Introduction
Evidence has accumulated that sex hormones (SexHs)
play a role in the development and progression of several
malignancies arising in the gonads (1), urogenital tract (2–4),
breast (5), skeletal muscles
(6) and hemato/lymphohematopoietic
tissues (7). Gonadal SexHs, and in
particular pituitary SexHs such as follicle-stimulating hormone
(FSH), luteinizing hormone (LH), and prolactin (PRL), are potent
mitogens, and their enhanced level in peripheral blood (PB) is
associated with prostate, colon, and lung cancer (8–11).
Based on this evidence, we became interested in the potential role
of pituitary SexHs in lung carcinoma and employed four non-small
cell lung cancer (NSCLC) cell lines (A549, HTB177, HTB183, and
CRL5803) and two small cell lung cancer (SCLC) cell lines (CRL2062
and CRL5853) to investigate this possible linkage. We also studied
tissue samples from lung cancer patients. An additional reason to
pursue this question is the fact that the level of FSH increases
with age as a result of aging and gradual dysfunction of the gonads
(12,13). This increase could be linked to an
increase in certain malignancies, including lung cancer, in the
aged population (14).
Lung cancer is the number one cause of
cancer-related deaths in industrialized countries (15) and is expected to cause ~200,000 and
~350,000 deaths yearly, respectively, in the United States and
European Union alone. These statistics are undoubtedly related to
the prevalence of cigarette smoking and air pollution (16). The four major histological types of
bronchial carcinoma are squamous cell carcinoma, adenocarcinoma,
large cell undifferentiated carcinoma, and small cell carcinoma,
and the first three can be grouped in the category termed NSCLC to
distinguish them from SCLC. SCLC is more common in men than in
women and is strongly associated with cigarette smoking (17) and, in contrast to NSCLC, is derived
from the neuroendocrine cells of the lung, which are characterized
by the expression of neuron-specific enolase, neurosecretory
granules, and neurofilaments and the ability to secrete a host of
polypeptide hormones (18,19). These tumors are composed of small,
dark, round-to-oval, lymphocyte-like cells (albeit larger than
lymphocytes) that have scant cytoplasm and hyperchromatic nuclei
(20). SCLCs are rapidly growing
lesions that tend to infiltrate widely and metastasize early in the
course of the disease and therefore are rarely resectable.
Migration and metastasis of cancer cells is
orchestrated by several signaling pathways involved in the change
of cell shape, and two major theories have been proposed to explain
their migration. The first, the so-called cytoskeletal model,
postulates a rapid polymerization of actin and formation of actin
filaments at the cell's leading edge, with a supportive role of
microtubules at the retracting trailing edge (21). The second somewhat competitive
membrane flow model, postulates extension of the leading edge by
addition of membrane at the front of the cell and endocytosis of
integrins toward the rear of the cell (22). We have recently demonstrated that
heme oxygenase-1 (HO-1) is a potent inhibitor of hematopoietic cell
migration and that downregulation of HO-1 enhances the migration of
cells, while its overexpression has the opposite effect (23,24).
Here, we demonstrate that human lung cancer cell
lines as well as primary tumor samples express pituitary SexH
receptors. These receptors are functional and may promote
migration, adhesion, and proliferation of lung cancer cells.
Moreover, priming of human lung cancer cells by pituitary SexHs
resulted in enhanced seeding efficiency of injected human cells to
bone marrow, liver, and lungs in an immunodeficient mouse model.
Finally, the enhanced chemotaxis of human lung cancer cells after
stimulation by FSH, LH, and PRL corresponded with downregulation of
HO-1 activity in a p38 MAPK-dependent manner, which suggests that
modulating HO-1 activity could be of therapeutic value.
Materials and methods
Cell lines
We used several human lung cancer cell lines
(obtained from ATCC) including four NSCLC cell lines (A549, HTB177,
HTB183, and CRL5803) and two SCLC cell lines (CRL2062 and CRL5853).
All NSCLC cell lines were cultured in Roswell Park Memorial
Institute (RPMI)-1640 medium containing L-glutamine (GE Healthcare)
and 10% heat-inactivated fetal bovine serum (FBS; VWR Life Science
Seradigm). Waymouth's MB 752/1 medium containing 10% FBS, was used
for cultivation of CRL2062 cells. CRL5853 cells were maintained in
DMEM/F12 medium supplemented with 5% FBS, 5 µg/ml insulin,
0.005 mg/ml transferrin, 30 nmol/l sodium selenite (ITS; Lonza,
Inc., Allendale, NJ, USA), 10 nmol/l hydrocortisone, 10 nmol/l
β-estradiol (both from Sigma-Aldrich, St. Louis, MO, USA), and 4
mmol/l L-glutamine. Penicillin (100 U/ml), and streptomycin (10
µg/ml; Corning) were added to all types of media. These
cells were cultured in a humidified atmosphere of 5% CO2
at 37°C with change of medium every 48 h. In some experiments, the
human ovarian cancer cell line A2780 was cultured in RPMI-1640
medium with 10% FBS under the same atmospheric conditions.
Human lung cancer samples
Lung cancer samples from eight patients suffering
from NSCLC were obtained during surgery after informed consent
according to the institutional IRB protocol, and the cDNA was
extracted for mRNA analysis.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was purified from NSCLC and SCLC cells as
well as primary lung cancer patients (n=8) using the RNeasy Mini
kit after treatment with DNase I (both from Qiagen, Inc.). The
purified mRNA was afterwards reverse-transcribed into cDNA using
TaqMan Reverse Transcription Reagents (Applied Biosystems/Life
Technologies, Foster City, CA, USA). Amplification of synthesized
cDNA fragments was carried out using AmpliTaq Gold DNA Polymerase
(Applied Biosystems/Life Technologies). The PCR conditions used
were 1 cycle of 8 min at 95°C; 2 cycles of 2 min at 95°C, 1 min at
60°C, and 1 min at 72°C; 40 cycles of 30 sec at 95°C, 1 min at
60°C, and 1 min at 72°C; and 1 cycle of 10 min at 72°C. The
sequence-specific primers employed for amplification were as
follows: human follicle-stimulating hormone receptor (hFSHR; sense,
5′-gcttctgagatctgtggaggtt-3′ and antisense,
5′-ggacaaacctcagttcaatggc-3′), human luteinizing
hormone/choriogonadotropin receptor (hLHCGR; sense,
5′-ccggtctcactcgactatcac-3′ and antisense,
5′-tgaggaggttgtcaaaggca-3′), human prolactin receptor (hPRLR;
sense, 5′-ctgggctttctgccttactca-3′ and antisense,
5′-ttctttagttttgccagggagca-3′). Samples without template controls
and reverse transcriptase were used in each run. All primers were
designed using the NCBI/Primer-BLAST program, and at least one
primer included an exon-intron boundary. Afterwards, all PCR
products were analyzed by 2% agarose gel electrophoresis.
Transwell migration assay
After cells were enzymatically dissociated using
0.25% trypsin and rendered quiescent by incubation in appropriate
pre-warmed medium supplemented with 0.5% (NSCLCs) or 0.2% (SCLCs)
bovine serum albumin (BSA; Sigma-Aldrich) at 37°C, they were seeded
onto the upper chambers of 1% gelatin-coated Transwell inserts
containing polycarbonate membranes with an 8-µm pore size
(Costar Transwell; Corning Costar, Lowell, MA, USA). The lower
Boyden chambers received different concentrations of FSH (0.01–10
mU/ml), LH (0.01–10 mU/ml), or PRL (0.05–5 µg/ml) in
serum-free assay medium (650 µl). All of these reagents were
purchased from Prospec-Tany TechnoGene, Ltd. (East Brunswick, NJ,
USA). The lower chambers containing 10% FBS and 0.5% or 0.2% BSA in
appropriate medium served as positive and negative controls,
respectively. After a 24-h stimulation at 37°C, the upper chambers
were carefully removed, the cells that had not migrated were
removed with a cotton applicator swab from the upper side, and the
cells that had transmigrated to the lower side of the membrane were
fixed and stained with Hema 3 reagent (PROTOCOL; Thermo Fisher
Scientific, Pittsburgh, PA, USA) and then counted using an inverted
microscope. In some experiments, the CRL2062 cell line was also
evaluated for cell migration toward these SexHs after inhibition or
stimulation of HO-1. The cells were exposed to the small-molecule
HO-1 inhibitor tin protoporphyrin (SnPP) IX dichloride (50
µmol/l), the HO-1 activator cobalt protoporphyrin (CoPP) IX
chloride (50 µmol/l) (both from Tocris Bioscience), or
vehicle alone for 2 h in serum-free medium. Two hours later, all
these cells were washed with PBS and evaluated for migration toward
FSH, LH, PRL, or medium alone. The loaded inserts were afterwards
carefully removed, and the migrated cells were stained and counted
24 h post-loading. The results are presented as a chemotactic ratio
(the number of cells that migrated toward the medium containing
test reagents/the number of cells that migrated toward the medium
alone ×100).
Signal transduction studies
Quiescent cells were stimulated with 0.5% BSA in
RPMI-1640 medium, FSH (1 mU/ml), LH (1 mU/ml), or PRL (0.5
µg/ml) for 5 min at 37°C. Harvested cells were then washed
with PBS, treated with RIPA lysis buffer supplemented with protease
and phosphatase inhibitors (Santa Cruz Biotechnology, Inc.) for 30
min on ice, and centrifuged at 15,000 rpm at −4°C for 15 min. The
protein concentration was measured using the Pierce BCA Protein
Assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA) and
Multimode Analysis Software (Beckman Coulter). The
concentration-adjusted extracted proteins were then separated on a
4–12% SDS-PAGE gel and transferred to a PVDF membrane (Bio-Rad).
