Introduction
Osteosarcoma is the most common primary malignant
bone tumor with a similar incidence worldwide. It is highly
aggressive and with early metastasis (1,2).
Osteosarcoma occurs mainly among children and adolescents with low
5-year survival rate, high amputation rate and poor postoperative
function recovery (3).
Osteosarcoma is a major death-causing disease in adolescence due to
its rapid progression and poor prognosis (4). The poor prognosis of osteosarcoma is
partially due to the lack of a better molecular biomarker to detect
it at early tumor stage. Therefore, it is necessary for us to
further understand the underlying molecular mechanisms and find a
useful biomarker of osteosarcoma to predict prognosis. Currently,
there are no effective target drugs for treating osteosarcoma.
Although chemotherapy largely improves the 5-year survival rate and
life quality of osteosarcoma patients (5), intrinsic or acquired chemoresistance
greatly impede further response to treatment (6). Similar to other tumors, osteosarcoma
is a complicated disease with multi-genetic variations (7). Systemic studies on gene regulation
network are essential for further understanding how osteosarcoma
initiates and develops. It is urgent to develop effective targeted
therapies to treat this disease. Hence, finding novel diagnostic
biomarkers and therapeutic strategies to improve the prognosis of
osteosarcoma is necessary.
Non-coding RNAs (ncRNAs) are divided based on their
size into three groups: long non-coding RNAs (lncRNAs), which are
longer than 200 base pairs; small ncRNAs, also knowns as microRNAs
(miRNAs), which are usually 18–25 nucleotides in length; circular
RNAs (circRNAs), which are small covalently closed circular loop
structures with either 5′ to 3′ polarity or polyadenylation at the
3′ ends (8). The well-known and
well-studied miRNAs are important post-transcriptional regulators
involved in various biological processes (9,10).
MicroRNAs regulate gene expression by inhibition of translation or
destabilization of mRNA transcript through base pairing to the
3′-UTR regions of mRNAs (11).
Although thousands of lncRNAs have been identified in the past
decade, only a small number have been functionally characterized.
However, emerging data demonstrate lncRNAs constitute a major part
of human transcriptome and involve in various critical biological
processes (12–15). Accumulating evidence has shown that
both lncRNAs and microRNAs participate in the regulation of cell
proliferation and apoptosis, and play key roles in tumori genesis
and progression (16,17).
Taurine upregulated gene 1 (TUG1), a 7.1-kb lncRNA,
was firstly identified as a transcript upregulated in response to
taurine treatment, which affects mouse retinal development
(18). TUG1 was found to be
upregulated in various human tumors, such as osteosarcoma, breast
cancer, urothelial carcinoma and esophageal squamous cell carcinoma
(19–22). Accumulating evidence has verified
that TUG1 expression was increased in osteosarcoma tissues and
correlates with poor prognosis and disease status in osteosarcoma
(21,23–26).
Experiment in vitro indicated TUG1 inhibition strongly
impaired proliferation of osteosarcoma cells and promoted apoptosis
(21,27). Another ncRNA, miR-144-3p was shown
to be downregulated in osteosarcoma (28). However, how and by which mechanism
lncRNA TUG1 and miR-144-3p regulate osteosarcoma initiation and
development is still poorly understood.
In this study, we aimed to elucidate the correlation
between TUG1 and miR-144-3p expression in osteosarcoma and
investigated their downstream targets. Our finding will provide new
insights into the molecular function of TUG1 in osteosarcoma and
find new biomarkers for osteosarcoma diagnosis and prognosis.
Materials and methods
Human osteosarcoma tissue collection
All the osteosarcoma and adjacent normal tissues
were surgically resected from patients in the Sixth People's
Hospital Affiliated to Shanghai Jiao Tong University with informed
consent from 2010 to 2012. All specimens were confirmed by
clinical, radiographic, and histological examination for
osteosarcoma, and were not subjected to radiotherapy, chemotherapy,
and blood transfusion therapy before the operation. All the
clinical information was collected including sex, age, tumor size,
tumor position, tumor stage, and initial metastasis. The present
study was approved by the Research Ethics Committee of Shanghai
Jiao Tong University Affiliated Sixth People's Hospital. All
specimens were handled and made anonymous according to the ethical
and legal standards.
Cell culture
Human normal osteoblast HFOB1.19 cells and
osteosarcoma cell lines MG63, U2OS, HOS and Saos-2 were obtained
from the Bank of Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China). HFOB1.19 cells were cultured in F12
containing 10% fetal bovine serum (FBS). The HOS cell line was
maintained in Eagle's minimum essential medium (MEM), and all other
cell lines were cultured in RPMI-1640 medium containing 10% FBS.
All the cell culture medium and FBS were purchased from Gibco,
Invitrogen Corp. (Grand Island, NY, USA). The cell lines were
incubated at 37°C in a 5% CO2 incubator.
Vector constructs
For shRNA-mediated TUG1 silencing, DNA
oligonucleotides targeting TUG1 was inserted into a lentiviral
pLKO.1 plasmid. DNA sequences were mainly obtained from Sigma
MISSION shRNA library. For the overexpression of EZH2, the open
reading frame of the EZH2 gene was amplified and cloned into a
lentiviral pCDH vector.
Lentivirus production and
transfection
pCDH-EZH2 or pLKO.1-shTUG1 was co-transfected with
the packaging plasmid psPAX2 and the envelop plasmid pMD2.G into
HEK-293FT cells using Calcium phosphate transfection. Virus
particles were harvested 48 h after transfection. MG63 and U2OS
cells were infected with lentivirus particles plus 4 g/ml Polybrene
(Sigma).
RNA extraction and q-PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen). RNA (1 μg) was reversely transcribed into cDNA
with M-MLV Reverse Transcriptase (Promega, Madison, WI, USA). q-PCR
was performed with SYBR Premix Ex Taq (Takara) on ABI 7500 fast
real-time PCR system (Applied Biosystems). GAPDH mRNA was used as
an endogenous control for mRNA.
Luciferase reporter assay
The luciferase assays were carried out using the
Dual-luciferase Reporter Assay System (Promega). Briefly, cells
were co-transfected with miR-144-3p mimics or miR-control and
pMIR-reporter luciferase vector containing a specific sequence of
wild-type or mutant TUG1 or EZH2 fragment, using Lipofectamine 2000
(Invitrogen). Cells were collected and lysed for luciferase
detection 48 h after transfection. The relative luciferase activity
was normalized against to the Renilla luciferase
activity.
To test Wnt signaling, osteosarcoma cell lines were
co-transfected with either the Wnt signaling reporter TOPFlash or
the negative control FOPFlash plasmids (Upstate Biotechnology),
together with Renilla plasmid. The cells were infected with
TUG1 shRNA or EZH2 overexpression lentiviruses. The data are
presented as normalized TOPFlash/FOPFlash values. After 48 h, the
luciferase activities were measured by Dual-luciferase Reporter
Assay System (Promega).
RNA-binding protein immunoprecipitation
and RNA pull-down assay
RIP and RNA pull-down assay were performed as
described by Lei et al (29).
Western blot analysis
The cells were suspended and lysed in RIPA buffer
(Beyotime, Beijing, China) supplemented with protease inhibitor
cocktail (Sigma). Protein extractions were separated by SDS-PAGE
and transfected to a PVDF membrane (Millipore). The membrane was
blocked with 5% (w/v) reagent-grade nonfat milk (Cell Signaling
Technology) and incubated with primary antibodies at 4°C overnight
followed by secondary antibody incubation. The protein bands were
visualized using Clarity™ Western ECL substrate (Bio-Rad). The
protein level was quantified using ImageJ software normalized with
GAPDH.
