Introduction
Hepatocellular carcinoma (HCC) is the third most
common cancer worldwide, and over 50% of patients emerge from Asia
(1). Although significant progress
has been made in the diagnosis and treatment of HCC, the overall
prognosis is still poor, and high metastasis and recurrence rates
are the main factors affecting the prognosis of HCC patients
(2,3). Therefore, it is important to
investigate and clarify the molecular mechanisms underlying HCC
metastasis and recurrence in order to find novel therapeutic
targets.
The Wnt signalling pathway is a highly conserved
signalling pathway that controls cell growth, differentiation,
apoptosis and self-renewal, and it is implicated in maintenance of
tumour progenitor cells, drug resistance, tumour progression,
invasion and metastasis (4,5).
Canonical Wnt signalling is mediated by ligand binding to Frizzled
and the low-density lipoprotein receptor-related protein-5/6
(LRP5/6) receptors, resulting in the accumulation of β-catenin in
the cytoplasm and β-catenin transfer to the nucleus, where it
interacts with T cell factor (TCF)/lymphatic enhancement factor
(LEF) to regulate the expression of various target genes (6,7).
Wnt3a is a ligand in the Wnt signalling pathway. It has been
reported that Wnt3a is activated and participates in the metastasis
process in many cancers, including non-small cell lung cancer,
colorectal cancer, gastric cancer and breast cancer (8–11).
Recently, some researchers have suggested that targeting the
Wnt/β-catenin signalling pathway can regulate HCC cell
proliferation, invasion and metastasis (12–14).
However, these studies primarily focused on β-catenin, and whether
Wnt3a is activated and involved in the occurrence and metastasis of
HCC has rarely been reported.
Given the effects of Wnt3a on progression,
recurrence and metastasis of other tumours, it is important to
analyse the function of Wnt3a in HCC. In the present study, we
assessed the critical role of Wnt3a in HCC. We also investigated
the effects of Wnt3a siRNA on cell cycle distribution and the
metastatic ability of HCC cells in vitroOur findings suggest
novel therapeutic strategies for HCC treatment.
Materials and methods
Clinical specimens
In total, 32 primary HCC patients, diagnosed by an
oncologist and a pathologist, were sampled from a prospectively
designed database. In accordance with the protocol approved by the
Affiliated Hospital of Guangdong Medical University (Ethics
Committee protocol no. PJ2013105), all the participants signed an
informed consent. We ensured that the patients understood the
information regarding the application of specimens. Cancerous
tissues and adjacent tissues from 32 patients were obtained from
the Hepatobiliary Surgery Center and were frozen in liquid nitrogen
before use. None of the patients received radiotherapy or
chemotherapy before sample collection.
Cell culture
Several hepatoma cell lines (SK-HEP-1, QGY7701,
HeP3B, QGY7703, HepG2, MHCC97H, MHCC97L, and Smmc7721) and one
immortalized liver cell line (HL7702) were used in this study. All
cells were purchased from the Cell Bank of Shanghai (China). The
HepG2, HL7702 and Smmc7721 cells were cultured in RPMI-1640 basic
medium supplemented with 10% fetal bovine serum (FBS, cat. no.
10100-147; Gibco, Australia). The other cells were cultured in DMEM
supplemented with 10% FBS.
siRNA transfection
MHcc97H and SK-Hep-1 cells were plated into 6-well
plates and transfection procedures were performed according to the
manufacturer's protocol. To confirm the validity of the experiment,
we screened the efficacy of Wnt3a knockdown and selected the best
performing siRNA transcript. Before each experiment, we transiently
transfected MHcc97H and SK-Hep-1 cells using an siRNA-Wnt3a gene
knockdown kit (Jima Biotechnology Co., Shanghai, China) and the
negative control siRNA (NC-si).
Cell inhibition and cytotoxicity assay
(MTT assay)
MHcc97H and SK-Hep-1 cells (1×104
cells/well) were seeded into 96-well plates. Following 24 h of
adherent culture at 37°C, the cells were treated with siRNA to
inhibit Wnt3a expression. Phosphate-buffered saline treatment
served as a blank control, and NC-si treatment acted as a negative
control. Then, 20 µl of MTT stock solution at a concentration of 5
mg/ml was transferred to each well to obtain a final volume. Next,
the cell supernatants were gently removed, and 200 µl of DMSO was
added to each well to solubilize the formed crystals. The
absorbance was detected at 570 nm with a spectrophotometer
(Perkin-Elmer, USA).
Flow cytometry analysis
The cells treated with siRNA were placed into 6-well
plates (1×105 cells per well). Following overnight
growth, cells were carefully collected, pelleted, washed with PBS,
and suspended in binding buffer. Then, 100 ml of cells was
incubated with 5 ml of Annexin V-FITC and 5 ml of PI for 15 min in
the dark. The fixed cells under each experimental condition were
examined, and DNA content was analysed using FlowJo software. Then,
cell cycle analysis was performed with a flow cytometer (BD
FACSCalibur). All measurements were performed as described under
the same instrumental settings following the manufacturer's
protocol.
Colony formation assay
Briefly, MHcc97H and SK-Hep-1 cells were initially
plated in 6-well culture dishes (BD) and cultured in DMEM
containing 10% FBS for seven days, and the medium was refreshed
every two days. After the incubation, we removed the medium and
washed the cells twice with PBS. Then, the cells were stained with
1% crystal violet for 15 min before being counted. The results were
photographed with a camera and analysed with ImageJ software. All
the studies were repeated three times.
Wound healing assay
The cells were cultured in a 24-well plate in DMEM
containing 10% FBS for 24 h until they grew to 100% confluence. A
straight scratch was created in the adherent cells with a pipette
tip after three washes with phosphate-buffered saline to remove
cellular debris. Then, the cells were cultured in DMEM containing
1% FBS. The wounds were observed under a microscope, and images
were captured at 0, 12 and 24 h. The data were analysed with ImageJ
software, and the experiments were conducted in triplicate
independently.
Transwell assay
MHcc97H and SK-Hep-1 cells were seeded in Transwell
chambers at a concentration of 1×105 cells/chamber in
the absence or presence of Wnt3a-siRNA treatment. Transwell inserts
(chambers) were placed in a 24-well plate, cells were diluted with
serum-free DMEM, and growth medium (DMEM with 10% FBS) was added to
the lower chambers. After incubation for 24 h at 37°C, the cells
that passed through the membrane to the bottom wells were fixed in
75% ethanol and stained with 0.1% crystal violet (Amresco, Solon,
OH, USA). The cells were observed under a microscope and imaged,
and the experiments were performed three times independently. Cell
invasion ability was also assessed. The cell invasion assay was
similar to the cell motility assay, with the exception that the
inserts were pretreated with Matrigel (1:10 diluted in DMEM).
qRT-PCR analysis
Total RNA was extracted from frozen tissues using
TRIzol reagent (Invitrogen, Guangzhou, China) according to the
manufacturer's protocol. Reverse transcription was performed with 1
µg of RNA using a PrimeScript RT reagent kit and a gDNA Eraser kit
(Takara, Dalian, China). We employed (10×(Log2
∆CT))−1 calculations to determine relative
mRNA expression. Data were evaluated using the comparative count
method and normalized to the corresponding 18S ribosomal RNA
value.
The primer sequences used were as follows: Wnt3a,
forward, 5′-AATTTGGAGGAATGGTCTCTCGG-3′ and reverse,
5′-CAGCAGGTCTTCACTTCACAG-3′; Wnt5a, forward,
5′-TCCGGACTACTGTGTGC-3′ and reverse, 5′-AGC AGCACCAGTGAAAC-3′;
Frizzled, forward, 5′-CGTACTGA GTGGAGTGTGTTTTG-3′ and reverse,
5′-TGAGCTTTTCCA GTTTCTCTGTC-3′; c-Myc, forward, 5′-CGTCCTGGGAAG
GGAGAT-3′ and reverse, 5′-CGCTGCTATGGGCAAAGT-3′; MMP-7, forward,
5′-CACCACACTATTTTGAGGTCTTCC GCAG-3′ and reverse,
5′-CATCCTAGGCTGAGGCTGGTAT GTTTTG-3′; 18S, forward,
5′-CGGCGACGACCCATTCG AAC-3′ and reverse, 5′-GAATCGAACCCTGATTCCCC
GTC-3′.
