Introduction
Osteosarcoma is well known as the most common
primary malignant bone tumor in clinic. It is strongly invasive and
has poor prognosis, and mainly occurs in children and adolescents
(1-3). Photodynamic therapy (PDT), a new
promising approach for tumor therapy, is featured by excellent
selectivity, less side reactions, and visible light of specific
wavelength to excite photosensitizer enriched in tumor tissue,
which can subsequently result in reactive oxygen, mainly singlet
oxygen to cause tumor cell death (4,5). In
addition, PDT has already been used in the treatment of skin and
esophageal cancer, and other tumors (6-8).
MPPa, a second generation photosensitizer derived from chlorophyll,
has many advantages including stability, single component, rapid
absorption and metabolism and strong photosensitivity (9). It has been reported that MPPa-PDT can
kill tumor cells such as nasopharyngeal carcinoma, prostate and
breast cancer cells (10-12).
However, photodynamic therapy may lead to the
resistance of tumor cells (13).
Numerous mechanisms are reported to mediate the resistance of tumor
cells (14). Furthermore, the
surviving tumor cells play an important role in the recurrence and
deterioration of tumor, and result in poor prognosis (15,16).
It has been reported that the increased expression of HO1, SOD1 and
other antioxidant proteins can protect tumor cells from ROS damage
(17). ATP-binding cassette (ABC)
transporters (e.g. ABCG2, MRP1 and MDR1) were found to mediate
tumor cell resistance, and alleviate cell damage through pumping
out intracellular toxins (18).
Furthermore, the overexpression of anti-apoptotic protein
P-survivin was responsible for chemoresistance and radio-resistance
of tumor cells (19). The Bcl-2
protein family can regulate the permeability of mitochondrial
membrane, and its expression can decrease the sensitivity of tumor
cells to antitumor therapy (20).
The present study aimed to establish human osteosarcoma cell lines
resistant to MPPa-PDT. The resistant cell lines can also be
employed to investigate the resistance mechanism of human
osteosarcoma cells to MPPa-PDT and explore the approach to overcome
MPPa-PDT resistance.
Materials and methods
Reagents and instruments
MPPa and 2′7′-dichlorofluorescin diacetate (DCFH-DA)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). BCA and
trypsin were purchased from Beyotime Biotech (Shanghai, China).
Cell viability and cytotoxicity test kits [Cell Counting Kit-8
(CCK-8)] were obtained from Dojindo Molecular Technologies
(Kumamoto, Japan). Extracellular matrix gel was obtained from BD
Biosciences (Franklin Lakes, NJ, USA). Annexin V-propidium iodide
(PI) double-staining test kit was purchased from KeyGen Biotech
(Nanjing, China). LED equipment was purchased from Chongqing Jingyu
Laser Technology Co. Ltd. (Chongqing, China).
Antibodies
Primary antibodies were: β-actin (1:1,000), cleaved
caspase-3 (1:1,000), SOD1 (1:1,000), cleaved PARP (1:1,000), Bcl-2
(1:1,000), Bcl-xL (1:1,000), Bax (1:1,000), CD133 (1:1,000), MDR1
(1:500), MRP1 (1:500) and P-survivin (1:1,000; all from Cell
Signaling Technology, Inc. Danvers, MA, USA), HO1 (1:500;
Proteintech Group, Inc., Wuhan, China) and CD133-APC (1:20; BD
Biosciences). Secondary antibodies were: HRP monoclonal antibody
anti-IgG of mouse and HRP monoclonal antibody anti-IgG of rabbit
(Cell Signaling Technology, Inc.).
Cell line and culture
MG63 and HOS cells were obtained from the Chinese
Academy of Sciences (Shanghai, China), and cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS) (both from HyClone, Beijing, China), 100 µg/ml
penicillin and 100 µg/ml streptomycin (Beyotime Biotech) at
37°C in a humidified atmosphere containing 5% CO2.
Resistance induction to MPPa-PDT
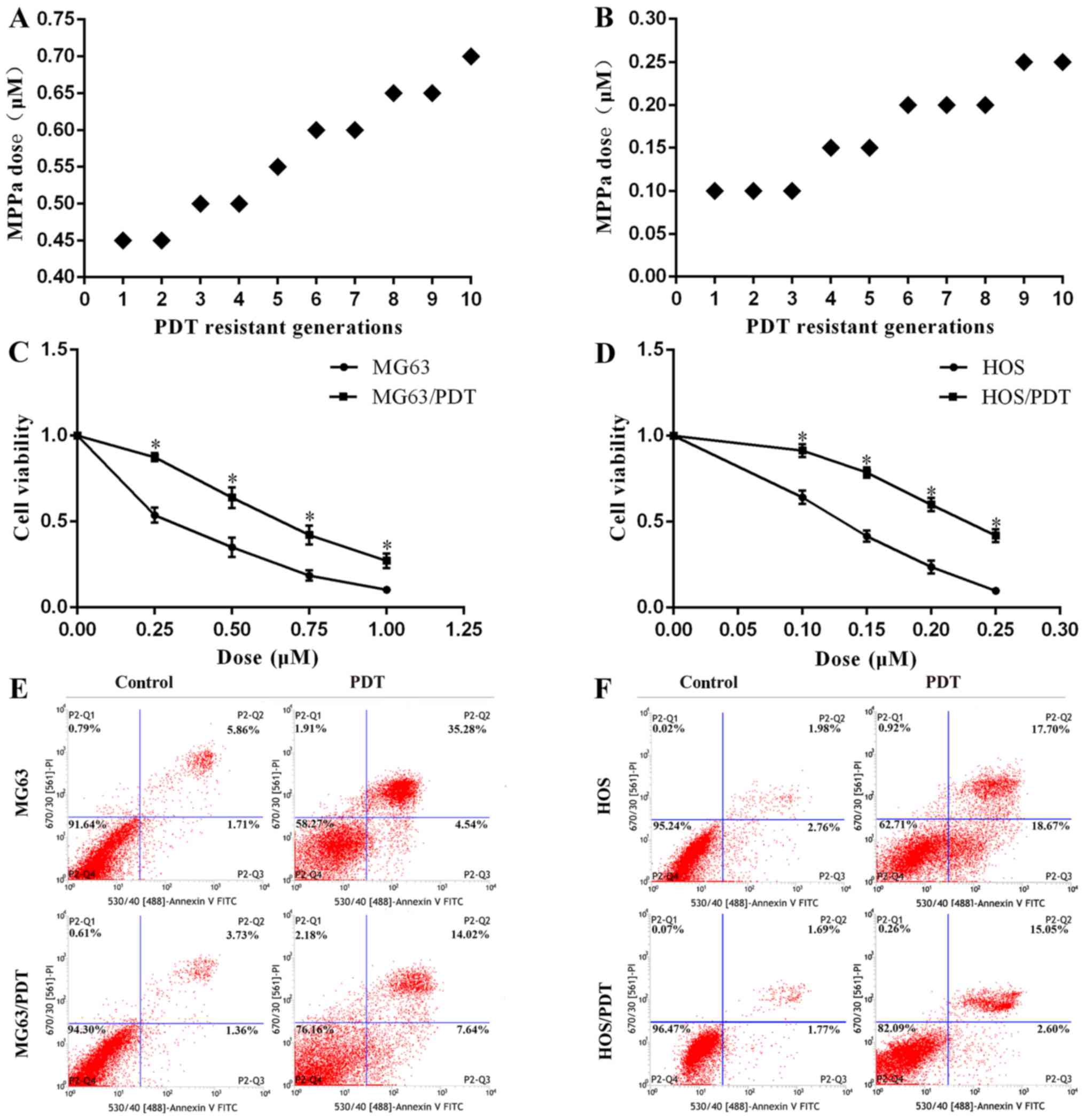
MG63 and HOS cells were cultured in the dark and
incubated with different MPPa concentrations (Fig. 1A and B) for 20 h, and then washed
twice with phosphate-buffered saline (PBS). The culture medium was
replaced, and the cells were exposed to red light (630 nm, 40
mW/cm2) in the continuous output mode. Treatment
conditions that caused survival rates of 40–60% were chosen. The
dead cells were wiped off and the surviving cells were cultured in
fresh complete medium continuously for 24 h. Twenty hours later,
the cells were harvested and replaced. They were subjected to a new
PDT with intermittently increased doses of MPPa. The final
populations were obtained following a total of 10 cycles of PDT,
and they were MG63/PDT and HOS/PDT.
MPPa-PDT sensitivity assay
CCK-8 was used to investigate the sensitivity of
cell lines MG63, MG63/PDT, HOS and HOS/PDT to MPPa-PDT. Cells were
plated in 96-well plates at a density of 5×103
cells/well with 3 duplications. After a 24-h incubation, the
culture medium was replaced with the fresh medium containing
different concentrations of MPPa (0, 0.25, 0.5, 0.75 and 1.0
µM for MG63 and MG63/PDT; 0, 0.1, 0.15, 0.2 and 0.25
µM for HOS and HOS/PDT cells). The cells were then cultured
for 20 h. The culture medium was replaced, and the cells were
exposed to red light (630 nm, 40 mW/cm2). Twenty-four
hours later, the cells were incubated for 1 h with 10 µl
CCK-8 in each well. A microplate reader was employed to detect the
absorption values of CCK-8 at 450 nm. The cell viability was
calculated according to the following formulation:
Cell viability(%)=Average OD in experiment group/average OD in control group×100%
Resistance indices(RIs)=IC50values for resistant cells/IC50values for parental cells
Based on the results of the cell viability test, we
chose an MPPa concentration of 0.45 µM for MG63 and MG63/PDT
cells and 0.15 µM for HOS and HOS/PDT cells with a light
energy density of 4.8 J/cm2 as the treatment
conditions.
Detection of apoptosis rate by Annexin
V-PI double staining and FCM
Cells were seeded in 6-well plates at a density of
1×105 cells/well. All cells were harvested after
corresponding treatments, and assessed by FCM after Annexin V-PI
double staining (KeyGen Biotech).
Cell proliferation assay by CCK-8
Cells were seeded in 96-well plates at a density of
5×103 cells/well. When all cells attached, the cell
viability was determined using the CCK-8 assay at 0, 12, 24 and 48
h.
Cell cycle analysis by FCM
Cells were seeded in 6-well plates at a density of
1×105 cells/well. After incubation for ~36 h, when
fusion was up to ~60–70%, all groups were collected at the same
time and washed twice, and fixed by suspending in 70% ethanol at
4°C for 24 h, and then subjected to FCM.
Assessment of intracellular ROS level by
DCFH-DA staining
Cells were inoculated in 6- and 24-well plates, at a
density of 1×105 and 5×104 cells/well,
respectively. Following the corresponding treatments, the cells
were further incubated for 2 h. Then, DCFH-DA (10 µM) was
added at 37°C for 20 min. Finally, cells in 24-well plates were
observed by fluorescence microscope (FM) after being washed 3
times, and the cells in 6-well plates were determined by FCM after
being trypsinized and collected.
