|
1
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Rev Cancer. 11:719–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ledermann JA, Marth C, Carey MS, Birrer M,
Bowtell DD, Kaye S, McNeish I, Oza A, Scambia G, Rustin G, et al
Gynecologic Cancer InterGroup: Role of molecular agents and
targeted therapy in clinical trials for women with ovarian cancer.
Int J Gynecol Cancer. 21:763–770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
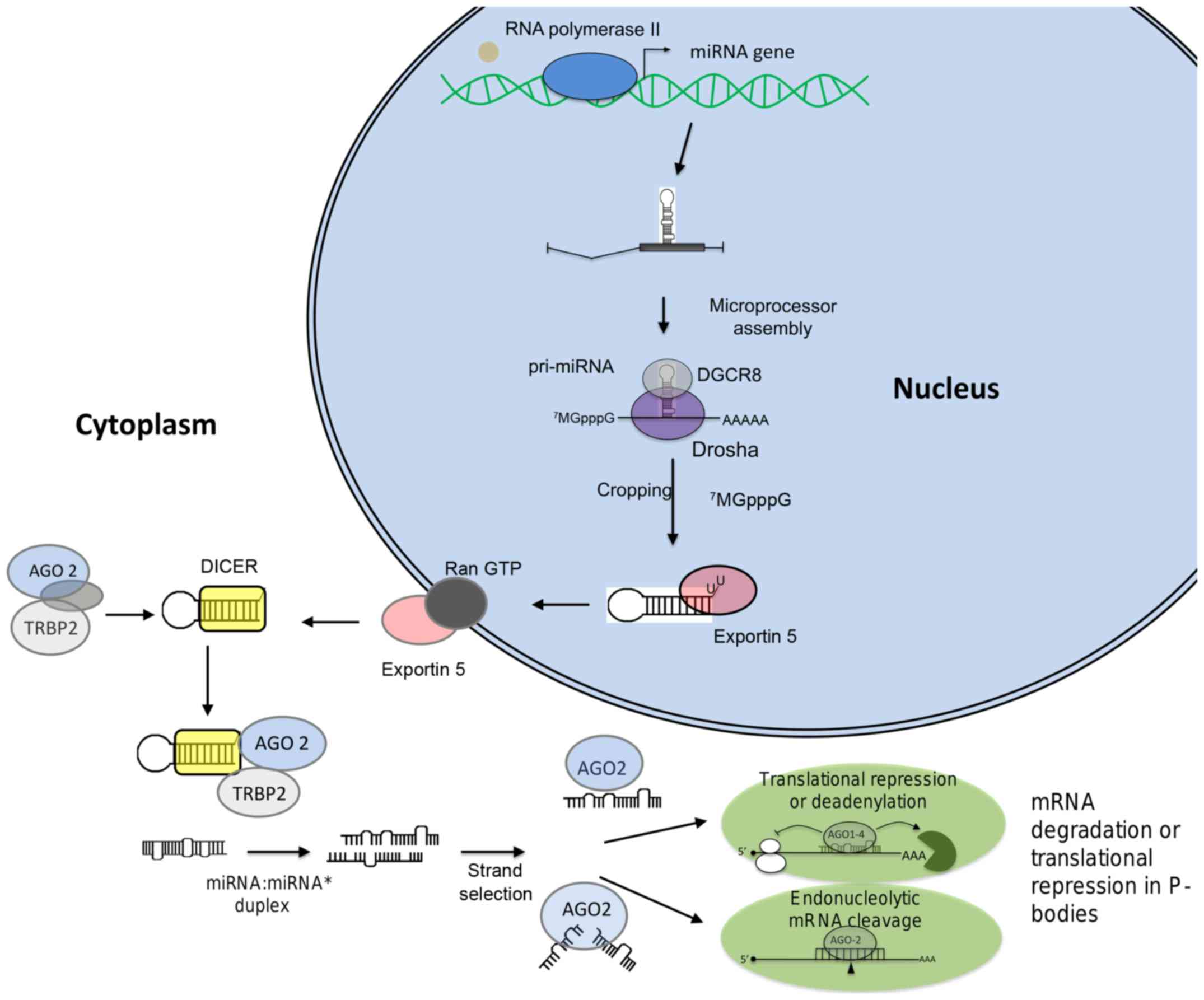
Kim VN: MicroRNA biogenesis: Coordinated
cropping and dicing. Nat Rev Mol Cell Biol. 6:376–385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
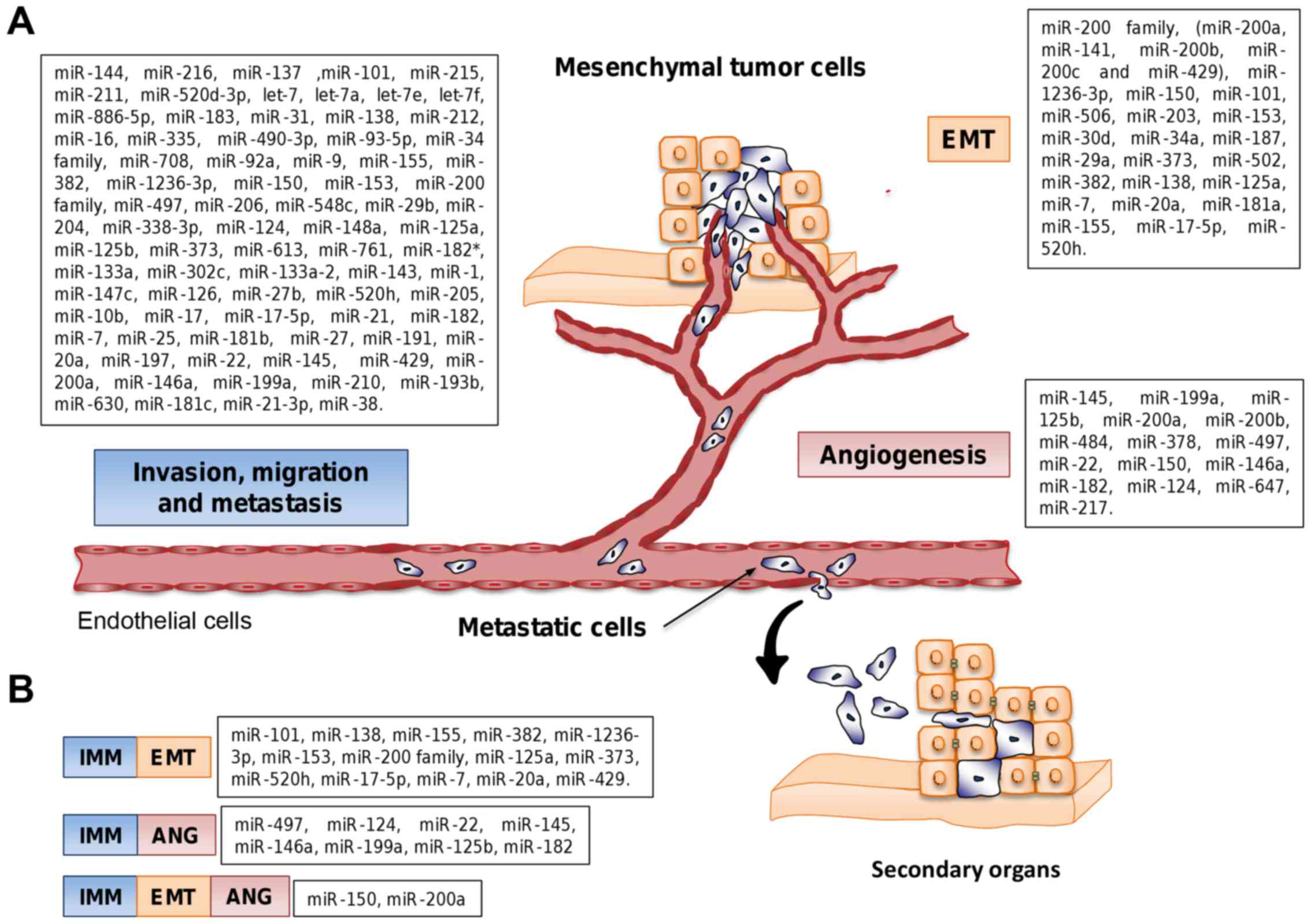
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lopez-Camarillo C, Marchat LA,
Arechaga-Ocampo E, Perez-Plasencia C, Del Moral-Hernandez O,
Castaneda-Ortiz EJ and Rodriguez-Cuevas S: MetastamiRs: Non-coding
MicroRNAs driving cancer invasion and metastasis. Int J Mol Sci.
13:1347–1379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
White NM, Fatoohi E, Metias M, Jung K,
Stephan C and Yousef GM: Metastamirs: A stepping stone towards
improved cancer management. Nat Rev Clin Oncol. 8:75–84. 2011.
View Article : Google Scholar
|
|
7
|
Cai X, Hagedorn CH and Cullen BR: Human
microRNAs are processed from capped, polyadenylated transcripts