Western blot analysis
Protein samples were prepared from cancerous and
adjacent tissues collected from eight patients using Fastprep-24
Sample Preparation equipment (MP, USA), and the Wnt3a and Notch3
protein levels were detected. The cells were harvested and lysed in
lysis buffer. Equal amounts of protein samples were loaded per
well. Membranes containing protein blots were incubated in blocking
buffer (5% non-fat milk) for 1 h at room temperature, and then, the
membranes were incubated with primary antibodies (Cell Signaling
Technology) diluted 1:1,000 at 4°C overnight. Following a wash with
TBS-T, the membranes were incubated with goat anti-rabbit secondary
antibodies diluted 1:3,000 for 1 h. Immunocomplexes were detected
with enhanced chemiluminescence reagent.
Statistical analysis
The data were obtained from at least three
independent experiments, and all analyses were performed with
GraphPad version 5.0 software. The results were evaluated using
Student's t-test, and Pearson correlation analyses were used to
examine the correlation between two parameters. The statistical
significance levels were defined as P<0.05, P<0.01 and
P<0.001.
Results
The Wnt3a pathway is activated in HCC
tissues
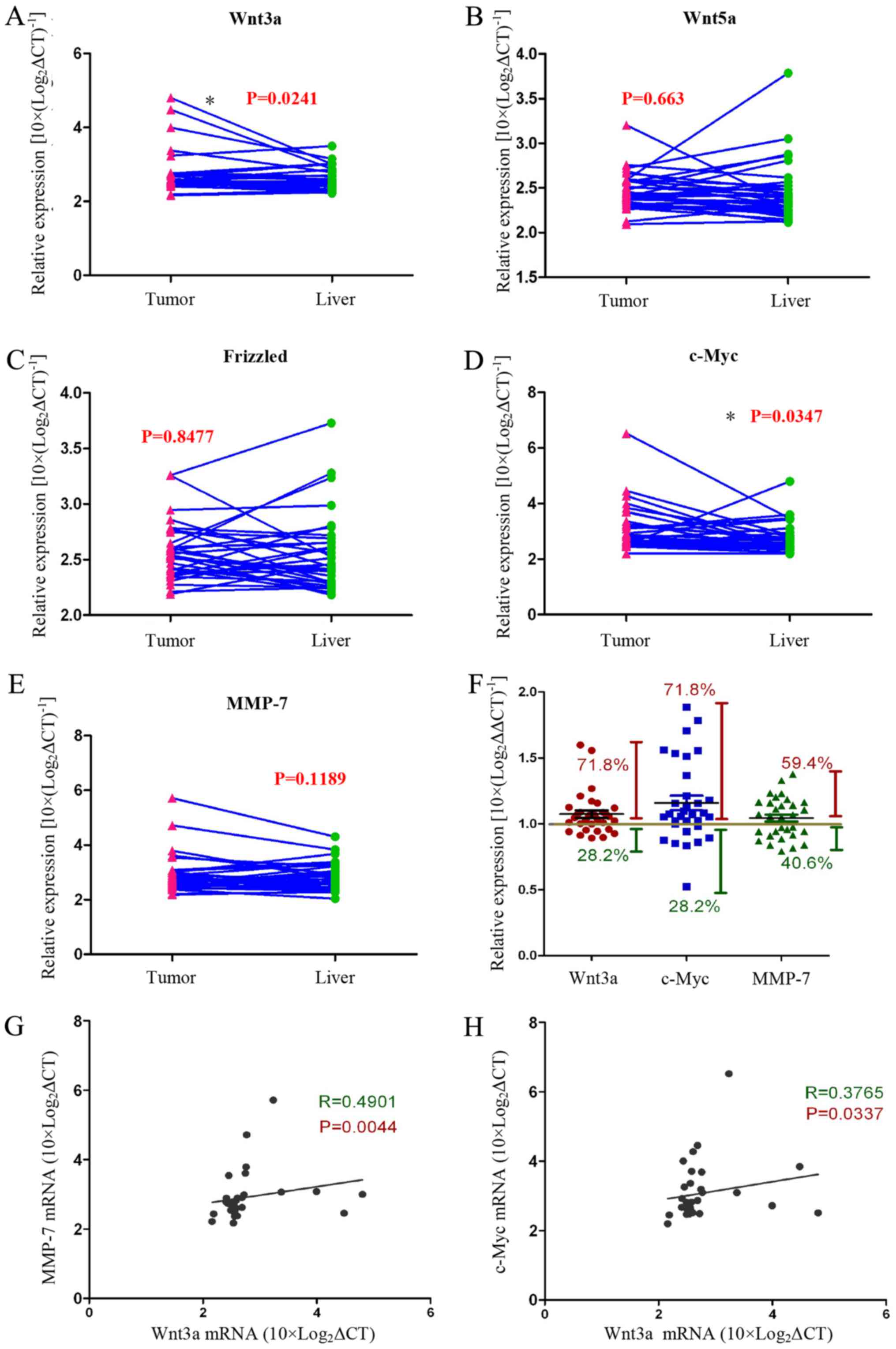
We assayed the expression of Wnt-related genes
[Wnt3a, Wnt5a, Frizzled, c-Myc, and matrix metalloproteinase
(MMP)-7] in HCC tissues and normal liver tissues with qRT-PCR. We
found that the expression of Wnt3a and c-Myc genes was
significantly increased in tumour tissues compared with normal
liver tissues from the same patient (Fig. 1A–E). The data showed that 71.8% of
all cases (n=32) expressed higher Wnt3a and Myc mRNA levels
compared with normal liver tissue. In addition, MMP-7 gene
expression was increased in tumour tissues compared with normal
liver tissues in >59.4% of the cases (n=32) (Fig. 1F). We analysed the relationship
between the Wnt3a and the Wnt pathway target genes c-Myc and MMP-7,
and the data showed that they are positively correlated (Fig. 3G and H). These experimental data
strongly support our view that the Wnt signalling pathway is
activated in tumour tissues of HCC patients.
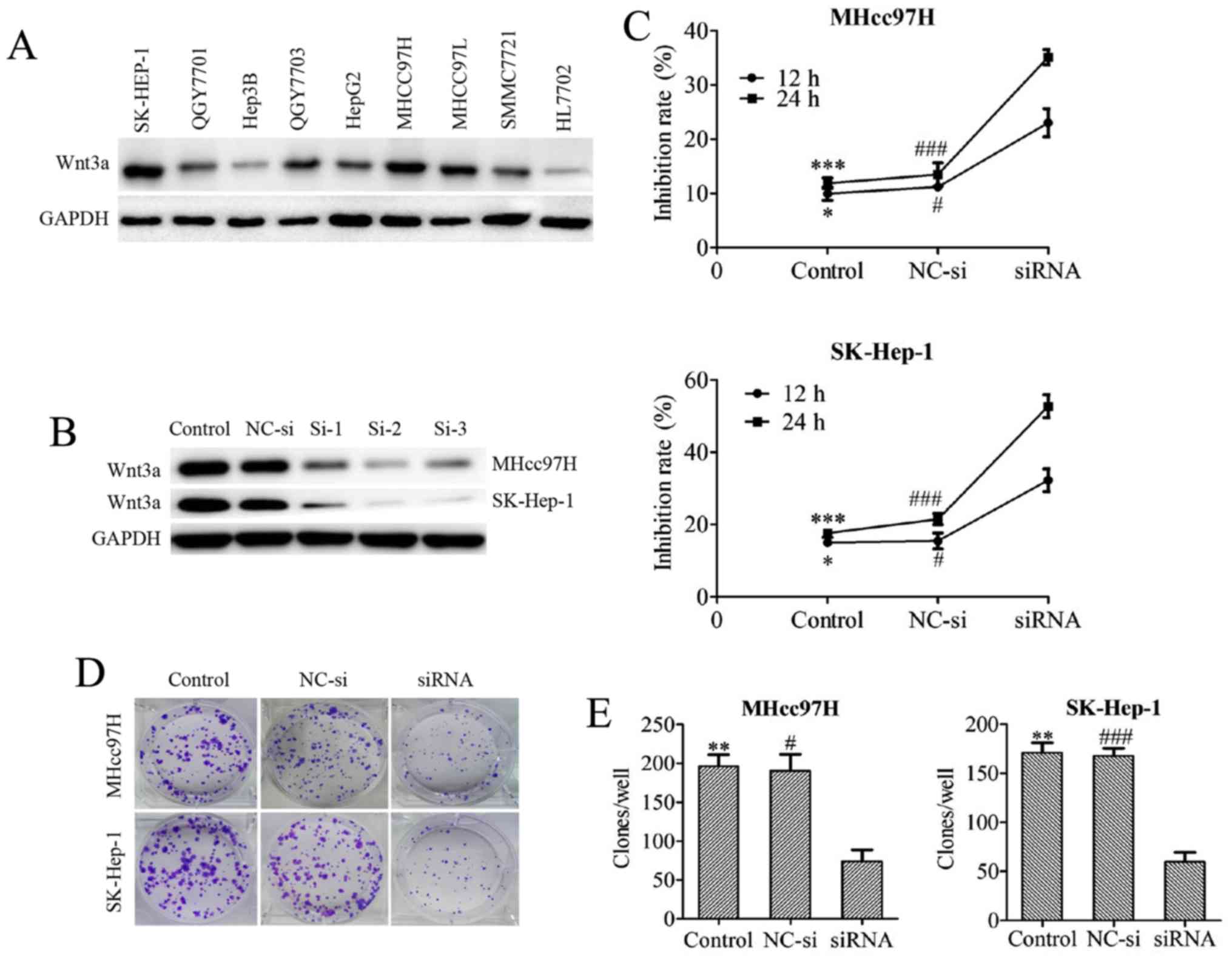 | Figure 3Downregulation of Wnt3a expression
inhibits the viability of MHcc97H and SK-Hep-1 cells. The Wnt3a
expression profiles of several HCC cell lines were screened by
western blotting (A). The knockdown efficiency of 3 different Wnt3a
siRNA sequences was tested in both MHcc97H and SK-Hep-1 cell lines
by western blotting (B). The viability of cells treated with
Wnt3a-siRNA for 12 and 24 h was determined using an MTT assay (C).
