Introduction
Endometrial cancer (EC) is one of the common
gynecologic malignancies which incidence is rapidly increasing all
over the world (1,2). Approximately 80% of endometrial
cancers are diagnosed at an early stage, hysterectomy combined with
chemotherapy is the primary treatment for EC (3). Because of the hope for survival and
pregnancy, prevention and treatment of endometrial cancer is of
great importance. Chemotherapy is a main treatment strategy for EC
but chemoresistance is a restrictive factor for its clinical
outcome. Endometrial cancer is considered to be a chemotherapy poor
responding cancer type. The effectiveness of conventional
chemotherapy with platinum-derived compounds, doxorubicin,
anthracyclines, and paclitaxel is only ranging from 25 to 57%
(4). Therefore promoting
chemosensitivity of endometrial carcinoma cells could lead to an
improved clinical curative effect (5).
Chemoresistance is considered to be the main cause
of the treatment failure in >90% of metastatic tumors (6). Chemoresistance is due to two
mechanisms, primary and acquired resistance (7). Tumors can be intrinsically resistant
prior to chemotherapy, or acquired resistance during treatment by
tumors which are initially sensitive to chemotherapy(6). Existence of cancer stem cells (CSCs)
is thought to be one of the main reasons for resistance to
chemotherapy and responsible for the clinical results. CSCs are
recognized as a small proportion of cells among the tumor cell
population which are capable of self-renewal, aberrant
differentiation, and escape homeostasis (8). It has been demonstrated that CSCs
stay quiescent and resist chemotherapy agents which target cycling
cells (9). In endometrial cancer,
the cancer stem cell hypothesis has been studied in vitro
using the isolation of colony forming units, side population with
dye efflux capacity, and tumor spheres (10,11).
Besides, the chemoresistance characteristic of stemness of CSCs in
endometrial cancer has been shown in CSC phenotype and associated
with CSC markers.
Activation of autophagy pro-survival pathways is a
mechanism of resistance to several therapies including radiotherapy
and chemotherapy using oxazaphosphorine, taxanes, and
platinum-derived agents in various human cancers (12,13).
Autophagy seems to be related to ALDH1 expression in breast cancer
and is fundamental for tumorigenesis in hypoxic environment
(14). Autophagy is involved in
endometrial tumor growth and cisplatin resistance (15), autophagy inhibition enhances
sensitivity of endometrial carcinoma cells to paclitaxel (5). In our previous study, we found
miR-218 inhibited HMGB1-mediated autophagy in endometrial carcinoma
cells during chemotherapy (16).
These results suggest that autophagy is closely related to
chemosensitivity in endometrial cancer.
It is proposed that there is a close relationship
between cancer stem cells and autophagy. Accumulating evidence
provides insight into the function of autophagy in maintenance,
plasticity and survival (17–19).
CSCs have higher autophagy than normal cancer cells and are more
sensitive to autophagy inhibition compared to cells not expressing
CSC markers (20). It was
demonstrated that autophagy is essential for CSC maintenance.
Autophagy enriched the population of colorectal and liver CSCs and
participated in maintaining the stemness of colorectal and liver
CSCs (21,22). Moreover, reduced 'stemness' were
observed after CQ-mediated autophagy inhibition in sorted
MDA-MB-231 CSCs (19). The
increasement of spheres could be reversed by 3-MA treatment
(23). Besides, CQ effectively
targets CSCs via autophagy inhibition in triple-negative breast
cancer (24). These results
suggested that autophagy is closely associated with the stemness of
CSCs and could be an important mechanism that drives the survival
and multidrug resistance of CSCs (25–27).
In addition, it was found that autophagy could be a key factor in
maintaining the homeostasis of cancer stem cells and normal cancer
cells and was able to induce the dynamic transformation between
normal tumor cells to CSC of pancreatic cancer (28). However, if CSC autophagy plays a
role in the poor chemosensitivity of endometrial cancer is still
unknown. We put forward the hypothesis that chemotherapy-induced
autophagy activation triggers the dynamic transformation from
ordinary tumor cells to cells possessing stemness properties then
improve the chemoresistance. Additionally, as the novel mediator of
endometrial cancer autophagy, EIG121 could play important role in
the stemness of ECSCs.
Materials and methods
Cell line models and spheriod culture
conditions
Human endometrial cancer cell line JEC was purchased
from the Cell Bank of Chinese Academy of Sciences (CAS). JEC cells
were cultured in DMEM medium with 10% fetal bovine serum, 100 U/ml
penicillin and 100 U/ml streptomycin at 37°C in a humidified
incubator with 5% CO2.
Conventional 2-D cultures was performed for the
self-renewal and successive spheriod generation experiments
according to related references (29–33).
Spheroid culture conditions: parental cell lines or the floating
spheres were plated in serum-free sphere formation media (SFM):
low-glucose (1 g/l) DMEM and supplemented with L-glutamine, sodium
pyruvate, penicillin/streptomycin (Wisent Inc.), 20 ng/ml basic
FGF, 20 ng/ml EGF, and B27 (Invitrogen, Grand Island, NY, USA), 100
mg/ml gentamycin using 6-well ultra-low attachment plates (Corning
Inc., Tewksbury, MA, USA) (31).
Spheres were dissociated with trypsin every 7 days and split at 1:3
ratio for the next passage generation. For sphere formation assay,
5×104 cells were seeded in ultra-low attachment 6-well
plate (Corning Inc.) in 2 ml SFM/well for 2 weeks. For self-renewal
assay, cells were diluted into ~1–3 cells/well in 96-well ultra-low
attachment plates (Corning Inc.) using SFM for 2 weeks, both the
media and the drugs were replaced every 3 days. Then the floating
spheres were photographed at ×100 magnification (Nikon Eclipse
TS100). Sphere number was determined by ImageJ program.
Microbead sorting and flow cytometric
analysis of CD133+/CD44+ cells
Microbead sorting was performed using CD133 and CD44
microbead kit (Miltenyi Biotec, Germany). JEC cells were adjusted
into 1×108/ml and centrifuged at 1,300 rpm for 5 min,
the supernatant was discarded and cells were resuspened in 300 µl
cold buffer, then mixed softly. The remaining steps were performed
according to the manufacturer's instructions. After CD133 sorting,
the appropriate number of CD133+ cells were expanded for
7 days by culture and then harvested, sorted with CD44 microbeads
for the enrichment of CD133+/CD44+ cells.
This two-step isolation enabled us to obtain a suffcient number of
CD133+/CD44+ CSCs for the experiment.
To verify the purity of the isolated
CD133+/CD44+ CSCs or spheres of different
generations, flow cytometric analysis of
CD133+/CD44+ cells was performed with
CD133-FITC and CD44-PE antibodies (Miltenyi Biotec) according to
the manufacturer's instructions using a FACSCalibur instrument
(Beckman Coulter Life Sciences, USA).
Lentivirus and cell infection
For EIG121 knockdown, lentiviral constructs
containing EIG121 shRNA (TRCN0000263309 and TRCN0000263310) were
purchased from Sigma. JEC cells stably expressing Lv-con-shRNA or
EIG121 shRNA were generated by lentiviral infection at MOI 25 and
selected with 2 mg/ml puromycin.
Quantitative real-time PCR (QPCR)
Total RNA was extracted with the TRIzol reagent
(Invitrogen, USA) and cDNA was reverse-transcribed using a reverse
transcription kit (Fermentans, Germany) according to the
manufacturer's protocol. Quantitative detection of RT products was
performed using SYBR Green qRCR Mix (Toyobo, Japan). The PCR was
performed using Fluorescence quantitative PCR instrument 7300 (ABI
PRISM, USA). The PCR reaction condition was as follows: 95°C for 3
min, 95°C for 10 sec, 58°C for 30 sec, and 72°C for 30 sec, 40
cycles, to obtain fluorescence intensity. β-actin was used as an
internal control. The specific primers are listed in Table I. Relative mRNA expression levels
were determined by the 2−ΔΔCt method in comparison with
control group.
 | Table ISequences of qRT-PCR primers. |
Table I
Sequences of qRT-PCR primers.
