Introduction
Breast cancer is a major health concern worldwide,
and a leading cause of cancer-related deaths among women in the
United States where over 245,000 new cases are diagnosed and 45,000
deaths occur each year (1). In the
majority of diagnoses, this disease presents as a hormone-sensitive
breast cancer that overexpressed estrogen receptor (ER),
progesterone receptor (PR) and/or human epidermal growth factor
receptor 2 (HER2/neu). These cancers typically depend on
hormone-receptor signaling for tumor growth and progression.
Consequently, conventional therapy against the majority of breast
cancers targets these receptors (e.g., tamoxifen, a selective
estrogen receptor modulator and herceptin, an antibody that
inhibits HER) or attempts to inhibit enzymes involved in estrogen
synthesis (e.g., aromatase inhibitors such as letrozole) (2–4). In
contrast, the most deadly subtype of this disease is known
clinically as triple-negative breast cancer (TNBC), and constitutes
approximately 15% of invasive breast cancers. TNBCs lack expression
of genes coding for ER, PR and HER2/neu, are not dependent on
hormonal signaling for progression, and do not respond to
conventional therapy (5).
TNBCs are frequently of the highly invasive and
recurrent basal-like subtype (6).
They are also associated with a variety of aberrations (e.g.,
higher incidence of TP53 mutations and the constitutive activation
of pro-survival and inflammatory pathways such as MAPK and NF-κB),
all of which may contribute to their growth, survival and
resistance to chemotherapy (7–10).
NF-κB signaling maintains an autocrine loop of pro-inflammatory
cytokines such as IL-6 and TNF-α, promotes expression of
pro-survival factors such as Bcl-xL, cIAP1 and cIAP2 and proteins
involved in cell cycle progression (e.g., cyclin D1), as well as
invasion and metastasis markers such as MMP-9 and vimentin
(11–17). Constitutive activation of NF-κB in
triple-negative breast cancer may contribute to its aggressiveness
and ability to maintain hormone-independent growth. Inhibition of
NF-κB signaling is thus a viable target for novel therapeutics
against TNBC.
Plant-derived molecules have been a critical source
of cancer therapeutic agents. Generally, there are four main
classes of plant-derived anticancer drugs currently in clinical
use: Vinca alkaloids, taxanes, epipodophyllotoxins and
camptothecins; the former two have entered clinical use against
breast cancer following successful trials (18,19).
Studies have also identified other natural plant compounds as
capable of exerting pleiotropic anticancer effects. Plumbagin, for
example, was shown to inhibit the DNA-binding activity of NF-κB and
induce apoptosis in triple-negative MDA-MB-231 breast cancer cells
(20). Further, halofuginone
inhibited the nuclear localization of NF-κB and AP1, which are
critical transcriptional activators of matrix metalloproteinase-9
(MMP-9), thereby reducing migration and invasiveness of these cells
(21). Curcumin, honokiol,
resveratrol and pinocembrin, among others, have also been shown to
possess potent anti-cancer effects (22–25).
These positive outcomes highlight the importance of fully
uncovering the untapped clinical potential of natural
compounds.
Herein, we report on an extract from Lippia
origanoides, a medicinal tropical plant native to South America
for its potential anticancer effects on TNBC. Also known as
'oregano de monte' or mountain oregano, infusions of the plant have
been used traditionally to alleviate gastrointestinal ailments and
as topical analgesics (26).
Towards a better understanding of L. origanoides actions and
health benefits, the novel L. origanoides extract (LOE) used
in this study was originally isolated by supercritical fluid
extraction and characterized using HPLC-MS (27,28).
The LOE was found to have numerous major components including
pinocembrin, carvacrol, thymol and trans-β-caryophyllene. Studies
using commercially available reagents of these compounds have shown
they have pro-apoptotic and anti-proliferative effects on various
cancer cell lines. For example, pinocembrin, a flavanone which
comprises nearly 55% of the total LOE, has been shown to decrease
viability and prevent epithelial-mesenchymal transition induced by
TGF-β in Y-79 retinoblastoma cells (25). Further, trans-β-caryophyllene and
α-humulene, two sesquiterpenes present in the extract, were shown
to synergize and inhibit cell growth and proliferation in MCF-7
breast cancer cells (29).
Strikingly, several components of LOE have shown inhibitory effects
on NF-κB signaling, a key survival and proliferative pathway in
TNBC (30–34). These reports support the idea that
L. origanoides may be a source of novel components that can
effectively target aggressive breast cancer.
Our results show that treatment with LOE leads to a
G0/G1 phase halt and apoptosis in MDA-MB-231 triple-negative breast
cancer cells without promoting necrotic cell death. Further, we
reveal that cell cycle proteins and apoptotic markers, as well as
key NF-κB regulatory molecules, are modulated by treatment with
LOE, thereby shedding light on a mechanism of action behind the
anticancer effects of LOE. These data provide an important first
step towards defining the potential utility of LOE in the
identification and development of novel therapeutic strategies for
TNBC.
Materials and methods
Plant material and extract
Lippia origanoides plants were collected from
the Chicamocha River Canyon (Los Santos, Santander, Colombia).
Taxonomic identification of L. origanoides was performed by
Dr José Luis Fernández Alonso (National University, Bogotá,
Colombia). The L. origanoides specimen (COL560259) was
placed in the Colombian National Herbarium (Bogotá). Fresh leaves
and flowers from L. origanoides were used for extraction as
previously described (28). The
extract was dissolved in methanol at a concentration of 50 mg/ml
(stock solution), and then different extract concentrations were
prepared in methanol for in vitro bioassays.
Cell culture
Triple-negative breast cancer (MDA-MB-231 and
CRL-2321) and normal mammary epithelial (MCF10A) cell lines were
obtained from the American Type Culture Collection. MCF10A-H cells,
derived via H-ras transformation of MCF10A cells, were a
kind gift from Dr Barbara Stefanska, Purdue University. MDA-MB-231
cells were cultured in Dublecco's modified essential medium (DMEM;
Life Technologies, Grand Island, NY, USA) supplemented with 10%
fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA, USA),
100 IU/ml penicillin and 100 µg/ml streptomycin (Life
Technologies). MCF10A and MCF10A-H cells were cultured in a 1:1
ratio of DMEM:Ham's F12 supplemented with 5% horse serum (HS;
Atlanta Biologicals), 20 ng/ml human epidermal growth factor
(Sigma-Aldrich, St. Louis, MO, USA), 0.5 mg/ml hydrocortisone
(Sigma-Aldrich), 100 ng/ml cholera toxin (Sigma-Aldrich), 10
µg/ml bovine insulin (Sigma-Aldrich), 100 IU/ml penicillin
and 100 µg/ml streptomycin. CRL2321 cells were cultured in
Roswell Park Memorial Institute 1640 medium (RPMI-1640, Mediatech
Inc., Herndon, VA, USA) supplemented with 10% FBS, 100 IU/ml
penicillin and 100 µg/ml streptomycin.
Assessment of metabolic activity via MTT
assay
MCF10A, MCF10A-H, MDA-MB-231 and CRL-2321 cells were
seeded at 105 cells/well in 96-well plates and allowed
to attach overnight. Spent media was then replaced with fresh media
containing treatment dosages between 0.15–0.2 mg/ml LOE. Media in
control wells was replaced with fresh media without additives (No
treatment, NT) or media containing the vehicle, methanol (Veh).
