Introduction
Angiogenesis is crucial to the growth of malignant
glioma. Anti-angiogenic therapy thus represents a promising
modality for treating this condition. Recently, reports were
published on large clinical trials for newly diagnosed glioblastoma
treated with standard chemoradiation therapy plus vascular
endothelial growth factor (VEGF) antibody, bevacizumab (1,2).
These reports demonstrated a significant improvement of
progression-free survival (PFS) with/without better quality of
life. However, there was no benefit for overall survival in either
trial. One reason for this is considered to be VEGF resistance. As
such, there is a need for further improvements in anti-angiogenic
treatment of glioma, for example, by identifying useful biomarkers
for VEGF antibody treatment, which was not achieved in these two
large clinical trials (3).
Biomarkers for bevacizumab have been reported in breast and
prostate cancer. Serum VEGF concentration was identified as a
predictive biomarker for breast cancer (4), but not for prostate cancer (5). However, a preliminary report on a
bevacizumab biomarker project described that serum VEGF
concentration, as well as various angiogenic factors, including
VEGFR-1, VEGFR-2, E-selectin, VEGFR-3, IL-8, bFGF, PDGF-C, VEGF-C,
PlGF and ICAM-1, cannot be used as biomarkers, and tissue vascular
density and area are also not predictive factors for bevacizumab
(3,6). Further anti-angiogenic clinical
trials (on cilengitide, cediranib and enzastaurin) also failed to
reveal a survival benefit for glioblastoma (7). To achieve a survival benefit for
gliomas with anti-angiogenic therapy, we need to establish a strong
and useful biomarker for anti-angiogenic therapy of gliomas and a
new strategy combining chemotherapy and/or immunotherapy with an
anti-angiogenic agent.
Current defined endogenous angiogenic stimulators
and inhibitors in neoplasms have been well described. In
particular, it has been shown that the balance between stimulators
and inhibitors regulates tumor angiogenesis, resulting in tumor
growth (8,9). Many endogenous angiogenesis
inhibitors have been discovered, and the list continues to grow
(10,11). Endogenous protein inhibitors have
the advantages of low toxicity, high tolerance, low risk of drug
resistance, and a higher likelihood of specifically blocking
pathological neovascularization without affecting the normal
vasculature (12). Some of them
have reached the clinical trial stage or are already on the market;
these include endostatin (endostar) and angiostatin (13–15).
Other inhibitors have served as parent molecules from which
derivative analogues have been developed and reached the clinical
trial stage, such as thrombospondin-1 and its analogue ABT-510
(16,17). Nevertheless, to date, the clinical
efficacy of these inhibitors in humans is still questionable. Only
endostar, a modified recombinant endostatin, has been approved as
an anticancer drug in China (18).
Soluble Flt-1 (sFlt1) is a soluble form of VEGF
receptor 1 (VEGFR-1), which is measurable in conditioned medium and
cell/tissue lysate and inhibits angiogenesis. The function of sFlt1
is assumed to be mainly inhibitory, complexing VEGF and thus acting
as a regulator of VEGF-A bioavailability. Flt1 has more than
10-fold higher affinity to VEGF-A, even in soluble form, but has
approximately a 10-fold lower thyrosine kinase activity than
VEGFR-2. In addition, sFlt1 can form heterodimers with
transmembrane VEGFR-2, preventing autophosphorylation, and thus,
abolishing signaling in a dominant-negative fashion (19). sFlt1, an endogenous angiogenesis
inhibitor, is a candidate for anti-angiogenic therapy for malignant
gliomas. Among several different approaches for transferring
anti-angiogenic genes (sFlt1, angiostatin and endostatin), sFlt1
gene transfer was found to be the most potent for inhibiting tumor
growth in a preclinical animal model (20).
In the present study, sFlt1 and VEGF were measured
in a series of human glioma tissues using specific enzyme-linked
immunosorbent assay (ELISA) in order to determine whether their
concentrations, and especially the net balance of VEGF/sFlt1,
predict patient survival and angiogenic potential. The
effectiveness of sFlt1 gene delivery as an endogenous angiogenic
inhibitor against glioma growth and angiogenesis was also
investigated in a mouse model.
Materials and methods
Tissue preparation
Tissues from 69 cases of human malignant glioma
(Table I) (glioblastoma 39,
anaplastic astrocytoma 21 and anaplastic oligoastrocytoma 4) were
obtained during surgery at the University of Tsukuba Hospital
between 1997 and 2004. The tissues were immediately stored at −80°C
until use. The tissues were thawed, 50-mg samples were homogenized
in 10-fold extraction buffer (25 mM Tris-HCl, pH 7.4, 100 mM NaCl,
20 mM NH4HCO3) and then they were again
stored at −80°C until use. The protein concentration was measured
by DC protein assays (Bio-Rad Laboratories, Hercules, CA, USA). The
rest of the tissue was fixed in formalin and embedded in
paraffin.
 | Table IPrognostic factors in malignant
gliomas (Hazard model). |
Table I
Prognostic factors in malignant
gliomas (Hazard model).
| Hazard ratio | 95% CI | P-value |
|---|
| Univariate |
| VEGF (pg/mg) | | | |
| >1000 vs.
<1000 | 2.17 | 1.28–3.70 | <0.01 |
| sVEGFR1
(pg/mg) | | | |
| <1000 vs.
>1000 | 0.95 | 0.56–1.61 | ns |
| VEGF/R1 ratio | | | |
| >1 vs.
<1 | 2.79 | 1.61–4.86 | <0.001 |
| Pathology | | | |
| Grade IV vs.
III | 2.65 | 1.53–4.58 | <0.001 |
| MIB1 (%) | | | |
| >20 vs.
<20 | 1.77 | 1.05–3.00 | <0.05 |
| p53 (%) | | | |
| >10 vs.
<10% | 0.85 | 0.48–1.50 | ns |
| Density | | | |
| >30 vs.
<30 | 1.18 | 0.70–1.97 | ns |
| Vessel area
(%) | | | |
| >7 vs.
<7 | 2.39 | 1.35–4.21 | <0.01 |
| Multivariate |
| VEGF/R1 ratio | | | |
| >1 vs.
<1 | 1.99 | 1.08–3.66 | <0.05 |
| Pathology | | | |
| Grade IV vs.
III | 1.81 | 0.98–3.33 | ns |
| MIB1 (%) | | | |
| >20 vs.
<20 | 1.47 | 0.86–2.49 | ns |
sFlt1 and VEGF ELISA
The concentrations of sFlt1 and VEGF were measured
in tumor extract supernatants using Quantikine™ Human sFlt1 and
VEGF immunoassays (DVR100 and DVE00, respectively; R&D Systems,
Minneapolis, MN, USA). sFlt1 and VEGF levels were normalized to
total extract protein concentrations, and are expressed as pg sFlt1
and VEGF/mg total extract protein.
Immunohistochemistry and angiogenic
profile
The Dako LSAB2 kit for mouse and rabbit primary
antibody (Dako, Glostrup, Denmark) was used (21). Tissue sections were deparaffinized
and incubated with 10% normal goat serum in phosphate-buffered
serum (PBS) for 20 min. The sections were then incubated with a
polyclonal anti-VEGF antibody, A-20 (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), at a concentration of 10 µg/ml,
a monoclonal MIB-1 antibody (Immunotech Laboratories, Inc.,
Monrovia, CA, USA) in phosphate-buffered saline (PBS) overnight at
4°C, a monoclonal p53 antibody (clone DO7; Dako) in PBS overnight
at 4°C, and a monoclonal CD34 antibody (BD Biosciences, San Jose,
CA, USA) at a dilution of 1/50 (10 µg/ml) in PBS for 60 min
at room temperature. Chromatographically purified mouse IgG and
rabbit IgG (Dako) at the same IgG concentration were used as
negative controls. Sections were incubated with biotin-conjugated
goat anti-mouse or anti-rabbit immunoglobulin for 10 min, followed
by washing in PBS for 10 min. The sections were then incubated with
peroxidase-conjugated streptavidin solution for 5 min, followed by
washing in PBS for 5 min. Sections were then stained with freshly
prepared aminoethylcarbazole solution for 10 min, followed by
washing for 5 min in tap water. Next, sections were counterstained
with hematoxylin and mounted with aqueous mounting media. The
intracellular VEGF immunostaining was assessed using a
semi-quantitative scale (−, not detected; +, moderate; and ++,
strong). The rates of nuclei positive for MIB-1 and p53 were
determined by counting at least 1000 tumor cells.
The number of vessels, area occupied with vessels,
and mean vessel diameter at a ×200 magnified field (1.0
mm2) were morphometrically measured in microvessel 'hot
spots' (i.e., microscopic areas containing the densest collections
of microvessels, as initially identified under low-power
magnification) using an Olympus microscope, AHBT3 (Olympus, Tokyo,
Japan) and WinROOF software (Mitani Corp., Tokyo, Japan) on
CD34-stained tissue sections. Vascular density and area were
determined by averaging the number of vessels and area in the three
most vascularized areas.
