Introduction
Non-small cell lung cancer (NSCLC) is a main type of
lung cancer, which accounts for approximately 85% of all lung
cancer patients in China (1).
Despite significant improvement in surveillance and targeted
therapy, the 5-year survival rate of patients after curative
resection is very low, which is reported to be only 30–60% mainly
because of tumor metastasis (2).
Therefore, understanding the potential molecular mechanism involved
in NSCLC metastasis may contribute to improve diagnosis and
treatment of NSCLC.
MicroRNA (miRNA) is a class of small
non-protein-coding RNAs which is composed of 21–23 bases that
negatively regulate protein-coding gene expression and/or repress
mRNA translation by binding to the 3′-untranslated region (3′-UTR)
(3,4). Previous studies have reported that
miRNAs play pivotal roles in a wide range of cellular processes
such as proliferation, cycle, differentiation, apoptosis and
metastasis (5). The miRNAs have
been confirmed to function either as oncomiRs or tumor suppressors
in lung cancer (6–8). For example, miR-383 is significantly
downregulated in NSCLC cell lines and NSCLC carcinomas tissues,
which is a functional tumor suppressor in NSCLC (9). Zeng et al reported that
ectopic expression of miR-205 in NSCLC cells suppressed cellular
viability and proliferation, accelerated the cell cycle, and
promoted tumor growth of lung carcinoma xenografts in nude mice
(10). Thus, identification of
tumor-suppressive or oncogenic miRNAs may be the first step in
construction of a new treatment strategy for NSCLC.
Epithelial-mesenchymal transition (EMT) is an
intricate process by which epithelial cells lose their epithelial
characteristics and adopt a mesenchymal-like phenotype (11). Increasing evidence suggest that EMT
plays a key role in tumor progression and metastasis (12,13).
The molecular mechanisms of EMT in tumor metastasis are very
complex. Multiple molecules are known to regulate EMT, including
miRNAs (14–16). Some miRNAs (for example, the
miR-200 family and miR-145) have been shown to regulate EMT in
cancers (17–19). However, studies of the roles of
other miRNAs in the regulation of EMT are limited.
In this study, we found that upregulation of miR-92a
could promote cell invasion in vitro and tumor growth in
vivo in NSCLC. Furthermore, we demonstrated that miR-92a
promoted the phosphoinositide 3-kinase (PI3K)/AKT signaling pathway
and induced EMT by targeting PTEN. The findings in the present
study revealed that miR-92a could induce EMT phenotype via
regulating PTEN-mediated AKT pathway, influencing cancer
progression, invasion and metastasis.
Materials and methods
Cell culture and tissue samples
One human bronchial epithelial cell line 16HBE and
four NSCLC cell lines (A549, H358, SPC-A1 and H1299) were used in
this study, all cell lines were obtained from the Cell Culture
Center of the Shanghai Institute (Shanghai, China) and cultured in
DMEM (Dulbecco's minimum essential medium) containing 10% fetal
bovine serum (Gibco, Grand Island, NY, USA) at 37°C in a humidified
incubator with 5% CO2.
Fifty pairs of NSCLC tissues were obtained randomly
from patients who underwent surgical resection at Changhai
Hospital, Second Military Medical University between April 2014 and
August 2015. None of the patients received chemotherapy or
radiotherapy before surgery. All human materials were obtained with
informed consent from patients and were approved by the ethics
committees of the Second Military Medical University. The tissues
were stored at −80°C.
MicroRNA expression profile data from
Gene Expression Omnibus (GEO)
We downloaded the microRNA data (accession number:
GSE29248) from GEO databases in NCBI (http://www.ncbi.nlm.nih.gov/geo/). The microarray data
were generated using Affymetrix U133A/B and Plus 2.0 platforms.
After inter array quantile normalization, the expression levels of
50 miRNAs were visually generated as a heat map using GeneSpring
GX, version 7.3 statistical software.
Transfection
Oligonucleotides including miR-92a mimics, miR-92a
inhibitor, PTEN siRNA and their negative control (NC) were
purchased from Shanghai GenePharma Co. Ltd. A549 and H358 cells
were plated in 6-well plates and transfected with 50 nM miRNAs or
PTEN siRNA with Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA,
USA) according to the manufacturer's protocol. The coding sequences
of PTEN were amplified by PCR and inserted into pcDNA3.0 vector to
construct the PTEN over expression vector. The transfection of
plasmids was conducted using Lipofectamine 2000 (Invitrogen). All
the transfections were repeated more than three times
independently.
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
Total RNA of the cultured cells and the tissues was
extracted using TRIzol (Invitrogen) according to the manufacturer's
instructions. Total RNA from each sample was reverse-transcribed to
cDNA using the PrimeScript RT reagent kit (Takara, Tokyo, Japan).
The procedure for qRT-PCR was previously described (20). To normalize the data for
quantifcation of mRNA and miRNAs, the GAPDH and U6 (Applied
Biosystems) were used as internal standards for miRNA and mRNA,
respectively. The sequences of the PCR primers were as follows:
PTEN forward, 5′-CGGCAGCATCAAATGTTTCAG-3′ and reverse
5′-AACTGGCAGGTAGAAGGCAACTC-3′; GAPDH forward,
5′-CTCCTCCTGTTCGACAGTCAGC-3′, and reverse
5′-CCCAATACGACCAAATCCGTT-3′. miR-92a forward
5′-CTGTCCTGTTATTGAGCACTGGTCTATGG-3′ and reverse
5′-AAGACATTAGTAACCCACCCCCATTCC-3′; U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′.
Cell viability assay
The effect of miR-92a on the viability of A549 and
H358 cells was examined using the MTT assay. Briefly, A549 and H358
cells were seeded in a 96-well plate (8×103 cells/well)
in 100 µl growth medium after transfection. After 48 h
incubation at 37°C, 20 µl of MTT solution (5 mg/ml) was
added to each well, and the cells were continuously incubated for 4
h before 200 µl DMSO was added. The absorbance was read with
a microplate reader (BioTek, Winooski, VT, USA) at 570 nm according
to the manufacturer's instructions.
Apoptosis assay
The effect of miR-92a on the apoptosis of A549 and
H358 cells was examined using an Annexin V-FITC apoptosis detection
kit (Life Technologies). After 24 h post-transfection, A549 and
H358 cells were collected, centrifuged and resuspended in 100
µl binding buffer. Propidium iodide (PI) (3 µl) and
Annexin V-FITC (6 µl) were added to each 100 µl
sample and incubated for 15 min at room temperature in the dark.
The samples were analyzed on a FACScalibur flow cytometer to
determine rate of apoptosis.
Cell invasion assays
The effect of miR-92a on cell invasion was evaluated
using Transwell chamber assay (BD Biosciences, Bedford, MA, USA)
according to the manufacturer's instructions. Briefly,
5×104 A549 and H358 cells were seeded on the top chamber
coated with Matrigel after transfection. The bottom chambers were
filled with 20% FBS in DMEM. After incubated at 37°C for 48 h, the
cells adherent to the upper surface of the filter were removed
using a cotton swab. Then the migration cells were stained with
crystal violet after fixed with 4% paraformaldehyde for 20 min, and
the migrated cells were counted by averaging the total number of
cells from triplicate determinations.