Following 24 h treatment, 12 mM of MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Life
Technologies), was added to the cells followed by incubation for 4
h at 37°C. Formazan crystals formed at the end of incubation period
were dissolved using dimethyl sulfoxide (DMSO) and plates were read
at 570 nm with a reference wavelength of 630 nm.
Assessment of cell cycle arrest via flow
cytometry
For cell cycle assay, MDA-MB-231 were seeded in
6-well plates at a concentration of 6×105 cells/well.
Cells were allowed to attach overnight followed by replacement of
spent media with serum-free media after 24 h to allow for
synchronization of cell cycle. Cells were then treated in fresh
media containing LOE at 0.06 and 0.15 mg/ml. Controls were treated
in triplicate with either serum-free media as a positive control or
media containing the vehicle, methanol. At the end of 36 h of
treatment, media was aspirated, cells were washed once with 1X
Phosphate Buffered Saline (PBS) and collected via trypsinization.
Samples were centrifuged at 3,000 rpm for 5 min and supernatant was
drained. Cell pellets were washed and resuspended in PBS containing
2.5 mM EDTA (1X PBS-EDTA buffer). Cells were then fixed by adding
cell suspension drop-wise into microcentrifuge tubes containing 1
ml ice-cold 70% ethanol. Fixed cells were stored at -20°C until
staining. For staining, fixed samples were centrifuged at 4000 rpm
for 5 min and supernatant was drained following which cells were
washed and resuspended in 200 µl 1X PBS-EDTA buffer. Samples
were centrifuged at 4000 rpm for 5 min and supernatant was drained.
Next, 200 µl Muse® Cell Cycle Staining Reagent
(EMD Millipore, Billerica, MA, USA) containing propidium iodide and
RNase A was added to each sample. Samples were stored at room
temperature for at least 30 min to allow for adequate staining
before being run through an FC500 flow cytometer (Beckman Coulter,
Brea, CA, USA) at the Bindley Bioscience Center, Purdue
University.
Assessment of apoptosis via flow
cytometry
MDA-MB-231 were seeded in 12-well plates at a
concentration of 2×105 cells/well. Cells were allowed to
attach overnight followed by synchronization in serum-free media.
Cells were then treated with fresh media containing LOE at 0.15 and
0.30 mg/ml for 24 h in triplicate while controls were treated with
media containing methanol. Cells collected via trypsinization and
centrifuged and supernatant was removed. Next, 100 µl Muse™
Annexin V Dead Cell Assay stain (EMD Millipore) was added to the
cell pellet and samples were resuspended by gentle pipetting.
Samples were incubated in the dark for 20 min, following which they
were run through a Muse™ Cell Analyzer (EMD Millipore) using
manufacturer's protocol.
Caspase-3/7 activation assay
MDA-MB-231 cells were seeded at 105
cells/well in a 96-well plate and allowed to attach overnight. LOE
and control treatments were prepared in fresh media containing 5
µM IncuCyte™ Caspase-3/7 apoptosis assay reagent and added
to the cells, following which the plate was placed in an
IncuCyte® ZOOM live-cell analyzer. Cells were imaged
recurrently for 24 h and quantified for changes in caspase-3/7
activation via measurement of fluorescence intensity at a
wavelength of 400 nm.
Western blotting
MDA-MB-231 cells were seeded at 106
cells/well in 6-well plates and allowed to attach overnight. Cells
were then treated with fresh media containing LOE at 0.06 or 0.15
mg/ml for 3, 6, 12 and 24 h intervals. Treated cells were collected
in RIPA buffer and sonicated to obtain lysates. Cell lysates were
cleared of debris by centrifugation and protein concentration of
lysates was estimated via the bicinchoninic acid (BCA) assay. Next,
20 µg of protein from each sample was subjected to
SDS-polyacrylamide gel electrophoresis, transferred onto a
polyvinyl difluoride (PVDF) membrane and immunoblotted. Primary
antibodies for cyclin D1, cellular Inhibitor of Apoptosis Protein 2
(cIAP2), RIP1, cleaved caspase-8 and cleaved Poly-ADP Ribose
Polymerase (PARP) were purchased from Cell Signaling Technologies,
Danvers, MA, USA. Cleaved caspase-3 antibody was purchased from
Epigenomics, WA. β-actin and β-tubulin antibodies were obtained
from Sigma-Aldrich and Abcam, Cambridge, MA, USA, respectively.
Quantification was carried out using ImageJ software (35).
Statistical analysis
Data are presented as mean values with corresponding
standard errors from various experiments. Analysis for statistical
significance for MTT assay was carried out using one-way analysis
of variance (ANOVA). Analysis for statistical significance for
western blotting, flow cytometry and caspase-3/-7 assay were
carried out using Student's unpaired two-tailed t-test.
Results
LOE decreases viability of
triple-negative breast cancer cells
In order to study the differential response of
normal, invasive, and malignant triple-negative cells to LOE
treatment, MCF10A, MCF10A-H, MDA-MB-231 and CRL-2321 cells were
treated with increasing dosages of LOE for 24 h and changes in
cellular metabolic activity were quantified using the MTT assay and
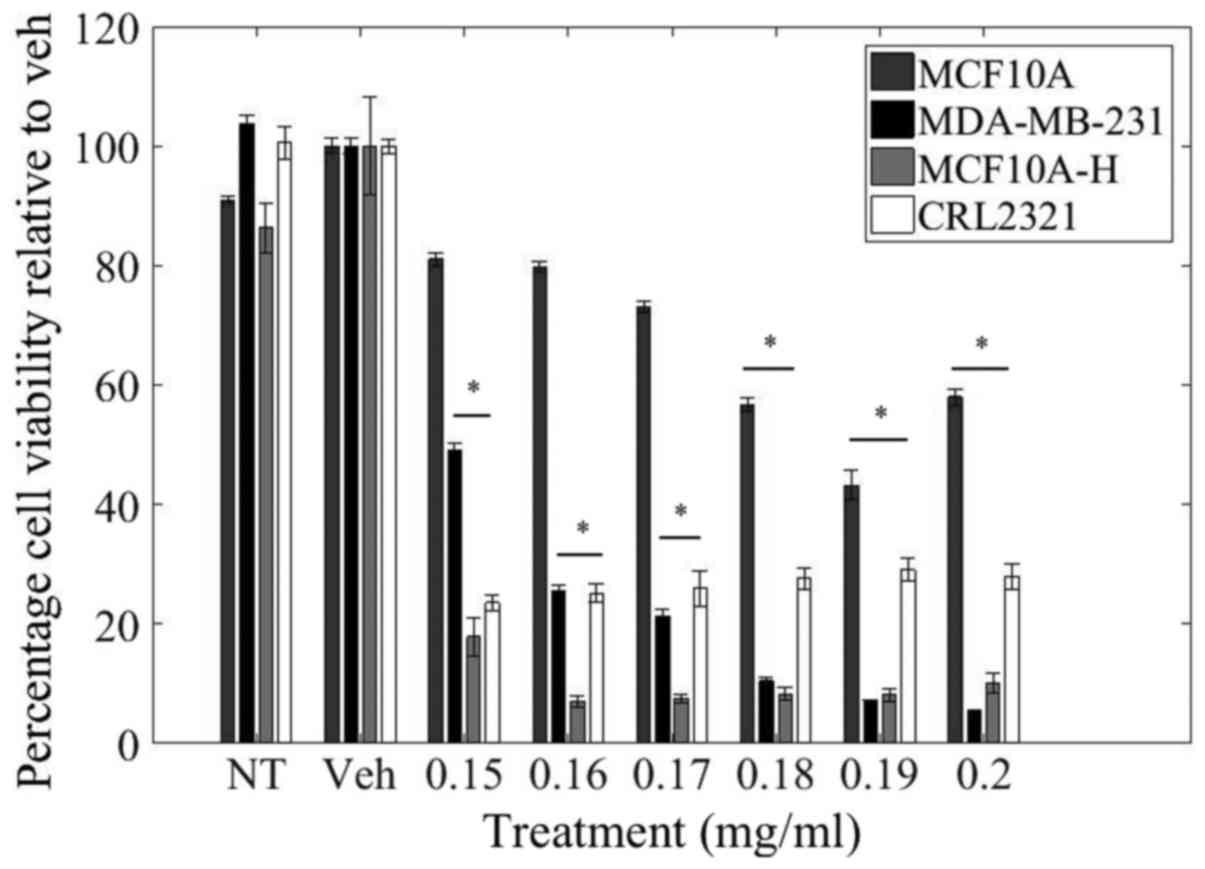
utilized as a reflection of cell viability (Fig. 1).