Assessment of CD133 by FCM
Cells were seeded in 6-well plates at a density of
1×105 cells/well. All cells were trypsinized,
centrifuged and resuspended in PBS. CD133-APC (1:20) was added to
corresponding groups and incubated in the dark for 10 min at 4°C.
After being washed, the cells were resuspended in PBS and analyzed
by FCM.
Measurement of intracellular MPPa
Cells were inoculated in 6- and 96-well plates at a
density of 1×105 and 5×103 cells/well,
respectively. After attachment, the cells were incubated with
different MPPa concentrations (2, 4 and 8 µM) for 20 h.
Then, an inverted FM was used to observe the fluorescence of cells
with 4 µM MPPa in 6-well plates, and a microplate reader was
adopted to examine the fluorescence value of cells in 96-well
plates (λexc 525 nm; λem 680 nm).
Colony formation assay
MG63 and MG63/PDT were seeded into 6-well plates at
a density of 200 cells/well. HOS and HOS/PDT were seeded in 6-well
plates at a density of 500 cells/well. The culture medium was
replaced every 3 days. Two weeks later, colonies were fixed with
polyformaldehyde (4%) for 20 min and visualized by crystal violet
solution.
Invasion and migration assays
The ECM (1:8, 100 µl) was added to the top
chamber and placed in an incubator for 2 h. When the ECM was dried,
4×104 MG63 and MG63/PDT or 8×104 HOS and
HOS/PDT cells in serum-free medium were added to the top chamber.
In the lower chamber, complete medium was added. After incubation
at 37°C in 5% CO2 for 48 h, the cells on the upper
surface of the membrane were removed using a cotton swab. The cells
on the lower surface of the membrane were fixed with
polyformaldehyde (4%) for 15 min and stained by crystal violet
solution (0.1%). Cells were observed by inverted phase contrast
microscope. Five randomized fields at a magnification of ×40 were
selected. For the migration assay, a protocol similar to the
invasive assay was performed, but without the ECM layer in the
chamber, and the cells added as well as the incubation time was
half of that in invasion assay, respectively.
Western blot analysis
Cells were rinsed with PBS and lysed by RIPA buffer
containing a phosphatase and protease inhibitor cocktail. Protein
concentration was assessed by BCA. Protein samples (40 µg)
were electrophoresed and blotted on polyvinylidene fluoride
membranes, which were blocked in Tris-buffered saline containing
Tween-20 and 5% non-fat milk for 1 h at room temperature, and
incubated with the corresponding primary antibodies overnight at
4°C. After being rinsed, the membranes were subjected to the
horseradish peroxidase-conjugated secondary antibody for 1 h and
developed by electrochemiluminescence. Quantity One software was
used to detect the gray values of some western bands. The relative
expression of target proteins was displayed using the ratio of
target protein/β-actin. Three independent experiments were
performed.
Statistical analysis
Data are expressed as the mean ± SD and analyzed by
SPSS (SPSS, Inc., Chicago, IL, USA). Differences between groups
were determined using the one-way or two-way ANOVA test for
intergroup and independent-sample t-test for two groups. At
P<0.05, the difference was considered significant.
Results
Establishment of the resistant cell
lines
Parental cells, MG63 and HOS cells, were treated by
PDT with increased concentration of MPPa for 10 cycles (Fig. 1A and B). The 10th generation of
resistant cells obtained were named MG63/PDT and HOS/PDT,
respectively. We employed MG63, HOS and MG63/PDT, HOS/PDT as
experimental objects for the following studies. In order to verify
the tolerance of resistant cells compared with their corresponding
parental cells, the cell viability and apoptosis rate were assessed
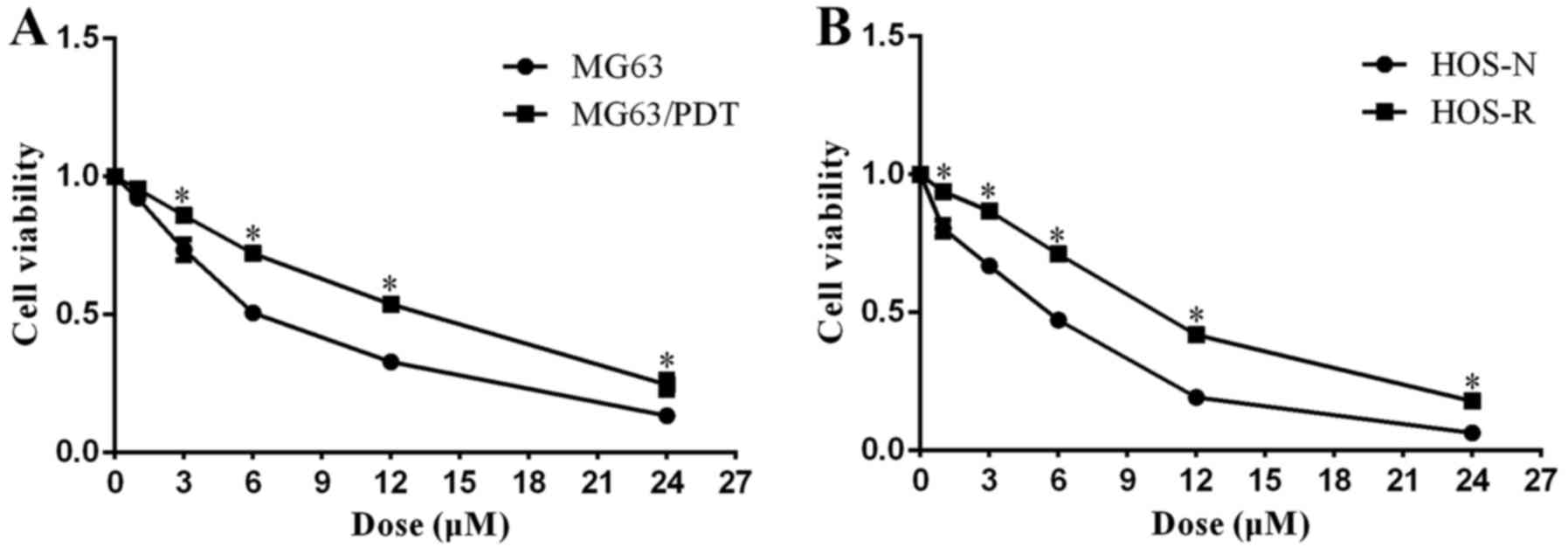
after PDT. A CCK-8 assay demonstrated that the concentration at 50%
inhibition (IC50) for MG63/PDT (0.704±0.016 µM)
was 1.67-fold more resistant than that for MG63 (0.421±0.028
µM; P<0.001), and the concentration at 50% inhibition
(IC50) for HOS/PDT (0.226±0.008 µM) was 1.61-fold
higher as compared to that for HOS (0.140±0.004 µM)
(P<0.001; Fig. 1C and D). FCM
indicated that the apoptosis rate of MG63/PDT (20.04±2.16%) was
markedly lower than that of MG63 (40.58±2.34%) (P<0.001;
Fig. 1E), when they were subjected
to PDT with 0.45 µM MPPa. The apoptosis rate of HOS/PDT
(17.80±1.26%) subjected to PDT with 0.15 µM MPPa was also
significantly lower than that of HOS (37.22±0.81%) (P<0.001;
Fig. 1F).
Measurement of intracellular ROS by FCM
and FM
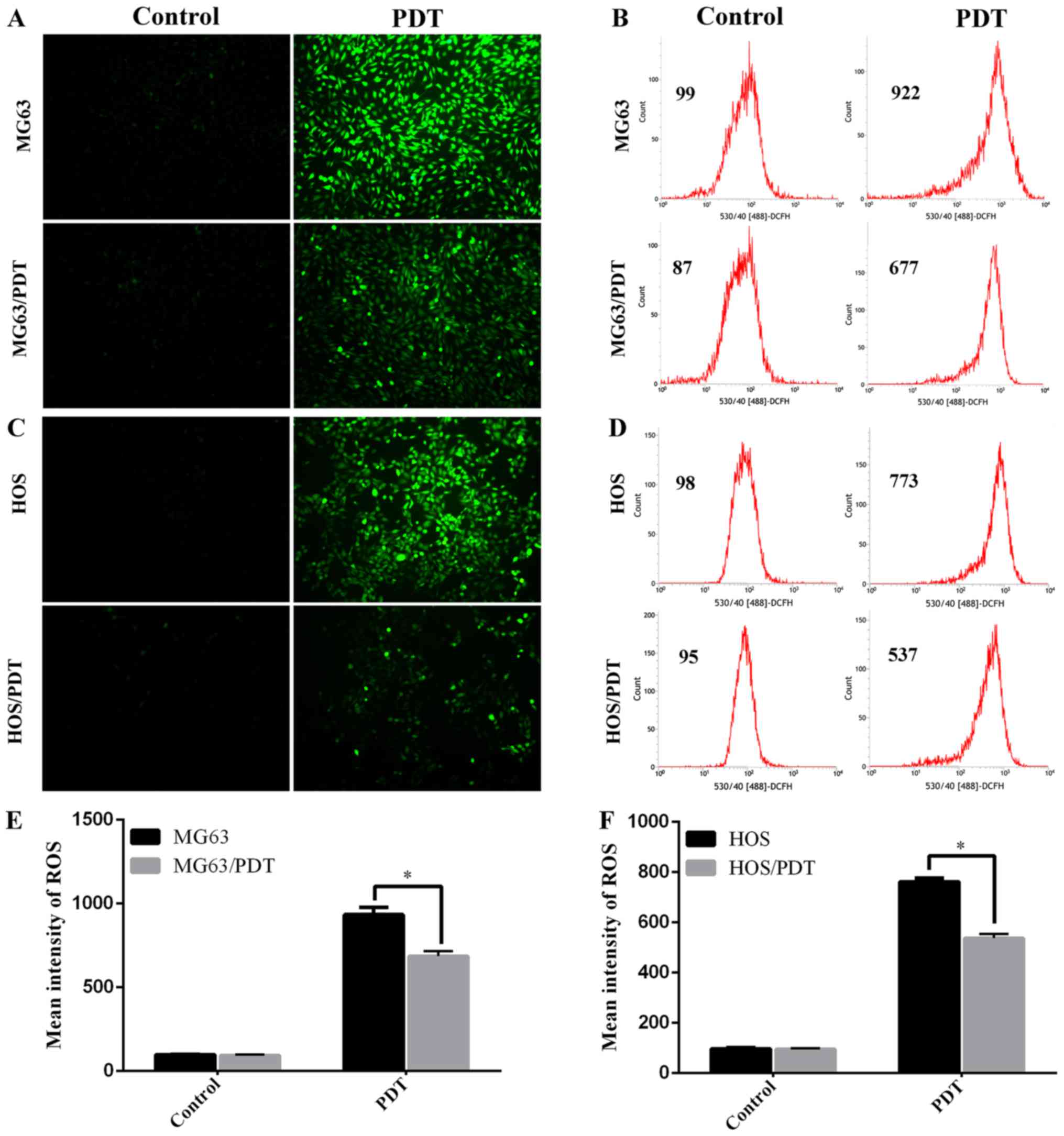
FCM and FM demonstrated that there was no
significant difference of intracellular ROS levels between MG63 and
MG63/PDT cells without PDT treatment (P=0.267). However, the ROS
level in MG63 cells was markedly higher than that in MG63/PDT after
PDT treatment (P=0.001) (Fig. 2A, B
and E). Similar results were observed between HOS and HOS/PDT
cells (Fig. 2C, D and F).
