Introduction
Compounds derived from natural products play an
important role in the discovery of clinically suitable therapeutic
agents. This is particularly true for anticancer medicines, with
almost 70% of the novel anticancer drugs approved, over the past
six decades, coming from natural products or based on the knowledge
gained from natural products (1).
Recently, a renewed interest for flavonoids as
anticancer agents has been catalyzed by flavopiridol (Alvocidib), a
potent cyclin-dependent kinase (CDK) inhibitor, which has been
granted orphan drug designation by the FDA in 2014 to treat
patients with acute myeloid leukemia (2). Flavonoids are found throughout the
plant kingdom and several epidemiological studies suggest that
dietary intake of flavonoids is responsible for chemoprevention
(3). Of particular interest are
the polymethoxyflavones (PMFs), flavones substituted with two or
more methoxy groups. This subclass of flavonoids is thought to be
superior to polyhydroxylated flavonoids due to their increased
metabolic stability, oral bioavailability and consequently improved
cancer chemopreventive activities (4).
PMFs can be found in high concentrations in the peel
of several Citrus species and in medicinal plants used in
traditional medicine (5–7). Studies on the anticancer activity of
PMFs have mostly been focused on nobiletin. This
5,6,7,8,3′,4′-hexamethoxyflavone has been shown to be effective
in vitro and in vivo by affecting several cellular
activities, including inhibition of cell proliferation, invasion
and migration, inducing cell cycle arrest as well as reducing
angiogenesis, signaling pathways and bioactivation by CYP1
(8–11). Notable also, is its predominant
anticancer activity in MDA-MB-468 cells which indicates a potential
role of nobiletin for the prevention of triple-negative breast
cancer (TNBC) (12), an aggressive
and highly metastatic subtype with poor prognosis for which
hormonal therapy is not beneficial and chemotherapy remains the
only treatment (13).
Studies with different Citrus species and
medicinal plants indicate a high structural variability in PMF
content, including the presence of smaller methoxyflavones and
structural isomers. While several reports suggest that the
anticancer activity from flavonoids is profoundly affected by their
composition and structure, limited studies are published on the
effect of these less known congeners (4), such as
5,6,7,3′,4′,5′-hexamethoxyflavone. This flavone has the same
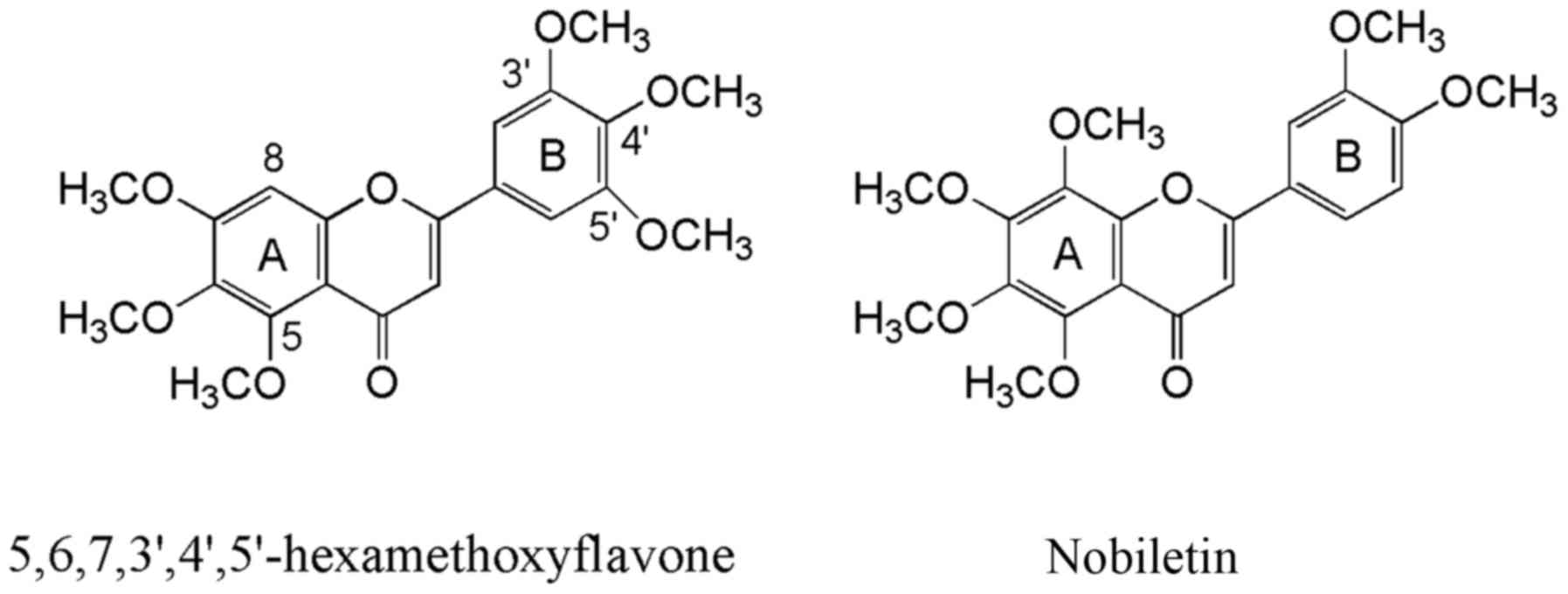
structural formula as nobiletin and has been isolated from
Citrus reticulate and Ageratum conyzoides (Fig. 1). The compound was found to be
cytotoxic against P-388 mouse leukemia cells, but not against the
HT-29 human colon adenocarcinoma cell line and to suppress the
degranulation from antigen-stimulated rat basophil RBL-2H3 cells
through its effect on signaling as Syk/PLCγ's/PKC and
mitogen-activated protein kinase (MAPK) pathways and
Ca2+ influx (14,15).
The present study aimed at investigating the
possible anticancer effects of 5,6,7,3′,4′,5′-hexamethoxyflavone
and comparison against the well-studied nobiletin in the Hs578T
progression model of TNBC. This in vitro cell system
comprises the Hs578T TNBC cell line and its more metastatic and
isogenic variant Hs578Ts(i)8 and embodies an elegant
experimental model for studying the anticancer activity of both
hexamethoxyflavones in TNBC and on TNBC progression (16).
Materials and methods
Antibodies and other reagents
Antibodies directed against p-ERK (D13.14.4E),
p-JNK/SAPK (81E11), p-Akt (D9E), p-p38 MAPK (D3F9), p-Chk2 (C13C1),
p-Chk1 (133D3), p-Cdc2 (10A11) and anti-β-actin (D6A8) or β-tubulin
(9F3) antibodies as well as camptothecin were from Cell Signaling
Technology (Danvers, MA, USA). Anti-mouse and anti-rabbit alkaline
phosphatase-labeled secondary antibodies, the BCA protein assay
reagent kit and trypan blue solution were from Thermo Fisher
Scientific (Waltham, MA, USA). Drug toxicity was evaluated through
measurement of mitochondrial dehydrogenase activities with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
reagent (Sigma-Aldrich, St. Louis, MO, USA). Nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone were obtained from Alkemist Labs
(Costa Mesa, CA, USA).
Cell culture
The human mesenchymal breast cancer Hs578T cells and
the derivative cell line Hs578Ts(i)8 were a kind gift
from Dr S. McDonnell (UCD School of Chemical and Bioprocess
Engineering, University College Dublin, Ireland) (16) and were grown in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% (v/v) fetal bovine
serum (FBS), 100 IU/ml penicillin, 100 µg/ml streptomycin
and 0.01 mg/ml bovine insulin (Thermo Fisher Scientific) at 37°C
equilibrated with 5% (v/v) CO2 in humidified air. The
TNBC cells used in the present study were frozen in liquid nitrogen
when not in use and were not passaged in our laboratory for >15
weeks.