that can also function as mRNAs. RNA. 10:1957–1966. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J,
Lee J, Provost P, Rådmark O, Kim S, et al: The nuclear RNase III
Drosha initiates microRNA processing. Nature. 425:415–419. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vasudevan S, Tong Y and Steitz JA:
Switching from repression to activation: microRNAs can up-regulate
translation. Science. 318:1931–1934. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eulalio A, Behm-Ansmant I and Izaurralde
E: P bodies: At the crossroads of post-transcriptional pathways.
Nat Rev Mol Cell Biol. 8:9–22. 2007. View Article : Google Scholar
|
|
11
|
Sen GL and Blau HM: Argonaute 2/RISC
resides in sites of mammalian mRNA decay known as cytoplasmic
bodies. Nat Cell Biol. 7:633–636. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
14
|
Nishimura M, Jung E-J, Shah MY, Lu C,
Spizzo R, Shimizu M, Han HD, Ivan C, Rossi S, Zhang X, et al:
Therapeutic synergy between microRNA and siRNA in ovarian cancer
treatment. Cancer Discov. 3:1302–1315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Mezencev R, Švajdler M, Benigno BB
and McDonald JF: Ectopic over-expression of miR-429 induces
mesenchymal-to-epithelial transition (MET) and increased drug
sensitivity in meta- stasizing ovarian cancer cells. Gynecol Oncol.