Relative to control treatment, there was a
dose-dependent decrease in cell viability of MDA-MB-231 cells. LOE
at a concentration of 0.15 mg/ml resulted in 50% reduction in
MDA-MB-231 cell viability while a higher dosage of 0.2 mg/ml
resulted in a 95% reduction in MDA-MB-231 cell viability.
Importantly, LOE impacted cell viability of MDA-MB-231, as well as
the other triple-negative invasive and malignant cell lines
(MCF10A-H and CRL-2321, respectively) to a much greater degree than
in normal MCF10A mammary epithelial cells. In contrast with
MDA-MB-231 cells, there was only a 20% decrease in cell viability
of MCF10A cells with 0.15 mg/ml LOE treatment and a 40% decrease
with 0.2 mg/ml LOE. Curiously, CRL-2321 cells demonstrated greater
sensitivity than MDA-MB-231 cells in their initial response to low
doses of LOE, but viability in this cell line did not decrease
further with increasing dosage.
LOE induces cell cycle arrest in
triple-negative breast cancer cells
To quantify changes in cell cycle progression
induced by LOE treatment, MDA-MB-231 cells were treated with LOE
for 36 h. Control cells were treated with either serum-free media
or growth media containing methanol. Cells were then collected and
stained with propidium iodide followed by analysis using an FC500
flow cytometer. Representative raw data showing shifts in cell
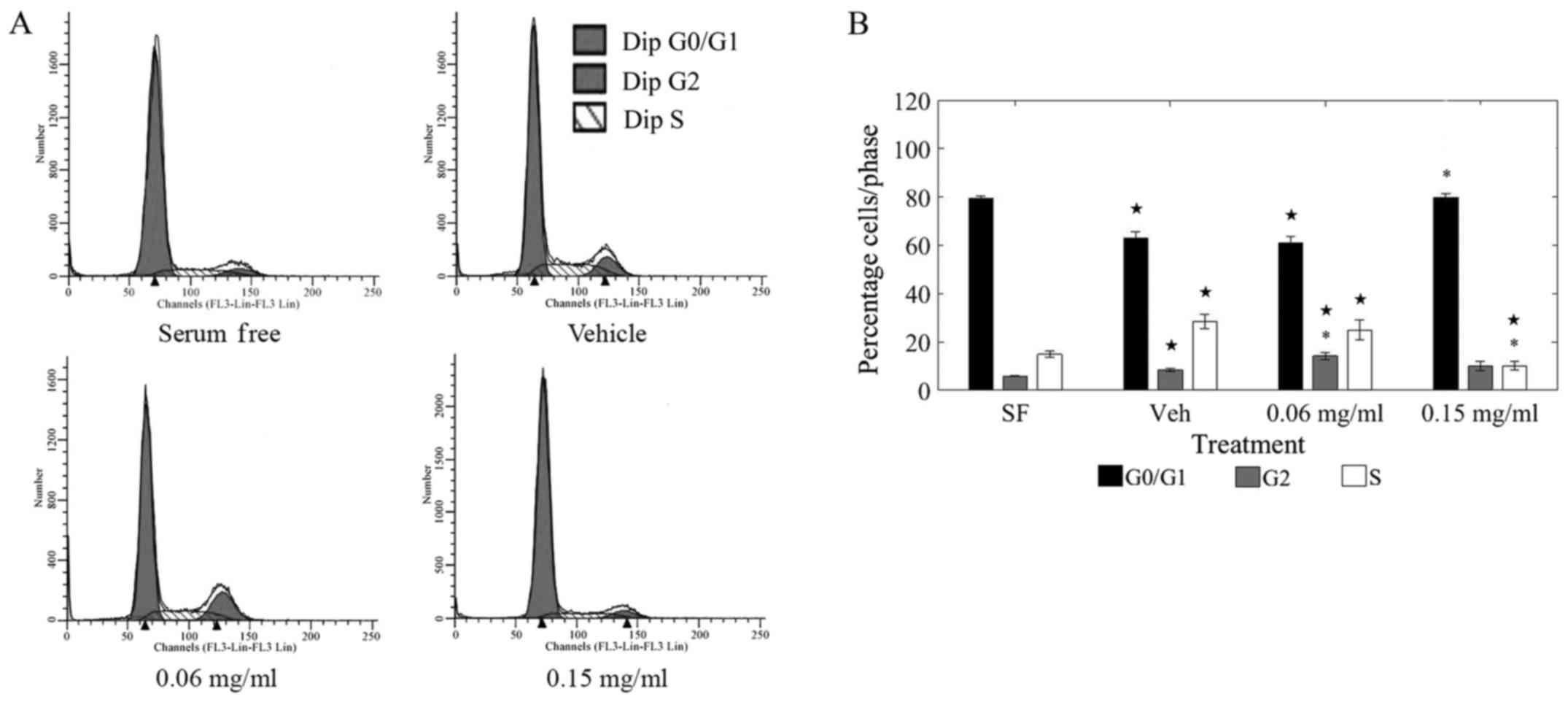
cycle phases under various treatments are shown in Fig. 2A.
Treatment with LOE at 0.15 mg/ml induced a
significant shift away from the replication stage (S-phase) of the
cell cycle as compared to vehicle-treated cells (Fig. 2B). Of the total cells counted,
almost 20% shifted away from the S-phase and toward the G0/G1 phase
upon LOE treatment at 0.15 mg/ml. Further, there was a striking
resemblance in the S-phase distribution between 0.15 mg/ml
LOE-treated cells and serum-starved cells (0.15 mg/ml LOE: 79.78%
G0/G1; SF: 79.27% G0/G1), corroborating that LOE induces a halt in
cell cycle progression in MDA-MB-231 cells.
