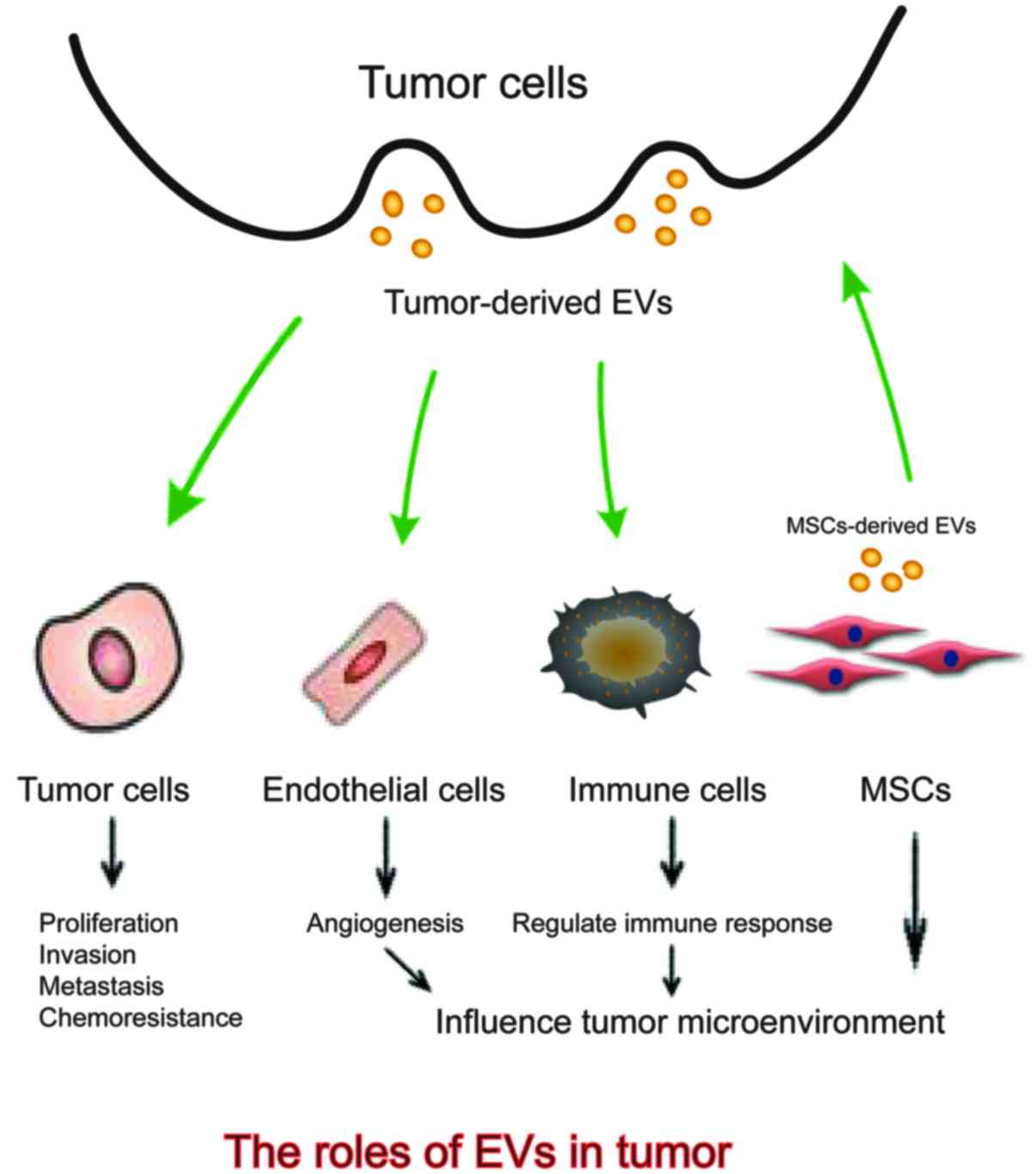
Intercellular communication is an essential hallmark
of multicellular organisms. In recent years, the intercellular
transfer of extracellular vesicles (EVs) have been discovered as a
remarkable new system for cell-to-cell communication (1,2). EVs
are a heterogeneous group of membrane-enclosed vesicles ranging
from 30 to 1,000 nm in size released by a variety of cells
including cancer cells into the extracellular milieu (3). Then, they can diffuse to neighboring
cells or be carried to distant locations where they may induce
signal transduction or mediate the horizontal transfer of molecular
information in recipient cells (4). Subsequent studies have shown that EVs
reflect the biological function of parental cells by containing a
variety of cargos such as proteins (including transmembrane and
enclosing cytosolic proteins), lipids, messenger RNA (mRNA),
microRNA (miRNA), non-coding RNAs and DNA (5). They can be detected in many human
body fluids, including plasma (6),
cerebrospinal fluid (7), urine
(8), bronchoalveolar lavage
(9), malignant ascites (10), saliva (11), semen (12), nasal lavage fluid and ascites
(13).
The composition and function of EVs depend on their
originating cells. Tumor-derived EVs have been recently discovered
to be involved in the transfer of oncogenic cargo through which
cancer cells can shape the tumor microenvironment and influence
tumor progression and metastasis (14–17).
Moreover, EVs derived from stromal cells in the tumor
microenvironment may contribute to tumor progression through the
transmission of their cargo to tumor cells (18,19).
This functional role of EVs in cancer development as well as their
ability to be easily isolated from body fluids such as serum makes
them an attractive candidate for biomarker development.
The present review will provide an overview of EVs
in nasopharyngeal carcinoma (NPC), with a focus on their role in
reprogramming tumor microenvironment and influencing tumor
progression. The potential role of the molecules within EVs in NPC
diagnosis and therapeutic targets will also be addressed.
Depending on the mode of release and the size, EVs
are currently classified into two general types: exosomes and
microvesicles (MVs) (20). They
are distinct in biogenesis, morphology, molecular composition, size
and buoyant density (20,21). In the present review, we provide a
brief description of exosomes and MVs prior to a discussion on
their roles in NPC.
A population of EVs formed from the inward budding
of intercellular endosomes result in multivesicular bodies (MVBs),
which are known as exosomes. After inward budding, the MVBs fuse
with the plasma membrane, releasing the exosomes into the
extracellular space (22). Another
release process involves the direct shedding by budding from the
plasma membrane and this process forms MVs (23). In addition to the differences in
the mode of release, the size of the vesicles is also used for
characterization. Although different scales are used, MVs are from
100 to 1,000 nm and exosomes are smaller with a diameter of 30 to
100 nm (24). EVs are mostly
isolated from body fluids and the supernatants of cultured cells by
performing differential ultracentrifugation (24). Then, varied sized classes of EVs
can be efficiently separated using their different floatation
velocity (25). Currently, with
the interest in their potential use in therapy or as biomarkers for
disease, commercially available kits are being developed and
marketed. Further characterization of isolated EVs requires
immunoblotting, mass spectrometry and imaging techniques. In
general, the specific EV marker proteins are typically used to show
the purity and enrichment, besides, mass spectrometry approach is
used to profile EV protein compositions. The morphology and size
can be determined by electronic microscopy. The number and size
distribution of EVs can be measured by nanoparticle tracking
analysis, flow cytometry and tunable resistive pulse sensing
(26). However, the distinctive
features, properties and functional roles of each subtype are still
under investigation.