The clonogenicity of cells after depletion of Wnt3a via siRNA
interference was measured with a colony formation assay (D and E).
The data are expressed as the means ± SD of 3 independent
experiments. *P<0.05, **P<0.01 and
***P<0.001 between the indicated groups, control vs.
siRNA; #P<0.05, ##P<0.01 and
###P<0.001 between the indicated groups, NC-si vs.
siRNA; NC-si, negative control siRNA; si-1, 2, 3, Wnt3a-siRNA-1, 2,
3. |
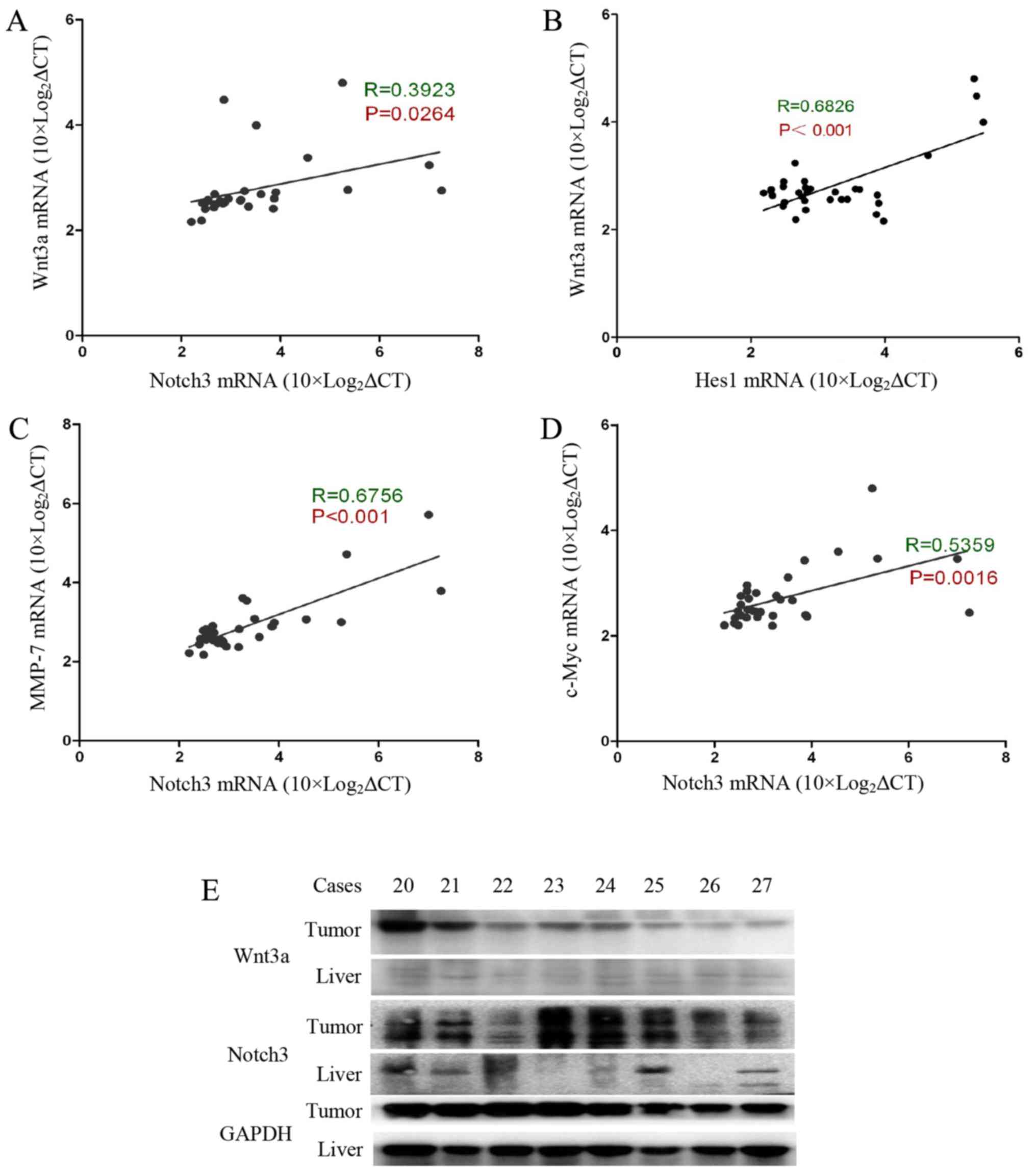
Wnt3a signalling activation is correlated
with activation of the Notch3 signalling pathway in HCC
Our previous experimental results indicated that
Notch3 and Hes1 gene expression levels are significantly increased
in tumour tissue, and there is a positive correlation between
Notch3 and Hes1 gene expression levels (15). Interestingly, Wnt3a was found to be
positively correlated with Notch3 (R=0.39, P=0.026) and Hes1
(R=0.68, P<0.001) (Fig. 2A and
B). We also evaluated the correlation between Notch3 and the
Wnt pathway target genes c-Myc and MMP-7. MMP-7 is involved in
cancer cell invasion and metastasis processes (16,17).
c-Myc is a well-known gene that determines the cell capacity for
self-renewal and proliferation (18). Our data show that Notch3 expression
is significantly correlated with the expression of c-Myc and MMP-7
(Fig. 2C and D). In addition, we
determined the Wnt3a and Notch3 protein contents in the tumour
specimens and the accumulation of Wnt3a and Notch3 in the tumour
tissues (Fig. 2E). Based on these
data, Wnt3a and Notch3 participate in the process of HCC
carcinogenesis. Wnt3a may influence hepatocarcinoma cell invasion,
metastasis and proliferation by targeting MMP-7 and c-Myc.
Downregulation of Wnt3a expression
inhibits MHcc97H and SK-Hep-1 cell viability
We first examined the baseline Wnt3a protein
expression level in several hepatoma cell lines. Wnt3a exhibited
higher expression levels in hepatoma cells compared with the normal
liver cell line HL7702. Among the hepatoma cell lines, we found
that MHcc97H and SK-Hep-1 cells highly express Wnt3a (Fig. 3A). Therefore, we silenced Wnt3a
mRNA expression via siRNA transfection in MHcc97H and SK-Hep-1
cells. The knockdown efficiency of 3 different Wnt3a siRNA
sequences was tested in both MHcc97H and SK-Hep-1 cell lines, and
the results were assessed with western blotting. Sequence 2 was
chosen for use in subsequent experiments because of its excellent
Wnt3a knockdown performance (Fig.
3B). The inhibitory effect of Wnt3a knockdown was measured with
MTT assays after depletion of Wnt3a via siRNA interference in
MHcc97H and SK-Hep-1 cells. Cell growth was potently inhibited in a
time-dependent manner (Fig. 3C). A
colony formation assay was used to evaluate the ability of MHcc97H
and SK-Hep-1 cells to form colonies after Wnt3a was downregulated
by siRNA, and we found that Wnt3a inhibition reduced the
colony-forming capability of the cells (Fig. 3D and E).