All membranes were blocked with 2.5% BSA in Tris-buffered saline
containing 0.1% Tween (TBST) for 1 h at room temperature. After
washing with TBST, phosphorylation of the intracellular kinase
p42/44 mitogen-activated protein kinase (p42/44 MAPK), p38 MAPK,
and AKT was detected by incubating the membranes overnight at 4°C
with phospho-specific anti-phospho-p42/44 MAPK (clone no. 9101,
diluted at 1:1,000), anti-phospho-AKT (Ser473; clone no. 9271,
diluted at 1:1,000) rabbit polyclonal antibodies, and
anti-phospho-p38 MAPK (Thr180/Thr182; clone no. 9216, diluted at
1:2,000) mouse monoclonal antibody (Cell Signaling Technology,
Inc.). Next, the PVDF membranes were incubated with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit or anti-mouse IgG
secondary antibodies (1:5,000; Santa Cruz Biotechnology, Inc.) for
2 h at RT. To confirm equal protein loading in all lanes, the blots
were stripped using stripping buffer (Thermo Fisher Scientific) and
then reprobed with appropriate anti-rabbit p42/44 MAPK (clone no.
9102), anti-rabbit p38 MAPK (clone no. 9212), and anti-rabbit AKT
(clone no. 9272) antibodies (all from Cell Signaling Technology,
Inc.). All membranes were then treated with enhanced
chemiluminescence (ECL) reagent and subsequently exposed to film
(Hyperfilm) (both from Amersham/GE Healthcare Life Sciences). For
band visualization, an automatic film developer supplied with fresh
warm developer and fixer solutions was used.
Expression of HO-1 by western blot
analysis
Cells (CRL5853, CRL2062) were cultured with FSH (1
mU/ml), LH (1 mU/ml), or PRL (0.5 µg/ml) in serum-free
RPMI-1640 medium for 6 h at 37°C. The harvested cells were
centrifuged and washed with ice-cold PBS. The total protein
extracts were collected, and their concentrations were then
measured. The concentration-adjusted extracted proteins (70
µg/each sample) were then separated on a 4–12% SDS-PAGE gel
and then transferred to a PVDF membrane. Next, the membranes were
blocked with 2.5% non-fat dry milk in Tris-buffered saline
containing 0.1% Tween (TBST) for 1 h at room temperature. After
washing with TBST, the membranes were incubated with rabbit
anti-HO-1 polyclonal antibody (diluted at 1:1,000; Enzo Life
Sciences, Inc., Farmingdale, NY, USA) overnight at 4°C. To assure
equal protein loading in all the lanes, the blots were then
reprobed with rabbit anti-β-actin monoclonal antibody (diluted at
1:1,000; Novus Biologicals, Littleton, CO, USA). All membranes were
treated with ECL reagent, and subsequently exposed to film for band
visualization.
Adhesion of human lung cancer cells to
fibronectin
Quiescent cells were incubated for 5 h in 0.5% BSA
RPMI-1640 medium in a humidified atmosphere of 5% CO2 at
37°C. Next, NSCLC (A549, HTB177) and SCLC (CRL2062, CRL5853) cell
lines were cultured in 0.5% BSA RPMI-1640 medium, or with FSH (1–10
mU/ml), LH (1–10 mU/ml), or PRL (0.05–5 µg/ml) in medium
containing BSA for a 5-min incubation at 37°C. Cells were then
added directly and allowed to adhere to the fibronectin-coated
wells (5,000 cells/well) in 96-well plates at 37°C. The wells were
coated first with 70 µl of fibronectin (10 µg/ml;
Sigma-Aldrich) overnight at 4°C and blocked before the experiment
with BSA for 2 h at 37°C. Following incubation of unstimulated and
stimulated cells at 37°C, the plates were vigorously washed three
times with PBS, and the adherent cells were counted under an
inverted microscope. The results are presented as an adhesion ratio
(the number of adherent cells stimulated with SexHs/the number of
adherent unstimulated cells ×100).
Cell proliferation
Cells were cultured in 24-well plates (CELLSTAR;
Greiner Bio-One) in RPMI-1640 culture medium containing 0.5%
(NSCLCs) or 0.2% (SCLCs) BSA for 72 h at an initial density of
1.25×104 cells/well (NSCLCs) or 6×104
cells/well (SCLCs) in the presence or absence of FSH (1 mU/ml), LH
(1 mU/ml), or PRL (0.5 µg/ml). RPMI-1640 medium containing
0.5% or 0.2% BSA was used as a negative control, while the full
medium containing 10% FBS was treated as a positive control. The
cell number was calculated directly after cell seeding (0 h) as
well as 24, 48, and 72 h after addition of the stimulants. At these
time points, the cells were harvested from the wells and counted
using FACS.
Quantitative real-time PCR (RT-qPCR)
RT-qPCR was performed to detect and quantify
relative levels of HO-1 mRNA in human lung cancer cells stimulated
in vitro with FSH (1 mU/ml), LH (1 mU/ml), or PRL (0.5
µg/ml) in serum-free medium for 6 h at 37°C. The purified
RNA was reverse-transcribed with MultiScribe Reverse Transcriptase,
oligo(dT), and a random hexamer primer mix (all from Applied
Biosystems/Life Technologies). Quantitative evaluation of the
target gene was then performed by using an ABI PRISM 7500 Sequence
Detection System (Applied Biosystems/Life Technologies) with Power
SYBR-Green PCR Master Mix reagent and specific primers (hHO-1
sense, 5′-gggtgatagaagaggccaagact-3′ and antisense,
5′-agctcctgcaactcctcaaga-3′). The PCR cycling conditions were 95°C
(15 sec), 40 cycles at 95°C (15 sec), and 60°C (1 min). According
to the melting point analysis, only one PCR product was amplified
under these conditions. The relative quantity of a target gene,
normalized to the β2-microglobulin gene as the endogenous control
and relative to a calibrator, was expressed as 2−ΔΔCt
(fold difference), where Ct is the threshold cycle, ΔCt = (Ct of
target genes) − (Ct of the endogenous control gene,
β2-microglobulin), and ΔΔCt = (ΔCt for target gene in test sample)
− (ΔCt for target gene in calibrator sample).
In vivo transplant into immunodeficient
mice
The care and use of mice was carried out in
accordance with the guidelines provided by the Institutional Animal
Care and Use Committee of the University of Louisville, which
conform to the Guide for the Care and Use of Laboratory Animals
(Department of Health and Human Services, NIH publication no.
86-23). Prior to in vivo transplantation, CRL2062 and
CRL5853 cells (10×105 per mouse) were treated ex
vivo with vehicle only, FSH (1 mU/ml), PRL (0.5 µg/ml),
or CoPP IX chloride, a small-molecule HO-1 activator (50
µmol/l; Tocris Bioscience), for 2 h at 37°C. In parallel,
cells were also pre-treated with SB203580, a p38 MAPK inhibitor (20
µmol/l), for 6 h and were afterwards subjected to FSH or PRL
for a further 2-h incubation at 37°C. All cells were then washed
and transplanted into severe combined immunodeficient (SCID)/beige
inbred mice (n=3 per group), which were initially irradiated with
350 cGy 24 h before transplantation. At 48 h post-transplantation,
bone marrows, livers, and lungs were collected, and the presence of
metastasized cancer cells (i.e., murine-human chimerism) was
evaluated as described (25).
Briefly, genomic DNA was purified from organs using the QIAamp DNA
Mini kit (Qiagen, Inc.). Next, detection of human α-satellite and
murine β-actin DNA levels was carried out using real-time PCR and
the ABI PRISM 7500 Fast Sequence Detection System (Applied
Biosystems/Life Technologies). A 25-µl reaction mixture
containing 12.5 µl SYBR-Green PCR Master Mix, 300 ng DNA
template, and specific primers (α-satellite DNA sense,
5′-ACCACTCTGTGTCCTTCGTTCG-3′ and antisense,
5′-ACTGCGCTCTCAAAAGGAGTGT-3′; β-actin DNA sense,
5′-TTCAATTCCAACACTGTCCTGTCT-3′ and antisense,
5′-CTGTGGAGTGACTAAATGGAAACC-3′) was used. Real-time PCR conditions
for the amplification process were as follows: 95°C (15 sec); 40
cycles at 95°C (15 sec); and 60°C (1 min). Samples without template
controls were used in each run, and the ΔCt values were determined.
For each cell line, the number of human cells present in the murine
organs (the degree of chimerism) was calculated according to a
standard curve generated by mixing different concentrations of
human cells with a constant number of murine cells in a linear
manner.
Data analysis
Statistical analysis was carried out using GraphPad
Prism 6 software (GraphPad Software, Inc., La Jolla, CA, USA). All
data are presented as means ± SD. Statistical analysis of the data
was done using one-way ANOVA and Tukey's test for post hoc pairwise
multiple comparison. In all analyses p≤0.05 and p≤0.01 were
considered significant.
Results
Human lung cancer cell lines express
functional pituitary SexH receptors
We employed RT-PCR analysis to evaluate the
expression of SexH receptors in four human NSCLC cell lines (A549,
HTB177, HTB183, and CRL5803) and two SCLC cell lines (CRL2062 and
CRL5853) and found that all these cell lines express FSH receptor
(FSHR), PRL receptor (PRLR), and LH receptor (LHR) (Fig. 1A). We also found that in human lung
cancer cell lines all these receptors responded to stimulation from
pituitary SexHs by phosphorylation of p42/44 MAPK and AKT (Fig. 1B). Expression of these receptors
was subsequently confirmed by immunofluorescence staining (data not
shown).
