Introduction
It is well-established that chronic inflammation is
a critical component of tumour development and progression
(1,2). However, many of the molecular and
cellular factors involved remain obscure. Infiltrating neutrophils
and eosinophils have been observed in most human cancers and are
often associated with poor survival (3–5).
Recently, neutrophils have gained considerable attention as
important players in cancer progression because of their role in
ECM remodeling, tumour angiogenesis and metastasis (6–8).
Moreover, Chen et al (5)
revealed that an elevated peripheral neutrophil/lymphocyte ratio
was associated with poor survival in breast cancer.
Within the tumour microenvironment, the accumulation
of neutrophils is reported within the invasive front of tumours
(9,10), while eosinophils abundantly
accumulate near blood vessels and infiltrate hypoxic regions
(11,12), where they are activated to release
an arsenal of factors from their cytoplasmic granules. A major
component of these released factors are myeloperoxidase (MPO) and
eosinophil peroxidase (EPO), which are released by infiltrating
neutrophils and eosinophils, respectively. Importantly, these
enzymes are abundantly deposited in breast cancer (4,13).
However, to date, no distinct role has been attributed to their
presence within the tumour microenvironment. We have recently
reported a new biological role for peroxidase enzymes as key
regulators of fibroblasts and endothelial cell functionality,
driving migration, collagen biosynthesis and blood vessel
development (14,15), processes which are major
contributors of cancer development and metastasis (16).
In the present study, we investigated for the first
time the pro-tumorigenic and pro-metastatic potential of MPO and
EPO using the 4T1 syngeneic breast cancer mouse model and
characterized the molecular mechanisms involved. In vitro,
peroxidase treatment stimulated primary human mammary fibroblast
migration, collagen type I and VI protein secretion, and modified
ECM architecture. In vivo, peroxidases enhanced mammary
tumour growth and progression. Taken together, our findings
identify peroxidase enzymes as key mediators of tumour development
and progression, and suggest that peroxidase inhibitors may have
therapeutic potential for the treatment of breast cancer and
metastasis.
Materials and methods
Reagents
Native human eosinophil peroxidase (EPO) was
obtained from Cell Sciences (Canton, MA, USA) and GenWay Biotech
Inc. (San Diego, CA, USA). Recombinant human myeloperoxidase (MPO)
was purchased from R&D Systems (Minneapolis, MN, USA).
Animals
Five week-old female BALB/c mice (Institute of
Medical and Veterinary Services Division, Gilles Plains, SA,
Australia) were acclimatized to the animal housing facility for a
minimum period of 1 week prior to the commencement of
experimentation. All mice were housed under pathogen free
conditions and all experimental procedures on animals were carried
out with strict adherence to the rules and guidelines for the
ethical use of animals in research and were approved by the Animal
Ethics Committees of the University of Adelaide and the Institute
of Medical and Veterinary Science, Adelaide, SA, Australia.
Mammary fat pad injection of breast
cancer cells
4T1 mouse breast cancer cells were cultured in
RPMI-1640 media, supplemented with 2 mM glutamine, 100 IU/ml
penicillin, 160 µg/ml gentamicin, HEPES (20 mM) and 10%
fetal bovine serum (FBS; Biosciences, Sydney NSW, Australia) in a
5% CO2-containing humidified atmosphere, until they
reached 70–80% confluent. Adherent cells were removed from flasks
with 0.5% trypsin/EDTA and resuspended in phosphate-buffered saline
(PBS) at 0.5×105 cells/10 µl and kept on ice in
an Eppendorf tube. An equal volume of Matrigel™-HC (BD Biosciences,
Bedford, MA, USA) was added to the cells and resuspended.
Seven-week old mice were divided into 3 groups (n=7) and
anaesthetized by isoflurane (Faulding Pharmaceuticals, Adelaide,
Australia). The mammary fat pad area of the mice was wiped with
ethanol and the skin was lifted over the left outermost nipple.
Finally, a 25-G needle was inserted and 20 µl of cells were
injected into the mammary fat pad. Mice were allowed to recover
under the heat lamp before being transferred into cages. Mice were
humanely sacrificed 23 days after the cancer cell transplantation,
due to high tumour load and the lungs were removed for assessment
of tumour burden.
In vivo bioluminescent imaging (BLI)
Non-invasive, whole body imaging to monitor
luciferase-expressing murine 4T1 mammary carcinoma cells in mice
was performed weekly using the IVIS Spectrum™ imaging system
(Xenogen Corp., Alameda, CA, USA). Mice were injected into the
intraperitoneal (i.p.) space with 100 µl of the D-Luciferin
solution at final dose of 3 mg/20 g mouse body weight (Xenogen
Corp.) and then gas-anaesthetized with isoflurane (Faulding
Pharmaceuticals). Images were acquired for 0.5–30 sec
(representative images are shown at 1 sec) from the front angle and
the photon emission transmitted from mice was captured and
quantitated in photons/sec using Living Image software (version
4.2).
Histochemistry analysis
Standard hematoxylin and eosin (H&E) staining of
paraffin embedded tissue was used for histological examination of
primary tumours. Stained sections were examined and photographed on
a Nikon Eclipse 90i microscope. Tumour necrosis was assayed on
H&E whole tumour cross-section and quantified by ImageJ
software (NIH, Bethesda, MD, USA) as previously described (17). Briefly, H&E histological slides
of whole tumour cross-sections were digitized, where areas of
tumour borders and necrosis were assessed and highlighted as a
separate pixelated area. The area of tumour necrosis was then
quantified as a percentage of total tumour area. For
immunohistochemistry, sections (6 µm thick) of 4T1 tumours
were probed with primary antibodies; rabbit anti-human collagen
type I polyclonal; rabbit anti-human collagen type VI polyclonal
(Rockland Immunochemicals, Inc., Pottstown, PA, USA), mouse
anti-human α-SMA (Sigma-Aldrich, St. Louis, MO, USA), rabbit
anti-human CD31 (Abcam, Cambridge, UK) as previously described
(14). Quantitative analysis of
the degree of positive staining was performed by digitized image
analysis with ImageJ software as previously described (18). Briefly, the image of the tissue
area was converted to grey scale and the threshold level of
staining was set for each image and measured and expressed as a
percentage of the total tissue surface.
Transwell migration assay
Mammary fibroblast cell migration was determined
using the 24-well Transwell plate (BD Falcon FluoroBlok™) system
with 8.0 µm pore PET membranes. Fibroblasts were starved in
serum-free Dulbecco's modified Eagle's medium (DMEM) overnight,
then seeded (1×105 cells) in 100 µl of serum-free
DMEM into the upper wells and incubated at 37°C in 5%
CO2 for 30 min to allow cell adhesion. Lower chambers
were then filled with 700 µl of serum-free DMEM with no
further supplementation as the negative control, or supplementation
with TGF-β (10 ng/ml) serving as the positive control, or with the
various peroxidase proteins. Cells were allowed to migrate for 18
h. Migrated cells were fixed in 6:1 ethanol/acetic acid for 10 min,
stained with DAPI (1 µg/ml), and then photographed and
quantified on a fluorescent inverted microscope (Axio Observer Z1;
Carl Zeiss AG, Oberkochen, Germany).
