Introduction
Cancer is one of most deadly diseases, while
hepatocellular carcinoma (HCC) lies in the third position in cancer
caused deaths. Resection is often used for certain patients, but
mostly transiently, the reason is lack of clinical marker for
surgeon due to tumor metastasis (1). Therefore, the chemotherapy is still
important procedure in clinical practice, but the side-effect and
drug-resistance limit it widely use. Looking for novel drug with
highly selectivity and efficiency is an urgent task for medical
research. It has been recognized that tumor microenvironment plays
an important role in the metastasis process (1,2), the
metalloenzymes, such as MMPs in tumor environment are required to
degrade extracellular matrix, thus, the metalloproteases are also
considered as targets in metastasis inhibition (3). Copper, iron and zinc are essential
trace metal elements for most organisms, those metal ions present
in either in metalloenzymes or metal labile pools. Generally,
cancer cell has higher demand for iron compared to normal cell,
meanwhile to acquire metastatic phenotype the vascularization is
also required for tumor cell escaping from local site, the copper
ion has critical role during the vascularization, copper-lowering
directly results in inhibition of angiogenesis and cancer cell
growth (4,5), therefore, use of chelators for cancer
treatment is prospective strategy.
Dithiocarbamates are an important class of
sulfur-containing compounds with various applications in medicine,
such as diethyldithiocarbamate and brassinin. They have been used
for the treatment of bacterial, fungal infections, AIDS and cancer
(6). To achieve better biological
activity, many studies of structural modification on compounds have
been conducted (7–11), some of which were found to have
excellent antitumor activity in vitro and in vivo.
Due to many metal complexes exhibiting significant
antiproliferative activity in clinic, many metal complexes of
dithiocarbamates have been prepared and investigated in antitumor
activity. Some of them, as such gold, platinum and palladium
complexes showed prospective use in cancer therapy and entered
preclinical observation (12,13).
Preliminary mechanistic studies revealed that dithiocarbamate
derivatives can be inhibitors of nuclear factor kappa B (14), proteasome inhibitor (11), DNA intercalator, and inactivator of
numerous metal-containing enzymes (15). However, whether the
antiproliferative activity of dithiocarbamate correlates with p53
activation remains to be elucidated (16–18).
To gain more details of dithiocarbamate against tumor cells, in the
present study we made a novel dithiocarbamate derivative
(dipyridylhydraone dithiocarbamate s-acetic acid, DpdtaA), which
contain both a heterozygous coordination unit and S-alkylated
group. The new derivative may achieve higher stability and less
undesirable consequences due to not directly inactivating enzymes
that cell growth required. To address the role of p53 in
antiproliferative activity of the dithiocarbamate, p53 expression
at different conditions was assessed. The preliminary mechanistic
study revealed that the antiproliferative effect of the new
dithiocarbamate derivative was partly involved in ROS production,
p53 mediated apoptosis and autophagy. The p53 upregulation was
attributed to downregulation of Mdm2 (mouse double minute 2
homolog), and potent disruption of the interaction between p53 and
Mdm2 based on molecular docking. Furthermore, p53 activation led to
alteration in apoptosis-related proteins and autophagy after
exposure of the agent to the cell lines. Besides, copper ion could
attenuate cytotoxicity of DpdtaA, which was similar to previously
observations.
Materials and methods
General information
MTT, di-2-pyridylketone, 3-methylad-enin (3-MA),
Pifithrin-α, RPMI-1640 and other chemicals were purchased from
Sigma-Aldrich. LC3 antibody was obtained from Proteintech Group
(Wuhan, China); antibodies p53, caspase-8, β-actin, cytochrome
c, Bax, Mdm2 and Bcl-2 were purchased from Wuhan Boster
Biological Technology, Ltd. (Wuhan, China).
Preparation of di-2-pyridylhydrazone
dithiocarbamate s-acetic acid (DpdtaA)
DpdtaA (generated by ACDLabs:
3-[({2-[di(pyridin-2-yl)methylidene] hydrazinyl} carbonothioyl)
sulfanyl] acetic acid) was made by three-step reactions as
indicated in Fig. 1: the first two
steps were as previously described (19). In addition, the final product
(DpdtaA) was prepared by reaction of di-2-pyridylhydrazone
dithiocarbamate (1 mmol) with 2-bromo acetic acid in absolute
ethanol (5 ml), the yellow solid was filtered and washed with
ethanol. TLC tracing (ethyl acetate/petroleum ether = 3:1) showed
the reaction completed. Following a flash chromatography the purity
of the compound was achieved to 98%. Yield: 80%; mp: 148.6°C;
composition:
C14H12N4O2S2;
1HNMR (Bruker, D6-DMSO): 8.86 (d, H, J = 4
Hz), 8.64 (d, H, J = 4 Hz), 8.02 (m, 3H, J = 4, 8 Hz), 7.63 (m, 2H,
J = 8 Hz), 7.55 (ddd, H, J = 4, 8 Hz), 4.12 (s, 2H).
13CNMR: 199.25, 169.87, 155.09, 151.14, 149.20, 148.94,
145.43, 138.24, 137.92, 128.04, 125.72, 124.93, 123.96, 36.80. IR
(cm−1): IR (KBr, cm−1): 3088, 1709, 1584,
1458, 1435, 1417, 1393, 1326, 1292, 1234, 1202, 1188, 1127, 1058,
1018, 959, 905, 802, 791, 750, 695, 654, 640, 596, 472. ESI-MS
(microTOF-Q III, Bruker): m/z: 371.0043 (M+K, calcd:
371.00389).
Cytotoxicity (MTT) assay
A 10 mM DpdtaA in 70% dimethyl sulfoxide (DMSO) was
diluted to the required concentration with culture. The copper
complex was prepared by mixing DpdtaA with CuCl2 (at
high concentration) based on 1:1 molar ratio and diluted to
required concentration with DMSO. The MTT assay was conducted as
previously described (20).
Briefly, the HepG2 (5×103/ml) cells were seeded
equivalently into 96-well plate and the various amount of DpdtaA
(or its copper complex) was added after the cells adhered.
Following 48-h incubation at 37°C in a humidified atmosphere of 5%
CO2, 10 μl MTT solution (5 mg/ml) was added to
each well, and incubated further for 4 h. After removing the cell
culture, 100 μl DMSO was added in each well to dissolve the
formed formazan. The absorption of the solution that was related to
the number of live cells was performed on a microplate reader (MK3;
Thermo Fisher Scientific, Waltham, MA, USA) at 490 nm. Percent
growth inhibition was defined as percent absorbance inhibition
within appropriate absorbance in each cell line. The same assay was
performed in triplicate. Morphologic study was conducted under
inverted microscope (Shanghai Batuo Instrument Co., Ltd., Shanghai,
China), the photographs of HepG2 cells exposed to DpdtaA (1.56 or
3.12 μM DpdtaA or DpdtaA-Cu for 24 or 48 h were recorded
(objective size: 10×20).
ROS detection in vitro and in vivo
ROS production assessment was conducted as
previously reported (21).
Briefly, H2DCF-DA was first converted to
dichlorofluorescin (DCF) by NaOH, following neutralization, the
hydrolysate was used for the assay. Reaction system contained
either single reagent or multicomponent in 50 mM sodium phosphate
buffer (pH 7.4) with total 4 ml volume, i.e., 0.4 μM DCF, or
with 6.25 μM
(NH4)2Fe(SO4)2 (or
CuCl2 or DpdtaA) and 200 μM
H2O2 (1 mM) for the Fenton reactions. The
fluorescence was detected by a FC-960 spectrofluorimeter
(excitation at 488 and emission at 525 nm; Shanghai Lengguang
Technology Co., Ltd., Shanghai, China) in a 10-min time course at
room temperature.
The intracellular ROS assay was measured as company
recommended (Beyotime Institute of Biotechnology, Beijing, China).
Briefly, ~106 HepG2 cells were collected and washed by
phosphate-buffered saline (PBS), then the cells were stained by
H2DCF-DA in serum-free culture medium for 30 min,
followed by washing of serum-free culture to remove excess
H2DCF-DA. Then, 100 μl the cell suspension was
transferred to individual PCR tube and the test compound (or
positive control) was added, following 1-h incubation, the cell
suspension was used directly for ROS detection on a FC-960
spectrofluorimeter (excitation at 488 nm and emission at 525 nm;
Shanghai Lengguang Technology).
Western blot analysis
Briefly, 1×107 HepG2 cells treated with
or without the DpdtaA or DpdtaA-Cu were scraped off in lysis buffer
(50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1.0% NP-40, 10% glycerol and
protease inhibitors) and subjected to sonication, following spin
down by centrifugation at 14,000 × g. The clear supernatant was
stored at −80°C. The protein concentration was determined using a
colorimetric Bio-Rad DC protein assay on a microplate reader MK3 at
570 nm. Proteins (50 μg) were separated on a 13% sodium
dodecyl sulfate-polyacrylamide gel at 200 V for 1 h. The separated
proteins were subsequently transferred onto a PVDF membrane at 60 V
for 1 h. The membrane was washed three times with Tris-buffered
saline (TBS) and was then blocked for 2 h in TBS containing 0.1%
Tween-20 and 5% non-fat skimmed milk. The membrane was incubated at
4°C overnight with the primary monoantibody used at a dilution of
1:300 in TBS plus 0.1% Tween-20 (TBST). The membrane was washed
several times with TBST and was subsequently incubated with
HRP-conjugated secondary antibody (1:2,000 in TBST) for 1 h at room
temperature. After another wash of the membrane with TBST, the
protein bands were detected using a super sensitive ECL solution
(Wuhan Boster Biological Technology), and visualized on an Amersham
Imager 600 (GE Healthcare Life Sciences, Fairfield, CT, USA).
