Introduction
Angiogenesis refers to the process of new blood
vessel formation, which constitutes a hallmark in physiological and
pathological contexts, such as embryo development, wound healing,
diabetic retinopathy and cancer metastasis (1,2).
Research on tumor angiogenesis progressed slowly until the
discovery of the key molecular initiators of angiogenesis, i.e.,
the vascular endothelial growth factor (VEGF), and its endothelial
cell specific tyrosine kinase receptor VEGFR (3). It is believed that the
anti-angiogenic therapy could prevent cancer growth and
metastasis.
VEGF has crucial and numerous effects on vascular
endothelial cells. It acts as both pro-survival factor and
stimulator of cell proliferation and migration, increases the
vascular permeability, and initiates the development of new blood
vessels (4). VEGF activates
endothelial cells through VEGFR1 and VEGFR2, while VEGFR2 mediates
the major angiogenic function of VEGF. VEGFR2 can lead to the
activation of multiple downstream signal transduction cascades
including the ERK1/2, the AKT and the focal adhesion kinase
pathways (5). Besides, VEGF
induces stress fiber formation to enhance vascular permeability and
facilitates cell migration. Therefore, intervention in one or more
of the pathways could downregulate the effect of VEGF signaling
pathways. Development of the inhibitors for the VEGF/VEGFR2
signaling pathway has led to several approved FDA drugs which
benefit hundreds of thousands of cancer patients. Bevacizumab
(Avastin), a VEGF-targeted antibody, and sunitinib (Sutent), a
VEGFR inhibitor, are some of the best-known drugs in this category
(6).
However, clinical experiments also revealed cases of
limited efficacy in the anti-VEGF therapy due to unknown causes
(7). One proposed mechanism of the
resistance involves tumors upregulating the alternate
pro-angiogenic molecules, such as placental growth factor (PIGF),
fibroblast growth factor 2 (FGF2) and angiopoietin 2 (Ang2). Due to
the unique mode of function and increased understanding of both
VEGF- and Ang2-induced pathways, tremendous effort has been made to
combine these two modalities to achieve enhanced outcomes (8).
As a ligand of the Tie2 receptor, Ang2 is
exclusively expressed and stored in endothelial cells and functions
as a partial Tie2 agonist. It can be rapidly released following
cytokine stimulation, such as VEGF (9,10).
The Ang2/Tie2 system is an important angiogenic switch to promote
vessel remodeling, sprouting and mural cell recruiting. Therefore,
the interference of Ang2/Tie2 and VEGF/VEFGFR2 axes has attracted
increasing attention for cancer prevention (11,12).
Indeed, several clinical trials of the dual target therapies have
shown a more potent anticancer activity (11,13).
For instance, Ang2 and VEGFA bispecific antibody CrossMab has been
clinically validated as an initial treatment for renal cell
carcinoma (13).
Laggera alata is a member of genus
Laggera (Compositae) and is a traditional herbal medicine
with both anti-inflammatory and anti-bacterial activities (14,15).
However, its anti-angiogenic efficacy is not known. Our group has
isolated several eudesmane-type sesquiterpenes (ETSs) from
Laggera alata (16). Two
high-yield ETSs were selected to be evaluated for their
anti-angiogenic activity. In the present study, both ETSs were
found to be effective as angiogenic inhibitors, not only in the
in vitro cell model but also in the in vivo zebrafish
model. The mechanistic study showed that their anti-angiogenic
effects were related to their interference in both the VEGF/VEGFR
and the Ang2/Tie2 axes.
Materials and methods
Chemicals and reagents
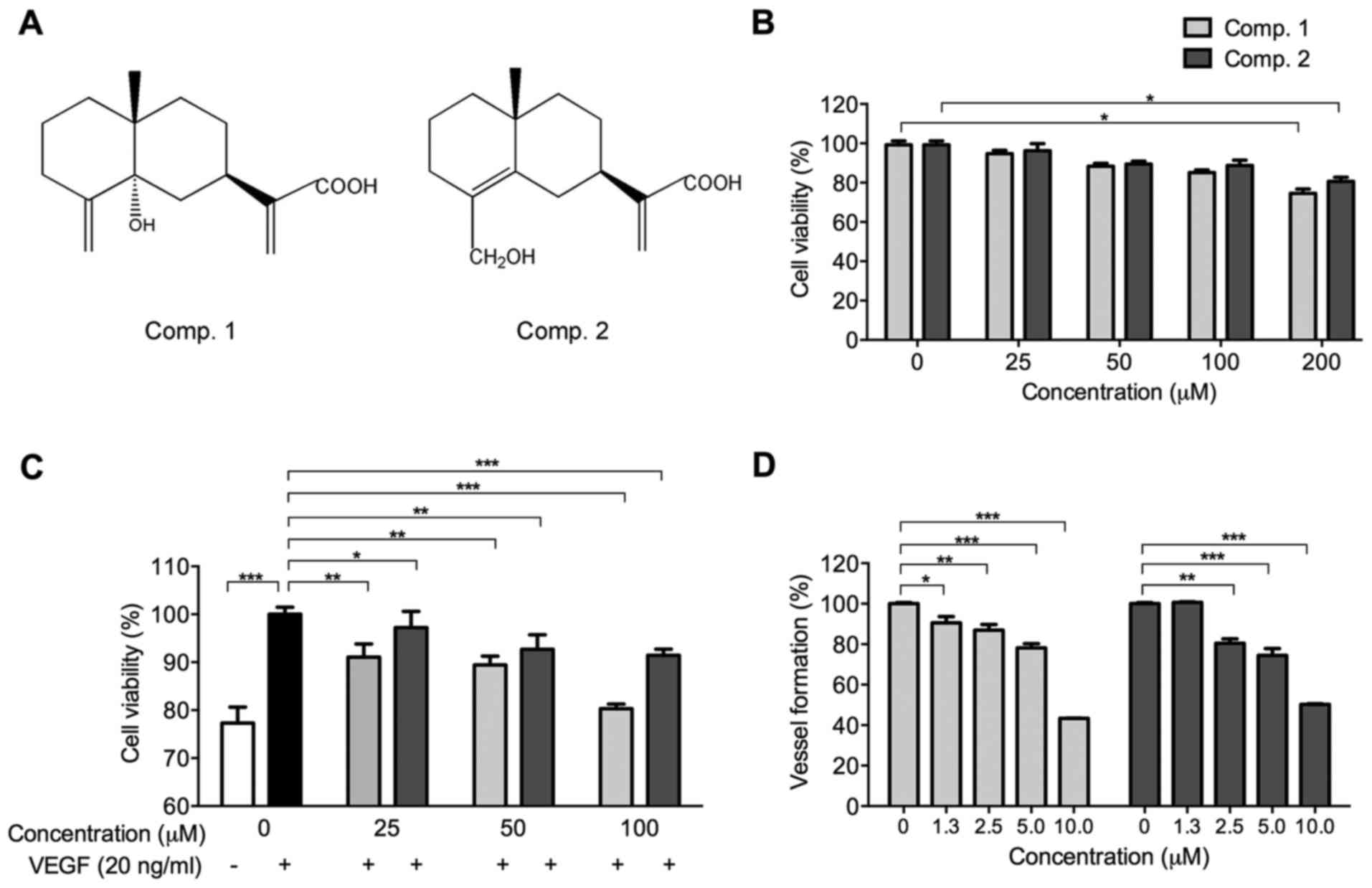
Extraction and isolation of the two ETSs,
5α-hydroxycostic acid (Comp. 1) and hydroxyisocostic acid (Comp.
2), from Laggera alata was carried out as described
previously (16). Their structures
are shown in Fig. 1A. The two
chemicals were dissolved in DMSO at a final concentration of 50 mM
respectively, and diluted in culture medium to the indicated
concentration for assigned assay. The vehicle group contained DMSO
at 0.1% in its culture medium.
The human Ang2 was purchased from Adipogen (San
Diego, CA, USA). VEGF (VEGFA-165) was obtained from Peprotech
(Rockyhill, NJ, USA). The concentrations of Ang2 and VEGF used in
the subsequent experiments were 400 and 20 ng/ml, respectively.
Fetal bovine serum (FBS), low serum growth supplement (LSGS),
Dulbecco's modified Eagle's medium (DMEM), phosphatase substrate
kit, BCA protein assay kit, Medium 200, Matrigel matrix, M-MLV and
TRIzol reagent were all purchased from Thermo Fisher Scientific
(Waltham, MA, USA), and Taq DNA polymerase was from Takara
(Tokyo, Japan). Evagreen dye was purchased from Biotium (Hayward,
CA, USA). Reagents including semaxanib (SU5416, a specific VEGFR
inhibitor), phalloidin, heparin, bull serum albumin (BSA), MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and
non-protein chemicals were obtained from Sigma Chemical Co. (St.
Louis, MO, USA). The pexmetinib (ARRY614, a specific inhibitor of
Tie2 and p38) was purchased from APExBIO (Houston, TX, USA).
