Introduction
Multiple myeloma (MM) is a B cell neoplasm
characterized by the aberrant clonal expansion of plasma cells
(PCs) within the bone marrow (1,2). It
is an age-dependent malignancy and leads to osteolytic lesions,
immunodeficiency and renal failure. Multiple myeloma accounts for
20% of all deaths from hematological cancers and is the second most
common hematological malignancy worldwide (3). Despite advances in novel therapeutics
targeting specific myeloma disease-driven pathways, multiple
myeloma remains a largely incurable disease with a median survival
of 62 months due to relapse and drug resistance (4). Therefore, understanding the
mechanisms associated with multiple myeloma pathogenesis is
necessary for the development of future therapeutic strategies.
The bone marrow (BM) microenvironment, an intricate
and dynamic niche composed of bone marrow stromal cells (BMSCs),
fibroblasts, hematopoietic stem cells, progenitor cells,
endothelial cells, immune cells, and the extracellular matrix
(5), supports myeloma cell growth
and contributes to disease progression. Previous studies reported
that stromal cells directly promoted the progression of multiple
myeloma and drug resistance through cell-to-cell contact and
cytokine stimulation (6). Adhesion
molecules on the surface of the myeloma cells bind to extracellular
matrix proteins and BMSCs (7,8).
Steroid receptor coactivator-3 (SRC3), a
multifunctional transcriptional coactivator, is a member of the
p160 steroid receptor coactivator family (9,10).
SRC3 acts as a bridge between the hormone-activated nuclear
receptors (NRs), other coregulators, and the basal transcriptional
machinery to regulate target gene transcription and impact multiple
growth factor pathways (11,12).
SRC3 plays an important role in physiological and pathological
functions, and physiological processes including somatic growth,
sexual maturation, energy metabolism, female reproductive function,
tumorigenesis (9,12). SRC3 is amplified in different
cancers and is speculated to be associated with the initiation
and/or progression of carcinomas (10–12).
In addition, SRC3 influences the radiosensitivity of hematopoietic
cells, hematopoietic ability and bone marrow microenvironment
(13,14). However, it remains unclear how SRC3
in BMSCs change the bone marrow microenvironment, and promote the
proliferation and migration of multiple myeloma.
Connexin 43 (Cx43), a predominant gap junction
protein expressed in BMSCs is phosphorylated by Mitogen-activated
protein kinase (MAPK) and MAPK is also thought to regulate Cx43
function (15). Gap junctions
(GJs) are transmembrane domains that allow direct intercellular
communication between neighboring cells. Aberrant function of GJs
and reduction in cell-cell coupling via GJs is associated with many
pathological conditions, including cancer (16). Cx43 is aberrantly expressed in
several cancers, such as liver cancer, prostate cancer, and breast
cancer. In breast cancer, therapeutic targeting of Cx43 exhibits
strong anticancer effects and few detrimental side effects
(17). In addition, Cx43 plays a
role in breast cancer cell proliferation, differentiation, and
migration (18,19). Therefore, in this study, we tested
our hypothesis that SRC3 in BMSCs mediate the bone marrow
microenvironment by regulating the expression of Cx43. Our in
vitro and in vivo studies suggest that overexpressed
SRC3 regulates Cx43 via the MAPK pathway to promote myeloma cell
growth.
Materials and methods
Multiple myeloma patients
Patients newly diagnosed (within 6 months) with
multiple myeloma (n=20, 14 male and 6 female) were recruited in
this study between April 2015 and March 2016 at The Third
Affiliated Daping Hospital. All patients had myeloma that was
classified as Durie-Salmon stage II or III and/or ISS stage ≥2. The
average age of all patients was 65 years. The basic characteristics
of multiple myeloma patients were as shown in Table I. This study was approved by the
Medical Ethics Committee of the Third Military Medical University.
Healthy donors were utilized as control samples. Serum from the
patients was collected for the following studies. All the patients
signed informed written consents in accordance with the Declaration
of Helsinki.
 | Table IBasic characteristics of MM
patients. |
Table I
Basic characteristics of MM
patients.
| Characteristic | No. of patients
(%) |
|---|
| Total | 20 (100) |
| Male | 14 (70) |
| Female | 6 (30) |
| Age >50
years | 20 (63.2) |
| Durie-Salmon stage
≥2 | 20 (100) |
| ISS stage ≥2 | 20 (100) |
Cell culture
The human multiple myeloma cell lines (RPMI-8226 and
U266) were purchased from American Type Culture Collection
(Manassas, VA, USA). The human bone marrow-mesenchymal stem cells
were obtained from Shanghai Cell Institute (Shanghai, China). The
cells were cultured at 37°C in a water-saturated atmosphere of 95%
air and 5% CO2 in RPMI-1640 supplemented with 10%
heat-inactivated Fetal Bovine Serum (FBS) (Gibco, Carlsbad, CA,
USA), 100 U/ml penicillin and 100 µg/ml streptomycin
(Amresco, Solon, OH, USA) or human bone marrow mesenchymal stem
cell complete medium (Guangzhou, China). For treatment with the P38
inhibitor SB202190, the cells were seeded at 2×105
cells/well in a 24-well plate, and incubated overnight at 37°C.
Then, the medium was replaced with fresh medium supplemented with
100 µM of SB202190 (Cell Signaling Technology, Danvers, MA,
USA), and the cells were cultured for an additional 4 h. Cells were
harvested for further studies.
Co-culture of myeloma cells and
BMSCs
The myeloma cells and BMSCs were cultured separately
until the fifth passage (50% confluence). Then, myeloma cells and
BMSC were co-cultured using a non-contact Transwell system (Corning
Inc., Corning, NY, USA). Myeloma cells (4×105 cells/ml)
were added to the top of BMSCs and incubated for 24 h. Then, cells
were separated and pelleted for the following studies.
Cell Counting Kit-8 (CCK-8) assay
CCK-8 assay was performed by Cell Counting Kit-8
(Dojindo). The RPMI-8226 cells were co-cultured and inoculated into
96-well plates at a density of 5×103 cells/ml, and 100
µl of culture medium was added into each well. After the
cells were cultured for 24, 48 and 72 h, 10 µl of CCK-8
reagent was added to each well and incubated for 2 h in 5%
CO2 at 37°C. The optical density (OD) was measured by a
microplate reader at 450/630 nm.
Lentivirus transfection
To knock down SRC3 expression in BMSCs, cells were
transfected with SRC3-specific short hairpin RNA (sh-SRC3) and
scrambled shRNA lentiviral vectors. The lentiviral vector green
fluorescent protein (GFP) was expressed in all lentiviruses and was
used to evaluate the transduction efficiency. The efficiency for
SRC3 knockdown was verified by detecting mRNA and protein levels of
SRC3. All lentiviral vectors were purchased from GeneChem
(Shanghai, China). Multiplicity of infection (MOI) refers to the
number of viral particles per cell. Transfections were performed in
BMSCs with MOI of 10:1 following the manufacturer's instructions.
The medium containing the vector was replaced by complete medium
after 10 h.
Cell transfection
The RPMI-8226 cells were transfected with control
plasmids (pcDNA3.1) or overexpression plasmids (pcDNA3.1-Cx43).
Both the pcDNA3.1 or pcDNA3.1-Cx43 plasmids were purchased from
Shanghai GenePharma, Co., Ltd. (Shanghai, China). Before
transfection, cells were cultured in 12-well plates till they
reached 70% confluency. Opti-MEM medium (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and Lipo 3000 reagent (Thermo Fisher
Scientific, Inc.) were used. After transfection for 48 h, cells
were collected for subsequent analysis.
