Introduction
Multidrug resistance (MDR) is the main cause of
chemotherapeutic treatment failure in cancer patients. As a
difficult problem, combating MDR cancer has always been an
important mission of oncotherapy. The mechanisms of MDR are complex
and obscure (1,2). The major mechanism of MDR involves
the overexpression of ATP-binding cassette (ABC) transporters. The
ABC family of transporters comprises 49 members that are subdivided
into seven subfamilies, ABC-A to ABC-G, based on their sequences
and structures. Among known ABC transporters, ABCB1, ABCC1 and
ABCG2 are the most influential members in MDR cancer (3,4).
ABCB1 (P-glycoprotein), encoded by the MDR1 gene, is a 170-kDa
apical plasma membrane protein comprising two homologous halves,
each containing an ATP-binding domain and six transmembrane helices
(5,6). ABCB1 is an ATP-dependent drug efflux
pump that can transport a wide range of amphipathic and hydrophobic
anticancer drugs, such as doxorubicin (DOX), vincristine (VCR),
paclitaxel, epipodophyllotoxins, anthracyclines and taxanes
(7). Thus, antitumor agents that
are ABCB1 substrates and are used in long-term treatment
subsequently stimulate cancer cells to upregulate the ABCB1 mRNA
copy number. The overexpression of ABCB1 results in decreased
intracellular antineoplastic drug concentrations to sub-toxic
levels. Thus, resensitizing MDR cancer cells to anticancer drugs
using inhibitors that block the efflux system may be a successful
chemotherapeutic strategy. However, in the past three decades, most
ABCB1 modulators have failed in clinical trials due to their low
binding affinities, excessive toxicity or non-specificity (8–12).
Thus, the identification of new ABCB1 transporter inhibitors with
lower toxicity but greater potency and specificity is extremely
urgent.
Uncaria, a traditional Chinese medicine, is a member
of the Uncaria rhynchophylla (Miq.) Jacks. family and is
used to treat neoplastic diseases, various cerebrovascular
diseases, nervous disorders and symptoms associated with
hypertension, epilepsy, stroke and preeclampsia (13,14).
Various active constituents have been isolated, such as alkaloids,
flavonoids, sterols, coumarins, terpenoids and terpenoid glycosides
(15). A number of alkaloids in
uncaria are used for cancer treatment and adjuvant chemotherapy,
among which rhynchophylline (Rhy), isorhynchophylline (Irhy),
corynoxeine (Cory), isocorynoxeine (Icory), hirsutine (HS) and
hirsuteine (HST) are the major components. The exact antitumor
activities and mechanisms of these uncaria alkaloids remain to be
identified.
Herein, six uncaria alkaloids were preliminarily
screened, and all these compounds exhibited potential MDR-reversal
activity. Among them, Icory had the strongest activity. Therefore,
we used Icory as a representative compound to study in depth its
MDR-reversal activity and to further clarify its underlying
mechanisms.
Materials and methods
Chemicals and reagents
Rhy, Irhy, Cory, Icory, HS and HST were purchased
from YUANYE (Shanghai, China), and their purities determined by
HPLC were >98%. VCR, DOX, paclitaxel and cisplatin were
purchased from Selleck Chemicals (Houston, TX, USA). The Pgp-Glo™
assay kit was purchased from Promega (Fitchburg, WI, USA). The
Quanscript RT kit and Multi-PCR kit were obtained from
Multisciences (Hangzhou, Zhejiang, China). Mouse anti-ABCB1
antibody was purchased from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). Mouse anti-β-actin antibody was acquired from Cell
Signaling Technology (Beverly, MA, USA). Verapamil (VRP),
Fumitremorgin C (FTC), Mitoxantrone (MX), MK-571,
3-(4,5-dimethylthiazol-yl)-2,5-diphenyllapatinibrazolium bromide
(MTT), 2-(4-amidinophenyl)-6-indolecarbamidene dihydrochloride
(DAPI), dimethyl sulfoxide (DMSO) and other chemicals were obtained
from Sigma-Aldrich (St. Louis, MO, USA).
Cell lines and cell culture
The human hepatocellular carcinoma cell line HepG2
and the ABCB1-overexpressing cell line HepG2/ADM were kindly
provided by Professor Kwok-Pui Fung (The Chinese University of Hong
Kong, Hong Kong, China). The human breast cancer cell line MCF-7
and its doxorubicin-selected ABCB1-overexpressing cell line
MCF-7/ADR were previously provided by Professor Li-Wu Fu (Sun
Yat-Sen University, Guangzhou, China). The human primary embryonic
kidney cell line HEK293 and its pcDNA3.1, ABCB1-transfected cell
lines HEK293/pcDNA3.1 and HEK293/MRP1 were kindly provided by Dr
Suresh Ambudkar (NCI, NIH, Bethesda, MD, USA). The colorectal
cancer cell line S1 and ABCG2 overexpressing S1-M1-80 cells were
kindly provided by Dr Susan Bates and Robert Robey (NCI, NIH,
Bethesda, MD, USA). All of the cell lines were maintained in DMEM
supplemented with 10% fetal bovine serum and 1%
penicillin-streptomycin in a humidified incubator containing 5%
CO2 at 37°C. HepG2/ADM and MCF-7/ADR cells were cultured
in media containing 1.2 μM DOX to maintain their resistance
features.
Cytotoxicity determination by the MTT
assay
The MTT assay was used to detect the cytotoxic
activity and MDR-reversal activity of uncaria alkaloids and VRP.
Cells (5×103/well) were distributed into 96-well plates
and were maintained overnight. For the cytotoxicity experiments,
cells were cultured with different concentrations of uncaria
alkaloids (Rhy, Irhy, Cory, Icory, HS and HST) or with VRP for 72
h. To evaluate the reversal activity of these alkaloids, various
concentrations of the chemotherapeutic drugs with or without
alkaloids at sub-toxic concentrations were added into the
designated wells. After 72 h of incubation, 30 μl of MTT (5
mg/ml) was added to each well, and the plates were further
incubated at 37°C for 4 h. The medium was then removed, and 100
μl of DMSO was added into each well to dissolve the formazan
crystals. The absorbance was read at 570 nm using a DTX 880
Multimode Detector (Beckman Coulter, Brea, CA, USA). The
IC50 value was calculated from the survival curves using
GraphPad Prism 5.0 software. The reversal fold was calculated by
dividing the IC50 of antineoplastic agents alone by the
IC50 of antineoplastic agents in the presence of
alkaloids. VRP was used as a positive control.
Microscopic observation of intracellular
DOX
HepG2/ADM and MCF-7/ADR cells were seeded into 35-mm
dishes for 24 h before being pretreated with Icory or VRP for 2 h,
then 10 μM DOX were added and further co-incubated with
modulators for 2 h. Subsequently, the cells were washed with PBS
and fixed with 4% paraformaldehyde. DAPI was used to stain the
nuclei. Cellular DOX fluorescence was detected by fluorescence
microscopy (Axio Imager A2, Zeiss, Jena, Germany).
DOX accumulation assay
To measure the accumulation of DOX, 2×104
cells/well were seeded in 96-well plates for 24 h. Cells were
pretreated with Icory (25 and 50 μM) or VRP (50 μM)
at 37°C for 2 h and were then incubated with DOX at a concentration
of 10 μM in the absence or presence of modulators for 2 h at
37°C. Subsequently, the medium was discarded, and the cells were
washed three times with ice-cold PBS and soaked in 50 μl of
PBS. The fluorescence value of DOX was detected using a DTX 880
Multimode Detector (Beckman Coulter), and the excitation and
emission wavelengths of DOX were 488 and 535 nm, respectively.
DOX efflux assay
Cells (5×103 per well) were distributed
into 96-well plates and incubated for 24 h before being pretreated
with 25 μM DOX at 37°C for 2 h, after which time the cells
were supplemented with reversal agents (Icory or VRP). After
various time points (0, 30, 70, 130 min), the cell culture
supernatant was discarded, and the cell cultures were immediately
washed three times with ice-cold PBS. The cells were fixed with
paraformaldehyde for the detection of radioactivity, in a routine
manner.