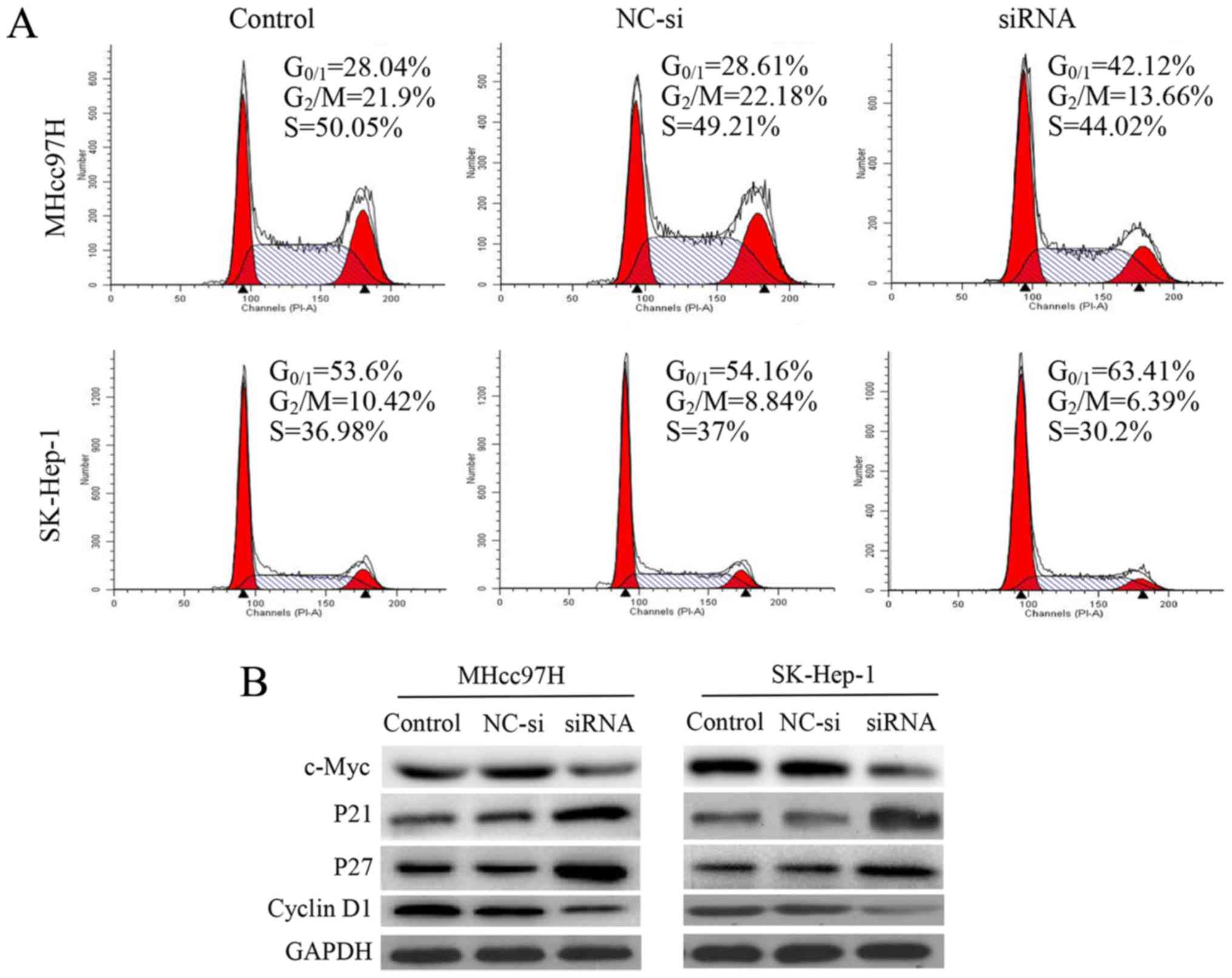
Silencing of Wnt3a expression induces
cell cycle arrest by regulating cell cycle regulatory proteins
To investigate whether the anti-proliferative effect
of decreased Wnt3a expression was triggered by cell cycle arrest,
we examined cell cycle distribution using flow cytometry. We
observed that after 24 h of siRNA interference, cell cycle
progression was arrested in MHcc97H cells at
G0/G1 phase. Compared with the control and
NC-si-transfected cells, the percentage of
G0/G1 cells was increased significantly from
28.04 and 28.61 to 42.12%. There was a similar effect on cell cycle
distribution in SK-Hep-1 cells, and the percentage of
G0/G1 cells increased significantly from 53.6
and 54.14 to 63.41%. Analysis of this mechanism revealed that
downregulation of Wnt3a was associated with downregulation of c-Myc
and cyclin D1 expression and upregulation of p21 and p27 (Fig. 4).
Downregulation of Wnt3a expression
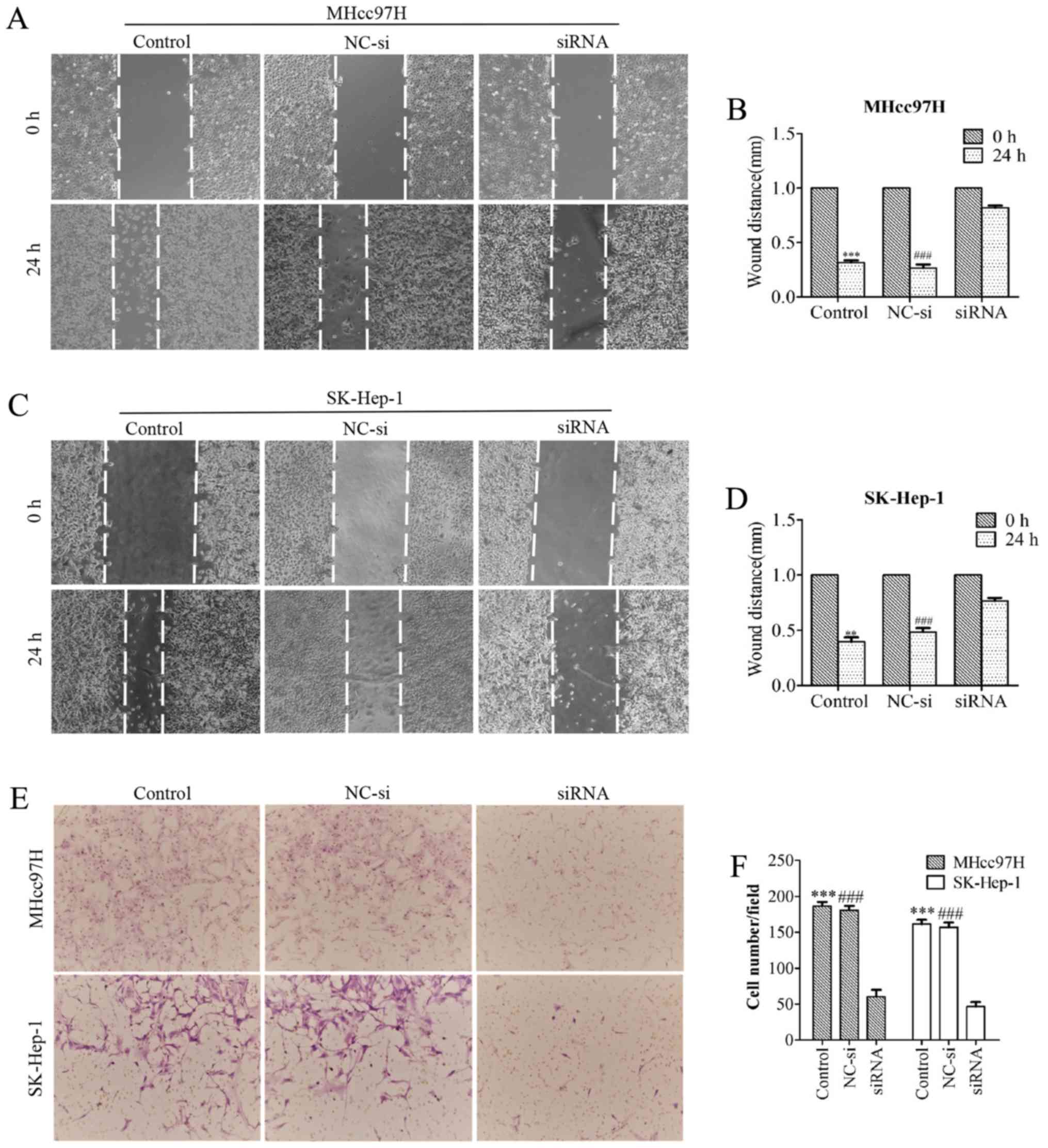
suppresses MHcc97H and SK-Hep-1 cell migration
To evaluate whether downregulation of Wnt3a
expression affects the metastatic ability of MHcc97H and SK-Hep-1
cells, we performed a wound healing assay. MHcc97H cell migration
was significantly inhibited by Wnt3a knockdown. The size of the
wound in the Wnt3a-siRNA group was 0.82±0.04 mm, which was
significantly larger than the wound size in either the control
group (0.32±0.04 mm) or the NC-si-transfected group (0.27±0.06 mm)
(Fig. 5A and B). Similar results
were obtained for SK-Hep-1 cells; the size of the wound in the
Wnt3a-siRNA group was 0.77±0.05 mm, compared to 0.40±0.07 mm in the
control group and 0.48±0.07 mm in the NC-si group (Fig. 5C and D). We used a Transwell Boyden
chamber system with porous polycarbonate membranes to further
quantify cell motility. The results were consistent with those of
the wound healing assay. After MHcc97H cells were transfected with
siRNA for 24 h, the number of cells was reduced from 186.33±10.26
and 180.67±10.07 to 60.33±16.65 compared with the control and NC-si
groups. Similarly, the number of migrated SK-Hep-1 cells per field
was reduced from 161.33±10.41 and 157±11.14 to 46.67±10.79
(Fig. 5E and F). Taken together,
our results support a role for Wnt3a in the migratory capability of
MHCC97L and SK-Hep-l cells.