Western blotting
Cells were lysed in RIPA buffer (Boston BioProducts,
Ashland, MA, USA) containing protease inhibitor cocktail (Roche
Diagnostics, Indianapolis, IN, USA). The protein concentration was
measured by BCA Protein Assay kit (Pierce, Rockford, IL, USA).
Equal amounts of protein samples (50 µg) were separated by
12% SDS-PAGE gel and transferred onto PVDF membranes (Millipore,
Billerica, MA, USA). The PVDF membranes were blocked in TBST
containing 2% BSA for 1 h, and then incubated with primary
antibodies specific for SRC3, Cx43, phosphorylated ERK (pERK), p38
(p-p38) and JNK (p-JNK). The antibodies were all from Cell
Signaling Technology. The bands were visualized using ECL Western
Blotting Detection Reagents (Kibbutz Beit Haemek, Israel) and
subjected to Alpha Innotech Flour Chem-FC2 imaging system (Alpha
Innotech).
Quantitative real-time PCR (qPCR)
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer's
instructions. A total of 1 µg RNA was used as the template
for single strand cDNA synthesis utilizing random primers and the
Primescript reverse transcriptase (M-MLV, Takara, Shiga, Japan).
The PCR amplification conditions were 95°C for 10 sec, 40 cycles of
94°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec on Applied
Biosystems (ABI) step-one plus sequence detection system (Applied
Biosystems, Foster City, CA, USA) with SYBR Green PCR Mix (iTAP,
Bio-Rad). The qPCR was performed for Cx43, SRC3 and GAPDH. And, the
related primer sequence information of Cx43, SRC3 and GAPDH is
listed in Table II. Analysis and
fold differences were determined using the comparative cycle
threshold (CT) method. Fold change was calculated from the ΔΔCT
values with the formula 2−ΔΔCT.
 | Table IIThe specific primer sequences for
qRT-PCR. |
Table II
The specific primer sequences for
qRT-PCR.
| ID | Sequence
(5′-3′) |
|---|
| Cx43 | F:
TGGTAAGGTGAAAATGCGAGG |
| R:
GCACTCAAGCTGAATCCATAGAT |
| SRC3 | F:
TTGTCTCAACCCACTTCCTT |
| R:
CCATACCTAGCTCCACTCATC |
| GAPDH | F:
TGTTCGTCATGGGTGTGAAC |
| R:
ATGGCATGGACTGTGGTCAT |
Immunohistochemistry
Immunohistochemistry (IHC) was performed as
previously described (20).
Tissues were fixed in 4% paraformaldehyde overnight, embedded in
paraffin and cut in 5 µm thickness for experiments. Tissue
sections were deparaffinized, hydrated, and then heated in a
steamer for 2.5 min to retrieve antigen. IHC was carried out with
primary monoclonal antibodies (anti-Cx43 or SRC3; Cell Signaling
Technology) at 1:500 dilutions for 50 min, followed by incubation
with goat anti-rabbit secondary antibody (Thermo Fisher Scientific,
Inc., Fremont, CA, USA). Negative control sections were incubated
with Phosphate Buffered Saline (PBS) instead of primary antibodies.
In this study, we set the negative control, but not the positive
control.
Transwell assay
RPMI-8226 cells (2×105) co-cultured with
either BMSC or MAPK inhibitor SB202190, were seeded into the
Transwell upper chamber (Corning Inc.). The upper chamber was added
with serum-free medium, and lower chamber was added with culture
medium containing 10% FBS. Moreover, we aseptically coated the
outside bottom surface of the inner chamber with Poly-D-lysine
polymers so as to promote cell adhesion to the solid substrate.
After 48 h, the non-migrated cells were removed from the chamber
using a cotton swab and migrated cells were fixed with 2%
paraformaldehyde and stained with crystal violet dye. Migrated
cells were observed under a phase contrast microscope, and
photographed.
Scratch-wound healing assay
Scratch-wound healing assays were performed using
RPMI-8226 cells after different co-culture. Cells
(5×105) were seeded into 6-well plates containing 5
µg/cm2 of surface area Poly-D-lysine polymers
solution and cultured. Scratches were made with a sterile 200
µl pipette tip to create a cell-free zone. Detached cells
were washed off with culture medium. Images of the scratch area
were captured after 24 h using an inverted phase contrast
microscope (Eclipse E400, Nikon Corp., Tokyo, Japan). The remaining
wound area was measured using ImageJ software [National Institutes
of Health (NIH), Bethesda, MD, USA] and normalized to time 0
wounds. Experiments were performed three times.
Immunofluorescence staining
PMI 8226 cells were stained according to previously
described protocols (21).
Briefly, cells were fixed in 4% paraformaldehyde and permeabilized
with 0.5% Triton X-100 for 15 min. The cells were washed with PBS,
blocked with 5% BSA in PBS for 1 h and incubated with primary
antibody overnight at 4°C. The primary rabbit polyoclonal anti-Cx43
antibody (dilution 1:1,000; Abcam, Cambridge, MA, USA) and
secondary goat anti-mouse IgG H&L (Texas Red®)
antibody (dilution 1:1,000; Abcam) were used. The coverslip was
subjected to DAPI (1:5,000, Sigma) nuclear counterstain. Cells were
visualized by a confocal laser fluorescence microscope (Zeiss LSM
700).
Hoechst staining
Cells were grown on coverslips and then rinsed with
PBS 3 times. Then, cells were incubated in the Hoechst labeling
solution for 30 min at room temperature. Cells were further rinsed
with PBS 3 times. The coverslips were mounted and images were
obtained at an excitation wavelength of 353 nm and an emission
wavelength of 483 nm.
In vivo tumor growth study
The animal study was approved by the Medical Ethics
Committee of the Third Military Medical University in accordance
with the Guide for the Care and Use of Laboratory Animals (NIH
publication no. 80-23, revised 1996) and animals were treated
according to the institutional guidelines. Male nude mice aged
between 6–8 weeks were purchased from Shanghai Laboratory Animal
Center of China. Each nude mouse was injected with 100 µl
cell suspension containing 3×106 RPMI-8226 cells and
3×105 MSC subcutaneously into the right flank for the
following groups: A, MM+MSC (RPMI-8226 cells, sh-SRC3-MSC); B,
MM+sh-SRC3-MSC+pcDNA3.1 (pcDNA3.1-RPMI-8226 cells, sh-SRC3-MSC); C,
MM+sh-SRC3-MSC+Cx43 (Cx43-RPMI-8226 cells, sh-SRC3-MSC); D,
MM+sh-SRC3-MSC+inhibitor (pcDNA3.1-RPMI-8226 cells+ SB202190,
sh-SRC3-MSC). The tumor size was measured by calipers and the tumor
volume calculated with the following formula: V =
(length)2 × (width)/2. After 28 days, mice were
sacrificed by intraperitoneal administration of an overdose of
anaesthetic drug cocktail (240 mg/kg) followed by cervical
dislocation. The xenografted tumor tissues were excised and
prepared for further analysis.
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) staining
To detect the number of apoptotic nuclei, TUNEL
staining was performed. The tumor tissue sections were incubated
with permeabilization solution and washed with PBS 2 times. Then,
the samples were incubated with TUNEL (Roche Applied Science,
Indianapolis, IN, USA) for 60 min at 37°C. The samples were further
stained with DAPI (1:5,000, Sigma) nuclear counterstain. Cells were
visualized by a confocal laser fluorescence microscope (Zeiss LSM
700).
Statistical analysis
All results are presented as means ± SEM of at least
three independent experiments. Student's t-test, Mann-Whitney U
test and Log-rank test were used to assess differences between two
groups. A P-value of <0.05 was considered to be statistically
significant.
