Introduction
Sarcomas constitute cancers of mesenchymal origin
and hence arise in tissue of the circulatory and lymphatic system
as well as connective tissue such as cartilage and bone. Sarcomas
represent ~20% of pediatric solid malignancies and <1% of adult
solid malignancies (1). Of all
sarcomas, ~13% comprises malignant bone tumors of which ~4% entails
osteosarcoma (OS) (1). Although
the incidence of OS is low, it has one of the most dismal survival
rates of the pediatric cancers. Approximately 10–20% of patients
present with metastatic disease at diagnosis and the 5-year overall
survival is ~70% for non-metastatic patients and ~30% for
metastatic patients (2–4). The survival rate is affected by the
tumor site, increasing proportionally with the degree of necrosis
from neoadjuvant chemotherapy, and decreases with increased age
(5), tumor mass, expression of
surface markers [P-glycoprotein (6), CXCR4 (7), and possibly HER2 (8)], number of metastases [particularly to
the lungs (9)], and time to
metastasis (10,11).
A plethora of prognostic markers has been unraveled
for OS in the last couple of years, although the intrinsic value of
some of these markers is questionable inasmuch as the tumors
exhibit a propensity to metastasize (12) and metastasis is detrimental to
prognosis (9,12,13).
Moreover, a large portion of the currently available prognostic
markers is derived from the primary tumor. However, these markers
do not account for metastatic potential per se and may, at
least in part, discount the critical effect of metastasis on
prognosis. Circulatory markers have also been identified, including
miR-29, miR-133b, miR-148a, miR-195, miR-196a/b, and miR-206
(14–18), but these also do not contain
information on the cells that are de facto responsible for
metastasis, i.e., the circulating tumor cells (CTCs) (19) and circulating cancer stem cells
(CCSCs) (20–23).
Accordingly, a method that detects CTCs and CCSCs
would be most useful in assessing metastatic potential in OS
patients and rendering an accurate prognosis, as previously
addressed for other types of cancer (24). However, the currently employed
FDA-approved method (CellSearch system from Veridex) utilizes
markers (EpCAM and cytokeratins) that are specific for CTCs of
epithelial origin but not mesenchymal origin. Since sarcomas lack
these markers, their detection with the CellSearch system is
impossible. Although alternative methods for detecting
sarcoma-derived CTCs have recently been developed, none exist for
OS CTCs (19).
Consequently, the present study describes a method
for the enumeration and quantification of CTCs from peripheral
blood (PB) of OS patients based on abnormal chromosome numbers
(aneuploidy) in CTCs rather than the surface epitopes. We recently
validated this method for the detection of CTCs in breast cancer
patients (25), and have therefore
extended the method for the detection of OS CTCs in this in
vitro and prospective clinical study. Accordingly, the CTCs
were characterized by fluorescence in situ hybridization
(FISH) in conjunction with immunocytochemistry for cytokeratin and
CD45 to exclude epithelial and lymphocytic cells, respectively.
Following methodological proof-of-concept, the number of CTCs in
patients with primary OS were compared to the number of CTCs in
patients with recurrent or metastatic OS. This analysis showed that
the PB of the latter group contained more CTCs than the PB of
primary OS patients. Finally, correlation analysis was performed on
the number of CTCs in the patient's PB and progression-free
survival (PFS), which revealed that OS patients with ≥2 CTCs per
7.5 ml of PB had worse PFS than patients whose PB contained <2
CTCs. We therefore concluded that the FISH/immunocytochemistry
method was suitable to quantitate CTCs in liquid biopsies of OS
patients and that the results may have prognostic value.
Materials and methods
The present study was conducted in accordance with
the CONSORT 2010 checklist.
Cell culture
Human OS (HOS) cells and human hepatocellular
carcinoma (HepG2) were purchased from the Cell Bank of the Chinese
Academy of Sciences (Shanghai, China). The cell lines were
authenticated and characterized by DNA fingerprinting, isozyme
analysis, cell vitality analysis, and mycoplasm by the supplier.
The cells were expanded and frozen in P10 aliquots to standardize
the experiments.
HOS and HepG2 cells were cultured in RPMI-1640
medium (cat. no. 45000-396; VWR, Radnor, PA, USA) supplemented with
10% fetal bovine serum (FBS; Gibco/Life Technologies, Carlsbad, CA,
USA) in T25 culture flasks (Corning, Manassas, VA, USA) at standard
culture conditions (humidified atmosphere of 5% CO2 and
95% air, 37°C). HOS and HepG2 cells were subcultured at a ratio of
1:6 and 1:4, respectively, and used in experiments after reaching
~95% confluence.
Leukocyte isolation from peripheral
blood
PB (4 ml) was collected from a healthy volunteer
(HZ) into EDTA tubes and mixed 1:3 (v/v) with red blood cell lysis
buffer (ACK lysis buffer; Beyotime Institute of Biotechnology,
Shanghai, China). After 5-min incubation at room temperature (RT),
the samples were centrifuged at 150 × g for 10 min at RT. The
leukocyte-containing pellet was washed twice with
phosphate-buffered saline (PBS) at the previously mentioned
settings and resuspended in 4 ml of PBS. The samples were directly
assayed by flow cytometry as described in 'Flow cytometry'.
Flow cytometry
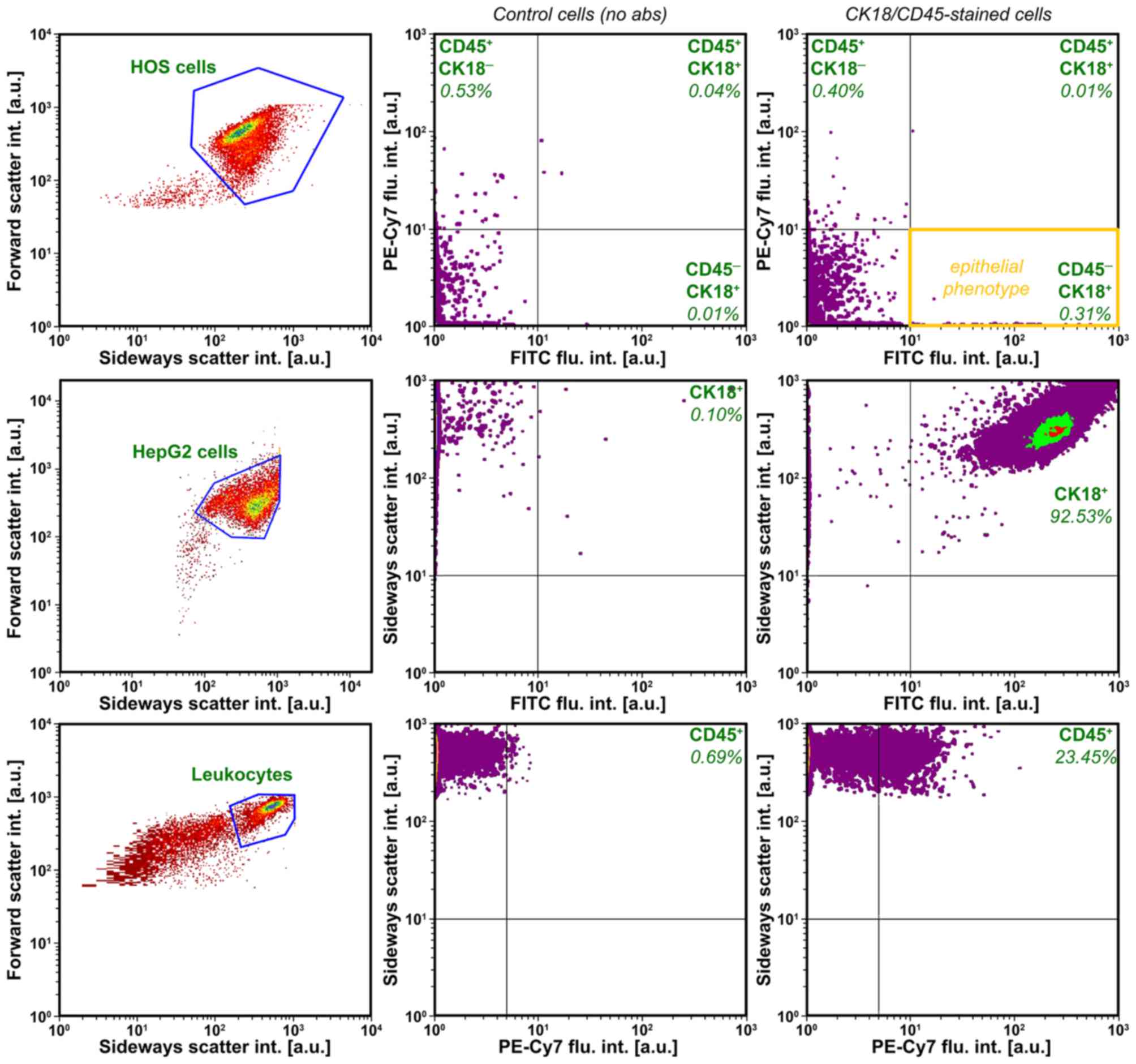
To show that HOS cells cannot be quantified with
putative epithelial markers (CK18 and CD45), flow cytometry was
performed on HOS cells following CK18 and CD45 antibody staining.