Real-time fluorescence quantitative PCR
assay
A real-time fluorescence quantitative PCR (qRT-PCR)
assay was used to determine the mRNA expression of ABCB1. HepG2/ADM
or MCF-7/ADR cells (5×105/well) were seeded in 6-well
plates and were treated with various concentrations of Icory for 24
h or 48 h. Total RNA was then collected using a TRIzol kit (Qiagen,
Hilden, Germany) according to the manufacturer's protocol. cDNA was
reverse transcribed from 1 μg of total RNA using a reverse
transcription kit (Roche, Mannheim, Germany), and the forward and
reverse primers used in the qRT-PCR were as follows:
5′-CAGAGTCAAGGAGCATGGCA-3 (MDR1 forward primer),
5′-TCAGAGTTCACTGGCGCTTT-3 (MDR1 reverse primer); GAPDH-F:
5′-GAAGGTGAAGGTCGGAGTC-3′, GAPDH-R: 5′-GAAGATGGTGATGGGATTTC-3′.
Amplification was performed according to the instructions for the
SYBR Green Master Mix (Roche) using a Roche LightCycler (Basel,
Basel-Stadt, Switzerland). PCR was performed at 95°C for 10 sec of
initial denaturation and then at 60°C for 20 sec, 72°C for 20 sec
for 45 cycles. Relative expression levels were calculated using the
2−ΔΔCt method for qRT-PCR.
Western blotting
Cells were collected after incubation with 25 or 50
μM Icory for 24 or 48 h, lysed with RIPA lysis buffer (10 mM
Tris HCl pH 7.5, 1 mM EDTA, 0.1% SDS, 150 mM NaCl, 1% Triton X-100
and 0.01% leupeptin) for 0.5 h and centrifuged at 14,000 × g at 4°C
for 10 min. The supernatant was stored at −80°C until gel
electrophoresis. Protein concentrations were detected using the
bicinchoninic acid (BCA)-based protein assay (Thermo Scientific,
Rockford, IL, USA). A total of 30 μg of protein from the
cell lysates was separated by 10% SDS-PAGE and transferred onto a
PVDF membrane (Millipore, Billerica, MA, USA). The membranes were
blocked in blocking buffer (5% non-fat dry milk in TBST buffer) and
were then immunoblotted with ABCB1 antibody (1:500) overnight at
4°C. After washing three times with TBST buffer, the membranes were
further incubated for 1 h at room temperature with HRP-conjugated
secondary antibody (1:2,000). Protein detection on the blot was
performed using an enhanced chemiluminescence detection kit
(Beyotime Institute of Biotechnology, Shanghai, China).
Immunofluorescence assay
Cells (1×105) were incubated with 50
μM of Icory for 24 and 48 h, respectively. Then cells were
washed thrice with PBS and fixed with 4% paraformaldehyde for 20
min at room temperature. Subsequently, cells were blocked with 5%
BSA for 1 h at room temperature, followed by monoclonal antibody
(1:50) against ABCB1 at 4°C overnight. After that cells were rinsed
with PBS and then stained with Alexa fluor 488-conjugated goat
anti-mouse secondary antibody (1:500) for 1 h at room temperature
in the dark. DAPI (1 μg/μl) was used to counterstain
the nuclei. Images were taken by a confocal microscope (LSM 700,
Zeiss) at 495-nm excitation and 519-nm emission wavelength (FITC
488) and 340-nm excitation and 488-nm emission wavelength
(DAPI).
ABCB1 ATPase assay
The ABCB1-Glo™ ATPase assay was used to detect the
effects of Icory on recombinant human ABCB1. The ABCB1 ATPase
inhibitor Na3VO4 (250 μM), VRP (500
μM) and Icory (25, 50 and 100 μM) were prepared and
added to untreated white polystyrene 96-well plates, and then
ABCB1-containing membranes were added to each well and incubated at
37°C for approximately 5 min. The reactions were initiated by
adding 10 μl of MgATP (25 mM) and were then incubated for 40
min at 37°C. The plate was removed from 37°C, and luminescence was
initiated by adding 50 μl of Detection Reagent. Next, the
plate was incubated at room temperature for 20 min to stabilize the
luminescent signal, and the luminescence was read on a DTX880
Multimode Detector (Beckman Coulter).
Duration of MDR-reversal effect
assay
The durations of Icory and VRP reversal effects were
measured as previously described (16). HepG2/ADM cells (5×103
cells) were pretreated with Icory or VRP at a concentration of 50
μM for 24 h. In the durative group, the MDR modulators were
removed, and the cells were washed with PBS and then treated with
various concentrations of DOX. In the unwashed group, different
concentrations of DOX were added to retain the MDR modulators. Cell
viability was detected using the MTT assay, as described above.
Docking assay
The crystal structure of mouse ABCB1 was obtain from
the protein data bank (PDB entry code: 3G5U), which was further
prepared by using Surflex-Dock in Sybyl 8.0. Halved structure of
3G5U was used as the receptor in the following docking experiment.
The low-energy 3D structure of Icory and VPR was generated based on
the random conformational search with restricted chirality, which
was performed using the standard MMFF94s molecular mechanics force
field and MMFF94 charge. Optimized 3D structures of VRP and Icory
were docked into the binding site of ABCB1 by using Surflex-dock in
Sybyl 8.0 to simulate the binding modes. The best docking score was
used in the docking process to identify the preferable conformation
of Icory and ABCB1. Icory and VRP were docked into the binding site
of ABCB1 according to their best docking scores, and further
interactions and binding energy were analyzed.
Data analysis
Every experiment was repeated at least three times,
and values are shown as the mean ± SD. The data were analyzed using
GraphPad Prism 5.0, and the differences between two groups were
analyzed by Student's t-test. The P-value significance threshold
was set at P<0.05.
Results
Cytotoxicity of uncaria alkaloids and VRP
in sensitive and resistant cells
The sub-cytotoxic concentrations (cell survival rate
of at least 90%) of uncaria alkaloids (Rhy, Irhy, Cory, Icory, HS
and HST) and VRP in sensitive and resistant cell lines were
determined. A concentration of 50 μM rather than 100
μM met the requirement for Rhy, Irhy, Cory and Icory
(Fig. 1B). A surprising finding
was that HS and HST showed potential antitumor activity in
HepG2/ADM cells and in the sensitive parental cell line HepG2, and
their sub-cytotoxic concentrations fell to 3 μM (Fig. 1B). To further test the reversal
activity and to explore the underlying mechanisms of Icory, a pair
of DOX-resistant and -sensitive cell lines, MCF-7/ADR and MCF-7,
were chosen. Similarly, a non-toxic concentration of Icory in
MCF-7/ADR and MCF-7 cells was 50 μM (Fig. 1C). Therefore, concentrations of 25
and 50 μM Icory were used for the subsequent MDR-reversal
experiments. As a positive control, VRP at 50 μM showed no
toxicity to the four cell lines (Fig.
1D).