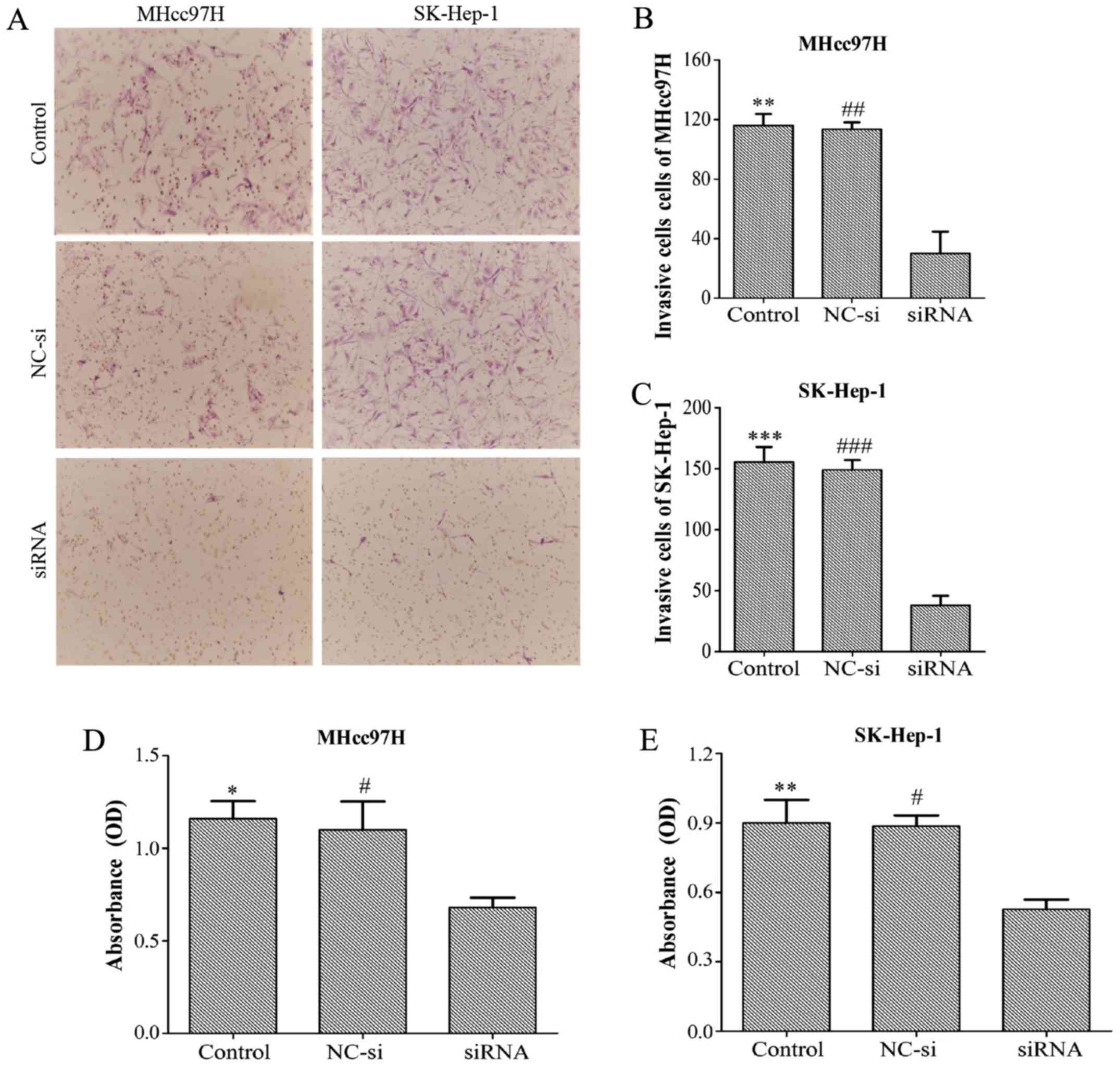
Downregulation of Wnt3a expression
inhibits the invasion and adhesion of MHcc97H and SK-Hep-1
cells
Transwell assay chambers with a Matrigel coating
were used to evalu ate tumour aggressiveness. The cells observed to
degrade the Matrigel and pass through the membrane were counted,
and the results revealed that the number of aggressive MHcc97H
cells was lower in the Wnt3a-siRNA group (30±25.51) than in both
the NC-si group (113.33±8.14) and the control group (116±13.23).
Similar results were obtained for SK-Hep-1 cells (Wnt3a-siRNA,
38±13.45; NC-si, 149±14; control, 155.33±21.73) (Fig. 6A–C). Whether Wnt3a affects the
adhesion ability of HCC cells was also investigated with an MTT
assay. The data demonstrated that depletion of Wnt3a dramatically
inhibited cell adhesion compared with the control group and NC-si
group (Fig. 6D and E). Overall,
these results support the proposal that downregulation of Wnt3a
expression can significantly suppress the invasion and adhesion
capability of MHCC97L and SK-Hep-l cells.