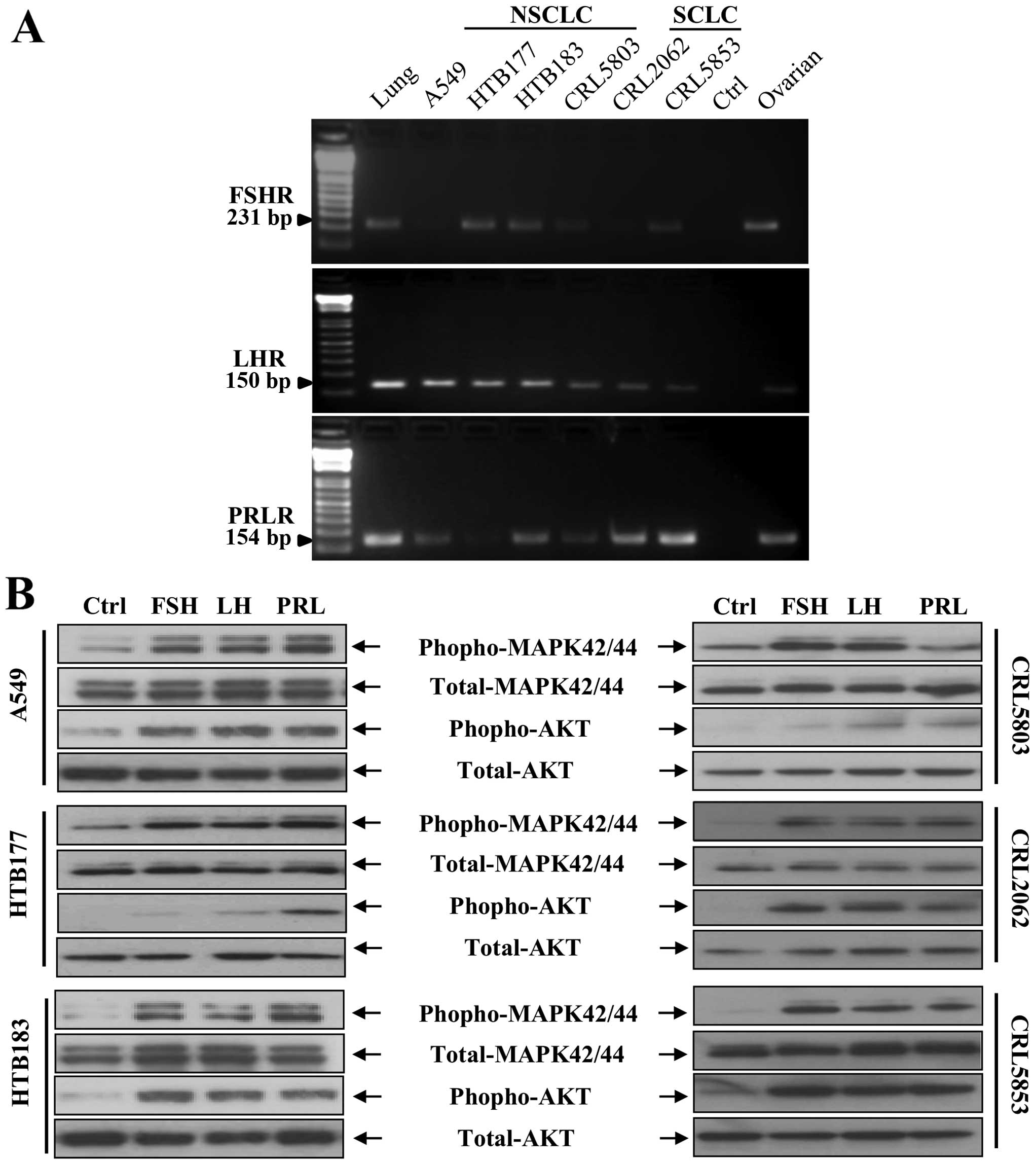 | Figure 1Human lung cancer cell lines express
functional pituitary SexH receptors. (A) Expression of FSHR, LHR,
and PRLR was detected in purified mRNA samples from both NSCLC cell
lines (A549, HTB177, HTB183, and CRL5803) and SCLC cell lines
(CRL2062 and CRL5853) by RT-PCR. Samples containing only water
instead of cDNA and samples with cDNA purified from established
human ovarian cancer cell lines were used in each run as negative
and positive controls, respectively. Representative agarose gels of
the RT-PCR amplicons are shown. (B) The effect of FSH, LH and PRL
on phosphorylation of the intracellular pathway proteins p42/44
MAPK and AKTser473 in both NSCLC and SCLC cell lines was
evaluated by western blot analysis. These cells were rendered
quiescent by incubation for 6 h in RPMI-1640 medium containing 0.5%
BSA at 37°C, and afterwards the protein lysates were harvested
after a 5-min stimulation with FSH (1 mU/ml), LH (1 mU/ml), PRL
(0.5 µg/ml), or serum-free medium containing vehicle. The
experiment was performed twice with similar results, and
representative blots are shown. SexH, sex hormone; FSHR, FSH
receptor; LHR, LH receptor; PRLR, PRL receptor; NSCLC, non-small
cell lung cancer; SCLC, small cell lung cancer; RT-PCR, reverse
transcription-polymerase chain reaction; FSH, follicle-stimulating
hormone; LH, luteinizing hormone; PRL, prolactin; p42/44 MAPK,
p42/44 mitogen-activated protein kinase; BSA, bovine serum
albumin. |
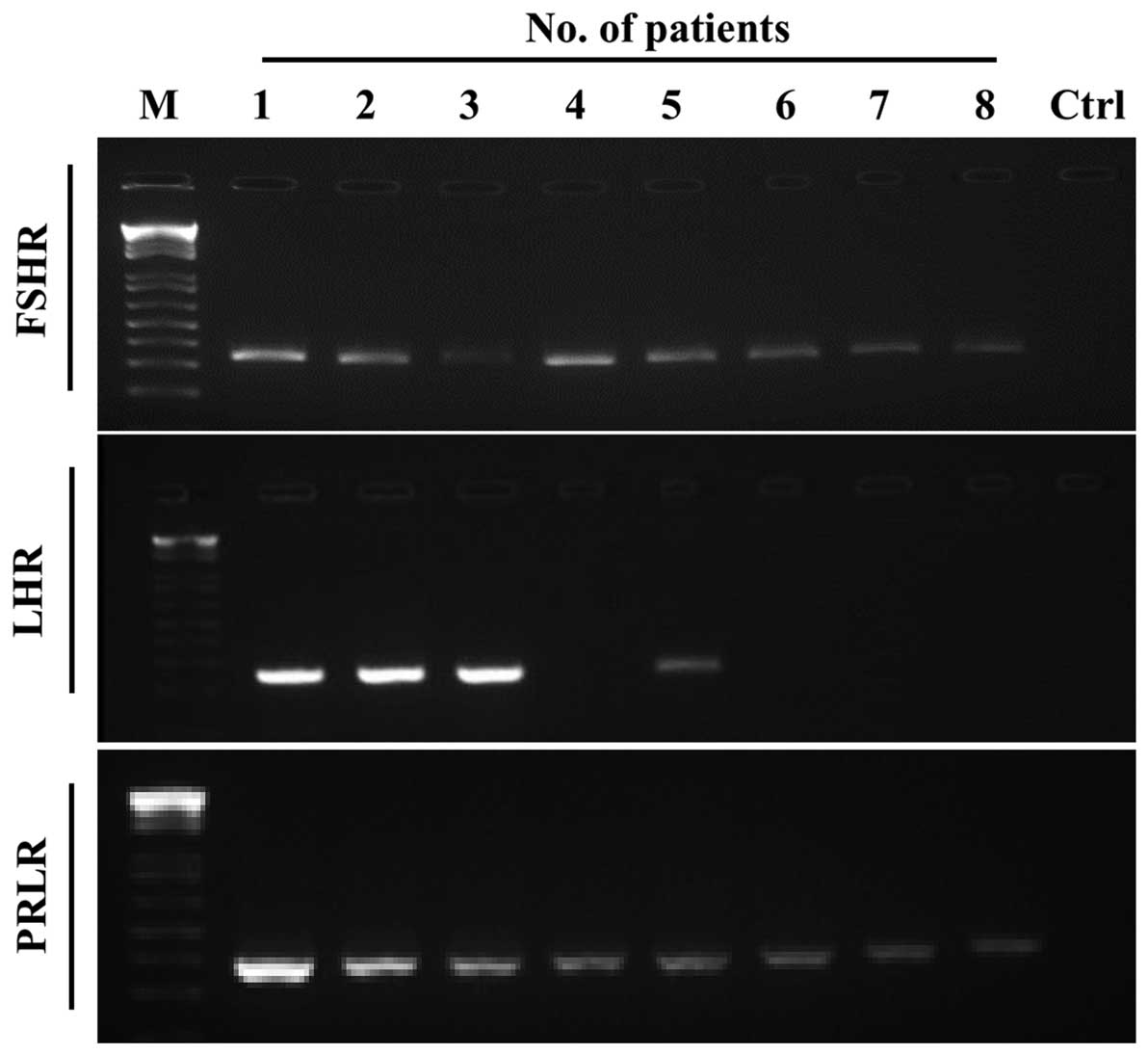
Similarly, we were able to detect FSHR and PRLR mRNA
in all eight patient NSCLC samples, and in four out of eight
patients we also detected the expression of LHR mRNA (Fig. 2).
