Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent malignancies worldwide, and its morbidity and mortality
rates have been increasing in recent years (1,2).
Currently, systemic therapies and anticancer drugs for HCC have
obtained considerable advances; however, the incidence rate of
resistance to chemotherapy, tumor recurrence and metastasis is
still increasing (3,4). Accumulating evidence has revealed
that one of the reasons for the resistance to anticancer drugs is
the existence of CSCs or tumor-initiating cells (TICs) (5).
The concept of CSCs or TICs was first proposed
approximately 50 years ago (6).
The existence of CSCs has been demonstrated in leukemia (7) and solid tumors, including breast,
colon, brain and liver cancers (8–13).
CSC is a small subpopulation of cancer cells with stem cell
properties, such as self-renewal, pluripotency, chemoresistance and
limitless proliferation (14).
Substantial evidence revealed that CSCs are responsible for
initiation and resistance to chemotherapy; and the cancer always
relapses and metastasizes due to the persistence of CSCs (14,15).
Hence, inhibiting CSC proliferation or even eradicating them may be
a pivotal strategy for overcoming chemoresistance and improving the
curative effect in liver cancer.
To better investigate CSC properties and novel
anticancer drugs, many studies have been created in vitro
model system using serum-free stem cell conditional medium to
select and expand CSCs. Tumor spheres formed in this condition
possess cancer stem cell characteristics, including self-renewal,
proliferation, chemoresistance and higher tumorigenicity. Thus,
they are considered to be CSCs (16,17).
This sphere cell formation method has been successfully used to
enrich CSCs from liver, brain, lung and ovarian cancers (17–20).
Thus, this method was also used to enrich CSCs, and the condition
of the culture medium was optimized to create a more suitable
microenvironment for better forming liver cancer stem cells
(LCSCs).
CD13/APN is a marker for semi-quiescent CSCs and a
therapeutic target in human liver CSCs (5). CD13 is a zinc-binding type 2
transmembrane ectopeptidase (150 kDa), which is related to
malignant behavior in many cancers, such as prostate, colon and
lung cancers (21–23). In in vivo transplantation
model of mice, CD13+ cells survived, and the expression
of CD13 are upregulated after 5-FU treatment alone, which is one of
the most common cytotoxic drugs in HCC treatment. Thus,
co-processing with a CD13 inhibitor could be helpful for radical
elimination of CD13+ cells or liver CSCs (24). Bestatin (ubenimex) is reported to
be a CD13 inhibitor, which is constantly used for adult acute
nonlymphocytic leukemia (25). As
expected, combining bestatin with 5-FU treatment efficiently
elicits tumor regression and improves the effect of liver cancer
treatment (5). Thus far, few
drugs, which could not only target liver CSCs but has cytotoxic
effects on the CSCs, may kill CSCs and reveal a promising cure for
liver cancer.
In the present study, the serum-free stem cell
conditional medium was optimized and sphere cells were successfully
enriched and expanded, which possessed the characteristics of stem
cells. The compound BC-02, an APN/CD13 inhibitor, showed a
significant effect on self-renewal and malignant proliferation of
liver CSCs compared to the control, bestatin, 5-FU and even 5-FU
plus bestatin. Subsequently, BC-02 could target CD13 and increase
the intracellular ROS and the ROS-induced DNA damage, thereby
inhibiting the liver CSCs and overcoming the chemoresistance in
liver cancer. Therefore, BC-02 might be a novel therapeutic drug
for improving the survival rate of liver cancer patients.
Materials and methods
Chemicals and reagents
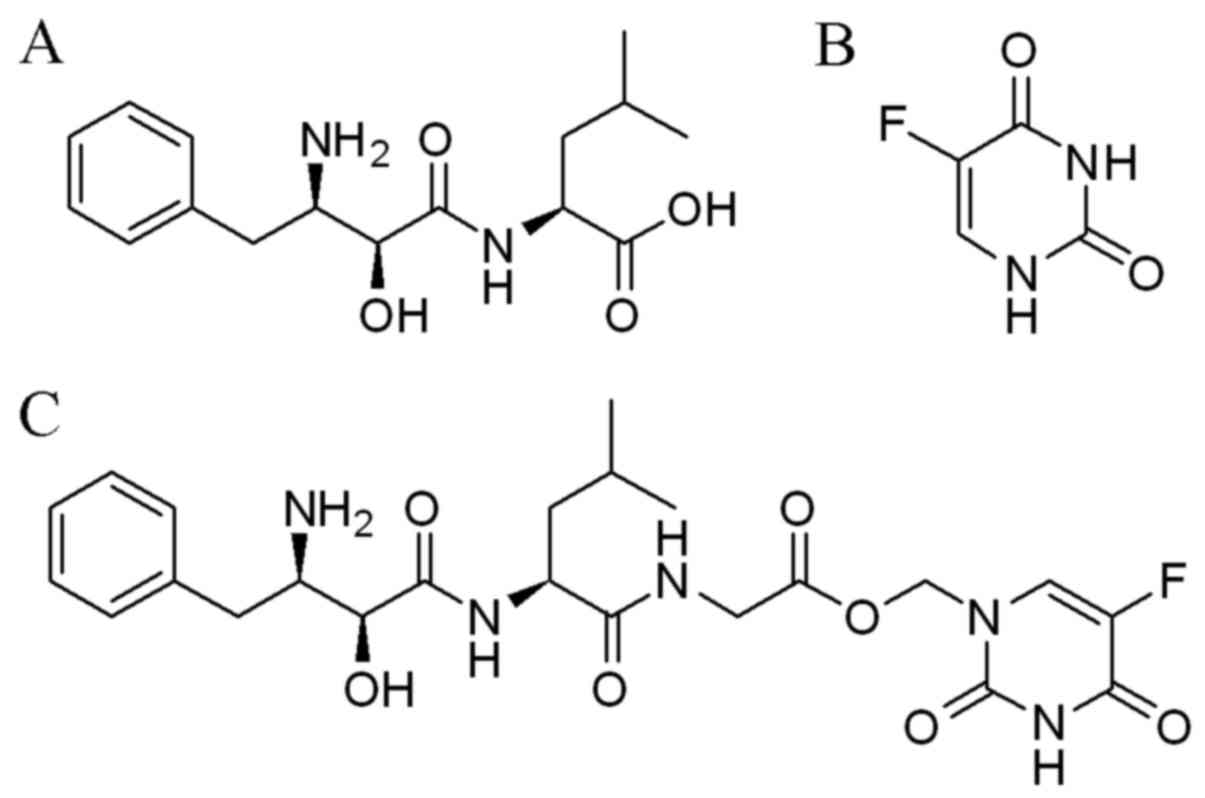
Bestatin (Fig. 1A)
and 5-FU (Fig. 1B) were obtained
from Shanghai Biochempartner Co., Ltd. (Shanghai, China). The
