Introduction
Many studies have demonstrated that a variety of
human malignancies, including head and neck squamous cell carcinoma
(HNSCC), contain subpopulations of cells called cancer stem-like
cells (CSCs) that exhibit stem cell-like properties, such as
self-renewal and tumor-initiating capabilities (1,2).
CSCs can lead to disease progression by giving rise to new tumors
despite therapeutic intervention. The resistance of CSCs to
conventional chemoradiotherapy may involve enhanced DNA damage
repair pathways and alterations in cell cycle kinetics (3).
CD44 has been recently recognized as one of the cell
surface markers associated with CSCs in HNSCC (4,5). A
previous study showed that a CD44+ cell subset in HNSCC
was predominantly in G2/M phase compared with the
CD44−cell subset associated with resistance to apoptosis
(6,7). The upregulation of CD44 serves as a
survival mechanism, allowing cells to escape apoptotic cell death
in response to DNA damage repair in HNSCC. Although the nature of
CSCs is still controversial, CD44-expressing subfractions of many
human carcinomas are highly malignant and share common properties
with CSCs (8,9).
Scutellaria root is a common component of
many preparations in traditional Chinese medicine (10). It is a multi-purpose treatment, for
inflammation, hypertension, cardiovascular diseases, and bacterial
and viral infections. Chinese herbal medicine is a mixture of many
herbs following the theory of traditional Chinese medicine
(11,12). Among these herbs, the main drugs
that contain Scutellaria root are Shosaikoto
(Xiao-Chai-Hu-Tang) (13),
Daisaikoto (Da-Chai-Hu-Tang) (14), Saireito (Chai-Ling-Tang) (15), Saikokeishito (Chai-Hu-Gui-Zhi-Tang)
(16), and Saikokaryukotsuboreito
(Chai-Hu-Jia-Long-Gu-Mu-Li-Tang) (17). The medicinal effects of these
medicines improve gastrointestinal, liver and breathing responses,
targets immune function, and relieves inflammation (18). The medicines use different
diagnostic depending on traditional Chinese medicine. Some research
reports have claimed that the herbal medicines that contain
Scutellaria root can inhibit cancer (10,19,20).
However, the effects of traditional medicines on CSCs are
unclear.
Here, we explored the Scutellaria root
ingredient of herbal medicine and its effects on CSCs of HNSCC. We
analyzed its effects on CD44, a marker of CSCs, and on the cell
cycle in HNSCC.
Materials and methods
Reagents and antibodies
Dimethyl sulfoxide (DMSO), sodium dodecyl sulfate
(SDS), baicalin, baicalein, and cisplatin were purchased from Wako
Pure Chemical Industries, Ltd. (Osaka, Japan). Dulbecco's modified
Eagle's medium (DMEM) was purchased from Invitrogen (Carlsbad, CA,
USA), and fetal bovine serum (FBS) was purchased from Nichirei
Bioscience (Tokyo, Japan). Primary antibodies against CD44 and
cPARP were purchased from Cell Signaling Technology (Danvers, MA,
USA), and primary antibodies against phospho-CHK1 (S301) and
β-actin were purchased from Sigma-Aldrich (St. Louis, MO, USA). The
horseradish peroxidase-conjugated secondary anti-mouse
immunoglobulin G (IgG) and anti-rabbit IgG antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The protein assay kit was purchased from Bio-Rad (Herndon,
VA, USA). Liquid chromatography-grade acetonitrile, acetic acid,
ethyl acetate, methanol, 1-butanol, 2-propanol, baicalein,
baicalin, and trifluoroacetic acid [for use in the high-performance
liquid chromatography (HPLC) experiments described below] were
purchased from Wako Pure Chemical Industries, Ltd. Milli-Q plus
water (Millipore, Bedford, MA, USA) was used in the present study.
All other chemicals were purchased from Wako Pure Chemical
Industries, Ltd., except where otherwise noted.
Plant materials
Dried powders of herbal medicine [Shosaikoto
(Xiao-Chai-Hu-Tang) (21),
Daisaikoto (Da-Chai-Hu-Tang) (22), Saireito (Chai-Ling-Tang) (23), Saikokeishito (Chai-Hu-Gui-Zhi-Tang)
(24), and Saikokaryukotsuboreito
(Chai-Hu-Jia-Long-Gu-Mu-Li-Tang) (25)] and Scutellaria root extract
were supplied by Tsumura Co., Ltd. (Tokyo, Japan). The herbal
medicines contained several dried herbs in fixed proportions, as
standardized by the Health, Labour and Welfare Ministry of Japan
(Table I). The quality of each
crude herb was tested in accordance with the guidelines set out by
the pharmacopoeia of Japan. The root drugs were extracted by
boiling, and the decoctions were lyophilized and stored at room
temperature under desiccated conditions until use. The dried
powders were reconstituted and employed as hot water extracts.
 | Table ICrude herbal constituents
(percentages) and clinical indications of five herbal
medicines. |
Table I
Crude herbal constituents
(percentages) and clinical indications of five herbal
medicines.
| Sample Crude
herb | Shosaikoto
Xiao-Chai-Hu-Tang | Daisaikoto
Da-Chai-Hu-Tang | Saireito
Chai-Ling-Tang | Saikokeishito
Chai-Hu-Gui-Zhi-Tang |
Saikokaryukotsuboreito
Chai-Hu-Jia-Long-Gu-Mu-Li-Tang |
|---|
| Bupleurum root | 7.0 | 6.0 | 7.0 | 5.0 | 5.0 |
| Scutellaria
root | 3.0 | 3.0 | 3.0 | 2.0 | 2.5 |
| Pinellia
tuber | 5.0 | 4.0 | 5.0 | 4.0 | 4.0 |
| Jujube fruit | 3.0 | 3.0 | 3.0 | 2.0 | 2.5 |
| Ginseng root | 3.0 | | 3.0 | 2.0 | 2.5 |
| Ginger rhizome | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Glycyrrhiza
root | 2.0 | | 2.0 | 2.0 | |
| Cinnamon bark | | | 2.0 | 2.0 | 3.0 |
| Peony root | | 3.0 | | 2.0 | |
| Hoelen | | | 3.0 | | 3.0 |
| Immature
orange | | 2.0 | | | |
| Rhubarb
rhizome | | 1.0 | | | |
| Alisma
rhizome | | | 5.0 | | |
| Atractylodes
lancea rhizome | | | 3.0 | | |
| Chuling | | | 3.0 | | |
| Oyster shell | | | | | 2.5 |
| Fossilized
bone | | | | | 2.5 |
| Percentage
(w/w) | | | | | |
| Scutellaria
root | 12.5% | 13.0% | 7.5% | 9.1% | 8.8% |
| Clinical
indications | Bronchial asthma,
common cold, chronic liver diseases, enterogastritis | Hyperlipidemia,
diabetes mellitus, cholelithiasis, jaundice | Diarrhea, edema,
enterogastritis, nephritic disease | Duodenal ulcers,
pancreatitis, chronic liver diseases | Psychotropic
stress, neurasthenia, hypertension, atherosclerosis,
hypercholesterolemia |
Cell culture
The HNSCC cell lines HSC-2 and HSC-3 were obtained
from Riken Cell Bank (Ibaraki, Japan). The human immortalized
non-tumorigenic keratinocyte cell line HaCaT was supplied by DKFZ
(Heidelberg, Germany). Cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Life Technologies Japan Ltd.) supplemented
with 10% FBS (Life Technologies Japan Ltd.) and antibiotics
[penicillin (100 U/ml), streptomycin (100 μg/ml) and
amphotericin B (25 μg/ml)] at 37°C in a humidified
atmosphere containing 5% CO2.