| Gene | Primer type | Sequence |
|---|
| CD133 | Sense |
TCAAGGACTTGCGAACTCTC |
| Antisense |
GTCTCCTTGATCGCTGTTG |
| CD44 | Sense |
AGCAGCACTTCAGGAGGTTAC |
| Antisense |
CCATGTGAGTGTCTGGTAGCA |
| OTC4 | Sense |
TTCAGCCAAACGACCATCT |
| Antisense |
GCTTTGCATATCTCCTGAAGA |
| c-Mcy | Sense |
CTGAGGAGGAACAAGAAGATGA |
| Antisense |
GCTGCGTAGTTGTGCTGATG |
| CK4 | Sense |
TCCTGAAGGTCCTCTATGATGCG |
| Antisense |
CGCTTGCTGTGGGCATCTTT |
| ABCG2 | Sense |
GCCGTGGAACTCTTTGTGGTAG |
| Antisense |
CTGCCTTTGGCTTCAATCCTAAC |
| ALDH1 | Sense |
TTACCTGTCCTACTCACCGATT |
| Antisense |
CAACATCCTCCTTATCTCCTTC |
| Beclin1 | Sense |
TGTGGAATGGAATGAAATCAA |
| Antisense |
CCCCCAGAACAGTACAACGGC |
| Atg5 | Sense |
TAAGTTTGGCTTTGGTTG |
| Antisense |
TTCCCTTTCAGTTATCTCAT |
| Atg7 | Sense |
ATGCCTGGGCATCCAGTGAACTT |
| Antisense |
CATCATTGCAGAAGTAGCAGCCA |
| EIG121 | Sense |
GGGACCAAGAACAACAAGAT |
| Antisense |
TGAGGGTAAAGTGATGGAAGTA |
| β-actin | Sense |
AGGGGCCGGACTCGTCATACT |
| Antisense |
GGCGGCACCACCATGTACCCT |
Western blotting
Cells were lysed in RIPA buffer and cleared by
centrifugation at 13,000 rpm for 10 min. The proteins (100 mg) were
resolved in a 12% SDS-PAGE gel and transferred to a nitrocellulose
membrane. Primary antibodies against the LC3B antibody (2 µg/ml,
ab81785, Abcam, USA), EIG121 (1:1,000, ab156275, Abcam), Beclin1
(1:1,000, ab14071, Abcam), CD133 (1:500, 18470-1-AP, Proteintech,
China), CD44 (1:500, 15675-1-AP, Proteintech), OCT4 (1:1,000,
ab181557, Abcam), c-Myc (1:500,10828-1-AP, Proteintech), CK4
(1:500, ab155406, Abcam), and ABCG2 (1:1,000, ab108312, Abcam), or
β-actin (1:2,000, LCA01, Auragene, Changsha, China) were added to
the membrane in 8% non-fat milk and incubated at 4°C overnight.
After extensive washing, the membrane was incubated in 8% non-fat
milk containing HRP conjugated anti-rabbit secondary antibody
(1:2,000, SA009, Auragene) or anti-mouse secondary antibody
(1:2,000, SA002, Auragene) for 30 min at room temperature. The
AuraECL detection kit (Auragene) was used to visualize the target
bands.
H&E and immunofluorescence
staining
Cells were cultured on cover glasses coated with
0.1% gelatin in PBS in 6-well tissue culture plates with DMEM. The
cells were incubated overnight then washed with PBS, and fixed in
4% paraformaldehyde for 15 min. They were then permeabilized in
0.2% Triton X-100 for 10 min prior to blocking in 6% bovine serum
albumin (BSA) for 30 min. For H&E staining, cells were washed
using PBS 3 times then stained with hematoxylin for 10 min. After
washing, stained with eosin for 15 min, dehydrated by anhydrous
alcohol for 10 sec and dried. Subsequently, stained cells were
photographed at ×400 magnifcation (Nikon Eclipse TS100). For
immunofluorescence staining, the cells were incubated overnight
with anti-LC3B (1:200, 12741S, Cell Signaling, USA) at 4°C,
followed by incubation with goat anti-rabbit IgG (H+L)-Cy3 (1:200,
SA012, Auragene) for 1 h at room temperature in dark. Nuclei were
counterstained with DAPI (10 µg/ml, C0065, Solarbio, China).
Finally, cells were analyzed using a confocal fluorescence
microscope (BX50; Olympus, Japan) and the percentage of cells with
LC3 puncta was calculated with the help of Image-Pro Plus 6.0.
Cell viability assay
ECSCs were dispersed and seeded into 96-well plates
at 5×103 cells/well and incubated with DMEM containing
PTX (20 µg/ml) alone or with CQ (10 µM)/3-MA (1 mM) respectively
for 24 h. MTT solution (10 µl) was added to each well and incubated
at 37°C for 4 h, then 150 µl DMSO was added to each well. Ten
minutes later, the absorbance at 570 nm was monitored using a
microplate reader (BioTek) to assess cell viability. Cell growth
inhibition was normalized and expressed relative to the absorbance
of cells treated with DMSO alone.
Phenotypic recovery assay
It was known that when the culture environment is
restored to normal culture condition of tumor cells, the spheres
would differentiate to normal adherent tumor cells and
proliferation. So, to some extent, the extension of cells in 6-well
plates reflect the cell viability. The phenotypic recovery assay
was performed to determine the re-differentiation ability of
different cells. P3 JEC spheres or
CD133+/CD44+ CSCs were digested with 0.05%
trypsinase to obtain single cell suspension, then the cells were
inoculated in 6-well normal cell culture plate filled with complete
DMEM medium containing CQ or 3-MA, respectively. When the cell was
completely adherent, the phenotypic change was observed and
photographed at ×100 magnification (Nikon Eclipse TS100). The
cell-free areas were filled with black using Photoshop and measured
by Image-Pro Plus 6.0.
Tumorigenicity assay and IHC
staining
Animal experiments were performed in accordance with
the Guide for the Care and Use of Laboratory Animals of The
Affiliated Tumour Hospital of Xiangya Medical School of Central
South University. The protocol was approved by the Committee on the
Ethics of Animal Experiments of The Affiliated Tumour Hospital.
NOD/SCID mice at age of 3–5 weeks, male, were maintained in
pathogen-free conditions at animal facility. The JEC, Lv-Con, and
Lv-EIG121-shRNA cells were resuspended in serum-free medium and
mixed with Matrigel at the ratio of 1:1. NOD/SCID mice were
randomly divided into 3 groups (n=4 per group). Cells
(1×106) were inoculated subcutaneously into the inguinal
folds of NOD/SCID mice. Tumor formation was evaluated regularly
after injection by palpation of injection sites. At the end of
experiment, the mice were sacrificed under deep anesthesia with
pentobarbital. The tumors were then dissected and captured. The
primary tumors were immunostained for PCNA and active caspase-3 as
previously described (34).
Statistical analysis
The data are presented as mean ± standard deviation.
The means of groups were compared with one-way analysis of
variance, and after checking for equal variance, comparisons
between two means were performed using the least significant
difference (LSD) method. Student's t-test was used for two group's
comparison. Analysis of variance was used for statistical analyses.
P<0.05 was considered as statistically significant.
