Introduction
Cervical cancer is known as the third most common
malignancy among the female, and about half million new cases are
diagnosed and >200,000 deaths are reported each year (1,2).
Although a large number of cervical cancer cases can be reduced by
traditional screening and precancerous lesions treatment, cervical
cancer is still the main reason contributing to cancer mortality
among females in many developing regions and countries (3). The high number of cervical cancer
death is due to the low survival rates of patients suffered from
advanced cervical cancer during diagnosis (4). The strategy for cervical cancer is
currently, stage-specific. Although early stage disease could be
cured with surgery or radiotherapy, the most useful strategy for
the local advanced-stage patients is with co-treatment of
chemotherapy and pelvic irradiation (5,6).
Recently, reports from large scale genomic sequencing of human
cervical tumors has illustrated that many targeted treatments may
possess a better choice (7).
Therefore, despite the progress made in prevention, patients with
cancers in metastasis and those who are experiencing recurrent or
persistent disease are without effective therapeutic options
(8).
MicroRNAs (miRNAs) are known as endogenous, small
18–25 ng nucleotides. They are non-coding RNAs, negatively
modulating gene expression at the level of post-transcription
through binding with 3′ untranslated region (UTR) involved in
specific messenger RNAs (mRNAs) (9–11).
Accompanied with sequence complementary to their target mRNAs
partially, miRNAs are of great importance in regulating (mostly
inhibiting) gene expression in different kinds of organisms
(12,13). Additionally, studies have evidenced
that miRNAs are included in a variety of biological processes, such
as cell growth, proliferation as well as metabolism, which is
dependent on mRNA translation inhibition (14). In addition, studies indicate that
~20–30% human genes are modulated by miRNAs, altering expression of
various genes (15). Studies
before have revealed the effects of miR-940 on different diseases.
Liang et al demonstrated that miR-940 downregulation leads
to human Tetralogy of Fallot development (16). Moreover, miR-940 was shown to
suppress progression of prostate cancer and pancreatic ductal
adenocarcinoma by suppressing migration and invasion enhancer 1
(17). Furthermore, previous
report revealed that miR-940 suppressed the migratory and invasive
activity of cells, attenuated anchorage-independent growth ability,
accompanied with increased E-cadherin activation in prostate cancer
of human (18). Taken together, by
targeting different genes under different pathological conditions,
miR-940 might exhibit different biological functions and clinical
impacts. Hence, we should explore the role of miR-940 in a
disease-specific manner.
Herein, we elucidated that miR-940 is markedly
increased in cervical cancer and that miR-940 expression is related
to the development and overall survival rate in cervical cancer.
Ectopic miR-940 promoted, while inhibition of miR-940 reduced the
proliferation, cell cycle arrest and tumorigenicity of cervical
cancer cells from human in vitro. Furthermore, we
demonstrated that miR-940 directly targets and down-regulates p27
and PTEN through recognizing their 3′ UTRs, and downregulation of
p27 and PTEN was essential for the miR-940-mediated effects in
cervical cancer cells. This study demonstrated that miR-940 shows
an essential role in human cervical cancer development and may be a
potential target for the treatment of cervical cancer.
Materials and methods
Cancer cell culture
Human cervical cancer cell lines, including SiHa,
HeLa, Caski, C4-1 and C-33a, and the normal cervical cells of CEC
were purchased from American Type Culture Collection, the Cell
Resource Center, Shanghai Institute of Biochemistry and Cell Bank
at the Chinese Academy of Sciences. Cell lines were routinely
authenticated by DNA-fingerprinting and isoenzyme analyses and
checked for contamination by mycoplasma using Hoechst staining. All
cell lines were maintained in Roswell Park Memorial Institute
(RPMI)-1640, Dulbecco's modified Eagle's medium or minimum
essential medium with 10% fetal bovine serum (FBS) and then were
cultured at 37°C with 5% CO2.
Tissue specimens and patient
information
The paraffin-embedded and -archived cervical cancer
specimens and freshly collected cervical cancer tissue specimens
evaluated in this study were histopathologically and clinically
diagnosed at the Xinjiang Medical University Affiliated Tumor
Hospital between 2010 and 2014. The disease stages of all patients
were divided following the International Federation of Gynecology
and Obstetrics (FIGO) guidelines. The clinical characteristics of
these 83 patients are provided in Table I. Normal cervical tissues were
obtained from individuals who underwent wedge biopsy of the cervix
and proved to be free of any pre-existing detectable situations
pathologically. All samples were detected along with prior written
informed consent from the patients. Prior donor consents, and
approval from Institutional Research Ethics Committee were
received.
 | Table IClinical parameters of 83 cervical
cancer patients. |
Table I
Clinical parameters of 83 cervical
cancer patients.
| Category | Subcategory | N=83 |
|---|
| Age (years) | ≤45 | 32 |
| >45 | 51 |
| Tumor size | ≤5 cm | 58 |
| >5 cm | 26 |
| FIGO stage | I | 22 |
| II | 12 |
| III | 34 |
| IV | 15 |
| Histology | Squamous | 75 |
| Adenocarcinoma | 6 |
| Adenosquamous | 2 |
| Pelvic node
involvement | Positive | 26 |
| Equivocal | 14 |
| Negative | 43 |
| Overall
survival | Death | 24 |
| Alive | 59 |
| miR-940
expression | | |
| Low
expression | I | 15 |
| II | 6 |
| III | 13 |
| IV | 4 |
| High
expression | I | 7 |
| II | 6 |
| III | 21 |
| IV | 11 |
RNA extraction and real-time quantitative
PCR
Total RNA from tumor tissue samples and cultured
cells was extracted through mirVana miRNA Isolation kit (Ambion)
according to the manufacturer's instructions. Then cDNA was
synthesized from total RNA through TaqMan miRNA reverse
transcription kit obtained from Applied Biosystems (USA). RT PCR
was performed by the Applied Biosystems 7500 Sequence Detection
system with iQ™ SYBR Green Supermix (Bio-Rad Laboratories, USA)
with 5 ng cDNA as well as 10 pM of each primer for assessment. The
data here were then normalized to geometric mean of the
housekeeping gene of GAPDH or U6 small nuclear RNA expression and
the results were evaluated with the 2-∆∆CT method.
Sequences of the primers used are as follows: miR-940 forward,
5′-GCA TCG TTC CTT CAA GCC GAT CT-3′ and reverse, 5′-TGG GTG AGT
CGT TCG G-3′; U6 forward, 5′-GTC CTG GCA GAT ATA CAC TAA ACA T-3′
and reverse, 5′-CTC ACG CTT GAA TTC ATG CGG CTT-3′; PTEN forward,
5′-TGT TTG GCA GAT CTT CCT TG-3′ and reverse, 5′-CTC GGT CGT CGC
TCA TAT-3′; p27 forward, 5′-TTG TTG AGC GTA TCT CTT CG-3′ and
reverse, 5′-CGT CGT CAG TCT ATC GCT-3′; cyclin D1 forward, 5′-GTT
GGT TCA TAG CTC TGT CAT-3′ and reverse, 5′-CTG CGT CGT TAC GTC
CTA-3′; Bax forward, 5′-GCT GAT TTA TAG CAC CGT CAT TG-3′ and
reverse, 5′-CAG CAT GGT TTC GAC CGA-3′; Bcl-xL forward, 5′-CTT GCT
TAA TCG CCC TAT CGC AT-3′ and reverse, 5′-TTG CAT GGT AAC CTC CTG
AAC-3′; GAPDH forward, 5′-CAT TCA AGA CCG GAC AGA GG-3′ and
reverse, 5′-ACA TAC TCA GCA CCA GCA TCA CC-3′.