Cell viability assay
Baicalin and baicalein stock solutions were prepared
in DMSO and added to the cultured HSC-2, HSC-3, and HaCaT cells in
DMEM/10% FBS to achieve the indicated concentrations. The cells
were incubated for the indicated periods of time. Cell viability
was determined by performing
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assays with the Cell Proliferation kit I (Roche
Diagnostics GmbH, Mannheim, Germany) according to the
manufacturer's instructions. The number of viable cells was
assessed by measuring the absorbance of the formazan crystals at
595 nm with a MultiSkan JX microplate reader and Ascent software
(Thermo Labsystems, Vantaa, Finland). All data are presented as the
mean ± standard deviation (SD) of at least three independent
experiments.
Quantitative analysis of baicalin and
baicalein contents in five herbal medicines
A Jasco HPLC system (Jasco, Inc., Tokyo, Japan),
equipped with a pump and a spectrophotometer suitable for
ultraviolet (UV)/visible light detection, was used to generate a
chromatogram at 277 nm for the analysis of baicalin and baicalein
contents in Shosaikoto, Daisaikoto, Saireito, Saikokeishito, and
Saikokaryukotsuboreito. Chromatographic conditions were adapted
from a previous report (26), with
the following minor modifications. The experiment used a Mightysil
RP-18 GP 150-4.6 column (Kanto Corp., Tokyo, Japan). Eluent A
corresponded to 0.05% trifluoroacetic acid (v/v), and eluent B
corresponded to acetonitrile. The linear gradient of A to B
consisted of 80–40% eluent A for 0–40 min, followed by 40–0% eluent
A for 40–50 min. The flow rate was 1.0 ml/min at ambient
temperature, and the injection volume was 10 μl. Calibration
curves were obtained from plots of the peak area versus the
concentration of the baicalin or the baicalein calibration standard
(10–250 μg/ml, r=0.999). All experiments were conducted in
triplicate.
CD44 knockdown
CD44 knockdown was achieved using short hairpin RNA
(shRNA) retrovirus particles. Oligonucleotides encoding shRNAs that
target standard exons in CD44 mRNA (shCD44) were cloned into the
plasmid vector pSINsi-hU6-Neo (Takara Bio Inc. Shiga, Japan). The
DNA sequences corresponding to the CD44 and control shRNAs were
5′-GTGTACATCCTCACATCCA-3′ (shCD44) and 5′-TCTTAATCGCGTATAAGGC-3′
(shCtrl), respectively. The plasmids were transfected into HSC-3
cells using Lipofectamine RNAiMAX (Invitrogen) according to the
manufacturer's instructions, and the cells were cultured for 2
weeks in the presence of neomycin (0.6 mg/ml; Roche Diagnostics,
IN, USA). CD44 knockdown in HSC-3 cells was confirmed by
immunoblotting and immunofluorescence.
Cell cycle analysis
HSC-3 cells or shCD44 HSC-3 cells were treated with
100 μM baicalin or baicalein for 48 h at 37°C. Appropriate
controls were also set up. Nuclei were labeled with propidium
iodide (PI) (BD Pharmingen, BD BioSciences, San Jose, CA, USA), and
the DNA contents of the PI-labeled nuclei were measured via flow
cytometry according to the manufacturer's instructions (BD
Pharmingen). Data acquisition and analysis were performed using a
Beckman Coulter EPICS Altra Flow Cytometer and Expo3 v1.2B analysis
software (Beckman Coulter, Brea, CA, USA).
Immunoblot analysis
HCS-3 cells were treated with DMSO, baicalin and
baicalein (100 μM), cisplatin (10 μM), five herbal
medicines (50 μg/ml), or Scutellaria root extract (50
μg/ml). Cells were scraped with a rubber policeman and
collected in 10X cell lysis buffer (Cell Signaling Technology,
Beverly, MA, USA). A solution containing phenylmethanesulfonyl
fluoride (1 mM) plus one tablet of protease inhibitor cocktail
(Complete, EDTA-free; Roche Diagnostics GmbH, Mannheim, Germany)
was added to each cell lysate. Protein contents in the lysates were
assayed, and equal amounts of protein for each sample were
subjected to SDS-polyacrylamide gel electrophoresis, followed by
immunoblotting with primary antibodies against anti-CD44 mouse
monoclonal antibody, anti-cleaved PARP rabbit monoclonal antibody
(all from Cell Signaling Technology), anti-phospho-Chk1 (pS301)
rabbit polyclonal antibody and anti-β-actin antibody
(Sigma-Aldrich) and secondary anti-IgG antibodies, as previously
described (27). When necessary,
membranes were stripped with Restore Western Blot Stripping Buffer
(Pierce).
Immunofluorescence
Monolayers of cells were cultured for 48 h in 4-well
cover glass chamber slides in medium containing 10% serum. Cells
were washed twice in PBS supplemented with 1% bovine serum albumin
(Sigma-Aldrich). The cell surface Fc receptor was blocked using IgG
(Santa Cruz Biotechnology, Inc.) on ice for 15 min. Cells were
stained for 30 min at 37°C with 1:100 dilution of anti-CD44 FITC
monoclonal antibody (BD Biosciences, Franklin Lakes, NJ, USA).
After washing, cells were analyzed using Nikon Eclipse TS100-F with
Nikon Intensilight C-HGFIE (Nikon Co., Ltd., Tochigi, Japan).
Digital images were processed with NIS Elements BR3.2 imaging
software (Nikon Co., Ltd.) and Adobe Photoshop 7.0 (San Jose, CA,
USA).
Apoptosis analysis
HSC-3 or shCD44 HSC-3 cells (1×105 cells)
were cultured on 12-well plates for 24 h and treated with baicalin
or baicalein for 24 h. Cells were stained using the FITC Annexin V
Apoptosis detection kit I (BD Pharmingen) according to the
manufacturer's instructions. Data acquisition and analysis were
performed using the EC800 Flow Cytometry Analyzer (Sony
Biotechnology, Tokyo, Japan) with EC800 analysis software (Sony
Biotechnology). Annexin V-positive cells were considered as
apoptotic cell death.