Wound healing assays
Wound healing assays were performed to detect cell
migration. MG63 and U2OS cells were seeded in 6-well plates and
allowed to reach confluence. An artificial wound was made using a
200 μl pipette tip across the cell monolayer. Cells were
rinsed with PBS and cultured in the medium. Wound closure was
detected at 0, 24 and 48 h, and imaging performed under a
microscope.
Transwell migration assays
Transwell migration assays were performed according
to the manufacturer's protocol (BD Biosciences). Briefly,
transfected cells in serum-free medium were added to the top
chamber and incubated at 37°C in a humidified incubator containing
5% CO2. Cells that migrated into the lower chamber were
stained with 10% crystal violet (Sigma) and quantitated by counting
in five different areas under a light microscope.
Statistical analysis
All data are presented as the mean ± SD and derived
from at least three independent experiments. Statistical analysis
was performed by SPSS 18.0 software (SPSS, Chicago, IL, USA) and
GraphPad Prism software (GraphPad Software, Inc., San Diego, CA,
USA). For all comparisons, differences were considered significant
at P<0.05.
Results
lnRNAs-TUG1 is upregulated in
osteosarcoma and correlated with disease status
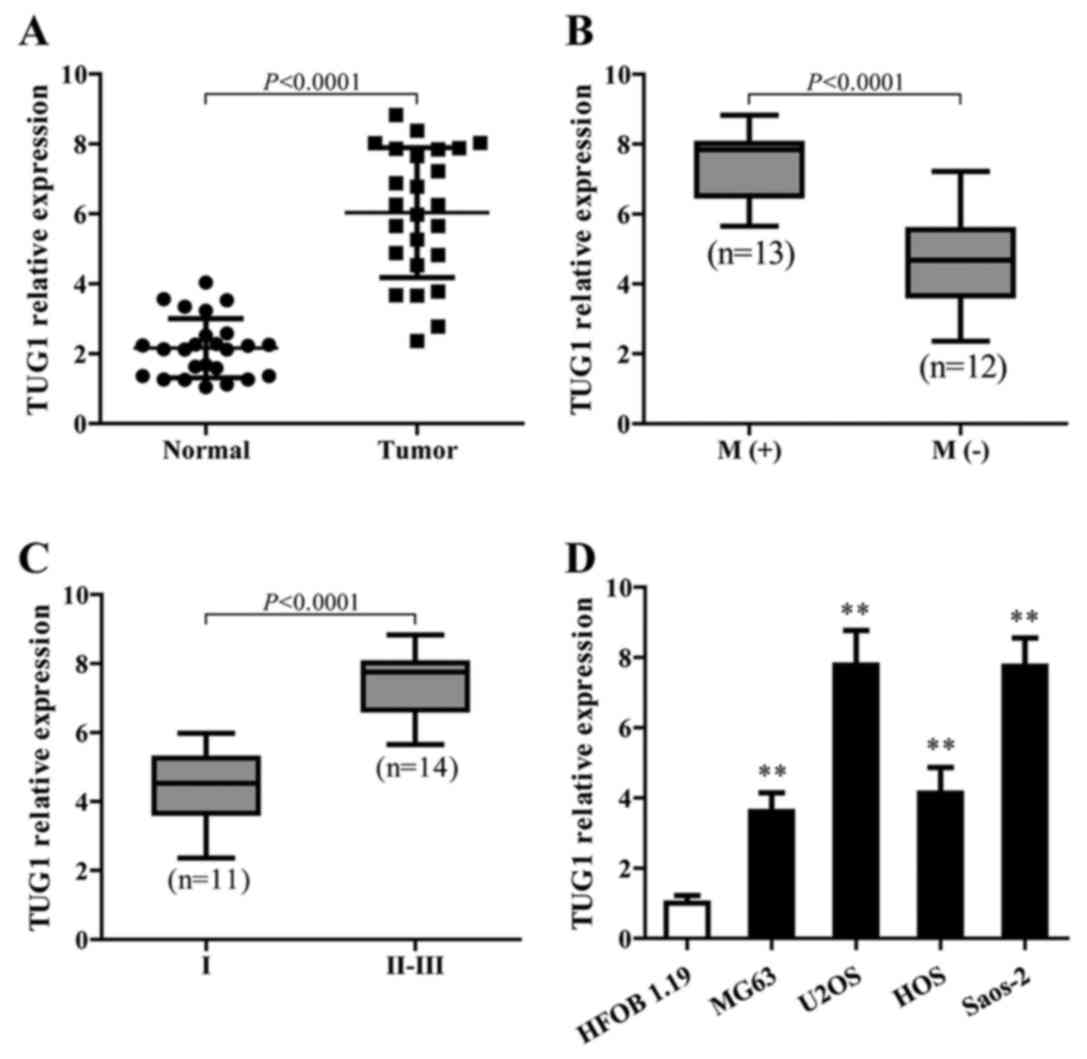
To identify the function of TUG1 in osteosarcoma,
TUG1 expression in cancerous and adjacent normal tissues from 25
patients with osteosarcoma in different stages was detected using
qPCR. The results showed that TUG1 levels were significantly higher
in cancer tissues than normal tissues (P<0.0001) (Fig. 1A). In addition, higher TUG1
expression showed a strong correlation with osteosarcoma metastasis
(Fig. 1B) and was associated with
the late stage of osteosarcoma (Fig.
1C). TUG1 also showed a higher expression level in different
osteosarcoma cell lines compared to the normal cells (Fig. 1D).