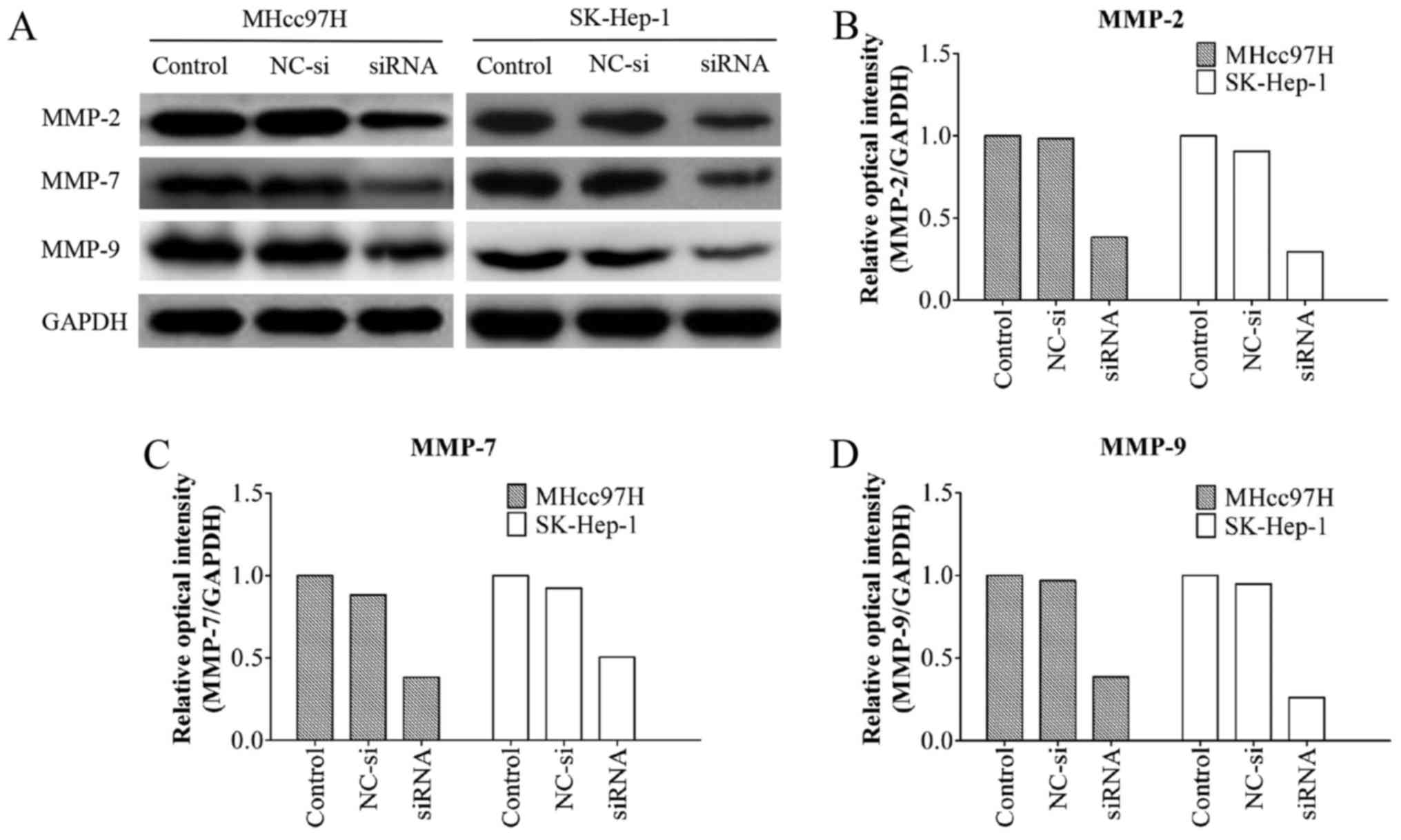
Wnt3a siRNA downregulates MMP-2, MMP-7
and MMP-9 protein expression in MHcc97H and SK-HEP-1 cells
MMPs are known to play an important role in tumour
metastasis (16,19). A western blotting assay showed that
the MMP-2/-9 protein levels in the Wnt3a-siRNA group were
significantly lower than those in the other groups. Notably, the
MMP-7 protein levels were also decreased after Wnt3a knockdown
(Fig. 7). These results indicated
that Wnt3a promotes cell migration and invasion by regulating the
expression of MMP-2/-7/-9.
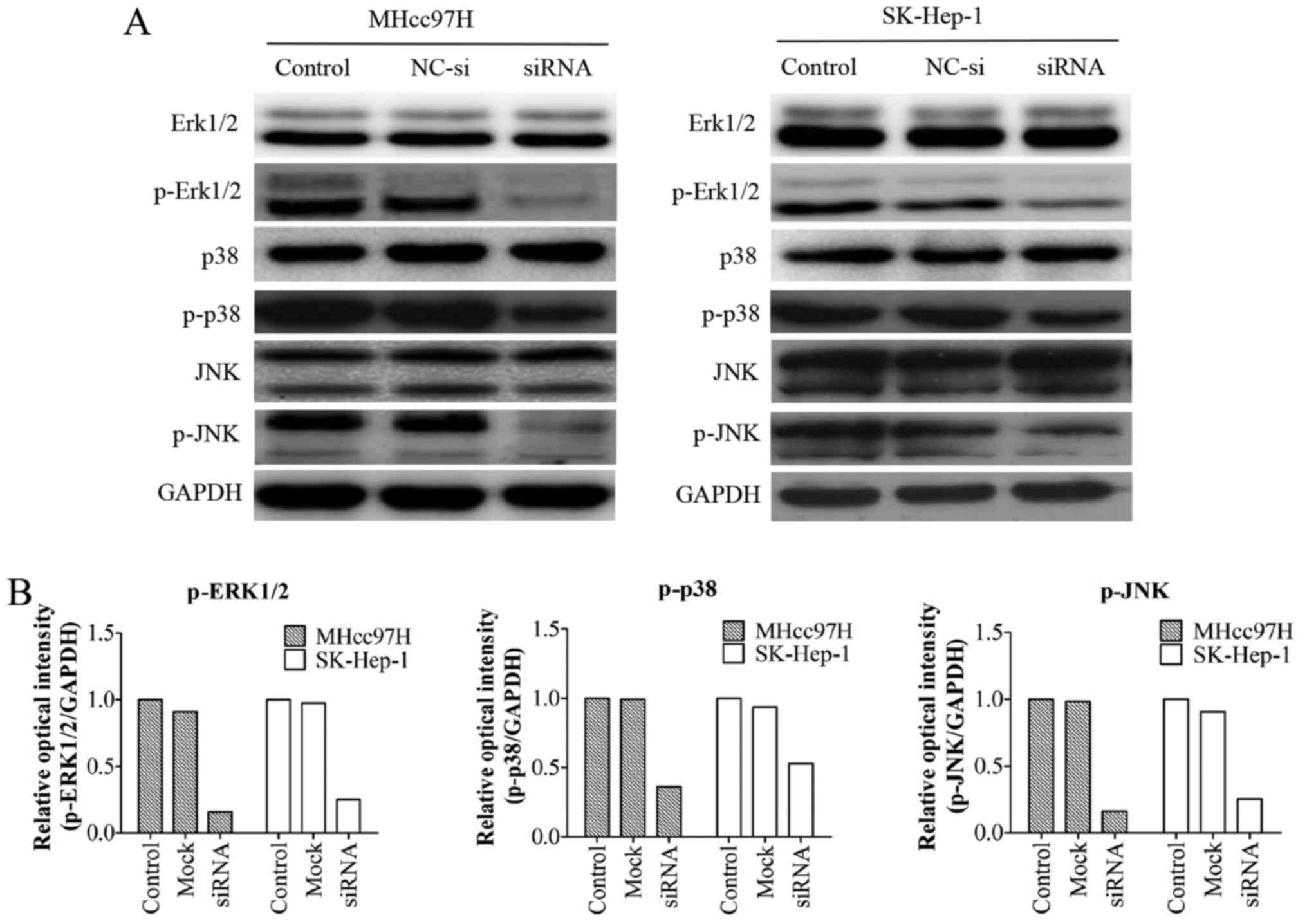
Wnt3a-altered MMP expression is
associated with the MAPK signalling pathway
We examined the expression of mitogen-activated
protein kinase (MAPK) pathway-associated proteins, including the
total protein levels of ERK1/2, p38, JNK and their phosphorylated
forms (p-ERK1/2, p-p38, and p-JNK). The results demonstrated that
downregulation of Wnt3a had no effect on the protein levels of
ERK1/2, p38 and JNK; however, the levels of the phosphorylated form
were markedly decreased (Fig.
8).
Discussion
HCC patients have a higher mortality rate and a
lower 5-year survival rate than patients with various other cancers
(20,21), mainly because of tumour cell
invasion and metastasis. Thus, novel therapies that specifically
inhibit these processes are critical. Some reports have suggested
that the Wnt signalling pathway is activated and involved in tumour
metastasis, but the detailed molecular mechanisms remain unclear.
Our experiments attempted to explore the role of Wnt3a in
pathogenesis and metastasis of HCC.
The expression of related genes in tumour specimens
from HCC patients were evaluated with qRT-PCR. We found that
expression of Wnt3a and the Wnt target gene c-Myc was upregulated.
Wnt ligands can promote β-catenin nuclear translocation and enable
β-catenin to interact with T-cell factor (TCF)/lymphoid enhancer
factor (LEF) transcription factors to regulate gene expression,
such as c-Myc, MMP-7 and cyclin D1 (5,22,23).
Wnt3a is a ligand in the Wnt signalling pathway. Driskell et
al reported that Wnt3a knockout mice lacked LEF1 protein
expression in submucosal gland (SMG) placodes, and confirmed that
Wnt3a can directly transcriptionally regulate the LEF1 gene, which
needs TCF4 to bind to the LEF1 promoter Wnt response region
(24). Galceran et al
showed that null mutations LEF1/TCF1 resulted in a defect in the
formation of paraxial mesoderm, which was virtually identical to
that seen in Wnt3a-deficient mice (25). Multiple studies reported that
inhibition of Wnt3a expression reduced LEF/TCF activity and
overexpression of wnt3a promoted LEF/TCF activity (26–28).
