Introduction
Increasing evidence from epidemiological studies and
randomized clinical trials have suggested that aspirin decreases
the incidence of colorectal cancers (CRC) (1–4).
Most of aspirin's chemopreventive effects came to light during the
course of its use as a cardio-protective and/or analgesic agent. It
was observed that aspirin at doses ranging from 75 to 325 mg, when
taken regularly over 5–10 years decreases the incidence and
mortality associated with CRC (1,4). The
low-dose 75 mg aspirin was just as effective as the high-dose in
preventing CRC. Studies also revealed that aspirin is more
effective against CRC as compared to the cancers of other tissues
such as breast, prostate, liver, lung, and skin (5–7).
Aspirin is mainly absorbed in the acidic environment
of the stomach and upper intestine. The oral bioavailability of
aspirin is 40–50% (8); it
undergoes hydrolysis in the intestine, liver, and plasma by
esterases (9). In contrast to the
short half-life of intact aspirin (20 min), salicylic acid,
depending upon the dose, has a half-life varying between 4 to 6 h,
and remains much longer in the plasma. In the liver, salicylic acid
undergoes further metabolism through glucuronide formation to
produce ester and ether glucuronides (1–42%), or conjugation with
glycine to produce salicyluric acid (20–65%). In addition, the
cytochrome P450 (CYP450) enzymes in the liver metabolize salicylic
acid to 2,5-dihydroxybenzoic acid (2,5-DHBA, genitisic acid) and
2,3-dihydrozybenzoic acid (2,3-DHBA), which accounts for less than
1% of the salicylic acid metabolites. The 2,5-DHBA can undergo
conjugation with glycine to form gentisuric acid. These metabolites
are eliminated from the body either through the kidneys or bile
(10–12).
Our laboratory has been investigating the hypothesis
that aspirin and salicylic acid may target cell cycle regulatory
proteins to exert their chemopreventive effects. Cyclin dependent
kinases (CDKs) are key regulators of the cell cycle, and are
activated by binding with temporally expressed cyclins during
various stages of the cell cycle. The important CDKs in mammalian
cell cycle regulation are: CDK1, CDK2, CDK4 and CDK6. CDK4 and
CDK6, through binding to cyclin D, facilitate the progression
through G1, whereas, CDK2 via binding to cyclin E helps to
transition from G1 to S phase. CDK1 and CDK2 get activated via
binding to cyclins A and B, which facilitate the progression
through G2 and M phases. In many cancers, CDKs are deregulated and
cyclins are over expressed (13).
Due to their important regulatory role in cell cycle progression,
CDKs have been attractive targets in cancer treatment (14–16),
in this regard only two CDK inhibitors (palbociclib and ribociclib)
specific to CDK4 and 6 have been approved for cancer treatment,
which are classified as ATP-competitive inhibitors (17,18).
In our previous study, we demonstrated that exposure
of colon cancer cells, to aspirin and salicylic acid caused
downregulation of cyclin A2, B1 and D3, and CDKs 1, 2, 4 and 6
(19). In that study, we also
demonstrated that salicylic acid potentially binds to CDK2 via
interactions with Asp145, and Lys33, both of which are found in the
active site of the enzyme. Despite these potential interactions,
salicylic acid failed to inhibit the CDK2 enzyme activity in an
in vitro kinase assays. In this study, we considered the
possibility that the two salicylic acid metabolites 2,3-DHBA and
2,5-DHBA, which are known to be produced through CYP450 catalyzed
reactions may inhibit CDK enzyme activity. The goal of the present
study was to determine the effect of salicylic acid metabolites
(2,3-DHBA and 2,5-DHBA) and also derivatives (2,4-DHBA, 2,6-DHBA
and 2,4,6-trihydroxybenzoic acid) on CDK activity in vitro,
and determine the potential sites of interactions with CDK-1.
Herein, we report that salicylic acid metabolites
2,3-DHBA and 2,5-DHBA, as well as the salicylic acid derivatives
2,4-DHBA and 2,6-DHBA, inhibited CDK1 enzyme activity. The
trihydroxy derivative of salicylic acid, 2,4,6-THBA, was also
highly effective. While 2,3-DHBA and 2,5-DHBA are produced from the
oxidation of salicylic acid through CYP450 metabolism, they are
also reported to be naturally present in several fruits and
medicinal herbs. This is the first report demonstrating the ability
of salicylic acid metabolites, and naturally occurring salicylic
acid derivatives to inhibit CDK activity, suggesting their
potential role in chemoprevention.
Materials and methods
Cell lines
HCT-116 human colorectal carcinoma cell line was
purchased from American Type Culture Collection (ATCC). The cells
were cultured in McCoy's 5A medium containing 10% FBS for 24 h
before treatment with specified compounds for indicated times
(20). Authentication of cell
lines was done by ATCC through their DNA-STR profile.
Reagents
Aspirin, salicylic acid, 2,4,6-THBA, 3,4,5-THBA and
trypsin-EDTA solution were purchased from Sigma (St. Louis, MO,
USA); Immobilon membranes, H1 Histones from EMD Millipore
(Billerica, MA, USA); 32P γ-ATP were from MP
Biochemical; Super Signal™ West Pico Chemiluminescent Substrate,
2,3-DHBA, 2,4-DHBA, 2,5-DHBA, 2,6-DHBA, 3,4-DHBA, 3,5-DHBA,
2,4,6-THBA and protease inhibitor tablets and all other chemicals
were obtained from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA).
Recombinant proteins and antibodies
Anti-CDK1 and anti-β actin antibodies were purchased
from Cell Signaling Technology (Danvers, MA, USA); Anti-acetyl
lysine conjugated agarose beads from Immune Chem (Burnaby, BC,
Canada); goat anti-rabbit and goat anti-mouse antibodies were
obtained from Bio-Rad (Hercules, CA, USA). CDK1/cyclin B1 active
enzyme from New England Biolabs (NEB) (Ipswich, MA, USA),
CDK1/cyclin B1, CDK2/cyclin A2, CDK4/cyclin D1, CDK6/cyclin D1,
Retinoblastoma (C-term) and kinase buffer were purchased from
SignalChem (Richmond, BC, Canada).
Cell lysate preparation and western
blotting
After treatment with aspirin at the indicated
concentrations, cells were washed with phosphate buffered saline
and scraped into lysis buffer and protein extracts were prepared as
previously described (21).
Samples containing 50 µg of protein were separated by 8%
polyacrylamide gel electrophoresis (PAGE) and immunoblotted with
respective antibodies. For immuno-precipitations, 500 µg of
the protein was immunoprecipitated with anti-acetyl lysine
conjugated agarose beads overnight, immunocomplexes were collected,
and immunoblotted with anti-CDK1 antibody. Intensities of the bands
were quantified using NIH ImageJ software.
In vitro CDK assay
In vitro CDK assays were performed as
described by the protocols from NEB and SignalChem. Briefly,
purified kinase was aliquoted into the reaction buffer provided by
the respective kits and incubated with indicated compounds at
various concentrations for 10 min at room temperature. Kinase
reaction were performed by incubating the enzyme with a kinase
buffer containing 15 µM ATP, 2 µCi of
[γ-32P]ATP, 5 µg of H1 Histone or retinoblastoma,
at 30°C for 20 min. The final volume of the reaction was 50
µl, the reactions were stopped by adding EDTA to a final
concentration of 20 mM and 4X loading buffer. The samples were
boiled for 10 min, loaded on to a 10% SDS-PAGE. The gel was stained
using Coomassie Brilliant blue (R), dried and exposed to X-ray
film. Intensities of the bands were quantified using NIH ImageJ
software.
MTT Assay
Cytotoxicity was determined by MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
assay. Briefly, HCT-116 cells were seeded in 24-well plates
overnight at a density of 20,000 cells/well and treated with
various compounds for 72 h. MTT assays were carried out as
previously described (22).
Molecular docking studies
The crystallographic three dimensional structures of
selected target proteins of CDK1 (4y72 A chain) and cyclin b1 (PDB
ID: 2b9r) were retrieved from the Protein Data Bank (PDB)
http://www.pdb.org. The human CDK1, Cyclin b1 and
CDK1_cyclin b1 complex (PDB ID: 2b9r and 4y72) were selected for
energy minimization using Gromacs 3.3.1 package with the GROMOS96
force field (23). These molecules
were used as the receptor for virtual small molecule docking with
ligand aspirin, salicylic acid, 2,3-DHBA, 2,5-DHBA, 2,6-DHAB and
2,4,6 THBA using AutoDockVina. The molecular results were
visualized by PYMOL molecular graphics system version 1.3.
Statistical analysis
All experiments were repeated 3–6 times
independently of each other. One-way ANOVA followed by Tukey's
post-hoc multiple comparison tests were adopted to compare group
differences to control, and significance was defined at
P<0.05.
Results
Effect of aspirin, salicylic acid and
salicylic acid metabolites and derivatives on CDK1 kinase activity
in vitro
In a previous study, we demonstrated that aspirin
potentially binds to CDK2 through Lys33 and salicylic acid via
Asp145 and Lys33. Despite the strong evidence of interactions of
these drugs with CDK2, both failed to inhibit CDK2 enzyme activity
in in vitro kinase assays (19). In the current study, we initially
extended these observations to determine the effect of aspirin and
salicylic acid on CDK1 enzyme activity in vitro. This was
tested using commercially obtained CDK1/cyclin B1 assay kits, and
by providing [γ-32P]ATP and H1 histones as substrates.
As a positive control, we used flavopiridol, a known inhibitor of
CDK1 (24). Fig. 1A demonstrates that neither aspirin
nor salicylic acid inhibited CDK1 activity as measured by the
ability to phosphorylate H1 histones; however, flavopiridol
dose-dependently inhibited CDK1 activity (upper panel). These
samples contained similar levels of H1 histones (lower panel,
Fig. 1A). Quantification of the
bands in Fig. 1A (upper panel) are
shown in Fig. 1B. These results
show that aspirin and salicylic acid do not inhibit CDK1,
consistent with the results previously obtained with CDK2 (19).