Wound healing assay
The effect of miR-92a on cell migration was
evaluated with wound healing assays. Cells were plated in 6-well
plates at 8×105 cells/well, and after 48 h of
transfection, the cell monolayer was scraped using a 10 µl
micropipette tip, and then cultured with serum-free medium for 24
h. The gap distances of migrating cells were calculated from
photomicrographs as previously described (21).
Western blotting
Total cellular proteins were lysed in RIPA buffer in
the presence of proteinase inhibitor (Sigma, St. Louis, MO, USA).
Concentrations of total cellular protein were determined using a
BCA assay kit (Pierce, Rockford, IL, USA). Protein samples (25
µg) were separated by 8% SDS-PAGE and then transferred onto
polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA,
USA). The membranes were incubated with primary antibodies at 4°C
overnight using the following concentrations of PTEN, β-catenin,
Vimentin, N-cadherin, E-cadherin, AKT, phospho-mTOR and mTOR
(1:1000; Cell Signaling Technology, Danvers, MA, USA), phospho-AKT
(Ser473) (1:500; Cell Signaling Technology) and anti-β-actin
(1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by
horseradish peroxidase-conjugated secondary antibody (anti-rabbit,
1:2000, Cell Signaling Technology). Anti-β-actin antibody was used
as an internal control. The detected protein signals were
visualized using the ECL method (22).
Luciferase assays
The wild-type PTEN 3′-UTR and mutated PTEN 3′-UTR
were amplified and cloned downstream of the luciferase gene in a
pGL3 reporter plasmid (Promega). The constructed vectors were named
as wt-PTEN-PGL3 and mut-PTEN-PGL3, respectively. For the luciferase
reporter assays, HEK293 cells were cultured in 24-well plates, and
each well was co-transfected with wt (mut)-PTEN-PGL3 and
miR-control, miR-195 mimics or inhibitor and cultured for 48 h. The
luciferase activities were measured using the Dual-Luciferase
Reporter Assay System (Promega, Madison, WI, USA), according to the
manufacturer's protocol.
Tumor xenograft animal model
A549 cells transfected with miR-92a inhibitor or the
inhibitor NC and H358 cells transfected with miR-92a mimic or mimic
NC were subcutaneously injected into four-week-old male athymic
nude mice at 3×106 cells in 0.2 ml PBS per mouse, 5 mice
per group. After 5 weeks, mice were sacrificed and tumor weight was
detected. All experiments were performed in the Animal Institute of
Second Military Medical University and approved by the Medical
Experimental Animal Care Commission of Second Military Medical
University.
Statistical analysis
Statistical analysis was performed with the GraphPad
Prism 5.0 software. Data are reported as mean ± SD. Statistical
significance was calculated by Student's t-test among different
groups. Pearson's or Spearman's analysis was used in correlation
analysis. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
miR-92a is upregulated in both NSCLC
cells and clinical specimens
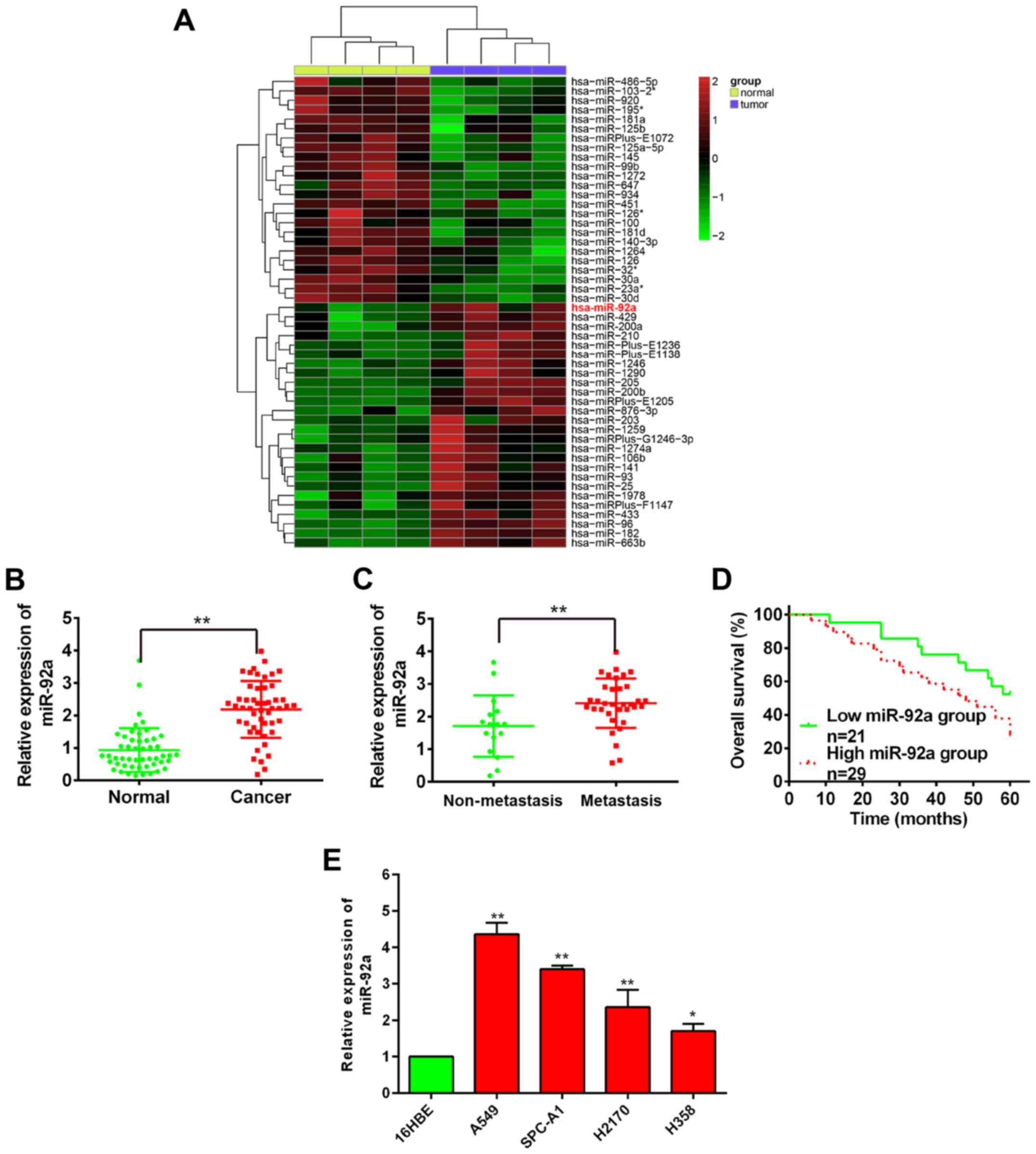
To explore the role of miRNAs in NSCLC, we first
analyzed the differential expressed miRNAs via retrieving the
microarray data in the GEO dataset (GSE29248). Cluster analysis
based on the miRNA expression pattern indicated a significant
difference between NSCLC cancer tissue and the adjacent normal
tissue (Fig. 1A). It is well known
that EMT play a key role in the metastasis of tumors (11,23),
while miR-92a is reported to promote EMT progress in several other
types of human cancer (24). For
this reason, miR-92a was chosen as the candidate for further
study.
To validate the expression of miR-92a obtained from
miRNA microarray assay, qRT-PCR was applied to examine miR-92a
expression in 50 pairs of NSCLC tissues, along with 34 metastasis
and 16 non-metastatic NSCLC tissues. The result showed that miR-92a
was significantly higher in NSCLC tissues than those of their
matched adjacent normal tissues (Fig.