miR-144-3p is regulated by TUG1 in
osteosarcoma cells
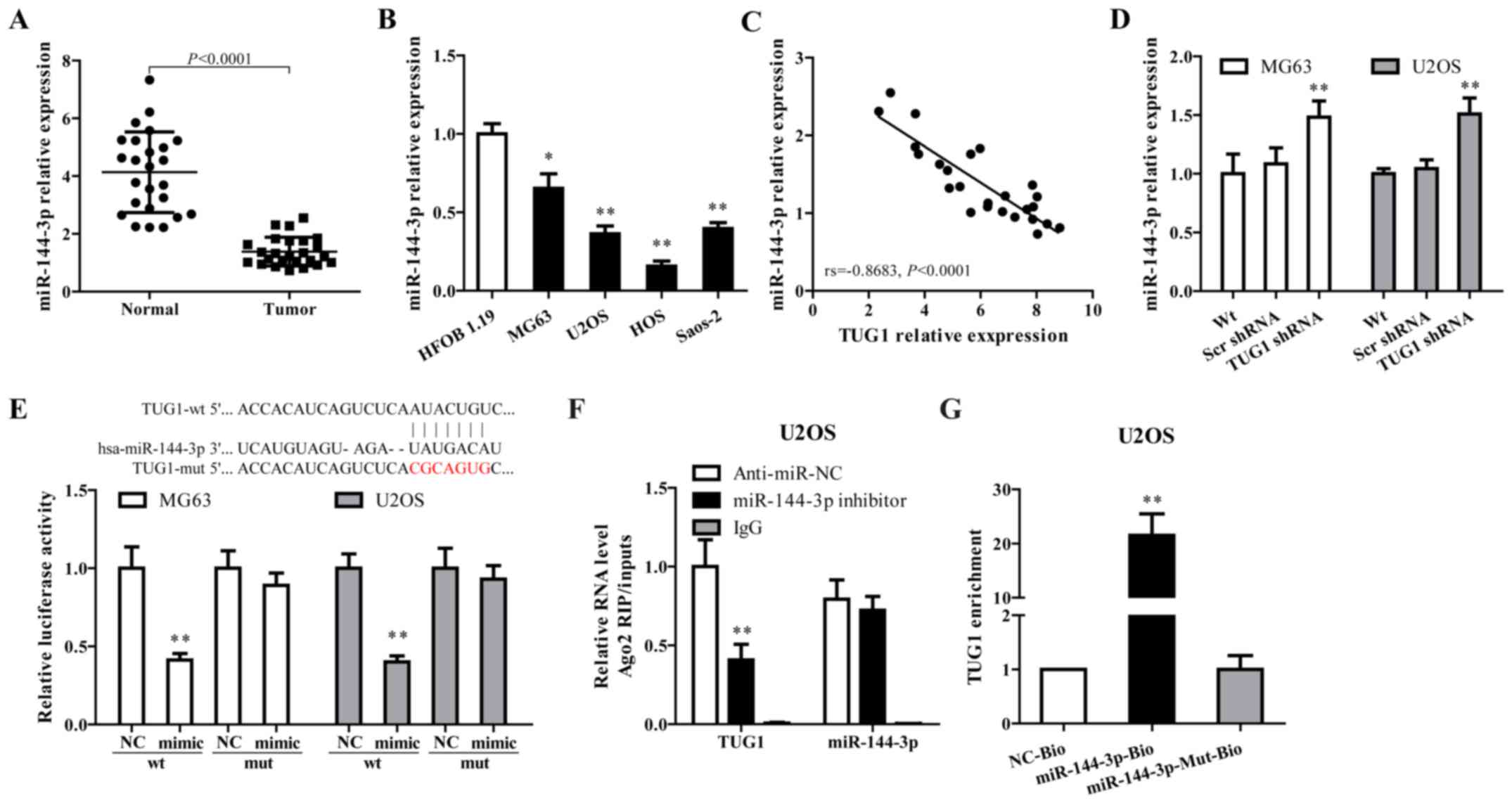
Growing evidence documented that TUG1 works
competing with endogenous (ce)RNAs by serving as sponges that bind
and sequester miRNAs (30,31). As a putative combining target of
TUG1 (Fig. 2E, upper panel),
miR-144-3p is downregulated in osteosarcoma and has been associated
with the progress of this disease (32,33).
To verify whether TUG1 functions through regulating miR-144-3p,
miR-144-3p expression in osteosarcoma tissues and cell lines were
determined. As shown in Fig. 2A–C,
miR-144-3p was downregulated in tumor tissues and cell lines, and
showed a negative correlation with TUG1 expression (rs= −0.8683,
P<0.0001). TUG1 knockdown using TUG1 shRNA in MG63 and U2OS
cells significantly increased miR-144-3p expression (Fig. 2D).
Dual luciferase reporter assay, RNA-binding protein
immuno precipitation (RIP) and applied biotin-avidin pull-down
system were performed to explore whether TUG1-mediated miR-144-3p
expression through function as a ceRNA. As shown in Fig. 2E, co-transfection of pMIR-TUG1
wild-type and miR-144-3p mimics greatly reduced the luciferase
activity compared with TUG1-wt+miR-144-3p NC group, whereas
mutation of the miR-144-3p-binding site within TUG1 abrogated the
inhibitory effect of miR-144-3p mimics on reporter gene expression.
Results from RIP assay showed that TUG1 was detected in Ago2
immunoprecipitates from the control group, but was drastically
reduced in Ago2 complexes purified from cells treated with
miR-144-3p inhibitor (Fig. 2F),
indicating that TUG1 is likely in the miR-144-3p RISC complex.
Results from RNA pull-down showed that TUG1 was pulled down by
miR-144-3p, while miR-144-3p-Mut with mutated binding site of TUG1
failed to pull-down TUG1 (Fig.
2G), indicating that the recognition of miR-144-3p to TUG1 is
in a sequence-specific manner. Together, our findings confirmed
miR-144-3p is regulated by TUG1 in osteosarcoma cells through
direct binding.
EZH2, a target of miR-144-3p, is
positively regulated by TUG1 in osteosarcoma
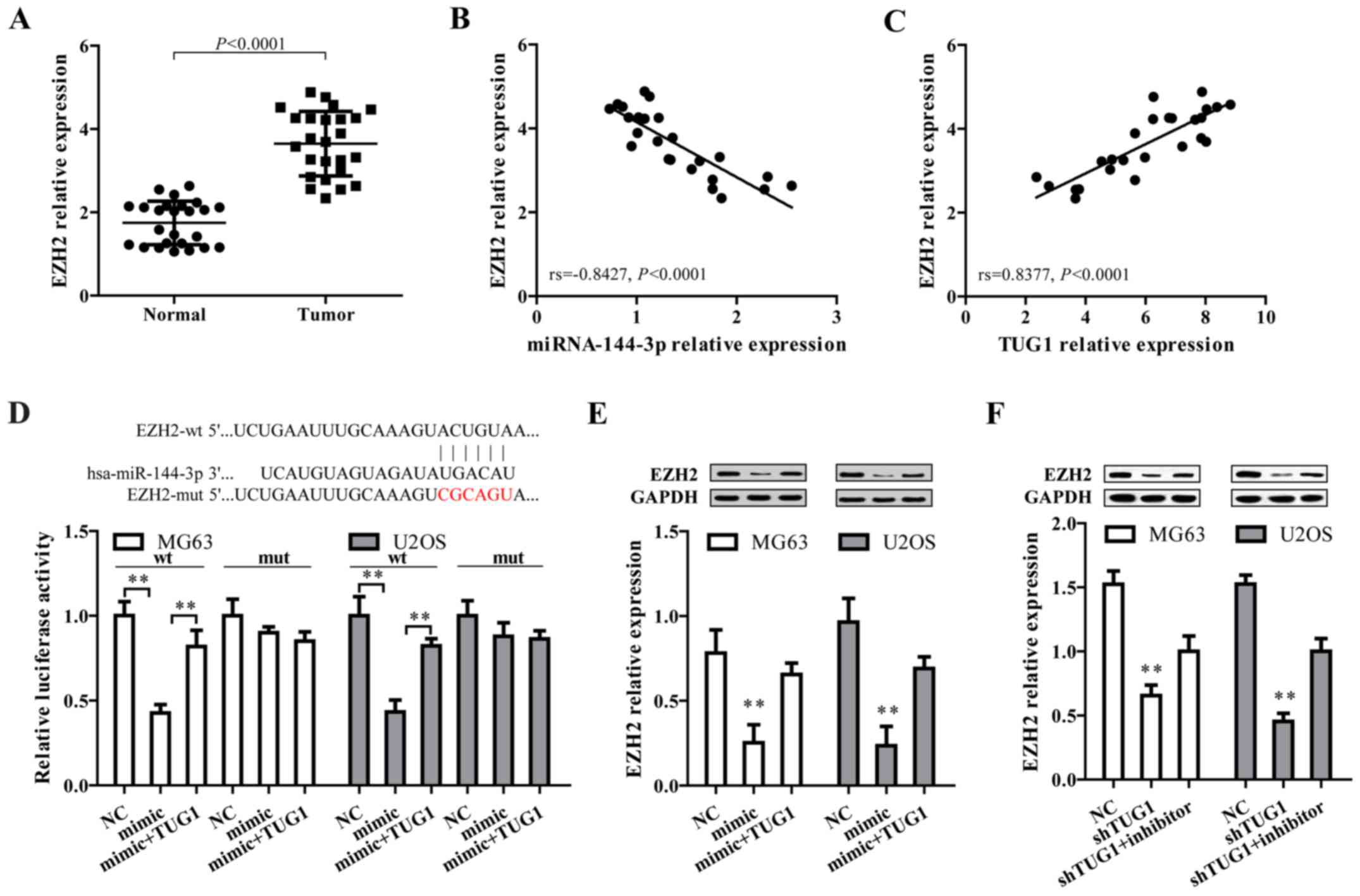
After the confirmation that TUG1 could regulate
miR-144-3p in osteosarcoma cells, we proposed TUG1 may exert their
function through regulating miR-144-3p target genes by functioning
as a ceRNA. To address this, three bioinformatic databases
(TargetScan, Miranda and PicTar) were employed to predict the
potential target genes of miR-144-3p. Targets that were predicted
in all databases and with PicTar score >2.40 are listed in
Table I. Among these, EZH2 was
selected as a target gene owing to its high expression and
promoting effect on tumorigenesis and cancer progression in
osteosarcoma (34–37). By comparing tumor and normal
tissues, we found EZH2 expression level was higher in osteosarcoma
tissues (Fig. 3A), and showed a
negative correlation with miR-144-3p level (Fig. 3B) but positively associated with
TUG1 expression (Fig. 3C).