EVs contain specific biologically active components
that could be transferred from the original cell to the recipient
cell to trigger downstream signaling events. They could directly
influence the recipient cells via cell surface interactions or by
manipulating the local and distant biological environment (27,28).
The above shows that EVs are implicated in a variety of tumor
malignant biological properties including modulating tumor
proliferation, invasion and metastasis, influencing angiogenesis,
regulating immune response and conferring chemoresistance (29–32)
(Fig. 1). Better understanding the
duality roles of EVs in intercellular communication will help to
gain further knowledge on the carcinogenic process.
Metastases refer to the concept that primary tumors
infiltrate through the basement membrane and disseminate to the
distant organs (33). Studies have
reported that the ability for a tumor cell moving to metastatic
colonization is not random (34).
Tumor cells travelling through the microcirculation is multistage
and complex, and more and more scientists have found that tumor
microenvironment plays an essential role in this progression.
Metastasis occurs when the tumor microenvironment is well suited
and tumor cells degrade connective tissue, extracellular matrix and
basement membrane components (35,36).
As this progression is via circulation, EVs also perform important
roles in nearly all the steps. EVs might transfer the signals to
other tumor cells and make EMT occur, which might help them to
invade the distant tissues easily. Then, EVs might be uptaken by
other cells to make the microenvironment more suitable for
metastatic cells arrest. Besides, EVs might also regulate
inflammation response pathways to help cell metastasis (15–19).
Angiogenesis is the new blood vessel formation from
pre-existing vasculature. It is a complex and multistep process
consisting of proliferation, migration, invasion, adhesion and
differentiation of endothelial cells (37). Angiogenesis occurs under various
physiological and pathological conditions (38) and pathological angiogenesis could
enhance tumor growth and metastasis by providing oxygen and
nutrients (39). Intriguingly,
previous studies have shown that genetic information can be
transferred to human umbilical vein endothelial cells (HUVECs) to
induce pathological angiogenesis (40). As EVs contain rich genetic
information and can mediate exchange of molecules (41), studies have been performed to know
the relationship between EVs and pathological angiogenesis.
Immunoescape has been considered as one of the most
common occurrences during tumor progression. Tumor cells might
employ different methods to evade immune surveillance, accelerating
their proliferation, migration and other biological malignant
behavior. Increasing evidence shows that the dysregulation of
immune system plays an essential role in tumor progression. As EVs
can mediate cell-to-cell communication, they are also known to
influence the immune system. EVs might contain receptors, proteins,
RNA and DNA, which can be uptaken by immune cells and impact the
immune microenvironment. This process could evoke immune responses,
either make cells escape the immune response or activate immune
suppression. Thus, investigating how tumor-derived EVs influence
immune system might help us find the mechanism of tumorigenesis and
develop new strategies for tumor therapy. Clayton et al
(46) reported that EVs secreted
by tumor cells might contribute to the production of extracellular
adenosine and modulate T cells in the tumor environment. Wieckowsk
et al (47) reported that
tumor-derived EVs might be able to induce immune suppression by
promoting T regulatory cell expansion and induce the apoptosis of
CD8(+) T cells via activating the Fas/Fas ligand pathway.
Tumor-derived EVs might also influence macrophages. EVs secreted by
prostate cancer contained high level of MFG-E8 (milk fat
globule-EGF factor 8 protein), when the macrophages were cocultured
with the tumor cells, MFG-E8 expression was elevated and possibly
polarized into M2 type tumor-associated macrophages. This type of
cells could accelerate tumor progression (48). Besides, EVs might have impact on
the complement system. Whitehead et al (49) showed that malignant cell-derived
EVs could increase complement activation via calcium-sensitive
pathways. Taken together, tumor derived EVs have important roles in
the immune system.
By playing a role in facilitating cell to cell
communication, EVs may mediate resistance to cytotoxic insults. In
lung cancer, Xiao et al (50) found that EVs could regulate the
sensitivity of tumor cells to cisplatin (DDP). They treated lung
cancer cells A549 with DDP, and more EVs secreted in the
supernatant were observed. Besides, in these EVs, the expression
levels of some miRNAs and mRNAs associated with DDP sensitivity
were dysregulated, transfer-ring DDP resistance to those untreated
cells. In breast cancer, drug-resistant breast cancer cells also
released EVs to confer adriamycin resistance to sensitive ones by
delivering specific miRNAs (51–53).
In prostate cancer, Corcoran et al (54) treated the cells with EVs derived
from sera of patients undergoing docetaxel treatment, and after the
treatment, these cells also showed response to docetaxel. These
results showed that EVs play an important role in drug resistance
and might act as new therapeutic targets.
An invasive biopsy of tumor is the golden standard
for most tumors, which might be difficult for the early detection
in some patients. As reported above, the physiological state of EVs
depends on their originating cells and EVs could be detected in
human body fluids, such as plasma, cerebrospinal fluid, saliva and
urine. This makes them easily isolated and act as new disease
biomarkers (24,55,56).
Tumor derived EVs contain specific proteins, mRNAs, miRNAs, which
could reflect the states of tumor cells, more and more scientists
are devoted to utilizing EVs for early diagnosis and assessment of
therapeutic responses or prognosis of tumors (57). Studies show that specific proteins
in EVs could be used for the early detection of tumors. Guan et
al (58) reported that the
expression level of MDA-9 and GRP78 were higher in EVs derived from
metastatic melanoma patients than those without metastases. Thus,
MDA-9 and GRP78 in EVs might be useful biomarkers for assessing the
prognosis of melanoma. In plasma samples from ovarian cancer
patients, EVs exhibited high expression of claudin-4, which might
be used as biomarker for ovarian cancer detection (59). Besides proteins, miRNAs in EVs have
also described as promising candidates as tumor biomarkers
(60). Cazzoli et al
(61) reported that miR-151a-5p,
miR-30a-3p, miR-200b-5p, miR-629, miR-100 and miR-154-3p in EVs
might be used to discriminate lung adenocarcinoma and granuloma.
Madhavan et al (62) showed
that miR-1246, miR-4644, miR-3976 and miR-4306 were upregulated in
83% of pancreatic cancer derived EVs, but rarely in control groups
and might act as highly sensitive biomarkers. Similar results were
also found in prostate cancer, glioblastoma, and colon cancer
(63–65). Not only EVs in serum could predict
the diagnosis of tumor, EVs in other body fluid might also act as
tumor biomarkers. Liu and colleagues (66) reported that miR-21 and miR-146a
were upregulated in EVs derived from the cervicovaginal lavage
specimens of cervical cancer patients. Therefore, EVs derived from
body fluids might take messages of the original cells and provide a
new biopsy technique for tumor diagnosis.
Recently, scientists are devoted to use EVs as the
therapeutic approach for tumor treatment. Hiltbrunner et al
(67) reported that peptide-loaded
EVs might be cancer treatment vehicles. They obtained
ovalbumin-loaded dendritic cell-derived EVs from MHCI−/−
mice, these EVs induce antigen-specific T cells response as
wild-type EVs, EVs lacking MHC class I could add tumor infiltrating
T cells and increase patients' overall survival. These results
confirmed the prospective of using impersonalized EVs for tumors
68). More and more evidence focused on the application of EVs in
tumor immunotherapy. Zhang et al (69) reported that EVs derived from
interleukin (IL)-12 expressing renal cancer cells might express
renal cell carcinoma-associated antigen G250 and GPI-IL-12, which
could promote the proliferation of T cells and increase the
immunogenicity and antitumor effects. This research is a novel way
of EV-based vaccine for tumor treatments. Besides, EVs could be
isolated from the stromal cells culturing media including MSCs,
which might exert similar functions to those of MSCs. MSC-derived
EVs could provide anticancer therapy via EV-mediated delivery of
anticancer drugs (70). Lou et
al (71) transfected adipose
tissue-derived MSCs (AMSC) with miR-122, which made the effectively
package of miR-122 into secreted EVs. After the communication
between AMSCs and HCC cells, the proliferation of HCC cells was
inhibited and the tumor cells were sensitive to the
chemotherapeutic agents. This research represented a promising
strategy for HCC chemotherapy. Shimbo et al (72) also reported that microRNA-143
containing MSCs could inhibit the migration of osteosarcoma
cells.