Assay for cell viability
The effect of nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone on cell viability was tested in
accordance with Romijn et al (17). Briefly, mitochondrial dehydrogenase
activities were measured by an MTT reagent. Cells were seeded in
96-well plates at an initial density of 1.5×104 cells in
100 µl culture medium. After overnight incubation, cells
were treated with nobiletin and 5,6,7,3′,4′,5′-hexamethoxy-flavone
at a final concentration of 100, 50 and 10 µM. After 24 and
72 h of incubation, 100 µl medium was removed prior to the
addition of MTT reagent, and formed formazan crystals were
dissolved in 200 µl dimethyl sulfoxide (DMSO). Four
independent experiments were completed to determine the mean
absorbance referring to cell viability, using a Cytation™ 3 Cell
Imaging Multi-mode reader with Gen5 software (BioTek Instruments,
Inc., Winooski, VT, USA) and were expressed in percentage as
compared to DMSO-treated control cells. In each experiment, eight
wells were used per condition.
Cell counting
Cells were seeded in 25-cm2 culture
flasks at an initial density of ~1.5×105 cells in 5 ml
culture medium and were treated with nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone at a final concentration of 100
µM after overnight incubation. The cells were allowed to
grow for 24, 48 and 72 h, harvested using trypsin/EDTA and counted
with a TC20™ automated cell counter (Bio-Rad Laboratories,
Hercules, CA, USA). At least three independent experiments were
performed to determine the mean value, which is presented as a
percentage compared to the DMSO-treated controls.
Wound healing assay
Cells were grown in 24-well plates until confluency
and washed twice with phosphate-buffered saline (PBS). A scratch
was made using a P200 pipette tip and 1 ml of medium in the
presence of DMSO, nobiletin or 5,6,7,3′,4′,5′-hexamethoxyflavone at
100 µM, was added. Cell migration was monitored and images
were collected after 17 h, with an EVOS® XL Core Cell
Imaging (Thermo Fisher Scientific). ImageJ software was used to
estimate the cell free area of the wounds (18). The distances over which the cells
migrated were measured in three independent experiments and
expressed as percentage compared to DMSO-treated Hs578T and
Hs578Ts(i)8 cells.
Proteome Profiler Human Phospho-kinase
Array
Cells at 70% confluency were washed three times,
serum-starved overnight, washed again and stimulated for 2 h with
complete culture medium, followed by a treatment of 10 min with 100
µM nobiletin and 5,6,7,3′,4′,5′-hexamethoxyflavone prior to
cell lysis. Lysates were made using kit components and the array
experiments were performed following the manufacturer's
instructions (R&D Systems, Inc., Minneapolis, MN, USA).
Briefly, aliquots of cell lysates, containing 250 µg of
protein, were incubated overnight with a human phospho-kinase array
membrane containing capture antibodies against 43 different kinase
phosphorylation sites. After washing, the membranes were incubated
with biotinylated antibodies, streptavidin-HRP and chemiluminescent
reagent for detection of phosphorylated protein at each capture
spot on the membranes. Images were taken with a LI-COR®
Odyssey Fc and analyzed with Image Studio 5.0 (LI-COR Biosciences,
Lincoln, NE, USA) for determination of mean pixel density and
further analyzed using Excel.
Western blotting
Confluent cell cultures (70%) were washed three
times and for MAPK and Akt signaling experiments serum-starved
overnight, washed again and stimulated for 2 h with complete
culture medium prior to treatment with 100 µM nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone for indicated times. For
investigation of cell cycle regulators, 70% confluent cultures were
not stimulated and washed three times. Subsequently, cells were
lysed using lysis buffer containing 1% Triton X-100 and 1% Halt™
Protease Inhibitor Cocktail (Thermo Fisher Scientific). Aliquots of
lysates containing 25–30 µg of proteins were boiled for 5
min in SDS-PAGE sample buffer supplemented with 5%
β-mercaptoethanol, electrophoresed on 10% or 4–15% gradient
Mini-PROTEAN® TGX™ gels and transferred to PVDF
membranes (Bio-Rad Laboratories). After transfer, membranes were
incubated with relevant antibodies against p-ERK, p-JNK/SAPK, p-p38
MAPK, p-Akt, p-Chk2 and Chk1, p-Cdc2 and β-tubulin or β-actin, as
loading controls, followed by incubation with a secondary
alkaline-phosphatase anti-rabbit antibody and stained with NBT/BCIP
(1:50 in 0.1 M Tris-HCl, 0.05 M MgCl2 and 0.1 M NaCl at
pH 9.5).
Flow cytometric analysis
Cells were treated for 72 h with 100 µM
nobiletin and 5,6,7,3′,4′,5′-hexamethoxyflavone. Floating cells and
trypsinized adherent cells were combined and washed with cold PBS
or cell culture medium. For detection of apoptosis, cells were
stained with an Annexin V/7-AAD kit (Beckman Coulter, Miami, FL,
USA) using the manufacturer's protocol. In brief, cells were
incubated with Annexin V and 7-AAD in ice-cold binding buffer in
the dark. After 15 min the samples were mixed with more binding
buffer and analyzed within 30 min. Positive controls for apoptosis
induction included Hs578T and Hs578Ts(i)8 cells treated
with 10 µM camptothecin for 72 h. Cell cycle analysis was
investigated by adding Vybrant® DyeCycle™ Green Stain
(Thermo Fisher Scientific) to 1 ml cell suspension at a final
concentration of 250 nM. After 30-min incubation at 37°C, the
samples were analyzed by flow cytometry and compared against
DMSO-treated cells. All these experiments were performed on a
CytoFLEX flow cytometer (Beckman Coulter) using CytExpert 2.0
software.
Statistics
All treatments were matched and carried out at least
3 times. Data were analyzed using Excel, for determination of mean,
standard deviation (SD) and Student's t-test (95%). The intensity
of the immunoblotted bands was quantified by densitometry, using
statistical software Scion Image (Scion Corp., Frederick, MD,
USA).
Results
Effect of nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone on cell viability
Hs578T and the more invasive Hs578Ts(i)8
cells were treated for 24 and 72 h with nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone at concentrations of 10, 50 and
100 µM and cytotoxicity was determined by the MTT-test. The
effects of these compounds on cell viability are shown in Table I. Overall, the compounds did not
have a major toxic effect toward the cell viability of the tested
cell lines at 100 µM, after 24 and 72 h and consequently no
IC50 values could be determined. Instead, nobiletin
generally reduced the cell viability of the Hs578T and
Hs578Ts(i)8 cell lines with ~30% after 24 h and 30–50%
after 72 h at its highest concentration, while no noteworthy effect
was observed for 5,6,7,3′,4′,5′-hexamethoxyflavone after 24 h
(upper panel), and a small drop after 72 h (lower panel). Notably,
we found that both nobiletin and 5,6,7,3′,4′,5′-hexame-thoxyflavone
at 100 µM showed a greater toxicity toward the more invasive
Hs578Ts(i)8 variant cell line, decreasing the cell
viability to 51.6 and 68.1%, respectively, after 72 h.
 | Table IPercentage of cell viability of
Hs578T and Hs578Ts(i)8 after 24 and 72 h nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone treatment. |
Table I
Percentage of cell viability of
Hs578T and Hs578Ts(i)8 after 24 and 72 h nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone treatment.