HepG2 cells, which have an epithelial phenotype
(CK18+/CD45−), and PB-derived leukocytes
(CK18−/CD45+) were used as positive controls
(N=3/cell line, N=3 for PB-derived leukocytes).
HOS and HepG2 cells were detached with trypsin/EDTA
(cat. no. R-001-100; Gibco/Life Technologies) and washed once with
medium at 300 × g for 5 min at RT. Cells were subsequently
resuspended in PBS. HOS and HepG2 cells were stained with
PE/Cy7-conjugated mouse anti-human CD45 monoclonal antibodies
(clone F10-89-4, cat. no. ab46729; Abcam, Cambridge, UK) and
FITC-conjugated mouse anti-human cytokeratin 18 (CK18) monoclonal
antibodies (clone DC-10, cat. no. ab72813; Abcam) for 30 min at RT
in the dark, using 10 µg of antibodies per 106
cells. HepG2 cells (106 cells/ml PBS) and leukocytes
(105 cells/ml PBS) were stained in a similar manner with
anti-CK18 and anti-CD45 antibodies, respectively.
Cells were assayed by flow cytometry (FACSAria III;
BD Biosciences, Franklin Lakes, NJ, USA). Ten thousand events were
collected in the gated region and data were analyzed in FCS Express
(De Novo Software, Glenndale, CA, USA).
In vitro CTC sample preparation
To establish in vitro proof-of-concept of the
CTC detection and quantification technique in a setting that
emulated the clinical scenario, PB was spiked with cultured HOS
cells. The cultured HOS cells, which provisionally functioned as
CTCs, were subsequently enumerated from the spiked sample.
HOS cells were cultured as described in 'Cell
culture', isolated as described in 'Flow cytometry', and diluted in
PBS to a density of 3×103 cells/ml. The cells were added
to blood within 5 min after harvesting.
PB was collected from healthy volunteers into 10-ml
EDTA Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ, USA).
Next, 5 ml of PB was spiked with 2.5 ml of the HOS cell suspension
to yield a final cell density of 103 cells/ml (N=3
replicates from 3 volunteers). The HOS cells were isolated as
described in 'Isolation of HOS from spiked venous blood' and
quantified as described in 'Enumeration of cultured HOS cells by
FISH'. Informed consent was obtained from healthy volunteers before
blood collection. The drawing of blood from healthy volunteers
(including the healthy volunteer in 'Leukocyte isolation from
peripheral blood' was approved by the Drug and Clinical Trial
Ethics Committee, First Affiliated Hospital of The Fourth Military
Medical University, and all experimental procedures were conducted
in accordance with the institute's guidelines and regulations.
Isolation of HOS from spiked venous
blood
The HOS-spiked blood samples (7.5 ml) were
centrifuged at 800 × g for 8 min at 25°C and the cell pellet was
resuspended in 4 ml of 1X hCTC buffer (Cytelligen, San Diego, CA,
USA). The cell suspension was centrifuged at 300 × g for 5 min at
25°C and the resulting cell pellet was resuspended in 3 ml of PBS.
The cell suspension was incubated with 150 µl of mouse
anti-human CD45 monoclonal antibody-coated magnetic beads (Human
CTC Immunofluorescence Staining kit, cat. no. IFH-LEA-001;
Cytelligen) for 30 min at 25°C to tag leukocytes and placed onto a
magnetic stand (MagneSphere Technology Magnetic Separation Stands,
cat. no. CD4002; Promega, Madison, WI, USA) positioned at a tilted
angle for 3 min at 25°C. The leukocyte-bound beads were
magnetically captured and the supernatant, i.e., the fraction
containing the CTC cells, was transferred to another centrifuge
tube and diluted with 1X hCTC buffer to a final volume of 45 ml.
This suspension was subsequently washed three times (650 × g for 5
min at 25°C) to retain the pellet containing the CTCs in ~100
µl of buffer. The cell pellet was resuspended as indicated
below and either subjected to FISH + immunocytochemistry or counted
under fluorescence microscope to determine the retrieval
efficiency.
The CTC-enriched cell pellet was resuspended in 200
µl of fixative solution (Human Circulating Rare Cell
Subtraction Enrichment kit, cat. no. SEH-001; Cytelligen) and CTCs
were characterized by FISH in conjunction with immunocytochemistry
as it is described in 'Enumeration of cultured HOS cells by
FISH'.
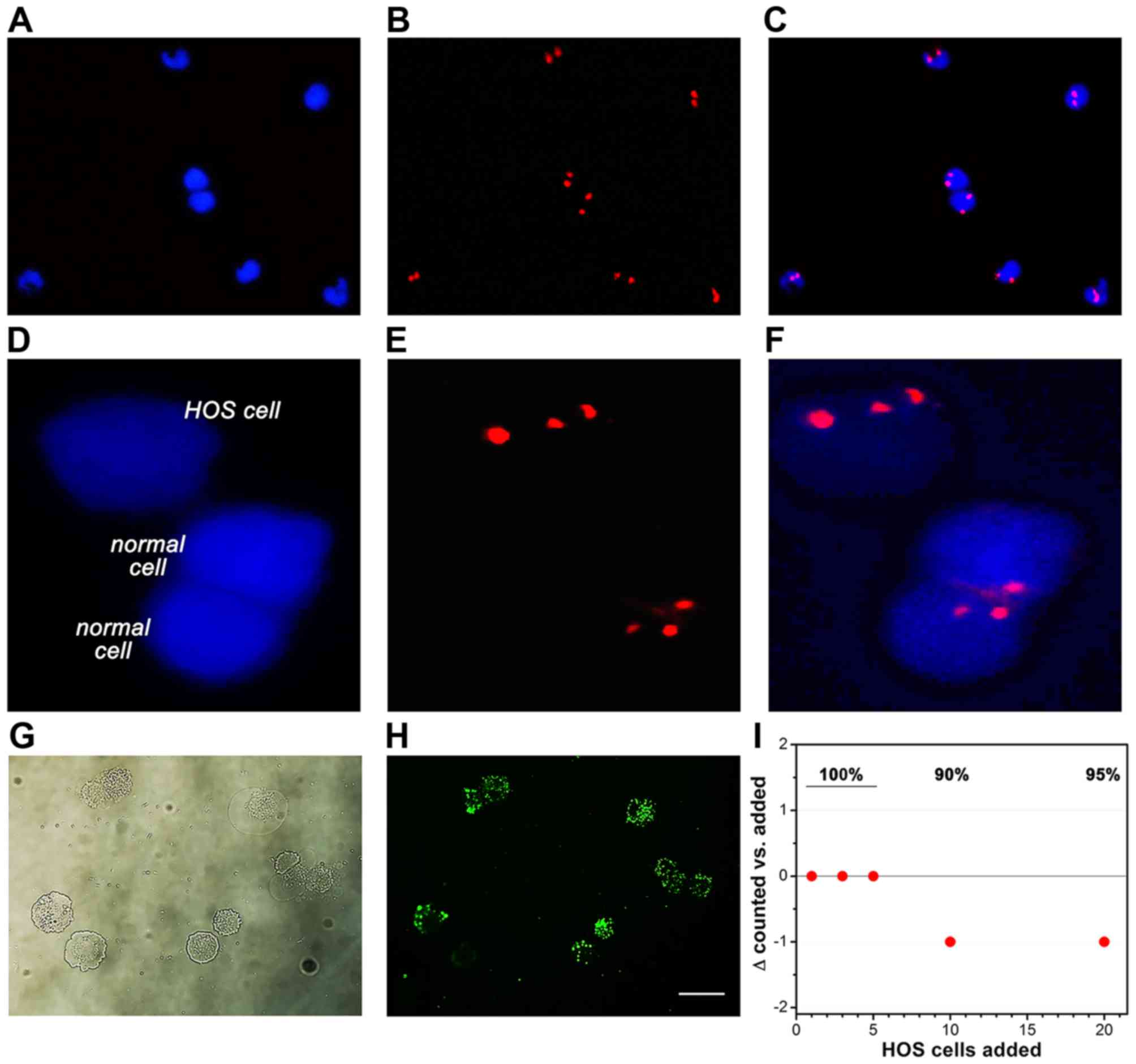
For the retrieval efficiency study, HOS cells were
cultured as described in 'Cell culture', isolated as described in
'Flow cytometry', and counted. The cells were diluted to a
concentration of 100, 300, 500, 1,000, and 2,000 cells/ml medium
and fluorescently labeled (CYTO-ID Green long-term cell tracer kit;
Enzo Life Sciences, Farmingdale, NY, USA) according to the
manufacturer's instructions. Blood samples (7.5 ml) were
subsequently spiked with the fluorescently labeled HOS cells to a
final count of 1, 3, 5, 10 and 20 cells/sample. Fluorescently
labeled HOS cells were isolated as described above but not fixed.