Statistical analysis
All quantitative data are presented as the mean ± SD
and were evaluated using one-way analysis of variance followed by
Dunnett's multiple comparison. In all cases, P<0.05 was
considered statistically significant.
Results
Quantification of principle active
constituents of Scutellaria root contents in five herbal
medicines
We analyzed the half maximal inhibitory
concentration (IC50) of the five complete herbal
medicines with human HNSCC cell lines (HSC-2 and HSC-3) and a human
skin keratinocyte cell line (HaCaT), and the IC50 values
ranged from 30.8 to 57.0 μg/ml (Table II). We next determined the
components in representatives of five widely used herbal medicines
containing Scutellaria root for evaluating potential
anticancer effects. These medicinal herbs are formulated from
several different herbs combined in a particular intrinsic mass
ratio. Table I shows the clinical
indications, composition of crude herbs, fixed proportions, and
percentage (w/w) of Scutellaria root in hot water extracts
of Shosaikoto, Daisaikoto, Saireito, Saikokeishito, and
Saikokaryukotsuboreito. The Scutellaria root is a component
of all of these five Chinese herbal medicines, and the percentage
of Scutellaria root in these medicines ranged from 7.5 to
13.0%.
 | Table IIBaicalin contents in extracts of each
of five herbal medicines (1 g) and IC50 values
(μg/ml) in HSC-2, HSC-3 and HaCaT cell lines. |
Table II
Baicalin contents in extracts of each
of five herbal medicines (1 g) and IC50 values
(μg/ml) in HSC-2, HSC-3 and HaCaT cell lines.
| Sample (Chinese
name) | Baicalin contents
(% ± SD) | IC50
(μg/ml)
|
|---|
| HSC-2 | HSC-3 | HaCaT |
|---|
| Shosaikoto
(Xiao-Chai-Hu-Tang) | 5.1±0.3 | 36.3±7.7 | 44.6±6.1 | 35.3±11 |
| Daisaikoto
(Da-Chai-Hu-Tang) | 4.0±0.5 | 37.2±4.0 | 57.0±1.8 | 40.4±7.1 |
| Saireito
(Chai-Ling-Tang) | 3.5±0.6 | 38.8±4.9 | 47.1±7.5 | 39.8±6.4 |
| Saikokeishito
(Chai-Hu-Gui-Zhi-Tang) | 3.1±0.2 | 41.2±5.2 | 52.3±3.8 | 30.8±5.1 |
|
Saikokaryukotsuboreito
(Chai-Hu-Jia-Long-Gu-Mu-Li-Tang) | 3.2±0.3 | 37.4±6.6 | 46.1±5.5 | 33.7±4.4 |
Baicalin is one of the main constituents in
Scutellaria root, and baicalein is its aglycone (10,28).
Table II shows the results of the
quantitation of baicalin (5,6,7-trihydroxyflavone-7-O-glucuronide)
contents in each sample by HPLC-UV analysis. Standard curves for
baicalin were linear (r=0.999) over a concentration range of 10–250
μg/ml (tR 10.3±0.15 min). Baicalein
(5,6,7-trihydroxyflavone) (tR 20.5±0.28 min) was not
detectable in any of the samples. All sample matrices contained a
large number of constituents, of which baicalin (30–50 mg),
comprising ~3–5% (w/w) of the total extract (1 g), was a common
component. They were equivalent to 2–5 μM content of
baicalin in the five herbal medicines. These data show that the
five herbal medicines contain a high proportion of baicalin (3–5%).
Baicalin is absorbed in the body, and it is metabolized to
baicalein (28). Some studies have
demonstrated that baicalin (10,29)
and baicalein (29–31) have potential anticancer activities.
Therefore, we focused on these two ingredients to predict the
effects of the five herbal medicines.
Baicalin and baicalein reduce HNSCC cell
viability
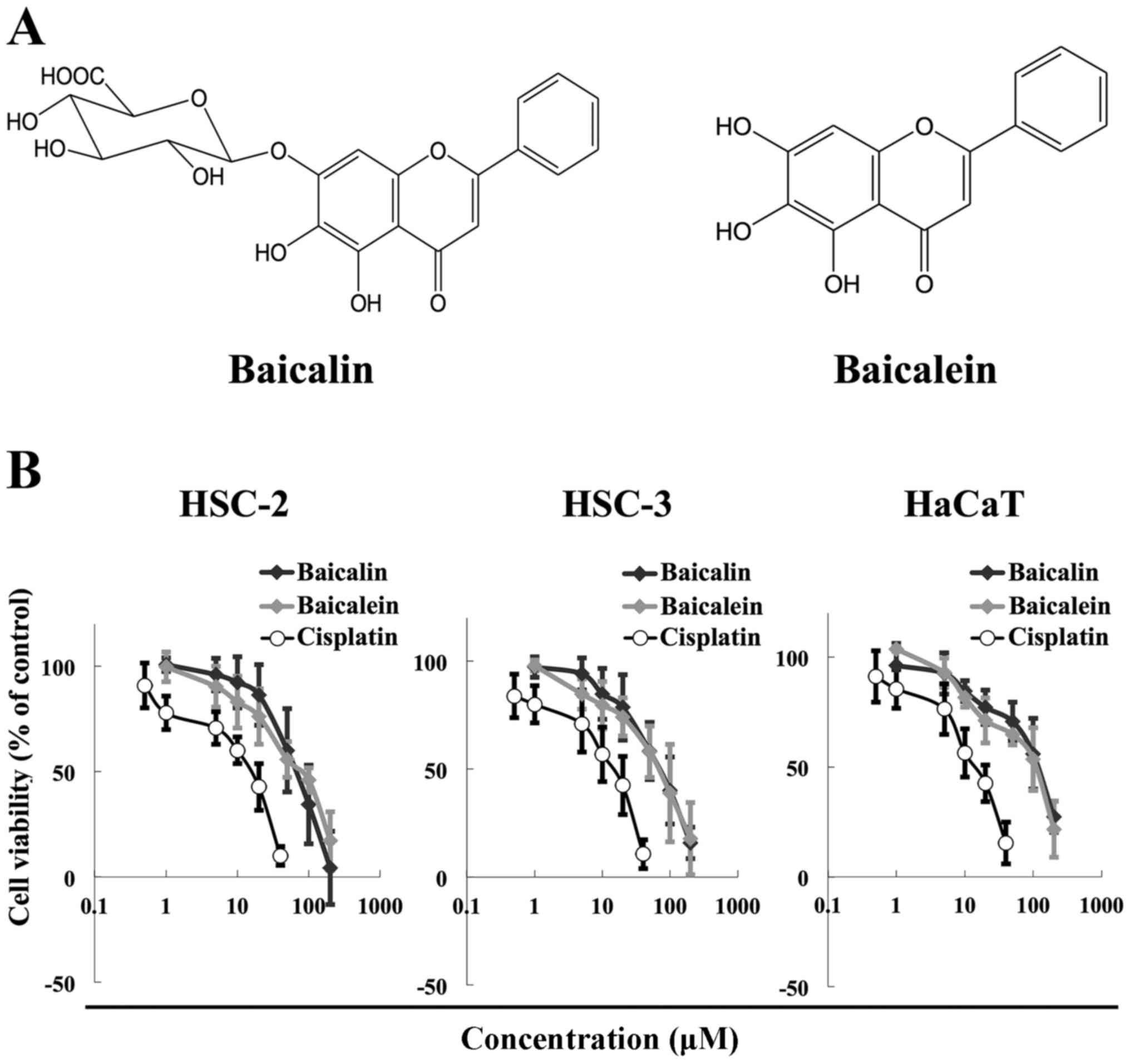
The chemical structures of baicalin and baicalein
are shown in Fig. 1A. The two
flavones differ in that baicalein does not contain the glucuronic
acid moiety at the C7 position on the flavone backbone. We next
examined possible anticancer effects of baicalin and baicalein
against HNSCC. The cytotoxicity of baicalin and baicalein in the
HSC-2 and HSC-3 HNSCC cell lines and the HaCaT normal human
keratinocyte cell line was evaluated by MTT assays. Both compounds
reduced the viability of all three cell lines at concentrations
ranging from 50 to 200 μM for 72 h in a dose-dependent
manner (Fig. 1B). The cytotoxicity
of baicalin and baicalein was similar. Cisplatin was used as a
positive control, a well-known anticancer agent (32,33).