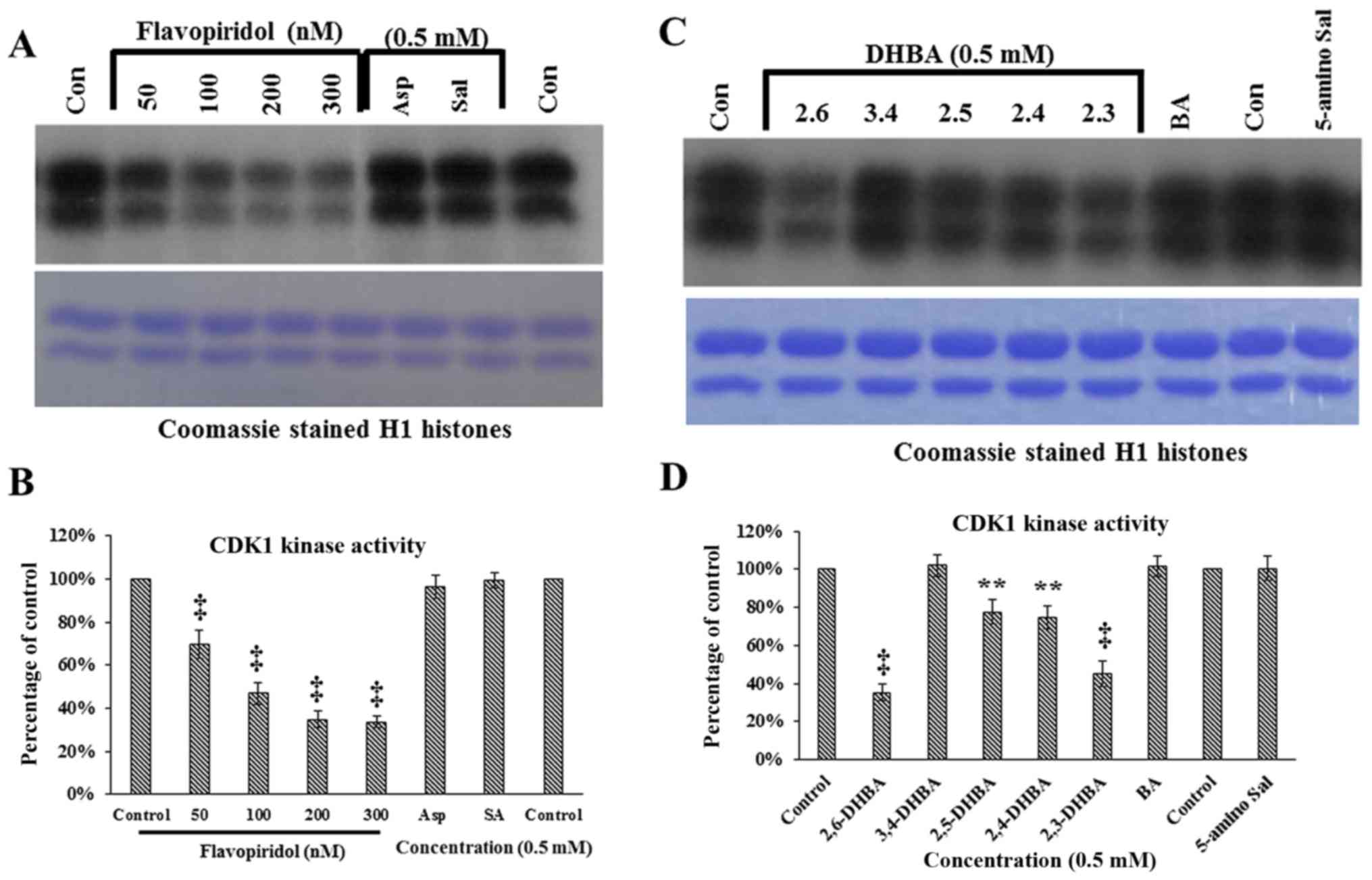 | Figure 1In vitro kinase assays showing
the effect of aspirin, salicylic acid metabolites and derivatives
on CDK1 enzyme activity. (A) Effect of aspirin (Asp), salicylic
acid (Sal) and flavopiridol on CDK1 enzyme activity. (B)
Quantification of the blot in (A). (C) Effect of salicylic acid
metabolites 2,3-DHBA, 2,5-DHBA and derivatives 2,4-DHBA, 2,6-DHBA,
3,4-DHBA, 5-amino salicylic acid (5-amino Sal), and benzoic acid
(BA) on CDK1 enzyme activity. The lower panels below (A and C)
shows coomassie stained H1 histones. (D) quantification of the blot
in (C). (E and F) The dose-dependent effect of 2,3-DHBA and
2,6-DHBA on CDK1 enzyme activity. (G) Dose-dependent effect of
2,4.6-THBA on CDK1. The lower panel of (G), shows coomassie stained
pattern of histone (H). (H) Quantification of the blot in (G). The
intensities of bands in various blots were quantified and expressed
as percentage of control. *P<0.05, **P<0.01,
†P<0.001, ‡P<0.001. |
We next determined the ability of salicylic acid
metabolites (2,3-DHBA and 2,5-DHBA) as we all as salicylic acid
derivatives (2,4-DHBA, 2,6-DHBA and 3,4-DHBA and 5-aminosalicylic
acid) and benzoic acid to inhibit CDK-1 enzyme activity. We
observed that, of the seven different compounds tested, all DHBA
compounds with a common -OH group at the 2nd carbon inhibited CDK1
enzyme activity to varying degrees (Fig. 1C, upper panel). It is interesting
to note that the salicylic acid metabolites, 2,3-DHBA and 2,5-DHBA,
both showed inhibitory effects on CDK1 activity. Quantification of
the intensities of the bands showed that, four compounds showed
varied levels of inhibition (2,6-DHBA, 65%; 2,5-DHBA, 22%;
2,4-DHBA, 25% and 2,3-DHBA, 55%) (Fig.
1D). Three compounds, 3,4-DHBA, 5-aminosalicylic acid and
benzoic acid did not show any inhibition. In addition, 3,5-DHBA
failed to inhibit the CDK1 enzyme activity (data not shown).
Dose-dependent inhibition of CDK1 enzyme
activity by 2,3-DHBA, 2,6-DHBA and 2,4,6-THBA
In experiments described in Fig. 1C, the salicylic acid metabolite
2,3-DHBA, and the salicylic acid derivative 2, 6-DHBA, showed
greater inhibition on CDK1 activity as compared to other compounds
tested. Therefore, we focused initially on these two compounds to
study their dose-dependent effects on CDK1 activity. Fig. 1E and F show that both 2,3-DHBA and