Oligonucleotides, siRNA and
transfection
In order to produce a miR-940 expression vector, the
miR-940 anti-sense (miRZip-940) plasmid used as miR-940 inhibitor,
and the vector control (miRZip-vector) were purchased from System
Biosciences (San Francisco, CA, USA) and used according to the
manufacturer's instructions. For depletion of p27-, and PTEN-siRNAs
were synthesized and purified by RiboBio. Transfection of
oligonucleotides and siRNAs were performed using the Lipofectamine
2000 reagent (Invitrogen, USA), according to the manufacturer's
instructions. miR-940 mimics, and the negative control were
obtained from Genecopoeia (USA) and transfected into cervical
cancer cells by the use of Lipofectamine 2000 reagent (Invitrogen),
according to the manufacturer's instructions.
Immunoblot analysis
Cell proteins were isolated by a T-PER Tissue
Protein Extraction reagent kit (Thermo) according to the
manufacturer's instructions. Different protein concentrations were
calculated through BCA protein assay kit. Then the equal mounts of
protein were loaded per well on a 10–12% sodium dodecyl
sulphatepolyacrylamide gel. Next, proteins were transferred onto
the polyvinylidene difluoride membrane (PVDF). Resulting membrane
was then blocked by the use of Tris-buffered saline with 0.05%
Tween-20 (TBS-T), dissolved in 5% skim milk (Sigma, USA) at room
temperature for 3 h on a rotary shaker, which was followed by TBS-T
for washing. Specific primary antibody used in our study, suspended
in TBST, was then incubated with the membrane at 4°C overnight.
Next, the membrane was washed by the TBS-T after incubation with
peroxidase-conjugated secondary antibody at room temperature for 2
h. The immunoactive proteins were detected through an enhanced
chemiluminescence western blotting detection kit. Western blotting
bands were calculated through GE Healthcare ECL western blot
analysis system and exposed to X-ray film (Kodak). The primary
antibodies were: p27 (Abcam), PTEN (Abcam, USA), caspase-3 (Cell
Signaling Technology), caspase-9 (Abcam), PARP (Abcam), Bcl-2 (Cell
Signaling Technology), Bcl-xL (Cell Signaling Technology), Mcl-1
(Abcam), Bak (Cell Signaling Technology), Bad (Cell Signaling
Technology), Bax (Abcam) and cyclin D1 (Cell Signaling Technology,
USA).
Colony formation assay
Cervical cancer cells per well in 60-mm plates were
cultured in 10% FBS RPMI-1640. Cells were treated under different
conditions. After another 7-day incubation, the cell colonies were
washed twice with PBS, fixed with 4% paraformaldehyde for 15 min
and then stained by Giemsa for 30 min. Each clone with >50 cells
were evaluated. Clone forming efficiency for cells was calculated
based on colonies/number of inoculated cells × 100%.
Transwell migration assay
Cervical cancer cells 1×105 cells/well
were seeded in the top chamber of 24-well Transwell micro-pore
polycarbonate membrane filter with pore size of 8-µm (Millipore,
USA). Then cells were suspended in serum-free medium. Forty-eight
hours later, the cells on the top surface of membrane were removed
by a cotton swab carefully. Finally, the migrated cells were
counted in five random fields for each treatment.
Flow cytometry analysis
All cancer cells in a culture dish were harvested by
trypsinization, washed in ice-cold PBS, and fixed in 80% ice-cold
ethanol in PBS. To evaluate the number of cells experiencing
apoptosis, the Annexin V-FITC kit (BD Biosciences, USA) was used
according to the manufacturer's instructions. Finally, 400 µl
binding buffer was added to analyze the cells immediately through
flow cytometry (BD Biosciences). All experiments were performed in
triplicate.
Immunohistochemistry
At the end of our study, the mice were sacrificed.
Tumors, kidneys and livers were carefully harvested and maintained
in 4% neutral formalin liquid, IHC staining for the measurement of
PTEN, P27 and 1 expression was carried out. The nuclei were
counterstained with Mayer's hematoxylin.
Immunofluorescent analysis
Cells were fixed in 4% paraformaldehyde and then
stained overnight with primary antibodies against. Afterwards,
cells were washed with phosphate-buffered saline and incubated for
0.5 h at room temperature with Alexa 488 (Invitrogen) secondary
antibodies. Cells were fixed with the ProLong gold antifade reagent
with 4′,6-diamidino-2-phenylindole (Invitrogen) at room temperature
for 24 h before visualizing. The coverslips were viewed through a
laser-scanning confocal microscope.
Luciferase assay
Cancer cells were seeded in triplicate in 24-well
plate and allowed to settle for ~12 h. One hundred nanograms of
pGL3-PTEN, or p27-luciferase plasmid was cotransfected into
cervical cancer cells with TK-Renilla plasmid as control
signals using the Lipofectamine 2000 reagent according to the
manufacturer's instructions. Luciferase and control signals were
measured at 48 h after transfection using the Dual Luciferase
Reporter assay kit (Promega, Madison, WI, USA), according to a
protocol provided by the manufacturer. Three independent
experiments were performed.
Xenografted tumor model
The mouse experiments were conducted in the Animal
Laboratory Center. Female, 4–5-week-old BALB/c-nude mice (18–20 g)
were purchased from the Center of Experimental Animal of Xinjiang
University. The BALB/c nude mice were randomly divided into two
groups. One group of mice was subcutaneously inoculated with
5×106 SiHa/vector cells in the left dorsal flank and
with 5×106 SiHa/miR-940 cells in the right dorsal flank
of each mouse. Another group was subcutaneously inoculated with
5×106 SiHa/miRZip-vector cells in the left dorsal flank
and with 5×106 SiHa/miRZip-940 cells in right dorsal
flank of a mouse. Tumor size was calculated with digital caliper.
The tumor volume was determined every 5 days and the experimantal
mice were sacrificed at the end of 6 weeks. Tumors were then
excised, weighed, fixed with 10% neutral formalin, and then
embedded in paraffin for further analysis.
Statistical analysis
The differences of data are stated by means ± SEM.
Different groups were compared through Graph Pad PRISM (Graph Pad
Software, USA). P<0.05 was considered as significant difference
between groups.