134:96–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
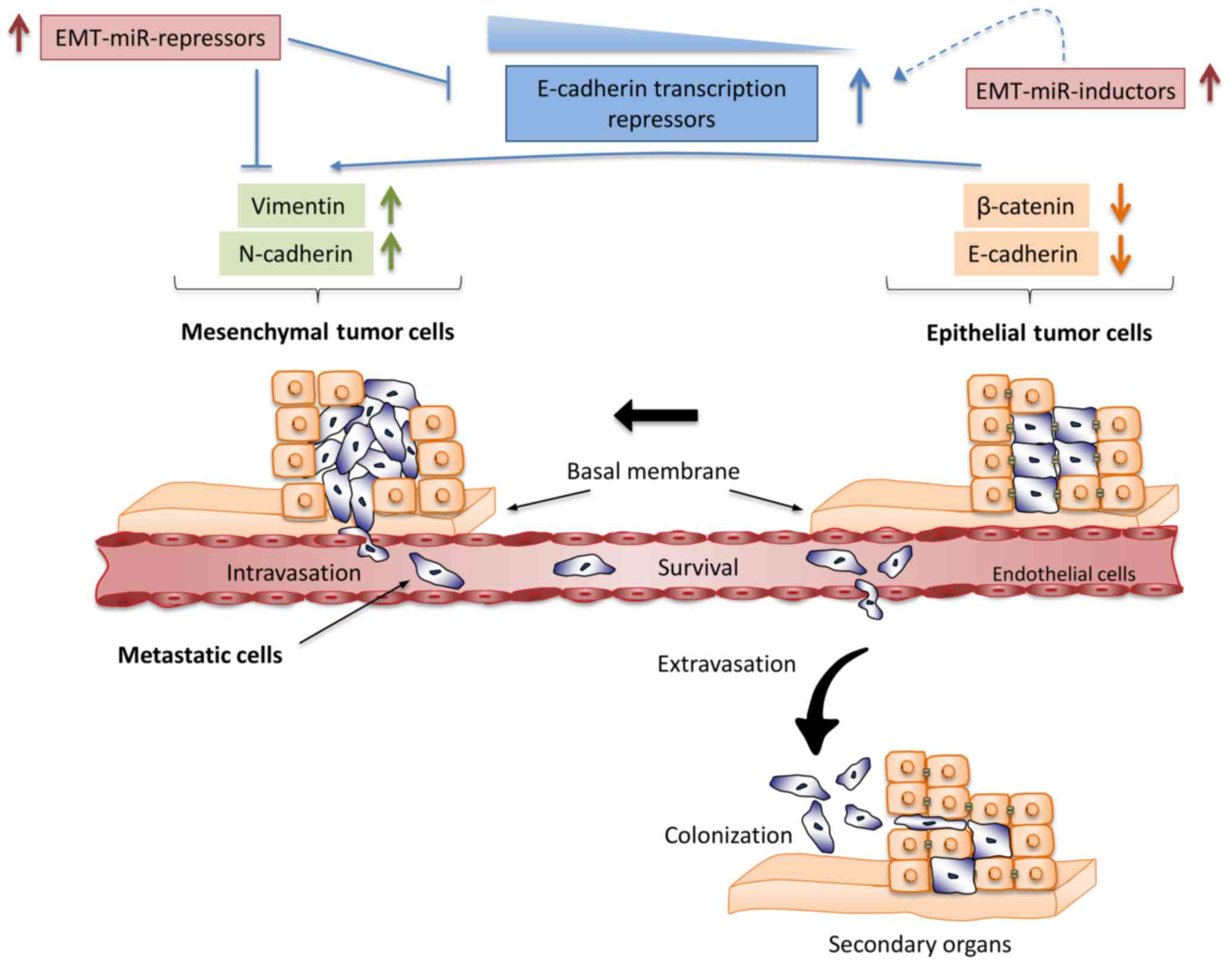
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Corallino S, Malabarba MG, Zobel M, Di
Fiore PP and Scita G: Epithelial-to-mesenchymal plasticity
harnesses endocytic circuitries. Front Oncol. 5:452015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oskarsson T, Batlle E and Massagué J:
Metastatic stem cells: Sources, niches, and vital pathways. Cell
Stem Cell. 14:306–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garg M: Targeting microRNAs in
epithelial-to-mesenchymal transition-induced cancer stem cells:
Therapeutic approaches in cancer. Expert Opin Ther Targets.
19:285–297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abba ML, Patil N, Leupold JH and Allgayer
H: MicroRNA regulation of epithelial to mesenchymal transition. J
Clin Med. 5:82016. View Article : Google Scholar :
|
|
22
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heuberger J and Birchmeier W: Interplay of
cadherin-mediated cell adhesion and canonical Wnt signaling. Cold
Spring Harb Perspect Biol. 2:a0029152010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arend RC, Londoño-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: A
review. Gynecol Oncol. 131:772–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ke Z, Caiping S, Qing Z and Xiaojing W:
Sonic hedgehog-Gli1 signals promote epithelial-mesenchymal
transition in ovarian cancer by mediating PI3K/AKT pathway. Med
Oncol. 32:3682015. View Article : Google Scholar
|
|
26
|
Fang D, Chen H, Zhu JY, Wang W, Teng Y,
Ding HF, Jing Q, Su SB and Huang S: Epithelial-mesenchymal
transition of ovarian cancer cells is sustained by Rac1 through
simultaneous activation of MEK1/2 and Src signaling pathways.
Oncogene Sep. 12:2016Epub ahead of print. View Article : Google Scholar
|
|
27
|
Talbot LJ, Bhattacharya SD and Kuo PC:
Epithelial-mesenchymal transition, the tumor microenvironment, and
metastatic behavior of epithelial malignancies. Int J Biochem Mol
Biol. 3:117–136. 2012.PubMed/NCBI
|
|
28
|
Fuxe J, Vincent T and Garcia de Herreros
A: Transcriptional crosstalk between TGF-β and stem cell pathways
in tumor cell invasion: Role of EMT promoting Smad complexes. Cell
Cycle. 9:2363–2374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moustakas A and Heldin CH: Signaling
networks guiding epithelial-mesenchymal transitions during
embryogenesis and cancer progression. Cancer Sci. 98:1512–1520.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gibbons DL, Lin W, Creighton CJ, Rizvi ZH,
Gregory PA, Goodall GJ, Thilaganathan N, Du L, Zhang Y,
Pertsemlidis A, et al: Contextual extracellular cues promote tumor
cell EMT and metastasis by regulating miR-200 family expression.
Genes Dev. 23:2140–2151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park SM, Gaur AB, Lengyel E and Peter ME:
The miR-200 family determines the epithelial phenotype of cancer
cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes
Dev. 22:894–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bendoraite A, Knouf EC, Garg KS, Parkin
RK, Kroh EM, O'Briant KC, Ventura AP, Godwin AK, Karlan BY,
Drescher CW, et al: Regulation of miR-200 family microRNAs and ZEB
transcription factors in ovarian cancer: Evidence supporting a
mesothelial-to-epithelial transition. Gynecol Oncol. 116:117–125.
