Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors in the world and causes significant
mortality, rapidly rising in incidence in China (1,2).
Surgical resection and transplantation are the cornerstone of
therapy in early-stage hepatocellular carcinoma, while locoregional
therapy and chemotherapy (such as sorafenib and oxaliplatin) are
beneficial in those with more advanced disease or those who are not
surgical candidates (3,4). Oxaliplatin is a new platinum
anticancer drug, commonly used in metastatic colorectal cancer
treatment, or adjuvant treatment in resection of primary colon
cancer (5). The mechanism of
oxaliplatin, is not yet entirely clear, but some research suggest
that oxaliplatin produced hydration derivatives, acting on DNA, to
form inter and intra chain cross-linking, which inhibits DNA
synthesis, induced cytotoxicity and antitumor activity (6). Oxaliplatin-based regimens were
permitted by CFDA (China Food and Drug Administration) as systemic
therapies for advanced HCC since 2013 in China, which bring more
benefit for patients with advanced HCC. Clinical research has shown
that oxaliplatin-based regimens are safe and efficacious in
patients with HCC (7–9).
It has long been recognized that tumor does not
generally respond to conventional chemotherapy, especially to P53
mutation or deletion tumors. With the development of cancer
genetics, gene therapy has stood out as a promising
multidisciplinary treatment approach against tumors (10). P53, TK and other gene products have
been used in clinical treatment of liver cancer, and achieved
certain therapeutic effect alone or in coordination with other
treatments (11,12). However, the treatment to tumors of
P53 mutation is not ideal, so it is necessary to develop new gene
therapy.
Aspp2 (apoptosis stimulating protein of p53-2) is
one of p53 binding proteins. Effects of inhibiting tumor cell
growth and inducing apoptosis are already confirmed. Aspp2 is lowly
expressed in a variety of tumors and is related to the occurrence
and development of tumors. These studies suggest that Aspp2 is a
very important tumor suppressor and may be a candidate gene for
gene therapy of HCC (13–17).
Combination of gene therapy with other therapeutic
approaches such as chemotherapy might provide more treatment
benefit (18,19). Our previous research showed that
Aspp2 enhanced oxaliplatin-induced colorectal cancer cell apoptosis
in a p53-independent manner by inhibiting cell autophagy (20). Moreover, we successfully prepared
the human Aspp2 recombinant adenovirus (Aspp2-ad) (21). In the present study, human Aspp2
recombinant adenovirus (Aspp2-ad) preparation was used to
investigate whether Aspp2 could enhance the inhibitory effect of
oxaliplatin on hepatocellular carcinoma. To confirm whether Aspp2
exerted the inhibitory effect in a p53-independent way, we chose
the cell lines of Hep3B (P53 natural deficiency) and
HepG2P53−/− (constructed by our laboratory) as the
objects of the study.
Materials and methods
Reagents and antibodies
Human Aspp2 recombinant adenovirus (Aspp2-ad) was
constructed by the Beijing Institute of Hepatology (Beijing,
China); oxaliplatin was obtained from Qilu Pharmaceutical Co., Ltd.
(Jinan, China); trypsin-ethylenediaminetetraacetic acid and
Dulbecco's modified Eagle's medium (DMEM) were purchased from Gibco
(Grand Island, NY, USA); fetal bovine serum (FBS) was from China
Hangzhou Sijiqing Biological Technology Co., Ltd. (Hangzhou,
China); 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium
bromide (MTT) and dimethyl sulfoxide (DMSO) were provided by
Sigma-Aldrich (St. Louis, MO, USA); p53 primary monoclonal antibody
was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA);
activation of caspase-3, Bax, Bcl-2, LC3B, Beclin1, ERK, p-ERK,
mTOR, p-mTOR, STAT3, p-STAT3 and GAPDH primary monoclonal antibody
were from Cell Signaling Technology (Boston, MA, USA); CD31 and
PCNA immunohistochemistry kits were obtained from Beijing Zhongshan
Gold Bridge Technology Co., Ltd. (Beijing, China); Annexin V
apoptosis Kit-PE kit was from SouthernBiotech (Birmingham, AL,
USA). Total/phospho ERK MAG kit, 2-Plx phospho/Total STAT3 MAG kit,
Total/Phospho mTOR MAG kit and tubulin MAG kit were purchased from
Merk Millipore (Billerica, MA, USA). Lentivirus-shRNAp53-GFP (shRNA
p53: 5′-CUACUUCCUGAAAACA ACGTT-3′ and 5′-CGUUGUUUUCAGGAAGUAGTT-3′)
were obtained from Shanghai Obio Technology Co., Ltd. (Shanghai,
China).
Cell line and cell culture
HepG2 and Hep3B cells were preserved in Beijing
Institute of Hepatology and parental generations of these cell
lines were from the American Type Culture Collection (ATCC;
Manassas, VA, USA). HepG2P53−/− liver cancer cell line
was constructed by the Beijing Institute of Hepatology.
HepG2P53−/− and Hep3B cells were cultured in DMEM medium
supplemented with 10% FBS and maintained at 37°C in a humidified
incubator with 5% CO2. The culture medium was changed
every 2 days. HepG2P53−/− cell construction method was
as follows: 500 µl 4×104/ml HepG2 cells were
added into 12-well paltes. After incubation for 12-20 h,
lenti-virus-shp53-GFP (virus dosage = cell number × MOI
×103=/original virus titer) and 2.5 µl 1 mg/ml
polybrene were added to help transfection. After 72 h, puromycin
(final concentration 6 µg/ml) was added to screen the
transfection for 24 h. Then, DMEM medium containing puromycin
(final concentration 2 µg/ml) was changed every 2-3 days.
Two weeks later, protein of cells was extracted to verify the P53
expression.
Animals
Four-weeks-old male balb/c nude mice were obtained
from Beijing Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China) (animal quality certificate, no. 11400700177504)
and were maintained in individually ventilated cages (IVC) under
specific pathogen free sterile condition. The animal study protocol
was approved by the Animal Welfare Committee of the Capital Medical
University.
MTT assay
Cells in the logarithmic growth phase were plated in
96-well plates in a seeding density of 5,000 cells/well and
incubated in a 37°C incubator with 5% CO2 overnight.
After cells were pretreated with Aspp2-ad for 24 h, the cells were
then incubated with oxaliplatin for 24-48 h. The culture medium in
each well was abandoned, incubating with 0.5 g/l MTT 100 µl
for 4 h. Then, each well was added with 150 µl DMSO and
vibrated for 10 min, then absorbance of each well was detected with
microplate reader (ELx800 type; BioTek Instruments Inc., Winooski,
VT, USA) at the 490 nm wavelength. The inhibition rate (IR) was
calculated as follows: IR (%) =
(1-ODtreatment/ODcontrol) × 100%.
Annexin-PE stain
Cell culture was as described above. After treated
with Aspp2-ad and oxaliplatin, the cells were stained with 10
µl Annexin-PE at 37°C for 15 min. The Annexin-PE stained
cells of 5 same visual fields were counted under inverted
fluorescence microscope (Cytation 3; BioTek Instruments).
Xenograft experiment
Hep3B cells (5×106) were inoculated in
nude mice subcutaneously to establish xenograft tumors to be
transplanted into nude mice. Three days after the transplantation,
the mice were divided into groups according to the tumor sizes.
Each group contained 6 mice. When the tumor sizes were ~100
mm3, the test mice were injected with 25 mg/kg
oxaliplatin intraperitoneally and/or Aspp2-ad intratumorally
(1×108–1×109 pfu/mouse according to the tumor
size) 2 times every week. The control mice were given the vehicle.