Effect of pituitary SexHs on
proliferation, migration, and adhesion of human lung cancer cell
lines
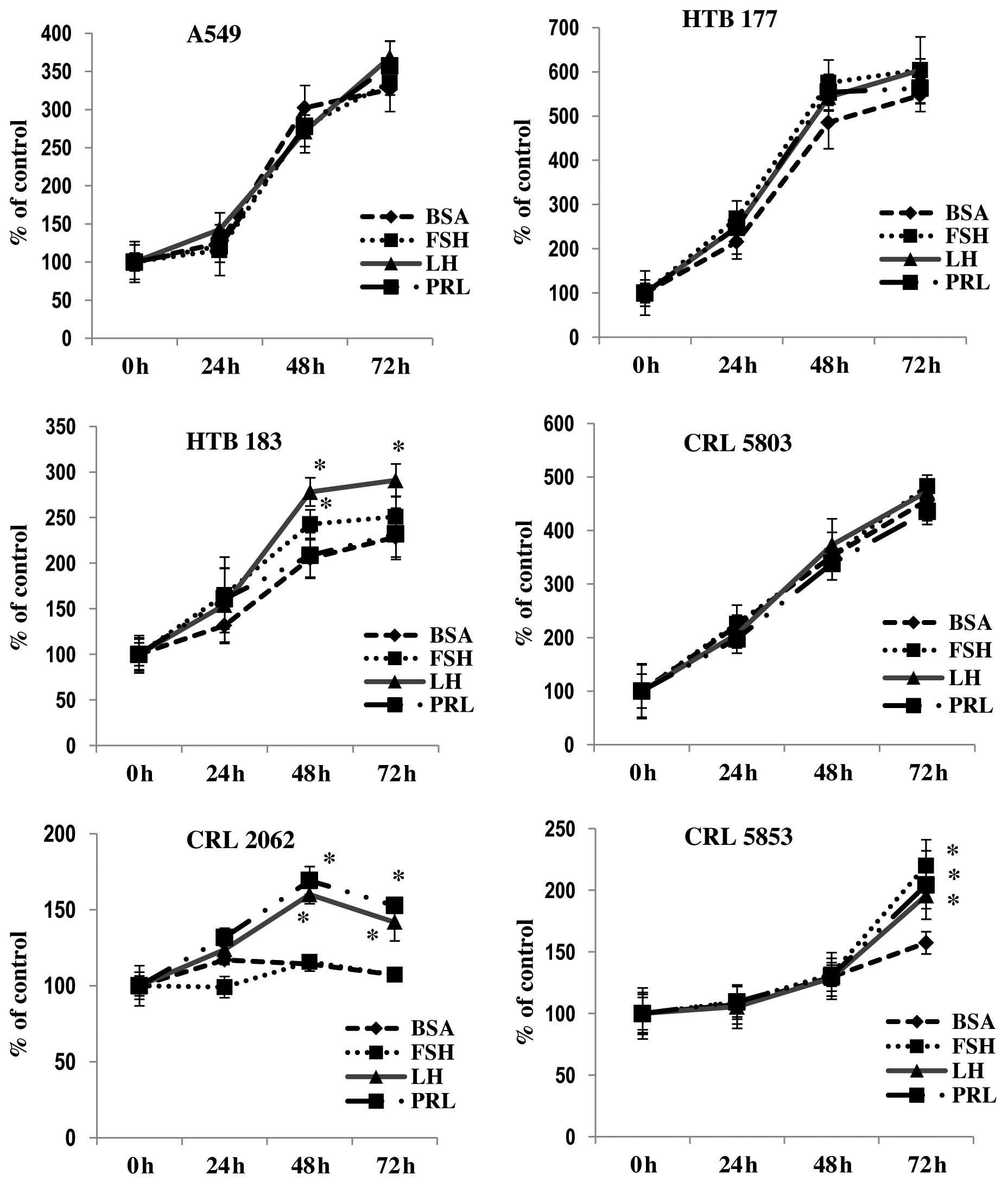
To study the effect of pituitary SexHs on
proliferation of lung cancer, we exposed lung cancer cell lines to
FSH, LH, or PRL in serum-free medium supplemented with 0.5% BSA
(Fig. 3). We found that one NSCLC
cell line (HTB183) responded to stimulation by LH and FSH and that
CRL2062, which is a SCLC cell line, responded to PRL or LH by
proliferation, while another SCLC cell line (CRL5853) responded to
all pituitary SexHs tested in our study.
In Transwell chemotaxis assays we found that lung
cancer cell lines, to different degrees, responded to pituitary
SexH gradients (Fig. 4). When we
employed FSH as a chemoattractant, we observed a chemotactic
response for three NSCLC cell lines (A549, HTB183, and CRL5803) and
both SCLC cell lines (CRL2062, CRL5853). A significant
responsiveness to LH was observed for the NSCLC cell lines HTB177,
HTB183, and CRL5803 and both SCLC cell lines (CRL2062, CRL5853).
Chemotactic responsiveness to PRL was particularly visible for both
SCLC cell lines (CRL2062, CRL5853) as well as for A549, HTB177, and
CRL5803 NSCLC cell lines.
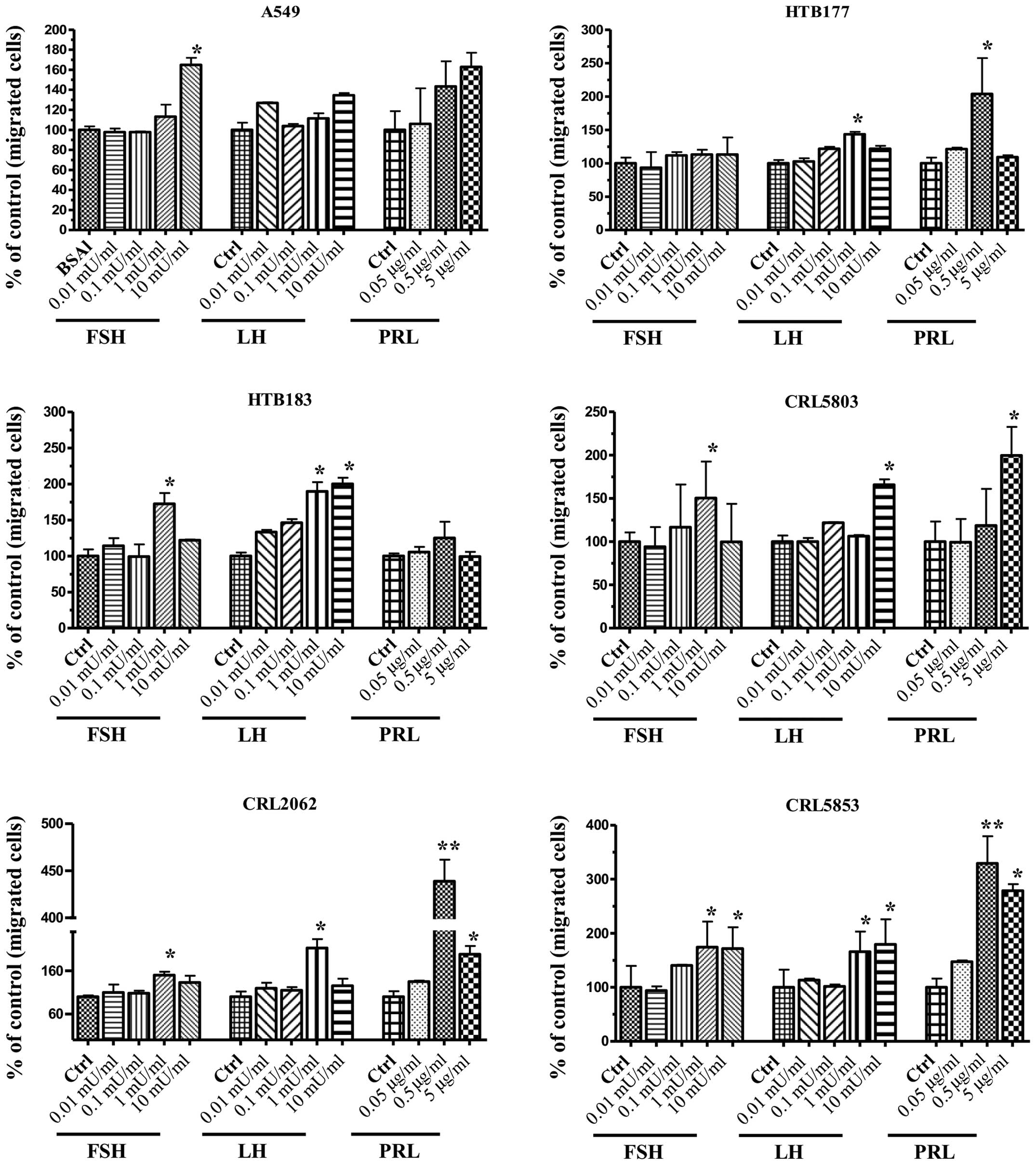 | Figure 4Pituitary SexHs stimulate the
chemotaxis of human NSCLC and SCLC cell lines. Chemotaxis of NSCLC
and SCLC cells through Transwell membranes (8-µm pore size)
coated with 1% gelatin toward different concentrations of FSH
(0.01–10 mU/ml), LH (0.01–10 mU/ml), or PRL (0.05–5 µg/ml)
was assessed. Before stimulation, cells were enzymatically
dissociated by digestion with 0.25% trypsin and then rendered
quiescent by incubation for 3 h in serum-free medium at 37°C. All
cell lines were also evaluated for migration in response to 10% FBS
and medium containing BSA as a positive and negative control,
respectively. Twenty-four hours post-stimulation, loaded inserts
were carefully removed, and the migrated cells were afterwards
stained and counted using an inverted microscope. Data are
extracted from at least triplicate samples from three independent
experiments. Significance levels: *p≤0.05, **p≤0.01 vs.
control (untreated) cells. SexHs, sex hormones; NSCLC, non-small
cell lung cancer; SCLC, small cell lung cancer; FSH,
follicle-stimulating hormone; LH, luteinizing hormone; PRL,
prolactin; FBS, fetal bovine serum; BSA, bovine serum albumin. |
In an adhesion assay (Fig. 5), we observed that pituitary SexHs,
depending on the type of hormone and its dose, enhanced the
adhesion of lung cancer cells to fibronectin-coated plates. The
only exception was a non-significant effect of LH on the adhesion
of HTB177 cells.