Cell culture and sFlt1 transfection
The human glioma cell line U-87 MG was obtained from
the American Type Culture Collection (ATCC; Rockville, MD, USA).
Cells were maintained in minimum essential medium (MEM)
supplemented with 10% fetal calf serum (FCS) in a humidified
atmosphere containing 5% CO2 at 37°C.
In order to investigate the effect of sFlt1 gene
overexpression in vitro and in vivo, a human sFlt1
gene (2223 bp)-encoding plasmid, pBLAST45-hsFLT1 (#pbla-hsflt1;
Invivogen, Inc., San Diego, CA, USA), was transfected into U87
malignant glioma cells using Fugene6 (Invivogen). Transfectants
were selected using blasticidine. Among the three clones obtained,
two were confirmed to express sFlt1 by reverse transcription
polymerase chain reaction (RT-PCR). mRNA expression of sFlt1 was
not observed in U87 cells, which were the parental cells, or in
empty vector transfectant (empty), which did not contain the sFlt1
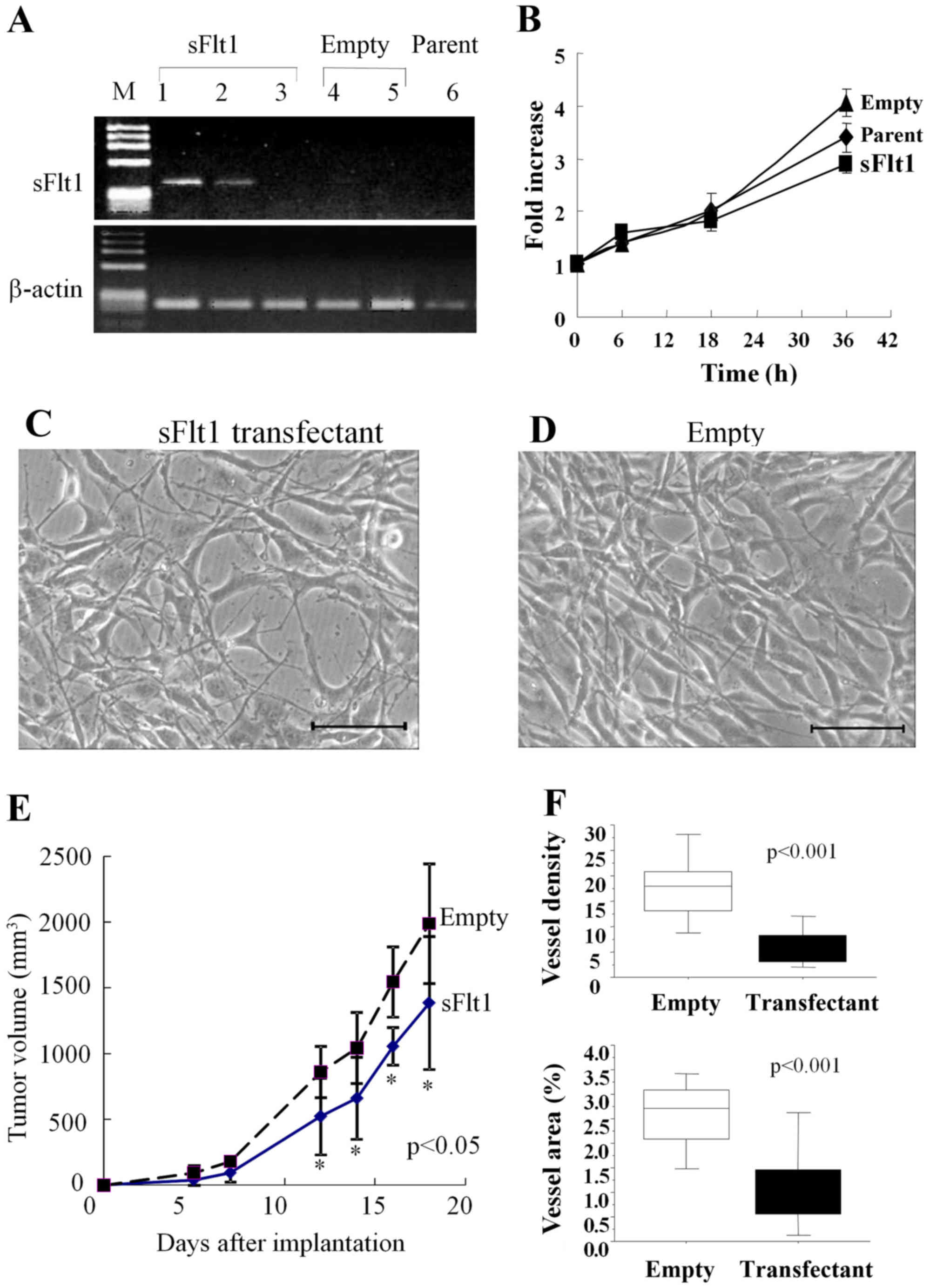
gene. One of the clones expressing sFlt1 (Fig. 3A, lane 1) was used in the
subsequent experiments.
The cells were incubated with 5% CO2 at
37°C for 24 h under hypoxic (0.1% O2) or normoxic
conditions (20% O2). Hypoxic conditions were established
by placing the cells in a molecular incubator chamber (Model,
APM-30D; Astec, Columbus, OH, USA) with a gas mixture consisting of
1% O2, 5% CO2 and balanced N2 for
the indicated period. Conditioned medium was collected for western
blot analysis and ELISA.
Cell proliferation assay (MTT assay)
Cell proliferation assays were performed using the
CellTiter 96™ Aqueous nonradioactive proliferation assay (Promega
Corp., Madison, WI, USA), as previously described (22). This assay measures the reduction of
a tetrazolium
compound,3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTT), by living cells to a formazan product. Briefly, 5000 glioma
cells, at 1×105/ml in MEM with 10% FCS, were plated in
96-well plates (Becton-Dickinson, Lincoln Park, NJ, USA). The cells
were incubated for 6, 18 or 36 h. At the end of the incubation
period, the microplate wells were supplemented with 20 µl of
a freshly prepared solution containing a mixture of a tetrazolium
compound and an electron coupling reagent (phenazine methosulfate)
and incubated for 2 h at 37°C. Then, the optical density at 490 nm
was read on an automatic microplate reader (Model 550; Bio-Rad
Laboratories). The experiment was repeated at least three times in
triplicate wells.
Western blot analysis
The tissues and the cell pellets were homogenized
with an ultrasonic homogenizer on ice in 1 ml of extraction buffer
[25 mM Tris, 100 mM NaCl, 20 mM NH4HCO3, pH
7.5, protease inhibitor cocktail; Complete Mini (Roche), one
tablet] per 100 mg wet weight of tissue and the protein lysates
were obtained after centrifugation at 50,000 × g for 30 min at 4°C.
Lysates containing 50 µg of total protein, as estimated by
the Bradford method using bovine serum albumin (BSA) as a standard,
were separated on 12% sodium dodecyl sulfate polyacrylamide gel,
electroblotted onto a 0.2-µm nitrocellulose membrane
(Bio-Rad Laboratories), and immunoassayed with rabbit polyclonal
anti-VEGF antibody (A-20, 100 µg/ml; Santa Cruz
Biotechnology) at a dilution of 1:200 and mouse monoclonal
anti-human sFlt1 antibody (ab9540; Abcam) at a dilution of 1:100.
The immunocomplexes formed were visualized with alkaline
phosphatase-conjugated anti-rabbit or anti-mouse immunoglobulin G
(IgG) using the ECL Western blotting analysis system (Amersham
Pharmacia Biotech, Piscataway, NJ, USA).
U87 severe combined immunodeficiency
(SCID) mouse subcutaneous model
After the implantation of 1×105 U87 cells
(sFlt1 transfectant: sFlt1, empty transfectant:empty) in the flank
of a 6-week-old male SCID mouse (Clea, Inc., Tokyo Japan), U87
transfectant tumor tissue fragments were removed and then
reimplanted into the flank of another SCID mouse. Harvested tumor
fragments 1 mm3 in size were implanted into the flank of
6 SCID mice for each transfectant. The size of the subcutaneous
tumor was measured using calipers. Eighteen days after the
implantation, the tumor tissue was removed. Some of the tissue was
immediately fixed in 10% phosphate-buffered formalin for 48 h,
embedded in paraffin, and used for routine pathological diagnosis
and immunohistochemistry. The rest of the tissue was immediately
homogenized for protein extraction, frozen with liquid nitrogen for
mRNA extraction, and stored at −70°C. Ethics approval was from the
University of Tsukuba, Tsukuba, Japan.
