Prostate cancer is the most commonly diagnosed
malignancy and the second leading cause of cancer death among men
in the United States. It is estimated that approximately 180,890
new cases of prostate cancer and 26,120 deaths from prostate cancer
occurred in the USA in 2016 (1).
The common risk factors for prostate cancer are age,
race/ethnicity, geography, family history and lifestyle (2). Depending on the severity of the
disease, current treatment options for prostate cancer include
single or a combination of therapies such as active surveillance,
surgery, radiation therapy, chemotherapy, hormone therapy or
vaccines (3). Although these
interventions have significantly improved the quality of life of
the patients and the overall survival rates, effective treatment of
prostate cancer is still limited due to the major challenges such
as genetic heterogeneity, tumor recurrence (~30% of the cases) and
resistance to conventional chemotherapeutic drugs (4–6).
Therefore, it is crucial to develop novel preventive and
therapeutic strategies that have the potential to improve outcomes
for prostate cancer patients.
Epidemiological studies have shown that there is a
significant disparity in incidence and mortality rates of prostate
cancer among different countries, with the highest rates in the USA
and European countries and the lowest rates in Asian countries such
as Japan and China (7,8). This wide variability in the prostate
cancer rates across countries suggests that several factors
including genetic, epigenetic and environmental differences play a
key role in the etiology of the disease. Notably, it has been shown
that Asian immigrants in the USA have an increased incidence of
prostate cancer compared to those individuals with the same genetic
background who live in Asia, indicating that environmental factors,
especially the diet, are major determinants of prostate cancer
incidence (9). One of the
remarkable dietary differences between Asian and Western countries
is the amount of soy-based food consumption. Asian populations
consume high quantities of soy food which is rich in isoflavones
(~2 g of isoflavones per kg of fresh soybean) (10). It has been shown that plasma and
prostatic fluid concentrations of isoflavones in Asian men are 10
to 100 times higher than those in Western men, with particularly
high levels of the isoflavone genistein (11,12).
An increasing body of population-based studies has demonstrated
that high intake of soy isoflavones are associated with a 25–30%
reduced risk of prostate cancer (13,14).
As the major biologically active isoflavone in the
soy diet, genistein has been extensively investigated for its
chemopreventive potential in various types of cancer, including
prostate cancer. The average daily intake of genistein in Asian
populations has been shown to be 20–80 mg whereas it is 1–3 mg in
the USA, supporting the protective effects of genistein against
prostate cancer in Asian men (15). Genistein reaches plasma
concentrations of 1–5 µM 6–8 h after intake of soy-rich diet
(11,16). The plasma half-life of genistein
has been reported as 7.9 h in adults. In addition, concentrations
of total soy isoflavones in prostate tissue have been shown ~6-fold
higher than serum levels of isoflavones (17). Safety and pharmacokinetic studies
of soy isoflavones have demonstrated that minimal clinical toxicity
was observed in healthy subjects administered with purified soy
isoflavones at doses that exceed normal dietary intakes (18).
Due to its structural similarity to the steroid
hormone 17β-estradiol, genistein binds to estrogen receptors, ER-α
and ER-β, with a higher affinity to ER-β, and acts as a natural
selective estrogen receptor modulator (16,19,20).
Genistein exerts its inhibitory effects on prostate cancer cells by
upregulating the expression of ER-β, which has anti-proliferative
and pro-apoptotic roles in prostate cells (21,22).
In addition to its estrogenic activities, genistein regulates
androgen receptor (AR)-mediated pathways in prostate cancer
(23,24). Of note, it has been shown that the
inhibitory effect of genistein on AR expression is also mediated by
ER-β (25). Several other
molecular mechanisms underlying the preventive effects of genistein
on prostate cancer include the inhibition of cell proliferation by
inducing G1 and/or G2/M cell cycle arrest (26–28),
angiogenesis (29,30) and metastasis (31–33)
and induction of apoptosis (34,35).
Genistein exerts its pleiotropic effects in the context of prostate
cancer through modulation of several cell signal transduction
pathways such as IGF-1 (36),
TGF-β (37), Wnt/β-catenin
(36), NF-κB (38), AKT and MAPK (39) signaling. This modulation could be
by direct binding to nuclear receptors or modification of the
phosphorylation state of signal transduction proteins. In addition,
genistein inhibits tyrosine kinase activities (40) and shows antioxidant properties
(41,42) in prostate cells. Swami et al
(43) demonstrated that genistein
reduces prostate cancer progression by inhibiting prostaglandin
synthesis and activity. Genistein has also been reported to have
possible effects on DNA damage and repair in prostate cancer cells
(42). Moreover, genistein
inhibits DNA methylation (44–48)
and histone modifications (47,48)
and regulates miRNAs (49–52) in prostate cancer. It is of interest
that genistein has been shown to enhance the efficacy of
radiotherapy and chemotherapy (53,54).
Prostate specimens from a clinical trial of
genistein supplementation prior to prostatectomy (56) were analyzed for global changes in
DNA methylation and gene expression. Participants were recruited
from the outpatient clinic at the Department of Urology, Oslo
University Hospital, Oslo, Norway between April 2007 and August
2008. The study was approved by the Norwegian Medicines Agency, the
Regional Ethics Committee, the Privacy Ombudsman and the Prostate
Biobank at the Oslo University Hospital, Aker.
Total DNA was isolated from frozen prostate tissues
using DNeasy Blood and Tissue kit (Qiagen, Valencia, CA, USA)
according to the manufacturer's instructions. DNA was submitted to
the Emory Integrated Genomics Core for DNA methylation analysis
using Illumina HumanMethylation450 BeadChip Microarrays.
Methylation data are available on GEO (accession number
GSE84749).
Total RNA was extracted from frozen prostate tissues
using the mirVana miRNA Isolation kit (Life Technologies, Grand
Island, NY, USA), followed by RNA clean-up using the RNeasy Mini
kit (Qiagen). Total RNA was submitted to the Emory Integrated
Genomics Core for gene expression analysis using the Illumina
HumanHT-12 v4 Expression BeadChip Microarray. Microarray data are
available on GEO (accession number GSE84748).
RNA was reverse-transcribed into cDNA using iScript
cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA).