2010. View Article : Google Scholar :
|
|
35
|
Chen J, Wang L, Matyunina LV, Hill CG and
McDonald JF: Overexpression of miR-429 induces
mesenchymal-to-epithelial transition (MET) in metastatic ovarian
cancer cells. Gynecol Oncol. 121:200–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jabbari N, Reavis AN and McDonald JF:
Sequence variation among members of the miR-200 microRNA family is
correlated with variation in the ability to induce hallmarks of
mesenchymal-epithelial transition in ovarian cancer cells. J
Ovarian Res. 7:122014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu YM, Shang C, Ou YL, Yin D, Li YN, Li X,
Wang N and Zhang SL: miR-200c modulates ovarian cancer cell
metastasis potential by targeting zinc finger E-box-binding
homeobox 2 (ZEB2) expression. Med Oncol. 31:1342014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen D, Zhang Y, Wang J, Chen J, Yang C,
Cai K, Wang X, Shi F and Dou J: MicroRNA-200c overexpression
inhibits tumorige-nicity and metastasis of
CD117+CD44+ ovarian cancer stem cells by
regulating epithelial-mesenchymal transition. J Ovarian Res.
6:502013. View Article : Google Scholar
|
|
39
|
Wang Y, Yan S, Liu X, Zhang W, Li Y, Dong
R, Zhang Q, Yang Q, Yuan C, Shen K, et al: miR-1236-3p represses
the cell migration and invasion abilities by targeting ZEB1 in
high-grade serous ovarian carcinoma. Oncol Rep. 31:1905–1910.
2014.PubMed/NCBI
|
|
40
|
Wu Q, Guo R, Lin M, Zhou B and Wang Y:
MicroRNA-200a inhibits CD133/1+ ovarian cancer stem
cells migration and invasion by targeting E-cadherin repressor
ZEB2. Gynecol Oncol. 122:149–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vang S, Wu HT, Fischer A, Miller DH,
Maclaughlan S, Douglass E, Comisar L, Steinhoff M, Collins C, Smith
PJ, et al: Identification of ovarian cancer metastatic miRNAs. PloS
One. 8:e582262013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jin M, Yang Z, Ye W, Xu H and Hua X:
MicroRNA-150 predicts a favorable prognosis in patients with
epithelial ovarian cancer, and inhibits cell invasion and
metastasis by suppressing transcriptional repressor ZEB1. PloS One.
9:e1039652014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Y, Luo J, Tian R, Sun H and Zou S:
miR-373 negatively regulates methyl-CpG-binding domain protein 2
(MBD2) in hilar cholangiocarcinoma. Dig Dis Sci. 56:1693–1701.
2011. View Article : Google Scholar
|
|
44
|
Oppenheimer H, Kumar A, Meir H, Schwartz
I, Zini A, Haze A, Kandel L, Mattan Y, Liebergall M and
Dvir-Ginzberg M: Set7/9 impacts COL2A1 expression through binding
and repression of SirT1 histone deacetylation. J Bone Miner Res.
29:348–360. 2014. View Article : Google Scholar
|
|
45
|
Zhou J, Xie M, Shi Y, Luo B, Gong G, Li J,
Wang J, Zhao W, Zi Y, Wu X, et al: MicroRNA-153 functions as a
tumor suppressor by targeting SET7 and ZEB2 in ovarian cancer
cells. Oncol Rep. 34:111–120. 2015.PubMed/NCBI
|
|
46
|
Kim YS, Yi BR, Kim NH and Choi KC: Role of
the epithelial-mesenchymal transition and its effects on embryonic
stem cells. Exp Mol Med. 46:e1082014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang D, Sun Y, Hu L, Zheng H, Ji P, Pecot
CV, Zhao Y, Reynolds S, Cheng H, Rupaimoole R, et al: Integrated
analyses identify a master microRNA regulatory network for the
mesenchymal subtype in serous ovarian cancer. Cancer Cell.
23:186–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun Y, Mezzanzanica D and Zhang W:
MiR-506: A Multitasker in suppression of the
epithelial-to-mesenchymal transition. RNA Dis.
1:e4472014.PubMed/NCBI
|
|
49
|
Sun Y, Hu L, Zheng H, Bagnoli M, Guo Y,
Rupaimoole R, Rodriguez-Aguayo C, Lopez-Berestein G, Ji P, Chen K,
et al: MiR-506 inhibits multiple targets in the
epithelial-to-mesenchymal transition network and is associated with
good prognosis in epithelial ovarian cancer. J Pathol. 235:25–36.
2015. View Article : Google Scholar
|
|
50
|
Zhao G, Guo Y, Chen Z, Wang Y, Yang C,
Dudas A, Du Z, Liu W, Zou Y, Szabo E, et al: miR-203 functions as a
tumor suppressor by inhibiting epithelial to mesenchymal transition
in ovarian cancer. J Cancer Sci Ther. 7:34–43. 2015.
|
|
51
|
Ye Z, Zhao L, Li J, Chen W and Li X:
miR-30d blocked transforming growth factor β1-induced
epithelial-mesenchymal transition by targeting Snail in ovarian
cancer cells. Int J Gynecol Cancer. 25:1574–1581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Siemens H, Jackstadt R, Hünten S, Kaller
M, Menssen A, Götz U and Hermeking H: miR-34 and SNAIL form a
double-negative feedback loop to regulate epithelial-mesenchymal
transitions. Cell Cycle. 10:4256–4271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Su JL, Chen PB, Chen YH, Chen SC, Chang
YW, Jan YH, Cheng X, Hsiao M and Hung MC: Downregulation of
microRNA miR-520h by E1A contributes to anticancer activity. Cancer
Res. 70:5096–5108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu J and Wei JJ: HMGA2 and high-grade
serous ovarian carcinoma. J Mol Med (Berl). 91:1155–1165. 2013.
View Article : Google Scholar
|
|
55
|
Wu J, Liu Z, Shao C, Gong Y, Hernando E,
Lee P, Narita M, Muller W, Liu J and Wei JJ: HMGA2
overexpression-induced ovarian surface epithelial transformation is
mediated through regulation of EMT genes. Cancer Res. 71:349–359.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tanaka T, Arai M, Wu S, Kanda T, Miyauchi
H, Imazeki F, Matsubara H and Yokosuka O: Epigenetic silencing of
microRNA-373 plays an important role in regulating cell
proliferation in colon cancer. Oncol Rep. 26:1329–1335.