2,6-DHBA dose-dependently inhibit CDK1 activity with an
IC50 of 386 µM and 365 µM, respectively.
The ability of 2,3-DHBA and 2,6-DHBA to inhibit CDK1 enzyme
activity suggested that the hydroxyl group at the 2nd and 6th
carbon in these compounds may be important, and that a compound
having these two -OH groups may more potently inhibit CDK1
activity. Therefore, in in vitro kinase assays, we
determined the effect of 2,4,6-THBA on CDK1 activity at various
concentrations. Fig. 1G (upper
panel) demonstrates that 2,4,6-THBA inhibited CDK1 activity with an
IC50 of 226 µM. Quantification of the bands are
shown in Fig. 1H. Of note,
3,4,5-THBA (gallic acid) did not show any inhibitory effect (data
not shown). This again suggests that the -OH group at the 2nd and
6th carbon may contribute to greater inhibition of CDK1 enzyme
activity.
Molecular docking studies show potential
interactions of aspirin, salicylic acid, salicylic acid metabolites
and derivatives with CDK1 and cyclin B1
We used AutoDockVina to understand the interactions
between aspirin, salicylic acid, 2,3-DHBA, 2,5-DHBA 2,6-DHBA and
2,4,6-THBA with CDK1 alone, cyclin B1 alone, and the CDK1/cyclinB1
complex. The binding free energy and hydrogen bond lengths were
also determined. The results of the docking studies are shown in
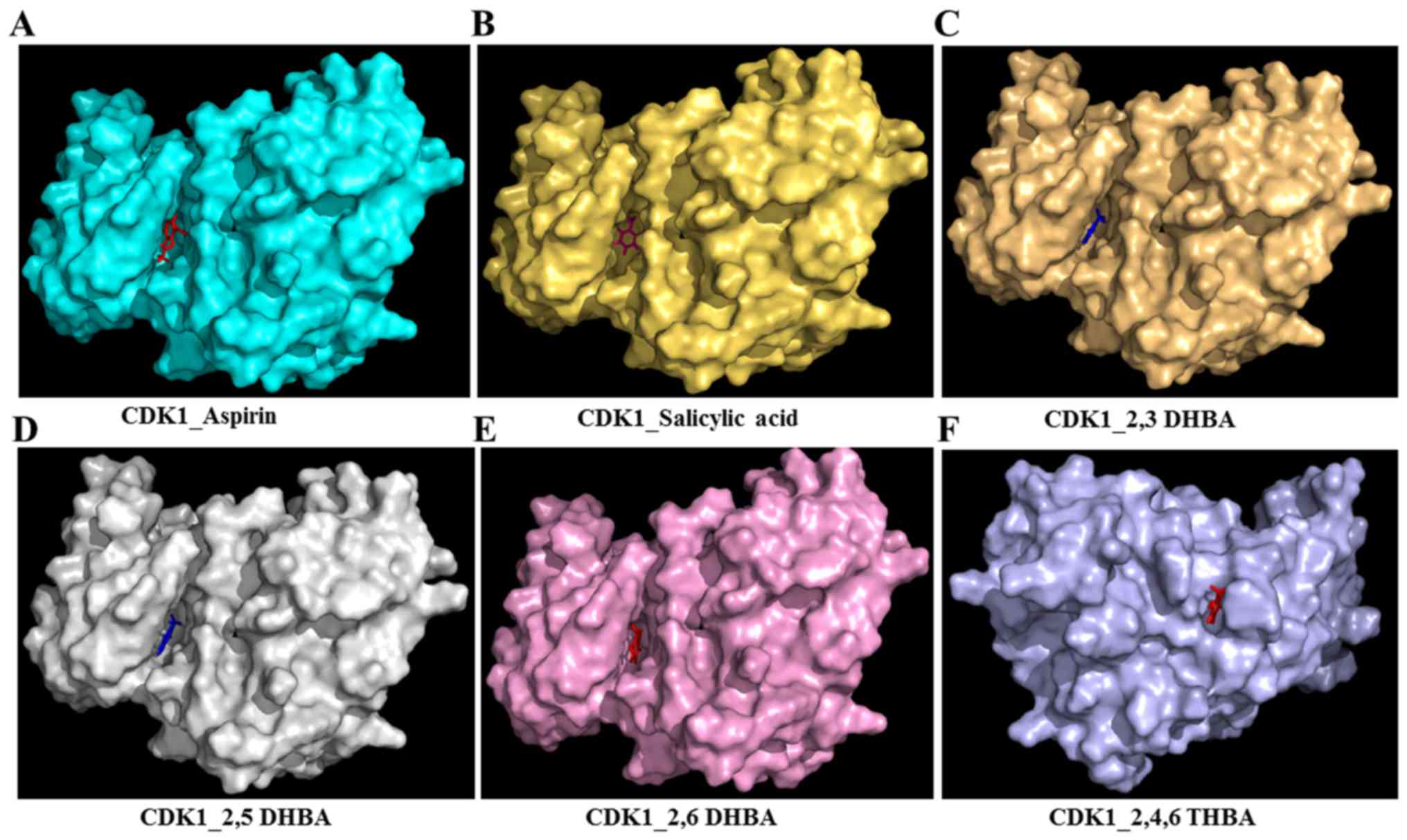
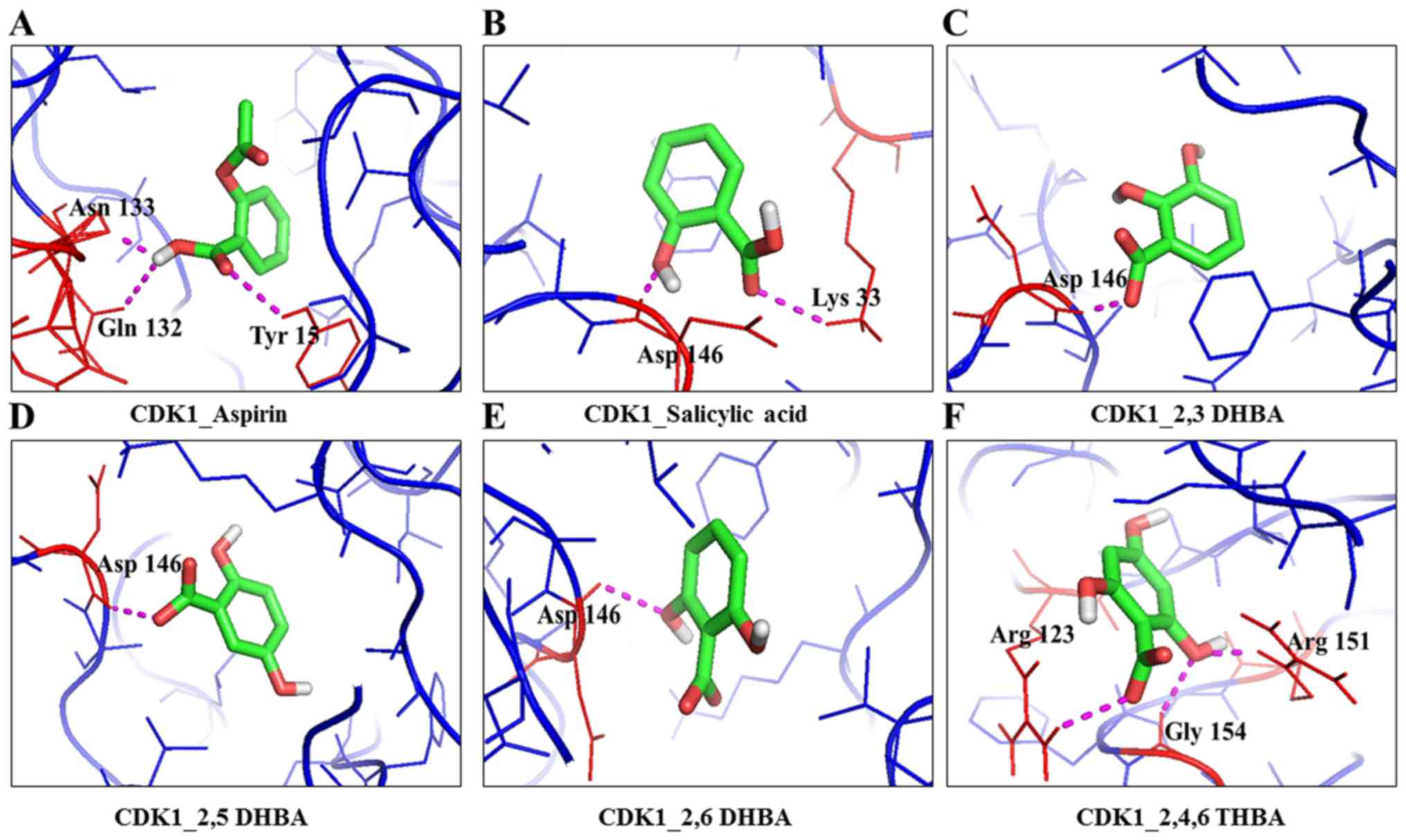
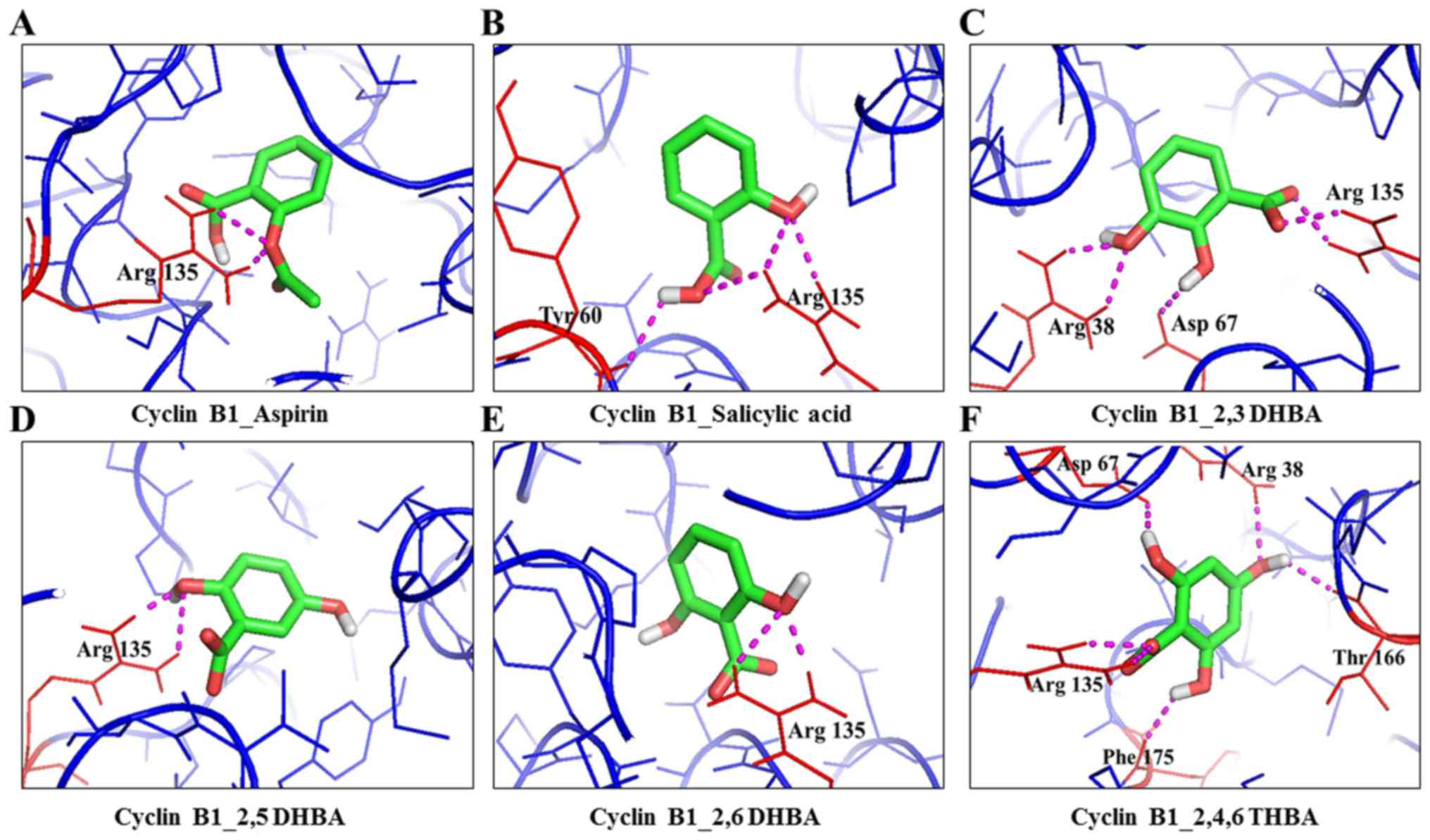
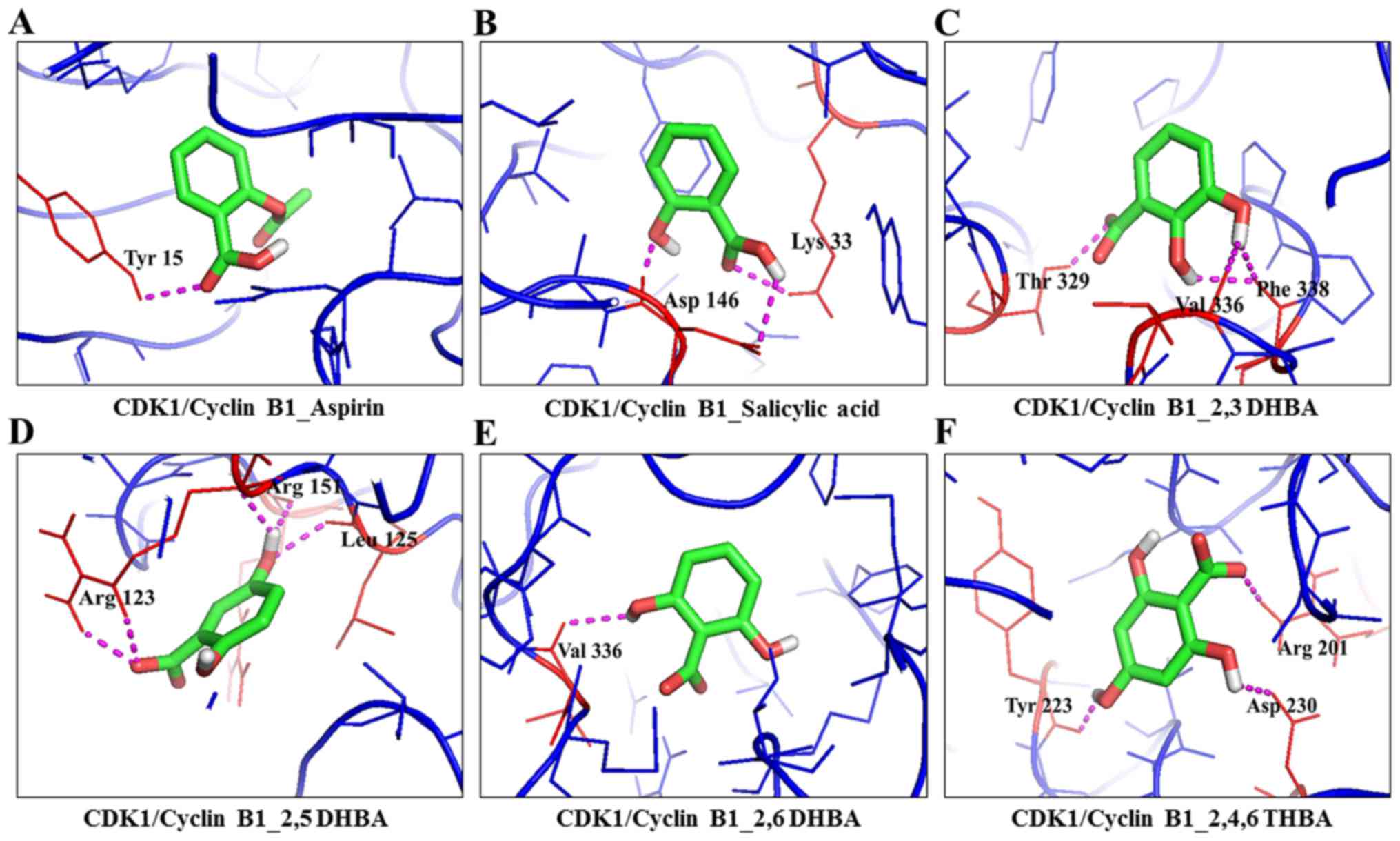
Table I, Fig. 2A–F (space-filling model) and
Figs. 3Figure 4–5. Among the potential interactions shown
in Table I, salicylic acid
interactions with CDK1 through Asp146 and Lys33 is significant as
these two amino acids are part of the active site of the enzyme and
are conserved among different CDK family members (25). Noteworthy, salicylic acid also
interacts with CDK1 through Asp146 and Lys33 when it is present as
part of the cyclin B1/CDK1 complex (Table I). Docking studies also revealed
that aspirin can potentially interact with Tyr-15 in the CDK1
monomer, or in the CDK1/cyclin B1 dimer. It is interesting to note
that Tyr15 is conserved in various CDKs, and its phosphorylation by
Wee1/Mik1 kinase family inactivates the enzyme activity (26). Despite these predicted interactions
of aspirin and salicylic acid with key amino acid residues in CDK1,
both failed to inhibit CDK1 enzyme activity (Fig. 1).