Finally, 200 µl of the eluted HOS-containing sample was
transferred to a microscope slide and coverslipped. Cells in the
entire sample were counted by fluorescence microscopy (Nikon
Eclipse 80i; Nikon Tokyo, Japan) using a FITC filter set. A
modified Bland-Altman plot was constructed to describe the
retrieval efficiency.
Enumeration of cultured HOS cells by
FISH
For FISH, cells were permeabilized by the addition
of 100 µl of 0.1% Triton X-100 (Sigma Aldrich, St. Louis,
MO, USA). Subsequently, the cells were gently vortexed and a
200-µl aliquot was transferred onto a microscope slide. The
slides were air-dried overnight at 37°C, thereby affixing the cells
onto the slide. Next, the cells were prehybridized (0.1 M HCl,
0.05% Triton X-100), washed in saline sodium citrate (2X SSC; 0.3 M
NaCl and 0.034 M sodium citrate) and subsequently with PBS, and
fixed with formaldehyde (1% in PBS). After washing (PBS, 2X SSC)
and dehydration in graded steps of ethanol (70, 85, and 100%), DNA
was denatured in formamide (70% in 2X SSC, 70°C, 5 min) and the
sample was again dehydrated via graded steps of ethanol.
Hybridization solution (Hybrisol VI; Oncor, Dallas, TX, USA)
containing fluorescence-labeled alpha satellite probes for the
centromeres of chromosome (CEP 8) (2 µg/ml, Vysis CEP;
Abbott Molecular, Abbott Park, IL, USA) was applied to each slide,
which was coverslipped, sealed, and incubated overnight at 37°C. A
detailed CEP 8 FISH protocol can be found at the Abbott Molecular
technical support website (http://www.abbottmolecular.com/contactus/fishtechsupport/keyproductinformation/vysisproducts/cep.html).
After the FISH procedure, cells were immunostained
with Alexa Fluor 488-conjugated mouse anti-human CK18 monoclonal
antibodies (cat. no. HAB-001R1; Cytelligen) and Alexa Fluor
594-conjugated mouse anti-human CD45 monoclonal antibodies (cat.
no. HAB-001R2; Cytelligen) for 1 h. Samples were washed three times
with PBS. Finally, nuclei were counterstained with
4-6-diamidino-2-phenylindole (DAPI)-containing mounting medium
(cat. no. FSH-001R7; Cytelligen) and subsequently analyzed by
fluorescence microscopy equipped with the respective filter sets
for the Alexa Fluor dyes and DAPI (Nikon Eclipse 80i). Light
microscopy was used to confirm the CTC morphology of aneuploidic
cells.
HOS cells were characterized based on aneuploidy,
DAPI-positive staining, negative staining for CD45 and CK18, and
cell size and morphology (intact cells were round to oval). All
cells on the microscope slide were analyzed by at least two
pathologists. Inasmuch as non-cancerous cells in PB are amphiploid
and mostly diploid, aneuploidic (triploidic, pentaploidic) cells
were classified as HOS cells in accordance with previous studies
(26–29).
Patients and study design
A prospective, cross-sectional, single center trial
was conducted at the Xijing Hospital to evaluate the diagnostic and
prognostic utility of CTCs in 23 patients from Shaanxi, Gansu, and
Ningxia province who had been diagnosed with recurrent or
metastatic OS between January 2010 and August 2014. The study was
approved by the Drug and Clinical Trial Ethics Committee, First
Affiliated Hospital of The Fourth Military Medical University and
registered in a clinical trial management public platform (Chinese
Clinical Trial Register, www.chictr.org.cn) under register ID
ChiCTR-OOC-15005925 (registration date: 28 January 2015). Written
informed consent was obtained from every patient before enrollment
in the trial. The clinical procedures were conducted in accordance
with the guidelines approved by the Drug and Clinical Trial Ethics
Committee.
Diagnosis and staging was predicated on histological
assessment of tissue biopsies by a pathologist. The disease status
was assessed according to World Health Organization criteria
(30) and the staging was
performed based on the Enneking classification system (31). Only patients with conventional and
small round-cell OS were included. The exclusion criteria were the
following: i) patients did not understand the research purpose or
did not give consent; ii) no or limited legal capacity to provide
consent; iii) pregnancy or breastfeeding; iv) concurrent non-OS
malignant tumors; v) serious complications, severe uncontrollable
medical condition, or acute infection; and vi) medical history that
could interfere with test results or increase the risk to patients.
Following OS diagnosis, patients were screened for lung metastases
using X-ray and chest CT.
Patient treatment and clinical
monitoring
Limb salvage surgery was performed in all enrolled
patients as previously described (32). All patients standardly received 4
cycles of first-line neoadjuvant chemotherapy with ifosfamide (12
g/m2) and lobaplatin-adriamycin (45 and 60
mg/m2, respectively), starting immediately after
diagnosis. After surgery patients received an additional 6–8 cycles
of chemotherapy (same regimen as prior to surgery). The patients
were followed up every 3 months for the first 2 years, and every 6
months thereafter for a maximum period of 5 years.
X-ray and chest CT scans were performed every 2
months during chemotherapy and every 3 months after the treatment
to screen for lung and other metastases. Upon diagnosis of lung
metastasis, the patients were scheduled for chemotherapy and local
radiotherapy (gamma knife). In case of recurrence or local or
single bone metastasis, the patients received surgery and
chemotherapy. Patients with multifocal recurrence or metastasis
were scheduled for either chemotherapy or treatment was withheld.
First line chemotherapy was administered in all instances as
described above.
Blood sampling and CTC enumeration
Blood samples were collected 1 h before the patients
commenced chemotherapy. Venous blood (7.5 ml) was collected into
CellSave preservative tubes (Veridex LLC, Raritan, NJ, USA). The
samples were stored at room temperature and processed according to
the manufacturer's instructions within 72 h of collection. A second
venous blood sample (2 ml) was collected into an EDTA Vacutainer
tube (BD Biosciences) to determine plasma alkaline phosphatase by
routine clinical chemistry. CTCs were enumerated as described in
'Isolation of HOS from spiked venous blood' and 'Enumeration of
cultured HOS cells by FISH'.
Data and statistical analysis
Data analysis was performed in GraphPad Prism
(GraphPad Software, San Diego, CA, USA). The normal distribution of
data sets was confirmed with a D'Agostino-Pearson omnibus test. An
unpaired homoscedastic Student's t-test was used to assess the
statistical significance between ordinal variables. A P≤0.05 was
considered statistically significant. Data are reported as mean ±
standard deviation (SD).
To determine a CTC cut-off level that best predicts
rapid progression of disease compared to slow progression, cut-off
values of 1–5 CTCs per 7.5 ml of PB were correlated with PFS for
the 23 patients and analyzed using the Cox proportional hazards
ratio. The 95% confidence interval (CI) was also calculated for
this statistic.
The time interval between diagnosis and recurrence
or metastasis was calculated on the basis of the patient's medical
history and was used as a measure of PFS. PFS was based on X-ray
and chest CT scans and encompassed the elapsed time between the
date on which OS was diagnosed and either the time of death or last
follow-up. Survival curves were plotted as Kaplan-Meier estimators
and compared using log-rank testing. The analyses of PFS were
performed according to the intention-to-treat principle (33).
Correlation analysis was performed in SPSS (IBM,
Armonk, NY, USA). Non-parametric Spearman rho analysis coupled with
a two-tailed significance test was performed between the number of
CTCs, gender, age, ethnic origin, alkaline phosphatase, recurrence
or metastasis, follow-up time and survival. Categorical string
variables were converted to numerical variables prior to the
analysis (gender, male=0, female=1; ethnicity, Han=0, Hui=1;
survival, yes=1, no=0; recurrence or metastasis, yes=1, no=0).