LOE induces apoptosis in triple-negative
breast cancer cells
Cell death may take the form of apoptosis, a
programmed and controlled process involving the degradation and
clearance of cellular constituents; or necrosis, a traumatic and
inflammatory process which leads to the expulsion of cellular
material into the extracellular environment (36). In order to analyze the impact of
LOE on cell death in triple-negative breast cancer cells,
MDA-MB-231 cells were treated with LOE for 24 h. Cells were stained
with Annexin V/7-Aminoactinomycin D and evaluated in a Muse Cell
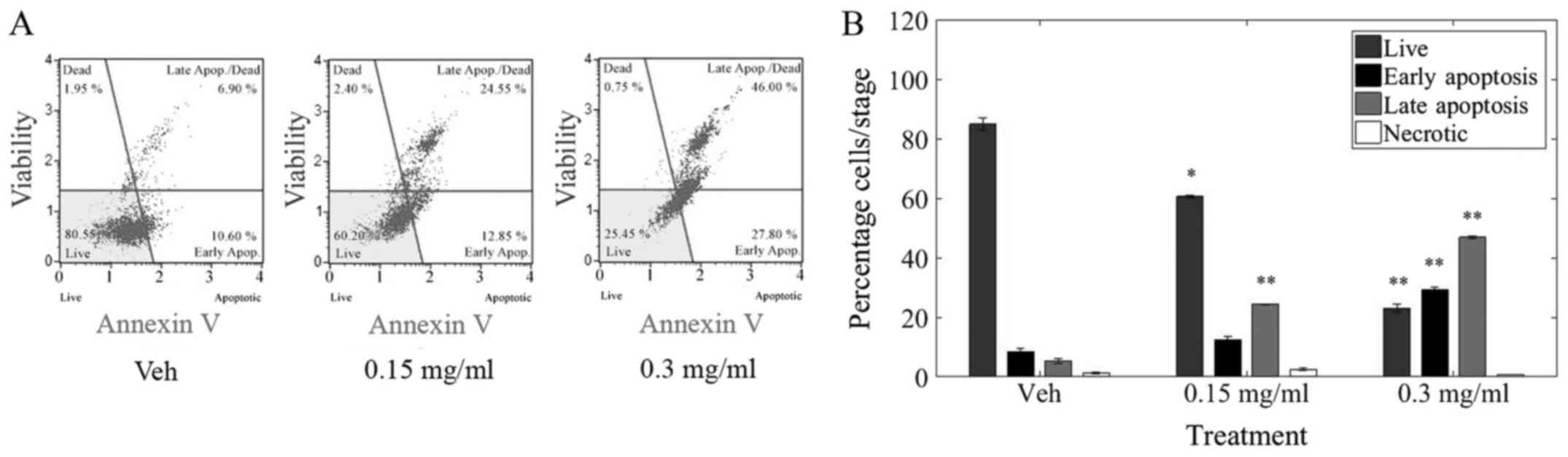
Analyzer (Merck Millipore, Billerica. MA, USA). Representative raw
data from various treatments indicating shifts from live to early
(EA) and late (LA) apoptosis as well as necrosis (Dead) is shown in
Fig. 3.
Treatment with LOE induced a significant decrease in
live cells as well a significant increase in apoptosis but not
necrosis in MDA-MB-231 cells. Furthermore, 40% of cells were
apoptotic after 24 h of 0.15 mg/ml LOE treatment while ~80% of
cells underwent apoptosis after 0.3 mg/ml LOE treatment over the
same time period.
LOE impacts critical regulators of cell
cycle progression
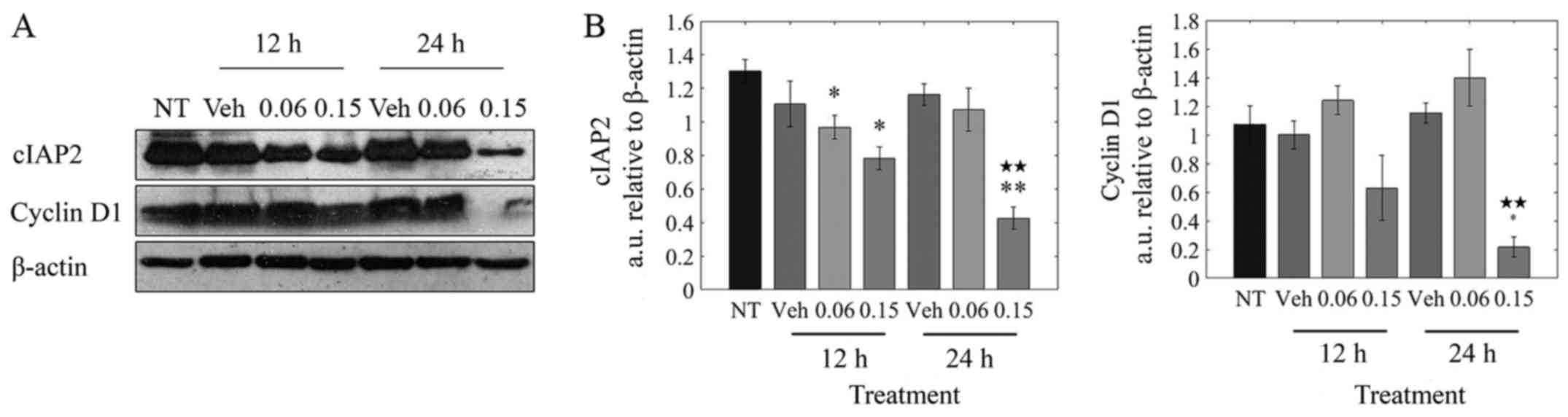
As seen from Fig.
2B, LOE treatment induced a shift toward the G0/G1 phase and
away from the S phase of the cell cycle in MDA-MB-231 cells. The
progression from G0/G1 is mediated by cyclin D1, with cellular
inhibitor of apoptosis (cIAP) proteins playing a supportive role by
suppressing apoptosis (37,38).
As observed in Fig. 4B, there was
a significant (5-fold) decrease in cyclin D1 protein at 24 h
post-treatment. cIAP2 levels were significantly reduced by 40%
after 12 h and by ~65% after 24 h.
Taken together, these results suggest that the halt
in cell cycle progression observed upon treatment of MDA-MB-231
cells with LOE is brought about by its impact on cyclin D1 and
cIAP2 expression.