 | Table IMolecular docking studies of
CDK1/Cyclin B1 with metabolites/derivatives of aspirin. |
Table I
Molecular docking studies of
CDK1/Cyclin B1 with metabolites/derivatives of aspirin.
| S. no. | Ligand | Receptor | PDB ID | Interacting amino
acids | No. of H-bonds | Measurement
(A0) | Energy value
(kcal/mol) | Interactions |
|---|
| 1 | Aspirin | CDK1 | 4y72 (A Chain) | Asn133, Gln132 and
Tyr15 | 3 | 2.7, 2.1 and
2.6 | −7.4 | -COOH - Asn133,
Gln132 and Tyr15 |
| 2 | Salicylic acid | CDK1 | 4y72 (A chain) | Asp146 and Lys
33 | 2 | 1.8 and 2.5 | −8.6 | -OH - Lys33; -COOH
- Asp146 |
| 3 | 2,3-DHBA | CDK1 | 4y72 (A Chain) | Asp146 | 1 | 2.0 | −6.8 | -COOH - Asp146 |
| 4 | 2,5-DHBA | CDK1 | 4y72 (A Chain) | Asp146 | 1 | 2.1 | −6.8 | -COOH - Asp146 |
| 5 | 2,6-DHBA | CDK1 | 4y72 (A Chain) | Asp146 | 1 | 2.0 | −7.1 | 6th -OH -
Asp146 |
| 6 | 2,4,6-THBA | CDK1 | 4y72 (A Chain) | Arg123, Arg151 and
Gly154 | 3 | 2.5, 2.0 and
2.4 | −5.8 | 2nd -OH - Arg151
and Gly154; -COOH -Arg123 |
| 7 | Aspirin | Cyclin B1 | 2b9r | Arg135 | 2 | 1.9 and 2.5 | −6.8 | -COOH Arg135 |
| 8 | Salicylic acid | Cyclin B1 | 2b9r | Arg135 and
Tyr60 | 4 | 2.2, 2.4, 2.7 and
2.3 A | −7.4 | -OH - Arg135; -COOH
- Tyr60, Arg135 |
| 9 | 2,3-DHBA | Cyclin B1 | 2b9r | Arg135, Arg38 and
Asp67 | 6 | 2.1, 2.2, 2.2, 2.2,
2.4 and 2.3 | −6.8 | 2nd -OH - Asp67;
3rd - OH -COOH - Arg38; Arg135 |
| 10 | 2,5-DHBA | Cyclin B1 | 2b9r | Arg135 | 2 | 2.0 and 2.4 | −6.7 | 2nd -OH -
Arg135 |
| 11 | 2,6-DHBA | Cyclin B1 | 2b9r | Arg135 | 2 | 2.1 and 2.5 | −5.4 | 6th -OH -
Arg135 |
| 12 | 2,4,6-THBA | Cyclin B1 | 2b9r | Arg135, Phe175,
Asp67, Arg38, Thr166 | 6 | 2.2, 2.4, 2.2, 2.2,
2.3 and 2.1 | −6.8 | 2nd -OH - Asp67;
4th -OH - Arg38, Thr166; 6th -OH - Asp67; -COOH - Arg135 |
| 13 | Aspirin | CDK1_ Cyclin
B1 | 4y72 (A, B
chain) | Tyr15 (CDK1) | 1 | 2.5 | −7.0 | -COOH - Tyr15
(CDK1) |
| 14 | Salicylic acid | CDK1_ Cyclin
B1 | 4y72 (A, B
chain) | Asp146 and Lys33
(CDK1) | 3 | 1.9, 2.5 and
2.6 | −9.6 | -OH - Asp146; -COOH
- Lys33, Asp146 (CDK1) |
| 15 | 2,3-DHBA | CDK1_Cyclin B1 | 4y72 (A, B
chain) | Thr329, Val336,
Phe338 (Cyclin B1) | 2 | 2.4, 2.1 and
2.4 | −6.1 | 2nd and 3rd -OH -
Phe338; 3rd -OH Val336; -COOH Thr329 (cyclin B1) |
| 16 | 2,5-DHBA | CDK1_ Cyclin
B1 | 4y72 (A, B
chain) | Arg123 (3), Leu125,
and Arg151 (CDK1) | 5 | 2.2, 2.2, 2.4, 2.4
and 2.4 | −6.0 | 5th -OH - Leu125
and Arg151; -COOH - Arg123 (CDK1) |
| 17 | 2,6-DHBA | CDK1_ Cyclin
B1 | 4y72 (A, B
chain) | Val336 (Cyclin
B1) | 1 | 2.6 | −5.6 | 6th -OH - Val336
(cyclin B1) |
| 18 | 2,4,6-THBA | CDK1_ Cyclin
B1 | 4y72 (A, B
chain) | Tyr223, Asp 230,
and Arg201 (Cyclin B1) | 2 | 2.3, 2.5 and
2.5 | −5.4 | 2nd -OH - Asp230;
4th -OH - Tyr223; -COOH - Arg201 (cyclin B1) |
Table I also shows
that all three DHBA compounds (2,3-DHBA, 2,5-DHBA and 2,6-DHBA)
showed potential interactions with CDK1 monomer through Asp 146,
whereas 2,4,6-THBA interacted via Arg123, Arg151 and Gly154.
Fig. 2A–F (space-filling model)
shows that 2,3-DHBA, 2,5-DHBA and 2,6-DHBA bind to the same pocket
in CDK1 (same as aspirin and salicylic acid); however, 2,4,6-THBA
binds at a different site. Interestingly, all four compounds
(DHBAs/THBA) inhibited CDK1 enzyme activity (Fig. 1C–H).
Aspirin acetylates recombinant CDK1, and
pre-incubation with salicylic acid, 2,3-DHBA, 2,6-DHBA or
2,4,6-THBA inhibits aspirin's ability to acetylate CDK1
We performed experiments to provide a second line of
evidence for the physical interactions of aspirin, salicylic acid,
2,3-DHBA, 2,6-DHBA and 2,4,6-THBA with CDK1. We hypothesized that
if aspirin binds to CDK1, it should be able to acetylate CDK1 and
this could be detected by immunoblotting with anti-acetyl lysine
antibody. If this is true, then pre-incubation of CDK1 with
salicylic acid, 2,3-DHBA, 2,6-DHBA or 2,4,6-THBA should prevent
aspirin-induced CDK1 acetylation. Therefore, first, we tested the
ability of aspirin at various concentrations to acetylate
recombinant CDK1 (Prospec) in vitro. For this, recombinant
CDK1 (300 ng) was incubated with aspirin at various concentrations
for 12 h, and then samples were immunoblotted with anti-acetyl
lysine antibody. Fig. 6A
demonstrates that aspirin dose-dependently acetylated recombinant
CDK1 beginning at 0.25 mM (upper panel). Stripping and reprobing
the blot showed similar amounts of CDK1 protein in all lanes
(Fig. 6A, lower panel).
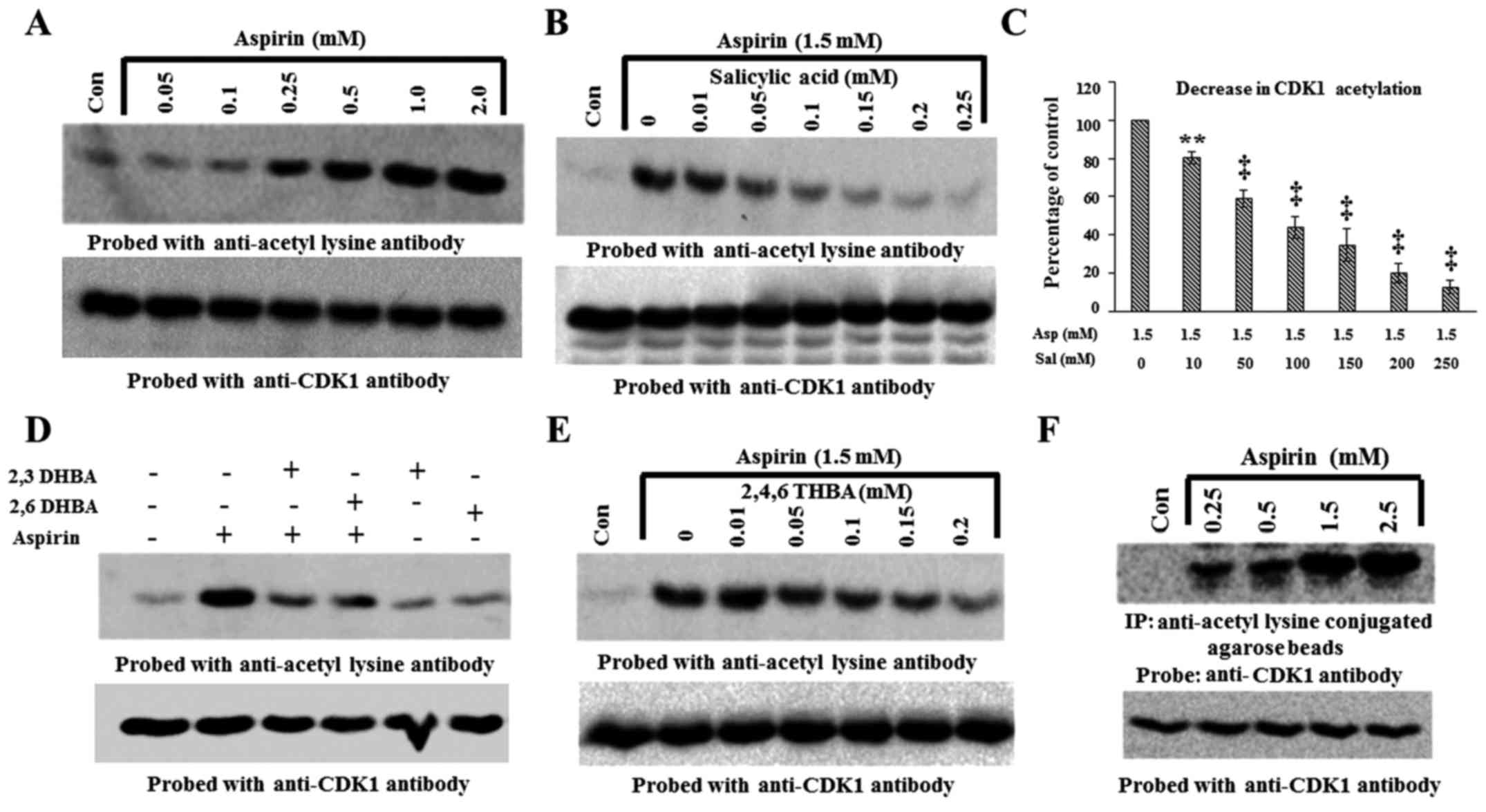 | Figure 6Aspirin acetylates recombinant CDK1
in vitro and cellular CDK1 in HCT116 cells. (A) Upper panel,
dose-dependent effect of aspirin on recombinant CDK1 acetylation.