compound BC-02 (Fig. 1C) was
synthesized by conjugating a CD13 inhibitor bestatin and 5-FU in
the Department of Medicinal Chemistry, School of Pharmacy, Shandong
University (26). The BC-02 could
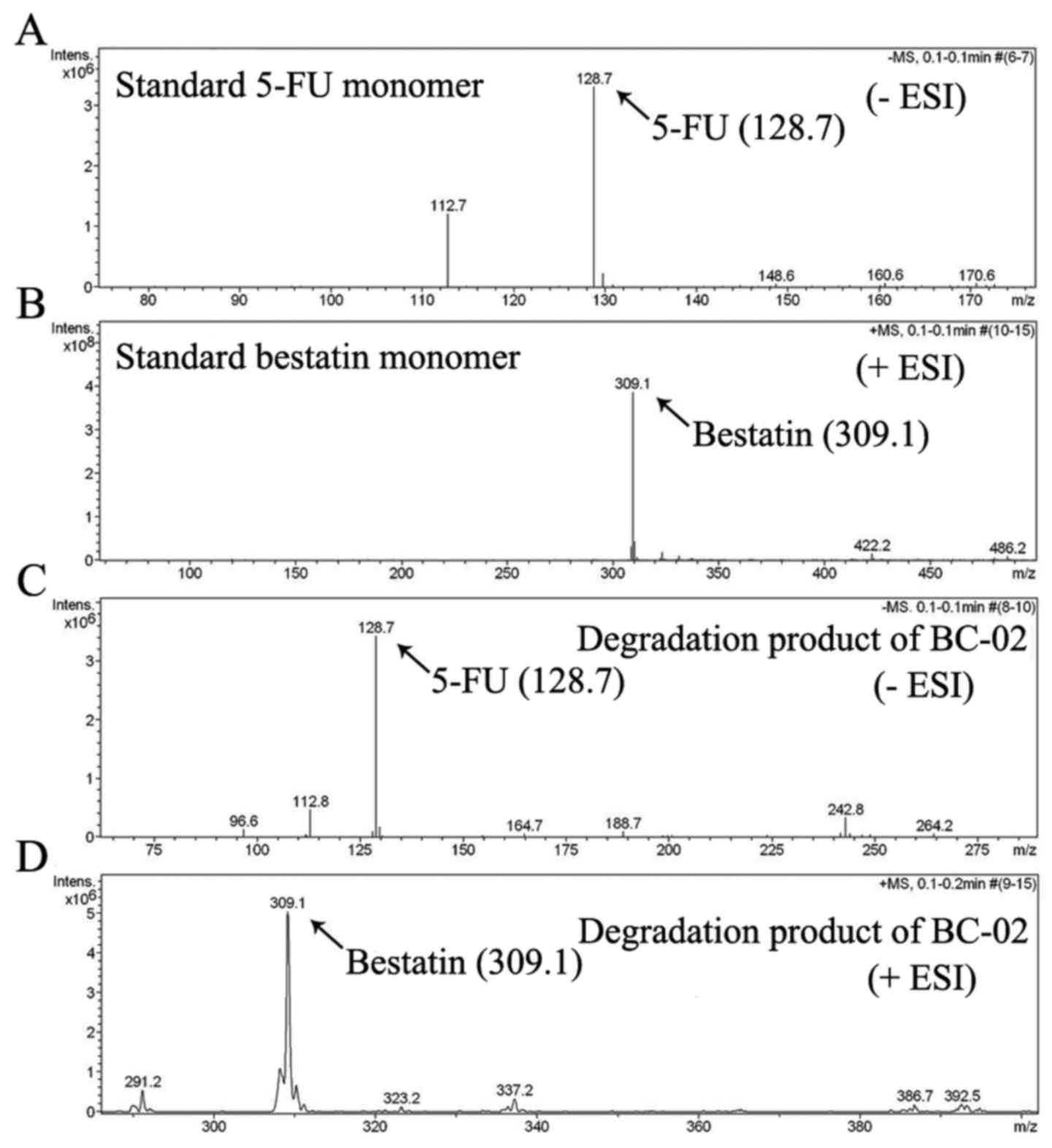
be decomposed into 5-FU and bestatin, when it was preliminary
analysed by electrospray ionization mass spectrometry (ESI-MS)
(Fig. 2). 5-FU, bestain and BC-02
were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St.
Louis, MO, USA) at 400 mM as stock solution. The 5-FU and bestatin
was mingled by equimolar concentration at 800 mM as stock solution,
it is represented by 1:1 in the subsequent experiments. The CD13
mouse anti-human antibody was obtained from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Methyl thiazolyl tetrazolium
(MTT) was purchased from Beijing Solarbio Science and Technology
Co., Ltd. (Beijing, China).
Cell culture
Human liver cancer cell lines PLC/PRF/5 and Huh7
were purchased from the Cell Bank of Shanghai (Shanghai, China) and
cultured in modified Eagle's medium (MEM) and Dulbecco's modified
Eagle's medium (DMEM; Life Techologies Corp., Carlsbad, CA, USA)
respectively, both supplemented with 10% fetal bovine serum (FBS;
Biological Industries, Kibbutz Beit Haemek, Israel). The cells were
cultured at 37°C in a humidified 5% CO2 incubator and
passaged every other day.
Sphere culture and self-renewal
assay
PLC/PRF/5 and Huh7 parental cells were collected and
washed with phosphate buffered saline (PBS) to remove serum,
respectively. Then the cells were plated in serum-free stem cell
conditional medium composed of DMEM/F12 (HyClone Laboratories,
Inc., South Logan, UT, USA), KnockOut™ serum replacement, 2% B-27
supplement, 1% N-2 supplement (Gibco/Invitrogen, Grand Island, NY,
USA), 20 ng/ml recombinant human insulin-like growth factor-I
(IGF-I), 20 ng/ml animal-free recombinant human epidermal growth
factor (EGF), 10 ng/ml recombinant human fibroblast growth
factor-basic (b-FGF), (PeproTech, Rocky Hill, NJ, USA), 2 mM
L-glutamine, 5 μg/ml heparin sodium and 1%
penicillin-streptomycin (Beijing Solarbio Science and Technology).
The cells were suspended in ultralow attachment 6-well plates
(Corning Inc., Corning, NY, USA) at a density of 10,000 cells/well.
When the spheres grew to 100–200 μm in diameter, the spheres
were dissociated with trypsin-EDTA. Then the single cells were
re-suspended in stem cell conditional medium to re-form spheres and
the medium was changed every other day. The PLC/PRF/5 spheres were
passaged every 5–7 days. The Huh7 spheres were passaged every 3–5
days. In addition, the isolated PLC/PRF/5 parental cells were
cultured in ultralow attachment 6-well plates at a density of 2,000
cells/well for 48 h. Then cells were treated with 400 μM
various chemicals (5-FU, bestatin, 1:1, BC-02, and BC-02 + NAC).
Cells were pretreated by 500 μM NAC for 2 h. Chemicals were
removed after 6 h. After further culturing for 3 days, the spheres
were analyzed by optical microscopy.
Colony formation assay
PLC/PRF/5 parental and sphere cells were dissociated
into single cells and the cells were seeded in MEM with 10% FBS on
6-well plates (1,000 cells/well). After 8–10 days, the cells grew
to visible colonies (>50 cells). The colonies were counted under
an ordinary optical microscope after crystal violet staining. To
analyze the effects of chemicals on colony forming of PLC/PRF/5 and
Huh7 sphere cells, single cells were cultured in 6-well plates at a
density of 10,000 cells/well for 24 h. The cells were treated with
various chemicals (5-FU, bestain, 1:1, BC-02 and BC-02 + NAC) for 6
h. Cells were pretreated by 500 μM NAC for 2 h. Then cell
medium was changed to remove chemicals. The colonies were counted
as mentioned and PLC/PRF/5 sphere cells were treated with 400
μM drugs, and the concentration of chemicals for Huh7 sphere
cells was 200 μM.