Results
Expression of Cx43 in multiple myeloma
samples and cell lines
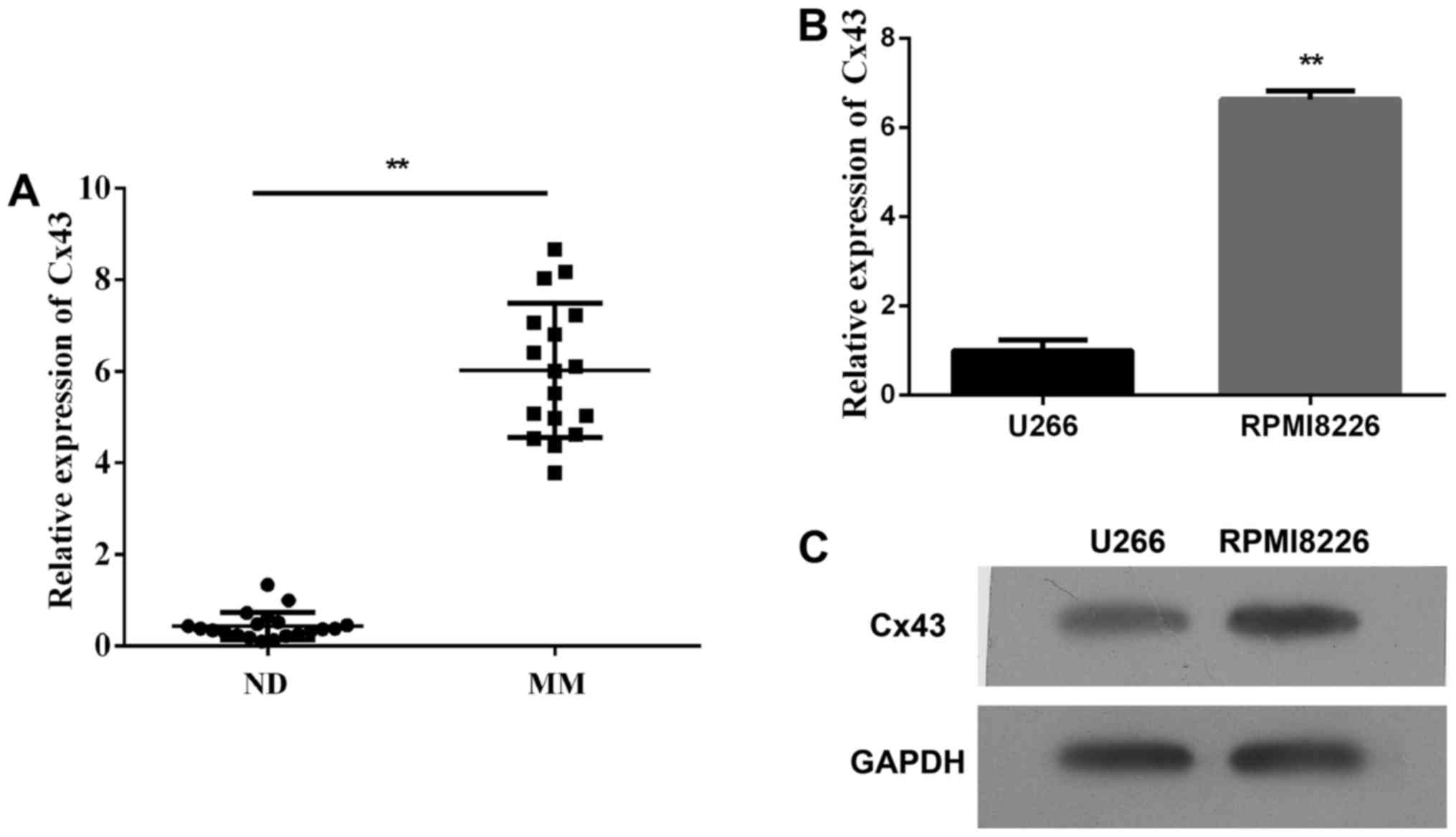
First, we detected the plasma expression levels of
Cx43 circulating in patients with multiple myeloma and found that
the plasma level of Cx43 significantly increased in patients with
newly diagnosed multiple myeloma within 6 months compared with
healthy donors (Fig. 1A,
P<0.01). Second, we assessed the expression of Cx43 in human
multiple myeloma cell lines (RPMI-8226 and U266). As shown in
Fig. 1B and C, both MM cell lines
expressed Cx43, and the mRNA and protein levels of Cx43 in
RPMI-8226 cells were higher than U266 cells. It could be that the
expression of Cx43 reduced in the late stages of B cell
differentiation. The results are consistent with the data published
by Zhang et al (22).
SRC3 expressed in BMSCs is involved in
the proliferation and migration of multiple myeloma cells
Evidence from the literature suggests that BMSCs
promote the proliferation and migration of multiple myeloma cells
and contribute to resistance to chemotherapy (23,24).
Furthermore, SRC3 influences the radiosensitivity of hematopoietic
cells, hematopoietic ability and bone marrow microenvironment
(13,14). We wanted to investigate if SRC3 in
BMSCs are involved in promoting the proliferation and migration of
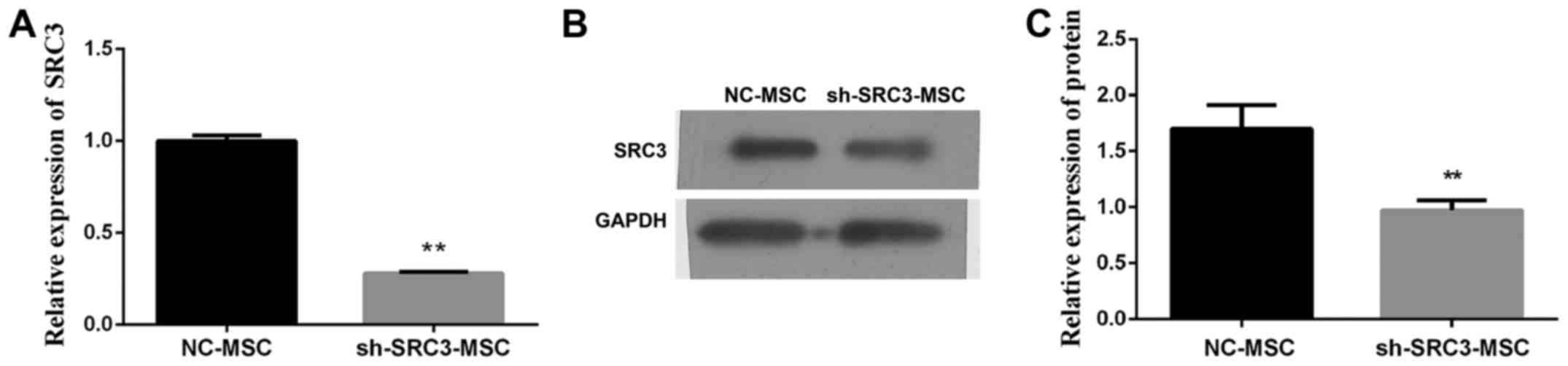
multiple myeloma cells. We transfected BMSCs with SRC3-specific
short hairpin RNA (sh-SRC3) lentiviral vector to knock down the