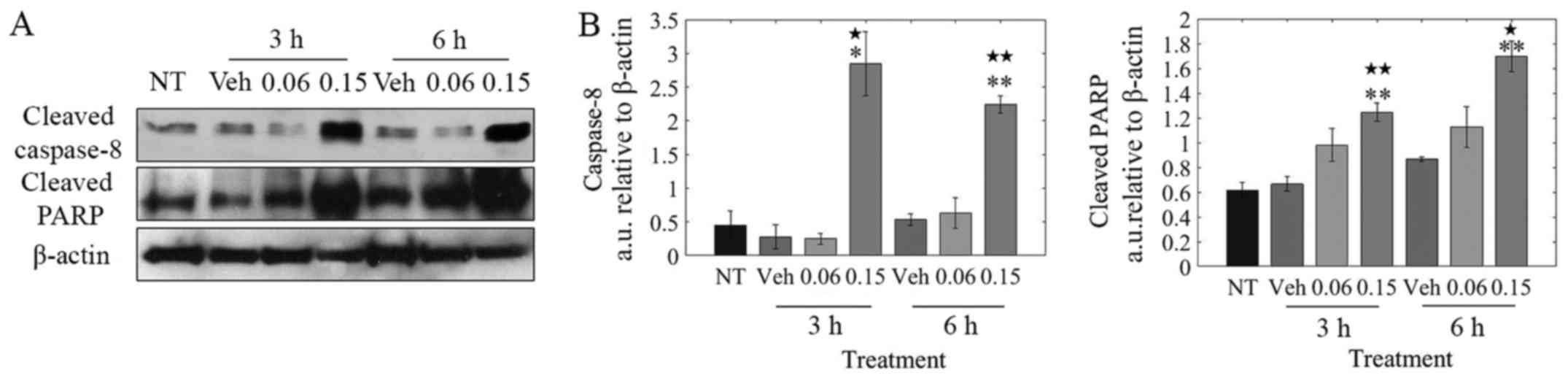
LOE induces apoptosis via caspase-8/-3
activation
Based on the observation that LOE induces apoptosis
and not necrosis in MDA-MB-231 cells (Fig. 3B), our goal was to determine if LOE
induces the extrinsic pathway of apoptosis via caspase-8
activation. As shown in Fig. 5B,
LOE induced the rapid cleavage of caspase-8 within 3 h
post-treatment with PARP cleavage peaking at 6 h
post-treatment.
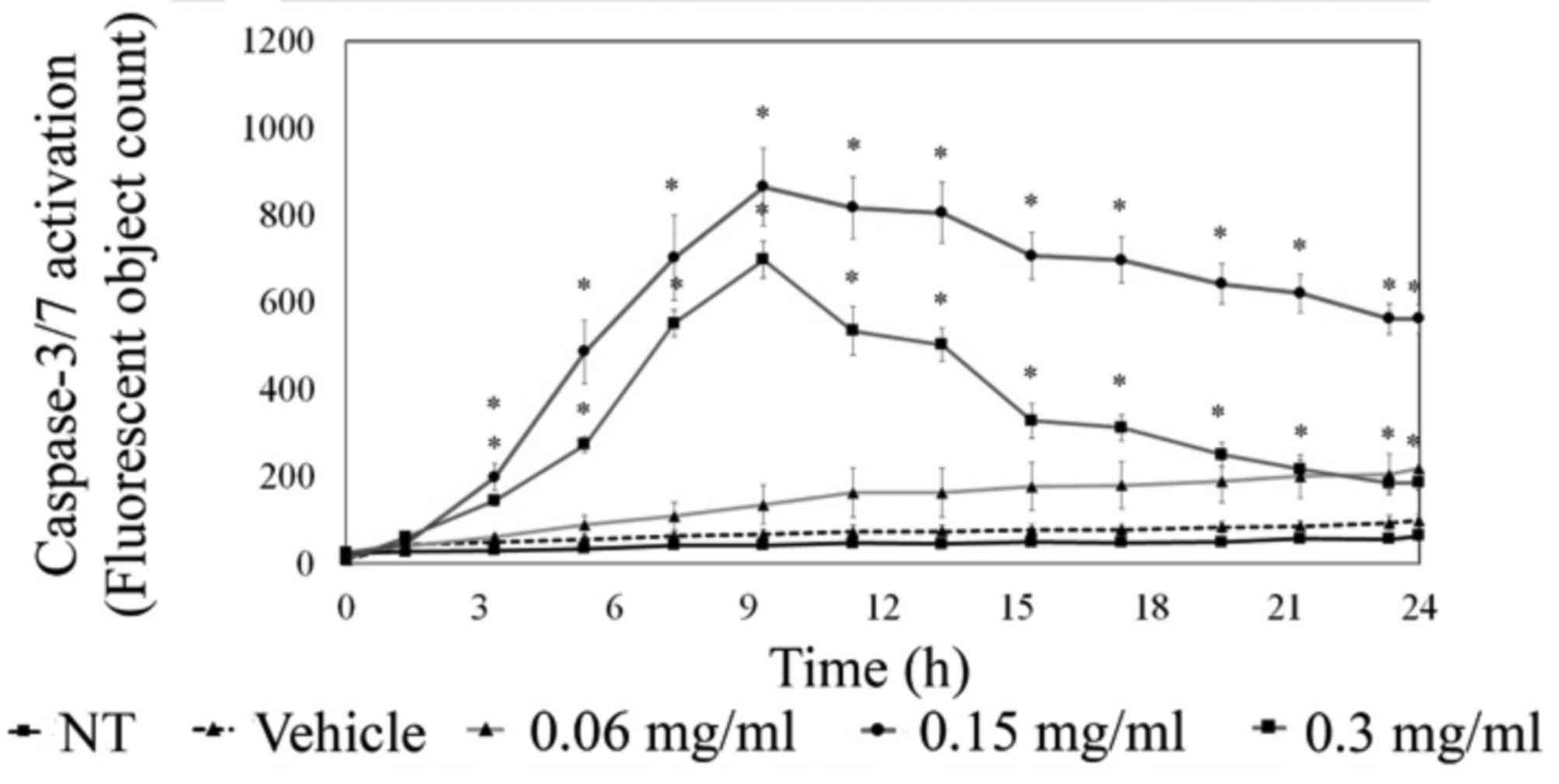
To further understand apoptotic signaling,
MDA-MB-231 cells were analyzed for executioner caspase-3/-7
activity via live cell imaging using the IncuCyte ZOOM system.
These studies showed a marked visible increase in caspase-3/-7
activation upon LOE treatment. As quantified in Fig. 6, there was a significant increase
in caspase-3/-7 activity 3 h post-treatment with 0.15 and 0.3 mg/ml
LOE, with peak activity at approximately 10 h post-treatment. This
was supported by western blotting time-course data, which indicated
a peak in caspase-3 activity at 12 h post-treatment with 0.15 mg/ml
LOE (data not shown).
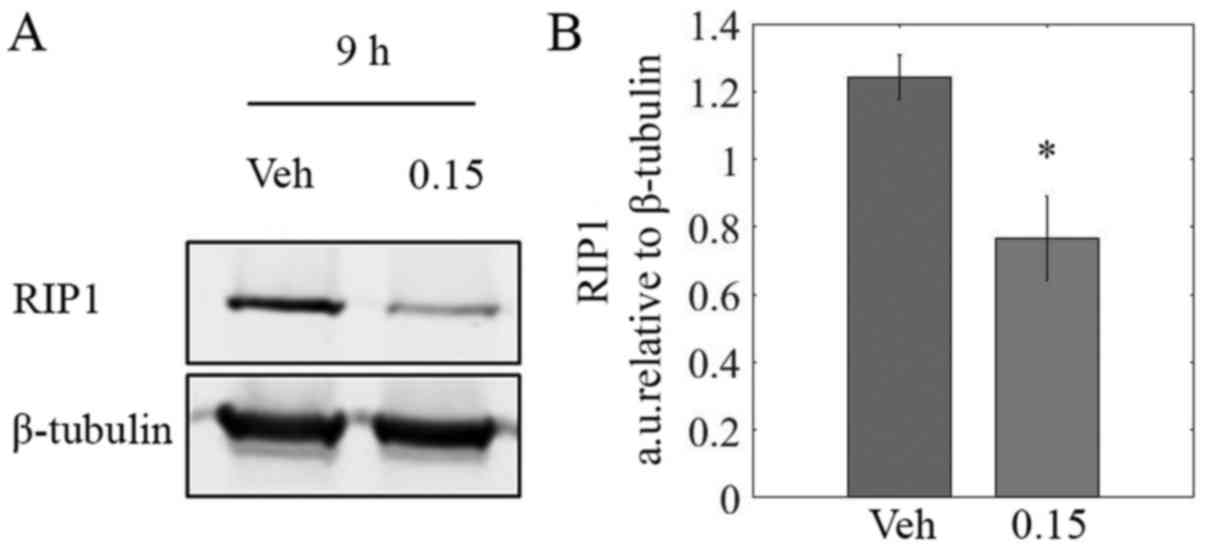
LOE reduces RIP1 protein levels
TNBC is often characterized by constitutive
activation of NF-κB signaling via recruitment of key effector
proteins by RIP1 (39,40). Immunoblotting revealed a
significant decrease of ~40% in RIP1 protein levels in lysates from
MDA-MB-231 cells treated with LOE for 9 h as compared to controls
(Fig. 7B).