Results
CD133+/CD44+ cells
exhibit higher level of autophagy
It has been reported that CD133+ cells
exhibit properties of CSCs and CD44 is another important marker of
ECSCs (35–37). To better understand the underlying
mechanism responsible for low chemosensitivity in EC,
CD133+/CD44+ cells were sorted from the JEC
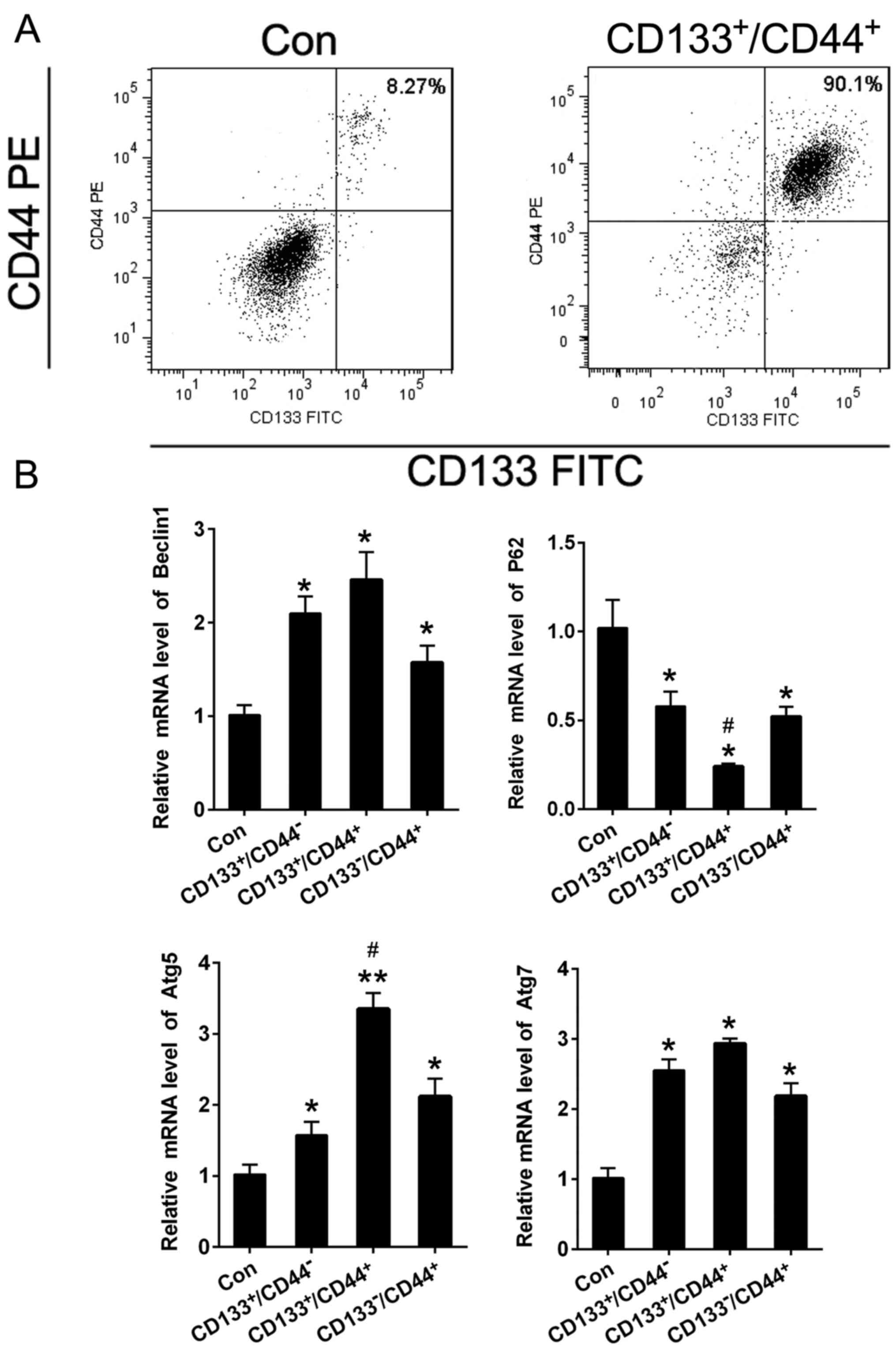
cell lines by CD133 and CD44 microBeads (38). The purity of
CD133+/CD44+ cells before and after sorting
from JEC was 8.27 and 90.1%, respectively (Fig. 1A). The QPCR was performed to detect
the autophagy markers in JEC control,
CD133+/CD44−,
CD133+/CD44+ and
CD133−/CD44+ cells. The results showed that
Beclin1, Atg5, Atg7 mRNA levels were significantly enhanced but the
P62 was obviously reduced in CD133+/CD44+
cells compared to JEC cells (Fig.
1B). Besides, the mRNA alteration of Atg5 and P62 exhibited
significance between CD133+/CD44− and
CD133+/CD44+ groups. The results demonstrated
that the CD133+/CD44+ cells which exhibit CSC
properties possess higher autophagy.
Stemness kept in line with autophagy in
successive spheroid JEC generations
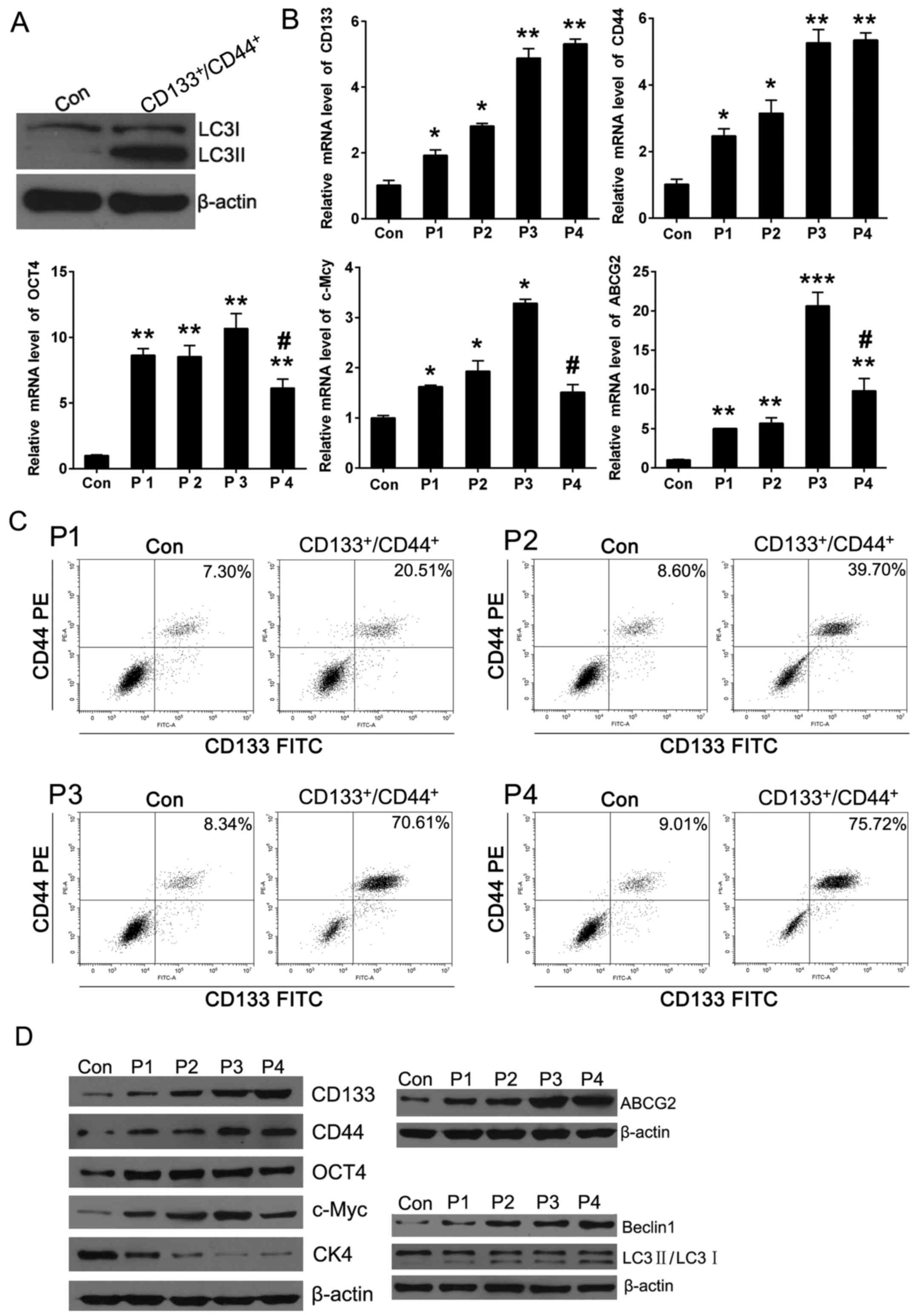
Firstly, it was found that bead sorted cells
(CD133+/CD44+ cells) are enriched for
autophagosomic LC3B II over parental cells (Con) alone which
suggeted the autophagy level of ECSCs would be higher than normal
cancer cells (Fig. 2A).
Spheroid culture is a mainstream method to obtain
cells exhibiting properties of CSCs. In order to observe the
autophagy dynamic changes in the process of stemness dynamic
changing, successive spheroid cultures were performed from passage
1 (P1) to passage 4 (P4). QPCR was performed to detect the relative
mRNA level of CSC markers and autophagy markers. The results showed
that the relative expression of CSC marker CD133, CD44, Oct4, c-Myc
and drug resistance marker ABCG2 were increased but the
differentiation marker CK4 decreased obviously from P0 (Con) to P3.
Besides, the mRNA level of ABCG2, c-Myc and CK4 were decreased from
the relative peak value when the spheroid generations have
undergone P4 culture but the CD133 and CD44 are still maintained at
peak level. On the other hand, flow cytometric staining was used to
detect the purity of CD133+/CD44+ cells in
spheres at different passages, the percentage of
CD133+/CD44+ cells increased significantly
from P1 to P3, but to the P4 generation, the increasement was not
obvious compared to P3 (Fig. 2B and
C). We thought the JEC P3 spheres could be partially considered
as ECSCs with strongest stemness using the successive spheroid
culture method. It was of great interest that the expression of
classic autophagy marker Beclin1 and LC3Π/I kept in line with the
stemness changing, in other words, autophagy was enhanced gradually
from P0 to P4 (Fig. 2D). These
results allowed to conclude that stemness kept in line with
autophagy in successive culture of JEC spheres and the synchronized
level suggested the inherent link between ECSCs and JEC
autophagy.