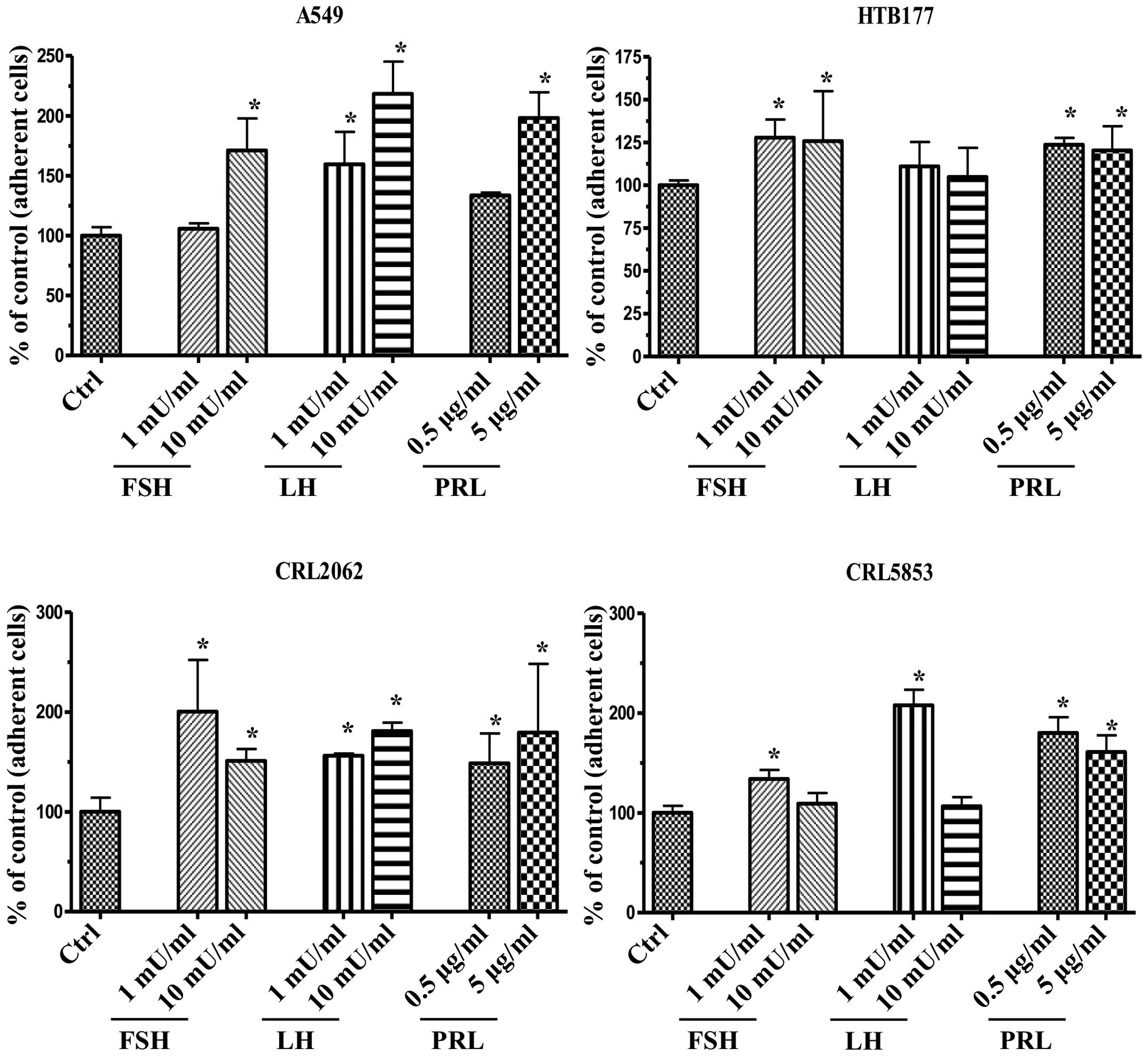 | Figure 5Pituitary SexHs promote the
adhesiveness of human lung cancer to fibronectin. Adhesion of NSCLC
(A549, HTB177) and SCLC (CRL2062, CRL5853) cells to
fibronectin-coated surfaces in response to FSH (1–10 mU/ml), LH
(1–10 mU/ml), or PRL (0.05–5 µg/ml). Quiescent cells (5,000
cells/100 µl) were stimulated in medium containing BSA for a
5-min incubation at 37°C. After the non-adherent cells were removed
via three consecutive washes with PBS, the number of adherent cells
was directly scored by microscopic analysis. Cells were also
evaluated for adhesion toward RPMI-1640 medium, with BSA as a
negative control. Data are extracted from at least triplicate
samples from three independent experiments. In all experiments, the
negative control values are normalized to 100%. Data are displayed
as means ± SD, with a statistical significance *p≤0.05
between cells exposed to SexHs vs. control (unstimulated) cells.
SexHs, sex hormones; NSCLC, non-small cell lung cancer; SCLC, small
cell lung cancer; FSH, follicle-stimulating hormone; LH,
luteinizing hormone; PRL, prolactin; BSA, bovine serum albumin. |
HO-1 is a negative regulator of lung
cancer cell migration
We recently reported that HO-1 is a negative
regulator of the migration of non-adherent cells and that the
activity of HO-1 is regulated by p38 MAPK, which is a negative
regulator of HO-1 expression (unpublished data). Here we asked
whether the positive effect of pituitary SexHs on the migration of
lung cancer cells could be explained by the downregulation of HO-1
and, based on the findings cited above, whether it corresponds with
the upregulation of p38 MAPK.
To address this issue we stimulated both SCLC cell
lines (CRL2062 and CRL5853), which respond robustly by chemotaxis
to pituitary SexHs, and observed that FSH, LH, and PRL downregulate
HO-1 expression in these cells, both at the mRNA and protein levels
(Fig. 6A and B). Based on these
findings, we exposed CRL5853 cells to FSH, LH, and PRL gradients to
a small-molecule inhibitor (SnPP) and stimulator (CoPP) of HO-1
before chemotaxis (Fig. 6C). We
found that, while the downregulation of HO-1 activity by SnPP
enhanced the migratory response of lung cancer cells, an increase
in HO-1 activity after exposure to CoPP led to a decrease in the
migratory potential of these cells. This result demonstrated that
HO-1 negatively regulates migration not only for non-adherent
cancer cells, as we demonstrated previously (23,24),
but also for adherent cancer cells. Moreover, a decrease in HO-1
activity after stimulation by SexH receptors correlated with
upregulation of p38 MAPK (Fig.
8A).
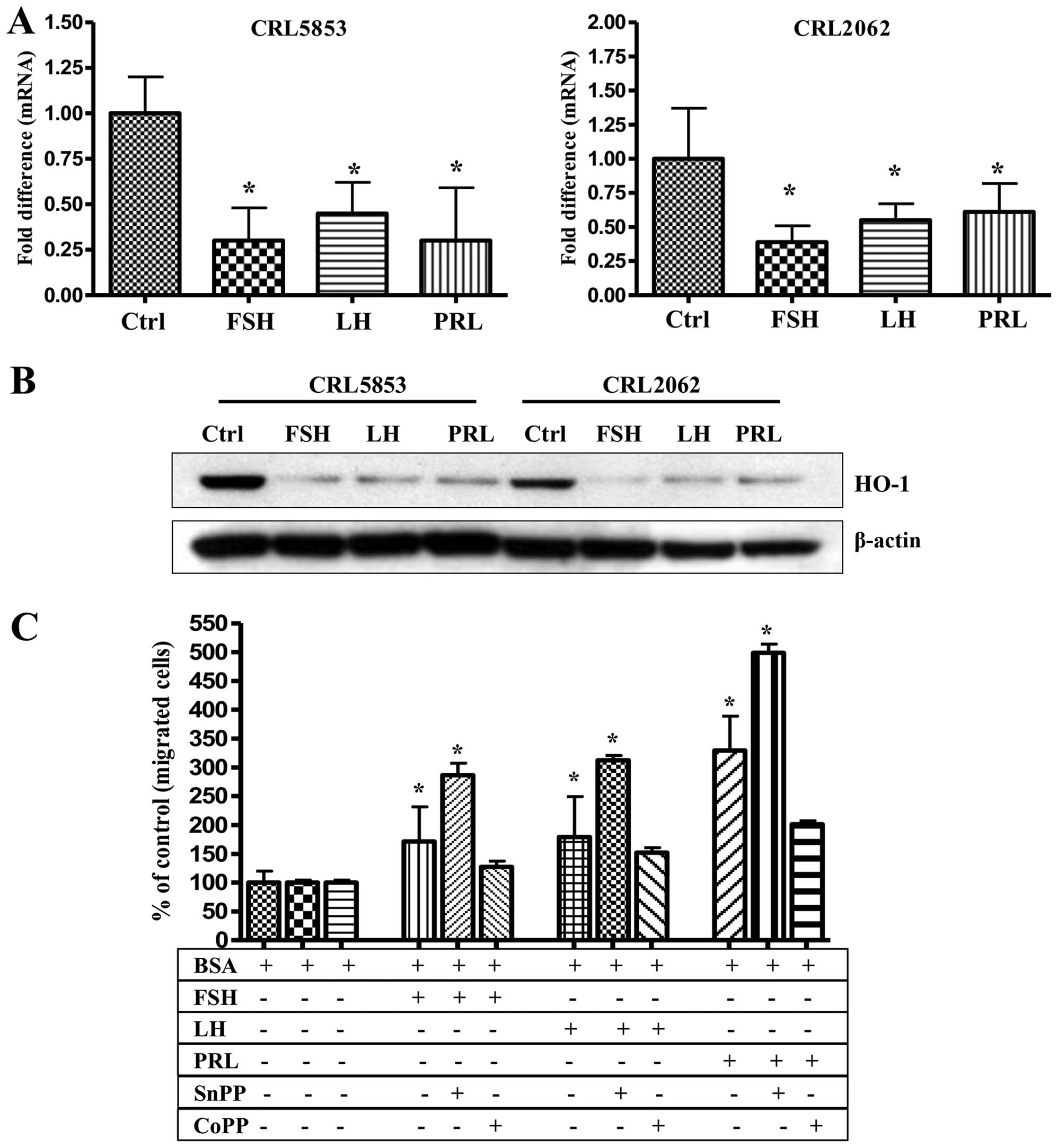 | Figure 6Pituitary SexHs enhance the migration
of lung cancer cell by downregulation of HO-1. (A) RT-qPCR analysis
of mRNA human HO-1 transcripts in mRNA samples purified from
CRL5853 (left) and CRL2062 (right) cell lines cultured with FSH (1
mU/ml), LH (1 mU/ml), or PRL (0.5 µg/ml) in serum-free
medium for 6 h at 37°C. β2-microglobulin was used as an endogenous
control. Samples containing only water instead of cDNA were used in
each run as a negative control. *P≤0.05 is considered
statistically significant between cells exposed to SexHs vs.