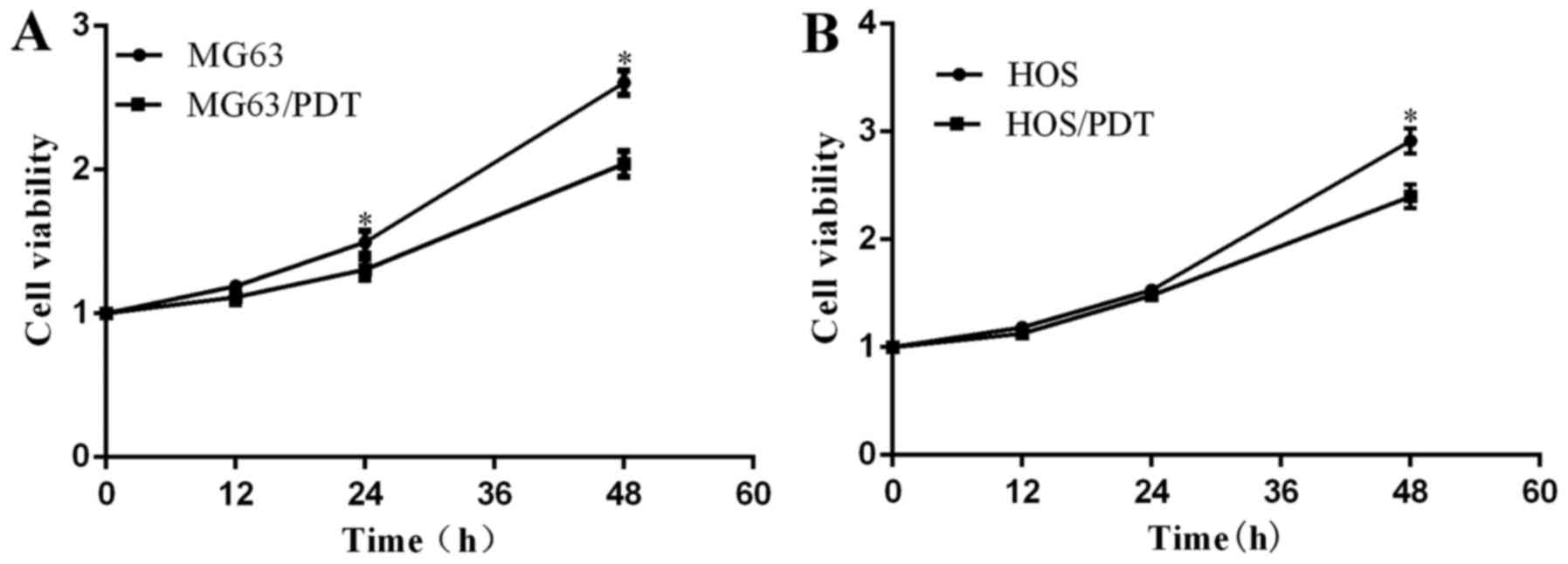
Cell proliferation test
After 12 h of culture, the MG63/PDT cells began to
grow slower compared with the MG63 cells and the difference of
growth speed was significant after 24 h (P=0.037; Fig. 3A). However, HOS/PDT began to grow
slower than the parental HOS cells after 24 h, and the difference
of growth speed was significant after 48 h (P=0.005; Fig. 3B). The proliferation curves of two
types of resistant cells were more flat compared with that of the
relatively primitive cells.
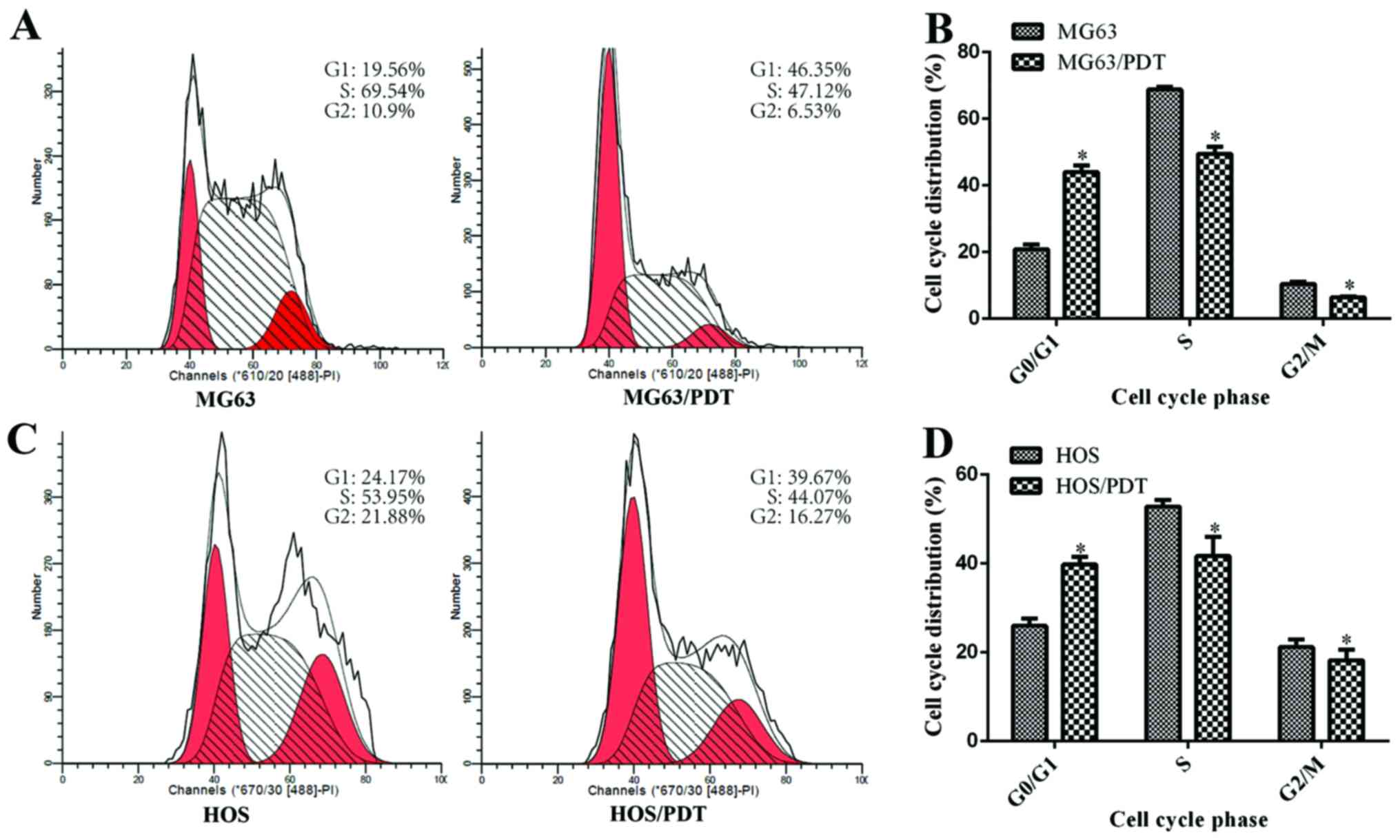
Examination of cell cycle distribution by
FCM
FCM revealed that the proportion of cells in the
G0/G1, S and G2/M phase were 19.56, 69.54 and 10.9% for MG63;
46.35, 47.12 and 6.53% for MG63/PDT (Fig. 4A); 24.17, 53.95 and 21.88% for HOS;
and 39.67, 44.07 and 16.27% for HOS/PDT (Fig. 4C), respectively. The MG63/PDT and
HOS/PDT cells exhibited shorter S and G2/M phases with a
concomitantly longer G0/G1 phase in cycle distribution than their
comparative cells (P<0.05; Fig. 4B
and D).
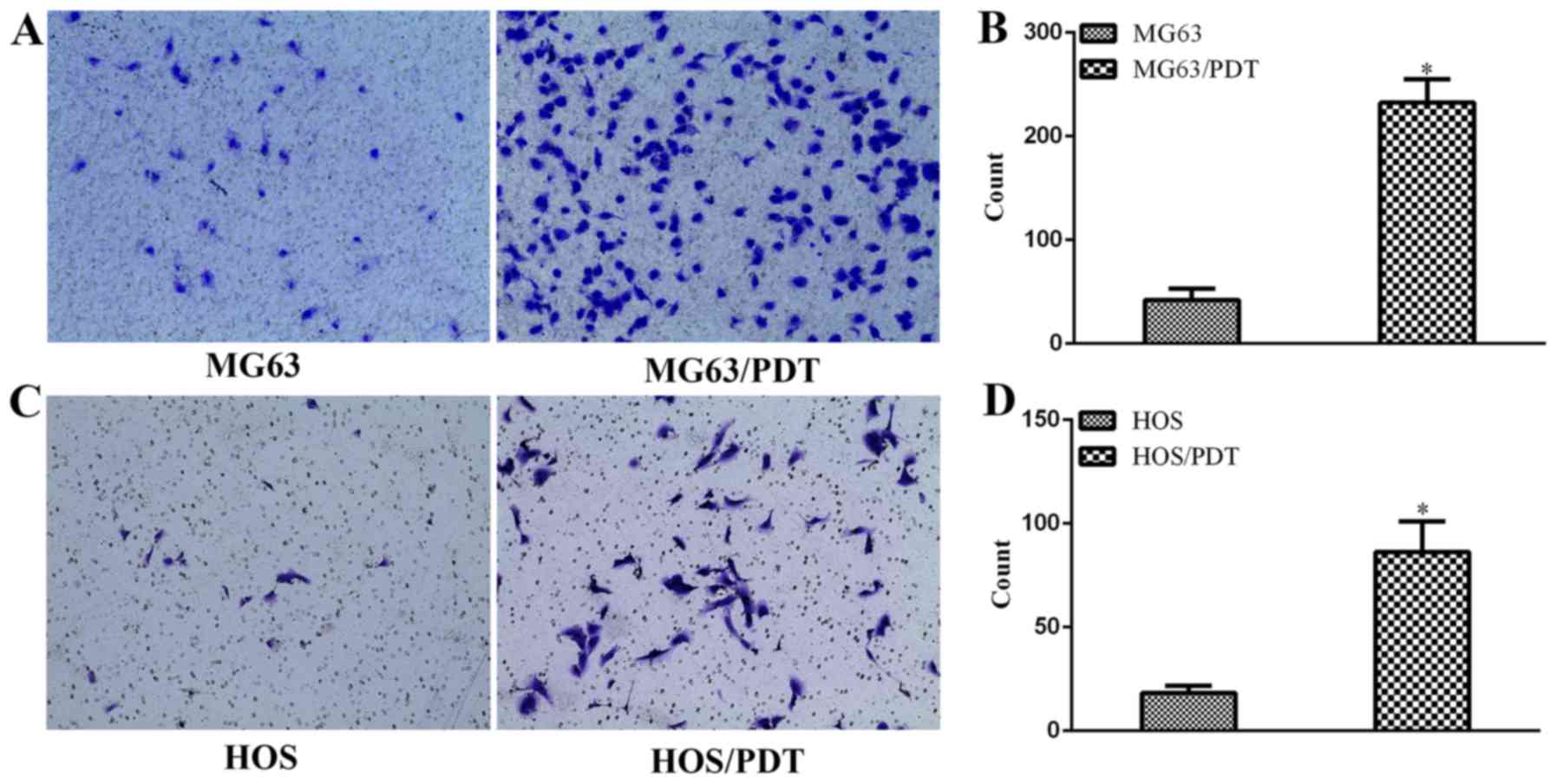
Invasion assay
Transwell assays with a layer of Matrigel on the top
inserts were employed to examine the invasive capability. The
results revealed that the number of cells penetrated both the
Matrigel and membrane was 232±22.7 for MG63/PDT cells, 42±11 for
MG63 cells (Fig. 5A and B),
86.33±14.64 for HOS/PDT cells and 18.33±3.51 for HOS cells
(Fig. 5C and D), demonstrating
that MG63/PDT and HOS/PDT had better ability of invasiveness
compared with their corresponding parental cells (P<0.05).
