|
1
|
Stępiński D: Nucleolus-derived mediators
in oncogenic stress response and activation of p53-dependent
pathways. Histochem Cell Biol. 146:119–139. 2016. View Article : Google Scholar
|
|
2
|
Merino D and Malkin D: p53 and hereditary
cancer. Subcell Biochem. 85:1–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muller PA and Vousden KH: p53 mutations in
cancer. Nat Cell Biol. 15:2–8. 2013. View
Article : Google Scholar
|
|
4
|
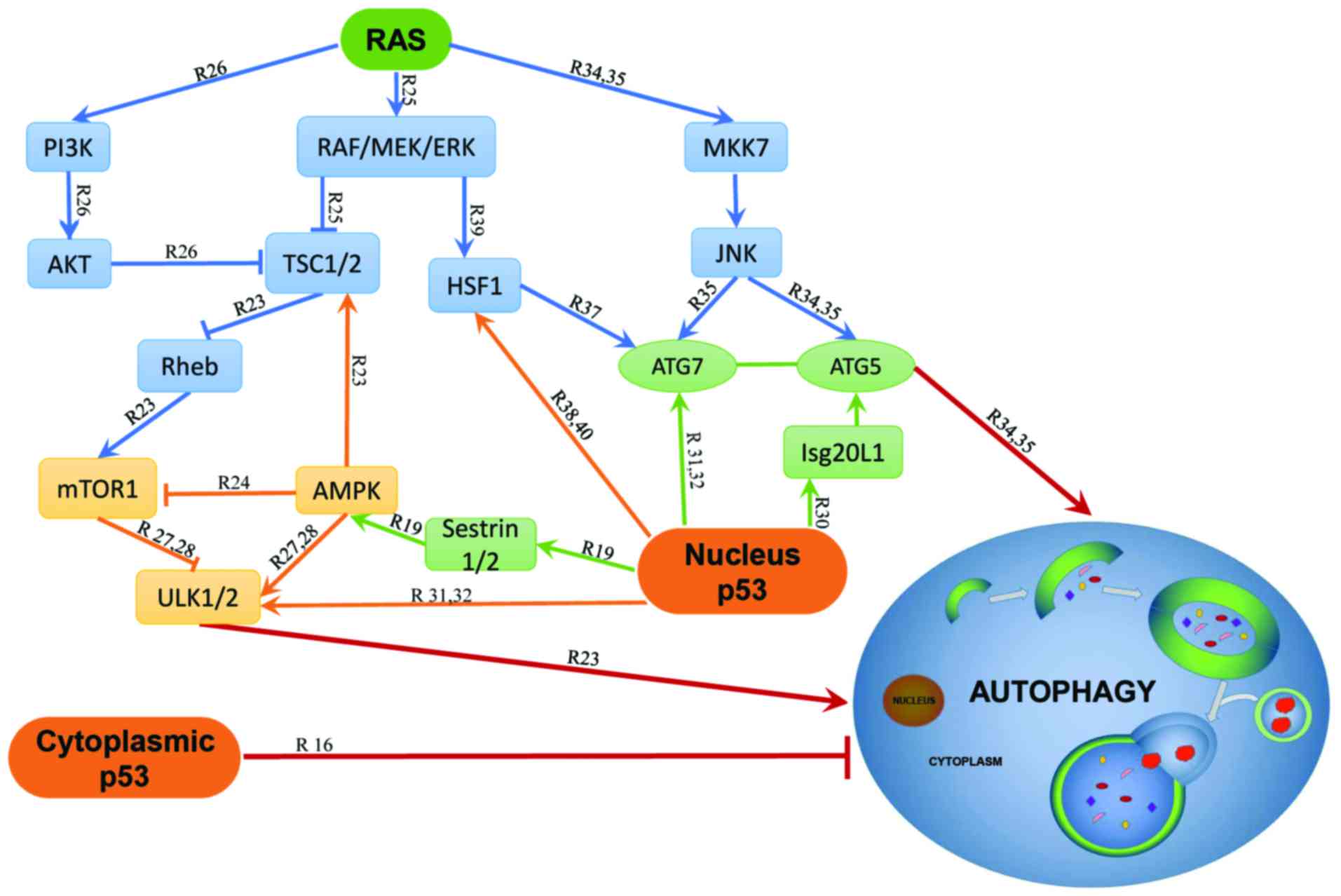
Freed-Pastor WA and Prives C: Mutant p53:
One name, many proteins. Genes Dev. 26:1268–1286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Silva JL, De Moura Gallo CV, Costa DC and
Rangel LP: Prion-like aggregation of mutant p53 in cancer. Trends
Biochem Sci. 39:260–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fang B: RAS signaling and anti-RAS
therapy: Lessons learned from genetically engineered mouse models,
human cancer cells, and patient-related studies. Acta Biochim
Biophys Sin (Shanghai). 48:27–38. 2016.