Cytoflow analysis of apoptosis
Cells were seeded into a 6-well flask and treated as
described above for the cell viability assay. The cells were
treated with different concentrations of the agents (0.78 and 1.56
μM DpdtaA or 3.12 and 6.25 μM DpdtaA-Cu) for 24 h.
Then the cell culture was removed, following PBS washing, trypsin
digestion, finally the Annexin V and propidium iodide (a kit from
Dojindo Laboratories, Kumamoto, Japan) were added as recommended by
the company. The stained cells were subjected to cytoflow
analysis.
Molecular docking studies
The structure of human type MDM2 (3jzk) was obtained
from the RCSB Protein Data Bank. The structure of DpdtaA was
generated by Chemdraw (Chemdraw Ultra 8.0; CambridgeSoft,
Cambridge, MA, USA). The energy minimization was conducted by
Chem3D (Ultra 8.0; CambridgeSoft). PyMol and LigPlot displayed the
conformation, interaction between bound ligands and residue around
the binding cavity (22,23).
Molecular docking studies were performed by AutoDock
Vina and AutoDock Tools based on the recommended procedure
(24). Grid box was set to the
center of Yin model, and the grid box size for DpdtaA was set to
22, 24 and 28 for x-, y- and z-axes, respectively. The DpdtaA was
set as a flexible ligand by using the default parameters of the
AutoDock Tools. The optimal conformation of the ligand was
generated by AutoDock Vina.
DpdtaA and its copper complex induced
autophagy and LMP
Cells were seeded into a 24-well flask and treated
as described above in the cell viability assay. The cells were
treated with different concentrations of the agents (0.78 and 1.56
μM DpdtaA or 3.12 and 6.25 μM DpdtaA-Cu) for 24 h.
For detection of the acidic cellular compartment, acridine orange
(or LysoTracker Red; Invitrogen) was used, which emits bright red
fluorescence in acidic vesicles but green fluorescence in the
cytoplasm and the nucleus. After treatment of the cells with the
agent, acridine orange was added at a final concentration of 1
μg/ml (the concentration of LysoTracker Red, as recommended)
for a period of 15 min. Following PBS washing, the fluorescent
micrographs were captured using an inverted fluorescence
microscope.
Results
Preparation and proliferation inhibition
of DpdtaA
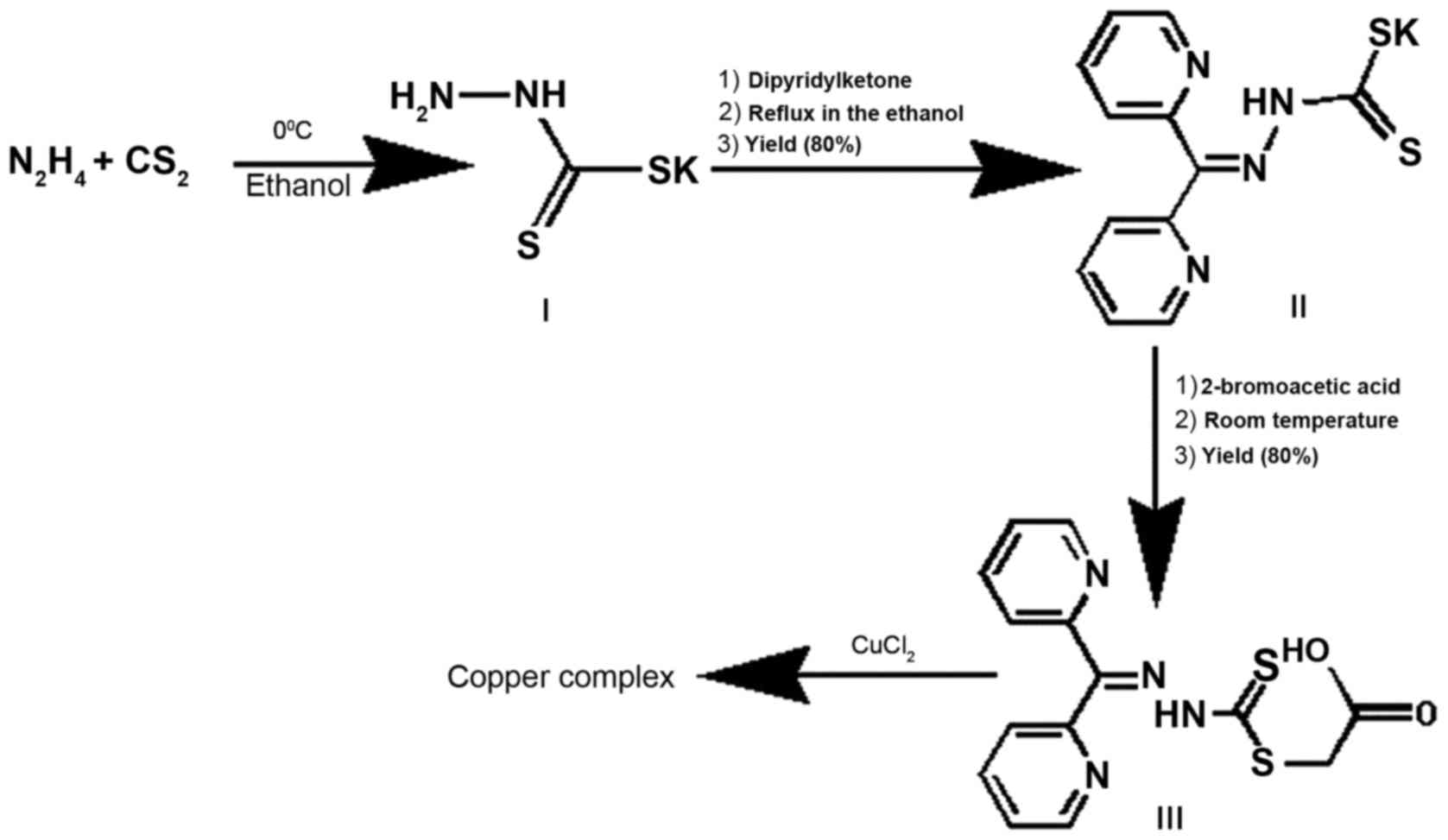
A three-step reaction as described in Fig. 1 was used to generate the DpdtaA.
Firstly, hydrazine reacted with carbon disulfide to form hydrazine
dithiocarbamate (HdtC, I), then following addition of
dipyridineketone, the dipyridylhydrazone dithiocarbamate (DpdtC,
II) was made as previously described (19). The DpdtaA (III) was prepared by
DpdtC reaction with 2-bromoacetic acid. The new compound was traced
by TLC, purified by flash chromatography. Finally, the chemical
structure of DpdtaA was characterized by NMR and HRMS spectra. HPLC
and NMR showed that agents have adequate purity (>98%, data not
shown), indicating that the new prepared DpdtaA can be used for
biological assay.
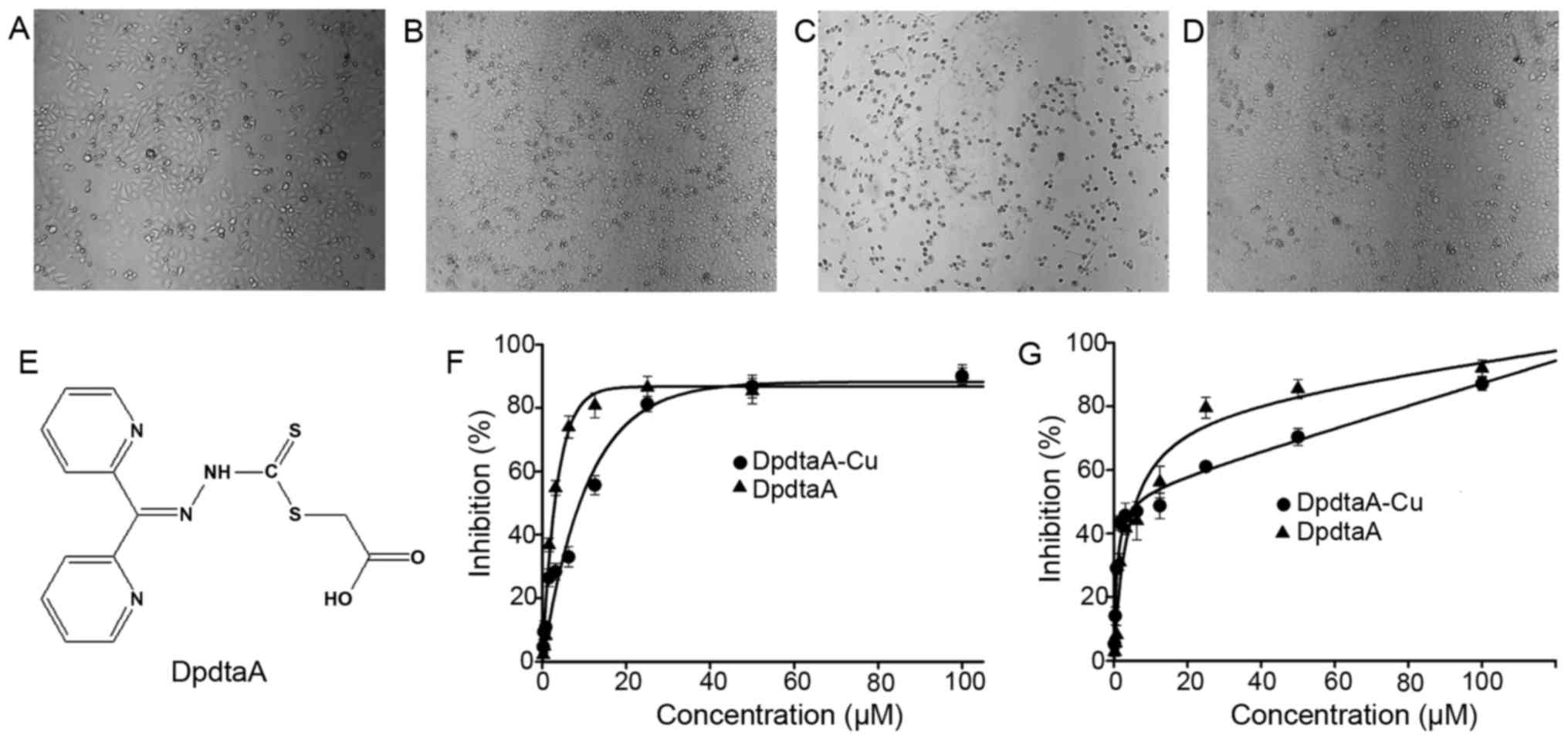
We are interested in the biological activity of new
synthetic DpdtaA, thus, antiproliferative activity of DpdtaA
against hepatocellular carcinoma cell lines was screened (Fig. 2F–G), the dose-response curves are
depicted in Fig. 2. As shown in
Fig. 2F–G, DpdtaA exhibited
significant growth inhibition for HepG2 and Bel-7402
(IC50 ≤6 μM), but the cell line dependence in
dose-response was also obvious. For HepG2 cells, the maximal
inhibition of ~80% can be achieved at 12.5 μM (Fig. 2F), but only 40% inhibition for
Bel-7402 at the same concentration of DpdtaA (Fig. 2G). In view of the important role of
copper ion in dithiocarbamates activity, the proliferation
inhibition of DpdtaA in the presence of copper ion was also
investigated. As previously reported, an antagonistic effect in
growth inhibitions was observed for both cell lines, leading to an
~3-fold decrease in HepG2 cells based on IC50 value, but
this was not evident for Bel-7402 cells except maximal inhibition,
which was few for dithiocarba-mate derivatives. To gain details,
the morphologic change was further investigated. As shown in
Fig. 2A–D, the DpdtaA caused
rounded HepG2 cells, but the addition of copper ion significantly
attenuated the action, showing an antagonistic effect as we
previously reported (19). We
speculated that the 'antagonistic effect' might stem from a new
species which was confirmed at DpdtaA/Cu =1:1 ratio in composition
by spectral titration (data not shown).
