Introduction
Blood hypercoagulability is a common systemic
alteration in patients with cancer that predisposes to thrombosis
which has a deteriorating effect on patients' quality of life and
survival (1–4). Cancer cells are directly involved in
the pathogenesis of thrombosis though several pathways which
implicate triggering and enhancement of thrombin generation and
imbalance of procoagulant and anticoagulant forces. However, the
biochemical basis of the activation of coagulation in cancer
patients is still not completely understood. Clinical and
experimental evidence support the paradigm that coagulation and
tumor growth form a vicious circle. Enhanced generation of serine
proteases of blood coagulation, promotes the aggressiveness of
cancer cells and vice versa (2).
Attention has been focused on tissue factor (TF), a transmembrane
receptor protein, which is not only the primary initiator of
coagulation but also promotes tumor growth, angiogenesis, and
metastasis (5). Cancer patients
exhibit heightened levels of circulating procoagulant
micropatricles that correlate with the risk of thrombosis (6–11).
Tumor cells may express procoagulant activity that
can directly induce thrombin generation. In addition, normal host
tissues may express procoagulant activity in response to the tumor.
Many tumor cells express high levels of TF. In several types of
cancer, including breast and pancreatic cancer, TF expression on
tumor cells is correlated with grade and tumor progression
(12–14). Recent studies from our group showed
that the contact of cancer cells with human plasma triggers and
enhances thrombin generation in a partially tissue factor-dependent
manner (15). Pancreas
adenocarcinoma cells BXPC3 and breast cancer cells MCF7 showed that
they accelerate the initiation and the propagation phase of
thrombin generation but they have different procoagulant potential
(15). To some extent, this is due
to different amount of TF expressed by the BXPC3 and MCF7 cells.
These studies raised the concept that the procoagulant potential
varies according to the histological type of cancer cells.
An additional mechanism that amplifies cancer
related hypercoagulability is the release of cancer cell-derived
procoagulant microparticles (CaCe-dMP) (16). Among the contributing factors,
CaCe-dMP has generated considerable interest since the discovery of
their proand anticoagulant properties, their fibrinolytic activity
and their ability to contribute to thrombosis in vivo
(17,18). It is suggested that CaCe-dMP
bearing TF is a trigger for thrombogenesis (6,7,19,20).
In addition, TF bearing CaCe-dMP appear to participate in
triggering thrombogenesis directly and play an important role in
cancerinduced coagulopathy (21).
Approximately 50% of MPs in the patients with cancer expressed the
tumor antigen mucin 1 on their surface, suggesting that they derive
from the tumor (20).
Consequently, TF bearing CaCe-dMP could be an important marker in
the consideration of prevention or therapy of cancer associated
thrombosis.
The present study aimed to identify the mechanism by
which hypercoagulabilty is produced in the presence of cancer
cells. We focused on the analysis of the procoagulant elements
carried by CaCe-dMP and we evaluated the impact of microparticles
associated with the cancer cells from which they stem on thrombin
generation. We also explored any potential differences on the
prothrombotic potential of the cancer cells and their
microenvironment which are related with the histological type of
the cancer cells.
Materials and methods
Cell cultures
Adhesive cell lines from human pancreatic primary
adenocarcinoma cell line BXPC3 (lot F-11067) was from American Type
Culture Collection (ATCC; Rockville, MD, USA). Breast
adenocarcinoma cell line MCF7 (Michigan Cancer Foundation-7) was
from ATCC (Rockville, MD, USA). Both cell lines were used for
thrombin generation experiments in human plasma.
Cells were expanded and cultured as described
elsewhere (15). A volume of 100
μl of cell suspension (50 cells/μl) was placed into
96-well plates and cells were cultured and adhered at 37°C in 100%
humidified atmosphere with 5% CO2 for 24 h.
The HUVEC were obtained from Clonetics (San Diego,
CA, USA) and cultured in endothelial cell growth media EGM-2
(Clonetics) containing 2% of foetal bovine serum and supplements.
Cells of 2nd passage were used in the experiments. Adhesive
cultures were developed on 25 cm2 culture flasks and
adhered at 37°C in 100% humidified atmosphere with 5%
CO2. Cells were used for experiments when they reached
80% confluence. For transfer to the 96-well plates HUVEC (a
suspension of 50 cells/μl) were treated with the same
protocol as that described above for BXPC3 and MCF7 cells.
Normal human plasma
Samples of normal platelet poor plasma (PPP) for
thrombin generation experiments were purchased from Stago (ref. no.
00539; Genevilliers, France) in compliance with Helsinki
Declaration.
Specific TF activity concentration
The BXPC3 and MCF7 cells were obtained from adhesive
cultures as described above. After three washing cycles with PBS,
cells were suspended in distilled water (at final concentrations
adjusted between 50 and 200 cells/μl) and incubated at 4°C
for 30 min. Then samples were centrifuged for 30 min at 1,000 × g,
afterwards, supernatants were collected and kept frozen at −80°C
until measurement of TF activity.
Tissue factor activity (TFa) was measured in normal
plasma, the same which was used for thrombin generation
experiments; in which cancer cells were suspended. Tissue factor
activity (TFa) was assessed with an in-house chromogenic method as
described elsewhere (22–24).
Calibrated Automated Thrombogram
assay
In each well of the micro-plate, 80 μl of PPP
samples were mixed with saline (20 μl). Thrombin generation
was initiated by adding 20 μl triggering solution containing
CaCl2 (16.7 mM final concentration) and fluorogenic substrate
(Z-Gly-Gly-Arg-AMC, 417 μM final concentration). Thrombin
generation was assessed with the Calibrated Automated Thrombogram
assay (Thrombinoscope b.v., Maastricht, The Netherlands) as
described elsewhere (25). Among
thrombogram parameters we analyzed the mean rate index (MRI), which
reflects the rate of the propagation phase of thrombin generation
[calculated by the formula MRI = Peak/(ttPeak − lag-time)]. This
parameter includes lag-time, the time to Peak (ttPeak) and the
Peak. These parameters of thrombogram as shown in previous studies,
reflect the biological activity of cancer cells on thrombin
generation, better than the endogenous thrombin potential.