|
|
7
|
Kimmelman AC: Metabolic dependencies in
RAS-driven cancers. Clin Cancer Res. 21:1828–1834. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stites EC and Ravichandran KS: A systems
perspective of ras signaling in cancer. Clin Cancer Res. 15(5):
1510–1513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vandal G, Geiling B and Dankort D: Ras
effector mutant expression suggest a negative regulator inhibits
lung tumor formation. PLoS One. 9:e847452014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia M and Land H: Tumor suppressor p53
restricts Ras stimulation of RhoA and cancer cell motility. Nat
Struct Mol Biol. 14:215–223. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meylan E, Dooley AL, Feldser DM, Shen L,
Turk E, Ouyang C and Jacks T: Requirement for NF-kappaB signalling
in a mouse model of lung adenocarcinoma. Nature. 462:104–107. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boiko AD, Porteous S, Razorenova OV,
Krivokrysenko VI, Williams BR and Gudkov AV: A systematic search
for downstream mediators of tumor suppressor function of p53
reveals a major role of BTG2 in suppression of Ras-induced
transformation. Genes Dev. 20:236–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song H, Hollstein M and Xu Y: p53
gain-of-function cancer mutants induce genetic instability by
inactivating ATM. Nat Cell Biol. 9:573–580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lorin S, Hamaï A, Mehrpour M and Codogno
P: Autophagy regulation and its role in cancer. Semin Cancer Biol.
23:361–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumar A, Singh UK and Chaudhary A:
Targeting autophagy to overcome drug resistance in cancer therapy.
Future Med Chem. 7:1535–1542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–53016. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang J, Di J, Cao H, Bai J and Zheng J:
p53-mediated autophagic regulation: A prospective strategy for
cancer therapy. Cancer Lett. 363:101–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tasdemir E, Maiuri MC, Galluzzi L, Vitale
I, Djavaheri-Mergny M, D'Amelio M, Criollo A, Morselli E, Zhu C,
Harper F, et al: Regulation of autophagy by cytoplasmic p53. Nat
Cell Biol. 10:676–687. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmukler E, Kloog Y and Pinkas-Kramarski
R: Ras and autophagy in cancer development and therapy. Oncotarget.
5:577–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lock R, Kenific CM, Leidal AM, Salas E and
Debnath J: Autophagy-dependent production of secreted factors
facilitates oncogenic RAS-driven invasion. Cancer Discov.
4:466–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Budanov AV: Stress-responsive sestrins
link p53 with redox regulation and mammalian target of rapamycin
signaling. Antioxid Redox Signal. 15:1679–1690. 2011. View Article : Google Scholar :
|
|
24
|
Huang J and Manning BD: The TSC1–TSC2
complex: A molecular switchboard controlling cell growth. Biochem
J. 412:179–190. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gwinn DM, Shackelford DB, Egan DF,
Mihaylova MM, Mery A, Vasquez DS, Turk BE and Shaw RJ: AMPK
phosphorylation of raptor mediates a metabolic checkpoint. Mol
Cell. 30:214–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kong B, Wu W, Cheng T, Schlitter AM, Qian
C, Bruns P, Jian Z, Jäger C, Regel I, Raulefs S, et al: A subset of
metastatic pancreatic ductal adenocarcinomas depends quantitatively
on oncogenic Kras/Mek/Erk-induced hyperactive mTOR signalling. Gut.