Additionally, bioinformatics analysis revealed that in the 3′UTR
region of EZH2 contains a binding site for miR-144-3p (Fig. 3D, upper panel). Results from dual
luciferase reporter assay showed that co-transfection of mature
miR-144-3p and EZH2-wt significantly limited the luciferase
activity in both MG63 and U2OS cells, which was abated by
transfection of TUG1 overexpression (Fig. 2E, lower panel). Furthermore,
miR-144-3p mimics markedly reduced EZH2 protein expression, which
was reversed by TUG1 overexpression (Fig. 3E). Moreover, shRNA-mediated TUG1
knockdown significantly represses EZH2 expression, which could be
reversed by miR-144-3p inhibitor (Fig.
3F). These data collectively demonstrated EZH2 is a target of
miR-144-3p and is positively regulated by TUG1.
 | Table IPutative target genes of miR-144-3p
predicted by TargetScan, Miranda and PicTar with PicTar score
>2.40. |
Table I
Putative target genes of miR-144-3p
predicted by TargetScan, Miranda and PicTar with PicTar score
>2.40.
| Putative
target | PicTar score | Putative
target | PicTar score | Putative
target | PicTar score |
|---|
| ARID1A | 9.44 | SHANK2 | 4.39 | ACBD3 | 3.10 |
| GLTSCR1 | 8.06 | BACH2 | 4.33 | ATP2B1 | 3.05 |
| SORCS3 | 8.01 | TRIO | 4.27 | USP38 | 3.04 |
| RARB | 7.99 | ALS2 | 4.24 | ANKRD17 | 3.03 |
| FBN2 | 7.89 | TJP1 | 4.23 | ATP2B2 | 3.02 |
| MAP3K4 | 7.67 | RBM12 | 4.23 | ZFX | 3.00 |
| UBE2D1 | 7.10 | PDE4D | 4.18 | GSPT1 | 2.92 |
| MYCN | 7.03 | STAG1 | 4.10 | ATP2B1 | 2.92 |
| QKI | 7.01 | ANK2 | 4.03 | SEMA6A | 2.87 |
| NFE2L2 | 6.82 | IDH2 | 3.82 | KCNH7 | 2.86 |
| MARK1 | 6.55 | FBXO32 | 3.75 | LHX2 | 2.86 |
| ADAMTSL3 | 6.53 | PDE7B | 3.75 | CAV2 | 2.86 |
| ABCA1 | 6.32 | NGFRAP1 | 3.61 | TFAP4 | 2.84 |
| ELL2 | 6.25 | STARD8 | 3.60 | CDH2 | 2.83 |
| MSI1 | 6.06 | CDYL | 3.59 | PPFIA1 | 2.81 |
| ZFHX4 | 6.05 | STC1 | 3.57 | MYB | 2.80 |
| DCBLD2 | 5.98 | ST18 | 3.54 | SLITRK4 | 2.78 |
| SOCS5 | 5.95 | C6orf62 | 3.51 | GDF10 | 2.78 |
| RIN2 | 5.84 | GOLGA4 | 3.50 | PAX3 | 2.78 |
| RNF111 | 5.82 | WTAP | 3.49 | STAT6 | 2.69 |
| SLC12A2 | 5.39 | SUCLA2 | 3.48 | MITF | 2.68 |
| SS18 | 5.37 | CXorf23 | 3.47 | MYO1E | 2.66 |