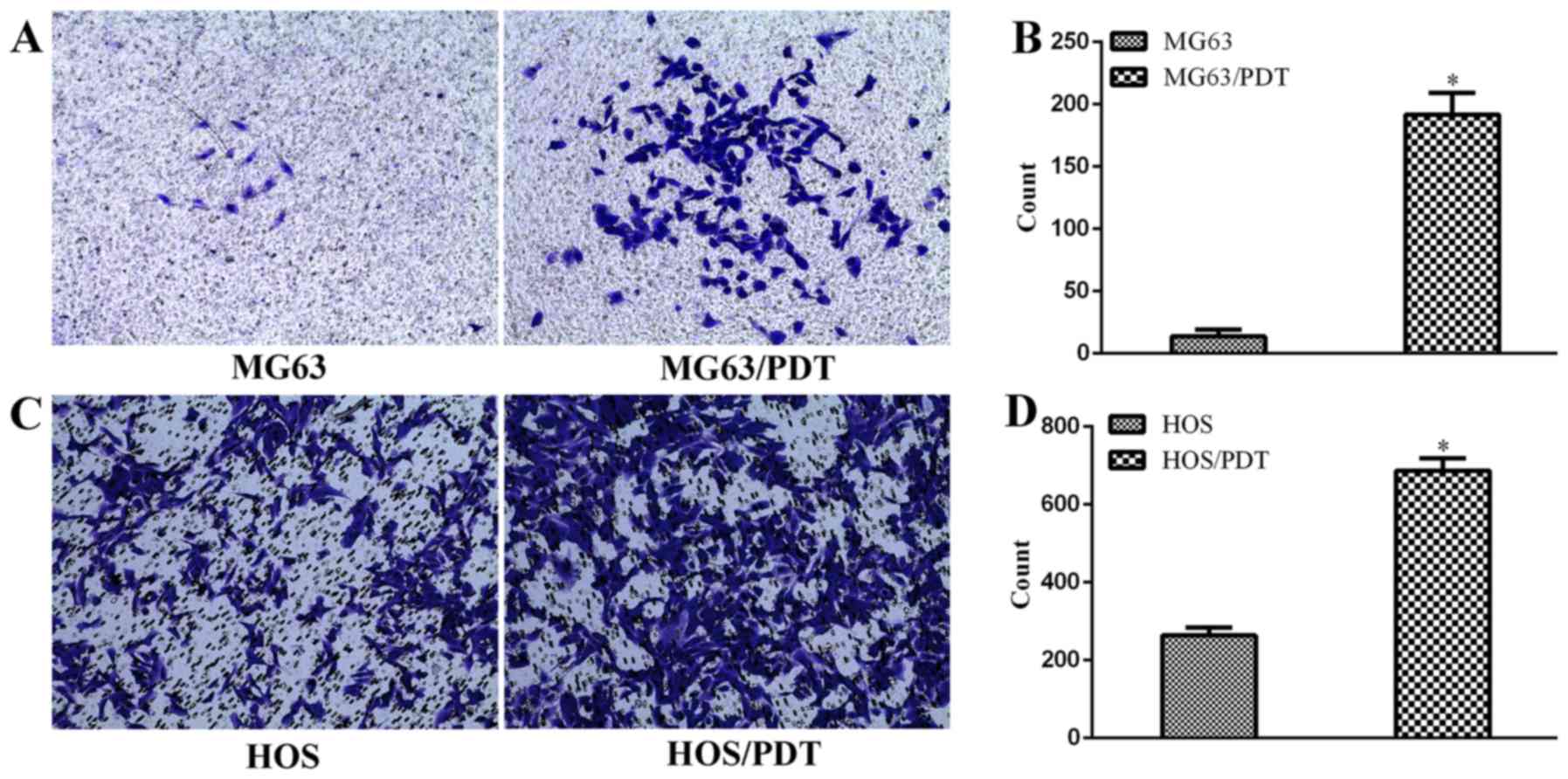
Migration assay
Transwell assay was performed to detect migration
ability. The results indicated that the number of cells penetrating
the membrane was 195±12.12 for MG63/PDT cells, 13.67±5.68 for MG63
cells (Fig. 6A and B),
687.33±31.72 for HOS/PDT cells and 263.67±20.84 for HOS cells
(Fig. 6C and D), revealing that
the migration ability of MG63/PDT and HOS/PDT cells were increased
compared with parental MG63 and HOS cells, respectively
(P<0.05).
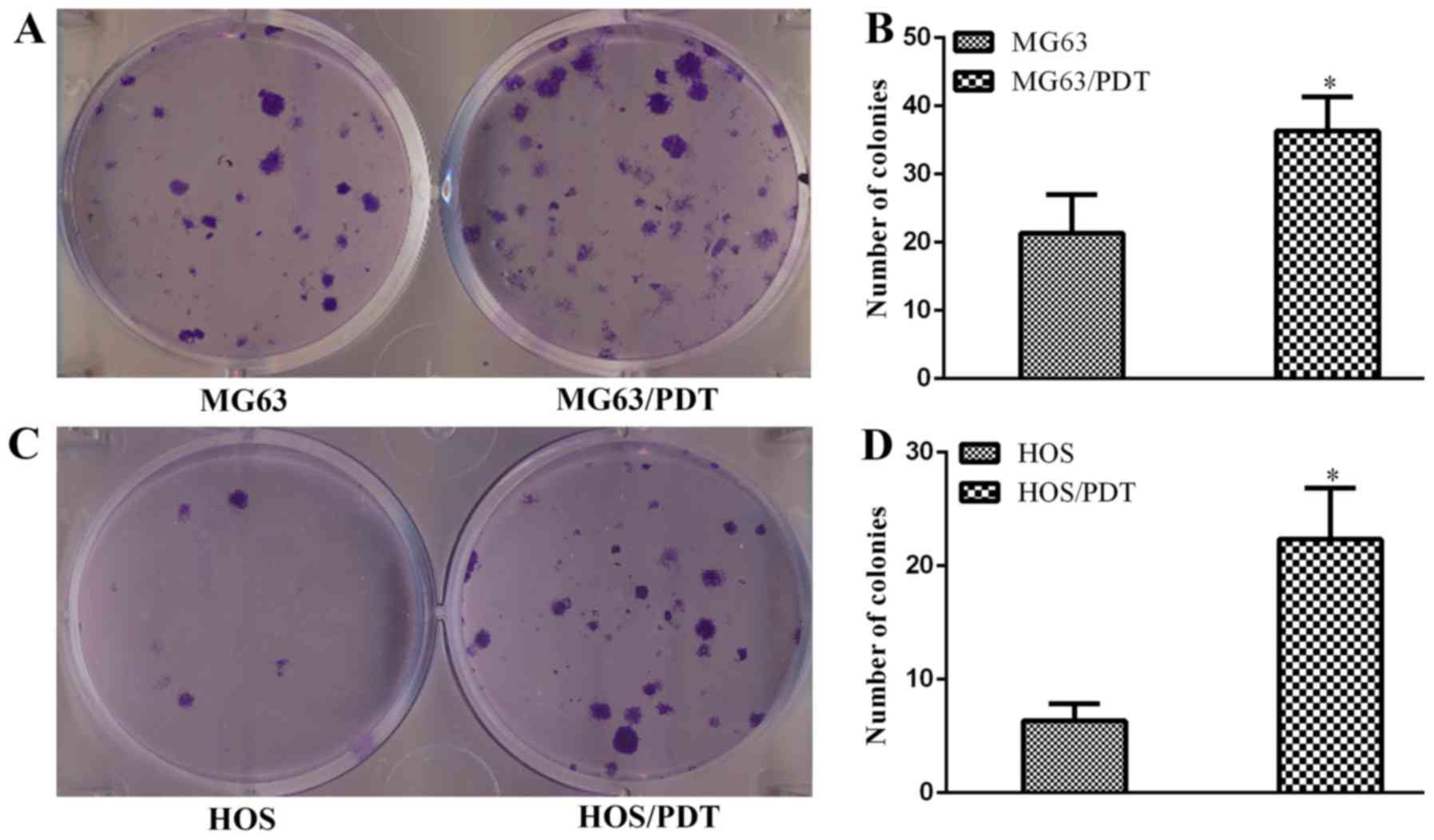
Colony formation assay
Both MG63/PDT and HOS/PDT cells had more potential
in forming colonies. The number of MG63/PDT cell colonies
(36.3±5.0) was significantly higher compared to MG63 cells
(21.3±5.7, P=0.027; Fig. 7A and
B). The number of HOS/PDT cell colonies (22.3±4.5) was also
markedly higher than HOS cells (6.3±1.5, P=0.004; Fig. 7C and D).