The sizes of subcutaneous tumors were measured with calipers and
internal constructions of tumors were observed with ultrasonic
machine (S-sharp Prospect). At the endpoint of the experiment, nude
mice were dissected and the tumor samples were collected according
to the follow-up experiments. The tumor growth inhibition rates and
relative tumor volumes (RTV) is calculated: RTV = Vt/V0 (where Vt
is the volume of the tumor after the initial administration and V0,
the volume of the tumor before the initial administration).
Histological observation
The tumor tissues were fixed in 4% paraformaldehyde
solution for conventional hematoxylin and eosin (H&E) staining
and immunohistochemistry. CD31, PCNA expression were measured
according to the kit instruction. Nuclear mitotic index (MI) was
calculated in 3 slices of each animal. MI (%) = number of mitotic
cells/1,000 cells.
Western blot analysis
Proteins of cells and tissues were conventionally
extracted. The protein concentration of lysates was determined
using the bicinchoninic acid method. Cell lysates (40 µg per
lane) were separated using 10% SDS-PAGE and transferred
electrophoretically to polyvinylidene difluoride membrane.
Membranes were blocked with Tris-buffered saline/0.1% Tween-20
containing 5% bovine serum albumin (BSA) and then incubated
overnight at 4°C with primary antibodies (1:1,000). Membranes were
washed three times with TBS/T and incubated for 1 h at room
temperature with the appropriate secondary antibody conjugated to
goat anti-rabbit horseradish peroxidase (1:2,000). Membranes were
then washed and immunoreactive band were developed with ECL and
visualized by autoradiography. Protein loading was normalized using
β-actin antibody.
Luminex assay
The protein concentrations of sample tissues were
diluted below 2 µg/ml. Assay buffer (200 µl) was
added into each well of 96-well microtiter plate to block for 10
min with shaking. Another 25 µl of assay buffer, 25
µl sample and 25 µl mixed antibody microspheres were
added into each plate after the assay buffer was removed. After
incubation overnight at 4°C, the residual liquid was discarded. A
total of 200 µl of wash buffer was used to wash the plate;
25 µl secondary antibody was applied and incubated for 2 h
at room temperature, then 25 µl streptavidin-phycoerythrin
was added and incubated for 30 min. All the liquid was discarded,
each plate was washed with 200 µl of wash buffer twice; 150
µl of sheath liquid was added with shaking for 5 min. The
mean fluorescence intensity was measured with the FLEXMAP 3D™
system (Luminex Corp., Austin, TX, USA).
Statistical analysis
The data were expressed as the mean ± SD. The
results were subjected to one-way ANOVA test using SPSS software
(17.0 version; SPSS, Inc., Chicago, IL, USA).
Results
P53 expression in Hep3B and
HepG2P53−/− cells
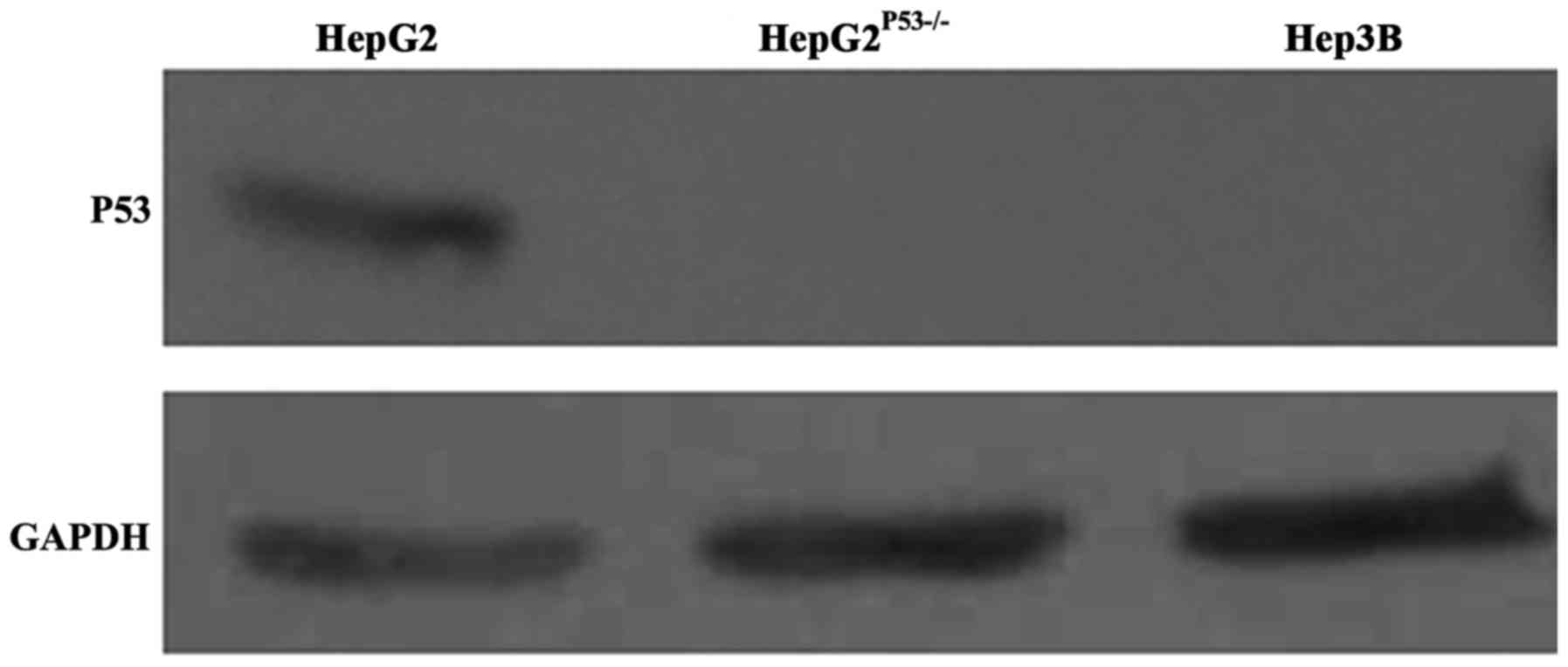
We constructed a HepG2 cell line with P53 deletion,
as shown in Fig. 1. P53 expression
in wild-type HepG2 cells was normal, but was absent in the Hep3B
and HepG2P53-/- cells (Fig.
1). This suggested we successfully constructed the P53
deficient cell line.
Aspp2-ad enhances the inhibition of
oxaliplatin on hepatocellular carcinoma cells
After pretreated with 1.25×108,
6.125×107 pfu/ml Aspp2-ad for 24 h, these hepatocellular
carcinoma cells were then added with oxaliplatin for 24 and 48
h.