In order to evaluate the hypoxic area in tumor
sections, pimonidazole hydrochloride (Hypoxyprobe-1; Chemicon
International, Temecula, CA, USA) was administered at 120 mg/kg
just before sacrifice. Paraffinized tissue sections were stained
with anti-pimonidazole antibody (Chemicon International) at a
dilution of 1:20 in PBS for 60 min at room temperature to visualize
the hypoxic area (22).
RNA isolation and RT-PCR
Total RNA was extracted from the transfectants and
from frozen tumor tissue derived from them (5 empty transfectants
and 6 sFlt1 transfectants) using RNeasy Mini kit (Qiagen GmbH,
Hilden, Germany). Quantitative RT-PCR for sFlt1 and VEGF mRNA in
glioma cells and glioma tissues was performed. We performed RT-PCR
with the GeneAmp™RNA PCR kit (Perkin-Elmer Cetus, Norwalk, CT,
USA). Briefly, 1 µg of total RNA was reverse-transcribed by
MuLV reverse transcriptase in the presence of random hexamer,
followed by the indicated cycles of PCR reaction (95°C for 1 min,
55°C for 1 min and 72°C for 1 min) in the presence of 2 µM
sFlt1-specific primers (32 cycles), VEGF-specific primers (28
cycles) or β-actin-specific primers (16 cycles) as a control. The
sFlt1 primers were designed so that the reverse primer
(5′-TATGTTTCTTCCC ACAGTCCCAAC-3′) corresponds to positions
2178–2201, and the forward primer (5′-CTAATTGTCAATGTGAAACC CCAG-3′)
corresponds to positions 1821–1844. The VEGF primers included a
reverse primer (5′-CCTGGTGAGAG ATCTGGTTC-3′) spanning bases 861-842
and a forward primer (5′-TCGGGCCTCCGAAACCATGA-3′) spanning bases
141-160. The β-actin primers included a reverse primer
(5′-GGAGTTGAAGGTAGTTTCGTG-3′) spanning bases 2429-2409 and a
forward primer (5′-CGGGAAATCGTGC GTGACAT-3′) spanning bases
2107-2126. The predicted sizes of the amplified sFlt1 and β-actin
DNA products were 380 and 214 bp, respectively. The VEGF primers
were chosen because they amplified exons 3–8 and allowed the
different VEGF splicing variants to be distinguished. PCR products
of 516 and 648 bp corresponded to VEGF121 and VEGF165,
respectively. Quantification of the levels of these RT-PCR products
was performed on a Macintosh computer using the public domain NIH
Image program (developed at the U.S. National Institutes of Health,
Bethesda, MD, USA).
Statistical analyses
Vascular density, vessel area, vessel diameter,
vessel perimeter, vessel roundness, MIB-1 positivity, tumor volume,
VEGF concentration and sFlt-1 concentration are expressed as mean ±
standard deviation. Statistically significant differences between
the groups were determined using a one-way analysis of variance and
the Tukey's test. All P-values are two-sided; values are considered
statistically significant at P<0.05. The survival time was
calculated by the Kaplan-Meier method. Differences in survival were
assessed by the log-rank test. For categorical variables,
two-tailed Fisher's exact test was used.
Results
Angiogenic profiles and
angioarchitectural features as prognostic factors in malignant
gliomas
Our treatment strategy for malignant gliomas in the
study period was total or subtotal removal, following by 40-Gy
whole-brain and 20-Gy local boost irradiation combined with PAV
(procarbazine, ACNU and vincristine) chemotherapy (23) and interferon-β administration. Upon
recurrence, PE (cisplatin and etoposide) and temozolomide
chemotherapy and/or immunotherapy, including locolesional natural
killer or cytotoxic T lymphocyte injection (24) were carried out. The median survival
time of all cases of malignant glioma was 19.2 months (grade IV,
glioblastoma: 11.2 months, grade III, anaplastic
astrocytoma/anaplastic oligodendroglioma: 30.9 months).
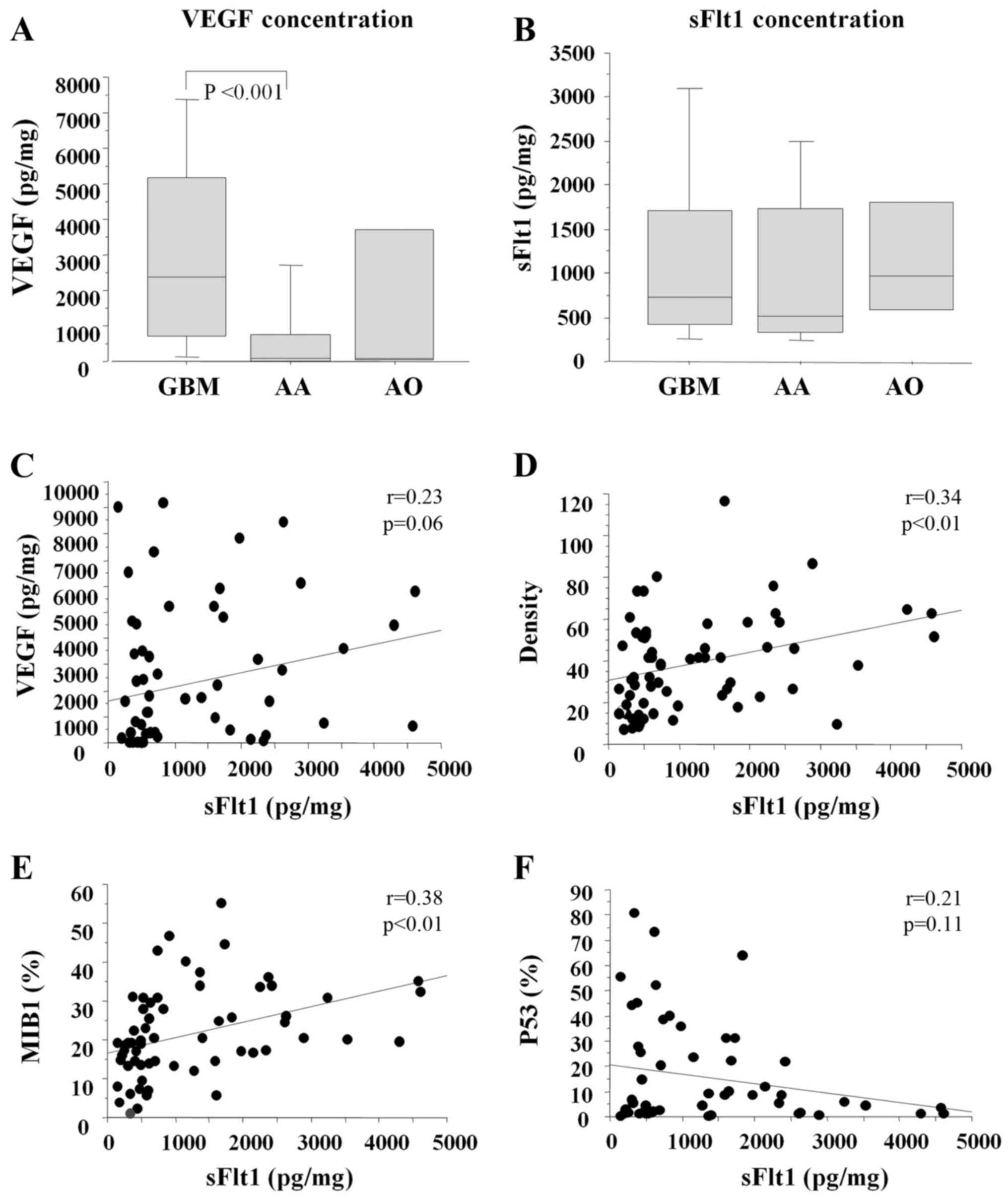
Fig. 1 and Table I show the angiogenic profiles of
each case. VEGF concentration was significantly high in
glio-blastoma compared with that in anaplastic astrocytoma, while
sFlt1 concentration was not. sFlt1 concentration was significantly
correlated with vessel density (r=0.34, P<0.01) and MIB1
positivity (r=0.38, P<0.01), and weakly correlated with VEGF
concentration (P=0.06). VEGF/sFlt1 ratio (>1), pathology (grade
IV), MIB1% (>20%) and vessel area (>7%) were poor prognostic
factors as determined by univariate analysis. Among these,
VEGF/sFlt1 ratio >1 was the strongest independent prognostic
factor by multivariate analysis (hazard ratio 1.99, 95% confidence
interval 1.08–3.66; P<0.05).
