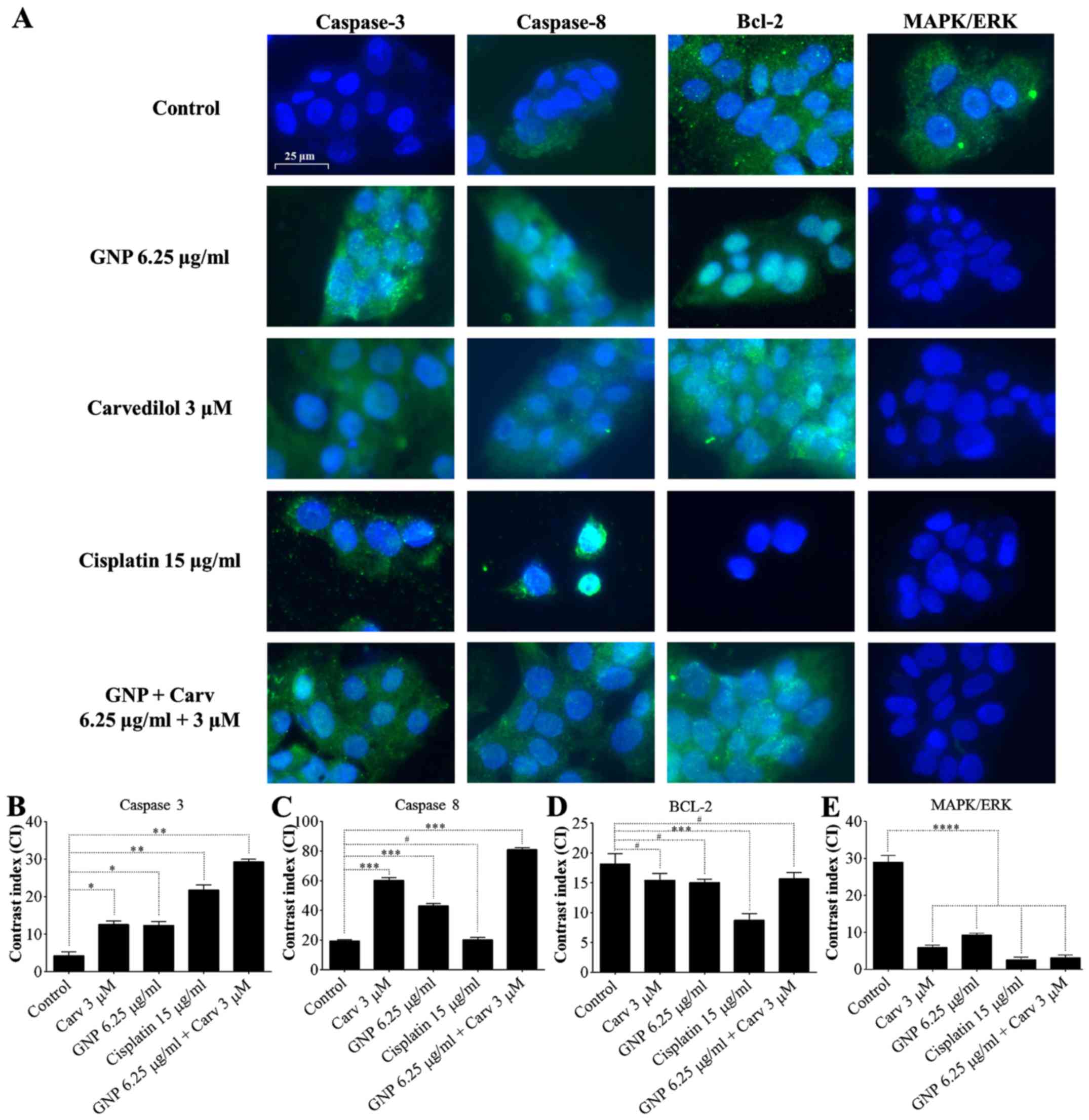
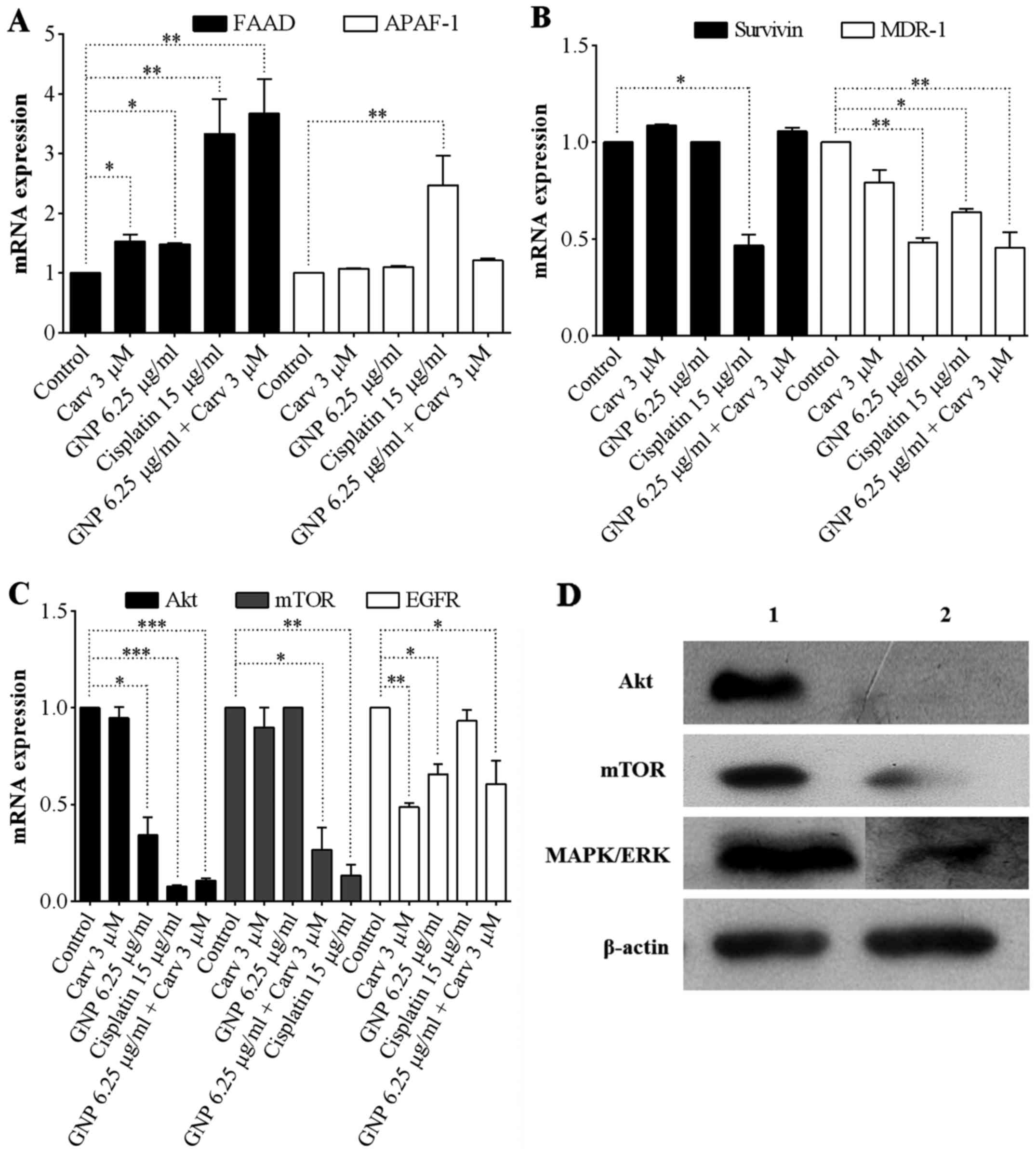
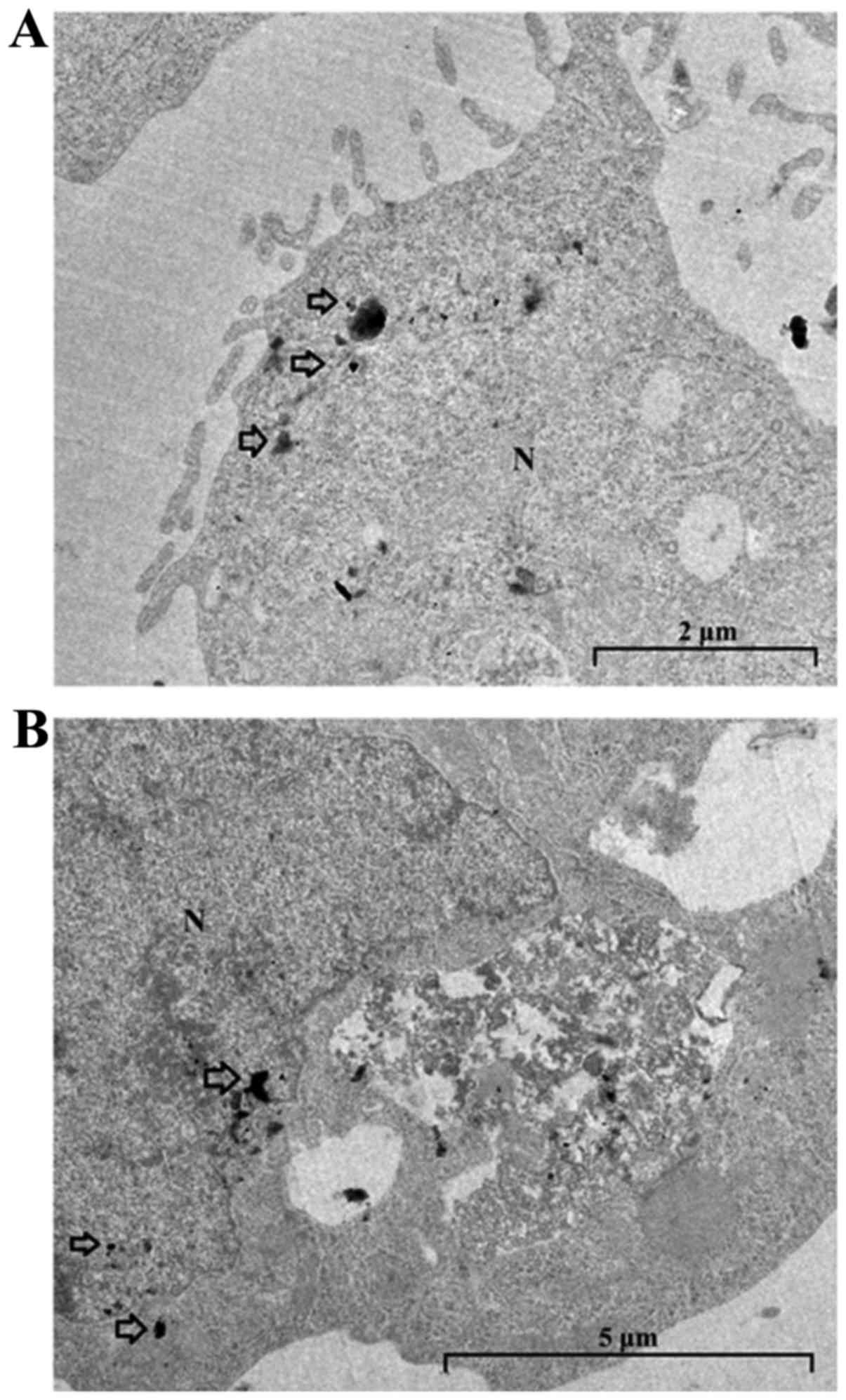
|
1
|
Hales S, Chiu A, Husain A, Braun M, Rydall
A, Gagliese L, Zimmermann C and Rodin G: The quality of dying and
death in cancer and its relationship to palliative care and place
of death. J Pain Symptom Manage. 48:839–851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
4
|
Özdemir F, Akalın G, Şen M, Önder NI,
Işcan A, Kutlu HM and Incesu Z: Towards novel anti-tumor strategies
for hepatic cancer: ε-viniferin in combination with vincristine
displays pharmacodynamic synergy at lower doses in HepG2 cells.
OMICS. 18:324–334. 2014. View Article : Google Scholar
|
|
5
|
Ling CQ: Problems in cancer treatment and
major research of integrative medicine. Zhong Xi Yi Jie He Xue Bao.
1:168–170. 2003.In Chinese. View Article : Google Scholar
|
|
6
|
Agrawal S: Late effects of cancer
treatment in breast cancer survivors. South Asian J Cancer.
3:112–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oberstein PE and Olive KP: Pancreatic
cancer: Why is it so hard to treat? Therap Adv Gastroenterol.
6:321–337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michaelson MD, Cotter SE, Gargollo PC,