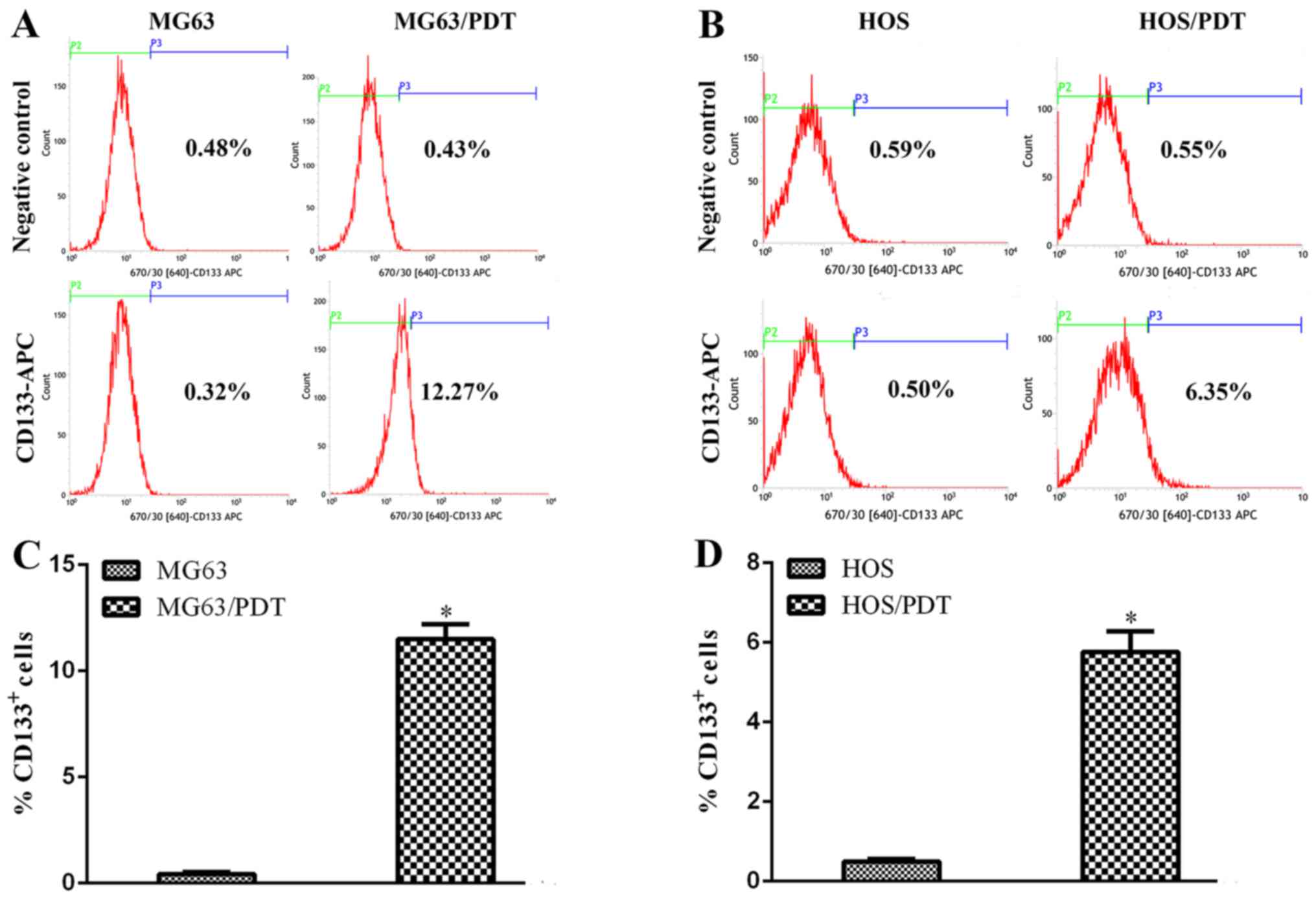
Determination of osteosarcoma stem marker
CD133 by FCM
FCM revealed that the proportion of
CD133+ cells in MG63/PDT, MG63, HOS/PDT and HOS cells
was 11.49±0.71, 0.43±0.09, 5.76±0.52 and 0.50±0.06%, respectively.
MG63/PDT and HOS/PDT exhibited more CD133+ cells than
their corresponding original cells (Fig. 8; P<0.05).
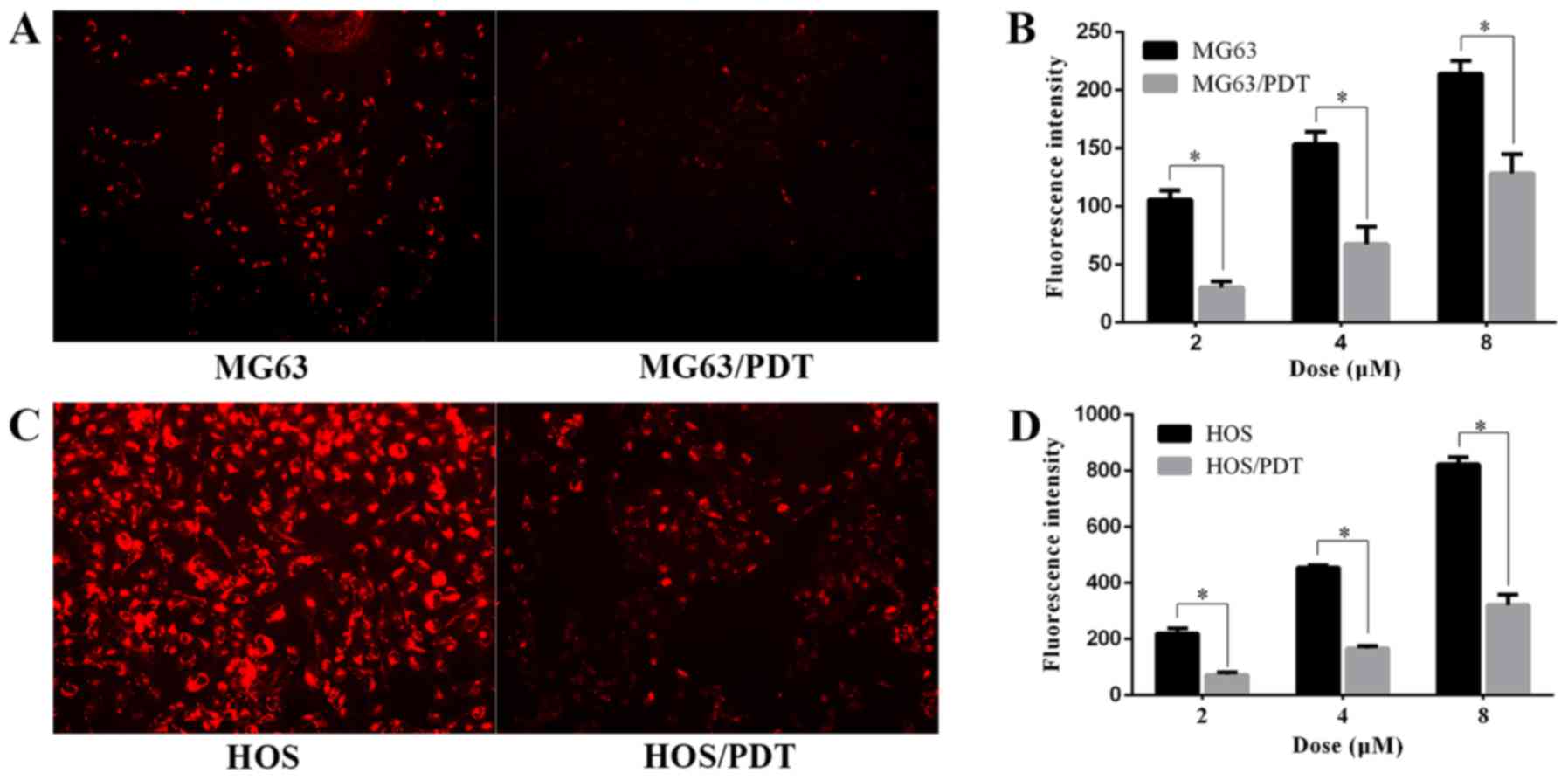
Measurement of intracellular MPPa
The fluorescence intensity of MPPa cells was
observed by FM. The results revealed that the fluorescence
intensity of MPPa in MG63 cells was stronger than that of MG63/PDT
(Fig. 9A), and the fluorescence
intensity of MPPa in HOS cells was stronger than that of HOS/PDT
cells (Fig. 9C). Microplate reader
was employed to quantify fluorescence intensity of MPPa in the
cells, revealing that MPPa content in MG63 and HOS cells was
significantly higher than that of MG63/PDT and HOS/PDT cells,
respectively (P<0.05; Fig. 9B and
D).
Cytotoxicity by CDDP
CCK-8 was adopted to assess the cyto-toxicity of
CDDP on PDT resistant and parental cells. Survival rates of
MG63/PDT and HOS/PDT were significantly higher than those of the
corresponding original MG63 and HOS cells (P<0.05; Fig. 10).
Western blot assays
WB was used to detect the expression of different
proteins. The results demonstrated that there was no significant
difference of the expression of antioxidant-related protein HO-1,
SOD1 and pro-apoptotic proteins cleaved-caspase 3, cleaved-PARP
between MG63 and MG63/PDT cells (P>0.05). After treatment with
MPPa-PDT for 12 h, the expression of HO-1, SOD1, cleaved-caspase 3
and cleaved-PARP in MG63 and MG63/PDT cells was increased, but
their expression in MG63/PDT cells was lower than those in MG63
cells (P<0.05; Fig. 11A and
B). The expression of HO-1, SOD1, cleaved-caspase 3 and
cleaved-PARP in HOS and HOS/PDT cells displayed similar results
(Fig. 11C and D).