Primers were designed using Primer3 tool. Sequences of the primers
are listed in Table I. qPCR was
performed using iQ SYBR-Green Supermix (Bio-Rad Laboratories) on a
Bio-Rad iCycler according to the manufacturer's protocols. Human
β-actin gene, which has been shown to be a valid reference gene for
normalization of qPCR in human tissue samples of prostate cancer,
was used as an internal control in the present study (57). Normal prostate tissue sample was
used as the calibrator. The relative changes in gene expression
data were analyzed by the 2−ΔΔCT method. Triplicates
were run for each sample. Data are presented as the mean ± standard
deviation.
Gene expression analysis was performed using
GenePattern ComparativeMarkerSelection module (58) comparing genistein-treated tumors to
placebo-treated tumors. Illumina Microarray data were filtered to
include genes that were detected (P<0.05) in at least one
experimental group to result in a dataset of 15918 genes for
analysis. The comparative marker selection module of GenePattern
was used to compute two-sided Student's t-tests between groups with
10,000 permutations to compute false discovery rates. The random
seed used was 779948241. Hierarchical clustering was performed
using Cluster software (59) and
Java TreeView (60). Methylation
microarray analysis was performed in R using CpGassoc module in
Bioconductor (61). Data from the
450K probes was filtered to those in which the maximum - minimum
β-value was >0.2 to result in 160K probes for differential
methylation analysis. CpGassoc was used to identify 162 significant
probes that were differentially methylated. Three probes were
differentially methylated between genistein-treated tumor samples
and placebo-treated tumor samples, three probes were significant
between genistein-treated tumor samples and normal samples and 156
were significantly different between placebo-treated tumor samples
and normal samples.
Mann-Whitney U test (two-tailed) was used to
determine significant differences between two groups of data.
P<0.05 was considered as statistically significant.
We analyzed prostate tissue samples from a previous
study, which was a randomized, placebo-controlled, double-blind
Phase 2 clinical trial on Norwegian patients with localized
prostate cancer who received 30 mg synthetic genistein or placebo
capsules daily for 3–6 weeks before radical prostatectomy (56). The clinical and pathological
characteristics of the cases were previously described (56). The availability of frozen tissue
limited the sample size in this study and we investigated the DNA
methylation and gene expression levels of prostate tumor samples