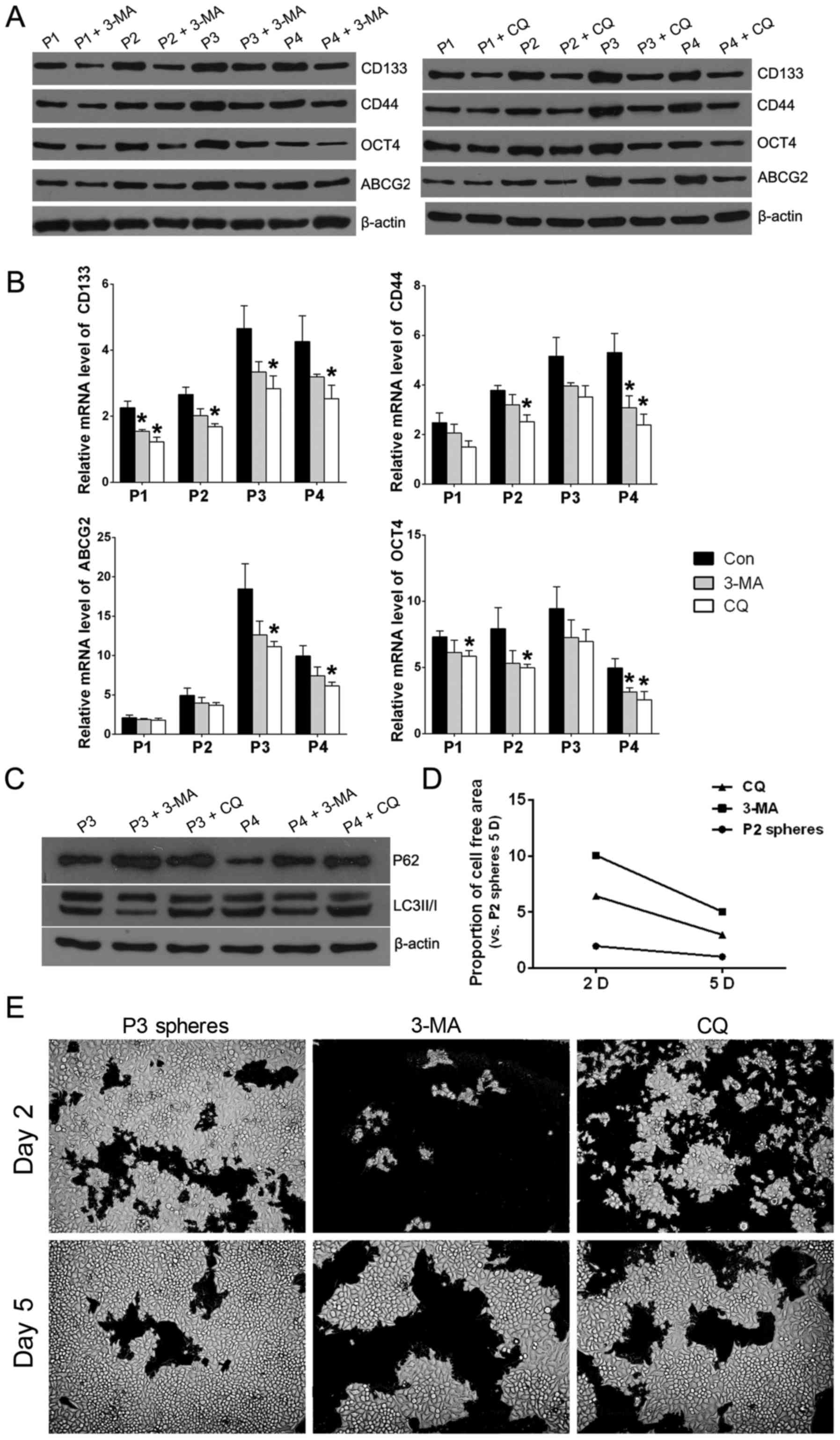
Autophagy inhibition decreases the
properties of CSCs in JEC spheres
It has been reported that autophagy plays an
important role in CSC maintenance, plasticity and survival
(17–19) and takes part in the dynamic
transformation between normal cancer cells and CSC in pancreatic
cancer (27,28). In order to clarify if autophagy
inhibition could influence the dynamic transformation of ECSCs,
conventional autophagy inhibitors 3-MA and CQ were added during the
successive spheroid culture, respectivly, then the relative
expression of CSC markers was detected by QPCR and western
blotting. Results showed when 3-MA or CQ was added, the CSC markers
(CD133, CD44, OCT4 and ABCG2 decreased compared to the control
group of the same sphere passage (Fig.
3A and B). To further confirm the effect of autophagy
inhibitors, western blotting was performed to detect expression of
P62 and LC3II/I in P3 and P4 spheres. The experimental results
showed that autophagy inhibitor 3-MA addition could increase P62
while decrease the ratio of LC3 II/I and CQ addition could increase
both P62 and the ratio of LC3 II/I compared to spheres without
autophagy inhibitor in P3 and P4 JEC spheres (Fig. 3C). Re-differentiation ability is
one of the important characteristics of CSCs. For the sake of
detecting the re-differentiation ability of JEC spheres, P3 spheres
were used to perform phenotypic recovery assay which aims to show
whether the addition of autophagy inhibitor could decrease the
re-differentiation of JEC spheres. The extension of cells in 6-well
plates reflect the cell viability. It was observed that when the P3
spheres were treated with 3-MA or CQ, the recovery was more
difficult to adherent phenotype and proliferation under routine
culture conditions (Fig. 3D and
E).
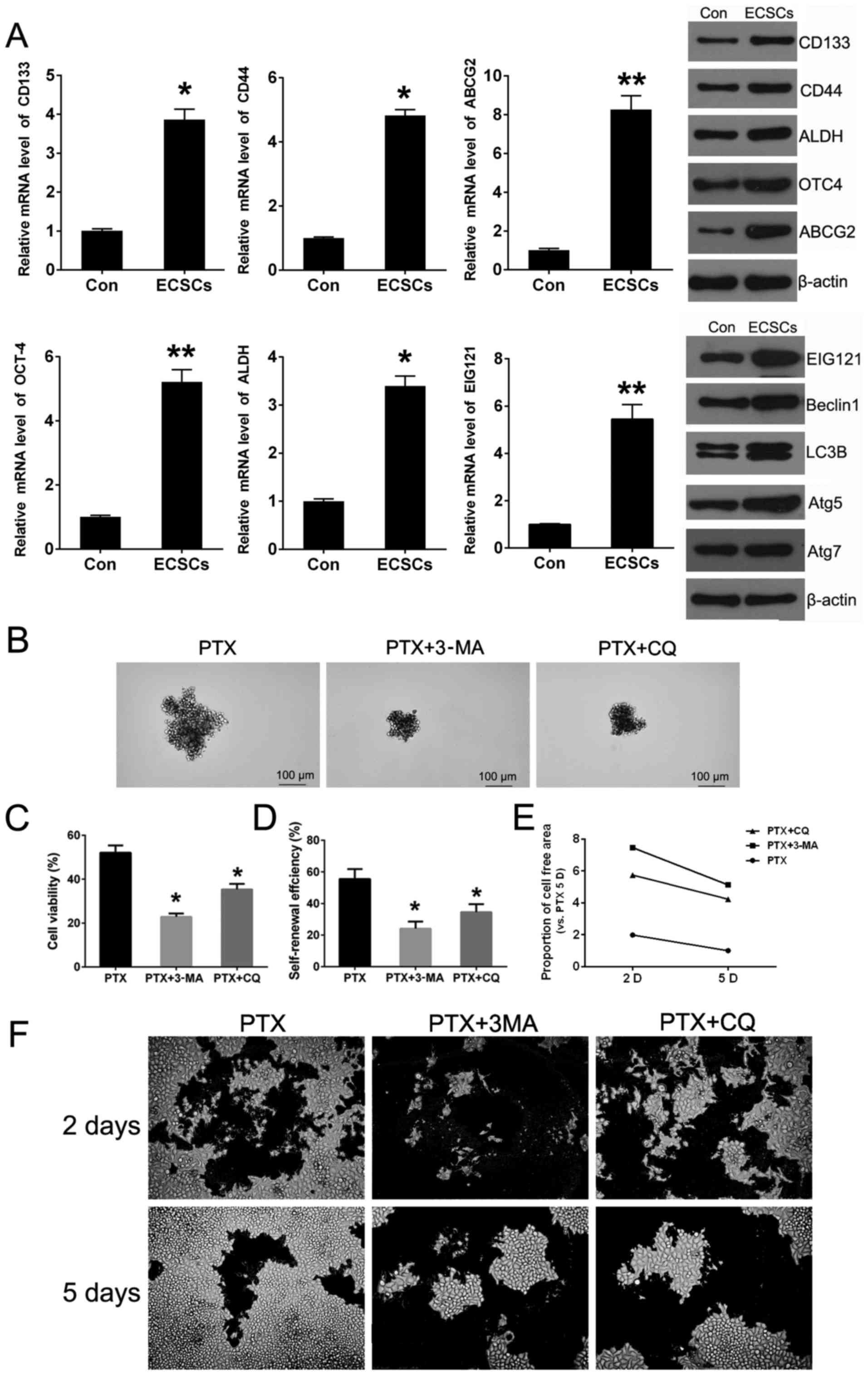
Autophagy inhibition enhances sensitivity
of ECSCs to paclitaxel
We obtained CD133+/CD44+ cells
by microBead sorting and CSC markers were detected by QPCR and
western blotting. The relative expression of CD133, CD44, ABCG2,
OCT4 and ALDH was significantly enhanced in
CD133+/CD44+ cells, so that
CD133+/CD44+ cells would be considered as
endometrial carcinoma stem cells (ECSCs) to some extent. Besides,
the expression levels of EC autophagy marker EIG121, Beclin1, Atg5,
Atg7 and LC3Π/I were obviously increased dependent on VS JEC cells
(Con). The above demonstrated that the ECSC cells exhibited higher
level of autophagy (Fig. 4A). For
evaluating the role of autophagy in the PTX chemoresis-tance of
ECSCs, cell viability, self-renewal and phenotypic recovery assays
were performed using PTX treatment with or without autophagy
inhibitors. The results showed when the ECSCs were treated in
combination with 3-MA or CQ, the cell viability attenuated
significantly (Fig. 4C).