2011.PubMed/NCBI
|
|
57
|
Zhang Y, Zhao FJ, Chen LL, Wang LQ, Nephew
KP, Wu YL and Zhang S: MiR-373 targeting of the Rab22a oncogene
suppresses tumor invasion and metastasis in ovarian cancer.
Oncotarget. 5:12291–12303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hsiao CP, Araneta M, Wang XM and Saligan
LN: The association of IFI27 expression and fatigue intensification
during localized radiation therapy: Implication of a
para-inflammatory bystander response. Int J Mol Sci.
14:16943–16957. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li S, Xie Y, Zhang W, Gao J, Wang M, Zheng
G, Yin X, Xia H and Tao X: Interferon alpha-inducible protein 27
promotes epithelial-mesenchymal transition and induces ovarian
tumorigenicity and stemness. J Surg Res. 193:255–264. 2015.
View Article : Google Scholar
|
|
60
|
Baskar S, Wiestner A, Wilson WH, Pastan I
and Rader C: Targeting malignant B cells with an immunotoxin
against ROR1. MAbs. 4:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tan H, He Q, Gong G, Wang Y, Li J, Wang J,
Zhu D and Wu X: miR-382 inhibits migration and invasion by
targeting ROR1 through regulating EMT in ovarian cancer. Int J
Oncol. 48:181–190. 2016.
|
|
62
|
Yeh YM, Chuang CM, Chao KC and Wang LH:
MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis
by targeting SOX4 and HIF-1α. Int J Cancer. 133:867–878. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cowden Dahl KD, Dahl R, Kruichak JN and
Hudson LG: The epidermal growth factor receptor responsive miR-125a
represses mesenchymal morphology in ovarian cancer cells.
Neoplasia. 11:1208–1215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bobbs A, Gellerman K, Hallas WM, Joseph S,
Yang C, Kurkewich J and Cowden Dahl KD: ARID3B directly regulates
ovarian cancer promoting genes. PloS One. 10:e01319612015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhou X, Hu Y, Dai L, Wang Y, Zhou J, Wang
W, Di W and Qiu L: MicroRNA-7 inhibits tumor metastasis and
reverses epithelial-mesenchymal transition through AKT/ERK1/2
inactivation by targeting EGFR in epithelial ovarian cancer. PloS
One. 9:e967182014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yuan ZQ, Sun M, Feldman RI, Wang G, Ma X,
Jiang C, Coppola D, Nicosia SV and Cheng JQ: Frequent activation of
AKT2 and induction of apoptosis by inhibition of
phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer.
Oncogene. 19:2324–2330. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Luo X, Dong Z, Chen Y, Yang L and Lai D:
Enrichment of ovarian cancer stem-like cells is associated with
epithelial to mesenchymal transition through an miRNA-activated AKT
pathway. Cell Prolif. 46:436–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Parikh A, Lee C, Joseph P, Marchini S,
Baccarini A, Kolev V, Romualdi C, Fruscio R, Shah H, Wang F, et al:
microRNA-181a has a critical role in ovarian cancer progression
through the regulation of the epithelial-mesenchymal transition.
Nat Commun. 5:29772014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fang L, Li H, Wang L, Hu J, Jin T, Wang J
and Yang BB: MicroRNA-17-5p promotes chemotherapeutic drug
resistance and tumour metastasis of colorectal cancer by repressing
PTEN expression. Oncotarget. 5:2974–2987. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fang Y, Xu C and Fu Y: MicroRNA-17-5p
induces drug resistance and invasion of ovarian carcinoma cells by
targeting PTEN signaling. J Biol Res (Thessalon). 22:122015.
View Article : Google Scholar
|
|
71
|
Qin W, Ren Q, Liu T, Huang Y and Wang J:
MicroRNA-155 is a novel suppressor of ovarian cancer-initiating
cells that targets CLDN1. FEBS Lett. 587:1434–1439. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Habata S, Iwasaki M, Sugio A, Suzuki M,
Tamate M, Satohisa S, Tanaka R and Saito T: BAG3 increases the
invasiveness of uterine corpus carcinoma cells by suppressing
miR-29b and enhancing MMP2 expression. Oncol Rep. 33:2613–2621.
2015.PubMed/NCBI
|
|
74
|
Sun X, Cui M, Zhang A, Tong L, Wang K, Li
K, Wang X, Sun Z and Zhang H: MiR-548c impairs migration and
invasion of endo-metrial and ovarian cancer cells via
downregulation of Twist. J Exp Clin Cancer Res. 35:102016.
View Article : Google Scholar
|
|
75
|
Su N, Qiu H, Chen Y, Yang T, Yan Q and Wan
X: miR-205 promotes tumor proliferation and invasion through
targeting ESRRG in endometrial carcinoma. Oncol Rep. 29:2297–2302.
2013.PubMed/NCBI
|
|
76
|
Mozos A, Catasús L, D'Angelo E, Serrano E,
Espinosa I, Ferrer I, Pons C and Prat J: The FOXO1-miR27 tandem
regulates myometrial invasion in endometrioid endometrial
adenocarcinoma. Hum Pathol. 45:942–951. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Dong M, Yang P and Hua F: MiR-191
modulates malignant transformation of endometriosis through
regulating TIMP3. Med Sci Monit. 21:915–920. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li S, Hu R, Wang C, Guo F, Li X and Wang
S: miR-22 inhibits proliferation and invasion in estrogen receptor
α-positive endometrial endometrioid carcinomas cells. Mol Med Rep.
9:2393–2399. 2014.PubMed/NCBI
|
|
79
|
Chen X, Yan Q, Li S, Zhou L, Yang H, Yang
Y, Liu X and Wan X: Expression of the tumor suppressor miR-206 is
associated with cellular proliferative inhibition and impairs
invasion in ERα-positive endometrioid adenocarcinoma. Cancer Lett.