Procoagulant potential of cancer cells
assessed with the calibrated automated thrombogram assay
The BXPC3 or MCF7 cells as well as the HUVEC
(control experiment) were expanded in the wells of microtiter
plates suitable for thrombin generation assessment (as described
above). Then, 80 μl of normal PPP was added in each well and
thrombin generation was assessed as described above. In the control
experiments, wells were filled with culture medium without cells
and treated in the same way as the experiments. Each experiment was
repeated several times. Saline (20 μl) was used in the
control experiment. In additional control experiments, thrombin
generation was assessed in plasma spiked with increasing
concentration of lymphocytes from healthy donors and was compared
to thrombin generation obtained after calcification of normal
plasma. No significant difference was found between the two
experimental procedures (data not shown). In preliminary
experiments, we also verified that the culture medium (solution of
RPMI, glutamine, penicillin, streptomycin and fetal calf serum) did
not influence thrombin generation process of normal PPP. Thrombin
generation was initiated and recorded as described above.
Thrombin generation in the presence of an
anti-TF antibody
In separate experiments, 50 μl of the working
suspension of BXPC3 or MCF7 cells (in a volume yielding a count of
100 cells/μl) were mixed with 50 μl of a solution
containing an anti-TF mouse monoclonal antibody 4509 (American
Diagnostica, Neuville-sur-Oise, France) or anti-human TF9-10H10 of
mouse origin (AbD Serotec, Bio-Rad Laboratories, Steenvoorde,
France). An isotype mouse IgG1 (100 μg/ml) or saline were
used in control experiments. Cells were incubated with the anti-TF
antibody or the isotype IgG1 for 15 min at 37°C and then 20
μl of this suspension were mixed with 180 μl of PPP.
The number of BXPC3 and MCF7 cells in plasma assessed for thrombin
generation was 50 per μl. The experimental conditions were
defined after conducting preliminary experiments on thrombin
generation, with variable concentrations of the cells and the
anti-TF monocolonal antibody. Cells were used at the lower active
concentration in plasma and antibody was employed at the
concentration of 25 μg/ml. At this concentration the anti-TF
antibody completely inhibited the effect of high TF concentrations
on thrombin generation. Impact of anti-TF antibody on thrombin
generation were triggered in normal PPP by BXPC3 or MCF7 cells
triggered in presence of MP-Reagent® to eliminate any
interactions of the phospholipid concentration.
Isolation of cancer cell-derived
microparticles
Cancer cell-derived microparticles were isolated
from conditioned media, containing 2% of foetal bovine serum and
supplements, from confluent BXPC3, MCF7, HUVEC cells by
differential centrifugation, as previously described (26). Briefly, culture supernatants (cell
conditioned media) were collected and centrifuged at 1,500 × g for
5 min to pellet whole cells and debris. The collected supernatant
was re-centrifuged at 15,000 × g for 1 h at 15°C to pellet the
CaCe-dMP. The final pellet was re-suspended in 0.5 ml PBS and
centrifuged at 2,000 × g for 1 min to remove debris. The clear
CaCe-dMP suspension was further centrifuged at 18,000 × g for 30
min at 15°C to pellet MPs. The number of MP was measured with flow
cytometry and was standardized for the mixing experiments.
Flow cytometric analysis on cells and
cancer cell-derived microparticles
BXPC3 or MCF7 cells were detached using Versene
Buffer (0.54 mM EDTA, 140 mM NaCl, 2.7 mM KCl, 8.1 mM
Na2HPO4, 1.46 mM
KH2PO4, and 1 mM glucose, pH 7.4), washed
once, and re-suspended in PBS supplemented with 1% BSA (PBS/BSA).
Cells suspended in phosphate-buffered saline (PBS), were
centrifuged at 300 × g for 5 min. For TF assessment, cells
re-suspended in PBS (3×105 cells/25 μl), were
incubated with 10 μl of a control mouse immunoglobulin IgG1
(Beckman Coulter, Villepinte, France, reference 731581) or with a
mouse monoclonal antibody against human TF (American Diagnostica,
product no. 4509), for 30 min at room temperature in the dark.
Anti-TF monoclonal antibody and IgG1 isotype control were used at
1/25 and 1/50 dilution, yielding a final concentration of 5 and 7
μg/ml, respectively. After washing twice, cells were
incubated at room temperature with a PE-conjugated antibody goat
anti mouse IgG1 (1/50 dilution; Beckman Coulter, ref. no. 731914)
for 30 min in the dark. After incubation, cells were washed with
PBS and suspended in PBS for tissue factor assessment. Sample data
were acquired and analyzed using an FC 500 flow cytometer (Beckman
Coulter, Villepinte, France) with CXP Acquisition and CXP Analysis
soTFware (Beckman Coulter, Miami, FL, USA). Forward scatter and
side scatter of light was set in logarithmic scale.
For the detection of microparticles by flow
cytometry, an initial microparticle-size gate was set with the help
of calibrating fluorescent 0.8 μm and 3.0 μm latex
beads (Sigma, St. Louis, MO, USA). This microparticle gate excludes
the electronic background noise through the threshold. In parallel,
we used Megamix (American Diagnostic Corp., Hauppauge, NY, USA), a
mixture of microbeads of three different sizes (0.5, 0.9, and 3.0
μm) which was developed to confirm the size of the
microparticles. Forward scatter and side scatter had a logarithmic
gain. The absolute count of microparticles was measured setting the
stop condition for TruCount beads at 10,000 events. In order to
separate true events from background noise and unspecific binding
of antibodies to debris, we defined microparticles as particles
that were less than 1.0 μm in diameter, had positive
staining for Annexin V and expressed surface antigens (CD31 or CD42
or both). For the measurement of procoagulant phospholipids
expression the microparticle suspension was incubated for 30 min
with 10 μl FITC-Annexin V or PE-anti-mouse monoclonal
antibodies. Annexin V-FITC with phosphate-buffered saline without
calcium were used as control. Analyses were performed on a flow
cytometer (Navios) using a Megamix bead-calibrated protocol
(BioCytex). Preliminary experiment verified that conditioned media
containing 2% of foetal bovine serum and supplements without any
exposure to the studied cell lines did not contain any detectable
amount of microparticles.