65:647–657. 2016. View Article : Google Scholar
|
|
27
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mizushima N, Yoshimori T and Ohsumi Y: The
role of Atg proteins in autophagosome formation. Annu Rev Cell Dev
Biol. 27:107–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eby KG, Rosenbluth JM, Mays DJ, Marshall
CB, Barton CE, Sinha S, Johnson KN, Tang L and Pietenpol JA:
ISG20L1 is a p53 family target gene that modulates genotoxic
stress-induced autophagy. Mol Cancer. 9:952010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kenzelmann Broz D, Spano Mello S, Bieging
KT, Jiang D, Dusek RL, Brady CA, Sidow A and Attardi LD: Global
genomic profiling reveals an extensive p53-regulated autophagy
program contributing to key p53 responses. Genes Dev. 27:1016–1031.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao W, Shen Z, Shang L and Wang X:
Upregulation of human autophagy-initiation kinase ULK1 by tumor
suppressor p53 contributes to DNA-damage-induced cell death. Cell
Death Differ. 18:1598–1607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo JY, Karsli-Uzunbas G, Mathew R, Aisner
SC, Kamphorst JJ, Strohecker AM, Chen G, Price S, Lu W, Teng X, et
al: Autophagy suppresses progression of K-ras-induced lung tumors
to oncocytomas and maintains lipid homeostasis. Genes Dev.
27:1447–1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim MJ, Woo SJ, Yoon CH, Lee JS, An S,
Choi YH, Hwang SG, Yoon G and Lee SJ: Involvement of autophagy in
oncogenic K-Ras-induced malignant cell transformation. J Biol Chem.
286:12924–12932. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kinsey C, Balakrishnan V, O'Dell MR, Huang
JL, Newman L, Whitney-Miller CL, Hezel AF and Land H: Plac8 links
oncogenic mutations to regulation of autophagy and is critical to
pancreatic cancer progression. Cell Rep. 7:1143–1155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Desai S, Liu Z, Yao J, Patel N, Chen J, Wu
Y, Ahn EE, Fodstad O and Tan M: Heat shock factor 1 (HSF1) controls
chemoresistance and autophagy through transcriptional regulation of
autophagy-related protein 7 (ATG7). J Biol Chem. 288:9165–9176.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vydra N, Toma A and Widlak W: Pleiotropic
role of HSF1 in neoplastic transformation. Curr Cancer Drug
Targets. 14:144–155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dai C, Santagata S, Tang Z, Shi J, Cao J,
Kwon H, Bronson RT, Whitesell L and Lindquist S: Loss of tumor
suppressor NF1 activates HSF1 to promote carcinogenesis. J Clin
Invest. 122:3742–3754. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Q and Martinez JD: P53 is transported
into the nucleus via an Hsf1-dependent nuclear localization
mechanism. Mol Carcinog. 50:143–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu YL, Jahangiri A, De Lay M and Aghi MK:
Hypoxia-induced tumor cell autophagy mediates resistance to
anti-angiogenic therapy. Autophagy. 8:979–981. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen C, Pore N, Behrooz A, Ismail-Beigi F
and Maity A: Regulation of glut1 mRNA by hypoxia-inducible
factor-1. Interaction between H-ras and hypoxia. J Biol Chem.
276:9519–9525. 2001. View Article : Google Scholar
|
|
44
|
Nieminen AL, Qanungo S, Schneider EA,
Jiang BH and Agani FH: Mdm2 and HIF-1alpha interaction in tumor
cells during hypoxia. J Cell Physiol. 204:364–369. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Robertson ED, Semenchenko K and Wasylyk B:
Crosstalk between Mdm2, p53 and HIF1-α: Distinct responses to
oxygen stress and implications for tumour hypoxia. Subcell Biochem.