(B) Salicylic acid pre-incubation prevents aspirin-induced
acetylation (upper panel). (C) Quantification of blot in (B), the
decrease in CDK1 acetylation is expressed as a percentage of the
control. (D) Pre-incubation of recombinant CDK1 with 2,3-DHBA and
2,6-DHBA at 0.5 mM prevents aspirin induced acetylation of CDK1
(upper panel). (E) Pre-incubation of recombinant CDK1 with
2,4,6-THBA prevents aspirin induced acetylation of CDK1 (upper
panel). (F) Aspirin acetylates cellular CDK1 in HCT-116 cells
(upper panel). (F) Lower panel, equal amounts of proteins was
immunoblotted with anti-CDK1 antibody. The lower panels in all
other blots (A, B, D and E), the upper panel blots were stripped
and reprobed with anti-CDK1 antibody. **P<0.01,
‡P<0.001. |
The ability of aspirin to acetylate CDK1 as shown in
Fig. 6A, supports the results
obtained from molecular docking studies which suggested that
aspirin potentially binds to CDK1 (Table I). Since salicylic acid also
potentially binds to CDK1, we next determined if pre-incubation of
CDK1 with salicylic acid would prevent aspirin's ability to
acetylate CDK1. For this, recombinant CDK1 was left untreated, or
treated with aspirin alone (1.5 mM), or first pre-incubated with
different concentrations of salicylic acid for 10 min (0.01–0.25
mM) and then treated with aspirin (1.5 mM) for 12 h. The samples
were then immunoblotted with anti-acetyl lysine antibody. Fig. 6B demonstrates that aspirin caused
acetylation of recombinant CDK1; however, this was dose-dependently
prevented by pre-incubation with salicylic acid (upper panel).
Reprobing the blot in Fig. 6B
showed that all lanes contained equal amounts of CDK1 protein
(lower panel).
In similar experiments as mentioned above, we next
determined whether pre-incubation of recombinant CDK1 with 2,3-DHBA
and 2,6-DHBA would prevent aspirin-mediated acetylation of CDK1.
Recombinant CDK1 was pre-incubated with 0.5 mM 2,3-DHBA and
2,6-DHBA for 10 min; then 1.5 mM, aspirin was added and the samples
were incubated for 12 h at room temperature. The samples were
analyzed by immunoblotting with anti-acetyl lysine antibodies.
Fig. 6D (upper panel),
demonstrates that pre-incubation of CDK1 with 2,3-DHBA or 2,6-DHBA
significantly prevented aspirin-mediated acetylation of CDK1. In
separate experiments, we also observed that pre-incubation of
recombinant CDK1 with 2,4,6-THBA prevented aspirin-mediated
acetylation of CDK1 (Fig. 6E,
upper panel). These results collectively demonstrate that aspirin,
salicylic acid, 2,3-DHBA, 2,6-DHBA and 2,4,6-THBA can all directly
bind to CDK1, and support the data obtained from molecular docking
studies (Table I).
Aspirin acetylates cellular CDK1
We also performed experiments to determine if
exposure of HCT-116 cells to aspirin would acetylate cellular CDK1.
Cells were treated with aspirin at various concentrations (0.25–2.5
mM) for 12 h, and lysates prepared and immunoprecipitated with
anti-acetyl lysine antibody agarose conjugates and immunoblotted
with anti-CDK-1 antibody. Fig. 6F,
upper panel, demonstrates that CDK1 was acetylated in aspirin
treated samples in a dose-dependent fashion. We also analyzed equal
amounts of proteins representing these samples by immunoblotting
with anti-CDK1 antibody. Fig. 6F,
lower panel, shows that these samples contained similar amounts of
CDK1 protein.
Effect of aspirin, salicylic acid,
2,3-DHBA, 2,6-DHBA and 2,4,6-THBA on CDK2, CDK4 and CDK6 enzyme
activity
Having demonstrated the ability of 2,3-DHBA,
2,6-DHBA and 2,4,6-THBA to inhibit CDK1 enzyme activity in
vitro, we sought to determine if these compounds would also
inhibit CDK2, 4 and 6 enzyme activity. Commercially available CDK2
enzymes were pre-incubated with these compounds for 10 min, and
then in vitro kinase assays were carried out by providing H1
histones as substrates. Fig. 7A and
B demonstrates that aspirin, salicylic acid, 2,3-DHBA and
2,6-DHBA did not inhibit CDK2 activity. However, 2,4,6-THBA showed
dose-dependent inhibition of CDK2 enzyme activity (Fig. 7C and D). The IC50 for
the inhibition of CDK2 by 2,4,6-THBA was ~300 µM (Fig. 7D).
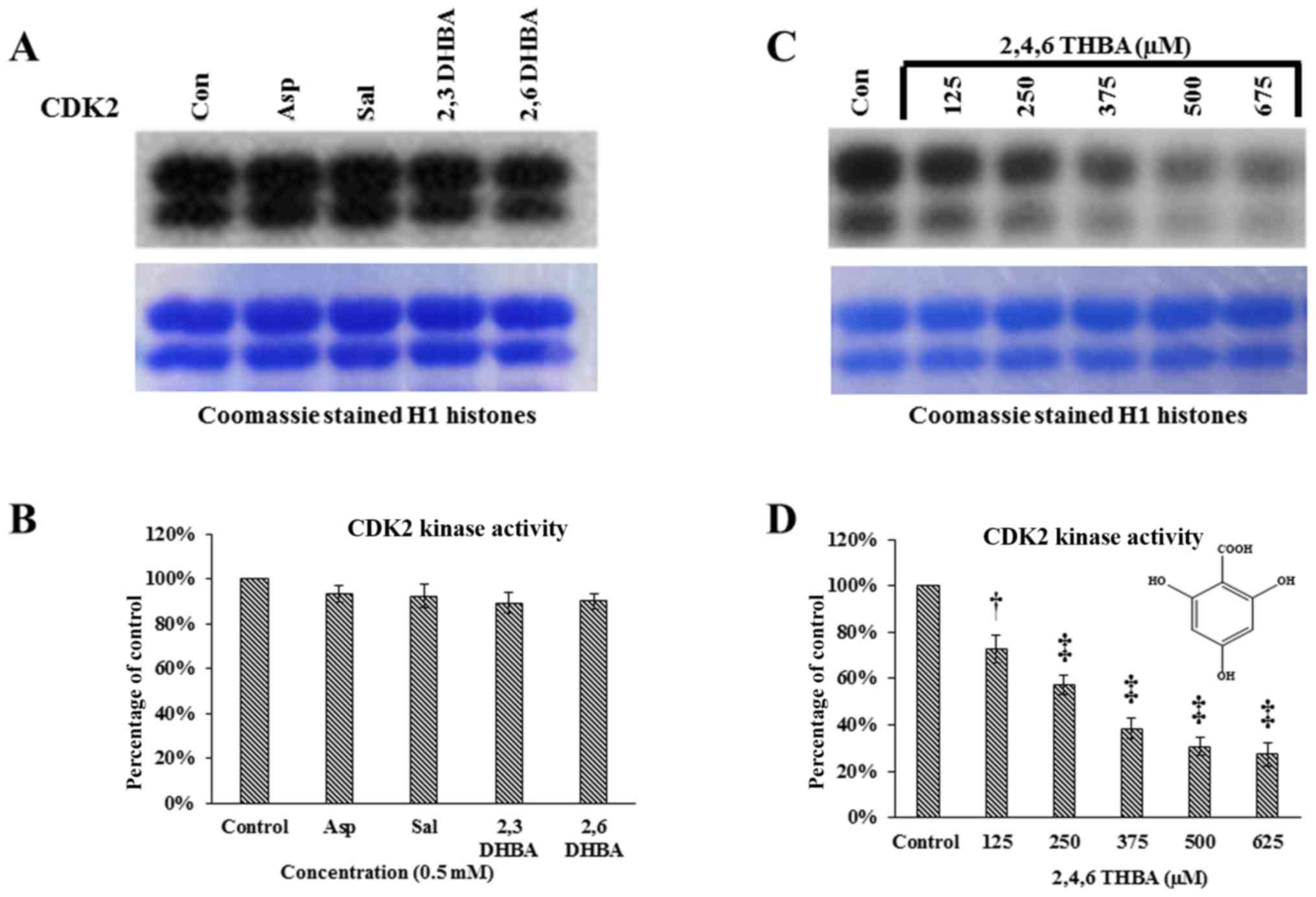 | Figure 7Effect of aspirin, salicylic acid,
2,3-DHBA, 2,6-DHBA and 2,4,6-THBA (at 0.5 mM) on CDK2, 4 and 6
enzyme activity. (A) Upper panel represents in vitro kinase
assays showing the effect of aspirin, salicylic acid, 2,3-DHBA and
2,6-DHBA on CDK2 activity. Lower panel shows coomassie stained H1
histones. (B) Quantification of the blot in (A). (C) Upper panel
represents in vitro kinase assays showing the dose-dependent
effect of 2,4,6-THBA on CDK2 activity. Lower panel shows coomassie
stained H1 histones. (D) Quantification of the blot in (C). (E)
Upper panel represents the effect of aspirin, salicylic acid,
2,3-DHBA and 2,6-DHBA and 2,4,6-THBA on CDK4 activity. Lower panel
shows coomassie stained retinoblastoma protein. (F) Quantification
of the blot in (E). (G) Upper panel represents the effect of
aspirin, salicylic acid, 2,3-DHBA and 2,6-DHBA (0.5 mM) and
2,4,6-THBA on CDK6 enzyme activity. Lower panel shows coomassie
stained retinoblastoma protein. (H) Quantification of the blot in
(G). *P<0.05, **P<0.01,
†P<0.001, ‡P<0.001. |
We next performed experiments to determine the
effect of aspirin, salicylic acid, 2,3-DHBA, 2,6-DHBA and
2,4,6-DHBA on CDK4 and CDK6 enzyme activity in in vitro
kinase assays similar to the experiments performed in Fig. 7A except that retinoblastoma (Rb)
protein was provided as substrate for phosphorylation. Fig. 7E and F demonstrates that aspirin,
salicylic acid, 2,3-DHBA and 2,6-DHBA, all failed to inhibit CDK4;
however, 2,4,6-DHBA inhibited CDK-4 activity (45%). Fig. 7G and H demonstrates that aspirin
and salicylic acid did not affect CDK6 activity; interestingly,
2,3-DHBA, 2,6-DHBA and 2,4,6-THBA significantly inhibited the CDK6
activity by 28, 37 and 40%, respectively.