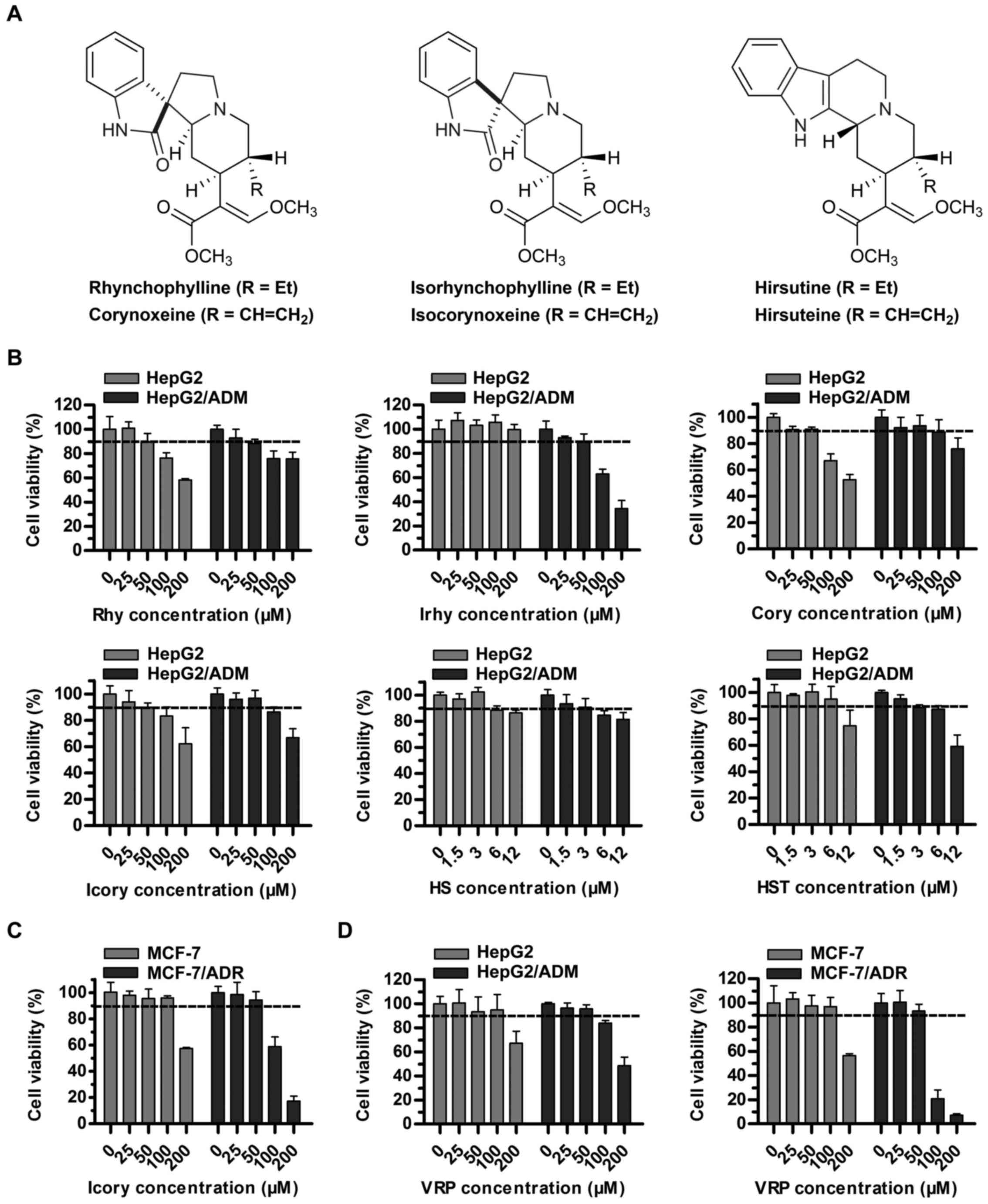 | Figure 1Cytotoxicity assays of individual
uncaria alkaloids (Rhy, Irhy, Cory, Icory, HS and HST) and VRP in
ABCB1-overexpressing cells and in their parental cells. (A) The
chemical structures of Rhy, Irhy, Cory, Icory, HS and HST. (B) The
percentage of surviving cells was measured after treatment with
Rhy, Irhy, Cory, Icory, HS and HST for 72 h in ABCB1-overexpressing
cells and their parental cells, namely, HepG2/ADM and HepG2 cells,
respectively. (C) The cell survival percentage was measured after
treatment with Icory for 72 h in MCF-7/ADR and MCF-7 cells. (D) The
cell survival percentage was measured after treatment with VRP for
72 h in HepG2/ADM, HepG2 cells, MCF-7/ADR and MCF-7 cells. The
results were presented as the means ± SD of three independent
experiments performed in six replicates. |
Uncaria alkaloids sensitize
ABCB1-overexpressing cells to DOX
To investigate whether uncaria alkaloids had the
ability to reverse ABCB1-mediated MDR, DOX was used as the
anticancer drug in HepG2/ADM cells and in the parental HepG2 cells.
The IC50 values of DOX with or without uncaria alkaloids
in the two cell lines are shown in Table I. The results showed that each of
the uncaria alkaloids variously increased the sensitivity of
HepG2/ADM cells to DOX and that Icory exhibited the most potent
activity, with a reversal-fold value of 31.09. Thus, Icory was
chosen for the subsequent experiments as a representative of
uncaria alkaloids.
 | Table IReversal activity of six uncaria
alkaloids in HepG2/ADM cells and their parental cells. |
Table I
Reversal activity of six uncaria
alkaloids in HepG2/ADM cells and their parental cells.
| Treatments | IC50 ±
SDa (μM)
(fold-reversalb)
|
|---|
| HepG2/ADM | HepG2 |
|---|
| DOX | 78.5314±9.7768
(1.00) | 0.3357±0.0193
(1.00) |
| + Rhy 50
μM | 5.4859±2.7101
(14.32) | 0.3852±0.1186
(0.87) |
| + Irhy 50
μM | 3.9646±1.1056
(19.81) | 0.3678±0.0261
(0.91) |
| + Cory 50
μM | 5.2865±0.7767
(14.86) | 0.3278±0.1221
(1.02) |
| + Icory 50
μM | 2.5256±0.6897
(31.09) | 0.3653±0.0849
(0.92) |
| + HS 3
μM | 15.2977±0.7666
(5.13) | 0.3982±0.0796
(0.84) |
| + HST 3
μM | 14.3615±2.0456
(5.47) | 0.4124±0.0153
(0.81) |
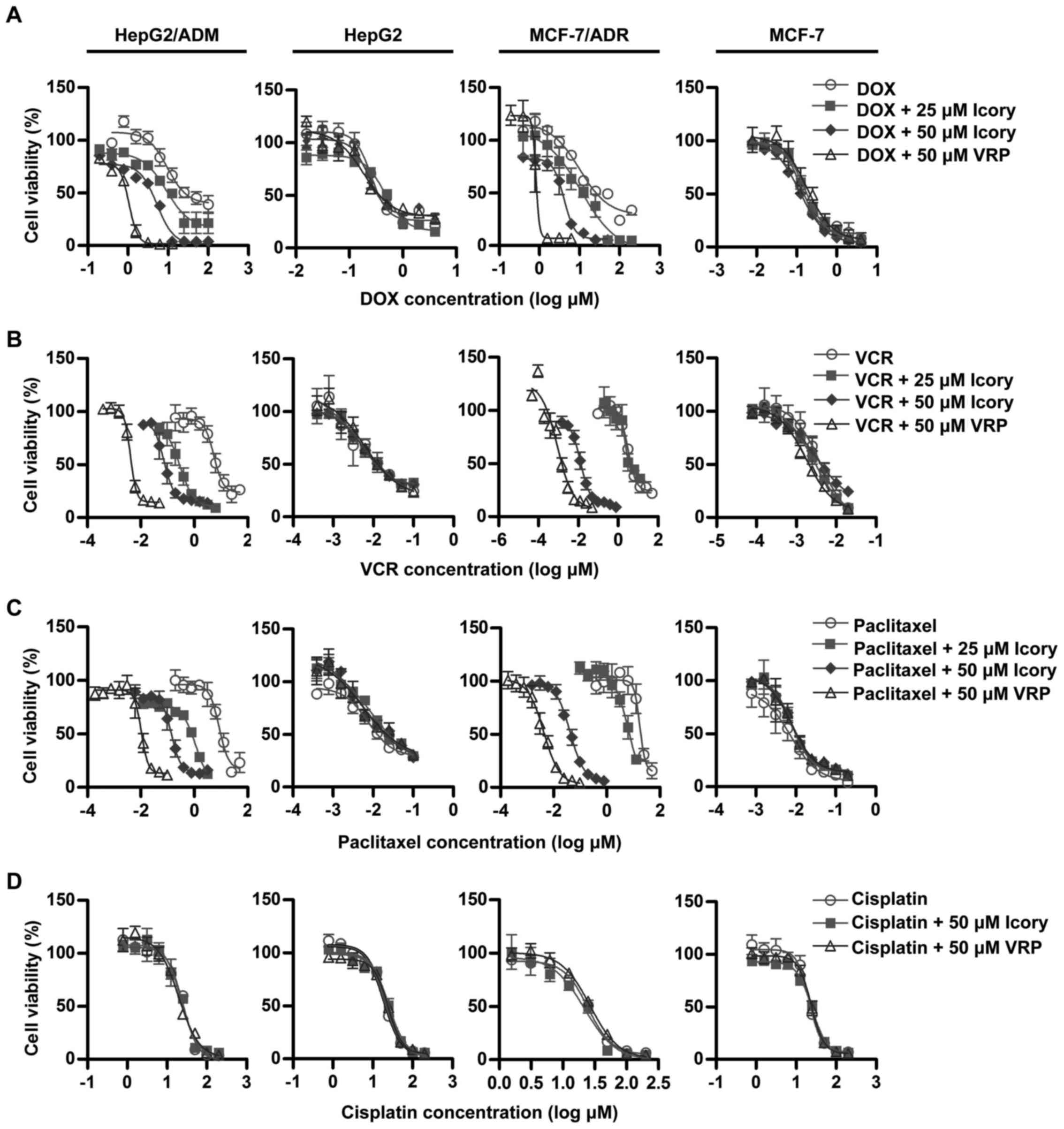
Icory reverses ABCB1-overexpressing cell
resistance to DOX, VCR and paclitaxel
The modulating effect of Icory was further examined
in MDR cancer cell lines (HepG2/ADM and MCF-7/ADR) and in their
corresponding sensitive cell lines (HepG2 and MCF-7). The
IC50 values of anticancer drugs (DOX, VCR, paclitaxel
and cisplatin) in the ABCB1-overexpressing cells and in the
parental cells with or without Icory are summarized in Table II. Consistent with the preliminary
screening, Icory greatly enhanced the activity of DOX in
DOX-resistant HepG2/ADM and MCF-7/ADR cells but not in their
parental cell lines. Icory also increased the sensitivity of
HepG2/ADM and MCF-7/ADR cells to VCR and paclitaxel, whereas this
effect did not occur in their respective parental cell lines.