Zietman AL, Dahl DM and Smith MR: Management of complications of
prostate cancer treatment. CA Cancer J Clin. 58:196–213. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fernald K and Kurokawa M: Evading
apoptosis in cancer. Trends Cell Biol. 23:620–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Labi V and Erlacher M: How cell death
shapes cancer. Cell Death Dis. 6:e16752015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ran LK, Chen Y, Zhang ZZ, Tao NN, Ren JH,
Zhou L, Tang H, Chen X, Chen K, Li WY, et al: SIRT6 verexpression
Potentiates apoptosis evasion in hepatocellular carcinoma via
BCL2-associated X protein-dependent apoptotic pathway. Clin Cancer
Res. 22:3372–3382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai Y, Tan X, Liu J, Shen Y, Wu D, Ren M,
Huang P and Yu D: Inhibition of PI3K/Akt/mTOR signaling pathway
enhances the sensitivity of the SKOV3/DDP ovarian cancer cell line
to cisplatin in vitro. Chin J Cancer Res. 26:564–572.
2014.PubMed/NCBI
|
|
13
|
Lu Z and Xu S: ERK1/2 MAP kinases in cell
survival and apoptosis. IUBMB Life. 58:621–631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang C, Cigliano A, Delogu S, Armbruster
J, Dombrowski F, Evert M, Chen X and Calvisi DF: Functional