The antibodies against VEGFR,
p-VEGFR2Tyr1175, p-Tie2Tyr992, AKT, p-AKTSer473, ERK1/2,
p-ERK1/2Thr202/Tyr204, p38, p-p38Thr180/Tyr182,
p-PLCγSer1248, p-SrcTyr416, p-FAKTyr397, p-eNOSSer1177, anti-mouse
and anti-rabbit horseradish perioxidase (HRP)-conjugated secondary
antibodies were purchased from Cell Signaling Technology (CST,
Danvers, MA, USA). The antibodies against Tie2, and p-Tie
(Tie2Tyr992 + Tie1Tyr1007) were from Abcam (Cambridge, UK). The
anti-β-actin antibody was obtained from Santa Cruz Biotechnology
(Dallas, TX, USA). The antibody against E-cadherin was from BD
Biosciences (BD, San Jose, CA, USA). Both DAPI and proteinase
inhibitor cocktail tablets were from Roche (Basel,
Switzerland).
Cell culture and zebrafish handling
Human umbilical vein endothelial cells (HUVECs) were
purchased from Life Technologies Corp. (Carlsbad, CA, USA), and
were cultured in the Medium 200 supplemented with LSGS, heparin
(0.1 mg/ml), and heat-inactivated FBS (20%, v/v). The human breast
cancer MCF-7 cells were obtained from the American Type Culture
Collection (ATCC, Manassas, VA, USA), and were cultured in DMEM
containing 10% FBS. Both cell types were cultured in a 5%
CO2 humidified atmosphere at 37°C. For some of the in
vitro experiments, HUVECs were starved in both serum- and
LSGS-free Medium 200 (maintenance medium) containing 0.5% BSA for 4
h, and MCF-7 cells were serum-starved in DMEM medium (maintenance
medium) overnight (~8 h). Then, the effects of ETSs were detected.
Wild-type zebrafish (AB strain) were bought from Hong Kong goldfish
market and maintained in flow-through aquaria at 28°C. The fish
were fed with general tropical fish food and brine shrimp. Embryos
were collected by natural pairwise mating, and transferred to
beakers (25 fish/300 ml beaker) with clean embryo water (60
µg/ml instant ocean salt).
Cell proliferation assay
The cellular viability of the HUVECs in the presence
of either ETS compound was assayed using the MTT method (17). In brief, HUVECs (5×103
cells/well) were seeded in 96-well plates and allowed to attach
overnight. Cells were then treated with ETSs (from 25 to 200
µM) for 48 h. The staining reagent of 30 µl MTT (5
mg/ml) was added to each well for 4 h. The medium was then removed
and 200 µl DMSO was added. After complete dissolution of the
precipitated formazan, optical density was measured at 570 nm using
a UV-VIS spectrophotometer (Genesys 5, Spectronic Instruments,
USA). The cell viability was expressed as percentage (%) of the
vehicle group.
The effect of ETSs on VEGF-induced HUVECs
proliferation was also evaluated by MTT assay. Starved HUVECs
(5×103 cells/well) were stimulated by VEGF in the
presence or absence of the ETSs for 24 h, then the effect of ETSs
on the proliferation of HUVECs was evaluated.
Real-time PCR analysis
Total RNA in HUVECs or zebrafish embryos was
isolated using TRIzol reagent and cDNA was conducted using M-MLV
(18). Real-time PCR was performed
on a Bio-Rad detection system CFX96 in a volume of 20 µl
containing Evagreen dye 1 µl, Taq polymerase 0.1
µl, cDNA 4 µl (200 ng), 1 µl of each primer
and H2O 12.9 µl. The reaction protocol was 40
cycles at 95°C for 20 sec, 57°C for 20 sec, and 72°C for 20 sec. A
melt curve analysis was performed at the end of the reaction to
assess the specificity of amplification. The mRNA levels of the
genes were normalized to the internal controls, namely,
GAPDH and ELF1α, in HUVECs and zebrafish,
respectively. The primer sequences for HUVECs are as follows:
hGAPDH forward, 5′-CCCACTCCTCCACCT TTGAC-3′ and reverse,
5′-TCTTCCTCTTGTGCTCTTGC-3′; hVEGFR2 forward,
5′-GGAACCTCACTATCCGCAGAGT-3′ and reverse,
5′-CCAAGTTCGTCTTTTCCTGGGC-3′; hTie1 forward,
5′-GACCCACACTGACCAACACA-3′ and reverse, 5′-GTTGTGCACGTAGATGACGC-3′;
hTie2 forward, 5′-CTGCAGTGCAATGAAGCATGC-3′ and reverse,
5′-CTGCAGACCCAAACTCCTGAG-3′. The primer sequences for zebrafish are
as follows: zELF1α forward, 5′-GGCTGACTGTGCTGTGCTGATTG-3′
and reverse, 5′-CTTGTCGGTGGGACGGCT AGG-3′; zVEGFA-121
forward, 5′-CTACCCAGTGACCAACGC-3′ and reverse,
5′-GCTCTACAGCTCCTGACGAT-3′; zVEGFA-165 forward,
5′-TGCTCCTGCAAATTCACACAA-3′ and reverse,
5′-ATCTTGGCTTTTCACATCTGCAA-3′; zVEGFR2 forward,
5′-GCCTGATCCACAACTGCTTCC-3′ and reverse,
5′-CTCTCCTCACACGACTCAATGC-3′; zAng2
5′-AGGTGGAGGCTGGACTGTC-3′ and reverse, 5′-GTGGTGAGCAGGTGGATGAC-3′;
zTie1 forward, 5′-CAAGAGGCACGGAAGGCTTA-3′ and reverse,
5′-AGTGACAGTAACGCAGAGCC-3′; zTie2 forward,
5′-CTACCCAGTGACCAACGC-3′ and reverse,
5′-GCTCTACAGCTCCTGACGAT-3′.
Wound-healing migration assay
The effect of the ETSs on cell migration was
evaluated by the wound-healing assay according to protocols
reported previously (19) with
some modifications. Briefly, HUVECs and MCF-7 cells were plated in
24-well plates at a density of 2×105 and
2.5×105 cells/well, respectively. When the cells reached
100% confluence, they were starved to inactivate cell proliferation
and a scrape was created with sterilized pipette tip. After
washings twice with PBS, the cells were overlaid with fresh medium
containing various concentrations of ETSs or SU5416 (4 µM),
co-treated with either VEGF or Ang2. Images were taken using an
inverted microscope (Nikon TE300, Japan) at 0 h and after 20-h
incubations. The extent of migration was analyzed using an
Image-Pro Plus software (Media Cybernetics, USA). Wound size of the
vehicle group was set as 100%.
Capillary-like tube formation assay
Tube formation assay was carried out as described
previously (20) with some
modifications. In brief, HUVECs (6×103 cells/well) in
the medium were seeded onto the Matrigel matrix-coated 96-well
plates along with VEGF. Various dilutions of ETSs or SU5416 (4
µM) were added to the wells and kept for 6 h. The tubular
structures were captured and quantified using an Image-Pro Plus
software.
Immunofluorescent staining
HUVECs (1×105 cells/ml) or MCF-7 cells
(2×105 cells/ml) were seeded to the clear cover
glass-bottom 35-mm Petri-dishes for confocal imaging overnight at
37°C before starvation. During cell starvation, ETSs (100
µM) were added to the cells 4 h before the cells were
treated with either VEGF or Ang2 for specified duration (20 min to
6 h) depending on the objective of the experiment. Collected cells
were washed twice with ice-cold PBS, fixed with 4% paraformaldehyde
for 15 min, and permeabilized with 0.1% Triton X-100 in PBS. After
rinsing twice with PBS, the cells were blocked in the 3% BSA in PBS
for 1 h and immunostained with corresponding primary antibody at
4°C overnight. Then, the cells were incubated with the secondary
fluorescent antibody for 1 h at room temperature. Finally, the cell
nucleus was visualized with DAPI, after washing with PBS, and
images were captured using a confocal microscope (Olympus FV1000
IX81, Tokyo, Japan) (21).
Western blotting
HUVECs or MCF-7 cells (1×106 cells/dish)
were seeded in 100-mm culture dishes for 24 h before starvation.
During cell starvation, ETSs were exposed to cells for 4 h and then
stimulated with VEGF or Ang2 for specified time interval.
Whole-cell extracts were collected using a lysis buffer
supplemented with proteinase inhibitors. Protein concentration was
measured using the BCA protein assay kit. Equal amounts of proteins
(40 µg) were resolved by electrophoresis on 12% SDS-PAGE
gels and then transferred to PVDF membrane (Millipore, Billerica,
MA, USA). The membrane was blocked with 3% BSA at room temperature
for 1 h, incubated with primary antibody at 4°C overnight followed
by HRP-conjugated goat secondary antibodies at room temperature for
1 h. Protein bands were visualized using enhanced chemiluminescence
detection reagents (Bio-Rad, Hercules, CA, USA) (22). The resulting images were scanned
using a scanner (Canon PIXMA MP150, Japan).