unstimulated cells. (B) Western blot analysis of human HO-1 in
protein lysates collected from CRL5853 and CRL2062 cell lines (70
µg per sample). After incubation of cells with these
hormones at the doses indicated above, the protein was immediately
extracted and afterwards quantified using the Pierce BCA Protein
Assay kit and Multimode Analysis Software. In parallel, β-actin was
also analyzed to ensure the equality of loading. Proteins extracted
from cells cultured in assay medium only served as a control. (C)
Chemotaxis of the CRL2062 cell line was also evaluated after
inhibition or stimulation of HO-1 by incubation of cells with SnPP
(50 µmol/l) or CoPP (50 µmol/l), respectively, in
serum-free medium. Two hours later, the cells were then washed with
PBS and evaluated for migration toward medium alone, FSH, LH, or
PRL. The loaded inserts were afterwards carefully removed, and the
migrated cells were stained and counted using an inverted
microscope 24 h post-loading. Data are extracted from at least
duplicate samples from three independent experiments. Significance
is indicated by *p≤0.05, where p represents the
statistical difference in migration between treated and untreated
cells. SexHs, sex hormones; HO-1, heme oxygenase-1; RT-qPCR,
quantitative real-time PCR; FSH, follicle-stimulating hormone; LH,
luteinizing hormone; PRL, prolactin; SnPP, tin protoporphyrin;
CoPP, cobalt protoporphyrin. |
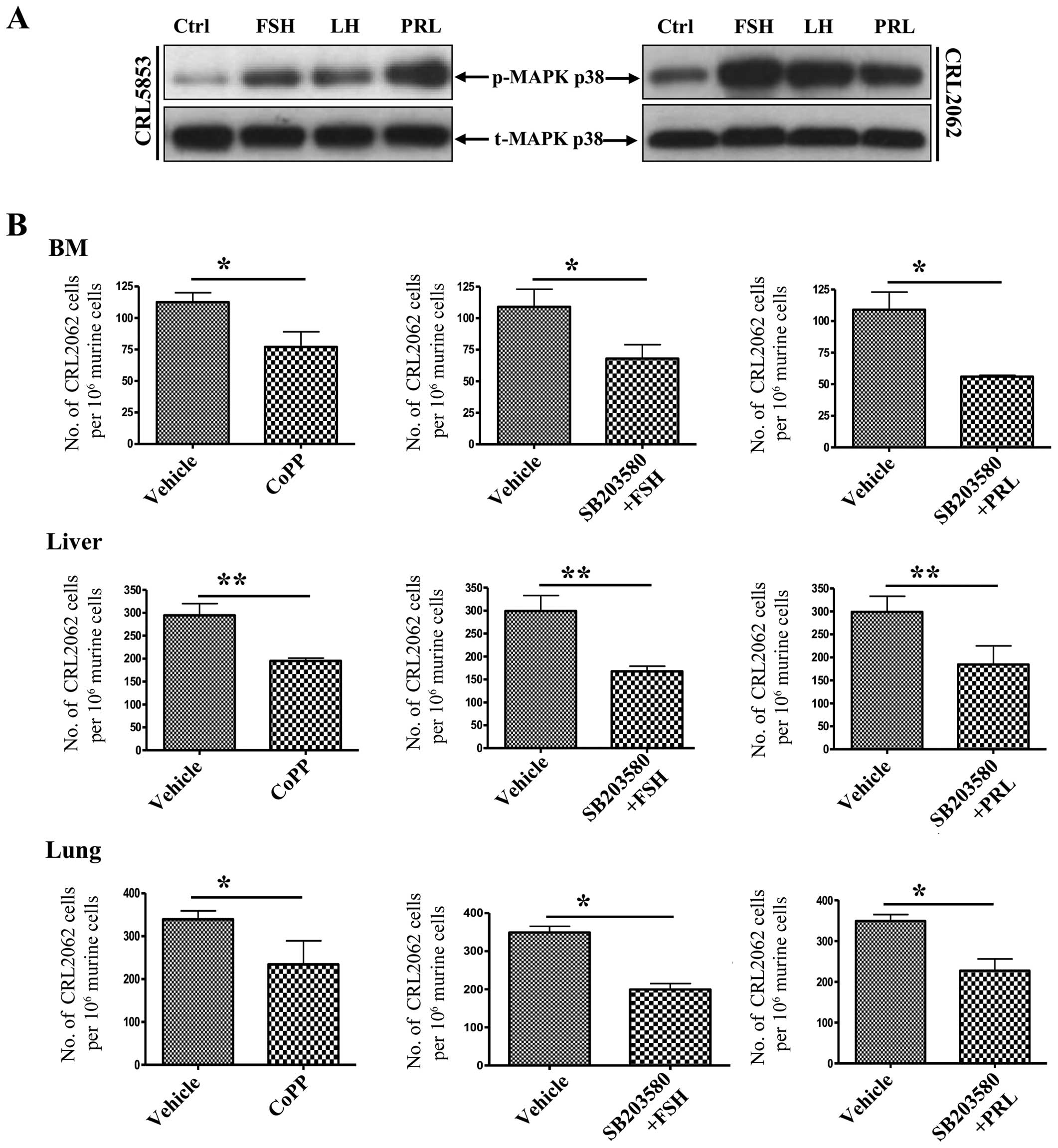 | Figure 8Pituitary SexHs enhance the migration
of lung cancer cells through phosphorylation of p38 MAPK-dependent
downregulation of HO-1. (A) Western blot analysis of phospho-p38
MAPK in protein lysates collected from quiescent CRL5853 (left) and
CRL2062 (right) lung cancer cell lines. Cells were stimulated with
0.5% BSA in appropriate medium, FSH, LH, or PRL for 5 min at 37°C.
Total p38 MAPK was also analyzed to ensure equal protein loading in
all lanes. (B) Evaluation of the spread of transplanted lung cells
(CRL2062) in vivo after stimulation of HO-1 levels via
pre-incubation of cells with the HO-1 activator CoPP (50
µmol/l) for 2 h at 37°C. In parallel, cells were also
pre-treated with SB203580, a p38 MAPK inhibitor (20 µmol/l),
for 6 h and subsequently subjected to FSH (1 mU/ml) or PRL (0.5
µg/ml) for a further 2 h. Forty-eight hours after in
vivo transplantation into irradiated immunodeficient
(SCID)/beige inbred mice (1×106 cells/mouse), the organs
were harvested, and detection and quantification of the human cells
were then analyzed by RT-qPCR. Significance levels are indicated by
*p≤0.05, **p≤0.01 vs. untreated cells
(vehicle only). SexHs, sex hormones; HO-1, heme oxygenase-1; BSA,
bovine serum albumin; FSH, follicle-stimulating hormone; LH,
luteinizing hormone; PRL, prolactin; CoPP, cobalt protoporphyrin;
SCID, severe combined immunodeficient; RT-qPCR, quantitative
real-time PCR. |
Priming of lung cancer cells with
pituitary SexHs enhances their in vivo seeding efficiency, and the
stimulation of HO-1 by CoPP reverses this effect
To address the role of the in vivo effect of
pituitary SexHs on the metastasis of lung cancer cells, we exposed
both SCLC cell lines to FSH or PRL, and after incubation the cells
were injected i.v. into immunodeficient NOD/SCID mice. Fig. 7 shows that the incubation of tumor
cells before injection with FSH or PRL enhanced the seeding
efficiency of lung cancer cells into bone marrow, liver, and
lung.
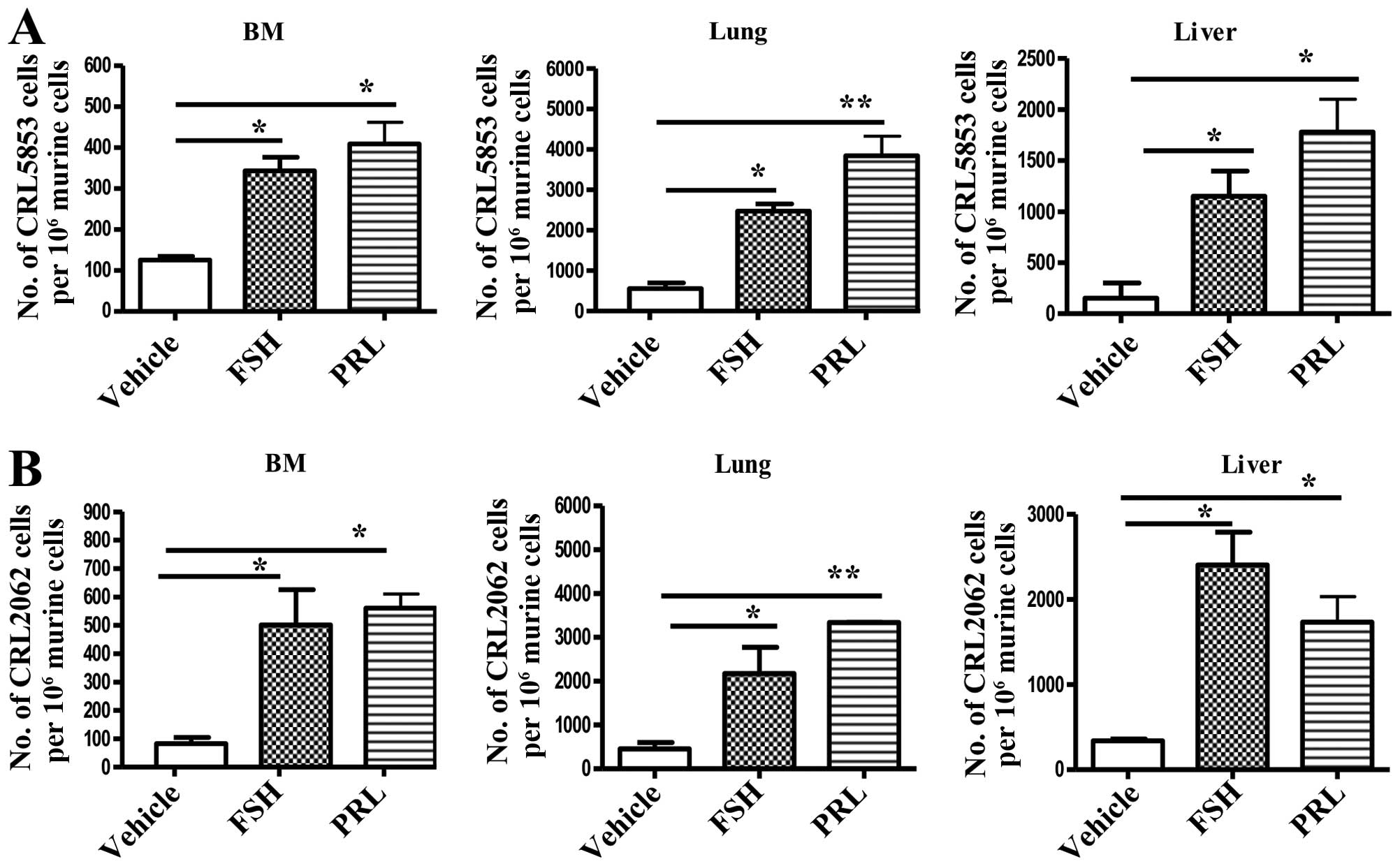 | Figure 7Pituitary SexHs accelerate the
metastasis of lung cancer cells in vivo. Detection of
transplanted human (A) CRL5853 and (B) CRL2062 cells
(1×105 cells/mouse) in the organs of irradiated
(SCID)/beige inbred mice post in vivo transplantation.