Discussion
Since the discovery of aspirin in 1897, many of its
therapeutic properties and mechanisms of actions have been
identified. It is used for a variety of illnesses including
inflammation, fever, pain, and for its anti-platelet properties.
These actions of aspirin have been attributed mainly to
acetylation-mediated inhibition of cyclooxygenases (COX). There are
two COX enzymes: COX-1 and COX-2. Aspirin's anti-platelet effect
occurs through inhibition of COX-1 (IC50-1.67
µM); whereas, its analgesic and anti-inflammatory effects
occur primarily through inhibition of COX-2 (IC50-278
µM) (27). In recent years,
evidence has emerged establishing aspirin's ability to decrease
cancer incidence, igniting a renewed interest in its research and
use. Despite extensive studies, aspirin's mode of action in cancer
prevention, as well as how it primarily prevents CRC as compared to
other cancers, is not clearly established. Although multiple
targets and signaling pathways have been proposed, a unifying
mechanism has not been identified till date, suggesting that
different mechanisms may be responsible in different cancers or
that the actual mechanism is yet to be discovered.
One of the hypotheses proposed for aspirin's
anticancer effects is acetylation-mediated inactivation of COX, as
upregulation of COX-2 is observed in nearly 80–90% of CRC; in
contrast, COX-1 expression is unaffected (3). The observation that low dose aspirin,
used for its cardio-protective effect, is also effective in the
prevention of CRC, led to the hypothesis that a common pathway
involving COX-1 inhibition in platelets may be central to both
effects (28–30). Since low dose aspirin is
insufficient to achieve complete inhibition of COX-2, it is argued
that aspirin-mediated inhibition of platelet COX-1 plays a role in
the prevention of CRC, and that aspirin's effects may occur through
sequential steps involving COX-1 and COX-2. It is hypothesized that
low dose aspirin by inhibiting COX-1 in platelets prevents their
activation and the release of growth factors and lipid mediators,
required for COX-2 expression and tumorigenesis in the colonic
tissue. While this is a tenable hypothesis, it is yet to be
confirmed. A recent study demonstrated in human intestinal mucosal
cells that low-dose aspirin caused acetylation of COX-1 and
inhibited PGE2 synthesis leading to decreased levels of S6 kinase,
implicating this in the prevention of early colorectal
carcinogenesis (31). In addition,
salicylic acid, the hydrolyzed product of aspirin, has also been
implicated in aspirin's chemopreventive effects. Salicylic acid was
shown to bind to a number of cellular proteins (Salicylic Acid
Binding Proteins or SABPs) such as IκB kinase (IKK), a component of
the NF-κB complex (32), AMP
activated protein kinase (33),
High Mobility Group Box 1 proteins and GAPDH (34), and CDK2 (19), affecting their levels and/or
functional activity.
Although specific protein targets have been
identified for both aspirin and salicylic acid, no studies have
reported on the cellular targets for salicylic acid metabolites.
One of the most important findings of this study is the ability of
salicylic acid metabolites, 2,3-DHBA and 2,5-DHBA, as well as other
derivatives such as 2,4-DHBA, and 2,6-DHBA to inhibit CDK1 enzyme
activity in vitro (Fig. 1).
Among these, 2,3-DHBA (IC50 386 µM) and 2,6-DHBA
(IC50 365 µM) showed greater degree of inhibition
as compared to 2,4-DHBA and 2,5-DHBA. The ability of 2,3-DHBA and
2,5-DHBA to inhibit CDK1 activity is highly significant, because
they are known to be generated from salicylic acid metabolism
through CYP450-mediated reactions (11). Additionally, in this study, we also
screened two THBA derivatives, 3,4,5-THBA and 2,4,6-THBAs, for
their ability to inhibit CDK1. We observed that 3,4,5-THBA failed
to inhibit; however, 2,4,6-THBA (IC50-226 µM)
significantly inhibited CDK1 activity in a dose-dependent fashion.
In fact, the degree of inhibition observed with 2,4,6-THBA was
greater than that of 2,3-DHBA and 2,6-DHBA (Fig. 1). Not all compounds tested
inhibited CDK1 activity in vitro. For example, aspirin,
salicylic acid, benzoic acid, 3,4-DHBA 3,5-DHBA, and
5-amino-salicylic acid failed to inhibit CDK1 activity. These
results collectively suggest that the DHBAs or THBAs with a common
-OH group at the 2nd carbon is likely important for the inhibitory
effect on CDK1.
We also performed limited studies to determine the
effect of 2,3-DHBA, 2,6-DHBA and 2,4,6-THBA on CDK2, 4 and 6 enzyme
activity. We observed that 2,3-DHBA and 2,6-DHBA had either no or
marginal effect on CDK2 and 4 (at 500 µM) activity (Figs. 7A and 4E); however, both inhibited CDK6 activity
(Fig. 7G). 2,4,6-THBA was highly
effective and inhibited all CDKs examined (CDK1, 2, 4 and 6)
(Figs. 1G, 7C, E and G). This suggests that although
CDK members share significant homology, the binding pockets for
these compounds in CDKs may differ, perhaps accounting for their
differential effects. A compilation of the inhibitory effect of
2,3-DHBA, 2,6-DHBA and 2,4,6-THBA on CDK-1, 2, 4 and 6 are shown in
Table II. Cytotoxicity assays in
HCT-116 cells showed that the IC50 for these compounds
differ. For 2,3-DHBA, the IC50 was ~1.8 mM; for
2,6-DHBA, it was 1.9 mM and for 2,4,6-THBA, it was 0.8 mM (Table III). The IC50 for
compound's cytotoxicity in HCT-116 cells is significantly higher
than observed for in vitro CDK1 inhibition (e.g., Fig. 1E–H); this may be related to a poor
uptake of polyphenolic compounds by cancer cells (35). Our pilot studies using HPLC showed
that the cellular uptake of dihydroxy and trihydroxy-benzoic acid
are lower as compared to the mono-hydroxy benzoic acid (salicylic
acid) (data not shown), possibly reflecting the presence of
multiple OH groups making them more hydrophilic and less
absorbable. The poor uptake of HBAs may also be related to the
significantly lower expression of monocarboxylate transporters
(MCTs, e.g., SLC5A8 and SLC5A12) in metastatic cancer cells
(36). Therefore, the effect of
these compounds on cellular functions, such as cell cycle
regulation, can be better studied after formulation with compounds
that can enhance cellular uptake, or in cell lines expressing MCTs.
Further work is required to determine, how DHBAs and THBAs affect
cell cycle regulation and functions.
 | Table IIEffect of aspirin, salicylic acid,
2,3-DHBA, 2,6-DHBA, 2,4,6-THBA and 3,4,5-THBA on CDK-1, 2, 4 and
6. |
Table II
Effect of aspirin, salicylic acid,
2,3-DHBA, 2,6-DHBA, 2,4,6-THBA and 3,4,5-THBA on CDK-1, 2, 4 and
6.
|  |  |  |  |  |  |
|---|
|
|---|
| CDKs | Aspirin | Salicylic acid | 2,3-DHBA | 2,6-DHBA | 2,4,6-THBA | 3,4,5-THBA |
|---|
| 1 | − | − | + | + | + | − |
| 2 | − | − | − | − | + | − |
| 4 | − | − | − | − | + | − |
| 6 | − | − | + | + | + | − |
 | Table IIIIn vitro IC50
determination for 2,3-DHBA, 2,6-DHBA and 2,4,6-THBA using purified
CDK1 enzyme and MTT assay in HCT-116 cells. |
Table III
In vitro IC50
determination for 2,3-DHBA, 2,6-DHBA and 2,4,6-THBA using purified
CDK1 enzyme and MTT assay in HCT-116 cells.
| Compounds | CDK1
IC50 (µM) | MTT assay HCT-116
(µM) 72 h |
|---|
| 2,3-DHBA | 386.1 | 1778.28 |
| 2,6-DHBA | 365.2 | 1949.84 |
| 2,4,6-THBA | 226.0 | 785.236 |
Molecular docking studies revealed that aspirin,
salicylic acid, 2,3-DHBA, 2,5-DHBA and 2,6-DHBA potentially bind at
the same pocket in CDK1; in contrast, 2,4,6-THBA appears to bind to
a different site (Fig. 2). Despite
the common binding pocket, aspirin and salicylic acid failed to
inhibit CDK1; however, 2,3-DHBA, 2,5-DHBA and 2,6-DHBA inhibited
CDK1 activity (Fig. 1A–D).
Inhibition was also observed with 2,4,6-THBA (Fig. 1G and H). The reason for the
inability of aspirin and salicylic acid to inhibit CDK1 activity is
not clear at this stage and requires further investigations. All
DHBAs potentially showed interaction with Asp146 in CDK1, either
through -COOH or -OH groups. 2,3-DHBA and 2,5-DHBA use -COOH group;
while, 2,6-DHBA uses the -OH group at the 6th carbon to interact
with CDK1. Salicylic acid, which did not show any inhibition of
CDK1 appears to interact with Asp146 via the -COOH group, and with
Lys33 via the -OH group at the 2nd carbon. It is also interesting
to note that 2,4,6-THBA which showed significant inhibition of CDK1
appears to interact with Arg123 via the -COOH group, and with
Arg151 and Gly154 via the -OH group at the 6th carbon (Table I, Fig.
2). These data suggest that the ability of these compounds to
inhibit CDK1 is likely determined by the interacting functional
groups, as well as their orientation at the binding pocket
(Fig. 2), which may affect CDK1
conformation and activity.
