kinoshita
I, Dosaka-Akita H and Nishimura M: B7-H1 expression on non-small
cell lung cancer cells and its relationship with tumor-infiltrating
lymphocytes and their PD-1 expression. Clin Cancer Res.
10:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ohigashi Y, Sho M, Yamada Y, Tsurui Y,
Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, et al:
Clinical significance of programmed death-1 ligand-1 and programmed
death-1 ligand-2 expression in human esophageal cancer. Clin Cancer
Res. 11:2947–2953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thompson RH and Kwon ED: Significance of
B7-H1 overexpression in kidney cancer. Clin Genitourin Cancer.
5:206–211. 2006. View Article : Google Scholar
|
|
42
|
Hamanishi J, Mandai M, Iwasaki M, Okazaki
T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N,
et al: Programmed cell death 1 ligand 1 and tumor-infiltrating
CD8+ T lymphocytes are prognostic factors of human
ovarian cancer. Proc Natl Acad Sci USA. 104:3360–3365. 2007.
View Article : Google Scholar
|
|
43
|
Nomi T, Sho M, Akahori T, Hamada K, Kubo
A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, et al:
Clinical significance and therapeutic potential of the programmed
death-1 ligand/programmed death-1 pathway in human pancreatic
cancer. Clin Cancer Res. 13:2151–2157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ghebeh H, Tulbah A, Mohammed S, Elkum N,
Bin Amer SM, Al-Tweigeri T and Dermime S: Expression of B7-H1 in
breast cancer patients is strongly associated with high
proliferative Ki-67-expressing tumor cells. Int J Cancer.
121:751–758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yao Y, Tao R, Wang X, Wang Y, Mao Y and
Zhou LF: B7-H1 is correlated with malignancy-grade gliomas but is
not expressed exclusively on tumor stem-like cells. Neuro-oncol.
11:757–766. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Okazaki T, Chikuma S, Iwai Y, Fagarasan S
and Honjo T: A rheostat for immune responses: The unique properties
of PD-1 and their advantages for clinical application. Nat Immunol.
14:1212–1218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nazareth MR, Broderick L, Simpson-Abelson
MR, Kelleher RJ Jr, Yokota SJ and Bankert RB: Characterization of
human lung tumor-associated fibroblasts and their ability to
modulate the activation of tumor-associated T cells. J Immunol.
178:5552–5562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Taube JM, Klein A, Brahmer JR, Xu H, Pan
X, Kim JH, Chen L, Pardoll DM, Topalian SL and Anders RA:
Association of PD-1, PD-1 ligands, and other features of the tumor
immune microenvironment with response to anti-PD-1 therapy. Clin
Cancer Res. 20:5064–5074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: KEYNOTE-001 Investigators: Pembrolizumab for the treatment
of non-small-cell lung cancer. N Engl J Med. 372:2018–2028. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Taube JM, Anders RA, Young GD, Xu H,
Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL,
et al: Colocalization of inflammatory response with B7-h1
expression in human melanocytic lesions supports an adaptive
resistance mechanism of immune escape. Sci Transl Med.
4:127ra372012. View Article : Google Scholar : PubMed/NCBI
|