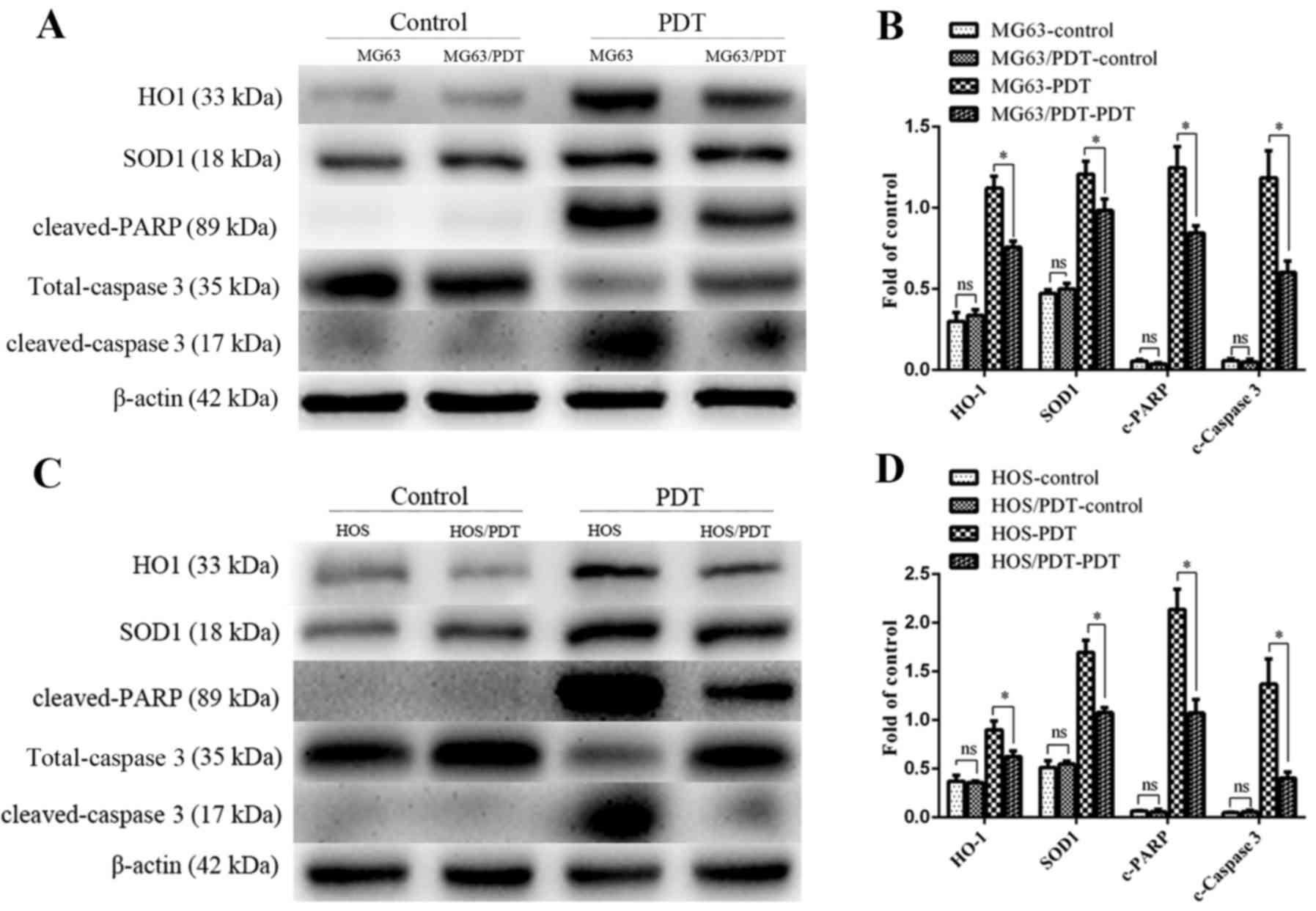 | Figure 11Changes in the expression of
antioxidant proteins HO-1, SOD1 and apoptosis proteins
cleaved-PARP, total-caspase 3, cleaved-caspase 3 following MPPa-PDT
were analyzed by western blotting as depicted in the Materials and
methods section. (A and C) WB images of HO-1, SOD1, cleaved-PARP,
total-caspase 3, cleaved-caspase 3 in cells. (B and D) The relative
protein expression levels of HO-1, SOD1, cleaved-PARP,
cleaved-caspase 3 to β-actin; *P0.05. |
To further study the characteristics of the
resistance and the mechanisms, the expression of drug
resistance-related proteins (e.g. ABCG2, MRP1, MDR,
apoptosis-related proteins, Bcl-2, Bcl-xL, P-survivin and Bax) and
cancer stemness markers CD133 in MG63, MG63/PDT, HOS and HOS/PDT
were detected. The results indicated that the levels of ABCG2,
MRP1, MDR and the anti-apoptotic proteins Bcl-2, Bcl-xL, P-survivin
in MG63/PDT and HOS/PDT cells were all more highly expressed than
those in MG63 and HOS cells (P<0.05; Fig. 12). However, the pro-apoptotic
protein Bax in MG63/PDT and HOS/PDT cells was significantly
downregulated compared with that in their parental cells
(P<0.05; Fig. 12).
Furthermore, the expression for the cancer stem marker CD133 in
MG63/PDT cells and HOS/PDT cells was markedly increased relative to
MG63 and HOS cells (P<0.05; Fig.
12).
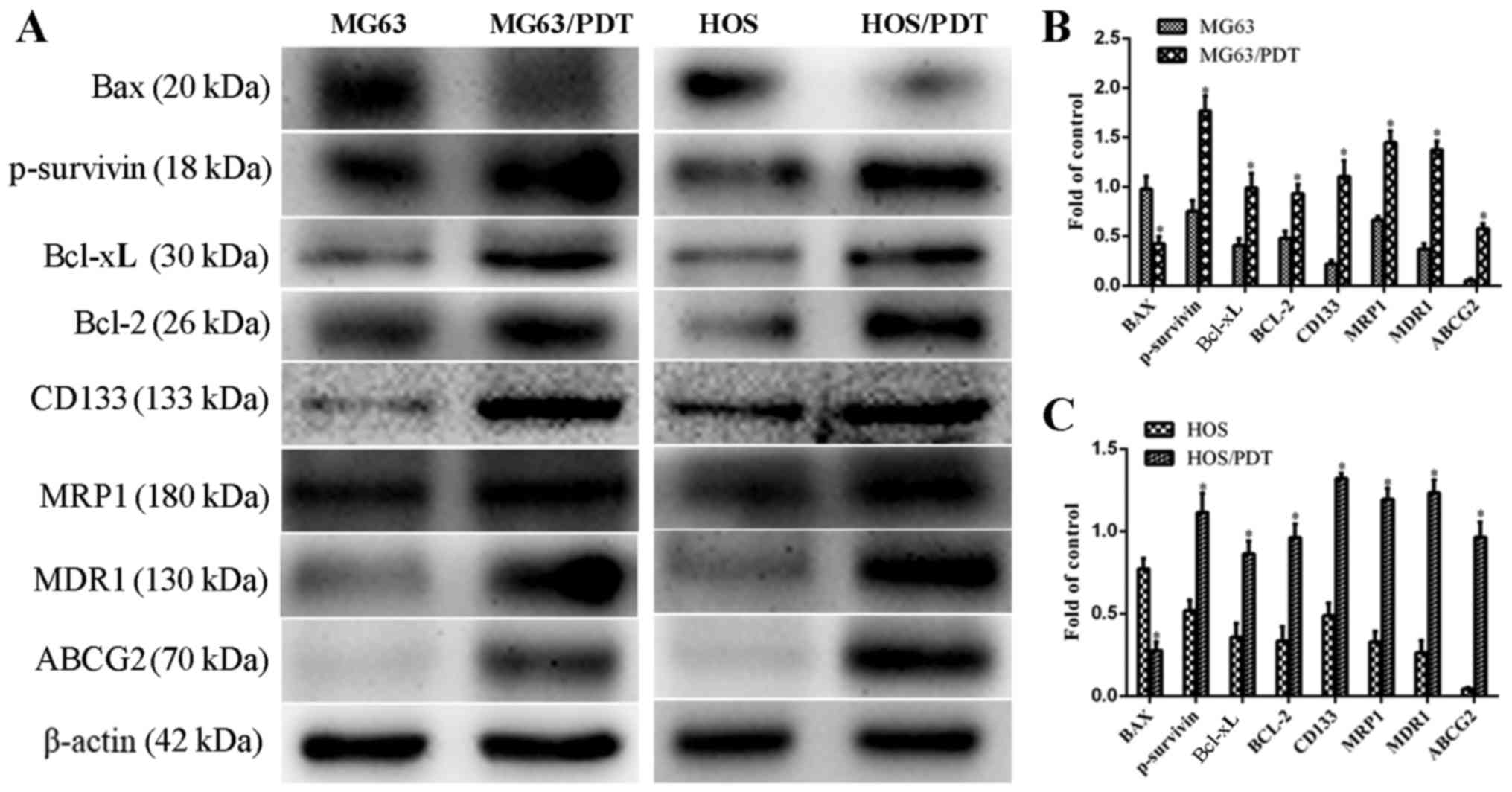 | Figure 12Different expression of Bax,
P-survivin, Bcl-xL, Bcl-2, CD133, MRP1, MDR1, ABCG2 between MG63
and MG63/PDT cells and between HOS and HOS/PDT cells. (A) WB images
of Bax, P-survivin, Bcl-xL, Bcl-2, CD133, MRP1, MDR1, ABCG2 and
β-actin in MG63, MG63/PDT cells, HOS and HOS/PDT cells. (B and C)
Protein expression levels of Bax, P-survivin, Bcl-xL, Bcl-2, CD133,
MRP1, MDR1 and ABCG2 after normalization relative to β-actin;
*P<0.05. |
Discussion
The 5-year survival rate of osteosarcoma patients
has reached 60-70% with the continuous improvement of treatment,
but the treatment is still not effective for the patients with
recurrence, metastasis and chemoresistance. PDT has emerged as an
important approach to treat tumors, with some advantages including
strong targeting, non-invasive and repeatable treatment (4). Our previous study found that
MPPa-mediated photodynamic therapy could significantly kill
osteosarcoma MG63 cells, suggesting that it may be used in the
clinical treatment of osteosarcoma patients (3). It is well known that resistance is
very common in chemotherapy, radiotherapy and other cancer therapy
(21,22). This may be related to the existence
of heterogeneous cells resistant to the therapy or the induction of
resistance in tumor cells (23).
Some surviving cells after the treatment become more aggravated,
more prone to invasion and metastasis (13,16).
However, PDT still encountered the problem of resistance due to the
expression of resistance-related proteins.
Milla et al successfully isolated squamous
carcinoma cells (SCCs) resistant to PDT by repeated methyl
d-aminolevulinic acid (Me-ALA-PDT) treatment of LD90 doses for
tumor cells (24). The present
study selected LD90 doses of MPPa-PDT for human osteosarcoma cell
lines MG63 and HOS to establish new human osteosarcoma cell lines.
However, after 3 days of treatment, all the cells died and failed
to form resistance. This may be related to mismatch speed of
resistance-related molecule expression. Thus, we chose a relatively
mild treatment condition of IC40-IC60. The MG63 and HOS cells were
subjected to 10 cycles of PDT by gradually increasing the dose of
MPPa, and finally MPPa-PDT-resistant cells were obtained, named
MG63/PDT and HOS/PDT, respectively. In order to verify the
resistance of newly constructed osteosarcoma cell lines MG63/PDT
and HOS/PDT to MPPa-PDT, we examined the expression of
cleaved-caspase 3 and cleaved-PARP, apoptosis, cell viability in
MG63, MG63/PDT, HOS and HOS/PDT cells after MPPa-PDT treatment. The
results revealed that MG63/PDT and HOS/PDT cells were more
resistant to MPPa-PDT compared to their corresponding parental
cells. There may be some mechanisms that protected them from the
damage of MPPa-PDT in osteosarcoma cells.
ROS is the main mechanism by which PDT kills
osteosar-coma cells (3,25). In the present study, ROS in
resistant cells MG63/PDT and HOS/PDT and parental cells MG63 and
HOS, was analyzed by FCM and FM. The results demonstrated that
there was no difference in the ROS level between resistant and
parental cells in the absence of treatment. However, after
treatment with PDT, the amount of ROS in resistant cells was
significantly lower than that in parental cells, suggesting that
the resistant cells changed some signal molecules to decrease the
production of ROS. The amount of ROS induced by PDT depends on the
type and the dose of the photosensitizer, irradiation time and the
ability of cells to antioxidative stress. HO-1 not only degrades
heme, but also promotes antioxidation, anti-inflammation and
anti-apoptosis (26,27). Ciesla et al found that
upregulation of HO-1 expression in rhabdomyosarcoma could reduce
intracellular ROS content and promote cell survival (28). Lv et al reported that
inhibition of HO-1 could increase the sensitivity of laryngeal
carcinoma to CDDP. Early studies also found that HO-1 expression
could decrease the damage of photodynamic therapy to tumors
(29). SOD1 is an important
antioxidant enzyme in cells, and is capable of decomposing
superoxide, and free cells of ROS damage. Soares et al
reported that inhibition of SOD1 increased the sensitivity of tumor
cells to photodynamic therapy (30,31).
