Introduction
Melatonin, mainly synthesized and secreted by the
pineal gland, is known for its oncostatic actions on
estrogen-dependent mammary tumors. Studies using both breast cancer
animal models and breast cancer cell lines have examined the
effects of nocturnal physiological concentrations of melatonin (1
nM), concluding that the pineal hormone, at this concentration,
impairs the growth of estrogen-responsive breast cancer cell lines
stimulated with estradiol through at least two different
mechanisms: i) the downregulation of the neuroendocrine axis
(therefore resulting in a reduction in estrogen levels); and ii)
direct effects on tumoral and peritumoral cells. At this level,
melatonin regulates the expression and activity of several enzymes
necessary for the local synthesis of estradiol, thus behaving as a
selective estrogen enzyme modulator (SEEM) (1–3).
Additionally, the pineal hormone interferes directly with the
effects of estrogens after their binding with the estrogen receptor
(ER), therefore behaving as a selective estrogen receptor modulator
(SERM). Melatonin impairs the transcriptional activation triggered
by the E2-ERα-calmodulin complex through destabilization
of its binding at both ERE-and AP1 containing promoters (4). Of note, melatonin does not alter the
recruitment of activators induced by the E2-ERα complex,
indicating that its actions differ from those of other
anti‑estrogens used in cancer therapy (5,6).
Several findings point to cyclic adenosine monophosphate (cAMP) as
the likely link between the melatonin and estradiol pathways. In
mammary tumor cells, estrogens activate adenylate cyclase,
increasing cAMP levels; the increase in cAMP synergizes with the
genomic actions of estradiol, promoting transcription (7). On the contrary, melatonin acting
through its membrane receptor, MT1, decreases cAMP levels (8). As a result, this indoleamine reduces
the expression of estrogen-regulated proteins, growth factors and
proto-oncogenes [transforming growth factor (TGF)α,
c-MYC, pS2, progesterone receptor (PGR),
cFOS and TGFβ] in human breast cancer cells (9,10).
Similar to the effects of tamoxifen, the treatment
of human breast cancer cells with melatonin has been shown to cause
a G1-S transition delay (11), probably through the differential
expression of proteins controlling the G1-S transition.
Thus, melatonin increases the expression of TP53 (12) and inhibits that of human telomerase
reverse transcriptase (hTERT) (13,14).
Additionally, melatonin blocks the invasion of cells induced by
estradiol (15), and importantly,
melatonin exerts a modulatory effect in the tumor microenvironment,
inhibiting the secretion of cytokines by breast cancer cells
(16–18).
Several clinical trials have been performed to
assess the value of melatonin as an adjuvant in human neoplasms and
have revealed multiple beneficial effects of melatonin when used
concomitantly with chemotherapeutic agents. Chemotherapy is better
tolerated when administered to cancer patients in conjunction with
melatonin. The pineal hormone protects from side-effects, such as
asthenia, cardiotoxicity and neurotoxicity, and it increases both
the 1- and 5-year survival rates and the objective tumor regression
in patients with metastatic solid tumors (19–23).
Melatonin exerts anticancer effects at different
phases of carcinogenesis, namely initiation, progression and
spreading from the primary tumor (24). Recently, studies have addressed the
potential benefits that melatonin could have on the effects of
chemotherapeutic agents (25). The
disruption of the nocturnal melatonin rhythm contributes to a
complete loss of tumor sensitivity to doxorubicin (26), whereas melatonin co-treatment
sensitizes cancer cells to this drug, increasing doxorubicin
intracellular concentrations, possibly through a downregulation in
the levels of P-glycoprotein (27). Melatonin induces Bim
upregulation and cyclooxygenase (COX)-2
down-regulation, thus enhancing the tunicamycin-induced apoptosis
of breast cancer cells (28), and
sensitizing non-small-cell lung cancer cells harbouring an
epidermal growth factor receptor (EGFR) mutation to
gefitinib, a specific tyrosine kinase inhibitor (29). The pineal hormone enhances
cisplatin-induced cytotoxicity and the apoptosis of lung cancer and
cervical cancer cells (30,31).
Additionally, co-treatment with melatonin and each of three
different chemotherapeutic agents (5‑fluorouracil, cisplatin and
doxorubicin) has been shown to result in the enhanced
chemotherapy-induced cytotoxicity and apoptosis of the rat
pancreatic tumor cell line, AR42J (32). In ER-responsive mammary cancer rat
models treated with adriamycin, melatonin co-treatment was shown to
result in lighter tumor weights, increased apoptosis, a higher
expression of E-cadherin and a higher survival rate (33). In MCF-7 cells, melatonin has been
shown to exert synergistic effects with doxorubicin on apoptosis
and the activation of transient receptor potential vanilloid
(TRPV)1 channels (34). In
prostate cancer cells, melatonin combined with doxorubicin,
docetaxel or etoposide, has been shown to make cells more sensitive
to these compounds (35). Finally,
as regards its ability to modulate global gene expression,
melatonin influences both microRNA (miRNA or miR) and gene
expression. In a global gene expression study on MCF-7 cells, 22
miRNAs were found to be differentially expressed in
melatonin-treated cells (36).
Thus, since melatonin: i) is associated with
oncostatic actions both in vivo and in vitro; ii)
sensitizes many cell lines to treatment with different
chemotherapeutic agents; and iii) seems to exert several beneficial
effects when administered concomitantly with chemotherapeutic
agents to patients bearing solid tumors, in the present study we
investigated the effects of melatonin on the proliferation, cell
cycle progression and gene transcription in estrogen-sensitive
MCF-7 human breast cancer cells treated with docetaxel, a
microtubule-interfering agent that stabilizes microtubules,
commonly used in chemotherapy. Docetaxel belongs to the family of
taxanes, a class of diterpenes used in the treatment of various
types of cancer, including breast cancer.
Materials and methods
Cells and culture conditions
MCF-7 human breast cancer cells were purchased from
the American Tissue Culture Collection (ATCC, Rockville, MD, USA).
They were maintained as monolayer cultures in 75-cm2
plastic culture flasks in Dulbecco's modified Eagle's medium (DMEM)
(Sigma‑Aldrich, Madrid, Spain) supplemented with 10% fetal bovine
serum (FBS) (PAA Laboratories, Pasching, Austria), penicillin (20
U/ml) and streptomycin (20 μg/ml) (Sigma‑Aldrich) at 37˚C in
a humid atmosphere containing 5% CO2.