Quantitative EAP assay
Wild-type zebrafish (AB strain) embryos were
selected to evaluate the anti-angiogenic ability of ETSs in
vivo, a quantitative endogenous alkaline phosphatase (EAP)
assay was performed as described previously (18). Briefly, healthy, limpid, and
regular embryos (24 h post fertilization, 24 hpf) were arrayed in a
96-well plate (1 embryo/well) and treated with ETSs (1–10
µM) until 48 hpf. Embryos were then stained according to the
manufacturer's instructions of the phosphatase substrate kit
(Pierce, Waltham, MA, USA). The optical density of soluble end
product was measured at 405 nm using a UV-VIS spectrophotometer.
Vessel growth is presented as the percentage in optical density
compared to that of the vehicle group: % vessel formation = (OD
treated at 48 hpf - OD vehicle at 24 hpf)/(OD vehicle at 48 hpf -
OD vehicle at 24 hpf) × 100%.
Statistical analysis
All experiments were performed at least three times.
Data are presented as the mean ± standard deviation (SD).
Statistical analysis was determined using one-way ANOVA.
Results
Effects of ETSs on the viability of
HUVECs
The chemical structures of ETSs are shown in
Fig. 1A. Before assessing the
anti-angiogenic properties of ETSs, the MTT assay was used to
evaluate their toxic effects on HUVECs in the normal growth medium.
As shown in Fig. 1B, various
concentrations (0–100 µM) of ETSs were non-toxic to HUVECs.
In this regard, the concentrations of ETSs (≤100 µM) used in
the subsequent in vitro experiments were considered
non-toxic.
ETSs inhibits the proliferation of
VEGF-induced HUVECs and vessel formation in zebrafish embryos
VEGF plays an important role during angiogenesis for
its mitogenic effect on vascular endothelial cells (23). Therefore, we measured the
inhibitory effect of ETSs on VEGF-induced HUVECs. The viability of
HUVECs was upregulated significantly from 77.32 to 100%
(p<0.005) when VEGF was added (Fig.
1C), while with the addition of ETSs, the viability was
suppressed dose-dependently (Fig.
1C). At 25 µM of Comp. 2, cell viability decreased to
97.21% (p<0.05). A stronger inhibitory effect was shown by Comp.
1 with viability of 91.07% (p<0.01). These data suggest that
VEGF-induced proliferation of the HUVECs is sensitive to the
presence of ETSs (Fig. 1B and C).
Next, the anti-angiogenic activities of ETSs were examined in the
wild-type zebrafish embryos. As shown in Fig. 1D, the two selected ETSs
dose-dependently inhibited the vessel formation in the zebrafish
embryos in the range of 1.3–10 µM. At the concentration of 5
µM, both ETSs have exhibited very significant
anti-angiogenic activities (p<0.005).
Both ETSs inhibit VEGF-induced HUVECs
migration, stress fibers and tube formation
The effects of ETSs on VEGF-mediated HUVECs
migration were investigated using a wound-healing assay (Fig. 2A). HUVECs migrated to a clear area
when stimulated with VEGF, and the stimulatory effect of VEGF was
inhibited by ETSs in a dose-dependent manner (Fig. 2A). Even at 25 µM, the two
compounds exhibited very significant inhibitory effects
(p<0.005) (Fig. 2A). These data
reveal that ETSs inhibit VEGF-stimulated migration of the
endothelial cells.
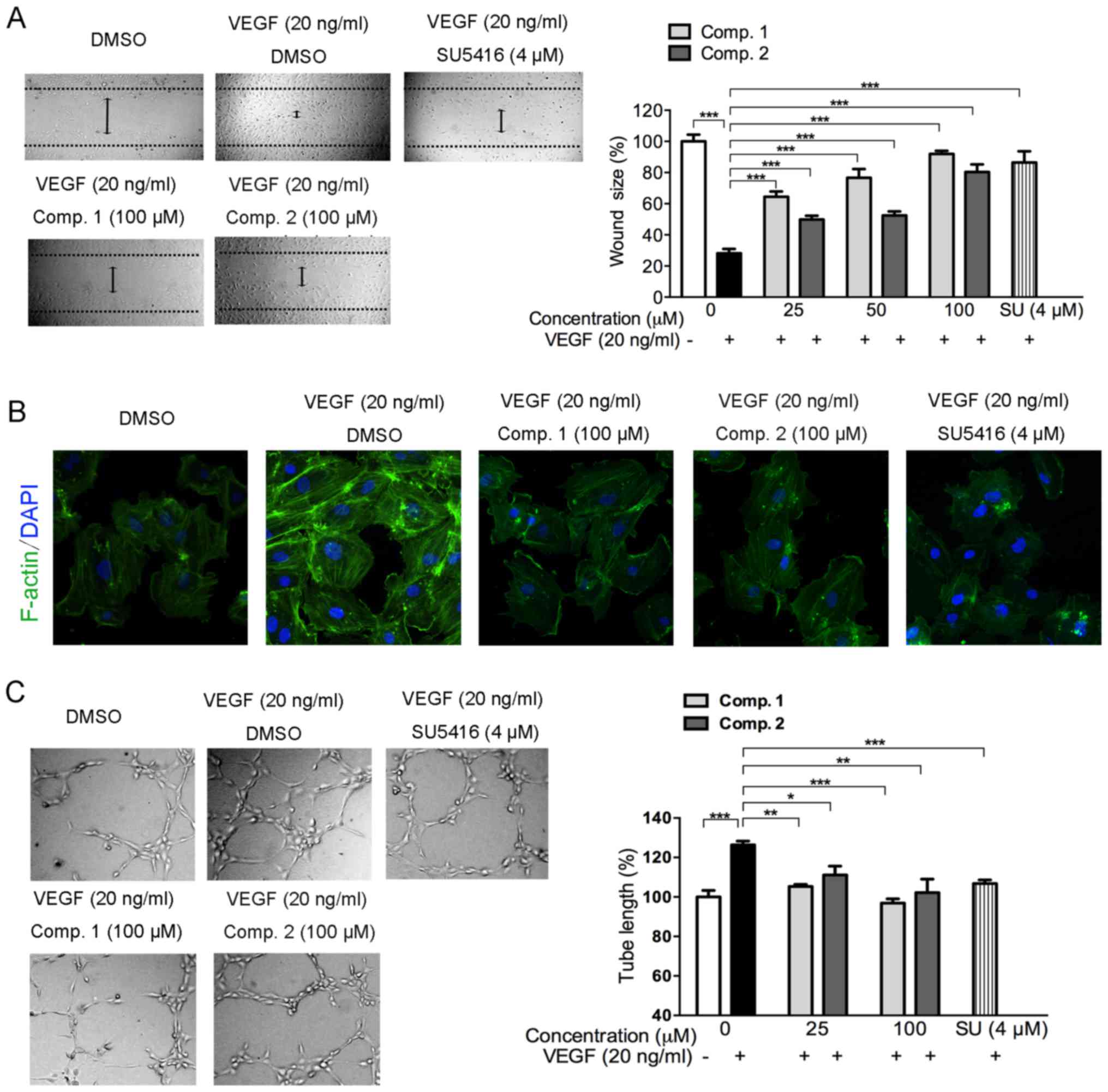 | Figure 2Effects of ETSs on VEGF-stimulated
HUVEC migration, stress fibers and tube formation. (A) Effect of
ETSs on VEGF-induced HUVECs migration. Starved HUVECs were
subjected to wound-healing assay as described in Materials and
methods (magnification, ×100). (B) Effect of ETSs on VEGF-induced
stress fibers formation in HUVECs. Immunofluorescent staining of
HUVECs with FITC-conjugated phalloidin for F-actin (green) and DAPI
(blue for nuclear). Starved HUVECs were treated with ETSs (100
µM) or SU (SU5416, 4 µM) for 4 h and then stimulated
with VEGF for 20 min, images were taken after immunostaining
processes by confocal microscope (magnification, ×1,200). (C)
Inhibition of VEGF-induced HUVECs tube formation by ETSs or SU
(SU5416). Cell tubular structures were captured (magnification,
×100) and tube length was quantified (n=3). Each bar represents the
mean ± SD, ***p<0.005, **p<0.01,
*p<0.05 for the comparison with cells exposed to VEGF
only. |
The activation of endothelial cell migration by VEGF
involves a massive remodeling of the actin cytoskeleton that
reorganizes into transcytoplasmic stress fibers (24). As shown in Fig. 2B, the immunofluorescent intensity
of F-actin (green) stained with FITC-conjugated phalloidin was
highly increased in the VEGF-stimulated HUVECs, which indicated an
increase of the stress fibers formation. However, such stress in
the endothelial cells was markedly attenuated in the presence of
ETSs (Fig. 2B). These results show
that ETSs might suppress the migration of the VEGF-induced HUVECs
by inhibiting the formation of the stress fiber.
During angiogenesis, a critical step is the
formation of the new capillary tube derived from the migrated
endothelial cells (4). Therefore,
we investigated whether ETSs could inhibit VEGF-induced endothelial
cell tube formation. As shown in Fig.
2C, a rough, but complete tube network was formed from the
VEGF-stimulated HUVECs. However, with the treatment of ETSs (100
µM), the tube formation pattern disassembled in a manner
similar to that of the vehicle group (p>0.05, data not shown).