The IC50 values of baicalin were 54.3 μM for
HSC-2 cells, 60.6 μM for HSC-3 cells, and 69.0 μM for
HaCaT cells. The corresponding IC50 values for baicalein
were 60.7 μM for HSC-2 cells, 58.4 μM for HSC-3
cells, and 64.5 μM for HaCaT cells. Together our results
show that baicalin and baicalein exhibit similar cytotoxic effects
in HNSCC cells as cisplatin.
Baicalin and baicalein exert differential
effects on the cell cycle
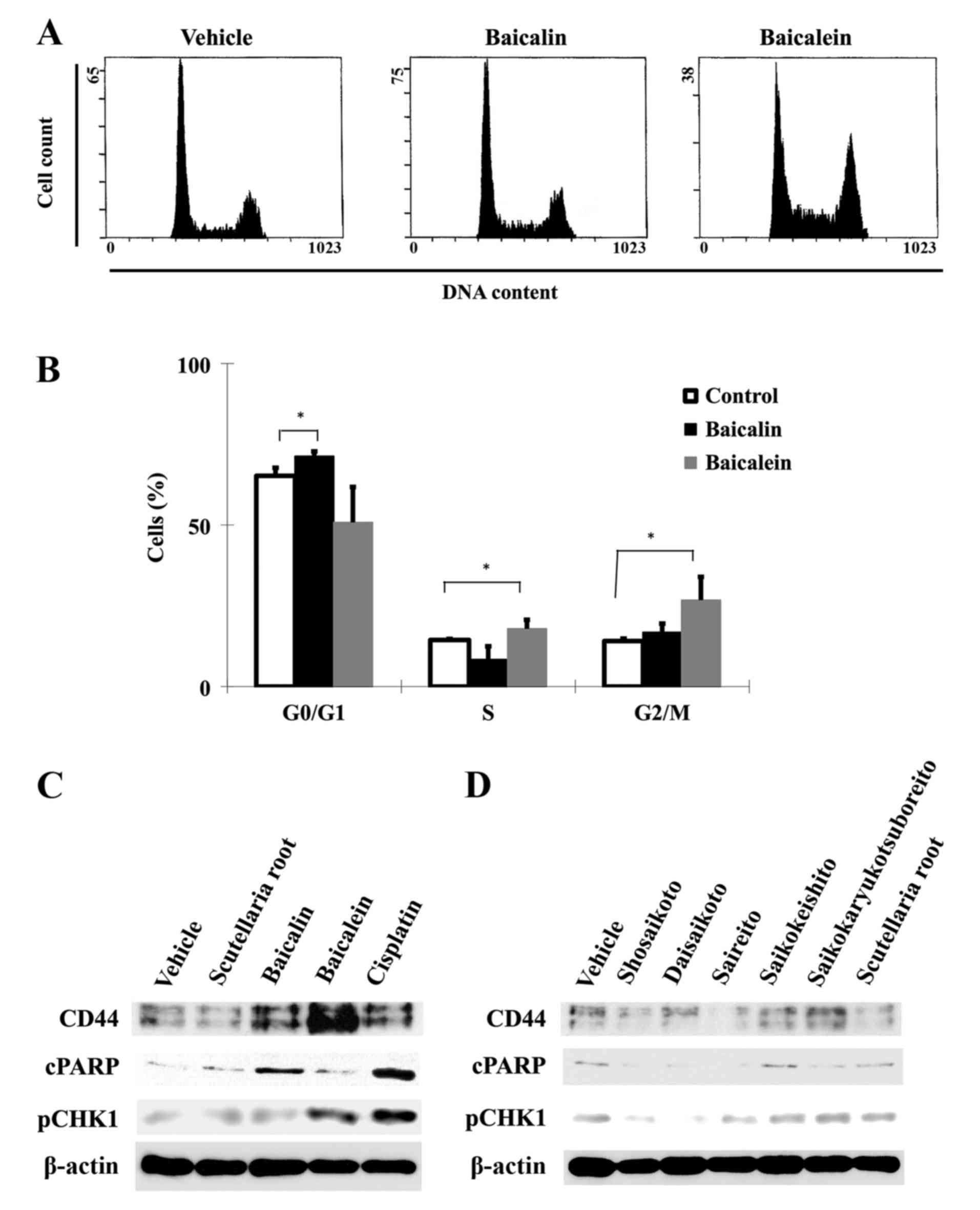
We next examined whether baicalin and baicalein
affect the cell cycle progression of cancer cells. Based on cell
viability results, baicalin and baicalein at concentrations of 100
μM were used for subsequent analyses. Cell cycle
distribution patterns were compared in control cells and cells
treated for 48 h by flow cytometric analysis (Fig. 2A and B). While the majority of
HSC-3 cells were in G0/G1 phase in the control group, only 6.1% of
the cells were arrested in G0/G1 phase in response to baicalin,
whereas 12.7% of cells were arrested in G2/M phase in response to
baicalein. Baicalein-induced G2/M phase arrest was accompanied with
a small increase (3.4%) in S phase cells and a concomitant decrease
in G0/G1 phase cells, suggesting that the S phase entry of the
cells was not significantly affected in response to baicalein, but
the exit from S phase might be partially impaired, causing a slight
increase in S phase cells. Together our results showed that
baicalin induces G0/G1 arrest and baicalein induces G2/M arrest in
HSC-3 cells.