crosstalk between AKT/mTOR and Ras/MAPK pathways in
hepatocarcinogenesis: Implications for the treatment of human liver
cancer. Cell Cycle. 12:1999–2010. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katayama K, Noguchi K and Sugimoto Y:
Regulations of P-glycoprotein/ABCB1/MDR1 in human cancer cells. New
J Sci. 2014:e4769742014. View Article : Google Scholar
|
|
16
|
Yang X, Uziely B, Groshen S, Lukas J,
Israel V, Russell C, Dunnington G, Formenti S, Muggia F and Press
MF: MDR1 gene expression in primary and advanced breast cancer. Lab
Invest. 79:271–280. 1999.PubMed/NCBI
|
|
17
|
Chiara F, Gambalunga A, Sciacovelli M,
Nicolli A, Ronconi L, Fregona D, Bernardi P, Rasola A and Trevisan
A: Chemotherapeutic induction of mitochondrial oxidative stress
activates GSK-3α/β and Bax, leading to permeability transition pore
opening and tumor cell death. Cell Death Dis. 3:e4442012.
View Article : Google Scholar
|
|
18
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marullo R, Werner E, Degtyareva N, Moore
B, Altavilla G, Ramalingam SS and Doetsch PW: Cisplatin induces a
mitochondrial-ROS response that contributes to cytotoxicity
depending on mitochondrial redox status and bioenergetic functions.