Results
Detection of HOS cells by FISH: in vitro
proof-of-concept
The first step was to demonstrate that HOS cells do
not express epithelial and lymphocytic markers standardly used by
the CellSearch system. Cultured HOS cells were assayed by flow
cytometry following incubation with anti-CK18 and anti-CD45
antibodies, whereby HepG2 cells and PB leukocytes were used as
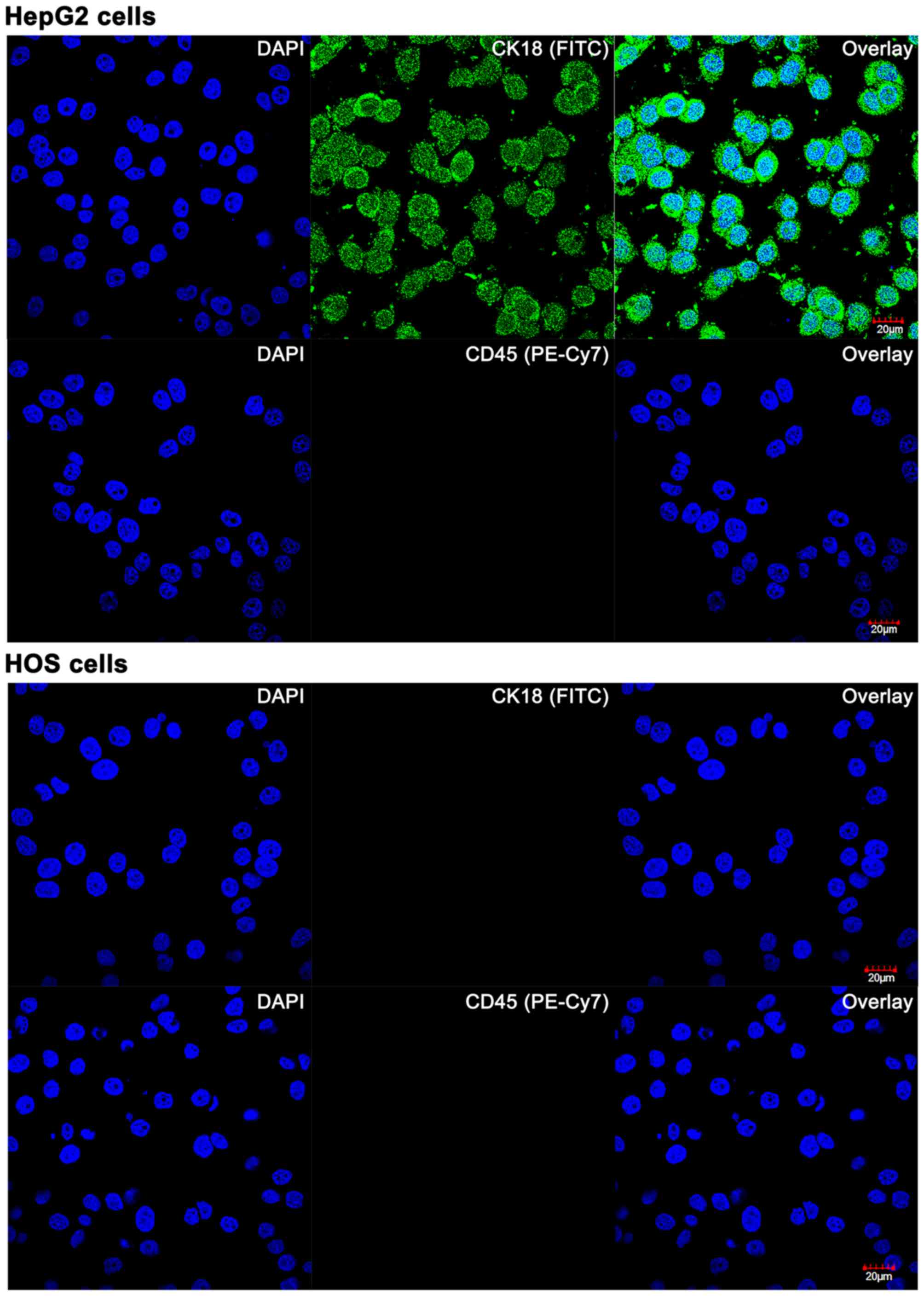
positive controls. As shown in Fig.
1, HOS cells were negative for both markers, whereas HepG2
cells (positive control for CK18, see also Fig. 2) and PB-derived leukocytes
(positive control for CD45) exhibited a CK18+ and
CD45+ phenotype, respectively. These data indicate that
the conventional CTC enumeration methods (usually based on
CK18+ cell isolation following CD45+ cell
depletion) are not suitable to isolate, detect and quantify CTCs of
mesenchymal origin and that alternative techniques must be
used.
The reliance on CK18 expression can be circumvented
by staining chromatin (CEP 8) by FISH after erythrocyte and
CD45+ cell depletion. To establish proof-of-concept of
this OS CTC detection and quantification method, PB of healthy
volunteers was spiked with cultured HOS cells and subjected to FISH
and microscopic analysis. In the non-spiked PB of healthy patients,
circulating non-leukocytic, non-erythrocytic cells were efficiently
labeled by CEP 8 FISH, as evidenced by the diploid chromatin
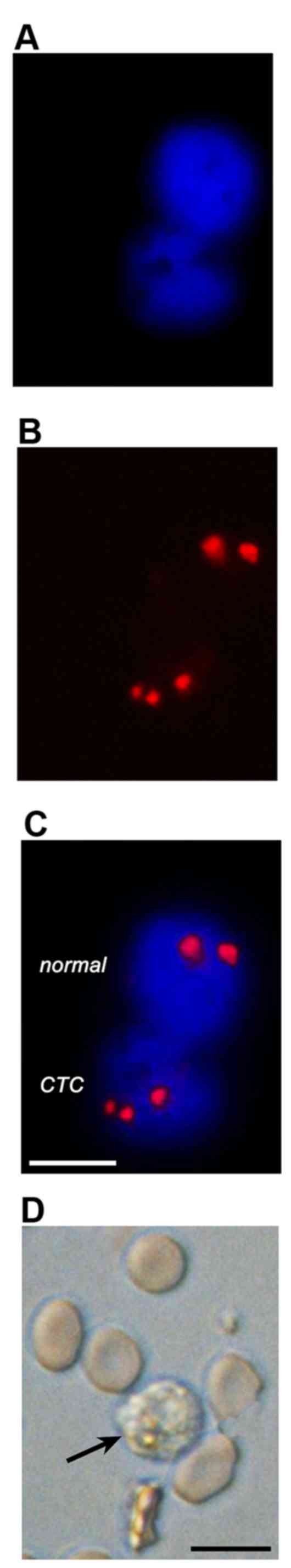
labeling (Fig. 3A–C) and stained
positively for CK18 (data not shown). The
CK18−/CD45− aneuploidic HOS cells (Fig. 1) in the spiked PB samples were
easily retrieved on the basis of an aberrant number of fluorescent
chromosomes (Fig. 3D–F) that
coincided with negative CK18 and CD45 staining (Fig. 2).
Patient demographics, medical history,
and disease characteristics
Next, the CTC enumeration method was validated in OS
patients. A total of 23 eligible patients were enrolled between
January 2010 and August 2014, of whom the demographics, medical
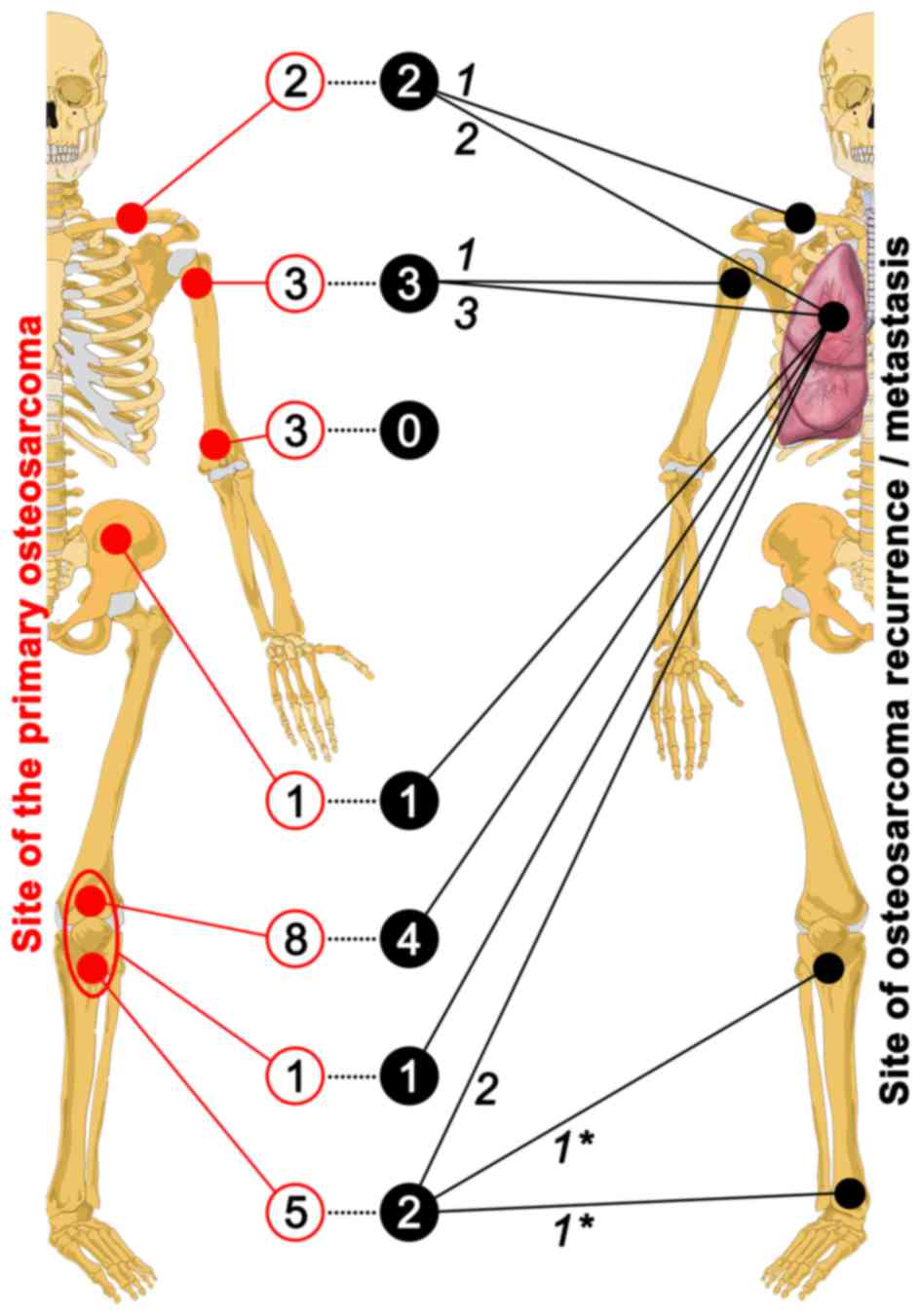
history, and disease characteristics are summarized in Table I. Information regarding the OS
primary site and the site of recurrence or metastasis is provided
in Fig. 4. Of the 23 patients, 16
patients (69.6%) comprised ethnic Han (population of ~1.25 billion
in China) and 7 patients (30.4%) comprised ethnic Hui (population
of ~10.6 million in China). These statistics suggest that ethnic
Hui Chinese have a more considerable predilection for developing
conventional and small round-cell OS than ethnic Han Chinese,
particularly since the ratio of ethnic Hui:Han Chinese in the three
provinces from which the patients were recruited is 0.5:99.4. The
follow-up period ranged from 22 to 219 weeks (median of 77 weeks).