DpdtaA and its copper complex (DpdtaA-Cu)
induces ROS generation
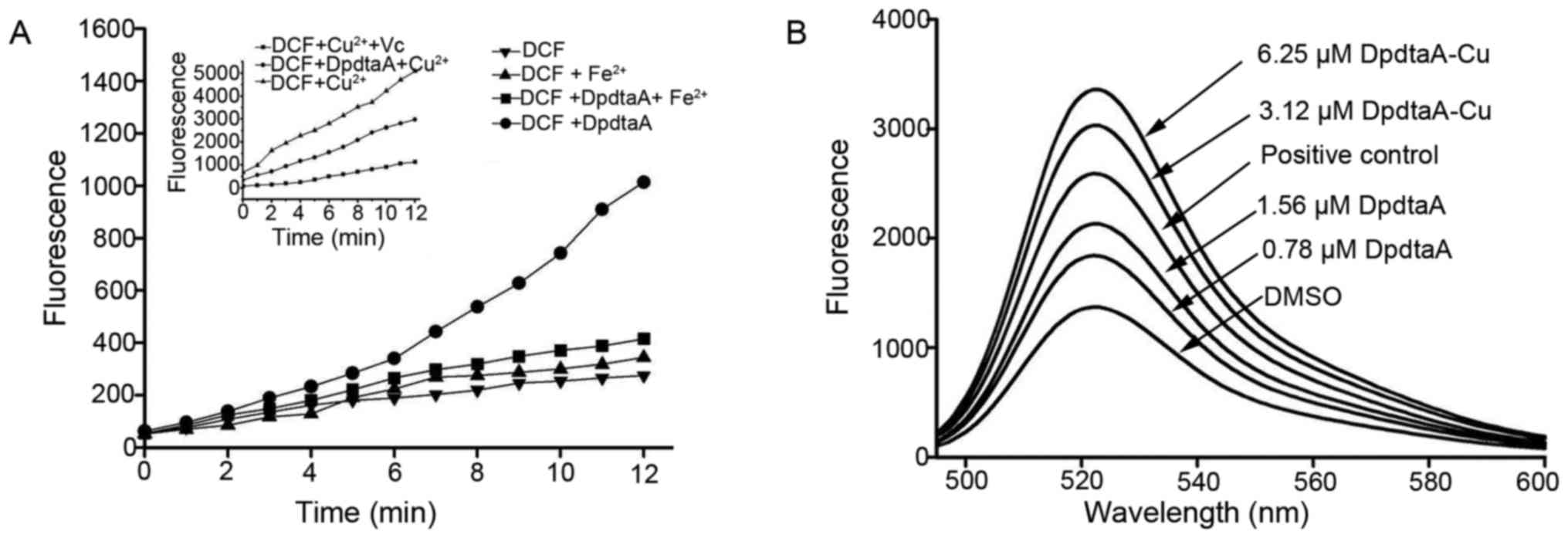
It is prevailingly accepted concept that
antiproliferation inhibition (or cytotoxicity) of many drugs stem
from generation of reactive oxygen species (ROS). DpdtaA may
chelate iron or copper, thus, involved in Fenton or Fenton-like
reaction. The ROS production in vitro is shown in Fig. 3A, fluorescence intensities of DCF
in the presence of DpdtaA and Fe2+ was higher than that
of Fe2+, indicating that iron DpdtaA complex is redox
active and could produce more ROS. In contrast, DpdtaA-Cu has
weaker ability to generate ROS, however, in the reduced environment
[presence of ascorbic acid (Vc)], the DpdtaA-Cu induced highest
content of ROS. To further confirm the in vitro results, the
drugs inducing intracellular ROS were investigated, as shown in
Fig. 3B, both DpdtaA and its
copper complex induced ROS generation in a concentration-dependent
manner. It was noted that the fluorescent intensities of DCF from
copper complex treated cells were significantly greater than that
of DpdtaA, which might be related to the redox feature of
Cu2+/1+ complexes, because the copper (I) complex could
also react with oxygen molecule to form superoxide radical except
with H2O2 (25).
ROS mediates proliferative
inhibition
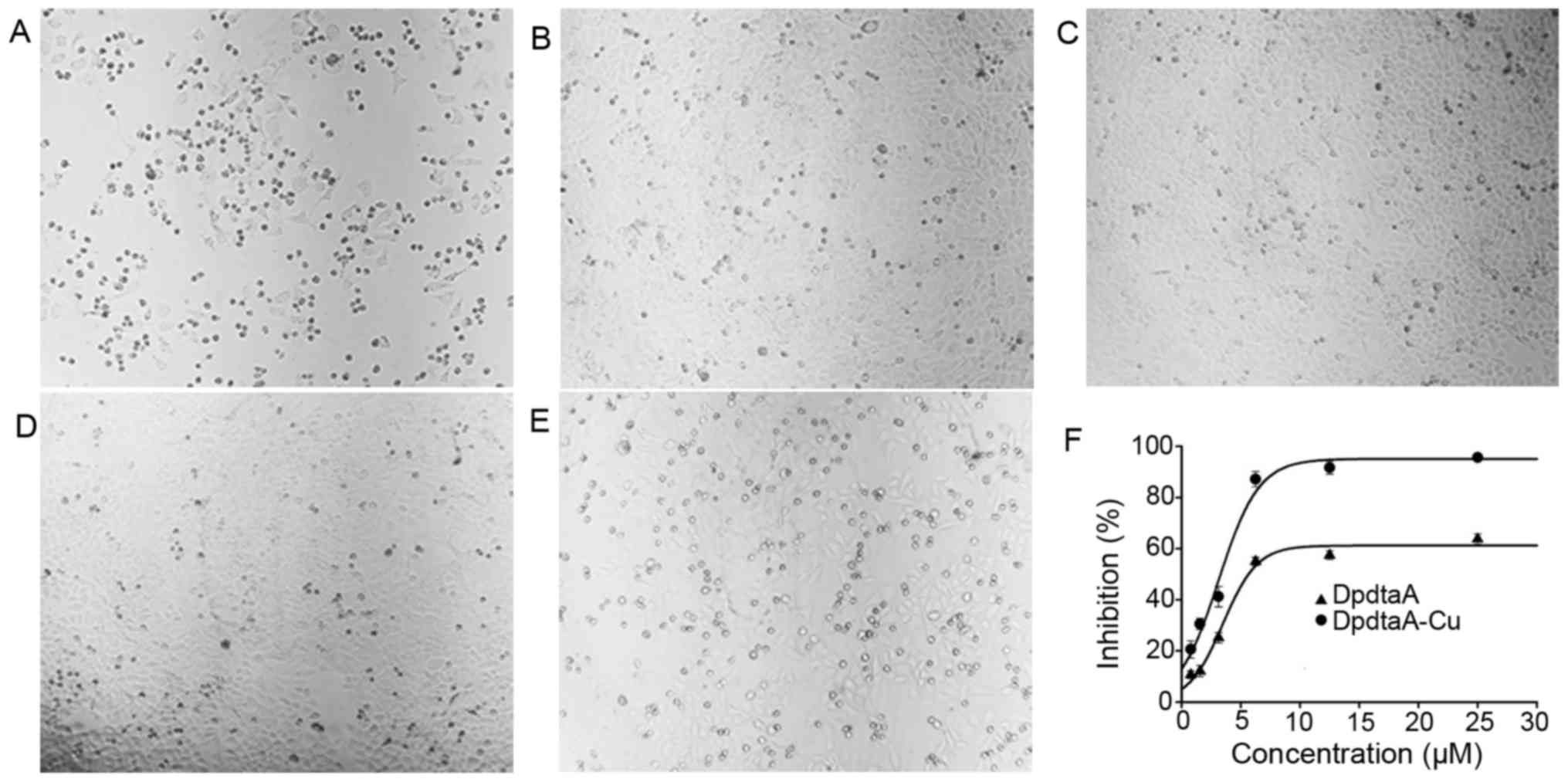
As mentioned above, both DpdtaA and its copper
complex induced ROS generation, whether the ROS generation was
involved in antiproliferative activity was unclear. Thus, the
correlation between ROS and growth inhibition, the effect of
reducing agent, N-acetylcysteine (NAC) on growth inhibition of
DpdtaA was investigated. The morphologic changes induced by DpdtaA
in the absence or presence of NAC are shown in Fig. 4. It was clear that the numbers of
rounded cells after addition of DpdtaA were decreased with
increasing NAC (Fig. 4A–C),
indicating that the NAC can protect the HepG2 cells from ROS
oxidative damage. However, a contrary phenomenon in the DpdtaA-Cu
treated cells was also observed, i.e., rounded cells increased
(Fig. 4D–E) with addition of NAC.