Statistical analysis
Non-parametric Mann-Withney test was applied to
control changes in thrombogram parameters in the presence or in the
absence of cancer cells in plasma as well as in the different
experimental conditions described above. Results are shown as mean
± SD. The level of statistical significance was set at 0.05. The
inhibition of thrombin generation (TG) was calculated by the
formula: Inhibition of TG = (1-TGcells/TGcontrol) %. Two-sided
values of P<0.05 were considered as statistically significant.
SPSS statistical soTFware package was used for statistical
analysis.
Results
After the initial screening of several cancer cell
lines, the BXPC3 and MCF7 cancer cell lines were selected because
they were from different organs (pancreas and breast) and induced
significantly different intensity of thrombin generation. For
technical reasons it was not possible to obtain cells from healthy
pancreatic or breast tissue. So we used primary human umbilical
vein cells (HUVEC) as normal control experiment.
In preliminary experiments we found that after 24 h
of incubation, the BXPC3 cells were at 70% confluence and the MCF7
cells were at 80% confluence. In these conditions the two cancer
cell lines gave similar intensity of thrombin generation. We also
ruled out any potential interference of trypsin on cell activity by
assessing thrombin generation either with cells which were moved by
the flasks mechanically or by exposure to trypsin. We also
confirmed that the concentration of fetal bovine serum (ranging
from 1 to 10%) and the incubation time of cells in the plaque
(ranging from 5 to 72 h) did not significantly influence their
procoagulant potential on thrombin generation. For the studied
cancer cell lines, a plateau effect on thrombin generation was
observed at cell numbers equal or higher than 50 cells/μl
(data not shown). Cells were used in the experiments only if the
apoptotic cell number was lower that 2% of the whole cells
count.
Enhancement of thrombin generation by
BXPC3 and MCF7
As previously showed, the contact of cancer cells
with normal PPP resulted in a significant increase of the Peak and
the MRI and a reduction of the lag-time and ttPeak as compared to
normal PPP without cancer cells. At equal numbers of cells the MCF7
had less potent procoagulant activity as compared to the BXPC3
cells. The parameters of thrombogram assessed in the presence of
HUVEC were not significantly different as compared to the control
experiment (PPP without cells) (Table
I).
 | Table IVariability of the pro-coagulant
effect of cancer cells on thrombin generation of normal human
plasma. Thrombin generation was triggered by addition of
CaCl2. |
Table I
Variability of the pro-coagulant
effect of cancer cells on thrombin generation of normal human
plasma. Thrombin generation was triggered by addition of
CaCl2.
| lag-time (min) | tt-Peak (min) | Peak (nM) | MRI (nM/min) |
|---|
| MCF7 cells | 6.1±0.9a | 9.6±1.1a | 121±22a | 34±6a |
| BXPC3 cells | 4.1±1.1a,b | 6.9±1.3a,b | 199±13a,b | 71±7a,b |
| HUVEC | 8.6±1.1 | 15.2±1.4 | 86±11 | 13±4 |
| Control | 9.6±1.2 | 16.3±1.5 | 118±9 | 17±4 |
Both BXPC3 and MCF7 cells expressed significantly
higher levels of TFa (1.42±0.10 and 0.82±0.08 pM, respectively) as
compared to the normal plasma and the HUVEC (0.20±0.05 and
0.23±0.02 pM, respectively; P<0.05). The BXPC3 cells expressed
significantly higher levels of TFa as compared to the MCF7 cells
(P<0.05). Data are summarized in Table II.
 | Table IITissue factor activity (measured by a
clotting based assay) and TF expression (measured by flow
cytometry) of the cells and the respective microparticles. |
Table II
Tissue factor activity (measured by a
clotting based assay) and TF expression (measured by flow
cytometry) of the cells and the respective microparticles.
| TFa (pM) | TF expression
(MIF) |
|---|
| BXPC3 cells | 1.42±0.1a | 395±132a |
| BXPC3 MPs | 33±2b,c | 491±24b,c |
| MCF7 cells | 0.82±0.08a | 95±20a |
| MCF7 MPs | 4.6±0.08b,c | 378±25b,c |
| HUVEC | 0.23±0.02a | 5±10 |
| HUVEC MPs | 0.34±0.1 | 8±2 |
| normal PPP | 0.2±0.05 | 0 |
Exposure of tissue factor and
phosphatidyserine by cancer cells
Flow cytometry analysis was performed to BXPC3 and
MCF7 cells. The labeling of cancer cells by an anti-TF antibody
showed the presence of TF on the membrane of cancer cells.
Fluorogenic intensity was significantly higher on BXPC3 cells than
on MCF7 cells. The mean index of fluorescence (MIF) was 395±132 and
95±20 for BXPC3 and MCF7 cells, respectively. In the same
experiment on HUVEC traces of TF were detected (MIF=5±10). Data are
summarized in Table II.
The proportion of cells carrying TF was slightly
higher in BXPC3 than MCF7 cells (95±13 vs. 78±10%, respectively;
P=0.001). A significantly lower number of HUVEC expressed TF
(10±2%). The labeling of cancer cells with Annexin V documented the
absence of procoagulant phospholipids on the surface of the BXPC3
or MCF7 cells or the HUVEC (control experiment). The study by a
functional test did not detect phospholipids on these three types
of cells.