In this study, using immunoblotting, we also
demonstrated that incubation of recombinant CDK1 (Prospec) monomer
with aspirin for 12 h dose-dependently acetylated CDK1 (Fig. 6A), which was also observed in
HCT-116 cells (Fig. 6F). An
initial mass spectrometry analysis of aspirin-acetylated CDK1
showed that Lys34 is targeted for acetylation (data not shown). It
is not clear at this stage whether this modification affects CDK1
activity; however, the Lys34 is adjacent to the amino acid Lys33
(in the active site). Our in vitro kinase assays using
CDK1/cyclin B1 (NEB) were designed to address how the binding of
aspirin affects CDK1 activity by preincubating with aspirin for 10
min, before assaying for H1 histone phosphorylation. The effect of
aspirin-mediated acetylation on CDK1 activity could not be
assessed, as the short incubation time (10 min) of CDK1/cyclin B1
complex with aspirin was insufficient to cause acetylation; longer
incubation (12 h) caused degradation (unpublished data). The
ability of aspirin to acetylate recombinant CDK1 monomer (Prospec)
was taken advantage of to demonstrate the binding of salicylic
acid, and its derivatives 2,3-DHBA, 2,6-DHBA and 2,4,6-TBHA to
CDK1, in competition experiments. Pre-incubation with all three
compounds prevented aspirin-mediated CDK1 acetylation (Fig. 6A–E), which supported the data from
molecular docking studies that all compounds potentially bind to
CDK1 (Table I). These experiments
also show that CDK1 is a salicylic acid binding protein.
It is well established that aspirin is more
effective in preventing CRC as compared to other cancers, but a
clear explanation for its preferential protective effect in
colorectal tissues has not emerged in any reports. Our observation
that salicylic acid metabolites 2,3-DHBA and 2,5-DHBA inhibit CDK1
activity provides an important insight into aspirin's
chemopreventive actions. This is particularly relevant to the
prevention of colon tumorigenesis, as expression of several CYP450
members, including CYP3A4/A5 implicated in aspirin/salicylic acid
metabolism in the liver, are also reported to be present in healthy
and cancerous colonic tissues (37–40).
Although the stomach and upper intestine are the major sites of
aspirin absorption, it is likely that some aspirin and its
hydrolyzed product, salicylic acid, is passed on to the lower
intestine and colon. Moreover, availability of aspirin in the
intestine and colon will be much higher after ingestion of enteric
coated tablets, as they are designed to resist dissolution in the
acidic environment of the stomach. Aspirin and salicylic acid
absorbed by the intestinal and colonic epithelial cells may be
metabolized by local CYP450s to produce 2,3-DHBA and 2,5-DHBA.
Although 2,3-DHBA and 2,5-DHBA are the minor metabolites generated
by CYP450 metabolism in the liver, the extent to which they are
generated in epithelial cells of the GI are unknown, and it is
possible that these metabolites may be produced at much higher
levels in GI epithelial cells. These intracellularly produced
hydroxyl-derivatives of salicylic acid are probably too hydrophilic
to cross the basolateral side of the epithelial cell membrane, and
within these cells, they may accumulate to pharmacologically
relevant concentrations sufficient to inhibit CDKs and act locally
to exert anticancer effects. Another source of salicylic acid
derivatives for epithelial cell uptake may be generated through GI
microflora. The epithelial cells of the intestine/colon are likely
to be exposed to higher concentrations of the salicylic acid
metabolites and derivatives (HBAs) as opposed to other tissues
resulting in the inhibition of CDKs. This additional layer of
protection against tumor development may be unique to the
colorectal tissue as compared to other tissues such as breast,
lung, ovary and skin, allowing aspirin to preferentially act
against CRC. A model depicting new insights into aspirin's effect
against CRC is shown in Fig. 8.
This model envisions that the ability of aspirin to inhibit tumor
formation in the intestinal/colonic mucosa is a local effect via
locally generated salicylic acid metabolites/derivatives leading to
inhibition of CDKs, and may not require systemic absorption into
the blood.
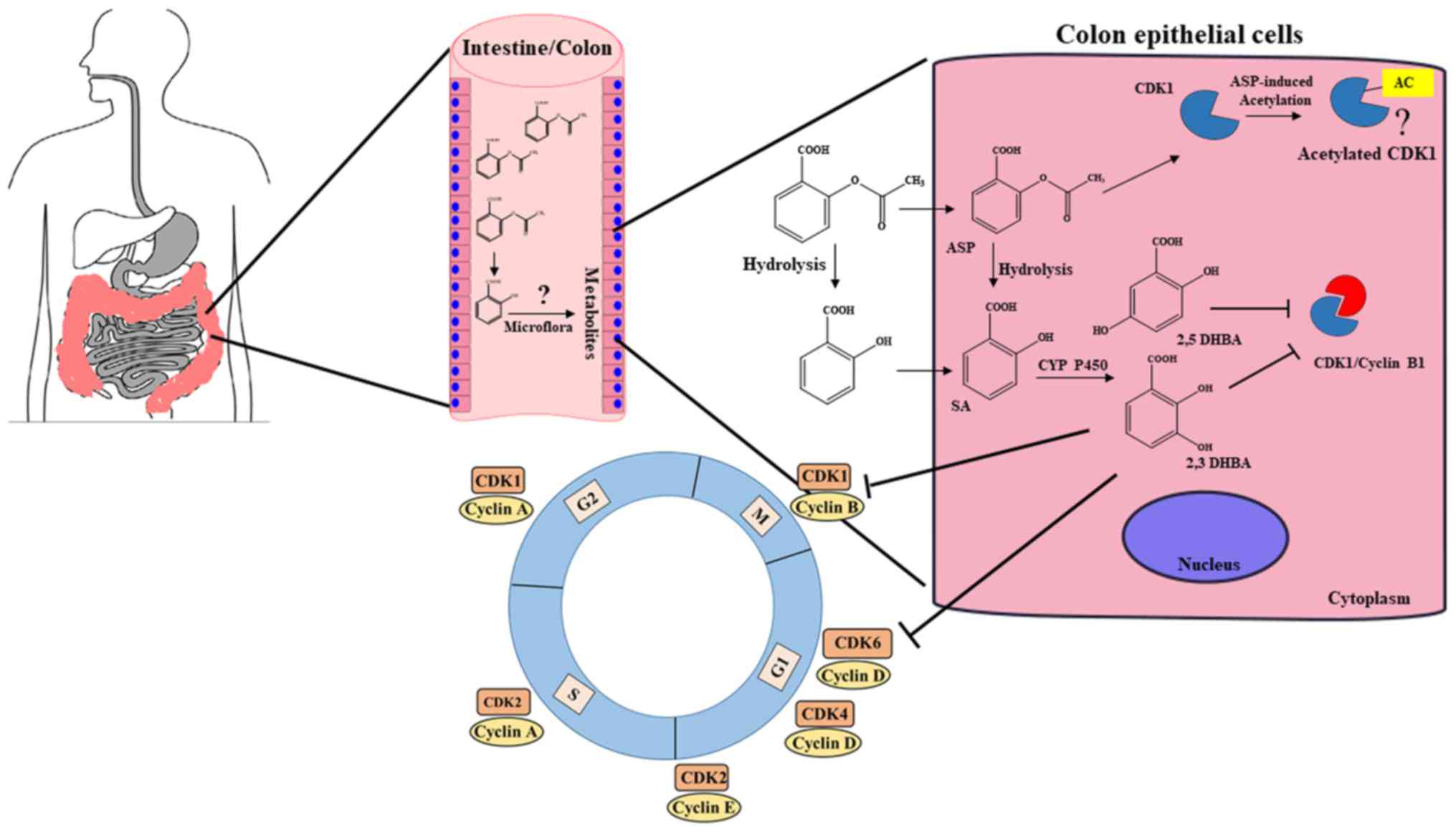 | Figure 8A model depicting how aspirin may
preferentially act on colonic tissue to protect against CRC. We
suggest that unabsorbed aspirin/salicylic acid in the stomach and
upper intestine is passed on to the colon, taken up by the colonic
epithelial cells, and metabolized by CYP450s to produce 2,3-DHBA
and 2,5-DHBAs. These hydrophilic DHBAs may not easily cross the
basolateral membrane of the epithelial cells and within these
cells, they may accumulate to pharmacologically relevant
concentrations, leading to the inhibition of CDK1 and CDK6, and
exert anticancer effects. Colonic epithelial cells may also get
exposed to salicylic acid metabolites generated by the gut
microflora, and uptake of these HBAs by cells may also cause CDK
inhibition. This additional layer of protection against tumor
development may be unique to the colonic tissue allowing aspirin as
a more effective drug against CRC. (?), Aspirin acetylates CDK1;
however, how this modification affects its function is not clear at
this stage. |
Salicylic acid metabolites 2,3-DHBA and 2,5-DHBA
were also reported to be present in the plasma of individuals even
when there has been no intake of aspirin, indicating a dietary
source (41). It is suggested that
diet containing fruits and vegetables may also provide a rich
source of HBAs. 2,3-DHBA is present in avocados, batoko plum and in
medicinal herbs such as Madagascar rosy periwinkle. 2,5-DHBA is
also widely present in fruits including kiwi fruit, aloe vera,
mushrooms, apple, bitter melon, blackberries, grapes and pears; it
is also present in wine (42). It
is important to note that 2,4,6-THBA was demonstrated as one of the
in vitro degradation product of catechins by the intestinal
bacterium, Acinetobacter calcoace-ticus (43,44).
In addition, 2,4-DHBA and 2,4,6-THBA were reported to be produced
through pH-dependent degradation of anthocyanins (45). The exposure of the colonic
epithelial cells to these pharmacologically active CDK inhibitors
may provide a link between diet rich in salicylic
acid/DHBAs/catechins/anthocyanins and decreased occurrences of CRC
(46–48).
We believe that our observations have opened a new
frontier in aspirin research by identifying CDKs as novel cellular
targets for salicylic acid metabolites and derivatives. The
potential contributions of locally generated HBAs either in the
colon or within the colonic epithelial cells, to aspirin's
chemopreventive actions against CRC via CDK inhibition represent an
important area for future research. Against the backdrop of our
in vitro observation that salicylic acid metabolites inhibit
CDKs, the question is raised whether aspirin through acetylation of
COX or other proteins (7,49), or salicylic acid through binding to
cellular targets (SABPs) (19,34),
or salicylic acid metabolites through inhibition of CDKs, are
responsible for the observed chemopreventive effects of aspirin. We
suggest that although aspirin's ability to prevent CRC may occur
through multiple mechanisms, salicylic acid metabolites formed
locally in the colon, or within the intestinal epithelial cells,
may contribute significantly to its protective effects. Other
interesting questions that need to be addressed include: are there
other cellular protein targets for salicylic acid metabolites
besides CDKs? Will daily supplements of DHBAs/THBAs capable of
inhibiting CDKs provide protection against CRCs? IC50
for the inhibition of CDKs by DHBAs and THBAs are although in
micromolar range (300 µM), it could be argued that these
concentrations are still physiologically relevant. Given the
abundance of the occurrences of DHBAs and THBAs in fruits and
vegetables (42), it is possible
that CDKs have been evolved to be less sensitive to inhibition for
these natural compounds avoiding cytotoxicity.
In recent times, attention is increasingly focused
on developing CDK inhibitors to arrest cell cycle as a therapeutic
intervention to treat cancer. Although several potential CDK
inhibitors are undergoing clinical trials, palbociclib and
ribociclib are the only FDA approved drugs used in cancer treatment
(17). We are hopeful that
structural modification to DHBAs and THBAs may potentially lead to
the development of novel class of CDK inhibitors for cancer
prevention and treatment.