Western blot analysis
Parental and sphere cells were collected, washed
with PBS, and lysed in RIPA buffer with protease inhibitor cocktail
(Sigma-Aldrich). Protein concentrations were measured using a BCA
protein assay kit (Beijing Solarbio Science and Technology). Then
quantified proteins were electrophoresed, separated in
SDS-polyacrylamide gel (SDS-PAGE) and transferred onto
polyvinylidene difluoride (PVDF) membrane (Millipore). Rabbit
anti-human N-cadherin antibodies (dilution 1:1,000; Abcam,
Cambridge, MA, USA), rabbit anti-human vimentin (dilution 1:1,000;
Cell Signaling Technology, Danvers, MA, USA), and mouse anti-human
beta actin (dilution 1:1,000; PeproTech) were used as the primary
antibodies, and horseradish peroxidase-conjugated anti-rabbit and
anti-mouse immunoglobulin (dilution 1:5,000; Beyotime Institute of
Biotechnology, Shanghai, China) were used as the secondary
antibody. Blots were acquired by enhanced chemiluminescence kit
(Millipore).
MTT assays
Cell viability was measured by the MTT assay.
PLC/PRF/5 parental and sphere cells were dissociated into single
cells. The dissociated parental cells were re-suspended in MEM with
10% FBS on 96-well plates at a density of 5,000 cells/well, and the
dispersed sphere cells were re-suspended in serum-free stem cell
conditional medium on ultralow attachment 96-well plates (5,000
cells/well). After 24 h, the cells were treated with various
concentrations of compounds. Two days after treatment, cells were
incubated with MTT solutions for 4 h at 37°C. Then the DMSO was
added to each well. After that, the absorbance was measured at 570
nm using a multifunctional microplate reader (SpectraMax M5;
Molecular Devices, Sunnyvale, CA, USA). Huh7 cells were treated
with the same method. The effects of CD13 inhibition on cell
proliferation of spheres were examined using this method.
Cell proliferation in vitro was also
measured by MTT method
The enzymatically dissociated cells were seeded in
ultralow attachment 96-well plates in 200 μl of serum-free
stem cell conditional medium at a density of 1,000 cells/well.
Cells were fed with stem cell conditional medium every day. The
absorbance were measured every 12 h for 6 consecutive days at 570
nm using a multifunctional microplate reader and the growth curves
were draw using the data.
Enzyme activity assay
The APN activity was determined as previously
described (24). PLC/PRF/5 cells
were re-suspended in PBS in a 96-well plate with specified
concentrations of bestatin or BC-02. Cells were incubated with
L-leucine-pnitroanilide (Sigma-Aldrich) for 30 min at 37°C. Then
the enzyme activity was obtained by measuring the absorbance at 405
nm using a multifunctional microplate reader. The APN enzyme
activity inhibition rates were calculated by (OD control − OD
tested)/OD control × 100%.
Cellular ROS detection
Parental and sphere cells were trypsinized with
trypsin-EDTA and the isolated cells were incubated at 37°C for 30
min in darkness with 2′,7′-dichlorofluorescein diacetate (DCFH-DA).
After washing with PBS, the fluorescence intensity of DCF inside
the cells was measured using a FACSCan flow cytometer.
Intracellular ROS levels were analyzed using FlowJo 7.6 software
and the data represented the mean fluorescence intensity of 30,000
events.
Alkaline comet assay
To determine the oxidative DNA damage, the alkaline
comet assay was performed according to the manufacturer's
instructions. Briefly, PLC/PRF/5 sphere cells were treated with
various drugs (400 μM of 5-FU, bestatin, 1:1, BC-02 and
BC-02 + NAC) for 12 h. Cells were pretreated by 500 μM NAC
for 2 h. Then the cells were dissociated into single cells. The
resulting cells were mixed with 0.7% low-melting agarose, added to
slides pre-coated with 0.55% normal-melting agarose, covered with
coverslips and allowed to solidify at 4°C for 30 min. Then the
coverslips were gently removed and the slides were kept in lysis
buffer (2.5 M NaCl, 0.1 M Na2EDTA, 10 mM Tris, 1% Triton
X-100, and 10% DMSO, pH 10.0) for 1.5 h, submerged in alkaline
electrophoresis solution (10 M NaOH, 0.2 M EDTA, pH >13) for 50
min, and subjected to electrophoresis in the same buffer at 25 V
for 25 min. After electrophoresis, cells were washed with neutral
buffer (pH 7.5) and stained with propidium iodide (PI) for 10 min.
The images were taken with a fluorescent microscope and the comets
were quantified by determination of the percentage of DNA in the
tail using TriTek Comet Score™ Freeware v1.5. Huh7 cells were
treated with the same method and the concentration of the chemicals
was 200 μM.
Nude mouse tumor formation assay
Four to five week-old female nude mice were
purchased from the Hunan SJA Laboratory Animal Co., Ltd.,2 (Hunan,
China) and maintained in a specific pathogen-free conditions. All
animal experiments were approved by the Guidelines of the Animal
Care and Use Committee at Weifang Medical University. The defined
number of viable parental and sphere cells was subcutaneously
injected into the left or right flank of mice. After the
inoculation, tumor formation and growth were monitored every day
and mice were sacrificed at 12 weeks.
Statistical analysis
Each experiment was performed at least three times,
and quantitative data were expressed as mean ± standard deviation
(SD). Statistical differences between the two groups were evaluated
by the Student's t-test using the SPSS 17.0 statistical software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was defined as
significant.
Results
PLC/PRF/5 and Huh7 cells form suspended
spheres
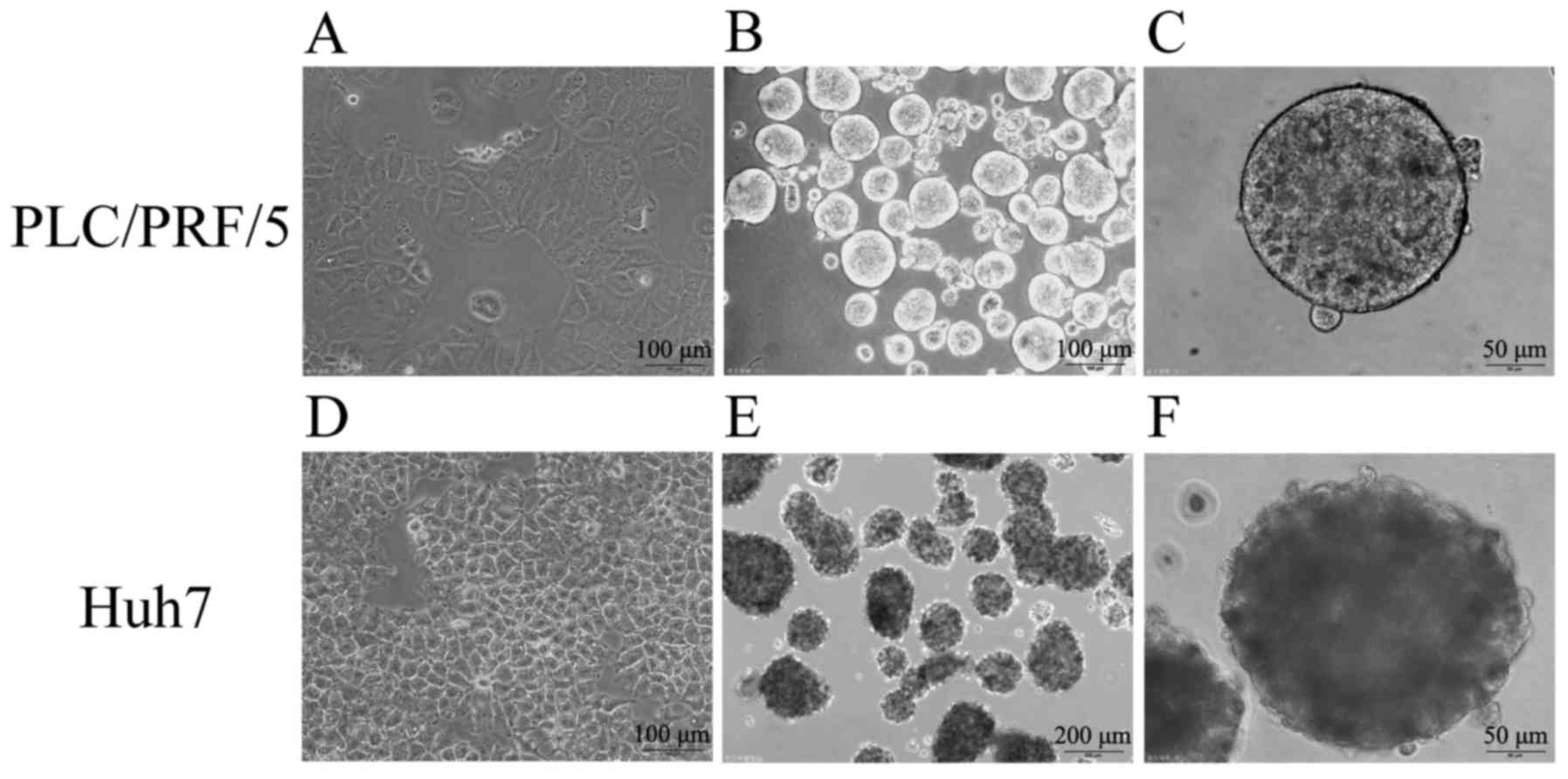
Previous studies demonstrated that the spheres,
which are suspended culture of cancer cells with growth factors in
serum-free medium, are enriched with CSCs. To successfully induct
CSCs, a more suitable culture system was first determined. Through
careful screening, the suitable culture, which contains DMEM/F12,
serum replacement, B-27 supplement, N-2 supplement, IGF-I, EGF,
b-FGF, L-glutamine, heparin sodium and penicillin-streptomycin, was
determined. In the culture system, two human HCC cell lines,
namely, PLC/PRF/5 and Huh7, successfully formed suspended spheres.