The situation was speculated to attribute to the redox feature of
Cu2+ which can be reduced to Cu1+ by NAC, and
the resulting DpdtaA-Cu1+ reacted with O2 to
generate more ROS. To confirm the speculation, the growth
inhibition in the presence of NAC was further evaluated. As shown
in Fig. 4F, the antiproliferative
activity of DpdtaA was significantly decreased from ~90 to 60%, but
the growth inhibition of DpdtaA-Cu dramatically increased,
indicating the antiproliferative action of the agents was ROS
mediated.
DpdtaA and its copper complex induce
partly cellular apoptosis
It has been well documented that the excess
intracellular ROS induce apoptosis whether the ROS induced by the
agents involved in apoptosis was required to be determined. To
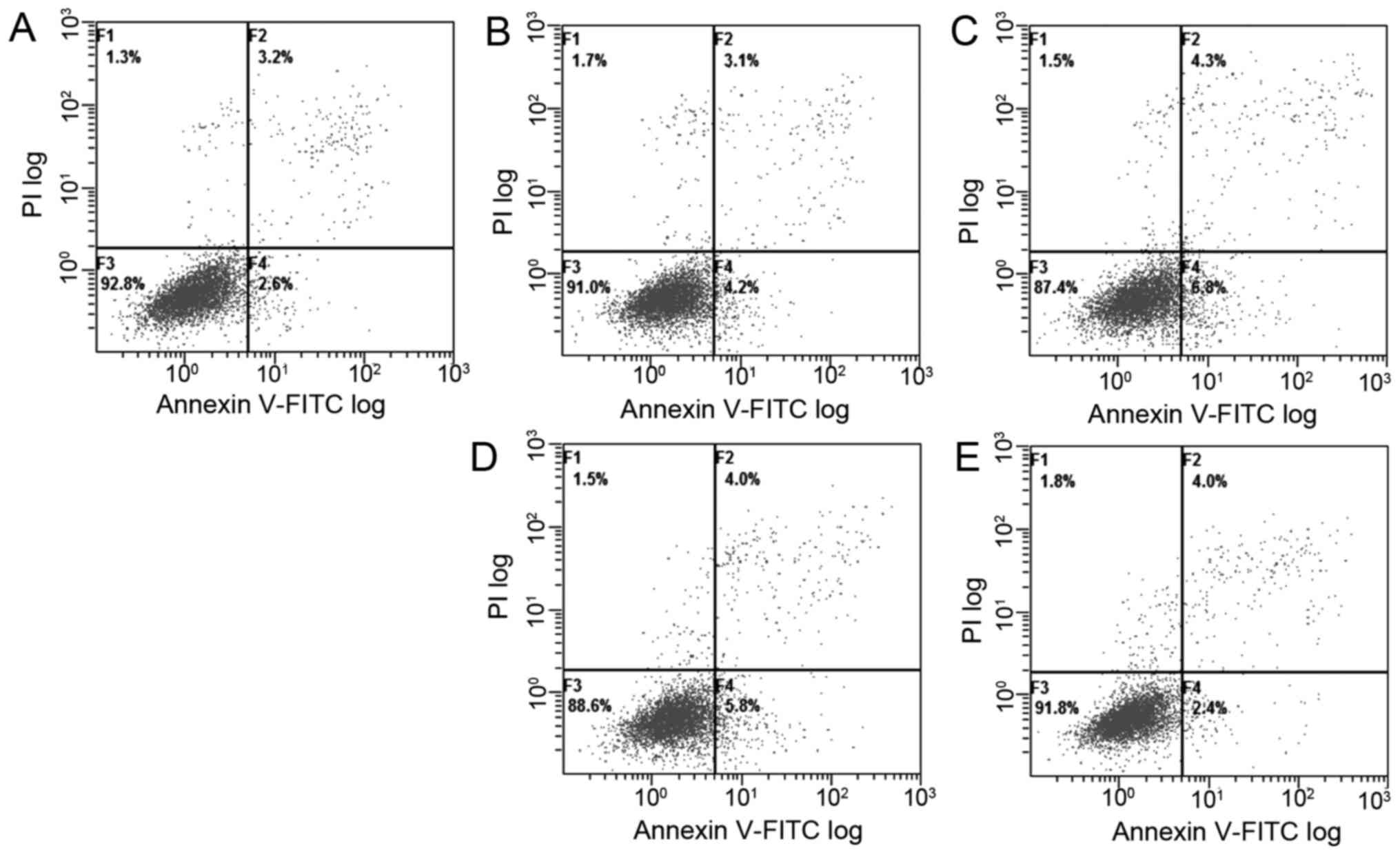
measure the apoptosis populations at early and late stages, the
Annexin V/propidium iodide (PI) staining was performed, which
measures externalization of phosphatidylserine on the cell surface
of apoptotic cells specifically. The flow cytometric analyses
showed that the DpdtaA induced early apoptosis and later apoptosis
in a concentration-dependent manner (Fig. 5A–C, from 5.8 to 11.1%), but the
apoptotic ratios were smaller after 24-h treatment of DpdtaA.
Similar situation for DpdtaA-Cu, just the percentage was less
(5.8–9.8%) even at higher concentration.
Generally the excess ROS lead to
apoptosis frequently signaling through changes in the expression of
bcl-2 family proteins
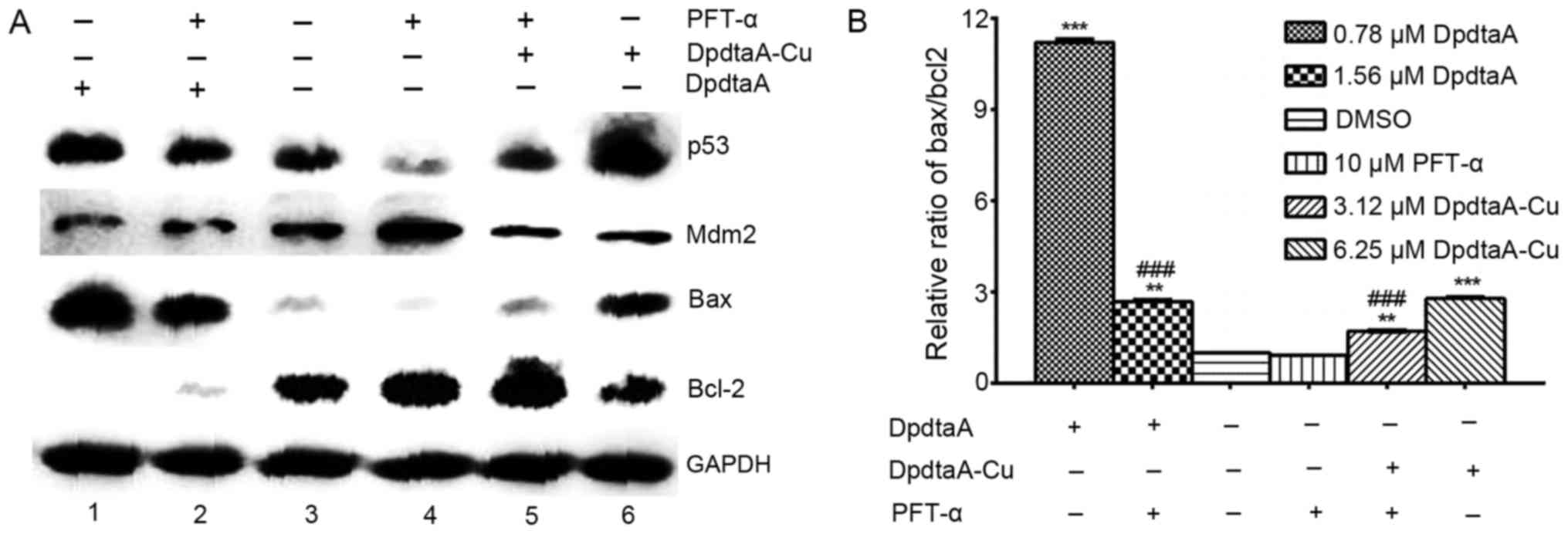
To further support that the growth inhibition may
involve apoptosis, the expressions of apoptosis related proteins
were investigated by western blotting. The alterations of bcl-2 and
bax proteins before and after treatment with DpdtaA at different
concentrations were recorded (Fig.