| Nobiletin
| Hexamethoxyflavone
|
|---|
| 100µM | 50µM | 10µM | 100µM | 50µM | 10µM |
|---|
| (24 h) | | | | | | |
| Hs578T | 71.4±6.1 | 98.4±2.7 | 99.0±0.9 | 92.7±4.4 | 97.5±1.3 | 95.9±4.3 |
|
Hs578Ts(i)8 | 70.0±9.0 | 88.5±2.0 | 93.0±3.9 | 85.9±7.2 | 88.4±6.7 | 92.6±7.6 |
| (72 h) | | | | | | |
| Hs578T | 73.2±13.4 | 98.9±12.1 | 99.7±2.8 | 81.9±1.6 | 84.5±3.6 | 90.2±13.0 |
|
Hs578Ts(i)8 | 51.6±7.7 | 84.7±4.6 | 103.7±3.6 | 68.1±7.7 | 80.9±5.3 | 93.7±11.1 |
Nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone inhibit cell growth
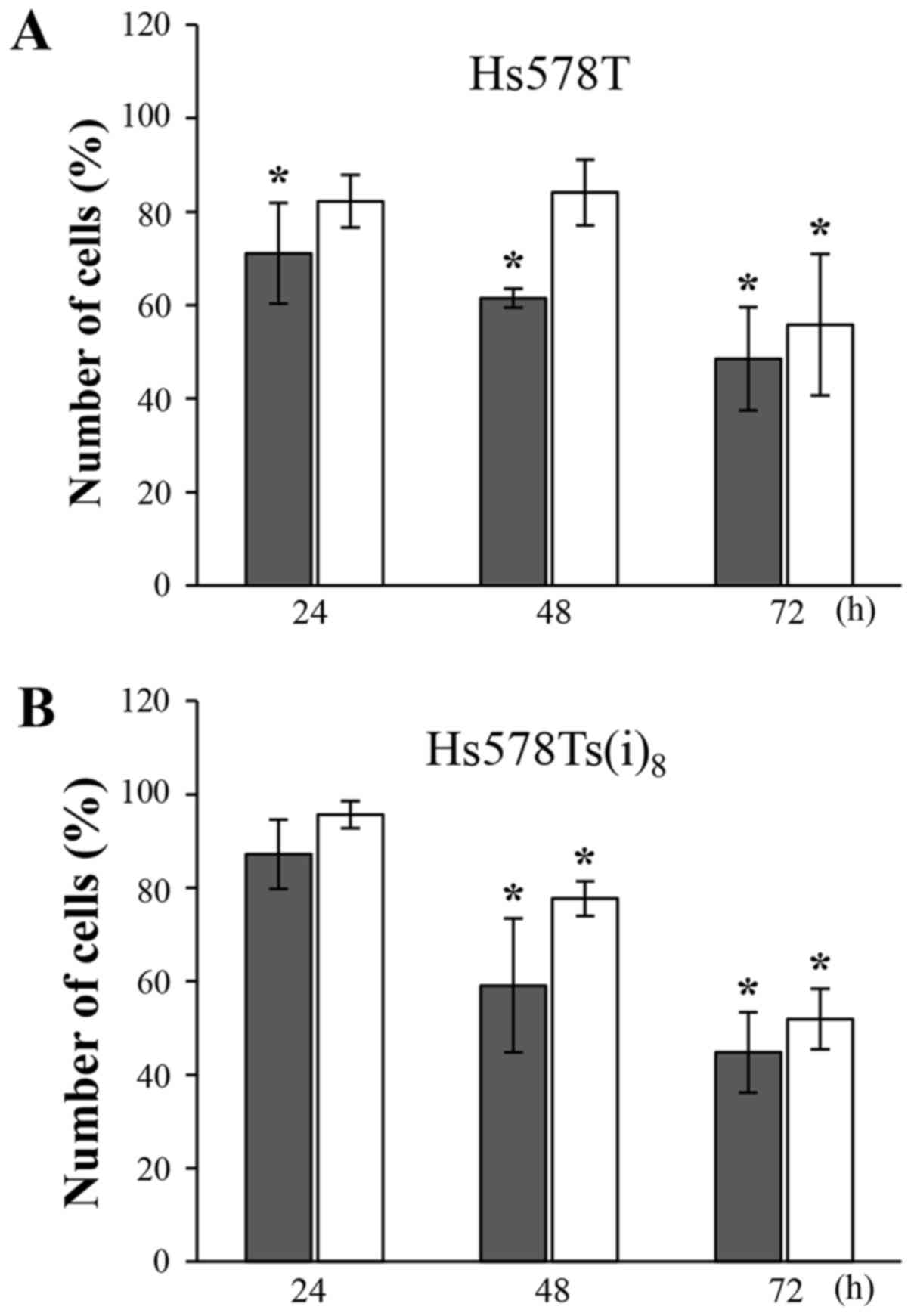
The growth inhibitory effects of the compounds at
100 µM were evaluated after 24, 48 and 72 h by cell
counting. Nobiletin and 5,6,7,3′,4′,5′-hexamethoxyflavone markedly
inhibited the growth of Hs578T (Fig.
2A) and Hs578Ts(i)8 cells (Fig. 2B) in a time-dependent manner. After
24 h, limited effects were observed. After 48 and 72 h, however,
nobiletin significantly reduced the amount of cells by roughly 40
and 50%, respectively. The growth inhibition following
5,6,7,3′,4′,5′-hexamethoxyflavone treatment was most pronounced
after 72 h, and reached a level (~50%) almost similar to nobiletin.
It should be noted, that the reduction in number of cells grown in
the presence of nobiletin is relatively comparable to the data
obtained in the MTT-assays (Table
I), which may suggest a confounding effect due to its effect on
cell viability.
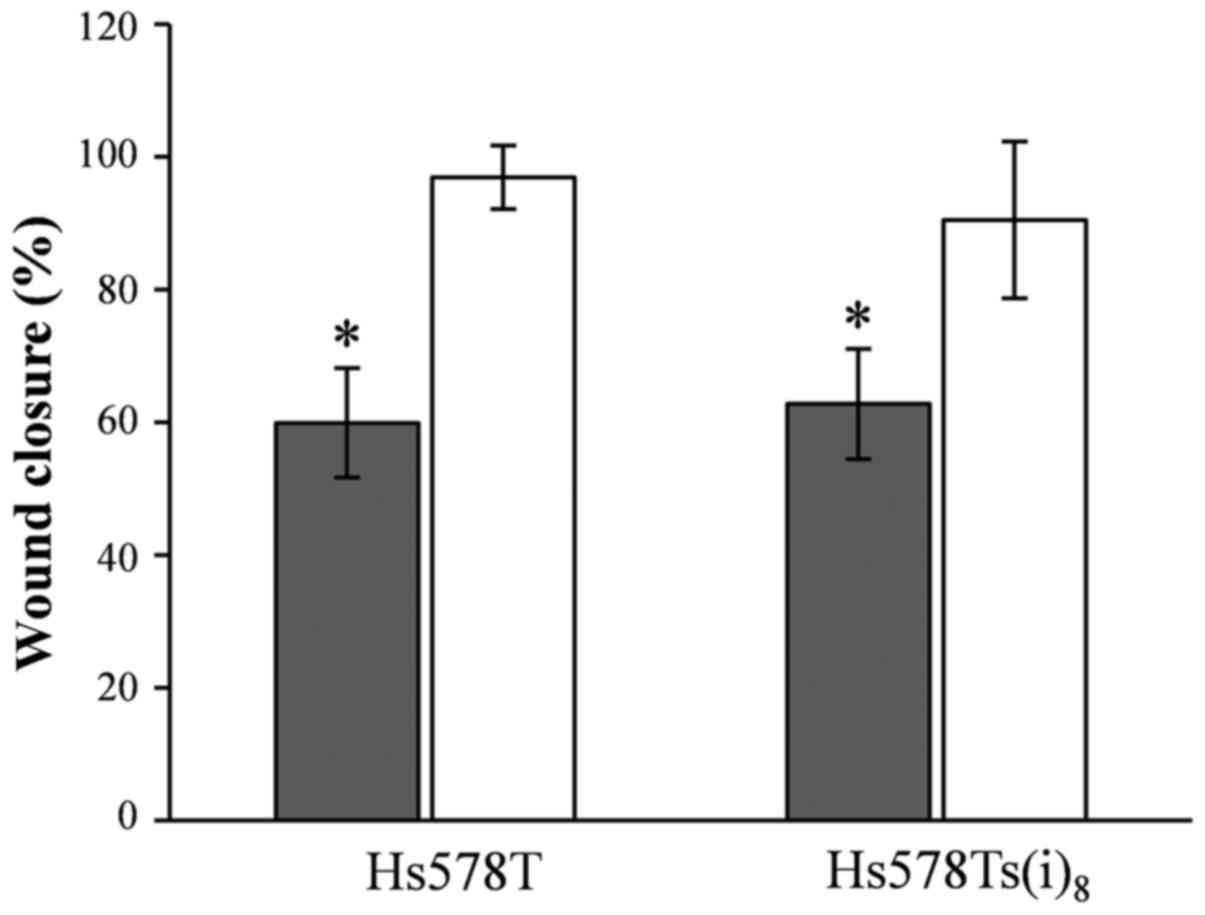
Nobiletin and cell migration
In our previous study, we published that the
Hs578Ts(i)8 cells migrate twice as fast compared to the
parental Hs578T cells in a wound healing assay under normal growth
conditions (19), which was in
line with the earlier published increased migratory capacity of the
more invasive Hs578Ts(i)8 cell line using the BD
Matrigel™Invasion Chamber assay system (16). Herein, we tested the potential of
nobiletin and 5,6,7,3′,4′,5′-hexamethoxyflavone to inhibit the
migratory behavior, and this particularly of the
Hs578Ts(i)8 cells. Results shown in Fig. 3 reveal that nobiletin at a
concentration of 100 µM significantly reduced the migration
by 40%, and that there was no selectivity toward the more migratory
and invasive Hs578Ts(i)8 cells. In contrast, no effect
was seen for 5,6,7,3′,4′,5′-hexamethoxyflavone and the wound was
closed after 17 h, similar to the DMSO-control conditions. Yet
again, the effect of nobiletin should be viewed with caution, as
the growth inhibitory activity may confound the effect of nobiletin
on cellular migration.