Cell proliferation assay
The cells were initially cultured for 24 h in DMEM
supplemented with 0.5% dextran-charcoal stripped FBS (csFBS) prior
to being seeded in 96-multi-well plates in DMEM supplemented with
10% FBS and incubated at 37˚C for 24 h to allow for cell
attachment. Melatonin pre-treated cells were incubated for 24 h in
DMEM supplemented with 10% FBS containing melatonin (1 nM).
Following pre-treatment, both the melatonin-treated and the control
cells were seeded into 96-multi-well plates at a density of
3×103 cells per well, and incubated at 37°C for 24 h to
allow for cell attachment. The media were replaced with fresh media
with 10% FBS containing docetaxel ranging from 1 μM to 10
pM, plus/minus melatonin (1 nM) and/or the vehicle (ethanol at a
final concentration <0.0001%). The cells were cultured for 3 or
6 days and cell proliferation was measured by
3(4,5dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay. Briefly, yellow MTT (5 mg/ml in PBS) is reduced to purple
formazan in the mitochondria of living cells. This reduction takes
place only when mitochondrial reductase enzymes are active, and
therefore conversion can be directly related to the number of
viable cells. The formazan crystals can be dissolved by the
addition of 4 mM HCl. An increase in cell number is directly
related to the increase in absorbance due to the amount of MTT
formazan formed (37). After 24 h,
the absorbance is read at 570 nm on a microplate reader (Labsystems
Multiskan RC 351; Thermo LabSystems Inc. Vienna, VA, USA). MTT
solution was obtained from Molecular Probes Inc. (Eugene, OR, USA).
Each result represents the median ± standard error of the mean
(SEM) of 3 independent experiments and data are presented as a
percentage of the untreated control cells.
Measurement of cell cycle kinetics
The cells were initially cultured for 24 h in DMEM
supplemented with 0.5% dextran-csFBS prior to being seeded in
96-multi-well plates in DMEM supplemented with 10% FBS and
incubated at 37°C for 24 h to allow for cell attachment. The
control cells and cells pre-treated with melatonin (1 nM) for 24 h
were seeded in 6-well plates at a density of 5×105 cells
per well, in DMEM supplemented with 10% FBS and incubated at 37°C
for 24 h to allow cell attachment. The media were then replaced
with fresh media with 10% FBS containing either docetaxel alone (1
nM) or in combination with melatonin (1 nM) and/or the vehicle
(ethanol at a final concentration <0.0001%). After 24 h of
incubation, the cells were harvested with trypsin, washed twice
with phosphate‑buffered saline (PBS), and fixed in 70% cold ethanol
for 30 min. Following the removal of ethanol by centrifugation for
5 min at 300 × g, the cells were stained with a solution containing
50 μg/ml propidium iodide (PI) (Sigma-Aldrich) and incubated
in the dark for 30 min at room temperature. In total, 10,000 cells
were acquired for each sample on a BD FACSCanto II analyzer (BD
Biosciences, San Jose, CA, USA). The cell cycle distribution was
determined using a DNA software program (FACSDiva software; BD
Biosciences).
Determination of cell apoptosis
The induction of apoptosis was determined using an
Annexin V-FITC apoptosis detection kit (Miltenyi Biotec GmbH,
Germany), according to the manufacturer's instructions. Briefly,
the control cells and cells pre-treated with melatonin (1 nM) were
seeded in 6-well plates at a density of 3×105 cells per
well in DMEM supplemented with 10% FBS and incubated at 37°C for 24
h to allow for cell attachment. The media were then replaced by
fresh media containing docetaxel (1 nM) plus/minus melatonin (1 nM)
and/or the vehicle (ethanol at a final concentration <0.0001%).
After 24 h of incubation, the cells were harvested, washed twice
with PBS, and centrifuged at 300 × g for 10 min; the cells were
resuspended in 100 μl binding buffer containing 5 μl
of Annexin V-FITC, incubated for 15 min at room temperature, washed
twice, and finally resuspended in 500 μl binding buffer
containing 5 μl of PI. Cells were immediately analyzed
following incubation with the probes using a flow cytometer (BD
FACSCanto II analyzer; BD Biosciences). A total of 10,000 events
were analyzed using the FL-1 (green; Annexin V-FITC) and FL-3 (red;
PI) detectors. Each sample was tested 3 times in independent
experiments. Under all conditions tested, the percentages of
Annexin-/PI− (alive) and
Annexin+/PI− cells (early apoptotic) cells
were compared.
RNA isolation and cDNA synthesis
Total RNA was isolated from the MCF-7 cells and
purified using the Nucleospin RNA II kit (Macherey-Nagel GmbH &
Co,. Düren, Germany) following the manufacturer's instructions. The
concentration and purity of the RNA was quantified by measuring the
absorbance on a spectrophotometer (Nanodrop 1000; Thermo Fisher
Scientific, Wilmington, DE, USA). The absorbance ratio A260 nm/A280
nm was always >1.9. For cDNA synthesis, 0.5 μg of total
RNA was used as template using the RT2 First Strand kit
(Qiagen, Valencia, CA, USA), following the manufacturer's
instructions. First, the genomic DNA was eliminated by incubating
the samples 5 min at 42°C. After mixing with the reverse
transcription mix, the samples were incubated for exactly 15 min at
42°C in a final volume of 20 μl. The reaction was terminated
by incubation at 95°C for 5 min. Subsequently, 91 μl of
RNA-free water was added to each reaction and the samples were kept
on ice until proceeding with the real-time PCR protocol.
RT2 Profiler™ PCR array
Pathway-focused gene expression profiling was
performed using a 96‑well human breast cancer PCR array
(RT2 Profiler PCR array ‑ PAHS‑131ZA, Human Breast
Cancer PCR array, Qiagen, Valencia, CA, USA). In this array, each
well contains all the components required and designed to generate
single, gene‑specific amplicons, testing the expression of 84 genes
related to breast cancer pathways (apoptosis, metabolism, cell
cycle and DNA repair), plus 5 housekeeping genes. Each
RT2 Profiler PCR array plate also includes controls for
data normalization, genomic DNA contamination detection, RNA sample
quality and general PCR performance.
