Introduction
Bladder cancer is thought to develop along two
major, independent pathways and these progression pathways have
different genetic abnormalities (1). One is the 'papillary pathway' that is
characterized as low-grade papillary cancer via epithelial
hyperplasia. Approximately 80% of bladder cancers arise from the
papillary pathway and these cancers can be treated by transurethral
resections and have a relatively good prognosis compared to other
types. This pathway is associated with genetic abnormalities such
as activating mutations of the oncogene, HRAS and the
receptor tyrosine kinase gene, FGFR3. The other pathway is
the 'carcinoma in situ (CIS) pathway'. This pathway is
associated with genetic abnormalities of the tumor suppressor
genes, TP53 and Rb. Approximately 80% of
muscle-invasive bladder cancer is thought to arise from this
pathway. CIS is a distinct entity with a high-grade malignancy and
a high tendency to progress to muscle-invasive bladder cancer
(2). CIS is commonly treated by
intravesical infusion therapy. Although therapy has been developed
for CIS, there remain therapy-resistant cases in ~30% of patients
treated with Bacillus Calmette-Guérin (BCG) therapy and in 50% of
cases treated with intravesical chemotherapy (3). Moreover, in more than 50% of cases,
residual CIS lesions are found in radical cystectomy specimens
after preoperative chemotherapy (4). Thus, CIS is considered to have the
potential to develop anti-cancer drug resistance.
Recently, gemcitabine combined with cisplatin (GC)
therapy has been accepted as a new standard treatment for advanced
bladder cancer because of fewer adverse events than those with
methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) therapy
(5). However, despite a reasonable
response rate after initial chemotherapy, 60–70% of patients
relapse within the first year probably because of drug resistance
to gemcitabine. The mechanism of drug resistance to gemcitabine has
not been elucidated. Recently, gemcitabine had been used not only
as a systemic chemotherapy for metastatic bladder cancer, but also
as intravesical therapy for CIS (6). It is therefore necessary to clarify
the mechanism of CIS drug-resistance to gemcitabine, which could
provide a basis for the development of novel strategies for bladder
cancer.
Y box binding-1 (YB-1) is a member of a family of
DNA-binding proteins that contain a cold shock domain and it is
directly involved in the cellular response to genotoxic stress,
such as DNA-damaging agents and UV irradiation (7–9).
YB-1 is predominantly localized to the cytoplasm in many cancer
cells but its translocation to the nucleus is directly induced by
phosphorylated Akt, in response to these environmental stresses
(10–14). Nuclear translocated YB-1
upregulates transcription of the multidrug resistance-1
(MDR-1) gene. YB-1 associates with p53 and p53 is thought to
be essential for nuclear translocation of YB-1 (15–17).
In the present study, we aimed to analyze YB-1
association with the drug resistance of TP53-mutated bladder
cancer, including immunohistochemical analysis of YB-1,
P-glycoprotein and p53 in vivo and analysis of the role of
p53 in the nuclear translocation and nuclear function of YB-1 in
vitro. Additionally, we examined the association between
gemcitabine and the nuclear translocation of YB-1.
Materials and methods
Patients
Transurethral resected specimens, and clinical data
were obtained retrospectively from 81 patients newly diagnosed with
CIS from 2000 to 2013 at the Department of Pathology of Saitama
Medical Center, Saitama Medical University. The examined specimens
were from patients previously untreated for CIS. The staging was
performed according to the TNM classification (7th edition). All
specimen collections were approved by the ethics committee of
Saitama Medical Center, Saitama Medical University (no. 992).
Immunohistochemical analysis of clinical
samples
The following antibodies were used for
immunohistochemical detection; anti-YB-1 mouse monoclonal IgG
antibody (clone: 21A3, 1:100; Immuno-Biological Laboratories,
Gunma, Japan), anti-p53 mouse monoclonal IgG antibody (clone: DO-7,
1:40; Dako, Tokyo, Japan) and anti-P-glycoprotein mouse monoclonal
IgG antibody (clone: C494, 1:100; Thermo Fisher Scientific,
Waltham, MA, USA) antibodies. Immunohistochemical staining for p53
was performed using the Ventana iVIEW DAB kit reagents (Ventana
Medical Systems, Inc., Tucson, AZ, USA) and an auto-immunostainer
(Ventana ULTRA). For YB-1 and P-glycoprotein detection, the BOND
polymer system refine kit and an auto-immunostainer (BOND III;
Leica Microsystems GmbH, Wetzlar, Germany) were used according to
the manufacturer's instructions. Protein expression was blindly
assessed by two pathologists (T.Y. and J.T.). Expression of YB-1
and p53 was categorized into two groups according to the proportion
of expressing cells in 5 hot spots of the specimens: positive, ≥30%
of cancer cells stained; negative, <30% of cancer cells stained.
Nuclear expression of YB-1 was evaluated according to the presence
or absence of staining in the nucleus. For P-glycoprotein
expression, positive staining of ≥50% of the cells was defined as
positive.
Cell culture, reagents and materials
The human cervical cancer cell line HeLa, the human
breast cancer cell line MCF-7 and the human skin cancer cell line
A431 were obtained from the American Type Culture Collection (ATCC;
Rockville, MD, USA). The human bladder cancer cell lines, 5637 and
T24 were obtained from the RIKEN BioResource Center (Saitama,
Japan). HeLa, MCF-7, T24 and A431 cells were maintained in
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific)
and 5637 cells were maintained in RPMI-1640 medium (Thermo Fisher
Scientific). Media were supplemented with 10% fetal bovine serum
(FBS; Nichirei Biosciences, Inc., Tokyo, Japan), L-glutamine and
antibiotics (penicillin and streptomycin). Gemcitabine was
purchased from Taiho Pharmaceutical, Co., Ltd. (Tokyo, Japan) and
cisplatin was purchased from Nichi-Iko Pharmaceutical, Co., Ltd.
(Toyama, Japan).