Procoagulant phospholipids expressed by
cancer cell-derived microparticles
Cancer cells in culture spontaneously release
procoagulant microparticles into the conditioned medium. Flow
cytometry assessment using Annexin V labeling showed the presence
of procoagulant phospholipids at both BXPC3 and MCF7 derived
microparticles, which showed similar MIF (30±4 and 49±6,
respectively; P>0.05). The percentage of phosphatidylserine
expression was the same in both BXPC3 and MCF7 derived
microparticles (56±3 and 57±5%). No procoagulant phospholipids were
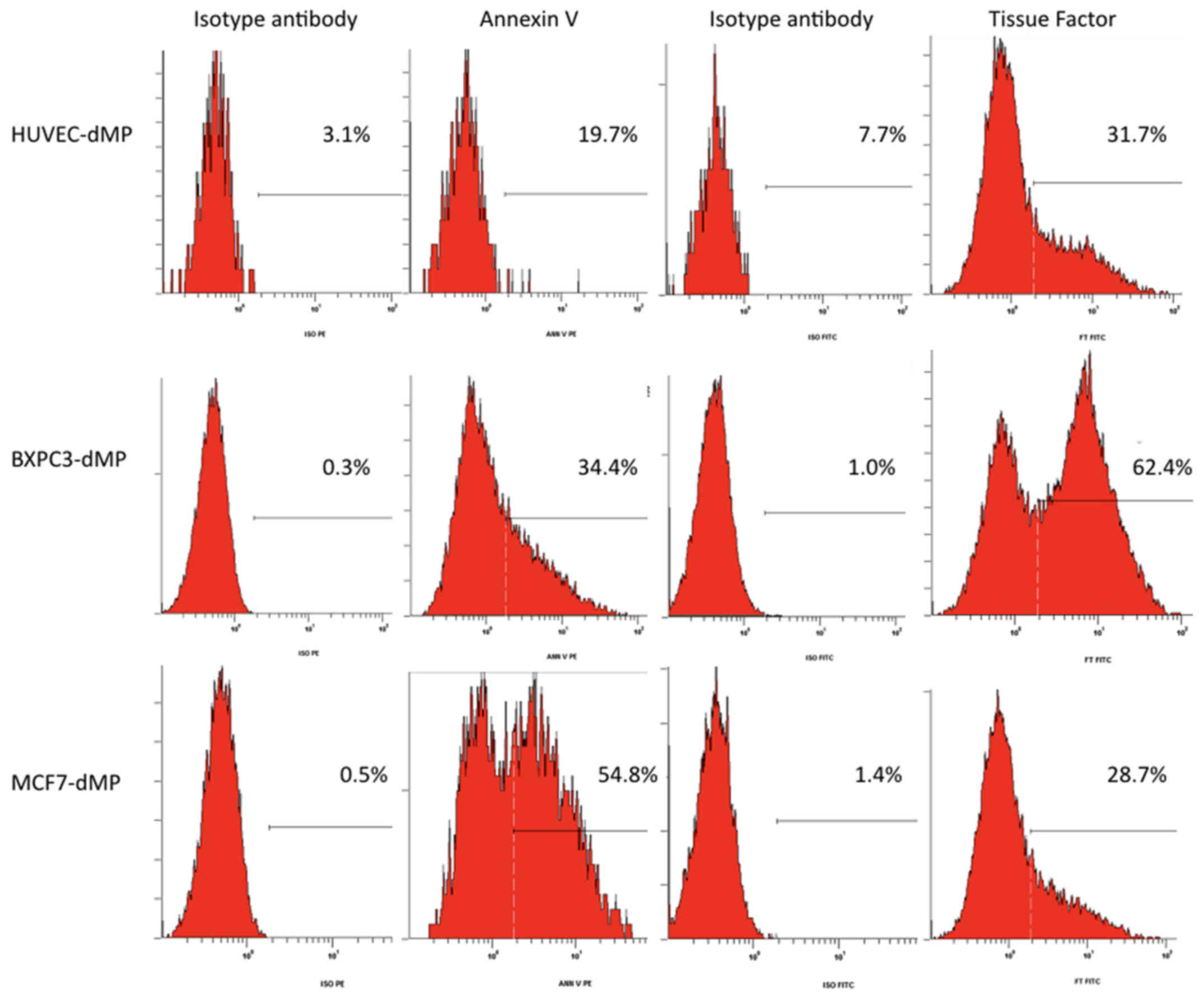
detected on microparticles derived from HUVEC (Fig. 1).
Tissue factor expression by cancer
cell-derived microparticles
Flow cytometry analysis showed the presence of TF on
microparticles from BXPC3 and MCF7 cells (MIF: 491±24 and 378±25,
respectively; P>0.05) (Fig. 1).
Only traces of TF were detected on HUVEC derived microparticles
(MIF=8±2). The proportion of carrier cells was identical for BXPC3
and MCF7. Data are summarized in Table II.
TF expressed by CaCe-dMP had procoagulant activity.
The levels of TFa expressed by BXPC3 and MCF7 derived
microparticles were 33±2 and 4.6±0.5 pM, respectively; P=0.001. The
levels of TFa expressed by HUVEC-derived microparticles was
0.34±0.1 pM. Data are summarized in Table II. The CaCe-dMP expressed
approximately 20-fold higher levels of TFa as compared to the
respective cells.