Quantification of the tube length revealed that 25 µM ETSs
could abolish the VEGF-stimulated tube formation significantly
(p<0.05) (Fig. 2C), suggesting
a potent inhibitory effect of ETSs on the tubulogenesis of the
VEGF-induced endothelial cells.
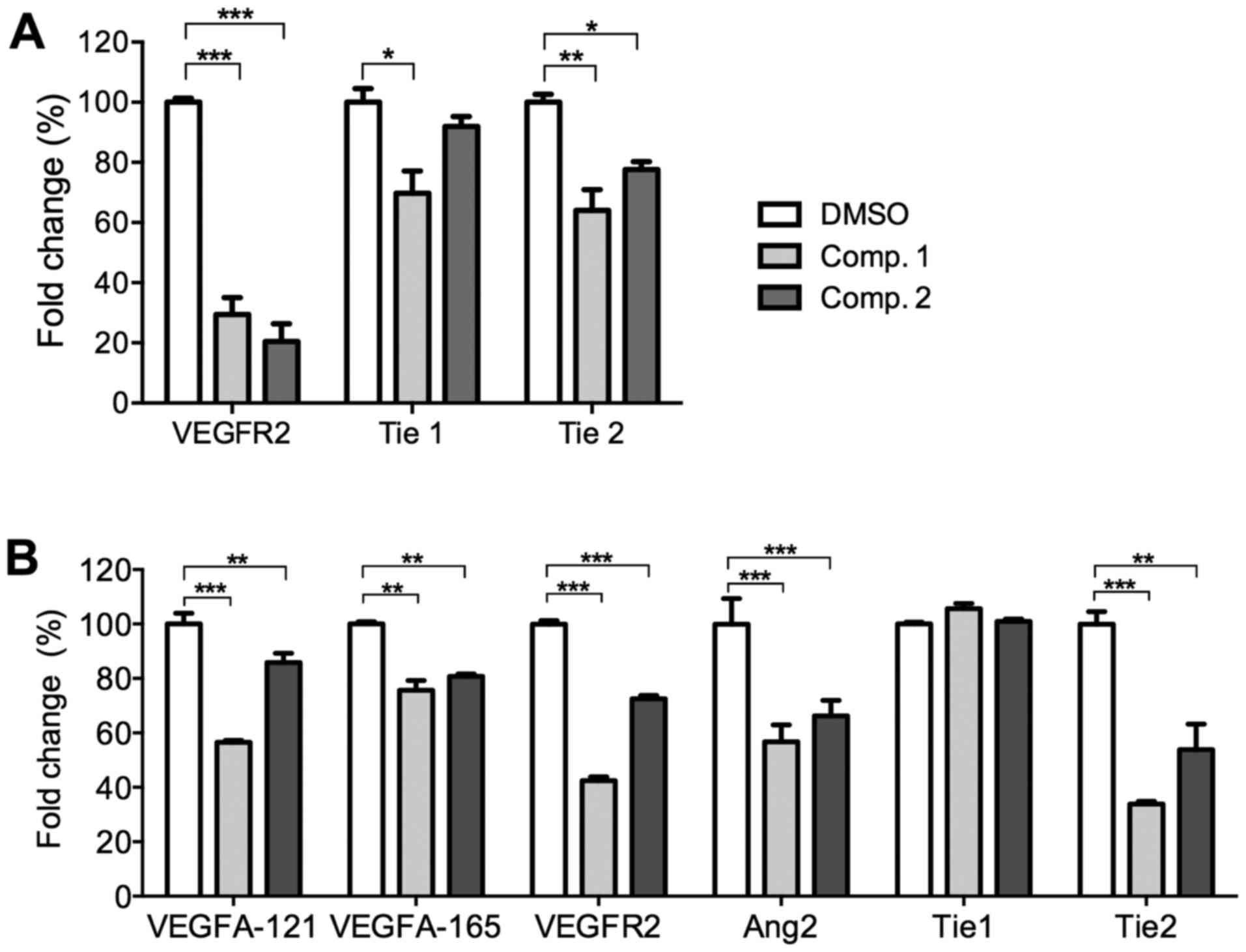
ETSs downregulate the
pro-angiogenic-related genes in HUVECs and zebrafish embryos
The effects of ETSs on the angiogenic-related gene
expression levels in HUVECs were evaluated by the real-time PCR
analysis. HUVECs were exposed to ETSs (50 µM) for 24 h, the
mRNA levels of VEGFR2 and Tie1/2 were then
determined. As shown in Fig. 3A,
both ETSs significantly inhibited the mRNA levels of VEGFR2
(p<0.005), the key receptor of VEGF. Only Comp. 1
downregulated the activity of the angiopoietin orphan receptor
Tie1 (p<0.01). However, both compounds significantly
reduced the mRNA level of angiopoietin receptor Tie2
(p<0.01 or 0.005). These data suggest that ETSs apparently acted
as inhibitors of angiogenesis by regulating VEGF/VEGFR2 and
Ang2/Tie2 pathways. Thus, the following results from in vivo
experiment focused on the two pathways.
To determine the effects of ETSs on the
pro-angiogenic-related gene expression levels in vivo,
zebrafish embryos were treated with ETSs (10 µM) for 24 h
and subjected to real-time PCR analysis. As shown in Fig. 3B, the mRNA levels of both
VEGFA and VEGFR2 were downregulated significantly in
the presence of ETSs. As for the Ang2/Tie axis, ETSs
downregulated the mRNA levels of Ang2 (p<0.01 or
p<0.005) and Tie2 receptor (p<0.01), but no
significance was found in Tie1 (Fig. 3B). The PCR data from the zebrafish
embryos further confirmed their anti-angiogenic activities.
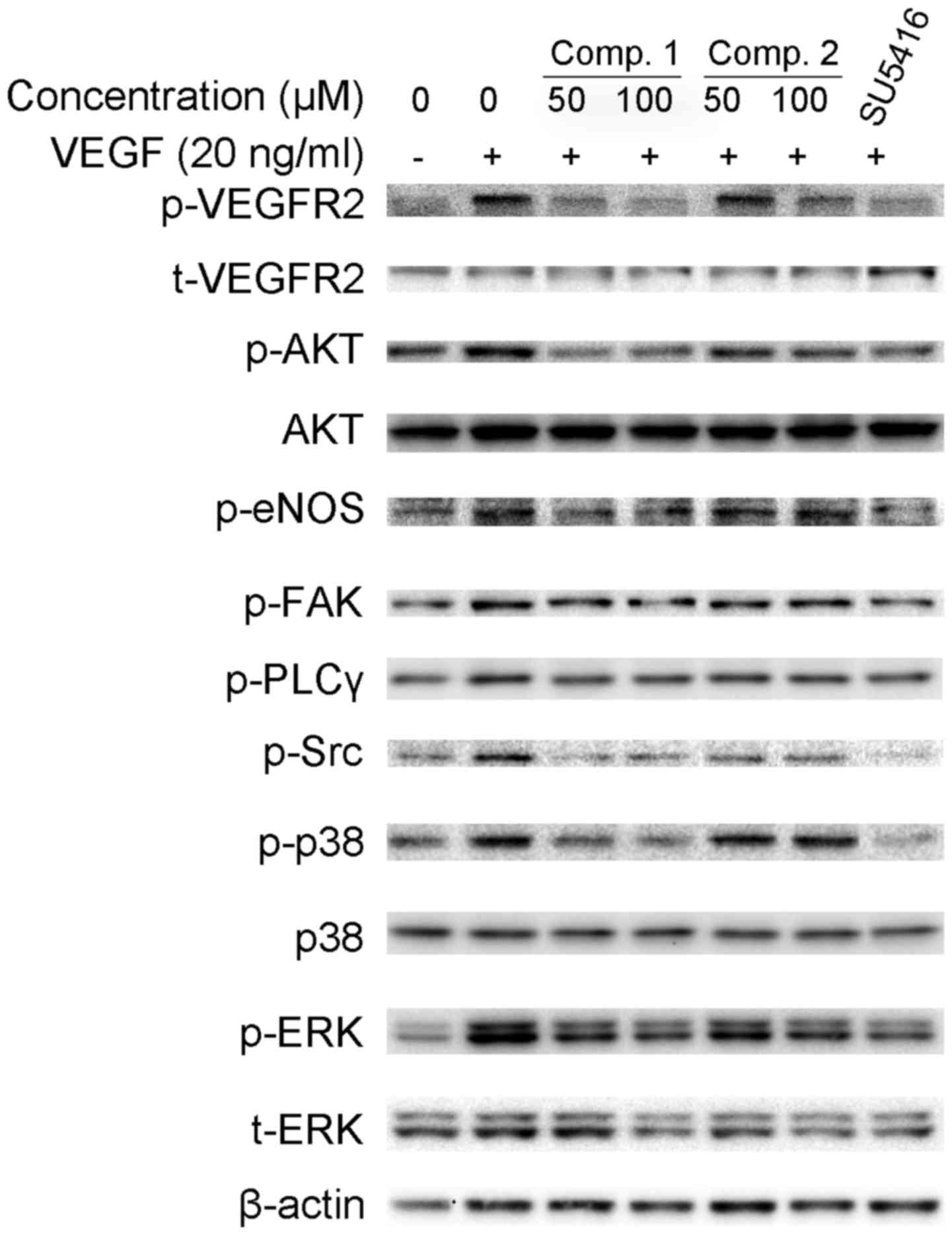
ETSs inhibit the activation of
VEGF/VEGFR2 signaling pathways in HUVECs
Our previous results proved that ETSs effectively
suppressed VEGF-stimulated angiogenesis both in vitro and
in vivo. To understand the molecular mechanism of
ETS-induced inhibition of angiogenesis, we investigated whether
ETSs suppressed the phosphorylation of VEGFR2, the critical
receptor tyrosine kinase on the surface of endothelial cells
(25). The addition of exogenous
VEGF to HUVECs strongly increased the phosphorylation of VEGFR2 at
Ser1175 site (Fig. 4). However,
pretreatment with ETSs suppressed the VEGF-stimulated VEGFR2
phosphorylation (Fig. 4).