Briefly, the MCF‑7 cells were seeded in 6‑well
plates in DMEM supplemented with 10% FBS and incubated at 37°C for
24 h to allow for cell attachment. The media were then replaced
with fresh media with 10% FBS and containing either docetaxel
(Sigma-Aldrich) alone (1 μM) or in combination with 1 nM
melatonin (Sigma-Aldrich) and/or the vehicle (ethanol at a final
concentration <0.0001%). After 6 h of incubation, total RNA was
extracted and reverse transcribed as explained in the 'RNA
isolation and cDNA synthesis' section. The cDNA template was mixed
with the appropriate amount of RT2 SYBR-Green qPCR
Mastermix (Qiagen GmbH, Hilden, Germany), aliquoted 25 μl to
each well of the same plate, and then the real-time PCR cycling
program was performed on an MX3005P (Agilent, CA, USA) following
the manufacturer's instructions. Amplification was initiated by 1
cycle at 95°C for 10 min and then performed for 40 cycles for
quantitative analysis using the following temperature profile: 95°C
for 30 sec (denaturation); 60°C for 60 sec (annealing/ extension).
Dissociation curves were performed to verify that only a single
product was amplified. The Ct data for each gene were analyzed
using the Qiagen RT2 profiler PCR array data analysis
software. Data are represented as the fold-regulation between the
experimental groups and the control cells. Fold change values <1
indicate a negative result or downregulation, and the fold
-regulation is the negative inverse of the fold change.
Measurement of specific mRNA gene
expression
The analysis of mRNA gene expression was carried out
by RT-qPCR following incubation of the cells for 6 h with docetaxel
(1 μM or 1 nM), in the presence or absence of melatonin (1
nM). Total cellular RNA was isolated and reverse transcribed as
indicated above and RT-PCR was performed on an Mx3005P QPCR System
(Agilent Technologies, Santa Clara, CA, USA) using the same
temperature profile. Reactions were run in triplicate and melting
curves were performed to verify that only a single product was
amplified. Each result represents the median of 3 to 5 independent
experiments and data are presented as the fold change between the
experimental groups and the control cells. The primers used for
amplification (Sigma Genosys Ltd., Cambridge, UK) are listed in
Table I.
 | Table IPrimers used for the amplification of
mRNA transcripts. |
Table I
Primers used for the amplification of
mRNA transcripts.
| mRNA | Sequence |
|---|
| β‑ACTIN FW |
5′‑TCCTGCGAGTGCTGTCAGAG‑3′ |
| β‑ACTIN RV |
5′‑TCACCGCCCTACACATCAAAC‑3′ |
| BAX FW |
5′‑AACTGGACAGTAACATGGAG‑3′ |
| BAX RV |
5′‑TTGCTGGCAAAGTAGAAAAG‑3′ |
| BAD FW |
5′‑ATCATGGAGGCGCTG‑3′ |
| BAD RV |
5′‑CTTAAAGGAGTCCACAAACTC‑3′ |
| BCL‑2 FW |
5′‑CCTTTGGAATGGAAGCTTAG‑3′ |
| BCL‑2 RV |
5′‑GAGGGAATGTTTTCTCCTTG‑3′ |
| CDKN1A (p21)
FW |
5′‑CAGCATGACAGATTTCTACC‑3′ |
| CDKN1A (P21)
RV |
5′‑CAGGGTATGTACATGAGGAG‑3′ |
| GATA3 FW |
5′‑CGGTCCAGCACAGGCAGGGAGT‑3 |
| GATA3 RV |
5′‑GAGCCCACAGGCATTGCAGACA‑3′ |
| MYC FW |
5′‑TGAGGAGGAACAAGAAGATG‑3′ |
| MYC RV |
5′‑ATCCAGACTCTGACCTTTTG‑3′ |
| MUC1 FW |
5′‑GCAAGAGCACTCCATTCTCAATT‑3′ |
| MUC1 RV |
5′‑TGGCATCAGTCTTGGTGCTATG‑3′ |
| TP53 FW |
5′‑CAGCCAAGTCTGTGACTTGCACGTAC‑3′ |
| TP53 RV |
5′‑CTATGTCGAAAAGTGTTTCTGTCATC‑3′ |
Analysis of RT-qPCR data
For the primers used there were no differences
between transcription efficiencies, and the fold change in each
sample was calculated using the 2−∆∆Cq method (38). The fractional cycle at which the
amount of amplified target becomes significant (Ct) was
automatically calculated by the PCR program. The relative
expression of β-actin was used to normalize gene expression.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software. The data are expressed as the means ± SEM.
Statistical differences between groups were analyzed using one-way
analysis of variance (ANOVA), followed by the Student-Newman-Keuls
test. Results were considered as statistically significant at
p<0.05. To confirm whether the effects of docetaxel and
melatonin are synergistic as regards gene expression (BAD
and BCL-2 expression) and apoptosis, linear regression
models were obtained in order to estimate the independent effects
of melatonin, docetaxel and their interaction.