Survival time was evaluated by the Kaplan-Meier
analysis (Table II and Fig. 2). This analysis again demonstrated
the survival benefit associated with VEGF concentration (<1000
pg/mg), pathology (grade III), MIB-1% (<20%) and vessel area
(<7%). Although there was no survival benefit associated with
sFlt1 concentration itself, VEGF/sFlt1 concentration ratio ≤1 was
the variable with the strongest survival benefit for cases of
malignant glioma. The median survival time was 11.3 months for
those with a ratio >1 and 28.7 months for those with a ratio
<1 (P<0.001). These results suggest that the balance of
stimulators and inhibitors of angiogenesis is particularly
important for the angiogenic evaluation of malignant gliomas. Each
parameter was then evaluated only for glioblastomas. VEGF,
VEGF/sFlt1 ratio, MIB1 and vessel density were not prognostic
factors for this condition, but vessel area >7% was a strong
prognostic factor for the glioblastoma group alone (data not
shown).
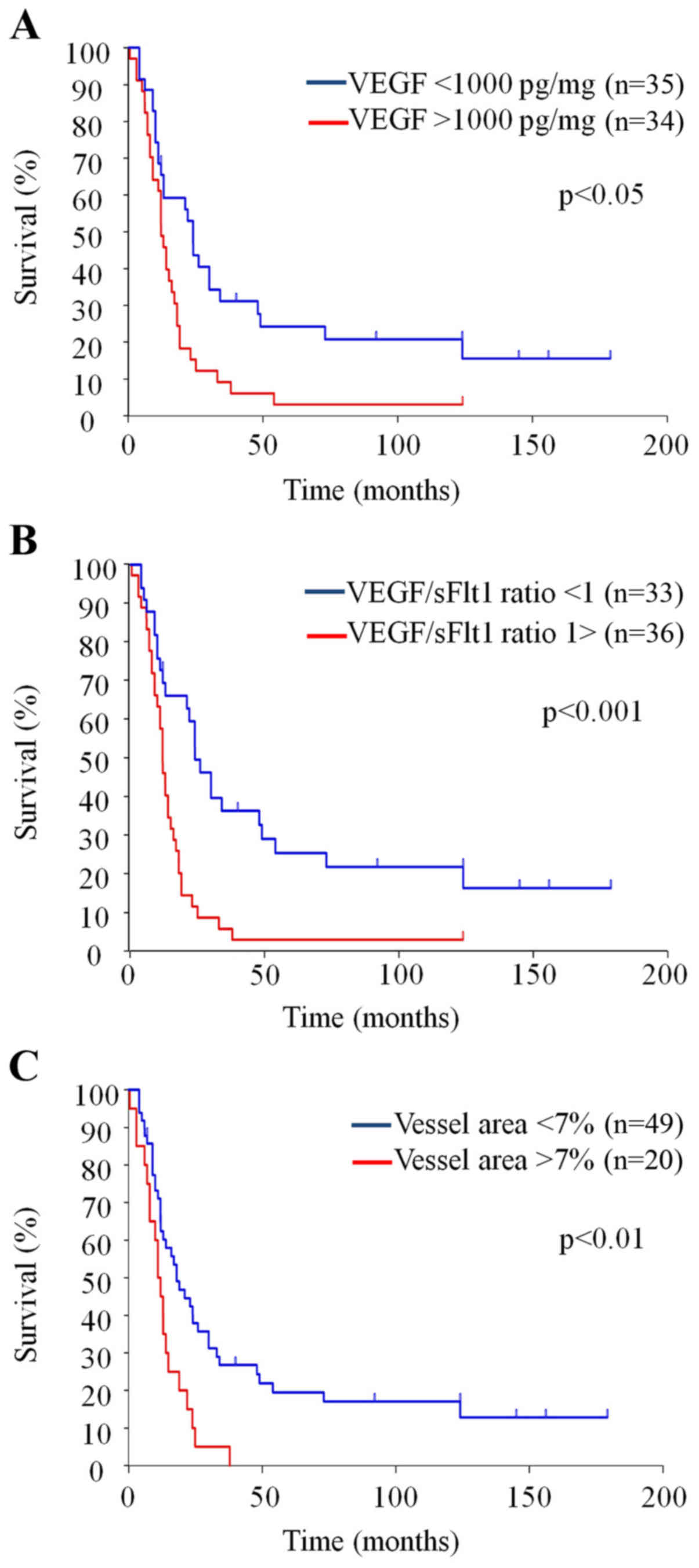 | Figure 2Survival curves with VEGF
concentration (A), VEGF/sFlt1 ratio (B) and vessel area (C). (A)
Overall survival is significantly longer with VEGF <1000 pg/mg
(51.3 mo) than VEGF >1000 pg/mg (18.0 mo) [Stratified hazard
ratio, 2.17 (95% CI, 1.28–3.70), P<0.05 by log-rank test]. (B)
Overall survival is significantly longer with VEGF/sVEGFR ratio
<1 (54.9 mo) than VEGF/sVEGFR ratio >1 (16.4 mo) [Stratified
hazard ratio, 2.79 (95% CI, 1.61–4.86), P<0.001 by log-rank
test]. (C) Overall survival is significantly longer with vessel
area <7% (44.8 mo) than vessel area >7% (13.1%) [Stratified
hazard ratio, 2.39 (95% CI, 1.35–4.21), P<0.01 by log-rank
test]. Please note statistically significant difference is stronger
with VEGF/sFlt1 ratio compared with VEGF concentration alone. |
 | Table IIPrognostic factors in malignant
gliomas (Kaplan-Meier). |
Table II
Prognostic factors in malignant
gliomas (Kaplan-Meier).
| Parameters | n | MST (month) | Log-rank |
|---|
| VEGF (pg/mg) |
| >1000 | 34 | 18.0 | <0.05 |
| <1000 | 35 | 51.3 | |
| sVEGFR1
(pg/mg) |
| <1000 | 40 | 32.1 | ns |
| >1000 | 29 | 36.0 | |
| VEGF/R1 ratio |
| >1 | 36 | 16.4 | <0.001 |
| <1 | 33 | 54.9 | |
| Pathology |
| Grade IV | 40 | 19.5 | <0.001 |
| Grade III | 29 | 53.7 | |
| MIB1 (%) |
| >20 | 34 | 20.2 | <0.05 |
| <20 | 35 | 48.2 | |
| p53 (%) |
| >10 | 24 | 26.2 | ns |
| <10 | 37 | 39.9 | |
| Density/0.1
mm2 |
| >30 | 38 | 31.8 | ns |
| <30 | 31 | 36.2 | |
| Vessel area
(%) |
| >7 | 20 | 13.1 | <0.01 |
| <7 | 49 | 44.8 | |
The effect of sFlt1 overexpression on
glioma angiogenesis and growth
Under a microscope, both sFlt1 transfectant and
empty vector transfectant showed a fusiform shape similar to that
of the parental U87 cells. The growth rates of these transfectants
and the parents were similar, as determined by the WST 8 assay
(Fig. 3B–D).