Collagen enzyme-linked immunosorbent
assay (ELISA)
To evaluate the effect of MPO and EPO on collagen
production, human mammary fibroblasts were seeded into 96-well
plates (Nunc, Roskilde, Denmark) at a density of 1.2×104
cells/well and cultured for 5 days in DMEM/10% FBS until reaching
confluence. Cells were starved overnight in serum-free DMEM and
then stimulated for an additional 72 h in serum-free DMEM
containing either ascorbic acid 2-phosphate at 10 µmol/l
(Wako Chemical Industries, Ltd., Osaka, Japan) as a positive
control, or with the peroxidase proteins in the absence of ascorbic
acid supplementation. At the end of the 72-h treatment period,
fibroblast-conditioned media were collected for measurement of
secreted soluble type I and VI collagen by ELISA. Cell
viability/growth was then assessed using the AlamarBlue™
fluorescent dye assay (Invitrogen Life Technologies, Melbourne,
Australia) as per manufacturer's instructions. The amount of
soluble type I and VI collagen in cell-conditioned medium was
measured by a direct coat ELISA method using standard curves
constructed from purified type I and VI human placental collagen
(BD Biosciences) as previously described (14).
3D matrix production
To assess the effect of MPO and EPO on 3D collagen
formation, 3D collagen matrices were generated by stimulated human
mammary fibroblasts as previously described (19). Briefly, 24-well plates were treated
with 0.2% gelatin in PBS overnight at 4°C, then cross-linked with
paraformaldehyde and washed multiple times with PBS. Human mammary
fibroblasts were seeded into plates and grown to confluency. When
cells reached 100% confluency, platting media were replaced with
reduced serum (2% FBS) DMEM supplemented with 1 µg/ml of MPO
or EPO. Ascorbic acid 2-phosphate at 10 µM and 2% FBS DMEM
were used as positive and vehicle controls, respectively. Treatment
media were replaced every second day for six days. Fibroblasts were
then removed using cell lysis buffer, and the remaining ECM was
washed multiple times with PBS prior to use in adhesion, invasion
and immunofluorescence assays.
Immunofluorescence assays
Human mammary fibroblasts were seeded onto circular
glass coverslips where 3D collagen matrices were generated.
Matrices were blocked with goat serum in PBS Triton-X, before
washing and immune-labeled overnight with antibodies against
collagen type I (Rocklands Immunochemicals). Labeled proteins were
detected using Alexa Fluor® 488 goat anti-mouse IgG
(Molecular Probes/Thermo Fisher Scientific Australia Pty Ltd.,
Scoresby VIC, Australia). Fluorescence was captured using a laser
confocal microscope (Zeiss LSM 700; Carl Zeiss AG).
4T1 mammary carcinoma adhesion assay
Using 3D-ECM matrices created from fibroblasts,
adherence of 4T1 cancer cells was assessed via adhesion assays
previously described (20).
Briefly, 2×104 cells were applied to 3D-ECMs in 24-well
plates and were allowed to adhere for 4 h. Cells were then gently
washed with PBS before the cell measurement via AlamarBlue™assay as
per manufacturer's instructions.
4T1 mammary carcinoma invasion assay
Three-dimensional (3D) ECM invasion assays were
adapted from a previously described study (21). Mammary fibroblast derived 3D-ECM
matrices were created in the upper chamber of a 24-well Transwell
plate (BD Falcon FluoroBlok™) system with 8.0 µm pore PET
membranes. 4T1 cells were then seeded (1×105 cells) in
100 µl of serum-free RPMI into the upper wells and incubated
at 37°C in 5% CO2 for 30 min to allow cell adhesion.
Lower chambers were filled with 700 µl of reduced serum (2%
FBS) RPMI as a chemo-attractant stimulus for 24 h. Migrated cells
on the opposite side of the Transwell membrane were fixed in 6:1
ethanol/acetic acid for 10 min, stained with DAPI (1 µg/ml),
and then photographed and quantified on a fluorescent inverted
microscope (Axio Observer Z1; Carl Zeiss AG).
4T1 mammary carcinoma proliferation
assay
Proliferation of sub-confluent 4T1 cultures was
assessed by alamarBlue, as previously described (22). Briefly, 4T1 (1×104)
cells were cultured in 96-well plates in 100 µl in 2% FBS
RPMI media. Cells were treated with 100 µl of increasing
concentrations of MPO and EPO (0.31–5 µg/ml) at the time of
cell plating and cultured for 48 h. FBS RPMI growth media (10%)
served as the positive control. The fluorescence was measured and
quantified at wavelengths of 530 nm excitation and 595 nm emission
using FLUOstar Optima microplate reader (BMG Labtech, Ortenberg,
Germany).
RNA isolation and quantitative real-time
PCR
To evaluate the effect on human mammary fibroblast
and 4T1 mammary carcinoma gene regulation by MPO and EPO (2
µg/ml), RT-PCR was performed. RNA isolation and cDNA
synthesis was performed as previously described (14). Messenger RNA expression of specific
genes was identified using real-time RT-PCR using SYBR-Green Fluor
qPCR Mastermix (Qiagen) in a CFX96 real-time system (Bio-Rad
Laboratories) as previously described (23). The primer combinations used are as
follows: cyclooxygenase-2 (COX-2): forward, 5′-CCTGTG
CCTGATGATTGC-3′ and reverse, 5′-CTGATGCGTGAAGTGCTG-3′; matrix
metalloproteinase 1 (MMP1): forward, 5′-GACGTTCCCAAAAATC-3′ and
reverse, 5′-GCTAGAAGGGATTG-3′; matrix metalloproteinase 3 (MMP3):
forward, 5′-AGAGGCATCCACACC-3′ and reverse,
5′-CTGGCTCCATGGAATTTCTCTTC-3′. Expression values were normalized to
the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase
(GAPDH): forward, 5′-ACCCAGAAGACTCTGTGGATGG-3′ and reverse,
5′-TCAGTGAGCTTCCCGTTCAG-3′
Data analysis and statistical
analysis
Data points derived from experiments are reported as
the mean ± standard error of the mean (SEM). Analysis of variance
to determine significant difference between samples was performed
using the paired Student's t-test, and one way ANOVA followed by
multiple comparison test (Dunnett's Method) where indicated, using
SigmaPlot 2011 (version 12.0; Systat Software, Inc., San Jose, CA,
USA).