expression of SRC3. We confirmed the efficiency by detecting mRNA
and protein levels of SRC3 in BMSCs (Fig. 2A and B). We, next co-cultured the
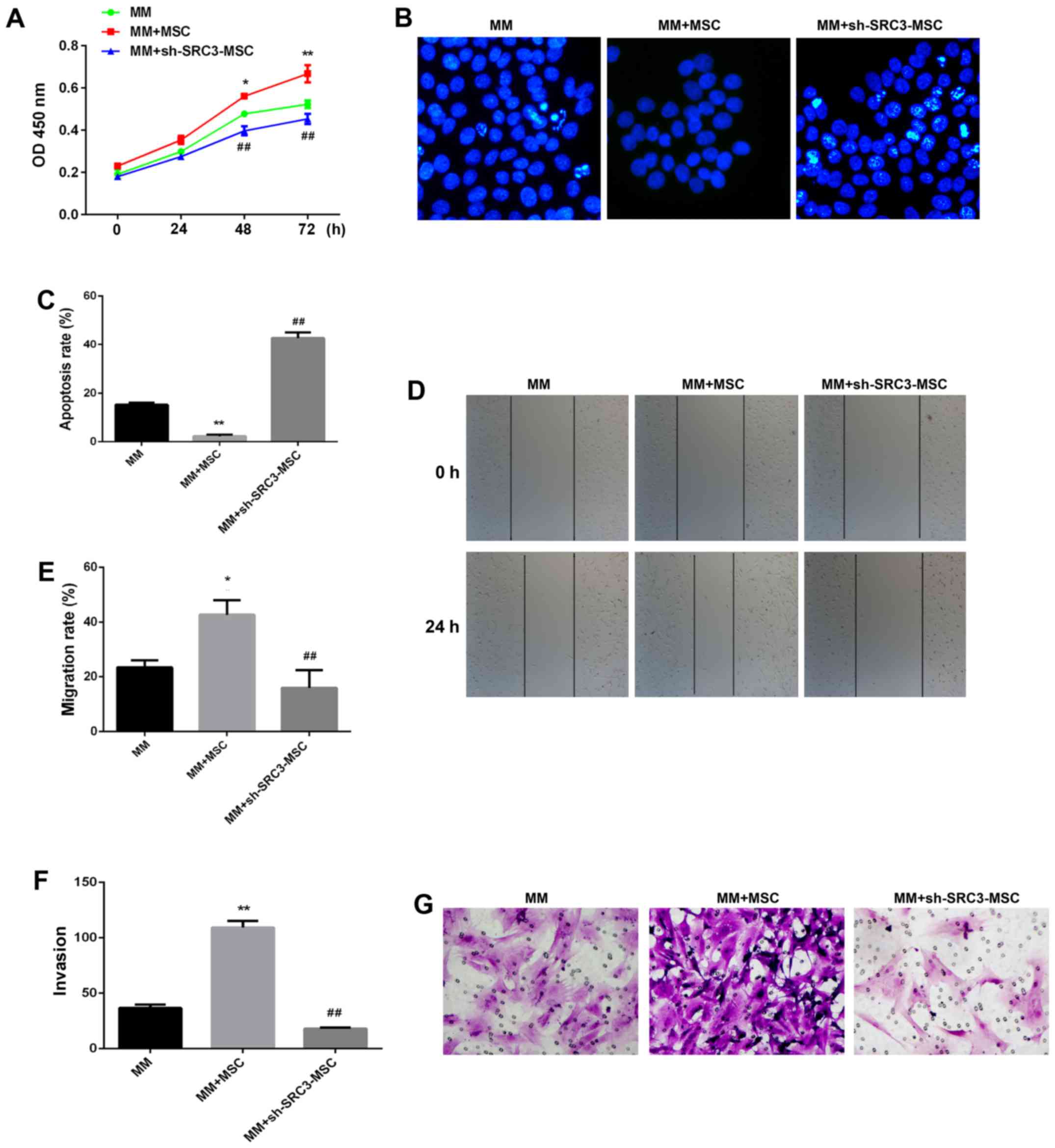
RPMI-8226 cells with either between April 2015 and March 2016 at
the third affiliated Daping Hospital control BMSCs or sh-SRC3-BMSCs
and evaluated the proliferation and migration ability of RPMI-8226
cells. As shown in Fig. 3A,
knocking down SRC3 expression in BMSCs significantly inhibited the
proliferation ability (P<0.01) and significantly decreased the
rate of apoptosis in RPMI-8226 cells (Fig. 3B and C, P<0.01). In addition,
knocking down SRC3 expression in BMSCs inhibited the migration of
RPMI-8226 cells assessed by both the wound healing assay (Fig. 3D and E, P<0.01) and Transwell
migration assay (Fig. 3F and G,
P<0.01).
SRC3 expressed in BMSCs regulates the
expression of Cx43 via the MAPK pathway in RPMI-8226 cells
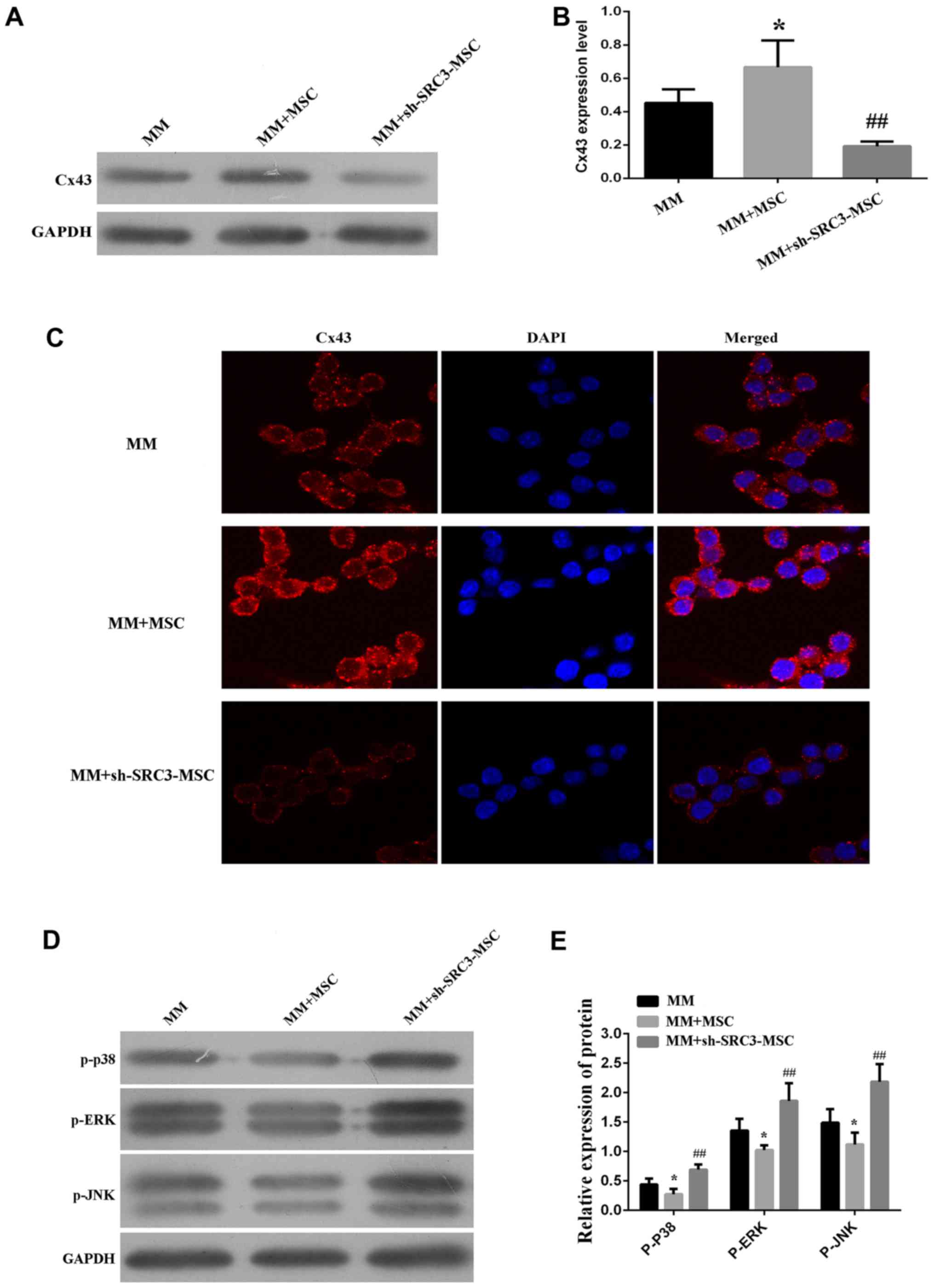
We next asked if SRC3 expression in BMSCs regulated
the expression of Cx43. We found that when RPMI-8226 cells were
co-cultured with BMSCs, the protein expression of Cx43 was
increased (P<0.05). Conversely, when RPMI-8226 cells were
co-cultured with BMSCs with knocked down SRC3 expression, the
protein level of Cx43 was decreased (Fig. 4A and B, P<0.01). We observed
similar results using the immunofluorescence assay (Fig. 4C). The p38 MAPK pathway is
implicated in the regulation of cell growth, migration,
differentiation, inflammation, survival or apoptosis (25–27).