from 10 patients who received genistein and 10 patients who
received placebo. Four adjacent normal prostate tissue samples were
also analyzed. Clinical data for the 20 patients analyzed here are
provided in Table II. There were
no statistically significant differences in age, levels of serum
PSA and Gleason score between the two treatment groups.
The genome-wide DNA methylation profiles of a total
of 24 prostate samples from tumor or normal tissues were generated
using Illumina HumanMethylation450 BeadChip kit. Methylation status
of each sample was analyzed for 485,577 sites, covering 21,231
genes. We compared the methylation profiles of genistein-treated
tumor samples with placebo-treated cases. In general, methylation
changes were modest, and there was no significantly differentially
methylated gene after correction for multiple hypothesis testing.
However, uncorrected P-values indicated that RBM28 and
CYTSB genes were demethylated in genistein-treated tumor
samples compared to placebo-treated samples. The lack of
statistical significance was likely due to the small numbers of
samples analyzed in this study. We did observe 156 probes with
significantly increased methylation in placebo-treated tumor
tissues vs. normal tissues that were not significant between
genistein-treated tumor tissues and normal tissues, suggesting that
genistein may have had some demethylation effects (available upon
request). These 156 probes corresponded to at least 92 separate
genes including ADCY4, ALOX12, HAAO,
LRRC4, NEU1, RAPGEFL1 and WNT7B
(Table III).
To identify molecular effects of genistein on mRNA
levels in prostate cancer, we compared gene expression profiles of
genistein-treated tumors with placebo-treated samples. Once again,
there were no differentially expressed probes that remained
statistically significant after correction for multiple hypothesis
testing. However, there were 628 probes that reached nominally
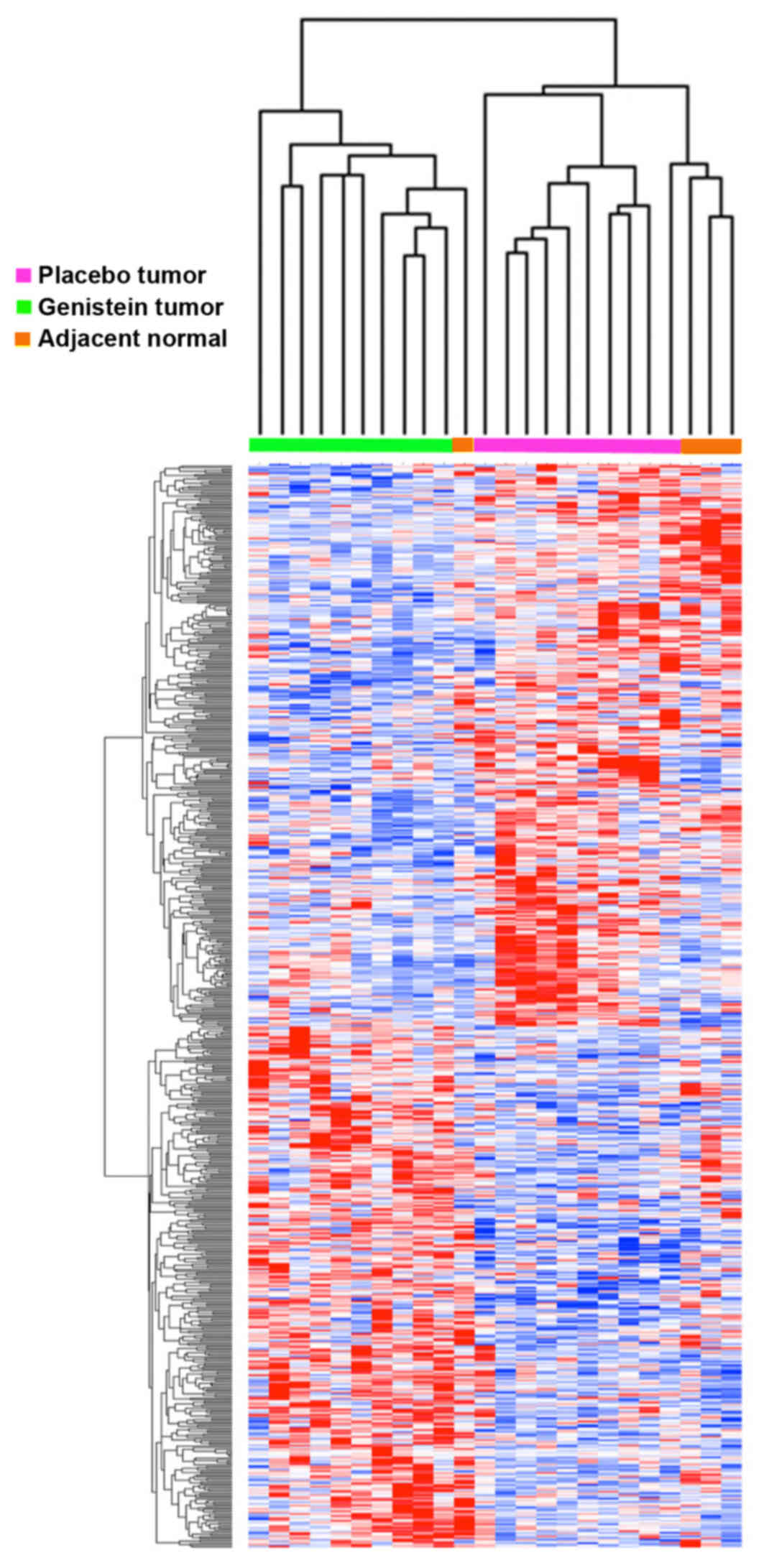
significant P-values (available upon request). Hierarchial
clustering of this dataset showed strong segregation of patients
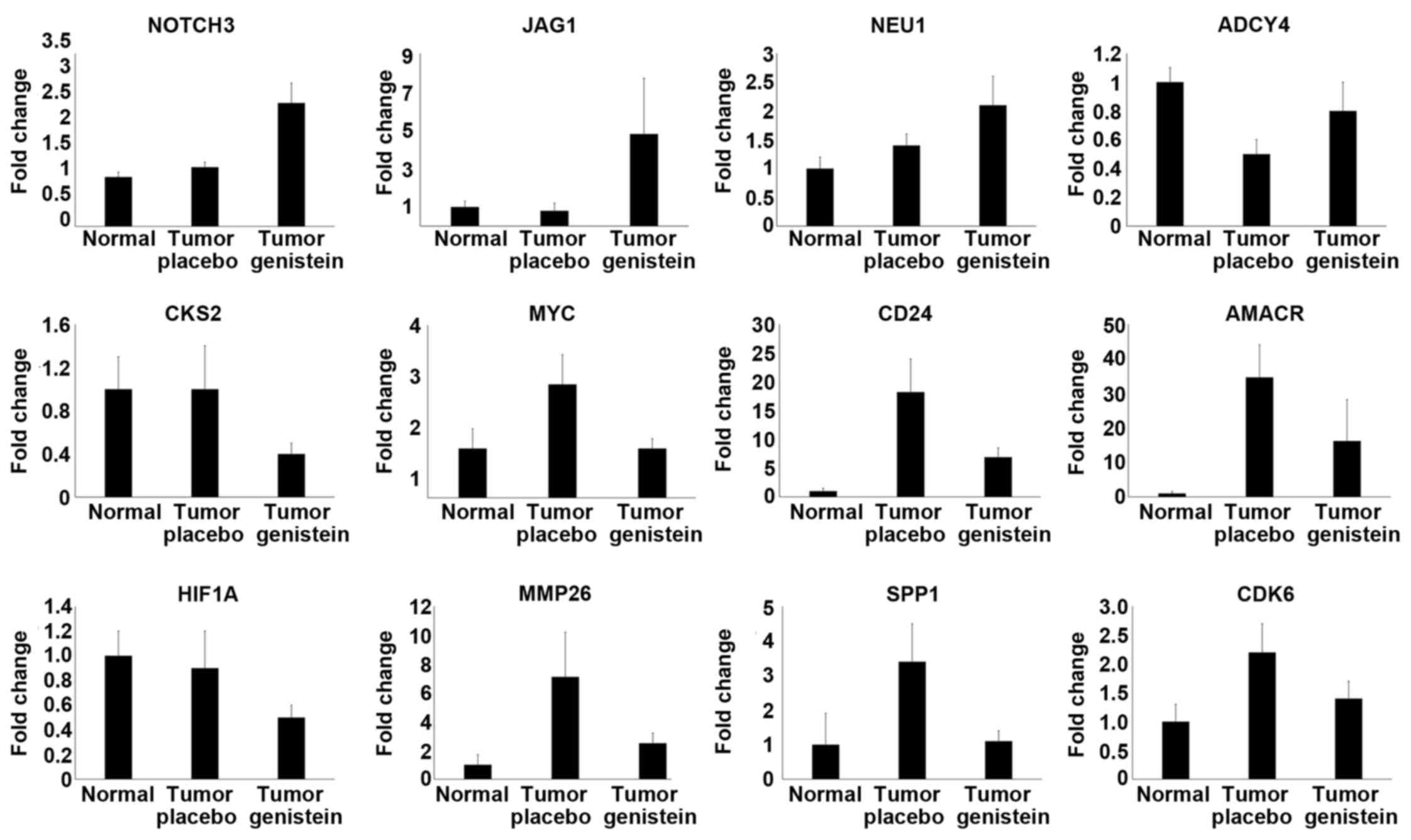
with and without genistein treatment (Fig. 1). The genes with nominally
significant P-values included NOTCH3, JAG1,
CKS2, HIF1A, CDK6, MYC, CD24,
AMACR, MMP26 and SPP1 genes (Table IV). NEU1 and ADCY4
did not reach nominal significance but had a trend towards
significance, and integration of the methylation data with the
paired gene expression profiling data indicated decreased
methylation status and increased expression levels of ADCY4
and NEU1 genes in genistein-treated cases.