Results
Peroxidases enhance primary tumour growth
in vivo
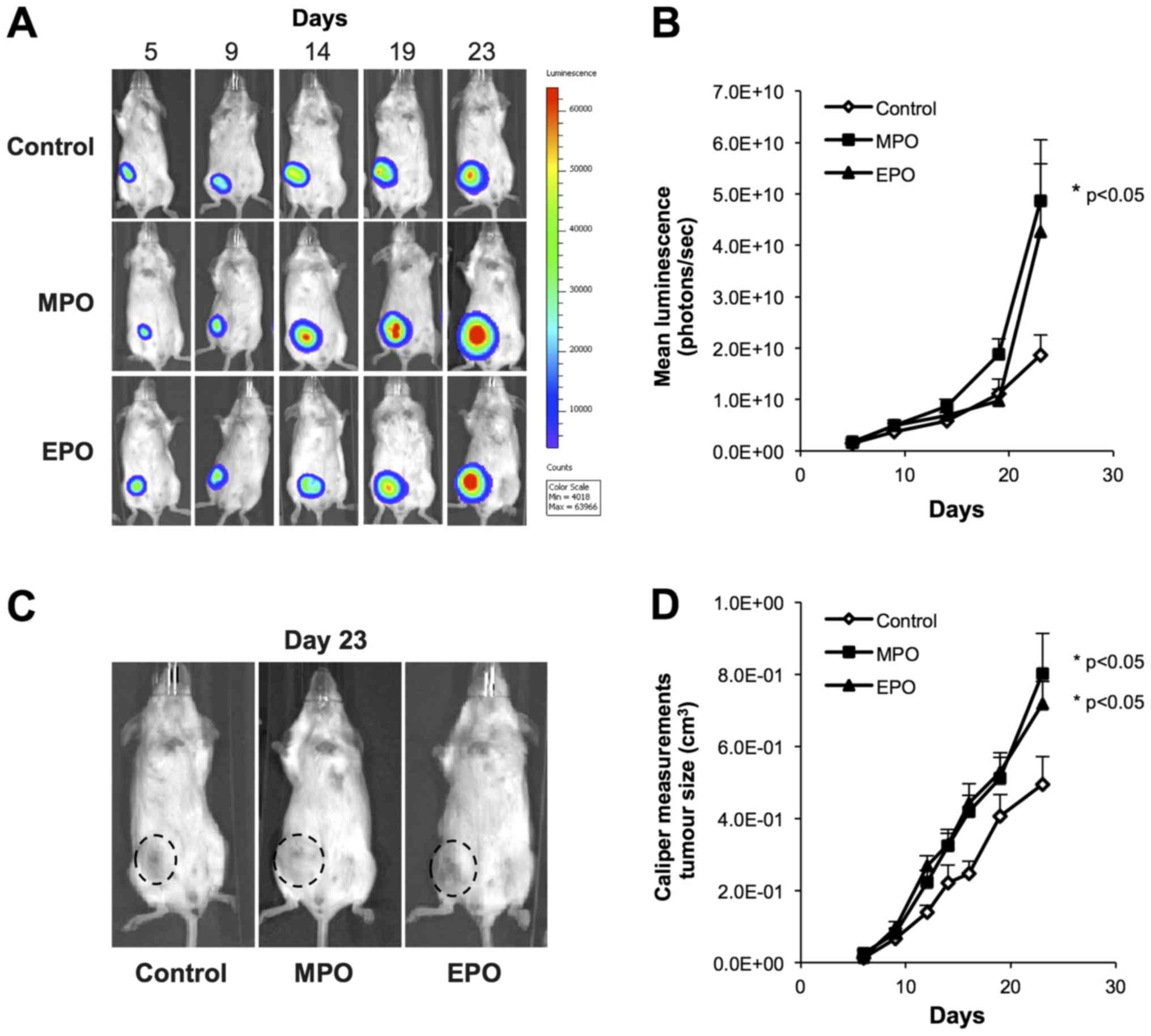
To evaluate the role of MPO and EPO in mammary
tumour growth and metastasis, we used a bioluminescent, luciferase
labeled 4T1 mouse mammary carcinoma cell line, and the subsequent
orthotopic 4T1 tumour metastasis model in BALB/c mice. 4T1 cells
were inoculated into the 4th mammary fat pad of BALB/c mice and
allowed one week for tumour establishment before once weekly
intratumoral injections of 5-µg doses of MPO or EPO, with
PBS serving as the vehicle control. Tumour growth was monitored
weekly with non-invasive bioluminescence imaging (BLI) (Fig. 1A), and quantified via luciferase
intensity (photons/sec) (Fig. 1B),
which showed that both MPO and EPO treatment increased primary
tumour growth (MPO, P=0.05), over 23 days, as compared to the
vehicle control. Tumour burden measured also by calipers and
expressed as a function of volume in mm3 confirmed the
BLI (P<0.05; Fig. 1C and
D).
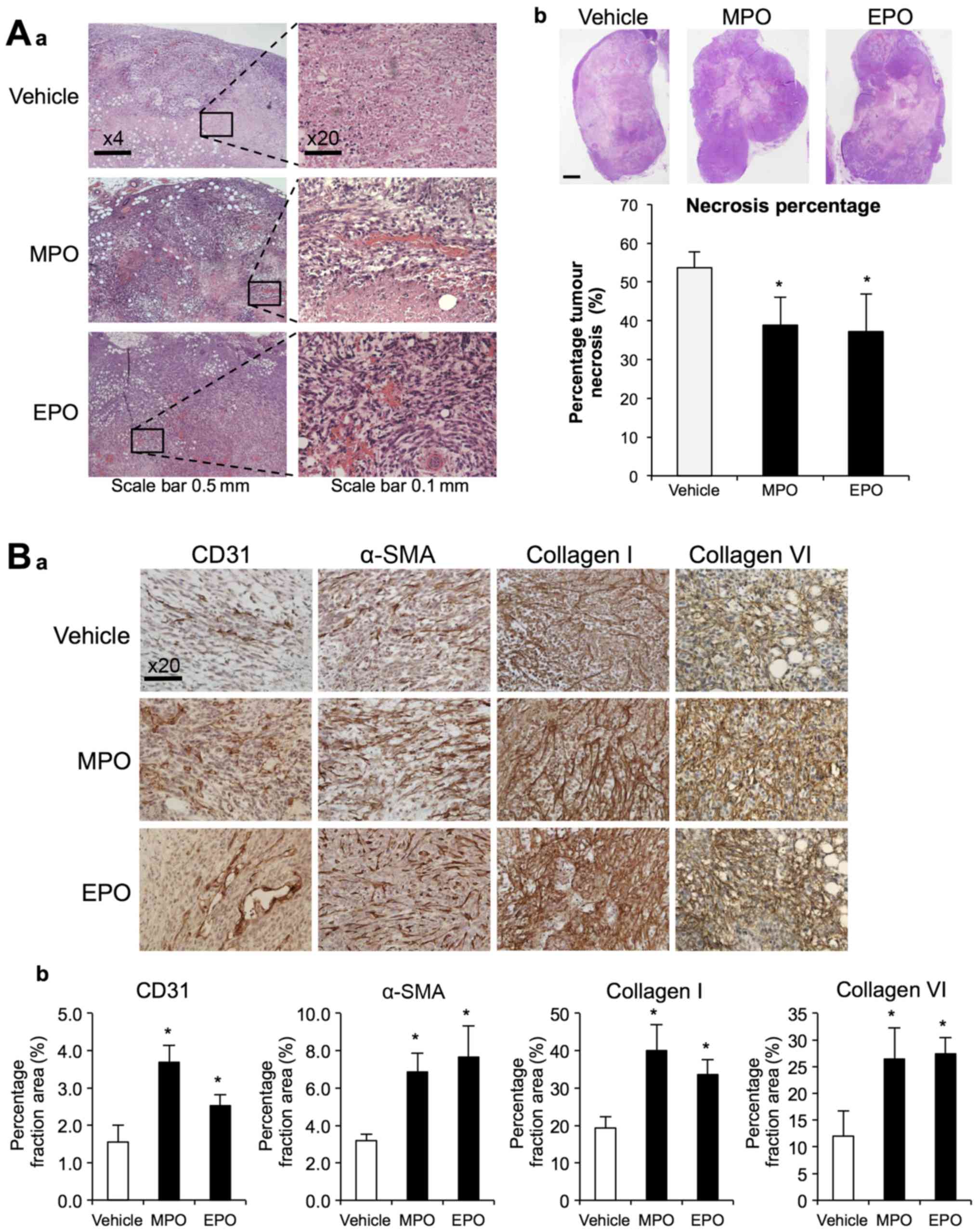
Histological examinations of the 4T1 tumours after
23 days (Fig. 2A) showed that
tumour necrosis was significantly decreased by MPO and EPO
treatment. Representative images of H&E staining clearly show
the inherent necrotic and avascular regions within the PBS treated
4T1 primary tumour in the control mice. In contrast, treatment with
either peroxidase, show diminished regions of necrotic areas that
were associated with the presence of viable tumour cells.