Results
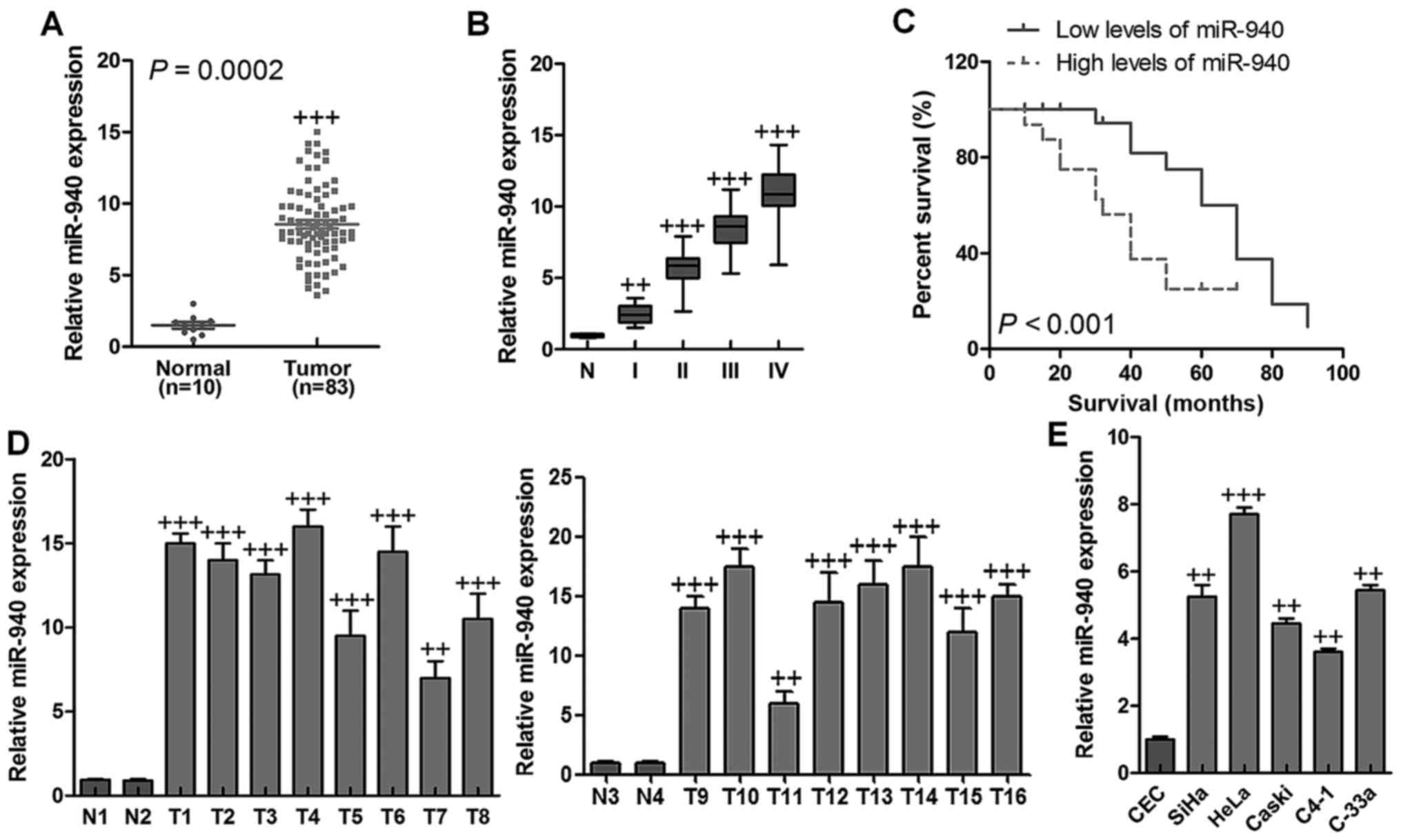
miR-940 is upregulated and related to
overall survival in human cervical cancer
In our study, miR-649, miR-338, miR-21, miR-222,
miR-133b, miR-20a, Mir-221, miR-942, miR-353, miR-370, miR-126, and
miR-940 were assessed, and miR-940 was upregulated significantly
via analyzing a published microarray-based high throughput
assessment (NCBI/E-MTAB-1067) (P=0.0002) in human cervical cancer
tissues in comparison to the normal cervical tissues (Fig. 1A). Expression of miR-940 was
further examined in archived clinical cervical cancer specimens. As
shown in Fig. 1B, miR-940
expression was low in stage I and II tumors, markedly increased in
stage III tumors and was further elevated in stage IV cervical
cancer (n=10). In addition, a high level of miR-940 expression was
significantly related to shorter overall survival (P<0.001)
(Fig. 1C). The data indicated a
possible link between high-level miR-940 expression and the
progression of human cervical cancer, and highlights miR-940 may
have potential value as a prognostic biomarker in human cervical
cancer. Real-time PCR analysis revealed that miR-940 was
significantly overexpressed in 16 freshly-collected cervical cancer
samples compared to the two normal cervical tissues (Fig. 1D). In line with these observations,
upregulation of miR-940 was confirmed in cervical cancer cell lines
compared with a control normal cervical cell line (Fig. 1E). Taken together, the results
above strongly indicated that miR-940 was upregulated in human
cervical cancer.
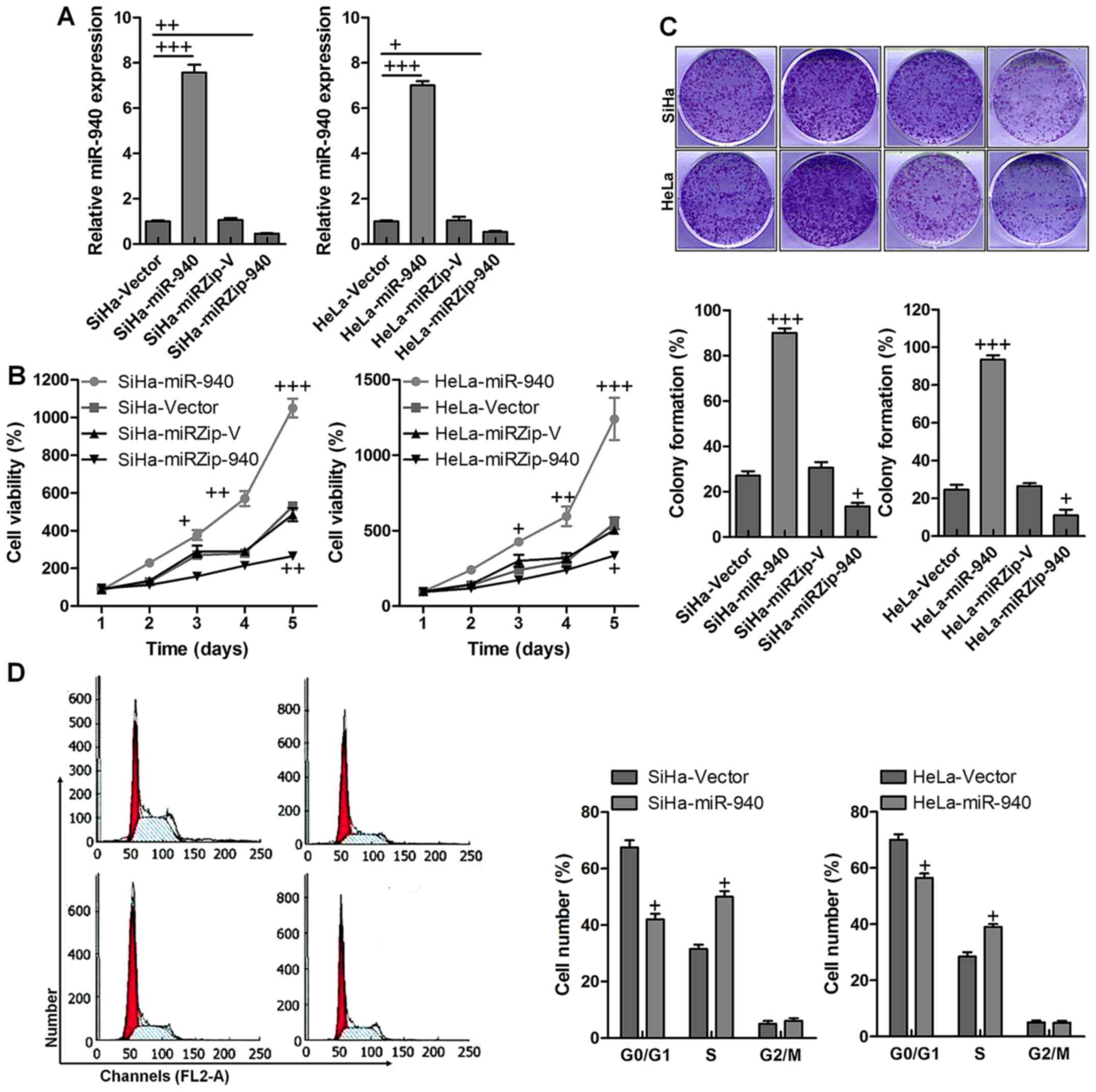
Overexpression of miR-940 promotes the
proliferation and cell cycle progression in human cervical cancer
cells
The data indicated that both in SiHa and HeLa cells,
miR-940 was highly expressed compared to the vector control,
indicating the stable transfectant expresses miR-940. Furthermore,
miR-940 was also reduced by knockdown with significant difference
compared to the vector control group (Fig. 2A). The MTT assay demonstrated that