Effect of nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone on apoptosis
Annexin V-FITC/7-AAD double staining was used to
examine the effect of the two hexamethoxyflavones on the induction
of cellular apoptosis. There are several compounds available to
induce apoptosis in cancer cells in studies, in vitro. In
vivo, taxanes have been shown to be beneficial in neoadjuvant,
adjuvant, and metastatic settings in TNBC (20), and this specifically toward the
basal-like (BL) 1 and 2 TNBC subtypes as compared to the
mesenchymal-like and luminal androgen receptor subtypes (21). Since the
Hs578T/Hs578Ts(i)8 breast cancer model represents
mesenchymal-like TNBC, the well-known and universally used inducer
of apoptosis, camptothecin, was used. While the addition of
camptothecin in a final concentration of 5–10 µM and
incubation in a time range of 4–24 h usually allows the evaluation
of apoptosis in many cell lines, Hs578T and Hs58Ts(i)8
cells did not respond to the treatment within that time frame or
after 48 h (data not shown). Instead, camptothecin-induced
apoptosis was observed after 72 h at a concentration of 10
µM. Consequently, Hs578T and Hs578Ts(i)8 cells
were exposed to nobiletin and 5,6,7,3′,4′,5′-hexamethoxyflavone for
72 h, and floating cells and adherent cells were combined prior to
staining. Fig. 4A and the
quantification in Fig. 4B indicate
no significant differences in the Annexin V-FITC positive and 7-AAD
positive or negative cells after 72-h treatment with nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone at a final concentration of 100
µM as compared to DMSO and this in both cell lines. These
results suggest that Hs578T and Hs578Ts(i)8 cells: i)
are quite resistant toward camptothecin-induced apoptosis; and ii)
do not undergo apoptosis after exposure to nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone (Fig. 4).
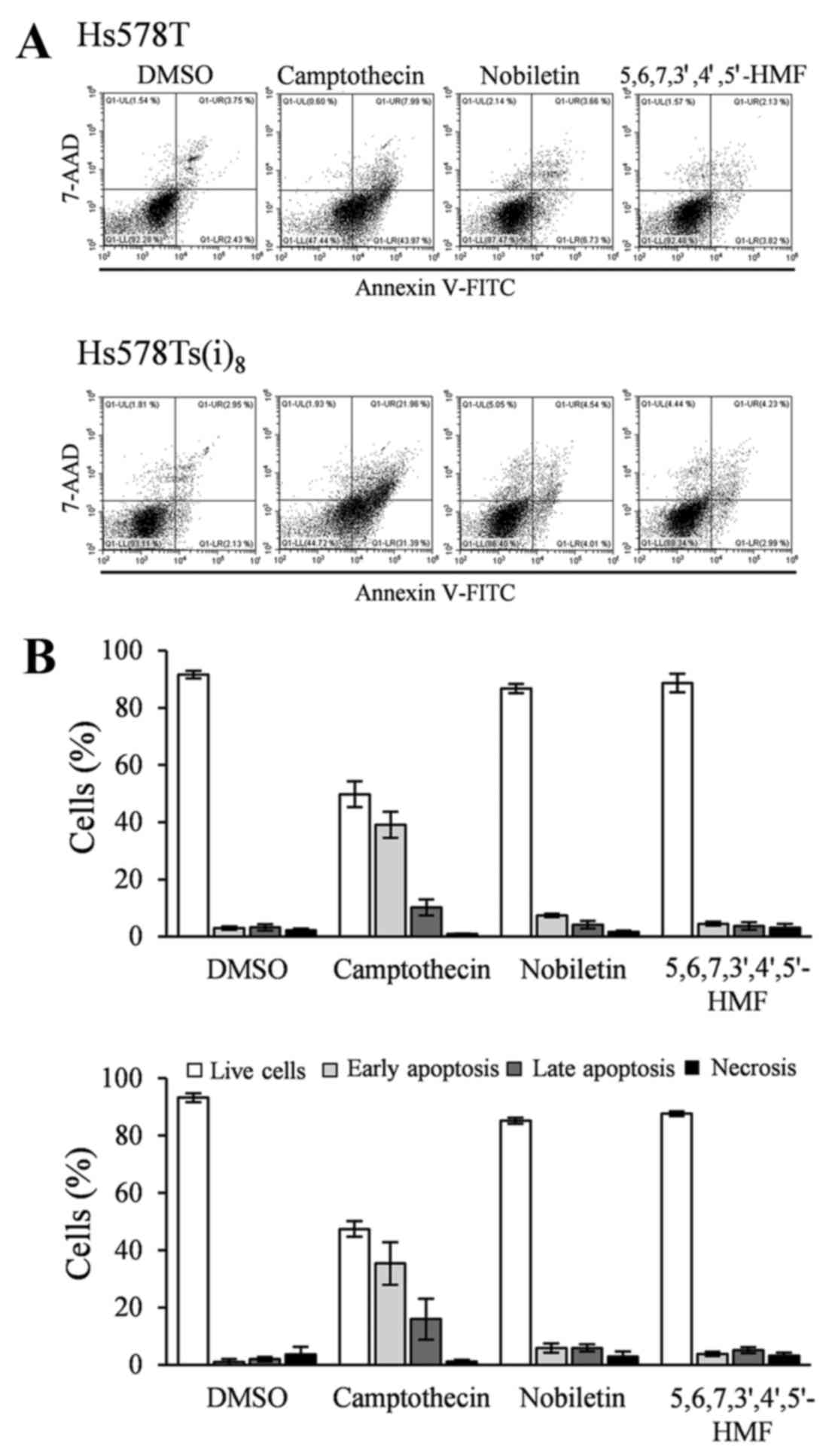 | Figure 4Effects of 100 µM nobiletin
and 5,6,7,3′,4′,5′-hexamethoxyflavone, as well as 10 µM
camptothecin treatment for 72 h on apoptosis, in Hs578T (upper
panel) and Hs578Ts(i)8 cells (lower panel). (A) Cells
were trypsinized, combined with floating cells prior to staining
with Annexin V-FITC/7-AAD. Profiles are representative examples of
four independent experiments. (B) Percentage of live (open bars),
early apoptotic (light grey bars), late apoptotic (grey bars) and
necrotic (closed bars) cells, expressed as mean % ± SD of four
independent experiments. |
Nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone inhibit activation of distinct
signaling molecules
With increasing knowledge on and complexity of
signaling pathways as well as the fact that these pathways are
highly cell-type specific, phospho-kinase arrays were performed
alongside western blotting, to take an unbiased approach for
analysis of affected signaling molecules and pathways upon
treatment with the two hexamethoxyflavones. This was particularly
important, given the uniqueness of the cell model system and the
limited information available. Initially, western blotting was
performed to determine an approximate optimal time-point in the
cell lines used, for nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone-mediated inhibition of
phosphorylated ERK and Akt. The phosphorylation of these two
signaling molecules is often found to be elevated after hormone or
growth factor stimulation and inhibited following exposure to PMFs,
such as nobiletin and tangeretin (8,22).