VEGF binding to VEGFR2 activates various downstream
signaling molecules with distinct and overlapping functions, which
is responsible for endothelial cell proliferation, migration and
tube formation (5,26). Therefore, we explored whether ETSs
inhibited VEGF/VEGFR2-mediated signaling pathways. Starved HUVECs
were pretreated with ETSs for 4 h and subsequently stimulated by
VEGF for 20 min. As shown in Fig.
4, an extensive downregulation of the activation of
Src/AKT/eNOS signaling pathway was observed at 50 µM ETSs
(Fig. 4). ETSs also suppressed the
activation of the VEGFR2 downstream signaling molecules, namely,
FAK, PLCγ, ERK1/2 and p38. Taken together, these data suggested
that ETSs suppressed angiogenesis by inhibiting the
VEGF/VEGFR2-mediated Src/AKT/eNOS, FAK, PLCγ/ERK1/2, and p38
signaling pathways.
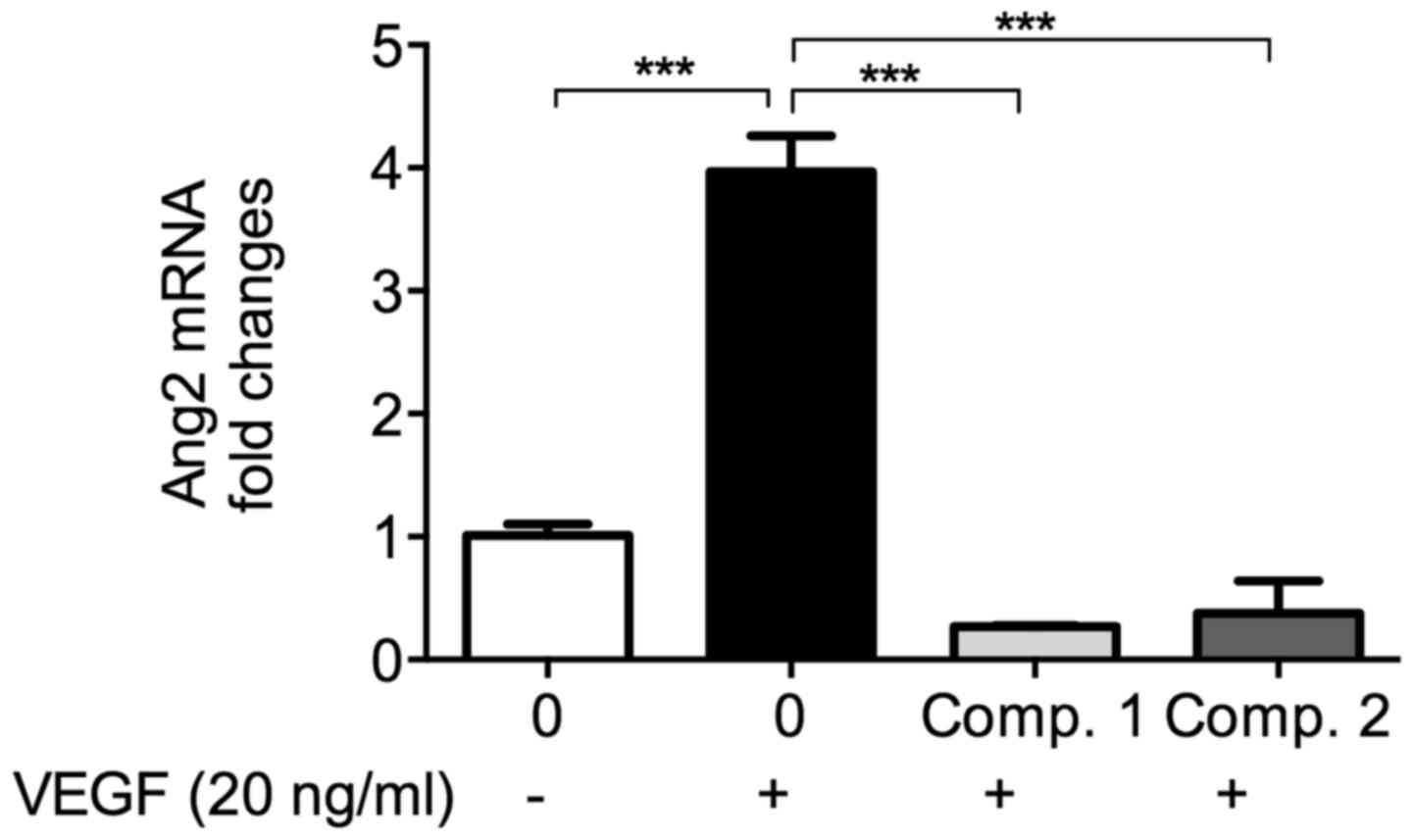
ETSs downregulate VEGF-enhanced Ang2 mRNA
level in HUVECs
With the stimulation of VEGF in endothelial cells,
the mRNA level of Ang2, another crucial pro-angiogenic factor,
increased (11). As shown in
Fig. 5, in the presence of VEGF,
the Ang2 mRNA level increased significantly compared to the
vehicle. Pretreatment by ETSs (50 µM) almost completely
reversed the increase induced by VEGF (p<0.001). VEGF induced
Ang2 secretion and synthesis, this Ang2 could bind with Tie2 and
promote the angiogenic activity of VEGF. The low Ang2 level of
ETS-treated group may indicate that Ang2/Tie2 might have taken part
in the ETS-induced anti-angiogenesis. To clarify the direct effects
of ETSs on Ang2/Tie2 axis, we completed the following
experiments.
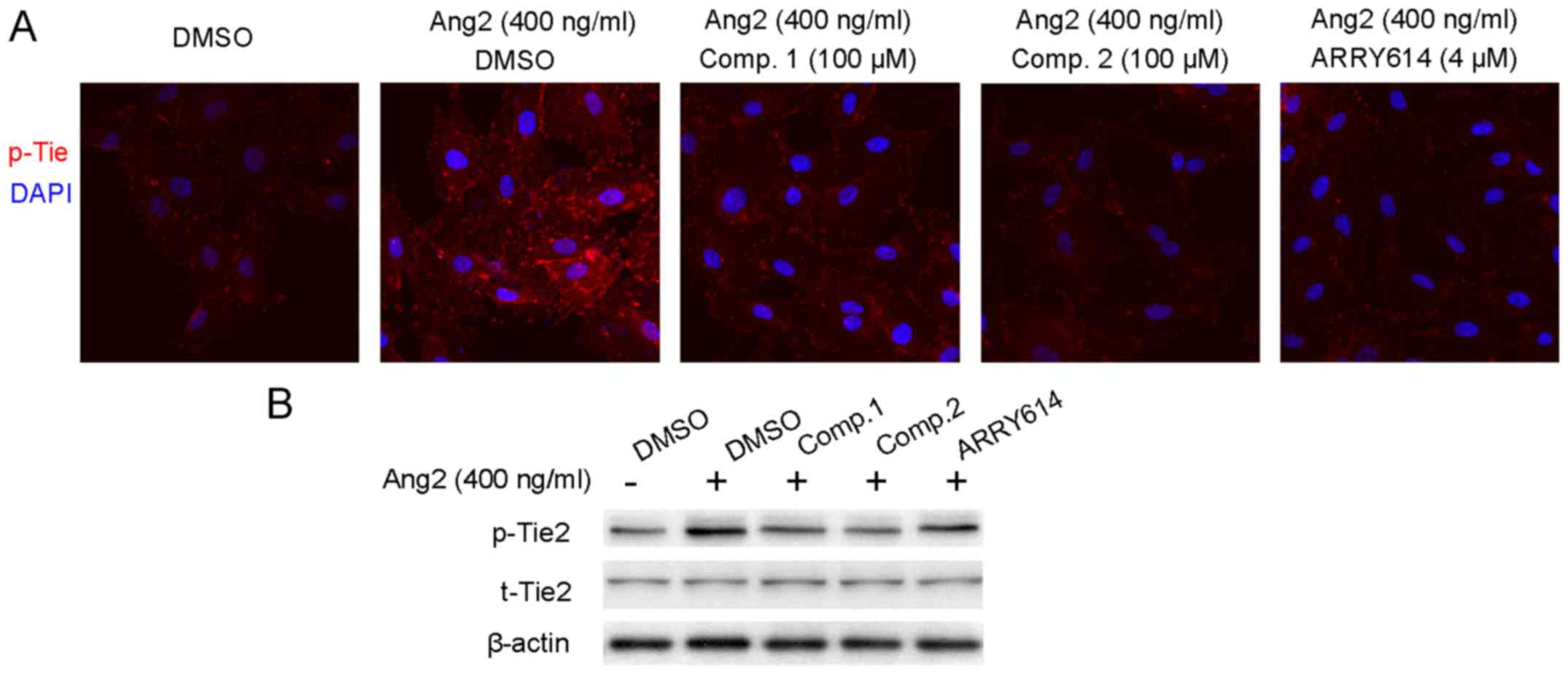
ETSs inhibit Ang2-induced phosphorylation
of Tie receptor
Ang2 acts as an agonist of Tie2 receptor and leads
to the phosphorylation of Tie2. This would result in vascular
destabilization and remodeling (22). Hence, we utilized exogenous Ang2 to
induce Tie2 phosphorylation, while the activation of Tie2 in the
presence of ETSs was evaluated by both immunofluorescent staining
and the western blotting. Starved HUVECs were pretreated with ETSs
(100 µM) for 4 h and stimulated with Ang2 for 30 min.