ectopic overexpression of miR-940 significantly increased the
growth rate of both SiHa and HeLa cells. Also, miR-940 at low
expression dispalyed lower SiHa and HeLa cell viability compared to
the control ones with significant difference (Fig. 2B). The colony formation assay
revealed that ectopic overexpression of miR-940 markedly enhanced
the growth ability of both SiHa and HeLa cells, as indicated by
increased colony numbers and sizes. Similarly, after miR-940
suppression, the percent of colony formation was significantly
reduced, which was comparable to the control group in both SiHa and
HeLa cancer cells (Fig. 2C).
Furthermore, cell cycle analysis showed ectopic overexpression of
miR-940 significantly increased the percentage of cells in the S
phase and decreased the percentage of cells in the G1/G0 peak
(Fig. 2D). Collectively, these
results demonstrated that miR-940 functioned to promote
proliferation and cell cycle progression in human cervical cancer
cells.
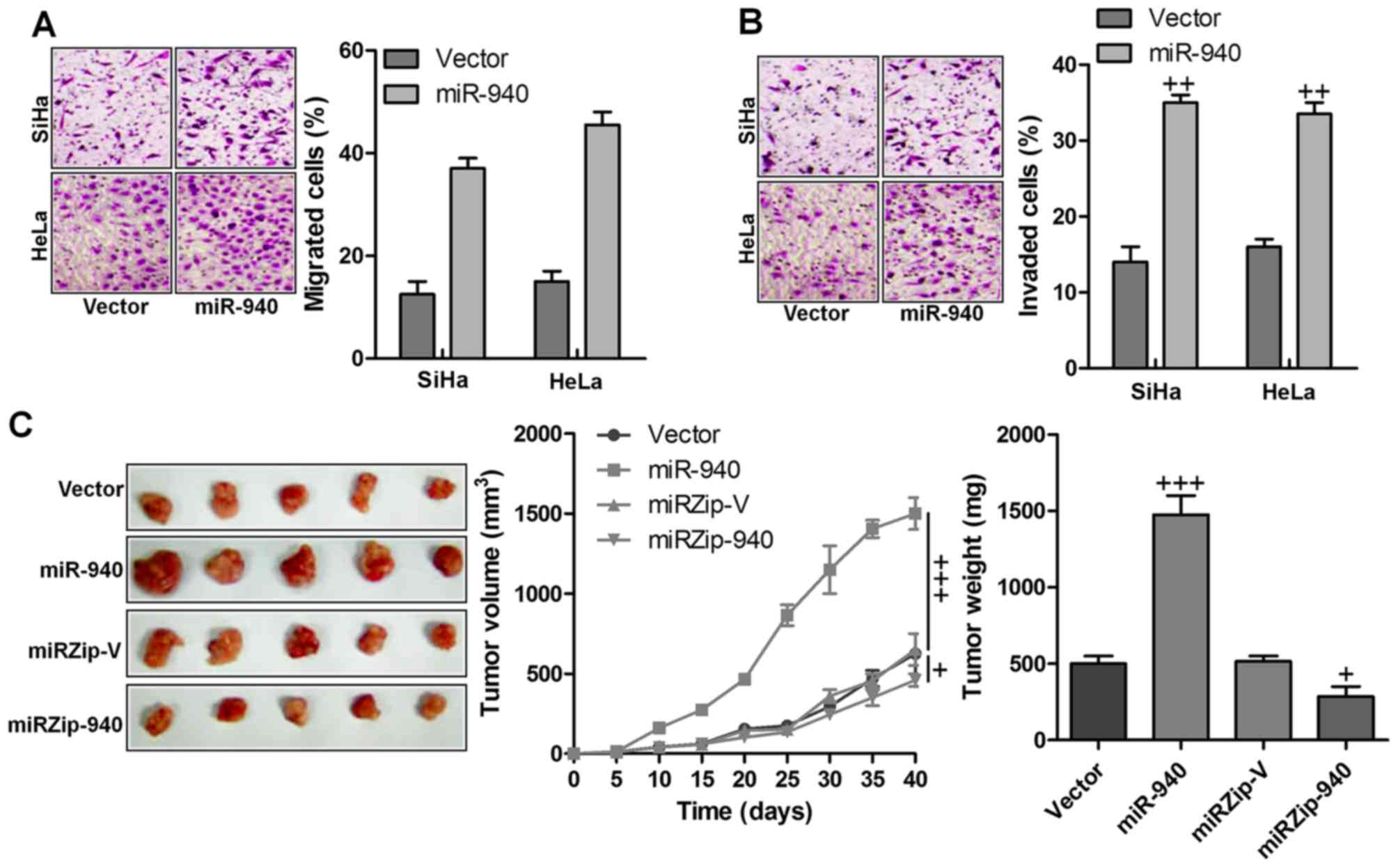
miR-940 enhances tumorigenicity of
cervical cancer cells both in vitro and in vivo
Next, we examined whether miR-940 has any effect on
cervical cancer cell migration and invasion. Cervical cancer cells
stably expressing miR-940 formed higher numbers and greater
migration and invasion than the control cells, while inhibition of
miR-940 led to the formation of fewer and less migration and
invasion (Fig. 3A and B). The
biological effect of miR-940 on cervical cancer progression was
further examined using an in vivo tumor model. The
miR-940-transduced cervical cancer cells and miR-940-silenced
cells, or the corresponding control cells, were subcutaneously
injected into the dorsal flank of nude mice. As shown in Fig. 3C, the tumors formed by
miR-940-transduced cervical cancer cells were larger, in both size
and weight, than the corresponding control tumors, whereas the
tumors formed by miR-940-silenced cervical cancer cells were
smaller in size and weight than the corresponding control tumors.