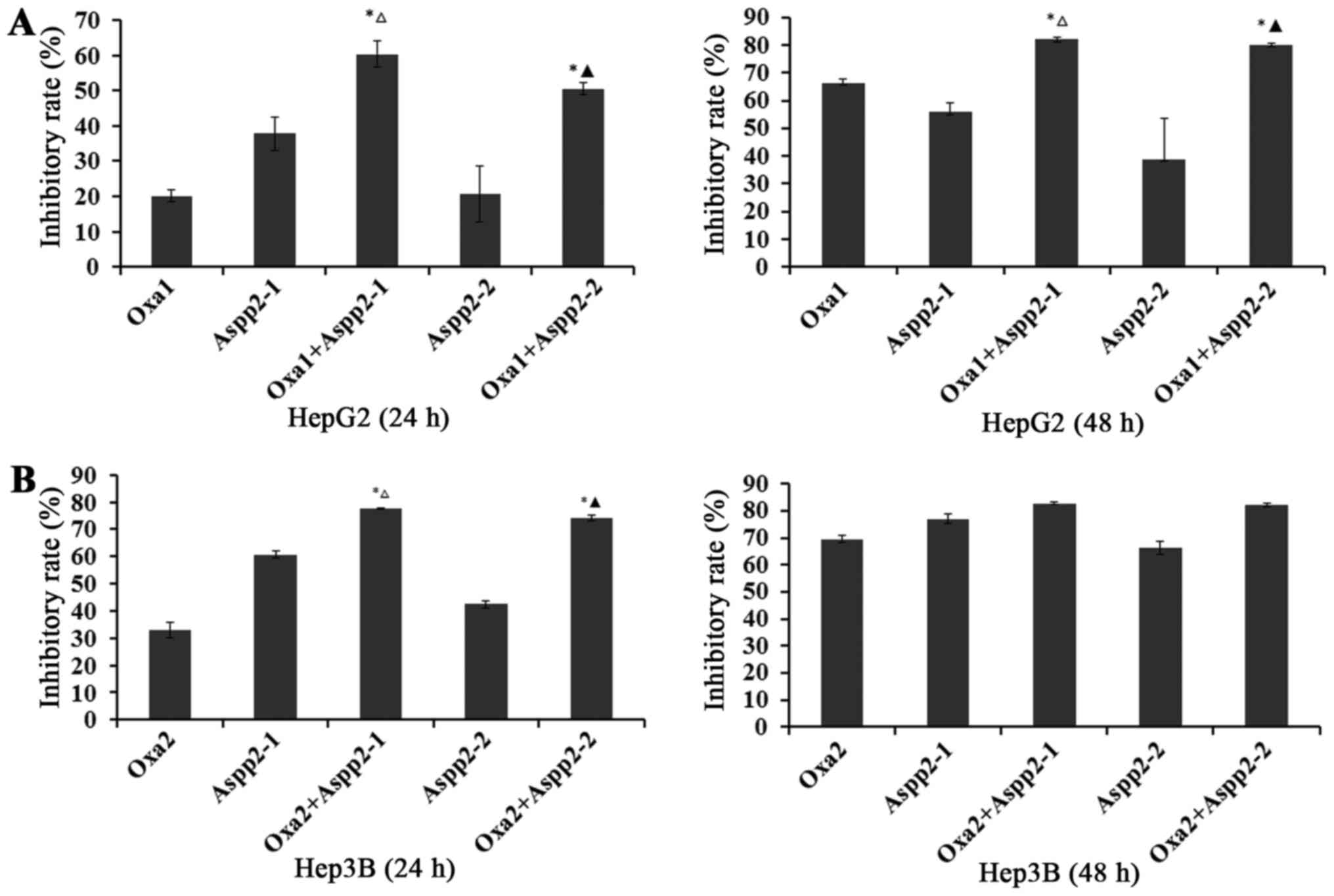
For 24 h in HepG2P53−/− cells, the
inhibitory rate (IR) of 20 µg/ml oxaliplatin and 1.25×108,
6.125×107 pfu/ml Aspp2-ad were 20.10, 37.87 and 20.62%,
respectively. Synergistic inhibitory rates of 1.25×108,
6.125×107 pfu/ml Aspp2-ad with oxaliplatin were 60.37
and 50.54%, respectively. For 48 h in HepG2P53−/− cells,
the IR of 20 µg/ml oxaliplatin and 1.25×108,
6.125×107 pfu/ml Aspp2-ad were 66.43, 56.09 and 39.00%,
respectively. Synergistic inhibitory rates of 1.25×108,
6.125×107 pfu/ml Aspp2-ad with oxaliplatin were 82.28
and 80.23%, respectively (Fig.
2A).
For 24 h in Hep3B cells, the IR of 10 µg/ml
oxaliplatin and 1.25×108, 6.125×107 pfu/ml
Aspp2-ad were 32.93, 60.71 and 42.46%, respectively. Synergistic
inhibitory rates of 1.25×108, 6.125×107
pfu/ml Aspp2-ad with oxaliplatin were 77.81 and 74.01%,
respectively. For 48 h in HepG2P53−/− cells, the IR of
20 µg/ml oxaliplatin and 1.25×108,
6.125×107 pfu/ml Aspp2-ad were 69.52, 76.91 and 66.15%,
respectively. Synergistic inhibitory rates of 1.25×108,
6.125×107 pfu/ml Aspp2-ad with oxaliplatin were 82.82
and 82.31%, respectively (Fig.
2B).
These results suggested that Aspp2-ad could
significantly enhance the inhibitory effect of οxaliplatin on
hepatocellular carcinoma cells.
Aspp2-ad enhances the apoptosis of
οxaliplatin on hepatocellular carcinoma cells
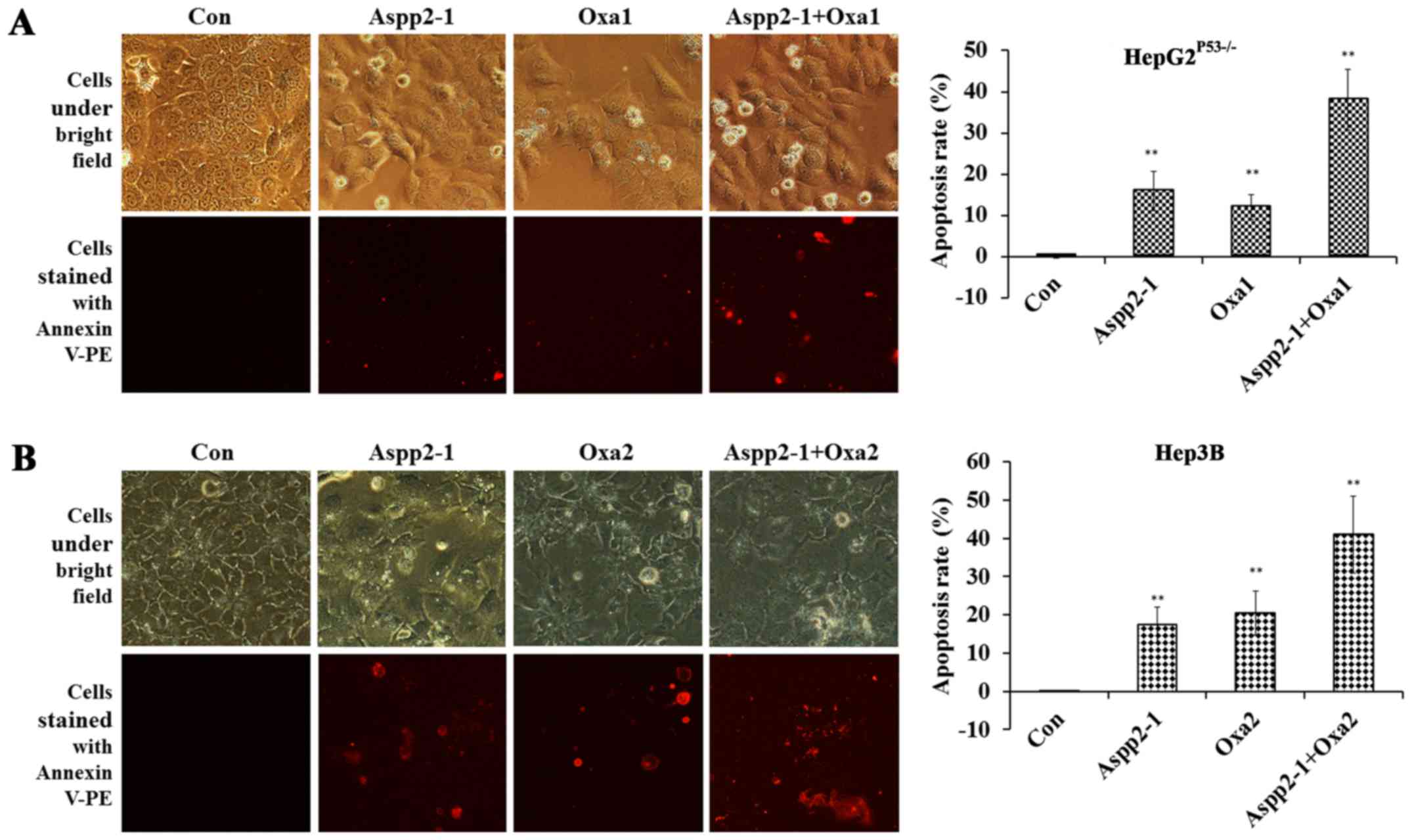
After the cells were stained with Annexin V-PE, we
found that the apoptotic cells in Aspp2-ad, οxaliplatin,
combination (Aspp2-ad+οxaliplatin) groups increased greatly. In
HepG2P53−/− cells, the apoptosis rates of control,
Aspp2-ad, οxaliplatin and combination groups were 0.01±0.30,
15.876±4.79, 12.09±3.02 and 38.09±7.33%, respectively (Fig. 3A). In Hep3B cells, the apoptosis
rates of control, Aspp2-ad, oxaliplatin and Aspp2-ad with
combination groups were 0.04±0.21, 17.33±4.57, 20.4±5.75 and
40.95±10.10%, respectively (Fig.