PLoS One. 8:e811622013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dreaden EC, Austin LA, Mackey MA and
El-Sayed MA: Size matters: Gold nanoparticles in targeted cancer
drug delivery. Ther Deliv. 3:457–478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jain S, Hirst DG and O'Sullivan JM: Gold
nanoparticles as novel agents for cancer therapy. Br J Radiol.
85:101–113. 2012. View Article : Google Scholar :
|
|
22
|
Lee J, Chatterjee DK, Lee MH and Krishnan
S: Gold nanoparticles in breast cancer treatment: Promise and
potential pitfalls. Cancer Lett. 347:46–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alkilany AM and Murphy CJ: Toxicity and
cellular uptake of gold nanoparticles: What we have learned so far?
J Nanopart Res. 12:2313–2333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alvarenga ÉC, Caires A, Ladeira LO, Gamero
EJP, Andrade LM and Paz MTL: Potenciais alvos terapêuticos contra o
câncer. Cienc Cult. 66:43–48. 2014. View Article : Google Scholar
|
|
25
|
Naha PC, Chhour P and Cormode DP:
Systematic in vitro toxicological screening of gold nanoparticles
designed for nanomedicine applications. Toxicol In Vitro.
29:1445–1453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Butterworth KT, Coulter JA, Jain S, Forker
J, McMahon SJ, Schettino G, Prise KM, Currell FJ and Hirst DG:
Evaluation of cytotoxicity and radiation enhancement using 1.9 nm
gold particles: Potential application for cancer therapy.
Nanotechnology. 21:2951012010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coulter JA, Jain S, Butterworth KT,
Taggart LE, Dickson GR, McMahon SJ, Hyland WB, Muir MF, Trainor C,
Hounsell AR, et al: Cell type-dependent uptake, localization, and
cytotoxicity of 1.9 nm gold nanoparticles. Int J Nanomedicine.
7:2673–2685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murawala P, Tirmale A, Shiras A and Prasad
BLV: In situ synthesized BSA capped gold nanoparticles: Effective
carrier of anticancer drug methotrexate to MCF-7 breast cancer
cells. Mater Sci Eng C. 34:158–167. 2014. View Article : Google Scholar
|
|
29
|
Patra HK, Banerjee S, Chaudhuri U, Lahiri
P and Dasgupta AK: Cell selective response to gold nanoparticles.
Nanomedicine (Lond). 3:111–119. 2007. View Article : Google Scholar
|
|
30
|
Budni P, Pedrosa RC, Dalmarco EM, Dalmarco
JB, Frode TS and Wilhelm Filho D: Carvedilol enhances the
antioxidant effect of vitamins E and C in chronic Chagas heart
disease. Arq Bras Cardiol. 101:304–310. 2013.PubMed/NCBI
|
|
31
|
Arozal W, Watanabe K, Veeraveedu PT, Ma M,
Thandavarayan RA, Sukumaran V, Suzuki K, Kodama M and Aizawa Y:
Protective effect of carvedilol on daunorubicin-induced
cardiotoxicity and nephrotoxicity in rats. Toxicology. 274:18–26.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arumanayagam M, Chan S, Tong S and
Sanderson JE: Antioxidant properties of carvedilol and metoprolol
in heart failure: A double-blind randomized controlled trial. J
Cardiovasc Pharmacol. 37:48–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dandona P, Ghanim H and Brooks DP:
Antioxidant activity of carvedilol in cardiovascular disease. J
Hypertens. 25:731–741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li YC, Ge LS, Yang PL, Tang JF, Lin JF,
Chen P and Guan XQ: Carvedilol treatment ameliorates acute
coxsackievirus B3-induced myocarditis associated with oxidative
stress reduction. Eur J Pharmacol. 640:112–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pasquier E, Street J, Pouchy C, Carre M,
Gifford AJ, Murray J, Norris MD, Trahair T, Andre N and Kavallaris
M: β-blockers increase response to chemotherapy via direct
antitumour and anti-angiogenic mechanisms in neuroblastoma. Br J
Cancer. 108:2485–2494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Erguven M, Yazihan N, Aktas E, Sabanci A,
Li CJ, Oktem G and Bilir A: Carvedilol in glioma treatment alone
and with imatinib in vitro. Int J Oncol. 36:857–866. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dezong G, Zhongbing M, Qinye F and Zhigang
Y: Carvedilol suppresses migration and invasion of malignant breast
cells by inactivating Src involving cAMP/PKA and PKCδ signaling
pathway. J Cancer Res Ther. 10:998–1003. 2014. View Article : Google Scholar
|
|
38
|
Chang A, Yeung S, Thakkar A, Huang KM, Liu
MM, Kanassatega RS, Parsa C, Orlando R, Jackson EK, Andresen BT, et
al: Prevention of skin carcinogenesis by the β-blocker carvedilol.
Cancer Prev Res (Phila). 8:27–36. 2015. View Article : Google Scholar
|
|
39
|
Hsieh YD, Chi CC, Chou CT, Cheng JS, Kuo
CC, Liang WZ, Lin KL, Tseng LL and Jan CR: Investigation of
carvedilolevoked Ca2+ movement and death in human oral
cancer cells. J Recept Signal Transduct Res. 31:220–228. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng JS, Huang CC, Chou CT and Jan CR:
Mechanisms of carvedilol-induced [Ca2+]i
rises and death in human hepatoma cells. Naunyn Schmiedebergs Arch
Pharmacol. 376:185–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cohen DJ and Hochster HS: Rationale for
combining biotherapy in the treatment of advanced colon cancer.