These data indicated that miR-940 played an important role in
cervical cancer development in vivo.
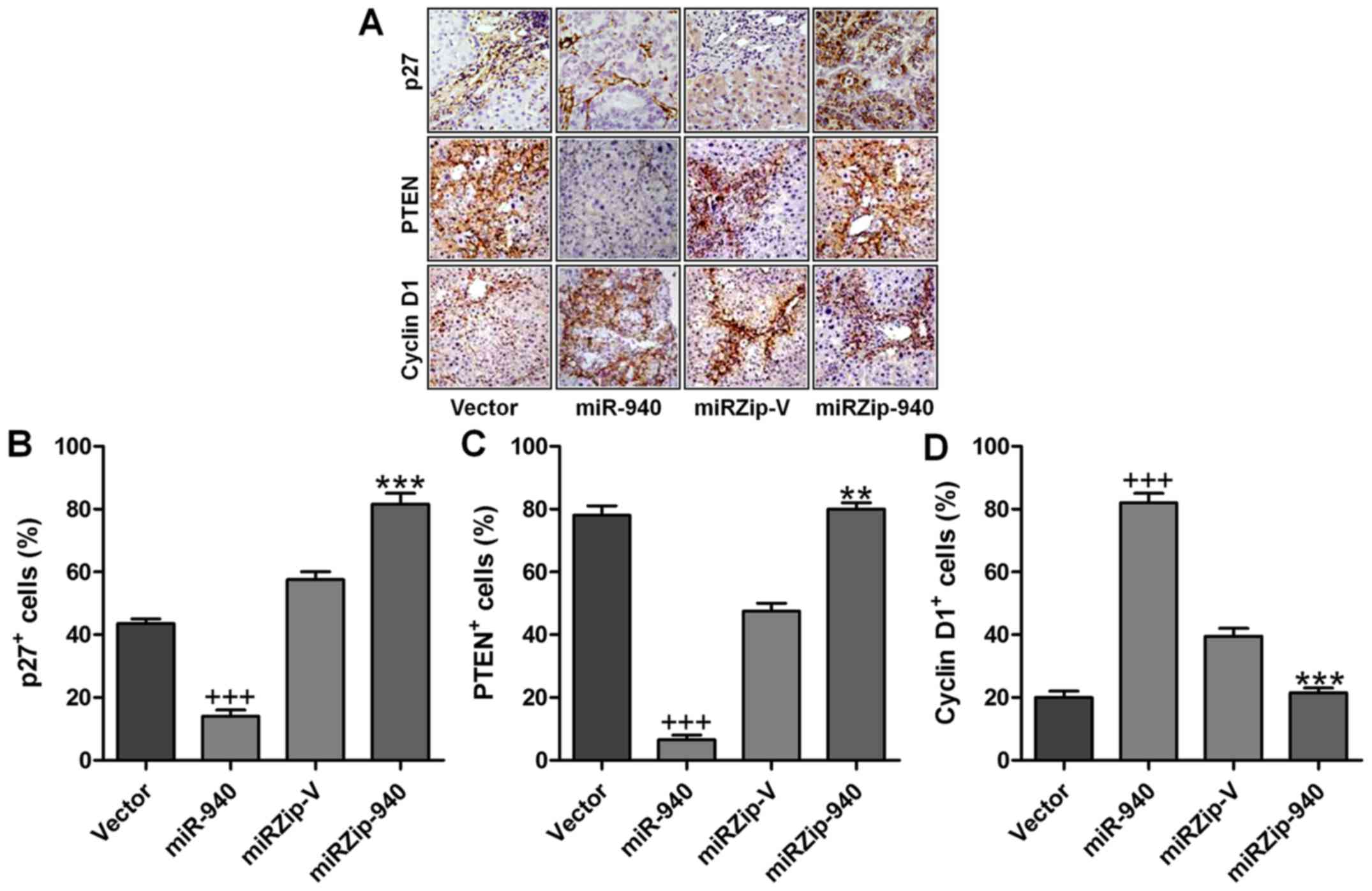
Expression of miR-940, p27, PTEN and
cyclin D1 in human cervical cancer tissues
Finally, to examine whether miR-940-mediated
suppression of p27 and PTEN in cervical cancer is clinically
relevant, eight freshly collected cervical cancer samples and two
normal cervical tissues were obtained for further study. As shown
in Fig. 4, the levels of miR-940
correlated with the protein expression levels of p27, PTEN and
cyclin D1. These results suggested that miR-940 decreased the
expression of p27 and PTEN and increased cyclin D1 expression,
consequently leading to an aggressive phenotype and poorer
prognosis in cervical cancer.
p27 and PTEN are essential for
miR-940-mediated proliferation in cervical cancer
In an attempt to identify the mRNA targets of
miR-940, we performed bioinformatic analysis using a publicly
available algorithm (TargetScan 6.2). Additionally, following
previous studies, p27 and PTEN have been suggested to be related
with miR-940 expression (19–23)
Thus, we suppossed that p27 and PTEN might be also potential
targets of miR-940 in human cervical cancer. Therefore, they were
specificly explored in our study. However, we do not exclude the
possibility that there are still other candidates modulated by
miR-940. On the contrary, p27 and PTEN are essential for tumor
progression. Hence, the two candidates were further explored in our
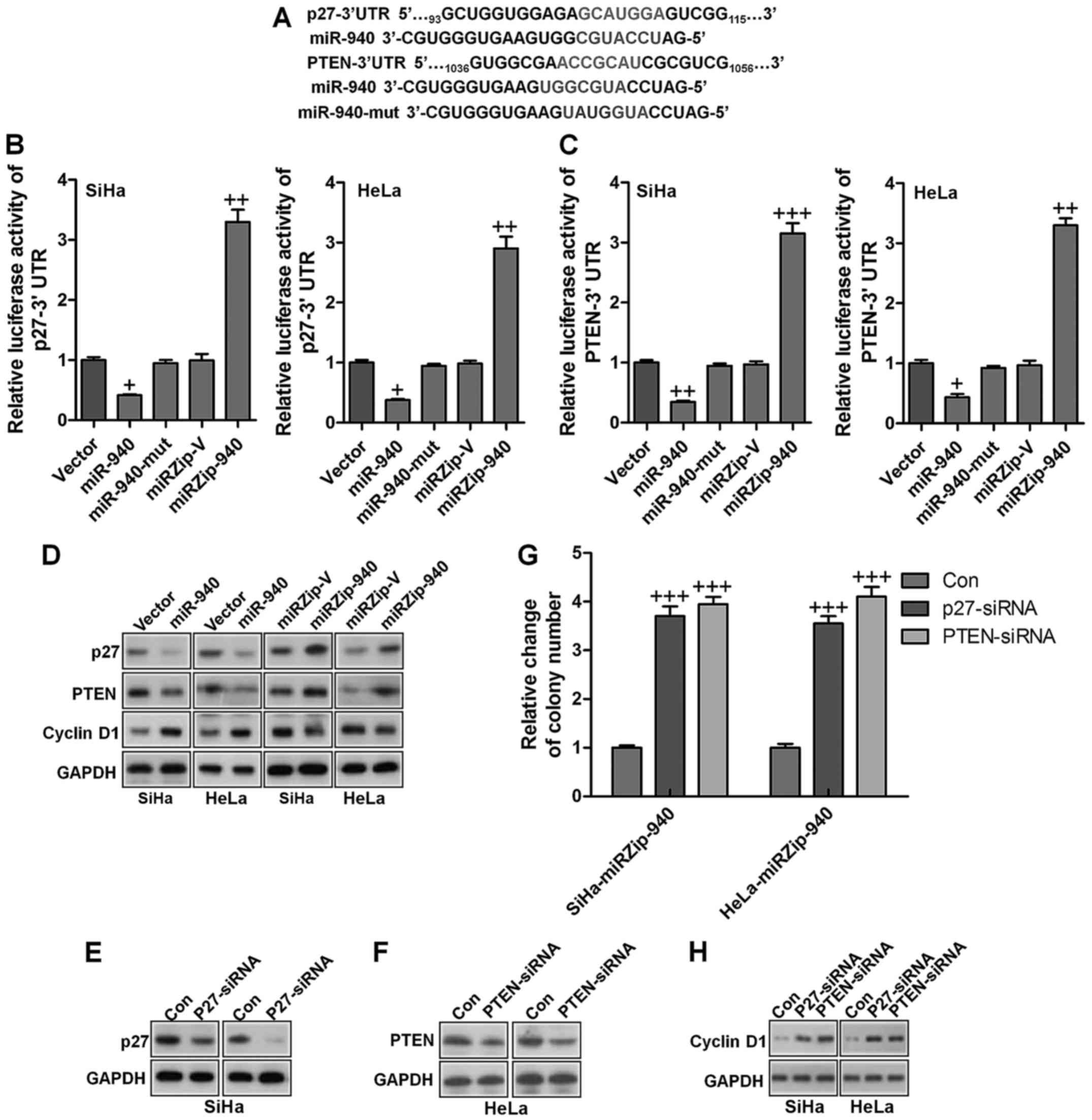
study. As shown in Fig. 5A, p27
and PTEN, which are critical attenuators of cell proliferation and
cell-cycle progression, were identified as potential targets of
miR-940. Luciferase reporter plasmids containing regions of the 3′
UTR of p27 and PTEN were constructed and cotransfected into
cervical cancer cells with miR-940, miR-940 inhibitor or the
corresponding negative controls. As shown in Fig. 5B and C, miR-940 significantly
reduced the luciferase activity of the p27 and PTEN reporter genes,