In addition, MAPKs are also involved in the expression of Cx43 in
mammary carcinoma and bladder carcinogenesis cells (28–30).
We, thus wanted to test if the MAPK pathway mediated SRC3-regulated
Cx43 in BMSCs. We co-cultured RPMI-8226 cells transfected with
either control BMSCs or sh-SRC3-BMSCs and evaluated the expression
of the different signaling molecules in the MAPK pathway, using
western blots. As shown in Fig. 4D and
E, we found that the protein levels of phosphorylated ERK
(pERK), p38 (p-p38) and JNK (p-JNK) were decreased in RPMI-8226
cells co-cultured with BMSCs and elevated in RPMI-8226 cells
co-cultured with sh-SRC3-MSC (knockdown of SRC3, P<0.01). These
results suggest that SRC3 in BMSCs regulates the expression of Cx43
via the MAPK pathway in RPMI-8226 cells.
MAPK pathway promotes Cx43-regulated
proliferation and migration of RPMI-8226 cells
To gain insight into the possible mechanism
associated with the expression of Cx43 regulated by the MAPK
pathway, we treated RPMI-8226 cells with the Cx43 overexpression
plasmid or with the MAPK inhibitor SB202190. These cells were then
co-cultured with either BMSCs or sh-SRC3-MSC. SB202190 inhibits p38
MAP kinase activity by competing with ATP and inhibiting p38 MAPK
phosphorylation (31). As shown in
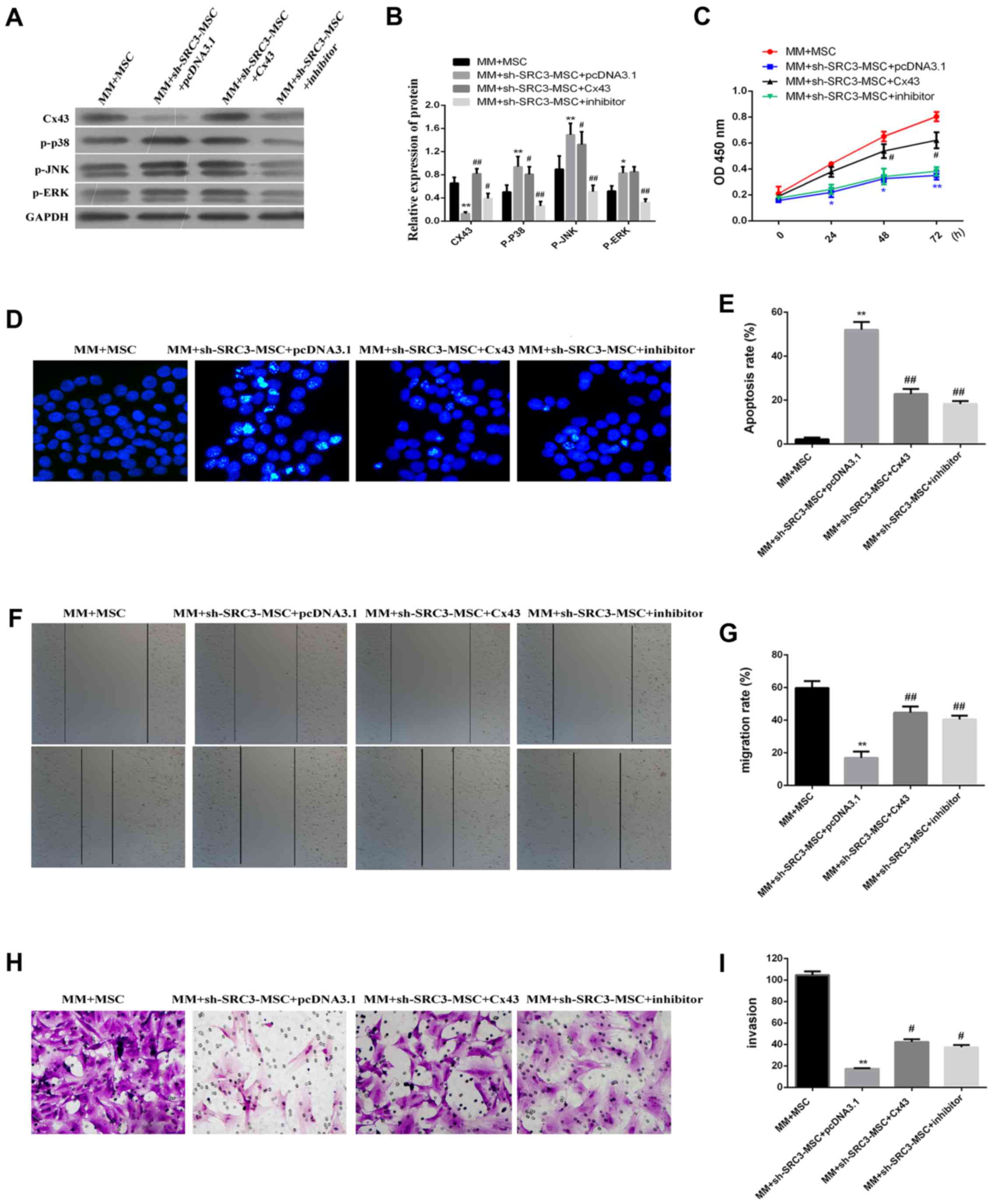
Fig. 5A and B, knocking down SRC3
in BMSCs significantly enhanced the protein levels of p-ERK, p-p38
and p-JNK and significantly decreased the protein level of Cx43
level (P<0.01 or P<0.05). We found that the protein levels of
p-p38 and p-JNK were down regulated in cells co-cultured with
Cx43-MM and sh-SRC3-MSC (P<0.05). We did not observe any
significant changes in p-ERK protein levels. SB202190 treatment
significantly decreased the expression levels of p-ERK, p-p38 and
p-JNK, and signifi-cantly enhanced the expression level of Cx43 in
RPMI-8226 cells (Fig. 5A and B,
P<0.01 or P<0.05). Knocking down SRC expression and
overexpressing Cx43 promoted cell proliferation in RPMI-8226 cells
(Fig. 5C, P<0.05) and decreased
the proportion of apoptosis in RPMI-8226 cells (Fig. 5D and E, P<0.05). Both the wound
healing assay and the Transwell migration assay showed that the
migration of RPMI-8226 cells was enhanced by over expressing Cx43
and treating with SB202190 (Fig. 5F
and G, P<0.05 and Fig. 5H and
I, P<0.05, respectively). Taken together, our data suggest
that inactive MAPK pathway enhances the expression of Cx43 and
promotes proliferation and migration of RPMI-8226 cells.