Histological staining and subsequent quantitative image analysis of
tumour sections with antibodies to CD31, α-SMA and collagen types I
and VI, identified and increase in vascularization, myofibroblasts
and ECM deposition, respectively (Fig.
2B).
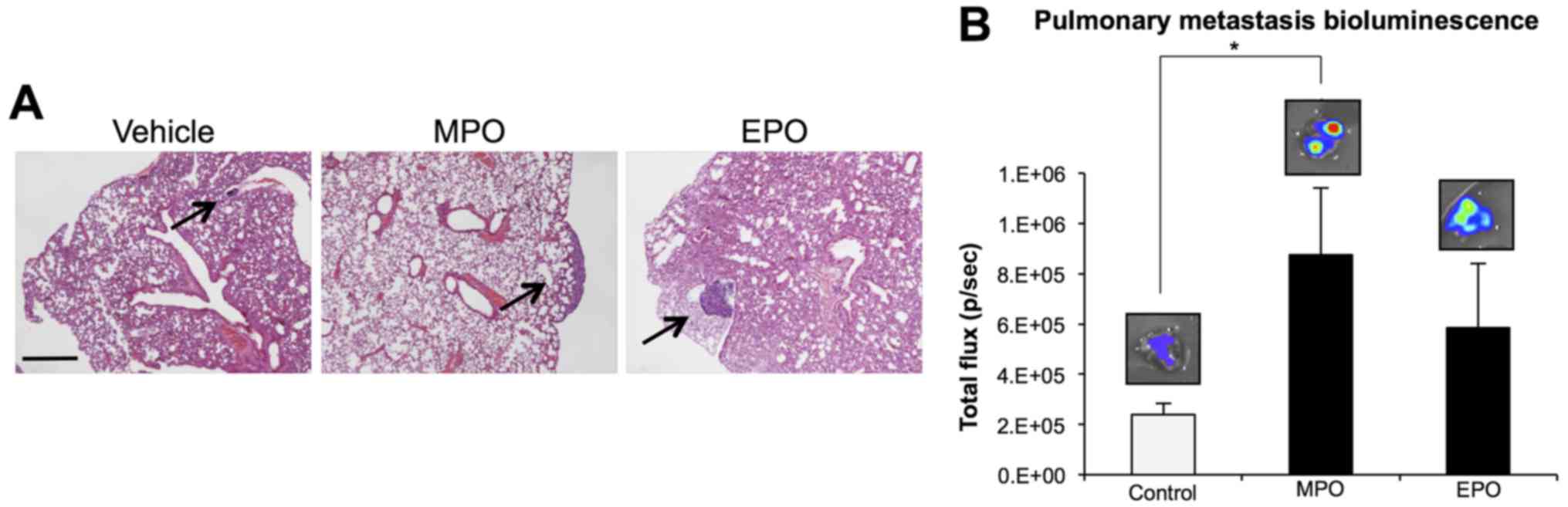
Peroxidases enhance lung metastasis
Mammary carcinoma 4T1 cells spontaneously
metastasize to the lungs from the mammary fat pad (24). Histological assessment of excised
lungs stained with H&E confirmed the presence of lung
metastasis indicating an increase in macrometastases in the lungs
of animals treated with peroxidases (Fig. 3A). Ex vivo BLI
quantification of excised lungs from animals in each group
confirmed these findings as a function of photon counts per sec.
Both MPO and EPO treatment groups revealed an increase in the
intensity (photons/sec) representative of increased spontaneous
lung metastasis (MPO, P<0.05; EPO, P=0.12) after 23 days
relative to vehicle control (Fig.
3B). Thus, our data indicate that an increase in peroxidase
levels within the primary tumour has the potential to promote
metastatic spread to the lungs in vivo.
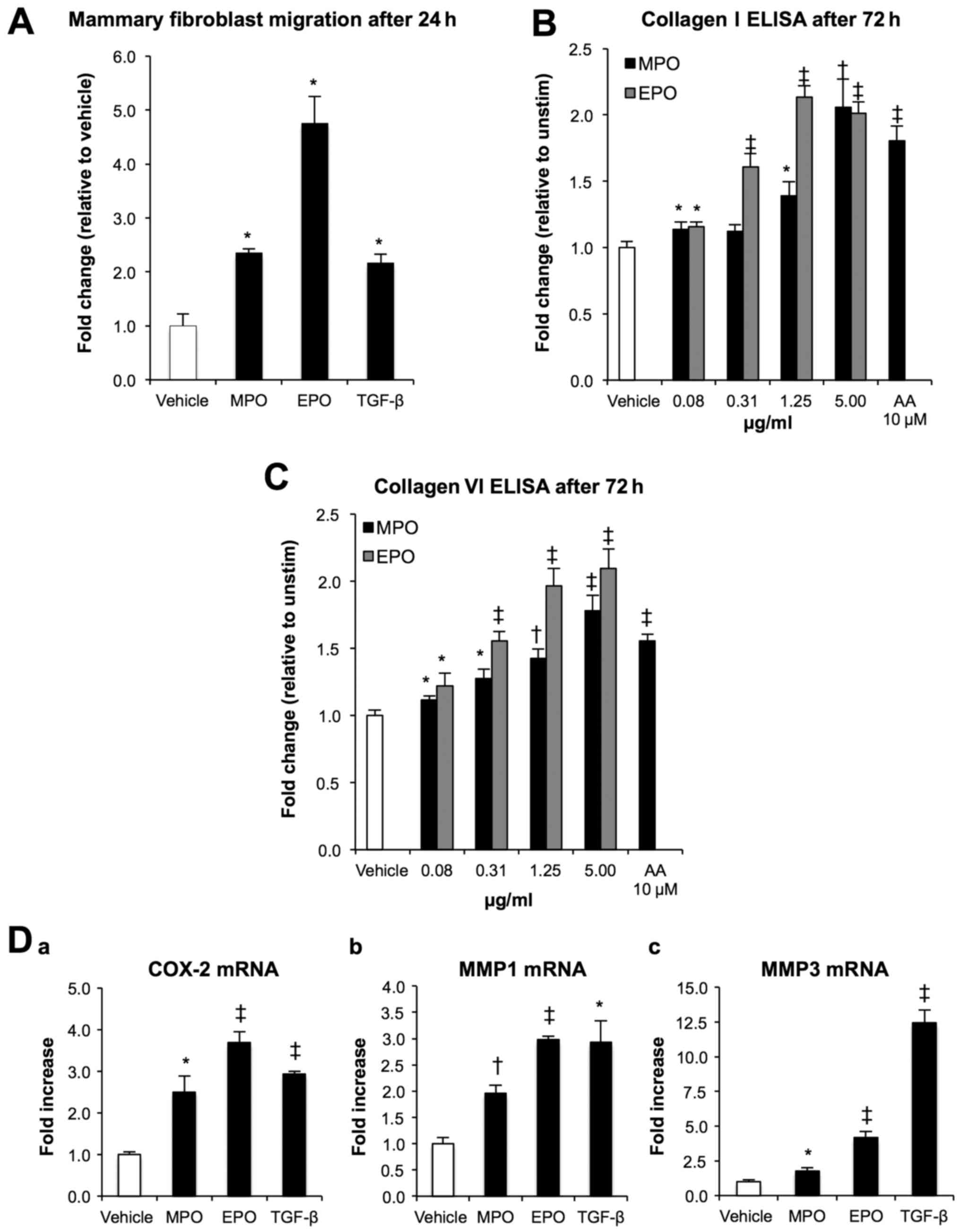
Peroxidases promote pro-fibrotic effects
in primary human mammary fibroblasts in vitro
The migratory ability of fibroblasts within the
tumour microenvironment plays a central role in tumour growth and
is causatively associated with increased metastatic behaviour of
cancer cells (25). To determine
the effect of peroxidases on primary human mammary fibroblasts, we
measured cellular movement toward MPO and EPO using a Transwell
migration assay. After 24 h in serum-free conditions, MPO and EPO
(5 µg/ml) significantly increased mammary fibroblast
migration by 2- and 4-fold, respectively, when compared to vehicle
control (P<0.05; Fig. 4A). The
stimulation of primary human mammary fibroblasts with increasing
concentrations of MPO and EPO in the absence of ascorbic acid (AA)
demonstrated a dose-dependent increase in collagen type I (Fig. 4B) and type VI (Fig. 4C) up to 2-and 1.6-fold,
respectively (P<0.0001, P<0.001 and P<0.05). At these
peroxidase concentrations, there was no impact on the proliferation
and/or viability of the confluent mammary fibroblasts. To determine