Plasmids
Total RNA was extracted from cells using TRIzol
reagent (Thermo Fisher Scientific) and reverse transcribed using
the SuperScript™ III First-Strand Synthesis SuperMix (Thermo Fisher
Scientific) for polymerase chain reaction (PCR), according to the
manufacturer's protocol. YB-1 and p53 cDNA sequence was obtained
from the National Center for Biotechnology Information (NCBI)
GeneBank database (http://www.ncbi.nlm.nih.gov/genbank/). The cDNAs
encoding YB-1 and wild-type p53 were cloned using PCR of normal
lymphocyte mRNA from a healthy donor, and that encoding mutant p53
(R273H) was cloned by PCR of the mRNA from A431 cells. The primers
for PCR were: YB-1 sense, 5′-GGACTCAGATCTCGAGCAACCATGAGCAGC GAGG-3′
and antisense, 5′-GTCGACTGCAGAATTTTTACTCAGCCCCGCCCTGC-3′; p53
sense, 5′-GGACTCAGATCTCGAGCCATGGAGGAGCCGC-3′ and antisense,
5′-GTCGACTGCAGAATTTGTCAGTCTGAGTCAGGCCCT-3′. PCR was perfomed with
an initial denaturation step at 98°C for 1 min, followed by 30
cycles of denaturation at 98°C for 10 sec, annealing at 55°C for 15
sec and extension at 68°C for 90 sec. The PCR fragments from p53
and YB-1 cDNAs were cloned in frame with the enhanced green
fluorescence protein (EGFP) or mCherry (Clontech Laboratories,
Inc., Mountain View, CA, USA) as a C-terminal fusion using
In-Fusion HD cloning kit (Clontech Laboratories). The insert sites
were released from EGFP and mCherry by digestion with XhoI
and EcoRI. Transfections were performed with Lipofectamine
3000 (Thermo Fisher Scientific) as directed by the
manufacturer.
Small interfering RNAs (siRNA)
Two siRNA species for knockdown of YB-1, YB-1 siRNA
I (cat. no. #6206) and II (cat. no. #6207), as well as control
siRNA (cat. no. #6568), were purchased from Cell Signaling
Technology (Danvers, MA, USA). Transfections were performed with
Lipofectamine 2000 (Thermo Fisher Scientific) as directed by the
manufacturer.
Gemcitabine cytotoxicity assay
The 5637 cells transfected with YB-1 siRNA or
control siRNA were incubated for 72 h with 100 nM of gemicitabine.
Viable cells were labeled using the Cell Counting kit-8 (CCK-8;
Dojindo Molecular Technologies Inc., Kumamoto, Japan) and were
quantified using a microplate spectrophotometer (Bio-Rad
Laboratories, Inc., Tokyo, Japan). Cell viability was determined
according to the manufacturer. One untreated control sample was
used for each sample.
Cell imaging
At 20 to 48 h after transfection the cells were
imaged using an inverted fluorescent microscope (TE2000-S Eclipse;
Nikon, Corp., Tokyo, Japan) equipped with a cooled CCD camera
(CoolSNAP HQ™; Roper Scientific GmbH, Ottobrunn, Germany), with a
precentered fiber illuminator as a light source (Intensilight
C-HGFI; Nikon) and controlled by Image-Pro Plus®
software (Media Cybernetics, Inc., Rockville, MD, USA). Oil
immersion objective lenses of ×40 or ×100 were used for all
imaging. For immunofluorescence analysis of YB-1 in cells, the
cells were incubated with anti-YB-1 antibody for 1 h after fixation
in 4% paraformaldehyde for 10 min and permeabilization in 0.2%
Triton X-100 for 10 min. Conjugated antibody was visualized with
Flour Alexa 594-conjugated anti-mouse secondary antibody (Cell
Signaling Technology).
Image analysis
Obtained images were analyzed with Fiji software
(https://fiji.sc/). The nuclear/cytosolic (N/C) ratio
of fluorescence intensity in the region of interest (ROI) of cells
was calculated from ~100 cells.
Nuclear/cytosolic protein extraction and
western blotting
For preparation of nuclear and cytosolic extracts,
cells were suspended in 350 µl of cytosol extraction (CE)
buffer (10 mM HEPES pH 7.6, 60 mM KCl, 1 mM EDTA, 0.075% NP-40, 1
mM dithiothreitol, protease inhibitor cocktail), incubated on ice
for 3 min and centrifuged at 1,500 × g for 4 min (18). After removal of the supernatant
(cytosolic extract), the pellet was obtained as a nuclear extract
after two washes in 1 ml of CE buffer. Nuclear and cytosolic
extracts were dissolved in 120 µl of 1× Laemmli buffer. For
whole protein extracts, cells were suspended in 1× Laemmli buffer,
followed by SDS-PAGE and western blotting. Protein concentrations
were determined using Pierce BCA Protein assay kit (Thermo Fisher
Scientific) according to the standard protocol of the manufacturer.
Equal amounts of protein (20–40 µg protein/lane) were
separated on a 6–12% sodium dodecyl sulfate (SDS) gel via
polyacrylamide gel electrophoresis (PAGE) and transferred onto
polyvinylidene difluoride (PVDF) membranes. The primary antibodies
used for western blotting were as follows; anti-YB-1 mouse
monoclonal IgG antibody (clone: 21A3, 1:100), anti-phospho-YB-1
rabbit monoclonal IgG antibody (clone: 34A2, 1:100; Cell Signaling
Technology), anti-P-glycoprotein mouse monoclonal IgG antibody
(sc-55510; Santa Cruz Biotechnology, Dallas, TX, USA), anti-p53
mouse monoclonal IgG antibody (clone: DO-7, 1:200; Dako), anti-Akt
goat polyclonal IgG antibody (clone: C-20, 1:2,000; Santa Cruz
Biotechnology), anti-phospho-Akt rabbit monoclonal IgG antibody
(clone: D9E, 1:1,000; Cell Signaling Technology), anti-Fibrillarin
rabbit monoclonal IgG antibody (clone: 13C3, 1:1,000; Cell
Signaling Technology), anti-α/β tubulin rabbit polyclonal IgG
antibody (1:1,000; Cell Signaling Technology), and anti-actin goat
polyclonal IgG antibody (clone: I-19, 1:1,000; Santa Cruz
Biotechnology). Conjugated antibodies were detected by the
appropriate secondary antibody: mouse IgG HRP-linked (1:5,000; Cell
Signaling Technology) or rabbit IgG HRP-linked (1:5,000; Cell
Signaling Technology) antibody or Peroxidase AffiniPure
F(ab')2 Fragment rabbit anti-goat IgG (1:5,000; JIR,
West Grove, PA, USA). Bands were visualized using the Lumi cube
(Liponics, Ltd., Tokyo, Japan).
Statistical analysis
Comparison between the two groups of the
immunohistochemical analysis was made using Fisher's exact test.
The N/C ratio and viability of cells were analyzed using a Wilcoxon
signed-rank test or a Steel's test. In all cases, results were
considered significant at P<0.05. Statistical testing was
performed using JMP 10 (SAS Institute Japan Ltd., Tokyo,
Japan).
Results
Localization of YB-1 and expression of
p53 and P-glycoprotein in CIS
We first investigated the expression of YB-1 and
P-glycoprotein in CIS (pTis) lesions in newly diagnosed bladder
cancer samples. Table I summarizes
the characteristics of the patients. Seventy-one (88%) out of the
81 patients were male and ten patients (12%) were female. Lesions
of non-invasive papillary carcinoma (pTa) were also observed in 3
patients (4%) whereas 78 patients (96%) had a pTis lesion only.