| APPBP2 | 5.35 | ZNF638 | 3.42 | VKORC1L1 | 2.64 |
| AEBP2 | 5.33 | ICK | 3.41 | PABPN1 | 2.64 |
| MEIS2 | 5.25 | HSF2 | 3.41 | ZDHHC17 | 2.64 |
| DLG5 | 5.24 | AHCY | 3.40 | PTHLH | 2.62 |
| APP | 5.21 | USP47 | 3.39 | CCNK | 2.57 |
| MBNL1 | 5.12 | EZH2 | 3.38 | ITSN2 | 2.53 |
| HDGFRP3 | 5.09 | ARRDC3 | 3.37 | CPEB1 | 2.52 |
| CCNT2 | 5.06 | SEMA6D | 3.37 | PPP2R2A | 2.51 |
| ATP1B1 | 5.00 | PHF3 | 3.35 | NID2 | 2.51 |
| PURA | 4.90 | STRN3 | 3.32 | EMP1 | 2.47 |
| KIF2A | 4.58 | SENP7 | 3.31 | DUSP1 | 2.46 |
| PDE4A | 4.58 | HAT1 | 3.29 | BTBD3 | 2.43 |
| FLRT3 | 4.47 | CPEB2 | 3.27 | MBNL2 | 2.43 |
| UBE2D2 | 4.42 | ACSL4 | 3.23 | CDH5 | 2.43 |
| BRPF1 | 4.42 | VLDLR | 3.17 | | |
| ATP5G2 | 4.40 | ESRRG | 3.16 | | |
TUG1-miR-144-3p-EZH2 axis is critical for
osteosarcoma cell migration
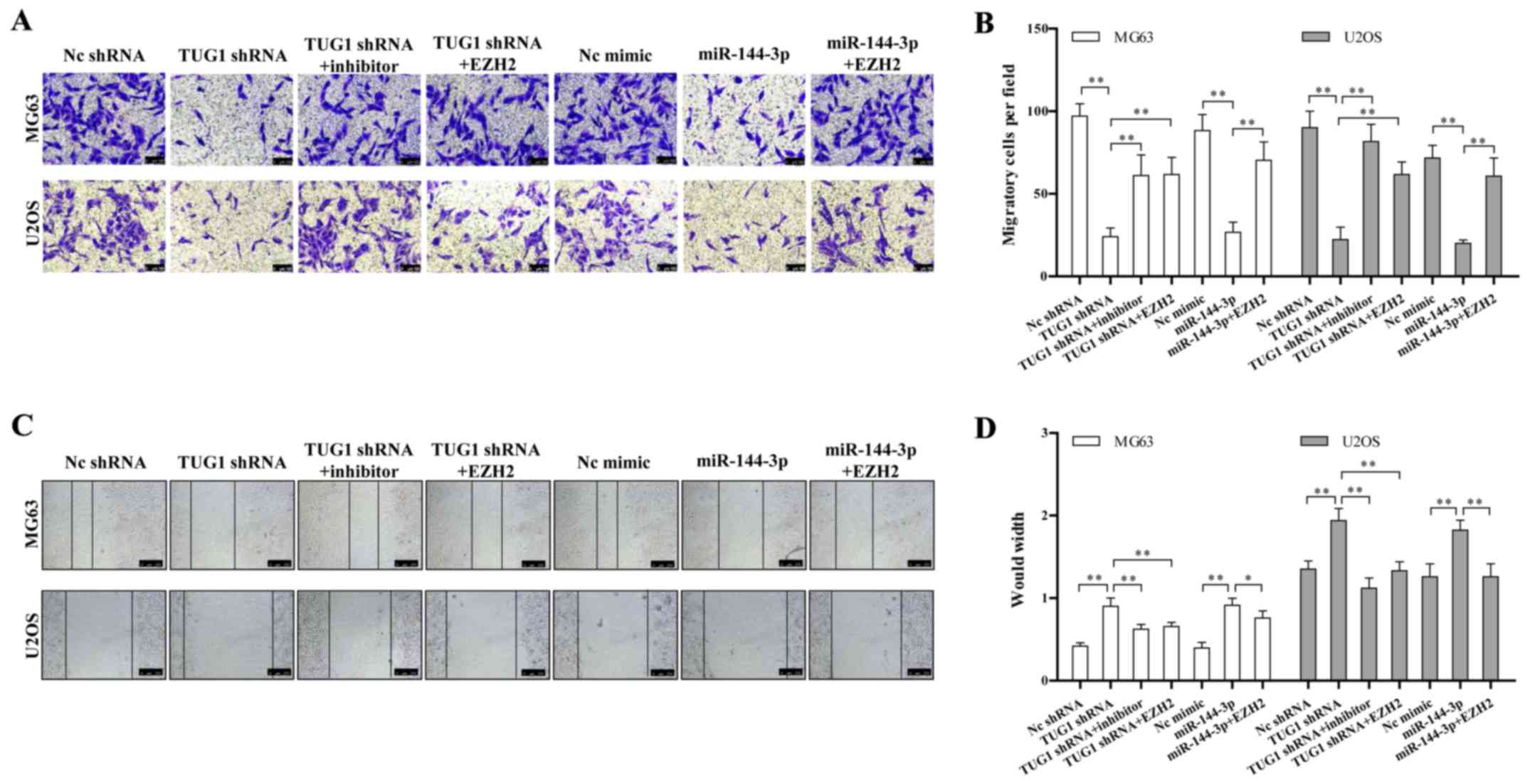
We determined whether the TUG1-miR-144-3p-EZH2 axis
is involved in regulating osteosarcoma migration. We found TUG1
knockdown or miR-144-3p overexpression significantly repressed the
migration of osteosarcoma cell line determined by both transwell
and wound healing assay (Fig. 4A and
C). The quantitative results are also shown in Fig. 4B and D. This migration inhibition
could be rescued by miR-144-3p inhibitor or EZH2 overexpression
(Fig. 4A–D). These data together
provided a critical role of the TUG1-miR-144-3p-EZH2 axis in
regulating osteosarcoma cell migration.
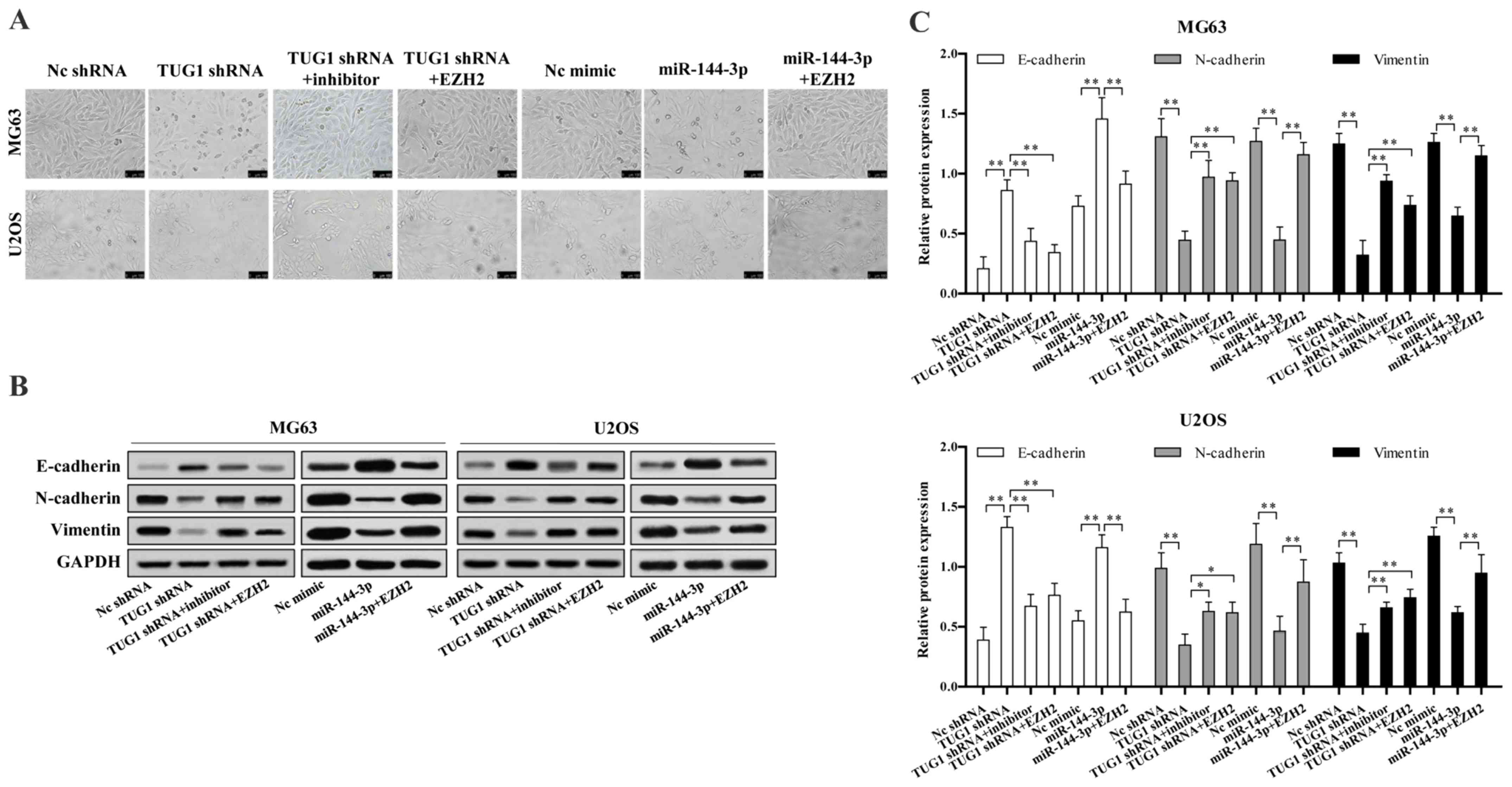
TUG1-miR-144-3p-EZH2 axis is critical for
osteosarcoma cell epithelial-mesenchymal transition (EMT)
We next investigated whether TUG1-miR-144-3p-EZH2
axis is involved in osteosarcoma EMT. We found TUG1 knockdown or
miR-144-3p overexpression significantly reduced osteosarcoma cell
EMT, which could be reversed upon EZH2 overexpression or
transfection of miR-144-3p inhibitor (Fig. 5A). TUG1 knockdown or miR-144-3p
overexpression largely up regulated the protein level of epithelial
marker E-cadherin, while decreased mesenchymal markers N-cadherin
and vimentin expression, which were reversed upon EZH2
overexpression or miR-144-3p inhibition (Fig. 5B and C).