the potential for other mechanisms by which peroxidases activate
fibroblasts, we next investigated the transcriptional regulation of
the key genes implicated in promoting a motile phenotype (Fig. 4D) including COX-2 and the matrix
modifying genes MMP1 and MMP3 via RT-PCR (26). Analysis of mRNA expression showed
that MPO and EPO increased COX-2 expression 2.5- and 3.7-fold, MMP1
expression 2- and 2.9-fold, and MMP3 expression 1.8-and 4.2-fold,
respectively, when compared to vehicle control (P<0.0001,
P<0.001 and P<0.05).
Peroxidases promote a functional
pro-tumorigenic ECM in vitro
Collagen fiber alignment plays a critical role in
tumour progression (27).
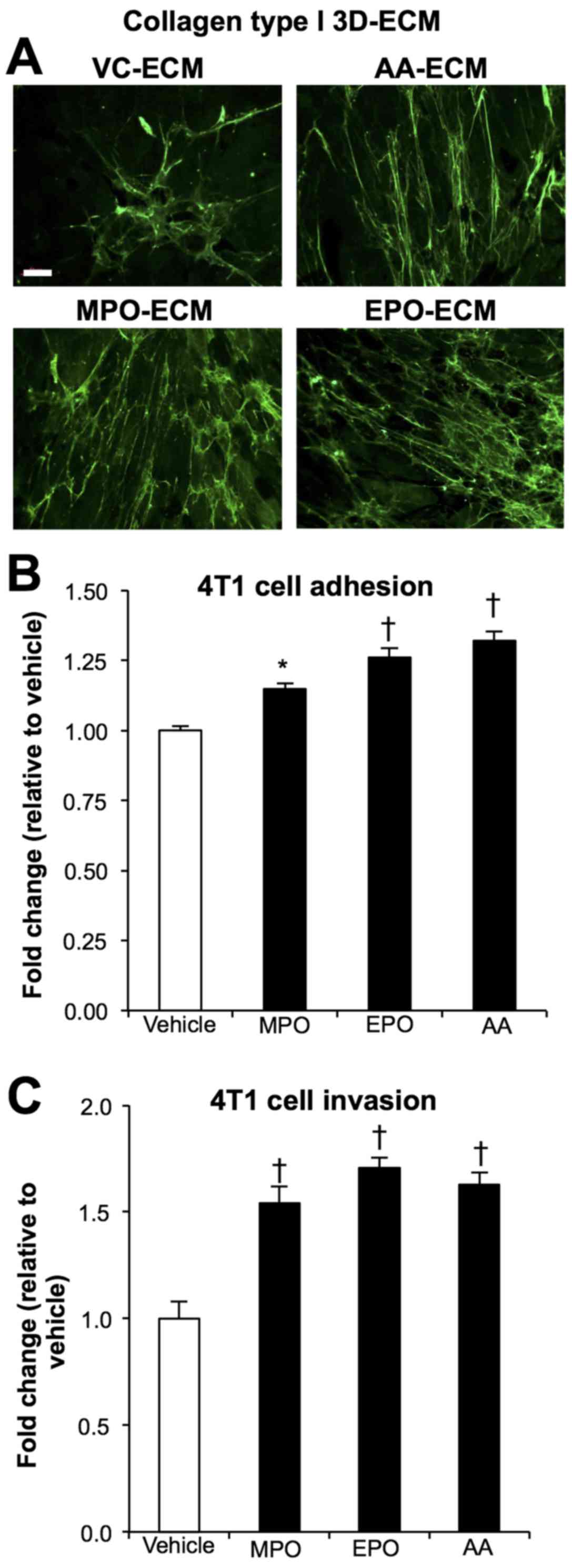
Immunofluorescent detection of collagen type I orientation within
cell derived 3D collagen matrices (Fig. 5A) showed that MPO (MPO-ECM) and EPO
(EPO-ECM) stimulation produced a matrix of parallel and/or
linearized collagen fibers comparable to the AA (AA-ECM) positive
control. In contrast, the unstimulated vehicle control matrices
(VC-ECM) display a mesh-like and disorganized organization of
random collagen fibers.
To determine whether this increase in the alignment
of collagenous matrix affects tumour cell behaviour, we next tested
the adherent properties of the different ECM by measuring 4T1
tumour cell adhesion to the matrices produced under different
conditions. After 4 h of co-incubation, MPO-ECM and EPO-ECM
significantly increased 4T1 adhesion up to 15% (P<0.01) and 25%
(P<0.001), respectively, when compared to VC-ECM. The response
was similar to that achieved by the AA-ECM (Fig. 5B). Collagen alignment facilitates
invasion, thus, to further understand how the reorganization of
aligned collagen fibers interacts with tumour cells, we tested the
effects of the peroxidase-modified matrices on the 4T1 cells in a
Transwell system designed to monitor cell movement through
fibroblast generated ECM. After a 24-h period in reduced serum
conditions, cell counts showed that the peroxidase induced 3D
matrices of MPO and EPO significantly increased 4T1 invasion
~1.5-fold (P<0.001) compared to vehicle control, a response
similar to that seen by the positive control, AA (Fig. 5C).
