Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common malignancy in the world and the second leading cause of
mortality among malignant tumors (1). At present, surgery is the main
strategy for long-term survival of patients with HCC. However, even
with radical resection, 60–70% of patients will present with
recurrence and metastasis within 5 years (2). In addition, ~80% of patients with HCC
have distant metastases at the time of diagnosis and surgical
resection may not be possible. Palliative care carries a 5-year
survival rate of only 5–7% (3).
The highly invasive nature of HCC, metastasis and recurrence are
the main causes of poor prognosis and high mortality. Therefore,
effective prevention and treatment for the invasion and metastasis
of HCC are important measures to improve its prognosis. However, at
present, the precise underlying molecular mechanisms of HCC
invasion and metastasis remain to be elucidated.
Epithelial-mesenchymal transition (EMT) refers to
the process of transformation of epithelial cells to mesenchymal
phenotypic cells resulting in increased motility and invasiveness.
During this process, cells lose polarity, cell-cell adhesion,
epithelial markers such as E-cadherin, and acquire mesenchymal
properties with high expression of mesenchymal molecular markers
including vimentin, Snail, Slug and Twist (4). A number of studies have shown that
EMT plays a crucial role in liver cancer invasion and metastasis
(5–7), nevertheless, there is a lack of clear
picture in HCC of the overall EMT signaling network. Recently, the
potential link between Connexin (Cx) (8) or gap junction (GJ) (9) and EMT has been rendered.
Cx, the structural protein of GJs, exerts its
biological and cellular functions through both GJ-dependent and
GJ-independent pathways (10). In
liver tissues and cell lines, Cx32 and Cx26 are predominantly
expressed and contribute to the major component of hepatocyte GJs
(11). Decreased GJ function
caused by not only the reduced Cx expression level but also their
aberrant cytoplasmic localization has been indicated in
carcinogenic processes (10,12).
Cx32 is often recognized as a tumor suppressor gene (13,14),
however, the paradigm that Cxs are of universal benefit by
restricting tumor progression has been challenged (10). Several reports suggest that Cxs
might facilitate tumor migration, invasion and metastasis (15–17).
Thus, the relationship between Cxs and tumor invasion, and also the
role of EMT in the process are needed to be clarified.
In the present study, we first defined the
expression of Cx32 in HCC tissue samples and its possible
relationship with clinicopathological parameters. The effect of
Cx32 on HCC invasion and metastasis was observed both in
vitro and in vivo. We also investigated whether Cx32
plays its role by regulating EMT as well as being a basis for a
possible molecular mechanism.
Materials and methods
HCC samples and cell lines
Archival normal liver (20 cases) and HCC (76 cases)
paraffin blocks were collected as described in an earlier study
(18) to determine the
relationship of Cx32 expression to clinicopathological parameters.
Another set of 34 cases of archival paraffin-embedded
formalin-fixed HCC tissues were also collected at our institution
from January 2014 to June 2015 to define the correlation between
Cx32 and other indicators. The use of the tissue samples was
approved by the Medical Ethics Committee of Bengbu Medical College
(Bengbu, China). Human normal hepatic cell line LO2, hepatocellular
carcinoma cell lines HepG2, Huh7 and SMMC-7721 were cultured at
37°C in 5% CO2 in Dulbecco's modified Eagle's medium
(DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS; HyClone Laboratories, Inc., Logan, UT, USA).
Hematoxylin and eosin (H&E) staining
and immunohistochemistry (IHC)
H&E and IHC staining was performed as previously
described (18). The Cx32
immunoreactivity was performed using a combined scoring system
based on the fraction of positive tumor cells and the predominant
staining intensity in the tumors as described by Regidor et
al (19). The cell membranous
staining of E-cadherin was also evaluated semi-quantitatively and
the tumors were divided into two groups: i) preserved pattern: ≥75%
of tumor cells showed equivalent membranous staining to adjacent
normal bile duct epithelium; and ii) reduced pattern: <75% of
tumor cells showed membranous staining (20). For Snail and β-catenin, nuclear
staining was considered as positive if at least one tumor cell had
a stained nucleus. The primary antibodies and dilutions used were
as follows: Cx32 (1:100; Sigma-Aldrich, St. Louis, MO, USA);
E-cadherin (1:50; Abcam, Cambridge, MA, USA); Snail (1:150; Abcam);
and β-catenin (1:200; Santa Cruz Biotechnology, Santa Cruz, CA,
USA).
Expression plasmids and gene
silencing
The pEX-2 plasmids containing the full-length cDNA
of human Cx32 (Gene ID: 2705) and Snail (Gene ID: 6615), were
purchased from Suzhou GenePharma Co., Ltd. (Suzhou, China). Empty
plasmid pEX-2 was used as a negative control. Cx32 and Snail siRNA
fragments were also synthesized and supplied by GenePharma. The
specific siRNA sequences are listed in Table I. Transfection reagent was
Lipofectamine™ 2000 (Invitrogen) and experiments were conducted
strictly according to the instructions.
 | Table IsiRNAs targeting specific genes. |
Table I
siRNAs targeting specific genes.
| Gene | Sequence
|
|---|
| Sense (5′-3′) | Antisense
(5′-3′) |
|---|
| Cx32 siRNA1 |
GCUCCCUGAAAGACAUACUTT |
AGUAUGUCUUUCAGGGAGCTT |
| Cx32 siRNA2 |
GCCGUCUUCAUGUAUGUCUTT |
AGACAUACAUGAAGACGGCTT |
| Cx32 siRNA3 |
GCAACACAUAGAGAAGAAATT |
UUUCUUCUCUAUGUGUUGCTT |
| Snail siRNA1 |
GCUGCAGGACUCUAAUCCATT |
UGGAUUAGAGUCCUGCAGCTT |
| Snail siRNA2 |
GCCUUCAACUGCAAAUACUTT |
AGUAUUUGCAGUUGAAGGCTT |
| Snail siRNA3 |
CAGAUGUCAAGAAGUACCATT |
UGGUACUUCUUGACAUCUGTT |
| Control |
UUCUCCGAACGUGUCACGUTT |
ACGUGACACGUUCGGAGAATT |
Establishment of stable cell line
overexpressing hCx32
Lentivirus particles expressing hCx32 and negative
control (NC) were purchased from GenePharma. Huh7 cells were seeded
in a 24-well plate until the cells grew to 40% confluency and were
then transduced with the lentivirus in the presence of polybrene (5
µg/ml; Sigma-Aldrich) using Lipofectamine 2000. After 2 days
of transfection, the cells were cultured in a selective medium
containing puromycin (0.75 µg/ml; Sigma-Aldrich). After 2–3
weeks of cultivation, the puromycin-resistant monoclonal cells were
selected and cultured under the selective pressure of 0.75
µg/ml puromycin to establish the stable overexpressed
LV5-hCx32 cell line (Huh7-hCx32) or LV5 lentiviral vector cell line
(Huh7-vec).
Cell proliferation study by MTT
assay
The Huh7 parental and transfected cells were seeded
at 3,000 cells/well into a 96-well plate and cultured for the
indicated time. MTT assay was performed as previously described
(18).
Clone formation assay
The Huh7 parental and transfected cells were
cultured in 6-well plates at 500 cells/well and grown for 10–14
days at 37°C in 5% CO2. After fixation with methanol,
colonies were stained with 0.1% crystal violet for 10 min and were
washed. The colonies was counted and imaged under a light
microscope.
Transwell invasion and wound healing
assay
Transwell invasion assay and wound healing assay
were performed as previously described (21). Invasion assay was performed using a
Transwell system (8 µm pore size; Millipore, Billerica, MA,
USA). Cells were plated onto the upper chamber with Matrigel (BD
Biosciences, San Jose, CA, USA) for 24 h. For the wound healing
assay, photographic images were taken from HCC and HCC transfected
cells at 0 and 24 h under an inverted microscope.