The status of the spheres was stably maintained even after several
generations, indicating their capability for self-renewal similarly
to TICs. Fig. 3 shows that cells
under conventional medium with 10% FBS formed epithelial
morphology, and the images of cells cultured in the serum-free stem
cell conditional medium formed suspended, self-renewing spheres,
which were observed by a microscope.
PLC/PRF/5 and Huh7 sphere cells possess
the characteristics of CSCs
Both PLC/PRF/5 and Huh7 spheres possessed the
characteristics of CSCs, including drug resistance, high
tumorigenicity, EMT phenotype, lower ROS levels, greater
colony-forming efficiency, and increased proliferation capacity
in vitro. MTT assays were performed to examine the
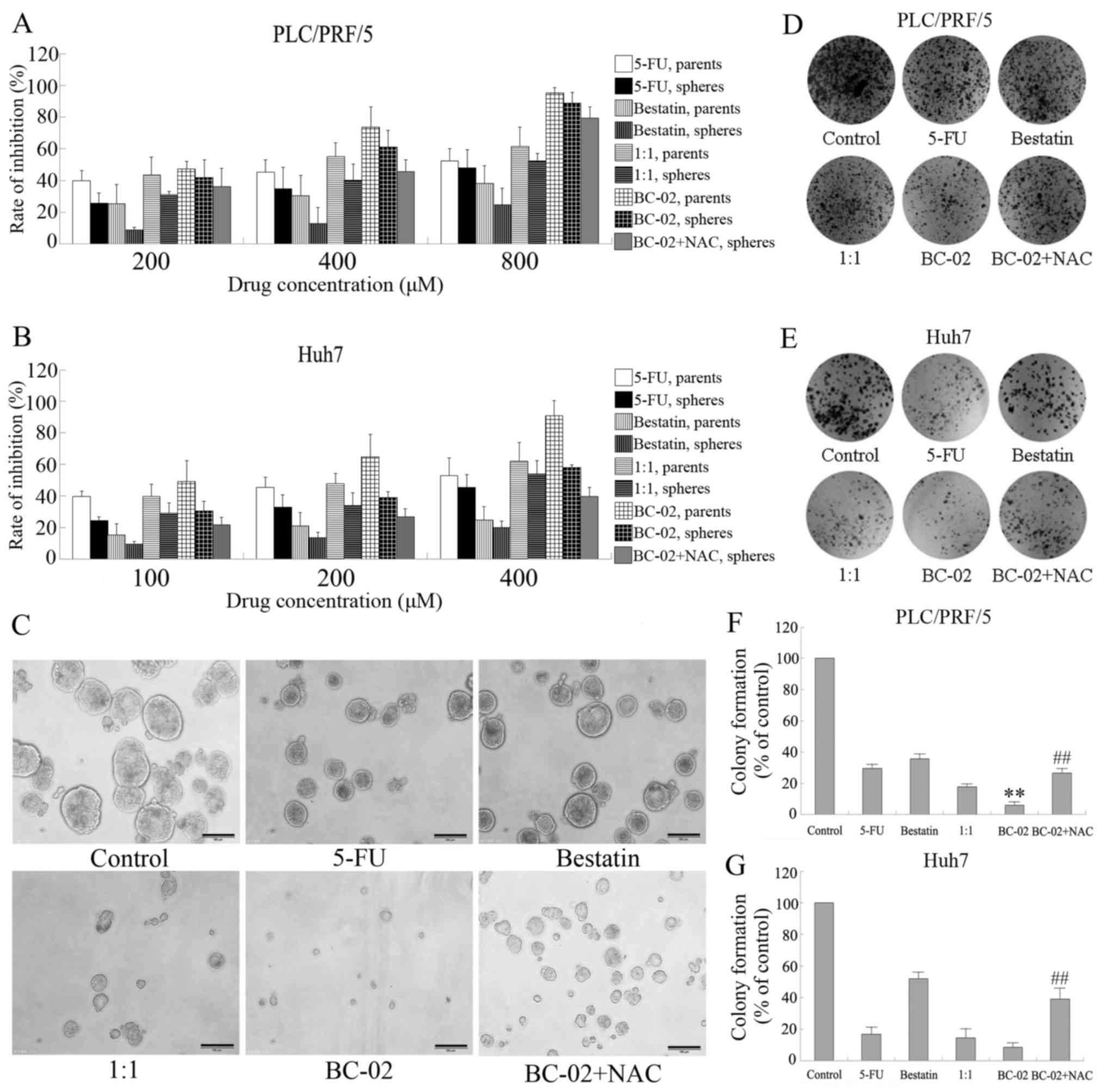
sensitivity of spheres and parental cells to our chemotherapy
drugs. Spheres and parental cells of liver cancer cell lines, such
as PLC/PRF/5 and Huh7, were treated with 5-FU, bestatin, 1:1, and
BC-02, respectively, with different concentrations for 48 h. As
shown in Fig. 5A and B, the
spheres showed much lower inhibition effect of the chemotherapy
drugs than their parental cells, which indicates that spheres had
increased chemoresistance. Tumorigenicity in vivo was
analyzed by tumor formation assay using nude mice. As shown in
Table I, 1×105 sphere
cells derived from PLC/PRF/5 cells successfully formed tumors in
one out of five mice, whereas similar number of PLC/PRF/5 parental
cells failed to form any tumors. Furthermore, although the number
of parental cells increased to 1×106, there was no tumor
generated. In addition, 1×106 sphere cells were capable
of forming tumors in three out of five mice. This result indicated
that the sphere cells possessed high tumorigenicity in vivo
and increased CSC properties. Recently, EMT has been considered to
be associated with CSC stemness characteristic. EMT-related
regulatory proteins, including N-cadherin and vimentin, were
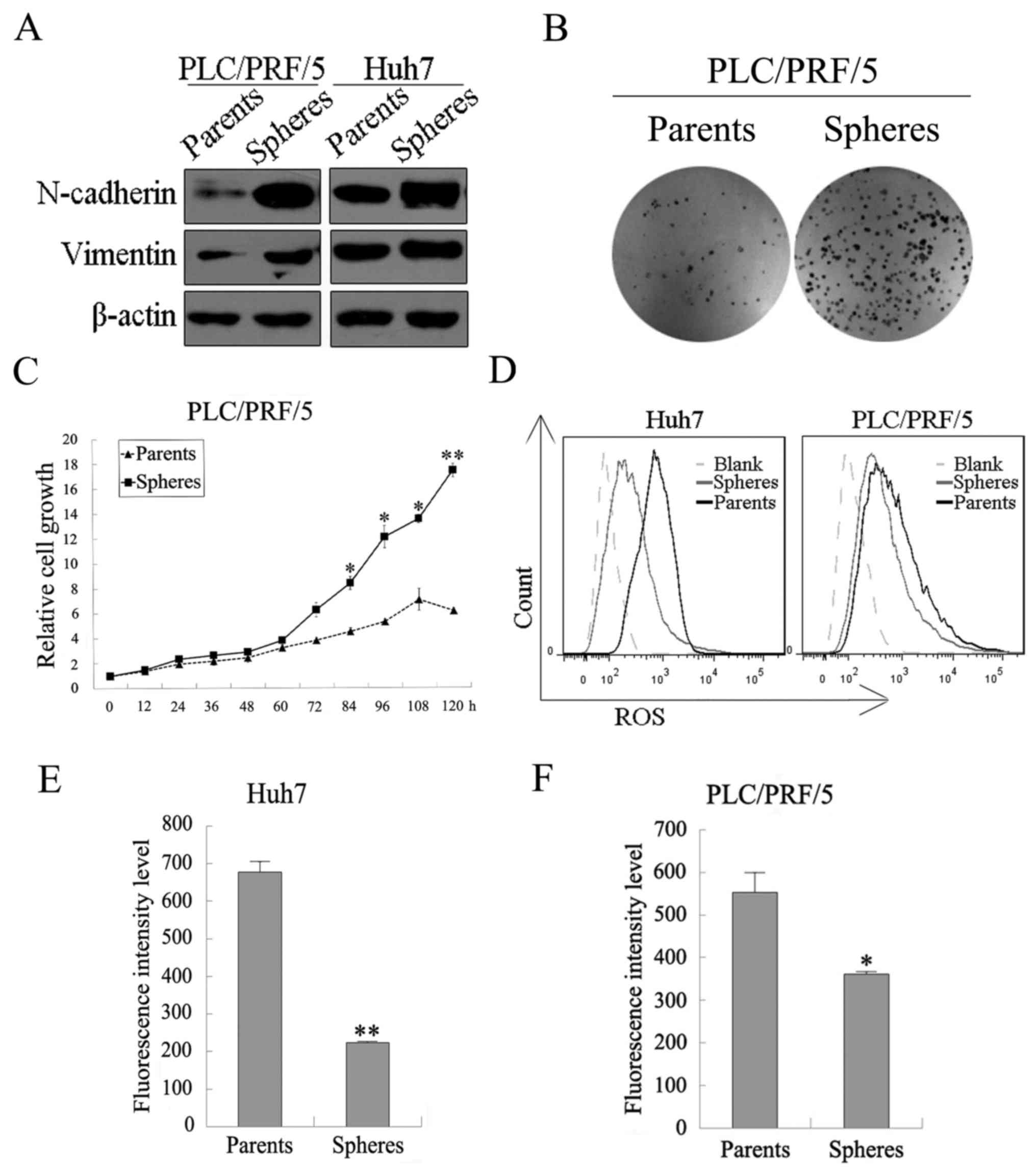
examined by western blotting. As shown in Fig. 4A, the mesenchymal markers
(N-cadherin and vimentin) were upregulated in PLC/PRF/5 and Huh7
spheres compared with their parental cancer cells. Cancer stem
cells which are similar to stem cells normally possess low levels
of intracellular ROS. Thus, flow cytometric analyses were performed
to examine intracellular ROS levels. Intracellular ROS levels both