Self-renewal capacity reduced both in sphere size and number
(Fig. 4B and D). Phenotypic
recovery assays were performed to imitate recurrence after
chemotherapy in vitro aiming to show that the addition of
autophagy inhibitor could enhance the growth inhibition of PTX of
JEC cells, and the extension of cells in 6-well plates reflect the
cell viability (Fig. 4E and F)
compared to ECSCs treated with PTX only, autophagy inhibition
attenuated the cell growth capacity to some extent after removal of
drugs and return to normal condition (Fig. 4E and F). Thus, the lower
self-renewal capacity was not only embodied in propagating clone
numbers but also the offspring sphere size.
EIG121 plays an autophagy-induced role in
JEC-obtained ECSCs
It was reported previously that the novel
estrogen-induced gene EIG121 regulates autophagy and promotes cell
survival under EC stress (39). In
this experiment, it was found that EIG121 was increased in ECSCs
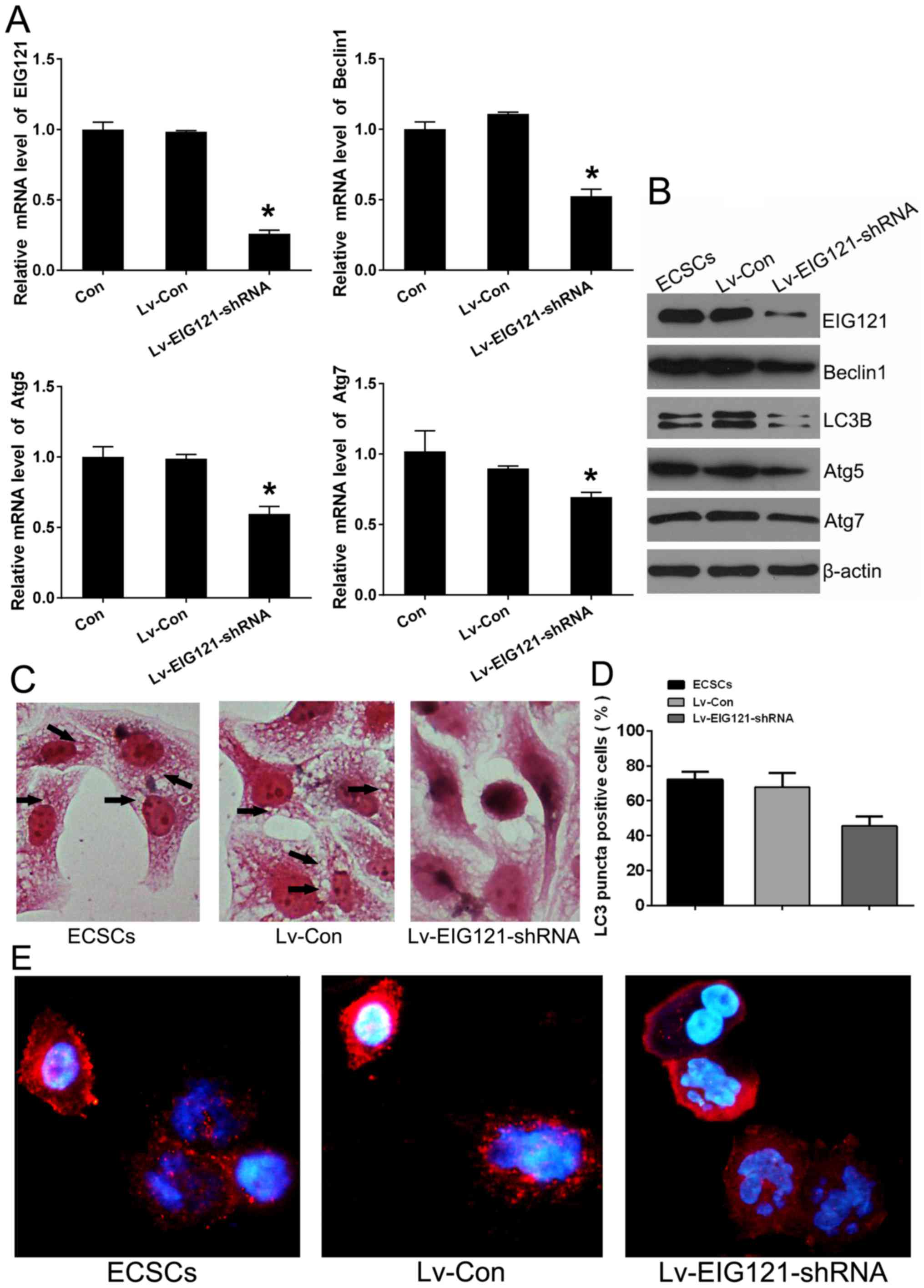
similarly to other autophagy-associated proteins (Fig. 4A). EIG121 loss-of-function stem
cell model was constructed to explore whether the EIG121 could
mediate the autophagy in JEC ECSCs. Lv-EIG121-shRNA lentivirus
infection of ECSCs was performed. When the relative expression of
EIG121 was effectively knocked down by Lv-EIG121-shRNA in ECSCs,
the expression of Beclin1, ATG5 and Atg7 were also down-regulated
in ECSCs (Fig. 5A and B).
Overexpression EIG121 would greatly increase cytoplasmic vacuole
accumulation in MDA-MB-213 (39).
As cytoplasmic vacuolation is a hallmark of autophagy, the H&E
staining was performed on JEC ECSCs at 12 h after lentivirus
infection and it was shown that the JEC-obtained ECSCs contained
numerous cytoplasmic vacuoles. However, when the EIG121 was knocked
down, cytoplasmic vacuoles became blurred (Fig. 5C). On the other hand, autophagy
induction is associated with LC3, conjugated LC3 moves into
autophagosomes and tightly binds to the autophagosome membrane.
Thus, LC3 translocation is a reliable biomarker of autophagy
(1,40). Immunofluorescence staining was
performed to detect the LC3 translocation. The results showed that
in ECSCs, LC3 expression occurred predominantly in punctuate
dot-like structures, consistent with autophagy induction. Whereas
in Lv-EIG121-shRNA infected ECSCs, LC3 puncta decreased obviously
and LC3 was uniformly distributed in the cytoplasm, the percentage
of cells with LC3 puncta was calculated with the help of Image-Pro
Plus 6.0 (Fig. 5D and E). These
results demonstrated that EIG121 played an autophagy-induced role
not only in EC normal cells, but also in JEC-obtained ECSCs.