314:41–53. 2012. View Article : Google Scholar
|
|
80
|
Li S, Li Y, Wen Z, Kong F, Guan X and Liu
W: microRNA-206 overexpression inhibits cellular proliferation and
invasion of estrogen receptor α-positive ovarian cancer cells. Mol
Med Rep. 9:1703–1708. 2014.PubMed/NCBI
|
|
81
|
Liang SH, Li J, Al-beit M, Zhang J, Ma D
and Lu X: Screening and identification of potential miRNA involved
in ovarian cancer invasion and metastasis. Zhonghua Zhong Liu Za
Zhi. 32:650–654. 2010.In Chinese. PubMed/NCBI
|
|
82
|
Li J, Liang S, Yu H, Zhang J, Ma D and Lu
X: An inhibitory effect of miR-22 on cell migration and invasion in
ovarian cancer. Gynecol Oncol. 119:543–548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li J, Liang S, Jin H, Xu C, Ma D and Lu X:
Tiam1, negatively regulated by miR-22, miR-183 and miR-31, is
involved in migration, invasion and viability of ovarian cancer
cells. Oncol Rep. 27:1835–1842. 2012.PubMed/NCBI
|
|
84
|
Wen C, Liu X, Ma H, Zhang W and Li H:
miR-338-3p suppresses tumor growth of ovarian epithelial carcinoma
by targeting Runx2. Int J Oncol. 46:2277–2285. 2015.PubMed/NCBI
|
|
85
|
Lin K-T, Yeh Y-M, Chuang C-M, Yang SY,
Chang JW, Sun SP, Wang YS, Chao KC and Wang LH: Glucocorticoids
mediate induction of microRNA-708 to suppress ovarian cancer
metastasis through targeting Rap1B. Nat Commun. 6:59172015.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang H, Wang Q, Zhao Q and Di W: MiR-124
inhibits the migration and invasion of ovarian cancer cells by
targeting SphK1. J Ovarian Res. 6:842013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wen Z, Zhao S, Liu S, Liu Y, Li X and Li
S: MicroRNA-148a inhibits migration and invasion of ovarian cancer
cells via targeting sphingosine-1-phosphate receptor 1. Mol Med
Rep. 12:3775–3780. 2015.PubMed/NCBI
|
|
88
|
Chen P, Zeng M, Zhao Y and Fang X:
Upregulation of Limk1 caused by microRNA-138 loss aggravates the
metastasis of ovarian cancer by activation of Limk1/cofilin
signaling. Oncol Rep. 32:2070–2076. 2014.PubMed/NCBI
|
|
89
|
Wang W, Ren F, Wu Q, Jiang D, Li H, Peng
Z, Wang J and Shi H: MicroRNA-497 inhibition of ovarian cancer cell
migration and invasion through targeting of SMAD specific E3
ubiquitin protein ligase 1. Biochem Biophys Res Commun.
449:432–437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lee M, Kim EJ and Jeon MJ: MicroRNAs 125a
and 125b inhibit ovarian cancer cells through post-transcriptional
inactivation of EIF4EBP1. Oncotarget. 7:8726–8742. 2016.
|
|
91
|
Meng X, Joosse SA, Müller V, Trillsch F,
Milde-Langosch K, Mahner S, Geffken M, Pantel K and Schwarzenbach
H: Diagnostic and prognostic potential of serum miR-7, miR-16,
miR-25, miR-93, miR-182, miR-376a and miR-429 in ovarian cancer
patients. Br J Cancer. 113:1358–1366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cao J, Cai J, Huang D, Han Q, Yang Q, Li
T, Ding H and Wang Z: miR-335 represents an invasion suppressor
gene in ovarian cancer by targeting Bcl-w. Oncol Rep. 30:701–706.
2013.PubMed/NCBI
|
|
93
|
Imam JS, Plyler JR, Bansal H, Prajapati S,
Bansal S, Rebeles J, Chen HI, Chang YF, Panneerdoss S, Zoghi B, et
al: Genomic loss of tumor suppressor miRNA-204 promotes cancer cell
migration and invasion by activating AKT/mTOR/Rac1 signaling and
actin reorganization. PloS One. 7:e523972012. View Article : Google Scholar
|
|
94
|
Chung TK, Lau TS, Cheung TH, Yim SF, Lo
KW, Siu NS, Chan LK, Yu MY, Kwong J, Doran G, et al: Dysregulation
of microRNA-204 mediates migration and invasion of endometrial
cancer by regulating FOXC1. Int J Cancer. 130:1036–1045. 2012.
View Article : Google Scholar
|
|
95
|
Vimalraj S, Miranda PJ, Ramyakrishna B and
Selvamurugan N: Regulation of breast cancer and bone metastasis by
microRNAs. Dis Markers. 35:369–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tang H, Yao L, Tao X, Yu Y, Chen M, Zhang
R and Xu C: miR-9 functions as a tumor suppressor in ovarian serous
carcinoma by targeting TLN1. Int J Mol Med. 32:381–388.
2013.PubMed/NCBI
|
|
97
|
Ohyagi-Hara C, Sawada K, Kamiura S, Tomita
Y, Isobe A, Hashimoto K, Kinose Y, Mabuchi S, Hisamatsu T,
Takahashi T, et al: miR-92a inhibits peritoneal dissemination of
ovarian cancer cells by inhibiting integrin α5 expression. Am J
Pathol. 182:1876–1889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Doberstein K, Bretz NP, Schirmer U, Fiegl
H, Blaheta R, Breunig C, Müller-Holzner E, Reimer D, Zeimet AG and
Altevogt P: miR-21-3p is a positive regulator of L1CAM in several
human carcinomas. Cancer Lett. 354:455–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chen S, Chen X, Xiu YL, Sun KX and Zhao Y:
MicroRNA-490-3P targets CDK1 and inhibits ovarian epithelial
carcinoma tumorigenesis and progression. Cancer Lett. 362:122–130.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chen X, Chen S, Xiu Y-L, Sun K-X, Zong Z-H
and Zhao Y: RhoC is a major target of microRNA-93-5P in epithelial
ovarian carcinoma tumorigenesis and progression. Mol Cancer.