YB-1 was expressed in 52 cases (64%). Nuclear expression of YB-1
was observed in 19 cases (23%), cytoplasmic expression in 37 cases
(46%) and both nuclear and cytoplasmic expression was observed in 4
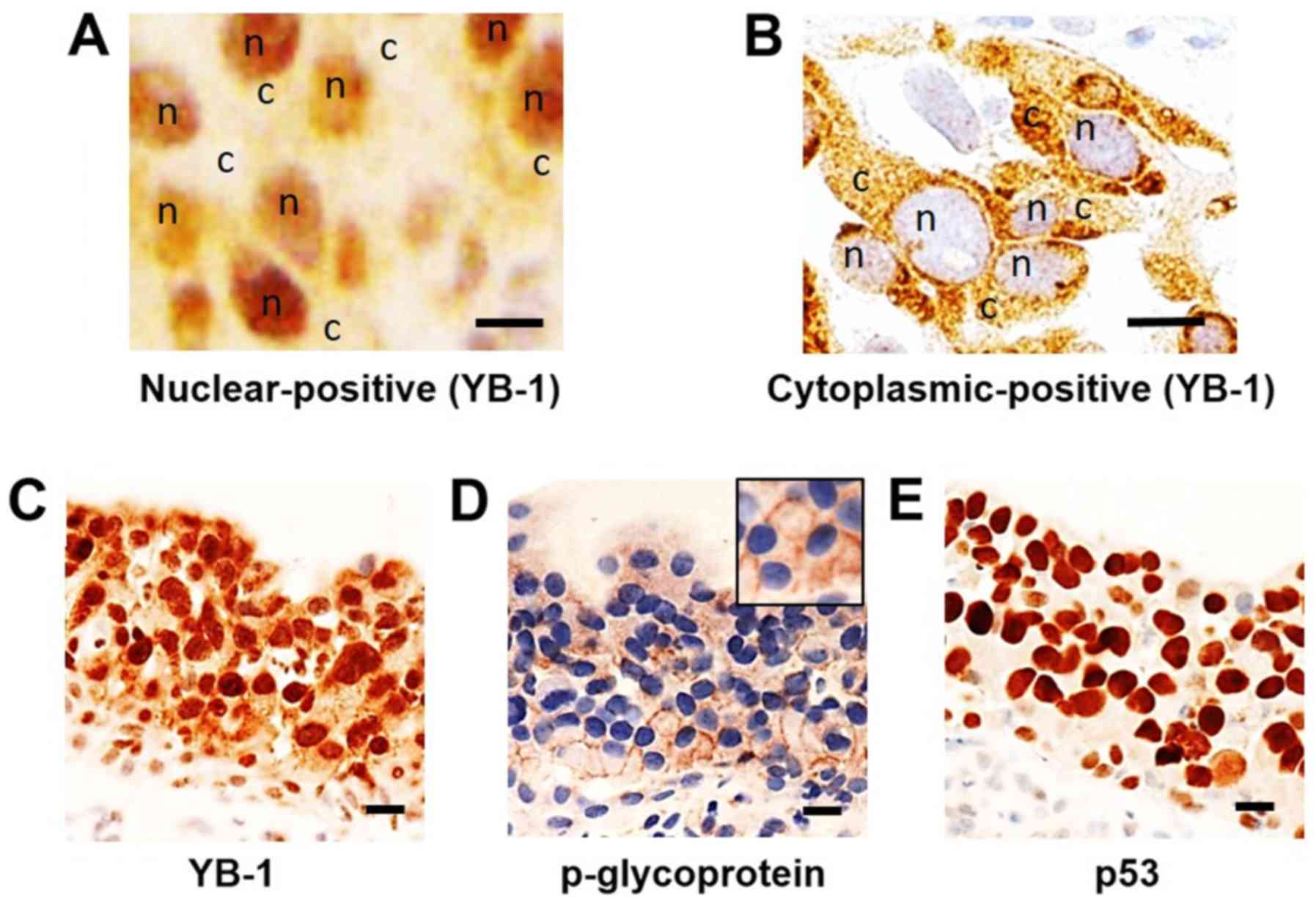
cases (5%) (Fig. 1A and B).
P-glycoprotein expression was observed in 31 cases (38%).
Expression of p-glycoprotein was significantly correlated with
nuclear expression of YB-1 (P<0.05; Table I and Fig. 1C and D). Next, to clarify the
involvement of p53, we investigated the expression of p53 in pTis
cancers. p53 was expressed in 41 cases (51%) of pTis lesions
(Fig. 1E) and its expression
correlated with the nuclear translocation of YB-1 (P<0.05;
Table II) and with the expression
of P-glycoprotein (P<0.05; Table
II). These data suggested that YB-1 coordinately translocates
to the nucleus with expression of p53 in CIS and thereby increases
p-glycoprotein expression.
 | Table IClinicopathological features and
immunohistochemical data of the study patients. |
Table I
Clinicopathological features and
immunohistochemical data of the study patients.
|
Characteristics | No. of patients
(%) |
|---|
| All patients | 81 (100) |
| Age (years) | |
| <70 | 47 (58) |
| ≥70 | 34 (42) |
| Sex | |
| Male | 71 (88) |
| Female | 10 (12) |
| Stage | |
| pTis | 78 (96) |
| pTis+pTa | 3 (4) |
|
Immunohistochemistry | |
| YB-1 | |
| Positive | 52 (64) |
| Nuclear
positive | 19 (23) |
| Cytoplasmic
positive | 37 (46) |
| Negative | 29 (36) |
| p53 | |
| Positive | 41 (51) |
| Negative | 40 (49) |
|
P-glycoprotein | |
| Positive | 31 (38) |
| Negative | 50 (62) |
 | Table IICorrelations between expression of
nuclear YB-1, P-glycoprotein and p53 in carcinoma in situ
cases. |
Table II
Correlations between expression of
nuclear YB-1, P-glycoprotein and p53 in carcinoma in situ
cases.