We performed gene enrichment analysis on the 628
nominally significant probes that were differentially expressed
between genistein and placebo samples (Table V) using Ingenuity Pathway Analysis
(62) and the DAVID Knowledgebase
(63). P-value indicates
hypergeometric distribution P-values of overlap for gene sets and
functional categories. FDR indicates false discovery rate corrected
P-values of overlap. Activation z-score is an indication of the
consistency of up and downregulated members of a gene set such as a
biological function (top table) or targets of an upstream regulator
(middle table). Activation z-scores >2 or <−2 are
statistically significant for consistency of activation or
inhibition. Molecules indicate the number of molecules in the set
of 628 analyzed probes that overlap with a given category.
Mechanistic network indicates the total number of target genes of
an upstream regulator, and the number of overlapping genes is
indicated in parentheses. We observed enrichment for terms
associated with angiogenesis, apoptosis, epithelial to mesenchymal
transition, tumor progression and PDGF binding. Analysis of
potential upstream regulators by IPA analysis suggested that PTEN
and PDGF were activated, while MYC, β-estradiol, glucocorticoid
receptor NR3C1 and interferon-γ were repressed in response to
genistein treatment.
To the best of our knowledge, the present study is
the first highlighting the effects of genistein on global changes
in DNA methylation and gene expression in patients from a clinical
trial of genistein in prostate cancer. Integrative analysis of
whole genome methylation and expression profiling identified a
number of candidate differentially methylated sites and expressed
sites between placebo and genistein groups. However, the
differences between placebo and genistein groups were not
statistically significant after correction for multiple hypothesis
testing, possibly due to the small number of the cases in this
study. Although the genistein-induced alterations are not
significant, these results may help to elucidate the molecular
mechanisms underlying the activities of genistein in prostate
cancer. Genome-wide DNA methylation arrays showed that a number of
genes, including RBM28 and CYTSB, appeared to be
demethylated in the genistein-treated tumor samples compared to the
samples in the placebo group. However, we did not observe any
alterations in the expression levels of these genes. Among the
differentially expressed genes identified by microarray analysis
were CKS2, NOTCH3, HIF1A, CDK6,
JAG1, NEU1, ADCY4, MYC, CD24,
AMACR, MMP26 and SPP1. Microarray data were
confirmed by qPCR analysis of these genes. Other genes with nominal
significance by microarray but not tested by qPCR included
ZNF639, CRIM1, PGC and USP54 (available
upon request).
It is of interest to note that DNA methylation
status was inversely correlated with gene expression for the
NEU1 and ADCY4 genes, which had decreased
methylation, and increased mRNA expression in the genistein group
in comparison with placebo group. Our finding showing the potential
of genistein for DNA demethylation is consistent with the
previously reported data that suggest genistein acts as a DNMT
inhibitor, thereby causing the demethylation of CpG islands in the
promoters of genes. For example, genistein has been shown to
reactivate the hypermethylated-silenced tumor suppressor genes,
including p16INK4a, retinoic acid receptor β (RARβ)
and O6-methylguanine methyltransferase (MGMT), in
prostate and esophageal cancer cells (46). Moreover, genistein has been
implicated in demethylation of WNT5a promoter in colon
cancer cells (64). One of the
genes shown to be demethylated by genistein in the present study is
ADCY4, which is a member of the family of adenylate cyclases, the
membrane-bound enzymes that catalyze formation of the secondary
messenger cyclic adenosine monophosphate (cAMP) (65). Consistent with our finding, it has
been recently shown that ADCY4 is a DNA methylation marker
representing early epigenetic events in prostate tumorigenesis,
supporting our hypothesis that genistein may reverse the pattern of
DNA methylation in ADCY4 in prostate cancer (66). The other gene that was modulated by
genistein intervention in the present study was NEU1, which is a
lysosomal sialidase involved in glycoconjugate catabolism and
cellular signaling, including immune responses and elastin
receptor-mediated signal transduction (67). In fact, NEU1 is critical for
desialylation of integrin β4 and inhibition of FAK, leading to
suppression of liver metastases in colon cancer (68). Kato et al (69) has reported that NEU1 overexpression
resulted in suppression of lung metastasis in melanoma. In
addition, suppression of NEU1 by miR-125b has been
shown to promote migration, invasion and metastasis in gastric
cancers (70). However, NEU1 can
also have pro-metastatic effects in pancreatic and ovarian cancers
(71), and thus it is not entirely
clear what the overall impact of increased NEU1 levels might be in
prostate cancer. Therefore, it is important to examine the NEU1
expression changes at the protein level, and molecular and cellular
studies are required to assess the functional consequences of
changes induced by NEU1 upregulation in prostate cancer cells.
Among the differentially expressed genes that were
validated by qPCR, only the expression of NOTCH3 and
JAG1 mRNAs were significantly higher in the genistein group
compared to the placebo group by qPCR. Based on our findings at
mRNA level without any confirmation at the protein or functional
level, it would be speculative to suggest that Notch signaling may
play a role in the mechanism of action of genistein on prostate
cancer. NOTCH3 is important for TGFβ-induced EMT in prostate cancer
(72), and is induced by hypoxia
and contributes to prostate cancer progression (73). The Notch ligand JAG1 is also
associated with more aggressive prostate cancer (74,75),
EMT and angiogenesis (76).
However, a tumor suppressive role of Notch signaling has also been
reported in hypoxia-induced neuroendocrine differentiation of
prostate cancer cells as well as in other cancer types including
bladder cancer, hematological malignancies, glioma, thyroid
carcinoma and lung cancer (77–82),
indicating the possibility that increased NOTCH3/JAG1
expression by genistein treatment may improve outcomes through its
tumor suppressor function. Our data suggest that further studies to
delineate the effect of genistein on the Notch signaling pathway in
prostate cancer may be warranted.
Enrichment analyses of mRNA changes induced by
genistein indicated that subtle changes in gene expression observed
between genistein and placebo samples are consistent with many
previously reported effects of genistein on critical tumor pathways
including PTEN, PDGF, MYC, β-estradiol, glucocorticoid receptor and
interferon-γ (41,83–89).
Genistein appeared to promote PTEN activity and inhibit MYC
activity, consistent with its potential utility in improving
outcomes in prostate cancer.