Gastrointest Cancer Res. 2:145–151. 2008.
|
|
42
|
Patutina OA, Mironova NL, Vlassov VV and
Zenkova MA: New approaches for cancer treatment: Antitumor drugs
based on gene-targeted nucleic acids. Acta Naturae. 1:44–60.
2009.PubMed/NCBI
|
|
43
|
Siddiqui M and Rajkumar SV: The high cost
of cancer drugs and what we can do about it. Mayo Clin Proc.
87:935–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tannock IF: Combined modality treatment
with radiotherapy and chemotherapy. Radiother Oncol. 16:83–101.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brito AF, Ribeiro M, Abrantes AM, Pires
AS, Teixo RJ, Tralhão JG and Botelho MF: Quercetin in cancer
treatment, alone or in combination with conventional therapeutics?
Curr Med Chem. 22:3025–3039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mierzwa ML, Nyati MK, Morgan MA and
Lawrence TS: Recent advances in combined modality therapy.
Oncologist. 15:372–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li J, Wang Y, Zhu Y and Oupický D: Recent
advances in delivery of drug-nucleic acid combinations for cancer
treatment. J Control Release. 172:589–600. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Collery P, Mohsen A, Kermagoret A,
D'Angelo J, Morgant G, Desmaele D, Tomas A, Collery T, Wei M and
Badawi A: Combination of three metals for the treatment of cancer:
Gallium, rhenium and platinum. 1. Determination of the optimal
schedule of treatment. Anticancer Res. 32:2769–2781.
2012.PubMed/NCBI
|
|
49
|
Law MR, Wald NJ, Morris JK and Jordan RE:
Value of low dose combination treatment with blood pressure
lowering drugs: Analysis of 354 randomised trials. BMJ.
326:14272003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Morton CO, Chau M and Stack C: In vitro
combination therapy using low dose clotrimazole and photodynamic
therapy leads to enhanced killing of the dermatophyte Trichophyton
rubrum. BMC Microbiol. 14:2612014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chang L, Graham PH, Ni J, Hao J, Bucci J,
Cozzi PJ and Li Y: Targeting PI3K/Akt/mTOR signaling pathway in the
treatment of prostate cancer radioresistance. Crit Rev Oncol
Hematol. 96:507–517. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chappell WH, Steelman LS, Long JM, Kempf
RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone
P, et al: Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors:
Rationale and importance to inhibiting these pathways in human
health. Oncotarget. 2:135–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
de Araújo RF, de Araújo AA, Pessoa JB,
Freire Neto FP, da Silva GR, Leitão Oliveira AL, de Carvalho TG,
Silva HF, Eugênio M, Sant'Anna C, et al: Anti-inflammatory,
analgesic and anti-tumor properties of gold nanoparticles.
Pharmacol Rep. 69:119–129. 2017. View Article : Google Scholar
|
|
54
|
de Araújo Júnior RF, Leitão Oliveira ALC,
de Melo Silveira RF, de Oliveira Rocha HA, de França Cavalcanti P
and de Araújo AA: Telmisartan induces apoptosis and regulates Bcl-2
in human renal cancer cells. Exp Biol Med (Maywood). 240:34–44.
2015. View Article : Google Scholar
|
|
55
|
Rahman I, Kode A and Biswas SK: Assay for
quantitative determination of glutathione and glutathione disulfide
levels using enzymatic recycling method. Nat Protoc. 1:3159–3165.
2006. View Article : Google Scholar
|
|
56
|
da Costa CM, dos Santos RC and Lima ES: A
simple automated procedure for thiol measurement in human serum
samples. J Bras Patol Med Lab. 42:345–350. 2006. View Article : Google Scholar
|
|
57
|
Esterbauer H and Cheeseman KH:
Determination of aldehydic lipid peroxidation products:
Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 186:407–421.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Al-Sheddi ES, Al-Oqail MM, Saquib Q,
Siddiqui MA, Musarrat J, Al-Khedhairy AA and Farshori NN: Novel all
trans-retinoic Acid derivatives: Cytotoxicity, inhibition of cell
cycle progression and induction of apoptosis in human cancer cell
lines. Molecules. 20:8181–8197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kimura H, Sakai K, Arao T, Shimoyama T,
Tamura T and Nishio K: Antibody-dependent cellular cytotoxicity of
cetuximab against tumor cells with wild-type or mutant epidermal
growth factor receptor. Cancer Sci. 98:1275–1280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Świątek Ł, Rajtar B, Pawlak K, Ludwiczuk
A, Głowniak K and Polz-Dacewicz M: In vitro evaluation of
cytotoxicity of n-hexane extract from Alnus sieboldiana male
flowers on VERO and HEK293 cell lines. JPCCR. 7:110–107. 2014.
|
|
61
|
Lapique N and Benenson Y: Digital
switching in a biosensor circuit via programmable timing of gene
availability. Nat Chem Biol. 10:1020–1027. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Selvaraj V, Bodapati S, Murray E, Rice KM,
Winston N, Shokuhfar T, Zhao Y and Blough E: Cytotoxicity and
genotoxicity caused by yttrium oxide nanoparticles in HEK293 cells.