85:199–214. 2014. View Article : Google Scholar
|
|
46
|
Hay ED: An overview of
epithelio-mesenchymal transformation. Acta Anat (Basel). 154:8–20.
1995. View Article : Google Scholar
|
|
47
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Puisieux A, Brabletz T and Caramel J:
Oncogenic roles of EMT-inducing transcription factors. Nat Cell
Biol. 16:488–494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fischer KR, Durrans A, Lee S, Sheng J, Li
F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al:
Epithelial-to-mesenchymal transition is not required for lung
metastasis but contributes to chemoresistance. Nature. 527:472–476.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang Y, Ngo VN, Marani M, Yang Y, Wright
G, Staudt LM and Downward J: Critical role for transcriptional
repressor Snail2 in transformation by oncogenic RAS in colorectal
carcinoma cells. Oncogene. 29:4658–4670. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
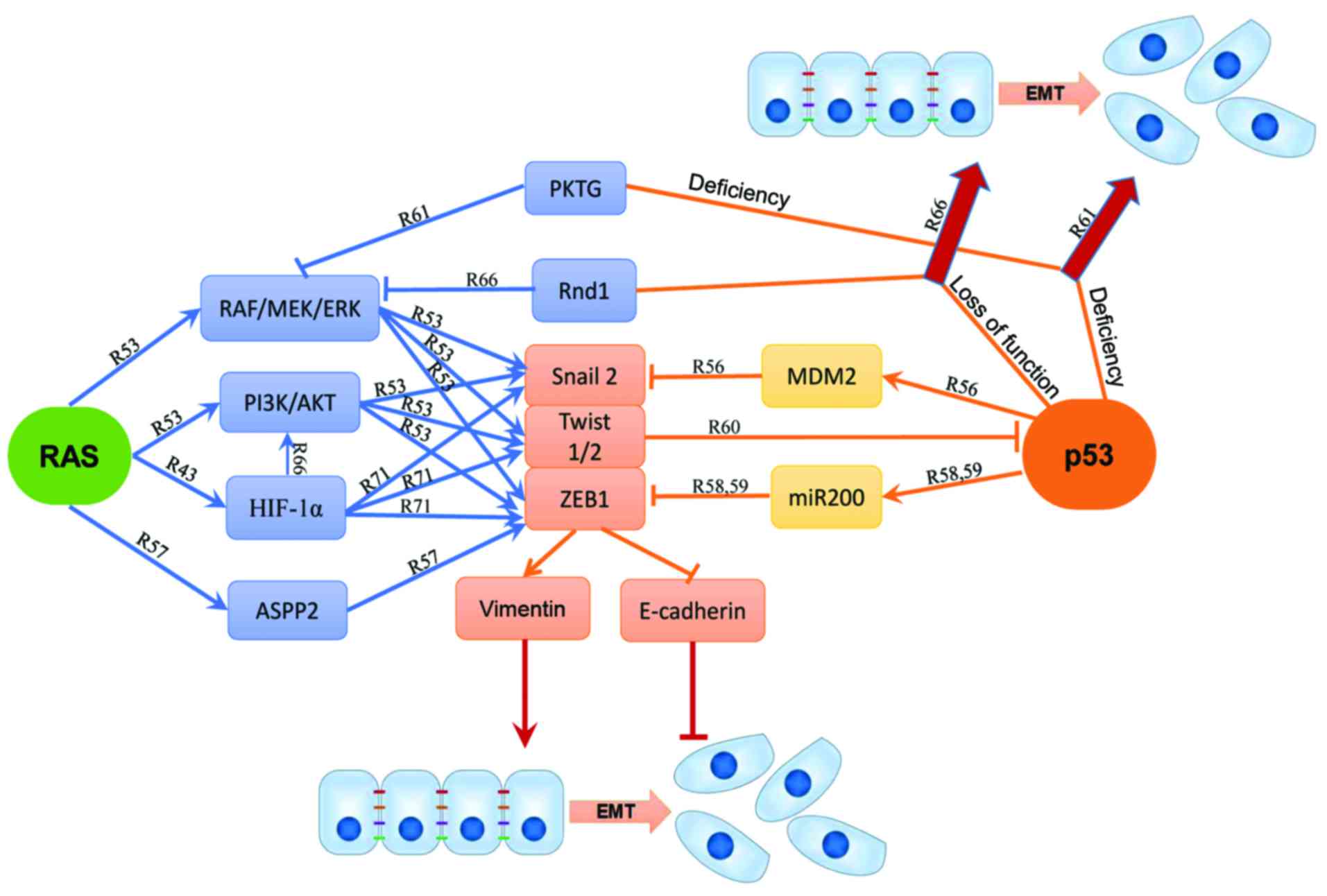
Zhang J, Lei Y, Gao X, Liang Q, Li L, Feng
J, Hou P, Han L, Zhang Y, Huang B, et al: p53 Attenuates the
oncogenic Ras-induced epithelial-mesenchymal transition in human
mammary epithelial cells. Biochem Biophys Res Commun. 434:606–613.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang Z, Wade P, Mandell KJ, Akyildiz A,
Parkos CA, Mrsny RJ and Nusrat A: Raf 1 represses expression of the
tight junction protein occludin via activation of the zinc-finger
transcription factor slug. Oncogene. 26:1222–1230. 2007. View Article : Google Scholar
|
|
55
|
Saegusa M, Hashimura M, Kuwata T and
Okayasu I: Requirement of the Akt/beta-catenin pathway for uterine
carcinosarcoma genesis, modulating E-cadherin expression through
the transactivation of slug. Am J Pathol. 174:2107–2115. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang SP, Wang WL, Chang YL, Wu CT, Chao