Discussion
TNBC continues to be a monumental concern in women's
health issues. As our understanding of the mechanisms that govern
the initiation and progression of breast cancer grows, it has
become apparent that conventional chemotherapeutics are limited by
their inability to target subtypes that are inherently more
aggressive while being drug resistant. One such subtype is TNBC,
which lacks expression of ER, PR and HER2/neu, thereby rendering
TNBC resistant to conventional endocrine-targeted therapy. In this
study, we provide support that an extract from the tropical South
American plant L. origanoides (LOE) may be a possible source
for therapeutic agents against TNBCs. In brief, we utilized the MTT
metabolic activity assay to show LOE induces a dose-dependent
decrease in the viability of MDA-MB-231 mammary adenocarcinoma
cells, a universal standard in vitro model of highly
invasive and drug resistant TNBC. This effect was much greater
compared to normal-like MCF10A mammary epithelial cells (Fig. 1). Our observation that LOE also
significantly reduced cell viability in triple-negative
Hras-transformed MCF10A invasive cells as well as the CRL2321
cell-line, recently shown to be the best in vitro phenotypic
representative of grade 3 TNBC tumors (41), further confirms its anti-TNBC
effects. This observation suggests the LOE induces molecular
actions that target pathways specific to cancer cells. We also note
that while CRL-2321 cells have a greater sensitivity to low doses
of LOE than MDA-MB-231 cells, higher dosages of LOE do not induce
decreases in CRL-2321 viability beyond 80%. Previous studies have
shown that cancer cell line subpopulations and sub-lines can
exhibit a high degree of transcriptome and phenotypic variability
leading to observable differences in metastatic activity (42) and drug sensitivity (43). This suggests the possibility of
heterogeneity within subpopulations of the CRL-2321 cell line
allowing for compensatory survival pathways providing modest
resistance to LOE treatment. The identification of novel mechanisms
targeted by new small molecular inhibitors is of major interest in
our continued studies of the actions of LOE.
Our study also quantified the molecular influences
of the extract within cancer cells. Cyclin D1 is a protein which
associates with cyclin-dependent kinase-4 and -6 (CDK-4 and -6) to
form heterodimers that progress the cell cycle from G0/G1 to S
phase (37). In addition, cellular
inhibitor of apoptosis proteins (cIAPs) prevent apoptosis and
permit cell cycle progression via binding and possibly inactivating
executioner caspases, i.e., caspase-3 and -7 (38). cIAP2, is known to be constitutively
expressed in MDA-MB-231 cells, and has been shown to protect
against death-inducing ligands such as TNF-α by possibly enhancing
NF-κB signaling through RIP1 activation as well as by binding and
inhibiting caspase-3 (39).
Herein, we observed that LOE induced G0/G1 phase halt in MDA-MB-231
cells (Fig. 2). Western blot
analysis revealed the effects of LOE on cell cycle progression
could be explained by a reduction in cyclin D1 and cIAP2 levels
upon treatment of the cells with the extract (Fig. 4). These experiments begin to build
an important framework for the actions of LOE on cell cycle
regulation.
We next studied LOE's impact on MDA-MB-231 cell
death. Upon triggering of the extrinsic pathway of apoptosis,
procas-pase-8 is activated via dimerization-induced auto-cleavage
and subsequently cleaves and activates the master 'executioner'
caspase, caspase-3 (44,45). We revealed a potential mechanism
behind the actions of the extract by showing LOE-induced apoptosis
involved activation of caspase-8/-3 and cleavage of PARP (Figs. 5 and 6), hallmarks of extrinsic apoptotic
pathway activation (36).
Additionally, LOE induced apoptosis in these cells unaccompanied by
necrotic cell death (Fig. 3). As
cancer therapy is focused on preventing damage to non-targeted
tissue areas, this result provides additional support for LOE as a
source for potential therapeutics with desirable
characteristics.
In previous work, Stashenko et al have
characterized the major components of LOE (28). However, it remains to be defined
which component, or combination of components, is responsible for
the extract's apoptotic effects. Furthermore, it is imperative that
major cellular pathways involved in LOE signal transduction be
delineated. Based on previous studies involving components present
in the LOE, one possible candidate pathway central to LOE-induced
apoptosis is NF-κB signaling. Constitutive activation of this
pathway has been previously linked to triple-negative breast cancer
and blocking NF-κB signaling can spontaneously induce cell death
via a caspase-8/-3-dependent mechanism or render cells susceptible
to pro-apoptotic signals (40,46,47).
In normal cells, ligand binding to TNFR1 is followed by rapid
activation of the extrinsic pathway of apoptosis. However, in
contrast to normal cells, ligand binding to TNFR1 in TNBC cells
instead activates NF-κB signaling via membrane-recruitment of the
scaffold protein RIP1, a Ser/Thr kinase (39). In brief, cIAP2 exerts E3 ubiquitin
ligase activity to polyubiquitinate and activate RIP1, which goes
on to phosphorylate the IKKα/β-NEMO complex. Active IKKs then
phosphorylate IκB proteins which sequester p50/RelA (NF-κB) dimers
to the cytoplasm, targeting the IκBs for polyubiquitination and
subsequent degradation. This frees p50/RelA to translocate to the
nucleus and act as a transcription factor for pro-survival genes
such as cyclin D1 and cIAPs (48,49).
We hypothesized that components of LOE could
interfere with NF-κB signaling, leading to a halt in the cell cycle
and induction of apoptosis. Indeed, we showed LOE treatment
resulted in a 40% decrease in protein levels of RIP1 in MDA-MB-231
cells (Fig. 7B). This may, at
least in part, be explained by our previous observation that LOE
treatment induced activation of caspase-8. Active caspase-8 has
previously been shown to cleave RIP-1, thereby inhibiting
pro-survival NF-κB signaling and inducing apoptosis (50). This result, coupled with our
findings that LOE inhibits cyclin D1 and cIAP2, proteins activated
by NF-κB signaling, while simultaneously stimulating caspase-8
dependent cell death, a characteristic of NF-κB inhibition, sheds
critical light on the mechanistic foreground of LOE action. All
together, these data highlight the potential of L.
origanoides extract and its major constituents as cancer
therapeutics.