Int J Nanomed. 9:1379–1391. 2014. View Article : Google Scholar
|
|
63
|
Jia J, Zhu F, Ma X, Cao Z, Cao ZW, Li Y,
Li YX and Chen YZ: Mechanisms of drug combinations: Interaction and
network perspectives. Nat Rev Drug Discov. 8:111–128. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Richardson PG, Siegel DS, Vij R,
Hofmeister CC, Baz R, Jagannath S, Chen C, Lonial S, Jakubowiak A,
Bahlis N, et al: Pomalidomide alone or in combination with low-dose
dexamethasone in relapsed and refractory multiple myeloma: A
randomized phase 2 study. Blood. 123:1826–1832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nijhof IS, Franssen LE, Levin M-D, Bos
GMJ, Broijl A, Klein SK, Koene HR, Bloem AC, Beeker A, Faber LM, et
al: Phase 1/2 study of lenalidomide combined with low-dose
cyclophosphamide and prednisone in lenalidomide-refractory multiple
myeloma. Blood. 128:2297–2306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gatoo MA, Naseem S, Arfat MY, Dar AM,
Qasim K and Zubair S: Physicochemical properties of nanomaterials:
Implication in associated toxic manifestations. BioMed Res Int.
2014:4984202014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Coelho M, Moz M, Correia G, Teixeira A,
Medeiros R and Ribeiro L: Antiproliferative effects of β-blockers
on human colorectal cancer cells. Oncol Rep. 33:2513–2520. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Baharara J, Ramezani T, Divsalar A,
Mousavi M and Seyedarabi A: Induction of apoptosis by green
synthesized gold nanoparticles Through activation of caspase-3 and
9 in human cervical cancer cells. Avicenna J Med Biotechnol.
8:75–83. 2016.PubMed/NCBI
|
|
69
|
Connor EE, Mwamuka J, Gole A, Murphy CJ
and Wyatt MD: Gold nanoparticles are taken up by human cells but do
not cause acute cytotoxicity. Small. 1:325–327. 2005. View Article : Google Scholar
|
|
70
|
Zhao Y, Xu Y, Zhang J and Ji T:
Cardioprotective effect of carvedilol: Inhibition of apoptosis in
H9c2 cardiomyocytes via the TLR4/NF-κB pathway following
ischemia/reperfusion injury. Exp Ther Med. 8:1092–1096. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Carvalho Rodrigues MA, Gobe G, Santos NA
and Santos AC: Carvedilol protects against apoptotic cell death
induced by cisplatin in renal tubular epithelial cells. J Toxicol
Environ Health A. 75:981–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lu SC: Glutathione synthesis. Biochim
Biophys Acta. 1830:3143–3153. 2013. View Article : Google Scholar :
|
|
73
|
Townsend DM, Tew KD and Tapiero H: The
importance of glutathione in human disease. Biomed Pharmacother.
57:145–155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kannan K and Jain SK: Oxidative stress and
apoptosis. Pathophysiology. 7:153–163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Takahashi A, Masuda A, Sun M, Centonze VE
and Herman B: Oxidative stress-induced apoptosis is associated with
alterations in mitochondrial caspase activity and Bcl-2-dependent
alterations in mitochondrial pH (pHm). Brain Res Bull. 62:497–504.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chang WK, Yang KD, Chuang H, Jan JT and
Shaio MF: Glutamine protects activated human T cells from apoptosis
by up-regulating glutathione and Bcl-2 levels. Clin Immunol.
104:151–160. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Estrela JM, Ortega A and Obrador E:
Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci.
43:143–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zubairi MB, Ahmed JH and Al-Haroon SS:
Effect of adrenergic blockers, carvedilol, prazosin, metoprolol and
combination of prazosin and metoprolol on paracetamol-induced
hepatotoxicity in rabbits. Indian J Pharmacol. 46:644–648. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Sgobbo P, Pacelli C, Grattagliano I,
Villani G and Cocco T: Carvedilol inhibits mitochondrial complex I
and induces resistance to H2O2-mediated
oxidative insult in H9C2 myocardial cells. Biochim Biophys Acta.
1767:222–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Barathmanikanth S, Kalishwaralal K, Sriram
M, Pandian SR, Youn HS, Eom S and Gurunathan S: Anti-oxidant effect
of gold nanoparticles restrains hyperglycemic conditions in
diabetic mice. J Nanobiotech. 8:162010. View Article : Google Scholar
|
|
81
|
Yakimovich NO, Ezhevskii AA, Guseinov DV,
Smirnova LA, Gracheva TA and Klychkov KS: Antioxidant properties of
gold nanoparticles studied by ESR spectroscopy. Russ Chem Bull.
57:520–523. 2008. View Article : Google Scholar
|
|
82
|
Nielsen F, Mikkelsen BB, Nielsen JB,
Andersen HR and Grandjean P: Plasma malondialdehyde as biomarker
for oxidative stress: Reference interval and effects of life-style
factors. Clin Chem. 43:1209–1214. 1997.PubMed/NCBI
|
|
83
|
Gaweł S, Wardas M, Niedworok E and Wardas
P: Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek.
57:453–455. 2004.In Polish.
|
|
84
|
Ho E, Karimi Galougahi K, Liu CC, Bhindi R
and Figtree GA: Biological markers of oxidative stress:
Applications to cardiovascular research and practice. Redox Biol.