YC, Kao SH, Yuan A, Lin CW, Yang SC, Chan WK, et al: p53 controls
cancer cell invasion by inducing the MDM2-mediated degradation of
Slug. Nat Cell Biol. 11:694–704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang Y, Bu F, Royer C, Serres S, Larkin
JR, Soto MS, Sibson NR, Salter V, Fritzsche F, Turnquist C, et al:
ASPP2 controls epithelial plasticity and inhibits metastasis
through β-catenin-dependent regulation of ZEB1. Nat Cell Biol.
16:1092–1104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim T, Veronese A, Pichiorri F, Lee TJ,
Jeon YJ, Volinia S, Pineau P, Marchio A, Palatini J, Suh SS, et al:
p53 regulates epithelial-mesenchymal transition through microRNAs
targeting ZEB1 and ZEB2. J Exp Med. 208:875–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chang CJ, Chao CH, Xia W, Yang JY, Xiong
Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH, et al: p53 regulates
epithelial-mesenchymal transition and stem cell properties through
modulating miRNAs. Nat Cell Biol. 13:317–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ansieau S, Bastid J, Doreau A, Morel AP,
Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S,
et al: Induction of EMT by twist proteins as a collateral effect of
tumor-promoting inactivation of premature senescence. Cancer Cell.
14:79–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jiang Y, Xie X, Li Z, Wang Z, Zhang Y,
Ling ZQ, Pan Y, Wang Z and Chen Y: Functional cooperation of RKTG
with p53 in tumorigenesis and epithelial-mesenchymal transition.
Cancer Res. 71:2959–2968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gibbons DL, Lin W, Creighton CJ, Rizvi ZH,
Gregory PA, Goodall GJ, Thilaganathan N, Du L, Zhang Y,
Pertsemlidis A, et al: Contextual extracellular cues promote tumor
cell EMT and metastasis by regulating miR-200 family expression.