6). It was clear that the increased bax was observed in both
DpdtaA and DpdtaA-Cu treated HepG2 and Bel-7402 cells compared to
that of control (Fig. 6A-a and
B-a), on the contrary bcl-2, a pro-survival protein was
downregulated in the agent treated cells. For comparative purposes,
the ratio of bax/bcl-2 was normalized (Fig. 6A-b and B-b). As shown in Fig. 6A-b the difference in ratio of
bax/bcl-2 was obvious, 23–35-fold increase for DpdtaA, 4- to
17-fold for DpdtaA-Cu. Similar results were observed in Bel-7402
cells except smaller folds (Fig.
6B-b). The increased bax/bcl2 determined that apoptosis
occurred when the cells were treated with the agents. To correlate
the apoptosis with ROS, the ROS scavenger NAC was added to the
Bel-7402 cells either individually or combined. As shown in
Fig. 6C-a, the NAC did decrease
the ratio of bax/bcl2 compared to the DpdtaA (DpdtaA-Cu) treated
group only, indicating that the agent-induced apoptosis was
ROS-dependent.
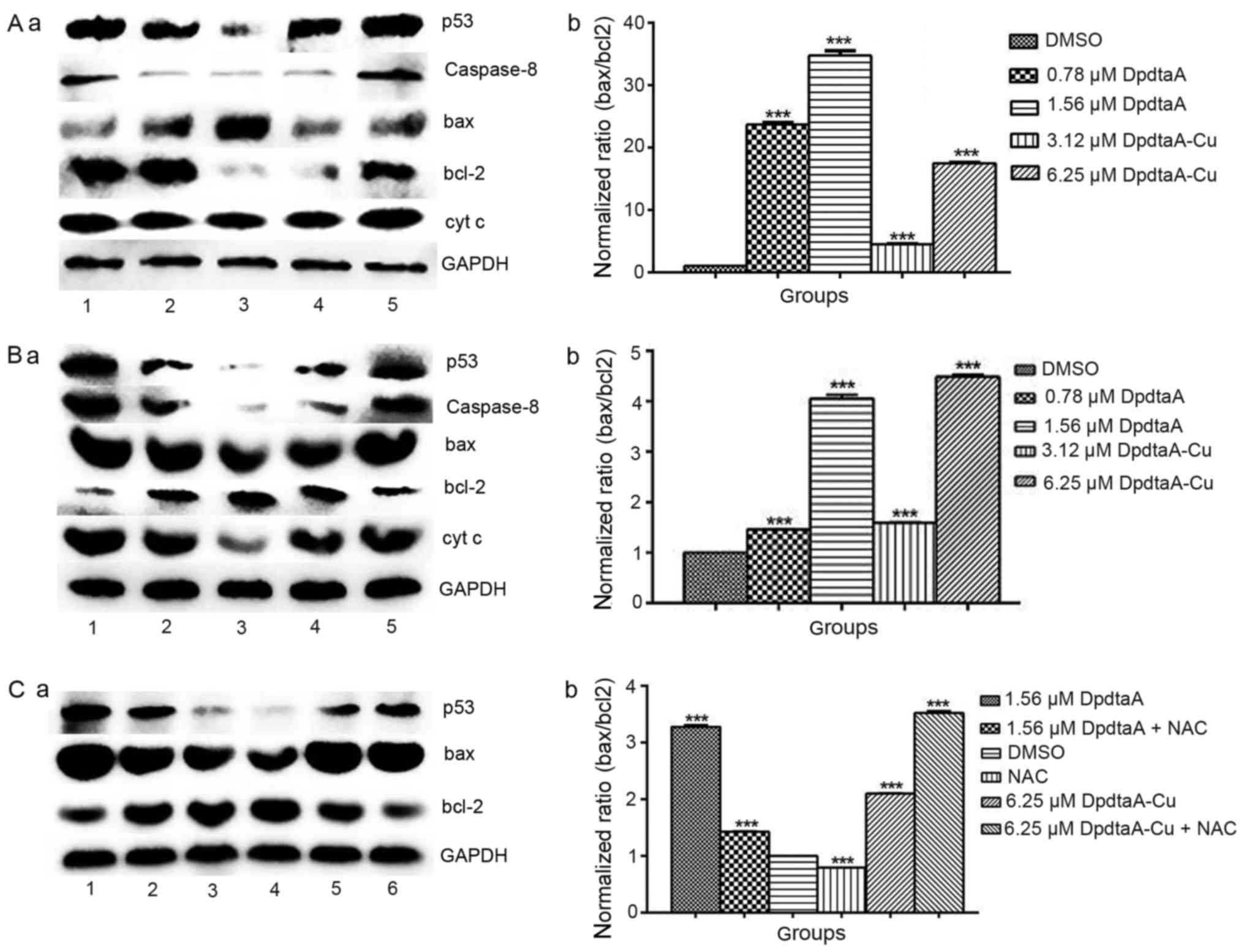 | Figure 6Western blot analysis of changes of
apoptosis-related genes. (A-a) The changes of apoptosis-related
protein in HepG2 cells, (A-b) normalized ratio of bax/bcl2; (B-a)
the expression of apoptosis-related protein in Bel-7402 cells,
(B-b) normalized ratio of bax/bcl2; lane 1, 1.56 μM DpdtaA;
lane 2, 0.78 μM DpdtaA; lane 3, DMSO; lane 4, 3.12 μM
DpdtaA-Cu; lane 5, 6.25 μM DpdtaA-Cu. (C-a) The effect of
NAC on apoptosis-related protein in Bel-7402 cells, (C-b)
normalized ratio of bax/bcl; lane 1, 1.56 μM DpdtaA; lane 2,
1.56 μM DpdtaA plus NAC; lane 3, DMSO; lane 4, NAC; lane 5,
6.25 μM DpdtaA-Cu; lane 6, 6.25 μM DpdtaA-Cu plus
NAC. (***P<0.01; one-way ANOVA). |
The change of permeability of the mitochondria and
cytochrome c release from the mitochondria are a key step in
the process of apoptosis and is one of markers in intrinsic
apoptosis, the increase of cytochrome c in cytoplasm
(Fig. 6A-a and B-a), indicating
that intrinsic apoptosis was involved in the growth inhibition
induced by the agents. To determine whether apoptosis was due to
p53 regulation, the expression of p53 was assessed, as shown in
Fig. 6, the upregulated p53 may
hint that there was a correlation between p53 and apoptosis. On the
other hand, the upregulation of caspase-8 may also indicate that an
extrinsic pathway protease was involved.
p53 upregulation correlate with
downregulation of Mdm2 and disruption interaction between p53 and
Mdm2 when exposing the agents to HepG2 cells
The DpdtaA and its copper complex induced
alterations in apoptosis related proteins in both cell lines were
similar, and could upregu-late p53. Hence, the role of p53 required
to be further investigated. Pifithrin-α (PFT-α) is a specific
wild-type p53 inhibitor, and the expression of wild-type p53 in
HepG2 cells was monitored in the presence or absence of 10 μM
PFT-α. Western blot analysis revealed that the expression of p53
was significantly attenuated by PFT-α treatment or combination with
the agents, compared with the treatment of agents alone (Fig. 7A). These results indicate that p53
played a role in agent-induced apoptosis. It has been accepted that
cellular p53 levels is primarily controlled through its
ubiquitin-mediated proteasomal degradation (26,27)
and the mouse double minute protein 2 (Mdm2) as the principal
endogenous E3-ligase with high specificity for p53 (28). The interaction of Mdm2 with p53 led
to p53 degradation. Thus, the assessment of expression of Mdm2 was
necessary. As shown in Fig. 7A,
the downregulation of Mdm2 was observed in treatment of the cell
with the agents, indicating that DpdtaA and its copper complex
upregulated p53 via Mdm2 downregulation pathway. Since p53 exerts
its induction of apoptosis mainly through the regulation of bcl-2
family proteins, the bcl-2 and bax protein levels were assessed.