Results
Effects of docetaxel and melatonin on
cell proliferation and cytotoxicity
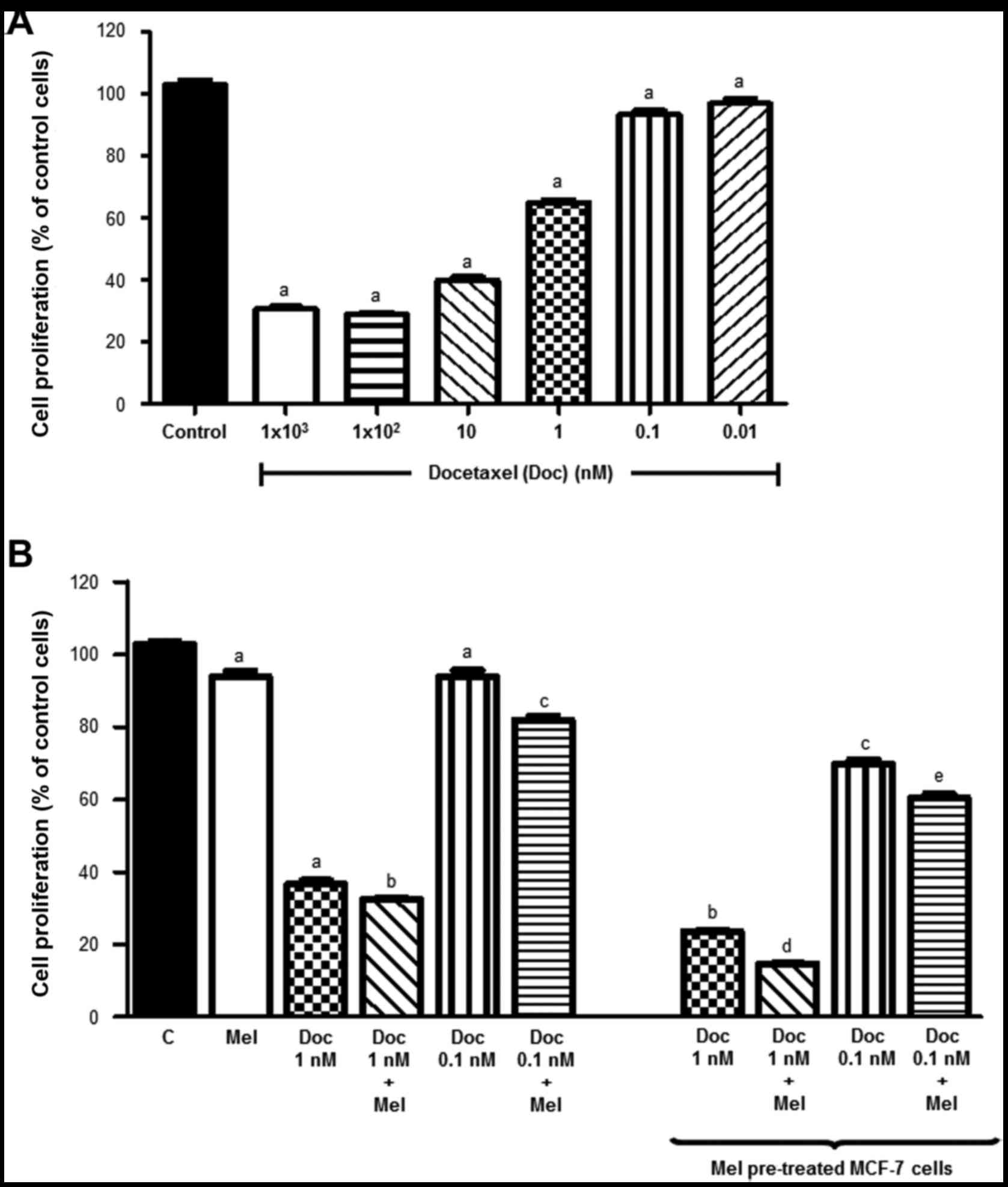
Our first goal was to determine whether melatonin
potentiates the anti-proliferative effects of docetaxel in MCF-7
cells. As expected, docetaxel inhibited the proliferation of the
MCF‑7 cells in a dose‑dependent manner. When the cells were treated
with docetaxel at 1 μM, proliferation was decreased by
almost 80% after 3 days, indicating that the majority of the cells
were dead (Fig. 1A). For this
reason, we examined the effect of physiological concentrations of
melatonin (1 nM) combined with low concentrations of docetaxel at 1
nM and 0.1 nM.
Consistent with previous findings of our group
(1), after 6 days of treatment,
melatonin alone induced an inhibitory effect on the proliferation
of MCF-7 cells (Fig. 1B).
Docetaxel (0.1 nM) also had a slight, but significant inhibitory
effect on cell proliferation (8%), whereas docetaxel (1 nM)
treatment resulted in a 62% decrease in cell viability. Of note,
co-treatment of the cells with docetaxel and a physiological
concentration of melatonin (1 nM) significantly potentiated the
inhibitory effects on cell proliferation induced at both docetaxel
concentrations. When the cancer cells were pre-treated with
melatonin for 24 h, a significant further decrease in cell
proliferation was obtained. For docetaxel (1 nM), a 77% decrease in
cell proliferation was obtained when the cells were pre-treated
with melatonin (whereas for non pre-treated cells the decrease in
cell proliferation observed was 62%). For docetaxel (0.1 nM), a 27%
decrease in cell proliferation was obtained when the cells were
pre-treated with 1 nM melatonin, (whereas an 8% decreased was
obtained in the in non pre‑treated cells). Moreover, significant
differences were observed when melatonin was maintained in the
medium following treatment with docetaxel, the effect of the
indolamine being a further reduction in cell proliferation.
Effects of docetaxel and melatonin on
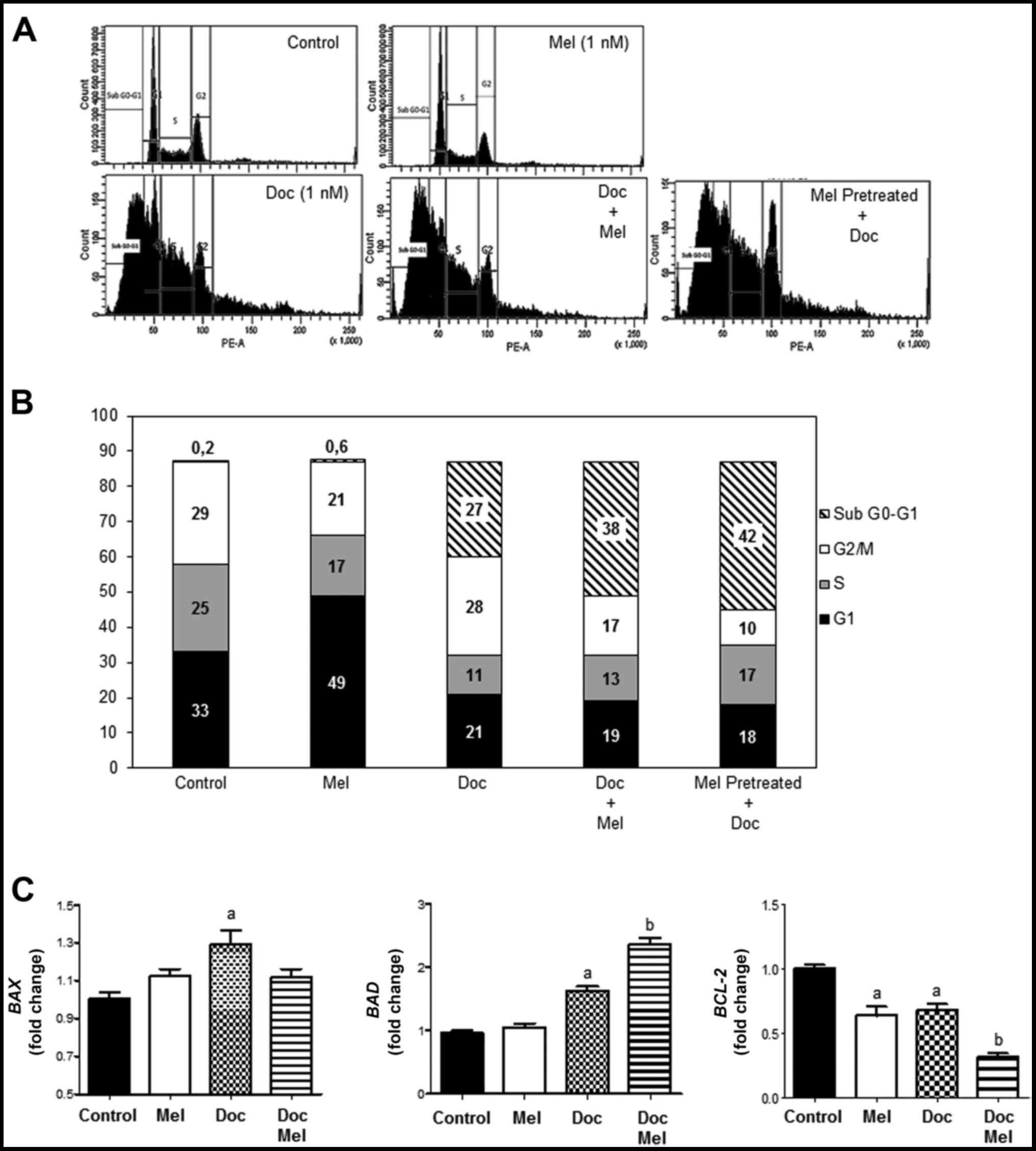
cell cycle phase distribution
The effects of melatonin, docetaxel and co-treatment
with both molecules on cell cycle phase distribution in the MCF-7
cells are shown in Fig. 2A, and
the percentage of cells in the sub G0-G1,
G1, S, and G2-M phases is represented in
Fig. 2B. As expected, treatment of
the cells with melatonin (1 nM) for 24 h induced an increase in the
proportion of cells in the G1 phase, thus causing a
delay in the transition to the S phase. As expected, a higher
number of cells in the sub G0–G1 phase was