Baicalin and baicalein exert regulatory
effects on CD44 expression and DNA damage response
We next explored the possible molecular responses
associated with the G0/G1 and G2/M cell cycle arrest induced by
baicalin and baicalein, respectively. We performed immunoblot
analysis of the protein levels of cleaved PARP (cPARP) as an
apoptosis marker, CD44 as a CSC surface marker, and phospho-Chk1
(S301) as a marker of DNA damage response to S-to-G2/M phase arrest
in HSC-3 cells. Both flavones (100 μM) increased the
expression of CD44 after 48 h of treatment, but the effect of
baicalein was more pronounced than that of baicalin (Fig. 2C). Baicalein-treated HSC-3 cells
also showed lower levels of cPARP than baicalin-treated cells,
suggesting that CD44 upregulation at G2/M phase may be an important
mechanism for cell survival. We also investigated the DNA damage
response upon treatment with these compounds. Baicalein and
cisplatin induced phosphorylation of CHK1, as a marker of robust
DNA damage response. Treatment with the five herbal medicines or
Scutellaria root (all used at 50 μg/ml for 48 h) did
not significantly affect phosphorylation of CHK1 (Fig. 2D). These results demonstrate that
HNSCC treated by baicalein can progressively escape apoptosis
regardless of the induction of DNA damage response, and that this
resistance phenomenon is correlated with inadequate expression of
CD44. However, the protein levels of CD44, cPARP, and pCHK1 were
not appreciably altered by any of the herbal medicines or by
Scutellaria root.
Together, these studies suggest that
baicalein-induced CD44 expression may cause apoptotic resistance,
leading to S-to-G2/M phase arrest regulated by the activation of
the CHK1 pathway.
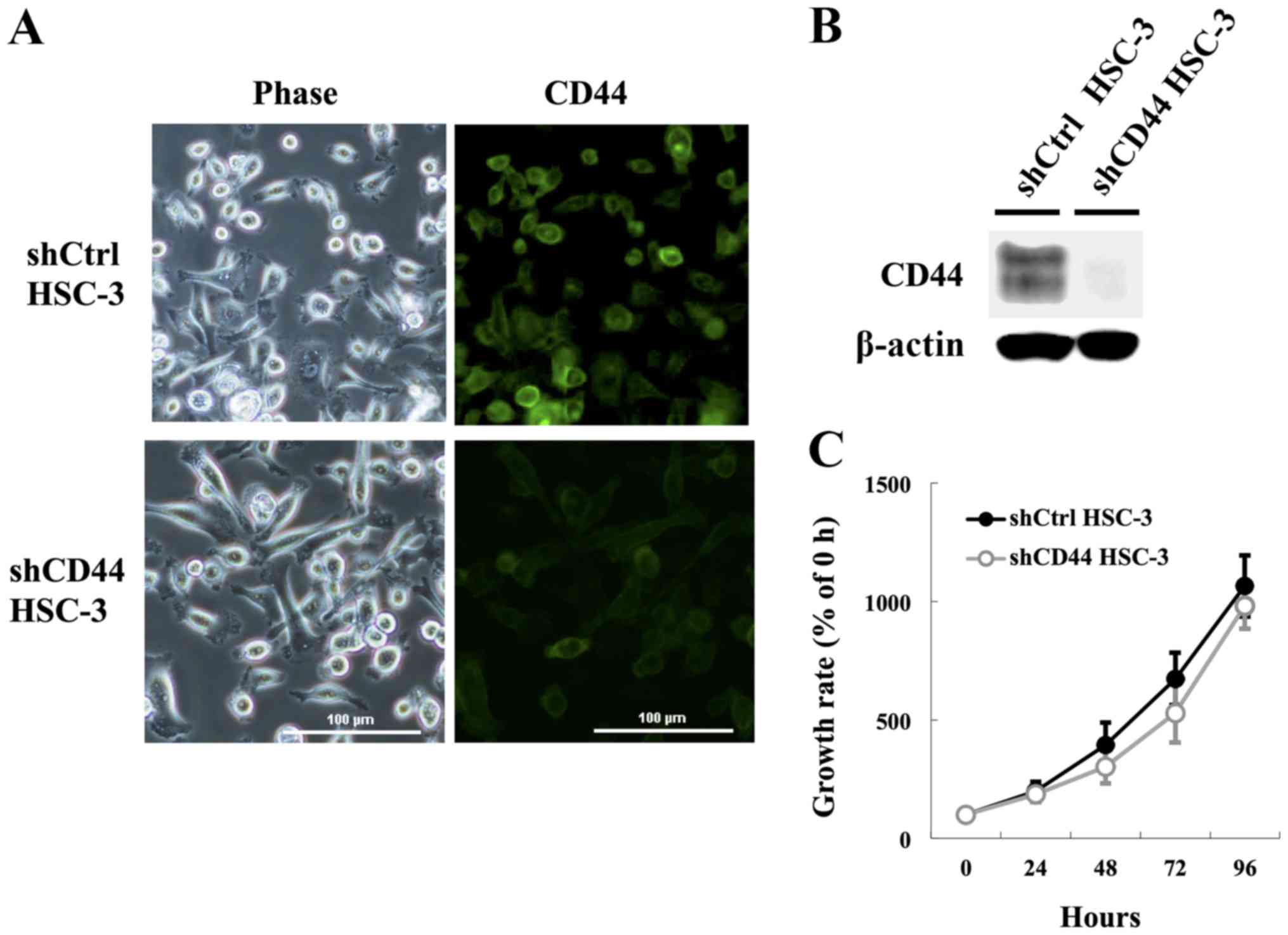
Generation of stable CD44-knockdown HSC-3
cells
Our above findings suggested that the CD44
upregulation may be correlated with resistance to apoptosis and DNA
damage response at G2/M arrest with activation of CHK1. Therefore,
we generated HSC-3 cells with knockdown of CD44 to further
investigate whether CD44 is involved in the proliferation and
apoptotic resistance in HNSCC cells. HSC-3 transfected control
(shCtrl HSC-3) and CD44 knockdown (shCD44 HSC-3) cell lines showed
no significant morphological changes, such as the appearance of
pseudopodia and spindle-shaped morphology (Fig. 3A). Immunostaining analyses for CD44
(Fig. 3A) corroborated the
immunoblotting analysis (Fig. 3B),
confirming downregulation of CD44 in the stable knockdown lines by
>95%. Additionally, some previous observations have suggested
that CD44 confers a decided growth advantage on certain types of
cancer cells (6,34); however, we observed no significant
attenuation in growth rate in monolayer cultures of shCD44 HSC-3
cells compared with shCtrl HSC-3 cells after 96 h (Fig. 3C). These findings indicated that
CD44 knockdown was not sufficient to mediate cell growth inhibition
in this study.