| P-glycoprotein
| P-value |
|---|
| Negative (%) | Positive (%) |
|---|
| Nuclear YB-1 |
| Negative
(n=62) | 42 (68) | 20 (32) | <0.05 |
| Positive
(n=19) | 7 (37) | 12 (63) | |
|
| Nuclear YB-1
| P-value |
| Negative (%) | Positive (%) |
|
| p53 |
| Negative
(n=40) | 38 (95) | 2 (5) | <0.05 |
| Positive
(n=41) | 24 (59) | 17 (41) | |
|
| P-glycoprotein
| P-value |
| Negative (%) | Positive (%) |
|
| p53 |
| Negative
(n=40) | 29 (73) | 11 (27) | <0.05 |
| Positive
(n=41) | 20 (49) | 21 (51) | |
Effect of p53 on YB-1 nuclear
translocation
To investigate whether p53 is involved in the
nuclear translocation of YB-1, we introduced exogenous P53
into a human cancer cell line and then observed the cellular
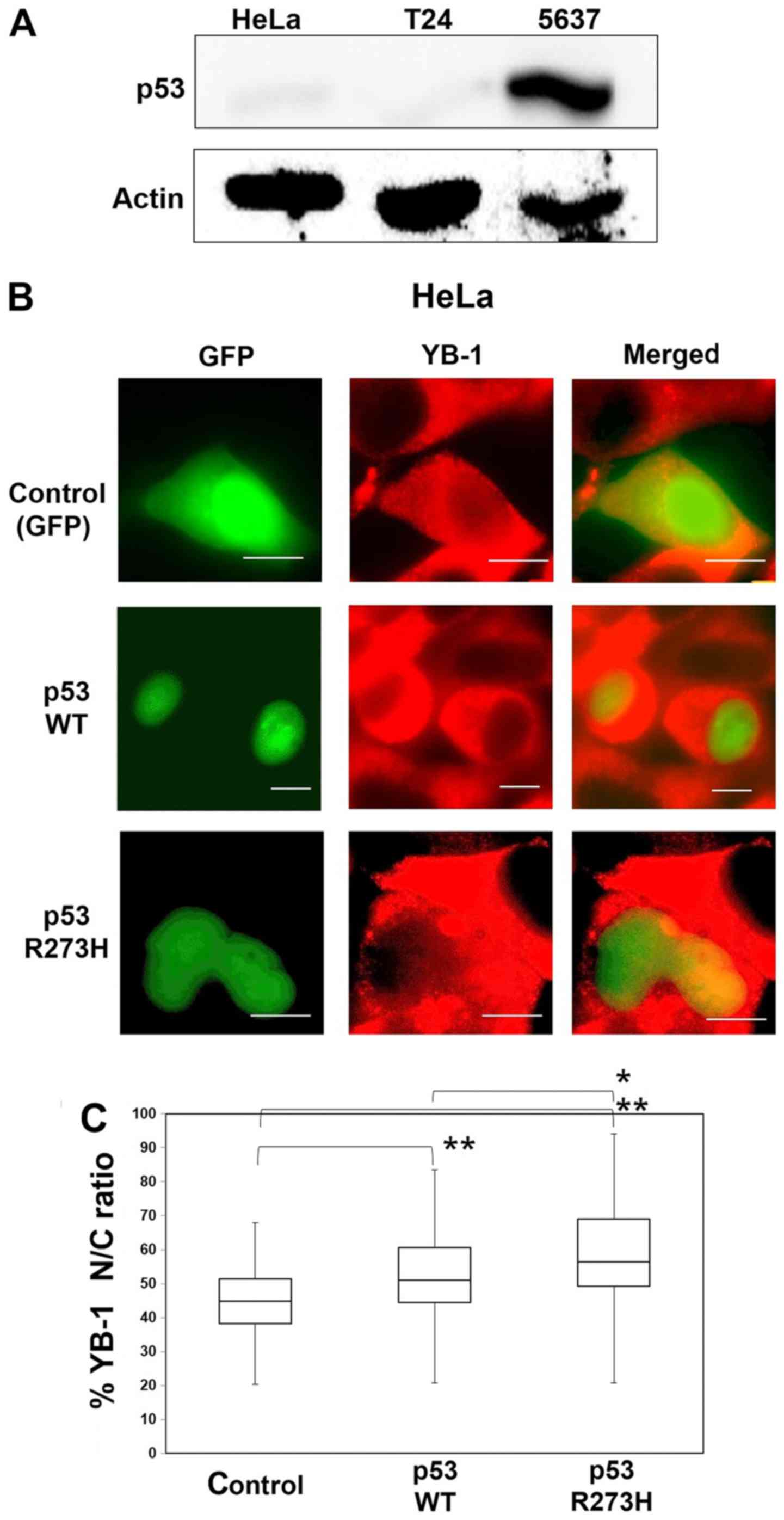
localization of YB-1. First, we assessed the expression levels of
p53 in the HeLa, T24 and 5637 cells by western blotting. It is
known that the expression level of the endogenous p53 protein is
suppressed to a low level in HeLa cells (Fig. 2A) because of its ubiquitination and
degradation by the E6 protein expressed in human papilloma virus
transformed HeLa cells (19). T24
cells contain a P53 mutant that has an in-frame deletion of
tyrosine 126 and this mutant p53 protein is expressed at a low
level compared to cancer cell lines with wild-type P53. The
5637 cells contain a P53 point mutant (R280T) and
overexpression of the p53 protein may correlate with P53
mutation (20,21). As expected, in western blotting the
p53 protein was undetectable in HeLa and T24 cells, whereas high
expression of p53 was seen in the 5637 cells (Fig. 2A). Next, we introduced P53
into HeLa cells. Thirty-six hours after transfection of
pEGFP-wild-type p53, some of the YB-1 population had translocated
to the nucleus (Fig. 2B) and the
nuclear/cytoplasmic (N/C) ratio was increased compared to that of
control cells (Fig. 2C). Moreover,
introduction of a mutant p53 (R273H), which is known to lead to
resistance to a variety of anticancer drugs, increased the N/C
ratio compared to that of control or cells in which wild-type p53
had been introduced (22). These
data suggested that p53 coordinates the nuclear translocation of
YB-1 and that a high amount of p53 protein is essential for this
translocation.
Drug-induced YB-1 nuclear translocation
and p-glycoprotein expression
Next, we examined whether gemcitabine induces the
nuclear translocation of YB-1. First, HeLa cells were transfected
with mCherry-fused YB-1 (mCherry-YB-1) and treated with
gemcitabine. Treatment with gemcitabine (35 µM) or with
cisplatin (13 µM) induced translocation of mCherry-YB-1 to
the nucleus (Fig. 3A) (23). Nuclear translocation of
mCherry-YB-1 with gemcitabine treatment was also observed in the
breast cancer MCF-7 cells and in the urinary bladder cancer 5637
and T24 cells, indicating that this phenomenon was not
cell-specific. The nuclear translocation of YB-1 was maintained for
up to 4 h after gemcitabine treatment. An immunohistochemical
analysis in the 5637 cells showed that endogenous YB-1 also
accumulated in the nucleus with gemcitabine treatment (Fig. 3B), while YB-1 was not accumulated
in T24 cells (data not shown). Since Akt-mediated phosphorylation
of YB-1 at Ser102 is required for nuclear translocation of YB-1
(24), we checked the
phosphorylation status of YB-1 in the nuclear fraction of
gemcitabine-treated cells. The phosphorylation of nuclear YB-1 was
increased 2 h after gemcitabine treatment (Fig. 4A–a). Furthermore, the increase of
the phosphorylated YB-1 corresponded to an increase in
P-glycoprotein expression (Fig.