3B).
Aspp2-ad enhances the growth inhibition
of οxaliplatin in hepatocellular carcinoma xenograft models
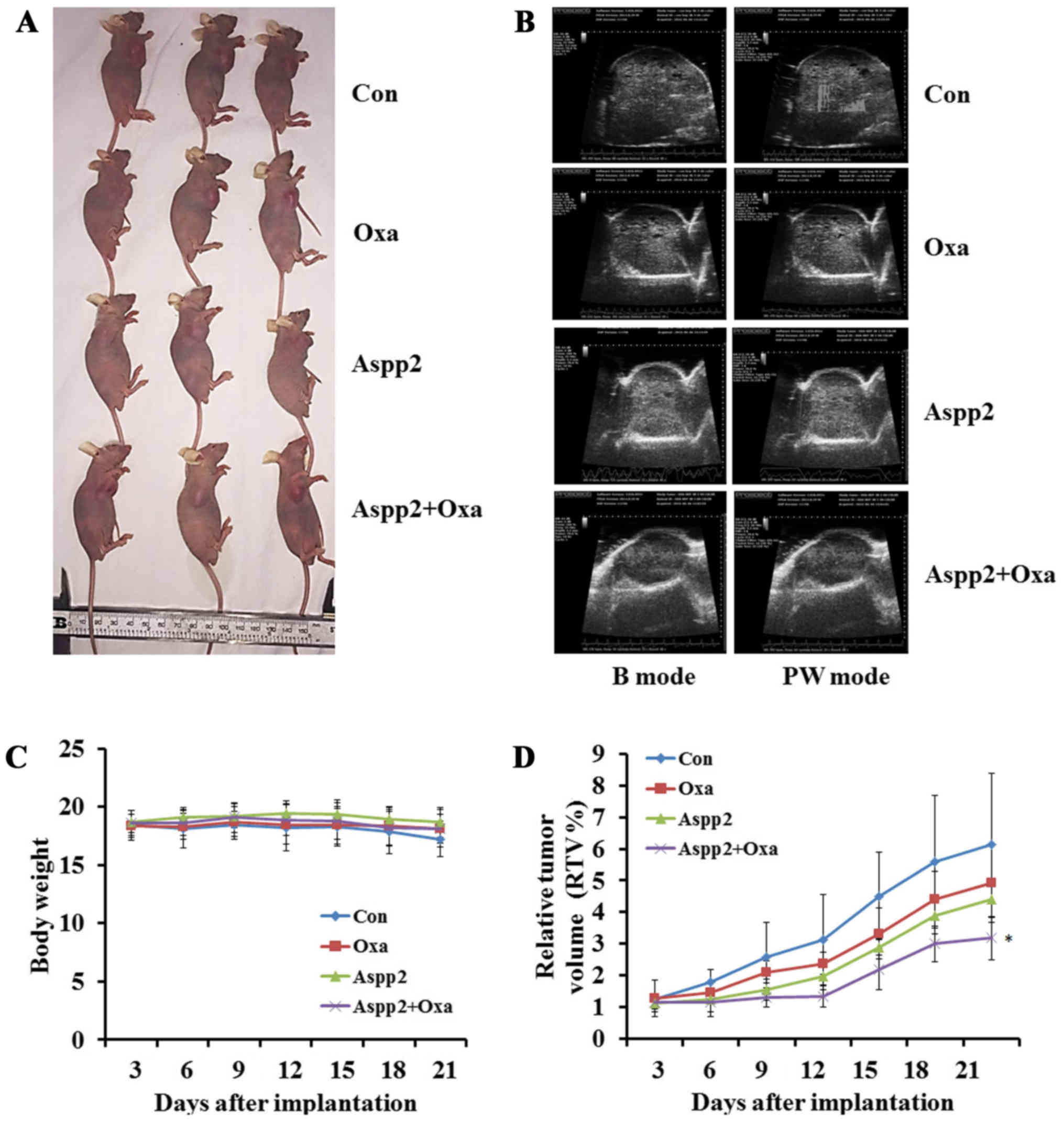
We successfully established the Hep3B subcutaneous
xenograft tumor models (Fig. 4A).
All the mice in different groups were tolerant to the treatment.
The body weights showed no significant difference in the groups
(Fig. 4C). The relative tumor
volume (RTV) was calculated to assess the efficacy of Aspp2-ad
and/or oxaliplatin. The results showed that RTVs of oxaliplatin,
Aspp2 and combination groups all tended to reduce, but only
significantly differed in combination groups compared with control
group (Fig. 4D). The ultrasonic
images showed that small vessel quantity in interior tumors were
also decreased in oxaliplatin, Aspp2 and combination groups
(Fig. 4B).
Synergistic influence of Aspp2-ad and
oxaliplatin on pathology and CD31, PCNA protein expression in
hepatocellular carcinoma xenograft models
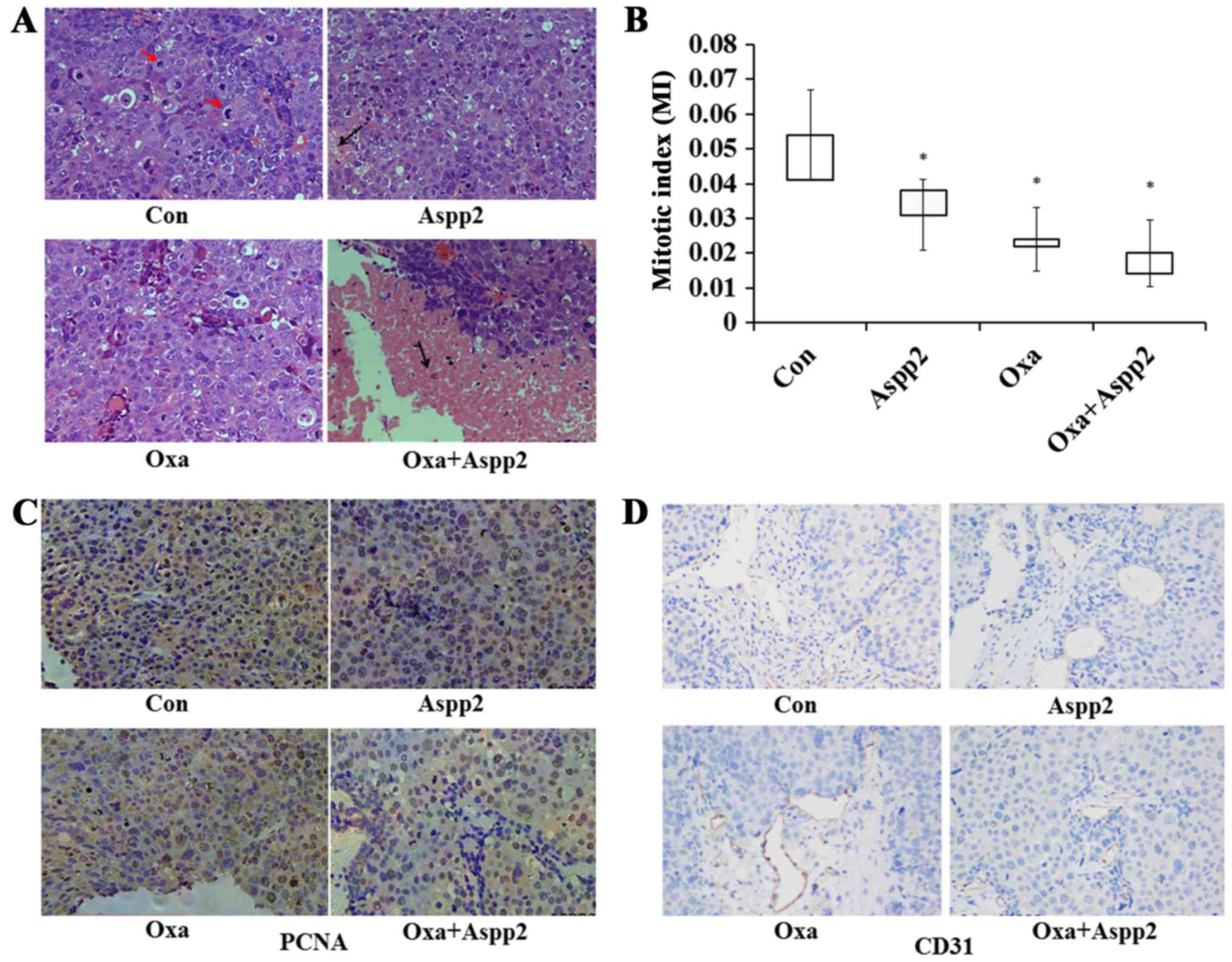
H&E results showed that MI decreased greatly in
Aspp2-ad, oxaliplatin and combination groups compared with control
group. The mitotic indexes of tumors in control, Aspp2-ad,
oxaliplatin and combination groups were 0.051, 0.030, 0.028 and
0.018, respectively. Necrosis areas of tumors in combination group
were increased compared to those in other groups (Fig. 5A and B).