14:312015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liu T, Qin W, Hou L and Huang Y:
MicroRNA-17 promotes normal ovarian cancer cells to cancer stem
cells development via suppression of the LKB1-p53-p21/WAF1 pathway.
Tumour Biol. 36:1881–1893. 2015. View Article : Google Scholar
|
|
102
|
Corney DC, Hwang CI, Matoso A, Vogt M,
Flesken-Nikitin A, Godwin AK, Kamat AA, Sood AK, Ellenson LH,
Hermeking H, et al: Frequent downregulation of miR-34 family in
human ovarian cancers. Clin Cancer Res. 16:1119–1128. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hwang C-I, Choi J, Zhou Z, Flesken-Nikitin
A, Tarakhovsky A and Nikitin AY: MET-dependent cancer invasion may
be preprogrammed by early alterations of p53-regulated feedforward
loop and triggered by stromal cell-derived HGF. Cell Cycle.
10:3834–3840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hwang C-I, Matoso A, Corney DC,
Flesken-Nikitin A, Körner S, Wang W, Boccaccio C, Thorgeirsson SS,
Comoglio PM, Hermeking H, et al: Wild-type p53 controls cell
motility and invasion by dual regulation of MET expression. Proc
Natl Acad Sci USA. 108:14240–14245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li R, Shi X, Ling F, Wang C, Liu J, Wang W
and Li M: MiR-34a suppresses ovarian cancer proliferation and
motility by targeting AXL. Tumour Biol. 36:7277–7283. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wu H, Xiao Z, Wang K, Liu W and Hao Q:
MiR-145 is downreg-ulated in human ovarian cancer and modulates
cell growth and invasion by targeting p70S6K1 and MUC1. Biochem
Biophys Res Commun. 441:693–700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ahmed AA, Etemadmoghadam D, Temple J,
Lynch AG, Riad M, Sharma R, Stewart C, Fereday S, Caldas C, Defazio
A, et al: Driver mutations in TP53 are ubiquitous in high grade
serous carcinoma of the ovary. J Pathol. 221:49–56. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Dong R, Liu X, Zhang Q, Jiang Z, Li Y, Wei
Y, Li Y, Yang Q, Liu J, Wei JJ, et al: miR-145 inhibits tumor
growth and metastasis by targeting metadherin in high-grade serous
ovarian carcinoma. Oncotarget. 5:10816–10829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Chen X, Dong C, Law PT, Chan MT, Su Z,
Wang S, Wu WK and Xu H: MicroRNA-145 targets TRIM2 and exerts
tumor-suppressing functions in epithelial ovarian cancer. Gynecol
Oncol. 139:513–519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kim TH, Song JY, Park H, Jeong JY, Kwon
AY, Heo JH, Kang H, Kim G and An HJ: miR-145, targeting
high-mobility group A2, is a powerful predictor of patient outcome
in ovarian carcinoma. Cancer Lett. 356B:937–945. 2015. View Article : Google Scholar
|
|
111
|
Yan L, Zhou J, Gao Y, Ghazal S, Lu L,
Bellone S, Yang Y, Liu N, Zhao X, Santin AD, et al: Regulation of
tumor cell migration and invasion by the H19/let-7 axis is
antagonized by metformin-induced DNA methylation. Oncogene.
34:3076–3084. 2015. View Article : Google Scholar
|
|
112
|
Gao Y, Meng H, Liu S, Hu J, Zhang Y, Jiao
T, Liu Y, Ou J, Wang D, Yao L, et al: LncRNA-HOST2 regulates cell
biological behaviors in epithelial ovarian cancer through a
mechanism involving microRNA let-7b. Hum Mol Genet. 24:841–852.
2015. View Article : Google Scholar
|
|
113
|
Guo Y, Tian P, Yang C, Liang Z, Li M, Sims
M, Lu L, Zhang Z, Li H, Pfeffer LM, et al: Silencing the
double-stranded RNA binding protein DGCR8 inhibits ovarian cancer
cell proliferation, migration, and invasion. Pharm Res. 32:769–778.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Rupaimoole R, Ivan C, Yang D, Gharpure KM,
Wu SY, Pecot CV, Previs RA, Nagaraja AS, Armaiz-Pena GN, McGuire M,
et al: Hypoxia-upregulated microRNA-630 targets Dicer, leading to
increased tumor progression. Oncogene. 35:4312–4320. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Tang R, Cui ZM and Lou YH: MicroRNA-16
regulates the proliferation, invasion and apoptosis of ovarian
epithelial carcinoma cells in vitro. Zhonghua Fu Chan Ke Za Zhi.
47:846–850. 2012.In Chinese.
|
|
116
|
Wei LQ, Liang HT, Qin DC, Jin HF, Zhao Y
and She MC: MiR-212 exerts suppressive effect on SKOV3 ovarian
cancer cells through targeting HBEGF. Tumour Biol. 35:12427–12434.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Li J, Li D and Zhang W: Tumor suppressor
role of miR-217 in human epithelial ovarian cancer by targeting
IGF1R. Oncol Rep. 35:1671–1679. 2016.
|
|
118
|
Li J, Li L, Li Z, Gong G, Chen P, Liu H,
Wang J, Liu Y and Wu X: The role of miR-205 in the VEGF-mediated
promotion of human ovarian cancer cell invasion. Gynecol Oncol.
137:125–133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhou J, Liu H, Chen Y, Wen J, Li L and Wu
X: Expression and significance of VEGF, miR-205 and target protein
Ezrin and Lamin A/C in ovarian cancer. Zhong Nan Da Xue Xue Bao Yi
Xue Ban. 39:142–150. 2014.0 (In Chinese). PubMed/NCBI
|
|
120
|
Fu X, Cui Y, Yang S, Xu Y and Zhang Z:
MicroRNA-613 inhibited ovarian cancer cell proliferation and
invasion by regulating KRAS. Tumour Biol. 37:6477–6483. 2016.