whereas transfection of the miR-940 inhibitor upregulated the
luciferase activity of the reporter genes. However, transfection of
miR-940-mut (miR-940 mutant) had no significant effect on the
luciferase activity of the reporter genes. Western blot analysis
showed that ectopic expression of miR-940 markedly decreased,
whereas inhibition of miR-940 increased, the protein expression
levels of p27 and PTEN in both SiHa and HeLa cervical cancer cells
(Fig. 5D). Moreover, the
expression of cyclin D1 was increased by ectopic expression of
miR-940, and decreased by miR-940 inhibition (Fig. 5D). These results confirmed that p27
and PTEN were direct targets of miR-940. To evaluate the effects of
p27 and PTEN on miR-940-induced cervical cancer progression, we
suppressed the expression of endogenous p27 and PTEN using specific
siRNAs (Fig. 5E and F). The colony
formation analysis illustrated that silencing p27 and PTEN
increased the proliferation of cervical cells transfected with the
miR-940 inhibitor (Fig. 5G). As
shown in Fig. 5H, silencing p27
and PTEN the miR-940-inhibitor transfected cells also increased the
mRNA and protein expression of cyclin D1, a well-characterized
regulator of cell proliferation. These results suggest that
silencing p27 and PTEN in miR-940-repressed cells reversed the
negative effect of the miR-940 inhibitor on cervical cancer cell
proliferation and tumorigenesis.
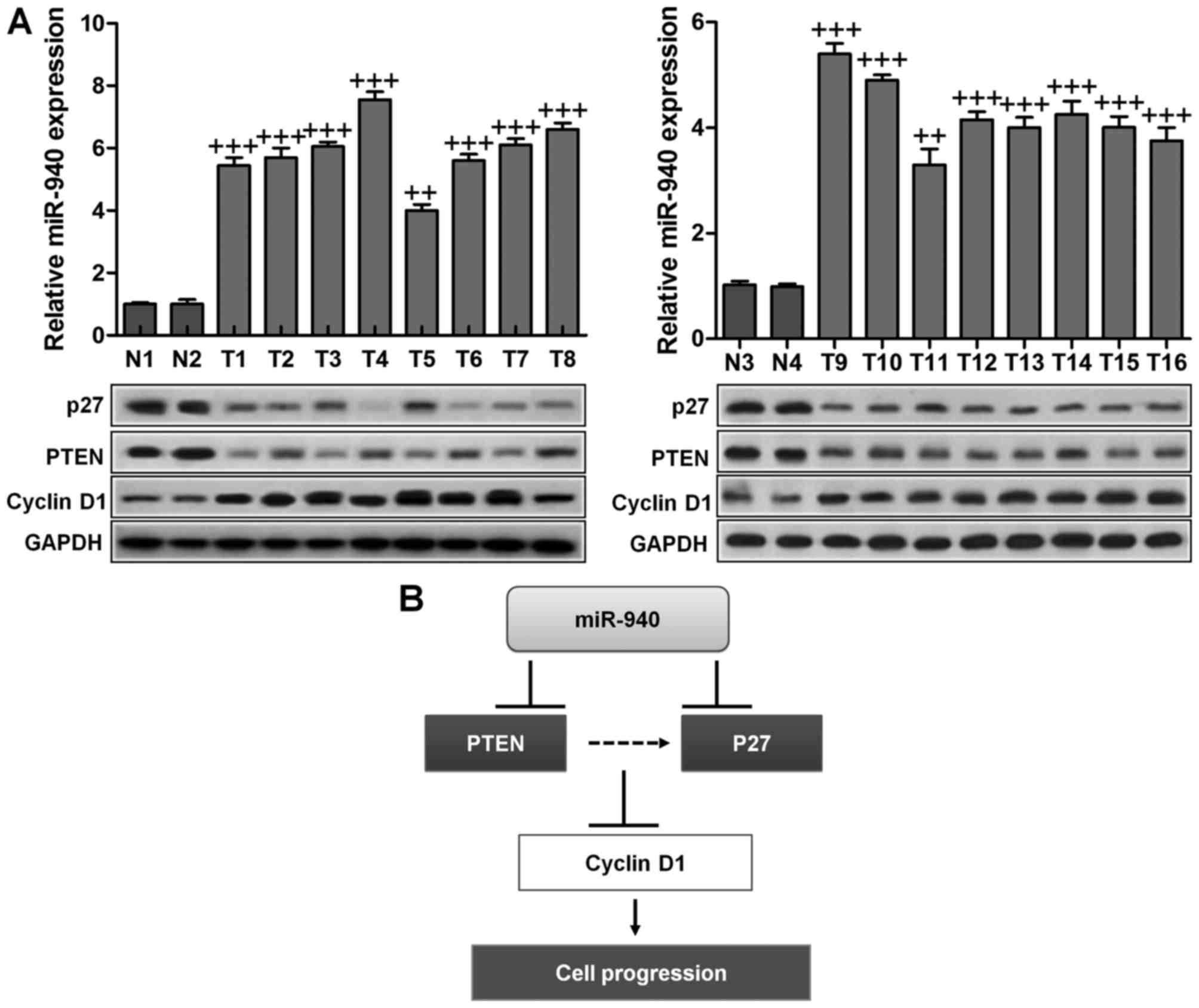
Expression of miR-940, p27, PTEN and
cyclin D1 in human cervical cancer tissues
Finally, to examine whether miR-940-mediated
suppression of p27 and PTEN in cervical cancer is clinically
relevant, 16 freshly collected cervical cancer samples and four
normal cervical tissues were obtained for further study. As shown
in Fig. 6A, the levels of miR-940
correlated with the protein expression levels of p27, PTEN and
cyclin D1. These results suggested that miR-940 decreased the
expression of p27 and PTEN and increased cyclin D1 expression,
consequently leading to an aggressive phenotype and poorer
prognosis in cervical cancer.