in PLC/PRF/5 and in HuH7 were lower in sphere cells than in
parental cells (Fig. 4D–F). Colony
formation and cell proliferation assays were also performed to
evaluate the proliferative ability in vitro of the PLC/PRF/5
sphere and parental cells. As shown in Fig. 4B, more clone numbers of the sphere
cells were significantly formed compared with the parental cells,
and the proliferation of the PLC/PRF/5 sphere cells was faster than
that of their parental cells (Fig.
4C). These results supported the greater proliferative ability
in vitro.
 | Table IComparison of the tumorigenesis of
PLC/PRF/5 parental cells and sphere cells using nude mice. |
Table I
Comparison of the tumorigenesis of
PLC/PRF/5 parental cells and sphere cells using nude mice.
| No. of cells
injected | Sphere | Parents |
|---|
|
1×105 | 1/5 | 0/5 |
|
2×105 | 2/5 | 0/5 |
|
1×106 | 3/5 | 0/5 |
BC-02 effectively inhibits proliferation
and self-renewal of liver cancer stem cell
Spheres cultured in stem cell conditional medium
have been proven to possess the characteristics of CSCs and to
enrich the CSCs. Then, the effect of BC-02 on PLC/PRF/5 or Huh7
spheres in vitro assays was examined. Both cell lines,
namely, PLC/PRF/5 and Huh7, were used to perform cell proliferation
assay. BC-02 significantly suppressed the proliferation not only of
parental cells but also sphere cells compared with other
chemotherapy drugs in a dose-dependent manner (Fig. 5A and B). Furthermore, sphere
formation experiments of PLC/PRF/5 cell line were performed using
different chemotherapy drugs, such as 5-FU, bestatin, 1:1 and
BC-02, with similar concentrations. As shown in Fig. 5C, BC-02 markedly inhibited the
number and size of tumor spheres compared with the control,
bestatin, 5-FU and even 1:1 groups. In addition, BC-02 also
effectively impaired the formation of colonies compared with other
groups not only of PLC/PRF/5 sphere cells but also of Huh7 sphere
cells (Fig. 5D–G). Overall, these
results indicate that BC-02 effectively suppresses malignant
proliferation and self-renewal of CSCs. The co-treatment of sphere
cells with the ROS scavenger NAC, which is an antioxidant,
attenuated the inhibitory effect on proliferation, self-renewal and
clone formation induced by BC-02. Those results prompted that lower
level of ROS may be a protection for cells and BC-02 may damage the
cells by increasing the intracellular ROS.