NPC has a variety of incidence rates throughout the
world, it is a squamous epithelial cancer arising from the
nasopharynx with an incidence of 30–80/100,000 each year in China
(73). The high mortality rate of
this disease arises from the lack of effective early diagnosis,
more importantly, most NPCs are poorly differentiated and have high
tendency to metastasize and invade adjacent regions, more than one
third of the patients will develop distant metastasis within 4
years (74). The prognosis may be
very poor as soon as NPC patients have metastatic disease (74,75).
Thus, the identification of the mechanisms associated with NPC
early diagnosis, metastasis and prognosis is of great significance.
Various research has shown that EVs, which play important roles in
tumor progression might also be present in NPC patient's serum and
be recognized and taken up by other cells in the microenvironment.
The intercellular communication between tumor cells and surrounding
cells could facilitate tumor proliferation, metastasis and immune
escape. Therefore, the functions of EVs in NPC progression and
NPC-derived EVs might serve as biomarkers for early diagnosis and
therapeutic targets.
The notion of tumor-associated microenvironment
refers to tumor-promoting and tumor-suppressing cells, soluble
molecules and extracellular matrix components (76). It is obvious that tumor cells and
stromal cells are in mutual dependence and in a sense, tumor
microenvironment has become 'the end of the cancer cell' (77). In the tumor microenvironment,
tumors release a variety of factors, which not only support tumor
proliferation but also facilitate tumor cells to metastasize to
distant organs (78). The factors
include single cells, EVs and cytokines and they can influence
distant tissues to have negative or positive feedback on themselves
(79,80). They might impact distant cell
signaling and maintain a better environment for tumor
progression.
Many studies have tried to understand the cellular
interactions within the tumor microenvironment (81). As important components of tumor
stromal cells, mesenchymal stem cells (MSCs) have received much
attention in recent years (82).
Studies have shown that MSCs could home to primary or metastatic
tumor sites and contribute to the formation of the tumor
microenvironment (83,84). As paracrine effectors of MSCs, EVs
have also been reported to play an important role during the
interaction between tumor microenvironment and tumor cells, they
could carry membrane and cytoplasmic components and mediate
interactions with target cells (85). Previous studies have reported that
EVs derived from MSCs might promote renal cancer cell growth and
EVs from multiple myeloma (MM) patient bone marrow-derived MSCs
promoted MM tumor growth (18,86).
A recent study reported that EVs also mediate the interaction
between MSCs and NPC cells. The data showed that NPC cells could
take up MSC-derived EVs and these EVs could promote tumor
proliferation, migration and the process of epithelial-mesenchymal
transition (EMT). Moreover, the study showed that FGF19 was highly
expressed in MSC-EVs. Besides, MSC-EVs could stimulate NPC
progression by activating the FGF19-FGFR4-dependent ERK signaling
cascade and by modulating the EMT. These data indicated that EVs
have an important role in NPC microenvironment and participate in
influencing NPC progression (19).
Recent clinical and preclinical findings in NPC
suggest that tumor hypoxia which occurs in >80% of NPC tumors
playing a key role in NPC progression and resistance to therapy
(87–89). Hypoxia, or oxygen deprivation, is
one of the most common phenomena in human solid tumors. The lack of
oxygen in the inner core of solid tumors, primarily due to
increasing distance of tumor cells from blood vessels and the
formation of aberrant blood vessels resulting in poor blood flow,
is believed to contribute to tumor progression, as well as
resistance to chemotherapy and radiotherapy (90,91).
Cancer cells can adapt to a hypoxic microenvironment
via multiple cellular mechanisms (92). EVs are among the most significant
tumor promoting factors stimulated by hypoxia that influence
adjacent tumor microenvironments (93). Hypoxia can remarkably stimulate EVs
secretion; for instance, nucleic acids and proteins as transmission
signals in EVs in the tumor microenvironment are involved in
various functions, such as inducing intratumoral heterogeneity,
altering immunological responses, producing cancer-associated
fibroblasts and promoting angiogenesis and metastasis (92). Park et al (94)found that hypoxia (1% O2)
is insufficient to induce apoptosis; nevertheless, hypoxia can
stimulate the release of EVs in human lung cancer cell line A549
and aid angiogenesis by chemo-tactically attracting endothelial
cells and fibroblasts and by stimulating stromal cells to release
angiogenesis-promoting cytokines. As in skin cancers, hypoxic A431
carcinoma cells released EVs enhancing angiogenesis in a
chorioallantoic membrane assay and metastasis. Aga et al
(95) demonstrated that endogenous
hypoxia-inducible factor 1 alpha (HIF1-α) is detectable in EVs and
that latent membrane protein 1 (LMP1) could increase the level of
HIF1-α in EVs. The present study found that in NPC, hypoxia
stimulated MMP-13 expression in EVs in a HIF-1α dependent manner.
Moreover, MMP-13 in EVs significantly upregulated vimentin
expression, while decreasing E-cadherin level in NPC cells, in
vitro and in vivo (96).
Heavy lymphoid infiltration in the primary tumor
sites is an important biologic feature of NPC (97). EVs, which act as intercellular
vehicles are also reported as important mediators in NPC
progression and immune escape. Ye et al (98) isolated EVs from the serum of NPC
patients and found the concentration of EVs was positively
correlated with lymph node stage and poor prognosis of NPC
patients. To further confirm the high level of EVs in patients with
lymph node metastasis was associated with T-cell immune response,
the scientists treated T cells with EVs derived from the
supernatant of NPC cells TW03. The results showed that TW03-derived
EVs could inhibit the proliferation of T-cell and the
differentiation of Th1 and Th17 and induce regulatory T cells (Treg
cells) by altering p-ERK and p-STAT. Besides, NPC derived EVs also
have anti-inflammatory effects and increase the expression of
proinflammatory cytokines. These findings suggested that EVs might
be potential targets for NPC immunotherapy. Some scientists also
focused on Treg cells within the tumor site and investigated the
mechanisms of Treg recruitment and the interaction between NPC-EVs
and Treg cells. The results showed that CCL20 was highly expressed
in NPC-EVs, which might play an important role in the recruitment
of human Treg into tumor sites. Besides, NPC-EVs could induce the
conversion of CD4+CD25-T cells into
CD4+CD25+ Treg, then promote their regulatory
phenotype and increase the suppressive function of Treg. The
results confirmed that NPC-EVs in the tumor microenvironment could
interact with Treg to exert immunoregulatory properties. In a word,
NPC-EVs might be a newly defined way to regulate the immune system
of NPC (99).
EVs are reported to contain a variety of mRNAs,
miRNAs and proteins, which play an essential role in tumor
malignant behavior. NPC derived EVs also interact with other cells
and are involved in NPC proliferation, invasion and metastasis.