Western blot analysis, RNA isolation and
qRT-PCR assay
Western blot analysis, RNA isolation and qRT-PCR
assay were described in our previous study (21). The primary antibodies and dilutions
used for western blot assay were as follows: Cx32 (1:500;
Sigma-Aldrich); E-cadherin (1:1,000; Abcam); vimentin (1:1,000;
Abcam); Snail (1:1,000; Abcam); Slug (1:2,000; Abcam); Twist-1
(1:2,000; Abcam); β-catenin (1:500; Santa Cruz Biotechnology);
p-β-catenin (Y654) (1:100; Abcam); Wnt-1 (1:200; Santa Cruz
Biotechnology); and β-actin (1:500; Santa Cruz Biotechnology). The
primers for qRT-PCR assay are listed in Table II. Gene and protein expression
levels were normalized to those of internal controls (β-actin).
 | Table IIThe primers used for qRT-PCR
analysis. |
Table II
The primers used for qRT-PCR
analysis.
| Gene | Sequence
| Product size
(bp) |
|---|
| Sense (5′-3′) | Antisense
(5′-3′) |
|---|
| Cx32 |
GCGTGAACCGGCATTCTA |
CCCTCAAGCCGTAGCATTT | 295 |
| Snail |
CGGAAGCCTAACTACAGCGA |
GGACAGAGTCCCAGATGAGC | 151 |
| β-actin |
TCCTCCTGAGCGCAAGTACTC |
GCATTTGCGGTGGACGAT | 130 |
Immunofluorescence
The experimental procedure was performed according
to our previous report (18). The
concentrations of primary antibodies were used all at 1:200
(diluted with 2% BSA). Alexa 488- or 568-conjugated secondary
anti-body was added for 1 h in the dark at room temperature. The
cells were examined and photographed under a fluorescence
microscope (Olympus).
In vivo metastasis analysis
Male BALB/c nu/nu nude mice (5–6-week old) of SPF
level were purchased from the Animal Center of the Chinese Academy
of Medical Sciences (Shanghai, China). Mice were first randomized
into two groups, and the Huh7-hCx32 cells and Huh7-vec cells
(1×108) were then respectively inoculated subcutaneously
into the right side of the backs of mice to imitate tumor
metastasis (n=8 for each group). The mice were sacrificed after 8
weeks of implantation, and the tumors, livers and bilateral lungs
were removed and embedded in paraffin for pathological examination.
All animal experiments were approved by the Animal Care and Use
Committee of Bengbu Medical College (Bengbu, China).
Statistical analysis
Results were analyzed with SPSS version 19.0
software (SPSS, Inc., Chicago, IL, USA). Differences between the
groups are illustrated in Table
III and evaluated by χ2 test. Correlation analysis
was performed using the Spearman analysis. Numerical data were
presented as means ± SEM and compared with unpaired Student's
t-test. Differences with P<0.05 were considered significant.
 | Table IIIRelationship between Cx32 expression
and clinicopathological parameters of 76 HCC samples. |
Table III
Relationship between Cx32 expression
and clinicopathological parameters of 76 HCC samples.
| Variables | n | Cx32
| χ2
value | P-value |
|---|
| − | + |
|---|
| Age (years) | | | | | |
| <60 | 60 | 33 | 27 | 0.289 | 0.778 |
| ≥60 | 16 | 10 | 6 | | |
| Sex | | | | | |
| Male | 61 | 34 | 27 | 0.089 | 1.000 |
| Female | 15 | 9 | 6 | | |
| Tumor size
(cm) | | | | | |
| ≤5 | 43 | 22 | 21 | 1.182 | 0.352 |
| >5 | 33 | 21 | 12 | | |
| Edmondson type | | | | | |
| I–II | 48 | 22 | 26 | 6.124 | 0.017a |
| III–IV | 28 | 21 | 7 | | |
| TNM stage | | | | | |
| I–II | 54 | 30 | 24 | 0.080 | 0.805 |
| III–IV | 22 | 13 | 9 | | |
| Hepatopathy
background | | | | | |
| Present | 60 | 35 | 25 | 0.357 | 0.581 |
| Absent | 16 | 8 | 8 | | |
| Lymph node
metastasis | | | | | |
| Negative | 62 | 31 | 31 | 5.930 | 0.018a |
| Positive | 14 | 12 | 2 | | |
| Intrahepatic
vascular embolism | | | | | |
| Present | 20 | 12 | 8 | 0.129 | 0.796 |
| Absent | 56 | 31 | 25 | | |
Results
Expression of Cx32 in HCC and its
clinical significance
Our previous study found that compared with normal
liver tissue, the expression of Cx32 in HCC tissue is
downregulated, and the positively expressed protein shows
significant ectopic expression from the cell membrane to the
cytoplasm (18). We further
analyzed its relationship to clinicopathological characteristics
and found that the expression of Cx32 was not correlated with age,
sex, tumor size, TNM stage, background of liver disease, or
vascular embolus (all P>0.05), but negatively correlated with
histological grade and lymph node metastasis (all P<0.05)
(Table III).
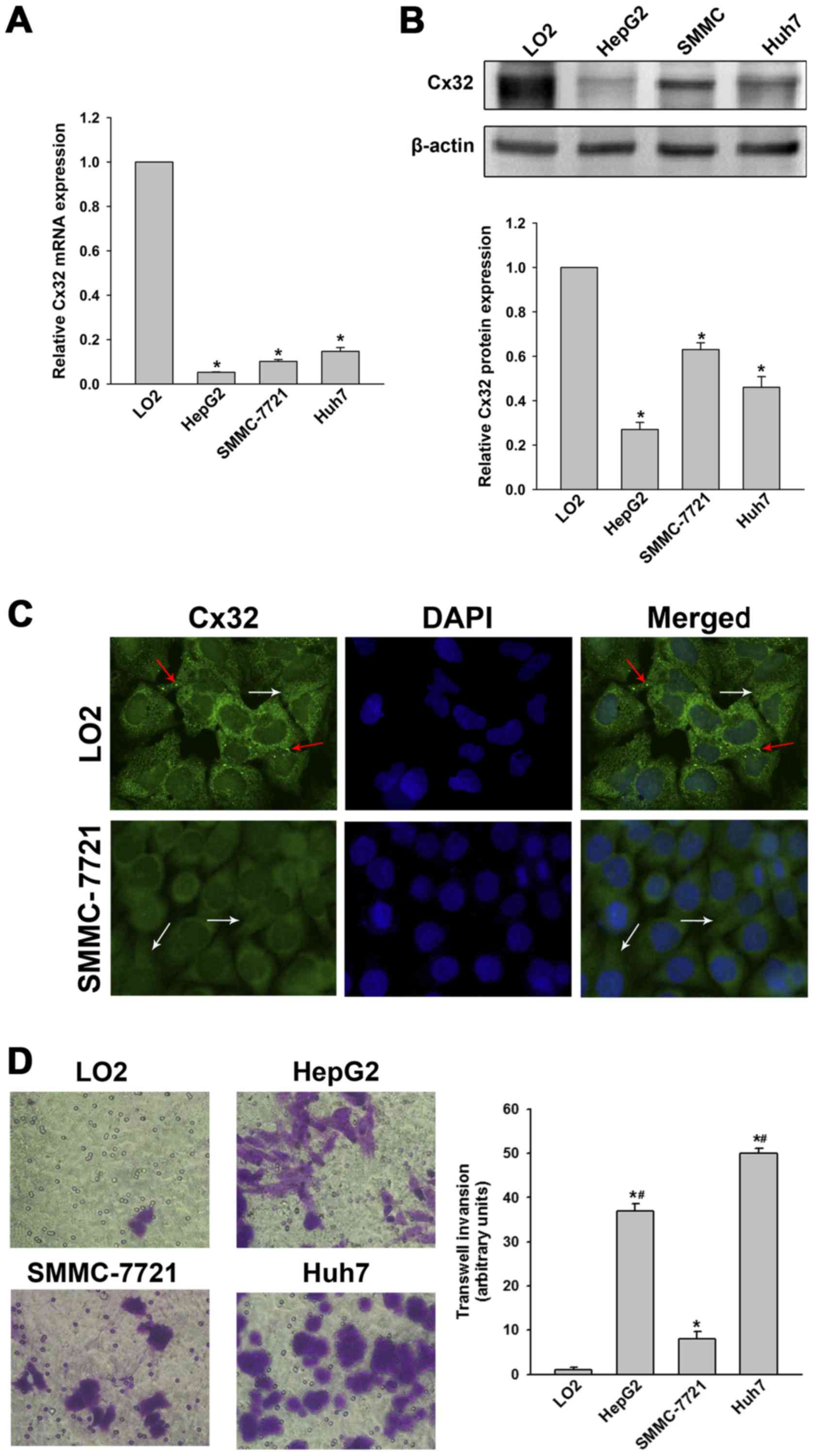
To verify the histological results, Cx32 expression
in three HCC cell lines (HepG2, SMMC-7721 and Huh7) and the normal
hepatic cell line (LO2) were examined. Results showed that Cx32 at
mRNA and protein levels in HCC cells were significantly decreased
compared with the LO2 cells (Fig. 1A
and B). Immunofluorescence assay further demonstrated that Cx32
was expressed in membrane and cytoplasm of LO2 cells, while Cx32
mainly located in the cytoplasm in SMMC-7721 cells (Fig. 1C). Thus, we confirmed that Cx32 was
downregulated as well as ectopically expressed during
hepatocarcinogenesis.