Compared with those in control group,
immunohistochemistry results showed that CD31 and PCNA expression
of tumors in treatment groups decreased greatly, which were
consistent with H&E and ultrasonic results (Fig. 5C and D).
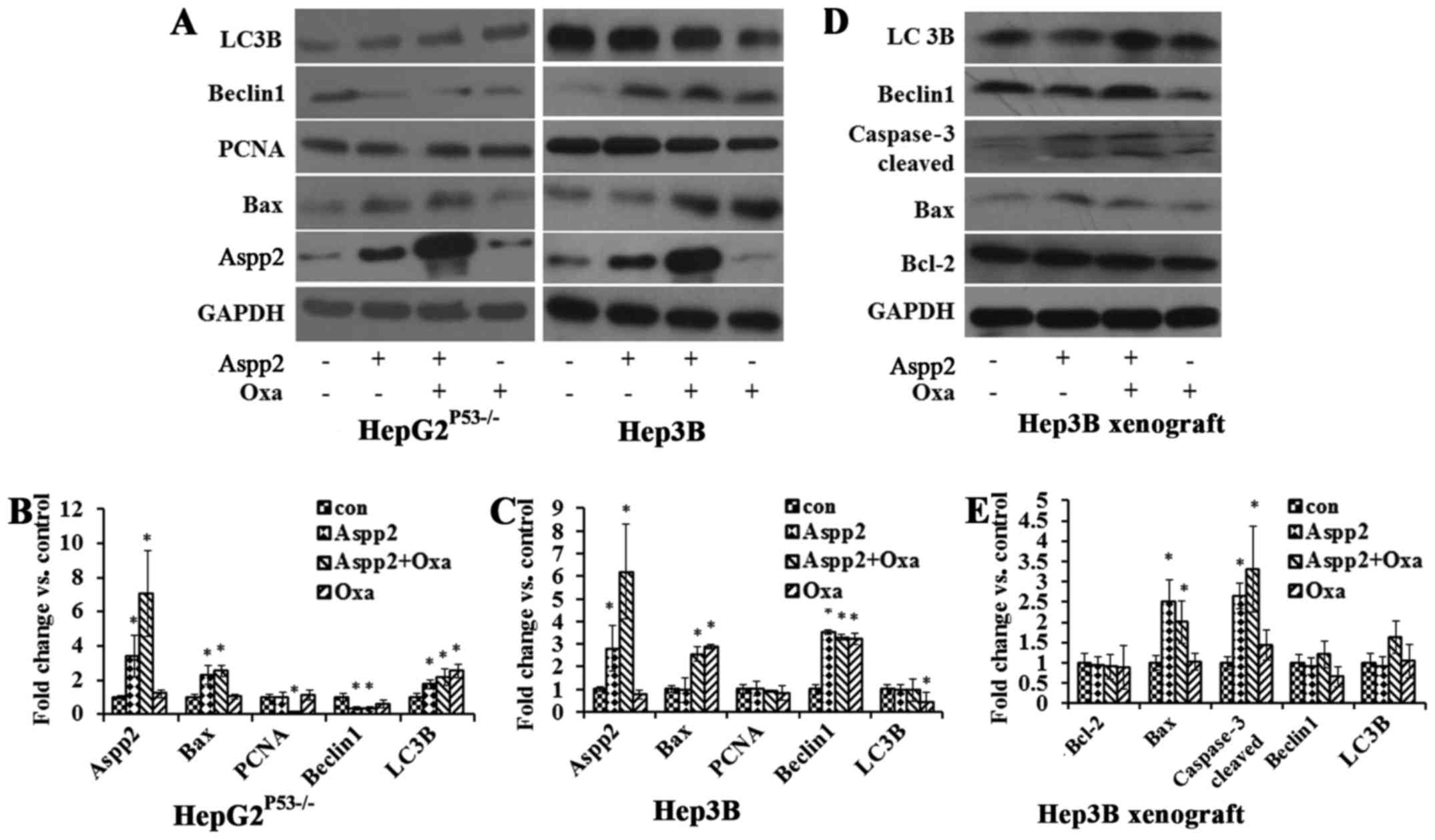
Synergistic influence of Aspp2-ad and
oxaliplatin on apoptosis and autophagy protein expression of
hepatocellular cells and hepatocarcinoma
In HepG2P53−/− cells, cells treated with
Aspp2-ad increased the Aspp2 protein expression, and oxaliplatin
enhanced the Aspp2 expression. Aspp2-ad increased the Bax protein
expression, while decreased the Beclin1 protein expression.
Aspp2-ad and oxaliplatin both increased the LC3B expression. PCNA
showed no significance in the treated cells. In Hep3B cells, cells
treated with Aspp2-ad increased the Aspp2 protein expression, and
oxaliplatin enhanced the Aspp2 expression. Oxaliplatin increased
the Bax protein expression while decreased LC3B expression.
Aspp2-ad and oxaliplatin both increased Beclin1 expression. PCNA
showed no significance in the different treatments of cells
(Fig. 6A–C).
In Hep3B xenograft tumor models, Aspp2-ad and
oxaliplatin both increased Bax, caspase-3 cleaved expression.
Aspp2-ad exerted oxaliplatin to regulate the LC3B and Beclin1
expression (Fig. 6D and E).