However, the IC50 values of cisplatin were not altered
in any of the cell lines when combined with Icory or VRP (Fig. 2). In the MCF-7/ADR cell line, Icory
strikingly enhanced the toxicity of VCR and paclitaxel with 584.10
and 336.36 reversal-fold values, respectively. Collectively, these
results indicated that Icory significantly reverses
ABCB1-overexpressing cell resistance to DOX, VCR and
paclitaxel.
 | Table IIReversal effect of Icory on
HepG2/ADM, MCF-7/ADR and their parental sensitive cells. |
Table II
Reversal effect of Icory on
HepG2/ADM, MCF-7/ADR and their parental sensitive cells.
| Treatments | IC50 ±
SDa (μM)
(fold-reversalb)
|
|---|
| HepG2/ADM | HepG2 | MCF-7/ADR | MCF-7 |
|---|
| DOX | 78.5314±9.7768
(1.00) | 0.3357±0.0193
(1.00) | 27.9157±0.8581
(1.00) | 0.1921±0.0184
(1.00) |
| + Icory 25
μM | 9.3748±0.6148
(8.38) | 0.3728±0.0467
(0.90) | 13.8451±0.5140
(2.02) | 0.1373±0.0064
(1.39) |
| + Icory 50
μM | 2.5256±0.6897
(31.09) | 0.3653±0.0849
(0.92) | 2.6711±0.6350
(10.45) | 0.1913±0.0414
(1.00) |
| + VRP 50
μM | 1.1914±0.2304
(65.92) | 0.3639±0.1987
(0.91) | 0.6437±0.1536
(43.37) | 0.1837±0.0415
(1.05) |
| VCR | 5.6795±0.6825
(1.00) | 0.0125±0.0018
(1.00) | 6.4485±3.7066
(1.00) | 0.0035±0.0013
(1.00) |
| + Icory 25
μM | 0.3408±0.2562
(16.67) | 0.0128±0.0019
(0.97) | 5.0590±1.3830
(1.27) | 0.0043±0.0003
(0.82) |
| + Icory 50
μM | 0.1384±0.0988
(41.04) | 0.0132±0.0030
(0.94) | 0.0110±0.0083
(584.10) | 0.0040±0.0014
(0.88) |
| + VRP 50
μM | 0.0037±0.0001
(1525.93) | 0.0132±0.0001
(0.94) | 0.0024±0.0016
(2633.12) | 0.0026±0.0007
(1.34) |
| Paclitaxel | 10.7748±2.2922
(1.00) | 0.0222±0.0154
(1.00) | 15.7079±2.6061
(1.00) | 0.0093±0.0071
(1.00) |
| + Icory 25
μM | 0.6820±0.3245
(15.80) | 0.0247±0.0008
(0.90) | 7.7363±0.7618
(2.03) | 0.0095±0.0012
(0.98) |
| + Icory 50
μM | 0.1462±0.0585
(73.70) | 0.0227±0.0034
(0.98) | 0.0467±0.0118
(336.36) | 0.0093±0.0009
(1.01) |
| + VRP 50
μM | 0.0105±0.0024
(1029.11) | 0.0207±0.0054
(1.07) | 0.0033±0.0010
(4783.16) | 0.0100±0.0013
(0.93) |
| Cisplatin | 26.9154±0.08765
(1.00) | 20.7555±0.5778
(1.00) | 25.0096±1.2170
(1.00) | 22.0492±1.0443
(1.00) |
| + Icory 50
μM | 26.0751±0.6919
(1.03) | 23.4144±3.5658
(0.87) | 20.6873±3.1638
(1.21) | 18.7218±6.9056
(1.18) |
| + VRP 50
μM | 23.0622±0.0826
(1.17) | 22.8127±1.4291
(0.91) | 29.4822±3.2336
(0.85) | 21.0994±4.1638
(1.05) |
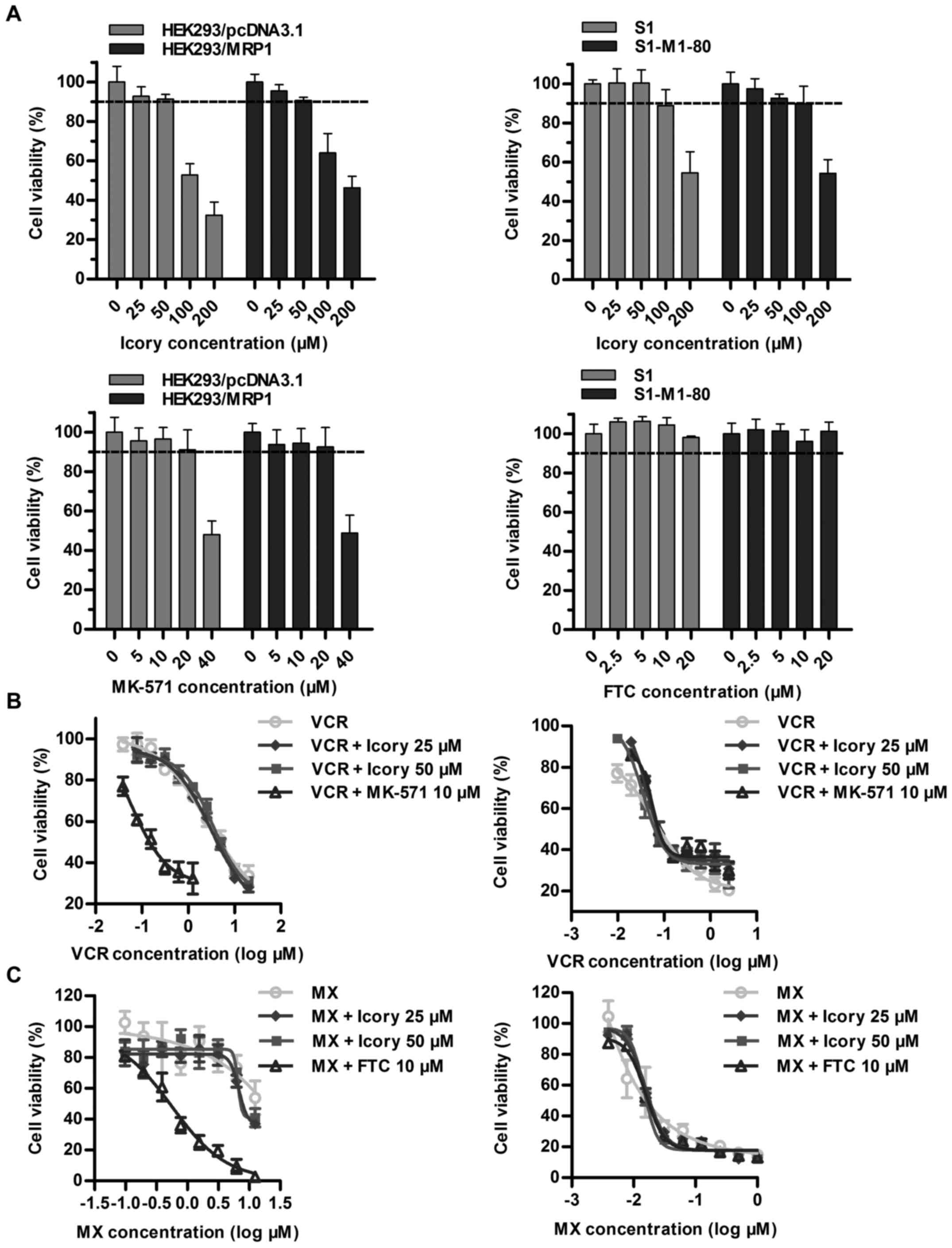
Icory does not reverse ABCC1- or
ABCG2-mediated MDR
Overexpressed ABCC1 and ABCG2 are two important
transporters in cancer MDR, and we further examined whether Icory
exerted reversal effects on ABCC1- and ABCG2-overexpressing cell
lines. First, we confirmed that Icory at 50 μM showed no
toxicity to the HEK293/MRP1 and S1-M1-80 cell lines or their
parental cell lines (Fig. 3A). The
ABCC1 modulator MK-571 significantly reversed the drug resistance
of HEK293/MRP1 cells. However, we did not observe similar activity
for Icory in HEK293/MRP1 cells when combined with the ABCC1
substrate VCR (Fig. 3B and
Table III). Analogously, Icory
had no reversal activity toward ABCG2-mediated MDR, as the
IC50 values of MX in ABCG2-overexpressing S1-M1-80 cells
and their parental S1 cells were unchanged when co-treated with
Icory. In stark contrast to Icory, FTC sensitized S1-M1-80 cells to
MX at a non-toxic concentration of 10 μM (Fig. 3C and Table IV). These results suggest that
Icory may be a specific ABCB1 modulator.