evident when the cells were treated with docetaxel (1 nM) for 24 h
(27%). When the cells were treated with a combination of melatonin
and the taxane, a significant increase in the number of cells in
this phase was observed (38%). Of note, treatment with melatonin
for 24 h prior to the addition of docetaxel resulted in the highest
percentage of cells in the sub G0–G1 phase
(42%), suggesting DNA fragmentation as a consequence of cell death
(Fig. 2B).
We then wished to determine whether the results
obtained by flow cytometric analysis correlated with the expression
of genes involved in apoptosis. The mRNA expression of the
pro-apoptotic genes, BAD and BAX, was stimulated,
whereas the mRNA levels of the anti-apoptotic BCL-2 gene
were significantly decreased by docetaxel (1 nM). When the cells
were treated with both compounds, melatonin further enhanced the
effects of the taxane on BAD and BCL-2 expression.
However, at this low concentration of docetaxel (together with
melatonin), the pineal hormone had no significant effect on
BAX expression. Melatonin alone induced a moderate, yet
significant downregulation in the levels of BCL-2 (Fig. 2C).
Effects of docetaxel and melatonin on
cell apoptosis
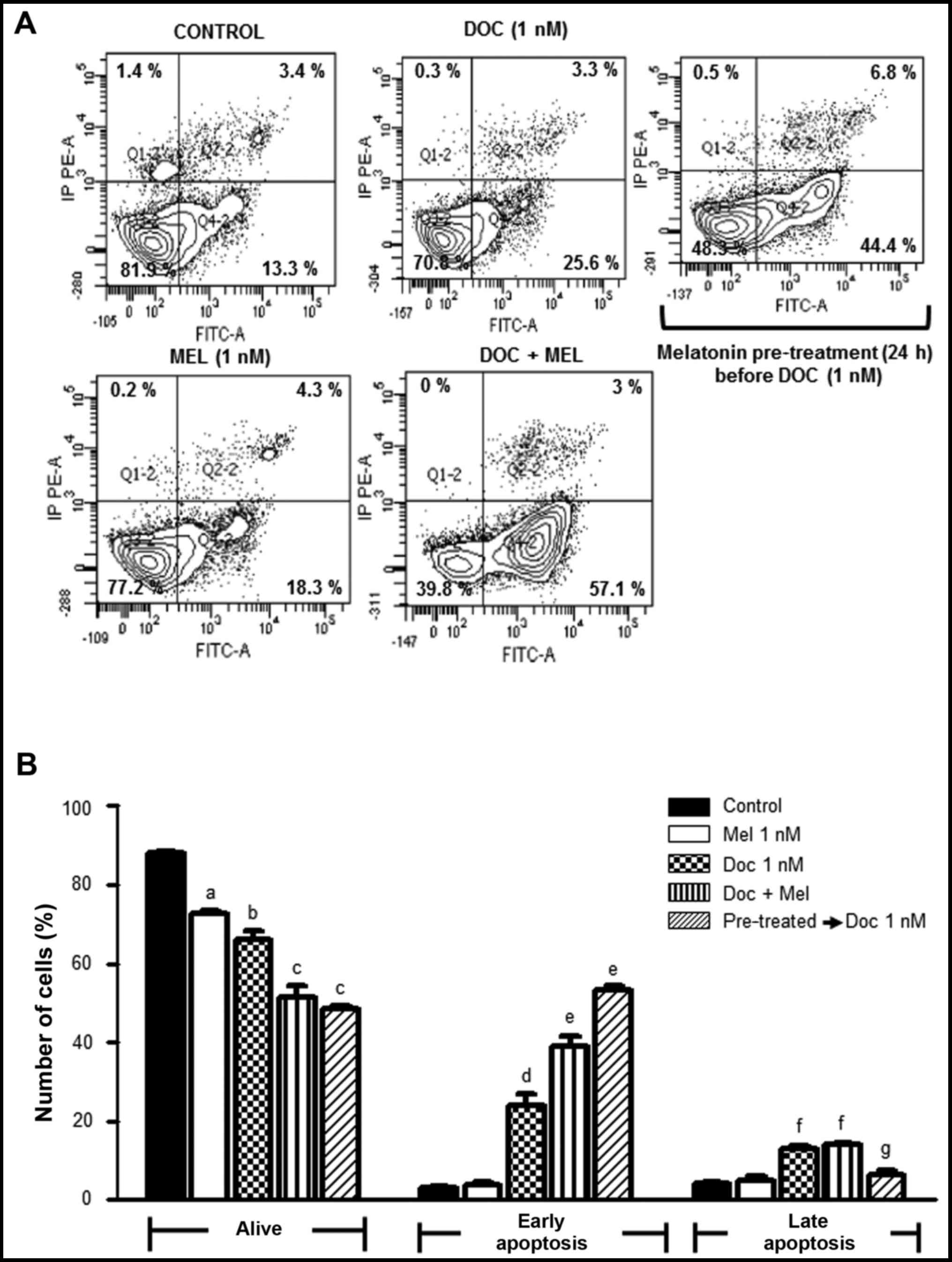
To examine whether the anti-proliferative effects
and the changes in the cell cycle phase distribution were related
to the induction of apoptosis, we treated the MCF-7 cells with
docetaxel (1 nM), alone or in combination with 1 nM melatonin for
24 h and used an Annexin V-FITC apoptosis detection kit, which
enables the differentiation of viable cells from apoptotic and
necrotic cells. The effects of melatonin pre-treatment for 24 h
were also examined. The results of Annexin V-FITC assay are shown
in Fig. 3A and the quantification
of the results obtained are shown in Fig. 3B. Following treatment with
melatonin alone, we observed a slight decrease in the percentage of
live cells compared to the control cells. Similarly, treatment with
docetaxel (1 nM) resulted in a decrease in the number of viable
cells parallel to an increase in the percentage of cells undergoing
early apoptosis. Of note, when the cells were simultaneously
treated with docetaxel and melatonin, the number of viable cells
was further decreased and the number of cells undergoing early
apoptosis was augmented, suggesting that melatonin enhanced the
apoptotic effects of docetaxel. Melatonin pre-treatment seemed to
sensitize the MCF-7 cells to this chemotherapeutic agent,
significantly further increasing the number of cells undergoing
early apoptosis.