Acknowledgments
The NIH (5RO3CA133061-02) and Faculty Excellence
Funds from the Office of Research, South Dakota State University to
G.J.B.; and Women and Giving-2015 Award to R.D. are gratefully
acknowledged. We thank Dr Jane Endicott of Newcastle University,
UK, for providing the CDK assay protocols, and Mrs. Daniela Paez
and members of the Proteomics Core Facility at SD-BRIN (NIH grant
no. P20GM103443), USD, for the mass spectrometry analysis. We thank
Ms. Mary Carlson for editing the manuscript.
References
|
1
|
Patrignani P and Patrono C: Aspirin and
Cancer. J Am Coll Cardiol. 68:967–976. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan AT, Arber N, Burn J, Chia WK, Elwood
P, Hul MA, Logan RF, Rothwell PM, Schrör K and Baron JA: Aspirin in
the chemoprevention of colorectal neoplasia: An overview. Cancer
Prev Res (Phila). 5:164–178. 2012. View Article : Google Scholar
|
|
3
|
Sostres C, Gargallo CJ and Lanas A:
Aspirin, cyclooxygenase inhibition and colorectal cancer. World J
Gastrointest Pharmacol Ther. 5:40–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rothwell PM, Wilson M, Elwin C-E, Norrving
B, Algra A, Warlow CP and Meade TW: Long-term effect of aspirin on
colorectal cancer incidence and mortality: 20-year follow-up of
five randomised trials. Lancet. 376:1741–1750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bosetti C, Rosato V, Gallus S, Cuzick J
and La Vecchia C: Aspirin and cancer risk: A quantitative review to
2011. Ann Oncol. 23:1403–1415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rothwell PM, Fowkes FGR, Belch JF, Ogawa
H, Warlow CP and Meade TW: Effect of daily aspirin on long-term
risk of death due to cancer: Analysis of individual patient data
from randomised trials. Lancet. 377:31–41. 2011. View Article : Google Scholar
|
|
7
|
Alfonso L, Ai G, Spitale RC and Bhat GJ:
Molecular targets of aspirin and cancer prevention. Br J Cancer.
111:61–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pedersen AK and FitzGerald GA:
Dose-related kinetics of aspirin. Presystemic acetylation of
platelet cyclooxygenase. N Engl J Med. 311:1206–1211. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bahar FG and Imai T: Aspirin hydrolysis in
human and experimental animal plasma and the effect of metal
cations on hydrolase activities. Drug Metab Dispos. 41:1450–1456.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hutt AJ, Caldwell J and Smith RL: The
metabolism of aspirin in man: A population study. Xenobiotica.
16:239–249. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bojić M, Sedgeman CA, Nagy LD and
Guengerich FP: Aromatic hydroxylation of salicylic acid and aspirin
by human cytochromes P450. Eur J Pharm Sci. 73:49–56. 2015.
View Article : Google Scholar
|
|
12
|
Grootveld M and Halliwell B:
2,3-Dihydroxybenzoic acid is a product of human aspirin metabolism.
Biochem Pharmacol. 37:271–280. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peyressatre M, Prével C, Pellerano M and
Morris MC: Targeting cyclin-dependent kinases in human cancers:
From small molecules to Peptide inhibitors. Cancers (Basel).
7:179–237. 2015. View Article : Google Scholar
|
|
15
|
Brown NR, Korolchuk S, Martin MP, Stanley
WA, Moukhametzianov R, Noble ME and Endicott JA: CDK1 structures
reveal conserved and unique features of the essential cell cycle
CDK. Nat Commun. 6:67692015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamilton E and Infante JR: Targeting
CDK4/6 in patients with cancer. Cancer Treat Rev. 45:129–138. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roskoski R Jr: Cyclin-dependent protein
kinase inhibitors including palbociclib as anticancer drugs.
Pharmacol Res. 107:249–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dachineni R, Ai G, Kumar DR, Sadhu SS,
Tummala H and Bhat GJ: Cyclin A2 and CDK2 as novel targets of
aspirin and salicylic acid: A potential role in cancer prevention.
Mol Cancer Res. 14:241–252. 2016. View Article : Google Scholar :
|
|
20
|
Ai G, Dachineni R, Kumar DR, Marimuthu S,
Alfonso LF and Bhat GJ: Aspirin acetylates wild type and mutant p53
in colon cancer cells: Identification of aspirin acetylated sites
on recombinant p53. Tumour Biol. 37:6007–6016. 2016. View Article : Google Scholar
|
|
21
|
Ai G, Dachineni R, Muley P, Tummala H and
Bhat GJ: Aspirin and salicylic acid decrease c-Myc expression in
cancer cells: A potential role in chemoprevention. Tumour Biol.
37:1727–1738. 2016. View Article : Google Scholar
|
|
22
|
Alfonso LF, Srivenugopal KS, Arumugam TV,
Abbruscato TJ, Weidanz JA and Bhat GJ: Aspirin inhibits
camptothecin-induced p21C IP1 levels and potentiates apoptosis in
human breast cancer cells. Int J Oncol. 34:597–608. 2009.PubMed/NCBI
|
|
23
|
Van Der Spoel D, Lindahl E, Hess B,
Groenhof G, Mark AE and Berendsen HJ: GROMACS: Fast, flexible, and
free. J Comput Chem. 26:1701–1718. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Senderowicz AM: Flavopiridol: The first
cyclin-dependent kinase inhibitor in human clinical trials. Invest
New Drugs. 17:313–320. 1999. View Article : Google Scholar
|
|
25
|
Sausville EA: Complexities in the
development of cyclin-dependent kinase inhibitor drugs. Trends Mol
Med. 8(Suppl): S32–S37. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Welburn JP, Tucker JA, Johnson T, Lindert
L, Morgan M, Willis A, Noble ME and Endicott JA: How tyrosine 15
phosphorylation inhibits the activity of cyclin-dependent kinase
2-cyclin A. J Biol Chem. 282:3173–3181. 2007. View Article : Google Scholar
|
|
27
|
Vane JR, Bakhle YS and Botting RM:
Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 38:97–120.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thun MJ, Jacobs EJ and Patrono C: The role
of aspirin in cancer prevention. Nat Rev Clin Oncol. 9:259–267.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dovizio M, Tacconelli S, Sostres C,
Ricciotti E and Patrignani P: Mechanistic and pharmacological
issues of aspirin as an anticancer agent. Pharmaceuticals (Basel).
5:1346–1371. 2012. View Article : Google Scholar
|
|
30
|
Lichtenberger LM, Fang D, Bick RJ,
Poindexter BJ, Phan T, Bergeron AL, Pradhan S, Dial EJ and Vijayan
KV: Unlocking aspirin's chemopreventive activity: Role of
irreversibly inhibiting platelet cyclooxygenase-1. Cancer Prev Res
(Phila). 10:142–152. 2017. View Article : Google Scholar
|
|
31
|
Patrignani P, Sacco A, Sostres C, Bruno A,
Dovizio M, Piazuelo E, Di Francesco L, Contursi A, Zucchelli M,
Schiavone S, et al: Low-dose aspirin acetylates cyclooxygenase-1 in
human colorectal mucosa: Implications for the chemoprevention of
colorectal cancer. Clin Pharmacol Ther. 102:52–61. 2017. View Article : Google Scholar
|
|
32
|
Kopp E and Ghosh S: Inhibition of NF-kappa
B by sodium salicylate and aspirin. Science. 265:956–959. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hawley SA, Fullerton MD, Ross FA,
Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green
KA, Mustard KJ, et al: The ancient drug salicylate directly
activates AMP-activated protein kinase. Science. 336:918–922. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Klessig DF, Tian M and Choi HW: Multiple
targets of salicylic acid and its derivatives in plants and
animals. Front Immunol. 7:2062016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Manach C, Scalbert A, Morand C, Rémésy C
and Jiménez L: Polyphenols: Food sources and bioavailability. Am J
Clin Nutr. 79:727–747. 2004.PubMed/NCBI
|
|
36
|
Ganapathy V, Thangaraju M, Gopal E, Martin
PM, Itagaki S, Miyauchi S and Prasad PD: Sodium-coupled
monocarboxylate transporters in normal tissues and in cancer. AAPS
J. 10:193–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gervot L, Carrière V, Costet P, Cugnenc
PH, Berger A, Beaune PH and de Waziers I: CYP3A5 is the major
cytochrome P450 3A expressed in human colon and colonic cell lines.
Environ Toxicol Pharmacol. 2:381–388. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thelen K and Dressman JB: Cytochrome
P450-mediated metabolism in the human gut wall. J Pharm Pharmacol.
61:541–558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Paine MF, Hart HL, Ludington SS, Haining
RL, Rettie AE and Zeldin DC: The human intestinal cytochrome P450
'pie'. Drug Metab Dispos. 34:880–886. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kumarakulasingham M, Rooney PH, Dundas SR,
Telfer C, Melvin WT, Curran S and Murray GI: Cytochrome P450
profile of colorectal cancer: Identification of markers of
prognosis. Clin Cancer Res. 11:3758–3765. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Paterson JR, Blacklock C, Campbell G,
Wiles D and Lawrence JR: The identification of salicylates as
normal constituents of serum: A link between diet and health? J
Clin Pathol. 51:502–505. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Juurlink BH, Azouz HJ, Aldalati AM,
AlTinawi BM and Ganguly P: Hydroxybenzoic acid isomers and the
cardiovascular system. Nutr J. 13:632014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Arunachalam M, Mohan N, Sugadev R,
Chellappan P and Mahadevan A: Degradation of (+)-catechin by
Acinetobacter calcoaceticus MTC 127. Biochim Biophys Acta.
1621:261–265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gao K, Xu A, Krul C, Venema K, Liu Y, Niu
Y, Lu J, Bensoussan L, Seeram NP, Heber D, et al: Of the major
phenolic acids formed during human microbial fermentation of tea,
citrus, and soy flavonoid supplements, only
3,4-dihydroxyphenylacetic acid has antiproliferative activity. J
Nutr. 136:52–57. 2006.
|
|
45
|
Seeram NP, Bourquin LD and Nair MG:
Degradation products of cyanidin glycosides from tart cherries and
their bioactivities. J Agric Food Chem. 49:4924–4929. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Paterson JR and Lawrence JR: Salicylic
acid: A link between aspirin, diet and the prevention of colorectal
cancer. QJM. 94:445–448. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kamiloglu S, Capanoglu E, Grootaert C and
Van Camp J: Anthocyanin absorption and metabolism by human
intestinal Caco-2 cells - A review. Int J Mol Sci. 16:21555–21574.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kresty LA, Mallery SR and Stoner GD: Black
raspberries in cancer clinical trials: Past, present and future. J
Berry Res. 6:251–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dovizio M, Bruno A, Tacconelli S and
Patrignani P: Mode of action of aspirin as a chemopreventive agent.
Prospects for Chemoprevention of Colorectal Neoplasia. Springer;
pp. 39–65. 2013, View Article : Google Scholar
|