 | Table IIIReversal effect of Icory on
HEK293/MRP1 and their parental sensitive cells. |
Table III
Reversal effect of Icory on
HEK293/MRP1 and their parental sensitive cells.
| Treatments | IC50 ±
SDa (μM)
(fold-reversalb)
|
|---|
| HEK293/MRP1 |
HEK293/pcDNA3.1 |
|---|
| VCR | 4.2113±1.8257
(1.00) | 0.0211±0.0062
(1.00) |
| + Icory 25
μM | 6.0003±1.1998
(0.70) | 0.0199±0.0055
(1.06) |
| + Icory 50
μM | 4.5762±2.0726
(0.92) | 0.0189±0.0089
(1.12) |
| + MK-571 10
μM | 0.1255±0.9248
(33.56) | 0.0201±0.0057
(1.05) |
 | Table IVReversal effect of Icory on SI-M1-80
and their parental sensitive cells. |
Table IV
Reversal effect of Icory on SI-M1-80
and their parental sensitive cells.
| Treatments | IC50±
SDa (μM)
(fold-reversalb)
|
|---|
| S1-M1-80 | S1 |
|---|
| MX | 14.3009±0.9842
(1.00) | 0.0135±0.0074
(1.00) |
| + Icory 25
μM | 6.3245±0.1678
(1.72) | 0.0129±0.0092
(1.05) |
| + Icory 50
μM | 8.3785±1.3797
(1.72) | 0.0142±0.0080
(0.95) |
| + FTC 10
μM | 0.4222±0.0231
(33.87) | 0.0126±0.0057
(1.07) |
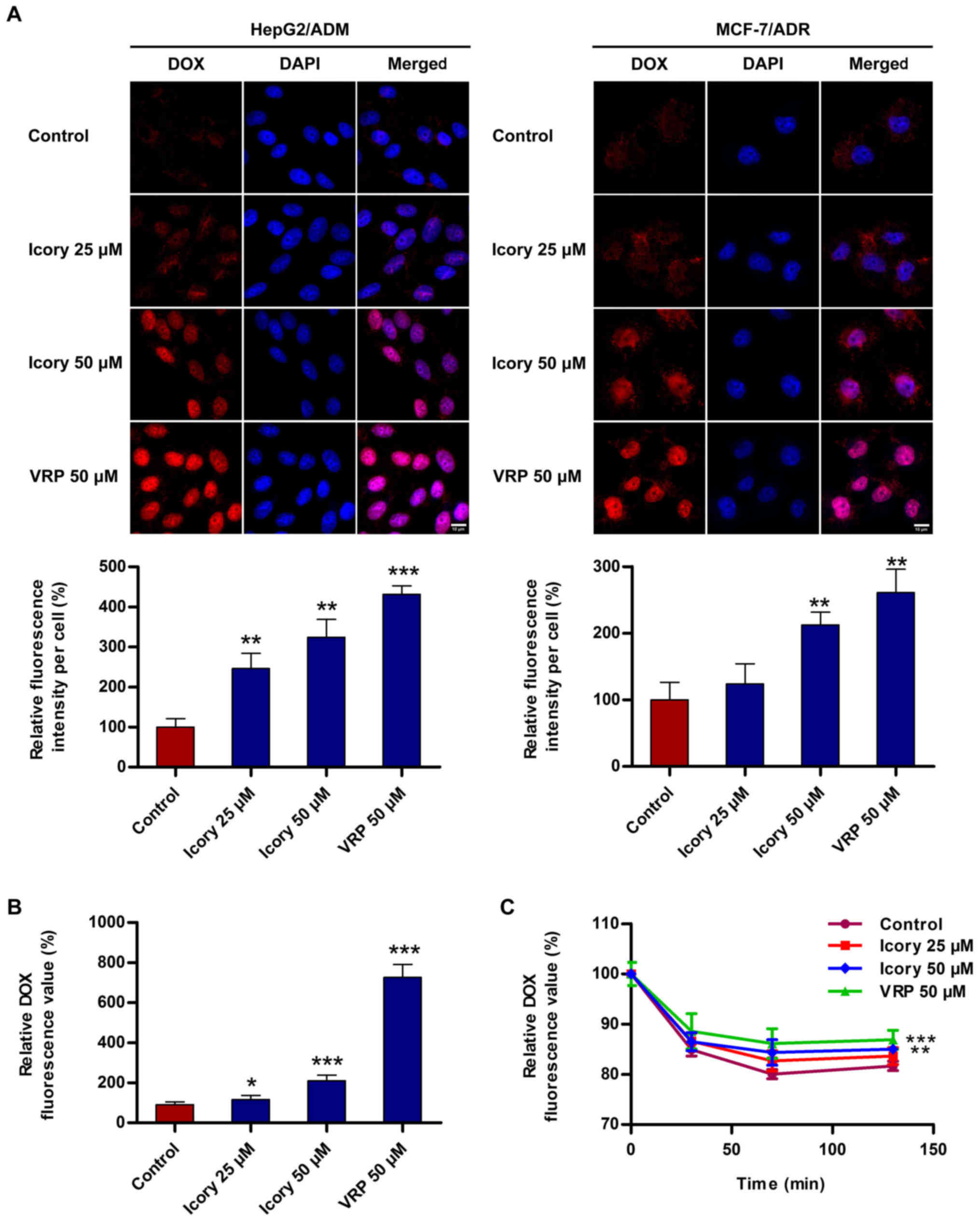
Icory increases the intracellular
accumulation of DOX in ABCB1-overexpressing cells
To investigate the reversal mechanism by which Icory
sensitizes ABCB1-mediated MDR in cancer cells, the accumulation
level of DOX in ABCB1-overexpressing cells was measured. As shown
in Fig. 4A, Icory enhanced the
intracellular fluorescence intensity of DOX in HepG2/ADM and
MCF-7/ADR cells. A quantitation of the fluorescence intensity is
presented with the images (Fig.