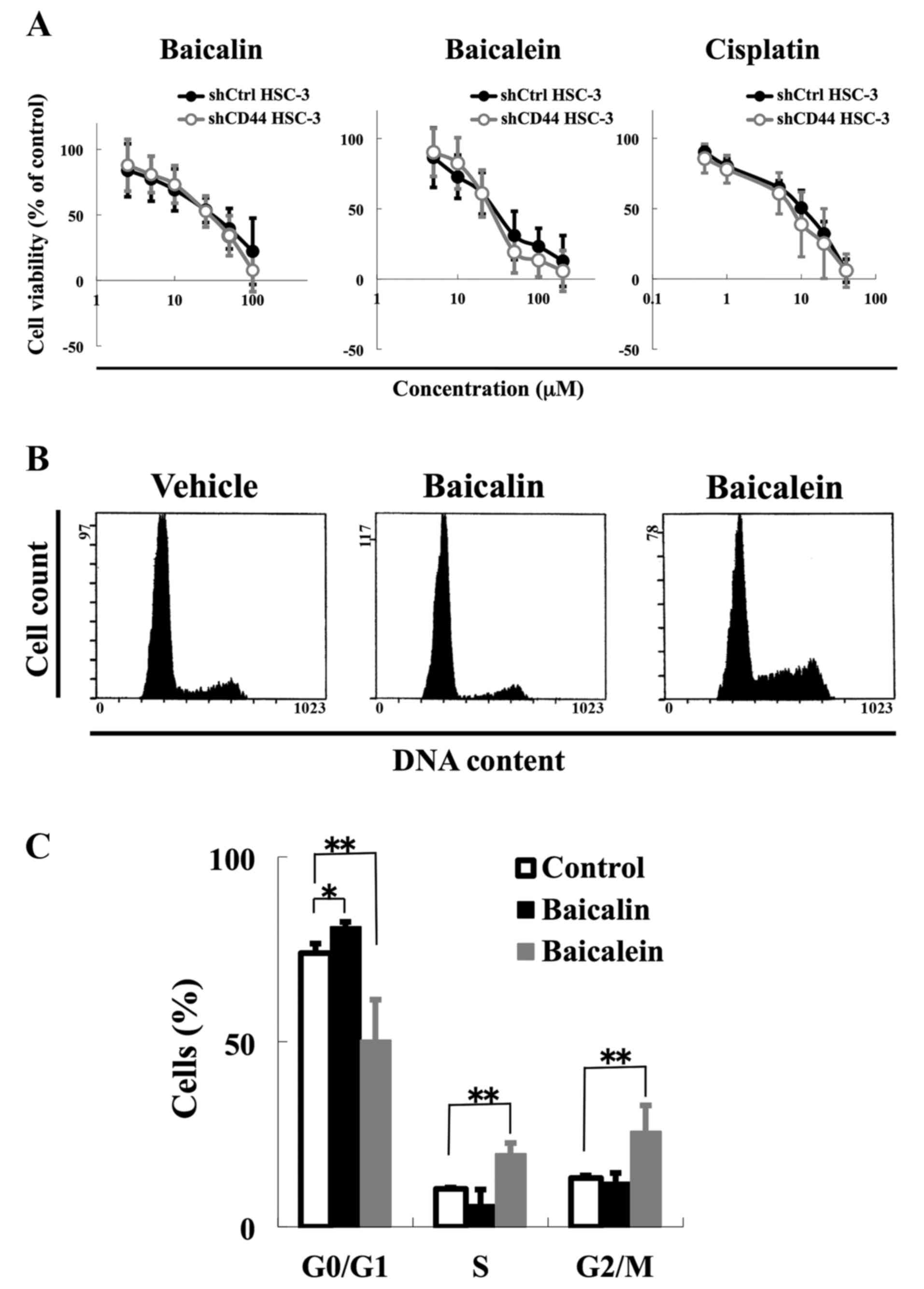
Apoptotic resistance at G2/M phase is
reduced in CD44 knockdown HSC-3 cells
Our next step was to examine the role of CD44
expression on apoptotic resistance by baicalein at G2/M phase. We
tested the ability of baicalein and baicalin to selectively kill
shCD44 HSC-3 cells. Despite their CD44 knockdown phenotype, shCD44
HSC-3 cells did not show any significant differences in response to
baicalein and baicalin compared with shCtrl HSC-3 cells (Fig. 4A). The IC50 values for
baicalin were 43.7 μM for shCtrl HSC-3 cells and 39.3
μM for shCD44 HSC-3 cells. The corresponding IC50
values for baicalein were 59.6 μM for shCtrl HSC-3 cells and
56.4 μM for shCD44 HSC-3 cells.
To understand the role of CD44 in DNA damage
response induction, we analyzed the effects of baicalin and
baicalein treatments on the cell cycle progression and using
immunoblotting. We found a similar trend in terms of effect of
baicalin and baicalein on cell cycle progression and DNA damage
response induction as compared with control cells (Figs. 2A, and B and 4B and C). Baicalein increased the number
of cells in S phase by 9.5% and G2/M phase by 12.6% compared with
the vehicle control, while baicalin increased the number of cells
in G0/G1 phase by 7.1% (P<0.001) (Fig. 4C). Furthermore, baicalein enhanced
cPARP, an apoptosis marker, in CD44 knockdown cells, while
phosphorylation of CHK1, a marker of DNA damage response to
S-to-G2/M phase arrest, was decreased by baicalein in CD44
knockdown cells (Fig. 4D). We also
confirmed that baicalein enhanced apoptosis by Annexin V staining
when CD44 was knocked down (Fig.
4E). These results suggest that CD44 is correlated with p-CHK1
expression and that CD44 expression plays a role in DNA damage
response in HNSCC cells.