The mean time between the first chest X-ray and CT scan and the
follow-up X-ray and CT scan was 13±5 weeks (range of 8–26 weeks,
median of 10 weeks). Correlation analysis revealed a positive
relationship between gender and survival (ϱ=0.505, P=0.014; female
patients exhibited better survival), age and recurrence/metastasis
(ϱ= 0.492, P= 0.017; older patients were more prone to
recurrence/metastasis), and the number of CTCs and
recurrence/metastasis (ϱ= 0.605, P=0.002). A negative correlation
was found between ethnicity and alkaline phosphatase (ϱ=−0.463,
P=0.026; ethnic Han OS patients exhibited higher levels of alkaline
phosphatase) (Table II).
 | Table IPatient demographics, medical
history, and disease characteristics (N=23 patients). |
Table I
Patient demographics, medical
history, and disease characteristics (N=23 patients).
| Demographics | Value | (%) (range) |
|---|
| Gender (N) | | |
| Male | 12 | 52.2 |
| Female | 11 | 47.8 |
| Age (years) | | |
| Mean ± SD | 16.1±4.4 | (9–26) |
| Median | 16.0 | |
| Male | | |
| Mean ± SD | 17.7±5.0a | (10–26) |
| Median | 17 | |
| Female | | |
| Mean ± SD | 13.5±3.1a | (9–18) |
| Median | 14 | |
| Tumor site
(N)b | | |
| Distal femur | 8 | (34.8) |
| Proximal
tibia | 6 | (26.1) |
| Distal
humerus | 3 | (13.0) |
| Proximal
humerus | 3 | (13.0) |
| Pelvis | 1 | (4.3) |
| Clavicle | 2 | (8.7) |
| Enneking grade
(N) | | |
| IIB | 21 | (91.3) |
| III | 2 | (8.7) |
| Type of OS (N) | | |
| Conventional
OS | 21 | (91.3) |
| Small round-cell
OS | 2 | (8.7) |
| Pathological
fracture (N) | | |
| Yes | 1 | (4.3) |
| No | 22 | (95.7) |
| Alkaline
phosphatase (N) | | |
| Elevated | 17 | (73.9) |
| Normal (male
45–125 IU/l; female 35–100 IU/l) | 6 | (26.1) |
| Male, mean ± SD
(IU/l) | 169±54 | (87–314) |
| Female, mean ± SD
(IU/l) | 148±87 | (66–340) |
| Treatment of
primary tumor (N) | | |
| Neoadjuvant
chemotherapy | | |
| Yes | 23 | (100.0) |
| No | 0 | (0.0) |
| Surgery | | |
| Amputation | 0 | (0.0) |
| Limb salvage | 23 | (100.0) |
| Postoperative
chemotherapy | | |
| Yes | 23 | (100.0) |
| No | 0 | (0.0) |
| Recurrence or
metastasis (N) | | |
| Yes | 13 | (56.5) |
| No | 10 | (43.5) |
| Site of recurrence
or metastasis (N) | | |
| Primary tumor
site |
3c | (23.1) |
| Lung | 13 | (100.0) |
| Other |
1d | (7.7) |
| Treatment of
recurrent or metastatic tumor (N) | 12 | (92.3) |
| Chemotherapy | 6 | (46.2) |
| Surgery and
chemotherapy | 3 | (23.1) |
| Gamma knife and
chemotherapy | 2 | (15.4) |
| Surgery and gamma
knife and chemotherapy | 1 | (7.7) |
| Mortality within
the follow-up period (N) | | |
| Alive | 18 | (78.3) |
| Deceased | 5 | (21.7) |
| Progression-free
survival (weeks, N=23) | | |
| Mean | 100.2 | |
| SD | 69.5 | |
| Median | 77.0 | |
 | Table IICorrelation analysis between
variables. |
Table II
Correlation analysis between
variables.
| | | Gender | Age | Ethnicity | CTCs | ALP | Follow-up |
Recurrence/metastasis | Survival |
|---|
| Spearman's rho | Gender | Correlation
coefficient | 1.000 | −0.429a | −0.066 | 0.013 | −0.230 | −0.033 | −0.389 | 0.505a |
| | Sig.
(2-tailed) | | 0.041 | 0.765 | 0.952 | 0.292 | 0.882 | 0.066 | 0.014 |
| | N | 23 | 23 | 23 | 23 | 23 | 23 | 23 | 23 |
| Age | Correlation
coefficient | | 1.000 | 0.208 | 0.357 | −0.093 | 0.214 | 0.492a | −0.232 |
| | Sig.
(2-tailed) | | | 0.342 | 0.095 | 0.672 | 0.327 | 0.017 | 0.288 |
| | N | | 23 | 23 | 23 | 23 | 23 | 23 | 23 |
| Ethnicity | Correlation
coefficient | | | 1.000 | 0.094 | −0.463a | 0.014 | 0.199 | 0.120 |
| | Sig.
(2-tailed) | | | | 0.669 | 0.026 | 0.949 | 0.363 | 0.587 |
| | N | | | 23 | 23 | 23 | 23 | 23 | 23 |
| CTCs | Correlation
coefficient | | | | 1.000 | −0.058 | 0.019 | 0.605b | 0.121 |
| | Sig.
(2-tailed) | | | | | 0.791 | 0.931 | 0.002 | 0.582 |
| | N | | | | 23 | 23 | 23 | 23 | 23 |
| ALP | Correlation
coefficient | | | | | 1.000 | −0.020 | 0.106 | −0.238 |
| | Sig.
(2-tailed) | | | | | | 0.927 | 0.631 | 0.273 |
| | N | | | | | 23 | 23 | 23 | 23 |
| Follow-up | Correlation
coefficient | | | | | | 1.000 | 0.126 | 0.088 |
| | Sig.
(2-tailed) | | | | | | | 0.567 | 0.691 |
| | N | | | | | | 23 | 23 | 23 |
|
Recurrence/metastasis | Ccorrelation
coefficient | | | | | | | 1.000 | −0.250 |
| | Sig.
(2-tailed) | | | | | | | | 0.251 |
| | N | | | | | | | 23 | 23 |
| Survival | Correlation
coefficient | | | | | | | | 1.000 |
| | Sig.
(2-tailed) | | | | | | | | |
| | N | | | | | | | | 23 |
Detection of CTCs in OS patients
To provide proof-of-concept of the enumeration
technique for CTCs in OS patients, FISH and microscopic analysis
were performed on OS patient-derived PB in a similar manner as
described for the HOS cell-spiked PB as described in 'Detection of
HOS cells by FISH: in vitro proof-of-concept'. The CTCs were
identified by means of aneuploidy and a DAPI-stained nucleus, which
was further confirmed by their size and round-to-oval morphology
using brightfield microscopy. As shown in Fig. 5, CTCs could be easily distinguished
from non-cancerous cells on the basis of the primary parameters
(fluorescence microscopy) alone.