1B). miR-92a was also overexpressed in metastatic tissues
compared with non-metastatic NSCLC tissues (Fig. 1C). To further investigate the
correlation between miR-92a expression and prognosis of NSCLC
patients, 50 patients were divided into 2 subgroups based on the
mean of all samples: low miR-92a group (n=21): miR-92a expression
ratio < median ratio; high miR-92a group (n=29): miR-92a
expression ratio > median ratio. From Kaplan-Meier survival
curve, we observed that patients with high miR-92a expression had
significantly shorter overall survival than those with low miR-92a
expression (P<0.001, log-rank test; Fig. 1D).
To validate whether upregulation of miR-92a was also
present in NSCLC cell lines, we examined miR-92a expression in four
NSCLC cell lines including A549, H358, SPC-A1, H1299, and a normal
human bronchial epithelial cell line 16HBE acted as a control. It
was found that the expression of miR-92a was also aberrantly
upregulated in four NSCLC cell lines, as compared to 16HBE
(Fig. 1E), and these results were
similar with the detection in NSCLC tissues. These results
indicated that miR-92a may be involved in the development of
NSCLC.
miR-92a promotes cell proliferation in
vitro and tumor growth in vivo
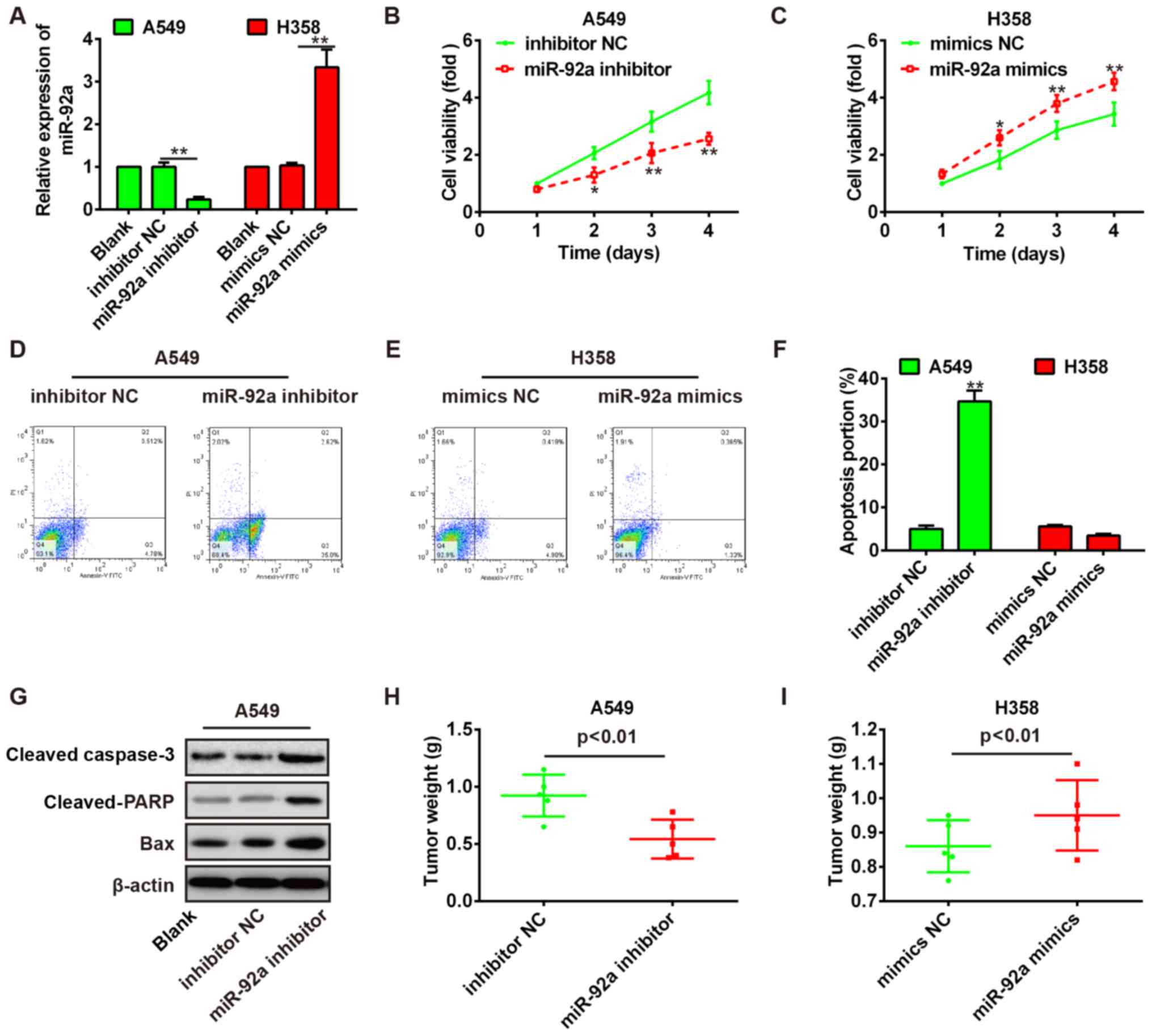
Given the upregulation of miR-92a in NSCLC tissues
and cell lines, we predicted that miR-92a may function as a tumor
oncogene. To verify our hypothesis, miR-92a inhibitor was
transfected into A549 cells and miR-92a mimic was transfected into
H358 cells because A549 and H358 cells exhibited the highest and
lowest expression levels of miR-92a in these NSCLC cell lines,
respectively. qRT-PCR analysis showed that miR-92a expression was
effectively reduced or enhanced by miR-92a inhibitor or mimic
(Fig. 2A). The results of MTT
revealed that miR-92a knockdown inhibited A549 cell proliferation,
whereas miR-92a overexpression promoted H358 cell proliferation
(Fig. 2B and C). Then, the
percentage of apoptotic cells in miR-92a-transfected A549 and H358
cells was evaluated by flow cytometry. As shown in Fig. 2D–F, the ratio of apoptotic cells in
A549 cells was increased, whereas decreased in H358 cells. To
further study possible mechanisms through which miR-92a alters
NSCLC cell apoptosis, we examined the expression of
apoptosis-relevant proteins. As shown in Fig. 2G, miR-92a knockdown resulted in an
obvious increased expression level of pro-apoptotic proteins
including the cleaved-caspase-3, caspase-PARP and Bax in A549
cells.