4A–b). In addition, Akt-induced YB-1 mediated P-glycoprotein
expression was more increased in the gemcitabine treated 5637 cells
vs. control cells (Fig. 4B).
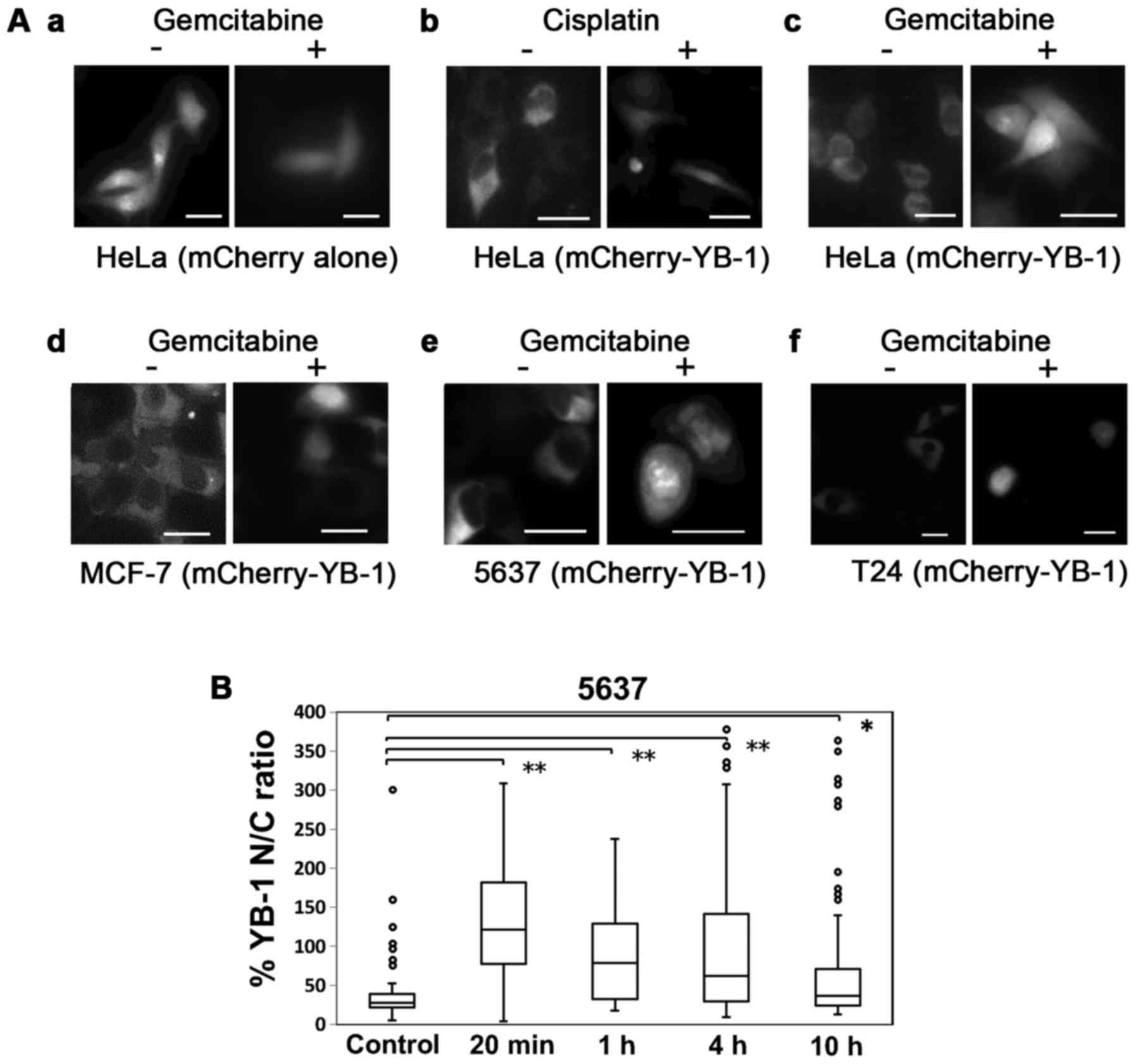 | Figure 3Gemcitabine treatment induces nuclear
translocation of YB-1. (A) Nuclear translocation of YB-1 with
gemcitabine treatment was observed in 4 cancer cell lines. HeLa,
MCF-7, 5637 and T24 cells were transfected with mCherry-YB-1. After
24 h, all cell lines were treated with an anticancer drug. HeLa
cells were treated with 13 µM cisplatin (b) or 35 µM
gemcitabine (c). MCF-7, 5637 and T24 cells were treated with 5 and
10 µM and 20 nM gemcitabine (d, e and f), respecitively. (a)
HeLa cells transfected with mCherry alone and treated with
gemcitabine were used as control. Left images, before anticancer
drug treatment (−). Right images, after anticancer drug treatment
(+). Bars, 10 µm. (B) Nuclear translocation of endogenous
YB-1 was increased relatively rapidly and was maintained for up to
4 h after gemcitabine treatment. The 5637 cells were treated for
the indicated time with 10 µM gemcitabine following which
the cells were fixed and stained with anti-YB-1 antibody. The
middle line of the box is the median. Brackets with asterisks
indicate statistically significant differences between the data
sets based on the Steel's test. *P<0.05,
**P<0.01. |
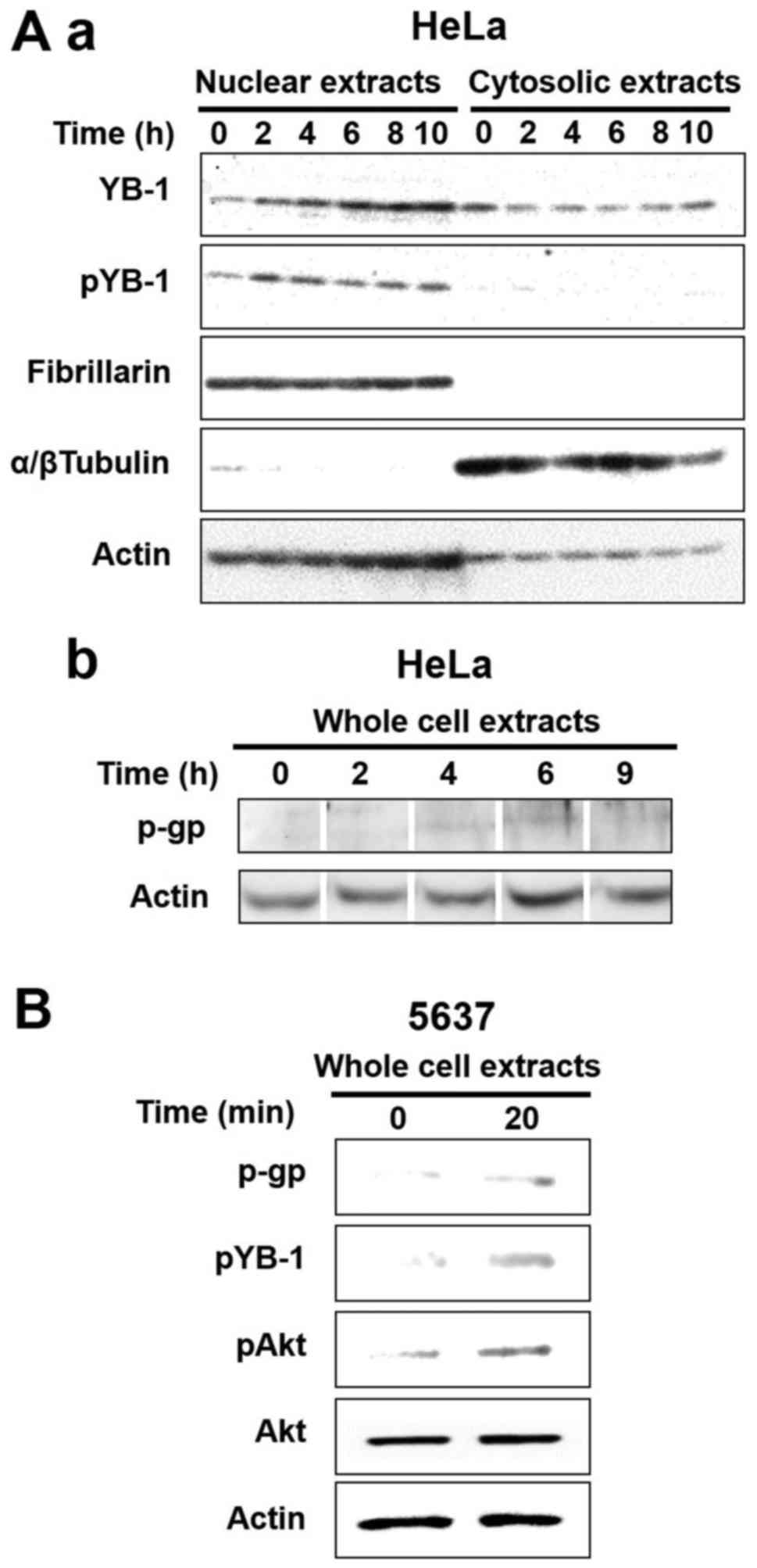 | Figure 4Both nuclear YB-1 and P-glycoprotein
were increased after gemcitabine treatment. (A) Subcellular
localization of endogenous YB-1 (a) and P-glycoprotein expression
(b) were analyzed by western blotting at the indicated times after
gemcitabine treatment. HeLa cells were treated with 35 µM
gemcitabine for the indicated times following which the cells were