The relative ratio of bax/bcl-2 is shown in Fig. 7B, such ratio may determine the cell
sensitivity or resistance to the apoptotic stimuli (7). On the other hand, p53 and Mdm2
interacted reciprocally, the interaction is hydrophobic
interaction, disrupting the interaction would benefit the
upregulation of p53. It has been demonstrated that some of small
molecules can disrupt their interaction (29), so the theoretical simulation by
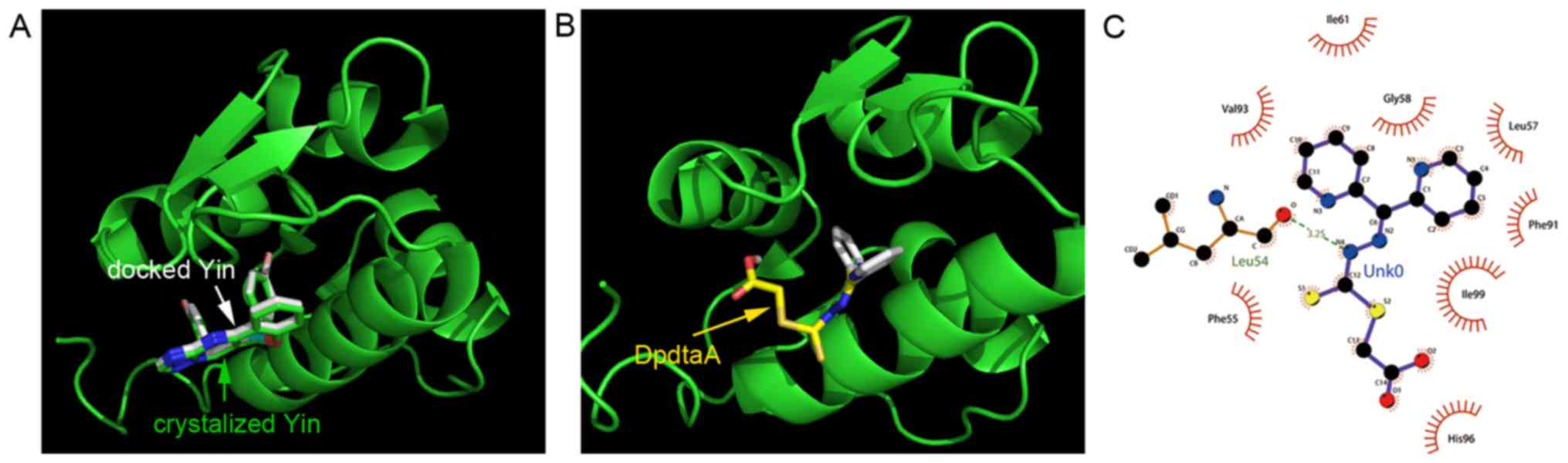
molecular docking was conducted. The crystal structure of human
type Mdm2 (PDB ID: 3jzk) was from the RCSB Protein Data Bank. In
order to evaluate the accuracy of our docking protocol, the Yin
(ligand in 3jzk) was re-docked into the Mdm2 complexes based on
recommended procedure (Fig. 8A).
As shown in Fig. 8, the docked Yin
was almost fully superimposed on the native co-crystallized one.
Thus, following the same protocol the DpdtaA was individually
docked into the Mdm2 (Fig. 8B),
the simulating affinity energies were −9.4 for Yin and −6.9
kcal/mol for DpdtaA, respectively. The interaction of DpdtaA with
residues of Mdm2 is depicted in Fig.
8C. The data implied that the medium interaction between DpdtaA
and Mdm2 might also contribute to the upregulation of p53.
DpdtaA and its copper complex leads to
autophagy response
Previous report revealed that bax could be
translocated from the cytosol to the lysosomal membrane, which led
to alteration in lysosomal membrane integrity (30). To test this hypothesis, LysoTracker
Red, which can accumulate within the lysosomes, was employed to
assess the LMP (31). As shown in
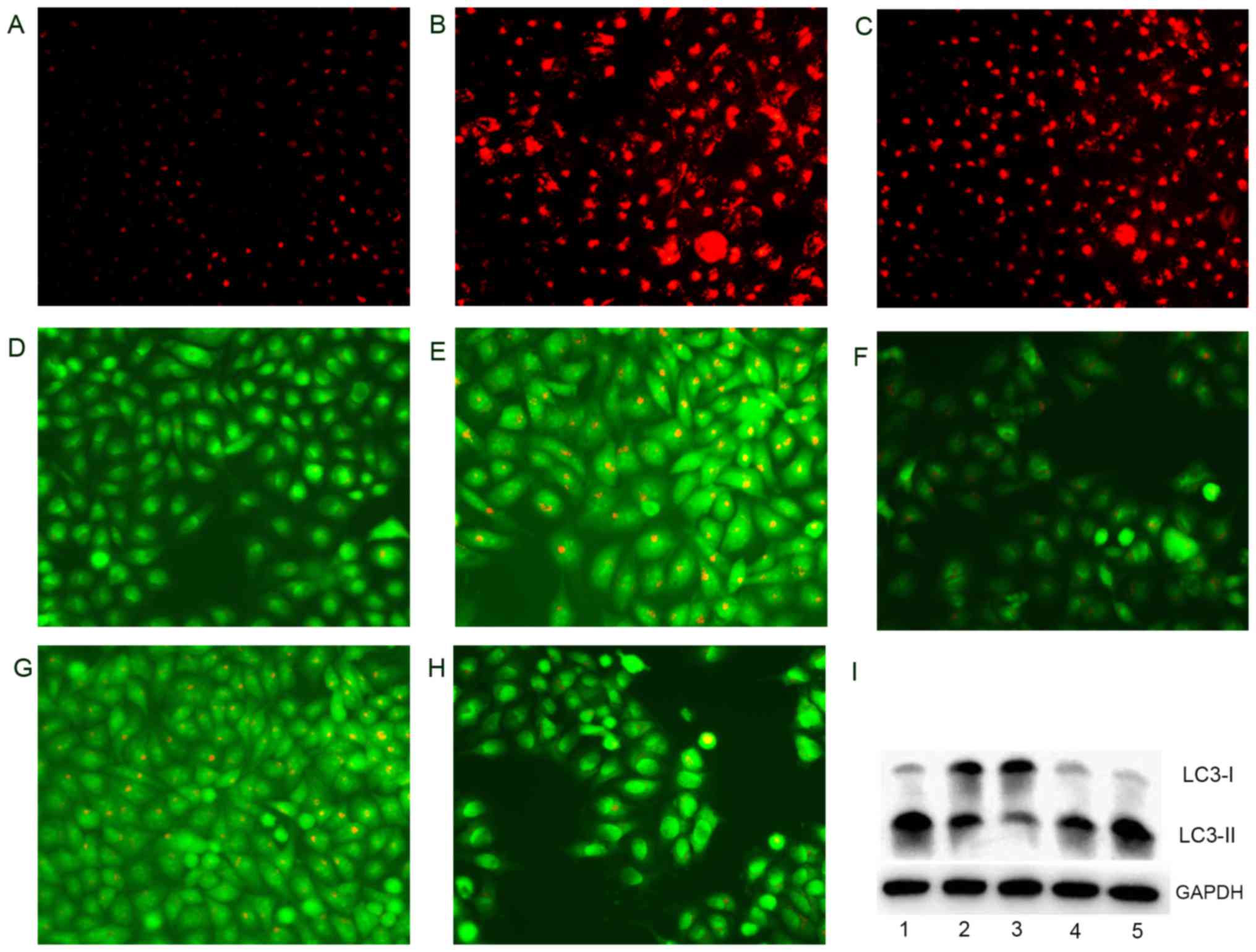
Fig. 9A–C, the treatments of
DpdtaA and DpdtaA-Cu significantly increased the red fluorescence
density in HepG2 cells compared to without treatment, indicating
that LMP was altered. Furthermore, the changes in LMP may be a
response to autophagy; thus, the formation of autophagosomes was
measured by acridine orange staining. As shown in Fig. 9D, E and G, the accumulated red
granular fluorescence in the acidic vacuoles was observed in the
agent-treated groups, implying that the formation of autophagosome
was increased, and autophagy occurred. To support the above
conclusion, an autophagy inhibitor, 3-methyladenin (3-MA) was added
to agent-treated cells and clearly the red granular fluorescence in
the acidic vacuoles decreased compared to that the agent only
(Fig. 9F and H). The LC3
(microtuble-associated protein light chain 3) is an autophagosome
molecular marker, its change was further assessed by western blot
analysis. As expected, the increase of cleaved LC3-II and decrease
of LC3-I were observed compared to that of control, indicating that
there was autophagy response after exposure of the agents (Fig. 9I). The changes of LC3-II were
parallel to the results from acridine orange staining and
demonstrated the DpdtaA and its copper complex had similar action
in induction of autophagy.
Discussion
Tumor microenvironment plays an important role in
tumor growth and metastasis (1,2),
many proteases, metal ions and cytokine are located in ECM and
maintain homeostasis of tumor environment. Once the homeostasis is
disturbed, either overgrowth or growth inhibition will occur. On
the other hand, cell proliferation requires some transition metals,
and the demand in cancer cells is much higher than normal cells,
therefore, moderate intervention of the metal homeostasis is
valuable strategy in cancer therapy. The antitumor activity of
dithiocarbamates, such as pyrrolidine dithiocarbamate (PDTC) is
partly attributed to their metal chelation. The free thiol in
dithiocarbamates is instable and thiol-alkylation may improve their
stability (32). In the present
study, pyridine ring and thioester structural units were introduced
to dithiocarbamate in order to neutralize chelating ability and
improve stability, and importantly enhance its antitumor activity.
Chemotherapeutic drugs in cancer treatment normally correlate with
the excess ROS production, that accordingly cause oxidative damage
of protein and nucleic acids, resulting in cell death. In the
present study the DpdtaA with similar ability to generate ROS both
in vitro and in vivo, led to growth inhibition. ROS
production induced by DpdtaA may involve in Fenton-like reaction
[redox active DpdtaA-Fe2+(Cu2+)] when
endogenous agent chelate iron from iron labile pool (33). To determine whether the growth
inhibition is ROS-dependent, an antioxidant, NAC was added during
the proliferative assay, the attenuated growth inhibition clearly
indicated that antiproliferative effect of DpdtaA was mediated by
ROS, which is similar to that previously reported (34–36).