Thus, the studies highlight the important roles of EVs as they
might contribute to NPC progression. Abundant research has been
carried out to characterize the content of EVs.
HS1-associated protein X-1 (HAX-1) was identied more
than 10 years ago as a novel protein with ubiquitous tissue
expression (100). It has been
shown that HAX-1 interacts with the 3′-untranslated regions (3′UTR)
of a variety of proteins and binds to the 3′-untranslated regions
of certain mRNAs involvement in multiple signaling pathways and
cellular processes (101–106). HAX-1 is reported to be associated
with biological processes such as cell apoptosis, cell motility and
endocytosis, so it also plays an important role in regulating tumor
cell apoptosis, proliferation and invasion. HAX-1 expression is a
predictor of tumorigenesis, growth, progression, invasion, and
metastasis of many human malignancies (107,108), and is overexpressed in many
tumors (107,109,110) such as esophageal squamous cell
carcinoma (111,112), colorectal cancer (113), oral squamous cell carcinoma, lung
cancer, lymphoma, melanoma (114), leukemia, myeloma, breast cancer
and hepatoma (115). In our
previous study, we also found that HAX-1 was highly expressed in
NPC tissues compared with normal tissues. Besides, its expression
level was correlated with lymph node metastasis and clinical stage
of NPC patients, it could also predict poor prognosis (45). As reported, tumor-derived EVs play
an important role in tumor progression and metastasis by acting as
intercellular communicators (116,117). The roles of NPC-derived EVs in
NPC tumor growth, migration and angiogenesis were also confirmed in
our previous study (45).
Moreover, we found surprisingly that HAX-1 was selectively packaged
in NPC-derived EVs. It is highly expressed in EVs derived from NPC
patients compared with healthy donors (45). To confirm the role of EVs regulated
by HAX-1, we stimulated HUVECs with EVs which contain different
levels of HAX-1 protein. As expected, HAX-1-containing EVs could
accelerate HUVECs proliferation, migration and angiogenesis.
Moreover, the intracellular downstream pathways were also activated
during the interaction between recipient HUVECs with
HAX-1-containing EVs. So the expression level of HAX-1 in NPC
patients derived EVs might be a biomarker for NPC diagnosis and act
as a therapy target (45).
MMPs are members of the metzincin superfamily which
comprises zinc- and calcium-dependent enzymes comprising more than
24 subtypes (118). It is widely
accepted that MMPs mediate degradation and modify most components
of the extracellular matrix (ECM) and the basal membrane (BM),
which is critical for cancer invasion and metastasis (119–121). As one of the most important MMP
genes, the MMP-13 gene, also known as collagenase-3, is located in
chromosome 11q22, spanning approximately 12.5 kb and consists of
ten exons and nine introns (122). MMP-13 has the ability to disrupt
collagen types I, II, III, IV, VI and X (123). Previous studies have supported
that MMP-13 is found to have high expression in various types of
tumors, including those from different parts of an individual's
body, such as the breast, stomach, head, neck, larynx and
colorectum (124). In this sense,
upregulation of MMP-13 expression has been associated with
increases in invasion and metastasis, and MMP-13 may have a
potential influence on risks of cancer development and progression
(125,126). Previous evidence showed that
MMP-13 acts as a potential intermediate between low expression of
microRNA-125b and increasing metastatic potential of non-small cell
lung cancer (118). Fan et
al (127) found that leptin
signaling enhances cell invasion and promotes the metastasis of
human pancreatic cancer via increasing MMP-13 production. Sedighi
et al (123) found that
MMP-13 level was an accurate diagnostic marker especially to
differentiate pre-invasive/invasive lesions from normal controls
(sensitivity and specificity: 100%). These findings indicate a
potential clinical significance of serum MMP-13 measurement for
early detection and prognostic assessment in ESCC patients. In the
present study, we first demonstrated that MMP-13 was overexpressed
in NPC cells and EVs purified from conditioned medium (CM) as well
as NPC patient plasma. Furthermore, MMP-13-containing EVs
facilitated the metastasis of NPC cells as well as angiogenesis
which provided novel insight into the vital role of
MMP-13-containing EVs in NPC progression which might offer unique
insights for potential therapeutic strategies for NPC progressions.
Then, we further investigated that NPC cells exposed to hypoxia
release EVs containing higher level of MMP-13 in HIF-1α dependency
that enhances metastases by inducing EMT in vitro and in
vivo. We further found overexpression of HIF-1α and MMP-13
might be involved in the carcinogenesis and development of NPC and
they were associated with poor patient prognosis. Thus, MMP-13
overexpression was triggered by hypoxia/HIF-1α as an important
mechanism that induced EMT and tumor invasion in NPC (96).
Current studies suggest that EVs are important
regulators of cell-cell communication. The growing knowledge on
their roles in urologic malignancies provides the basis for novel
therapeutic strategies. In addition, nucleic acid and the protein
content of EVs hold promise for tumor therapy. For NPC, more and
more studies have showed the importance of EVs in tumor
proliferation, metastasis, angiogenesis, immune regulation and so
on. Besides, EVs could act as potential biomarkers in the early
diagnosis of NPC and be used in NPC treatments. However, there are
still many questions to be answered including its deeper
mechanisms. So further fundamental researches and pre-clinical
trials need to be carried out to help better understanding of the
role of EVs in NPC. We hope the future studies will give evidence
for the large-scale clinical utilization of EVs.
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81672682,
81602385 and 81702707), the Major Scientific Research Project of
Jiangsu province (grant no. BE2017680), the Natural Science
Foundation of Jiangsu Province (grant no. BK20151266), the Nantong
Application Research Project (grant nos. MS32015020 and HS2016001),
the Innovative Research Project for postgraduate students of
Jiangsu province (no. SJLX15_0645 to L.B.).
|
1
|
Frühbeis C, Fröhlich D and Krämer-Albers
EM: Emerging roles of exosomes in neuron-glia communication. Front
Physiol. 3:1192012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frühbeis C, Fröhlich D, Kuo WP, Amphornrat
J, Thilemann S, Saab AS, Kirchhoff F, Möbius W, Goebbels S, Nave
KA, et al: Neurotransmitter-triggered transfer of exosomes mediates
oligodendrocyte-neuron communication. PLoS Biol. 11:e10016042013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abels ER and Breakefield XO: Introduction
to extracellular vesicles: Biogenesis, RNA cargo selection,
content, release, and uptake. Cell Mol Neurobiol. 36:301–312. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Syn N, Wang L, Sethi G, Thiery JP and Goh
BC: Exosome-mediated metastasis: From epithelial-mesenchymal
transition to escape from immunosurveillance. Trends Pharmacol Sci.
37:606–617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zaborowski MP, Balaj L, Breakefield XO and
Lai CP: Extracellular vesicles: Composition, biological relevance,
and methods of study. Bioscience. 65:783–797. 2015. View Article : Google Scholar
|
|
6
|
Grant R, Ansa-Addo E, Stratton D,
Antwi-Baffour S, Jorfi S, Kholia S, Krige L, Lange S and Inal J: A
filtration-based protocol to isolate human plasma membrane-derived
vesicles and exosomes from blood plasma. J Immunol Methods.
371:143–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Street JM, Barran PE, Mackay CL, Weidt S,
Balmforth C, Walsh TS, Chalmers RT, Webb DJ and Dear JW:
Identification and proteomic profiling of exosomes in human
cerebrospinal fluid. J Transl Med. 10:52012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pisitkun T, Shen RF and Knepper MA:
Identification and proteomic profiling of exosomes in human urine.