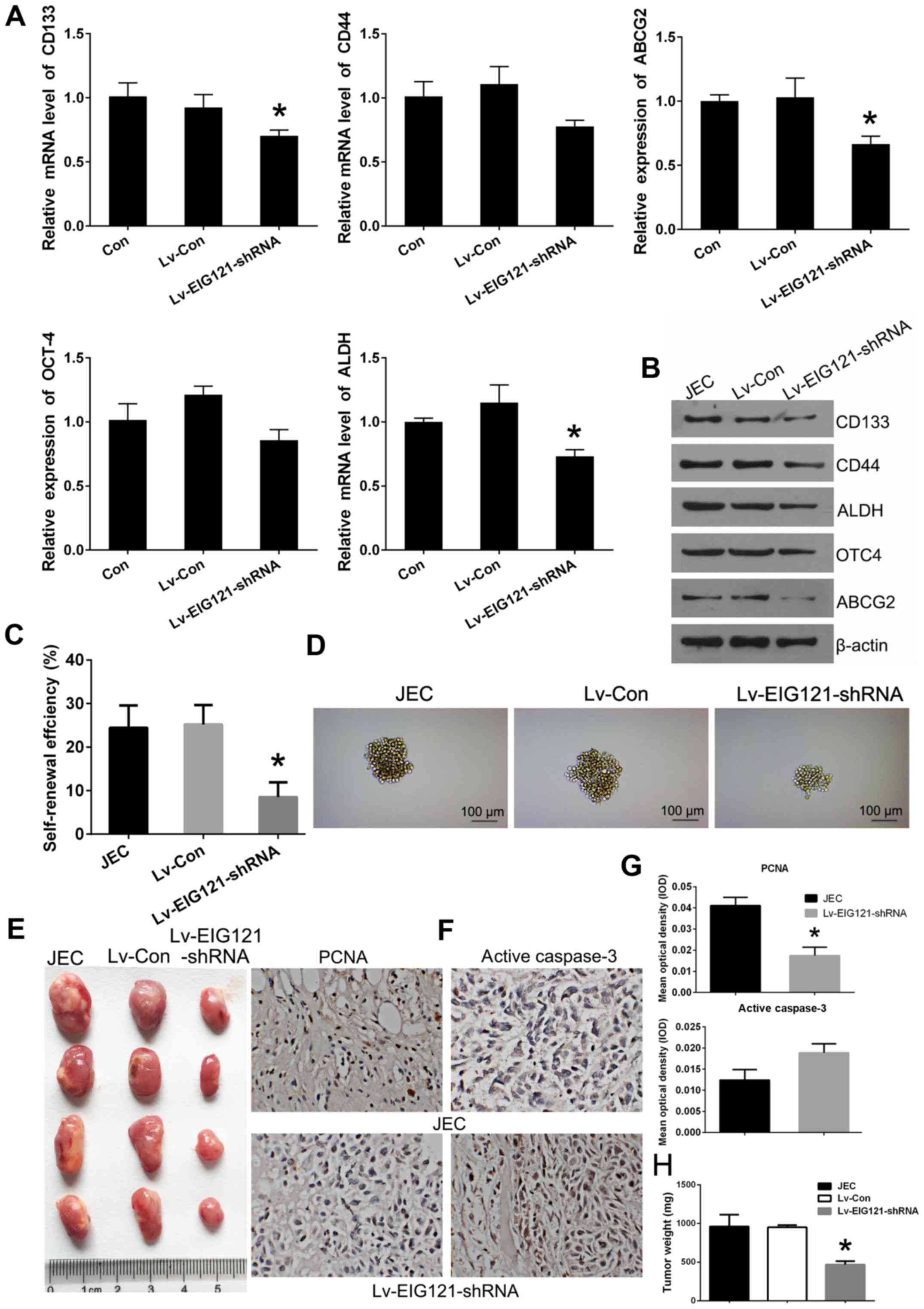
EIG121 knockdown in JEC cells results in
attenuated stemness and tumorigenesis
We considered that autophagy could play an important
role in the transformation between JEC cells and JEC-obtained
cancer stem cells. Besides, EC novel autophagy-induction protein
EIG121 was found to exert autophagy-induction function in ECSCs.
Based on this information, it was hypothesized that the novel
autophagy-inducing gene EIG121 would mediate the stemness of JEC
cells. To explore whether EIG121 expression could affect stemness
and tumorigenesis, EIG121 loss-of-function cell model was
constructed in JEC cells. Lentivirus (Lv-EIG121-shRNA) infected JEC
cells exhibited lower relative expression of stemness marker genes
of CD133, CD44, ABCG2, OCT4 and ALDH at both mRNA and protein
levels (Fig. 6A and B). Besides,
Lv-EIG121-shRNA infected JEC cells showed reduced self-renewal
capacity in both colony size and number (Fig. 6C and D). Moreover, cells were
inoculated into nude mice and all mice developed xenograft tumors
at the injection site 20 days after injection. It was found that
the tumors formed of the Lv-EIG121-shRNA group were generally
smaller than JEC and Lv-Con group (Fig. 6E). Additionally, the average tumor
weight was obviously lower in the Lv-EIG121-shRNA group compared to
other groups (Fig. 6H). The tumors
developed from Lv-EIG121-shRNA cells displayed weaker PCNA staining
and stronger active caspase-3 staining than that in JEC and Lv-Con
cells (Fig. 6F and G). These
results indicate that EIG121 knockdown in JEC cells resulted in
reduction of stemness and tumorigenesis.
Discussion
Endometrial cancer is one of the most common
gynecologic malignancies. Chemotherapy is the main method of
systemic therapy for endometrial cancer (15). Unfortunately, EC is a typical low
chemosensitive cancer type. Therefore, exploring the underlying
molecular mechanism for the EC chemo-resistance is of great
importance for novel therapeutic drug exploration. Low
chemosensitivity relates to two important mechanisms that might be
involved in autophagy and cancer stem cells (CSCs).
CSCs are undifferentiated cells with self-renewal
ability that can differentiate into multiple lineages. Once CSCs
are forced to differentiate, these cells lose their quiescent
properties and become more sensitive to chemotherapy (41). It has been hypothesized that the
reason for failure of chemotherapy is due to limited eradication of
the CSCs. Mounting evidence reinforced the foundation for emergence
of CSC-targeted therapeutic strategies that may help to enhance the
efficacy of conventional anticancer therapies (27). The existence of CSCs has been
confirmed in different tumor types including EC.
CD133+/CD44+ carcinoma stem cells from human
EC cell lines play a crucial role in proliferation, metastasis,
recurrence, and development of chemotherapy resistance possessing
high proliferation, migration, multidrug resistance and tumorigenic
capacity (42,43). In this study, we chose endometrial
adenocarcinoma cell line JEC which has low sensitivity to
paclitaxel as the parental cells, and
CD133+/CD44+ cells obtained by microbead
sorting were used as ECSCs which were verified using relative
expression of stemness marker genes.
ECSCs are thought as plausible root cause of low
chemosensitivity in EC, however, the mechanisms are still not
understood. Autophagy is a highly regulated conserved catabolic
process that functions as a cell survival mechanism during cellular
stress such as starvation, hypoxia, and chemo/radiotherapy
(44). Autophagy-mediated stress
tolerance can facilitate cell survival by sustaining energy
production which could lead to tumor growth and drug resistance
(45). Previous studies have shown
that autophagy inhibition restored chemosensitivity in various
cancer types which consolidated autophagy as a therapeutic target
(27).
CSCs are believed to be dependent on their own
microenvironment to sustain the population and autophagy has been
found as an important mechanism for CSC survival and drug
resistance (25,26). Accumulating evidence provides
insight into the function of autophagy in CSC maintenance,
plasticity and survival (17–19).
The autophagy level is higher in CSCs than normal cancer cells and
CSCs are more sensitive to autophagy inhibition compared to cells
not expressing CSC markers (20).
It was demonstrated that autophagy is essential for CSC
maintenance. Autophagy enriched the population of colorectal and
liver CSCs and participated in maintaining the stemness of
colorectal and liver CSCs (21,22).
Furthermore, reduced 'stemness' were observed afterCQ-mediated
autophagy inhibition in sorted MDA-MB-231 CSCs (19). The increasement of spheres could be
reversed by a 3MA treatment (23).
Besides, CQ effectively targets CSCs via autophagy inhibition in
triple-negative breast cancer (24). These results suggested that
autophagy is closely associated with the stemness of CSCs and could
be a important mechanism that drives the growth of cancer stem
cells.