To evaluate the effect of miR-92a on NSCLC tumor
growth in vivo, A549 cells transfected with miR-92a
inhibitor and H358 cells transfected with miR-92a mimic were
subcutaneously injected into nude mice, and the growth of the
resultant primary tumors were monitored. As expected, the tumor
growth was significantly inhibited in miR-92a inhibitor transfected
A549 cells (Fig. 2H), whereas
significantly promoted in miR-92a mimic transfected H358 cells
(Fig. 2I). All these results
suggested that miR-92a executed an oncogenic effect in NSCLC by
potentiating cell growth ability of NSCLC cells.
miR-92a promotes the migration and
invasion of NSCLC cells in vitro
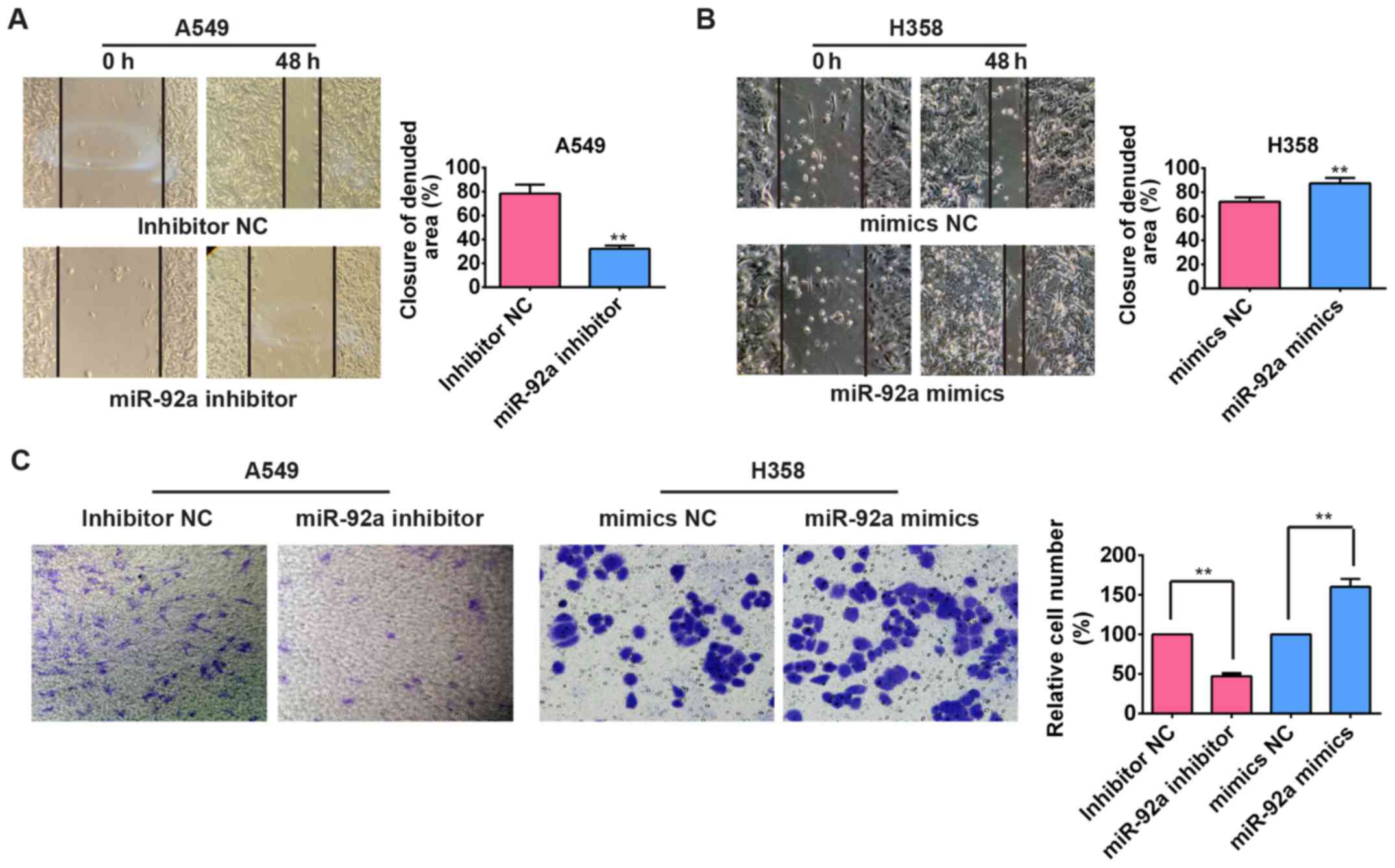
To explore whether miR-92a affects migration and
invasion of NSCLC cells, wound healing and Transwell invasion
assays were performed in A549 and H358 cells. Transfection of
miR-92a inhibitor significantly attenuated the capacity of
migration in A549 cells compared with the control cells without
transfection (Fig. 3A), whereas
the capacity of wound healing in H358 cells was significantly
enhanced after treated with miR-92a mimic (Fig. 3B). Subsequently, the relative
invaded cell number of A549 cells transfected with miR-92a
inhibitor was significantly decreased compared with the control
cells while the relative cell number was significantly increased in
the miR-92a mimic transfected H358 cells (Fig. 3C), suggesting that miR-92a plays an
important role in the regulation of NSCLC cellular motility,
including the cell invasive and metastatic capacity.
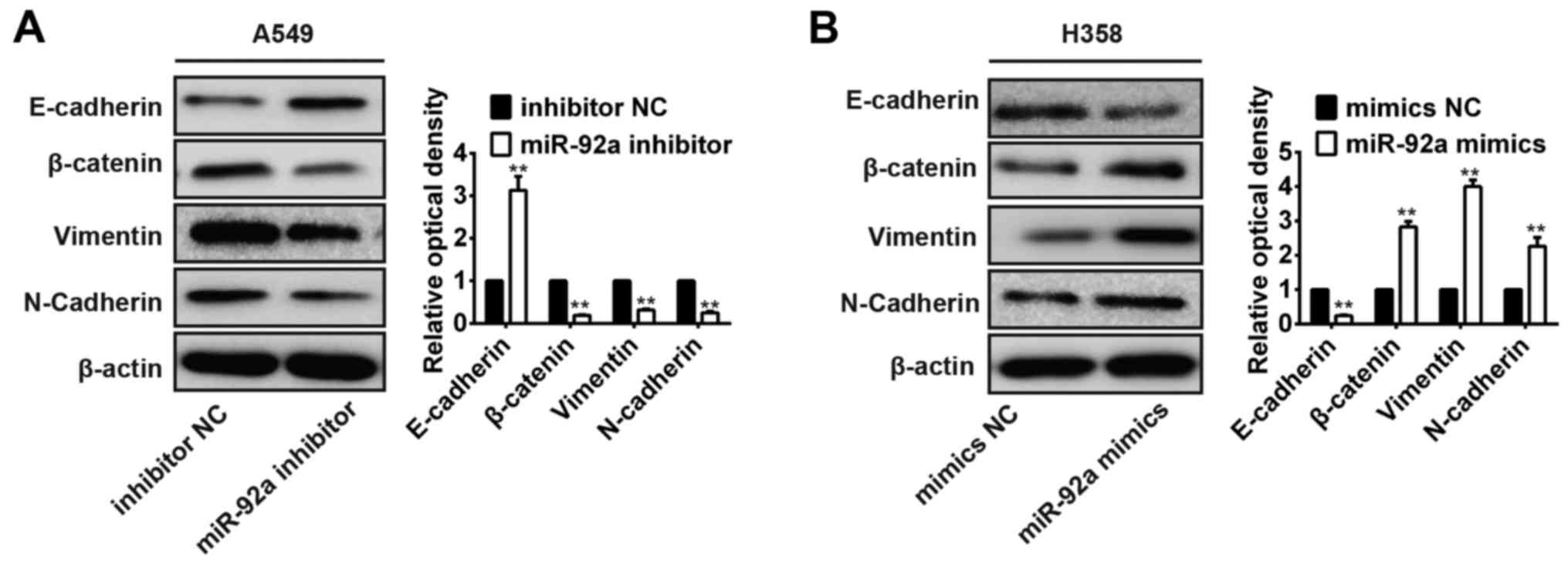
miR-92a regulates EMT in NSCLC cells
Recent studies indicate that EMT plays an important
role in the invasion and metastasis of NSCLC (25) and miRNAs have been recognized as
key regulators of EMT (26), thus
we assessed the ability of miR-92a to promote EMT in NSCLC cells.