In summary, our results indicate that genistein
intervention induces modulation of several genes, including
NOTCH3, JAG1, ADCY4 and NEU1,
suggesting that these genes may have the potential to be novel
molecular targets of genistein in prostate cancer. These genes are
involved in many critical biological processes including cell
cycle, angiogenesis, cellular immune response and intracellular
signal transduction, providing additional insight into the multiple
molecular pathways involved in prostate tumorigenesis. However,
further mechanistic studies are required to investigate the effects
of genistein on the regulation of the expression of these genes at
the protein level and cellular functions. These findings may then
contribute towards designing novel strategies for prevention and
treatment of prostate cancer. One caveat of gene expression
profiling studies is the incapability of identification of
mechanisms of action that are modulated at post-transcriptional
level, suggesting the possibility that genistein may alter
additional cellular processes. Another point that needs to be made
is timing and duration of exposure to genistein. Case control
studies have demonstrated that high consumption of soy early in
life (during childhood and/or adolescence) is associated with
25–60% reductions in breast cancer risk (90,91).
Similarly, high soy intake at puberty, the period during which
prostate undergoes androgen-induced growth, might be more effective
in prevention of prostate cancer. A limitation of the present study
is the small number of patient samples. Further large randomized
controlled clinical trials would provide more definitive results of
the effects of genistein on patient prostate tissues.
The present study was supported in part by the Emory
Integrated Genomics Core (EIGC), which is subsidized by the NCI
Cancer Center Support Grant P30CA138292 and Emory University School
of Medicine and is one of the Emory Integrated Core Facilities. It
was further supported by a Soy Research Award from the Soy Health
Research Program of the United Soybean Board.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prostate Cancer Facts and Figures 2016.
American Cancer Society Inc; Atlanta, GA: 2016
|
|
3
|
Dunn MW and Kazer MW: Prostate cancer
overview. Semin Oncol Nurs. 27:241–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sartor AO: Progression of metastatic
castrate-resistant prostate cancer: Impact of therapeutic
intervention in the post-docetaxel space. J Hematol Oncol.
4:182011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Skolarus TA, Wolf AMD, Erb NL, Brooks DD,
Rivers BM, Underwood W III, Salner AL, Zelefsky MJ, Aragon-Ching
JB, Slovin SF, et al: American Cancer Society prostate cancer
survivorship care guidelines. CA Cancer J Clin. 64:225–249. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hieronymus H, Schultz N, Gopalan A, Carver
BS, Chang MT, Xiao Y, Heguy A, Huberman K, Bernstein M, Assel M, et
al: Copy number alteration burden predicts prostate cancer relapse.
Proc Natl Acad Sci USA. 111:11139–11144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferlay J, Bray F, Pisani P and Parkin DM:
Globocan 2000: Cancer Incidence, Mortality and Prevalence
Worldwide. IARC Press; Lyon: 2001
|
|
8
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kimura T: East meets West: Ethnic
differences in prostate cancer epidemiology between East Asians and
Caucasians. Chin J Cancer. 31:421–429. 2012. View Article : Google Scholar
|
|
10
|
Reinli K and Block G: Phytoestrogen
content of foods–a compendium of literature values. Nutr Cancer.
26:123–148. 1996. View Article : Google Scholar
|
|
11
|
Adlercreutz H, Markkanen H and Watanabe S:
Plasma concentrations of phyto-oestrogens in Japanese men. Lancet.
342:1209–1210. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morton MS, Chan PS, Cheng C, Blacklock N,
Matos-Ferreira A, Abranches-Monteiro L, Correia R, Lloyd S and
Griffiths K: Lignans and isoflavonoids in plasma and prostatic
fluid in men: Samples from Portugal, Hong Kong, and the United
Kingdom. Prostate. 32:122–128. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang YW, Kim SY, Jee SH, Kim YN and Nam
CM: Soy food consumption and risk of prostate cancer: A
meta-analysis of observational studies. Nutr Cancer. 61:598–606.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van Poppel H and Tombal B: Chemoprevention
of prostate cancer with nutrients and supplements. Cancer Manag
Res. 3:91–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barnes S, Peterson TG and Coward L:
Rationale for the use of genistein-containing soy matrices in
chemoprevention trials for breast and prostate cancer. J Cell
Biochem (Suppl). S22:181–187. 1995. View Article : Google Scholar
|
|
16
|
Takimoto CH, Glover K, Huang X, Hayes SA,
Gallot L, Quinn M, Jovanovic BD, Shapiro A, Hernandez L, Goetz A,
et al: Phase I pharmacokinetic and pharmacodynamic analysis of
unconjugated soy isoflavones administered to individuals with
cancer. Cancer Epidemiol Biomarkers Prev. 12:1213–1221.
2003.PubMed/NCBI
|
|
17
|
Gardner CD, Oelrich B, Liu JP, Feldman D,
Franke AA and Brooks JD: Prostatic soy isoflavone concentrations
exceed serum levels after dietary supplementation. Prostate.
69:719–726. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bloedon LT, Jeffcoat AR, Lopaczynski W,
Schell MJ, Black TM, Dix KJ, Thomas BF, Albright C, Busby MG,
Crowell JA, et al: Safety and pharmacokinetics of purified soy
isoflavones: Single-dose administration to postmenopausal women. Am
J Clin Nutr. 76:1126–1137. 2002.PubMed/NCBI
|
|
19
|
Yildiz F: Phytoestrogens in functional
foods. CRC Press; Boca Raton, FL: 2005, https://doi.org/10.1201/9781420027594.
View Article : Google Scholar
|
|
20
|
Morito K, Hirose T, Kinjo J, Hirakawa T,
Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M and Masamune Y:
Interaction of phytoestrogens with estrogen receptors alpha and
beta. Biol Pharm Bull. 24:351–356. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang WY and Prins GS: Estrogen
receptor-beta: Implications for the prostate gland. Prostate.
40:115–124. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumar R, Verma V, Jain A, Jain RK,
Maikhuri JP and Gupta G: Synergistic chemoprotective mechanisms of
dietary phytoestrogens in a select combination against prostate
cancer. J Nutr Biochem. 22:723–731. 2011. View Article : Google Scholar
|
|
23
|
Wang J, Eltoum IE and Lamartiniere CA:
Genistein chemoprevention of prostate cancer in TRAMP mice. J
Carcinog. 6:32007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davis JN, Kucuk O and Sarkar FH:
Expression of prostate-specific antigen is transcriptionally
regulated by genistein in prostate cancer cells. Mol Carcinog.