In our study, we verified enhanced autophagy in
CD133+/CD44+ JEC cells compared with JEC
parental cells. Then successive JEC spheroid culture was performed
and the relative expression of CSC marker was measured. It was
obviously that with the increase of stemness, autophagy also
increased gradually. These results confirmed the close relationship
between ECSCs and autophagy to some extent. Besides, when the
autophagy inhibitors (3-MA and CQ) were added in the successive
spheroid culture medium, the self-renewal capacity reduced
obviously. It demonstrated that autophagy would partially play a
role in stemness-mediation. In addition, autophagy was reported to
take part in the maintenance dynamic equilibrium between CSCs and
normal cancer cells (28). So we
performed phenotypic recovery assay using P3 spheres to detect the
re-differentiation ability of JEC spheres.
Remarkably, when the autophagy inhibitor (3-MA or
CQ) was added in the normal medium, it was more difficult to
restore the adherent growth status of the P3 spheres. This result
indicated that when autophagy was repressed, the re-differentiation
ability of JEC spheres was reduced which was consistent with the
above point of view. When the PTX was used in combination of
autophagy inhibitors (3-MA or CQ), the sensitivity of ECSCs was
increased significantly. Our data suggest that autophagy in ECSCs
help in their survival and inhibition of autophagy can alleviate
ECSC resistance to some extent and loss of stemness would mean a
decrease in anti-apoptosis capacity. CSCs contain multiple
mechanisms to control cell death, which aids to protect these
crucial cells from cytotoxic insults. In CSCs, elevated
anti-apoptotic protein expression increases the threshold for
apoptosis induction and thereby directly protects the cells against
apoptosis (46). For example, in
breast and AML CSCs, BCL2 and BCLXL are highly expressed (47,48).
Besides an elevated apoptotic threshold, CSCs display high
expression of drug efflux pumps, such as ATP-binding cassette (ABC)
transporter family proteins (49).
These proteins are important for efflux of chemotherapy across the
plasma membrane. However, a potentially more challenging problem is
the recent observation that CSCs may exist that display quiescent
properties which could escape classical chemotherapy and
subsequently induce re-growth of the tumor (50). Our data establish autophagy as a
novel therapeutic target whose modulation presents new
opportunities for the low chemosensitivity of EC.
It was previously reported that the novel
estrogen-induced gene EIG121, which is overexpressed in endometrial
hyper-plasia and endometrioid-type endometrial carcinoma, was
identified as a important autophagy regulator protecting cells from
cell death in EC (51,52). As stated above, we have clarified
that autophagy could be one of the important reasons for
chemoresistance in ECSCs. Thus, we speculated that as an autophagy
regulator of EC, EIG121 played a role in regulation of ECSCs
autophagy and EC cell stemness. The lentivirus mediated EIG121
loss-of-function cell models were constructed in ECSCs and JEC
cells, respectively, and we found EIG121 played an
autophagy-induced role in JEC-obtained ECSCs and its knockdown in
JEC cells resulted in stemness reduction. The experimental results
agreed with our conjecture.
This study also has deficiencies, endometrial
carcinoma can be classified into estrogen-dependent type I and
-independent type II. In contrast, type II endometrial carcinoma is
usually ER-negative, and poorly differentiated, of high grade and
poor prognosis which account for ~15% of cases and responsible for
~50% of all relapses. Thus, studies targeting ECSCs of type II EC
seems more meaningful. CD133+/CD44+ carcinoma
stem cells from human type II EC cell lines (KLE, AN3CA) have been
used in previous research (27).
In order to explore the functional role of EIG121 in ECSCs, we
finally chose type I EC cell line JEC which has low sensitivity to
PTX and is ER-positive to perform our study. As to why EIG121
upregulated in the absence of estrogen, this may be related to its
multiple functional roles other than for estrogen induced gene
which need further exploration and the relationship between
autophagy and stemness alteration in type II EC would be the next
research topic.
In conclusion, this study uncovered the close
relationship between the regulation of autophagy and stemness in
ECSCs for the first time and clarified autophagy played an
important role in stemness alteration of ECSCs. Moreover, it was
found that EIG121 exerted dual functions in the regulation of
autophagy and stemness not only in normal EC cells but also ECSCs.
These findings provide useful information for developing targeted
therapies for endometrial carcinoma.
Abbreviations:
|
CSCs
|
cancer stem cells
|
|
EC
|
endometrial cancer
|
|
ECSCs
|
endometrial cancer stem cells
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ushijima K: Current status of gynecologic
cancer in Japan. J Gynecol Oncol. 20:67–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takahashi A, Kimura F, Yamanaka A,
Takebayashi A, Kita N, Takahashi K and Murakami T: Metformin
impairs growth of endometrial cancer cells via cell cycle arrest
and concomitant autophagy and apoptosis. Cancer Cell Int.
14:532014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burke WM, Orr J, Leitao M, Salom E, Gehrig
P, Olawaiye AB, Brewer M, Boruta D and Herzog TJ: Endometrial
cancer: a review and current management strategies: part II.
Gynecol Oncol. 134:393–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu S and Li X: Autophagy inhibition
enhances sensitivity of endometrial carcinoma cells to paclitaxel.
Int J Oncol. 46:2399–2408. 2015.PubMed/NCBI
|
|
6
|
Wilson TR, Longley DB and Johnston PG:
Chemoresistance in solid tumours. Ann Oncol (Suppl). 10:x315–x324.
2006. View Article : Google Scholar
|
|
7
|
Ayers D and Nasti A: Utilisation of
nanoparticle technology in cancer chemoresistance. J Drug Deliv.
2012:2656912012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carvalho MJ, Laranjo M, Abrantes AM,
Torgal I, Botelho MF and Oliveira CF: Clinical translation for
endometrial cancer stem cells hypothesis. Cancer Metastasis Rev.
34:401–416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Friel AM, Sergent PA, Patnaude C, Szotek
PP, Oliva E, Scadden DT, Seiden MV, Foster R and Rueda BR:
Functional analyses of the cancer stem cell-like properties of
human endometrial tumor initiating cells. Cell Cycle. 7:242–249.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kato K, Takao T, Kuboyama A, Tanaka Y,
Ohgami T, Yamaguchi S, Adachi S, Yoneda T, Ueoka Y, Kato K, et al:
Endometrial cancer side-population cells show prominent migration
and have a potential to differentiate into the mesenchymal cell
lineage. Am J Pathol. 176:381–392. 2010. View Article : Google Scholar :
|
|
12
|
Singh S, Brocker C, Koppaka V, Chen Y,
Jackson BC, Matsumoto A, Thompson DC and Vasiliou V: Aldehyde
dehydrogenases in cellular responses to oxidative/electrophilic
stress. Free Radic Biol Med. 56:89–101. 2013. View Article : Google Scholar :
|
|
13
|
Tang DG: Understanding cancer stem cell
heterogeneity and plasticity. Cell Res. 22:457–472. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cojoc M, Mäbert K, Muders MH and Dubrovska
A: A role for cancer stem cells in therapy resistance: Cellular and
molecular mechanisms. Semin Cancer Biol. 31:16–27. 2015. View Article : Google Scholar
|
|
15
|
Fukuda T, Oda K, Wada-Hiraike O, Sone K,
Inaba K, Ikeda Y, Miyasaka A, Kashiyama T, Tanikawa M, Arimoto T,
et al: The anti-malarial chloroquine suppresses proliferation and
overcomes cisplatin resistance of endometrial cancer cells via
autophagy inhibition. Gynecol Oncol. 137:538–545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ran X, Yang J, Liu C, Zhou P, Xiao L and
Zhang K: MiR-218 inhibits HMGB1-mediated autophagy in endometrial
carcinoma cells during chemotherapy. Int J Clin Exp Pathol.
8:6617–6626. 2015.PubMed/NCBI
|
|
17
|
Lei Y, Zhang D, Yu J, Dong H, Zhang J and
Yang S: Targeting autophagy in cancer stem cells as an anticancer
therapy. Cancer Lett. 393:33–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharif T, Martell E, Dai C, Kennedy BE,
Murphy P, Clements DR, Kim Y, Lee PW and Gujar SA: Autophagic
homeostasis is required for the pluripotency of cancer stem cells.
Autophagy. 13:264–284. 2017. View Article : Google Scholar
|
|
19
|
Sun R, Shen S, Zhang YJ, Xu CF, Cao ZT,
Wen LP and Wang J: Nanoparticle-facilitated autophagy inhibition
promotes the efficacy of chemotherapeutics against breast cancer
stem cells. Biomaterials. 103:44–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pellegrini P, Dyczynski M, Sbrana FV,
Karlgren M, Buoncervello M, Hägg-Olofsson M, Ma R, Hartman J,
Bajalica-Lagercrantz S, Grander D, et al: Tumor acidosis enhances
cytotoxic effects and autophagy inhibition by salinomycin on cancer
cell lines and cancer stem cells. Oncotarget. 7:35703–35723.