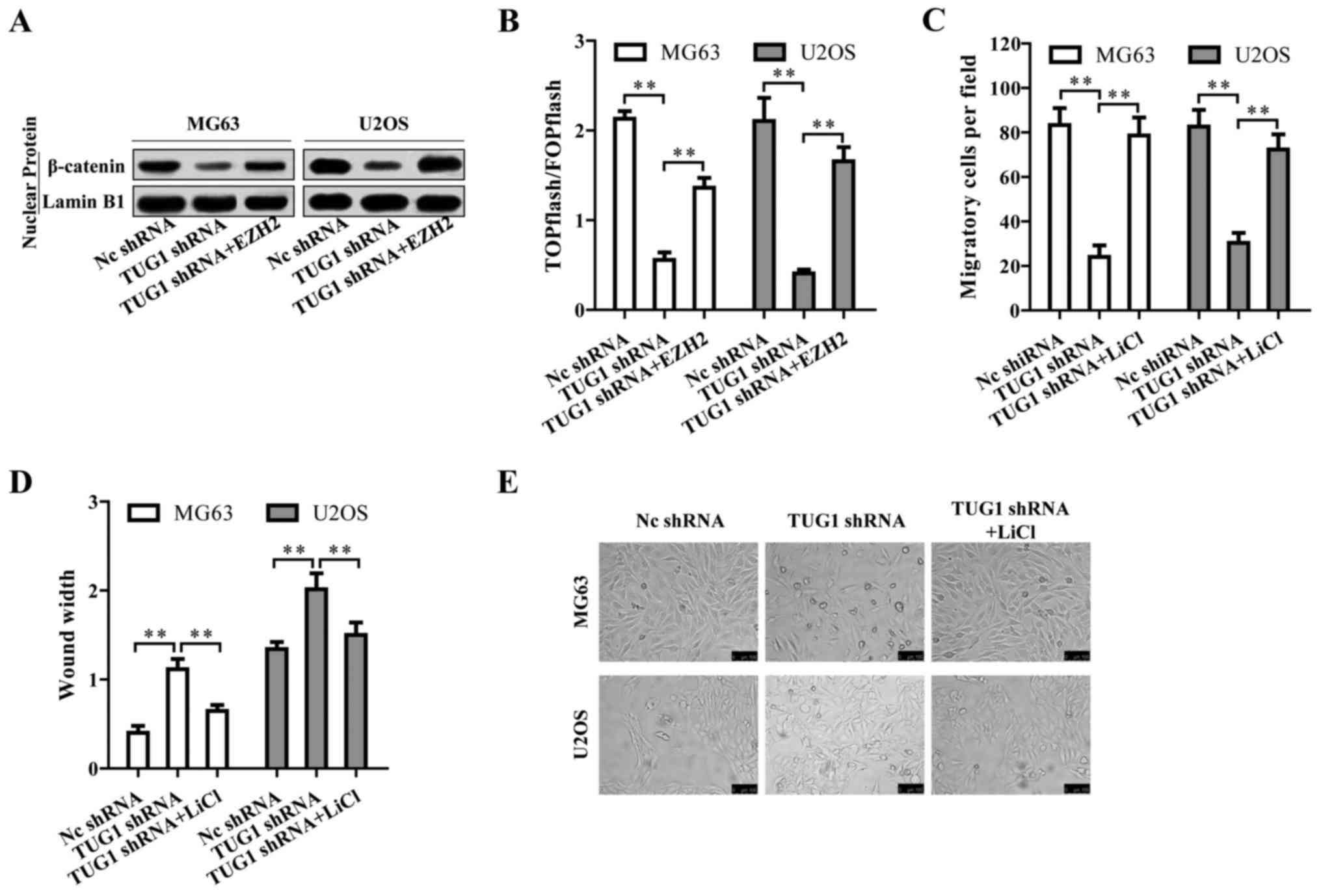
Wnt/β-catenin pathway is regulated by
TUG1 through EZH2 and contributes to osteosarcoma
tumorigenesis
We then investigated by which mechanism TUG1
regulates osteosarcoma progression. Surprisingly, TUG1 knockdown
inactivated Wnt/β-catenin pathway indicated by the reduced nuclear
β-catenin protein level (Fig. 6A).
The TUG1-mediated inactivation of Wnt/β-catenin pathway could be
reversed by EZH2 overexpression (Fig.
6A), suggesting that TUG1 promoted Wnt/β-catenin pathway
activation through EZH2. This TUG1-EZH2 mediated Wnt/β-catenin
pathway activation was also confirmed by TOPFlash luciferase
reporter assay (Fig. 6B). We then
treated the osteosarcoma cells with Wnt/β-catenin activator LiCl
upon TUG1 knockdown. LiCl reversed the repressive cell migration
and EMT caused by TUG1 knockdown (Fig.
6C–E). Based on the above results, TUG1 promotes osteosarcoma
progression by Wnt/β-catenin pathway activation via upregulating
EZH2.
Discussion
lncRNAs are a diverse set of RNA transcripts more
than 200 nucleotides, but contain no substantial open reading
frame, possessing no potential protein-coding capacity (38). lncRNAs are essential regulators at
epigenetic, transcriptional, and post-transcriptional levels.
Increasing studies have revealed that lncRNAs are expressed in a
tissue-specific manner and play fundamental roles in the
pathological processes related to tumorigenesis, angiogenesis,
invasion, and metastasis (39).
These properties make them valid candidates as diagnostic or
prognostic cancer biomarkers. Osteosarcoma is the most common
primary malignant bone tumor in adolescents (40). The high degree of malignancy makes
the 5-year survival rate to decrease to approximately 20% for
patients with metastasis (41,42).
The majority of patients eventually died from complications related
to pulmonary metastases. Previous studies have demonstrated that
lncRNAs are key regulators in the initiation, development, and
progression of osteosarcoma (43–45).
However, the molecular mechanisms are still poorly understood.
Moreover, powerful biomarkers to diagnose and predict osteosarcoma
are strongly needed. The data presented in our study give a better
understanding of how lncRNA-TUG1 regulates osteosarcoma metastasis
and provide some potential biomarkers.
In this study, we revealed that lncRNA-TUG1 was up
regulated in both osteosarcoma tissues and cell lines. The higher
lncRNA-TUG1 expression was significantly correlated with metastasis
and clinical stage. However, due to the difficulty of sample
collection, the sample size of this study is relatively small,
which is a limitation of our study. Fortunately, some other large
sample research also verified that TUG1 expression was increased in
osteosarcoma tissues and correlates with poor prognosis and disease
status in osteosarcoma (21,23–26).
Taken together, TUG1 may be a potential biomarker for osteosarcoma
diagnosis, prognosis and stage classification.
Several studies have shown that lncRNA can influence
post-transcriptional regulation by interfering with microRNAs
through molecular sponge effect so as to silence miRNA expression
and biological functions (46).
These lncRNAs contain miRNA responsive elements (MRE), function as
miRNA sponges to downregulated miRNAs, thus reducing the
miRNA-induced repression of their target mRNAs (47). TUG1 has been shown to contribute to
human osteosarcoma tumorigenesis by sponging miR-9-5p and
regulating POU2F1 expression (10). It also functions as a miR-26a
sponge in human glioma cells (48). TUG1 also targets miR-144/145 in
bladder cancer and gastric cancer (48,49).