Externalization of phosphatidylserine on the cell surface is one of
apoptotic features, however, unexpectedly, the lower ratio of
externalization was observed, which might be due to weaker activity
of phospholipid scramblase induced by the agents. It is well known
that in response to oxidative stress, the tumor suppressor p53
regulated expression of bcl-2 family and induced apoptosis
(37). In the present study,
DpdtaA upregulated p53 protein expression, down-regulated
antiapoptotic protein bcl-2 expression, promoting pro-apoptotic
protein bax expression, triggering cell apoptosis via intrinsic and
extrinsic pathway (Fig. 6)
(38). Moreover, p53 can directly
induce bax and bak oligomerization (39), thus, the higher ratio of bax/bcl-2
would be favorable for bax oligomerization, consequently
translocating the oligomerized bax to mitochondrial membrane, which
lead to release of cytochrome c and caused mitochondrial
cell death. p53 is a transcription factor, regulating many genes,
including Mdm2, a p53 specific E3-ubiquitin-protein ligase. Thus,
it was necessary to determine the expression of Mdm2. The results
obtained from this study showed that the DpdtaA could down-regulate
Mdm2 expression, indicating that upregulation of p53 was partly due
to Mdm2 feedback effect. Reversely blocking the p53 transcriptional
activity by PFT-α could downregulate Mdm2 expression (Fig. 7). In addition to retroregulation of
Mdm2, the dithiocarbamate bearing aromatic group might be a
disruptor to intervene interaction between p53 and Mdm2. To probe
the possibility, molecular docking was used, the simulation
revealed the DpdtaA has a moderate affinity to Mdm2, implying that
the disruption of the agents may not be excluded. The above
indicated that DpdtaA-induced apoptosis in HepG2 cells mainly
depended on p53-dependent pathway.
Autophagy occurs though degrading damaged proteins
and/or organelles and recycling the materials to maintain the cell
survival (40) and the acidic
vesicular organelles (AVOs) is characteristic marker in the process
(41). DpdtaA generated excess ROS
that may trigger autophagy. The acidic vesicular organelles in
stain of acridine orange was increased, the addition of an
autophagy inhibitor (3-MA) could reverse this situation, clearly
demonstrating that the antiproliferative activity of DpdtaA may be
involved in autophagy. Further molecular evidence was gained from
immunoblotting of LC3, the increased LC3-II were indicative of
autophagy (Fig. 9F). It should be
noted that autophagy can be either a physiological stress response
or cytotoxically exerted, to distinguish the roles of autophagy in
DpdtaA treatment, the autophagy inhibitor, 3-MA was employed, we
found that the inhibitor (3-MA) could significantly enhance
antiproliferative activity of DpdtA (data not shown), implying that
the autophagy was stress induced, which was similar to that
previously reported (42–44).
There are many catabolic enzymes in lysosome, its
rupture can potentially be harmful to the cells. Massive lysosomal
rupture causes release of lysosomal contents to cytoplasm and
cytoplasmic acidification, consequently resulting in cell death [or
lysosomal cell death (LCD)] (45).
Therefore, the integrity of lysosomal membrane or LMP has important
role in regulation of cell demise (46). Although the mechanism in LMP is not
fully solved, some ways to destabilize the lysosomal membrane have
been proposed: i) the compounds with detergent-like properties; ii)
excess ROS; and iii) bax that can be translocated to lysosome
membrane for pore formation, can destroy lysosomal membrane and
lead to alteration in LMP (46,47).
In the present study, the higher ratio of bax/bcl in DpdtaA group
(Fig. 6) and more lysosomal dye in
lysosome (Fig. 9) may indicate
that antiproliferative effect of DpdtaA may also partly involve
lysosomal cell death. In addition, there seemed to be a correlation
between antiproliferation and LMP although it still questions the
bax translocation to lysosome membrane (46,48).
Dithiocarbamates possess diverse biological
activities, partly stemming from their metal chelating ability.
In vitro copper complexes generally show an enhanced
antiproliferative activity (13,20,21,49),
but antagonistic effect was rarely reported. Dithiocarbamates can
inhibit the canonical NF-κB pathway (50), but upon chelating with copper
exhibit distinct biological activity (51). Dithiocarbamate copper complexes
also show proteasome inhibition to display their cytotoxicity
(52–54). However, the studies showed
dithiocarbamates enhanced cytotoxicity upon chelation with copper
ion. In the present study, we demonstrated that copper ion
significantly attenuated antiproliferative activity of DpdtaA
against HepG2 cells (Fig. 2),
which was similar to that previously reported for DpdtpA (19). The two dithiocarbamtes (DpdtaA and
DpdtpA) have similar structures, only one carbon difference, their
anti-proliferative effect against HepG2 cells was also similar, but
upon chelation of copper, the propionic acid thiol ester showed
greater fold decrease than acetate in antiproliferation. In
addition, the copper complexes in composition were also different,
ratio of DpdtpA/Cu was 2:1, but 1:1 for DpdtaA. Similar to
previously reported, there was no correlation between cellular ROS
level and its cytotoxicity (antiproliferation). DpdtaA-Cu has
stronger ROS inducing ability in vitro and in vivo,
but the changes in apoptotic proteins was not parallel, which
violate currently accepted concept in ROS-based cancer therapy.
This situation was also observed in the study by Zhu et al
(55). However, the attenuated
antiproliferative effect upon chelation of copper correlated with
the lower ratio of bax/bcl and alteration in LMP (same
concentration), indicating that copper ion may influence regulation
of DpdtaA on bax gene expression.
In conclusion, DpdtaA stimuli caused ROS generation.
In response to the oxidative stress, p53 was activated. More
details revealed the upregulation of p53 stemmed from Mdm2
downregulation, attenuating degradation of ubiquitination of p53.
Therefore antiproliferative activity exhibited by DpdtaA (or
DpdtaA-Cu) was partly via p53 meditated apoptosis. In addition, the
investigated agents also induced autophagy to respond the oxidative
stress. Taken together, the autophagy lysosomal cell death and
apoptosis were partly involved in antiproliferation of the agents.
Yet, the paradoxical issue between ROS and cytotoxicity for
DpdtaA-Cu and lesser extent of externalization of
phosphatidylserine were not fully solved in mechanism and required
further investigation.
Acknowledgments
The present study was supported by grants awarded by
the Natural Science Foundation of China (no. 21571153), the Henan
Science and Technology Agency (nos. 114300510012, 132102310250 and
152300410118), the Plan of Health Scientific and Technological
Innovation Talents of Henan Province (no. 2109901) to S.L. and the
Graduate innovation project of Xinxiang Medical University
(YJSCX201613Y).
References
|
1
|
Spano D and Zollo M: Tumor
microenvironment: A main actor in the metastasis process. Clin Exp
Metastasis. 29:381–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Catalano V, Turdo A, Di Franco S, Dieli F,
Todaro M and Stassi G: Tumor and its microenvironment: A
synergistic interplay. Semin Cancer Biol. 23:522–532. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sounni NE and Noel A: Targeting the tumor
microenvironment for cancer therapy. Clin Chem. 59:85–93. 2013.
View Article : Google Scholar
|
|
4
|
Khan G and Merajver S: Copper chelation in
cancer therapy using tetrathiomolybdate: An evolving paradigm.
Expert Opin Investig Drugs. 18:541–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bogaard HJ, Mizuno S, Guignabert C, Al
Hussaini AA, Farkas D, Ruiter G, Kraskauskas D, Fadel E, Allegood
JC, Humbert M, et al: Copper dependence of angioproliferation in
pulmonary arterial hypertension in rats and humans. Am J Respir
Cell Mol Biol. 46:582–591. 2012. View Article : Google Scholar :
|
|
6
|
Buac D, Schmitt S, Ventro G, Kona FR and
Dou QP: Dithiocarbamate-based coordination compounds as potent
proteasome inhibitors in human cancer cells. Mini Rev Med Chem.
12:1193–1201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Qi H, Li X, Hou X, Lu X and Xiao X:
A novel dithiocarbamate derivative induces cell apoptosis through
p53-dependent intrinsic pathway and suppresses the expression of
the E6 oncogene of human papillomavirus 18 in HeLa cells.
Apoptosis. 20:787–795. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang XJ, Xu HW, Guo LL, Zheng JX, Xu B,
Guo X, Zheng CX and Liu HM: Synthesis and in vitro antitumor
activity of new butenolide-containing dithiocarbamates. Bioorg Med
Chem Lett. 21:3074–3077. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mansouri-Torshizi H, Saeidifar M, Khosravi
F, Divsalar A, Saboury AA and Hassani F: DNA binding and antitumor
activity of α-diimineplatinum(II) and palladium(II) dithiocarbamate
complexes. Bioinorg Chem Appl. 2011:3945062011. View Article : Google Scholar
|
|
10
|
Milacic V, Chen D, Ronconi L,
Landis-Piwowar KR, Fregona D and Dou QP: A novel anticancer
gold(III) dithiocarbamate compound inhibits the activity of a
purified 20S proteasome and 26S proteasome in human breast cancer
cell cultures and xenografts. Cancer Res. 66:10478–10486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nardon C, Schmitt SM, Yang H, Zuo J,
Fregona D and Dou QP: Gold(III)-dithiocarbamato peptidomimetics in
the forefront of the targeted anticancer therapy: Preclinical
studies against human breast neoplasia. PLoS One. 9:e842482014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cattaruzza L, Fregona D, Mongiat M,
Ronconi L, Fassina A, Colombatti A and Aldinucci D: Antitumor
activity of gold(III)-dithiocarbamato derivatives on prostate
cancer cells and xenografts. Int J Cancer. 128:206–215. 2011.
View Article : Google Scholar
|
|
13
|
Schreck R, Meier B, Männel DN, Dröge W and
Baeuerle PA: Dithiocarbamates as potent inhibitors of nuclear
factor kappa B activation in intact cells. J Exp Med.
175:1181–1194. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ronconi L, Marzano C, Zanello P, Corsini
M, Miolo G, Maccà C, Trevisan A and Fregona D: Gold(III)
dithiocarbamate derivatives for the treatment of cancer: Solution
chemistry, DNA binding, and hemolytic properties. J Med Chem.