We found that the expression of E-cadherin known as the epithelial
marker was significantly upregulated, whereas that of the
mesenchymal marker N-cadherin, vimentin and β-catenin were
significantly downregulated in A549 cells transfected with miR-92a
inhibitor (Fig. 4A). In contrast,
overexpression of miR-92a markedly decreased E-cadherin expression
and increased N-cadherin, vimentin and β-catenin expression levels
in miR-92a mimic transfected H358 cells (Fig. 4B). These results suggest that
miR-92a controls NSCLC invasion and metastasis by regulating
EMT.
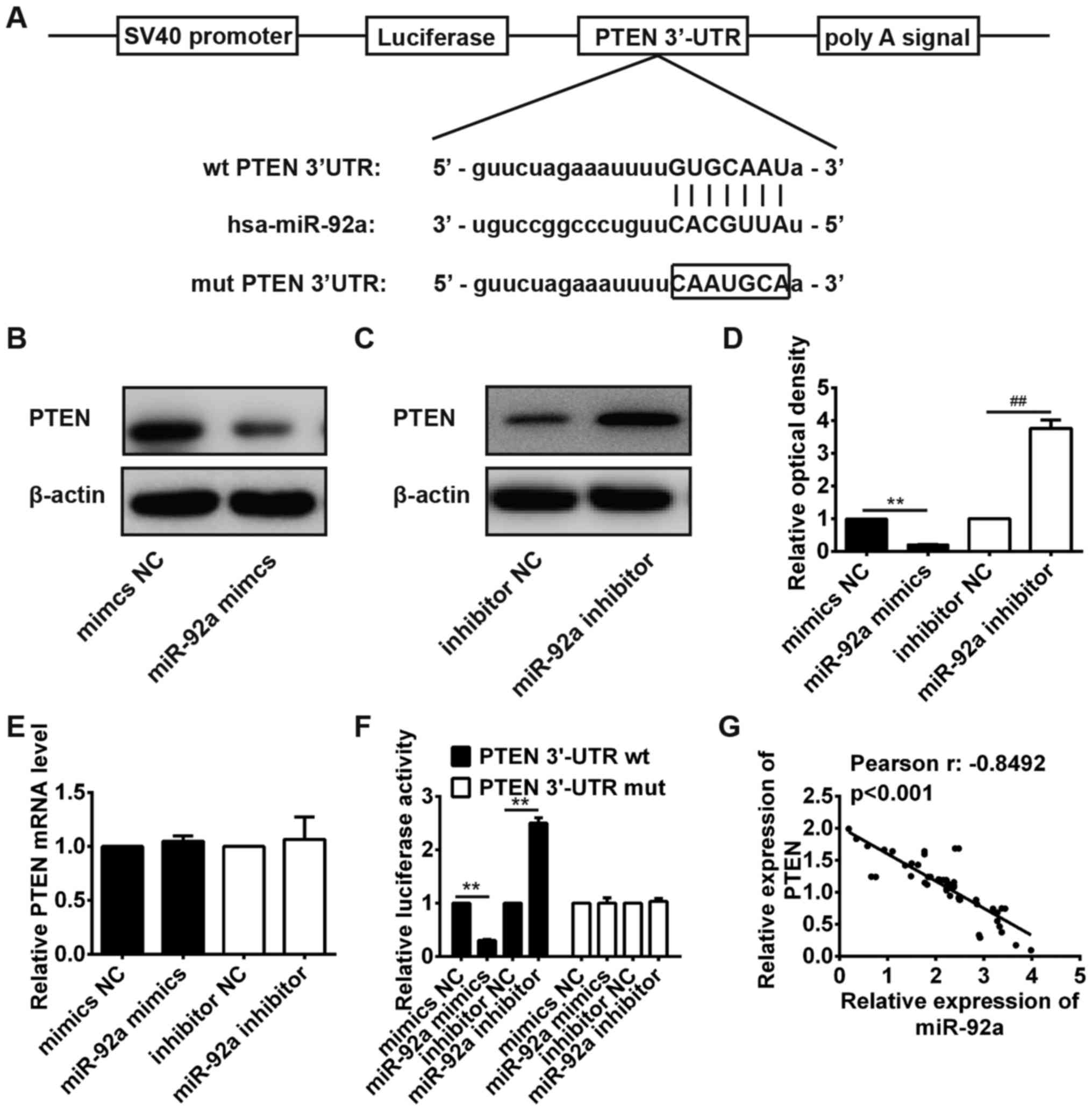
PTEN is a direct target of miR-92a in
NSCLC cells
To further characterize the molecular mechanisms
involved in the oncogenic role of miR-92a in NSCLC cells, we
searched for potential target genes of miR-92a using TargetScan and
microRNA.org. PTEN was chosen as a target gene of
miR-92a because its level is related with poor prognosis in lung
cancer and facilitated EMT in colorectal cancer (CRC) (24,27–29).
The predicted binding sites for miR-92a in the PTEN sequence are
illustrated in Fig. 5A. Then,
western blot analysis confirmed that overexpression of miR-92a
markedly inhibited PTEN expression on protein level in H358 cells
(Fig. 5B and D). In contrast,
inhibition of miR-92a markedly promoted PTEN expression on protein
level in A549 cells (Fig. 5C and
D). Notably, overexpression of miR-92a in H358 cells or
knockdown of miR-92a in A549 cells did not change PTEN mRNA level,
which indicated miR-92a targeted PTEN mainly via translational
inhibition (Fig. 5E). This finding
agrees with the results reported by Zhang et al in
colorectal cancer (24).
To determine whether PTEN is a direct target of
miR-92a in NSCLC cells, luciferase reporter assay was performed.
The result showed that overexpression of miR-92a significantly
inhibited the luciferase activity of wild-type PTEN 3′UTR, while
inhibition of miR-92a obviously promoted the luciferase activity of
wild-type PTEN 3′UTR, but the activity of the mutant-type PTEN
3′UTR was not changed (Fig.
5F).
To further investigate the relationship between
miR-92a and PTEN in NSCLC tissues, the level of PTEN mRNA in 50
NSCLC patients was measured by qRT-PCR (data not shown). As shown
in Fig. 5G, Pearson's correlation
analysis revealed that the expression of miR-92a was inversely
correlated with the expression of PTEN in the 50 patients with
NSCLC (r= −0.8492; P<0.001). These results indicated that
miR-92a could directly bind anti-oncogenic PTEN and inhibited its
expression in NSCLC.