In the present study, HO-1 and SOD1 expression were examined after
MPPa-PDT treatment by same MPPa and light dose. However, the
results were contrary to our expectation. The expression of HO-1
and SOD1 in resistant cells was significantly lower than those in
parental cells, though both of them were induced by MPPa-PDT. In
addition, there was no significant difference in the expression of
HO-1 and SOD1 between resistant and parental cells without MPPa-PDT
treatment. The results indicated that there may be another pathway
in resistant cells that induces the resistance to MPPa-PDT. Higher
expression of antioxidant machinery of cells definitely should
result in low ROS levels in response to a particular treatment.
Primitively, we hypothesized that MPPa-PDT-resistant osteosarcoma
cells may produce more antioxidant proteins than original cells in
order to clean out ROS. However, the results demonstrated that
MPPa-PDT-resistant osteosarcoma cells had less antioxidant proteins
than original osteosarcoma cells. Tian et al found that the
inhibition of antioxidants may increase ROS and the damage of
MPPa-PDT on tumor cells (32).
There may be another reason for the high expression of antioxidants
in parental cells. In one treatment, the expression of antioxidant
proteins was adjusted in osteosarcoma cells according to the amount
of ROS and formed tolerance to treatment, however this warrants
further exploration. Concomitantly, the expression of other
anti-oxidative stress kinases in resistant cells was also worthy of
further study.
Our previous study revealed that the cytotoxicity of
MPPa-PDT on osteosarcoma cells was in a dose-dependent manner
(3). Milla et al found that
the amount of Me-ALA in resistant SCC was less than that in
parental SCC cells after only Me-ALA treatment for 4 h (24). We hypothesized that certain
intracellular content after MPPa treatment resulted in the
difference of ROS between resistant and parental cells. By
detecting the fluorescence intensity of intracellular MPPa, we
found that the content of MPPa in the resistant cells was
significantly less than that in parental cells after the identical
MPPa treatment, suggesting that the resistant cells could decrease
the production of ROS induced by PDT owing to the less content of
intracellular photosensitizer compared with that in parental cells.
The ABC transporter family proteins with ATP enzyme activity (i.e.
ABCG2, MDR1 and MRP1) can diminish intracellular drug concentration
by accelerating drug efflux. Ishikawa et al found that
inhibition of ABCG2 increased the uptake of photosensitizer PpIX by
tumor cells (33). Liu et
al reported that inhibition of MDR1 or MRP1 could increase the
sensitivity of osteosarcoma cells to chemotherapy (34,35).
In the present study, WB results implied that the expression of
ABCG2, MDR1 and MRP1 in the resistant cells was signifi-cantly
unregulated compared to parental cells. The decrease of MPPa
content in the tolerant cells may be linked to the high expression
of ABC transporter protein. However, the definite relationship
between their high expression and the resistance to MPPa-PDT of
MG63/PDT and HOS/PDT cells warrants further studies.
In addition to the upregulation of the ABC
transporter, the anti-apoptotic and pro-apoptotic proteins may also
be involved in the formation of cell resistance. The BCL-2 protein
family is widely involved in the process of cell apoptosis. The
pro-apoptotic protein Bax can increase the permeability of the
mitochondrial membrane to facilitate the release of cytochrome
c from the mitochondria into the cytoplasm. On the contrary,
anti-apoptotic protein Bcl-2 and Bax-xL can stabilize the
mitochondrial membrane by inhibiting Bax (20). Previous studies have found that
MPPa-PDT can induce apoptosis of osteosarcoma MG63 cells by
downregulation of Bcl-2 and the promotion of the expression of Bax
(3). Survivin is a member of the
inhibitor of apoptosis protein, which can bind caspase 3, an
apoptosis executor, thereby inhibiting its activity (19). Ferrario et al found that a
reduction of survivin can increase the cytotoxicity of PDT on
breast cancer cells (36). The
expression of P-survivin is unregulated in PDT-resistant squamous
carcinoma cells (24). Our results
revealed that the expression of anti-apoptotic protein Bcl-2,
Bcl-xL and P-survivin was significantly increased in the resistant
cells MG63/PDT and HOS/PDT compared with the parental cells MG63
and HOS, and the expression of pro-apoptotic protein Bax was
notably decreased. The results demonstrated that upregulation of
anti-apoptotic proteins and downregulation of pro-apoptotic
proteins may be one of the mechanisms responsible for
PDT-resistance of MG63/PDT and HOS/PDT cells.
Under normal circumstances, the resistant cells may
accelerate proliferation to overcome the cytotoxicity of drugs
(37). In our experiments, we
found that the proliferation ability of the resistant cells
MG63/PDT and HOS/PDT was weaker than that of their primary MG63 and
HOS cells, respectively. The results are similar to those found in
the CDDP resistant osteosarcoma cells SOSP-9607/CDDP (38). The slow proliferation of the
resistant cells may be caused by the increased quiescent cells
(16). In line with this, our
results also demonstrated that there were more cells in the G0/G1
phase in resistant cells MG63/PDT and HOS/PDT compared with their
corresponding parental cells. PDT can cause DNA damage, which can
activate or inhibit some signal molecules to block the cell cycle
in the G1 phase and promote DNA repair (39,40).
The results revealed that more cells in the G0/G1 phase of the
resistant cells MG63/PDT and HOS/PDT could enhance their DNA repair
ability, which also may be one of the mechanisms responsible for
the PDT-resistance.
Casas et al found that the ability of
invasion and migration in PDT-resistant cells was significantly
weaker than that of their primary cells (41). However, Milla et al found
that the migration ability of PDT-resistant SCC cells was stronger
than that of their original cells (24). Han et al found that the
invasion ability of CDDP-resistant osteosarcoma cells was also
significantly enhanced compared to their primary cells (37). Our results revealed that the
invasion, migration and clone formation capacity of resistant cells
MG63/PDT and HOS/PDT were also significantly stronger than those of
parental cells. The invasion, migration and clone formation may
vary depending on the treatment methods, and the type of cells and
photosensitizers. The results revealed that the recurrence of
osteosarcoma after PDT may become more troublesome.
Cancer stem cells (CSCs) have the capacity of
self-renewal and differentiation, and are accountable for
proliferation, metastasis, drug resistance and recurrence of tumors
(42). CD133 is currently
recognized as a tumor stem cell marker, and many researchers have
chosen CD133 as a marker for osteosarcoma stem cells to screen
osteosarcoma stem cells (43-47).
ABCG2 is not only associated with drug resistance, but also as a
surface marker of CSC (48,49).
It was reported that osteosarcoma-cancer-stem cells exhibited
higher expression of ABCG2 and CD133 (50). In the present study, ABCG2 was
upregulated in resistant cells. The present study also revealed
that MG63/PDT and HOS/PDT cells presented higher expression of
CD133 and possessed more CD133+ cells compared to their
parental cells, demonstrating that the stemness of osteosarcoma
cells was enhanced during resistant cell construction by MPPa-PDT,
and osteosarcoma stem cells may be involved in PDT-resistance of
MG63/PDT and HOS/PDT cells. However, the proportion of
CD44+ cells, another cancer stem cell maker (51), between parental and resistant cells
did not exhibit any significant difference and the slow
proliferation and less cells in the G2/M phase of MG63/PDT and
HOS/PDT cells were inconsistent with the appearance of cancer stem
cells. It has been reported that PDT can conquer the resistance of
tumor cells to chemotherapeutic drugs (52). CCK-8 assay was used to detect the
viability of the cells treated with different doses of CDDP. The
results revealed that the PDT-resistant osteosarcoma cells also
appeared to be more resistant to CDDP, suggesting that MG63/PDT and
HOS/PDT cells were not only resistant to PDT, but also to
chemotherapy.
In conclusion, we successfully constructed two new
PDT-resistant osteosarcoma cell lines MG63/PDT and HOS/PDT which
featured by strong invasiveness, migration and stemness, and high
expression of anti-apoptotic proteins as well as the ABC
transporter family proteins, and low expression of pro-apoptotic
protein and CDDP tolerance. The newly constructed PDT-resistant
cell lines will be beneficial in the exploration of the biological
characteristics of recurrent osteosarcoma, the methods of
conquering PDT-resistance and to clarify possibly related
mechanisms.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (81572634), and the Graduate
Scientific Innovation Project of Chongqing Education Committee
(CYS15141). The authors wish to thank Mr. Yong Zhu, of the
Department of Orthopedics, The First Affiliated Hospital of
Chongqing Medical University (Chongqing, China), for their advice
and supervision with regards to the statistical analysis and
modification of the manuscript.
References
|
1
|
Wang W, Yang J, Wang Y, Wang D, Han G, Jia
J, Xu M and Bi W: Survival and prognostic factors in Chinese
patients with osteosarcoma: 13-year experience in 365 patients
treated at a single institution. Pathol Res Pract. 213:119–125.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Whelan J, McTiernan A, Cooper N, Wong YK,
Francis M, Vernon S and Strauss SJ: Incidence and survival of
malignant bone sarcomas in England 1979–2007. Int J Cancer.
131:E508–E517. 2012. View Article : Google Scholar
|
|
3
|
Huang Q, Ou YS, Tao Y, Yin H and Tu PH:
Apoptosis and autophagy induced by pyropheophorbide-α methyl
ester-mediated photodynamic therapy in human osteosarcoma MG-63
cells. Apoptosis. 21:749–760. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, et al: Photodynamic therapy of cancer: An update. CA Cancer J
Clin. 61:250–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khaled YS, Wright KE, Melcher A and Jayne
D: Anti-cancer effects of oncolytic viral therapy combined with
photodynamic therapy in human pancreatic cancer cell lines. Spring
Meeting for Clinician Scientists in Training 2015. 26–Feb. 2015,
https://doi.org/10.1016/S0140-6736(15)60371-3.
|
|
6
|
Cheng Y, Chang Y, Feng Y, Liu N, Sun X,
Feng Y, Li X and Zhang H: Simulated sunlight-mediated photodynamic
therapy for melanoma skin cancer by
titanium-dioxide-nanoparticle-gold-nanocluster-graphene
heterogeneous nanocomposites. Small. 13:16039352017. View Article : Google Scholar
|
|
7
|
Kuzyniak W, Schmidt J, Glac W, Berkholz J,
Steinemann G, Hoffmann B, Ermilov EA, Gürek AG, Ahsen V, Nitzsche
B, et al: Novel zinc phthalocyanine as a promising photosensitizer
for photodynamic treatment of esophageal cancer. Int J Oncol.