Compared to the vehicle group (DMSO-treated), Ang2 increased the
phosphorylation of Tie which occurred on the cell membrane
(Fig. 6A). However, for the group
treated with ETSs (100 µM), they showed weaker fluorescence
intensity than that with only the Ang2 stimulation, indicative of
the inhibition of the phosphorylation of Tie receptor in the
presence of ETSs. In the western blotting results (Fig. 6B), the data showed the inhibitory
effects of ETSs on Ang2-induced Tie2 phosphorylation.
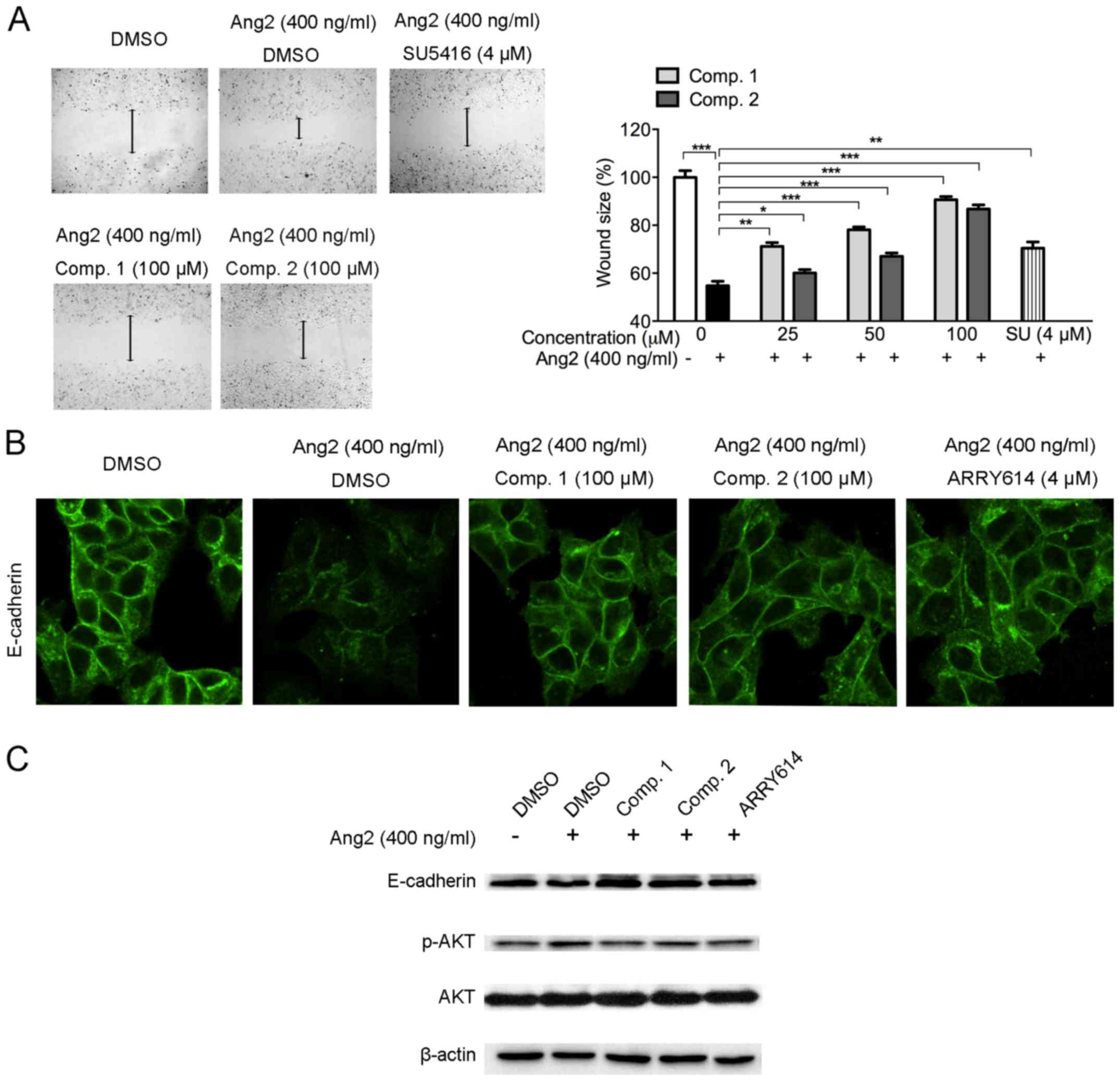
ETSs inhibit Ang2-induced MCF-7 cell
migration
Ang2 promotes the proangiogenic action of VEGF
leading to blood vessel growth. Besides, Ang2 also enhances tumor
growth and cancerous metastasis (27). In order to evaluate whether ETSs
could inhibit Ang2 stimulated cancer cell migration, we conducted
wound-healing analysis on breast cancer MCF-7 cells. Data revealed
that stimulation with Ang2 (400 ng/ml) highly enhanced cell
mobility, leading to smaller wound area (Fig. 7A). However, ETSs inhibited
Ang2-induced MCF7 cells migration dose-dependently from 25 to 100
µM. At 25 µM of ETSs, Comp. 1 exhibited stronger
inhibitory effect with larger wound area (p<0.005) than that of
Comp. 2 (Fig. 7A).
E-cadherin is a cell-cell adhesion molecule with
pivotal roles in tissue formation, epithelial cells behavior, and
is linked to the invasion of cancer cells (28). With the stimulation of Ang2 on
MCF-7 cells, we observed the loss of E-cadherin (Fig. 7B). Western blot assay of the
E-cadherin protein level also confirmed the loss of E-cadherin
induced by Ang2 (Fig. 7C).
However, ETS treatment suppressed the E-cadherin loss, both
immunofluorescent staining and western blot analysis showed the
inhibitory effect of ETSs. Besides, the p-AKT level was upregulated
after Ang2 stimulation in MCF-7 cells, while the ETS exposed cells
showed a sharp reduction of the p-AKT level (Fig. 7C). These data suggested that ETSs
inhibited Ang2-induced MCF-7 cell migration by suppressing
E-cadherin loss and AKT phosphorylation.
Discussion
Tumor growth is initially fed by the nearby blood
vessels in order to support the removal of waste from and supply of
nutrients to a tumor (29).
Therefore, anti-angiogenic therapy has been considered as a
promising method to treat cancer. Search for the appropriate
angiogenic inhibitors is a key step towards achieving this goal
(6). In the present study, to the
best of our knowledge, we show for the first time that ETSs
exhibited anti-angiogenic properties both in vivo and in
vitro.
Several activators in the angiogenic process have
been identified in many pro-angiogenic mechanisms. The VEGF/VEGFR
signaling pathway has been implicated as a critical regulator of
tumor neovasculation (7). In this
study, VEGF-stimulated HUVEC proliferation, migration, and tube
formation were inhibited dose-dependently by the ETSs at the
non-toxic concentrations (Figs. 1C
and 2). Moreover, ETSs inhibited
the VEGF-induced VEGFR2 phosphorylation in HUVECs (Fig. 4), suggesting that the ETSs may
likely function as VEGF/VEGFR2 inhibitors suppressing the
VEGF-stimulated angiogenesis.
Due to the strong tyrosine kinase activity of
VEGFR2, it transduces a series of downstream cascades in the
angiogenic process (30). AKT is a
serine/threonine kinase, a well-known VEGFR2 signaling downstream
mediator that plays a central role in a range of cellular functions
including cancer cell growth, migration, and angiogenesis (31). AKT/eNOS signaling has been
identified as a functional mediator in the VEGF-stimulated
angiogenesis, through the production of NO to mediate cell
cytoskeleton reorganization and increase vascular permeability
(31–33). Moreover, the function of AKT/eNOS
in the activation of VEGFR2 signaling pathway depends on its
upstream Src activation (34).