SRC3 expressed in BMSCs promoted tumor
growth of multiple myeloma cells by regulating the expression of
Cx43
To further validate the molecular mechanism in
vivo, we established murine models of multiple myeloma. The
BMSCs were treated with sh-SRC3 to knock down the expression of
SRC3 and RPMI-8226 cells were overexpressed with either Cx43 or
treated with SB202190. The cells were then co-injected into nude
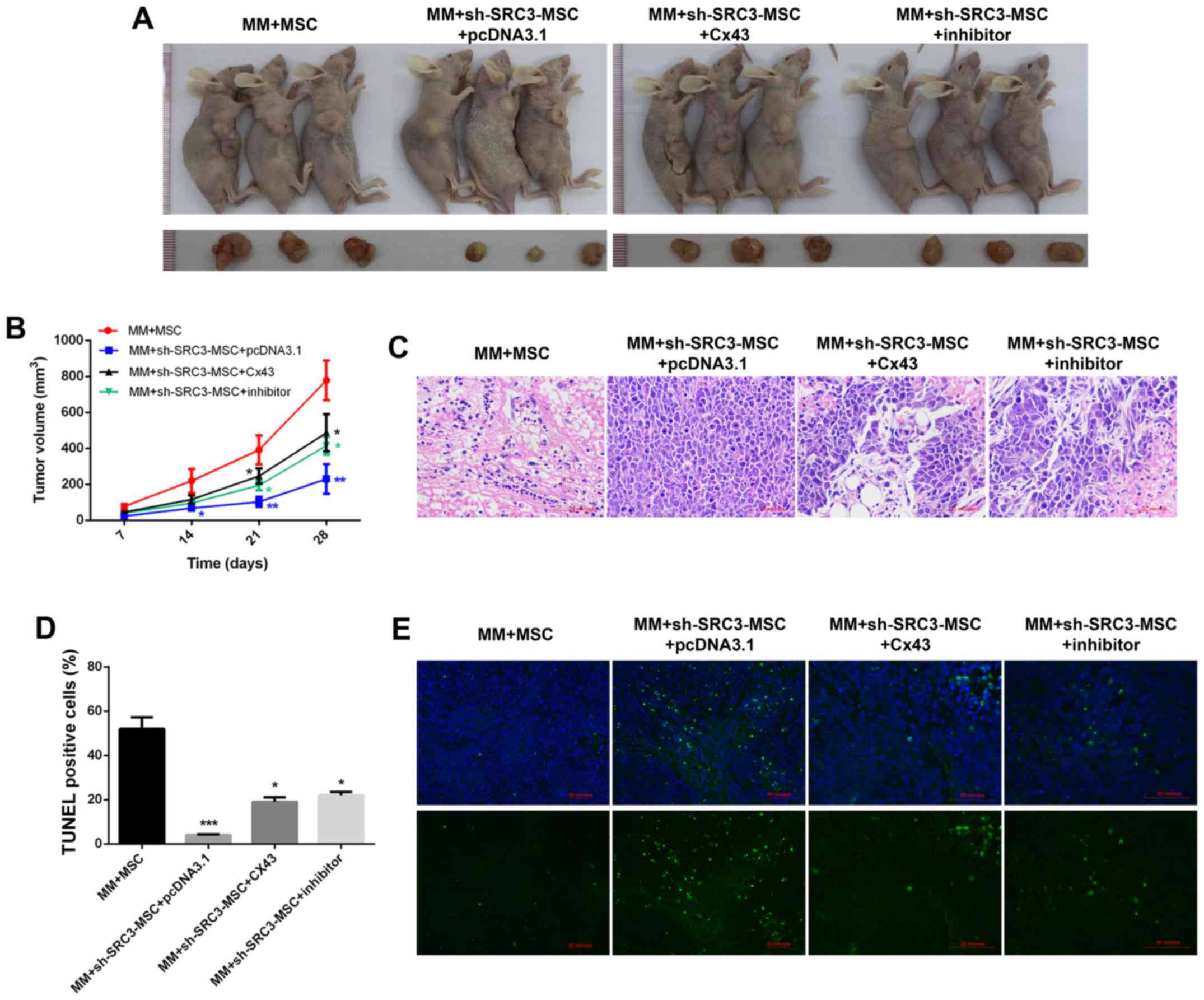
mice to establish murine multiple myeloma models. As shown in
Fig. 6A and B, compared with the
MM+MSCs group, knocking down SRC3 in MSCs (MM+sh-SRC3-MSC+pcDNA3.1)
significantly decreased the tumor growth (P<0.01). Both,
overexpressing Cx43 and treating with SB202190 promoted the tumor
growth (Fig. 6A and B, P<0.05).
Histological analysis showed fewer intratumoral leukocyte
populations in the MM+sh-SRC3-MSC+pcDNA3.1 group compared with the
MM+MSCs group. Intratumoral leukocyte populations increased after
overexpressing Cx43 and treating with SB202190 in RPMI-8226 cells
(Fig. 6C). Furthermore, TUNEL
staining showed that knocking down SRC3 in MSCs
(MM+sh-SRC3-MSC+pcDNA3.1) significantly increased cell apoptosis in
tumor tissue. Both, overexpressing Cx43 and treating with SB202190
decreased cell apoptosis in tumor tissue (Fig. 6D and E, P<0.05). Moreover, we
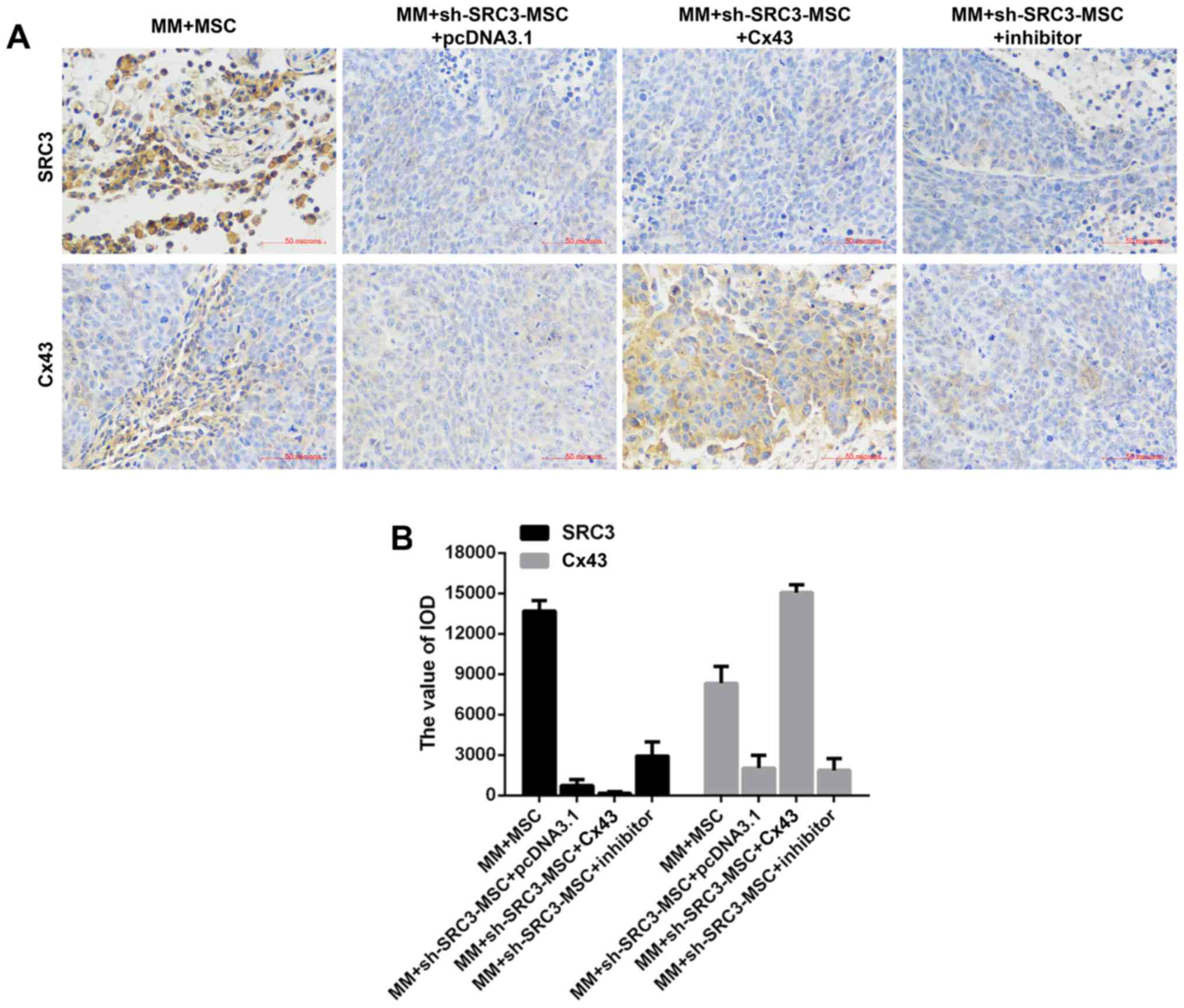
used immunohis-tochemical staining and found fewer SRC3 positive
cells in the MM+sh-SRC3-MSC+pcDNA3.1 group; thus, overexpressing
Cx43 and treating with SB202190 influenced the level of SRC3 in
tumor tissue. The protein level of Cx43 decreased when SRC3
expression in MSCs was knocked down and increased when cells were
overexpressed with Cx43 and treated with SB202190 (Fig. 7).