1:483–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sangeetha P, Das UN, Koratkar R and
Suryaprabha P: Increase in free radical generation and lipid
peroxidation following chemotherapy in patients with cancer. Free
Radic Biol Med. 8:15–19. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Esfahani A, Ghoreishi Z, Nikanfar A,
Sanaat Z and Ghorbanihaghjo A: Influence of chemotherapy on the
lipid peroxidation and antioxidant status in patients with acute
myeloid leukemia. Acta Med Iran. 50:454–458. 2012.PubMed/NCBI
|
|
87
|
Cabello CM, Bair WB III and Wondrak GT:
Experimental therapeutics: Targeting the redox Achilles heel of
cancer. Curr Opin Investig Drugs. 8:1022–1037. 2007.PubMed/NCBI
|
|
88
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
García M and Vecino E: Vías de
señalización intracelular que conducen a la apoptosis de las
células de la retina. Arch Soc Esp Oftalmol. 78:351–364. 2003.
View Article : Google Scholar
|
|
90
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
Targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ahmad S, White CW, Chang LY, Schneider BK
and Allen CB: Glutamine protects mitochondrial structure and
function in oxygen toxicity. Am J Physiol Lung Cell Mol Physiol.
280:L779–L791. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Drake J, Sultana R, Aksenova M, Calabrese
V and Butterfield DA: Elevation of mitochondrial glutathione by
gamma-glutamylcysteine ethyl ester protects mitochondria against
peroxynitrite-induced oxidative stress. J Neurosci Res. 74:917–927.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Marí M, Morales A, Colell A, García-Ruiz C
and Fernández-Checa JC: Mitochondrial glutathione, a key survival
antioxidant. Antioxid Redox Signal. 11:2685–2700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Cheng J, Wang F, Yu DF, Wu PF and Chen JG:
The cytotoxic mechanism of malondialdehyde and protective effect of
carnosine via protein cross-linking/mitochondrial
dysfunction/reactive oxygen species/MAPK pathway in neurons. Eur J
Pharmacol. 650:184–194. 2011. View Article : Google Scholar
|
|
95
|
Ayala A, Muñoz MF and Argüelles S: Lipid
peroxidation: Production, metabolism, and signaling mechanisms of
malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev.
2014:3604382014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Qin J, Kang Y, Xu Z, Zang C, Fang B and
Liu X: Dioscin prevents the mitochondrial apoptosis and attenuates
oxidative stress in cardiac H9c2 cells. Drug Res (Stuttg).
64:47–52. 2014.
|
|
97
|
Bang S, Jeong EJ, Kim IK, Jung YK and Kim
KS: Fas- and tumor necrosis factor-mediated apoptosis uses the same
binding surface of FADD to trigger signal transduction. A typical
model for convergent signal transduction. J Biol Chem.
275:36217–36222. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Osborn SL, Sohn SJ and Winoto A:
Constitutive phosphorylation mutation in Fas-associated death
domain (FADD) results in early cell cycle defects. J Biol Chem.
282:22786–22792. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Xerri L, Devilard E, Bouabdallah R, Stoppa
AM, Hassoun J and Birg F: FADD expression and caspase activation in
B-cell lymphomas resistant to Fas-mediated apoptosis. Br J
Haematol. 106:652–661. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Campioni M, Santini D, Tonini G, Murace R,
Dragonetti E, Spugnini EP and Baldi A: Role of Apaf-1, a key
regulator of apoptosis, in melanoma progression and
chemoresistance. Exp Dermatol. 14:811–818. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Brazil DP, Yang ZZ and Hemmings BA:
Advances in protein kinase B signalling: AKTion on multiple fronts.
Trends Biochem Sci. 29:233–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Daniele S, Costa B, Zappelli E, Da Pozzo
E, Sestito S, Nesi G, Campiglia P, Marinelli L, Novellino E,
Rapposelli S, et al: Combined inhibition of AKT/mTOR and MDM2
enhances glioblastoma multiforme cell apoptosis and differentiation
of cancer stem cells. Sci Rep. 5:99562015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Li C, Xin P, Xiao H, Zheng Y, Huang Y and
Zhu X: The dual PI3K/mTOR inhibitor NVP-BEZ235 inhibits
proliferation and induces apoptosis of burkitt lymphoma cells.
Cancer Cell Int. 15:652015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Liu Z, Ruan HJ, Gu PQ, Ding WY, Luo XH,
Huang R, Zhao W and Gao LJ: The roles of p38 MAPK and ERK1/2 in
coplanar polychlorinated biphenyls-induced apoptosis of human
extravillous cytotrophoblast-derived transformed cells. Cell
Physiol Biochem. 36:2418–2432. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Freudlsperger C, Burnett JR, Friedman JA,
Kannabiran VR, Chen Z and Van Waes C: EGFR-PI3K-AKT-mTOR signaling
in head and neck squamous cell carcinomas: Attractive targets for
molecular-oriented therapy. Expert Opin Ther Targets. 15:63–74.