Effect of cancer cell-derived
microparticles on thrombin generation
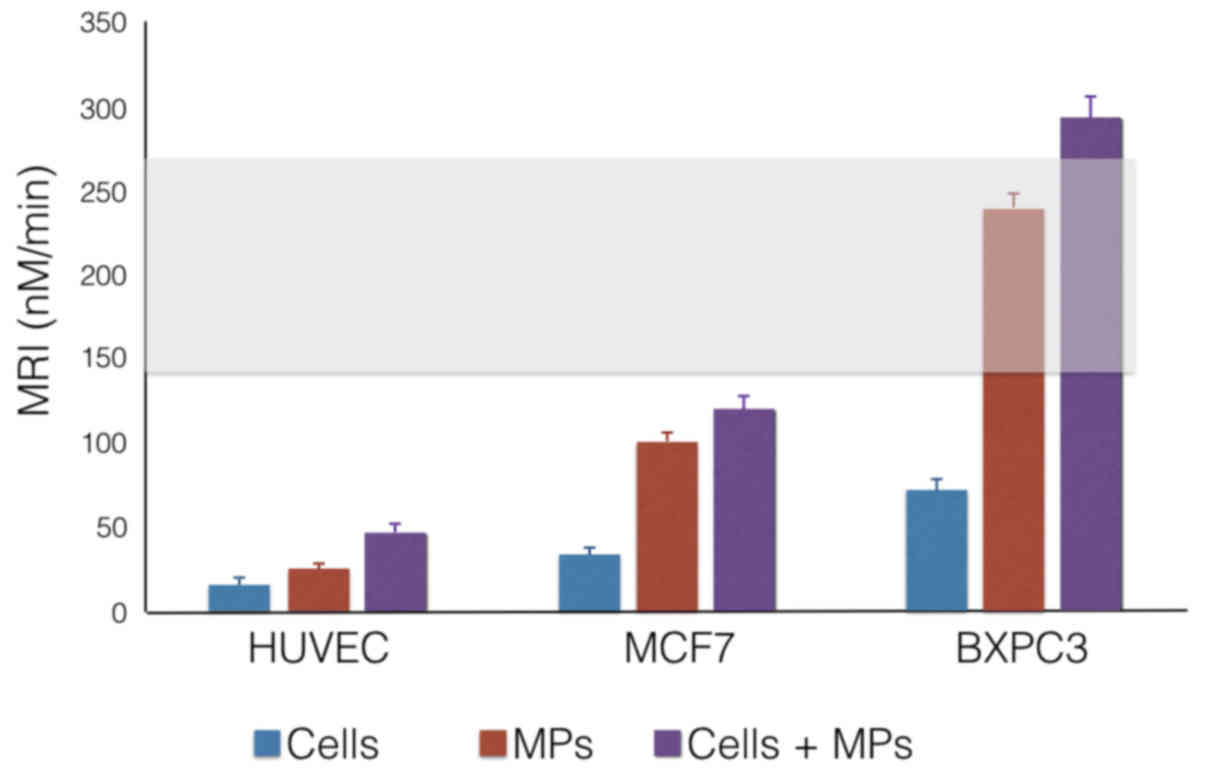
The addition of CaCe-dMP to normal PPP led to a
significant increase of the Peak and MRI, as well as a reduction of
the lag-time and ttPeak in comparison to the control (Table III). This effect was more
important for BXPC3 than MCF7 derived microparticles. No
significant effect was observed for HUVEC-derived microparticles
which did not show any significant effect on thrombin generation as
compared to the control.
 | Table IIIImpact of CaCe-dMP on thrombin
generation in normal PPP. |
Table III
Impact of CaCe-dMP on thrombin
generation in normal PPP.
| Pool | HUVEC | BXPC3 cell | MCF7 cells | MPs HUVEC | CaCe-dMP BXPC3 | CaCe-dMP MCF7 | HUVEC + MPsHUV | BXPC3 +CaCe-dMP
BXPC3 | MCF7 +CaCe-dM
MCF7 |
|---|
| Lag-time (min) | 8.9±1.6 | 7.7±0.9 | 4.8±0.6 | 7.5±0.7 | 8.2±0.9 | 2.2±0.6f | 6.1±0.4e | 7.9±0.9 | 1.2±0.5c | 5.1±0.7b |
| tt-Peak (min) | 14.6±1.3 | 12.1±0.9 | 5.7±08 | 9.3±1.0 | 12.3±1.4 | 3.3±0.4f | 8.2±0.8e | 11.7±0.9 | 2.6±0.6c | 6.9±1.1b |
| Peak (nM) | 136±11 | 150±11 | 220±12 | 179±11 | 142±11 | 380±14f | 210±11e | 166±10 | 410±12c | 220±10a |
| MRI (nM/min) | 24±9 | 35±10 | 244±13 | 99±12 | 32±10 | 247±14f | 100±13e | 45±10 | 294±11a | 120±12b |
Effect of the association of cancer cells
and cancer cell-derived microparticles on thrombin generation
Thrombin generation induced by cancer cells in the
presence of CaCe-dMP from the same cell type in normal PPP showed a
significant increase in Peak and MRI, as well as a reduction of the
lag-time and ttPeak as compared to the control experiment (Table III). A significant variability in
the effect of CaCe-dMP of different types of cancer cells on
thrombin generation in human plasma was observed. At an equal
number of cells, MCF7 derived microparticles had lower procoagulant
activity than BXPC3 derived microparticles. Under the same
conditions, the parameters of the thrombogram in the presence of
HUVEC (control) did not differ significantly from the control
experiments without cells or in the presence of microparticles
(Table III).
Thrombin generation triggered by cancer cells in
association with their respective microparticles amplified the
procoagulant efficiency of BXPC3 and MCF7 cancer cells. In contrast
to cancer cells, the normal cells (HUVEC) in association with
HUVEC-derived microparticles had no effect on thrombin generation.
The association of cancer cells with their homologus microparticles
resulted in a significant increase in thrombin generation Peak and
MRI, as well as a reduction of lag-time and ttPeak as compared to
the control. At an equal number of cells, MCF7 derived
microparticles had a lower procoagulant activity as compared to
BXPC3 derived microparticles. Under the same conditions, the
parameters of the thrombogram in the presence of HUVEC did not
differ significantly from the control experiments (Fig. 2).