Discussion
The BMSCs in the bone microenvironment promote the
proliferation of multiple myeloma cells (5). Interleukin-6 (IL-6), a key
inflammatory cytokine required for the growth and survival of
multiple myeloma cells, is mostly secreted by BMSCs (32). Adhesion of myeloma cells to BMSCs
triggers NF-κB activation, and induces secretion of vascular
endothelial growth factor, insulin-like growth factor, and other
factors, which support cell growth and chemoresistance of multiple
myeloma (33–35). VCAM-1, a cell-surface protein that
is highly expressed on BMSCs, promotes the adhesion between BMSCs
and multiple myeloma (36).
Similarly, in a co-culture system, we found that BMSCs promoted the
proliferation and migration of multiple myeloma cells. Knocking
down the expression of SRC3 in BMSCs reduced the proliferation and
migration of multiple myeloma cells. These results suggested that
SRC3 in BMSCs can modify the bone marrow microenvironment, and
promote the proliferation and migration of multiple myeloma cells.
Moreover, we found that SRC3 in BMSCs promoted the proliferation
and migration of multiple myeloma cells by upregulating the
expression of Cx43.
We showed that Cx43 is highly expressed in both
primary isolated multiple myeloma samples and in multiple myeloma
cell lines. Both MM cell lines expressed Cx43, and the mRNA and
protein levels of Cx43 in RPMI-8226 cells were higher than U266
cells. Consistently, Fu (37)
reported that Cx43 expression was heterogeneous and aberrant in MM
cells (RPMI 8266, U266, and XG7 cells). Work from other studies
demonstrated that all BMSCs from multiple myeloma patients
expressed higher levels of Cx43 than those from normal controls
(37), and that Cx43 expressed on
BMSCs played an essential role in multiple myeloma cell survival,
adhesion, migration and drug resistance (22,38).
In our study, we found that Cx43 expressed on multiple myeloma
cells promoted the proliferation and migration of multiple myeloma
cells, and decreased their cell apoptosis.
In addition, our results revealed that SRC3 in BMSCs
regulated the expression of Cx43 via the MAPK pathway in RPMI-8226
cells. The p38 mitogen-activated protein kinase (MAPK) is a
subgroup of the MAPK pathway and phosphorylates serine and/or
threonine residues of target proteins, subsequently regulating a
number of biological processes, including cell growth,
differentiation, apoptosis and inflammation (38). p38 MAPK plays a dual role as a
regulator of cell death, and can either mediate cell survival or
cell death depending on the type of stimulus and the type of the
cell (38). In mammary carcinoma
cells, over-expressing Cx43 increased the level of the
phosphorylated form of p38-MAPK (30). Mechanical stress increased the
expression of Cx43 and promoted the osteoblastic differentiation of
ligament fibroblasts via ERK1/2 and p38 MAPK pathway (39). In bladder cancer, the highly
expressed and cytoplasmic localized Cx43 contributed to oncogenic
and aberrant activation of JNK and ERK signaling (28). In our study, knocking down the
expression of SRC3 in BMSCs enhanced the protein levels of p-ERK,
p-p38 and p-JNK and decreased the protein level of Cx43. The
overexpressed Cx43 in RPMI-8226 cells did not influence the protein
levels of p-ERK, p-p38 and p-JNK. The inactive MAPK pathway
enhanced the expression of Cx43 and promoted cell proliferation and
migration of RPMI-8226 cells. In conclusion, SRC3 expressed in
BMSCs enhanced the expression of Cx43 via the MAPK pathway and the
increased Cx43 promoted cell proliferation and migration of
multiple myeloma cells. Our results contribute to a better
understanding of the effect of BMSCs in the bone marrow
microenvironment and its impact on disease progression.
Acknowledgments
The study was supported by the National Natural
Science Foundation of China for Young Scholars (no. 81500175) and
the Basic Science and Advanced Technology Research Project of
Chongqing City (no. cstc2016jcyjA0381).
References
|
1
|
Falank C, Fairfield H and Reagan MR:
Signaling interplay between bone marrow adipose tissue and multiple
myeloma cells. Front Endocrinol (Lausanne). 7(Suppl): 672016.
|
|
2
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zweegman S, Palumbo A, Bringhen S and
Sonneveld P: Age and aging in blood disorders: Multiple myeloma.
Haematologica. 99:1133–1137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
American Cancer Society: Cancer Facts and
Figures 2017. American Cancer Society; Atlanta, GA: 2017,
https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html.
|
|
5
|
Scheideler M, Elabd C, Zaragosi LE,
Chiellini C, Hackl H, Sanchez-Cabo F, Yadav S, Duszka K, Friedl G,
Papak C, et al: Comparative transcriptomics of human multipotent
stem cells during adipogenesis and osteoblastogenesis. BMC
Genomics. 9:3402008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mahindra A, Hideshima T and Anderson KC:
Multiple myeloma: Biology of the disease. Blood Rev. 24(Suppl 1):
S5–S11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Podar K, Chauhan D and Anderson KC: Bone
marrow microenvironment and the identification of new targets for
myeloma therapy. Leukemia. 23:10–24. 2009. View Article : Google Scholar
|
|
8
|
Hideshima T, Bergsagel PL, Kuehl WM and
Anderson KC: Advances in biology of multiple myeloma: Clinical
applications. Blood. 104:607–618. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu J and Li Q: Review of the in vivo
functions of the p160 steroid receptor coactivator family. Mol
Endocrinol. 17:1681–1692. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manier S, Sacco A, Leleu X, Ghobrial IM
and Roccaro AM: Bone marrow microenvironment in multiple myeloma
progression. J Biomed Biotechnol. 2012:1574962012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
York B, Yu C, Sagen JV, Liu Z, Nikolai BC,
Wu RC, Finegold M, Xu J and O'Malley BW: Reprogramming the
posttranslational code of SRC-3 confers a switch in mammalian
systems biology. Proc Natl Acad Sci USA. 107:11122–11127. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan J, Tsai SY and Tsai MJ: SRC-3/AIB1:
Transcriptional coactivator in oncogenesis. Acta Pharmacol Sin.