Proc Natl Acad Sci USA. 101:13368–13373. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prado N, Marazuela EG, Segura E,
Fernández-García H, Villalba M, Théry C, Rodríguez R and Batanero
E: Exosomes from bronchoalveolar fluid of tolerized mice prevent
allergic reaction. J Immunol. 181:1519–1525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Runz S, Keller S, Rupp C, Stoeck A, Issa
Y, Koensgen D, Mustea A, Sehouli J, Kristiansen G and Altevogt P:
Malignant ascites-derived exosomes of ovarian carcinoma patients
contain CD24 and EpCAM. Gynecol Oncol. 107:563–571. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ogawa Y, Miura Y, Harazono A, Kanai-Azuma
M, Akimoto Y, Kawakami H, Yamaguchi T, Toda T, Endo T, Tsubuki M,
et al: Proteomic analysis of two types of exosomes in human whole
saliva. Biol Pharm Bull. 34:13–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aalberts M, van Dissel-Emiliani FM, van
Adrichem NP, van Wijnen M, Wauben MH, Stout TA and Stoorvogel W:
Identification of distinct populations of prostasomes that
differentially express prostate stem cell antigen, annexin A1, and
GLIPR2 in humans. Biol Reprod. 86:822012. View Article : Google Scholar
|
|
13
|
Andre F, Schartz NE, Movassagh M, Flament
C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier
T, et al: Malignant effusions and immunogenic tumour-derived
exosomes. Lancet. 360:295–305. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rak J and Guha A: Extracellular vesicles -
vehicles that spread cancer genes. BioEssays. 34:489–497. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khan S, Jutzy JM, Aspe JR, McGregor DW,
Neidigh JW and Wall NR: Survivin is released from cancer cells via
exosomes. Apoptosis. 16:1–12. 2011. View Article : Google Scholar :
|
|
16
|
Dutta S, Warshall C, Bandyopadhyay C,
Dutta D and Chandran B: Interactions between exosomes from breast
cancer cells and primary mammary epithelial cells leads to
generation of reactive oxygen species which induce DNA damage
response, stabilization of p53 and autophagy in epithelial cells.
PLoS One. 9:e975802014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hoshino A, Costa-Silva B, Shen TL,
Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di
Giannatale A, Ceder S, et al: Tumour exosome integrins determine
organotropic metastasis. Nature. 527:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roccaro AM, Sacco A, Maiso P, Azab AK, Tai
YT, Reagan M, Azab F, Flores LM, Campigotto F, Weller E, et al: BM
mesenchymal stromal cell-derived exosomes facilitate multiple
myeloma progression. J Clin Invest. 123:1542–1555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi S, Zhang Q, Xia Y, You B, Shan Y, Bao
L, Li L, You Y and Gu Z: Mesenchymal stem cell-derived exosomes
facilitate nasopharyngeal carcinoma progression. Am J Cancer Res.
6:459–472. 2016.PubMed/NCBI
|
|
20
|
Colombo M, Raposo G and Théry C:
Biogenesis, secretion, and intercellular interactions of exosomes
and other extracellular vesicles. Annu Rev Cell Dev Biol.
30:255–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tkach M and Théry C: Communication by
extracellular vesicles: Where we are and where we need to go. Cell.
164:1226–1232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mathivanan S, Ji H and Simpson RJ:
Exosomes: Extracellular organelles important in intercellular
communication. J Proteomics. 73:1907–1920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martins VR, Dias MS and Hainaut P:
Tumor-cell-derived microvesicles as carriers of molecular
information in cancer. Curr Opin Oncol. 25:66–75. 2013. View Article : Google Scholar
|
|
24
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles, and friends. J Cell Biol.
200:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Taylor DD, Zacharias W and Gercel-Taylor
C: Exosome isolation for proteomic analyses and RNA profiling.
Methods Mol Biol. 728:235–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Momen-Heravi F, Balaj L, Alian S, Tigges
J, Toxavidis V, Ericsson M, Distel RJ, Ivanov AR, Skog J and Kuo
WP: Alternative methods for characterization of extracellular
vesicles. Front Physiol. 3:3542012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Al-Nedawi K, Meehan B and Rak J:
Microvesicles: Messengers and mediators of tumor progression. Cell
Cycle. 8:2014–2018. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ratajczak J, Wysoczynski M, Hayek F,
Janowska-Wieczorek A and Ratajczak MZ: Membrane-derived
microvesicles: Important and underappreciated mediators of
cell-to-cell communication. Leukemia. 20:1487–1495. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Azmi AS, Bao B and Sarkar FH: Exosomes in
cancer development, metastasis, and drug resistance: A
comprehensive review. Cancer Metastasis Rev. 32:623–642. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu DD, Wu Y, Shen HY, Lv MM, Chen WX,
Zhang XH, Zhong SL, Tang JH and Zhao JH: Exosomes in development,
metastasis and drug resistance of breast cancer. Cancer Sci.
106:959–964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Robinson SM, Fan L, White SA, Charnley RM
and Mann J: The role of exosomes in the pathogenesis of pancreatic
ductal adenocarcinoma. Int J Biochem Cell Biol. 75:131–139. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kahlert C and Kalluri R: Exosomes in tumor
microenvironment influence cancer progression and metastasis. J Mol
Med (Berl). 91:431–437. 2013. View Article : Google Scholar
|
|
33
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
35
|
Funasaka T and Raz A: The role of
autocrine motility factor in tumor and tumor microenvironment.
Cancer Metastasis Rev. 26:725–735. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Funasaka T and Wong RW: The role of
nuclear pore complex in tumor microenvironment and metastasis.
Cancer Metastasis Rev. 30:239–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoon YJ, Kim DK, Yoon CM, Park J, Kim YK,
Roh TY and Gho YS: Egr-1 activation by cancer-derived extracellular
vesicles promotes endothelial cell migration via ERK1/2 and JNK
signaling pathways. PLoS One. 9:e1151702014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Luo F, Wang B, Li H, Xu Y, Liu X,
Shi L, Lu X, Xu W, Lu L, et al: STAT3-regulated exosomal miR-21
promotes angiogenesis and is involved in neoplastic processes of
transformed human bronchial epithelial cells. Cancer Lett.
370:125–135. 2016. View Article : Google Scholar
|
|
41
|
Zomer A, Maynard C, Verweij FJ, Kamermans
A, Schäfer R, Beerling E, Schiffelers RM, de Wit E, Berenguer J,
Ellenbroek SIJ, et al: In Vivo imaging reveals extracellular
vesicle-mediated phenocopying of metastatic behavior. Cell.
161:1046–1057. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fleury A, Martinez MC and Le Lay S:
Extracellular vesicles as therapeutic tools in cardiovascular
diseases. Front Immunol. 5:3702014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kosaka N: Decoding the secret of cancer by
means of extracellular vesicles. J Clin Med. 5:52016. View Article : Google Scholar
|
|
44
|
Kosaka N, Iguchi H, Hagiwara K, Yoshioka
Y, Takeshita F and Ochiya T: Neutral sphingomyelinase 2
(nSMase2)-dependent exosomal transfer of angiogenic microRNAs
regulate cancer cell metastasis. J Biol Chem. 288:10849–10859.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
You B, Cao X, Shao X, Ni H, Shi S, Shan Y,
Gu Z and You Y: Clinical and biological significance of HAX-1
overexpression in nasopharyngeal carcinoma. Oncotarget.