Discussion
The discovery of effective diagnostic and prognostic
biomarkers and therapeutic methods is urgently required for the
diagnosis and treatment of cervical cancer. Numerous studies have
shown that miRNAs may represent valuable diagnostic and prognostic
markers for cancer (24–26). miR-940 is overexpressed in the
serum of patients of various carcinoma, and was used to construct a
miRNA signature for patients prognosis (16). However, the expression of miR-940
in cervical cancer has not previously been investigated. This study
demonstrated that miR-940 is significantly upregulated in cervical
cancer. In addition, the expression of miR-940 correlated with
cervical cancer progression and overall patient survival,
indicating that upregulation of miR-940 might contribute to the
development of cervical cancer and might have potential as a
diagnostic and prognostic biomarker for human cervical cancer.
In this study, p27 and PTEN were both identified as
direct targets of miR-940 and could be suppressed by overexpression
of miR-940, which in turn increased the proliferation and cell
cycle progression of cervical cancer cells in vitro and
promoted tumorigenesis in an in vivo model of cervical
cancer. Phosphatase and tensin homolog on chromosome 10 (PTEN) is
known as a dual-specificity phosphatase, which functions as a tumor
suppressor, possessing protein phosphatase and lipid phosphatase
activities, disturbing PI3K activation (27,28).
In addition, PTEN is functionally related to numerous human
cancers. Cells in absence of PTEN have significantly increased
levels of PIP3, activating down-streaming signals of PI3K/AKT
targets (29). PTEN overexpression
has a close relationship with the activation of proteolytic
cascade, contributing to apoptosis, which could be linked to
inactivation of AKT (30).
Furthermore, PTEN plays an important role in modulating genomic
instability as well as DNA repairing signaling pathway (31,32).
Recently, a study has indicated that PTEN negatively responds to
DNA damage response and has interaction with Chk signaling pathway,
leading to cancer progression regulation. Accumulating PTEN could
suppress AKT activity, and consequently inhibit cancer cell growth,
proliferation and impede apoptosis eventually (27). The effects of p27, a crucial tumor
suppressor, are well known including its ability to enhance cancer
cell death or suppress cell proliferation permanently. p27 tumor
suppressor is a crucial component of a complex network that helps
organisms to protect themslves against propagation of cells, which
could carry oncogenic mutations (33). Furthermore, p27 is reported to
suppress kinase activity and disturb cancer cell cycle development
and progression via G1 to S phase (34). Cyclin D1 was overexpressed in a
variety of cancers and has a close relationship with different
cancer cell proliferation. It mediated cancer cell proliferation
through cell cycle progression activation at the point of G1/S
restriction (35). p53-induced
upregulation of p27 in response to DNA damage induces cell cycle
blockade in G1, followed by DNA repair or induction of apoptosis
(36,37).
The evidence discussed above indicates that
downregulation of PTEN and p27 may play essential roles in
miR-940-induced tumor progression in cervical cancer. However, the
detailed regulatory network for miR-940, PTEN and p27, and the
related signaling pathways are likely to be complicated and need
further investigation. Our study demonstrated that miR-940, which
was overexpressed in human cervical cancer, could target and
suppress both PTEN and p27, leading to cervical cancer progression.
Thus, our results indicated the potential value of miR-940 in
cervical cancer development and miR-940 could be considered as
useful biomarker for cervical cancer diagnosis and prognosis
(Fig. 6B). According to previous
studies, target prediction suggested that miR-940 regulated cell
signaling, as well as pathways of cell communication and adhesion.
These signaling pathways have important effects on cancer
initiation and progression, and the downregulation of miR-940 in
plasma may perform as a significant biomarker for cancer detection
(17,18). These results suggested that the
same microRNA can exert distinct biological activities under
different cellular contexts.
In conclusion, miR-940 is overexpressed in human
cervical cancer and upregulation of miR-940 promoted cervical
cancer cell proliferation, cell cycle progression and
tumorigenicity both in vitro and in vivo, while
inhibition of miR-940 lead to the opposite effects. Furthermore,
the function of miR-940 in cervical cancer may be exerted via
downregulation of the target genes of PTEN and p27, which play an
essential role in the function of miR-940 in cervical cancer. Thus,
this study demonstrated that miR-940 might show an important effect
on human cervical cancer progression and might represent a
potential therapeutic target for cervical cancer therapy.
References
|
1
|
World Health Organization: International
Agency for Research on Cancer: Cervical Cancer - Estimated
Incidence. Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp.
Accession date: 2016-9-10.
|
|
2
|
National Comprehensive Cancer Network:
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines).
Cervical Cancer Version 1. 2013, https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
Accession date: 2016-9-10.