In a subcutaneous model, glioma growth was
significantly inhibited for the sFlt1 transfectant compared with
that for the empty vector transfectant (Fig. 3E; P<0.05). Tumor volume
reduction for the sFlt1 transfectant was only 31%. In tissue
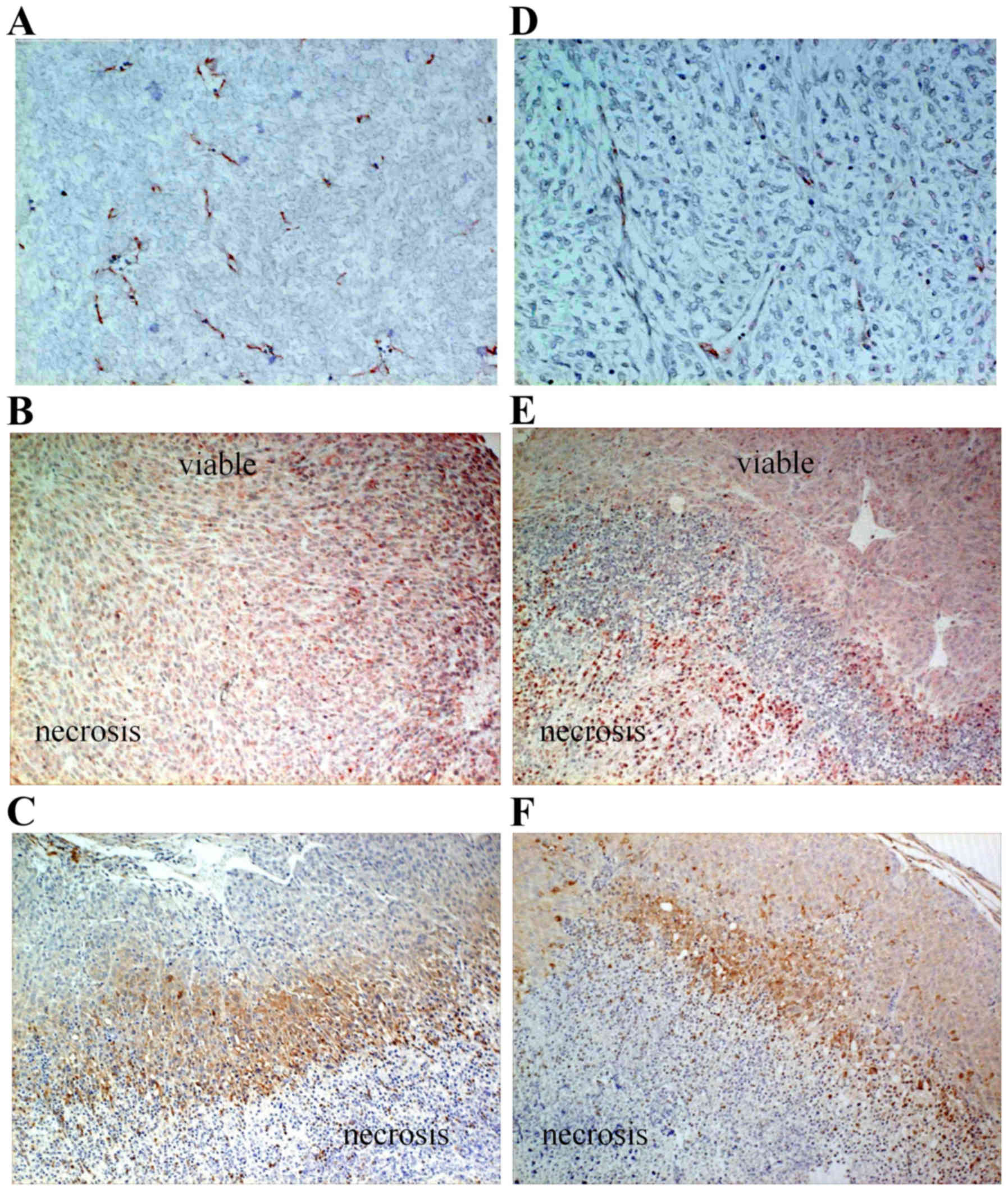
sections, vessel density and area were significantly decreased for
the sFlt1 transfectant (Figs. 3F
and 4A and D; P<0.001). VEGF
expression was also inhibited in the viable tumor area for the
sFlt1 transfectant (Fig. 4B and
E). However, even for the sFlt1 transfectant, there was still a
pimonidazole-stained hypoxic area that was very similar to that in
the empty transfec-tant, in which positive VEGF staining was
observed (Fig. 4B, C, E and F).
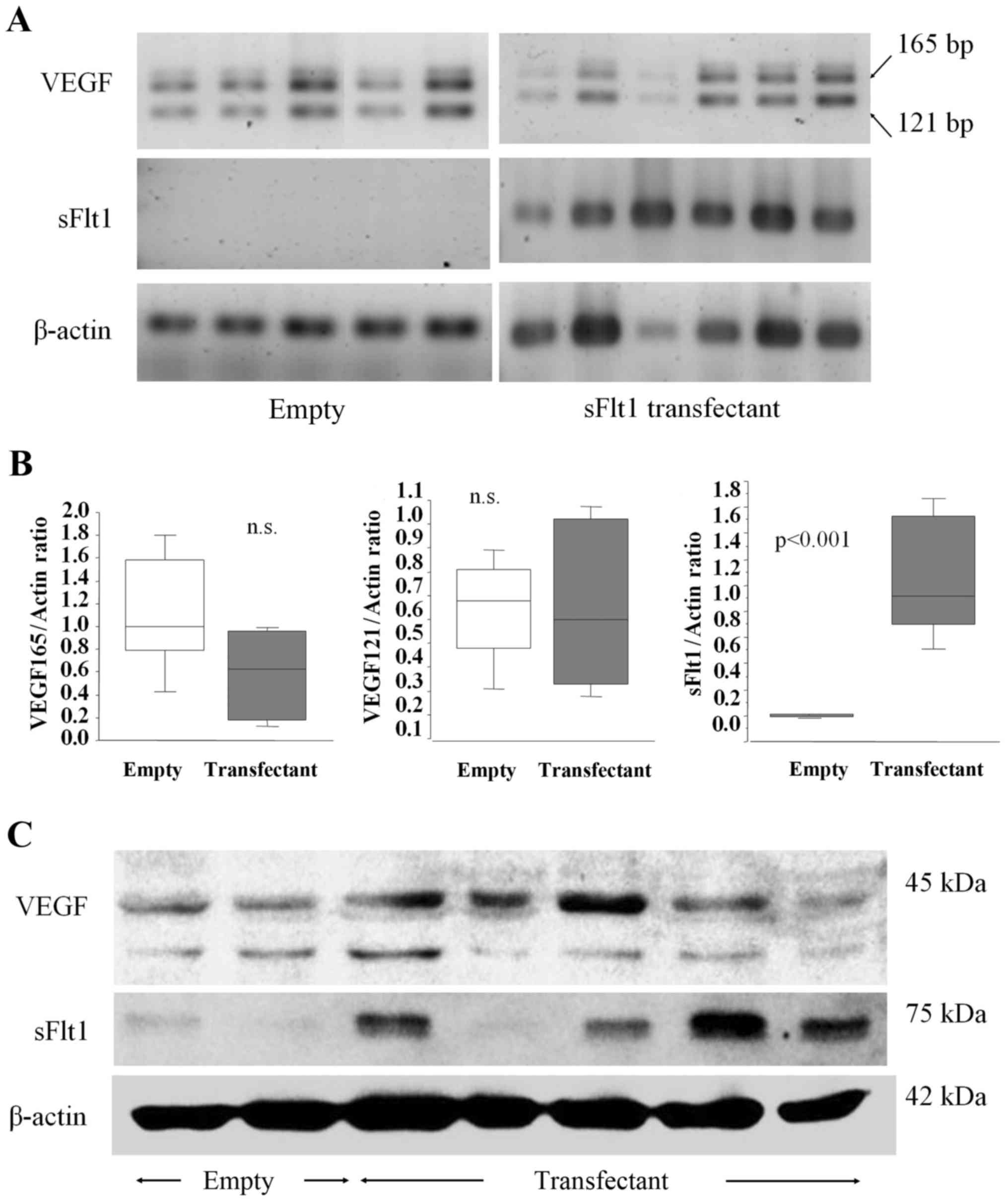
RT-PCR analysis of tumor tissues demonstrated that sFlt1 mRNA
expression was significantly upregulated for the sFlt1 transfectant
(Fig. 5A and B). Western blot
analysis of tumor tissues also demonstrated that sFlt1 protein
expression was significantly upregulated (Fig. 5C). By contrast, VEGF mRNA and
protein expression levels were not changed by sFlt1 transfection
(Fig. 5A–C).