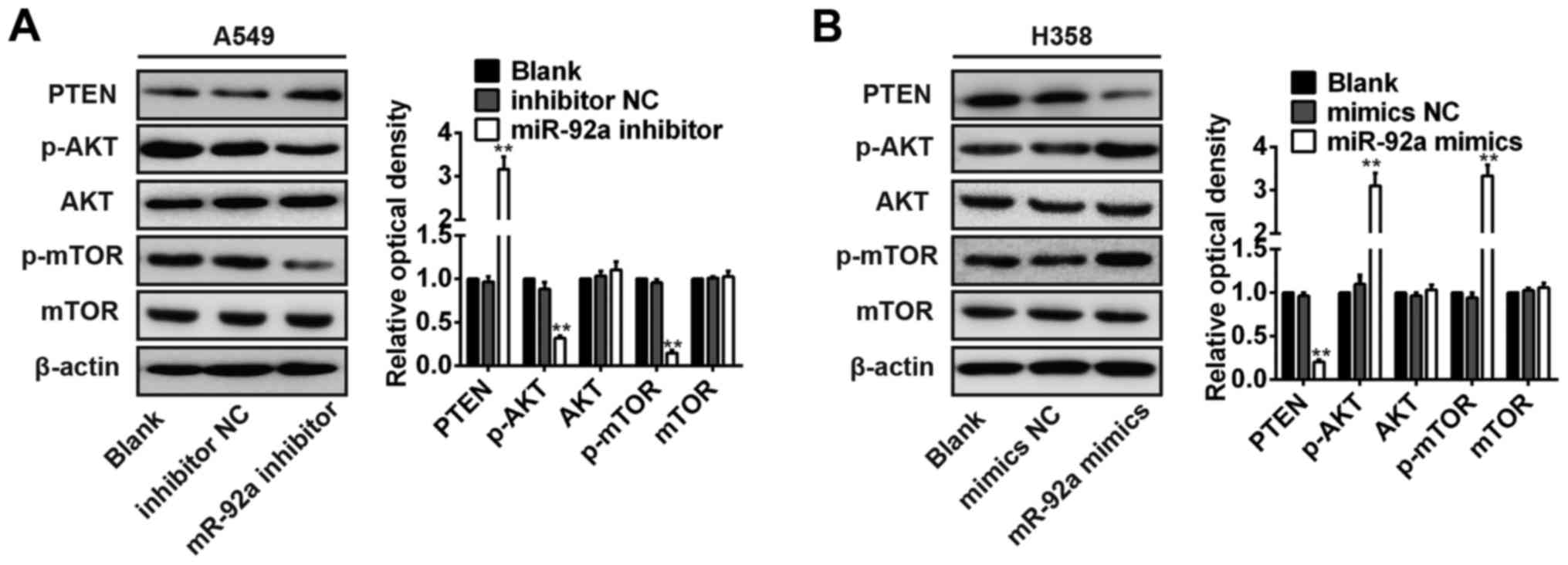
miR-92a activates the PTEN mediated AKT
signal pathway
It is reported that PTEN can negatively regulate the
activity of Akt (30,31), and the PI3K/Akt pathway plays an
important role in the invasion and metastasis of tumor cells
(32–34). However, the effect of miR-92a on
PTEN-mediated AKT signaling was not reported previously in NSCLC.
Western blot analysis revealed that miR-92a knockdown in A549 cells
robustly increased PTEN protein expression levels, inhibited the
phosphorylated AKT (Ser 473) and phosphorylated-mTOR (Fig. 6A), whereas the inhibition of
miR-92a in H358 cells exerted the opposite effect (Fig. 6B). However, miR-92a over expression
or miR-92a knockdown did not change the expression levels of total
AKT and mTOR protein (Fig. 6A and
B). These results indicate that miR-92a promotes the activity
of PI3K/AKT pathway in NSCLC cells.
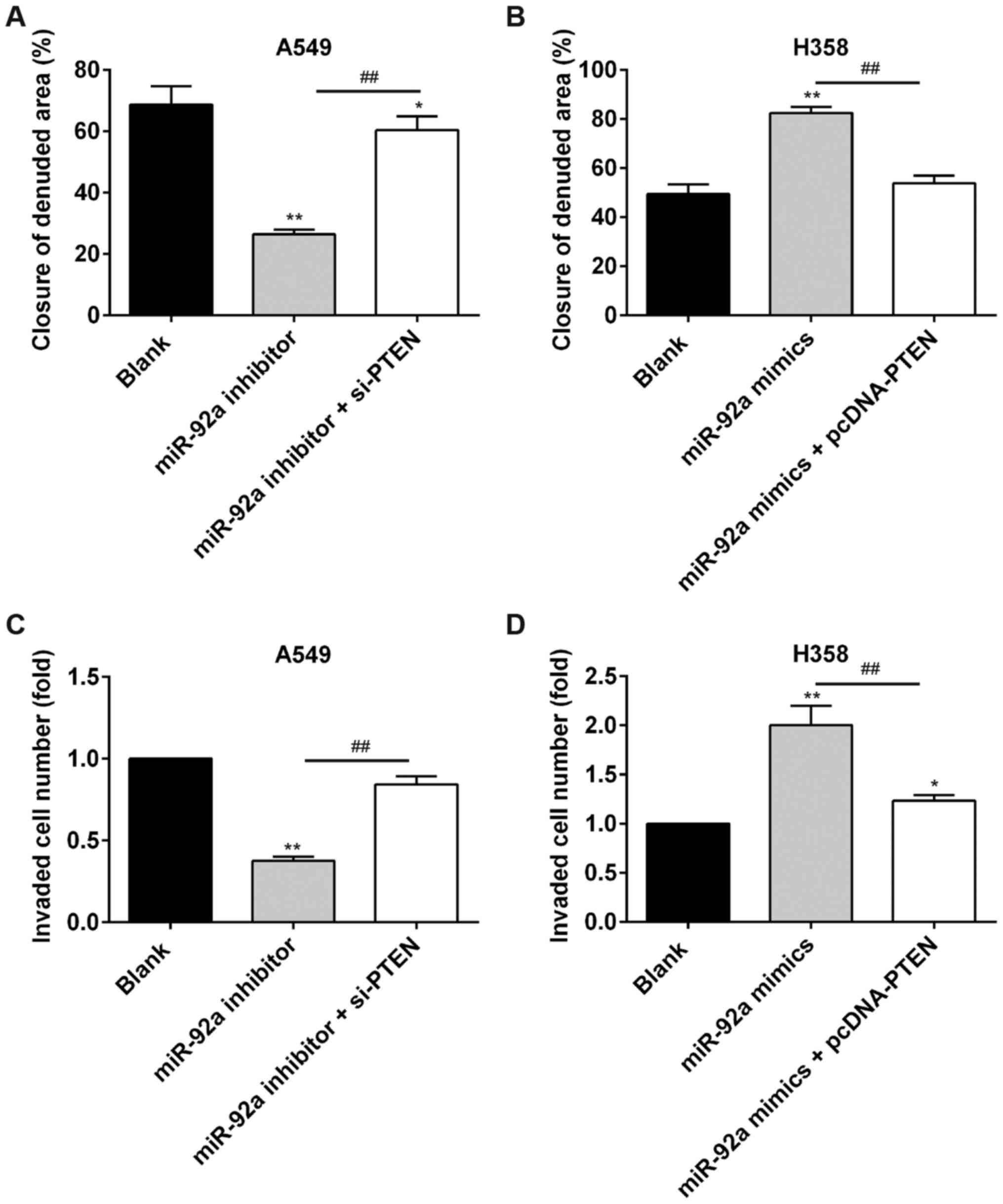
PTEN is required for the miR-92a-mediated
promotion of cell migration and invasion in NSCLC
To evaluate if PTEN is responsible for the oncogenic
potential of miR-92a in NSCLC cells, gain- and loss-of-function
studies of PTEN were performed. As expected, PTEN silencing by
si-PTEN partially reversed the effects of miR-92a knockdown on cell
migration and invasion in A549 cells (Fig. 7A and C). Conversely,
over-expression of PTEN significantly alleviated the enhancement of
cell migration and invasion induced by miR-92a mimic in H358 cells
(Fig. 7B and D). Together, these
data suggest that miR-92a exerts an oncogenic role by targeting
PTEN/PI3K/AKT pathway in NSCLC.