7:12505–12524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Clayton A, Al-Taei S, Webber J, Mason MD
and Tabi Z: Cancer exosomes express CD39 and CD73, which suppress T
cells through adenosine production. J Immunol. 187:676–683. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wieckowski EU, Visus C, Szajnik M,
Szczepanski MJ, Storkus WJ and Whiteside TL: Tumor-derived
microvesicles promote regulatory T cell expansion and induce
apoptosis in tumor-reactive activated CD8+ T
lymphocytes. J Immunol. 183:3720–3730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Soki FN, Koh AJ, Jones JD, Kim YW, Dai J,
Keller ET, Pienta KJ, Atabai K, Roca H and McCauley LK:
Polarization of prostate cancer-associated macrophages is induced
by milk fat globule-EGF factor 8 (MFG-E8)-mediated efferocytosis. J
Biol Chem. 289:24560–24572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Whitehead B, Wu L, Hvam ML, Aslan H, Dong
M, Dyrskjøt L, Ostenfeld MS, Moghimi SM and Howard KA: Tumour
exosomes display differential mechanical and complement activation
properties dependent on malignant state: Implications in
endothelial leakiness. J Extracell Vesicles. 4:296852015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xiao X, Yu S, Li S, Wu J, Ma R, Cao H, Zhu
Y and Feng J: Exosomes: Decreased sensitivity of lung cancer A549
cells to cisplatin. PLoS One. 9:e895342014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen WX, Liu XM, Lv MM, Chen L, Zhao JH,
Zhong SL, Ji MH, Hu Q, Luo Z, Wu JZ, et al: Exosomes from
drug-resistant breast cancer cells transmit chemoresistance by a
horizontal transfer of microRNAs. PLoS One. 9:e952402014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhong S, Chen X, Wang D, Zhang X, Shen H,
Yang S, Lv M, Tang J and Zhao J: MicroRNA expression profiles of
drug-resistance breast cancer cells and their exosomes. Oncotarget.
7:19601–19609. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yu DD, Wu Y, Zhang XH, Lv MM, Chen WX,
Chen X, Yang SJ, Shen H, Zhong SL, Tang JH, et al: Exosomes from
adriamycin-resistant breast cancer cells transmit drug resistance
partly by delivering miR-222. Tumour Biol. 37:3227–3235. 2016.
View Article : Google Scholar
|
|
54
|
Corcoran C, Rani S, O'Brien K, O'Neill A,
Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J, et
al: Docetaxel-resistance in prostate cancer: Evaluating associated
phenotypic changes and potential for resistance transfer via
exosomes. PLoS One. 7:e509992012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Brinton LT, Sloane HS, Kester M and Kelly
KA: Formation and role of exosomes in cancer. Cell Mol Life Sci.
72:659–671. 2015. View Article : Google Scholar
|
|
56
|
Yáñez-Mó M, Siljander PR, Andreu Z, Zavec
AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J,
et al: Biological properties of extracellular vesicles and their
physiological functions. J Extracell Vesicles. 4:270662015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Silva M and Melo SA: Non-coding RNAs in
exosomes: New players in cancer biology. Curr Genomics. 16:295–303.
2015. View Article : Google Scholar
|
|
58
|
Guan M, Chen X, Ma Y, Tang L, Guan L, Ren
X, Yu B, Zhang W and Su B: MDA-9 and GRP78 as potential diagnostic
biomarkers for early detection of melanoma metastasis. Tumour Biol.
36:2973–2982. 2015. View Article : Google Scholar
|
|
59
|
Li J, Sherman-Baust CA, Tsai-Turton M,
Bristow RE, Roden RB and Morin PJ: Claudin-containing exosomes in
the peripheral circulation of women with ovarian cancer. BMC
Cancer. 9:2442009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fujita Y, Kuwano K, Ochiya T and Takeshita
F: The impact of extracellular vesicle-encapsulated circulating
microRNAs in lung cancer research. BioMed Res Int. 2014:4864132014.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cazzoli R, Buttitta F, Di Nicola M,
Malatesta S, Marchetti A, Rom WN and Pass HI: microRNAs derived
from circulating exosomes as noninvasive biomarkers for screening
and diagnosing lung cancer. J Thorac Oncol. 8:1156–1162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Madhavan B, Yue S, Galli U, Rana S, Gross
W, Müller M, Giese NA, Kalthoff H, Becker T, Büchler MW, et al:
Combined evaluation of a panel of protein and miRNA serum-exosome
biomarkers for pancreatic cancer diagnosis increases sensitivity
and specificity. Int J Cancer. 136:2616–2627. 2015. View Article : Google Scholar
|
|
63
|
Bryant RJ, Pawlowski T, Catto JW, Marsden
G, Vessella RL, Rhees B, Kuslich C, Visakorpi T and Hamdy FC:
Changes in circulating microRNA levels associated with prostate
cancer. Br J Cancer. 106:768–774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Manterola L, Guruceaga E, Gállego
Pérez-Larraya J, González-Huarriz M, Jauregui P, Tejada S,
Diez-Valle R, Segura V, Samprón N, Barrena C, et al: A small
noncoding RNA signature found in exosomes of GBM patient serum as a
diagnostic tool. Neuro Oncol. 16:520–527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ogata-Kawata H, Izumiya M, Kurioka D,
Honma Y, Yamada Y, Furuta K, Gunji T, Ohta H, Okamoto H, Sonoda H,
et al: Circulating exosomal microRNAs as biomarkers of colon
cancer. PLoS One. 9:e929212014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu J, Sun H, Wang X, Yu Q, Li S, Yu X and
Gong W: Increased exosomal microRNA-21 and microRNA-146a levels in
the cervicovaginal lavage specimens of patients with cervical
cancer. Int J Mol Sci. 15:758–773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hiltbrunner S, Larssen P, Eldh M,
Martinez-Bravo MJ, Wagner AK, Karlsson MC and Gabrielsson S:
Exosomal cancer immunotherapy is independent of MHC molecules on
exosomes. Oncotarget. 7:38707–38717. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rafi MA and Omidi Y: A prospective
highlight on exosomal nanoshuttles and cancer immunotherapy and
vaccination. Bioimpacts. 5:117–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang Y, Luo CL, He BC, Zhang JM, Cheng G
and Wu XH: Exosomes derived from IL-12-anchored renal cancer cells
increase induction of specific antitumor response in vitro: A novel
vaccine for renal cell carcinoma. Int J Oncol. 36:133–140.
2010.
|
|
70
|
Pashoutan Sarvar D, Shamsasenjan K and
Akbarzadehlaleh P: Mesenchymal stem cell-derived exosomes: New
opportunity in cell-free therapy. Adv Pharm Bull. 6:293–299. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lou G, Song X, Yang F, Wu S, Wang J, Chen
Z and Liu Y: Exosomes derived from miR-122-modified adipose
tissue-derived MSCs increase chemosensitivity of hepatocellular
carcinoma. J Hematol Oncol. 8:1222015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Shimbo K, Miyaki S, Ishitobi H, Kato Y,
Kubo T, Shimose S and Ochi M: Exosome-formed synthetic microRNA-143
is transferred to osteosarcoma cells and inhibits their migration.
Biochem Biophys Res Commun. 445:381–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Brennan B: Nasopharyngeal carcinoma.