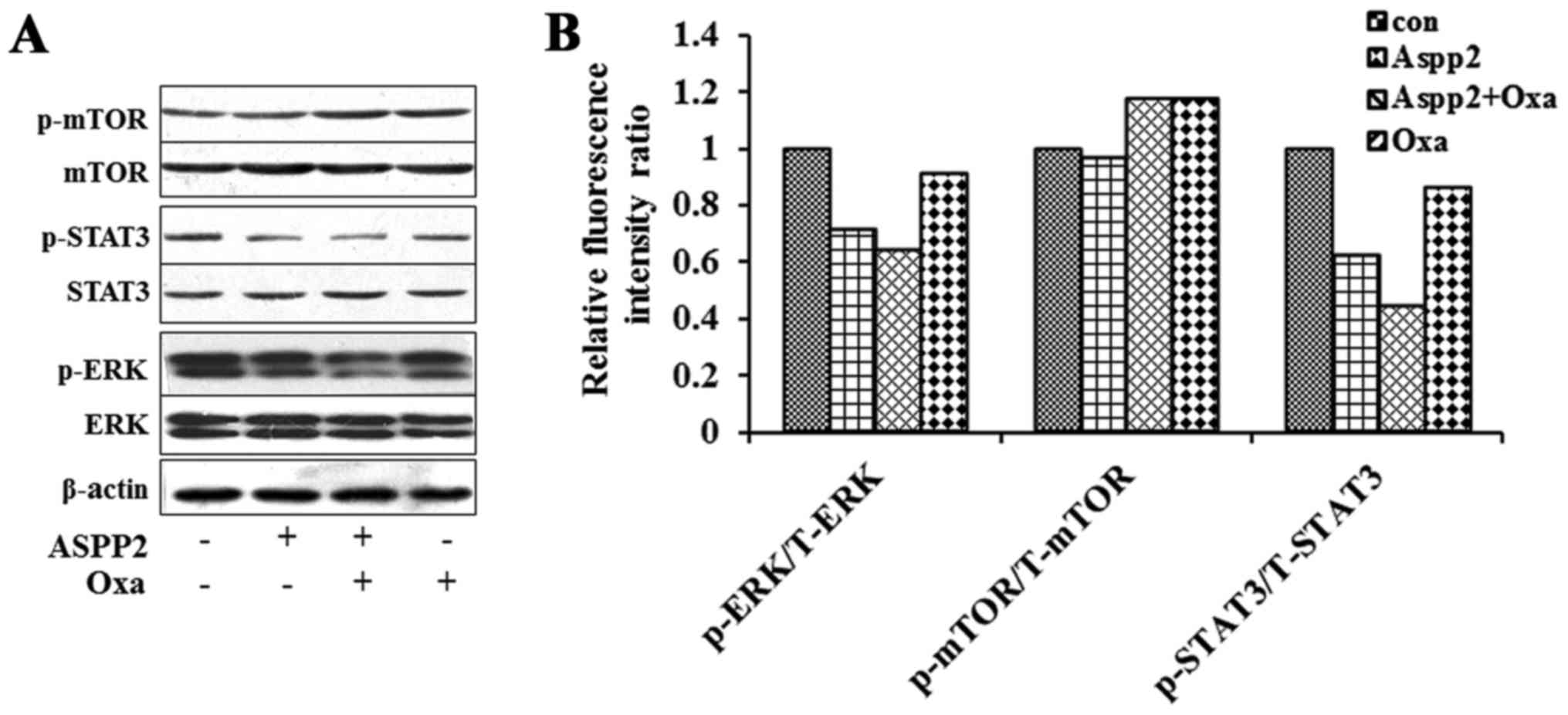
ASPP2-ad enhances the inhibitory effect
of oxaliplatin on hepatocellular carcinoma via regulating the cell
signal pathways
In Hep3B xenograft mice, the western blot results
showed that the expression of p-STAT3, p-ERK of Aspp2-ad and
combination group decreased greatly. The radio of p-ERK/T-ERK and
p-STAT3/T-STAT3 were also decreased in these two groups with
multiplexed bead-based immunoassay. Synergistic inhibition of
Aspp2-ad and oxaliplatin on these two signal molecules was
observed. No changes of p-mTOR/T-mTOR were shown in the different
groups (Fig. 7).
Discussion
Gene therapy is a biological treatment method which
can transfer the foreign gene into the target cells by gene
transfer technology, correcting or compensating for the disease
caused by the gene defect and abnormality (22). The first gene therapy approved by
FDA in the United States occurred in 1990 for a patient with severe
combined immunodeficiency disorder (23). In 2004, Gendicine (Recombinant
Human Ad-p53) as the first world's tumor gene therapy drug was
successfully developed in China (18,24).
Since then, various gene therapy research and several commercially
approved medications for cancer have been reported (25–28).
Although the research and development of antitumor gene drugs have
developed rapidly, the most serious problem of gene therapy is the
selection of effective genes. Overexpression of Aspp2 was found to
have antitumor effect independently and dependent on P53 pathways
(29). Aspp2 was considered an
appropriate gene for antitumor treatment in our previous study
(20).
In HCC, the frequency of p53 gene mutation/deletion
is as high as 50.0% (average, 30.0%). Therefore, analysis of this
gene and its products is of practical importance. Several studies
have reported that alterations of the p53 gene are correlated with
tumor differentiation, vascular invasion and tumor stage in HCC.
Moreover, aberrations of the p53 gene have been shown to be
prognostic indicators associated with recurrence-free survival and
overall survival in HCC patients (30,31).
The present study explored the potential treatment of Aspp2-ad
against hepatocarcinoma of P53 deletion by combination of
oxaliplatin in vitro and in vivo experiments to
further confirm the antitumor effects of Aspp2-ad and its
synergistic inhibitory effects with oxaliplatin via p53-independent
pathway.
After being treated with Aspp2-ad and/or oxaliplatin
for 24-48 h, HepG2P53−/− and Hep3B cells showed a
significant growth inhibition compared with vehicle control.
Combination group showed a synergetic effect, the inhibitory rates
were all above 80% at 48 h point in these cells. Annexin V PE stain
results showed that the apoptotic cell numbers of Aspp2-ad and/or
oxaliplatin treatment groups were decreased remarkably, especially
for the combined therapy group. The Hep3B xenograft experiment also
showed similar inhibition of Aspp2-ad and/or oxaliplatin to the
in vitro experiment. H&E results showed that combination
group had the lowest mitotic indexes and the most necrosis.
Proliferating cell nuclear antigen (PCNA) was initially considered
to be expressed during cell proliferation, with peak expression
occurring during late G1 and S phases (32). A wide range of functions of PCNA
involved in genome maintenance, duplication, transmission and
cell-cycle regulation were found later (33). Indeed, PCNA was increased in many
tumor tissues involved in the prognosis of cancer patients
(34). Platelet endothelial cell
adhesion molecule (PECAM-1) also known as cluster of
differentiation 31 (CD31). CD31 is mainly used to demonstrate the
presence of endothelial cells for assessing tumor angiogenesis,
which may imply a rapid increase in the extent of the tumor
(35). The immunohistochemistry
results showed that PCNA, and CD31 expression decreased greatly in
treatment groups. These results suggested that Aspp2-ad might
inhibit proliferation and vascular growth of hepatocarcinoma.
Physiological processes, such as autophagy and
apoptosis are affected in tumors. Most agents might regulate
progress of autophagy and apoptosis to influence tumor growth. The
regulation of autophagy on cell death is 2-fold: mild autophagy
protects cells from harmful conditions and promotes cell survival;
severe or rapid autophagy can induce programmed cell death, known
as autophagic cell death (autophagy-mediated cell death, ACD).
However, the regulation of apoptosis on cell death is
unidirectional, and the defect of apoptosis will lead to the
occurrence of tumor cell death (36–39).
Aspp2 induced apoptosis protein expression in Aspp2-ad and
combination groups, Bax and activation of caspase-3 expression
increased greatly both in vitro and in vivo. It is
worth noting that the intrinsic Aspp2 had no change after treatment
with oxaliplatin, but the Aspp2 expression increased greatly in
combination group, possibly oxaliplatin exerted the extrinsic Aspp2
to promote intrinsic Aspp2 expression. Notably, the autophagy
proteins showed different responses not only in
HepG2P53−/− and Hep3B cells but also in vitro and
in vivo, which might relate to the autophagy level of
samples.