Limiting factors to use this approach include the fact that these
cell lines are triple-negative and the poor comprehension of the
importance of growth factors in the behavior of the Hs578T and more
migratory and invasive Hs578Ts(i)8 cells. The MAPK and
Akt pathways are known to be activated by a variety of factors,
among them receptor tyrosine kinases, integrin, cytokine and
G-protein coupled receptors. Therefore, the method of overnight
serum-starvation and serum stimulation was applied to achieve
phosphorylation and demonstrate inhibition by nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone. Responsiveness to serum
stimulation, after overnight serum-starvation, with complete
culture media was tested over time and 2 h proved to be an optimal
stimulus, while a significant inhibition of the two
hexamethoxyflavones could be observed as early as ~5–10 min after
100 µM treatment, with 10 min used in the subsequent array
experiments.
The phospho-kinase array revealed a considerable
amount of information, with most notably the decrease of p-ERK
phosphorylation by nobiletin and 5,6,7,3′,4′,5′-hexamethoxyflavone
as compared to DMSO-treated cells. A similar trend was observed for
p-JNK1/2/3, although the effect was less pronounced. Moreover, both
hexamethoxyflavones inhibited the phosphorylation levels of Akt,
while the phosphorylation of checkpoint kinase 2 (Chk2) seemed to
be solely reduced upon 10 min of nobiletin treatment. Additionally,
phosphorylated p38 MAPK was only detected in the Hs578T cell line,
and was inhibited by the two hexamethoxyflavones (HMFs) (Fig. 5A and B), while it was below the
detection limit of the array for the Hs578Ts(i)8 cells.
It must be mentioned that only two arrays per cell line and per
component were performed to obtain initial data. Even though these
arrays would allow for the quantification of the relative
phosphorylation levels, the obtained results were further confirmed
by western blotting in kinetics experiments in Hs578T (Fig. 5C) and Hs578Ts(i)8
(Fig. 5D) cells. Serum stimulation
induced the phosphorylation of all kinases as compared to
unstimulated cells, except for Chk2. Chk2 was not only found highly
phosphorylated in these cell lines after serum-starvation but also
under normal growth conditions (data not shown). Nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone were able to inhibit the
serum-induced phosphorylation of ERK, SAPK/JNK and Akt in a time
range of 5–20 min in Hs578T and Hs578Ts(i)8 cells.
Additionally, significant Chk2 phosphorylation inhibition was
evident for nobiletin after 5 min, whereas
5,6,7,3′,4′,5′-hexamethoxyflavone significantly reduced the
phosphorylation levels after treatments of 20 min and longer. Also
in the western blotting experiments, phosphorylated p38 MAPK could
only be clearly detected in the Hs578T cells under the experimental
conditions used, and was reduced by nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone over the investigated time range
(Fig. 5C and D).
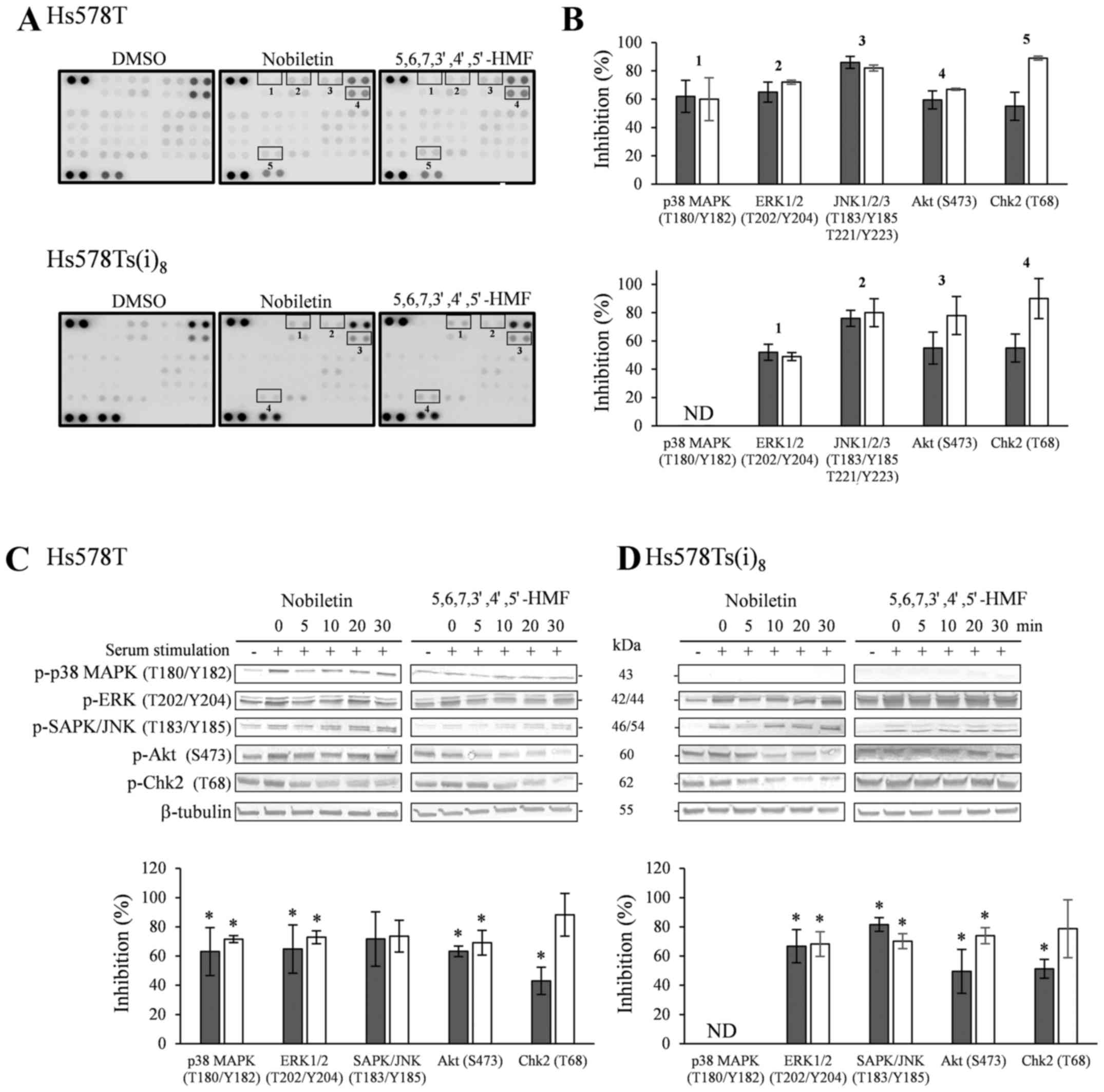 | Figure 5(A) Effect of a 10-min treatment with
100 µM nobiletin and 5,6,7,3′,4′,5′-hexamethoxyflavone,
after overnight serum-starvation and stimulation for 2 h with
complete culture media, on kinase phosphorylation sites using
phospho-kinase arrays in Hs578T (upper panel) and
Hs578Ts(i)8 cells (lower panel). (B) Quantification by
mean pixel density, expressed as means of % inhibition of nobiletin
(closed bars) and 5,6,7,3′,4′,5′-hexamethoxyflavone (open bars) in
Hs578T and Hs578Ts(i)8 cells vs DMSO-treated cells.