To further clarify the underlying mechanism, we found that
lncRNA-TUG1 could directly bind to the miR-144-3p and downregulate
miR-144-3p expression in osteosarcoma cells. miR-144 was previously
shown to be a tumor suppressor in osteosarcoma cells. Our results
confirmed miR-144-3p was downregulated in osteosarcoma tissues.
Moreover, the expression of miR-144-3p and TUG1 was negatively
correlated. Specific shRNA-mediated TUG1 knockdown resulted in the
upregulation of miR-144-3p.
By systemic analysis, we found miR-144-3p functioned
by directly targeting EZH2, which was upregulated in osteosarcoma
cells and showed a positive correlation with TUG1 expression.
miR-144-3p mimics increased EZH2 expression at both mRNA and
protein level. Furthermore, EZH2 overexpression enhanced migration
and EMT of osteosarcoma even upon TUG1 knockdown, which confirmed
TUG1 promoted tumor progression through EZH2. Consistent with our
results, EZH2 was shown to promote osteosarcoma cell metastasis in
several studies (37,38,50).
EZH2 is the catalytic subunits of polycomb
repressive complex 2 (PRC2), acting as an H3K27me3
methyltransferase, leading to epigenetic silencing of target genes,
mainly tumor suppressor genes (51,52).
EZH2 has been proven to promote cell metastasis and malignancy
(53–56). However, the downstream events
involving EZH2 and osteosarcoma progression remains unclear. As a
methyltransferase, EZH2 mainly functions in the nucleus, however,
recent research revealed that EZH2 has been detected in the
cytoplasm and implicated in the migration of cancer cells (54,57,58).
In our study, we found EZH2 enhanced osteosarcoma migration by
activation of Wnt/β-catenin pathway. It seems that EZH2 has other
functions rather than as a methyltransferase functions in the
nucleus. We proposed it would be of great interest to further check
whether EZH2 affects tumor suppressor gene or EMT gene expression
through the change of epigenetic modification in osteosarcoma
cells.
In summary, we demonstrated that TUG1 and EZH2 were
increased in osteosarcoma while miR-144-3p was down regulated. Our
study further identified TUG1-miR-144-3p-EZH2 axis is critical for
osteosarcoma cell migration and EMT by activating Wnt/β-catenin
pathway. Hence, we elucidated the underlying mechanism and
confirmed the role of TUG1 in osteosarcoma progression. Further
studies need to be carried out to verify whether there are more
TUG1 target genes and to determine how EZH2 affects osteosarcoma.
Our study indicates TUG1 and EZH2 may be potential biomarkers for
osteosarcoma diagnosis that need to be further developed.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
International osteosarcoma incidence patterns in children and
adolescents, middle ages and elderly persons. Int J Cancer.
125:229–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valery PC, Laversanne M and Bray F: Bone
cancer incidence by morphological subtype: A global assessment.
Cancer Causes Control. 26:1127–1139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qureshi A, Ahmad Z, Azam M and Idrees R:
Epidemiological data for common bone sarcomas. Asian Pac J Cancer
Prev. 11:393–395. 2010.PubMed/NCBI
|
|
4
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar
|
|
6
|
Ferrari S and Serra M: An update on
chemotherapy for osteosarcoma. Expert Opin Pharmacother.
16:2727–2736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akiyama T, Dass CR and Choong PF: Novel
therapeutic strategy for osteosarcoma targeting osteoclast
differentiation, bone-resorbing activity, and apoptosis pathway.
Mol Cancer Ther. 7:3461–3469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X, Fan S and Song E: Noncoding RNAs:
New players in cancers. Adv Exp Med Biol. 927:1–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie CH, Cao YM, Huang Y, Shi QW, Guo JH,
Fan ZW, Li JG, Chen BW and Wu BY: Long non-coding RNA TUG1
contributes to tumorigenesis of human osteosarcoma by sponging
miR-9-5p and regulating POU2F 1 expression. Tumour Biol.
37:15031–15041. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khorkova O, Hsiao J and Wahlestedt C:
Basic biology and therapeutic implications of lncRNA. Adv Drug
Deliv Rev. 87:15–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prensner JR, Iyer MK, Sahu A, Asangani IA,
Cao Q, Patel L, Vergara IA, Davicioni E, Erho N, Ghadessi M, et al:
The long noncoding RNA SChLAP1 promotes aggressive prostate cancer
and antagonizes the SWI/SNF complex. Nat Genet. 45:1392–1398. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wilusz JE: Long noncoding RNAs: Re-writing
dogmas of RNA processing and stability. Biochim Biophys Acta.
1859:128–138. 2016. View Article : Google Scholar
|
|
16
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kapranov P, Cheng J, Dike S, Nix DA,
Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermüller J,
Hofacker IL, et al: RNA maps reveal new RNA classes and a possible
function for pervasive transcription. Science. 316:1484–1488. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Young TL, Matsuda T and Cepko CL: The
noncoding RNA taurine upregulated gene 1 is required for
differentiation of the murine retina. Curr Biol. 15:501–512. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han Y, Liu Y, Gui Y and Cai Z: Long
intergenic non-coding RNA TUG1 is overexpressed in urothelial
carcinoma of the bladder. J Surg Oncol. 107:555–559. 2013.
View Article : Google Scholar
|
|
20
|
Xu Y, Wang J, Qiu M and Xu L, Li M, Jiang
F, Yin R and Xu L: Upregulation of the long noncoding RNA TUG1
promotes proliferation and migration of esophageal squamous cell
carcinoma. Tumour Biol. 36:1643–1651. 2015. View Article : Google Scholar
|
|
21
|
Zhang Q, Geng PL, Yin P, Wang XL, Jia JP
and Yao J: Downregulation of long non-coding RNA TUG1 inhibits
osteosarcoma cell proliferation and promotes apoptosis. Asian Pac J
Cancer Prev. 14:2311–2315. 2013. View Article : Google Scholar
|
|
22
|
Zhao XB and Ren GS: LncRNA
taurine-upregulated gene 1 promotes cell proliferation by
inhibiting microRNA-9 in MCF-7 cells. J Breast Cancer. 19:349–357.
2016. View Article : Google Scholar
|
|
23
|
Wang Y, Yang T, Zhang Z, Lu M, Zhao W,
Zeng X and Zhang W: Long non-coding RNA TUG1 promotes migration and
invasion by acting as a ceRNA of miR-335-5p in osteosarcoma cells.
Cancer Sci. 108:859–867. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Yu Y, Fan S and Luo L: Knockdown
of long noncoding RNA TUG1 inhibits the proliferation and cellular
invasion of osteosarcoma cells by sponging miR-153. Oncol Res.
Apr;12:2017Epub ahead of print.
|
|
25
|
Wang Q and Chen Q: Role of taurine
upregulated gene 1 as a predictor of poor outcome in osteosarcoma.
J Cancer Res Ther. (In press). http://www.cancerjournal.net/preprintarticle.asp?id=172585;type=0.
|
|
26
|
Ma B, Li M, Zhang L, Huang M, Lei JB, Fu
GH, Liu CX, Lai QW, Chen QQ and Wang YL: Upregulation of long
non-coding RNA TUG1 correlates with poor prognosis and disease
status in osteosarcoma. Tumour Biol. 37:4445–4455. 2016. View Article : Google Scholar
|
|
27
|
Feng YB, Liu XP, Li XL, Cao GL, Zhang P
and Tian FM: LncRNA TUG1 is upregulated and promotes cell
proliferation in osteosarcoma. Open Med (Wars). 11:163–167.