49:1648–1657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nobel CSI, Burgess DH, Zhivotovsky B,
Burkitt MJ, Orrenius S and Slater AF: Mechanism of dithiocarbamate
inhibition of apoptosis: Thiol oxidation by dithiocarbamate
disulfides directly inhibits processing of the caspase-3 proenzyme.
Chem Res Toxicol. 10:636–643. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu HH, Thomas JA and Momand J: p53 protein
oxidation in cultured cells in response to pyrrolidine
dithiocarbamate: A novel method for relating the amount of p53
oxidation in vivo to the regulation of p53-responsive genes.
Biochem J. 351:87–93. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu HH and Momand J: Pyrrolidine
dithiocarbamate prevents p53 activation and promotes p53 cysteine
residue oxidation. J Biol Chem. 273:18898–18905. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Verhaegh GW, Richard MJ and Hainaut P:
Regulation of p53 by metal ions and by antioxidants:
Dithiocarbamate down-regulates p53 DNA-binding activity by
increasing the intracellular level of copper. Mol Cell Biol.
17:5699–5706. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang T, Fu Y, Huang T, Liu Y, Wu M, Yuan
Y, Li S and Li C: Copper ion attenuated the antiproliferative
activity of di-2-pyri-dylhydrazone dithiocarbamate derivative;
however, there was a lack of correlation between ROS generation and
antiproliferative activity. Molecules. 21:10882016. View Article : Google Scholar
|
|
20
|
Yang Y, Li C, Fu Y, Liu Y, Zhang Y, Zhang
Y, Zhou P, Yuan Y, Zhou S, Li S, et al: Redox cycling of a copper
complex with benz-aldehyde nitrogen mustard-2-pyridine carboxylic
acid hydrazone contributes to its enhanced antitumor activity, but
no change in the mechanism of action occurs after chelation. Oncol
Rep. 35:1636–1644. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang T and Li C, Sun X, Zhu Z, Fu Y, Liu
Y, Yuan Y, Li S and Li C: The antitumor mechanism of
di-2-pyridylketone 2-pyridine carboxylic acid hydrazone and its
copper complex in ROS generation and topoisomerase inhibition, and
hydrazone involvement in oxygen-catalytic iron mobilization. Int J
Oncol. 47:1854–1862. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
DeLano WL: The PyMOL Molecular Graphics
System. DeLano Scientific; San Carlos, CA, USA: 2002
|
|
23
|
Laskowski RA and Swindells MB: LigPlot+:
Multiple ligand-protein interaction diagrams for drug discovery. J
Chem Inf Model. 51:2778–2786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trott O and Olson AJ: AutoDock Vina:
Improving the speed and accuracy of docking with a new scoring
function, efficient optimization, and multithreading. J Comput
Chem. 31:455–461. 2010.
|
|
25
|
Paris C, Bertoglio J and Bréard J:
Lysosomal and mitochondrial pathways in miltefosine-induced
apoptosis in U937 cells. Apoptosis. 12:1257–1267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brooks CL and Gu W: p53 ubiquitination:
Mdm2 and beyond. Mol Cell. 21:307–315. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kruse JP and Gu W: Modes of p53
regulation. Cell. 137:609–622. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haupt Y, Maya R, Kazaz A and Oren M: Mdm2
promotes the rapid degradation of p53. Nature. 387:296–299. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shangary S and Wang S: Small-molecule
inhibitors of the MDM2-p53 protein-protein interaction to
reactivate p53 function: A novel approach for cancer therapy. Annu
Rev Pharmacol Toxicol. 49:223–241. 2009. View Article : Google Scholar :
|
|
30
|
Johansson AC, Appelqvist H, Nilsson C,
Kågedal K, Roberg K and Ollinger K: Regulation of
apoptosis-associated lysosomal membrane permeabilization.
Apoptosis. 15:527–540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rehman SU, Zubair H, Sarwar T, Husain MA,
Ishqi HM, Nehar S and Tabish M: Redox cycling of Cu(II) by
6-mercaptopurine leads to ROS generation and DNA breakage: Possible
mechanism of anticancer activity. Tumour Biol. 36:1237–1244. 2015.
View Article : Google Scholar
|
|
32
|
Fussell KC, Udasin RG, Gray JP, Mishin V,
Smith PJ, Heck DE and Laskin JD: Redox cycling and increased oxygen
utilization contribute to diquat-induced oxidative stress and
cytotoxicity in Chinese hamster ovary cells overexpressing
NADPH-cytochrome P450 reductase. Free Radic Biol Med. 50:874–882.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kello M, Drutovic D, Chripkova M, Pilatova
M, Budovska M, Kulikova L, Urdzik P and Mojzis J: ROS-dependent
antiproliferative effect of brassinin derivative homobrassinin in
human colorectal cancer Caco2 cells. Molecules. 19:10877–10897.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Donadelli M, Dando I, Zaniboni T, Costanzo
C, Dalla Pozza E, Scupoli MT, Scarpa A, Zappavigna S, Marra M,
Abbruzzese A, et al: Gemcitabine/cannabinoid combination triggers
autophagy in pancreatic cancer cells through a ROS-mediated
mechanism. Cell Death Dis. 2:e1522011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chripkova M, Zigo F and Mojzis J:
Antiproliferative effect of indole phytoalexins. Molecules.
21:16262016. View Article : Google Scholar
|
|
36
|
Gillardon F, Wickert H and Zimmermann M:
Up-regulation of bax and down-regulation of bcl-2 is associated
with kainate-induced apoptosis in mouse brain. Neurosci Lett.
192:85–88. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Amaral JD, Xavier JM, Steer CJ and
Rodrigues CM: The role of p53 in apoptosis. Discov Med. 9:145–152.
2010.PubMed/NCBI
|
|
38
|
Chen Y, Azad MB and Gibson SB: Methods for
detecting autophagy and determining autophagy-induced cell death.
Can J Physiol Pharmacol. 88:285–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2001.PubMed/NCBI
|
|
40
|
Lin J, Huang Z, Wu H, Zhou W, Jin P, Wei
P, Zhang Y, Zheng F, Zhang J, Xu J, et al: Inhibition of autophagy
enhances the anticancer activity of silver nanoparticles.
Autophagy. 10:2006–2020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Donohue E, Thomas A, Maurer N, Manisali I,
Zeisser-Labouebe M, Zisman N, Anderson HJ, Ng SS, Webb M, Bally M,
et al: The autophagy inhibitor verteporfin moderately enhances the
antitumor activity of gemcitabine in a pancreatic ductal
adenocarcinoma model. J Cancer. 4:585–596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang X, Xu Q, Zhang Z, Cheng W, Cao W,
Jiang C, Han C, Li J and Hua Z: Chloroquine enhanced the anticancer
capacity of VNP20009 by inhibiting autophagy. Sci Rep. 6:297742016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Serrano-Puebla A and Boya P: Lysosomal
membrane permeabilization in cell death: New evidence and
implications for health and disease. Ann N Y Acad Sci. 1371:30–44.
2016. View Article : Google Scholar
|
|
44
|
Aits S and Jäättelä M: Lysosomal cell
death at a glance. J Cell Sci. 126:1905–1912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bové J, Martínez-Vicente M, Dehay B,
Perier C, Recasens A, Bombrun A, Antonsson B and Vila M: BAX
channel activity mediates lysosomal disruption linked to Parkinson
disease. Autophagy. 10:889–900. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guan JJ, Zhang XD, Sun W, Qi L, Wu JC and
Qin ZH: DRAM1 regulates apoptosis through increasing protein levels
and lysosomal localization of BAX. Cell Death Dis. 6:e16242015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fu Y, Yang Y, Zhou S, Liu Y, Yuan Y, Li S
and Li C: Ciprofloxacin containing Mannich base and its copper
complex induce antitumor activity via different mechanism of
action. Int J Oncol. 45:2092–2100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Oberle C, Huai J, Reinheckel T, Tacke M,
Rassner M, Ekert PG, Buellesbach J and Borner C: Lysosomal membrane
permeabilization and cathepsin release is a Bax/Bak-dependent,
amplifying event of apoptosis in fibroblasts and monocytes. Cell
Death Differ. 17:1167–1178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang Y, Huang T, Zhou S, Fu Y, Liu Y, Yuan
Y, Zhang Q, Li S and Li C: Antitumor activity of a
2-pyridinecarboxaldehyde 2-pyridinecarboxylic acid hydrazone copper
complex and the related mechanism. Oncol Rep. 34:1311–1318. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cvek B, Milacic V, Taraba J and Dou QP:
Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show
various activities against the proteasome in breast cancer cells. J
Med Chem. 51:6256–6258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cvek B and Dvorak Z: Targeting of nuclear
factor-kappaB and proteasome by dithiocarbamate complexes with
metals. Curr Pharm Des. 13:3155–3167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yu Z, Wang F, Milacic V, Li X, Cui QC,
Zhang B, Yan B and Dou QP: Evaluation of copper-dependent
proteasome-inhibitory and apoptosis-inducing activities of novel
pyrrolidine dithiocarbamate analogues. Int J Mol Med. 20:919–925.
2007.PubMed/NCBI
|
|
53
|
Skrott Z and Cvek B:
Diethyldithiocarbamate complex with copper: The mechanism of action
in cancer cells. Mini Rev Med Chem. 12:1184–1192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang H, Wu JS and Peng F: Potent
anticancer activity of pyrrolidine dithiocarbamate-copper complex
against cisplatin-resistant neuroblastoma cells. Anticancer Drugs.
19:125–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhu C, Hu W, Wu H and Hu X: No evident
dose-response relationship between cellular ROS level and its
cytotoxicity - a paradoxical issue in ROS-based cancer therapy. Sci
Rep. 4:50292014. View Article : Google Scholar
|