Cx32 negatively regulates HCC migration
and invasion in vitro and metastases in vivo
Histological results suggested a potential link
between Cx32 and HCC invasive and metastatic capacities, therefore,
relevant in vitro and in vivo experiments were
conducted. As shown in Fig. 1D,
LO2 cells had the highest expression of Cx32 but did not show
significant invasion ability. SMMC-7721 cells had a higher
expression of Cx32 than HepG2 and the HuH-7 cells, but the invasive
potential was significantly lower than that of the latter two HCC
cell lines. To investigate whether Cx32 inhibits malignant
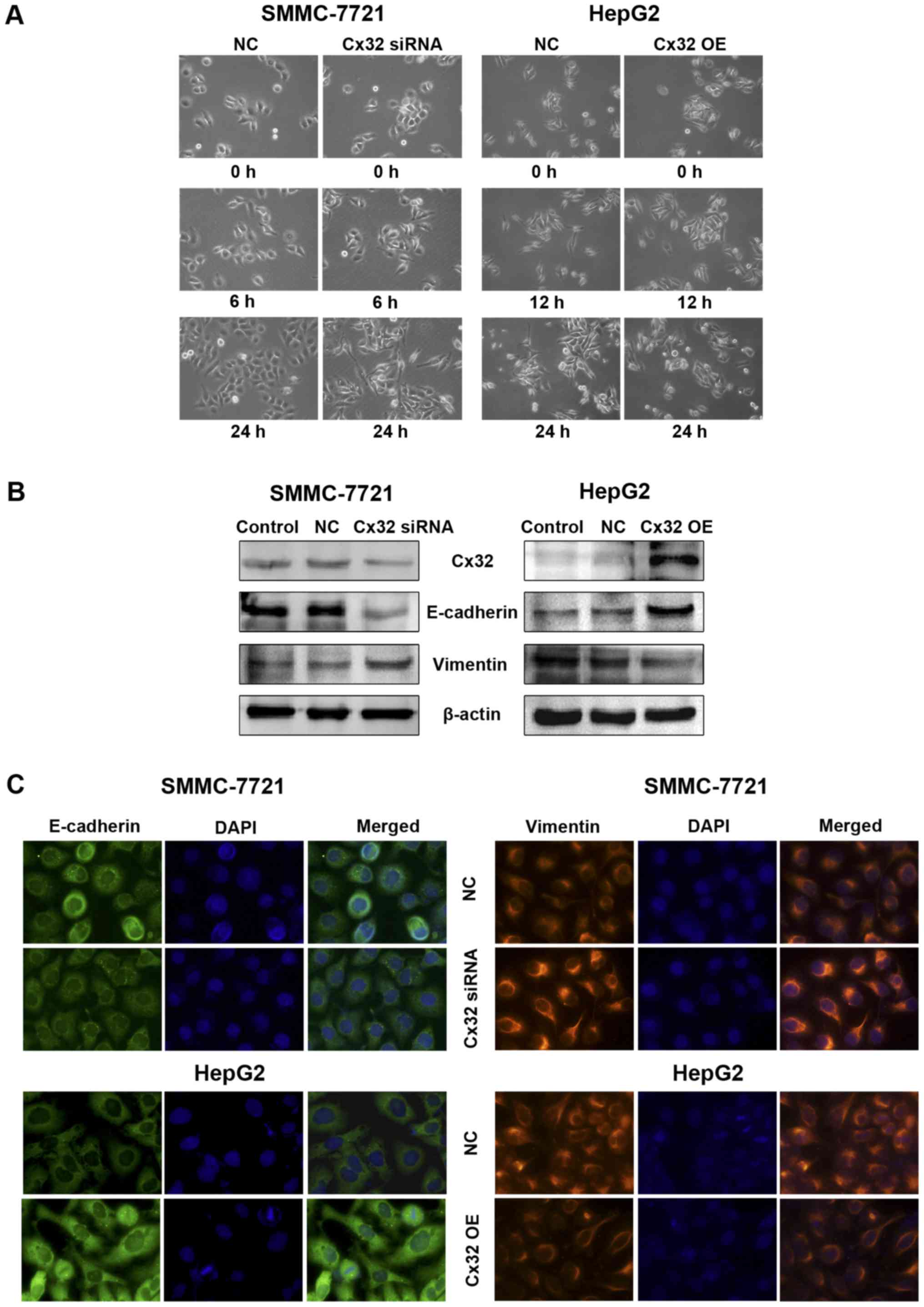
phenotype of HCC cells, we first established SMMC-7721 cells by
silencing the Cx32 expression by siRNA. On the contrary, HepG2
cells were transfected with Cx32 cDNA to upregulate Cx32
expression. Western blot analysis showed that the expression of
Cx32 was significant downregulated in SMMC-7721 cells with siRNA1
exhibiting the most inhibition, while the expression of Cx32 was
significantly upregulated in HepG2 cells following transfection
with Cx32 cDNA (Fig. 2A).
Immunofluorescence also confirmed the successful establishment of
Cx32 expression-regulated HCC cell models (Fig. 2B). The invasion ability of
SMMC-7721 cells was significantly enhanced by Cx32 downregulation,
and the invasive ability of HepG2 cells was significantly decreased
by Cx32 upregulation (Fig. 2C).
Similar results were also shown in wound healing assay (Fig. 2D).