Addition of an anti-TF monoclonal antibody in PPP in
the presence of CaCe-dMP or HUVEC derived microparticles
significantly increased the lag-time and ttPeak with decreased MRI
and Peak of thrombin compared to the assay without anti-TF
antibody. In the presence of BXPC3 derived MP, inhibition of
thrombin generation by the anti-TF antibody was 74% as compared to
the control experiment (without any antibody addition). The anti-TF
antibody also partially reversed the thrombin generation triggered
by MCF7 cells (42%; P<0.05 vs. BXPC3 derive microparticles). The
anti-TF antibody reduced by 10% thrombin generation triggered by
HUVEC (Table IV).
 | Table IVEffect of an anti-TF antibody on the
generation of thrombin in the presence of MPs of BXPC3, MCF7 and
HUVEC (n=3). |
Table IV
Effect of an anti-TF antibody on the
generation of thrombin in the presence of MPs of BXPC3, MCF7 and
HUVEC (n=3).
| Thrombin generation
of MPs in PPP in presence of anti-TF antibody with MP reagent (no
TF/PPL 4 μM)
|
|---|
Lag-time (min)
| tt-Peak(min)
| Peak (nM)
| MRI (nM/ml)
|
|---|
| MP of HUVEC | MP of BXPC3 | MP of MCF7 | MP of HUVEC | MP of BXPC3 | MP of MCF7 | MP of HUVEC | MP of BXPC3 | MP of MCF7 | MP of HUVEC | MP of BXPC3 | MP of MCF7 |
|---|
| Pool | 11.1±0.9 | 2.2±0.6 | 6.1±0.4 | 17.1±0.9 | 3.3±0.4 | 8.2±0.8 | 146±11 | 380±14 | 210±11 | 35±10 | 247±11 | 100±13 |
| Pool +
TF9-10H10 | 13.2±1.3 | 9.2±1.0b | 8.7±0.7a | 20.3±0.9 | 12.3±0.9b | 13.6±1.8a | 132±12 | 98±10b | 121±12b | 22±8 | 31±9b | 26±7b |
Discussion
Cancer associated thrombosis is a common
complication of malignancy representing the second most frequent
cause of death in cancer patients (1,2).
However, the pathophysiology CAT is not entirely understood.
Microparticles produced by tumour cells and their microenvironment
generate considerable interest since they modulate blood
coagulation and fibrinolysis and may contribute to thrombosis
(17,18,27–31).
The link between cancer and hypercoagulability is surrounded by
some critical questions: i) Are the mechanisms of CAT specific for
the type of cancer? ii) Is there any link between this cancer
type-specific thrombosis with the biological properties of the
cancer cells? iii) Which are the implications of blood borne
cancer-derived microparticles on the hypercoagulable state?
It is well established that cancer cells express TF;
the major trigger of blood coagulation (8,9,32).
Using an original and validated experimental system that allows the
study of thrombin generation triggered directly by cancer cells we
investigated if the release of microparticles by cancer cells
modify the procoagulant potential of plasma. We previously
demonstrated that the pancreas adenocarcinoma cells BXPC3 express
significantly higher amounts of TF as compared to the breast cancer
cells MCF7 and the HUVEC cells (15). The levels of TF expressed by each
type of cancer cells are correlated with their effect on thrombin
generation (15). We recently
showed that cancer cells, i.e. BXPC3 and MCF7 activate blood
coagulation via TF as well as via the activation of FXII (33). However, the procoagulant activity
of cancer cells is not sufficient to induce hypercoagulability
(33). Cancer cell-derived TF can
activate coagulation by binding the serine protease FVII/VIIa to
form an activating complex that promotes thrombin generation close
to the tumor (17). Microparticles
released by tumor cells could exhibit negatively charged
phospholipids and TF which enhance thrombin generation (34–37).
Zwicker et al observed elevated levels of TF-positive
microparticles in the plasma of patients with pancreatic, breast,
colorectal, ovarian, and non-small cell lung cancer (9,38).
Furthermore, approximately 50% of microparticles in the patients
with cancer expressed the tumor antigen mucin 1 (MUC-1) on their
surface, which suggests that they derived from the tumor.
In order to estimate the specific role of CaCe-dMP
in coagulation activation initiated by cancer cells we isolated the
microparticles present in the conditioned medium from the two
cancer cell lines (BXPC3 and MCF7) and normal cells (HUVEC). On
these microparticles, we first studied the expression of
procoagulant phospholipids and TF using Annexin V and an anti-TF
antibody using flow cytometry method. We also determined the
expression of TFa using a clotting based assay. Subsequently, we
studied thrombin generation induced by cultures of BXPC3 or MCF7
cells in the presence of the corresponding microparticles (isolated
from the respective conditioned medium). Phosphatidylserine was
identified on the surface of CaCe-dMP, unlike the cancer cells
themselves. Microparticle-derived MCF7 cells exhibited lower
amounts of phosphatidylserine as compared to BXPC3 derived
microparticles.
Both BXPC3 and MCF7 cells express abundant amounts
of TF. The TF density at the membrane of the BXPC3 cells and the
BXPC3 derived microparticles was significantly higher than that
measured at the membrane of the MCF7 cells and MCF7 derived
microparticles. This difference was directly correlated with the
difference in the procoagulant potential of BXPC3 and MCF7 cells.
Expression of TFa by BXPC3 cells was significantly superior as
compared to MCF7 cells. The expression of TFa by the microparticles
was significantly higher to that observed on the cancer cells, in a
ratio of approximately 30 for BXPC3 and 5 for MCF7.
In summary, CaCe-dMP provide abundantly procoagulant
phospholipids and active TF allowing the initiation and
amplification of thrombin generation. Culture of the cancer cells
with their microparticles resulted in a significant acceleration of
the initiation phase and amplification of the propagation phase of
thrombin generation. In accordance to the experiments with the
corresponding cancer cells the BXPC3-derived microparticles showed
higher procoagulant potential as compared to the microparticles
derived from MCF7 cells. Noteworthy, excessive increase of thrombin
generation at levels which correspond to a hypercoagulable state
were observed when BXPC3 cells and the corresponding microparticles
were combined. Microparticles, when they are cell-free present in
the plasma, result in a significant increase in thrombin generation
as compared to the control (normal PPP without MPs). This effect is
more pronounced for the BXPC3 than the MCF7 CaCe-dMP; a phenomenon
that we can relate to the expression of TF.