From the data above, we concluded that Aspp2-ad
exerted oxaliplatin to regulate the proliferation, apoptosis,
vascular growth and autophagy to inhibit hepatocarcinoma.
To clarify which signal pathway Aspp2 affected, we
detected the ERK1/2, m-TOR and STAT3 pathways with western blot
analysis and multiplexed bead-based immunoassay. ERK signal
transduction pathway is the classical MAPK signaling pathway, ERK
could phosphorylate cytoplasmic protein, but also phosphorylated in
the nucleus of some transcription factors, which were involved in
cell proliferation, differentiation, apoptosis and regulation of
aging, migration (40). In the
past 30 years, it has become evident that the
Ras/Raf/MEK/extracellular signal-regulated kinase (ERK) signaling
pathway plays a significant role in the occurrence and development
of HCC. ERK phosphorylation activates a variety of target molecules
to promote the development of liver cancer (41). The mammalian TOR (mTOR) pathway is
a key regulator of cell growth and proliferation and increasing
evidence suggests that its deregulation is associated with human
diseases, including cancer and diabetes. The mTOR pathway
integrates signals from nutrients, energy status and growth factors
to regulate many processes, including autophagy, ribosome
biogenesis and metabolism (42).
mTOR activation can promote angiogenesis, tumor invasion,
metastasis and the cell cycle. It plays a very important role in
the occurrence, development and treatment of liver cancer (43–46).
Signal transducers and activators of transcription3 (STAT3),
activation leads to increased expression of downstream target
genes, leading to increased cell proliferation, cell survival,
angiogenesis and immune system evasion. In normal cells, STAT3
activation is tightly controlled to prevent dysregulated gene
transcription, whereas constitutively activated STAT3 plays an
important role in tumorigenesis through the upregulation of genes
involved in anti-apoptosis, proliferation and angiogenesis
(47–49). We found that Aspp2-ad downregulated
the p-ERK1/2, p-STAT3 expression, the synergistic effects were
observed in combination group, while there was no response of mTOR
to Aspp2-ad. These results were in further confirmed that Aspp2-ad
exerted oxaliplatin inhibiting hepatocarcinoma via P53-independent
pathway by regulating ERK and STAT3 pathway.
In conclusion, Aspp2-ad, P53-independently,
regulated ERK and STAT3 signal molecules to inhibit hepatocarcinoma
in coordination with oxaliplatin by influencing the protein
expression of proliferation, apoptosis, autophagy and vascular
growth. Aspp2-ad has the potential to be developed as a gene
therapy for HCC, especially for P53 deletion or mutation in
HCC.
Acknowledgments
The present study was supported by the Training Plan
for High Level of Health Technical Personnel of Beijing Health
System (2015-3-101), the Foundation of Beijing Institute of
Hepatology (no. BJIH-01712), the National Natural Science
Foundation of China (no. 81272266), the National Natural Science
Foundation of China (no. 81361120401), the Beijing Municipal
Institute of Public Medical Research Development and Reform Pilot
Project (no. 2016-2) and the Beijing Precision Medicine and
Transformation Engineering Technology Research Center of Hepatitis
and Liver Cancer.
References
|
1
|
Lamarca A, Mendiola M and Barriuso J:
Hepatocellular carcinoma: Exploring the impact of ethnicity on
molecular biology. Crit Rev Oncol Hematol. 105:65–72. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Connell LC, Harding JJ and Abou-Alfa GK:
Advanced hepatocellular cancer: The currentstate of future
research. Curr Treat Options Oncol. 17:432016. View Article : Google Scholar
|
|
3
|
Grandhi MS, Kim AK, Ronnekleiv-Kelly SM,
Kamel IR, Ghasebeh MA and Pawlik TM: Hepatocellular carcinoma: From
diagnosis to treatment. Surg Oncol. 25:74–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao C, Fan L, Qi F, Ou S, Yu L, Yi X, Ni
B, Zheng Z, Lu J, Zhang C, et al: Raltitrexed plus
oxaliplatin-based transarterial chemoembolization in patients with
unresectable hepatocellular carcinoma. Anticancer Drugs.
27:689–694. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vijayvergia N, Li T, Wong YN, Hall MJ,
Cohen SJ and Dotan E: Chemotherapy use and adoption of new agents
is affected by age and comorbidities in patients with metastatic
colorectal cancer. Cancer. 122:3191–3198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ihara K, Yamaguchi S, Ueno N, Tani Y,
Shida Y, Ogata H, Domeki Y, Okamoto K, Nakajima M, Sasaki K, et al:
Expression of DNA double-strand break repair proteins predicts the
response and prognosis of colorectal cancer patients undergoing
oxaliplatin-based chemotherapy. Oncol Rep. 35:1349–1355. 2016.
View Article : Google Scholar
|
|
7
|
Zhao C, Fan L, Qi F, Ou S, Yu L, Yi X, Ni
B, Zheng Z, Lu J, Zhang C, et al: Raltitrexed plus
oxaliplatin-based transarterial chemoembolization in patients with
unresectable hepatocellular carcinoma. Anticancer Drugs.
27:689–694. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao S, Zhang PJ, Guo JH, Chen H, Xu HF,
Liu P, Yang RJ and Zhu X: Chemoembolization alone vs combined
chemoembolization and hepatic arterial infusion chemotherapy in
inoperable hepatocellular carcinoma patients. World J
Gastroenterol. 21:10443–10452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu L, Zheng YH, Han L and Qin SK:
Efficacy and safety of the oxaliplatin-based chemotherapy in the
treatment of advanced primary hepatocellular carcinoma: A
meta-analysis of prospective studies. Medicine (Baltimore).
95:e49932016. View Article : Google Scholar
|
|
10
|
Xie YS, Zhang YH, Liu SP, Liu SQ, Peng CW,
Wu L, Luo HS and Li Y: Synergistic gastric cancer inhibition by
chemogenetherapy with recombinant human adenovirus p53 and
epirubicin: An in vitro and in vivo study. Oncol Rep. 24:1613–1620.
2010.PubMed/NCBI
|
|
11
|
Chen S, Chen J, Xi W, Xu W and Yin G:
Clinical therapeutic effect and biological monitoring of p53 gene
in advanced hepatocellular carcinoma. Am J Clin Oncol. 37:24–29.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sangro B, Mazzolini G, Ruiz M, Ruiz J,
Quiroga J, Herrero I, Qian C, Benito A, Larrache J, Olagüe C, et
al: A phase I clinical trial of thymidine kinase-based gene therapy
in advanced hepatocellular carcinoma. Cancer Gene Ther. 17:837–843.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu ZJ, Lu X, Zhang Y, Zhong S, Gu SZ,
Zhang XB, Yang X and Xin HM: Downregulated mRNA expression of ASPP
and the hypermethylation of the 5′-untranslated region in cancer
cell lines retaining wild-type p53. FEBS Lett. 579:1587–1590. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mori T, Okamoto H, Takahashi N, Ueda R and
Okamoto T: Aberrant overexpression of 53BP2 mRNA in lung cancer
cell lines. FEBS Lett. 465:124–128. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu WK, Jiang XY, Ren JK and Zhang ZX:
Expression pattern of the ASPP family members in endometrial
endometrioid adenocarcinoma. Onkologie. 33:500–503. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mak VC, Lee L, Siu MK, Wong OG, Lu X, Ngan
HY, Wong ES and Cheung AN: Downregulation of ASPP2 in
choriocarcinoma contributes to increased migratory potential
through Src signaling pathway activation. Carcinogenesis.
34:2170–2177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schittenhelm MM, Illing B, Ahmut F, Rasp
KH, Blumenstock G, Döhner K, Lopez CD and Kampa-Schittenhelm KM:
Attenuated expression of apoptosis stimulating protein of p53-2
(ASPP2) in human acute leukemia is associated with therapy failure.