Pre-implantation, the cells were incubated ex vivo with
vehicle only, FSH (1 mU/ml), or PRL (0.5 µg/ml) for 2 h.
Under all conditions, serum-free medium was used. Detection of
human cells in BM, lung, and liver was evaluated by RT-qPCR for the
presence of human Alu sequences in purified genomic DNA samples.
Significance levels are indicated by *p≤0.05, **p≤0.01
vs. untreated cells. SexHs, sex hormones; SCID, severe combined
immunodeficient; FSH, follicle-stimulating hormone; PRL, prolactin;
RT-qPCR, quantitative real-time PCR. |
Finally, we repeated this experiment with CRL2062
cells with the modification that, before priming with FSH or PRL,
the cells were exposed to the small-molecule HO-1 activator CoPP or
the small-molecule p38 MAPK inhibitor SB203580 (Fig. 8B). By upregulating HO-1 activity,
both strategies decreased the seeding efficiency of lung cancer
cells to the BM, liver, and lungs of immunodeficient mice.
Discussion
Evidence has accumulated that several types of
malignancies share certain markers with germ cells and respond to
stimulation by SexHs (1,4–6). In
support of this connection, some tumors express pluripotency
markers (e.g., Oct-4), secrete carcinoembryonic antigen (CEA),
express cancer-testis antigens (CTAs), and respond by proliferation
after stimulation by both pituitary and gonadal SexHs (26–30).
Interestingly, it has been reported that human lung cancer cells
may express Oct-4, CEA, as well as several CTAs, including Sp17,
PTTG1, and AKAP-4, at the protein level. However, it is known that
the expression of these markers may vary between histological
subtypes of lung cancer (SCLC vs. NSCLC).
We became interested in the question of whether
human lung cancer cell lines express pituitary SexH receptors and
whether they respond to stimulation by FSH, LH, or PRL. The lung
cancer cell lines investigated in this study as well as tumor cells
from lung cancer patients all express pituitary SexH mRNAs.
Moreover, studies performed with human cancer cell lines
demonstrated that these receptors are functional. What is
intriguing, some of the lung cancer cell lines responded to SexHs
by enhanced proliferation. This observation suggests that pituitary
SexH therapy should be avoided in lung cancer patients, even if
they have achieved stable remission. Based on our results, there is
a risk that such treatment could activate dormant cancer cells.
There is another important question related to this
topic. One could ask whether elevated SexH levels could contribute
to lung cancer development as has been postulated in other types of
malignancies such as breast or ovarian cancer (1,31).
Lung cancer incidence increases with age, and it is well known that
the FSH level also increases with age as a compensatory feedback
loop in response to a decrease in gonadal function (12,14).
However, this hypothetical causal relationship requires more direct
experimental evidence and well-designed epidemiological studies. On
the other hand, while SCLC cells may produce some hormones
ectopically as part of the endocrine paraneoplastic syndrome, they
usually secrete other hormones such as adrenocorticotropic hormone
(ACTH) or PTH (9,32). Thus, the effect of SexHs on lung
cancer cell growth seems not to be autocrine but rather of an
endocrine nature.
We also found that, in addition to pro-proliferative
effects, pituitary SexHs chemoattract lung cancer cells and
increase their adhesion. These results are supported by clinical
observations showing that lung cancer cells may metastasize to
PRL-producing pituitary adenomas (33,34).
In fact, in our studies PRL was the most potent of all pituitary
SexHs in chemottracting SCLC cells. Additional evidence supporting
a pro-metastatic effect of pituitary SexHs on lung cancer cells are
in vivo results showing that a short exposure of these cells
ex vivo to pituitary SexHs enhances their seeding efficiency
in BM, liver, and lung in an immunodeficient mouse model.
Lung cancer cells may respond by chemotaxis to
several factors; therefore, an anti-metastatic strategy to block
only one type of receptor would be of very limited benefit. Thus,
while designing an anti-metastatic strategy, it is more important
to look for a molecular target that is employed by other
pro-metastatic factors (e.g., chemokines or certain pro-metastatic
growth factors). To address this issue, we have recently determined
that upregulation of the stress-induced enzyme HO-1 is an efficient
method for inhibiting cell migration (23,24).
In support of this finding in the current study, the enhanced
chemotaxis of lung cancer cell lines in response to FSH, LH, and
PRL gradients corresponded with decreases in HO-1 activity. Based
on this observation, we tested CoPP, a small-molecule stimulator of
HO-1, as a means to inhibit migration of lung cancer cells in both
in vitro and in vivo models and found that exposure
to CoPP significantly inhibited the trafficking of lung cancer
cells.
This strategy has an obvious advantage, since
upregulation of HO-1 in tumor cells will simultaneously decrease
their migratory responsiveness to other pro-metastatic factors
besides SexHs, such as SDF-1 or HGF/SF. Moreover, since HO-1 is
positively regulated in a p38 MAPK-dependent manner, we also
employed SB203580, a small-molecule inhibitor of p38 MAPK, and
observed a similar anti-metastatic effect. Therefore, we believe
that our observations generated with CoPP and SB203580 are highly
relevant for developing new anti-metastatic strategies.
These investigations also have another implication.
Namely, since pituitary SexH receptors are expressed by cells from
the germ lineage beginning at the very early stages of
embryogenesis (35,36), and, as demonstrated in this study,
lung cancer cells also express pituitary SexH rec eptors, there may
be a developmental link between these cells, as suggested at the
beginning of this section. Interestingly, 150 years ago Virchow
(37) and Cohnheim (38) proposed that some malignancies
develop from dormant embryonic or germ cells residing in adult
tissues. We have recently reported the existence of very small
embryonic-like stem cells (VSELs), which express several
embryonic/germline markers residing in adult tissues, including
lung tissue (27,39–41).
One can speculate that these or closely related cells could
theoretically be the source of some tumors. In support of this
possibility, VSELs express several SexH receptors as well as genes
involved in primordial germ cell development (35,36,39,41).
This working hypothesis, however, requires more experimental
evidence.
Finally, it is well known that the persistent
activation of the hypothalamic-pituitary-adrenal (HPA) axis as seen
for example in the chronic stress response and in depression may
impair the immune response and contribute to the development and
progression of some types of cancer (42). Our data indicate that in endocrine
system in addition to ACTH also SexH may play an important role.
Based on this, more study is needed on the bidirectional
communication between the endocrine and immune systems for the
development of a new clinical and treatment strategies.
In conclusion, lung cancer cells are responsive to
pituitary SexH stimulation, and these hormones may play an
important role in lung cancer progression and metastasis. However,
more experimental work is needed to see whether an increased level
of pituitary SexHs with age correlates with a predisposition to
lung cancer development. Finally, we propose that upregulation of
HO-1 and downregulation of p38 MAPK by small-molecule modulators
may provide the basis for new anti-metastatic strategies for lung
cancer patients.
Acknowledgments
This study was supported by NIH grants 2R01 DK074720 and R01HL112788, the Stella and Henry Endowment, and NCN Harmonia grant UMO‑2014/14/M/NZ3/00475 to M.Z.R., and OPUS grant UMO‑2016/21/B/NZ4/00201 to M.K.
References
|
1
|
van Kruchten M, van der Marel P, de Munck
L, Hollema H, Arts H, Timmer-Bosscha H, de Vries E, Hospers G and
Reyners A: Hormone receptors as a marker of poor survival in
epithelial ovarian cancer. Gynecol Oncol. 138:634–639. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
García-Cruz E, Piqueras M, Huguet J, Peri
L, Izquierdo L, Musquera M, Franco A, Alvarez-Vijande R, Ribal MJ
and Alcaraz A: Low testosterone levels are related to poor
prognosis factors in men with prostate cancer prior to treatment.