View Article : Google Scholar
|
|
121
|
Zhang L, Li Z, Gai F and Wang Y:
MicroRNA-137 suppresses tumor growth in epithelial ovarian cancer
in vitro and in vivo. Mol Med Rep. 12:3107–3114. 2015.PubMed/NCBI
|
|
122
|
Shi C and Zhang Z: miR-761 inhibits tumor
progression by targeting MSI1 in ovarian carcinoma. Tumour Biol.
37:5437–5443. 2016. View Article : Google Scholar
|
|
123
|
Yao L, Wang L, Li F, Gao X, Wei X and Liu
Z: MiR181c inhibits ovarian cancer metastasis and progression by
targeting PRKCD expression. Int J Clin Exp Med. 8:15198–15205.
2015.PubMed/NCBI
|
|
124
|
Feng S, Pan W, Jin Y and Zheng J: MiR-25
promotes ovarian cancer proliferation and motility by targeting
LATS2. Tumour Biol. 35:12339–12344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Xia Y and Gao Y: MicroRNA-181b promotes
ovarian cancer cell growth and invasion by targeting LATS2. Biochem
Biophys Res Commun. 447:446–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Lou Y, Yang X, Wang F, Cui Z and Huang Y:
MicroRNA-21 promotes the cell proliferation, invasion and migration
abilities in ovarian epithelial carcinomas through inhibiting the
expression of PTEN protein. Int J Mol Med. 26:819–827. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Lou Y, Cui Z, Wang F, Yang X and Qian J:
miR-21 down-regulation promotes apoptosis and inhibits invasion and
migration abilities of OVCAR3 cells. Clin Invest Med.
34:E2812011.PubMed/NCBI
|
|
128
|
Wang YQ, Guo RD, Guo RM, Sheng W and Yin
LR: MicroRNA-182 promotes cell growth, invasion, and
chemoresis-tance by targeting programmed cell death 4 (PDCD4) in
human ovarian carcinomas. J Cell Biochem. 114:1464–1473. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Xu X, Ayub B, Liu Z, Serna VA, Qiang W,
Liu Y, Hernando E, Zabludoff S, Kurita T, Kong B, et al:
Anti-miR182 reduces ovarian cancer burden, invasion, and
metastasis: An in vivo study in orthotopic xenografts of nude mice.
Mol Cancer Ther. 13:1729–1739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Liu Z, Liu J, Segura MF, Shao C, Lee P,
Gong Y, Hernando E and Wei JJ: MiR-182 overexpression in
tumourigenesis of high-grade serous ovarian carcinoma. J Pathol.
228:204–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Nakayama I, Shibazaki M, Yashima-Abo A,
Miura F, Sugiyama T, Masuda T and Maesawa C: Loss of HOXD10
expression induced by upregulation of miR-10b accelerates the
migration and invasion activities of ovarian cancer cells. Int J
Oncol. 43:63–71. 2013.PubMed/NCBI
|
|
132
|
Fan X, Liu Y, Jiang J, Ma Z, Wu H, Liu T,
Liu M, Li X and Tang H: miR-20a promotes proliferation and invasion
by targeting APP in human ovarian cancer cells. Acta Biochim
Biophys Sin (Shanghai). 42:318–324. 2010. View Article : Google Scholar
|
|
133
|
Zou D, Wang D, Li R, Tang Y, Yuan L, Long
X and Zhou Q: MiR-197 induces Taxol resistance in human ovarian
cancer cells by regulating NLK. Tumour Biol. 36:6725–6732. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Taylor MA and Schiemann WP: Therapeutic
opportunities for targeting microRNAs in cancer. Mol Cell Ther.
2:1–13. 2014. View Article : Google Scholar
|
|
135
|
Krützfeldt J, Rajewsky N, Braich R, Rajeev
KG, Tuschl T, Manoharan M and Stoffel M: Silencing of microRNAs in
vivo with 'antagomirs'. Nature. 438:685–689. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Elmén J, Lindow M, Silahtaroglu A, Bak M,
Christensen M, Lind-Thomsen A, Hedtjärn M, Hansen JB, Hansen HF,
Straarup EM, et al: Antagonism of microRNA-122 in mice by
systemically administered LNA-antimiR leads to up-regulation of a
large set of predicted target mRNAs in the liver. Nucleic Acids
Res. 36:1153–1162. 2008. View Article : Google Scholar :
|
|
137
|
Hutvágner G and Zamore PD: A microRNA in a
multiple-turnover RNAi enzyme complex. Science. 297:2056–2060.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Kota J, Chivukula RR, O'Donnell KA,
Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P,
Torbenson M, Clark KR, et al: Therapeutic microRNA delivery
suppresses tumorigenesis in a murine liver cancer model. Cell.
137:1005–1017. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: Rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Lu Y, Xiao J, Lin H, Bai Y, Luo X, Wang Z
and Yang B: A single anti-microRNA antisense
oligodeoxyribonucleotide (AMO) targeting multiple microRNAs offers
an improved approach for microRNA interference. Nucleic Acids Res.
37:e242009. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Dai F, Zhang Y, Zhu X, Shan N and Chen Y:
Anticancer role of MUC1 aptamer-miR-29b chimera in epithelial
ovarian carcinoma cells through regulation of PTEN methylation.
Target Oncol. 7:217–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Cittelly DM, Dimitrova I, Howe EN,
Cochrane DR, Jean A, Spoelstra NS, Post MD, Lu X, Broaddus RR,
Spillman MA, et al: Restoration of miR-200c to ovarian cancer
reduces tumor burden and increases sensitivity to paclitaxel. Mol
Cancer Ther. 11:2556–2565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Gambari R, Brognara E, Spandidos DA and
Fabbri E: Targeting oncomiRNAs and mimicking tumor suppressor
miRNAs: New trends in the development of miRNA therapeutic
strategies in oncology (Review). Int J Oncol. 49:5–32.
2016.PubMed/NCBI
|