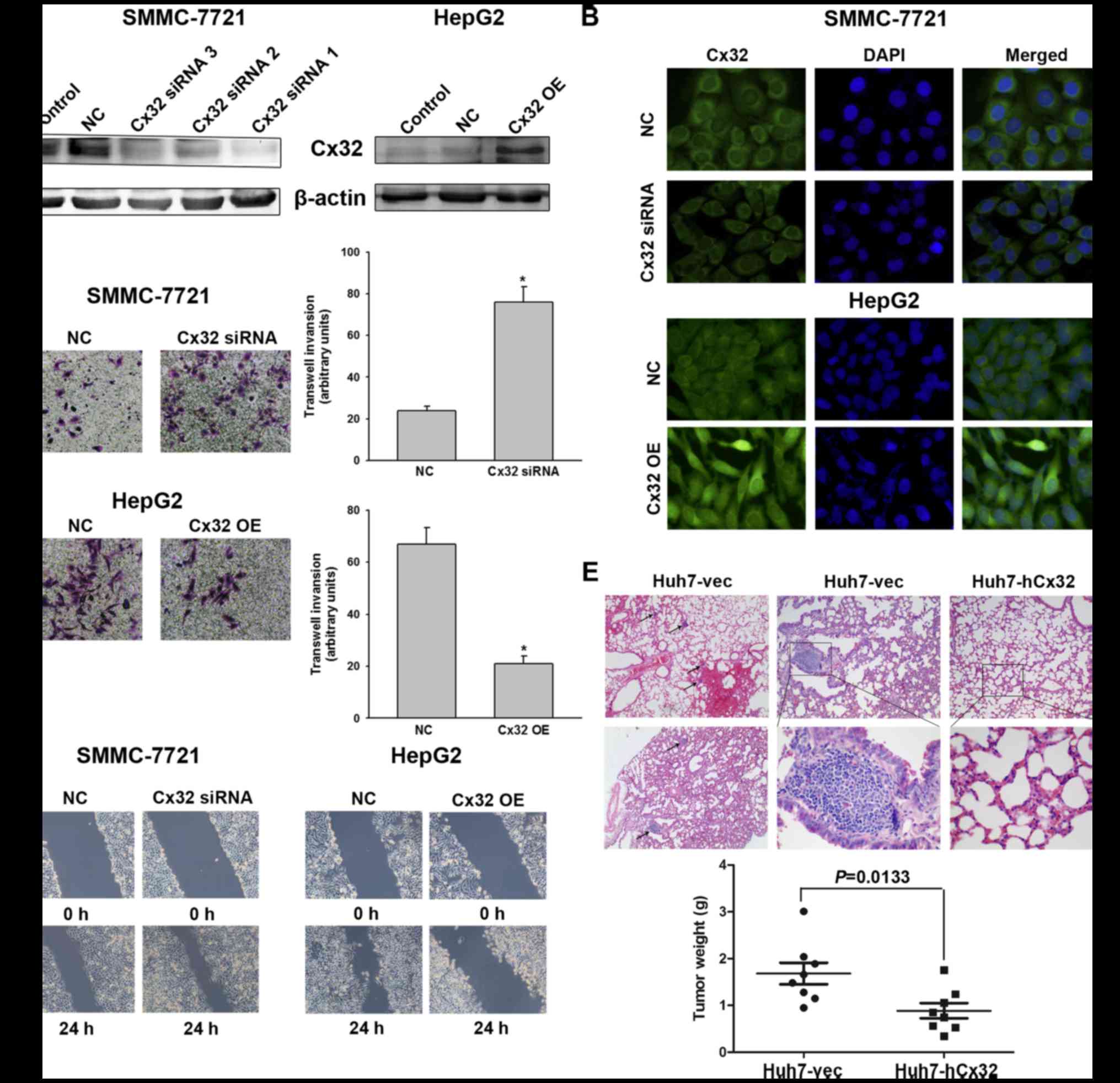 | Figure 2Cx32 inhibits HCC cell migration and
invasion in vitro and tumor metastases in vivo. (A)
Left panel, western blot analysis was conducted to detect the
inhibitory efficacy of Cx32 siRNA in SMMC-7721 cells. Right panel,
western blot analysis was performed to confirm an overexpression of
Cx32 in HepG2 cells following transfection by pEX-2/hCx32. (B)
Fluorescence images showed decreased Cx32 expression in SMMC-7721
cells transfected with Cx32 siRNA, and enhanced Cx32 expression in
HepG2 cells with Cx32 cDNA transfection, by the immunofluorescence
assay (original magnification, ×400). (C) Transwell invasion assay
was conducted to measure the invasive capacity of Cx32
downregulated SMMC-7721 and Cx32 overexpressed HepG2 cells
(original magnification, ×200). (D) Wound healing assay was
performed to investigate the migratory potential of Cx32
downregulated SMMC-7721 and Cx32 overexpressed HepG2 cells
(original magnification, ×100). (E) Upper panel, the pulmonary
metastatic nodules with H&E staining were observed under a
microscope (original magnification, ×40, ×100 and ×400,
respectively). Blank arrows were used to indicate multiple lung
metastases. Lower panel, the mean tumor weight of different groups.
NC, negative control; OE, overexpression. Data represent the mean ±
SEM of three independent experiments. *P<0.01 vs.
NC. |
To further explore whether Cx32 could inhibit the
HCC metastatic potential in vivo, Huh7-hCx32 and Huh7-vec
cells were transplanted into BALB/c nude mice by subcutaneous
implantation as described in Materials and methods. Consistent with
previous reports that Cx32 inhibited hepatocellular proliferation
(22,23), our in vitro experiments
showed that cell growth and proliferation of Huh7-hCx32 were
significantly slower than those of Huh7-vec cells (data not shown).
In vivo experiments showed the mice in both groups developed
tumors, however, the Huh7-hCx32 tumors were significantly smaller
than the Huh7-vec tumors. More importantly, 6 out of 8 mice in the
Huh7-vec group developed lung metastasis, while no metastasis was
observed in the Huh-hCx32 group (Fig.
2E).
Cx32 affects EMT and MET process in HCC
cells
Since the migration and invasion of tumor cells are
closely related to EMT, we then tested whether Cx32 could affect
EMT of HCC cells. Compared with the NC group, Cx32 downregulation
in SMMC-7721 cells led to apparent changes in the morphology
compatible with EMT, which included elongated, spindle-shaped
morphology, pseudopodia formation and increased cell scattering. In
contrast, HepG2 cells overexpressing Cx32 became more rounded and
showed no/decreased filamentous or lamellipodia and increased
intercellular connectivity (Fig.
3A). With the downregulation of Cx32, a decreased expression of
E-cadherin and an increased expression of vimentin were detected in
the SMMC-7721 cells. For the HepG2 cells transfected with Cx32
cDNA, the EMT markers showed opposite changes (Fig. 3B). Immunofluorescence assay further
confirmed the changes of expression of E-cadherin and vimentin by
Cx32 (Fig. 3C). These results
suggest that downregulation of Cx32 in HCC accelerated cell
migration and invasion accompanied by induction of EMT.