27:387–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin J, Wang Y, Wang J, Xu Y, Chen S, Wang
J, Ran X and Su Y: Increased radiosensitivity and radiation-induced
apoptosis in SRC-3 knockout mice. J Radiat Res. 55:443–450. 2014.
View Article : Google Scholar :
|
|
14
|
Jin J, Wang Y, Wang J, Xu Y, Chen SL, Wang
JP and Su YP: Impaired hematopoiesis and delayed thrombopoietic
recovery following sublethal irradiation in SRC-3 knockout mice.
Mol Med Rep. 9:1629–1633. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Warn-Cramer BJ, Cottrell GT, Burt JM and
Lau AF: Regulation of connexin-43 gap junctional intercellular
communication by mitogen-activated protein kinase. J Biol Chem.
273:9188–9196. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nimlamool W, Andrews RMK and Falk MM:
Connexin43 phosphorylation by PKC and MAPK signals VEGF-mediated
gap junction internalization. Mol Biol Cell. 26:2755–2768. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shishido SN, Delahaye A, Beck A and Nguyen
TA: The anticancer effect of PQ1 in the MMTV-PyVT mouse model. Int
J Cancer. 134:1474–1483. 2014. View Article : Google Scholar :
|
|
18
|
Kanczuga-Koda L, Sulkowska M, Koda M,
Rutkowski R and Sulkowski S: Increased expression of gap junction
protein -connexin 32 in lymph node metastases of human ductal
breast cancer. Folia Histochem Cytobiol. 45(Suppl 1): S175–S180.
2007.
|
|
19
|
Kanczuga-Koda L, Sulkowski S, Tomaszewski
J, Koda M, Sulkowska M, Przystupa W, Golaszewska J and Baltaziak M:
Connexins 26 and 43 correlate with Bak, but not with Bcl-2 protein
in breast cancer. Oncol Rep. 14:325–329. 2005.PubMed/NCBI
|
|
20
|
Zhou Y, Miao J, Wu H, Tang H, Kuang J,
Zhou X, Peng Y, Hu D, Shi D, Deng W, et al: PD-1 and PD-L1
expression in 132 recurrent nasopharyngeal carcinoma: The
correlation with anemia and outcomes. Oncotarget. 8:51210–51223.
2017.PubMed/NCBI
|
|
21
|
Morsing M, Klitgaard MC, Jafari A,
Villadsen R, Kassem M, Petersen OW and Rønnov-Jessen L: Evidence of
two distinct functionally specialized fibroblast lineages in breast
stroma. Breast Cancer Res. 18:1082016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Sun Y, Wang Z, Huang Z, Li B and
Fu J: Up-regulation of connexin-43 expression in bone marrow
mesenchymal stem cells plays a crucial role in adhesion and
migration of multiple myeloma cells. Leuk Lymphoma. 56:211–218.
2015. View Article : Google Scholar
|
|
23
|
Basak GW, Srivastava AS, Malhotra R and
Carrier E: Multiple myeloma bone marrow niche. Curr Pharm
Biotechnol. 10:345–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen X, Guo Y, Yu J, Qi J, Shi W, Wu X, Ni
H and Ju S: miRNA-202 in bone marrow stromal cells affects the
growth and adhesion of multiple myeloma cells by regulating B
cell-activating factor. Clin Exp Med. 16:307–316. 2016. View Article : Google Scholar
|
|
25
|
Khorasanizadeh M, Eskian M, Gelfand EW and
Rezaei N: Mitogen-activated protein kinases as therapeutic targets
for asthma. Pharmacol Ther. 174:112–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mugami S, Dobkin-Bekman M, Rahamim-Ben
Navi L and Naor Z: Differential roles of PKC isoforms (PKCs) in
GnRH stimulation of MAPK phosphorylation in gonadotrope derived
cells. Mol Cell Endocrinol. Apr 6–2017.Epub ahead of print.
PubMed/NCBI
|
|
27
|
Segalés J, Perdiguero E and Muñoz-Cánoves
P: Regulation of muscle stem cell functions: A focus on the p38
MAPK signaling pathway. Front Cell Dev Biol. 4:912016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ai XL, Chi Q, Qiu Y, Li HY, Li DJ, Wang JX
and Wang ZY: Gap junction protein connexin43 deregulation
contributes to bladder carcinogenesis via targeting MAPK pathway.
Mol Cell Biochem. 428:109–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Losa D, Köhler T, Bellec J, Dudez T,
Crespin S, Bacchetta M, Boulanger P, Hong SS, Morel S, Nguyen TH,
et al: Pseudomonas aeruginosa-induced apoptosis in airway
epithelial cells is mediated by gap junctional communication in a
JNK-dependent manner. J Immunol. 192:4804–4812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shishido SN and Nguyen TA: Induction of
apoptosis by PQ1 a gap junction enhancer that upregulates connexin
43 and activates the MAPK signaling pathway in mammary carcinoma
cells. Int J Mol Sci. 17:1782016. View Article : Google Scholar
|
|
31
|
Geiger PC, Wright DC, Han DH and Holloszy
JO: Activation of p38 MAP kinase enhances sensitivity of muscle
glucose transport to insulin. Am J Physiol Endocrinol Metab.
288:E782–E788. 2005. View Article : Google Scholar
|
|
32
|
Uchiyama H, Barut BA, Mohrbacher AF,
Chauhan D and Anderson KC: Adhesion of human myeloma-derived cell
lines to bone marrow stromal cells stimulates interleukin-6
secretion. Blood. 82:3712–3720. 1993.PubMed/NCBI
|
|
33
|
Hideshima T, Mitsiades C, Tonon G,
Richardson PG and Anderson KC: Understanding multiple myeloma
pathogenesis in the bone marrow to identify new therapeutic
targets. Nat Rev Cancer. 7:585–598. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kumar S, Witzig TE, Timm M, Haug J, Wellik
L, Fonseca R, Greipp PR and Rajkumar SV: Expression of VEGF and its
receptors by myeloma cells. Leukemia. 17:2025–2031. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nefedova Y, Cheng P, Alsina M, Dalton WS
and Gabrilovich DI: Involvement of Notch-1 signaling in bone marrow
stroma-mediated de novo drug resistance of myeloma and other
malignant lymphoid cell lines. Blood. 103:3503–3510. 2004.
View Article : Google Scholar
|
|
36
|
Mori Y, Shimizu N, Dallas M, Niewolna M,
Story B, Williams PJ, Mundy GR and Yoneda T: Anti-alpha4 integrin
antibody suppresses the development of multiple myeloma and
associated osteoclastic osteolysis. Blood. 104:2149–2154. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fu J: Cx43 expressed on bone marrow
stromal cells plays an essential role in multiple myeloma cell
survival and drug resistance. Arch Med Sci. 13:236–245. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Koul HK, Pal M and Koul S: Role of p38 MAP
kinase signal transduction in solid tumors. Genes Cancer.
4:342–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen D, Liu Y, Yang H, Chen D, Zhang X,
Fermandes JC and Chen Y: Connexin 43 promotes ossification of the
posterior longitudinal ligament through activation of the ERK1/2
and p38 MAPK pathways. Cell Tissue Res. 363:765–773. 2016.
View Article : Google Scholar
|