Treatment with ETSs has shown a sharp decrease in the
phosphorylation of SrcTyr416, AKTSer473 and eNOSSer1177 in the
endothelial cells (Fig. 4),
suggesting that the Src/AKT/eNOS pathway was involved in the
ETS-mediated anti-angiogenic molecular mechanism. VEGF/VEGFR2
system activates its downstream molecules, including FAK and PLCγ
to facilitate proliferation, migration and morphogenic
differentiation of endothelial cells into capillary-like structures
with vascular permeability (35,36).
VEGF relies on the activation of FAK to promote tumor vascular
permeability, progression, and metastasis (37). On the other hand, FAK partially
promotes ERK1/2 signaling cascade which is very important for
vascular endothelial cell proliferation after VEGF-recruited PLCγ
is activated (35,38). In our study, we observed that ETSs
inhibited endothelial cell proliferation and tube formation in
response to VEGF treatment, and with a sharp decrease in FAK,
ERK1/2 and PLCγ (Fig. 4),
indicating that ETSs suppressed the key steps of VEGF-stimulated
endothelial cells by inactivating FAK and PLCγ/ERK1/2 signaling
pathways. p38 was identified as an important player in VEGF-induced
endothelial cell mobility by promoting the formation of stress
fibers (24). Our results showed
that ETSs reduced significantly the formation of VEGF-induced
stress fibers and the phosphorylation of p38 (Figs. 2B and 4), suggesting that ETSs inhibited p38
activation to disrupt VEGF-induced stress fiber formation. These
mechanistic results reveal that ETSs inhibited the activation of
VEGFR2 and thereby suppressed the VEGFR2 downstream signaling
pathways in the endothelial cells.
Anti-VEGF and VEGFR2 drugs have been used clinically
and were shown to slow tumor growth, improve drug delivery, and
more importantly, to extend the lives of cancer patients, but drug
resistance of the inhibitors in the VEGF/VEGFR2 system limits their
clinical application (39). Thus,
other strategies for inhibiting the tumor neovascularization are
being sought. Mechanistic studies of the resistance showed that
anti-VEGF therapy involved the adaptive enforcement of Ang2/Tie2
system (12,40). Mounting evidence suggests that
combination of both anti-VEGF/VEGFR and Ang2/Tie2 agents targeting
the blood vessel network can lead to outcomes superior to those
using either agent alone (8,41).
It is well documented that VEGF upregulates the mRNA
level of Ang2 in endothelial cells and leads to Ang2 secretion
(9,11). The secreted Ang2 acts as a partial
agonist of Tie2 receptor leading to Tie2 phosphorylation,
disturbances of vascular endothelial cell junction integrity and
vascular sprouting (11,40,42).
In the present study, VEGF upregulated the mRNA level of Ang2 but
was suppressed by ETSs (Fig. 5).
Besides, ETSs reduced exogenous Ang2-induced Tie2 phosphorylation
in endothelial cells (Fig. 6). Our
results indicated that ETSs not only suppressed VEGF/VEGFR2 axis
but also the Ang2/Tie2 system in the endothelial cells. This makes
ETSs promising anti-angiogenic candidates with dual targets.
Higher circulating amount of Ang2 was detected in
the plasma of cancer patients at the advanced stage, and Ang2
facilitated the metastatic ability of cancer cells, such as breast
and ovarian cancers (43). MCF-7
cells have a weak metastatic ability, but Ang2 can upregulate their
mobility in tumor-bearing mice and cells. E-cadherin is a cell-cell
adhesion molecule with pivotal roles in tissue formation,
epithelial cell behavior and suppression of cancer, and it is
linked to the invasion of breast cancer cells (28). We have observed that ETSs showed a
sharp reduction in the migration of Ang2-induced MCF-7 cells,
suppression of the loss in E-cadherin, and decrease in the
expression of p-AKT (Fig. 7).
To further ascertain the anti-angiogenic effects by
ETSs, in vivo experiments were conducted in the zebrafish
embryo which is a successful animal model for studying angiogenesis
and evaluating anti-angiogenic agents (44,45).
Small molecules added directly to the culture media containing the
zebrafish embryos could diffuse into the embryos and induce
angiogenic effect (44). Our
results demonstrated that exposure of ETSs altered the process of
angiogenesis in zebrafish embryos (Figs. 1D and 3B). Therefore, the two compounds
exhibited anti-angiogenic activities in vivo.
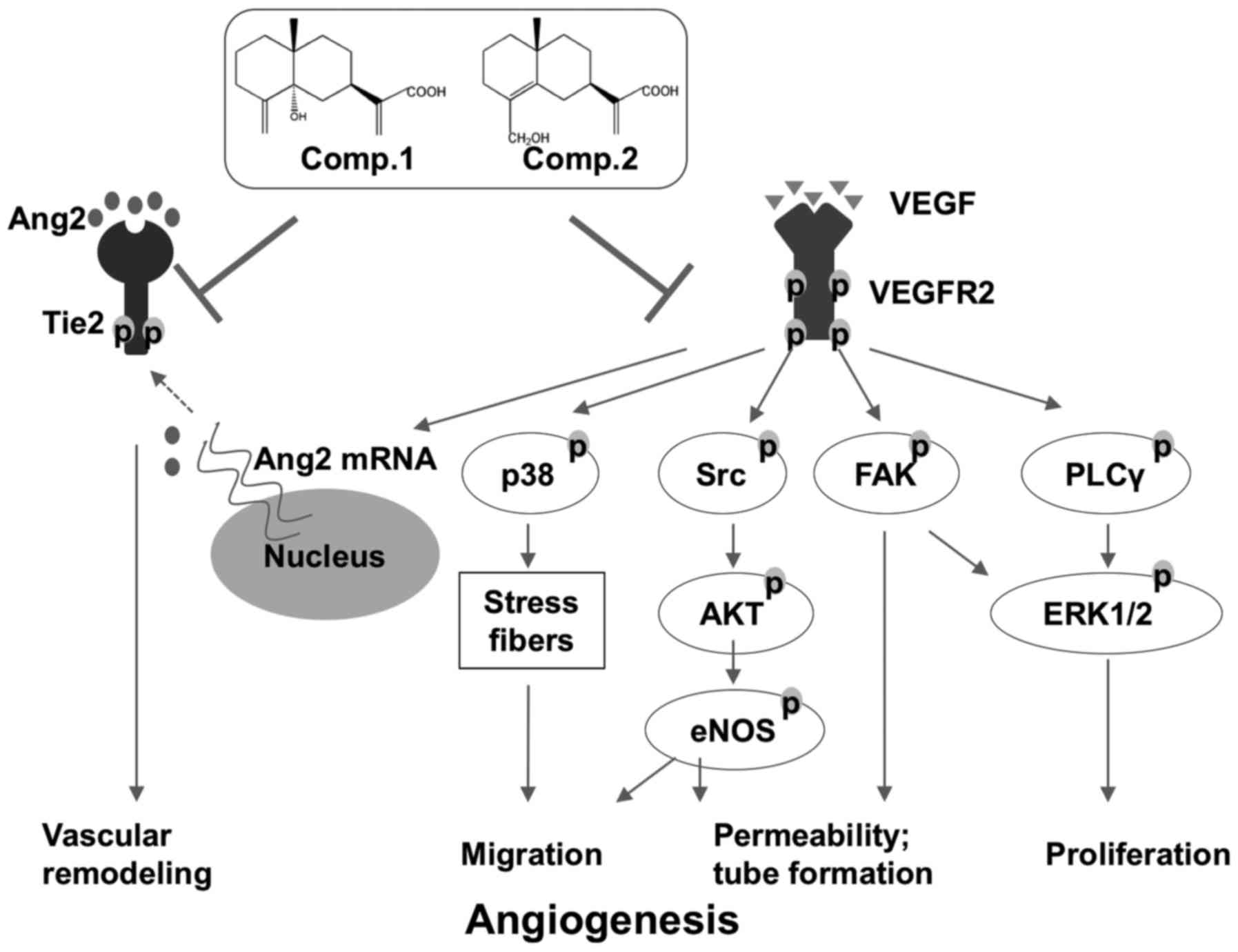
In conclusion, we provide evidence in the present
study that ETSs inhibited the proliferation, migration and tube
formation in VEGF-induced HUVECs. Suppressing the VEGF/VEGFR2
system following the treatment with ETSs could result in the
multiple inhibitions of a number of downstream VEGF-dependent
pathways, including Src/AKT/eNOS, FAK, PLCγ/ERK1/2 and p38
pathways, and contribute to ETS-mediated anti-angiogenic property
(Fig. 8). Furthermore, ETSs
effectively block Ang2/Tie2 axis, implying that ETSs may function
as angiogenic inhibitors with dual targets (Fig. 8). Collectively, these findings
suggest that the ETSs may be developed into a new type of
anti-angiogenic agent for the treatment of breast cancer.
Acknowledgments
This study was supported in part by the CUHK grants
(3132789 and FACULTY-P17179).