Discussion
In the present study, we demonstrated that miR-92a
is frequently upregulated in human NSCLC tissues and cell lines,
and significantly correlated with poor prognosis. Furthermore,
miR-92a can regulate NSCLC cellular metastasis by inducing EMT
through activating PI3K/AKT pathways, at least partially by
downregulating the level of PTEN. These results suggest that
miR-92a functions as an oncogene in NSCLC and may serve as a novel
and promising therapeutic target for NSCLC.
Increasing evidence has demonstrated that miRNAs
play a crucial role in NSCLC invasion and metastasis (35,36).
Some miRNAs, for instance miR-663a, miR-361-3p and miR-491-5p, have
been proved to contribute to the metastasis of NSCLC (37–39).
Recent studies showed that the expression of miR-92a was increased
in NSCLC tissues, and overexpression of miR-92a in NSCLC cells
promoted growth, metastasis, and chemoresistance by targeting PTEN
(40). Noteworthy, miR-92a was
found upregulated in human NSCLC tissues and cell lines via
retrieving the microarray data in the GEO dataset (GSE29248), which
was consistent with previous results (24). Thus, we chose miR-92a for further
study. Moreover, the in vitro and in vivo assay
results confirmed that miR-92a could promote proliferation,
migration in NSCLC cells and tumor formation in nude mice. In
addition, we found that up regulation of miR-92a was significantly
correlated with shorter overall survival. These results suggested
that miR-92a plays an oncogenic role in NSCLC.
EMT is a crucial event in tumor migration and
metastasis, which is the first indication of cancer development
(41). MicroRNAs recently emerged
as important regulators of EMT in various cancers (26,42).
For example, the miR-221/222 miRNA cluster has been found to induce
EMT in breast cancer cells (43).
miR-27 is upregulated in gastric cancer metastasis and enhances EMT
through regulation of Zeb1, Zeb2 and Slug (44). These studies support a crucial role
of microRNAs in controlling EMT and metastasis. For NSCLC, the role
of miRNAs in EMT is being explored. Our results showed that the
inhibition of miR-92a could suppress EMT of NSCLC cells whereas
overexpression of miR-92a promoted EMT of NSCLC cells. Thus, our
findings implicate miR-92a promote the metastasis of NSCLC cells by
regulating EMT phenotype.
PTEN, a tumor suppressor, is a well-known regulator
of EMT and inhibits tumor cell growth and invasion by blocking the
PI3K/Akt pathway (45–48). In NSCLC, PTEN was found to be
upregulated and associated with the metastatic phenotype of NSCLC
cells (29). In this study, our
results showed that miR-92a directly targeted PTEN in NSCLC cells
and the expression of PTEN was inversely correlated with miR-92
expression in NSCLC tissues. Furthermore, we found that
upregulation of miR-92a could promote activation of PI3K/AKT
signaling pathway through downregulation of PTEN. Of note,
overexpression of PTEN effectively inhibited the tumor promotional
functions of miR-92a on NSCLC migration and invasion.
In conclusion, our results reinforce the role of
miR-92a as a tumor oncogene in NSCLC. Importantly, we identified a
likely novel potential mechanism of miR-92a promoting EMT and tumor
metastasis by inhibiting PTEN and indirectly regulating PI3K/AKT
signaling pathway. In particular, the important role of miR-92a in
NSCLC suggests that the upregulation of miR-92a may have diagnostic
and therapeutic value for NSCLC.
References
|
1
|
Spira A and Ettinger DS: Multidisciplinary
management of lung cancer. N Engl J Med. 350:379–392. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mercier O, Fadel E, de Perrot M, Mussot S,
Stella F, Chapelier A and Dartevelle P: Surgical treatment of
solitary adrenal metastasis from non-small cell lung cancer. J
Thorac Cardiovasc Surg. 130:136–140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boeri M, Sestini S, Fortunato O, Verri C,
Suatoni P, Pastorino U and Sozzi G: Recent advances of
microRNA-based molecular diagnostics to reduce false-positive lung
cancer imaging. Expert Rev Mol Diagn. 15:801–813. 2015.PubMed/NCBI
|
|
7
|
Skrzypski M, Dziadziuszko R and Jassem J:
MicroRNA in lung cancer diagnostics and treatment. Mutat Res.
717:25–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guan P, Yin Z, Li X, Wu W and Zhou B:
Meta-analysis of human lung cancer microRNA expression profiling
studies comparing cancer tissues with normal tissues. J Exp Clin
Cancer Res. 31:542012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shang Y, Zang A, Li J, Jia Y, Li X, Zhang
L, Huo R, Yang J, Feng J, Ge K, et al: MicroRNA-383 is a tumor
suppressor and potential prognostic biomarker in human non-small
cell lung cancer. Biomed Pharmacother. 83:1175–1181. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng Y, Zhu J, Shen D, Qin H, Lei Z, Li W,
Liu Z and Huang JA: MicroRNA-205 targets SMAD4 in non-small cell
lung cancer and promotes lung cancer cell growth in vitro and in
vivo. Oncotarget. Jun 30–2016.Epub ahead of print.
|
|
11
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mahmood MQ, Ward C, Muller HK, Sohal SS
and Walters EH: Epithelial mesenchymal transition (EMT) and
non-small cell lung cancer (NSCLC): A mutual association with
airway disease. Med Oncol. 34:452017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin Q, Wei F, Zhang J and Li B: miR-134
suppresses the migration and invasion of non-small cell lung cancer
by targeting ITGB1. Oncol Rep. 37:823–830. 2017.PubMed/NCBI
|
|
14
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hao J, Zhang Y, Deng M, Ye R, Zhao S, Wang
Y, Li J and Zhao Z: MicroRNA control of epithelial-mesenchymal
transition in cancer stem cells. Int J Cancer. 135:1019–1027. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu M, Xue H, Wang Y, Shen Q, Jiang Q,
Zhang X, Li K, Jia M, Jia J, Xu J, et al: miR-345 inhibits tumor
metastasis and EMT by targeting IRF1-mediated mTOR/STAT3/AKT
pathway in hepatocellular carcinoma. Int J Oncol. 50:975–983.
2017.PubMed/NCBI
|
|
17
|
Sathyanarayanan A, Chandrasekaran KS and
Karunagaran D: microRNA-145 modulates epithelial-mesenchymal
transition and suppresses proliferation, migration and invasion by
targeting SIP1 in human cervical cancer cells. Cell Oncol (Dordr).
40:119–131. 2017. View Article : Google Scholar
|
|
18
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Korpal M and Kang Y: The emerging role of
miR-200 family of microRNAs in epithelial-mesenchymal transition
and cancer metastasis. RNA Biol. 5:115–119. 2008. View Article : Google Scholar
|
|
20
|
Song CL, Liu B, Shi YF, Liu N, Yan YY,
Zhang JC, Xue X, Wang JP, Zhao Z, Liu JG, et al: MicroRNA-130a
alleviates human coronary artery endothelial cell injury and
inflammatory responses by targeting PTEN via activating
PI3K/Akt/eNOS signaling pathway. Oncotarget. 7:71922–71936.