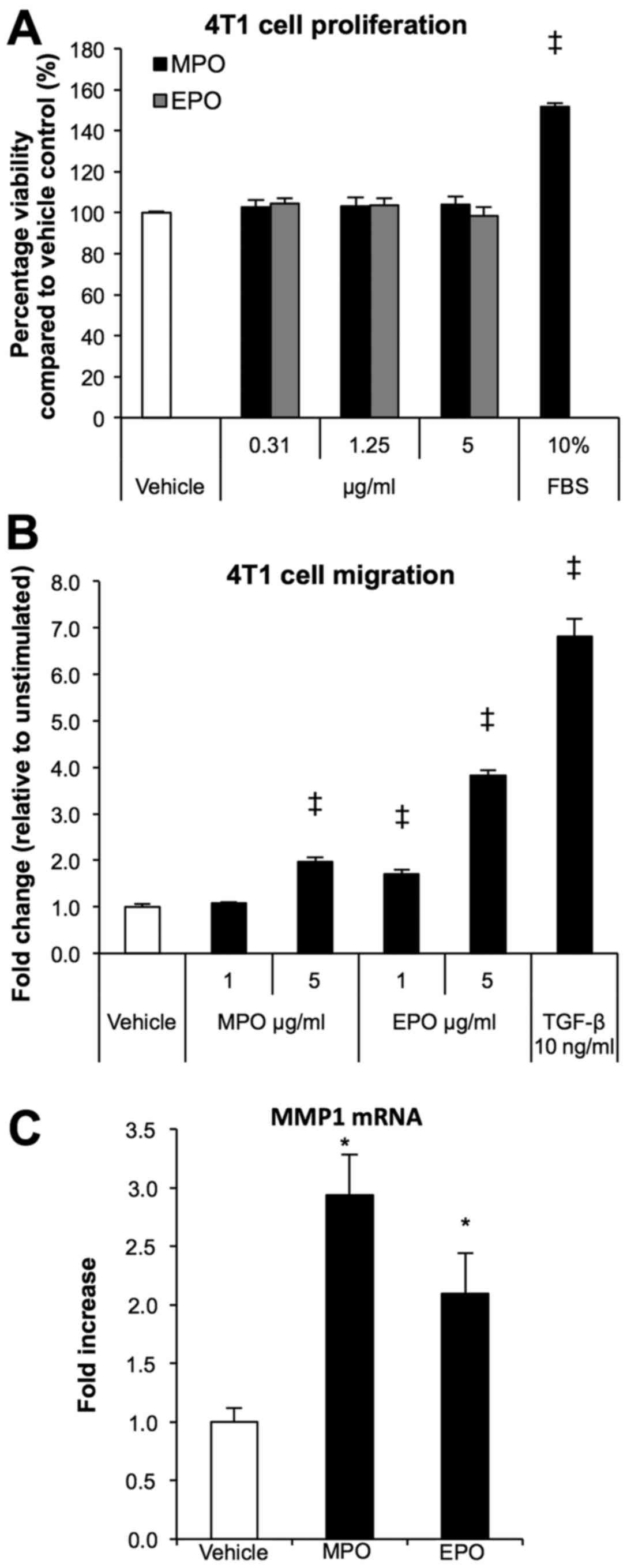
Peroxidases exhibit direct effects on 4T1
tumour cells
To explore further the role of increased peroxidase
presence within the tumour microenvironment, we investigated the
proliferation, migration/invasion, and mechanistic aspects of 4T1
tumour cells stimulated with either MPO or EPO. Treatment of 4T1
cells with various concentrations of peroxidases had no effect on
proliferation after 48 h when compared to vehicle control (Fig. 6A), while 10% FBS containing media
served as the positive control. However, using the Transwell
migration system, the chemo-attractant ability of MPO and EPO
significantly increased migration almost 2-fold (P<0.0001) and
4-fold (P<0.0001) respectively when compared to vehicle control
(Fig. 6B). MMP1 gene expression
belongs to a subset of genes that are associated with organ
specific metastasis to the lungs (28). To investigate the transcription
regulation of MMP1, we analyzed peroxidase treated 4T1 tumour cells
via RT-PCR. Analysis of mRNA expression showed that MPO and EPO
increased MMP1 expression 3- and 2-fold, respectively when compared
to vehicle control (P<0.05).
Discussion
It is well established that inflammatory cells have
commanding effects on tumour development and that both MPO and EPO
are found at high levels within the stroma of human breast cancers
and breast tumours in mouse models (4,29).
The present study is the first to uncover a mechanism linking
peroxidases with facilitating tumour growth and metastasis via the
regulation of ECM components and angiogenesis. Studies have shown
that the depletion of neutrophils can significantly reduce
collagen-dense mammary tumour progression (6) and angiogenesis (30), suggesting that these immune cells
and their constituents are key players in tumour formation and
metastasis.
Using the murine 4T1 breast cancer model we showed
that when injected into the tumour microenvironment, MPO and EPO
significantly increased tumour growth and evidence of enhanced
metastasis to the lungs. Our unique findings are supported by a
recent study showing that specific inhibition of MPO catalytic
activity during the early stages of tumour development reduced
tumour burden by 50% in a model of lung carcinogenesis (31). This finding suggests a causative
role for MPO during tumorigenesis, and identifies MPO inhibitors as
novel therapeutic agents. Furthermore, earlier studies have
identified that women with a genetic polymorphism responsible with
reduced MPO expression is associated with reduced breast cancer
risk (32,33). Taken together, these studies
suggest a more sinister role for these inflammatory enzymes. Our
histological analysis of the peroxidase-treated tumours, showed a
reduction of necrosis within the necrotic cores inherent of the 4T1
tumours (34). This suggests that
MPO and EPO may promote the survival of cancer cells likely via the
activation of the stroma compartment since peroxidases had no
direct effects on tumour cells themselves.
Peroxidases induced an increase in stromal
fibroblasts within the primary tumour and collagen I and VI
deposition. Enhanced deposition of collagens and the aberrant
changes through the remodeling of ECM composition and function
within the tumour microenvironment are crucial for facilitating
primary tumour growth and escape (35). Specifically, increased collagen
type I is a prognostic factor and is associated with breast cancer
recurrence in human breast cancer patients (36), while collagen type VI knockout mice
were shown to have reduced primary tumour formation and growth
(37). Importantly, the peroxidase
effects on primary human mammary fibroblasts in vitro appear
to correlate with our histological observations in vivo, as
we find that both MPO and EPO regulate fibroblast migration and/or
recruitment shown by an increase in α-SMA staining within the
primary tumours, as wells as increased collagen type I and VI
deposition.
Studies have shown that ECM configuration in
particular, collagen fiber alignment (27) and tissue stiffness (38) are functionally associated with
tumour invasion. In this study, we find that both MPO and EPO, in
addition to increasing collagen biosynthesis, alter collagen
alignment in a 3D matrix. Our results further show, that the
combination of increased collagen biosynthesis with the structural
shift in the alignment of collagen fibers in the absence of AA,
significantly increased tumour cell adhesion and invasion, however,
due to the dramatic ability of peroxidases to stimulate collagen,
it may be conceivable that more collagen is sufficient to exert
these effects. To accurately recapitulate these effects in
vivo, further studies are needed to confirm whether the
peroxidase-induced alignment observed in the present study affects
overall tumour stiffness as reported by Acerbi and colleagues
(39). Intriguingly, the authors
of this study revealed that a correlation between the invasive
regions of aggressive human breast cancer and ECM remodeling and
stiffening was linked to an increase in innate immune infiltrate.