4A, bottom). Furthermore, a significant increase in the DOX
intensity in the presence of Icory was detected using a Multimode
Detector (Fig. 4B). The increase
in DOX intensity mediated by Icory was less than that of the
positive-control VRP, in accord with their relative reversal
activities. These results showed that Icory treatment elevates the
intracellular DOX concentration in ABCB1-overexpressing cells.
Icory inhibits the intracellular efflux
of DOX in ABCB1-overexpressing cells
To investigate whether Icory can inhibit the efflux
activity of ABCB1, a DOX efflux assay was performed with HepG2/ADM
cells. As shown in Fig. 4C, the
pumping rate of DOX in HepG2/ADM cells treated with Icory was lower
than that in untreated cells. Consistent with their activities, VRP
showed a stronger effect than Icory on the retention level of
intracellular DOX. The results suggest that Icory promotes DOX
accumulation by blocking DOX efflux in ABCB1-overexpressing
cells.
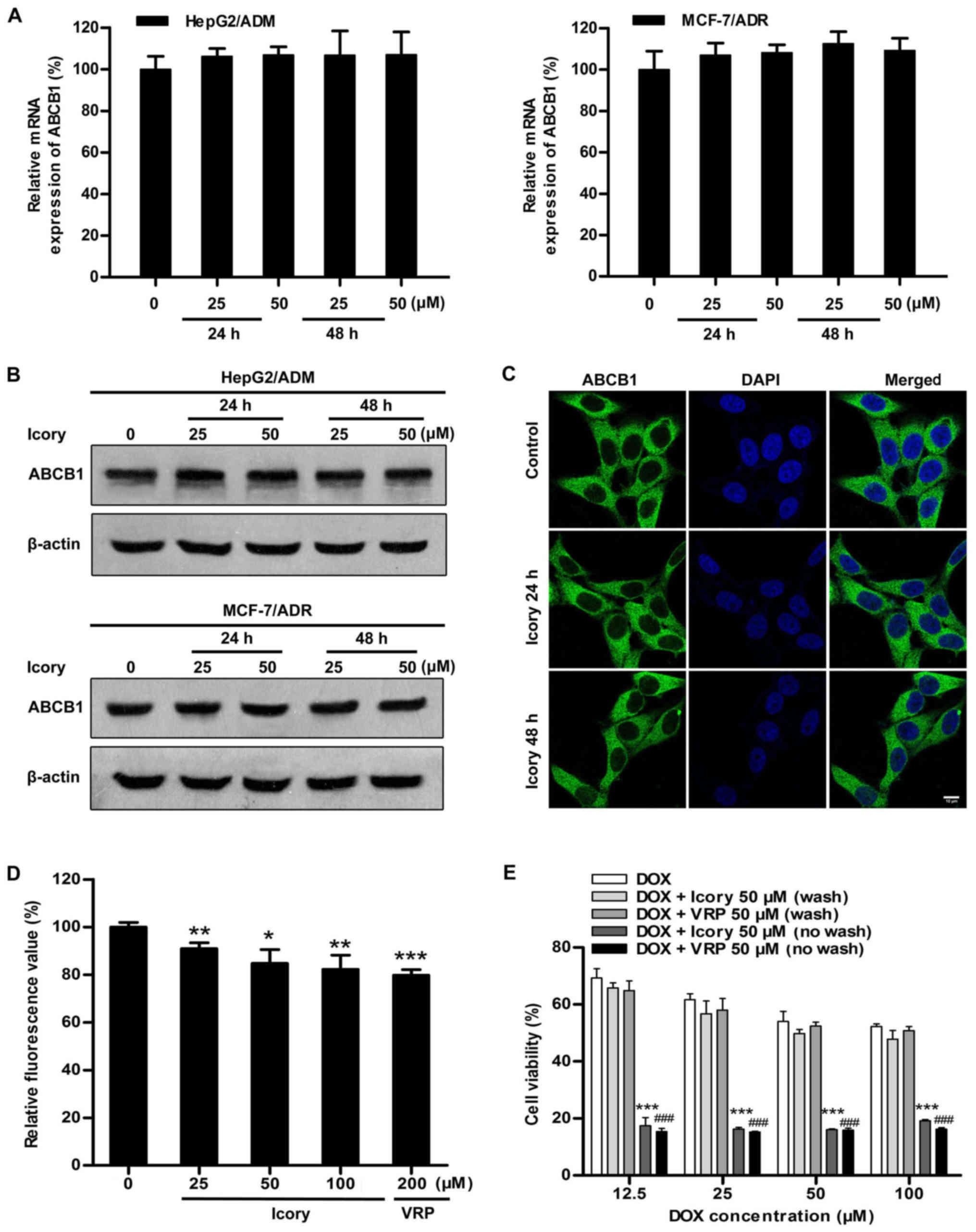
Icory has no effect on the expression of
ABCB1
Considering that blocking ABCB1 transport and
downregulating ABCB1 expression would both result in the retention
of intracellular DOX, we further examined the mRNA and protein
expression levels of ABCB1. As shown in Fig. 5A and B, HepG2/ADM and MCF-7/ADR
cells were treated with Icory at 25 μM and 50 μM for
two time periods (24 and 48 h). However, no significant alterations
were observed in the ABCB1 mRNA or protein levels, confirming that
the reversal effect of Icory is not related to the downregulation
of ABCB1 expression.
Icory does not alter the localization of
ABCB1
In order to further investigate whether Icory can
change cellular localization of ABCB1, the immunofluorescence assay
was conducted in HepG2/ADM cells. As shown in Fig. 5C, Icory treatment for 24 or 48 h at
50 μM did not alter the cellular localization of ABCB1.
Icory stimulates ABCB1 ATPase
activity
We reasoned that Icory might induce DOX retention by
inhibiting the efflux function of ABCB1. Thus, an ABCB1 ATPase
activity assay was performed to investigate whether Icory is a
substrate of ABCB1. According to the Pgp-Glo™ assay system, ABCB1
is an ATP-dependent drug efflux pump, and ATP consumption reflects
its transport activity. As shown in Fig. 5D, similar to VRP, Icory strikingly
stimulated ABCB1 ATPase activity in a concentration-dependent
manner. The results indicate that Icory is likely a substrate of
ABCB1 that blocks its efflux function.
The MDR-reversal effect of Icory depends
on its durative concentration
A characteristic of an ABCB1 competitive inhibitor
is that the reversal effect disappears following drug washout. The
persistence and reversibility of MDR-reversal effects can be
assessed in a therapeutically relevant way by assaying residual
activity after washout of the reversal agents. As shown in Fig. 5E, the reversal effect of Icory on
HepG2/ADM cells was no longer present after the washout of Icory,
similar to VRP, a known ABCB1 competitive inhibitor. These results
suggest that Icory may be a competitive inhibitor of ABCB1.