2016.
|
|
28
|
Namløs HM, Meza-Zepeda LA, Barøy T,
Østensen IH, Kresse SH, Kuijjer ML, Serra M, Bürger H,
Cleton-Jansen AM and Myklebost O: Modulation of the osteosarcoma
expression phenotype by microRNAs. PLoS One. 7:e480862012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lei H, Gao Y and Xu X: LncRNA TUG1
influences papillary thyroid cancer cell proliferation, migration
and EMT formation through targeting miR-145. Acta Biochim Biophys
Sin (Shanghai). 22:1–10. 2017.
|
|
30
|
Liu L, Chen X, Zhang Y, Hu Y, Shen X and
Zhu W: Long non-coding RNA TUG1 promotes endometrial cancer
development via inhibiting miR-299 and miR-34a-5p. Oncotarget.
8:31386–31394. 2017.PubMed/NCBI
|
|
31
|
Li J, An G, Zhang M and Ma Q: Long
non-coding RNA TUG1 acts as a miR-26a sponge in human glioma cells.
Biochem Biophys Res Commun. 477:743–748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao M, Huang J, Gui K, Xiong M, Cai G, Xu
J, Wang K, Liu D, Zhang X and Yin W: The downregulation of miR-144
is associated with the growth and invasion of osteosarcoma cells
through the regulation of TAGLN expression. Int J Mol Med.
34:1565–1572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang W, Zhou X and Wei M: MicroRNA-144
suppresses osteosarcoma growth and metastasis by targeting ROCK1
and ROCK2. Oncotarget. 6:10297–10308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang K, Zhang Y, Ren K, Zhao G, Yan K and
Ma B: MicroRNA-101 inhibits the metastasis of osteosarcoma cells by
downregulation of EZH2 expression. Oncol Rep. 32:2143–2149. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu Z, Tang J, Wang J, Duan G, Zhou L and
Zhou X: MiR-138 acts as a tumor suppressor by targeting EZH2 and
enhances cisplatin-induced apoptosis in osteosarcoma cells. PLoS
One. 11:e01500262016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun R, Shen J, Gao Y, Zhou Y, Yu Z,
Hornicek F, Kan Q and Duan Z: Overexpression of EZH2 is associated
with the poor prognosis in osteosarcoma and function analysis
indicates a therapeutic potential. Oncotarget. 7:38333–38346. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lv Y-F, Yan G-N, Meng G, Zhang X and Guo
Q-N: Enhancer of zeste homolog 2 silencing inhibits tumor growth
and lung metastasis in osteosarcoma. Sci Rep. 5:129992015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma L, Bajic VB and Zhang Z: On the
classification of long non-coding RNAs. RNA Biol. 10:925–933. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar
|
|
40
|
Nagarajan R, Kamruzzaman A, Ness KK,
Marchese VG, Sklar C, Mertens A, Yasui Y, Robison LL and Marina N:
Twenty years of follow-up of survivors of childhood osteosarcoma: A
report from the Childhood Cancer Survivor Study. Cancer.
117:625–634. 2011. View Article : Google Scholar
|
|
41
|
Bielack S, Carrle D and Casali PG; ESMO
Guidelines Working Group: Osteosarcoma: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20(Suppl 4): 137–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Eccles SA and Welch DR: Metastasis: Recent
discoveries and novel treatment strategies. Lancet. 369:1742–1757.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chan LH, Wang W, Yeung W, Deng Y, Yuan P
and Mak KK: Hedgehog signaling induces osteosarcoma development
through Yap1 and H19 overexpression. Oncogene. 33:4857–4866. 2014.
View Article : Google Scholar
|
|
44
|
Dong Y, Liang G, Yuan B, Yang C, Gao R and
Zhou X: MALAT1 promotes the proliferation and metastasis of
osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol.
36:1477–1486. 2015. View Article : Google Scholar
|
|
45
|
Wang Y, Yao J, Meng H, Yu Z, Wang Z, Yuan
X, Chen H and Wang A: A novel long non-coding RNA,
hypoxia-inducible factor-2α promoter upstream transcript, functions
as an inhibitor of osteosarcoma stem cells in vitro. Mol Med Rep.
11:2534–2540. 2015. View Article : Google Scholar
|
|
46
|
Lv J, Fan HX, Zhao XP, Lv P, Fan JY, Zhang
Y, Liu M and Tang H: Long non-coding RNA Unigene56159 promotes
epithelial-mesenchymal transition by acting as a ceRNA of
miR-140-5p in hepatocellular carcinoma cells. Cancer Lett.
382:166–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ji TT, Huang X, Jin J, Pan SH and Zhuge
XJ: Inhibition of long non-coding RNA TUG1 on gastric cancer cell
transference and invasion through regulating and controlling the
expression of miR-144/c-Met axis. Asian Pac J Trop Med. 9:508–512.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tan J, Qiu K, Li M and Liang Y:
Double-negative feedback loop between long non-coding RNA TUG1 and
miR-145 promotes epithelial to mesenchymal transition and
radioresistance in human bladder cancer cells. FEBS Lett.
589:3175–3181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chochi Y, Kawauchi S, Nakao M, Furuya T,
Hashimoto K, Oga A, Oka M and Sasaki K: A copy number gain of the
6p arm is linked with advanced hepatocellular carcinoma: An
array-based comparative genomic hybridization study. J Pathol.
217:677–684. 2009. View Article : Google Scholar
|
|
51
|
Cao R and Zhang Y: The functions of
E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr
Opin Genet Dev. 14:155–164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cha TL, Zhou BP, Xia W, Wu Y, Yang CC,
Chen CT, Ping B, Otte AP and Hung MC: Akt-mediated phosphorylation
of EZH2 suppresses methylation of lysine 27 in histone H3. Science.
310:306–310. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Croonquist PA and Van Ness B: The polycomb
group protein enhancer of zeste homolog 2 (EZH 2) is an oncogene
that influences myeloma cell growth and the mutant ras phenotype.
Oncogene. 24:6269–6280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kleer CG, Cao Q, Varambally S, Shen R, Ota
I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, et al: EZH2
is a marker of aggressive breast cancer and promotes neoplastic
transformation of breast epithelial cells. Proc Natl Acad Sci USA.
100:11606–11611. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Richter GH, Plehm S, Fasan A, Rössler S,
Unland R, Bennani-Baiti IM, Hotfilder M, Löwel D, von Luettichau I,
Mossbrugger I, et al: EZH2 is a mediator of EWS/FLI1 driven tumor
growth and metastasis blocking endothelial and neuroectodermal
differentiation. Proc Natl Acad Sci USA. 106:5324–5329. 2009.
View Article : Google Scholar
|
|
56
|
Varambally S, Dhanasekaran SM, Zhou M,
Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt
RG, Otte AP, et al: The polycomb group protein EZH2 is involved in
progression of prostate cancer. Nature. 419:624–629. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nolz JC, Gomez TS and Billadeau DD: The
Ezh2 methyltransferase complex: Actin up in the cytosol. Trends
Cell Biol. 15:514–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH,
Chen SP, Bian XW, Guan XY, Lin MC, Zeng YX, et al: The putative
tumour suppressor microRNA-124 modulates hepatocellular carcinoma
cell aggressiveness by repressing ROCK2 and EZH2. Gut. 61:278–289.
2012. View Article : Google Scholar
|