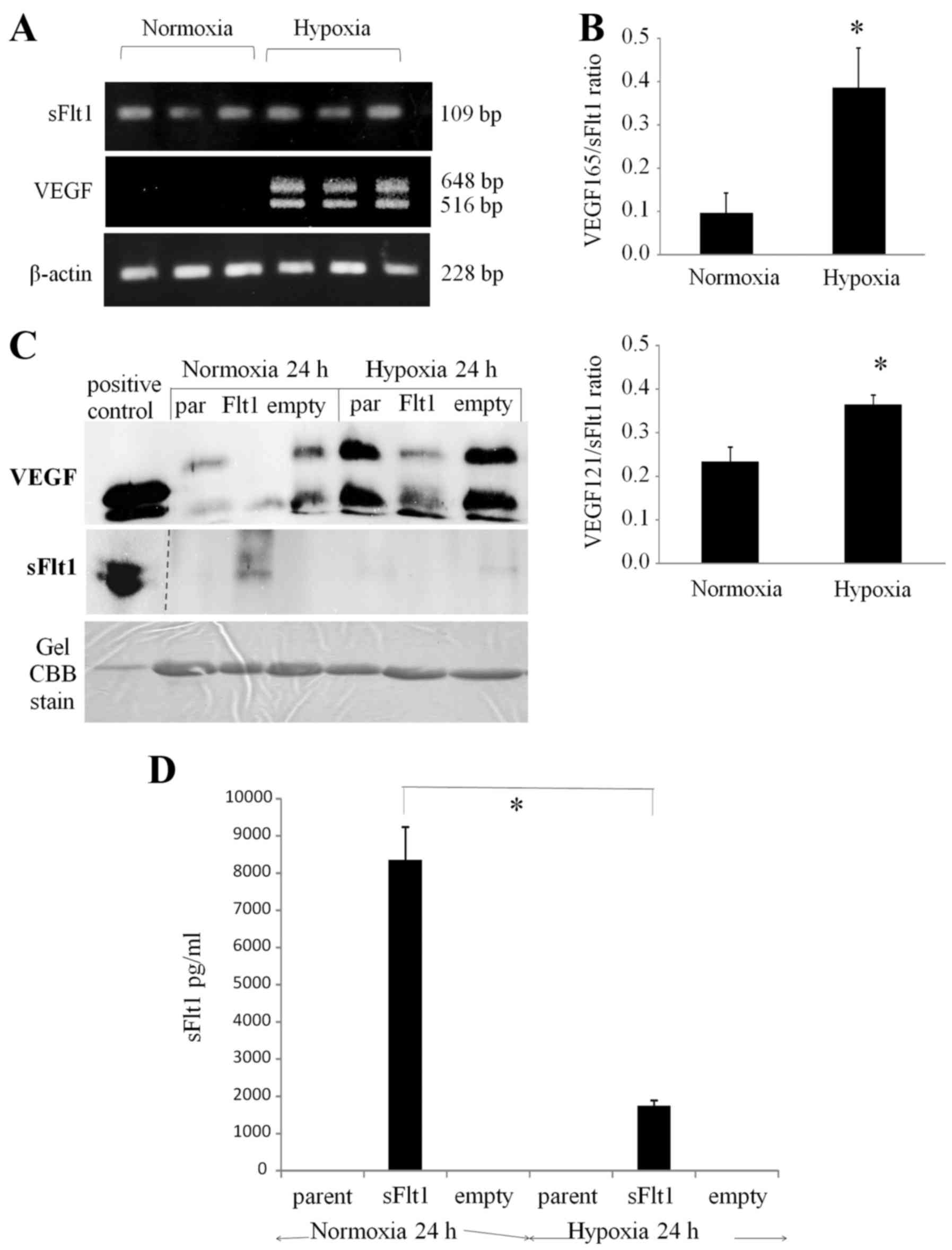
In order to investigate the mechanism by which the
hypoxic area was maintained in tissue sections, the transfectant
profile under hypoxic conditions was evaluated in vitro.
First, RT-PCR analysis showed the upregulation of both
VEGF121 and VEGF165 expression, but not sFlt1
expression, under hypoxic conditions (1% O2, 24 h)
compared with that under normoxic ones (20% O2, 24 h)
(Fig. 6A and B). Second, the
levels of VEGF and sFlt1 secretion into conditioned medium for the
sFlt1 transfectant, empty transfectant, and parent were measured by
western blotting under normoxic and hypoxic conditions (1%
O2, 24 h). As expected, VEGF secretion was upregulated
for the three types of cells under hypoxic conditions. The sFlt1
transfectant secreted sFlt1 into the conditioned medium under
normoxic conditions, but secreted little or none under hypoxia
(Fig. 6C). sFlt1 concentration in
the conditioned medium was precisely measured by sFlt1 ELISA and
was detected only for the sFlt1 transfectant. The sFlt1
concentration was 8340.3±890.4 pg/ml under normoxic conditions and
1735.3±140.1 pg/ml under hypoxia, which were significantly
different (Fig. 6D;
P<0.01).
Discussion
In the present study, we searched for prognostic
factors for the survival of patients with malignant gliomas by
measuring VEGF and sFlt1 concentrations, proliferation rate and
vascular architecture. VEGF concentration, VEGF/sFlt1 concentration
ratio, pathological grade of tumor, and vessel area were all
identified as prognostic factors. Among these variables, VEGF/sFlt1
ratio was the strongest independent prognostic factor. The
anti-angiogenic role of sFlt1 was then evaluated in vitro
and in vivo by introducing the sFlt1 gene into glioma cells.
This resulted in the inhibition of glioma growth and angiogenesis,
but such effects were only moderate because angiogenic inhibition
was not achieved under hypoxic conditions.
sFlt1 as a biomarker of glioma
angiogenesis
For the potential biomarker sFlt1, two different
variables can be measured, namely, its levels in tissue and
circulation. It is important to verify this candidate for
anti-angiogenic treatment in malignant gliomas by considering both
of these alternatives. Concerning the tissue biomarker, the present
study clearly showed that, among the variables in angiogenic
profiles, vessel area and the ratio of VEGF to its endogenous
inhibitor sFlt-1 strongly predict the prognosis of patients with
malignant gliomas. Although the concentration of VEGF, but not that
of sFlt1, was also a prognostic factor, the VEGF/sFlt1
concentration ratio should be considered a stronger independent
prognostic factor. These results are useful for the further
development of individualized anti-angiogenic therapy because
gliomas with a high VEGF/sFlt1 ratio should be responsive to
anti-angiogenic treatment.
Lamszus et al (25) reported that the concentrations of
sFlt1 protein were markedly increased in glioblastomas compared
with those in low-grade gliomas and normal brain. The concentration
of sFlt1 correlated with the malignancy grade and was 12-fold
higher in glioblastomas than in diffuse astrocytomas, with
intermediate levels being exhibited in anaplastic astrocytomas.
Although the absolute levels of sVEGFR-1 were increased in more
malignant gliomas, the sVEGFR-1:VEGF-A ratio was decreased 2.6-fold
in glioblastomas compared with that in diffuse astrocytomas,
suggesting that the ensuing increased bioavailability of VEGF-A
promotes angiogenesis. sFlt1 could be useful as an angiogenesis
inhibitor in the specific context of human gliomas. In this study,
the concentration of sFlt1 did not differ between glioblastoma and
grade III glioma (anaplastic astrocytoma and anaplastic
oligodendroglioma). The net balance between VEGF and sFlt1 would
still be tilted towards angiogenesis in malignant gliomas. In the
previous study mentioned above, no survival data related to the net
balance of VEGF/sFlt1 were presented. However, we demonstrated a
survival benefit associated with a lower VEGF/sFlt1 ratio in
malignant gliomas.
Several studies have focused on tissue biomarkers
associated with bevacizumab treatment. It has been observed in
tissue studies conducted in patients with recurrent high-grade
glioma treated with bevacizumab and irinotecan that high expression
of VEGF was associated with a higher likelihood of achieving a
radiographic response, but not increased survival (26). It was also observed in this same
study that elevated levels of carbonic anhydrase 9, a hypoxic
marker, were significantly associated with poor one-year survival.
In contrast, in a study of patients with glioblastoma treated with
bevacizumab and irinotecan with or without cetuximab (an EGFR
inhibitor), no biomarker was predictive of response to treatment or
prolongation of PFS (27).
Finally, a retrospective autopsy study of patients with recurrent
glioblastoma treated with various anti-VEGF agents including
bevacizumab showed that elevated numbers of CD68+ and
CD11+ tumor-associated macrophages were associated with
poor survival, indicating a potential biomarker of escape (28). In summary, several studies have
identified different tumor tissue markers that may serve as
biomarkers for response to treatment. However, the ratio of
VEGF/sFlt1 concentrations, which we identified as an independent
prognostic factor in malignant gliomas, has not yet been
investigated in terms of response to anti-VEGF agents.
The incorporation of bevacizumab and other anti-VEGF
agents into the early treatment of cancer patients would be
significantly enhanced by the discovery of circulating biomarkers
that can be used to establish rational guidelines for the selection
and use of these agents. In rectal cancer, baseline serum sFlt-1
was found to be a predictive biomarker for therapies that include
bevacizumab (29). However,
reports of circulating biomarkers for glioma are limited. The
AVAglio study, which included the evaluation of pretreatment plasma
VEGF and sVEGFR-2, found no association with PFS (6). A similar lack of associations between
pretreatment biomarkers, including VEGF and sVEGFR-2, and treatment
outcome in patients with GBM was reported for cediranib, vatalanib
and vandetanib (30–33). However, increases in serum sVEGFR-1
(sFlt1) have been found to be associated with poor survival in
patients treated with cediranib (31). Duda et al (29) also reported that serum sFlt1
increased during anti-VEGF therapy in rectal cancer cases; they
proposed that an increase of sFlt1 is a potential biomarker of
resistance to anti-VEGF therapy. Unfortunately, the significance of
circulating biomarkers in gliomas has still not been clarified.
sFlt1 as a target of anti-angiogenic
molecules in malignant gliomas
We demonstrated that a higher sFlt1 concentration
was strongly associated with higher vascular density in malignant
glioma tissues. The levels of sFlt1 present in human glioma tissues
were clearly insufficient to prevent neovascularization in
malignant gliomas. A molar concentration of sFlt1 that exceeds that
of VEGF by 2- to 200-fold was reported to be required to obtain
significant effects on endothelial chemotaxis in vitro
(25). Therefore, considerably
higher levels appear to be required to effectively inhibit the
angiogenic effects of VEGF on a glioma xenograft model in
vivo. We planned to perform sFlt1 gene transfer for glioma
cells that have no sFlt1 expression in vitro.