2016.PubMed/NCBI
|
|
21
|
Song YJ, Zhang SS, Guo XL, Sun K, Han ZP,
Li R, Zhao QD, Deng WJ, Xie XQ, Zhang JW, et al: Autophagy
contributes to the survival of CD133+ liver cancer stem
cells in the hypoxic and nutrient-deprived tumor microenvironment.
Cancer Lett. 339:70–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang HZ, Ma Y, Zhou Y, Xu LM, Chen XJ,
Ding WB and Zou HB: Autophagy contributes to the enrichment and
survival of colorectal cancer stem cells under oxaliplatin
treatment. Cancer Lett. 361:128–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Berardi DE, Flumian C, Rodriguez CE,
Bessone MI, Cirigliano SM, Joffé ED, Fiszman GL, Urtreger AJ and
Todaro LB: PKCδ inhibition impairs mammary cancer proliferative
capacity but selects cancer stem cells, involving autophagy. J Cell
Biochem. 117:730–740. 2016. View Article : Google Scholar
|
|
24
|
Liang DH, Choi DS, Ensor JE, Kaipparettu
BA, Bass BL and Chang JC: The autophagy inhibitor chloroquine
targets cancer stem cells in triple-negative breast cancer by
inducing mitochondrial damage and impairing DNA break repair.
Cancer Lett. 376:249–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bellodi C, Lidonnici MR, Hamilton A,
Helgason GV, Soliera AR, Ronchetti M, Galavotti S, Young KW, Selmi
T, Yacobi R, et al: Targeting autophagy potentiates tyrosine kinase
inhibitor-induced cell death in Philadelphia chromosome-positive
cells, including primary CML stem cells. J Clin Invest.
119:1109–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang H, Gomez-Manzano C, Aoki H, Alonso
MM, Kondo S, McCormick F, Xu J, Kondo Y, Bekele BN, Colman H, et
al: Examination of the therapeutic potential of Delta-24-RGD in
brain tumor stem cells: Role of autophagic cell death. J Natl
Cancer Inst. 99:1410–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ojha R, Bhattacharyya S and Singh SK:
Autophagy in cancer stem cells: A potential link between
chemoresistance, recurrence, and metastasis. Biores Open Access.
4:97–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu H, Wang D, Liu Y, Su Z, Zhang L, Chen
F, Zhou Y, Wu Y, Yu M, Zhang Z, et al: Role of the
hypoxia-inducible factor-1 alpha induced autophagy in the
conversion of non-stem pancreatic cancer cells into
CD133+ pancreatic cancer stem-like cells. Cancer Cell
Int. 13:1192013. View Article : Google Scholar
|
|
29
|
Chen Z, Che Q, He X, Wang F, Wang H, Zhu
M, Sun J and Wan X: Stem cell protein Piwil1 endowed endometrial
cancer cells with stem-like properties via inducing
epithelial-mesenchymal transition. BMC Cancer. 15:8112015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ciavardelli D, Rossi C, Barcaroli D, Volpe
S, Consalvo A, Zucchelli M, De Cola A, Scavo E, Carollo R,
D'Agostino D, et al: Breast cancer stem cells rely on fermentative
glycolysis and are sensitive to 2-deoxyglucose treatment. Cell
Death Dis. 5:e13362014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van der Zee M, Sacchetti A, Cansoy M,
Joosten R, Teeuwssen M, Heijmans-Antonissen C, Ewing-Graham PC,
Burger CW, Blok LJ and Fodde R: IL6/JAK1/STAT3 signaling blockade
in endometrial cancer affects the ALDHhi/CD126+
stem-like component and reduces tumor burden. Cancer Res.
75:3608–3622. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang L and Lai D: Ovarian cancer stem
cells enrichment. Methods Mol Biol. 1049:337–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhong Y, Guan K, Guo S, Zhou C, Wang D, Ma
W, Zhang Y, Li C and Zhang S: Spheres derived from the human
SK-RC-42 renal cell carcinoma cell line are enriched in cancer stem
cells. Cancer Lett. 299:150–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liao WT, Wang X, Xu LH, Kong QL, Yu CP, Li
MZ, Shi L, Zeng MS and Song LB: Centromere protein H is a novel
prognostic marker for human nonsmall cell lung cancer progression
and overall patient survival. Cancer. 115:1507–1517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Elbasateeny SS, Salem AA, Abdelsalam WA
and Salem RA: Immunohistochemical expression of cancer stem cell
related markers CD44 and CD133 in endometrial cancer. Pathol Res
Pract. 212:10–16. 2016. View Article : Google Scholar
|
|
36
|
Kato K: Endometrial cancer stem cells: A
new target for cancer therapy. Anticancer Res. 32:2283–2293.
2012.PubMed/NCBI
|
|
37
|
Kyo S and Kato K: Endometrial cancer stem
cell as a potential therapeutic target. Semin Reprod Med.
33:341–349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao Y, Liu T and Huang Y: MicroRNA-134
suppresses endometrial cancer stem cells by targeting POGLUT1 and
Notch pathway proteins. FEBS Lett. 589:207–214. 2015. View Article : Google Scholar
|
|
39
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics for Hispanics/Latinos, 2012. CA Cancer J Clin.
62:283–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gómez-López S, Lerner RG and Petritsch C:
Asymmetric cell division of stem and progenitor cells during
homeostasis and cancer. Cell Mol Life Sci. 71:575–597. 2014.
View Article : Google Scholar :
|
|
42
|
Gehrig PA and Bae-Jump VL: Promising novel
therapies for the treatment of endometrial cancer. Gynecol Oncol.
116:187–194. 2010. View Article : Google Scholar
|
|
43
|
Jiang F, Liu T, He Y, Yan Q, Chen X, Wang
H and Wan X: MiR-125b promotes proliferation and migration of type
II endometrial carcinoma cells through targeting TP53INP1 tumor
suppressor in vitro and in vivo. BMC Cancer. 11:4252011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sui X, Chen R, Wang Z, Huang Z, Kong N,
Zhang M, Han W, Lou F, Yang J, Zhang Q, et al: Autophagy and
chemotherapy resistance: A promising therapeutic target for cancer
treatment. Cell Death Dis. 4:e8382013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nagelkerke A, Sweep FC, Geurts-Moespot A,
Bussink J and Span PN: Therapeutic targeting of autophagy in
cancer. Part I: Molecular pathways controlling autophagy. Semin
Cancer Biol. 31:89–98. 2015. View Article : Google Scholar
|
|
46
|
Colak S and Medema JP: Cancer stem cells -
important players in tumor therapy resistance. FEBS J.
281:4779–4791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Konopleva M, Zhao S, Hu W, Jiang S, Snell
V, Weidner D, Jackson CE, Zhang X, Champlin R, Estey E, et al: The
anti-apoptotic genes Bcl-X(L) and Bcl-2 are over-expressed and
contribute to chemoresistance of non-proliferating leukaemic
CD34+ cells. Br J Haematol. 118:521–534. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Madjd Z, Mehrjerdi AZ, Sharifi AM,
Molanaei S, Shahzadi SZ and Asadi-Lari M: CD44+ cancer
cells express higher levels of the anti-apoptotic protein Bcl-2 in
breast tumours. Cancer Immun. 9:42009.
|
|
49
|
Wilson BJ, Schatton T, Zhan Q, Gasser M,
Ma J, Saab KR, Schanche R, Waaga-Gasser AM, Gold JS, Huang Q, et
al: ABCB5 identifies a therapy-refractory tumor cell population in
colorectal cancer patients. Cancer Res. 71:5307–5316. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao MQ, Choi YP, Kang S, Youn JH and Cho
NH: CD24+ cells from hierarchically organized ovarian
cancer are enriched in cancer stem cells. Oncogene. 29:2672–2680.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Deng L, Broaddus RR, McCampbell A, Shipley
GL, Loose DS, Stancel GM, Pickar JH and Davies PJ: Identification
of a novel estrogen-regulated gene, EIG121, induced by hormone
replacement therapy and differentially expressed in type I and type
II endometrial cancer. Clin Cancer Res. 11:8258–8264. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Deng L, Feng J and Broaddus RR: The novel
estrogen-induced gene EIG121 regulates autophagy and promotes cell
survival under stress. Cell Death Dis. 1:e322010. View Article : Google Scholar : PubMed/NCBI
|