50:953–963. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yano T, Kasai H, Horimatsu T, Yoshimura K,
Teramukai S, Morita S, Tada H, Yamamoto Y, Kataoka H, Kakushima N,
et al: A multicenter phase II study of salvage photodynamic therapy
using talaporfin sodium (ME2906) and a diode laser (PNL6405EPG) for
local failure after chemoradiotherapy or radiotherapy for
esophageal cancer. Oncotarget. 8:22135–22144. 2017.PubMed/NCBI
|
|
9
|
Luo T, Wilson BC and Lu QB: Evaluation of
one- and two-photon activated photodynamic therapy with
pyropheophorbide-a methyl ester in human cervical, lung and ovarian
cancer cells. J Photochem Photobiol B. 132:102–110. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li KM, Sun X, Koon HK, Leung WN, Fung MC,
Wong RN, Lung ML, Chang CK and Mak NK: Apoptosis and expression of
cytokines triggered by pyropheophorbide-a methyl ester-mediated
photodynamic therapy in nasopharyngeal carcinoma cells. Photodiagn
Photodyn Ther. 3:247–258. 2006. View Article : Google Scholar
|
|
11
|
Tian Y, Leung W, Yue K and Mak N: Cell
death induced by MPPa-PDT in prostate carcinoma in vitro and in
vivo. Biochem Biophys Res Commun. 348:413–420. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian YY, Hu XY, Leung WN, Yuan HQ, Zhang
LY, Cui FA and Tian X: Investigation of photodynamic effect caused
by MPPa-PDT on breast cancer Investigation of photodynamic effect
caused by MPPa-PDT. Laser Phys Lett. 9:754–758. 2012. View Article : Google Scholar
|
|
13
|
Gilaberte Y, Milla L, Salazar N,
Vera-Alvarez J, Kourani O, Damian A, Rivarola V, Roca MJ, Espada J,
González S, et al: Cellular intrinsic factors involved in the
resistance of squamous cell carcinoma to photodynamic therapy. J
Invest Dermatol. 134:2428–2437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zamarrón A, Lucena SR, Salazar N,
Sanz-Rodríguez F, Jaén P, Gilaberte Y, González S and Juarranz Á:
Isolation and charac-terization of PDT-resistant cancer cells.
Photochem Photobiol Sci. 14:1378–1389. 2015. View Article : Google Scholar
|
|
15
|
Anderson SJ, Wapnir I, Dignam JJ, Fisher
B, Mamounas EP, Jeong JH, Geyer CE Jr, Wickerham DL, Costantino JP
and Wolmark N: Prognosis after ipsilateral breast tumor recurrence
and locoregional recurrences in patients treated by
breast-conserving therapy in five National Surgical Adjuvant Breast
and Bowel Project protocols of node-negative breast cancer. J Clin
Oncol. 27:2466–2473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang C, Guo LB, Ma JY, Li YM and Liu HM:
Establishment and characterization of a paclitaxel-resistant human
esophageal carcinoma cell line. Int J Oncol. 43:1607–1617. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jeon M, Rahman N and Kim YS:
Cytoprotective effect of Makgeolli lees on paraquat induced
oxidative stress in A549 cells via activation of NRF2 and
antioxidant genes. J Microbiol Biotechnol. 26:277–286. 2016.
View Article : Google Scholar
|
|
18
|
Dean M: ABC transporters, drug resistance,
and cancer stem cells. J Mammary Gland Biol Neoplasia. 14:3–9.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang B, Pan JS, Liu JY, Han SP, Hu G and
Wang B: Effects of chemotherapy and/or radiotherapy on survivin
expression in ovarian cancer. Methods Find Exp Clin Pharmacol.
28:619–625. 2006. View Article : Google Scholar
|
|
20
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View Article : Google Scholar
|
|
21
|
Restifo NP, Smyth MJ and Snyder A:
Acquired resistance to immunotherapy and future challenges. Nat Rev
Cancer. 16:121–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wicki A, Mandalà M, Massi D, Taverna D,
Tang H, Hemmings BA and Xue G: Acquired resistance to clinical
cancer therapy: A Twist in physiological signaling. Physiol Rev.
96:805–829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Solyanik GI: Multifactorial nature of
tumor drug resistance. Exp Oncol. 32:181–185. 2010.
|
|
24
|
Milla LN, Cogno IS, Rodríguez ME,
Sanz-Rodríguez F, Zamarrón A, Gilaberte Y, Carrasco E, Rivarola VA
and Juarranz A: Isolation and characterization of squamous
carcinoma cells resistant to photodynamic therapy. J Cell Biochem.
112:2266–2278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tu P, Huang Q, Ou Y, Du X, Li K, Tao Y and
Yin H: Aloe-emodin-mediated photodynamic therapy induces autophagy
and apoptosis in human osteosarcoma cell line MG-63 through the
ROS/JNK signaling pathway. Oncol Rep. 35:3209–3215. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chau LY: Heme oxygenase-1: Emerging target
of cancer therapy. J Biomed Sci. 22:222015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Furfaro AL, Traverso N, Domenicotti C,
Piras S, Moretta L, Marinari UM, Pronzato MA and Nitti M: The
Nrf2/HO-1 axis in cancer cell growth and chemoresistance. Oxid Med
Cell Longev. 2016:19581742016. View Article : Google Scholar
|
|
28
|
Ciesla M, Marona P, Kozakowska M, Jez M,
Seczynska M, Loboda A, Bukowska-Strakova K, Szade A, Walawender M,
Kusior M, et al: Heme oxygenase-1 controls an HDAC4-miR-206 pathway
of oxidative stress in rhabdomyosarcoma. Cancer Res. 76:5707–5718.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lv X, Song DM, Niu YH and Wang BS:
Inhibition of heme oxygenase-1 enhances the chemosensitivity of
laryngeal squamous cell cancer Hep-2 cells to cisplatin. Apoptosis.
21:489–501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soares HT, Campos JR, Gomes-da-Silva LC,
Schaberle FA, Dąbrowski JM and Arnaut LG: Pro-oxidant and
antioxidant effects in Photodynamic Therapy: Cells recognize that
not all exogenous ROS are alike. ChemBioChem. 17:836–842. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wright KE, MacRobert AJ and Phillips JB:
Inhibition of specific cellular antioxidant pathways increases the
sensitivity of neurons to meta-tetrahydroxyphenyl chlorin-mediated
photodynamic therapy in a 3D co-culture model. Photochem Photobiol.
88:1539–1545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian S, Yong M, Zhu J, Zhang L, Pan L,
Chen Q, Li KT, Kong YH, Jiang Y, Yu TH, et al: Enhancement of the
effect of methyl pyropheophorbide-a-mediated photodynamic therapy
was achieved by increasing ROS via inhibition of Nrf2-HO-1 or
Nrf2-ABCG2 signaling. Anticancer Agents Med Chem. 17:12017.
View Article : Google Scholar
|
|
33
|
Ishikawa T, Nakagawa H, Hagiya Y,
Nonoguchi N, Miyatake S and Kuroiwa T: Key role of human ABC
transporter ABCG2 in photodynamic therapy and photodynamic
diagnosis. Adv Pharmacol Sci. 2010:5873062010.PubMed/NCBI
|
|
34
|
Li C, Guo D, Tang B, Zhang Y, Zhang K and
Nie L: Notch1 is associated with the multidrug resistance of
hypoxic osteosarcoma by regulating MRP1 gene expression. Neoplasma.
63:734–742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu T, Li Z, Zhang Q, De Amorim Bernstein
K, Lozano-Calderon S, Choy E, Hornicek FJ and Duan Z: Targeting
ABCB1 (MDR1) in multi-drug resistant osteosarcoma cells using the
CRISPR-Cas9 system to reverse drug resistance. Oncotarget.
7:83502–83513. 2016.PubMed/NCBI
|
|
36
|
Ferrario A, Rucker N, Wong S, Luna M and
Gomer CJ: Survivin, a member of the inhibitor of apoptosis family,
is induced by photodynamic therapy and is a target for improving
treatment response. Cancer Res. 67:4989–4995. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Otto T and Sicinski P: Cell cycle proteins
as promising targets in cancer therapy. Nat Rev Cancer. 17:93–115.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han T, Zhu X, Wang J, Zhao H, Ma Q, Zhao
J, Qiu X and Fan Q: Establishment and characterization of a
cisplatin-resistant human osteosarcoma cell line. Oncol Rep.
32:1133–1139. 2014.PubMed/NCBI
|
|
39
|
Baldea I, Olteanu DE, Bolfa P, Tabaran F,
Ion RM and Filip GA: Melanogenesis and DNA damage following
photodynamic therapy in melanoma with two meso-substituted
porphyrins. J Photochem Photobiol B. 161:402–410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Borgstahl GEO, Brader K, Mosel A, Liu S,
Kremmer E, Goettsch KA, Kolar C, Nasheuer HP and Oakley GG:
Interplay of DNA damage and cell cycle signaling at the level of
human replication protein A. DNA Repair. 21:12–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Casas A, Di Venosa G, Vanzulli S, Perotti
C, Mamome L, Rodriguez L, Simian M, Juarranz A, Pontiggia O, Hasan
T, et al: Decreased metastatic phenotype in cells resistant to
aminolevulinic acid-photodynamic therapy. Cancer Lett. 271:342–351.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stuckey DW and Shah K: Stem cell-based
therapies for cancer treatment: Separating hope from hype. Nat Rev
Cancer. 14:683–691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tirino V, Desiderio V, d'Aquino R, De
Francesco F, Pirozzi G, Graziano A, Galderisi U, Cavaliere C, De
Rosa A, Papaccio G, et al: Detection and characterization of
CD133+ cancer stem cells in human solid tumours. PLoS
One. 3:e34692008. View Article : Google Scholar
|
|
44
|
Veselska R, Hermanova M, Loja T, Chlapek
P, Zambo I, Vesely K, Zitterbart K and Sterba J: Nestin expression
in osteosarcomas and derivation of nestin/CD133 positive
osteosarcoma cell lines. BMC Cancer. 8:3002008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He A, Qi W, Huang Y, Feng T, Chen J, Sun
Y, Shen Z and Yao Y: CD133 expression predicts lung metastasis and
poor prognosis in osteosarcoma patients: A clinical and
experimental study. Exp Ther Med. 4:435–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li J, Zhong XY, Li ZY, Cai JF, Zou L, Li
JM, Yang T and Liu W: CD133 expression in osteosarcoma and
derivation of CD133+ cells. Mol Med Rep. 7:577–584.
2013. View Article : Google Scholar
|
|
47
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar
|
|
48
|
Robey RW, Polgar O, Deeken J, To KW and
Bates SE: ABCG2: Determining its relevance in clinical drug
resistance. Cancer Metastasis Rev. 26:39–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ding XW, Wu JH and Jiang CP: ABCG2: A
potential marker of stem cells and novel target in stem cell and
cancer therapy. Life Sci. 86:631–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Di Fiore R, Santulli A, Ferrante RD,
Giuliano M, De Blasio A, Messina C, Pirozzi G, Tirino V, Tesoriere
G and Vento R: Identification and expansion of human
osteosarcoma-cancer-stem cells by long-term 3-aminobenzamide
treatment. J Cell Physiol. 219:301–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jaggupilli A and Elkord E: Significance of
CD44 and CD24 as cancer stem cell markers: An enduring ambiguity.
Clin Dev Immunol. 2012:7080362012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Spring BQ, Rizvi I, Xu N and Hasan T: The
role of photodynamic therapy in overcoming cancer drug resistance.
Photochem Photobiol Sci. 14:1476–1491. 2015. View Article : Google Scholar : PubMed/NCBI
|