34:91–101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bektic J, Berger AP, Pfeil K, Dobler G,
Bartsch G and Klocker H: Androgen receptor regulation by
physiological concentrations of the isoflavonoid genistein in
androgen-dependent LNCaP cells is mediated by estrogen receptor
beta. Eur Urol. 45:245–251; discussion 251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen JC, Klein RD, Wei Q, Guan Y, Contois
JH, Wang TT, Chang S and Hursting SD: Low-dose genistein induces
cyclin-dependent kinase inhibitors and G(1) cell-cycle arrest in
human prostate cancer cells. Mol Carcinog. 29:92–102. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Davis JN, Singh B, Bhuiyan M and Sarkar
FH: Genistein-induced upregulation of p21WAF1, downregulation of
cyclin B, and induction of apoptosis in prostate cancer cells. Nutr
Cancer. 32:123–131. 1998. View Article : Google Scholar
|
|
28
|
Raffoul JJ, Wang Y, Kucuk O, Forman JD,
Sarkar FH and Hillman GG: Genistein inhibits radiation-induced
activation of NF-kappaB in prostate cancer cells promoting
apoptosis and G2/M cell cycle arrest. BMC Cancer. 6:1072006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y and Sarkar FH: Down-regulation of
invasion and angiogenesis-related genes identified by cDNA
microarray analysis of PC3 prostate cancer cells treated with
genistein. Cancer Lett. 186:157–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo Y, Wang S, Hoot DR and Clinton SK:
Suppression of VEGF-mediated autocrine and paracrine interactions
between prostate cancer cells and vascular endothelial cells by soy
isoflavones. J Nutr Biochem. 18:408–417. 2007. View Article : Google Scholar
|
|
31
|
Li Y, Che M, Bhagat S, Ellis KL, Kucuk O,
Doerge DR, Abrams J, Cher ML and Sarkar FH: Regulation of gene
expression and inhibition of experimental prostate cancer bone
metastasis by dietary genistein. Neoplasia. 6:354–363. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang LL, Li L, Wu DP, Fan JH, Li X, Wu
KJ, Wang XY and He DL: A novel anti-cancer effect of genistein:
Reversal of epithelial mesenchymal transition in prostate cancer
cells. Acta Pharmacol Sin. 29:1060–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kumi-Diaka JK, Hassanhi M, Merchant K and
Horman V: Influence of genistein isoflavone on matrix
metalloproteinase-2 expression in prostate cancer cells. J Med
Food. 9:491–497. 2006. View Article : Google Scholar
|
|
34
|
Kazi A, Daniel KG, Smith DM, Kumar NB and
Dou QP: Inhibition of the proteasome activity, a novel mechanism
associated with the tumor cell apoptosis-inducing ability of
genistein. Biochem Pharmacol. 66:965–976. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kumi-Diaka J, Sanderson NA and Hall A: The
mediating role of caspase-3 protease in the intracellular mechanism
of genistein-induced apoptosis in human prostatic carcinoma cell
lines, DU145 and LNCaP. Biol Cell. 92:595–604. 2000. View Article : Google Scholar
|
|
36
|
Lee J, Ju J, Park S, Hong SJ and Yoon S:
Inhibition of IGF-1 signaling by genistein: Modulation of
E-cadherin expression and downregulation of β-catenin signaling in
hormone refractory PC-3 prostate cancer cells. Nutr Cancer.
64:153–162. 2012. View Article : Google Scholar
|
|
37
|
Xu L and Bergan RC: Genistein inhibits
matrix metalloproteinase type 2 activation and prostate cancer cell
invasion by blocking the transforming growth factor beta-mediated
activation of mitogen-activated protein kinase-activated protein
kinase 2-27-kDa heat shock protein pathway. Mol Pharmacol.
70:869–877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Davis JN, Kucuk O and Sarkar FH: Genistein
inhibits NF-kappaB activation in prostate cancer cells. Nutr
Cancer. 35:167–174. 1999. View Article : Google Scholar
|
|
39
|
Li Y and Sarkar FH: Inhibition of nuclear
factor kappaB activation in PC3 cells by genistein is mediated via
Akt signaling pathway. Clin Cancer Res. 8:2369–2377.
2002.PubMed/NCBI
|
|
40
|
Akiyama T, Ishida J, Nakagawa S, Ogawara
H, Watanabe S, Itoh N, Shibuya M and Fukami Y: Genistein, a
specific inhibitor of tyrosine-specific protein kinases. J Biol
Chem. 262:5592–5595. 1987.PubMed/NCBI
|
|
41
|
Park CE, Yun H, Lee EB, Min BI, Bae H,
Choe W, Kang I, Kim SS and Ha J: The antioxidant effects of
genistein are associated with AMP-activated protein kinase
activation and PTEN induction in prostate cancer cells. J Med Food.
13:815–820. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Raschke M, Rowland IR, Magee PJ and
Pool-Zobel BL: Genistein protects prostate cells against hydrogen
peroxide-induced DNA damage and induces expression of genes
involved in the defence against oxidative stress. Carcinogenesis.
27:2322–2330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Swami S, Krishnan AV, Moreno J,
Bhattacharyya RS, Gardner C, Brooks JD, Peehl DM and Feldman D:
Inhibition of prostaglandin synthesis and actions by genistein in
human prostate cancer cells and by soy isoflavones in prostate
cancer patients. Int J Cancer. 124:2050–2059. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Adjakly M, Bosviel R, Rabiau N, Boiteux
JP, Bignon YJ, Guy L and Bernard-Gallon D: DNA methylation and soy
phytoestrogens: Quantitative study in DU-145 and PC-3 human
prostate cancer cell lines. Epigenomics. 3:795–803. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vardi A, Bosviel R, Rabiau N, Adjakly M,
Satih S, Dechelotte P, Boiteux JP, Fontana L, Bignon YJ, Guy L, et
al: Soy phytoestrogens modify DNA methylation of GSTP1, RASSF1A,
EPH2 and BRCA1 promoter in prostate cancer cells. In Vivo.