fractionated into nuclear and cytosolic components for analysis of
YB-1 (a) or were extracted to whole cell lysate for analysis of
P-glycoprotein (b). Nuclear extracts (20 µg), cytoplasmic
extracts (40 µg) and whole cell extracts (20 µg) were
applied to each well for SDS-PAGE and electroblotted membranes were
stained with anti-YB-1 (a, top), anti-pYB-1 (a, 2nd from the top),
anti-Fibrillarin (a, 3rd from the top; control for nuclear
extracts), anti-α/β tubulin (a, 4th from the top; control for
cytosolic extracts), anti-actin (a and b, bottom; loading control)
and anti-P-glycoprotein (b, top) antibodies. (B) P-glycoprotein
protein expression of the bladder cancer cell line 5637 was
analyzed by western blotting at the indicated times after
gemcitabine treatment. The 5637 cells were treated with 10
µM gemcitabine for the indicated times following which cell
extracts were made and 20 µg of the total extracted proteins
were applied to each well of a gel for SDS-PAGE. Electroblotted
membranes were stained with anti-P-glycoprotein (p-gp), anti-pYB-1,
anti-pAkt, anti-Akt and anti-actin (loading control)
antibodies. |
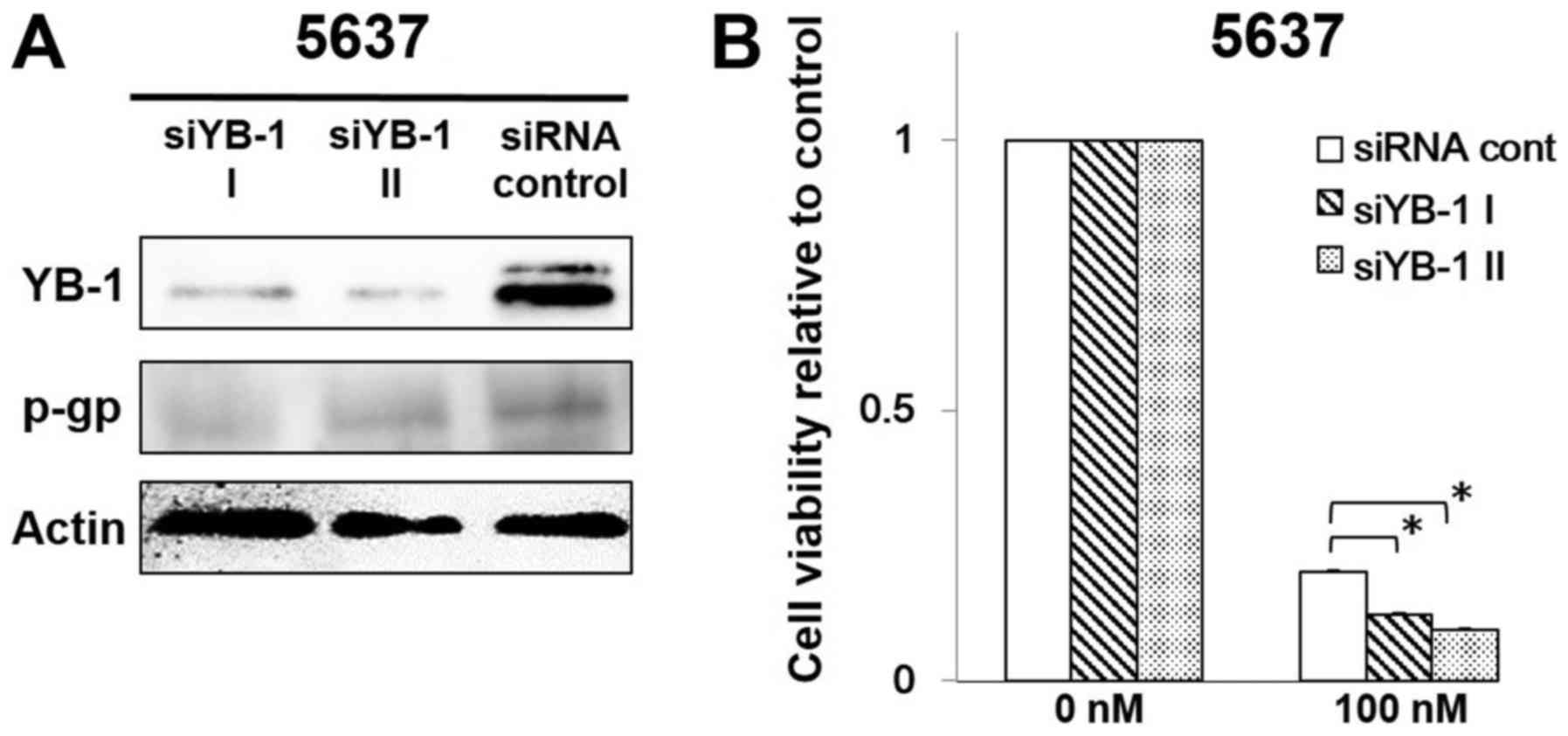
YB-1 knockdown decreased P-glycoprotein
expression and sensitized 5637 cells to gemcitabine
To examine the effect of YB-1 knockdown on
P-glycoprotein expression, the 5637 cells were transfected with
siRNA YB-1 I, siRNA YB-1 II or siRNA control. Western blotting
indicated that P-glycoprotein expression was significantly
decreased in YB-1 silenced cells compared with the siRNA
transfected control cells (at 3 days after siRNA transfection)
(Fig. 5A). Moreover, the 5637
cells transfected with YB-1 siRNA showed decreased resistance to
gemcitabine compared with the control (Fig. 5B).