Accordingly, CTCs were quantified in liquid biopsies
of 23 eligible OS patients, the distribution of which is provided
in Table III. The PB of 12 of
the 13 (92.3%) patients with recurrence or metastasis contained
CTCs, with a mean ± SD of 2.9±1.6 CTCs, a median of 3 CTCs, and a
range of 0–5 CTCs per 7.5 ml PB. In contrast, 3 of the 10 (30.0%)
patients with primary OS had no CTCs in their PB, with a mean ± SD
of 1.6±1.3 CTCs, a median of 1 CTC, and a range of 0–4 CTCs per 7.5
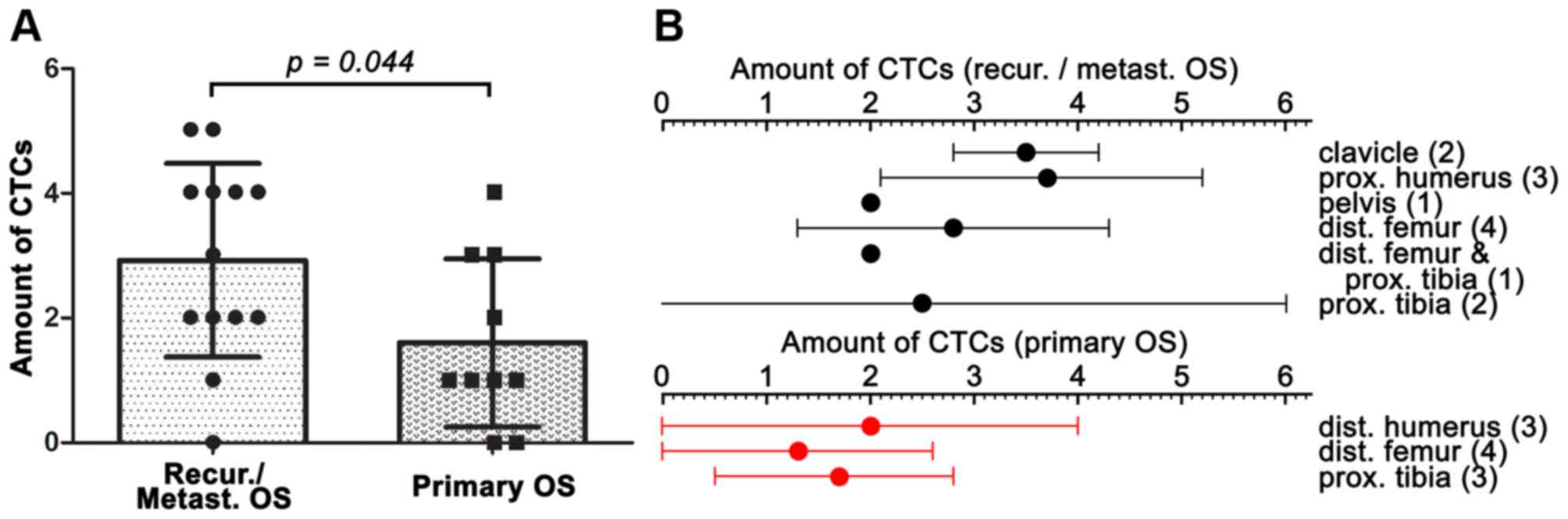
ml PB (P=0.044 vs. The recurrence/metastasis group) (Fig. 6A). The amount of CTCs was further
specified per primary OS site for the patients with no
recurrence/metastasis and the patients with recurrence/metastasis
in Fig. 6B.
 | Table IIIDistribution of the number of CTCs
detected in OS patients. |
Table III
Distribution of the number of CTCs
detected in OS patients.
| Nο. of CTCs | Nο. of cases | % |
|---|
| 0 | 3 |
13.0 |
| 1 | 5 |
21.7 |
| 2 | 5 |
21.7 |
| 3 | 3 |
13.0 |
| 4 | 5 |
21.7 |
| ≥5 | 2 (5,5)a |
8.7 |
| Total | 23 | 100.0 |
Prognostic value of CTC-based liquid
biopsies for progression-free survival
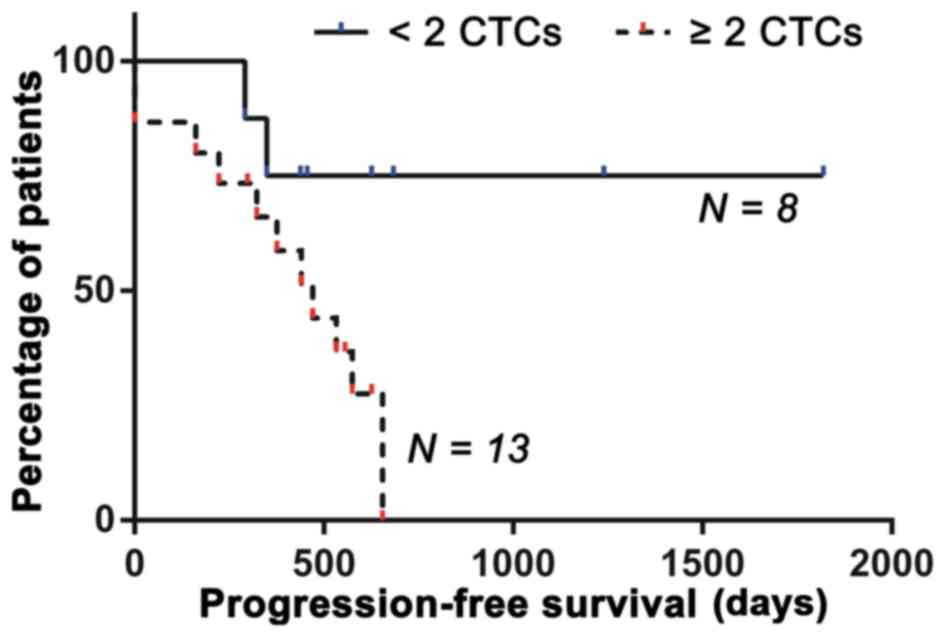
The analysis revealed that, at a cut-off of 2 CTCs
per 7.5 ml of PB, the Cox proportional hazards ratio reached a
plateau relative to the higher cut-off values, indicating the level
at which the cut-off should be set. At this cut-off, the hazard
ratio (log-ranked) was 4.091 and the 95% CI of the hazards ratio
was 1.075–9.684. Patients with ≥2 CTCs per 7.5 ml of PB had a
median PFS of 440 days compared to 547 days in patients with 0 or 1
CTCs per 7.5 ml PB (P=0.0416) (Fig.
7). Consequently, a cut-off of 2 CTCs per 7.5 ml of PB should
be employed to distinguish patients with unfavorable prognosis from
patients with a more favorable prognosis.
Discussion
A method was developed to enumerate CTCs with a
non-epithelial phenotype from liquid biopsies and to quantify the
OS CTCs on the basis of aneuploidy by fluorescence microscopy. The
FISH technique proved a viable alternative for the CellSearch
method, which is based on the depletion of CD45+ cells
and subsequent quantification of CK18+ cells, for the
quantification of CTCs of epithelial origin. The presented
technique is straightforward and can be clinically standardized,
given that an outcome can be rendered within a day, all reagents
are commercially available, standard clinical laboratories are
equipped with the necessary equipment, and the quantification does
not require special training or expertise. In addition to
validating the FISH method in a population of OS patients, it was
demonstrated that: i) the PB of recurrent/metastatic OS patients
contained more CTCs than the PB of primary OS patients; and ii) the
number of CTCs in liquid biopsies has prognostic value, whereby ≥2
CTCs per 7.5 ml of PB was associated with significantly shorter PFS
than when the liquid biopsy contained 1 or no CTCs.
Numerous tumor biopsy-based markers have been found
for OS and include miR-9, miR-132, miR-145, miR-183, miR-223, and
miR-128 in combination with transcript levels of phosphatase and
tensin homolog (PTEN) (34–39),
the DNA mismatch repair proteins MutS protein homolog 2 (MSH2) and
MSH6 (40), hypermethylated DNA of
ARF tumor suppressor (p14ARF) and estrogen receptor α
(41), the transcription factor
SOX9 (42), RAC-β
serine/threonine-protein kinase (AKT2) (43), periostin (44), neuropilin-1 (45), glucose transporter protein 1
(GLUT-1) (46), and polymeric
immunoglobulin receptor (pIgR) (47), amongst others. However, these
markers are derived from the primary OS site and therefore discount
metastases, which are important determinants for PFS (9,12,13).
The same applies to the circulatory markers (mainly miRs) alluded
to in the introduction. Although these markers confirm the
diagnosis rendered with standard imaging- and histological
techniques (that are already very accurate for the diagnosis of OS)
and have prognostic value, they do not contain information on the
cells that are mainly responsible for PFS, namely the CTCs and
CCSCs (19–23). CTC enumeration from liquid biopsies
does contain this information, has prognostic value, and merely
requires minimally invasive sampling and a simple enumeration and
quantification protocol.
In addition to establishing and validating a novel
CTC quantification method, the study demonstrated that a strong
relationship exists between the amount of CTCs and OS
recurrence/metastasis (ϱ= 0.605, P=0.002) and that CTCs are
predictive for PFS in OS patients. This is in agreement with
published literature on the predictive capacity of CTCs regarding
recurrence/metastasis and PFS in other types of cancer, including
(metastatic) breast cancer. Studies have demonstrated that CTCs are
a strong independent predictor of survival among women with
recurrent metastatic breast cancer (48) and that the presence and elevated
levels of CTCs are associated with poorer prognosis (49). Moreover, CTCs have been employed to
monitor disease status following an intervention (50), which is now theoretically possible
for OS as well with the FISH/microscopy method.