Results were obtained from 2 independent experiments. Lower panel:
western blot analysis of phosphorylated proteins in Hs578T (C) and
Hs578Ts(i)8 cells (D). Cells were serum-starved
overnight, stimulated with complete culture medium for 2 h and
treated for indicated times with 100 µM nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone. Cell lysates were analyzed by
TGXTM gels, transferred to PVDF membranes and use of the
corresponding primary antibodies against kinase phosphorylation
sites and tubulin as loading controls. Blots are representative
examples of at least three independent experiments. Scion Image
densitometry analysis of bands comparing the relative levels of
phosphorylation after 10 min 100 µM nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone as compared to DMSO-treatment.
Bar graphs are means ± SD from at least three independent
experiments. *P<0.05, statistical difference from
DMSO-treated cells. ND, not detected. |
Nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone affect the cell cycle
As a result of the significant nobiletin- and
5,6,7,3′,4′,5′-hexamethoxyflavone-mediated inhibition of Chk2
phosphorylation in the previous phosphokinase arrays and western
blot experiments, the effect of both hexamethoxyflavones on the
cell cycle was evaluated by performing a Vybrant®
DyeCycle™ green staining. Comparison with DMSO-treated cells
indicated that both hexamethoxyflavones upon treatment with 100
µM for 72 h, but not 48 h (data not shown), were able to
cause a slight increase in G2/M arrest (Fig. 6A and B). While
5,6,7,3′,4′,5′-hexamethoxyflavone elevated the subpopulation of
Hs578T and Hs578Ts(i)8 cells by 16.1 and 13.4%,
respectively, nobiletin only significantly enhanced the G2/M arrest
in Hs578T cells by 14.3%. Western blot results in Fig. 6C and D revealed that Chk2
phosphorylation at T68 decreased after 10 min in the presence of
nobiletin (P<0.05) and 30 min with
5,6,7,3′,4′,5′-hexamethoxyflavone (P<0.05), without prior
serum-starvation and stimulation, in both cell lines. Additionally,
Chk1 phosphorylation levels were below or near the detection limit
in the Hs578T cell lines, whereas weak levels were detected in the
more invasive variant, which were not notably altered in the
presence of the HMFs. Furthermore, phosphorylation levels of the
cell division cycle 2 (Cdc2), known as CDK1, which acts downstream
from Chk1 and regulates G2/M arrests, were found significantly
decreased after 30-min exposure to nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone in the Hs578T and after 30 to 60
min in Hs578Ts(i)8 cells (P<0.05).
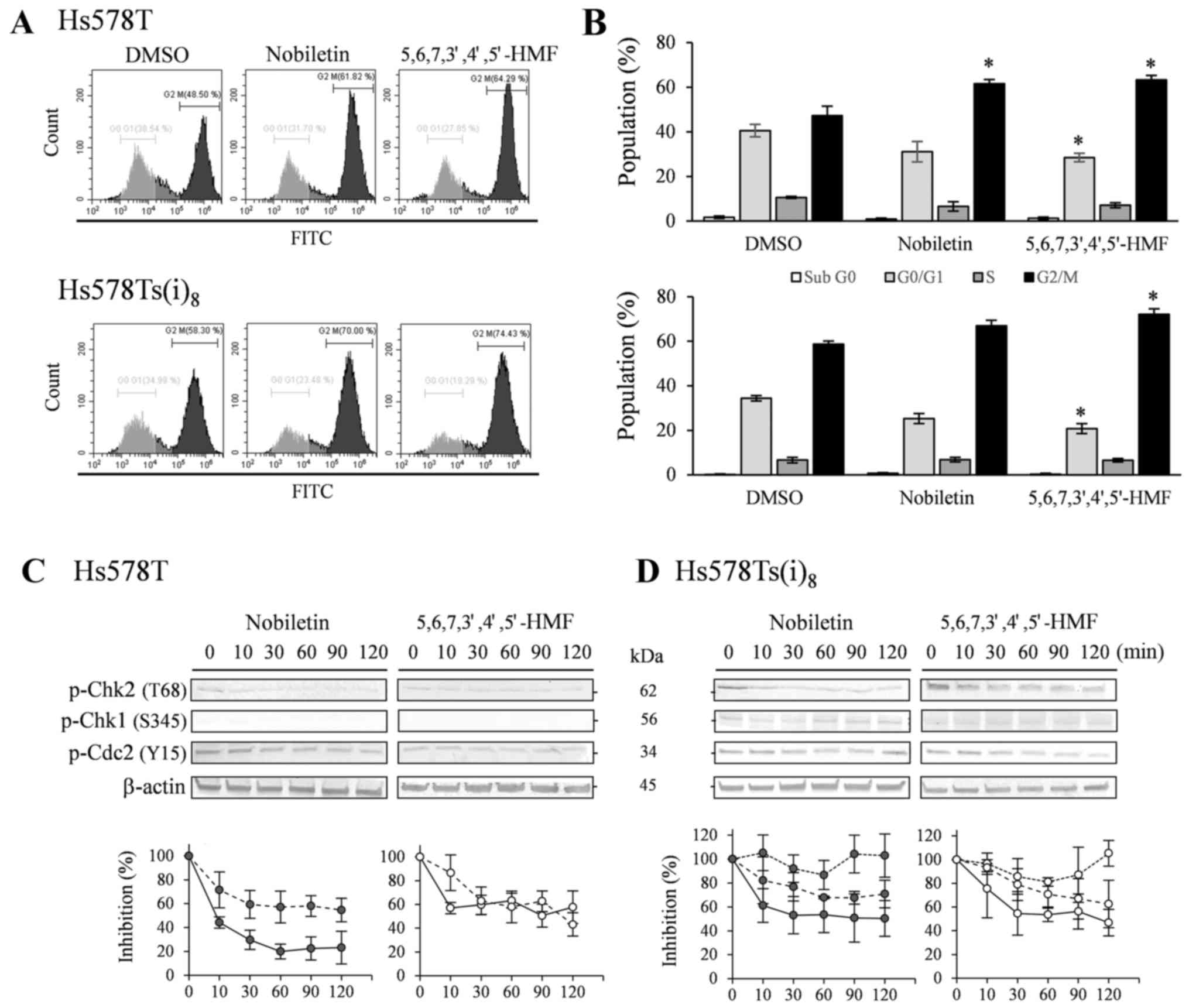 | Figure 6Effects on the cell cycle after
treatment with 100 µM nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone for 72 h in Hs578T (upper panel)
and Hs578Ts(i)8 cells (lower panel). (A) Cells were
trypsinized, combined with floating cells prior to staining with
Vybrant® DyeCycle™ Green. Profiles are representative
examples of four independent experiments. (B) Percentage of
population in sub G0 (open bars), G0/G1 (light grey bars), S (grey
bars) and G2/M (closed bars) cells, expressed as mean % ± SD of
three independent experiments. *P<0.05, statistical
difference from DMSO-treated cells. Lower panel: western blot
analysis of phosphorylated Chk2 and Cdc2 in Hs578T (C) as well as
Chk1 in Hs578Ts(i)8 cells (D). Cells were treated for
indicated times with 100 µM nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone. Cell lysates were analyzed by
TGX™ gels, transferred to PVDF membranes and use of the
corresponding primary antibodies against kinase phosphorylation
sites and β-actin as loading controls. Blots are representative
examples of at least three to five independent experiments. Scion
Image densitometry analysis of bands comparing the relative levels
of phosphorylated p-Chk2 (solid line) and p-Cdc2 (dashed line) in
Hs578T cells as well as p-Chk1 (dotted line) in
Hs578Ts(i)8 cells, after 100 µM nobiletin (closed
circles) and 5,6,7,3′,4′,5′-hexamethoxyflavone (open circles) as
compared to DMSO-treatment. Line graphs are means ± SD from three
to five independent experiments. |
Discussion
The present study reports for the first time on the
anticancer activity of 5,6,7,3′,4′,5′-hexamethoxyflavone. This
polymethoxyflavone is a structural isomer of
5,6,7,8,3′,4′-hexamethoxyflavone, known as nobiletin. They differ
in the position of one methoxy group either on ring A or B
(Fig. 1). While nobiletin is
widely studied and known for its beneficial effects on cancer
cells, 5,6,7,3′,4′,5′-hexamethoxyflavone is not, despite being
isolated from the same sources and the recognized concept of
isomerism affecting biological activity (4,13,15,23).