Discussion
We present data indicating that YB-1 translocation
to the nucleus correlates significantly with p53 expression in CIS.
Nuclear translocation of YB-1 is thought to be involved in drug
resistance by increasing MDR-1 transcription and its product,
P-glycoprotein expression (9). The
present study showed that YB-1 siRNA transfected into a bladder
cancer cell line decreased P-glycoprotein expression is consistent
with the involvement of YB-1 in P-glycoprotein expression. Indeed,
our data of clinical samples showed that nuclear expression of YB-1
correlated with the expression of P-glycoprotein. Moreover, YB-1
translocation to the nucleus correlates significantly with p53
expression in CIS. Nuclear expression of YB-1 was observed more
often in p53-positive cases than in p53 negative cases (41 and 5%,
respectively). These data indicated that p53 may regulate the
nuclear transport of YB-1 in bladder CIS.
Further we showed that the R273H mutation of p53
induced more YB-1 nuclear translocation compared to wild-type p53
in vitro. The R273H mutation of p53 is thought to be a
'gain-of-function' mutation that drives oncogenesis (25). Zhang et al (15) reported that some tumor-derived
mutants of p53 including R273H also induce nuclear translocation of
YB-1 and that nuclear expression of YB-1 is not seen in p53 null
cell lines. These data are consistent with recent reports that p53
upregulates YB-1 nuclear import in different cell types (11,15–17).
Nuclear accumulation of p53 in bladder cancer is thought to be a
result of mutation of p53, including the R273H mutation (26,27).
Combined with these previous reports, our findings indicate that
urinary CIS may acquire the property of drug resistance during its
oncogenesis through overexpression of p53 or mutation of p53.
Third, we showed that gemcitabine induced both
nuclear translocation of YB-1 and increased the expression of
P-glycoprotein and, in addition, YB-1 knockdown increased the
effect of gemcitabine in bladder cancer. To the best of our
knowledge, this is the first report that YB-1 is involved in
gemcitabine chemoresistance in bladder cancer. We showed that
gemcitabine treatment increased the nuclear translocation of YB-1
in 5637 cells that have a high expression level of p53, but not in
T24 cells that have a low expression level of p53. These results
support the notion that a high expression level of mutant p53
protein is necessary for nuclear translocation of YB-1 in bladder
cancer. Nowadays, GC therapy is a standard therapy for aggressive
bladder cancer. However, gemcitabine-induced drug resistance in
bladder cancer compromises the therapeutic efficacy. Although it
has been proposed that genes required for gemcitabine transport and
metabolism such as human equilibrative nucleoside transporter-1 and
deoxycytidine kinase are involved in the mechanism of cellular
resistance to gemcitabine, the mechanisms by which gemcitabine
resistance occurs are not fully understood (28,29).
Finally, gemcitabine has recently been used for
intravesical chemotherapy and it is expected that gemcitabine will
be more frequently used for bladder cancer treatment in the future.
Our in vitro findings indicate that further investigation of
the association of p53 expression and P53 gene status and
YB-1 expression in bladder cancer with drug-resistance, including
gemcitabine resistance, is warranted.
Glossary
Abbreviations
Abbreviations:
|
BCG
|
Bacillus Calmette-Guérin
|
|
CE
|
cytosol extraction
|
|
CIS
|
carcinoma in situ
|
|
GC
|
gemcitabine+cisplatin
|
|
mCherry-YB-1
|
mCherry-fused YB-1
|
|
MDR-1
|
multidrug resistance-1
|
|
N/C ratio
|
nuclear/cytosolic ratio
|
|
ROI
|
region of interest
|
|
YB-1
|
Y box binding-1
|
Acknowledgments
The present study was supported by a Grant-in-Aid
for Young doctor of SMC (grant no. 26-F-1-05). We thank Kazuko
Matsuno, Yuko Ohno, Kumiko Ohsawa and Tomoaki Aoki for their
technical assistance, and Akiko Murata of Leica Microsystems for
technical advice regarding immunohistochemistry.
References
|
1
|
Zieger K, Marcussen N, Borre M, Orntoft TF
and Dyrskjot L: Consistent genomic alterations in carcinoma in situ
of the urinary bladder confirm the presence of two major pathways
in bladder cancer development. Int J Cancer. 125:2095–2103. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knowles MA and Hurst CD: Molecular biology
of bladder cancer: New insights into pathogenesis and clinical
diversity. Nat Rev Cancer. 15:25–41. 2015. View Article : Google Scholar
|
|
3
|
Lamm D, Herr H, Jakse G, Kuroda M, Mostofi
FK, Okajima E, Sakamoto A, Sesterhenn I and da Silva FC: Updated
concepts and treatment of carcinoma in situ. Urol Oncol. 4:130–138.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parker WP, Ho PL, Melquist JJ, Scott K,
Holzbeierlein JM, Lopez-Corona E, Kamat AM and Lee EK: The effect
of concomitant carcinoma in situ on neoadjuvant chemotherapy for
urothelial cell carcinoma of the bladder: Inferior pathological
outcomes but no effect on survival. J Urol. 193:1494–1499. 2015.
View Article : Google Scholar
|
|
5
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shelley MD, Jones G, Cleves A, Wilt TJ,
Mason MD and Kynaston HG: Intravesical gemcitabine therapy for
non-muscle invasive bladder cancer (NMIBC): A systematic review.