Discussion
In this study, we first examined whether traditional
herbal medicines that contain Scutellaria root enhanced
apoptosis of HNSCC cells. Our results showed that the five herbal
medicines did not enhance apoptosis of HNSCC cells in cell culture
experiments (Table II and
Fig. 2D). We hypothesized that
some ingredients of herbal medicine could inhibit the expression of
CSC markers or enhance cancer cell apoptosis, thus we next examined
whether ingredients of herbal medicine affected CD44 levels, as a
cancer stem cell marker, or induced apoptosis. However, our
findings showed that baicalin and baicalein, which are present in
herbal medicine at high proportions, enhanced expression of CD44,
and the cytostatic activities were similar to that of cisplatin in
HNSCC cells. We next turned our attention to the potential
induction of apoptosis by the two flavone compounds. Apoptosis is
characterized by increased expression of cleaved PARP. PARP
initiates multiple cellular responses, including DNA repair, cell
cycle checkpoint control, apoptosis, and nuclear gene transcription
(35), while cPARP is selectively
involved in programmed cell death. Notably, baicalin increased
cPARP level in HSC-3 cells, whereas baicalein did not, despite its
induction of DNA damage response at G2/M cell cycle arrest and
inhibition of HSC-3 cell growth. In contrast, CD44 protein levels
were markedly increased by baicalein, but not by baicalin.
Therefore, we hypothesized that CD44 expression engaged with cPARP
level and G2/M cell cycle arrest in the HSC-3 HNSCC cell line. We
validated this hypothesis using CD44-knockdown HSC-3 cells. We
found that cPARP level, as an apoptotic response marker, was
significantly increased by CD44 knockdown at G2/M phase initiating
the DNA damage response. These results demonstrated that inadequate
expression of CD44 has a role in the transient apoptotic resistance
to the DNA damage response.
HNSCC tumors contain a subpopulation of CSCs that
are distinguished by a high level of CD44 expression (4). CSCs are illustrious for their
resistance to DNA damage and high DNA repair capacity (3), and CD44high cells are less
sensitive to apoptosis-inducing agents than CD44low
cells. CD44high cells in HNSCC tumors also spend a
consistently longer amount of time in G2 phase than
CD44low cells (7).
Furthermore, the proportion of CD44+ cells relative to
CD44− cells in G2 phase is markedly increased by
treatment with certain apoptosis-inducing stimuli (6). Our data demonstrated that
baicalein-mediated upregulation of CD44 was linked to an extended
G2/M phase in HNSCC cells and a possible maintenance of the
stemness to a CSC-like phenotype, potentially providing cells with
the opportunity and capacity to repair DNA damage. We propose that
the metabolism of baicalin to baicalein, followed by the
upregulation of CD44, might be involved in aberrant repair of
damaged cells to enable their survival, potentially contributing to
apoptotic resistance in HNSCC tumors. These results suggest that,
although both molecular structures of baicalin and baicalein appear
similar, the cellular environment to DNA damage response may differ
by CD44 upregulation, especially cell cycle progression, explaining
their differential apoptotic threshold to DNA damage repair.
It is important to emphasize that the five herbal
medicines and Scutellaria root extract had no clear effect
on the levels of CD44, cPARP or DNA damage response. It is possible
that the cell cycle stage at the time of drug treatment or some
other as yet unknown mechanism might modulate the switch between
cell death and survival in HNSCC-derived CSCs.
Together our results indicate that
baicalin/baicalein may potentially induce CD44 upregulation to
initiate the stemness of HNSCCs. Moreover, upregulated CD44 may
render the stem cells resistant to programmed cell death, perhaps
through G2/M arrest. The potential induction of CD44 expression may
be partly responsible for resistance to DNA-damaging
treatments.
Our results showed that baicalein could
significantly induce cell cycle arrest at G2/M phase and increased
level of cleaved PARP-1 (an apoptotic marker) in CD44 knockdown
cells without altering growth inhibitory effect and cell cycle
distribution. This indicates that transient expression of CD44
could enhance DNA repair damage response and contribute to survival
ability by apoptotic resistance by baicalein in HSC-3 cells.
In conclusion, knockdown and functional evaluation
of CD44, a cell surface marker of cancer stem-like cells, suggested
that induction of CD44 by baicalein, as an herbal ingredient, is
involved, at least in part, in an initial survival advantage,
resulting in efficient DNA damage repair. Overexpression of CD44
provides relative protection of HNSCC cells in terms of cell death
responses, but it is unclear whether the rescued cells convert DNA
damage to mutations and/or translocations during treatment of
cancer. However, the present study indicated that the function of
CD44 as a CSC marker of HNSCC is involved in the DNA damage
response in G2/M arrest.
Acknowledgments
This study was supported in part by JSPS KAKENHI
(grant nos. 24791982, 26462854 and 26861748).
References
|
1
|
Magee JA, Piskounova E and Morrison SJ:
Cancer stem cells: Impact, heterogeneity, and uncertainty. Cancer
Cell. 21:283–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Visvader JE and Lindeman GJ: Cancer stem
cells: Current status and evolving complexities. Cell Stem Cell.
10:717–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prince ME, Sivanandan R, Kaczorowski A,
Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF and Ailles
LE: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zöller M: CD44: Can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chikamatsu K, Ishii H, Takahashi G,
Okamoto A, Moriyama M, Sakakura K and Masuyama K: Resistance to
apoptosis-inducing stimuli in CD44+ head and neck
squamous cell carcinoma cells. Head Neck. 34:336–343. 2012.
View Article : Google Scholar
|
|
7
|
Harper LJ, Costea DE, Gammon L, Fazil B,
Biddle A and Mackenzie IC: Normal and malignant epithelial cells
with stem-like properties have an extended G2 cell cycle phase that
is associated with apoptotic resistance. BMC Cancer. 10:1662010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Joshua B, Kaplan MJ, Doweck I, Pai R,
Weissman IL, Prince ME and Ailles LE: Frequency of cells expressing
CD44, a head and neck cancer stem cell marker: Correlation with
tumor aggressiveness. Head Neck. 34:42–49. 2012. View Article : Google Scholar
|
|
9
|
Orian-Rousseau V: CD44, a therapeutic
target for metastasising tumours. Eur J Cancer. 46:1271–1277. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li-Weber M: New therapeutic aspects of
flavones: The anti-cancer properties of Scutellaria and its main
active constituents Wogonin, Baicalein and Baicalin. Cancer Treat
Rev. 35:57–68. 2009. View Article : Google Scholar
|
|
11
|
Zha LH, He LS, Lian FM, Zhen Z, Ji HY, Xu
LP and Tong XL: Clinical strategy for optimal traditional Chinese
medicine (TCM) herbal dose selection in disease therapeutics:
Expert consensus on classic TCM herbal formula dose conversion. Am
J Chin Med. 43:1515–1524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng H, He Y, Zheng G, Zhang W, Yao Z and
Xie W: Meta-analysis of traditional herbal medicine in the
treatment of nonalcoholic fatty liver disease. Cell Mol Biol
(Noisy-le-grand). 62:88–95. 2016.
|
|
13
|
Sumino M, Saito Y, Ikegami F, Hirasaki Y
and Namiki T: Extraction efficiency of shosaikoto (xiaochaihu tang)
and investigation of the major constituents in the residual crude
drugs. Evid Based Complement Alternat Med. 2012:8905242012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He JX, Ohno K, Tang J, Hattori M, Tani T
and Akao T: Da-Chaihu-Tang alters the pharmacokinetics of
nifedipine in rats and a treatment regimen to avoid this. J Pharm
Pharmacol. 66:1623–1630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kato S, Hayashi S, Kitahara Y, Nagasawa K,
Aono H, Shibata J, Utsumi D, Amagase K and Kadowaki M: Saireito
(TJ-114), a Japanese traditional herbal medicine, reduces
5-fluorouracil-induced intestinal mucositis in mice by inhibiting
cytokine-mediated apoptosis in intestinal crypt cells. PLoS One.