In the present study, we used the
Hs578T/Hs578Ts(i)8 breast cancer progression model
(16,19) for several reasons, including the
potential role of nobiletin against TNBC (12) and in prevention of metastasis
(8–10). Our results indicated that
5,6,7,3′,4′,5′-hexamethoxyflavone is less toxic than nobiletin
against Hs578T and Hs578Ts(i)8 cells, while exerting a
nearly similar effect as nobiletin in the cell counting experiments
after 72-h treatments. Additionally, only nobiletin reduced the
migratory behavior with almost 40% within 17 h in both cell lines.
The effects of nobiletin on cell viability are in the same range as
previously published studies using MCF-7, T47D, MDA-MB-231 and
SKBR3 breast cancer cells. Notably, these studies also reported
that nobiletin reduced the migratory behavior of MCF-7 cells by 40%
in wound-healing experiments and invasion by 30% using the Matrigel
invasion assay, while a 60% reduction in invasion was observed in
MDA-MB-231 cells (24,25). These findings as well as the
observations presented in this study should be interpreted and used
with caution, given that nobiletin decreased the cell viability and
number of cells by 20–30% within 24 h which may confound the effect
on migration and invasion. This idea is confirmed by the fact that
5,6,7,3′,4′,5′-hexamethoxyflavone did not influence migration,
while showing limited to no effect on cell viability and cell
counts after 24 h. Other structural congeners with methoxy groups
at position 3′,4′,5′ of the B-ring include sinensetin and
5,6,7,8,3′,4′,5′-heptamethoxyflavone. Unfortunately, few studies
are published on the potential anticancer activity of the
structurally related penta- and heptamethoxyflavone. A recent study
suggested that sinensetin has an antiproliferative effect and the
ability to induce apoptosis at concentrations of 50 µM and
higher (26). These results are
quite remarkable given the poor solubility in water and the typical
solvents as DMSO, ethanol or methanol and consequently questions
the validity of the results.
Further investigations on the possible mechanisms of
action of 5,6,7,3′,4′,5′-hexamethoxyflavone and nobiletin resulting
in a decreased number of cells suggested that 72 h treatments with
either compounds were not able to induce apoptosis in the Hs578T
and Hs578Ts(i)8 cells as compared to camptothecin. It
must be mentioned though that nobiletin studies are controversial
and inconclusive when it comes to its effect on apoptosis. One
reasoning could be that certain cell lines, such as the Hs578T cell
line and its more invasive variant Hs578Ts(i)8 as well
as glioma cell lines (8) are more
resistant to apoptosis, and that nobiletin or other PMFs are not
potent enough to induce an apoptotic effect similar to
camptothecin.
The use of Proteome Profiler Human phospho-kinase
arrays allowed for an objective screening of altered
phosphorylation levels of 43 different kinases. The array data as
well as the confirming western blotting results, using pathway
activation and potential inhibition as described by Burkhard and
Shapiro (27), revealed that
5,6,7,3′,4′,5′-hexamethoxyflavone treatment mainly inhibited MAPK
and Akt signaling pathways, recognized for influencing cell
proliferation, cell differentiation and cell death. These findings
were comparable to nobiletin and were also obtained without pathway
stimulation for which a significant inhibition was detected after
30-min exposure to both hexamethoxyflavones (data not shown).
Numerous studies link the effect of nobiletin to the inhibition of
MAPK and Akt signaling pathways and the small non-receptor tyrosine
kinases involved. This study contributes to this knowledge and adds
TNBC cell lines to the list, while the effect of
5,6,7,3′,4′,5′-hexamethoxyflavone on signaling is novel and puts
this flavone in a similar group as nobiletin and tangeretin, as
potential chemopreventive or therapeutic agent.
Additionally, both hexamethoxyflavones reduced the
phosphorylation levels of cell cycle checkpoint kinase 2 (Chk2) and
induced a subtle arrest at G2/M in the TNBC cell lines. Usually,
Chk2 is phosphorylated at T68 after DNA damage, such as ionizing
radiation and UV irradiation, however in the
Hs578T/Hs578Ts(i)8 cell model high phosphorylation
levels were seen in untreated or solvent treated conditions, which
suggests that repair mechanisms are constitutively active in these
cells. Furthermore, several reports indicate that Chk2 induces a
cell cycle arrest at G1/S and G2/M (28). Our results do not show a G1/S
arrest, instead the hexamethoxyflavones cause a G2/M arrest. This
outcome is in contrast to the G1/S arrest of nobiletin on
MDA-MB-468 TNBC cells reported by Chen et al (12). However, this study included not
only the effect on G2/M but also Chk2 phosphorylation and is
supported by previous studies in which particularly Chk2 was proven
to be required for the G2/M arrests triggered by
naturally-occurring chemopreventive agents (29). On the other hand, G2/M arrests
typically dependent on a Chk1-associated signaling pathway leading
to the inhibition of cyclin B1/Cdc2 activity, with Cdc2 also known
as cyclin dependent kinase 1 (CDK1). Chk1 is activated by
phosphorylation on S345 and subsequently inhibits Cdc25C
phosphatase by phosphorylating S216. This Cdc25C plays an important
role in the dephosphorylation and activation of CDK1/Cdc2 on
T14/Y15 needed for G2/M transition (30,31).
In this study, Chk1 phosphorylation was difficult to detect in the
Hs578T, whereas very weak levels were observed in
Hs578Ts(i)8 cells. Nobiletin and
5,6,7,3′,4′,5′-hexamethoxyfla-vone did not seem to significantly
affect the phosphorylation levels of Chk1, while the
phosphorylation of Cdc2 was found to be suppressed after 30 min and
more, which suggests that both hexamethoxyflavones induce the
activation of Cdc2 needed for G2/M transition. These results are
not in accordance with the observed G2/M arrest, thus, it seems
that Chk1 and Chk2 may have additional roles in the nobiletin and
5,6,7,3′,4′,5′-hexamethoxyflavone-mediated G2/M arrest.
Interestingly, these observations are not uncommon, several groups
have demonstrated a G2/M cell cycle arrest following the use of
herbal derivatives. These studies mentioned that the mechanism of
G2/M arrest may be secondary to antimitotic effects in contrast to
checkpoint modulation late in G2 (32). Furthermore, research using
flavopiridol or silibinin with other chemotherapeutic agents showed
increased cytotoxicity associated with G1 and G2 arrests, while
flavopiridol is known to mediate its effect via inhibition of cdks,
silibinin in combination with doxorubicin was found to decrease the
expression of Cdc25C and Cdc2 (32). These correlations hint that
downregulation of G2/M cell cycle regulators resulting in increased
G2/M arrest could be a mechanism as well. Further investigations
will be needed to clarify the mechanisms of G2/M arrest and
determine whether nobiletin and 5,6,7,3′,4′,5′-hexamethoxyflavone
have an antimitotic effect on the Hs578T and Hs578Ts(i)8
cells or suppress cell cycle regulators.
In conclusion, our results indicate that
5,6,7,3′,4′,5′-hexamethoxyflavone has several anticancer properties
and could be a more valuable component than nobiletin. This initial
study showed that 5,6,7,3′,4′,5′-hexamethoxyflavone is less toxic
than nobiletin, and shows comparable growth inhibition of TNBC
cells. This effect could be ascribed in part to a suppression of
MAPK and Akt signaling pathways and to the induction of a G2/M cell
cycle arrest. Taken together, the present results suggest that
5,6,7,3′,4′,5′-hexamethoxyflavone may possess potential as a novel
preventive or therapeutic agent in the treatment of TNBC.
Acknowledgments
The present study was supported by the National
Science Foundation/EPSCoR Cooperative Agreement #IIA-1355423, the
South Dakota Research and Innovation Center, BioSNTR, and by the
State of South Dakota.