BJU Int. 110:E541–E546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
García-Cruz E, Piqueras M, Ribal MJ,
Huguet J, Serapiao R, Peri L, Izquierdo L and Alcaraz A: Low
testosterone level predicts prostate cancer in re-biopsy in
patients with high grade prostatic intraepithelial neoplasia. BJU
Int. 110:E199–E202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
García-Cruz E, Carrión Puig A,
García-Larrosa A, Sallent A, Castañeda-Argáiz R, Piqueras M, Ribal
MJ, Leibar-Tamayo A, Romero-Otero J and Alcaraz A: Higher sex
hormone-binding globulin and lower bioavailable testosterone are
related to prostate cancer detection on prostate biopsy. Scand J
Urol. 47:282–289. 2013. View Article : Google Scholar
|
|
5
|
Lønning PE: Poor-prognosis estrogen
receptor-positive disease: Present and future clinical solutions.
Ther Adv Med Oncol. 4:127–137. 2012. View Article : Google Scholar
|
|
6
|
Poniewierska-Baran A, Schneider G, Sun W,
Abdelbaset-Ismail A, Barr FG and Ratajczak MZ: Human
rhabdomyosarcoma cells express functional pituitary and gonadal sex
hormone receptors: Therapeutic implications. Int J Oncol.
48:1815–1824. 2016.PubMed/NCBI
|
|
7
|
Abdelbaset-Ismail A, Borkowska S,
Janowska-Wieczorek A, Tonn T, Rodriguez C, Moniuszko M, Bolkun L,
Koloczko J, Eljaszewicz A, Ratajczak J, et al: Novel evidence that
pituitary gonadotropins directly stimulate human leukemic
cells-studies of myeloid cell lines and primary patient AML and CML
cells. Oncotarget. 7:3033–3046. 2016.
|
|
8
|
Taggart DP, Gray CE, Bowman A, Faichney A
and Davidson KG: Serum androgens and gonadotrophins in bronchial
carcinoma. Respir Med. 87:455–460. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huchon G and Akoun G: Endocrine secretions
by bronchial tumors. Sem Hop. 55:180–188. 1979.In French.
PubMed/NCBI
|
|
10
|
Siraj A, Desestret V, Antoine M, Fromont
G, Huerre M, Sanson M, Camparo P, Pichon C, Planeix F, Gonin J, et
al: Expression of follicle-stimulating hormone receptor by the
vascular endothelium in tumor metastases. BMC Cancer. 13:2462013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen M, Xu Y, Hao X, Zhang Y, Xu W and Lu
B: The significance of serum sexual hormones' level in male
patients with lung cancer. Zhongguo Fei Ai Za Zhi. 8:300–303.
2005.In Chinese. PubMed/NCBI
|
|
12
|
Klein NA, Illingworth PJ, Groome NP,
McNeilly AS, Battaglia DE and Soules MR: Decreased inhibin B
secretion is associated with the monotropic FSH rise in older,
ovulatory women: A study of serum and follicular fluid levels of
dimeric inhibin A and B in spontaneous menstrual cycles. J Clin
Endocrinol Metab. 81:2742–2745. 1996.PubMed/NCBI
|
|
13
|
Wang YJ, Wu JC, Lee SD, Tsai YT and Lo KJ:
Gonadal dysfunction and changes in sex hormones in postnecrotic
cirrhotic men: A matched study with alcoholic cirrhotic men.
Hepatogastroenterology. 38:531–534. 1991.PubMed/NCBI
|
|
14
|
Aasebø U, Bremnes RM, de Jong FH, Aakvaag
A and Slørdal L: Pituitary-gonadal dysfunction in male patients
with lung cancer. Association with serum inhibin levels. Acta
Oncol. 33:177–180. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mulshine JL, Treston AM, Brown PH, Birrer
MJ and Shaw GL: Initiators and promoters of lung cancer. Chest.
103(Suppl): 4S–11S. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schnabel PA and Junker K: Neuroendocrine
tumors of the lungs. From small cell lung carcinoma to diffuse
idiopathic pulmonary neuroendocrine cell hyperplasia. Pathologe.
35:557–564. 2014.In German. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Idowu MO and Powers CN: Lung cancer
cytology: Potential pitfalls and mimics - a review. Int J Clin Exp
Pathol. 3:367–385. 2010.PubMed/NCBI
|
|
21
|
Hall A: The cytoskeleton and cancer.
Cancer Metastasis Rev. 28:5–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caswell P and Norman J: Endocytic
transport of integrins during cell migration and invasion. Trends
Cell Biol. 18:257–263. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adamiak M, Iv JB, Zhao J,
Abdelbaset-Ismail A, Grubczak K, Borkowska S, Wysoczynski M and
Ratajczak MZ: Downregulation of heme oxygenase 1 (HO-1) activity in
hematopoietic cells enhances their engraftment after
transplantation. Cell Transplant. Oct 16–2015.Epub ahead of print.
PubMed/NCBI
|
|
24
|
Wysoczynski M, Ratajczak J, Pedziwiatr D,
Rokosh G, Bolli R and Ratajczak MZ: Identification of heme
oxygenase 1 (HO-1) as a novel negative regulator of mobilization of
hematopoietic stem/progenitor cells. Stem Cell Rev. 11:110–118.
2015. View Article : Google Scholar :
|
|
25
|
Abdelbaset-Ismail A, Pedziwiatr D,
Suszyńska E, Sluczanowska-Glabowska S, Schneider G, Kakar SS and
Ratajczak MZ: Vitamin D3 stimulates embryonic stem cells but
inhibits migration and growth of ovarian cancer and teratocarcinoma
cell lines. J Ovarian Res. 9:262016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhatt S, Stender JD, Joshi S, Wu G and
Katzenellenbogen BS: OCT-4: A novel estrogen receptor-α
collaborator that promotes tamoxifen resistance in breast cancer
cells. Oncogene. Apr 11–2016.Epub ahead of print. View Article : Google Scholar
|
|
27
|
Ratajczak MZ, Shin DM and Kucia M: Very
small embryonic/epiblast-like stem cells: A missing link to support
the germ line hypothesis of cancer development? Am J Pathol.
174:1985–1992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sławek S, Szmyt K, Fularz M, Dziudzia J,
Boruczkowski M, Sikora J and Kaczmarek M: Pluripotency
transcription factors in lung cancer - a review. Tumour Biol.
37:4241–4249. 2016. View Article : Google Scholar
|
|
29
|
Reiter MJ, Costello JE, Schwope RB,
Lisanti CJ and Osswald MB: Review of commonly used serum tumor
markers and their relevance for image interpretation. J Comput
Assist Tomogr. 39:825–834. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Barger CJ, Link PA,
Mhawech-Fauceglia P, Miller A, Akers SN, Odunsi K and Karpf AR: DNA
hypomethylation-mediated activation of Cancer/Testis Antigen 45
(CT45) genes is associated with disease progression and reduced
survival in epithelial ovarian cancer. Epigenetics. 10:736–748.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Key TJ, Appleby PN, Reeves GK, Travis RC,
Alberg AJ, Barricarte A, Berrino F, Krogh V, Sieri S, Brinton LA,
et al Endogenous Hormones and Breast Cancer Collaborative Group:
Sex hormones and risk of breast cancer in premenopausal women: A
collaborative reanalysis of individual participant data from seven
prospective studies. Lancet Oncol. 14:1009–1019. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sorenson GD, Pettengill OS, Brinck-Johnsen
T, Cate CC and Maurer LH: Hormone production by cultures of
small-cell carcinoma of the lung. Cancer. 47:1289–1296. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rotondo F, Kovacs K, Macdonald RL,
Prud'homme GJ, Latta E and Munoz D: Non-small cell bronchial
carcinoma metastasizing into a prolactin-producing pituitary
adenoma. Int J Surg Pathol. 21:68–71. 2013. View Article : Google Scholar
|
|
34
|
Hanna FW, Williams OM, Davies JS, Dawson
T, Neal J and Scanlon MF: Pituitary apoplexy following metastasis
of bronchogenic adenocarcinoma to a prolactinoma. Clin Endocrinol
(Oxf). 51:377–381. 1999. View Article : Google Scholar
|
|
35
|
Abdelbaset-Ismail A, Suszynska M,
Borkowska S, Adamiak M, Ratajczak J, Kucia M and Ratajczak MZ:
Human haematopoietic stem/progenitor cells express several
functional sex hormone receptors. J Cell Mol Med. 20:134–146. 2016.
View Article : Google Scholar :
|
|
36
|
Mierzejewska K, Borkowska S, Suszynska E,
Suszynska M, Poniewierska-Baran A, Maj M, Pedziwiatr D, Adamiak M,
Abdel-Latif A, Kakar SS, et al: Hematopoietic stem/progenitor cells
express several functional sex hormone receptors - novel evidence
for a potential developmental link between hematopoiesis and
primordial germ cells. Stem Cells Dev. 24:927–937. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Virchow R: Editorial Archive fuer
pathologische. Anatomie und Physiologie fuer klinische Medizin.
8:23–54. 1855.In German.
|
|
38
|
Conheim J: Congenitales, quergestreiftes
muskelsarkon der nireren. Virchows Arch. 65:64–69. 1875.In German.
View Article : Google Scholar
|
|
39
|
Ratajczak MZ, Shin DM, Liu R, Marlicz W,
Tarnowski M, Ratajczak J and Kucia M: Epiblast/germ line hypothesis
of cancer development revisited: Lesson from the presence of
Oct-4+ cells in adult tissues. Stem Cell Rev. 6:307–316.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ratajczak MZ, Zuba-Surma EK, Wysoczynski
M, Ratajczak J and Kucia M: Very small embryonic-like stem cells:
Characterization, developmental origin, and biological
significance. Exp Hematol. 36:742–751. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shin DM, Liu R, Klich I, Wu W, Ratajczak
J, Kucia M and Ratajczak MZ: Molecular signature of adult bone
marrow-purified very small embryonic-like stem cells supports their
developmental epiblast/germ line origin. Leukemia. 24:1450–1461.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Reiche EM, Nunes SO and Morimoto HK:
Stress, depression, the immune system, and cancer. Lancet Oncol.
5:617–625. 2004. View Article : Google Scholar : PubMed/NCBI
|