PLoS One. 8:e801932013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Li B, Li CJ and Li LJ: Key points of
basic theories and clinical practice in rAd-p53 (Gendicine™) gene
therapy for solid malignant tumors. Expert Opin Biol Ther.
15:437–454. 2015. View Article : Google Scholar
|
|
19
|
Chen GX, Zhang S, He XH, Liu SY, Ma C and
Zou XP: Clinical utility of recombinant adenoviral human p53 gene
therapy: Current perspectives. Onco Targets Ther. 7:1901–1909.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi Y, Han Y, Xie F, Wang A, Feng X, Li N,
Guo H and Chen D: ASPP2 enhances oxaliplatin (L-OHP)-induced
colorectal cancer cell apoptosis in a p53-independent manner by
inhibiting cell autophagy. J Cell Mol Med. 19:535–543. 2015.
View Article : Google Scholar :
|
|
21
|
Wang S, XU JJ, Liu XN and Chen DX: Quality
control of human ASPP2 recombinant adenovirus, 2016. Zhongguo Yao
Li Xue Tong Bao. 32:885–888. 2016.In Chinese.
|
|
22
|
Amer MH: Gene therapy for cancer: Present
status and future perspective. Mol Cell Ther. 2:272014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sheridan C: Gene therapy finds its niche.
Nat Biotechnol. 29:121–128. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tazawa H, Kagawa S and Fujiwara T:
Advances in adenovirus-mediated p53 cancer gene therapy. Expert
Opin Biol Ther. 13:1569–1583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiocca EA, Abbed KM, Tatter S, Louis DN,
Hochberg FH, Barker F, Kracher J, Grossman SA, Fisher JD, Carson K,
et al: A phase I open-label, dose-escalation, multi-institutional
trial of injection with an E1B-Attenuated adenovirus, ONYX-015,
into the peritumoral region of recurrent malignant gliomas, in the
adjuvant setting. Mol Ther. 10:958–966. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Block SL, Nolan T, Sattler C, Barr E,
Giacoletti KE, Marchant CD, Castellsagué X, Rusche SA, Lukac S,
Bryan JT, et al Protocol 016 Study Group: Comparison of the
immunogenicity and reactogenicity of a prophylactic quadrivalent
human papillomavirus (types 6, 11, 16, and 18) L1 virus-like
particle vaccine in male and female adolescents and young adult
women. Pediatrics. 118:2135–2145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kantoff PW, Schuetz TJ, Blumenstein BA,
Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R,
Schlom J, et al: Overall survival analysis of a phase II randomized
controlled trial of a Poxviral-based PSA-targeted immunotherapy in
metastatic castration-resistant prostate cancer. J Clin Oncol.
28:1099–1105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Putten EH, Dirven CM, van den Bent MJ
and Lamfers ML: Sitimagene ceradenovec: A gene-based drug for the
treatment of operable high-grade glioma. Future Oncol. 6:1691–1710.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu J, Li W, Deng M, Liu D, Ma Q and Feng
X: Immunohistochemical determination of p53 protein overexpression
for predicting p53 gene mutations in hepatocellular carcinoma: A
meta-analysis. PLoS One. 2016.11:e01596362016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J, Ma Q, Zhang M, Wang X, Zhang D, Li
W, Wang F and Wu E: Alterations of TP53 are associated with a poor
outcome for patients with hepatocellular carcinoma: Evidence from a
systematic review and meta-analysis. Eur J Cancer. 48:2328–2338.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qi LN, Bai T, Chen ZS, Wu FX, Chen YY, De
Xiang B, Peng T, Han ZG and Li LQ: The p53 mutation spectrum in
hepatocellular carcinoma from Guangxi, China: Role of chronic
hepatitis B virus infection and aflatoxin B1 exposure. Liver Int.
35:999–1009. 2015. View Article : Google Scholar
|
|
32
|
Bravo R, Fey SJ, Bellatin J, Larsen PM,
Arevalo J and Celis JE: Identification of a nuclear and of a
cytoplasmic polypeptide whose relative proportions are sensitive to
changes in the rate of cell proliferation. Exp Cell Res.
136:311–319. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin S, Li Z, Huang J, Miao Z, Zhang J, Lu
C and Xu H and Xu H: Prognostic value and clinicopathological
significance of proliferating cell nuclear antigen expression in
gastric cancer: A systematic review and meta-analysis. Onco Targets
Ther. 10:319–327. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma S, Yang J, Li J and Song J: The
clinical utility of the proliferating cell nuclear antigen
expression in patients with hepatocellular carcinoma. Tumour Biol.
37:7405–7412. 2016. View Article : Google Scholar
|
|
35
|
Pantanowitz L, Moses AV and Früh K: CD31
immunohistochemical staining in Kaposi Sarcoma. Arch Pathol Lab
Med. 136:1329author reply 1330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Delou JM, Biasoli D and Borges HL: The
complex link between apoptosis and autophagy: A promising new role
for RB. An Acad Bras Cienc. 88:2257–2275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ryter SW, Mizumura K and Choi AM: The
impact of autophagy on cell death modalities. Int J Cell Biol.
2014:5026762014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Su M, Mei Y and Sinha S: Role of the
crosstalk between autophagy and apoptosis in cancer, 2013. J Oncol.
2013:1027352013. View Article : Google Scholar
|
|
39
|
Mariño G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li L, Zhao GD, Shi Z, Qi LL, Zhou LY and
Fu ZX: The Ras/Raf/MEK/ERK signaling pathway and its role in the
occurrence and development of HCC. Oncol Lett. 12:3045–3050.
2016.PubMed/NCBI
|
|
42
|
Sarbassov DD, Ali SM and Sabatini DM:
Growing roles for the mTOR pathway. Curr Opin Cell Biol.
17:596–603. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cao Z, Liu LZ, Dixon DA, Zheng JZ,
Chandran B and Jiang BH: Insulin-like growth factor-I induces
cyclooxygenase-2 expression via PI3K.MAPK and PKC sign aling
pathways in human ovarian cancer cells, 2007. Cel Signal.
19:1542–1553. 2007. View Article : Google Scholar
|
|
44
|
Matei D, Kelich S, Cao L, Menning N,
Emerson RE, Rao J, Jeng MH and Sledge GW: PDGF BB induces VEGF
secretion in ovarian cancer. Cancer Biol Ther. 6:1951–1959. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park CM, Park MJ, Kwak HJ, Lee HC, Kim MS,
Lee SH, Park IC, Rhee CH and Hong SI: Ionizing radiation enhances
matrix metal-loproteinase-2 secretion and invasion of glioma cells
through Src/epidermal growth factor receptor-mediated p38/Akt and
phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res.
66:8511–8519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou Q, Wong CH, Lau CP, Hui CW, Lui VW,
Chan SL and Yeo W: Enhanced antitumor activity with combining
effect of mTOR inhibition and microtubule stabilization in
hepatocellular carcinoma. Int J Hepatol. 2013:1038302013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mali SB: Review of STAT3 (Signal
Transducers and Activators of Transcription) in head and neck
cancer. Oral Oncol. 51:565–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li CJ, Li YC, Zhang DR and Pan JH: Signal
transducers and activators of transcription 3 function in lung
cancer. J Cancer Res Ther. 9(Suppl 2): S67–S73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Subramaniam A, Shanmugam MK, Perumal E, Li
F, Nachiyappan A, Dai X, Swamy SN, Ahn KS, Kumar AP, Tan BK, et al:
Potential role of signal transducer and activator of transcription
(STAT)3 signaling pathway in inflammation, survival, proliferation
and invasion of hepatocellular carcinoma. Biochim Biophys Acta.
1835:46–60. 2013.
|