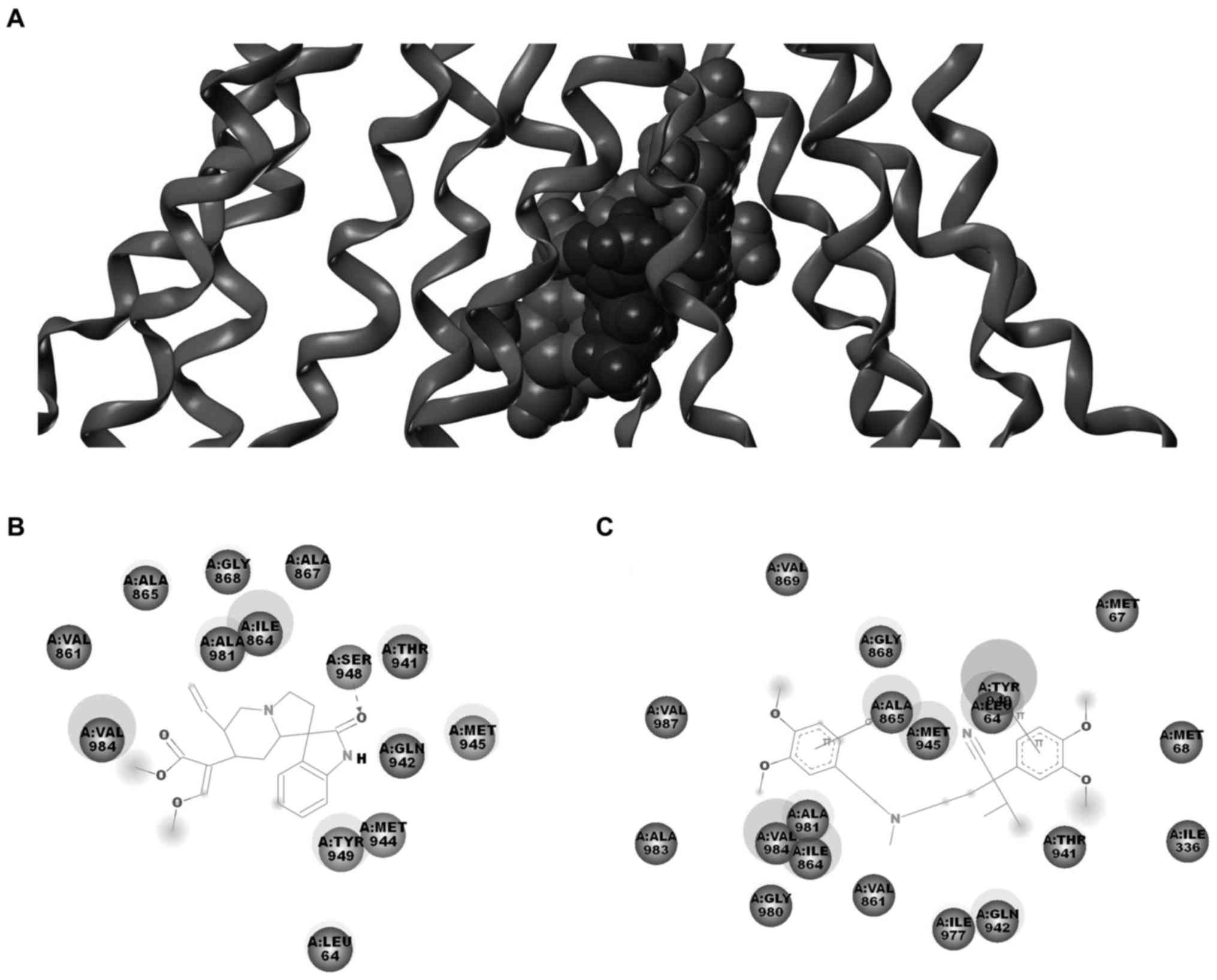
Molecular docking model of Icory binding
to ABCB1
The above results suggested that the reversal effect
of Icory may occur through a direct interaction with ABCB1. To
further explore the probable binding conformation with ABCB1, Icory
was computationally docked into ABCB1. As shown in Fig. 6, Icory bound to ABCB1 at the same
site as VRP but did not completely overlap. The residues of ABCB1
surrounding Icory were LEU64, VAL861, ILE864, ALA865, ALA867,
GLY868, THR941, GLN942, MET944, MET945, SER948, TYR949, ALA981,
VAL984. Among these residues, LEU64, VAL861, ILE864, ALA865,
GLY868, THR941, GLN942, MET945, TYR949, ALA981, VAL984 were also
around VRP. These residues were well consistent with the key
residues. The docking result further confirmed that Icory binds to
ABCB1 at the same site as VRP but that the binding site does not
completely overlap with that of VRP. In addition, the binding
energy between icory or VRP and ABCB1 was 46.55 kcal/mol and 123.5
kcal/mol, respectively, which suggested the affinity of Icory
binding to ABCB1 may be weaker than that of VRP.
Discussion
In the past three decades, researchers have devoted
much effort to the search for effective and clinically applicable
ABCB1 modulators. Searching for more effective, low-toxicity ABCB1
modulators from traditional Chinese medicine has become a promising
road. Uncaria alkaloids, the major naturally active substances of
uncaria, were reported to have anticancer activity, including
cytotoxicity and MDR-reversal effects. Uncaria is a common
traditional Chinese medicine, and there are different constituents
and contents with the change of uncaria species. The six alkaloids
are principal components which exist in great quantities ranging
from 0.1% to 1% in uncaria (17,18).
In this investigation, we demonstrated for the first time that all
six alkaloids significantly reduced the resistance of
ABCB1-mediated MDR cells to chemotherapeutics, also, the total
uncaria alkaloid extract exhibits MDR reversal activity with 8.74
reversal-fold value in HepG2/ADM cells when combined with DOX.
Furthermore, HS and HST showed higher cytotoxicity
against ABCB1-overexpressing cells than the other four agents,
likely due to differences in their chemical structures. Compared
with HS and HST, which are non-oxindole alkaloids, Rhy, Irhy, Cory
and Icory are oxindole alkaloids characterized by oxidization at
the indole ring, this distinction may explain their weaker toxicity
than HS and HST. By contrast, Rhy/Irhy and Cory/Icory are two pairs
of isomerides, and they contain a spirocyclic ring on the indole
ring but have varying stereochemistry on the ring skeleton, which
may be connected with their MDR-reversal effect. Therefore, the
selection of a suitable uncaria alkaloid chemotype may be very
important for MDR-reversal activity because it differs from the
chemical structure and conformation.
Since some MDR transporter inhibitors not only bind
to ABCB1 but also influence the function of other ABC transporters,
they do not exhibit specificity. Icory sensitized
ABCB1-overexpressing cells to DOX, VCR and paclitaxel, but it did
not have similar effect on their parental cells. Icory did not
alter the sensitivity of ABCB1-overexpressing cells to cisplatin,
which is not an ABCB1 substrate, suggesting that the reversal
potency of Icory is closely associated with ABCB1. As previously
reported, ABCC1 can remove internal noxious substances, including
important endogenous substances, xenobiotics and their metabolites,
to protect normal tissue from cytotoxicity (19). ABCG2 confers a protective effect to
hypoxic cells, and ABCG2 is also responsible for cellular
homeostasis and influences medicine absorption, distribution, and
excretion in the human body (20-23).
Thus, inhibiting ABCC1 and ABCG2 may disrupt cellular homeostasis
and normal drug metabolism, potentially causing toxicity and other
side effects. Fortunately, Icory had no significant reversal
effects on ABCC1- and ABCG2-overexpressing cells or their parental
cells. Therefore, the use of Icory would minimize any side effects
caused by the inhibition of ABCC1 and ABCG2 transporters.
Icory significantly increased intracellular DOX
accumulation in ABCB1-overexpressing cells, and the efflux assay
confirmed that the DOX accumulation was due to the inhibition of
ABCB1 efflux function. As previously reported, downregulation of
ABCB1 expression would result in the inhibition of ABC
transporter-mediated efflux (24).
However, our results showed that after treatment with Icory,
neither the expression levels nor localization of ABCB1 in
HepG2/ADM or MCF-7/ADR cells were significantly altered, suggesting
that Icory influences the drug transport function rather than the
expression and localization of ABCB1. It is well known that the
substrates of ABCB1 activate the ATPase to hydrolyze ATP and
provide the energy needed by the ABCB1 transporter. Therefore, a
change in ABCB1 ATPase activity directly reflects whether a drug is
the substrate of ABCB1. Our results showed that Icory markedly
stimulated the activity of the ABCB1 ATPase in a
concentration-dependent manner, indicating that Icory may be a
substrate of the ABCB1 transporter. As previously reported, an ATP
modulator can act as a competitive inhibitor by binding to the
drug-binding pocket in TMD or as a non-competitive inhibitor by
suppressing ATP binding by nucleotide-binding domains (25). Our results showed that the reversal
activity of Icory disappeared after being washed out, resembling
the positive-control VRP and indicating that Icory acts as a
competitive inhibitor of ABCB1. Moreover, our molecular docking
analysis indicated that the residues of ABCB1 surrounding Icory
also partly overlapped with those around VRP. The result further
supports the idea that Icory binds to important binding sites of
ABCB1 and acts as a substrate of ABCB1.