In this study, we demonstrated that TF specifically
released from tumor cells exhibits procoagulant activity in
vitro. We found that the procoagulant activities derived from
cancer cells in vitro was mainly associated with MP. The
procoagulant activity associated with these MP was completely
dependent on TF (and PS), as it was abolished by anti-TF (or
Annexin V). These results indicate that it is important to measure
levels of MP TF activity, and not simply levels of PS-positive MPs.
To the best of our knowledge, the present study demonstrates for
the first time that cancer related hypercoagulablity is the
resultant of the combined procoagulant effect of cancer cells with
the procoagulant microparticles and active TF which are released by
the cancer cells themselves. In addition, we show that the
intensity of the hypercoagulablity is related with the histological
type of the cancer cells. This concept is valuable for the cancer
cells as well as for the cancer cell derived elements present into
the microenvironment.
In conclusion, all these data show that: i) cancer
cells by themselves do not possess sufficient power to generate a
state of hypercoagulability; ii) cancer cells 'enrich' the
microenvironment with procoagulant elements, especially
procoagulant microparticles which express both TF and procoagulant
phospholipids, iii) the association of cancer cells and
microparticles is necessary for a state of hypercoagulability, at
the level of the tumor microenvironment and that the intensity of
this hypercoagulability depends on the histological type of the
cancer cells.
Glossary
Abbreviations
Abbreviations:
|
VTE
|
venous thromboembolism
|
|
TF
|
tissue factor
|
|
MCF7
|
Michigan Cancer Foundation 7, human
breast cancer cells
|
|
BXPC3
|
human pancreatic adenocarcinoma
cells
|
|
FXII
|
coagulation factor XII
|
|
TFa
|
tissue factor activity
|
|
PPP
|
platelet poor plasma
|
|
MRI
|
mean rate index
|
|
HUVEC
|
primary human umbilical vein
endothelial cells
|
|
TG
|
thrombin generation
|
|
UNL
|
upper normal limit
|
|
ttPeak
|
time to peak
|
|
PPL
|
procoagulant phospholipids
|
|
CaCe-dMP
|
cancer cells derived
microparticles
|
|
Peak
|
maximal concentration of thrombin
|
|
CAT
|
cancer associated thrombosis
|
|
MP
|
microparticles
|
References
|
1
|
Khorana AA, Francis CW, Culakova E,
Kuderer NM and Lyman GH: Thromboembolism is a leading cause of
death in cancer patients receiving outpatient chemotherapy. J
Thromb Haemost. 5:632–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Falanga A and Russo L: Epidemiology, risk
and outcomes of venous thromboembolism in cancer. Hamostaseologie.
32:115–125. 2012. View
Article : Google Scholar
|
|
3
|
Trousseau A: Phlegmasia Alba Dolens.
Clinique Medicale de l'Hotel-dieu de Paris. 3. JB Balliere et Fils;
Paris: pp. 654–712. 1865
|
|
4
|
Sørensen HT, Mellemkjaer L, Olsen JH and
Baron JA: Prognosis of cancers associated with venous
thromboembolism. N Engl J Med. 343:1846–1850. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Riewald M and Ruf W: Mechanistic coupling
of protease signaling and initiation of coagulation by tissue
factor. Proc Natl Acad Sci USA. 98:7742–7747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aharon A and Brenner B: Microparticles,
thrombosis and cancer. Best Pract Res Clin Haematol. 22:61–69.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Del Conde I, Bharwani LD, Dietzen DJ,
Pendurthi U, Thiagarajan P and López JA: Microvesicle-associated
tissue factor and Trousseau's syndrome. J Thromb Haemost. 5:70–74.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tilley RE, Holscher T, Belani R, Nieva J
and Mackman N: Tissue factor activity is increased in a combined
platelet and microparticle sample from cancer patients. Thromb Res.
122:604–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zwicker JI: Predictive value of tissue
factor bearing microparticles in cancer associated thrombosis.
Thromb Res. 125(Suppl 2): S89–S91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Castellana D, Toti F and Freyssinet JM:
Membrane microvesicles: Macromessengers in cancer disease and
progression. Thromb Res. 125(Suppl 2): S84–S88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Falanga A: Thrombophilia in cancer. Semin
Thromb Hemost. 31:104–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kakkar AK, Lemoine NR, Scully MF, Tebbutt
S and Williamson RC: Tissue factor expression correlates with
histological grade in human pancreatic cancer. Br J Surg.
82:1101–1104. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ueno T, Toi M, Koike M, Nakamura S and
Tominaga T: Tissue factor expression in breast cancer tissues: Its
correlation with prognosis and plasma concentration. Br J Cancer.
83:164–170. 2000.PubMed/NCBI
|
|
14
|
Vrana JA, Stang MT, Grande JP and Getz MJ:
Expression of tissue factor in tumor stroma correlates with
progression to invasive human breast cancer: Paracrine regulation
by carcinoma cell-derived members of the transforming growth factor
beta family. Cancer Res. 56:5063–5070. 1996.PubMed/NCBI
|
|
15
|
Gerotziafas GT, Galea V, Mbemba E,
Khaterchi A, Sassi M, Baccouche H, Prengel C, van Dreden P, Hatmi
M, Bernaudin JF, et al: Tissue factor over-expression by human
pancreatic cancer cells BXPC3 is related to higher prothrombotic
potential as compared to breast cancer cells MCF7. Thromb Res.