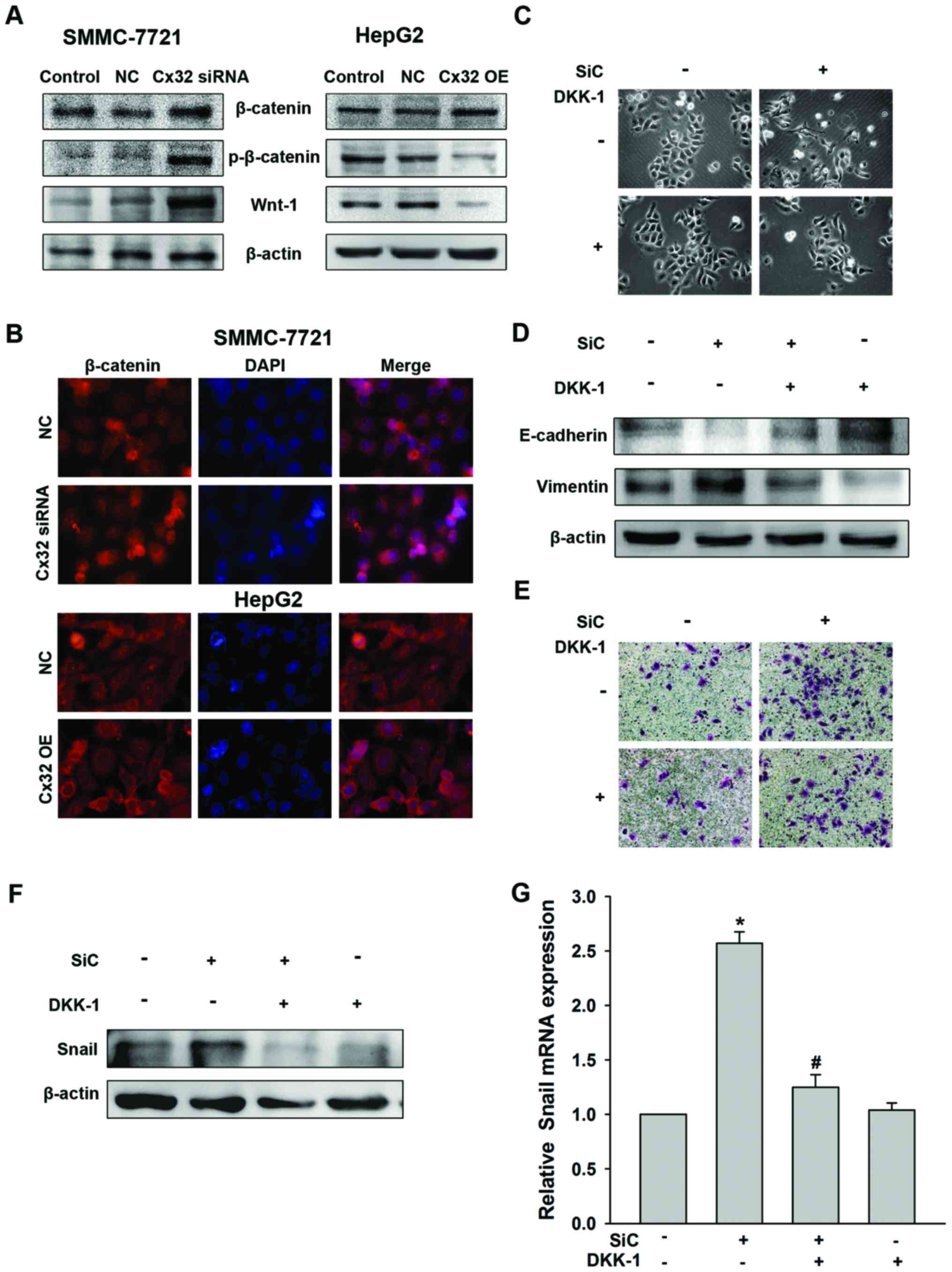
Snail mediates Cx32-regulated EMT in HCC
cells
In order to further clarify the molecular mechanism
of EMT regulated by Cx32, we subsequently investigated the effect
of Cx32 on EMT-related transcription factors Snail, Slug and
Twist-1. As shown in Fig. 4A, Cx32
downregulation in the SMMC-7721 cells led to a significant increase
of Snail expression, but not of Slug and Twist-1. Whereas,
overexpression of Cx32 in HepG2 cells resulted in a significant
decrease of Snail with no alteration in Slug or Twist-1 expression.
Immunofluorescence assay further demonstrated that Cx32 negatively
regulated Snail expression in both HCC cell lines (Fig. 4B). To assess whether the expression
change of Snail was due to transcriptional regulation, Snail mRNA
level was evaluated by qRT-PCR analysis. As shown in Fig. 4C, the expression of Snail mRNA was
also negatively regulated by Cx32.
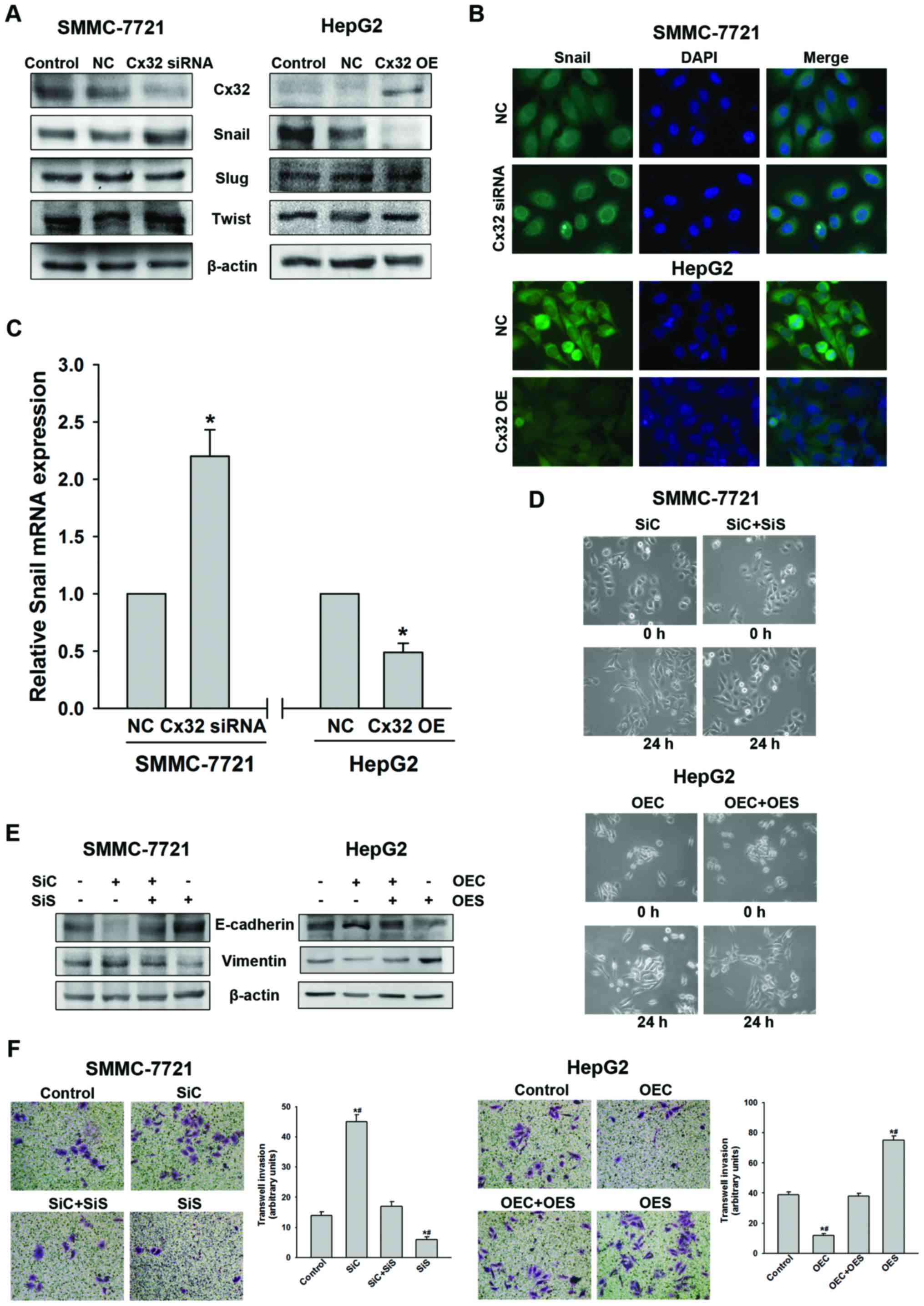 | Figure 4Cx32 exerts its effect through
negative regulation of Snail expression. (A) Knockdown of Cx32
increased Snail but not Slug or Twist-1 expression in SMMC-7721
cells, while overexpression of Cx32 decreased Snail but not Slug or
Twist-1 expression in HepG2 cells, as evidenced by western blot
analysis. (B) Expression of Snail was negatively regulated by Cx32
as confirmed by immunofluorescence assay (original magnification,
×400). (C) Snail mRNA was detected by real-time PCR in different
groups. (D) Cell morphological changes of SMMC-7721 and HepG2 cells
in the presence of both Cx32 and Snail modulation (original
magnification, ×200). (E) Silencing of Snail in SMMC-7721 cells
rescued Cx32 knockdown-induced EMT-related protein changes while
upregulation of Snail in HepG2 cells abrogated Cx32
overexpression-induced EMT-related proteins modulation. (F)
Invasive capacity of SMMC-7721 and HepG2 cells in the presence of
both Cx32 and Snail modulation (original magnification, ×200). NC,
negative control; OE, overexpression; SiC, siRNA of Cx32; SiS,
siRNA of Snail; OEC, overexpression of Cx32; OES, overexpression of
Snail. Data represent the mean ± SEM of three to four independent
experiments. *P<0.01 vs. NC (C);
*P<0.01 vs. control, #P<0.01 vs.