Icory has several particular characteristics
comparing to other MDR inhibitors. The six uncaria alkaloids all
derive from the low toxic traditional Chinese medicine uncaria,
which has been used in clinic for a long time, and they exist in
great quantities in uncaria; Icory can specifically reverse ABCB1
other than ABCC1 or ABCG2 transporter-mediated MDR cancer. However,
compared with the tyrosine kinase inhibitors, the reversal activity
of Icory is still weaker. Thus, in order to improve the efficacy of
uncaria alkaloid, further structural modification studies are
warranted.
In this study, we demonstrated that uncaria
alkaloids, including Rhy, Irhy, Cory, Icory, HS and HST, reverse
ABCB1-mediated MDR at non-toxic concentrations. Among them, Icory
showed the strongest activity. Instead of altering the expression
level and localization of ABCB1, Icory acts as a substrate of
ABCB1, competitively binding to the drug-binding sites of ABCB1 and
thus inhibiting its drug efflux function. The uncaria alkaloids
exhibit potential to be reversal agents for ABCB1
transporter-mediated MDR, and further in vivo studies are
warranted.
Acknowledgments
Special thanks to Professor Kwok-Pui Fung (The
Chinese University of Hong Kong, Hong Kong) for HepG2 and HepG2/ADM
cells, Professor Li-Wu Fu (Sun Yat-Sen University, China) for MCF-7
and MCF-7/ADR cells, Dr Suresh Ambudkar (NCI, NIH, Bethesda, MD,
USA) for HEK293/pcDNA3.1 and HEK293/MRP1 cells, Dr Susan Bates and
Robert Robey (NCI, NIH) for S1 and S1-M1-80 cells. This work was
supported by the National Science Foundation of China (81573455),
the Science and Technology Cooperation Research Project of Chinese
Traditional Medicine Industry for Jianhe County, Guizhou Province,
2014 (sponsored by the Bureau of Science and Technology of Jianhe
county, no. 4/no. 5).
Glossary
Abbreviations
Abbreviations:
|
ABC transporter
|
ATP-binding cassette transporter
|
|
Cory
|
corynoxeine
|
|
DOX
|
doxorubicin
|
|
FTC
|
fumitremorgin C
|
|
HS
|
hirsutine
|
|
HST
|
hirsuteine
|
|
Icory
|
isocorynoxeine
|
|
Irhy
|
isorhynchophylline
|
|
MDR
|
multidrug resistance
|
|
MX
|
mitoxantrone
|
|
qRT-PCR
|
quantitative real-time PCR
|
|
Rhy
|
rhynchophylline
|
|
VCR
|
vincristine
|
|
VRP
|
verapamil
|
References
|
1
|
Li W, Zhang H, Assaraf YG, Zhao K, Xu X,
Xie J, Yang DH and Chen ZS: Overcoming ABC transporter-mediated
multidrug resistance: Molecular mechanisms and novel therapeutic
drug strategies. Drug Resist Updat. 27:14–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang K, Mack P and Wong KP:
Glutathione-related mechanisms in cellular resistance to anticancer
drugs. Int J Oncol. 12:871–882. 1998.PubMed/NCBI
|
|
3
|
Dean M, Hamon Y and Chimini G: The human
ATP-binding cassette (ABC) transporter superfamily. J Lipid Res.
42:1007–1017. 2001.PubMed/NCBI
|
|
4
|
Tiwari AK, Sodani K, Dai CL, Ashby CR Jr
and Chen ZS: Revisiting the ABCs of multidrug resistance in cancer
chemotherapy. Curr Pharm Biotechnol. 12:570–594. 2011. View Article : Google Scholar
|
|
5
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarkadi B, Homolya L, Szakács G and Váradi
A: Human multidrug resistance ABCB and ABCG transporters:
Participation in a chemoimmunity defense system. Physiol Rev.
86:1179–1236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ambudkar SV, Dey S, Hrycyna CA,
Ramachandra M, Pastan I and Gottesman MM: Biochemical, cellular,
and pharmacological aspects of the multidrug transporter. Annu Rev
Pharmacol Toxicol. 39:361–398. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yanagisawa T, Newman A, Coley H, Renshaw
J, Pinkerton CR and Pritchard-Jones K: BIRICODAR (VX-710; Incel):
An effective chemosensitizer in neuroblastoma. Br J Cancer.
80:1190–1196. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rowinsky EK, Smith L, Wang YM, Chaturvedi
P, Villalona M, Campbell E, Aylesworth C, Eckhardt SG, Hammond L,
Kraynak M, et al: Phase I and pharmacokinetic study of paclitaxel
in combination with biricodar, a novel agent that reverses
multidrug resistance conferred by overexpression of both MDR1 and
MRP. J Clin Oncol. 16:2964–2976. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leonard GD, Fojo T and Bates SE: The role
of ABC transporters in clinical practice. Oncologist. 8:411–424.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leonard GD, Polgar O and Bates SE: ABC
transporters and inhibitors: New targets, new agents. Curr Opin
Investig Drugs. 3:1652–1659. 2002.PubMed/NCBI
|
|
13
|
Cui Y: Advances in study on chemical
compositions and pharmacological activities of Uncaria
rhynchophylla. J Xi'an Univ Natural Science Edition). 18:16–18.
2015.In Chinese.
|
|
14
|
He Y: Pharmacological development of
ramulus Uncariae cum uncis and its active ingredients. Shanghai
Univ Tradit Chin Med. 37:57–60. 2003.In Chinese.
|
|
15
|
Ndagijimana A, Wang X, Pan G, Zhang F,
Feng H and Olaleye O: A review on indole alkaloids isolated from
Uncaria rhynchophylla and their pharmacological studies.
Fitoterapia. 86:35–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu DL, Li YJ, Yao N, Xu J, Chen ZS, Yiu
A, Zhang CX, Ye WC and Zhang DM: Acerinol, a cyclolanstane
triterpenoid from Cimicifuga acerina, reverses ABCB1-mediated
multidrug resistance in HepG2/ADM and MCF-7/ADR cells. Eur J
Pharmacol. 733:34–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Yang CJ and Wu DG: Studies on the
chemical constituents of sharpleaf gambir plant (Uncaria
rhynchophylla) (II). Chin Tradit Herbal Drugs. 29:649–651.
1998.
|
|
18
|
Yamanaka E, Kimizuka Y, Aimi N, Sakai S
and Haginiwa J: Studies of plants containing indole alkaloids. IX.
Quantitative analysis of tertiary alkaloids in various parts of
Uncaria rhynchophylla MIQ. Yakugaku Zasshi. 103:1028–1033. 1983.In
Japanese. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He SM, Li R, Kanwar JR and Zhou SF:
Structural and functional properties of human multidrug resistance
protein 1 (MRP1/ABCC1). Curr Med Chem. 18:439–481. 2011. View Article : Google Scholar
|
|
20
|
Krishnamurthy P and Schuetz JD: The role
of ABCG2 and ABCB6 in porphyrin metabolism and cell survival. Curr
Pharm Biotechnol. 12:647–655. 2011. View Article : Google Scholar
|
|
21
|
Ye L, Lu L, Li Y, Zeng S, Yang X, Chen W,
Feng Q, Liu W, Tang L and Liu Z: Potential role of ATP-binding
cassette transporters in the intestinal transport of rhein. Food
Chem Toxicol. 58:301–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Babu K, Zhang J, Moloney S, Pleasants T,
McLean CA, Phua SH and Sheppard AM: Epigenetic regulation of ABCG2
gene is associated with susceptibility to xenobiotic exposure. J
Proteomics. 75:3410–3418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kummu M, Sieppi E, Wallin K, Rautio A,
Vähäkangas K and Myllynen P: Cadmium inhibits ABCG2 transporter
function in BeWo choriocarcinoma cells and MCF-7 cells
overexpressing ABCG2. Placenta. 33:859–865. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang SJ, Chen LK, Wang F, Zhang YK, Huang
ZC, To KK, Wang XK, Talele TT, Chen ZS, Chen WQ, et al: CEP-33779
antagonizes ATP-binding cassette subfamily B member 1 mediated
multidrug resistance by inhibiting its transport function. Biochem
Pharmacol. 91:144–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aller SG, Yu J, Ward A, Weng Y,
Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL,
et al: Structure of P-glycoprotein reveals a molecular basis for
poly-specific drug binding. Science. 323:1718–1722. 2009.
View Article : Google Scholar : PubMed/NCBI
|