24:393–400. 2010.PubMed/NCBI
|
|
46
|
Fang MZ, Chen D, Sun Y, Jin Z, Christman
JK and Yang C: Reversal of hypermethylation and reactivation of
16INK4a, RARbeta, and MGMT genes by genistein and other
isoflavones from soy. Clin Cancer Res. 11:7033–7041. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Majid S, Dar AA, Shahryari V, Hirata H,
Ahmad A, Saini S, Tanaka Y, Dahiya AV and Dahiya R: Genistein
reverses hyper-methylation and induces active histone modifications
in tumor suppressor gene B-Cell translocation gene 3 in prostate
cancer. Cancer. 116:66–76. 2010.
|
|
48
|
Kikuno N, Shiina H, Urakami S, Kawamoto K,
Hirata H, Tanaka Y, Majid S, Igawa M and Dahiya R: Genistein
mediated histone acetylation and demethylation activates tumor
suppressor genes in prostate cancer cells. Int J Cancer.
123:552–560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Y, Kong D, Ahmad A, Bao B, Dyson G and
Sarkar FH: Epigenetic deregulation of miR-29a and miR-1256 by
isoflavone contributes to the inhibition of prostate cancer cell
growth and invasion. Epigenetics. 7:940–949. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rabiau N, Trraf HK, Adjakly M, Bosviel R,
Guy L, Fontana L, Bignon YJ and Bernard-Gallon DJ: miRNAs
differentially expressed in prostate cancer cell lines after soy
treatment. In Vivo. 25:917–921. 2011.PubMed/NCBI
|
|
51
|
Chen Y, Zaman MS, Deng G, Majid S, Saini
S, Liu J, Tanaka Y and Dahiya R: MicroRNAs 221/222 and
genistein-mediated regulation of ARHI tumor suppressor gene in
prostate cancer. Cancer Prev Res (Phila). 4:76–86. 2011. View Article : Google Scholar
|
|
52
|
Chiyomaru T, Yamamura S, Fukuhara S,
Hidaka H, Majid S, Saini S, Arora S, Deng G, Shahryari V, Chang I,
et al: Genistein up-regulates tumor suppressor microRNA-574-3p in
prostate cancer. PLoS One. 8:e589292013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hillman GG, Forman JD, Kucuk O, Yudelev M,
Maughan RL, Rubio J, Layer A, Tekyi-Mensah S, Abrams J and Sarkar
FH: Genistein potentiates the radiation effect on prostate
carcinoma cells. Clin Cancer Res. 7:382–390. 2001.PubMed/NCBI
|
|
54
|
Li Y, Kucuk O, Hussain M, Abrams J, Cher
ML and Sarkar FH: Antitumor and antimetastatic activities of
docetaxel are enhanced by genistein through regulation of
osteoprotegerin/receptor activator of nuclear factor-kappaB
(RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res.
66:4816–4825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Spagnuolo C, Russo GL, Orhan IE,
Habtemariam S, Daglia M, Sureda A, Nabavi SF, Devi KP, Loizzo MR,
Tundis R, et al: Genistein and cancer: Current status, challenges,
and future directions. Adv Nutr. 6:408–419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Katoh M: Network of WNT and other
regulatory signaling cascades in pluripotent stem cells and cancer
stem cells. Curr Pharm Biotechnol. 12:160–170. 2011. View Article : Google Scholar
|
|
57
|
Mori R, Wang Q, Danenberg KD, Pinski JK
and Danenberg PV: Both beta-actin and GAPDH are useful reference
genes for normalization of quantitative RT-PCR in human FFPE tissue
samples of prostate cancer. Prostate. 68:1555–1560. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Reich M, Liefeld T, Gould J, Lerner J,
Tamayo P and Mesirov JP: GenePattern 2.0. Nat Genet. 38:500–501.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Eisen MB, Spellman PT, Brown PO and
Botstein D: Cluster analysis and display of genome-wide expression
patterns. Proc Natl Acad Sci USA. 95:14863–14868. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Saldanha AJ: Java Treeview - extensible
visualization of micro-array data. Bioinformatics. 20:3246–3248.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Barfield RT, Kilaru V, Smith AK and
Conneely KN: CpGassoc: An R function for analysis of DNA
methylation microarray data. Bioinformatics. 28:1280–1281. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
http://www.qiagen.com/ingenuity.
Qiagen's Ingenuity Pathway Analysis.
|
|
63
|
Dennis G Jr, Sherman BT, Hosack DA, Yang
J, Gao W, Lane HC and Lempicki RA: DAVID: Database for Annotation,
Visualization, and Integrated Discovery. Genome Biol. 4:32003.
View Article : Google Scholar
|
|
64
|
Wang Z and Chen H: Genistein increases
gene expression by demethylation of WNT5a promoter in colon cancer
cell line SW1116. Anticancer Res. 30:4537–4545. 2010.PubMed/NCBI
|
|
65
|
Gao BN and Gilman AG: Cloning and
expression of a widely distributed (type IV) adenylyl cyclase. Proc
Natl Acad Sci USA. 88:10178–10182. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Brikun I, Nusskern D, Gillen D, Lynn A,
Murtagh D, Feczko J, Nelson WG and Freije D: A panel of DNA
methylation markers reveals extensive methylation in histologically
benign prostate biopsy cores from cancer patients. Biomark Res.
2:252014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Monti E, Bonten E, D'Azzo A, Bresciani R,
Venerando B, Borsani G, Schauer R and Tettamanti G: Sialidases in
vertebrates: A family of enzymes tailored for several cell
functions. Adv Carbohydr Chem Biochem. 64:403–479. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Uemura T, Shiozaki K, Yamaguchi K,
Miyazaki S, Satomi S, Kato K, Sakuraba H and Miyagi T: Contribution
of sialidase NEU1 to suppression of metastasis of human colon
cancer cells through desialylation of integrin beta4. Oncogene.