Effects of docetaxel and melatonin on
gene expression
The changes in gene expression induced by
chemotherapeutic agents employed at clinical doses remain largely
unknown. In this study, we employed the human breast cancer
RT2 Profiler PCR Array, to determine the changes induced
by docetaxel (1 μM) either alone or in combination with
melatonin (1 nM) on the gene expression profiles in MCF‑7 cells.
The RT2 Profiler PCR Array allows the simultaneous
analysis of 84 genes relevant to a specific pathway or disease
state and includes genes involved in one or more processeses, such
as angiogenesis, adhesion, proteolysis, cell cycle progression and
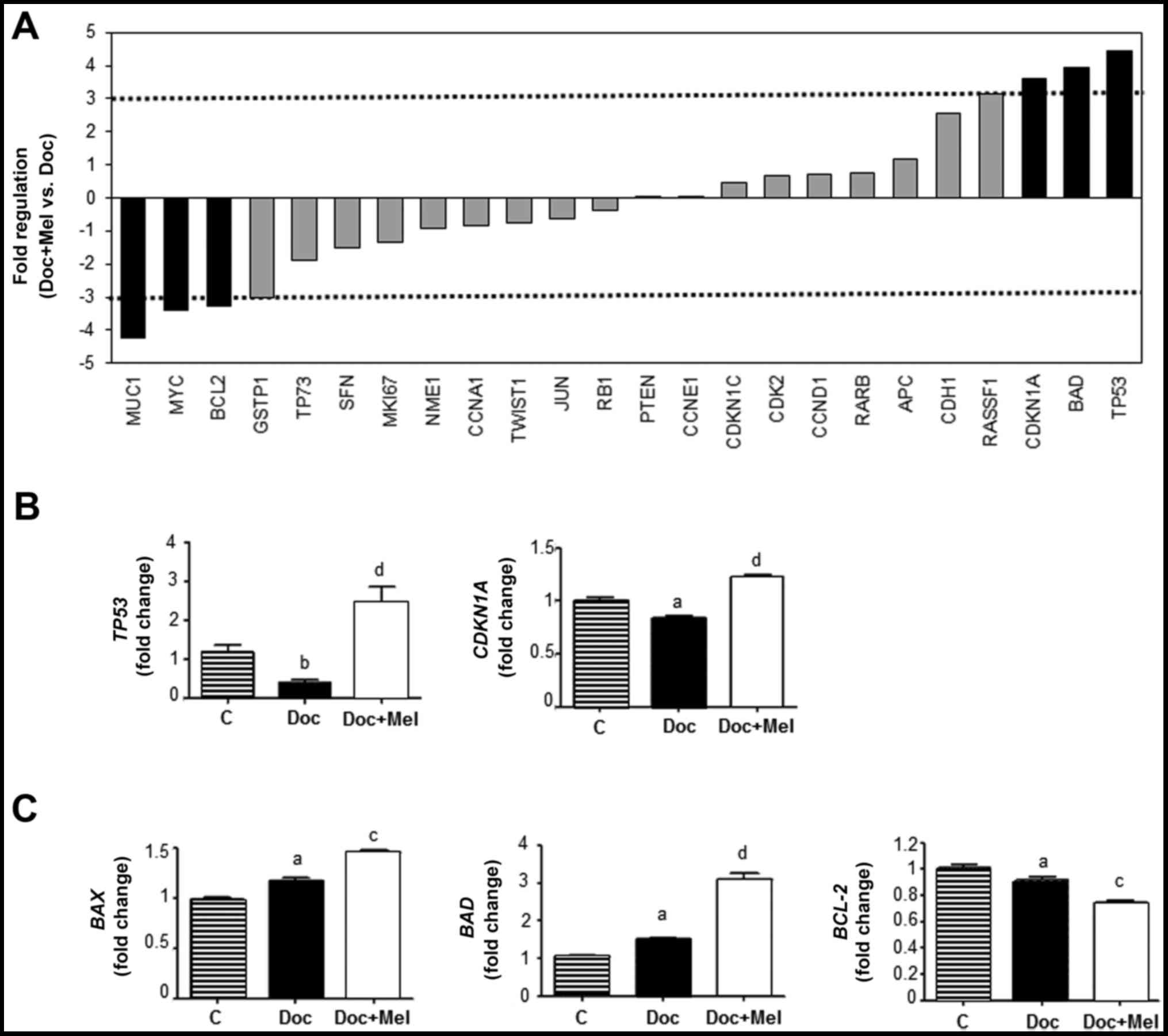
apoptosis. Melatonin alone significantly enhanced the expression of
some genes, such as TP53, cyclin-dependent kinase inhibitor
1A (CDKN1A), cadherin 13 (CDH13) or PGR,
whereas it diminished the expression of other genes, such as
c-MYC, interleukin (IL-6) or BCL-2 (data not
shown). Establishing as criteria a change of at least 1.5-fold
either with docetaxel alone or in combination with melatonin (in
comparison with expression of the untreated cells), we found that
treatment with docetaxel upregulated the expression of 8 genes and
downregulated the expression of 36 genes. When docetaxel was used
in combination with melatonin, we observed the upregulation of 15