Orphanet J Rare Dis. 1:232006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lee AW, Yau TK, Wong DH, Chan EW, Yeung
RM, Ng WT, Tong M, Soong IS and Sze WM: Treatment of stage IV(A-B)
nasopharyngeal carcinoma by induction-concurrent chemoradiotherapy
and accelerated fractionation. Int J Radiat Oncol Biol Phys.
63:1331–1338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Strazzulla A, Barreca GS, Giancotti A,
Pisani V, Costa C, Zicca E, La Boria A, Roveda L, Liberto MC, Tucci
L, et al: Nasopharyngeal carcinoma: Review of the literature with a
focus on therapeutical implications. Infez Med. 23:224–229.
2015.PubMed/NCBI
|
|
76
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sonnenschein C and Soto AM: The death of
the cancer cell. Cancer Res. 71:4334–4337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Robinson BD, Sica GL, Liu YF, Rohan TE,
Gertler FB, Condeelis JS and Jones JG: Tumor microenvironment of
metastasis in human breast carcinoma: A potential prognostic marker
linked to hematogenous dissemination. Clin Cancer Res.
15:2433–2441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Redon CE, Dickey JS, Nakamura AJ, Kareva
IG, Naf D, Nowsheen S, Kryston TB, Bonner WM, Georgakilas AG and
Sedelnikova OA: Tumors induce complex DNA damage in distant
proliferative tissues in vivo. Proc Natl Acad Sci USA.
107:17992–17997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Critchley-Thorne RJ, Simons DL, Yan N,
Miyahira AK, Dirbas FM, Johnson DL, Swetter SM, Carlson RW, Fisher
GA, Koong A, et al: Impaired interferon signaling is a common
immune defect in human cancer. Proc Natl Acad Sci USA.
106:9010–9015. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gourzones C, Barjon C and Busson P:
Host-tumor interactions in nasopharyngeal carcinomas. Semin Cancer
Biol. 22:127–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Salem HK and Thiemermann C: Mesenchymal
stromal cells: Current understanding and clinical status. Stem
Cells. 28:585–596. 2010.
|
|
83
|
Droujinine IA, Eckert MA and Zhao W: To
grab the stroma by the horns: From biology to cancer therapy with
mesenchymal stem cells. Oncotarget. 4:651–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kidd S, Spaeth E, Dembinski JL, Dietrich
M, Watson K, Klopp A, Battula VL, Weil M, Andreeff M and Marini FC:
Direct evidence of mesenchymal stem cell tropism for tumor and
wounding microenvironments using in vivo bioluminescent imaging.
Stem Cells. 27:2614–2623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Vlassov AV, Magdaleno S, Setterquist R and
Conrad R: Exosomes: Current knowledge of their composition,
biological functions, and diagnostic and therapeutic potentials.
Biochim Biophys Acta. 1820:940–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Du T, Ju G, Wu S, Cheng Z, Cheng J, Zou X,
Zhang G, Miao S, Liu G and Zhu Y: Microvesicles derived from human
Wharton's jelly mesenchymal stem cells promote human renal cancer
cell growth and aggressiveness through induction of hepatocyte
growth factor. PLoS One. 9:e968362014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yeh SH, Liu RS, Wu LC, Yang DJ, Yen SH,
Chang CW, Yu TW, Chou KL and Chen KY: Fluorine-18
fluoromisonidazole tumour to muscle retention ratio for the
detection of hypoxia in nasopharyngeal carcinoma. Eur J Nucl Med.
23:1378–1383. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zheng YJ, Fan W, Zhao C, Yang XC, Cui NJ
and Chen FJ: Clinical application of 99mTc-HL91 hypoxia imaging in
nasopharyngeal carcinoma. Ai Zheng. 25:378–381. 2006.In Chinese.
PubMed/NCBI
|
|
89
|
Zheng YJ, Zhao C, Fan W, Liu H, Cui NJ and
Chen FJ: Changes of hypoxia in primary lesion of nasopharyngeal
carcinoma during the treatment course and the clinical value
thereof. Zhonghua Yi Xue Za Zhi. 87:2698–2702. 2007.In Chinese.
|
|
90
|
Hong B, Lui VWY, Hashiguchi M, Hui EP and
Chan ATC: Targeting tumor hypoxia in nasopharyngeal carcinoma. Head
Neck. 35:133–145. 2013. View Article : Google Scholar
|
|
91
|
Janssen HL, Haustermans KM, Balm AJ and
Begg AC: Hypoxia in head and neck cancer: How much, how important?
Head Neck. 27:622–638. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yang Y, Yang X, Yang Y, Zhu H, Chen X,
Zhang H, Wang F, Qin Q, Cheng H and Sun X: Exosomes: A promising
factor involved in cancer hypoxic microenvironments. Curr Med Chem.
22:4189–4195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yang X, Zhu H, Ge Y, Liu J, Cai J, Qin Q,
Zhan L, Zhang C, Xu L, Liu Z, et al: Melittin enhances
radiosensitivity of hypoxic head and neck squamous cell carcinoma
by suppressing HIF-1α. Tumour Biol. 35:10443–10448. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Park JE, Tan HS, Datta A, Lai RC, Zhang H,
Meng W, Lim SK and Sze SK: Hypoxic tumor cell modulates its
microenvironment to enhance angiogenic and metastatic potential by
secretion of proteins and exosomes. Mol Cell Proteomics.
9:1085–1099. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Aga M, Bentz GL, Raffa S, Torrisi MR,
Kondo S, Wakisaka N, Yoshizaki T, Pagano JS and Shackelford J:
Exosomal HIF1α supports invasive potential of nasopharyngeal
carcinoma-associated LMP1-positive exosomes. Oncogene.
33:4613–4622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
You Y, Shan Y, Chen J, Yue H, You B, Shi
S, Li X and Cao X: Matrix metalloproteinase 13-containing exosomes
promote nasopharyngeal carcinoma metastasis. Cancer Sci.
106:1669–1677. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ferradini L, Miescher S, Stoeck M, Busson
P, Barras C, Cerf-Bensussan N, Lipinski M, von Fliedner V and Tursz
T: Cytotoxic potential despite impaired activation pathways in T
lymphocytes infiltrating nasopharyngeal carcinoma. Int J Cancer.
47:362–370. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS,
Zhang XS, Cui J, Zeng YX and Li J: Tumor-derived exosomes promote
tumor progression and T-cell dysfunction through the regulation of
enriched exosomal microRNAs in human nasopharyngeal carcinoma.
Oncotarget. 5:5439–5452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Mrizak D, Martin N, Barjon C,
Jimenez-Pailhes AS, Mustapha R, Niki T, Guigay J, Pancré V, de
Launoit Y, Busson P, et al: Effect of nasopharyngeal
carcinoma-derived exosomes on human regulatory T cells. J Natl
Cancer Inst. 107:3632014.PubMed/NCBI
|
|
100
|
Simmen T: Hax-1: A regulator of calcium
signaling and apoptosis progression with multiple roles in human
disease. Expert Opin Ther Targets. 15:741–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Fadeel B and Grzybowska E: HAX-1: A
multifunctional protein with emerging roles in human disease.
Biochim Biophys Acta. 1790:1139–1148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Sharp TV, Wang HW, Koumi A, Hollyman D,
Endo Y, Ye H, Du MQ and Boshoff C: K15 protein of Kaposi's
sarcoma-associated herpesvirus is latently expressed and binds to
HAX-1, a protein with antiapoptotic function. J Virol. 76:802–816.