28:1218–1229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kato T, Wang Y, Yamaguchi K, Milner CM,
Shineha R, Satomi S and Miyagi T: Overexpressing of lysosomal-type
sialidase leads to suppression of metastasis associated with
reversion of malignant phenotype in murine B16 melanoma cells. Int
J Cancer. 92:797–804. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chang S, He S, Qiu G, Lu J, Wang J, Liu J,
Fan L, Zhao W and Che X: MicroRNA-125b promotes invasion and
metastasis of gastric cancer by targeting STARD13 and NEU1. Tumour
Biol. 37:12141–12151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Haxho F, Neufeld RJ and Szewczuk MR:
Neuraminidase-1: A novel therapeutic target in multistage
tumorigenesis. Oncotarget. 7:40860–40881. 2016.PubMed/NCBI
|
|
72
|
Liu L, Chen X, Wang Y, Qu Z, Lu Q, Zhao J,
Yan X, Zhang H and Zhou Y: Notch3 is important for TGF-β-induced
epithelial-mesenchymal transition in non-small cell lung cancer
bone metastasis by regulating ZEB-1. Cancer Gene Ther. 21:364–372.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Danza G, Di Serio C, Ambrosio MR, Sturli
N, Lonetto G, Rosati F, Rocca BJ, Ventimiglia G, del Vecchio MT,
Prudovsky I, et al: Notch3 is activated by chronic hypoxia and
contributes to the progression of human prostate cancer. Int J
Cancer. 133:2577–2586. 2013.PubMed/NCBI
|
|
74
|
Terada N, Shiraishi T, Zeng Y, Aw-Yong KM,
Mooney SM, Liu Z, Takahashi S, Luo J, Lupold SE, Kulkarni P, et al:
Correlation of Sprouty1 and Jagged1 with aggressive prostate cancer
cells with different sensitivities to androgen deprivation. J Cell
Biochem. 115:1505–1515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pedrosa AR, Graça JL, Carvalho S,
Peleteiro MC, Duarte A and Trindade A: Notch signaling dynamics in
the adult healthy prostate and in prostatic tumor development.
Prostate. 76:80–96. 2016. View Article : Google Scholar
|
|
76
|
Pedrosa AR, Trindade A, Carvalho C, Graça
J, Carvalho S, Peleteiro MC, Adams RH and Duarte A: Endothelial
Jagged1 promotes solid tumor growth through both pro-angiogenic and
angiocrine functions. Oncotarget. 6:24404–24423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Danza G, Di Serio C, Rosati F, Lonetto G,
Sturli N, Kacer D, Pennella A, Ventimiglia G, Barucci R, Piscazzi
A, et al: Notch signaling modulates hypoxia-induced neuroendocrine
differentiation of human prostate cancer cells. Mol Cancer Res.
10:230–238. 2012. View Article : Google Scholar
|
|
78
|
Rampias T, Vgenopoulou P, Avgeris M,
Polyzos A, Stravodimos K, Valavanis C, Scorilas A and Klinakis A: A
new tumor suppressor role for the Notch pathway in bladder cancer.
Nat Med. 20:1199–1205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hernandez Tejada FN, Galvez Silva JR and
Zweidler-McKay PA: The challenge of targeting notch in hematologic
malignancies. Front Pediatr. 2:542014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Giachino C, Boulay JL, Ivanek R, Alvarado
A, Tostado C, Lugert S, Tchorz J, Coban M, Mariani L, Bettler B, et
al: A tumor suppressor function for Notch signaling in forebrain
tumor subtypes. Cancer Cell. 28:730–742. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jaskula-Sztul R, Eide J, Tesfazghi S,
Dammalapati A, Harrison AD, Yu XM, Scheinebeck C, Winston-McPherson
G, Kupcho KR, Robers MB, et al: Tumor-suppressor role of Notch3 in
medullary thyroid carcinoma revealed by genetic and pharmacological
induction. Mol Cancer Ther. 14:499–512. 2015. View Article : Google Scholar
|
|
82
|
Sriuranpong V, Borges MW, Ravi RK, Arnold
DR, Nelkin BD, Baylin SB and Ball DW: Notch signaling induces cell
cycle arrest in small cell lung cancer cells. Cancer Res.
61:3200–3205. 2001.PubMed/NCBI
|
|
83
|
Liu YL, Zhang GQ, Yang Y, Zhang CY, Fu RX
and Yang YM: Genistein induces G2/M arrest in gastric cancer cells
by increasing the tumor suppressor PTEN expression. Nutr Cancer.
65:1034–1041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Su SJ, Yeh TM, Chuang WJ, Ho CL, Chang KL,
Cheng HL, Liu HS, Cheng HL, Hsu PY and Chow NH: The novel targets
for anti-angiogenesis of genistein on human cancer cells. Biochem
Pharmacol. 69:307–318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jagadeesh S, Kyo S and Banerjee PP:
Genistein represses telomerase activity via both transcriptional
and posttranslational mechanisms in human prostate cancer cells.
Cancer Res. 66:2107–2115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mahmoud AM, Al-Alem U, Ali MM and Bosland
MC: Genistein increases estrogen receptor beta expression in
prostate cancer via reducing its promoter methylation. J Steroid
Biochem Mol Biol. 152:62–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Whirledge S, Senbanjo LT and Cidlowski JA:
Genistein disrupts glucocorticoid receptor signaling in human
uterine endometrial Ishikawa cells. Environ Health Perspect.
123:80–87. 2015.
|
|
88
|
Bhamre S, Sahoo D, Tibshirani R, Dill DL
and Brooks JD: Gene expression changes induced by genistein in the
prostate cancer cell line LNCaP. Open Prostate Cancer J. 3:86–98.
2010. View Article : Google Scholar
|
|
89
|
Ghaemi A, Soleimanjahi H, Razeghi S, Gorji
A, Tabaraei A, Moradi A, Alizadeh A and Vakili MA: Genistein
induces a protective immunomodulatory effect in a mouse model of
cervical cancer. Iran J Immunol. 9:119–127. 2012.PubMed/NCBI
|
|
90
|
Korde LA, Wu AH, Fears T, Nomura AM, West
DW, Kolonel LN, Pike MC, Hoover RN and Ziegler RG: Childhood soy
intake and breast cancer risk in Asian American women. Cancer
Epidemiol Biomarkers Prev. 18:1050–1059. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lee SA, Shu XO, Li H, Yang G, Cai H, Wen
W, Ji BT, Gao J, Gao YT and Zheng W: Adolescent and adult soy food
intake and breast cancer risk: Results from the Shanghai Women's
Health Study. Am J Clin Nutr. 89:1920–1926. 2009. View Article : Google Scholar : PubMed/NCBI
|