10:e01162132015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su SB, Xie MJ, Sawabu N and Motoo Y:
Suppressive effect of herbal medicine saikokeishito on acinar cell
apoptosis in rat spontaneous chronic pancreatitis. Pancreatology.
7:28–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iijima H, Daikonya A, Takamatsu S, Kanno
A, Magariyama K, Yoshikawa K, Takamiya T, Ueda Y, Yakubo S,
Matsumoto T, et al: Effects of the herbal medicine composition
'Saiko-ka-ryukotsu-borei-To' on the function of endothelial
progenitor cells in hypertensive rats. Phytomedicine. 20:196–201.
2013. View Article : Google Scholar
|
|
18
|
Nakayama T, Suzuki S, Kudo H, Sassa S,
Nomura M and Sakamoto S: Effects of three Chinese herbal medicines
on plasma and liver lipids in mice fed a high-fat diet. J
Ethnopharmacol. 109:236–240. 2007. View Article : Google Scholar
|
|
19
|
Ben-Arye E, Mahajna J, Aly R, Ali-Shtayeh
MS, Bentur Y, Lev E, Deng G and Samuels N: Exploring an herbal
'wonder cure' for cancer: A multidisciplinary approach. J Cancer
Res Clin Oncol. 142:1499–1508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung VC, Wu X, Lu P, Hui EP, Zhang Y,
Zhang AL, Lau AY, Zhao J, Fan M, Ziea ET, et al: Chinese herbal
medicine for symptom management in cancer palliative care:
Systematic review and meta-analysis. Medicine (Baltimore).
95:e27932016. View Article : Google Scholar
|
|
21
|
Ohtake N, Nakai Y, Yamamoto M, Sakakibara
I, Takeda S, Amagaya S and Aburada M: Separation and isolation
methods for analysis of the active principles of Sho-saiko-to (SST)
oriental medicine. J Chromatogr B Analyt Technol Biomed Life Sci.
812:135–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshie F, Iizuka A, Komatsu Y, Matsumoto
A, Itakura H and Kondo K: Effects of Dai-saiko-to (Da-Chai-Hu-Tang)
on plasma lipids and atherosclerotic lesions in female heterozygous
heritable Kurosawa and Kusanagi-hypercholesterolemic (KHC) rabbits.
Pharmacol Res. 50:223–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kishida Y, Miki H, Nishii T, Inoue T,
Nishida S, Yoshikawa H and Sugano N: Therapeutic effects of
Saireito (TJ-114), a traditional Japanese herbal medicine, on
postoperative edema and inflammation after total hip arthroplasty.
Phytomedicine. 14:581–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohta Y, Kongo-Nishimura M, Hayashi T,
Kitagawa A, Matsura T and Yamada K: Saikokeishito extract exerts a
therapeutic effect on α-naphthylisothiocyanate-induced liver injury
in rats through atenuation of enhanced neutrophil infiltration and
oxidative stress in the liver tissue. J Clin Biochem Nutr.
40:31–41. 2007. View Article : Google Scholar
|
|
25
|
Yoshie F, Iizuka A, Kubo M, Komatsu Y,
Matsumoto A, Itakura H, Takeda H, Matsumiya T and Kondo K:
Protective effects of Saiko-ka-ryukotsu-borei-to
(Chai-Hu-Jia-Long-Gu-Mu-Li-Tang) against atherosclerosis in
Kurosawa and Kusanagi-hypercholesterolemic (KHC) rabbits. Pharmacol
Res. 43:481–488. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohkoshi E, Nagashima T, Sato H, Fujii Y,
Nozawa K and Nagai M: Simple preparation of baicalin from
Scutellariae Radix. J Chromatogr A. 1216:2192–2194. 2009.
View Article : Google Scholar
|
|
27
|
Umemura N, Zhu J, Mburu YK, Forero A,
Hsieh PN, Muthuswamy R, Kalinski P, Ferris RL and Sarkar SN:
Defective NF-κB signaling in metastatic head and neck cancer cells
leads to enhanced apoptosis by double-stranded RNA. Cancer Res.
72:45–55. 2012. View Article : Google Scholar
|
|
28
|
Trinh HT, Joh EH, Kwak HY, Baek NI and Kim
DH: Anti-pruritic effect of baicalin and its metabolites, baicalein
and oroxylin A, in mice. Acta Pharmacol Sin. 31:718–724. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takahashi H, Chen MC, Pham H, Angst E,
King JC, Park J, Brovman EY, Ishiguro H, Harris DM, Reber HA, et
al: Baicalein, a component of Scutellaria baicalensis, induces
apoptosis by Mcl-1 down-regulation in human pancreatic cancer
cells. Biochim Biophys Acta. 1813:1465–1474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Donald G, Hertzer K and Eibl G: Baicalein
- an intriguing therapeutic phytochemical in pancreatic cancer.
Curr Drug Targets. 13:1772–1776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Naveenkumar C, Asokkumar S,
Raghunandhakumar S, Jagan S, Anandakumar P, Augustine TA, Kamaraj S
and Devaki T: Potent antitumor and antineoplastic efficacy of
baicalein on benzo(a) pyrene-induced experimental pulmonary
tumorigenesis. Fundam Clin Pharmacol. 26:259–270. 2012. View Article : Google Scholar
|
|
32
|
Weiss JM, Bagley S, Hwang WT, Bauml J,
Olson JG, Cohen RB, Hayes DN and Langer C: Capecitabine and
lapatinib for the first-line treatment of metastatic/recurrent head
and neck squamous cell carcinoma. Cancer. 122:2350–2355. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kurokawa M, Ise N, Omi K, Goishi K and
Higashiyama S: Cisplatin influences acquisition of resistance to
molecular-targeted agents through epithelial-mesenchymal
transition-like changes. Cancer Sci. 104:904–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chappell J and Dalton S: Altered cell
cycle regulation helps stem-like carcinoma cells resist apoptosis.
BMC Biol. 8:632010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schreiber V, Dantzer F, Ame JC and de
Murcia G: Poly(ADP-ribose): Novel functions for an old molecule.
Nat Rev Mol Cell Biol. 7:517–528. 2006. View Article : Google Scholar : PubMed/NCBI
|