BC-02 prevents the properties of hepatoma
stem cells by targeting CD13 and increasing the ROS-dependent DNA
damage
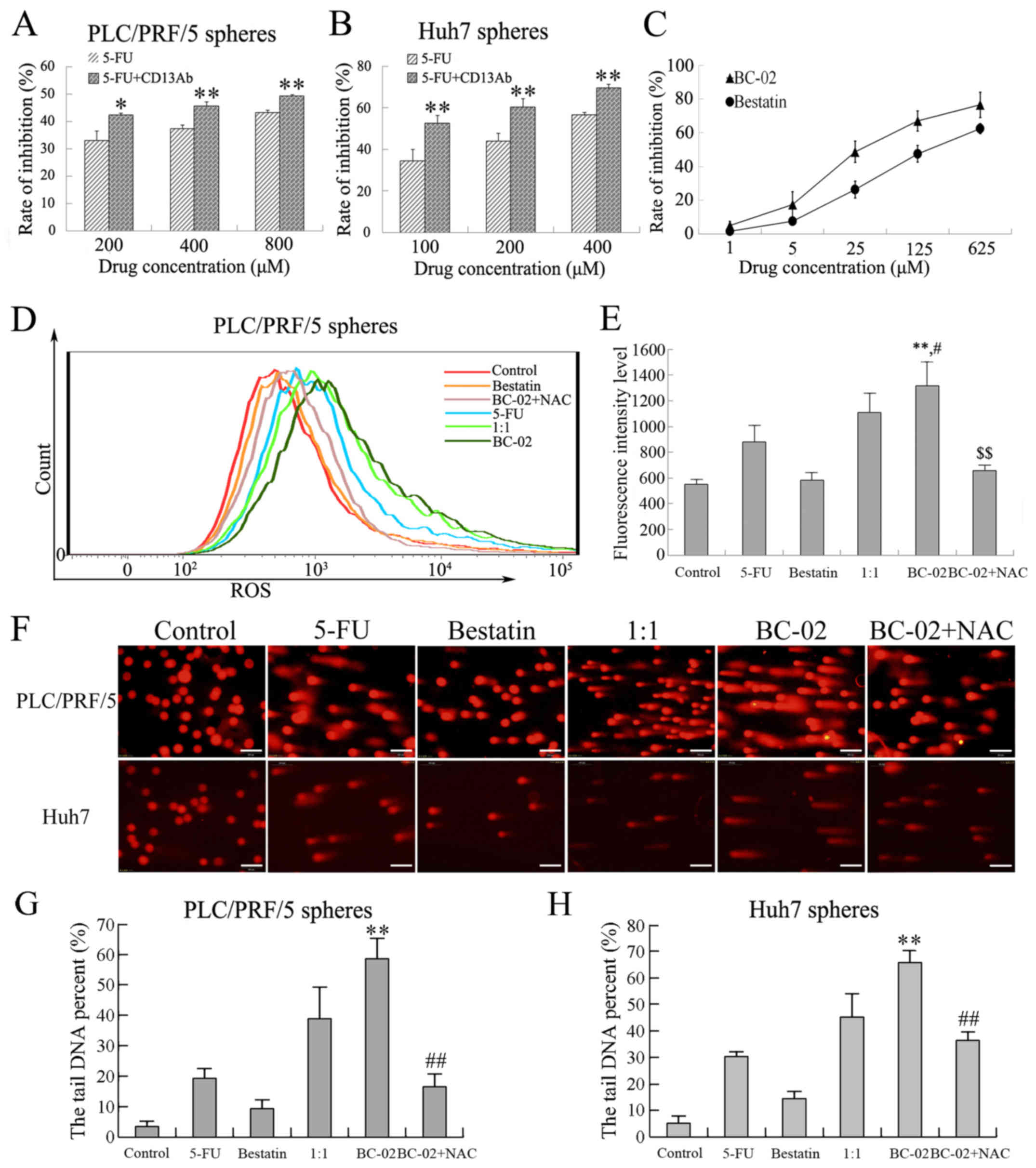
CD13 is a therapeutic target in human LCSCs. The
effect of CD13 inhibition on cell proliferation was confirmed by
cell proliferation assay in PLC/PRF/5 and Huh7 sphere cells. Cell
proliferation was effectively suppressed after exposure to 5-FU
plus CD13 Ab compared with 5-FU alone (Fig. 6A and B). In addition, bestatin was
recognized as CD13 inhibitor, which specifically blocks CD13 and
antagonizes with the zinc-binding site of the CD13 domain. The
inhibitory effects of bestatin and BC-02 on CD13 activity were
measured using PLC/PRF/5 cells, which had high expression levels of
CD13. As shown in Fig. 6C, CD13
activity was inhibited by bestatin as reported. BC-02 even showed
higher inhibition rate than bestatin in a concentration-dependent
manner. These results indicated that BC-02 was more effective to
target CD13 than bestatin; thus, inhibition of CD13 by BC-02
contributes to the inhibition of CSCs.
To investigate further the mechanism by which BC-02
suppresses self-renewal and malignant proliferation of CSCs, the
intracellular ROS levels were detected by prooxidants using the
fluorescence dye DCF-DA. In addition, ROS-induced DNA damage after
genotoxic chemo-stress was detected by an alkaline comet assay. As
mentioned, ROS played an important role in CSC proliferation. The
intracellular ROS levels were evaluated when cells were exposed to
different chemotherapeutic drugs (200 μM of 5-FU, bestatin,
1:1, BC-02 and BC-02 + NAC) for 15 h. A significant increase in ROS
level was observed in PLC/PRF/5 sphere cells treated with BC-02
compared with control, bestatin, 5-FU, and even 1:1 group (Fig. 6D and E), and the co-treatment with
NAC canceled the level of ROS induced by BC-02. In addition, the
most significant DNA damage with the longest cometic tail of DNA in
both PLC/PRF/5 and Huh7 sphere cells was induced by BC-02 compared
with other groups. The DNA damage was significantly reduced when
NAC was co-treated with BC-02 (Fig.
6F–H). Overall, BC-02 targeted CD13 and caused an increase in
intracellular ROS and ROS-induced DNA damage in hepatoma stem
cells.
Discussion
CSCs with stem cell properties are a minor
population of cancer cells. Their presence is responsible for the
chemoresistance to the systemic chemotherapy in HCC. Therefore,
searching for a novel agent that can target CSCs and exhibit
cytotoxic effects on CSCs for eradicating liver CSCs and improving
the prognosis of patients with HCC were indispensable. In this
study, BC-02 can potently inhibit the self-renewal and
proliferation of liver CSCs by targeting CD13 and increasing the
intracellular ROS and the ROS-induced DNA damage.
PLC/PRF/5 and Huh7 spheres formed and acquired
stemness in the serum-free medium. The sphere cells had several
characteristics of CSCs, including drug resistance, high
tumorigenicity in nude mice, EMT phenotype, lower ROS levels,
greater colony-forming efficiency, and increased proliferation
capacity in vitro. Similar to previous studies, spheres
possessed the stemness that was expanded by sphere culture methods
(27–30). What was interesting was that the
proliferation of the Huh7 sphere cells was slower than their
parental cells (data not shown), which may be because the culture
medium was not completely appropriate to the Huh7 cell line, and
the cells could not enter a rapid proliferation period. Thus,
different CSCs had different microenvironments that should be
adaptive to their survival. Efforts are still necessary for
exploring more suitable CSC media for various cell lines.
5-FU is one of the most common anticancer drugs in
HCC treatment. It has toxicity to the hematopoietic function of
bone marrow (31,32). Bestatin is a recognized CD13
inhibitor that is constantly used as an adjuvant, which can enhance
the immunity and prolong the lifetime of patients with acute adult
non-lymphocytic leukemia. Studies have reported that the
combination therapy of 5-FU plus bestatin efficiently enhanced the
antitumor effects of liver cancer (5,33).