SiC+SiS (or OEC+OES) (F). |
To further investigate whether Snail mediates the
Cx32-induced EMT, Snail was knocked down in SMMC-7721 cells using
siRNA and overexpressed in HepG2 cells using cDNA. Knockdown of
Snail resulted in reversal of the Cx32 inhibition induced
EMT-associated phenotype changes including EMT-like morphology,
downregulation of E-cadherin, upregulation of vimentin, and an
enhanced ability of cell invasion in the SMMC-7721 cells (Fig. 4D–F). Similarly, overexpression of
Snail can counteract the biological effects of upregulation of Cx32
in HepG2 cells (Fig. 4D–F). These
data indicated that Cx32 regulated EMT-associated invasion in HCC
cells by affecting the expression of Snail.
Wnt signaling is involved in
Snail-mediated EMT in HCC cells
Considering the general function of Cx32 acting as a
membrane protein (10), we
hypothesized that Cx32 was more likely to regulate Snail expression
indirectly. β-catenin is an important epithelial marker which links
E-cadherin and α-catenin to the cytoskeleton to form a complex
maintaining epithelial polarity and intercellular adhesion and is
associated closely to EMT (24).
As shown in Fig. 5A, there was no
significant change in the total protein expression of β-catenin by
Cx32 regulation, in SMMC-7721 or HepG2 cells. However, expression
of phosphorylated β-catenin (Y654), a status indicating
transcriptional activity and nuclear translocation of β-catenin
(25), was increased in Cx32
downregulated SMMC-7721 cells and decreased in Cx32 overexpressed
HepG2 cells. A consistent change was also shown for Wnt-1 (Fig. 5A). The results were confirmed by
immunofluorescence assay by showing a change of nuclear
translocation of β-catenin (Fig.
5B).
In an effort to establish that Snail-mediated EMT in
HCC cells was due to the Wnt signaling pathway activation, the
effect of DKK-1, the inhibitor of the Wnt signaling pathway
(26), was determined. As
expected, DKK-1 reversed the EMT phenotype changes and the
enhancement of cell invasion induced by Cx32 downregulation in
SMMC-7721 cells (Fig. 5C–E).
Moreover, DKK-1 addition abolished the upregulation of Snail
induced by Cx32 downregulation both in protein and mRNA levels
(Fig. 5F). Taken together, our
data indicated that downregulation of Cx32 upregulates Snail
expression and promotes EMT through activation of the Wnt signaling
pathway in HCC cells.
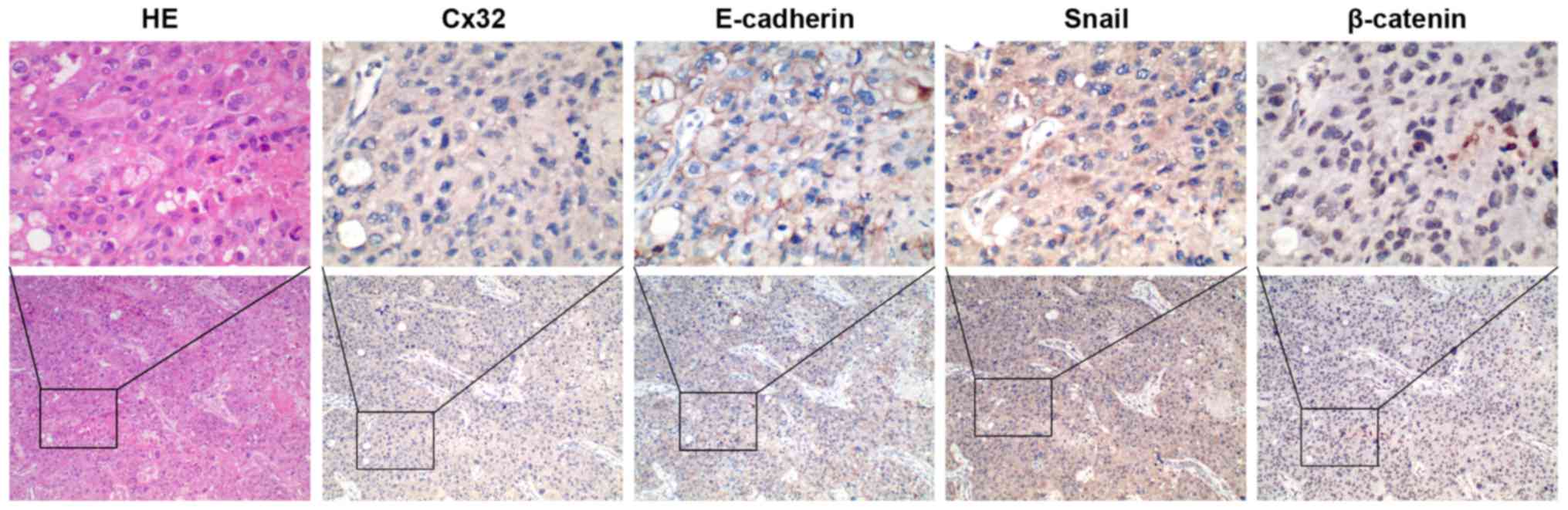
Cx32 is associated with EMT markers in
HCC tissues
To obtain clinical evidence of the correlation
between Cx32, E-cadherin, β-catenin and Snail, we tested the
expression of these proteins in an additional set of 34 HCC tissues
by IHC staining. Cx32 was identified in 14 of 34 HCC tissues and
the positive particles were weakly stained and mainly located in
the cytoplasm. A significant reduction or loss of E-cadherin
expression was detected in 18 cases. Snail stained both in
cytoplasm and nucleus and was recorded as positive in the nucleus
in 21 cases. For β-catenin staining, 41.18% (14/34) of HCC cases
was positive for nuclear accumulation with concurrent cytoplasmic
staining, while all the other samples showed membranous
localization. When further analyzed in comparison with expression
of EMT markers, Cx32 expression showed a strong correlation with
expression of the loss or reduction of E-cadherin (r=0.528,
P=0.001), nuclear Snail (r=−0.448, P=0.008), and nuclear
accumulation of β-catenin (r=−0.457, P=0.007) (Table IV, Fig. 6).
 | Table IVThe correlation between Cx32 and EMT
markers (E-cadherin, Snail and β-catenin) staining in an additional
set of 34 HCC samples. |
Table IV
The correlation between Cx32 and EMT
markers (E-cadherin, Snail and β-catenin) staining in an additional
set of 34 HCC samples.