BJU Int. 109:496–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uchiumi T, Kohno K, Tanimura H, Hidaka K,
Asakuno K, Abe H, Uchida Y and Kuwano M: Involvement of protein
kinase in environmental stress-induced activation of human
multidrug resistance 1 (MDR1) gene promoter. FEBS Lett. 326:11–16.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asakuno K, Kohno K, Uchiumi T, Kubo T,
Sato S, Isono M and Kuwano M: Involvement of a DNA binding protein,
MDR-NF1/YB-1, in human MDR1 gene expression by actinomycin D.
Biochem Biophys Res Commun. 199:1428–1435. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohga T, Uchiumi T, Makino Y, Koike K, Wada
M, Kuwano M and Kohno K: Direct involvement of the Y-box binding
protein YB-1 in genotoxic stress-induced activation of the human
multidrug resistance 1 gene. J Biol Chem. 273:5997–6000. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koike K, Uchiumi T, Ohga T, Toh S, Wada M,
Kohno K and Kuwano M: Nuclear translocation of the Y-box binding
protein by ultraviolet irradiation. FEBS Lett. 417:390–394. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okamoto T, Izumi H, Imamura T, Takano H,
Ise T, Uchiumi T, Kuwano M and Kohno K: Direct interaction of p53
with the Y-box binding protein, YB-1: A mechanism for regulation of
human gene expression. Oncogene. 19:6194–6202. 2000. View Article : Google Scholar
|
|
12
|
Stein U, Jürchott K, Walther W, Bergmann
S, Schlag PM and Royer HD: Hyperthermia-induced nuclear
translocation of transcription factor YB-1 leads to enhanced
expression of multidrug resistance-related ABC transporters. J Biol
Chem. 276:28562–28569. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Holm PS, Bergmann S, Jurchott K, Lage H,
Brand K, Ladhoff A, Mantwill K, Curiel DT, Dobbelstein M, Dietel M,
et al: YB-1 relocates to the nucleus in adenovirus-infected cells
and facilitates viral replication by inducing E2 gene expression
through the E2 late promoter. J Biol Chem. 277:10427–10434. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sutherland BW, Kucab J, Wu J, Lee C,
Cheang MC, Yorida E, Turbin D, Dedhar S, Nelson C, Pollak M, et al:
Akt phosphorylates the Y-box binding protein 1 at Ser102 located in
the cold shock domain and affects the anchorage-independent growth
of breast cancer cells. Oncogene. 24:4281–4292. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang YF, Homer C, Edwards SJ, Hananeia L,
Lasham A, Royds J, Sheard P and Braithwaite AW: Nuclear
localization of Y-box factor YB1 requires wild-type p53. Oncogene.
22:2782–2794. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guay D, Gaudreault I, Massip L and Lebel
M: Formation of a nuclear complex containing the p53 tumor
suppressor, YB-1, and the Werner syndrome gene product in cells
treated with UV light. Int J Biochem Cell Biol. 38:1300–1313. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Homer C, Knight DA, Hananeia L, Sheard P,
Risk J, Lasham A, Royds JA and Braithwaite AW: Y-box factor YB1
controls p53 apoptotic function. Oncogene. 24:8314–8325. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Birbach A, Gold P, Binder BR, Hofer E, de
Martin R and Schmid JA: Signaling molecules of the NF-kappa B
pathway shuttle constitutively between cytoplasm and nucleus. J
Biol Chem. 277:10842–10851. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomas M, Pim D and Banks L: The role of
the E6-p53 interaction in the molecular pathogenesis of HPV.
Oncogene. 18:7690–7700. 1999. View Article : Google Scholar
|
|
20
|
Cooper MJ, Haluschak JJ, Johnson D,
Schwartz S, Morrison LJ, Lippa M, Hatzivassiliou G and Tan J: p53
mutations in bladder carcinoma cell lines. Oncol Res. 6:569–579.
1994.PubMed/NCBI
|
|
21
|
Hinata N, Shirakawa T, Zhang Z, Matsumoto
A, Fujisawa M, Okada H, Kamidono S and Gotoh A: Radiation induces
p53-dependent cell apoptosis in bladder cancer cells with
wild-type-p53 but not in p53-mutated bladder cancer cells. Urol
Res. 31:387–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wong RP, Tsang WP, Chau PY, Co NN, Tsang
TY and Kwok TT: p53-R273H gains new function in induction of drug
resistance through down-regulation of procaspase-3. Mol Cancer
Ther. 6:1054–1061. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahmed M and Jamil J: Cytotoxicity of
neoplastic drugs Gefitinib, Cisplatin, 5-FU, Gemcitabine, and
Vinorelbine on human cervical cancer cells (HeLa). Biol Med.
3:60–71. 2012.
|
|
24
|
Evdokimova V, Ruzanov P, Anglesio MS,
Sorokin AV, Ovchinnikov LP, Buckley J, Triche TJ, Sonenberg N and
Sorensen PH: Akt-mediated YB-1 phosphorylation activates
translation of silent mRNA species. Mol Cell Biol. 26:277–292.
2006. View Article : Google Scholar :
|
|
25
|
Zhu J, Sammons MA, Donahue G, Dou Z,
Vedadi M, Getlik M, Barsyte-Lovejoy D, Al-awar R, Katona BW,
Shilatifard A, et al: Gain-of-function p53 mutants co-opt chromatin
pathways to drive cancer growth. Nature. 525:206–211. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Esrig D, Spruck CH III, Nichols PW,
Chaiwun B, Steven K, Groshen S, Chen SC, Skinner DG, Jones PA and
Cote RJ: p53 nuclear protein accumulation correlates with mutations
in the p53 gene, tumor grade, and stage in bladder cancer. Am J
Pathol. 143:1389–1397. 1993.PubMed/NCBI
|
|
27
|
Grimm MO, Jürgens B, Schulz WA, Decken K,
Makri D and Schmitz-Dräger BJ: Inactivation of tumor suppressor
genes and deregulation of the c-myc gene in urothelial cancer cell
lines. Urol Res. 23:293–300. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsumura N, Nakamura Y, Kohjimoto Y,
Inagaki T, Nanpo Y, Yasuoka H, Ohashi Y and Hara I: The prognostic
significance of human equilibrative nucleoside transporter 1
expression in patients with metastatic bladder cancer treated with
gemcitabine-cisplatin-based combination chemotherapy. BJU Int.
108:E110–E116. 2011. View Article : Google Scholar
|
|
29
|
Nakano Y, Tanno S, Koizumi K, Nishikawa T,
Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T and Kohgo
Y: Gemcitabine chemoresistance and molecular markers associated
with gemcitabine transport and metabolism in human pancreatic
cancer cells. Br J Cancer. 96:457–463. 2007. View Article : Google Scholar : PubMed/NCBI
|