Most importantly, CTCs may be used to rule out false
negative results obtained with X-ray and chest CT, which are
limited in spatial resolution and therefore unable to detect
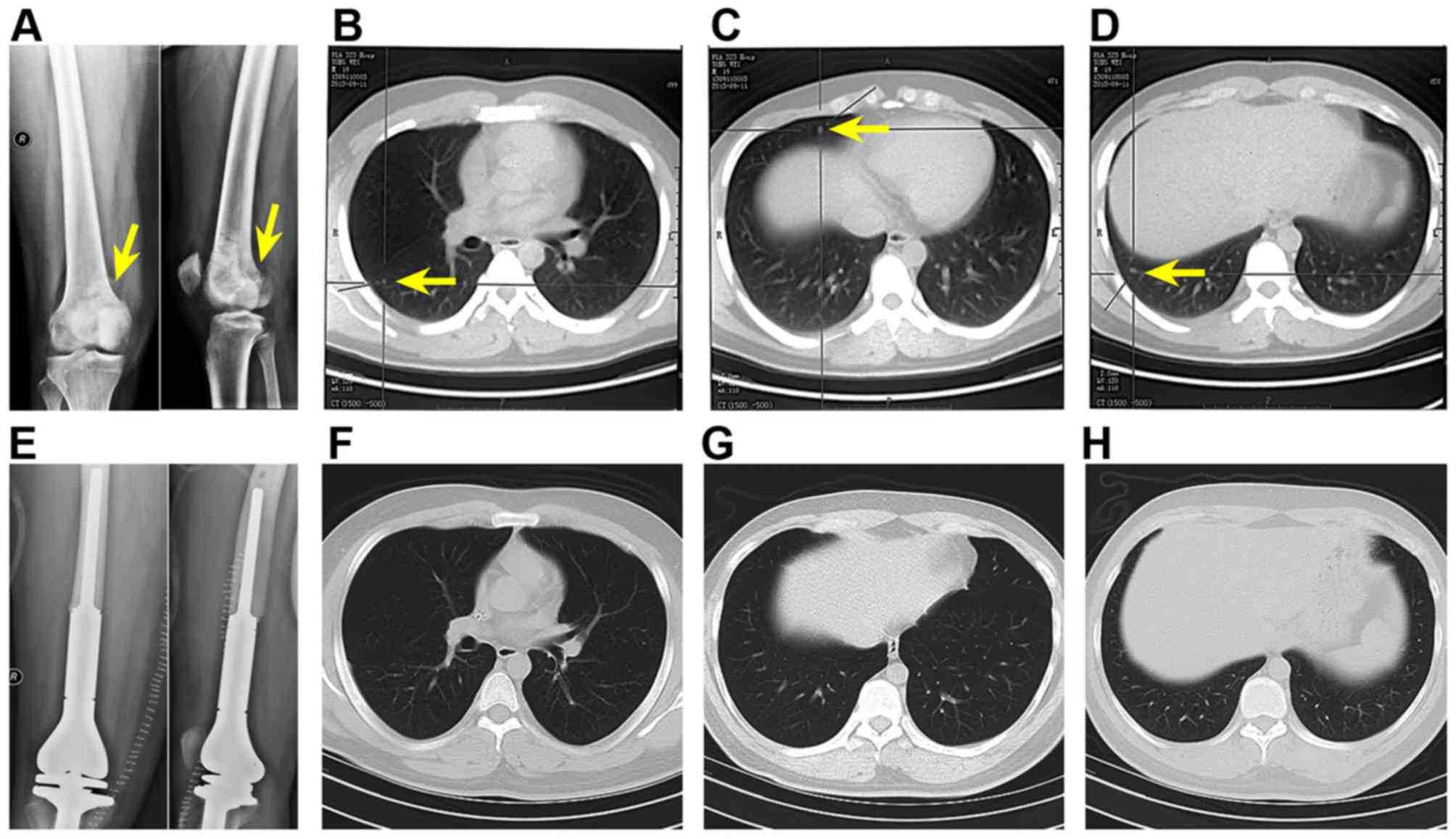
small-volume metastases. To exemplify this, two patients in the
study cohort with primary metastatic OS to the lungs (Fig. 8A–D) exhibited negative chest CT
following surgery and chemotherapy (Fig. 8E–H), but their PB contained CTCs.
Despite the favorable diagnosis, the patients were by no means
disease-free. Evidently, the gold standard methods are incapable of
providing enough information of the state of the malignancy or
accurately predict the clinical outcome, which is in conformity
with the fact that primary metastatic OS patients have poor
prognosis despite the good response to chemotherapy. To improve the
survival of OS patients, it is important to employ feasible
diagnostic methods with which recurrence and/or metastatic
potential can be determined and the antitumor treatment efficacy
can be accurately monitored.
The CTC enumeration and quantification method from
liquid biopsies fits well in a personalized medicine approach,
where information on each patient's unique clinical condition is
exploited to implement early disease intervention based on more
informed medical decisions. The presence of CTCs in PB of OS
patients is associated with poor clinical outcome in both primary
and metastatic malignancies. The CTC profile may therefore steer
the selection of effective treatment strategies in early and
advanced OS. Moreover, CTC quantification in OS patients may be
utilized as a (personalized) therapy monitoring tool (51) inasmuch as changes in CTC count
generally reflect therapeutic response after already the first
cycle of chemotherapy.
Readers should be aware that this prospective study
is associated with some limitations. First, the method should be
validated in a multi-center prospective clinical trial and include
larger patient cohorts. Specifically, the ≥2 CTC cut-off should be
subjected to more robust analysis in both primary OS and
metastatic/recurrent OS patient populations. Furthermore, the
sensitivity and specificity of the technique should be addressed.
Finally, the relationship between the number of CTCs and tumor
stage should be determined and the follow-up times should be
increased and entail a higher monitoring frequency to unequivocally
corroborate that CTC detection can eliminate false negatives
obtained with gold standard diagnostic techniques and to establish
its true prognostic power. After these steps have been undertaken,
the CTC method can be expanded towards other applications, such as
therapeutic response monitoring, elucidation of chemoresistance
mechanisms, and CTC genomics and proteomics studies to gain insight
into OS CTC biology and biochemistry.
Acknowledgments
We are grateful for the technological support
provided by the Shanghai Biotecan Clinical Laboratory. The present
study was supported by the National Natural Science Fund of China
(nos. 31170914 and 81272899) and the Discipline Booster Plan of Xi
Jing Hospital (no. XJZT12Z07).
References
|
1
|
Burningham Z, Hashibe M, Spector L and
Schiffman JD: The epidemiology of sarcoma. Clin Sarcoma Res.
2:142012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrari S and Palmerini E: Adjuvant and
neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr
Opin Oncol. 19:341–346. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jaffe N: Adjuvant chemotherapy in
osteosarcoma: An odyssey of rejection and vindication. Cancer Treat
Res. 152:219–237. 2009. View Article : Google Scholar
|
|
4
|
Chou AJ, Geller DS and Gorlick R: Therapy
for osteosarcoma: Where do we go from here? Paediatr Drugs.
10:315–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haddox Cl, Han G, Anijar L, Binitie O,
Letson GD, Bui MM and Reed DR: Osteosarcoma in pediatric patients
and young adults: a single institution retrospective review of
presentation, therapy, and outcome. Sarcoma. 2014:4025092014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Serra M, Scotlandi K, Manara MC, Maurici
D, Benini S, Sarti M, Campanacci M and Baldini N: Analysis of
P-glycoprotein expression in osteosarcoma. Eur J Cancer.
31A:1998–2002. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perissinotto E, Cavalloni G, Leone F,
Fonsato V, Mitola S, Grignani G, Surrenti N, Sangiolo D, Bussolino
F, Piacibello W, et al: Involvement of chemokine receptor 4/stromal
cell-derived factor 1 system during osteosarcoma tumor progression.
Clin Cancer Res. 11:490–497. 2005.PubMed/NCBI
|
|
8
|
Gill J, Geller D and Gorlick R: HER-2
involvement in osteosarcoma. Adv Exp Med Biol. 804:161–177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeffree GM, Price CH and Sissons HA: The
metastatic patterns of osteosarcoma. Br J Cancer. 32:87–107. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moore DD and Luu HH: Osteosarcoma. Cancer
Treat Res. 162:65–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xing D, Qasem SA, Owusu K, Zhang K, Siegal
GP and Wei S: Changing prognostic factors in osteosarcoma: Analysis
of 381 cases from two institutions. Hum Pathol. 45:1688–1696. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dunn D and Dehner LP: Metastatic
osteosarcoma to lung: A clinicopathologic study of surgical
biopsies and resections. Cancer. 40:3054–3064. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salah S, Ahmad R, Sultan I, Yaser S and
Shehadeh A: Osteosarcoma with metastasis at initial diagnosis:
Current outcomes and prognostic factors in the context of a
comprehensive cancer center. Mol Clin Oncol. 2:811–816.