Genes Dev. 23:2140–2151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Roger L, Jullien L, Gire V and Roux P:
Gain of oncogenic function of p53 mutants regulates E-cadherin
expression uncoupled from cell invasion in colon cancer cells. J
Cell Sci. 123:1295–1305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ohashi S, Natsuizaka M, Wong GS,
Michaylira CZ, Grugan KD, Stairs DB, Kalabis J, Vega ME, Kalman RA,
Nakagawa M, et al: Epidermal growth factor receptor and mutant p53
expand an esophageal cellular subpopulation capable of
epithelial-to-mesenchymal transition through ZEB transcription
factors. Cancer Res. 70:4174–4184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jiang Z, Deng T, Jones R, Li H,
Herschkowitz JI, Liu JC, Weigman VJ, Tsao MS, Lane TF, Perou CM, et
al: Rb deletion in mouse mammary progenitors induces luminal-B or
basal-like/EMT tumor subtypes depending on p53 status. J Clin
Invest. 120:3296–3309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Okada T, Sinha S, Esposito I, Schiavon G,
López-Lago MA, Su W, Pratilas CA, Abele C, Hernandez JM, Ohara M,
et al: The Rho GTPase Rnd1 suppresses mammary tumorigenesis and EMT
by restraining Ras-MAPK signalling. Nat Cell Biol. 17:81–94. 2015.
View Article : Google Scholar
|
|
67
|
Gao T, Li JZ, Lu Y, Zhang CY, Li Q, Mao J
and Li LH: The mechanism between epithelial mesenchymal transition
in breast cancer and hypoxia microenvironment. Biomed Pharmacother.
80:393–405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Imai T, Horiuchi A, Wang C, Oka K, Ohira
S, Nikaido T and Konishi I: Hypoxia attenuates the expression of
E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells.
Am J Pathol. 163:1437–1447. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang L, Huang G, Li X, Zhang Y, Jiang Y,
Shen J, Liu J, Wang Q, Zhu J, Feng X, et al: Hypoxia induces
epithelial-mesenchymal transition via activation of SNAI1 by
hypoxia-inducible factor-1α in hepatocellular carcinoma. BMC
Cancer. 13:1082013. View Article : Google Scholar
|
|
70
|
Cui Y, Li YY, Li J, Zhang HY, Wang F, Bai
X and Li SS: STAT3 regulates hypoxia-induced epithelial mesenchymal
transition in oesophageal squamous cell cancer. Oncol Rep.
36:108–116. 2016.PubMed/NCBI
|
|
71
|
Tsai YP and Wu KJ: Hypoxia-regulated
target genes implicated in tumor metastasis. J Biomed Sci.
19:1022012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gugnoni M, Sancisi V, Manzotti G, Gandolfi
G and Ciarrocchi A: Autophagy and epithelial-mesenchymal
transition: An intricate interplay in cancer. Cell Death Dis.
7:e25202016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Qin W, Li C, Zheng W, Guo Q, Zhang Y, Kang
M, Zhang B, Yang B, Li B, Yang H, et al: Inhibition of autophagy
promotes metastasis and glycolysis by inducing ROS in gastric
cancer cells. Oncotarget. 6:39839–39854. 2015.PubMed/NCBI
|
|
75
|
Qiang L and He YY: Autophagy deficiency
stabilizes TWIST1 to promote epithelial-mesenchymal transition.
Autophagy. 10:1864–1865. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Catalano M, D'Alessandro G, Lepore F,
Corazzari M, Caldarola S, Valacca C, Faienza F, Esposito V,
Limatola C, Cecconi F, et al: Autophagy induction impairs migration
and invasion by reversing EMT in glioblastoma cells. Mol Oncol.
9:1612–1625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lv Q, Hua F and Hu ZW: DEDD, a novel tumor
repressor, reverses epithelial-mesenchymal transition by activating
selective autophagy. Autophagy. 8:1675–1676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li J, Yang B, Zhou Q, Wu Y, Shang D, Guo
Y, Song Z, Zheng Q and Xiong J: Autophagy promotes hepatocellular
carcinoma cell invasion through activation of
epithelial-mesenchymal transition. Carcinogenesis. 34:1343–1351.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wei SC and Yang J: Forcing through tumor
metastasis: The interplay between tissue rigidity and
epithelial-mesenchymal transition. Trends Cell Biol. 26:111–120.