2016.PubMed/NCBI
|
|
21
|
Fukumoto I, Kinoshita T, Hanazawa T,
Kikkawa N, Chiyomaru T, Enokida H, Yamamoto N, Goto Y, Nishikawa R,
Nakagawa M, et al: Identification of tumour suppressive
microRNA-451a in hypopharyngeal squamous cell carcinoma based on
microRNA expression signature. Br J Cancer. 111:386–394. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
da Silva Xavier G, Leclerc I, Varadi A,
Tsuboi T, Moule SK and Rutter GA: Role for AMP-activated protein
kinase in glucose-stimulated insulin secretion and preproinsulin
gene expression. Biochem J. 371:761–774. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng H and Kang Y: Multilayer control of
the EMT master regulators. Oncogene. 33:1755–1763. 2014. View Article : Google Scholar
|
|
24
|
Zhang G, Zhou H, Xiao H, Liu Z, Tian H and
Zhou T: MicroRNA-92a functions as an oncogene in colorectal cancer
by targeting PTEN. Dig Dis Sci. 59:98–107. 2014. View Article : Google Scholar
|
|
25
|
Zhang JX, Zhai JF, Yang XT and Wang J:
MicroRNA-132 inhibits migration, invasion and
epithelial-mesenchymal transition by regulating TGFβ1/Smad2 in
human non-small cell lung cancer. Eur Rev Med Pharmacol Sci.
20:3793–3801. 2016.PubMed/NCBI
|
|
26
|
Lamouille S, Subramanyam D, Blelloch R and
Derynck R: Regulation of epithelial-mesenchymal and
mesenchymal-epithelial transitions by microRNAs. Curr Opin Cell
Biol. 25:200–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao J, Hu CP, He BX, Chen X, Lu XX, Xie
MX, Li W, He SY, You SJ and Chen Q: PTEN expression is a prognostic
marker for patients with non-small cell lung cancer: A systematic
review and meta-analysis of the literature. Oncotarget.
7:57832–57840. 2016.PubMed/NCBI
|
|
28
|
Gu J, Ou W, Huang L, Wu J, Li S, Xu J,
Feng J, Liu B and Zhou Y: PTEN expression is associated with the
outcome of lung cancer: Evidence from a meta-analysis. Minerva Med.
107:342–351. 2016.PubMed/NCBI
|
|
29
|
Zhu DY, Li XN, Qi Y, Liu DL, Yang Y, Zhao
J, Zhang CY, Wu K and Zhao S: MiR-454 promotes the progression of
human non-small cell lung cancer and directly targets PTEN. Biomed
Pharmacother. 81:79–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chetram MA and Hinton CV: PTEN regulation
of ERK1/2 signaling in cancer. J Recept Signal Transduct Res.
32:190–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng X, Jiang J, Shi S, Xie H, Zhou L and
Zheng S: Knockdown of miR-25 increases the sensitivity of liver
cancer stem cells to TRAIL-induced apoptosis via PTEN/PI3K/Akt/Bad
signaling pathway. Int J Oncol. 49:2600–2610. 2016.PubMed/NCBI
|
|
32
|
Xia H, Li Y and Lv X: MicroRNA-107
inhibits tumor growth and metastasis by targeting the BDNF-mediated
PI3K/AKT pathway in human non-small lung cancer. Int J Oncol.
49:1325–1333. 2016.PubMed/NCBI
|
|
33
|
Wang R, Zhang Q, Peng X, Zhou C, Zhong Y,
Chen X, Qiu Y, Jin M, Gong M and Kong D: Stellettin B induces G1
arrest, apoptosis and autophagy in human non-small cell lung cancer
A549 cells via blocking PI3K/Akt/mTOR pathway. Sci Rep.
6:270712016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiao L, Zhou H, Li XP, Chen J, Fang C, Mao
CX, Cui JJ, Zhang W, Zhou HH, Yin JY, et al: MicroRNA-138 acts as a
tumor suppressor in non small cell lung cancer via targeting YAP1.
Oncotarget. 7:40038–40046. 2016.PubMed/NCBI
|
|
36
|
Pastorkova Z, Skarda J and Andel J: The
role of microRNA in metastatic processes of non-small cell lung
carcinoma. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
160:343–357. 2016.PubMed/NCBI
|
|
37
|
Zhang Y, Xu X, Zhang M, Wang X, Bai X, Li
H, Kan L, Zhou Y, Niu H and He P: MicroRNA-663a is downregulated in
non-small cell lung cancer and inhibits proliferation and invasion
by targeting JunD. BMC Cancer. 16:3152016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen W, Wang J, Liu S, Wang S, Cheng Y,
Zhou W, Duan C and Zhang C: MicroRNA-361-3p suppresses tumor cell
proliferation and metastasis by directly targeting SH2B1 in NSCLC.
J Exp Clin Cancer Res. 35:762016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gong F, Ren P, Zhang Y, Jiang J and Zhang
H: MicroRNAs-491-5p suppresses cell proliferation and invasion by
inhibiting IGF2BP1 in non-small cell lung cancer. Am J Transl Res.
8:485–495. 2016.PubMed/NCBI
|
|
40
|
Ren P, Gong F, Zhang Y, Jiang J and Zhang
H: MicroRNA-92a promotes growth, metastasis, and chemoresistance in
non-small cell lung cancer cells by targeting PTEN. Tumour Biol.
37:3215–3225. 2016. View Article : Google Scholar
|
|
41
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zaravinos A: The regulatory role of
microRNAs in EMT and cancer. J Oncol. 2015:8658162015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Stinson S, Lackner MR, Adai AT, Yu N, Kim
HJ, O'Brien C, Spoerke J, Jhunjhunwala S, Boyd Z, Januario T, et
al: miR-221/222 targeting of trichorhinophalangeal 1 (TRPS1)
promotes epithelial-to-mesenchymal transition in breast cancer. Sci
Signal. 4:pt52011.PubMed/NCBI
|
|
44
|
Zhang Z, Liu S, Shi R and Zhao G: miR-27
promotes human gastric cancer cell metastasis by inducing
epithelial-to-mesenchymal transition. Cancer Genet. 204:486–491.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Castellino RC and Durden DL: Mechanisms of
disease: The PI3K-Akt-PTEN signaling node - an intercept point for
the control of angiogenesis in brain tumors. Nat Clin Pract Neurol.
3:682–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Grille SJ, Bellacosa A, Upson J,
Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN and
Larue L: The protein kinase Akt induces epithelial mesenchymal
transition and promotes enhanced motility and invasiveness of
squamous cell carcinoma lines. Cancer Res. 63:2172–2178.
2003.PubMed/NCBI
|
|
47
|
Vogt PK, Gymnopoulos M and Hart JR: PI
3-kinase and cancer: Changing accents. Curr Opin Genet Dev.
19:12–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tu K, Liu Z, Yao B, Han S and Yang W:
MicroRNA-519a promotes tumor growth by targeting PTEN/PI3K/AKT
signaling in hepatocellular carcinoma. Int J Oncol. 48:965–974.
2016.
|