2014.PubMed/NCBI
|
|
14
|
Hong Q, Fang J, Pang Y and Zheng J:
Prognostic value of the microRNA-29 family in patients with primary
osteosarcomas. Med Oncol. 31:372014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang C, Yao C, Li H, Wang G and He X:
Serum levels of microRNA-133b and microRNA-206 expression predict
prognosis in patients with osteosarcoma. Int J Clin Exp Pathol.
7:4194–4203. 2014.PubMed/NCBI
|
|
16
|
Ma W, Zhang X, Chai J, Chen P, Ren P and
Gong M: Circulating miR-148a is a significant diagnostic and
prognostic biomarker for patients with osteosarcoma. Tumour Biol.
35:12467–12472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai H, Zhao H, Tang J and Wu H: Serum
miR-195 is a diagnostic and prognostic marker for osteosarcoma. J
Surg Res. 194:505–510. 2015. View Article : Google Scholar
|
|
18
|
Zhang C, Yao C, Li H, Wang G and He X:
Combined elevation of microRNA-196a and microRNA-196b in sera
predicts unfavorable prognosis in patients with osteosarcomas. Int
J Mol Sci. 15:6544–6555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang L, Asatrian G, Dry SM and James AW:
Circulating tumor cells in sarcomas: A brief review. Med Oncol.
32:4302015. View Article : Google Scholar
|
|
20
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang ZF, Ngai P, Ho DW, Yu WC, Ng MN, Lau
CK, Li Ml, Tam KH, Lam CT, Poon RT, et al: Identification of local
and circulating cancer stem cells in human liver cancer.
Hepatology. 47:919–928. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schatton T, Murphy GF, Frank NY, Yamaura
K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM,
Weishaupt C, et al: Identification of cells initiating human
melanomas. Nature. 451:345–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sampieri K and Fodde R: Cancer stem cells
and metastasis. Semin Cancer Biol. 22:187–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Budd GT, Cristofanilli M, Ellis MJ,
Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV,
Terstappen LW, et al: Circulating tumor cells versus imaging -
predicting overall survival in metastatic breast cancer. Clin
Cancer Res. 12:6403–6409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sheng Y, Wang T, Li H, Zhang Z, Chen J, He
C, Li Y, Lv Y, Zhang J, Xu C, et al: Comparison of analytic
performances of Cellsearch and iFISH approach in detecting
circulating tumor cells. Oncotarget. 2015:52015. View Article : Google Scholar
|
|
26
|
Satelli A, Mitra A, Cutrera JJ, Devarie M,
Xia X, Ingram DR, Dibra D, Somaiah N, Torres KE, Ravi V, et al:
Universal marker and detection tool for human sarcoma circulating
tumor cells. Cancer Res. 74:1645–1650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ran R, Li L, Wang M, Wang S, Zheng Z and
Lin PP: Determination of EGFR mutations in single cells
microdissected from enriched lung tumor cells in peripheral blood.
Anal Bioanal Chem. 405:7377–7382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Q, Ge F, Cui W, Wang F, Yang Z, Guo
Y, Li L, Bremner RM and Lin PP: Lung cancer circulating tumor cells
isolated by the EpCAM-independent enrichment strategy correlate
with cyto-keratin 19-derived CYFRA21-1 and pathological staging.
Clin Chim Acta. 419:57–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ning N, Zhan T, Zhang Y, Chen Q, Feng F,
Yang Z, Liu Z, Xu D, Wang F, Guo Y, et al: Improvement of specific
detection of circulating tumor cells using combined CD45 staining
and fluorescence in situ hybridization. Clin Chim Acta. 433:69–75.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miller AB, Hoogstraten B, Staquet M and
Winkler A: Reporting results of cancer treatment. Cancer.
47:207–214. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Enneking WF: Musculoskeletal tumor
staging: 1988 update. Cancer Treat Res. 44:39–49. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Russell Wl, Sailors DM, Whittle TB, Fisher
DF Jr and Burns RP: Limb salvage versus traumatic amputation. A
decision based on a seven-part predictive index. Ann Surg.
213:473–480; discussion 480–481. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Detry MA and Lewis RJ: The
intention-to-treat principle: How to assess the true effect of
choosing a medical treatment. JAMA. 312:85–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu SH, Yang YL, Han SM and Wu ZH:
MicroRNA-9 expression is a prognostic biomarker in patients with
osteosarcoma. World J Surg Oncol. 12:1952014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang J, Gao T, Tang J, Cai H, Lin L and Fu
S: Loss of microRNA-132 predicts poor prognosis in patients with
primary osteosarcoma. Mol Cell Biochem. 381:9–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang M, Lin L, Cai H, Tang J and Zhou Z:
MicroRNA-145 downregulation associates with advanced tumor
progression and poor prognosis in patients suffering osteosarcoma.
Onco Targets Ther. 6:833–838. 2013.PubMed/NCBI
|
|
37
|
Mu Y, Zhang H, Che L and Li K: Clinical
significance of microRNA-183/Ezrin axis in judging the prognosis of
patients with osteosarcoma. Med Oncol. 31:8212014. View Article : Google Scholar
|
|
38
|
Zhang H, Yin Z, Ning K, Wang L, Guo R and
Ji Z: Prognostic value of microRNA-223/epithelial cell transforming
sequence 2 signaling in patients with osteosarcoma. Hum Pathol.
45:1430–1436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tian Z, Guo B, Yu M, Wang C, Zhang H,
Liang Q, Jiang K and Cao L: Upregulation of micro-ribonucleic
acid-128 cooperating with downregulation of PTEN confers metastatic
potential and unfavorable prognosis in patients with primary
osteosarcoma. Onco Targets Ther. 7:1601–1608. 2014.PubMed/NCBI
|
|
40
|
Jentzsch T, Robl B, Husmann M,
Bode-Lesniewska B and Fuchs B: Expression of MSH2 and MSH6 on a
tissue microarray in patients with osteosarcoma. Anticancer Res.
34:6961–6972. 2014.PubMed/NCBI
|
|
41
|
Sonaglio V, de Carvalho AC, Toledo SR,
Salinas-Souza C, Carvalho AL, Petrilli AS, de Camargo B and Vettore
AL: Aberrant DNA methylation of ESR1 and p14ARF genes could be
useful as prognostic indicators in osteosarcoma. Onco Targets Ther.
6:713–723. 2013.PubMed/NCBI
|
|
42
|
Zhu H, Tang J, Tang M and Cai H:
Upregulation of SOX9 in osteosarcoma and its association with tumor
progression and patients' prognosis. Diagn Pathol. 8:1832013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu Y, Zhou J, Ji Y and Yu B: Elevated
expression of AKT2 correlates with disease severity and poor
prognosis in human osteosarcoma. Mol Med Rep. 10:737–742.
2014.PubMed/NCBI
|
|
44
|
Hu F, Wang W, Zhou HC and Shang XF: High
expression of periostin is dramatically associated with metastatic
potential and poor prognosis of patients with osteosarcoma. World J
Surg Oncol. 12:2872014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu H, Cai H, Tang M and Tang J:
Neuropilin-1 is overexpressed in osteosarcoma and contributes to
tumor progression and poor prognosis. Clin Transl Oncol.
16:732–738. 2014. View Article : Google Scholar
|
|
46
|
Kubo T, Shimose S, Fujimori J, Furuta T,
Arihiro K and Ochi M: Does expression of glucose transporter
protein-1 relate to prognosis and angiogenesis in osteosarcoma?
Clin Orthop Relat Res. 473:305–310. 2015. View Article : Google Scholar :
|
|
47
|
Wang X, Du J, Gu P, Jin R and Lin X:
Polymeric immunoglobulin receptor expression is correlated with
poor prognosis in patients with osteosarcoma. Mol Med Rep.
9:2105–2110. 2014.PubMed/NCBI
|
|
48
|
Dawood S, Broglio K, Valero V, Reuben J,
Handy B, Islam R, Jackson S, Hortobagyi GN, Fritsche H and
Cristofanilli M: Circulating tumor cells in metastatic breast
cancer: From prognostic stratification to modification of the
staging system? Cancer. 113:2422–2430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hayes DF and Smerage J: Is there a role
for circulating tumor cells in the management of breast cancer?
Clin Cancer Res. 14:3646–3650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu MC, Shields PG, Warren RD, Cohen P,
Wilkinson M, Ottaviano YL, Rao SB, Eng-Wong J,
Seillier-Moiseiwitsch F, Noone AM, et al: Circulating tumor cells:
A useful predictor of treatment efficacy in metastatic breast
cancer. J Clin Oncol. 27:5153–5159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Krawczyk N, Banys M, Hartkopf A, Hagenbeck
C, Melcher C and Fehm T: Circulating tumour cells in breast cancer.
E Cancer Med Sci. 7:3522013.
|