In this study, BC-02 was synthesized with the 5-FU and bestatin
monomer. BC-02 could target CD13 and enter the cells accurately
with cytotoxic effects. Thus, it might reduce the drug side-effects
by cutting down the dosage and increasing the efficacy of
chemotherapeutics. BC-02 can effectively inhibit the activity of
CD13 compared with bestatin in a dose-dependent manner. Therefore,
BC-02 is a potential molecular targeting agent to CD13. BC-02 also
can slowly release bestatin and 5-FU (Fig. 2). Thus, BC-02 which is similar to
5-FU can possibly exploit the advantages of cytotoxicity to cancer
cells. As expected, BC-02 effectively suppressed the number and
size of tumor spheres, the formation of colonies, and the malignant
proliferation of the CSCs compared with 5-FU, bestatin, and even
the combination 5-FU with bestatin. In brief, the newfangled CD13
inhibitor BC-02 could efficiently inhibit self-renewal and
proliferation of liver CSCs. Experiments in vivo must be
conducted for further research on this novel antineoplastic
agent.
Previous findings indicated that CSCs could always
resist chemotherapy drugs and maintain long-term survival. Evidence
showed that those characters of CSCs are related to cell cycle
dormancy, lowering the ROS levels in CSCs, and DNA repair
mechanisms (34). In the present
study, the spheres of PLC/PRF/5 and Huh7 contained lower
intracellular ROS levels than their parental cells. Previous
studies suggested that chemotherapeutic drugs were used to cause
DNA damage through ROS accumulation (35), and the combination therapy of 5-FU
plus bestatin efficiently enhanced the antitumor effects by
upregulating intracellular ROS levels and inducing DNA injury
(5,33). Whether generating similar processes
of liver CSCs after exposure to BC-02 was examined. As expected,
BC-02 effectively inhibited self-renewal and proliferation of CSCs,
increased the intracellular ROS and promoted the DNA oxidative
damage. The treatment of CSCs with NAC recovered the proliferation
of CSCs, canceled the increase of the ROS level and repaired the
DNA damage. The molecular mechanism of BC-02 to CSCs was consistent
with previous studies. However, further studies are required to
determine the molecular mechanism of increasing production of
intracellular ROS or reducing the scavenger of ROS when CSCs are
exposed to BC-02.
A recent series of studies provided further evidence
that cancer progress is dependent not only on the malignant cells
but also on the microenvironment. This study demonstrated that
glioblastoma, one of the malignant brain tumors, maintains their
CSCs in vascular niches. Targeting cancer stem cell niches may be a
powerful approach for the treatment of cancer (36). Recent data showed that
anti-angiogenic strategies combined with conventional cytotoxic
drugs reduce the tumor stem-like cell fraction (37). Sorafenib is always used in the
systemic treatment of HCC, and it is the inhibitor the receptors of
the anti-angiogenic multi-kinase. Thus, BC-02 combined with
sorafenib might effectively provide curative effect for patients
with HCC.
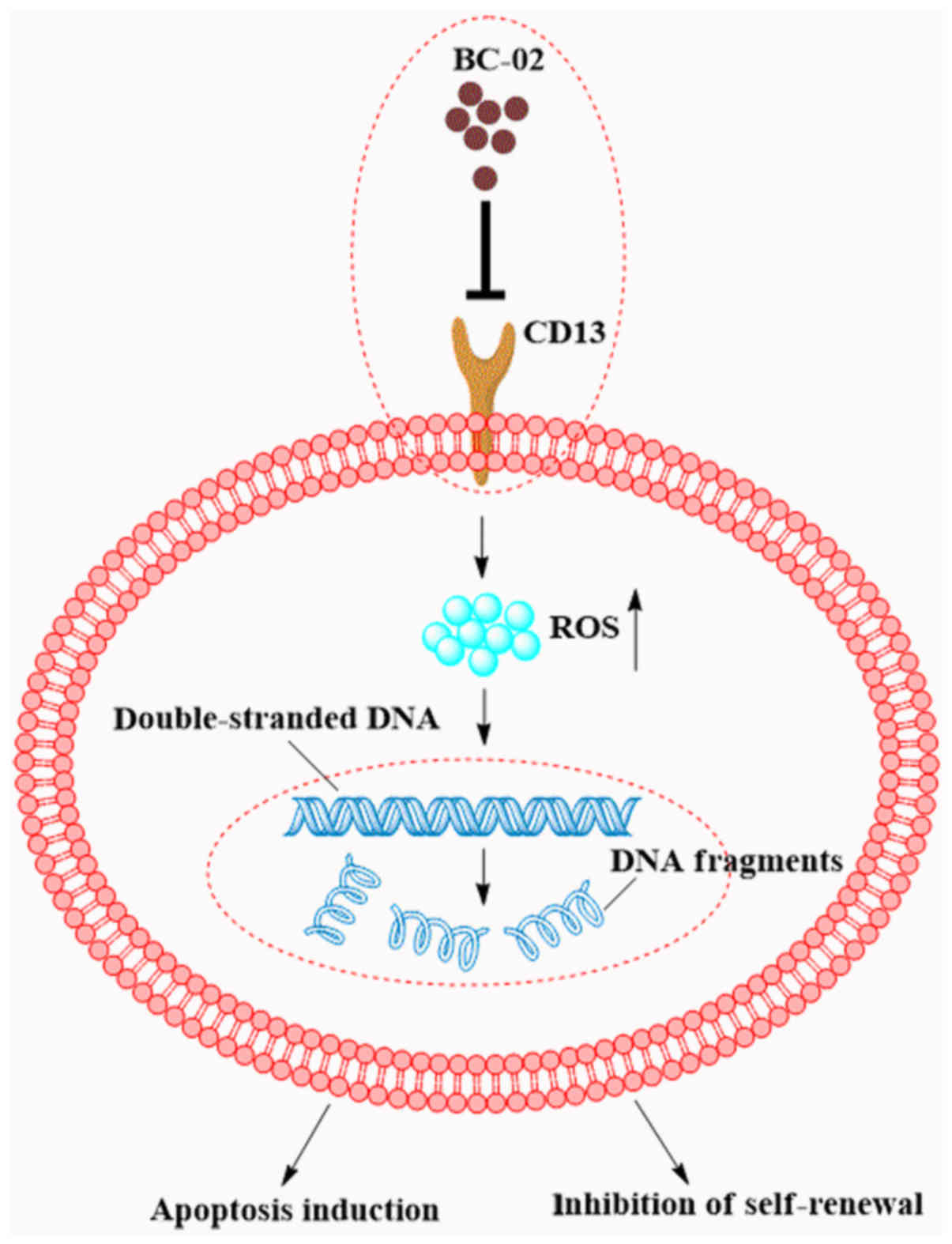
In conclusion, we indicated that combination of
BC-02 may efficiently inhibit the self-renewal and proliferation of
liver CSCs compared with 5-FU, bestatin, and even the combination
of 5-FU and bestatin. Furthermore, as summarized in Fig. 7, BC-02 was able to target CD13 and
then increase the intracellular ROS and the ROS-induced DNA damage.
This will lead to the inhibition of self-renewal and induction of
apoptosis of LCSCs. Therefore, the novel CD13 inhibitor BC-02 may
be a potential strategy for eradicating liver CSCs and overcoming
chemoresistance in liver cancer.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81503108), the Project of
Shandong Province Higher Educational Science and Technology Program
(J15LM58), the Research Award Fund for Outstanding Young and
Middle-aged Scientists of Shandong Province (no. BS2015YY016), and
the National Natural Science Foundation of China (nos. 81373282,
81201262 and 81471048).