In several animal models, adenovirus-mediated
overexpression of sFlt1 in tumor cells was shown to inhibit the
growth of melanoma and lung cancer in vivo (34,35)
and a mammalian cell-mediated approach effectively delivered sFlt-1
gene therapy and inhibited the angiogenesis and growth of thyroid
cancer in vivo (36).
However, some reports suggest that intravenous delivery of the
sFlt-1 gene via adenoviral vectors results in the overexpression of
sFlt-1 in the liver, leading to unacceptable hepatotoxicity
(37). Therefore, tumor-specific
targeting of vectors and tumor-specific expression strategies
should be used to ensure clinically useful anti-angiogenic gene
therapy. Previously, Goldman et al (38) reported that the growth of tumor
cells transfected with sFlt1-expressing plasmid was inhibited in
fibrosarcoma cells in a subcutaneous tumor model and prolonged the
survival time of glioma cells in a transcranial model. In our in
vitro study, sFlt1 was successfully expressed in glioma cells
at the gene and protein levels and we confirmed its secretion into
conditioned medium by transfectants in vitro. However, our
in vivo study demonstrated that the growth inhibitory effect
of sFlt1-transfected glioma cells was only 31%, even with
significant upregulation of sFt1 gene and protein expression in
tumor tissues. We found that the target molecule VEGF was still
present in the hypoxic area of tumor tissue in vivo. We also
confirmed the downregulation of sFlt1 secretion of sFlt1
transfectants under hypoxic conditions in vitro. VEGF
expression in the sFlt1 transfectants was upregulated similarly to
that in the parental cells, resulting in the net balance of
VEGF/sFlt1 being increased under hypoxic conditions. This increase
was considered to limit the growth inhibitory effect in brain
tumors in vivo. Downregulation of sFlt1 overexpression in
lentiviral-constructed human endothelial cells under hypoxic
conditions has been reported to occur by a mechanism involving mRNA
alternative processing (39). In
this study, under hypoxic conditions, mRNA expression was
maintained, but protein secretion was inhibited.
Most solid tumors develop regions of low oxygen
tension because of an imbalance between oxygen supply and
consumption (40,41). Several ideas have been proposed to
overcome the downregulation of VEGF/sFlt-1 ratio under hypoxic
conditions. For example, strategies to treat tumors have been
developed in which tumor cells are targeted with drugs or gene
therapy vectors specifically activated under hypoxic conditions
(42). Hypoxia in the tumor
microenvironment provides ideal conditions for anaerobic microbes
to survive. For example, Bifidobacterium infantis is a type
of bifidobacteria that is non-pathogenic and anaerobic; thus, it
can be transfected with an anti-angiogenic gene and selectively
localize and proliferate in the hypoxic environment in several
types of solid tumor after systemic application (43,44).
A hypoxia-responsive glial cell-specific gene therapy vector for
targeting pathological neovascularization has also been utilized
(45). A wide variety of
nanomedicine has been designed for cancer therapy, of which
hypoxia-responsive copolymer for siRNA delivery is one example
(46). New strategies for the
delivery of anti-angiogenic agents, such as sFlt1, into hypoxic
areas are required to ensure the long-term maintenance of an
anti-angiogenic effect.
In conclusion, a VEGF/sFlt1 ratio of >1 was
identified as a predictor of poor survival in cases of malignant
glioma, suggesting that the sFlt1 activity as an endogenous
angiogenesis inhibitor regulates glioma angiogenesis. sFlt1 is
thus, a candidate molecular target for glioma angiosuppression.
However, future work should focus on the regulation of sFlt1
overexpression under hypoxic conditions.
Acknowledgments
We are grateful to Yoshiko Tsukada and Makiko
Miyakawa for their excellent technical assistance. The present
study was supported in part by a Grant-in-Aid for Scientific
Research from the Ministry of Education, Culture, Sports, Science,
and Technology of Japan (no. 15H04947 to S.T.), the Japan Brain
Foundation (to S.T.) and the Japanese Foundation for
Multidisciplinary Treatment of Cancer (to S.T.).
References
|
1
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu-Emerson C, Duda DG, Emblem KE, Taylor
JW, Gerstner ER, Loeffler JS, Batchelor TT and Jain RK: Lessons
from anti-vascular endothelial growth factor and anti-vascular
endothelial growth factor receptor trials in patients with
glioblastoma. J Clin Oncol. 33:1197–1213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shivakumar S, Prabhakar BT, Jayashree K,
Rajan MG and Salimath BP: Evaluation of serum vascular endothelial
growth factor (VEGF) and microvessel density (MVD) as prognostic
indicators in carcinoma breast. J Cancer Res Clin Oncol.
135:627–636. 2009. View Article : Google Scholar
|
|
5
|
Botelho F, Pina F and Lunet N: VEGF and
prostatic cancer: A systematic review. Eur J Cancer Prev.
19:385–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishikawa R, Saran F, Mason W, Wick W,
Cloughesy TF, Henriksson R, Hilton M, Garcia J, Vogt T, Pallaud C,
et al: Biomarker (BM) evaluations in the phase III AVAglio study of
bevacizumab (Bv) plus standard radiotherapy (RT) and temozolomide
(T) for newly diagnosed glioblastoma (GBM). J Clin Oncol.
31:20232013.
|
|
7
|
Batchelor TT, Reardon DA, de Groot JF,
Wick W and Weller M: Antiangiogenic therapy for glioblastoma:
Current status and future prospects. Clin Cancer Res. 20:5612–5619.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prea SM, Chan EC, Dusting GJ, Vingrys AJ,
Bui BV and Liu GS: Gene therapy with endogenous inhibitors of
angiogenesis for neovascular age-related macular degeneration:
Beyond anti-VEGF therapy. J Ophthalmol. 2015:2017262015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rao N, Lee YF and Ge R: Novel endogenous
angiogenesis inhibitors and their therapeutic potential. Acta
Pharmacol Sin. 36:1177–1190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Volpert OV, Zaichuk T, Zhou W, Reiher F,
Ferguson TA, Stuart PM, Amin M and Bouck NP: Inducer-stimulated Fas
targets activated endothelium for destruction by anti-angiogenic
thrombospondin-1 and pigment epithelium-derived factor. Nat Med.
8:349–357. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beerepoot LV, Witteveen EO, Groenewegen G,
Fogler WE, Sim BK, Sidor C, Zonnenberg BA, Schramel F, Gebbink MF
and Voest EE: Recombinant human angiostatin by twice-daily
subcutaneous injection in advanced cancer: A pharmacokinetic and
long-term safety study. Clin Cancer Res. 9:4025–4033.
2003.PubMed/NCBI
|
|
14
|
Kurup A, Lin C, Murry DJ, Dobrolecki L,
Estes D, Yiannoutsos CT, Mariano L, Sidor C, Hickey R and Hanna N:
Recombinant human angiostatin (rhAngiostatin) in combination with
paclitaxel and carboplatin in patients with advanced non-small-cell
lung cancer: A phase II study from Indiana University. Ann Oncol.
17:97–103. 2006. View Article : Google Scholar
|
|
15
|
Rong B, Yang S, Li W, Zhang W and Ming Z:
Systematic review and meta-analysis of Endostar (rh-endostatin)
combined with chemotherapy versus chemotherapy alone for treating
advanced non-small cell lung cancer. World J Surg Oncol.
10:1702012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uronis HE, Cushman SM, Bendell JC, Blobe
GC, Morse MA, Nixon AB, Dellinger A, Starr MD, Li H, Meadows K, et
al: A phase I study of ABT-510 plus bevacizumab in advanced solid
tumors. Cancer Med. 2:316–324. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Westphal JR: Technology evaluation:
ABT-510, Abbott. Curr Opin Mol Ther. 6:451–457. 2004.PubMed/NCBI
|
|
18
|
Cui C, Mao L, Chi Z, Si L, Sheng X, Kong
Y, Li S, Lian B, Gu K, Tao M, et al: A phase II, randomized,
double-blind, placebo-controlled multicenter trial of Endostar in
patients with metastatic melanoma. Mol Ther. 21:1456–1463. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Davis-Smyth T, Chen H, Park J, Presta LG
and Ferrara N: The second immunoglobulin-like domain of the VEGF
tyrosine kinase receptor Flt-1 determines ligand binding and may
initiate a signal transduction cascade. EMBO J. 15:4919–4927.