|
|
3
|
Monk BJ, Sill MW, McMeekin DS, Cohn DE,
Ramondetta LM, Boardman CH, Benda J and Cella D: Phase III trial of
four cisplatin-containing doublet combinations in stage IVB,
recurrent, or persistent cervical carcinoma: A Gynecologic Oncology
Group study. J Clin Oncol. 27:4649–4655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thigpen T: The role of chemotherapy in the
management of carcinoma of the cervix. Cancer J. 9:425–432. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kato R, Hasegawa K, Achiwa Y, Okamoto H,
Torii Y, Oe S and Udagawa Y: Predicting nedaplatin sensitivity of
cervical cancer using the histoculture drug response assay. Eur J
Gynaecol Oncol. 32:381–386. 2011.PubMed/NCBI
|
|
6
|
Yamamoto K, Kokawa K, Umesaki N, Nishimura
R, Hasegawa K, Konishi I, Saji F, Nishida M, Noguchi H and Takizawa
K: Phase I study of combination chemotherapy with irinotecan
hydrochloride and nedaplatin for cervical squamous cell carcinoma:
Japanese gynecologic oncology group study. Oncol Rep. 21:1005–1009.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watanabe Y, Nakai H, Etoh T, Kanemura K,
Tsuji I, Ishizu A and Hoshiai H: Feasibility study of docetaxel and
nedaplatin for recurrent squamous cell carcinoma of the uterine
cervix. Anticancer Res. 28(4C): 2385–2388. 2008.PubMed/NCBI
|
|
8
|
Sultana H, Kigawa J, Kanamori Y, Itamochi
H, Oishi T, Sato S, Kamazawa S, Ohwada M, Suzuki M and Terakawa N:
Chemosensitivity and p53-Bax pathway-mediated apoptosis in patients
with uterine cervical cancer. Ann Oncol. 14:214–219. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Y and Srivastava D: A developmental
view of microRNA function. Trends Biochem Sci. 32:189–197. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stefani G and Slack FJ: Small non-coding
RNAs in animal development. Nat Rev Mol Cell Biol. 9:219–230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taipaleenmäki H, Bjerre Hokland L, Chen L,
Kauppinen S and Kassem M: Mechanisms in endocrinology: Micro-RNAs:
targets for enhancing osteoblast differentiation and bone
formation. Eur J Endocrinol. 166:359–371. 2012. View Article : Google Scholar
|
|
13
|
Vimalraj S and Selvamurugan N: MicroRNAs:
Synthesis, gene regulation and osteoblast differentiation. Curr
Issues Mol Biol. 15:7–18. 2013.
|
|
14
|
Ni CW, Qiu H and Jo H: MicroRNA-663
upregulated by oscillatory shear stress plays a role in
inflammatory response of endothelial cells. Am J Physiol Heart Circ
Physiol. 300:H1762–H1769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guan YJ, Yang X, Wei L and Chen Q:
miR-365: A mechanosensitive microRNA stimulates chondrocyte
differentiation through targeting histone deacetylase 4. FASEB J.
25:4457–4466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang D, Xu X, Deng F, Feng J, Zhang H,
Liu Y, Zhang Y, Pan L, Liu Y, Zhang D, et al: miRNA-940 reduction
contributes to human Tetralogy of Fallot development. J Cell Mol
Med. 18:1830–1839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rajendiran S, Parwani AV, Hare RJ,
Dasgupta S, Roby RK and Vishwanatha JK: MicroRNA-940 suppresses
prostate cancer migration and invasion by regulating MIEN1. Mol
Cancer. 13:2502014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song B, Zhang C, Li G, Jin G and Liu C:
miR-940 inhibited pancreatic ductal adenocarcinoma growth by
targeting MyD88. Cell Physiol Biochem. 35:1167–1177. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang HW, Liu GH, Liu YQ, Zhao HC, Yang Z,
Zhao CL, Zhang XF and Ye H: Over-expression of microRNA-940
promotes cell proliferation by targeting GSK3β and sFRP1 in human
pancreatic carcinoma. Biomed Pharmacother. 83:593–601. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang K, Tang Y, He L and Dai Y:
MicroRNA-340 inhibits prostate cancer cell proliferation and
metastasis by targeting the MDM2-p53 pathway. Oncol Rep.
35:887–895. 2016.PubMed/NCBI
|
|
21
|
Lin SY, Chang CH, Wu HC, Lin CC, Chang KP,
Yang CR, Huang CP, Hsu WH, Chang CT and Chen CJ: Proteome profiling
of urinary exosomes identifies alpha 1-antitrypsin and H2B1K as
diagnostic and prognostic biomarkers for urothelial carcinoma. Sci
Rep. 6:344462016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma J, Sun F, Li C, Zhang Y, Xiao W, Li Z,
Pan Q, Zeng H, Xiao G, Yao K, et al: Depletion of intermediate
filament protein Nestin, a target of microRNA-940, suppresses
tumorigenesis by inducing spontaneous DNA damage accumulation in
human nasopharyngeal carcinoma. Cell Death Dis. 5:e13772014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weber CEM, Luo C, Hotz-Wagenblatt A,
Gardyan A, Kordass T, Holland-Letz T, Osen W and Eichmüller SB:
miR-339-3p is a tumor suppressor in melanoma. Cancer Res.
76:3562–3571. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hasegawa K, Kato R, Torii Y, Ichikawa R,
Oe S and Udagawa Y: The relationship between ERCC1 expression and
clinical outcome in patients with FIGO stage I to stage II uterine
cervical adenocarcinoma. Int J Gynecol Cancer. 21:1479–1485. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chung HH, Kim MK, Kim JW, Park NH, Song
YS, Kang SB and Lee HP: XRCC1 R399Q polymorphism is associated with
response to platinum-based neoadjuvant chemotherapy in bulky
cervical cancer. Gynecol Oncol. 103:1031–1037. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ozen M, Creighton CJ, Ozdemir M and
Ittmann M: Widespread deregulation of microRNA expression in human
prostate cancer. Oncogene. 27:1788–1793. 2008. View Article : Google Scholar
|
|
27
|
Tamguney T and Stokoe D: New insights into
PTEN. J Cell Sci. 120:4071–4079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martin J and Dufour JF: Tumor suppressor
and hepatocellular carcinoma. World J Gastroenterol. 14:1720–1733.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao YJ, Ping XL, Zhang H, Chen FF, Lee PK,
Ahsan H, Chen CJ, Lee PH, Peacocke M, Santella RM, et al:
PTEN/MMAC1 mutations in hepatocellular carcinomas. Oncogene.
18:3181–3185. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Slipicevic A, Holm R, Nguyen MT, Bøhler
PJ, Davidson B and Flørenes VA: Expression of activated Akt and
PTEN in malignant melanomas: Relationship with clinical outcome. Am
J Clin Pathol. 124:528–536. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu Z, Stokoe D, Kane LP and Weiss A: The
inducible expression of the tumor suppressor gene PTEN promotes
apoptosis and decreases cell size by inhibiting the PI3K/Akt
pathway in Jurkat T cells. Cell Growth Differ. 13:285–296.
2002.PubMed/NCBI
|
|
32
|
Weng LP, Brown JL and Eng C: PTEN
coordinates G(1) arrest by down-regulating cyclin D1 via its
protein phosphatase activity and up-regulating p27 via its lipid
phosphatase activity in a breast cancer model. Hum Mol Genet.
10:599–604. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chu IM, Hengst L and Slingerland JM: The
Cdk inhibitor p27 in human cancer: Prognostic potential and
relevance to anticancer therapy. Nat Rev Cancer. 8:253–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
le Sage C, Nagel R and Agami R: Diverse
ways to control p27Kip1 function: miRNAs come into play. Cell
Cycle. 6:2742–2749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chu I, Sun J, Arnaout A, Kahn H, Hanna W,
Narod S, Sun P, Tan CK, Hengst L and Slingerland J: p27
phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell.
128:281–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pateras IS, Apostolopoulou K, Koutsami M,
Evangelou K, Tsantoulis P, Liloglou T, Nikolaidis G, Sigala F,
Kittas C, Field JK, et al: Downregulation of the KIP family members
p27(KIP1) and p57(KIP2) by SKP2 and the role of methylation in
p57(KIP2) inactivation in nonsmall cell lung cancer. Int J Cancer.
119:2546–2556. 2006. View Article : Google Scholar : PubMed/NCBI
|