In summary, we have shown that LOE promotes
apoptosis in TNBC cells, while having a much reduced impact on cell
death in normal mammary cells. We also found that LOE reduces cell
viability, alters the cell cycle and primarily leads to tumor cell
apoptosis and not necrosis. Further, we established some of the
mechanisms of LOE actions, showing that it suppresses markers of
cell cycle progression and cell survival while inducing the
extrinsic pathway of apoptosis via caspase-8/-3 activation.
Finally, we revealed that levels of RIP1, an upstream effector of
pro-survival NF-κB signaling, are reduced upon LOE treatment. These
data collectively support that LOE can be a valuable source for
development of novel anticancer agents. Moving forward, beyond
fully describing the cellular mechanisms of LOE action, a top
priority will be identifying the major component or combination of
components responsible for its bioactivity. Plant-derived compounds
offer a vast source of small molecule inhibitors that could be used
both in their native state as well as chemically modified to
optimize their pharmacological activity. The bio-reactivity of the
LOE-treatment in TNBC cells in vitro supports the idea that
future treatments for individual cancer subtypes lie in
naturally-derived chemicals.
Acknowledgments
The authors would like to thank Ms. Gabriela Sincich
and Ms. Shelli Taylor from the Department of Biological Sciences,
Purdue University, for their help in editing this manuscript. The
authors gratefully acknowledge the Flow Cytometry and Cell
Separation Facility and support from the Purdue University Center
for Cancer Research, NIH grant P30 CA023168. The LOE used in this
work was obtained within contract no. RC-0572-2012 supported by
'Patrimonio Autónomo Fondo Nacional de Financiamiento para la
Ciencia, la Tecnología y la Innovación, Francisco José de Caldas'.
The Ministerio de Ambiente y Desarrollo Sostenible (MADS) of
Colombia supported the project through permits to access genetic
resources and derivatives products during scientific research
(contract no. 101, resolution no. 0812).
Glossary
Abbreviations
Abbreviations:
|
cIAP
|
cellular inhibitor of apoptosis
|
|
ER
|
estrogen receptor
|
|
Her2/neu
|
human epidermal growth factor receptor
2
|
|
LOE
|
Lippia origanoides extract
|
|
NF-κB
|
nuclear factor κB
|
|
PARP
|
poly-ADP ribose polymerase
|
|
PR
|
progesterone receptor
|
|
TNBC
|
triple-negative breast cancer
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jordan VC: The role of tamoxifen in the
treatment and prevention of breast cancer. Curr Probl Cancer.
16:129–176. 1992.PubMed/NCBI
|
|
3
|
Buzdar A and Howell A: Advances in
aromatase inhibition: Clinical efficacy and tolerability in the
treatment of breast cancer. Clin Cancer Res. 7:2620–2635.
2001.PubMed/NCBI
|
|
4
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman
PA, et al: Trastuzumab plus adjuvant chemotherapy for operable
HER2-positive breast cancer. N Engl J Med. 353:1673–1684. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dent R, Hanna WM, Trudeau M, Rawlinson E,
Sun P and Narod SA: Pattern of metastatic spread in triple-negative
breast cancer. Breast Cancer Res Treat. 115:423–428. 2009.
View Article : Google Scholar
|
|
6
|
Brenton JD, Carey LA, Ahmed AA and Caldas
C: Molecular classification and molecular forecasting of breast
cancer: Ready for clinical application? J Clin Oncol. 23:7350–7360.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi X, Yin N, Ma S, Lepp A, Tang J, Jing W,
Johnson B, Dwinell MB, Chitambar CR and Chen G: p38γ MAPK is a
therapeutic target for triple-negative breast cancer by stimulation
of cancer stem-like cell expansion. Stem Cells. 33:2738–2747. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakshatri H, Bhat-Nakshatri P, Martin DA,
Goulet RJ Jr and Sledge GW Jr: Constitutive activation of NF-kappaB
during progression of breast cancer to hormone-independent growth.
Mol Cell Biol. 17:3629–3639. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sero JE, Sailem HZ, Ardy RC, Almuttaqi H,
Zhang T and Bakal C: Cell shape and the microenvironment regulate
nuclear translocation of NF-κB in breast epithelial and tumor
cells. Mol Syst Biol. 11:7902015. View Article : Google Scholar
|
|
11
|
Khoshnan A, Tindell C, Laux I, Bae D,
Bennett B and Nel AE: The NF-kappa B cascade is important in Bcl-xL
expression and for the anti-apoptotic effects of the CD28 receptor
in primary human CD4+ lymphocytes. J Immunol.
165:1743–1754. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang CY, Mayo MW, Korneluk RG, Goeddel DV
and Baldwin AS Jr: NF-kappaB antiapoptosis: Induction of TRAF1 and
TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation.
Science. 281:1680–1683. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huber MA, Azoitei N, Baumann B, Grünert S,
Sommer A, Pehamberger H, Kraut N, Beug H and Wirth T: NF-kappaB is
essential for epithelial-mesenchymal transition and metastasis in a
model of breast cancer progression. J Clin Invest. 114:569–581.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hartman ZC, Poage GM, den Hollander P,
Tsimelzon A, Hill J, Panupinthu N, Zhang Y, Mazumdar A, Hilsenbeck
SG, Mills GB, et al: Growth of triple-negative breast cancer cells
relies upon coordinate autocrine expression of the proinflammatory
cytokines IL-6 and IL-8. Cancer Res. 73:3470–3480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hinz M, Krappmann D, Eichten A, Heder A,
Scheidereit C and Strauss M: NF-kappaB function in growth control:
Regulation of cyclin D1 expression and G0/G1-to-S-phase transition.
Mol Cell Biol. 19:2690–2698. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yousef EM, Tahir MR, St-Pierre Y and
Gaboury LA: MMP-9 expression varies according to molecular subtypes
of breast cancer. BMC Cancer. 14:6092014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kagoya Y, Yoshimi A, Kataoka K, Nakagawa
M, Kumano K, Arai S, Kobayashi H, Saito T, Iwakura Y and Kurokawa
M: Positive feedback between NF-κB and TNF-α promotes
leukemia-initiating cell capacity. J Clin Invest. 124:528–542.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gill PS, Rarick M, McCutchan JA, Slater L,
Parker B, Muchmore E, Bernstein-Singer M, Akil B, Espina BM, Krailo
M, et al: Systemic treatment of AIDS-related Kaposi's sarcoma:
Results of a randomized trial. Am J Med. 90:427–433. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saville MW, Lietzau J, Pluda JM,
Feuerstein I, Odom J, Wilson WH, Humphrey RW, Feigal E, Steinberg
SM, Broder S, et al: Treatment of HIV-associated Kaposi's sarcoma
with paclitaxel. Lancet. 346:26–28. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahmad A, Banerjee S, Wang Z, Kong D and
Sarkar FH: Plumbagin-induced apoptosis of human breast cancer cells
is mediated by inactivation of NF-kappaB and Bcl-2. J Cell Biochem.