genes and the downregulation of 29 genes (Table II). TP53, BAD and
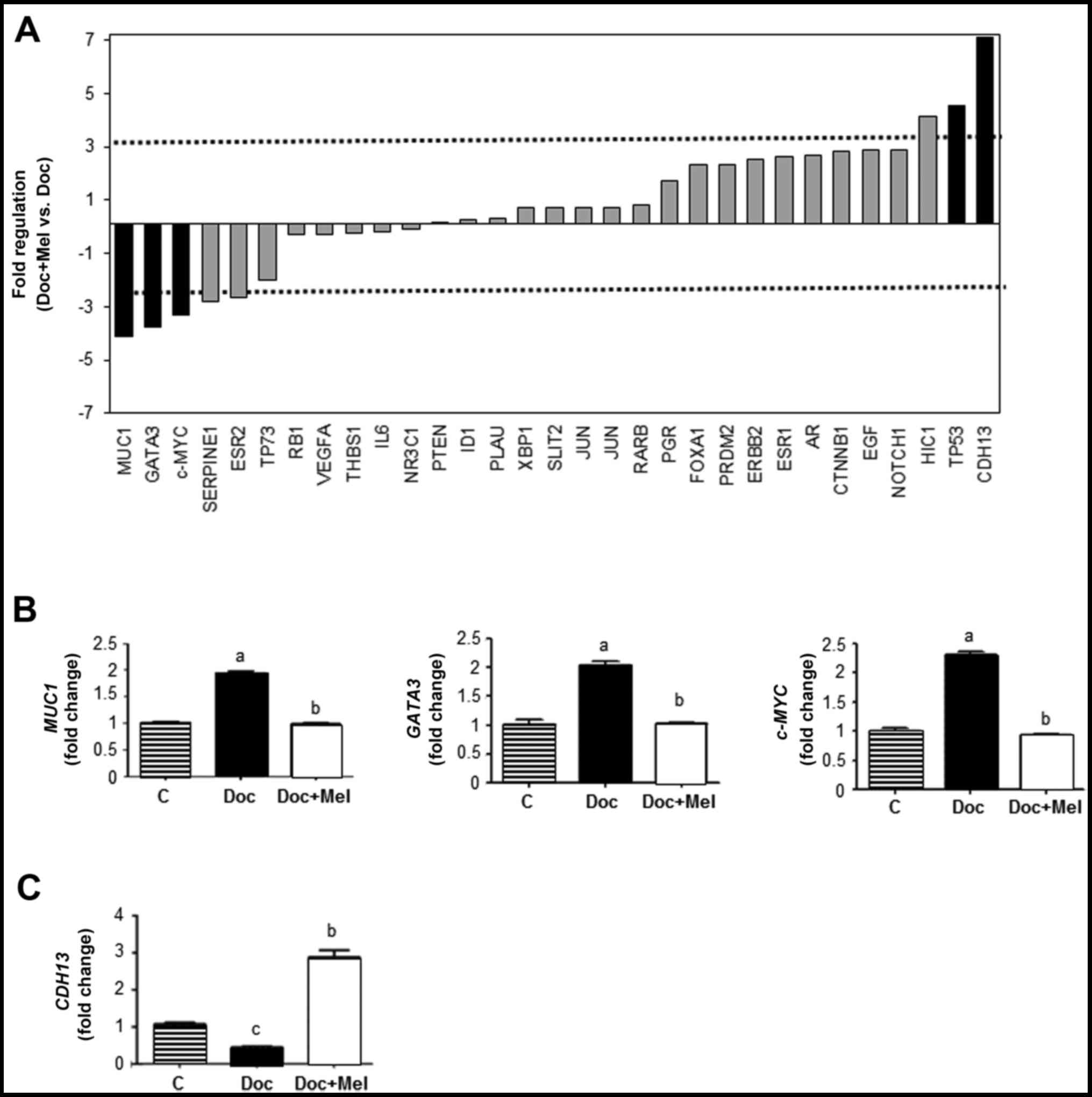
CDKN1A were upregulated >3-fold and mucin 1
(MUC1), BCL-2 and c-MYC were downregulated
>3-fold (Fig. 4A). The
following genes were selected for further analysis by specific
RT‑qPCR: TP53 and CDKN1A as cell cycle regulators,
and BAD and BCL-2 as genes involved in apoptosis.
Docetaxel (1 μM) significantly inhibited the expression of
TP53 and CDKN1A (Fig.
4B), and treatment with melatonin (1 nM) counteracted this
inhibitory effect. The expression of the pro-apoptotic gene
BAD was stimulated by docetaxel and co-treatment with
melatonin further increased its expression, whereas the expression
of the anti-apoptotic BCL-2 gene was significantly
decreased. Treatment with melatonin enhanced this inhibitory effect
(Fig. 4C). We analyzed the
expression of BAX, another pro-apoptotic member of the
family; similar to BAD, BAX expression was stimulated
by docetaxel and melatonin enhanced this stimulatory effect
(Fig. 4C).
 | Table IIList of the genes induced or
repressed at least 1.5-fold in MCF-7 cells treated either with
docetaxel (1 μM) or docetaxel (1 μM) + melatonin (1
nM) for 6 h. |
Table II
List of the genes induced or
repressed at least 1.5-fold in MCF-7 cells treated either with
docetaxel (1 μM) or docetaxel (1 μM) + melatonin (1
nM) for 6 h.