Glossary
Abbreviations
Abbreviations:
|
TNBC
|
triple-negative breast cancer
|
|
PMF
|
polymethoxyflavones
|
|
HMF
|
hexamethoxyflavones
|
|
FDA
|
Food and Drug Administration
|
|
MAPK
|
mitogen-activated protein kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
SAPK
|
stress-activated protein kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
HRP
|
horseradish peroxidase
|
|
CDK
|
cyclin-dependent kinase
|
|
Chk
|
checkpoint kinase, Cdc, cell division
cycle
|
References
|
1
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the last 25 years. J Nat Prod.
70:461–477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Orphan Drug status for alvocidib. Oncology
Times. 25–May. 2014, https://doi.org/10.1097/01.COT.0000450374.31680.1d.
|
|
3
|
Sak K: Cytotoxicity of dietary flavonoids
on different human cancer types. Pharmacogn Rev. 8:122–146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walle T: Methoxylated flavones, a superior
cancer chemopreventive flavonoid subclass? Semin Cancer Biol.
17:354–362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mizuno M, Iinuma M, Ohara M, Tanaka T and
Iwamasa M: Chemotaxonomy of the genus Citrus based on
polymethoxyflavones. Chem Pharm Bull (Tokyo). 39:945–949. 1991.
View Article : Google Scholar
|
|
6
|
Yenjai C, Prasanphen K, Daodee S,
Wongpanich V and Kittakoop P: Bioactive flavonoids from Kaempferia
parviflora. Fitoterapia. 75:89–92. 2004. View Article : Google Scholar
|
|
7
|
Faqueti LG, Brieudes V, Halabalaki M,
Skaltsounis AL, Nascimento LF, Barros WM, Santos AR and Biavatti
MW: Antinociceptive and anti-inflammatory activities of
standardized extract of polymethoxyflavones from Ageratum
conyzoides. J Ethnopharmacol. 194:369–377. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lien LM, Wang MJ, Chen RJ, Chiu HC, Wu JL,
Shen MY, Chou DS, Sheu JR, Lin KH and Lu WJ: Nobiletin, a
polymethoxylated flavone, inhibits glioma cell growth and migration
via arresting cell cycle and suppressing MAPK and Akt pathways.
Phytother Res. 30:214–221. 2016. View
Article : Google Scholar
|
|
9
|
Huang H, Li L, Shi W, Liu H, Yang J, Yuan
X and Wu L: The multifunctional effects of nobiletin and its
metabolites in vivo and in vitro. Evid Based Complement Alternat
Med. 2016:29187962016. View Article : Google Scholar :
|
|
10
|
Chien SY, Hsieh MJ, Chen CJ, Yang SF and
Chen MK: Nobiletin inhibits invasion and migration of human
nasopharyngeal carcinoma cell lines by involving ERK1/2 and
transcriptional inhibition of MMP-2. Expert Opin Ther Targets.
19:307–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Surichan S, Androutsopoulos VP, Sifakis S,
Koutala E, Tsatsakis A, Arroo RR and Boarder MR: Bioactivation of
the citrus flavonoid nobiletin by CYP1 enzymes in MCF7 breast
adenocarcinoma cells. Food Chem Toxicol. 50:3320–3328. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen C, Ono M, Takeshima M and Nakano S:
Antiproliferative and apoptosis-inducing activity of nobiletin
against three subtypes of human breast cancer cell lines.
Anticancer Res. 34:1785–1792. 2014.PubMed/NCBI
|
|
13
|
Andreopoulou E, Schweber SJ, Sparano JA
and McDaid HM: Therapies for triple negative breast cancer. Expert
Opin Pharmacother. 16:983–998. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adebayo AH, Jig CJ, Zhang YM, He WJ, Zeng
GZ, Han HJ, Xu JJ, Akindahunsi AA and Tana NH: A new chromene
isolated from Ageratum conyzoides. Nat Prod Commun. 6:1263–1265.
2011.PubMed/NCBI
|
|
15
|
Itoh T, Ohguchi K, Iinuma M, Nozawa Y and
Akao Y: Inhibitory effects of polymethoxy flavones isolated from
Citrus reticulate on degranulation in rat basophilic leukemia
RBL-2H3: Enhanced inhibition by their combination. Bioorg Med Chem.
16:7592–7598. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hughes L, Malone C, Chumsri S, Burger AM
and McDonnell S: Characterisation of breast cancer cell lines and
establishment of a novel isogenic subclone to study migration,
invasion and tumourigenicity. Clin Exp Metastasis. 25:549–557.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Romijn JC, Verkoelen CF and Schroeder FH:
Application of the MTT assay to human prostate cancer cell lines in
vitro: Establishment of test conditions and assessment of
hormone-stimulated growth and drug-induced cytostatic and cytotoxic
effects. Prostate. 12:99–110. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rasband WS: ImageJ. U. S. National
Institutes of Health; Bethesda, Maryland, USA: https://imagej.nih.gov/ij/.
1997–2016
|
|
19
|
Payan I, McDonnell S, Torres HM, Steelant
WF and Van Slambrouck S: FAK tyrosine 407 organized with integrin
αVβ5 in Hs578Ts(i)8 advanced triple-negative breast
cancer cells. Int J Oncol. 48:2043–2054. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mustacchi G and De Laurentiis M: The role
of taxanes in triple-negative breast cancer: Literature review.
Drug Des Devel Ther. 9:4303–4318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Van Slambrouck S, Parmar VS, Sharma SK, De
Bondt B, Foré F, Coopman P, Vanhoecke BW, Boterberg T, Depypere HT,
Leclercq G, et al: Tangeretin inhibits
extracellular-signal-regulated kinase (ERK) phosphorylation. FEBS
Lett. 579:1665–1669. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McConathy J and Owens MJ: Stereochemistry
in drug action. Prim Care Companion J Clin Psychiatry. 5:70–73.
2003. View Article : Google Scholar
|
|
24
|
Baek SH, Kim SM, Nam D, Lee JH and Ahn KS,
Choi SH, Kim SH, Shim BS, Chang IM and Ahn KS: Antimetastatic
effect of nobiletin through the down-regulation of CXC chemokine
receptor type 4 and matrix metallopeptidase-9. Pharm Biol.
50:1210–1218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sp N, Kang DY, Joung YH, Park JH, Kim WS,
Lee HK, Song KD, Park YM and Yang YM: Nobiletin inhibits
angiogenesis by regulating Src/FAK/STAT3-mediated signaling through
PXN in ER+ breast cancer cells. Int J Mol Sci.
18:9352017. View Article : Google Scholar
|
|
26
|
Dong Y, Ji G, Cao A, Shi J, Shi H, Xie J
and Wu D: Effects of sinensetin on proliferation and apoptosis of
human gastric cancer AGS cells. Zhongguo Zhong Yao Za Zhi.
36:790–794. 2011.In Chinese. PubMed/NCBI
|
|
27
|
Burkhard K and Shapiro P: Use of
inhibitors in the study of MAP kinases. Methods Mol Biol.
661:107–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zannini L, Delia D and Buscemi G: CHK2
kinase in the DNA damage response and beyond. J Mol Cell Biol.
6:442–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singh SV, Herman-Antosiewicz A, Singh AV,
Lew KL, Srivastava SK, Kamath R, Brown KD, Zhang L and Baskaran R:
Sulforaphane-induced G2/M phase cell cycle arrest involves
checkpoint kinase 2-mediated phosphorylation of cell division cycle
25C. J Biol Chem. 279:25813–25822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K
and Linn S: Molecular mechanisms of mammalian DNA repair and the
DNA damage checkpoints. Annu Rev Biochem. 73:39–85. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou BB and Bartek J: Targeting the
checkpoint kinases: Chemosensitization versus chemoprotection. Nat
Rev Cancer. 4:216–225. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
DiPaola RS: To arrest or not to G2-M
cell-cycle arrest. Clin Cancer Res. 8:3512–3519. 2002.
|