1996.PubMed/NCBI
|
|
20
|
Kuo CJ, Farnebo F, Yu EY, Christofferson
R, Swearingen RA, Carter R, von Recum HA, Yuan J, Kamihara J, Flynn
E, et al: Comparative evaluation of the antitumor activity of
antiangiogenic proteins delivered by gene transfer. Proc Natl Acad
Sci USA. 98:4605–4610. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takano S, Yoshii Y, Kondo S, Suzuki H,
Maruno T, Shirai S and Nose T: Concentration of vascular
endothelial growth factor in the serum and tumor tissue of brain
tumor patients. Cancer Res. 56:2185–2190. 1996.PubMed/NCBI
|
|
22
|
Takano S, Kamiyama H, Mashiko R, Osuka S,
Ishikawa E and Matsumura A: Metronomic treatment of malignant
glioma xenografts with irinotecan (CPT-11) inhibits angiogenesis
and tumor growth. J Neurooncol. 99:177–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Levin VA, Uhm JH, Jaeckle KA, Choucair A,
Flynn PJ, Prados MD, Bruner JM, Chang SM, Kyritsis AP, Gleason MJ,
et al: Yung WKA: Phase III randomized study of postradiotherapy
chemotherapy with alpha-difluoromethylornithine-procarbazine,
N-(2-chloroethyl)-N′-cyclohexyl-N-nitrosurea, vincristine
(DFMO-PCV) versus PCV for glioblastoma multiforme. Clin Cancer Res.
6:3878–3884. 2000.PubMed/NCBI
|
|
24
|
Tsuboi K, Saijo K, Ishikawa E, Tsurushima
H, Takano S, Morishita Y and Ohno T: Effects of local injection of
ex vivo expanded autologous tumor-specific T lymphocytes in cases
with recurrent malignant gliomas. Clin Cancer Res. 9:3294–3302.
2003.PubMed/NCBI
|
|
25
|
Lamszus K, Ulbricht U, Matschke J,
Brockmann MA, Fillbrandt R and Westphal M: Levels of soluble
vascular endothelial growth factor (VEGF) receptor 1 in astrocytic
tumors and its relation to malignancy, vascularity, and VEGF-A.
Clin Cancer Res. 9:1399–1405. 2003.PubMed/NCBI
|
|
26
|
Sathornsumetee S, Cao Y, Marcello JE,
Herndon JE II, McLendon RE, Desjardins A, Friedman HS, Dewhirst MW,
Vredenburgh JJ and Rich JN: Tumor angiogenic and hypoxic profiles
predict radiographic response and survival in malignant astrocytoma
patients treated with bevacizumab and irinotecan. J Clin Oncol.
26:271–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hasselbalch B, Eriksen JG, Broholm H,
Christensen IJ, Grunnet K, Horsman MR, Poulsen HS, Stockhausen MT
and Lassen U: Prospective evaluation of angiogenic, hypoxic and
EGFR-related biomarkers in recurrent glioblastoma multiforme
treated with cetuximab, bevacizumab and irinotecan. APMIS.
118:585–594. 2010.PubMed/NCBI
|
|
28
|
Lu-Emerson C, Snuderl M, Kirkpatrick ND,
Goveia J, Davidson C, Huang Y, Riedemann L, Taylor J, Ivy P, Duda
DG, et al: Increase in tumor-associated macrophages after
antiangiogenic therapy is associated with poor survival among
patients with recurrent glioblastoma. Neuro Oncol. 15:1079–1087.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duda DG, Willett CG, Ancukiewicz M, di
Tomaso E, Shah M, Czito BG, Bentley R, Poleski M, Lauwers GY,
Carroll M, et al: Plasma soluble VEGFR-1 is a potential dual
biomarker of response and toxicity for bevacizumab with
chemoradiation in locally advanced rectal cancer. Oncologist.
15:577–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Batchelor TT, Sorensen AG, di Tomaso E,
Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M,
et al: AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor,
normalizes tumor vasculature and alleviates edema in glioblastoma
patients. Cancer Cell. 11:83–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Batchelor TT, Duda DG, di Tomaso E,
Ancukiewicz M, Plotkin SR, Gerstner E, Eichler AF, Drappatz J,
Hochberg FH, Benner T, et al: Phase II study of cediranib, an oral
pan-vascular endothelial growth factor receptor tyrosine kinase
inhibitor, in patients with recurrent glioblastoma. J Clin Oncol.
28:2817–2823. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gerstner ER, Eichler AF, Plotkin SR,
Drappatz J, Doyle CL, Xu L, Duda DG, Wen PY, Jain RK and Batchelor
TT: Phase I trial with biomarker studies of vatalanib (PTK787) in
patients with newly diagnosed glioblastoma treated with enzyme
inducing anti-epileptic drugs and standard radiation and
temozolomide. J Neurooncol. 103:325–332. 2011. View Article : Google Scholar
|
|
33
|
Quant EC, Batchelor T, Lassman AB, Schiff
D, Kaley TJ, Wong E, Mikkelsen T, Drappatz J, Norden AD, Beroukhim
R, et al: Preliminary results from a multicenter, phase II,
randomized, noncomparative clinical trial of radiation and
temozolomide with or without vandetanib in newly diagnosed
glioblastoma. J Clin Oncol. 29:20692011.
|
|
34
|
Shiose S, Sakamoto T, Yoshikawa H, Hata Y,
Kawano Y, Ishibashi T, Inomata H, Takayama K and Ueno H: Gene
transfer of a soluble receptor of VEGF inhibits the growth of
experimental eyelid malignant melanoma. Invest Ophthalmol Vis Sci.
41:2395–2403. 2000.PubMed/NCBI
|
|
35
|
Takayama K, Ueno H, Nakanishi Y, Sakamoto
T, Inoue K, Shimizu K, Oohashi H and Hara N: Suppression of tumor
angiogenesis and growth by gene transfer of a soluble form of
vascular endothelial growth factor receptor into a remote organ.
Cancer Res. 60:2169–2177. 2000.PubMed/NCBI
|
|
36
|
Ye C, Feng C, Wang S, Wang KZQ, Huang N,
Liu X, Lin Y and Li M: sFlt-1 gene therapy of follicular thyroid
carcinoma. Endocrinology. 145:817–822. 2004. View Article : Google Scholar
|
|
37
|
Mahasreshti PJ, Kataram M, Wang MH,
Stockard CR, Grizzle WE, Carey D, Siegal GP, Haisma HJ, Alvarez RD
and Curiel DT: Intravenous delivery of adenovirus-mediated soluble
FLT-1 results in liver toxicity. Clin Cancer Res. 9:2701–2710.
2003.PubMed/NCBI
|
|
38
|
Goldman CK, Kendall RL, Cabrera G,
Soroceanu L, Heike Y, Gillespie GY, Siegal GP, Mao X, Bett AJ,
Huckle WR, et al: Paracrine expression of a native soluble vascular
endothelial growth factor receptor inhibits tumor growth,
metastasis, and mortality rate. Proc Natl Acad Sci USA.
95:8795–8800. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ikeda T, Sun L, Tsuruoka N, Ishigaki Y,
Yoshitomi Y, Yoshitake Y and Yonekura H: Hypoxia down-regulates
sFlt-1 (sVEGFR-1) expression in human microvascular endothelial
cells by a mechanism involving mRNA alternative processing. Biochem
J. 436:399–407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jain RK: Antiangiogenesis strategies
revisited: From starving tumors to alleviating hypoxia. Cancer
Cell. 26:605–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mashiko R, Takano S, Ishikawa E, Yamamoto
T, Nakai K and Matsumura A: Hypoxia-inducible factor 1α expression
is a prognostic biomarker in patients with astrocytic tumors
associated with necrosis on MR image. J Neurooncol. 102:43–50.
2011. View Article : Google Scholar
|
|
42
|
Kung AL, Wang S, Klco JM, Kaelin WG and
Livingston DM: Suppression of tumor growth through disruption of
hypoxia-inducible transcription. Nat Med. 6:1335–1340. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu B, Kou L, Li C, Zhu LP, Fan YR, Wu ZW,
Wang JJ and Xu GX: Bifidobacterium longum as a delivery system of
TRAIL and endostatin cooperates with chemotherapeutic drugs to
inhibit hypoxic tumor growth. Cancer Gene Ther. 16:655–663. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu H, Li Z, Mao S, Ma B, Zhou S, Deng L,
Liu T, Cui D, Zhao Y, He J, et al: Antitumor effect of sFlt-1 gene
therapy system mediated by Bifidobacterium Infantis on Lewis lung
cancer in mice. Cancer Gene Ther. 18:884–896. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Biswal MR, Prentice HM, Dorey CK and
Blanks JC: A hypoxia-responsive glial cell-specific gene therapy
vector for targeting retinal neovascularization. Invest Ophthalmol
Vis Sci. 55:8044–8053. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Perche F, Biswas S, Patel NR and Torchilin
VP: Hypoxia-responsive copolymer for siRNA delivery. Methods Mol
Biol. 1372:139–162. 2016. View Article : Google Scholar
|