References
|
1
|
Folkman J: Fighting cancer by attacking
its blood supply. Sci Am. 275:150–154. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folkman J: Angiogenesis. Annu Rev Med.
57:1–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herbert SP and Stainier DY: Molecular
control of endothelial cell behaviour during blood vessel
morphogenesis. Nat Rev Mol Cell Biol. 12:551–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taimeh Z, Loughran J, Birks EJ and Bolli
R: Vascular endothelial growth factor in heart failure. Nat Rev
Cardiol. 10:519–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jayson GC, Kerbel R, Ellis LM and Harris
AL: Antiangiogenic therapy in oncology: Current status and future
directions. Lancet. 388:518–529. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Biel NM and Siemann DW: Targeting the
angiopoietin-2/Tie-2 axis in conjunction with VEGF signal
interference. Cancer Lett. 380:525–533. 2016. View Article : Google Scholar
|
|
9
|
Augustin HG, Koh GY, Thurston G and
Alitalo K: Control of vascular morphogenesis and homeostasis
through the angiopoietin-Tie system. Nat Rev Mol Cell Biol.
10:165–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fagiani E and Christofori G: Angiopoietins
in angiogenesis. Cancer Lett. 328:18–26. 2013. View Article : Google Scholar
|
|
11
|
Gerald D, Chintharlapalli S, Augustin HG
and Benjamin LE: Angiopoietin-2: An attractive target for improved
antiangiogenic tumor therapy. Cancer Res. 73:1649–1657. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rigamonti N, Kadioglu E, Keklikoglou I,
Wyser Rmili C, Leow CC and De Palma M: Role of angiopoietin-2 in
adaptive tumor resistance to VEGF signaling blockade. Cell Rep.
8:696–706. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kienast Y, Klein C, Scheuer W, Raemsch R,
Lorenzon E, Bernicke D, Herting F, Yu S, The HH, Martarello L, et
al: Ang-2-VEGF-A CrossMab, a novel bispecific human IgG1 antibody
blocking VEGF-A and Ang-2 functions simultaneously, mediates potent
antitumor, antiangiogenic, and antimetastatic efficacy. Clin Cancer
Res. 19:6730–6740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu YH, Zhang XM, Hu MH, Wu XM and Zhao Y:
Effect of Laggera alata on hepatocyte damage induced by carbon
tetrachloride in vitro and in vivo. J Ethnopharmacol. 126:50–56.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu Y, Zhou C, Song L, Li X, Shi S, Mo J,
Chen H, Bai H, Wu X, Zhao J, et al: Effect of total phenolics from
Laggera alata on acute and chronic inflammation models. J
Ethnopharmacol. 108:243–250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang GC, Li GQ, Geng HW, Li T, Xu JJ, Ma
F, Wu X, Ye WC and Li YL: Eudesmane-type sesquiterpene derivatives
from Laggera alata. Phytochemistry. 96:201–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pratheeshkumar P, Budhraja A, Son YO, Wang
X, Zhang Z, Ding S, Wang L, Hitron A, Lee JC, Xu M, et al:
Quercetin inhibits angiogenesis mediated human prostate tumor
growth by targeting VEGFR- 2 regulated AKT/mTOR/P70S6K signaling
pathways. PLoS One. 7:e475162012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang W, Wang J, Liang Y, Ge W, Wang G, Li
Y and Chung HY: Potent anti-angiogenic component in Croton
crassifolius and its mechanism of action. J Ethnopharmacol.
175:185–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li M, Wu S, Liu Z, Zhang W, Xu J, Wang Y,
Liu J, Zhang D, Tian H, Li Y, et al: Arenobufagin, a bufadienolide
compound from toad venom, inhibits VEGF-mediated angiogenesis
through suppression of VEGFR-2 signaling pathway. Biochem
Pharmacol. 83:1251–1260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin S, Ching LT, Chen J and Cheung PCK:
Antioxidant and anti-angiogenic effects of mushroom phenolics-rich
fractions. J Funct Foods. 17:802–815. 2015. View Article : Google Scholar
|
|
21
|
Li H, Li M, Wang G, Shao F, Chen W, Xia C,
Wang S, Li Y, Zhou G and Liu Z: EM23, A natural sesquiterpene
lactone from Elephantopus mollis, induces apoptosis in human
myeloid leukemia cells through thioredoxin- and reactive oxygen
species-mediated signaling pathways. Front Pharmacol. 7:772016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bogdanovic E, Nguyen VP and Dumont DJ:
Activation of Tie2 by angiopoietin-1 and angiopoietin-2 results in
their release and receptor internalization. J Cell Sci.
119:3551–3560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robert S and Kerbel PD: Tumor
angiogenesis. N Engl J Med. 358:2039–2049. 2008. View Article : Google Scholar
|
|
24
|
Rousseau S, Houle F, Kotanides H, Witte L,
Waltenberger J, Landry J and Huot J: Vascular endothelial growth
factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and
requires concerted activation of stress-activated protein kinase 2
(SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal
adhesion kinase. J Biol Chem. 275:10661–10672. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shibuya M: VEGF-VEGFR signals in health
and disease. Biomol Ther (Seoul). 22:1–9. 2014. View Article : Google Scholar
|
|
26
|
Ha CH, Bennett AM and Jin ZG: A novel role
of vascular endothelial cadherin in modulating c-Src activation and
downstream signaling of vascular endothelial growth factor. J Biol
Chem. 283:7261–7270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eroglu Z, Stein CA and Pal SK: Targeting
angiopoietin-2 signaling in cancer therapy. Expert Opin Investig
Drugs. 22:813–825. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wahl O, Oswald M, Tretzel L, Herres E,
Arend J and Efferth T: Inhibition of tumor angiogenesis by
antibodies, synthetic small molecules and natural products. Curr
Med Chem. 18:3136–3155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen X, Zhao M, Hao M, Sun X, Wang J, Mao
Y, Zu L, Liu J, Shen Y, Wang J, et al: Dual inhibition of PI3K and
mTOR mitigates compensatory AKT activation and improves tamoxifen
response in breast cancer. Mol Cancer Res. 11:1269–1278. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ying L and Hofseth LJ: An emerging role
for endothelial nitric oxide synthase in chronic inflammation and
cancer. Cancer Res. 67:1407–1410. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lim KH, Ancrile BB, Kashatus DF and
Counter CM: Tumour maintenance is mediated by eNOS. Nature.
452:646–649. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Di Lorenzo A, Lin MI, Murata T,
Landskroner-Eiger S, Schleicher M, Kothiya M, Iwakiri Y, Yu J,
Huang PL and Sessa WC: eNOS-derived nitric oxide regulates
endothelial barrier function through VE-cadherin and Rho GTPases. J
Cell Sci. 126:5541–5552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sulzmaier FJ, Jean C and Schlaepfer DD:
FAK in cancer: Mechanistic findings and clinical applications. Nat
Rev Cancer. 14:598–610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu G, Luo J, Rana JS, Laham R, Sellke FW
and Li J: Involvement of COX-2 in VEGF-induced angiogenesis via P38
and JNK pathways in vascular endothelial cells. Cardiovasc Res.
69:512–519. 2006. View Article : Google Scholar
|
|
37
|
Chen XL, Nam JO, Jean C, Lawson C, Walsh
CT, Goka E, Lim ST, Tomar A, Tancioni I, Uryu S, et al:
VEGF-induced vascular permeability is mediated by FAK. Dev Cell.
22:146–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mariappan MM, Senthil D, Natarajan KS,
Choudhury GG and Kasinath BS: Phospholipase Cgamma-Erk Axis in
vascular endothelial growth factor-induced eukaryotic initiation
factor 4E phosphorylation and protein synthesis in renal epithelial
cells. J Biol Chem. 280:28402–28411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shojaei F: Anti-angiogenesis therapy in
cancer: Current challenges and future perspectives. Cancer Lett.
320:130–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moss A: The angiopoietin:Tie 2
interaction: a potential target for future therapies in human
vascular disease. Cytokine Growth Factor Rev. 24:579–592. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Scholz A, Plate KH and Reiss Y:
Angiopoietin-2: A multifaceted cytokine that functions in both
angiogenesis and inflammation. Ann NY Acad Sci. 1347:45–51. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yuan HT, Khankin EV, Karumanchi SA and
Parikh SM: Angiopoietin 2 is a partial agonist/antagonist of Tie2
signaling in the endothelium. Mol Cell Biol. 29:2011–2022. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cascone T and Heymach JV: Targeting the
angiopoietin/Tie2 pathway: Cutting tumor vessels with a
double-edged sword? J Clin Oncol. 30:441–444. 2012. View Article : Google Scholar
|
|
44
|
Serbedzija GN, Flynn E and Willett CE:
Zebrafish angiogenesis: A new model for drug screening.
Angiogenesis. 3:353–359. 1999. View Article : Google Scholar
|
|
45
|
Tobia C, Gariano G, De Sena G and Presta
M: Zebrafish embryo as a tool to study tumor/endothelial cell
cross-talk. Biochim Biophys Acta. 1832:1371–1377. 2013. View Article : Google Scholar : PubMed/NCBI
|