105:1461–1471. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin ML, Park SY, Kim YH, Park G and Lee
SJ: Halofuginone induces the apoptosis of breast cancer cells and
inhibits migration via downregulation of matrix
metalloproteinase-9. Int J Oncol. 44:309–318. 2014. View Article : Google Scholar
|
|
22
|
Pozo-Guisado E, Merino JM, Mulero-Navar ro
S, Lorenzo-Benayas MJ, Centeno F, Alvarez-Barrientos A and
Fernandez-Salguero PM: Resveratrol-induced apoptosis in MCF-7 human
breast cancer cells involves a caspase-independent mechanism with
downregulation of Bcl-2 and NF-kappaB. Int J Cancer. 115:74–84.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh S and Aggarwal BB: Activation of
transcription factor NF-kappa B is suppressed by curcumin
(diferuloylmethane) [corrected]. J Biol Chem. 270:24995–25000.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tse AK, Wan CK, Shen XL, Yang M and Fong
WF: Honokiol inhibits TNF-alpha-stimulated NF-kappaB activation and
NF-kappaB-regulated gene expression through suppression of IKK
activation. Biochem Pharmacol. 70:1443–1457. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen KS, Shi MD, Chien CS and Shih YW:
Pinocembrin suppresses TGF-β1-induced epithelial-mesenchymal
transition and metastasis of human Y-79 retinoblastoma cells
through inactivating αvβ3 integrin/FAK/p38α signaling pathway. Cell
Biosci. 4:412014. View Article : Google Scholar
|
|
26
|
Pascual ME, Slowing K, Carretero E,
Sánchez Mata D and Villar A: Lippia: traditional uses, chemistry
and pharmacology: a review. J Ethnopharmacol. 76:201–214. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stashenko EE, Martínez JR, Ruíz CA, Arias
G, Durán C, Salgar W and Cala M: Lippia origanoides chemotype
differentiation based on essential oil GC-MS and principal
component analysis. J Sep Sci. 33:93–103. 2010. View Article : Google Scholar
|
|
28
|
Stashenko EE, Martínez JR, Cala MP, Durán
DC and Caballero D: Chromatographic and mass spectrometric
characterization of essential oils and extracts from Lippia
(Verbenaceae) aromatic plants. J Sep Sci. 36:192–202. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Legault J and Pichette A: Potentiating
effect of beta-caryophyllene on anticancer activity of
alpha-humulene, isocaryophyllene and paclitaxel. J Pharm Pharmacol.
59:1643–1647. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aristatile B, Al-Assaf AH and Pugalendi
KV: Carvacrol suppresses the expression of inflammatory marker
genes in D-galactosamine-hepatotoxic rats. Asian Pac J Trop Med.
6:205–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Greiner JF, Müller J, Zeuner MT, Hauser S,
Seidel T, Klenke C, Grunwald LM, Schomann T, Widera D, Sudhoff H,
et al: 1,8-Cineol inhibits nuclear translocation of NF-κB p65 and
NF-κB-dependent transcriptional activity. Biochim Biophys Acta.
1833:2866–2878. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee JC, Kundu JK, Hwang DM, Na HK and Surh
YJ: Humulone inhibits phorbol ester-induced COX-2 expression in
mouse skin by blocking activation of NF-kappaB and AP-1: IkappaB
kinase and c-Jun-N-terminal kinase as respective potential upstream
targets. Carcinogenesis. 28:1491–1498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang D, Li F, Fu Y, Cao Y, Song X, Wang
T, Wang W, Guo M, Zhou E, Li D, et al: Thymol inhibits
LPS-stimulated inflammatory response via down-regulation of NF-κB
and MAPK signaling pathways in mouse mammary epithelial cells.
Inflammation. 37:214–222. 2014. View Article : Google Scholar
|
|
34
|
Kim C, Cho SK, Kim KD, Nam D, Chung WS,
Jang HJ, Lee SG, Shim BS, Sethi G and Ahn KS: β-Caryophyllene oxide
potentiates TNFα-induced apoptosis and inhibits invasion through
down-modulation of NF-κB-regulated gene products. Apoptosis.
19:708–718. 2014. View Article : Google Scholar
|
|
35
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Portt L, Norman G, Clapp C, Greenwood M
and Greenwood MT: Anti-apoptosis and cell survival: A review.
Biochim Biophys Acta. 1813:238–259. 2011. View Article : Google Scholar
|
|
37
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Salvesen GS and Duckett CS: IAP proteins:
Blocking the road to death's door. Nat Rev Mol Cell Biol.
3:401–410. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bertrand MJ, Milutinovic S, Dickson KM, Ho
WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ and
Barker PA: cIAP1 and cIAP2 facilitate cancer cell survival by
functioning as E3 ligases that promote RIP1 ubiquitination. Mol
Cell. 30:689–700. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yamaguchi N, Ito T, Azuma S, Ito E, Honma
R, Yanagisawa Y, Nishikawa A, Kawamura M, Imai J, Watanabe S, et
al: Constitutive activation of nuclear factor-kappaB is
preferentially involved in the proliferation of basal-like subtype
breast cancer cell lines. Cancer Sci. 100:1668–1674. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Grigoriadis A, Mackay A, Noel E, Wu PJ,
Natrajan R, Frankum J, Reis-Filho JS and Tutt A: Molecular
characterisation of cell line models for triple-negative breast
cancers. BMC Genomics. 13:6192012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nguyen A, Yoshida M, Goodarzi H and
Tavazoie SF: Highly variable cancer subpopulations that exhibit
enhanced transcriptome variability and metastatic fitness. Nat
Commun. 7:112462016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Leung E, Kannan N, Krissansen GW, Findlay
MP and Baguley BC: MCF-7 breast cancer cells selected for tamoxifen
resistance acquire new phenotypes differing in DNA content,
phospho-HER2 and PAX2 expression, and rapamycin sensitivity. Cancer
Biol Ther. 9:717–724. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Peter ME and Krammer PH: The
CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10:26–35. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Beaudouin J, Liesche C, Aschenbrenner S,
Hörner M and Eils R: Caspase-8 cleaves its substrates from the
plasma membrane upon CD95-induced apoptosis. Cell Death Differ.
20:599–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yamamoto M, Taguchi Y, Ito-Kureha T, Semba
K, Yamaguchi N and Inoue J: NF-κB non-cell-autonomously regulates
cancer stem cell populations in the basal-like breast cancer
subtype. Nat Commun. 4:22992013. View Article : Google Scholar
|
|
47
|
Micheau O and Tschopp J: Induction of TNF
receptor I-mediated apoptosis via two sequential signaling
complexes. Cell. 114:181–190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guttridge DC, Albanese C, Reuther JY,
Pestell RG and Baldwin AS Jr: NF-kappaB controls cell growth and
differentiation through transcriptional regulation of cyclin D1.
Mol Cell Biol. 19:5785–5799. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
You M, Ku PT, Hrdlicková R and Bose HR Jr:
ch-IAP1, a member of the inhibitor-of-apoptosis protein family, is
a mediator of the antiapoptotic activity of the v-Rel oncoprotein.
Mol Cell Biol. 17:7328–7341. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lin Y, Devin A, Rodriguez Y and Liu ZG:
Cleavage of the death domain kinase RIP by caspase-8 prompts
TNF-induced apoptosis. Genes Dev. 13:2514–2526. 1999. View Article : Google Scholar : PubMed/NCBI
|