Although, it is widely accepted that elevated collagen deposition
coupled with the alteration of linear collagen fibers lead to an
increase in ECM stiffness (40,41),
further exploration is needed to determine the stromal features
that contribute to tumour stiffness.
Aberrant MMP activities within the stromal
compartment have a causative role in tumour incidence by affecting
tumour cell motility and invasiveness. In the present study, we
showed that both MPO and EPO modulate MMP1 and MMP3 gene expression
in primary human mammary fibroblasts, while increasing the
transcription of MMP1 in 4T1 mammary carcinoma epithelial cells.
Elevated MMP1 expression in CAFs has been linked to vital
metastatic processes that facilitate angiogenesis, matrix
degradation and the conversion of normal resident fibroblasts to
activated CAFs (42). In addition,
several independent studies reported that the overexpression of
MMP3 within the mammary stroma, increases mammary tumour incidence
and invasion (43–45), while the overexpression of MMP1 in
mammary epithelial cells is associated with increased invasion, and
metastasis to the lungs, brain and the bone (28,46–48).
We have recently shown that MPO and EPO modulate the
transcriptional regulation of COX-2 in primary human endothelial
cells (15). Here, we show that
peroxidases also regulated COX-2 gene expression in primary human
fibroblasts. However, no significant effect was found in murine 4T1
carcinoma cells (data not shown). Based on the increased expression
in a large portion of breast carcinomas, the activation of COX-2
and MMPs have been implicated to have a significant role in
accelerating breast tumorigenesis and necessary for the initiation
of metastasis, particularly to the lungs (49). Emerging evidence reveals that
stromal COX-2 expression can also function as a mediator of tumour
progression (50,51).
In summary, our findings demonstrate for the first
time that the peroxidase enzymes MPO and EPO confer a broader range
of action than previously thought and exhibit potent
pro-tumorigenic effects in the tumour milieu altering matrix
composition and function, angiogenesis and invasion. Taken together
with our previous reported observations, we propose that both MPO
and EPO are causatively involved in breast cancer progression and
identify them as potential therapeutic targets whereby specific
novel inhibitors may reduce tumour growth and limit the occurrence
of metastasis.
Abbreviations:
|
AA
|
ascorbic acid
|
|
ANOVA
|
analysis of variance
|
|
BLI
|
bioluminescence imaging
|
|
COX-2
|
cyclooxygenase-2
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
ECM
|
extracellular matrix
|
|
ELISA
|
enzyme linked immunosorbent assay
|
|
EPO
|
eosinophil peroxidase
|
|
FBS
|
fetal bovine serum
|
|
G
|
gauge
|
|
H&E
|
hematoxylin and eosin
|
|
i.p
|
intraperitoneal
|
|
MMP
|
matrix metalloproteinase
|
|
MPO
|
myeloperoxidase
|
|
PBS
|
phosphate-buffered saline
|
|
PET
|
polyethylene terephthalate
|
|
RPMI
|
Roswell Park Memorial Institute
|
|
SEM
|
standard error of the mean
|
|
α-SMA
|
alpha-smooth muscle actin
|
Acknowledgments
We thank Matthew Iasiello for his excellent
technical assistance. The present study was supported in part by
The Hospital Research Foundation and the National Health and
Medical Research Council (Career Development Fellowship/627015;
Project Grant/1050694).
References
|
1
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galdiero MR, Bonavita E, Barajon I,
Garlanda C, Mantovani A and Jaillon S: Tumor associated macrophages
and neutrophils in cancer. Immunobiology. 218:1402–1410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Samoszuk MK, Nguyen V, Gluzman I and Pham
JH: Occult deposition of eosinophil peroxidase in a subset of human
breast carcinomas. Am J Pathol. 148:701–706. 1996.PubMed/NCBI
|
|
5
|
Chen J, Deng Q, Pan Y, He B, Ying H, Sun
H, Liu X and Wang S: Prognostic value of neutrophil-to-lymphocyte
ratio in breast cancer. FEBS Open Bio. 5:502–507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
García-Mendoza MG, Inman DR, Ponik SM,
Jeffery JJ, Sheerar DS, Van Doorn RR and Keely PJ: Neutrophils
drive accelerated tumor progression in the collagen-dense mammary
tumor microenvironment. Breast Cancer Res. 18:492016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Swierczak A, Mouchemore KA, Hamilton JA
and Anderson RL: Neutrophils: Important contributors to tumor
progression and metastasis. Cancer Metastasis Rev. 34:735–751.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wculek SK and Malanchi I: Neutrophils
support lung colonization of metastasis-initiating breast cancer
cells. Nature. 528:413–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tabariès S, Ouellet V, Hsu BE, Annis MG,
Rose AA, Meunier L, Carmona E, Tam CE, Mes-Masson AM and Siegel PM:
Granulocytic immune infiltrates are essential for the efficient
formation of breast cancer liver metastases. Breast Cancer Res.
17:452015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stockmann C, Schadendorf D, Klose R and
Helfrich I: The impact of the immune system on tumor: Angiogenesis
and vascular remodeling. Front Oncol. 4:692014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Samoszuk M, Lin F, Rim P and Strathearn G:
New marker for blood vessels in human ovarian and endometrial
cancers. Clin Cancer Res. 2:1867–1871. 1996.PubMed/NCBI
|
|
12
|
Cormier SA, Taranova AG, Bedient C, Nguyen
T, Protheroe C, Pero R, Dimina D, Ochkur SI, O'Neill K, Colbert D,
et al: Pivotal Advance: Eosinophil infiltration of solid tumors is
an early and persistent inflammatory host response. J Leukoc Biol.
79:1131–1139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ambrosone CB, Barlow WE, Reynolds W,
Livingston RB, Yeh IT, Choi JY, Davis W, Rae JM, Tang L, Hutchins
LR, et al: Myeloperoxidase genotypes and enhanced efficacy of
chemotherapy for early-stage breast cancer in SWOG-8897. J Clin
Oncol. 27:4973–4979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DeNichilo MO, Panagopoulos V, Rayner TE,
Borowicz RA, Greenwood JE and Evdokiou A: Peroxidase enzymes
regulate collagen extracellular matrix biosynthesis. Am J Pathol.
185:1372–1384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Panagopoulos V, Zinonos I, Leach DA, Hay
SJ, Liapis V, Zysk A, Ingman WV, DeNichilo MO and Evdokiou A:
Uncovering a new role for peroxidase enzymes as drivers of
angiogenesis. Int J Biochem Cell Biol. 68:128–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer Cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keskin D, Kim J, Cooke VG, Wu CC, Sugimoto
H, Gu C, De Palma M, Kalluri R and LeBleu VS: Targeting vascular
pericytes in hypoxic tumors increases lung metastasis via
angio-poietin-2. Cell Rep. 10:1066–1081. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rangan GK and Tesch GH: Quantification of
renal pathology by image analysis. Nephrology (Carlton).
12:553–558. 2007. View Article : Google Scholar
|
|
19
|
Castelló-Cros R and Cukierman E:
Stromagenesis during tumorigenesis: Characterization of
tumor-associated fibroblasts and stroma-derived 3D matrices.