2011. View Article : Google Scholar
|
|
106
|
Gan Y, Shi C, Inge L, Hibner M, Balducci J
and Huang Y: Differential roles of ERK and Akt pathways in
regulation of EGFR-mediated signaling and motility in prostate
cancer cells. Oncogene. 29:4947–4958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Seshacharyulu P, Ponnusamy MP, Haridas D,
Jain M, Ganti AK and Batra SK: Targeting the EGFR signaling pathway
in cancer therapy. Expert Opin Ther Targets. 16:15–31. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Kidger AM and Keyse SM: The regulation of
oncogenic Ras/ERK signalling by dual-specificity mitogen activated
protein kinase phosphatases (MKPs). Semin Cell Dev Biol.
50:125–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Gao Y, Moten A and Lin HK: Akt: A new
activation mechanism. Cell Res. 24:785–786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Liu P, Begley M, Michowski W, Inuzuka H,
Ginzberg M, Gao D, Tsou P, Gan W, Papa A, Kim BM, et al:
Cell-cycle-regulated activation of Akt kinase by phosphorylation at
its carboxyl terminus. Nature. 508:541–545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Aeder SE, Martin PM, Soh JW and Hussaini
IM: PKC-eta mediates glioblastoma cell proliferation through the
Akt and mTOR signaling pathways. Oncogene. 23:9062–9069. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Fan QW, Cheng C, Knight ZA, Haas-Kogan D,
Stokoe D, James CD, McCormick F, Shokat KM and Weiss WA: EGFR
signals to mTOR through PKC and independently of Akt in glioma. Sci
Signal. 2:ra42009. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mendoza MC, Er EE and Blenis J: The
Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends
Biochem Sci. 36:320–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Denduluri SK, Idowu O, Wang Z, Liao Z, Yan
Z, Mohammed MK, Ye J, Wei Q, Wang J, Zhao L, et al: Insulin-like
growth factor (IGF) signaling in tumorigenesis and the development
of cancer drug resistance. Genes Dis. 2:13–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Farabaugh SM, Boone DN and Lee AV: Role of
IGF1R in breast cancer subtypes, stemness and lineage
differentiation. Front Endocrinol (Lausanne). 6:592015.
|
|
116
|
Kapse-Mistry S, Govender T, Srivastava R
and Yergeri M: Nanodrug delivery in reversing multidrug resistance
in cancer cells. Front Pharmacol. 5:1592014.PubMed/NCBI
|
|
117
|
Salomon JJ and Ehrhardt C: Nanoparticles
attenuate P-glycoprotein/MDR1 function in A549 human alveolar
epithelial cells. Eur J Pharm Biopharm. 77:392–397. 2011.
View Article : Google Scholar
|
|
118
|
Callaghan R, Luk F and Bebawy M:
Inhibition of the multidrug resistance P-glycoprotein: Time for a
change of strategy? Drug Metab Dispos. 42:623–631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kakumoto M, Sakaeda T, Takara K, Nakamura
T, Kita T, Yagami T, Kobayashi H, Okamura N and Okumura K: Effects
of carvedilol on MDR1-mediated multidrug resistance: Comparison
with verapamil. Cancer Sci. 94:81–86. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wessler JD, Grip LT, Mendell J and
Giugliano RP: The P-glycoprotein transport system and
cardiovascular drugs. J Am Coll Cardiol. 61:2495–2502. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Mitsiades CS, Treon SP, Mitsiades N, Shima
Y, Richardson P, Schlossman R, Hideshima T and Anderson KC:
TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug
resis-tance in multiple myeloma: Therapeutic applications. Blood.
98:795–804. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Huang X, Kang B, Qian W, Mackey MA, Chen
PC, Oyelere AK, El-Sayed IH and El-Sayed MA: Comparative study of
photothermolysis of cancer cells with nuclear-targeted or
cytoplasm-targeted gold nanospheres: Continuous wave or pulsed
lasers. J Biomed Opt. 15:0580022010. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yang CJ and Chithrani DB: Nuclear
targeting of gold nanoparticles for improved therapeutics. Curr Top
Med Chem. 16:271–280. 2016. View Article : Google Scholar
|
|
124
|
Kodiha M, Wang YM, Hutter E, Maysinger D
and Stochaj U: Off to the organelles - killing cancer cells with
targeted gold nanoparticles. Theranostics. 5:357–370. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Yanes RE, Tarn D, Hwang AA, Ferris DP,
Sherman SP, Thomas CR, Lu J, Pyle AD, Zink JI and Tamanoi F:
Involvement of lysosomal exocytosis in the excretion of mesoporous
silica nanoparticles and enhancement of the drug delivery effect by
exocytosis inhibition. Small. 9:697–704. 2013. View Article : Google Scholar
|
|
126
|
Han SO, Xiao K, Kim J, Wu JH, Wisler JW,
Nakamura N, Freedman NJ and Shenoy SK: MARCH2 promotes endocytosis
and lysosomal sorting of carvedilol-bound β2-adrenergic
receptors. J Cell Biol. 199:817–830. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
El-Deiry WS: Insights into cancer
therapeutic design based on p53 and TRAIL receptor signaling. Cell
Death Differ. 8:1066–1075. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Sayers TJ: Targeting the extrinsic
apoptosis signaling pathway for cancer therapy. Cancer Immunol
Immunother. 60:1173–1180. 2011. View Article : Google Scholar : PubMed/NCBI
|