2002. View Article : Google Scholar
|
|
103
|
Lee AY, Lee Y, Park YK, Bae KH, Cho S, Lee
DH, Park BC, Kang S and Park SG: HS 1-associated protein X-1 is
cleaved by caspase-3 during apoptosis. Mol Cells. 25:86–90.
2008.PubMed/NCBI
|
|
104
|
Vafiadaki E, Arvanitis DA, Pagakis SN,
Papalouka V, Sanoudou D, Kontrogianni-Konstantopoulos A and Kranias
EG: The anti-apoptotic protein HAX-1 interacts with SERCA2 and
regulates its protein levels to promote cell survival. Mol Biol
Cell. 20:306–318. 2009. View Article : Google Scholar :
|
|
105
|
Al-Maghrebi M, Brulé H, Padkina M, Allen
C, Holmes WM and Zehner ZE: The 3′ untranslated region of human
vimentin mRNA interacts with protein complexes containing eEF-1
gamma and HAX-1. Nucleic Acids Res. 30:5017–5028. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sarnowska E, Grzybowska EA, Sobczak K,
Konopinski R, Wilczynska A, Szwarc M, Sarnowski TJ, Krzyzosiak WJ
and Siedlecki JA: Hairpin structure within the 3′UTR of DNA
polymerase beta mRNA acts as a post-transcriptional regulatory
element and interacts with Hax-1. Nucleic Acids Res. 35:5499–5510.
2007. View Article : Google Scholar :
|
|
107
|
Ramsay AG, Keppler MD, Jazayeri M, Thomas
GJ, Parsons M, Violette S, Weinreb P, Hart IR and Marshall JF:
HS1-associated protein X-1 regulates carcinoma cell migration and
invasion via clathrin-mediated endocytosis of integrin alphavbeta6.
Cancer Res. 67:5275–5284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Radhika V, Onesime D, Ha JH and
Dhanasekaran N: Galpha13 stimulates cell migration through
cortactin-interacting protein Hax-1. J Biol Chem. 279:49406–49413.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Jiang Y, Zhang W, Kondo K, Klco JM, Martin
TB St, Dufault MR, Madden SL, Kaelin WG Jr and Nacht M: Gene
expression profiling in a renal cell carcinoma cell line:
Dissecting VHL and hypoxia-dependent pathways. Mol Cancer Res.
1:453–462. 2003.PubMed/NCBI
|
|
111
|
Li M, Tang Y, Zang W, Xuan X, Wang N, Ma
Y, Wang Y, Dong Z and Zhao G: Analysis of HAX-1 gene expression in
esophageal squamous cell carcinoma. Diagn Pathol.
8:472013.PubMed/NCBI
|
|
112
|
Sun SJ, Feng L, Zhao GQ and Dong ZM: HAX-1
promotes the chemoresistance, invasion, and tumorigenicity of
esophageal squamous carcinoma cells. Dig Dis Sci. 57:1838–1846.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wei XJ, Li SY, Yu B, Chen G, Du JF and Cai
HY: Expression of HAX-1 in human colorectal cancer and its clinical
significance. Tumour Biol. 35:1411–1415. 2014. View Article : Google Scholar
|
|
114
|
Li WB, Feng J, Geng SM, Zhang PY, Yan XN,
Hu G, Zhang CQ and Shi BJ: Induction of apoptosis by Hax-1 siRNA in
melanoma cells. Cell Biol Int. 33:548–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Banerjee A, Saito K, Meyer K, Banerjee S,
Ait-Goughoulte M, Ray RB and Ray R: Hepatitis C virus core protein
and cellular protein HAX-1 promote 5-fluorouracil-mediated
hepatocyte growth inhibition. J Virol. 83:9663–9671. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Janowska-Wieczorek A, Wysoczynski M,
Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J and
Ratajczak MZ: Microvesicles derived from activated platelets induce
metastasis and angiogenesis in lung cancer. Int J Cancer.
113:752–760. 2005. View Article : Google Scholar
|
|
117
|
Soldevilla B, Rodríguez M, San Millán C,
García V, Fernández-Periañez R, Gil-Calderón B, Martín P,
García-Grande A, Silva J, Bonilla F, et al: Tumor-derived exosomes
are enriched in ΔNp73, which promotes oncogenic potential in
acceptor cells and correlates with patient survival. Hum Mol Genet.
23:467–478. 2014. View Article : Google Scholar
|
|
118
|
Yu X, Wei F, Yu J, Zhao H, Jia L, Ye Y, Du
R, Ren X and Li H: Matrix metalloproteinase 13: A potential
intermediate between low expression of microRNA-125b and increasing
metastatic potential of non-small cell lung cancer. Cancer Genet.
208:76–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Hadler-Olsen E, Fadnes B, Sylte I,
Uhlin-Hansen L and Winberg JO: Regulation of matrix
metalloproteinase activity in health and disease. FEBS J.
278:28–45. 2011. View Article : Google Scholar
|
|
120
|
Radisky ES and Radisky DC: Matrix
metalloproteinase-induced epithelial-mesenchymal transition in
breast cancer. J Mammary Gland Biol Neoplasia. 15:201–212. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Hwang BM, Chae HS, Jeong YJ, Lee YR, Noh
EM, Youn HZ, Jung SH, Yu HN, Chung EY and Kim JS: Protein tyrosine
phosphatase controls breast cancer invasion through the expression
of matrix metalloproteinase-9. BMB Rep. 46:533–538. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Balbín M, Pendás AM, Uría JA, Jiménez MG,
Freije JP and López-Otín C: Expression and regulation of
collagenase-3 (MMP-13) in human malignant tumors. APMIS. 107:45–53.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Sedighi M, Aledavood SA, Abbaszadegan M,
Memar B, Montazer M, Rajabian M and Gholamin M: Matrix
metalloproteinase-13: A potential biomarker for detection and
prognostic assessment of patients with esophageal squamous Cell
Carcinoma. Asian Pac J Cancer Prev. 17:2781–2785. 2016.
|
|
124
|
Vairaktaris E, Yapijakis C, Nkenke E,
Serefoglou ZC, Chatzitheofylaktou A, Vassiliou S, Derka S,
Vylliotis A, Perrea D, Neukam FW, et al: A metalloproteinase-13
polymorphism affecting its gene expression is associated with
advanced stages of oral cancer. Anticancer Res. 27:4027–4030.
2007.
|
|
125
|
González-Arriaga P, López-Cima MF,
Fernández-Somoano A, Pascual T, Marrón MG, Puente XS and Tardón A:
Polymorphism +17 C/G in matrix metalloprotease MMP8 decreases lung
cancer risk. BMC Cancer. 8:3782008. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Li Y, Jia JH, Kang S, Zhang XJ, Zhao J,
Wang N, Zhou RM, Sun DL, Duan YN and Wang DJ: The functional
polymorphisms on promoter region of matrix metalloproteinase-12,
-13 genes may alter the risk of epithelial ovarian carcinoma in
Chinese. Int J Gynecol Cancer. 19:129–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Fan Y, Gan Y, Shen Y, Cai X, Song Y, Zhao
F, Yao M, Gu J and Tu H: Leptin signaling enhances cell invasion
and promotes the metastasis of human pancreatic cancer via
increasing MMP-13 production. Oncotarget. 6:16120–16134. 2015.
View Article : Google Scholar : PubMed/NCBI
|