Methods Mol Biol. 522:275–305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kenny HA, Krausz T, Yamada SD and Lengyel
E: Use of a novel 3D culture model to elucidate the role of
mesothelial cells, fibroblasts and extra-cellular matrices on
adhesion and invasion of ovarian cancer cells to the omentum. Int J
Cancer. 121:1463–1472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fisher KE, Pop A, Koh W, Anthis NJ,
Saunders WB and Davis GE: Tumor cell invasion of collagen matrices
requires coordinate lipid agonist-induced G-protein and
membrane-type matrix metalloproteinase-1-dependent signaling. Mol
Cancer. 5:692006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Villa JC, Chiu D, Brandes AH, Escorcia FE,
Villa CH, Maguire WF, Hu CJ, de Stanchina E, Simon MC, Sisodia SS,
et al: Nontranscriptional role of Hif-1α in activation of
γ-secretase and notch signaling in breast cancer. Cell Rep.
8:1077–1092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leach DA, Need EF, Trotta AP, Grubisha MJ,
DeFranco DB and Buchanan G: Hic-5 influences genomic and
non-genomic actions of the androgen receptor in prostate
myofibroblasts. Mol Cell Endocrinol. 384:185–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pulaski BA and Ostrand-Rosenberg S:
Reduction of established spontaneous mammary carcinoma metastases
following immunotherapy with major histocompatibility complex class
II and B7.1 cell-based tumor vaccines. Cancer Res. 58:1486–1493.
1998.PubMed/NCBI
|
|
25
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cirri P and Chiarugi P: Cancer associated
fibroblasts: The dark side of the coin. Am J Cancer Res. 1:482–497.
2011.PubMed/NCBI
|
|
27
|
Provenzano PP, Eliceiri KW, Campbell JM,
Inman DR, White JG and Keely PJ: Collagen reorganization at the
tumor-stromal interface facilitates local invasion. BMC Med.
4:382006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu
W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang N, Francis KP, Prakash A and Ansaldi
D: Enhanced detection of myeloperoxidase activity in deep tissues
through luminescent excitation of near-infrared nanoparticles. Nat
Med. 19:500–505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nozawa H, Chiu C and Hanahan D:
Infiltrating neutrophils mediate the initial angiogenic switch in a
mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA.
103:12493–12498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rymaszewski AL, Tate E, Yimbesalu JP,
Gelman AE, Jarzembowski JA, Zhang H, Pritchard KA Jr and Vikis HG:
The role of neutrophil myeloperoxidase in models of lung tumor
development. Cancers (Basel). 6:1111–1127. 2014. View Article : Google Scholar
|
|
32
|
Ahn J, Gammon MD, Santella RM, Gaudet MM,
Britton JA, Teitelbaum SL, Terry MB, Neugut AI, Josephy PD and
Ambrosone CB: Myeloperoxidase genotype, fruit and vegetable
consumption, and breast cancer risk. Cancer Res. 64:7634–7639.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pabalan N, Jarjanazi H, Sung L, Li H and
Ozcelik H: Menopausal status modifies breast cancer risk associated
with the myeloperoxidase (MPO) G463A polymorphism in Caucasian
women: A meta-analysis. PLoS One. 7:e323892012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Serganova I, Rizwan A, Ni X, Thakur SB,
Vider J, Russell J, Blasberg R and Koutcher JA: Metabolic imaging:
A link between lactate dehydrogenase A, lactate, and tumor
phenotype. Clin Cancer Res. 17:6250–6261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cox TR and Erler JT: Remodeling and
homeostasis of the extracellular matrix: Implications for fibrotic
diseases and cancer. Dis Model Mech. 4:165–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van 't Veer LJ, Dai H, van de Vijver MJ,
He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ,
Witteveen AT, et al: Gene expression profiling predicts clinical
outcome of breast cancer. Nature. 415:530–536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iyengar P, Espina V, Williams TW, Lin Y,
Berry D, Jelicks LA, Lee H, Temple K, Graves R, Pollard J, et al:
Adipocyte-derived collagen VI affects early mammary tumor
progression in vivo, demonstrating a critical interaction in the
tumor/stroma micro-environment. J Clin Invest. 115:1163–1176. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Paszek MJ, Zahir N, Johnson KR, Lakins JN,
Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M,
Boettiger D, et al: Tensional homeostasis and the malignant
phenotype. Cancer Cell. 8:241–254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Acerbi I, Cassereau L, Dean I, Shi Q, Au
A, Park C, Chen YY, Liphardt J, Hwang ES and Weaver VM: Human
breast cancer invasion and aggression correlates with ECM
stiffening and immune cell infiltration. Integr Biol. 7:1120–1134.
2015. View Article : Google Scholar
|
|
40
|
Riching KM, Cox BL, Salick MR, Pehlke C,
Riching AS, Ponik SM, Bass BR, Crone WC, Jiang Y, Weaver AM, et al:
3D collagen alignment limits protrusions to enhance breast cancer
cell persistence. Biophys J. 107:2546–2558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Egeblad M, Rasch MG and Weaver VM: Dynamic
interplay between the collagen scaffold and tumor evolution. Curr
Opin Cell Biol. 22:697–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Eck SM, Côté AL, Winkelman WD and
Brinckerhoff CE: CXCR4 and matrix metalloproteinase-1 are elevated
in breast carcinoma-associated fibroblasts and in normal mammary
fibroblasts exposed to factors secreted by breast cancer cells. Mol
Cancer Res. 7:1033–1044. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sternlicht MD, Safarians S, Rivera SP and
Barsky SH: Characterizations of the extracellular matrix and
proteinase inhibitor content of human myoepithelial tumors. Lab
Invest. 74:781–796. 1996.PubMed/NCBI
|
|
44
|
Sympson CJ, Bissell MJ and Werb Z: Mammary
gland tumor formation in transgenic mice overexpressing
stromelysin-1. Semin Cancer Biol. 6:159–163. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Holliday DL, Hughes S, Shaw JA, Walker RA
and Jones JL: Intrinsic genetic characteristics determine
tumor-modifying capacity of fibroblasts: Matrix metalloproteinase-3
5A/5A genotype enhances breast cancer cell invasion. Breast Cancer
Res. 9:R672007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu H, Kato Y, Erzinger SA, Kiriakova GM,
Qian Y, Palmieri D, Steeg PS and Price JE: The role of MMP-1 in
breast cancer growth and metastasis to the brain in a xenograft
model. BMC Cancer. 12:5832012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kang Y, Siegel PM, Shu W, Drobnjak M,
Kakonen SM, Cordón-Cardo C, Guise TA and Massagué J: A multigenic
program mediating breast cancer metastasis to bone. Cancer Cell.
3:537–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gupta GP, Nguyen DX, Chiang AC, Bos PD,
Kim JY, Nadal C, Gomis RR, Manova-Todorova K and Massagué J:
Mediators of vascular remodelling co-opted for sequential steps in
lung metastasis. Nature. 446:765–770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Erez N, Truitt M, Olson P, Arron ST and
Hanahan D: Cancer-associated fibroblasts are activated in incipient
neoplasia to orchestrate tumor-promoting inflammation in an
NF-kappaB-dependent manner. Cancer Cell. 17:135–147. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Park SW, Kim HS, Choi MS, Jeong WJ, Heo
DS, Kim KH and Sung MW: The effects of the stromal cell-derived
cyclo-oxygenase-2 metabolite prostaglandin E2 on the proliferation
of colon cancer cells. J Pharmacol Exp Ther. 336:516–523. 2011.
View Article : Google Scholar
|