| E-cadherin
| r | P-value | Snail (nuclear)
| r | P-value | β-catenin (nuclear)
| r | P-value |
|---|
| Loss/reduction | Normal | − | + | − | + |
|---|
| Cx32 | − | 15 | 5 | 0.528 | 0.001 | 4 | 16 | −0.448 | 0.008 | 8 | 12 | −0.457 | 0.007 |
| + | 3 | 11 | | | 9 | 5 | | | 12 | 2 | | |
Discussion
While it has been established that Cx and
Cx-mediated GJ suppress tumor development during
hepatocarcinogenesis (11), the
role of Cxs in tumor progression, including invasion and metastasis
is still controversial. Zhao et al (23) demonstrated the exogenously
overexpressed Cx32 protein suppressed the metastatic ability of
human HCC cells both in vitro and in vivo. The
anti-invasive effect of Cx32 has also been reported in other type
of tumors such as kidney (13) and
lung (14) cancer, indicating Cx32
as a tumor suppressor gene. However, accumulating evidence has
shown that Cx26 (15) and Cx32
(16) are highly expressed in
lymph node metastases of patients with lung or breast cancer and
correlated with a poor prognosis. Breast cancer and melanoma cells
utilize Cxs to initiate brain metastasis by enhancing vascular
invasion (17). One reason for the
discrepancy in the role of Cxs in tumor progression is the fact
that cancer involves multiple stages from onset to progression and
metastasis and Cxs cannot provide benefit at all of these stages
(10).
The present study observed a negative relationship
between Cx32 expression and lymph node metastasis from clinical
data, and further confirmed the suppressive role of Cx32 in HCC
invasion and metastasis both in vitro and in vivo.
Considering the tumorigenicity and Cx32 expression pattern among
the different cell lines in vitro, we performed in
vivo metastasis analysis using Huh7 cells overexpressing Cx32.
However, using Huh7 Tet-off Cx32 cells, Li et al (27) obtained the opposite result by
showing that overexpression of cytoplasmic Cx32 protein induced
metastasis in vivo. Regarding the difference between these
two results, it was speculated that it might be related to the
different Cx32 inducible system and cell culture condition. Cx32
was induced to localize only in cytoplasm in the latter system,
however, in the present study, besides the increase in amount of
Cx32, the possible more formation of Cx32-mediated GJ that may
contribute to the suppression of metastasis could also play a role.
Moreover, the inhibitory role of Cx32 was also observed in the
other two HCC cell lines, including the SMMC-7721 cells and the
HepG2 cells, which is in line with the recently reported results
from Zhao et al (23). Our
findings are highly favorable from a therapeutic perspective,
because it is difficult to design strategies that could
specifically upregulate Cx32 in a primary HCC tumor while
decreasing off-target adverse effect due to the cytoplasmic
localization. Thus, the data support the notion of utilizing Cx32
as a therapeutic target in HCC treatment.
Clarification of the molecular mechanism of Cx32 in
inhibiting invasion and metastasis can help to fully understand the
biological effects of this gene in HCC. EMT is an important basis
for obtaining the malignant phenotype of hepatocytes (4). Moreover, Cx-mediated GJ has recently
been proposed to serve as an intercellular glue to suppress EMT and
cancer metastasis (9), we thus
explored the role of EMT in Cx32-mediated function. Upon the
downregulation of Cx32 expression, SMMC-7721 cells gained
characteristics of EMT including apparent changes in morphology,
downregulation of E-cadherin, upregulation of vimentin, enhancement
of cell migration and invasion ability. In contrast, after
upregulating Cx32 in HepG2 cells, the cells exhibited opposite
biological behavior. The ability of Cx43 to inhibit EMT has been
demonstrated by Yu et al (8), thus, it is not surprising that Cx32,
as an another important member of the Cx family, can also play a
role in EMT process. In the present study, we identified EMT as
novel target for Cx32 action, and for the first time provided a
mechanistic link between Cx32 anti-metastatic activity and EMT
modulation.
Molecular switches for the EMT program are multiple,
in which transcription factors play an important regulatory role
(28). We subsequently found that
Cx32 negatively regulate the expression of Snail but not Slug and
Twist-1, and Snail could counteract Cx32-mediated in vitro
biological effects. Snail is a zinc-finger transcriptional
repressor, which has been identified as a potential oncogene in
various tumors (29), capable of
triggering EMT and promoting metastasis in HCC (5,6). In
the present study, the positive rate of nuclear Snail in clinical
specimens of HCC was up to 61.76% (21/34), and was negatively
correlated with the expression of Cx32 and E-cadherin. The data
were consistent with a previous report showing Snail, rather than
Slug, downregulates E-cadherin expression and promotes human HCC
invasion (20). Thus, these data
clearly demonstrated that downregulation of Cx32 facilitates HCC
invasion and metastasis through Snail-mediated EMT.
Wnt signaling is shown to activate the
transcription, protein stability, as well as nuclear localization
of Snail through the inhibition of GSK3β (30,31).
Furthermore, during hepatocarcinogenesis, it has been indicated
that the Wnt/β-catenin signaling is abnormally activated (32), and GSK-3β inactivation is
associated with low expression of Cx32 (33). Thus, there is a possible link
between Cx32, Wnt signaling and Snail. We then explored whether
Wnt/β-catenin pathway was involved in the Cx32-mediated Snail
regulation. Together with Wnt-1, phosphorylated β-catenin (Y654)
expression was negatively regulated by Cx32 in HCC cells, but the
total β-catenin protein level was not changed significantly.
Inhibition of canonical Wnt pathway attenuated the upregulation of
Snail and induction of EMT response to Cx32 downregulation.
Moreover, nuclear accumulation of β-catenin in HCC tissues was
directly correlated with the reduced Cx32. These results provide
molecular and clinical evidence to support the fact that Cx32
regulates Snail expression through the Wnt/β-catenin pathway in
HCC.
In conclusion, the present study provides new
insight into the role of Cx32 in EMT and metastasis of HCC. We
demonstrated that downregulation of Cx32 upregulates Snail
expression and induces EMT in HCC cells through activation of
Wnt/β-catenin pathway. Thus, our data not only provide further
evidence of targeted increase of Cx32 as a beneficial strategy to
control HCC progression and metastasis, but also reveals the
underlying mechanism of a novel Cx32/β-catenin/Snail pathway as a
promising new molecular target against advanced HCC.
Abbreviations:
|
Cx32
|
connexin32
|
|
EMT
|
epithelial-mesenchymal transition
|
|
GJ
|
gap junction
|
|
HCC
|
hepatocellular carcinoma
|
|
H&E
|
hematoxylin and eosin
|
|
IHC
|
immunohistochemistry
|
|
NC
|
negative control
|
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81402514 to Y.Y. and
81572458 to Q.W.), the grant from the Natural Science Foundation of
Anhui Province (no. 1408085QH166 to Y.Y.), and the Natural Science
Research key Project of Education Office of Anhui Province (no.
KJ2014A152 to Q.W.). We thank the Biochemical and Medical
Engineering Research Center of Anhui Province and the Scientific
Research Platform of Bengbu Medical College for instrument
support.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahbari NN, Mehrabi A, Mollberg NM, Müller
SA, Koch M, Büchler MW and Weitz J: Hepatocellular carcinoma:
Current management and perspectives for the future. Ann Surg.
253:453–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cauchy F, Soubrane O and Belghiti J: Liver
resection for HCC: Patient's selection and controversial scenarios.
Best Pract Res Clin Gastroenterol. 28:881–896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cervantes-Arias A, Pang LY and Argyle DJ:
Epithelial-mesenchymal transition as a fundamental mechanism
underlying the cancer phenotype. Vet Comp Oncol. 11:169–184. 2013.