In conclusion, Wnt3a can directly transcriptionally regulate the
LEF/TCF gene activity. Our data confirmed that Wnt3a plays an
essential role in HCC progression. Activation of Wnt3a pathway is
accompanied by higher expression of Notch3. Hyperactive Wnt3a could
cause its target gene transcription activation. Stemness gene
(c-Myc and MMP-7) expressed in HCC always suggest poor prognosis
(29,30). c-Myc is known as the stemness gene
which determines the cell capacity of self-renewal and cell
proliferation. MMPs are involved in the process of cancer cell
invasion and metastasis (16,17,30).
The Wnt pathway is implicated in HCC pathogenesis through its
interaction with many signalling pathways, including Hippo,
AKT/PKB, ERK1/2, and NF-κB signal transduction pathways (31–34).
In addition, Sun et al (35) reported that Wnt and Notch1 pathways
promote hepatitis B virus X protein-induced hepatocarcino-genesis.
Our previous study found that Notch3 expression reflects
differentiation properties and correlates with a poor prognosis in
HCC and plays a role in modulating the stemness of HCC cells.
Notch3 is a distinct biomarker of cancer stem cells in HCC
progression. Therefore, HCC development may be associated with
activation of multiple signalling pathways. Our data demonstrated
that Wnt3a is positively correlated with Notch3 and Hes1
expression, and Notch3 is positively correlated with c-Myc and
MMP-7 expression. Furthermore, we measured the protein levels of
Wnt3a and Notch3 in the same specimen and confirmed that Wnt3a and
Notch3 were highly expressed. Based on these data, we concluded
that team work of Wnt pathway with Notch pathway contributed to
regulate the maintenance of cellular stemness and recurrence as
well as metastasis of HCC. However, their specific cooperation
mechanism still needs to be clarified in the future.
Some studies have reported that the expression of
Wnt3a was inhibited by the inhibitors including AXIN2, DKK-1,
NKD-1, β-TRCP and HS20 in liver cancers (36,37),
and these reports primarily elaborated the biological capability of
these antagonists. However, the effect on Wnt pathway target gene
and the capacity of proliferation and metastasis of the tumour
cells were not further investigated after the reduction of Wnt3a.
In our study, silencing of Wnt3a expression via siRNA transfection
suppressed cell proliferation and induced
G0/G1 cell cycle arrest in MHcc97H and
SK-HEP-1 cells. Deregulation of cell cycle progression is a
well-known and common feature of cancer (38). c-Myc is a key transcription factor
involved in several cellular processes, including growth and
proliferation in different cells (39,40).
The p21, p27 and Cyclin D1 proteins function as regulators of cell
cycle progression through G1 and S phases (41–43).
Thus, altered expression levels of these proteins indicate cell
cycle arrest. Our data suggested that downregulating the expression
of Wnt3a can reduce the expression of the target gene c-Myc. In
addition, the protein levels of p21 and p27 were increased and
cyclin D1 was decreased, indicating that Wnt3a siRNA treatment
induce p21- and p27-dependent cell cycle arrest. In conclusion,
these results clearly indicate that the Wnt3a pathway is involved
in cell cycle progression in HCC cells.
Based on a functional analysis, we further examined
the potential molecular mechanism of metastasis progression
mediated by Wnt3a. MMPs, including MMP-2/-7/-9, play an important
role in tumour metastasis (9,17,19).
Suppression of MMPs decreases cancer cell invasion and migration
(44,45). Accordingly, potential inhibitors of
MMPs will undoubtedly be valuable in our attempts to treat HCC. Our
studies demonstrated that inhibition of Wnt3a expression suppressed
HCC cell invasion and metastasis by decreasing MMP-2/-7/-9
expression. The MAPK signalling pathway, including JNK, ERK1/2 and
p38, has been reported to be the essential upstream signalling
pathway that activates MMP expression and participates in
regulation of cell migration and invasion in various cancers
(46–48). To determine whether the
downregulation of MMP expression is associated with MAPK signalling
pathways, we assessed the effects of Wnt3a siRNA on JNK, ERK1/2,
and p38 levels and those of their phosphorylated forms. The results
demonstrated that cooperation of JNK, ERK1/2 and p38 plays a
crucial role in Wnt3a-mediated cell migration and invasion in
SK-Hep-1 and MHCC97L cells.
In conclusion, we confirmed that Wnt3a plays an
essential role in HCC progression. Activation of the Wnt3a pathway
is accompanied by higher expression of Notch3. Furthermore, Wnt3a
may be critical for HCC cell cycle and metastasis by regulating
cell cycle regulatory proteins and the MAPK (p38, ERK1/2 and JNK)
pathway. Wnt3a may represent a novel therapeutic target for
treatment and inhibition of HCC metastasis.
Acknowledgments
This study was supported by the National Natural
Science Funds 81041099 and Guangdong Province Natural Science Funds
S2011010003750 (to M.L.). This study was also supported by the
Institute of Hepatobiliary Surgery, Affiliated Hospital of
Guangdong Medical University. We thank Professor Ningping Chen for
advice and support.
References
|
1
|
Fitzmaurice C, Dicker D, Pain A, Hamavid
H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R,
Wolfe C, et al Global Burden of Disease Cancer Collaboration: The
global burden of cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Du ZG, Wei YG, Chen KF and Li B: Risk
factors associated with early and late recurrence after curative
resection of hepatocellular carcinoma: A single institution's
experience with 398 consecutive patients. Hepatobiliary Pancreat
Dis Int. 13:153–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang LY, Chang RM, Lau WY, Ou DP, Wu W and
Zeng ZJ: Mesohepatectomy for centrally located large hepatocellular
carcinoma: Indications, techniques, and outcomes. Surgery.
156:1177–1187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oishi N, Yamashita T and Kaneko S:
Molecular biology of liver cancer stem cells. Liver Cancer.
3:71–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cadigan KM and Nusse R: Wnt signaling: A
common theme in animal development. Genes Dev. 11:3286–3305. 1997.
View Article : Google Scholar
|
|
7
|
Pai SG, Carneiro BA, Mota JM, Costa R,
Leite CA, Barroso-Sousa R, Kaplan JB, Chae YK and Giles FJ:
Wnt/beta-catenin pathway: Modulating anticancer immune response. J
Hematol Oncol. 10:1012017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eterno V, Zambelli A, Villani L, Tuscano
A, Manera S, Spitaleri A, Pavesi L and Amato A: AurkA controls
self-renewal of breast cancer-initiating cells promoting wnt3a
stabilization through suppression of miR-128. Sci Rep. 6:284362016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee MA, Park JH, Rhyu SY, Oh ST, Kang WK
and Kim HN: Wnt3a expression is associated with MMP-9 expression in
primary tumor and metastatic site in recurrent or stage IV
colorectal cancer. BMC Cancer. 14:1252014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li C, Song G, Zhang S, Wang E and Cui Z:
Wnt3a increases the metastatic potential of non-small cell lung
cancer cells in vitro in part via its upregulation of Notch3. Oncol
Rep. 33:1207–1214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka M, Kuriyama S, Itoh G, Maeda D,
Goto A, Tamiya Y, Yanagihara K, Yashiro M and Aiba N: Mesothelial
cells create a novel tissue niche that facilitates gastric cancer
invasion. Cancer Res. 77:684–695. 2017. View Article : Google Scholar
|
|
12
|
Leung WK, He M, Chan AW, Law PT and Wong
N: Wnt/β-catenin activates MiR-183/96/182 expression in
hepatocellular carcinoma that promotes cell invasion. Cancer Lett.
362:97–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng YY, He YH, Chen C, Xu T, Li L, Ni MM,
Meng XM, Huang C and Li J: NLRC5 regulates cell proliferation,
migration and invasion in hepatocellular carcinoma by targeting the
Wnt/β-catenin signaling pathway. Cancer Lett. 376:10–21. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao CH, Yeh CT, Huang YH, Wu SM, Chi HC,
Tsai MM, Tsai CY, Liao CJ, Tseng YH, Lin YH, et al: Dickkopf 4
positively regulated by the thyroid hormone receptor suppresses
cell invasion in human hepatoma cells. Hepatology. 55:910–920.