Glossary
Abbreviations
Abbreviations:
|
CSCs
|
cancer stem cells
|
|
LCSCs
|
liver cancer stem cells
|
|
APN or CD13
|
aminopeptidase N
|
|
5-FU
|
fluorouracil
|
|
EMT
|
epithelial-mesenchymal transition
|
|
CD13 Ab
|
CD13-neutralizing antibody
|
|
HCC
|
hepatocellular carcinoma
|
|
TICs
|
tumor-initiating cells
|
|
ESI-MS
|
electrospray ionization mass
spectrometry
|
|
MTT
|
methyl thiazolyl tetrazolium
|
|
PI
|
propidium iodide
|
References
|
1
|
Mlynarsky L, Menachem Y and Shibolet O:
Treatment of hepatocellular carcinoma: Steps forward but still a
long way to go. World J Hepatol. 7:566–574. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei KR, Yu X, Zheng RS, Peng XB, Zhang SW,
Ji MF, Liang ZH, Ou ZX and Chen WQ: Incidence and mortality of
liver cancer in China, 2010. Chin J Cancer. 33:388–394.
2014.PubMed/NCBI
|
|
4
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haraguchi N, Ishii H, Mimori K, Tanaka F,
Ohkuma M, Kim HM, Akita H, Takiuchi D, Hatano H, Nagano H, et al:
CD13 is a therapeutic target in human liver cancer stem cells. J
Clin Invest. 120:3326–3339. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bruce WR and Van Der Gaag H: A
quantitative assay for the number of murine lymphoma cells capable
of proliferation in vivo. Nature. 199:79–80. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar
|
|
11
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheung ST, Cheung PF, Cheng CK, Wong NC
and Fan ST: Granulin-epithelin precursor and ATP-dependent binding
cassette (ABC)B5 regulate liver cancer cell chemoresistance.
Gastroenterology. 140:344–355. 2011. View Article : Google Scholar
|
|
13
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nguyen LV, Vanner R, Dirks P and Eaves CJ:
Cancer stem cells: An evolving concept. Nat Rev Cancer. 12:133–143.
2012.PubMed/NCBI
|
|
15
|
Pang RW and Poon RT: Cancer stem cell as a
potential therapeutic target in hepatocellular carcinoma. Curr
Cancer Drug Targets. 12:1081–1094. 2012.PubMed/NCBI
|
|
16
|
Yu SC, Ping YF, Yi L, Zhou ZH, Chen JH,
Yao XH, Gao L, Wang JM and Bian XW: Isolation and characterization
of cancer stem cells from a human glioblastoma cell line U87.
Cancer Lett. 265:124–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao L, Zhou Y, Zhai B, Liao J, Xu W, Zhang
R, Li J, Zhang Y, Chen L, Qian H, et al: Sphere-forming cell
subpopulations with cancer stem cell properties in human hepatoma
cell lines. BMC Gastroenterol. 11:712011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
19
|
Pan Z, Hooley J, Smith DH, Young P,
Roberts PE and Mather JP: Establishment of human ovarian serous
carcinomas cell lines in serum free media. Methods. 56:432–439.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roberts PE: Isolation and establishment of
human tumor stem cells. Methods Cell Biol. 86:325–342. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishii K, Usui S, Sugimura Y, Yoshida S,
Hioki T, Tatematsu M, Yamamoto H and Hirano K: Aminopeptidase N
regulated by zinc in human prostate participates in tumor cell
invasion. Int J Cancer. 92:49–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hashida H, Takabayashi A, Kanai M, Adachi
M, Kondo K, Kohno N, Yamaoka Y and Miyake M: Aminopeptidase N is
involved in cell motility and angiogenesis: Its clinical
significance in human colon cancer. Gastroenterology. 122:376–386.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tokuhara T, Hattori N, Ishida H, Hirai T,
Higashiyama M, Kodama K and Miyake M: Clinical significance of
aminopeptidase N in non-small cell lung cancer. Clin Cancer Res.
12:3971–3978. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun ZP, Zhang J, Shi LH, Zhang XR, Duan Y,
Xu WF, Dai G and Wang XJ: Aminopeptidase N inhibitor 4cc synergizes
antitumor effects of 5-fluorouracil on human liver cancer cells
through ROS-dependent CD13 inhibition. Biomed Pharmacother.
76:65–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kobayashi T, Miyawaki S, Tanimoto M,
Kuriyama K, Murakami H, Yoshida M, Minami S, Minato K, Tsubaki K,
Ohmoto E, et al The Japan Leukemia Study Group: Randomized trials
between behenoyl cytarabine and cytarabine in combination induction
and consolidation therapy, and with or without ubenimex after
maintenance/intensification therapy in adult acute myeloid
leukemia. J Clin Oncol. 14:204–213. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang Y: School of Pharmacy, Shandong
University, PhD Dissertations, Development of novel anti-cancer
drugs base on aminopeptidase N/CD13. 2016
|
|
27
|
Huang YJ and Hsu SH: Acquisition of
epithelial-mesenchymal transition and cancer stem-like phenotypes
within chitosan-hyaluronan membrane-derived 3D tumor spheroids.
Biomaterials. 35:10070–10079. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han M, Wang Y, Liu M, Bi X, Bao J, Zeng N,
Zhu Z, Mo Z, Wu C and Chen X: MiR-21 regulates
epithelial-mesenchymal transition phenotype and hypoxia-inducible
factor-1α expression in thirdsphere forming breast cancer stem
cell-like cells. Cancer Sci. 103:1058–1064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rybak AP, He L, Kapoor A, Cutz JC and Tang
D: Characterization of sphere-propagating cells with stem-like
properties from DU145 prostate cancer cells. Biochim Biophys Acta.
1813:683–694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qiu X, Wang Z, Li Y, Miao Y, Ren Y and
Luan Y: Characterization of sphere-forming cells with stem-like
properties from the small cell lung cancer cell line H446. Cancer
Lett. 323:161–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Di Lorenzo G, Rea A, Carlomagno C, Pepe S,
Palmieri G, Labianca R, Chirianni A, De Stefano A, Esposito V, De
Placido S, et al: Activity and safety of pegylated liposomal
doxorubicin, 5-fluorouracil and folinic acid in inoperable
hepatocellular carcinoma: A phase II study. World J Gastroenterol.
13:6553–6557. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amstutz U, Froehlich TK and Largiadèr CR:
Dihydropyrimidine dehydrogenase gene as a major predictor of severe
5-fluorouracil toxicity. Pharmacogenomics. 12:1321–1336. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamashita M, Wada H, Eguchi H, Ogawa H,
Yamada D, Noda T, Asaoka T, Kawamoto K, Gotoh K, Umeshita K, et al:
A CD13 inhibitor, ubenimex, synergistically enhances the effects of
anticancer drugs in hepatocellular carcinoma. Int J Oncol.
49:89–98. 2016.PubMed/NCBI
|
|
34
|
Naka K, Muraguchi T, Hoshii T and Hirao A:
Regulation of reactive oxygen species and genomic stability in
hematopoietic stem cells. Antioxid Redox Signal. 10:1883–1894.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chetram MA, Bethea DA, Odero-Marah VA,
Don-Salu-Hewage AS, Jones KJ and Hinton CV: ROS-mediated activation
of AKT induces apoptosis via pVHL in prostate cancer cells. Mol
Cell Biochem. 376:63–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gilbertson RJ and Rich JN: Making a
tumour's bed: Glioblastoma stem cells and the vascular niche. Nat
Rev Cancer. 7:733–736. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Folkins C, Man S, Xu P, Shaked Y, Hicklin
DJ and Kerbel RS: Anticancer therapies combining antiangiogenic and
tumor cell cytotoxic effects reduce the tumor stem-like cell
fraction in glioma xenograft tumors. Cancer Res. 67:3560–3564.
2007. View Article : Google Scholar : PubMed/NCBI
|