129:779–786. 2012. View Article : Google Scholar
|
|
16
|
Thomas GM, Panicot-Dubois L, Lacroix R,
Dignat-George F, Lombardo D and Dubois C: Cancer cell-derived
microparticles bearing P-selectin glycoprotein ligand 1 accelerate
thrombus formation in vivo. J Exp Med. 206:1913–1927. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gheldof D, Mullier F, Bailly N, Devalet B,
Dogné JM, Chatelain B and Chatelain C: Microparticle bearing tissue
factor: A link between promyelocytic cells and hypercoagulable
state. Thromb Res. 133:433–439. 2014. View Article : Google Scholar
|
|
18
|
Lacroix R and Dignat-George F:
Microparticles as a circulating source of procoagulant and
fibrinolytic activities in the circulation. Thromb Res. 129(Suppl
2): S27–S29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hron G, Kollars M, Weber H, Sagaster V,
Quehenberger P, Eichinger S, Kyrle PA and Weltermann A: Tissue
factor-positive microparticles: Cellular origin and association
with coagulation activation in patients with colorectal cancer.
Thromb Haemost. 97:119–123. 2007.PubMed/NCBI
|
|
20
|
Tesselaar ME, Romijn FP, Van Der Linden
IK, Prins FA, Bertina RM and Osanto S: Microparticle-associated
tissue factor activity: A link between cancer and thrombosis? J
Thromb Haemost. 5:520–527. 2007. View Article : Google Scholar
|
|
21
|
Wang JG, Geddings JE, Aleman MM, Cardenas
JC, Chantrathammachart P, Williams JC, Kirchhofer D, Bogdanov VY,
Bach RR, Rak J, et al: Tumor-derived tissue factor activates
coagulation and enhances thrombosis in a mouse xenograft model of
human pancreatic cancer. Blood. 119:5543–5552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Van Dreden P, Rousseau A, Savoure A,
Lenormand B, Fontaine S and Vasse M: Plasma thrombomodulin
activity, tissue factor activity and high levels of circulating
procoagulant phospholipid as prognostic factors for acute
myocardial infarction. Blood Coagul Fibrinolysis. 20:635–641. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rousseau A, Favier R and Van Dreden P:
Elevated circulating soluble thrombomodulin activity, tissue factor
activity and circulating procoagulant phospholipids: New and useful
markers for pre-eclampsia? Eur J Obstet Gynecol Reprod Biol.
146:46–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schneider P, Van Dreden P, Rousseau A,
Kassim Y, Legrand E, Vannier JP and Vasse M: Increased levels of
tissue factor activity and procoagulant phospholipids during
treatment of children with acute lymphoblastic leukaemia. Br J
Haematol. 148:582–592. 2010. View Article : Google Scholar
|
|
25
|
Gerotziafas GT, Depasse F, Busson J,
Leflem L, Elalamy I and Samama MM: Towards a standardization of
thrombin generation assessment: The influence of tissue factor,
platelets and phospholipids concentration on the normal values of
Thrombogram-Thrombinoscope assay. Thromb J. 3:162005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lacroix R, Judicone C, Poncelet P, Robert
S, Arnaud L, Sampol J and Dignat-George F: Impact of pre-analytical
parameters on the measurement of circulating microparticles:
Towards standardization of protocol. J Thromb Haemost. 10:437–446.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bouvy C, Gheldof D, Chatelain C, Mullier F
and Dogné JM: Contributing role of extracellular vesicles on
vascular endothelium haemostatic balance in cancer. J Extracell
Vesicles. 3:102014.
|
|
28
|
Key NS, Chantrathammachart P, Moody PW and
Chang JY: Membrane microparticles in VTE and cancer. Thromb Res.
125(Suppl 2): S80–S83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chew HK, Wun T, Harvey D, Zhou H and White
RH: Incidence of venous thromboembolism and its effect on survival
among patients with common cancers. Arch Intern Med. 166:458–464.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prandoni P, Falanga A and Piccioli A:
Cancer and venous thromboembolism. Lancet Oncol. 6:401–410. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thaler J, Ay C, Mackman N, Metz-Schimmerl
S, Stift J, Kaider A, Müllauer L, Gnant M, Scheithauer W and
Pabinger I: Microparticle-associated tissue factor activity in
patients with pancreatic cancer: Correlation with
clinicopathological features. Eur J Clin Invest. 43:277–285. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thaler J, Koder S, Kornek G, Pabinger I
and Ay C: Microparticleassociated tissue factor activity in
patients with metastatic pancreatic cancer and its effect on fibrin
clot formation. Transl Res. 163:145–150. 2014. View Article : Google Scholar
|
|
33
|
Rousseau A, Van Dreden P, Larsen A, Sabbah
M, Elalamy I and Gerotziafas GT: Differential contribution of
tissue factor and Factor XII to thrombin generation triggered by
breast and pancreatic cancer cells. Int J Oncol. In press.
|
|
34
|
Castellana D, Kunzelmann C and Freyssinet
JM: Pathophysiologic significance of procoagulant microvesicles in
cancer disease and progression. Hamostaseologie. 29:51–57.
2009.PubMed/NCBI
|
|
35
|
Yu JL and Rak JW: Shedding of tissue
factor (TF)-containing microparticles rather than alternatively
spliced TF is the main source of TF activity released from human
cancer cells. J Thromb Haemost. 2:2065–2067. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Geddings JE, Hisada Y, Boulaftali Y, Getz
TM, Whelihan M, Fuentes R, Dee R, Cooley BC, Key NS, Wolberg AS, et
al: Tissue factor-positive tumor microvesicles activate platelets
and enhance thrombosis in mice. J Thromb Haemost. 14:153–166. 2016.
View Article : Google Scholar :
|
|
37
|
Falanga A, Panova-Noeva M and Russo L:
Procoagulant mechanisms in tumour cells. Best Pract Res Clin
Haematol. 22:49–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zwicker JI, Liebman HA, Neuberg D, Lacroix
R, Bauer KA, Furie BC and Furie B: Tumor-derived tissue
factor-bearing microparticles are associated with venous
thromboembolic events in malignancy. Clin Cancer Res. 15:6830–6840.
2009. View Article : Google Scholar : PubMed/NCBI
|