2016. View Article : Google Scholar :
|
|
80
|
Tojkander S, Gateva G and Lappalainen P:
Actin stress fibers-assembly, dynamics and biological roles. J Cell
Sci. 125:1855–1864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
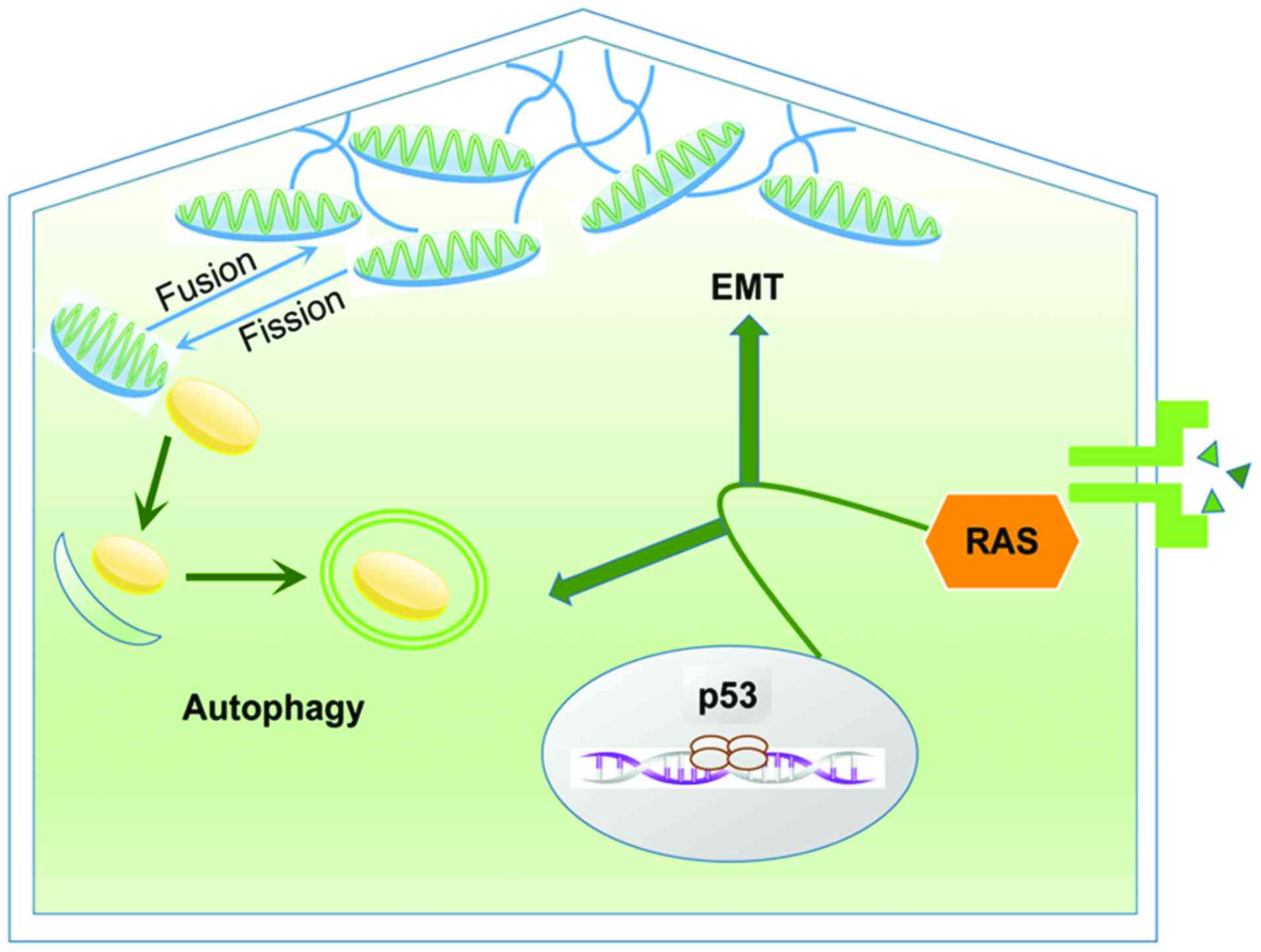
Ni HM, Williams JA and Ding WX:
Mitochondrial dynamics and mitochondrial quality control. Redox
Biol. 4:6–13. 2015. View Article : Google Scholar :
|
|
82
|
Youle RJ and van der Bliek AM:
Mitochondrial fission, fusion, and stress. Science. 337:1062–1065.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhao J, Zhang J, Yu M, Xie Y, Huang Y,
Wolff DW, Abel PW and Tu Y: Mitochondrial dynamics regulates
migration and invasion of breast cancer cells. Oncogene.