View Article : Google Scholar
|
|
5
|
Zhou ZJ, Dai Z, Zhou SL, Hu ZQ, Chen Q,
Zhao YM, Shi YH, Gao Q, Wu WZ, Qiu SJ, et al: HNRNPAB induces
epithelial-mesenchymal transition and promotes metastasis of
hepatocellular carcinoma by transcriptionally activating SNAIL.
Cancer Res. 74:2750–2762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu XT, Dai Z, Song K, Zhang ZJ, Zhou ZJ,
Zhou SL, Zhao YM, Xiao YS, Sun QM, Ding ZB, et al:
Macrophage-secreted IL-8 induces epithelial-mesenchymal transition
in hepatocellular carcinoma cells by activating the
JAK2/STAT3/Snail pathway. Int J Oncol. 46:587–596. 2015.
|
|
7
|
Wu Y, Liu H, Weng H, Zhang X, Li P, Fan
CL, Li B, Dong PL, Li L, Dooley S, et al: Glypican-3 promotes
epithelial-mesenchymal transition of hepatocellular carcinoma cells
through ERK signaling pathway. Int J Oncol. 46:1275–1285.
2015.PubMed/NCBI
|
|
8
|
Yu M, Zhang C, Li L, Dong S, Zhang N and
Tong X: Cx43 reverses the resistance of A549 lung adenocarcinoma
cells to cisplatin by inhibiting EMT. Oncol Rep. 31:2751–2758.
2014.PubMed/NCBI
|
|
9
|
Mao XY, Li QQ, Gao YF, Zhou HH, Liu ZQ and
Jin WL: Gap junction as an intercellular glue: Emerging roles in
cancer EMT and metastasis. Cancer Lett. 381:133–137. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Naus CC and Laird DW: Implications and
challenges of connexin connections to cancer. Nat Rev Cancer.
10:435–441. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vinken M, Henkens T, De Rop E, Fraczek J,
Vanhaecke T and Rogiers V: Biology and pathobiology of gap
junctional channels in hepatocytes. Hepatology. 47:1077–1088. 2008.
View Article : Google Scholar
|
|
12
|
Mesnil M, Crespin S, Avanzo JL and
Zaidan-Dagli ML: Defective gap junctional intercellular
communication in the carcinogenic process. Biochim Biophys Acta.
1719:125–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujimoto E, Sato H, Shirai S, Nagashima Y,
Fukumoto K, Hagiwara H, Negishi E, Ueno K, Omori Y, Yamasaki H, et
al: Connexin32 as a tumor suppressor gene in a metastatic renal
cell carcinoma cell line. Oncogene. 24:3684–3690. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
King TJ and Lampe PD: The gap junction
protein connexin32 is a mouse lung tumor suppressor. Cancer Res.
64:7191–7196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ito A, Koma Y, Uchino K, Okada T,
Ohbayashi C, Tsubota N and Okada M: Increased expression of
connexin 26 in the invasive component of lung squamous cell
carcinoma: Significant correlation with poor prognosis. Cancer
Lett. 234:239–248. 2006. View Article : Google Scholar
|
|
16
|
Kanczuga-Koda L, Sulkowska M, Koda M,
Rutkowski R and Sulkowski S: Increased expression of gap junction
protein-connexin 32 in lymph node metastases of human ductal breast
cancer. Folia Histochem Cytobiol. 45(Suppl 1): S175–S180. 2007.
|
|
17
|
Stoletov K, Strnadel J, Zardouzian E,
Momiyama M, Park FD, Kelber JA, Pizzo DP, Hoffman R, VandenBerg SR
and Klemke RL: Role of connexins in metastatic breast cancer and
melanoma brain colonization. J Cell Sci. 126:904–913. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Y, Zhu J, Zhang N, Zhao Y, Li WY,
Zhao FY, Ou YR, Qin SK and Wu Q: Impaired gap junctions in human
hepatocellular carcinoma limit intrinsic oxaliplatin
chemosensitivity: A key role of connexin 26. Int J Oncol.
48:703–713. 2016.
|
|
19
|
Regidor PA, Regidor M, Schindler AE and
Winterhager E: Aberrant expression pattern of gap junction
connexins in endometriotic tissues. Mol Hum Reprod. 3:375–381.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sugimachi K, Tanaka S, Kameyama T, Taguchi
K, Aishima S, Shimada M, Sugimachi K and Tsuneyoshi M:
Transcriptional repressor snail and progression of human
hepatocellular carcinoma. Clin Cancer Res. 9:2657–2664.
2003.PubMed/NCBI
|
|
21
|
Wu Q, Wang R, Yang Q, Hou X, Chen S, Hou
Y, Chen C, Yang Y, Miele L, Sarkar FH, et al: Chemoresistance to
gemcitabine in hepatoma cells induces epithelial-mesenchymal
transition and involves activation of PDGF-D pathway. Oncotarget.
4:1999–2009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edwards GO, Jondhale S, Chen T and Chipman
JK: A quantitative inverse relationship between connexin32
expression and cell proliferation in a rat hepatoma cell line.
Toxicology. 253:46–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao B, Zhao W, Wang Y, Xu Y, Xu J, Tang
K, Zhang S, Yin Z, Wu Q and Wang X: Connexin32 regulates hepatoma
cell metastasis and proliferation via the p53 and Akt pathways.
Oncotarget. 6:10116–10133. 2015. View Article : Google Scholar :
|
|
24
|
Heuberger J and Birchmeier W: Interplay of
cadherin-mediated cell adhesion and canonical Wnt signaling. Cold
Spring Harb Perspect Biol. 2:–a002915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van Veelen W, Le NH, Helvensteijn W,
Blonden L, Theeuwes M, Bakker ER, Franken PF, van Gurp L, Meijlink
F, van der Valk MA, et al: β-catenin tyrosine 654 phosphorylation
increases Wnt signalling and intestinal tumorigenesis. Gut.
60:1204–1212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aguilera O, Fraga MF, Ballestar E, Paz MF,
Herranz M, Espada J, García JM, Muñoz A, Esteller M and
González-Sancho JM: Epigenetic inactivation of the Wnt antagonist
DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene.
25:4116–4121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Q, Omori Y, Nishikawa Y, Yoshioka T,
Yamamoto Y and Enomoto K: Cytoplasmic accumulation of connexin32
protein enhances motility and metastatic ability of human hepatoma
cells in vitro and in vivo. Int J Cancer. 121:536–546. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moreno-Bueno G, Portillo F and Cano A:
Transcriptional regulation of cell polarity in EMT and cancer.
Oncogene. 27:6958–6969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of Snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim
NH, Cha SY, Ryu JK, Choi YJ, Kim J, et al: A Wnt-Axin2-GSK3beta
cascade regulates Snail1 activity in breast cancer cells. Nat Cell
Biol. 8:1398–1406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bachelder RE, Yoon SO, Franci C, de
Herreros AG and Mercurio AM: Glycogen synthase kinase-3 is an
endogenous inhibitor of Snail transcription: Implications for the
epithelial-mesenchymal transition. J Cell Biol. 168:29–33. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim M, Lee HC, Tsedensodnom O, Hartley R,
Lim YS, Yu E, Merle P and Wands JR: Functional interaction between
Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin
signaling pathway in hepatocellular carcinoma cells. J Hepatol.
48:780–791. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Plante I, Charbonneau M and Cyr DG:
Activation of the integrin-linked kinase pathway downregulates
hepatic connexin32 via nuclear Akt. Carcinogenesis. 27:1923–1929.
2006. View Article : Google Scholar : PubMed/NCBI
|