2012. View Article : Google Scholar
|
|
15
|
Zhang Q, Lu C, Fang T, Wang Y, Hu W, Qiao
J, Liu B, Liu J, Chen N, Li M, et al: Notch3 functions as a
regulator of cell self-renewal by interacting with the β-catenin
pathway in hepatocellular carcinoma. Oncotarget. 6:3669–3679. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jia S, Lu J, Qu T, Feng Y, Wang X, Liu C
and Ji J: MAGI1 inhibits migration and invasion via blocking
MAPK/ERK signaling pathway in gastric cancer. Chin J Cancer Res.
29:25–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shao Q, Luo X, Yang D, Wang C, Cheng Q,
Xiang T and Ren G: Phospholipase Cδ1 suppresses cell migration and
invasion of breast cancer cells by modulating KIF3A-mediated
ERK1/2/β-catenin/MMP7 signalling. Oncotarget. 8:29056–29066.
2017.PubMed/NCBI
|
|
18
|
Cheng Q, Yuan F, Lu F, Zhang B, Chen T,
Chen X, Cheng Y, Li N, Ma L and Tong T: CSIG promotes
hepatocellular carcinoma proliferation by activating c-MYC
expression. Oncotarget. 6:4733–4744. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okazaki I and Inagaki Y: Novel strategies
for hepatocellular carcinoma based on MMPs science. Anticancer
Agents Med Chem. 12:753–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gai JQ, Sheng X, Qin JM, Sun K, Zhao W and
Ni L: The effect and mechanism of bufalin on regulating
hepatocellular carcinoma cell invasion and metastasis via
Wnt/β-catenin signaling pathway. Int J Oncol. 48:338–348. 2016.
View Article : Google Scholar
|
|
23
|
Lian J, Tang J, Shi H, Li H, Zhen T, Xie
W, Zhang F, Yang Y and Han A: Positive feedback loop of
hepatoma-derived growth factor and β-catenin promotes
carcinogenesis of colorectal cancer. Oncotarget. 6:29357–29374.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Driskell RR, Goodheart M, Neff T, Liu X,
Luo M, Moothart C, Sigmund CD, Hosokawa R, Chai Y and Engelhardt
JF: Wnt3a regulates Lef-1 expression during airway submucosal gland
morphogenesis. Dev Biol. 305:90–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Galceran J, Fariñas I, Depew MJ, Clevers H
and Grosschedl R: Wnt3a−/−-like phenotype and limb
deficiency in Lef1 (−/−)Tcf1 (−/−) mice. Genes Dev. 13:709–717.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qiang YW, Chen Y, Brown N, Hu B, Epstein
J, Barlogie B and Shaughnessy JD Jr: Characterization of
Wnt/beta-catenin signalling in osteoclasts in multiple myeloma. Br
J Haematol. 148:726–738. 2010. View Article : Google Scholar
|
|
27
|
Wallace K, Marek CJ, Hoppler S and Wright
MC: Glucocorticoid-dependent transdifferentiation of pancreatic
progenitor cells into hepatocytes is dependent on transient
suppression of WNT signalling. J Cell Sci. 123:2103–2110. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang SH, Li N, Wei Y, Li QR and Yu ZP:
β-catenin deacetylation is essential for WNT-induced proliferation
of breast cancer cells. Mol Med Rep. 9:973–978. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brabletz T, Jung A, Dag S, Hlubek F and
Kirchner T: beta-catenin regulates the expression of the matrix
metalloproteinase-7 in human colorectal cancer. Am J Pathol.
155:1033–1038. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu D, Nakano J, Ishikawa S, Yokomise H,
Ueno M, Kadota K, Urushihara M and Huang CL: Overexpression of
matrix metalloproteinase-7 (MMP-7) correlates with tumor
proliferation, and a poor prognosis in non-small cell lung cancer.
Lung Cancer. 58:384–391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Erdal E, Ozturk N, Cagatay T,
Eksioglu-Demiralp E and Ozturk M: Lithium-mediated downregulation
of PKB/Akt and cyclin E with growth inhibition in hepatocellular
carcinoma cells. Int J Cancer. 115:903–910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim W, Khan SK and Yang Y: Interacting
network of Hippo, Wnt/β-catenin and Notch signaling represses liver
tumor formation. BMB Rep. 50:1–2. 2017. View Article : Google Scholar :
|
|
33
|
Li J, Dai W, Xia Y, Chen K, Li S, Liu T,
Zhang R, Wang J, Lu W and Zhou Y: et al Astaxanthin inhibits
proliferation and induces apoptosis of human hepatocellular
carcinoma cells via Inhibition of NF-κB P65 and Wnt/β-catenin in
vitro. Mar Drugs. 13:6064–6081. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang R, Sun Q, Wang P, Liu M, Xiong S, Luo
J, Huang H, Du Q, Geller DA and Cheng B: Notch and Wnt/β-catenin
signaling pathway play important roles in activating liver cancer
stem cells. Oncotarget. 7:5754–5768. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun Q, Wang R, Luo J, Wang P, Xiong S, Liu
M and Cheng B: Notch1 promotes hepatitis B virus X protein-induced
hepatocarcinogenesis via Wnt/β-catenin pathway. Int J Oncol.
45:1638–1648. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao W, Kim H, Feng M, Phung Y, Xavier CP,
Rubin JS and Ho M: Inactivation of Wnt signaling by a human
antibody that recognizes the heparan sulfate chains of glypican-3
for liver cancer therapy. Hepatology. 60:576–587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koch A, Waha A, Hartmann W, Hrychyk A,
Schüller U, Waha A, Wharton KA Jr, Fuchs SY, von Schweinitz D and
Pietsch T: Elevated expression of Wnt antagonists is a common event
in hepatoblastomas. Clin Cancer Res. 11:4295–4304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Akinyeke T, Matsumura S, Wang X, Wu Y,
Schalfer ED, Saxena A, Yan W, Logan SK and Li X: Metformin targets
c-MYC oncogene to prevent prostate cancer. Carcinogenesis.
34:2823–2832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Liu H, Lai C, Du X, Su Z and Gao S:
The Lin28/let-7a/c-Myc pathway plays a role in non-muscle invasive
bladder cancer. Cell Tissue Res. 354:533–541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bruyère C and Meijer L: Targeting
cyclin-dependent kinases in anti-neoplastic therapy. Curr Opin Cell
Biol. 25:772–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang ST, Ho HJ, Lin JT, Shieh JJ and Wu
CY: Simvastatin-induced cell cycle arrest through inhibition of
STAT3/SKP2 axis and activation of AMPK to promote p27 and p21
accumulation in hepatocellular carcinoma cells. Cell Death Dis.
8:e26262017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoon MK, Mitrea DM, Ou L and Kriwacki RW:
Cell cycle regulation by the intrinsically disordered proteins p21
and p27. Biochem Soc Trans. 40:981–988. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gao J, Ding F, Liu Q and Yao Y: Knockdown
of MACC1 expression suppressed hepatocellular carcinoma cell
migration and invasion and inhibited expression of MMP2 and MMP9.
Mol Cell Biochem. 376:21–32. 2013. View Article : Google Scholar
|
|
45
|
Ha TY, Hwang S, Moon KM, Won YJ, Song GW,
Kim N, Tak E, Ryoo BY and Hong HN: Sorafenib inhibits migration and
invasion of hepatocellular carcinoma cells through suppression of
matrix metalloproteinase expression. Anticancer Res. 35:1967–1976.
2015.PubMed/NCBI
|
|
46
|
Guo JR, Li W, Wu Y, Wu LQ, Li X, Guo YF,
Zheng XH, Lian XL, Huang HF and Chen YZ: Hepatocyte growth factor
promotes proliferation, invasion, and metastasis of myeloid
leukemia cells through PI3K-AKT and MAPK/ERK signaling pathway. Am
J Transl Res. 8:3630–3644. 2016.PubMed/NCBI
|
|
47
|
Luo Y, Wu JY, Lu M-H, Shi Z, Na N and Di
J-M: Carvacrol alleviates prostate cancer cell proliferation,
migration, and invasion through regulation of PI3K/Akt and MAPK
signaling pathways. Oxid Med Cell Longev. 2016:14696932016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang Y, Ye Y, Qiu Q, Xiao Y, Huang M, Shi
M, Liang L, Yang X and Xu H: Triptolide inhibits the migration and
invasion of rheumatoid fibroblast-like synoviocytes by blocking the
activation of the JNK MAPK pathway. Int Immunopharmacol. 41:8–16.
2016. View Article : Google Scholar : PubMed/NCBI
|