32:4814–4824. 2013. View Article : Google Scholar
|
|
84
|
Ketschek A and Gallo G: Nerve growth
factor induces axonal filopodia through localized microdomains of
phosphoinositide 3-kinase activity that drive the formation of
cytoskeletal precursors to filopodia. J Neurosci. 30:12185–12197.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Parada LF, Land H, Weinberg RA, Wolf D and
Rotter V: Cooperation between gene encoding p53 tumour antigen and
ras in cellular transformation. Nature. 312:649–651. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Jenkins JR, Rudge K and Currie GA:
Cellular immortalization by a cDNA clone encoding the
transformation-associated phosphoprotein p53. Nature. 312:651–654.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Eliyahu D, Raz A, Gruss P, Givol D and
Oren M: Participation of p53 cellular tumour antigen in
transformation of normal embryonic cells. Nature. 312:646–649.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
DuPage M, Dooley AL and Jacks T:
Conditional mouse lung cancer models using adenoviral or lentiviral
delivery of Cre recombinase. Nat Protoc. 4:1064–1072. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hingorani SR, Wang L, Multani AS, Combs C,
Deramaudt TB, Hruban RH, Rustgi AK, Chang S and Tuveson DA:
Trp53R172H and KrasG12D cooperate to promote
chromosomal instability and widely metastatic pancreatic ductal
adenocarcinoma in mice. Cancer Cell. 7:469–483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tsumura H, Yoshida T, Saito H,
Imanaka-Yoshida K and Suzuki N: Cooperation of oncogenic K-ras and
p53 deficiency in pleomorphic rhabdomyosarcoma development in adult
mice. Oncogene. 25:7673–7679. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zheng S, El-Naggar AK, Kim ES, Kurie JM
and Lozano G: A genetic mouse model for metastatic lung cancer with
gender differences in survival. Oncogene. 26:6896–6904. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Muñoz DM, Tung T, Agnihotri S, Singh S,
Guha A, Zadeh G and Hawkins C: Loss of p53 cooperates with K-ras
activation to induce glioma formation in a region-independent
manner. Glia. 61:1862–1872. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Solomon H, Brosh R, Buganim Y and Rotter
V: Inactivation of the p53 tumor suppressor gene and activation of
the Ras oncogene: Cooperative events in tumorigenesis. Discov Med.
9:448–454. 2010.PubMed/NCBI
|
|
94
|
Jackson JG and Lozano G: The mutant p53
mouse as a preclinical model. Oncogene. 32:4325–4330. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Bertheau P, Turpin E, Rickman DS, Espié M,
de Reyniès A, Feugeas JP, Plassa LF, Soliman H, Varna M, de
Roquancourt A, et al: Exquisite sensitivity of TP53 mutant and
basal breast cancers to a dose-dense epirubicin-cyclophosphamide
regimen. PLoS Med. 4:e902007. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Cassinelli G, Zuco V, Gatti L, Lanzi C,
Zaffaroni N, Colombo D and Perego P: Targeting the Akt kinase to
modulate survival, invasiveness and drug resistance of cancer
cells. Curr Med Chem. 20:1923–1945. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Gurpinar E and Vousden KH: Hitting
cancers' weak spots: Vulnerabilities imposed by p53 mutation.
Trends Cell Biol. 25:486–495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Bournet B, Buscail C, Muscari F, Cordelier
P and Buscail L: Targeting KRAS for diagnosis, prognosis, and
treatment of pancreatic cancer: Hopes and realities. Eur J Cancer.
54:75–83. 2016. View Article : Google Scholar : PubMed/NCBI
|