| Docetaxel | Fold
regulation | Docetaxel +
melatonin | Fold
regulation |
|---|
| GSTP1 - | 7.31 | GSTP1 | −10.10 |
| PGR | −6.50 | BIRC5 - | 7.36 |
| KRT8 | −4.08 | TP73 | −5.66 |
| TP73 | −3.97 | PGR | −4.89 |
| CST6 | −3.71 | CST6 | −3.94 |
| HIC1 | −3.43 | SFN | −3.76 |
| MAPK1 | −2.96 | TWIST1 | −2.97 |
| PTGS2 | −2.85 | NR3C1 | −2.77 |
| NR3C1 | −2.75 | MUC1 | −2.61 |
| TP53 | −2.69 | THBS1 | −2.53 |
| BRCA1 | −2.60 | NME1 | −2.48 |
| GRB7 | −2.51 | BRCA1 | −2.46 |
| JUN | −2.45 | PTGS2 | −2.46 |
| SRC | −2.28 | MKI67 | −2.41 |
| CDK2 | −2.23 | RB1 | −2.33 |
| SFN | −2.23 | GATA3 | −2.28 |
| TWIST1 | −2.22 |
SERPINE1 | −2.15 |
| THBS1 | −2.17 | KRT8 | −2.08 |
| FOXA1 | −2.14 | MYC | −2.06 |
| VEGFA | −1.96 | KRT18 | −2.03 |
| RB1 | −1.95 | MAPK1 | −1.96 |
| NME1 | −1.87 | IL6 | −1.95 |
| TGFB1 | −1.87 | JUN | −1.82 |
| CDKN1C | −1.84 | ABCB1 | −1.79 |
| RARB | −1.83 | KRT19 | −1.79 |
| SLIT2 | −1.80 | VEGFA | −1.75 |
| RASSF1 | −1.79 | AKT1 | −1.74 |
| CDKN1A | −1.75 | BCL-2 | −1.65 |
| CTNNB1 | −1.70 | BRCA2 | −1.52 |
| CDH13 | −1.69 | IGF1R | 1.55 |
| CDKN2A | −1.69 | CCNA1 | 1.60 |
| CSF1 | −1.68 | TP53 | 1.86 |
| KRT5 | −1.67 | ID1 | 1.88 |
| SFRP1 | −1.65 | CCND1 | 1.95 |
| XBP1 | −1.61 | ATM | 2.04 |
| KRT18 | −1.54 | IGFBP3 | 2.14 |
| ESR2 | 1.69 | CDKN1A | 2.17 |
| ID1 | 1.71 | APC | 2.55 |
| SNAI2 | 1.77 | EGF | 2.71 |
| GATA3 | 1.91 | BAD | 2.83 |
| MUC1 | 1.92 | CCND2 | 3.29 |
| GLI1 | 2.00 | CDH13 | 5.35 |
| MYC | 2.17 | SNAI2 | 5.86 |
| CCNA1 | 2.43 | IGF1 | 8.86 |
Among the genes classified as transcription factors
or involved in angiogenesis and/or cell adhesion, MUC1, GATA
binding protein 3 (GATA3) and c-MYC were
downregulated (>3-fold), and TP53 and CDH13 were
upregulated (>4-fold) when the cells were treated simultaneously
with melatonin and docetaxel in comparison with the cells treated
only with the taxane (Fig. 5A).
Specific RT‑PCR analyses confirmed that docetaxel (1 μM)
stimulated the expression of the transcription factors,
MUC1, GATA3 and c-MYC, whereas a physiological
concentration of melatonin counteracted this stimulatory effect of
the chemotherapeutic agent (Fig.
5B). The expression of CDH13 (involved in angiogenesis)
was inhibited by docetaxel and melatonin reversed this effect
(Fig. 5C).
Discussion
In patients diagnosed with locally advanced breast
cancer and a positive estrogen receptor status, chemotherapy is
often recommended when cancer spreads outside the breast and
axillary area. Several chemotherapy protocols for breast cancer
include docetaxel, a mitotic inhibitor acting as a spindle poison
(39).
Apart from undesirable side-effects, the molecular
changes induced by chemotherapy in cancer cells remain largely
unknown. The identification of such alterations may contribute to
an improved efficacy and may help to elucidate the mechanisms
responsible for undesirable processes, such as drug resistance.
Gene expression and post-translational modification profiles have
recently been obtained in all types of tumors and cancer cell lines
treated with chemotherapeutic drugs (25,40).
Melatonin has oncostatic actions, counteracting the
estrogen-mediated activation and modulating the expression and
activity of enzymes involved in the synthesis of estrogens
(2,6,41–43).
Melatonin treatment sensitizes MCF-7 cells to radiation, decreasing
cell proliferation, increasing the proportion of cells in the
G0–G1 phase of the cell cycle and
downregulating the expression of proteins implicated in
double-strand DNA break repair (12). Melatonin regulates global gene
expression in human breast cancer cells (36). However, the molecular mechanisms
through which melatonin modulates changes in gene expression, cell
proliferation and cell cycle progression triggered by
chemotherapeutic drugs in estrogen-responsive breast tumors remain
largely unknown. Therefore, in the present study, we examined the
effects of the combination of docetaxel and melatonin using MCF-7
cells as a model.
Since MCF-7 cells are very sensitive to
concentrations of docetaxel in the range of those used in
chemotherapy protocols, we decided to test low concentrations of
the taxane (1 nM and 0.1 nM) to examine the effects of melatonin
combined with the chemotherapeutic agent on cell proliferation. Our
results strongly suggested that the combination of melatonin and
low doses of docetaxel resulted in the cooperative enhancement of
cytotoxicity. Of note, treatment of the cells with melatonin prior
to docetaxel treatment resulted in a higher inhibition of cell
proliferation. These results point to melatonin as an enhancer of
the anti-proliferative effects of docetaxel when the taxane is
administered at lower doses than those administered to cancer
patients.
We then analysed the changes that melatonin and
docetaxel cause on the cell cycle. As previously reported (44), melatonin induces cell cycle arrest
in the G1 phase. Low concentrations of docetaxel induced
a higher proportion of cells in the sub G0–G1
phase and of note, co‑treatment with melatonin significantly
increased the number of cells in the sub
G0–G1 phase, indicating that melatonin
potentiated the effects of docetaxel. Low concentrations of
docetaxel decreased the number of viable/live cells and increased
the percentage of cells undergoing early apoptosis. Melatonin
enhanced this effect, more potently when added prior to treatment
with the taxane. Our results are in agreement with those of a
recent study reporting that melatonin induced cell inhibition and
the apoptosis of MCF-7 cells through an increase in p53 acetylation
and the inhibition of Akt/PI3K, modulating the MDM2/MDMX/ p300
pathway (45). Finally, we examine
the expression of the apoptosis-related genes, BAD,
BAX and BCL-2, and found that melatonin potentiated
the stimulatory effects of docetaxel on the expression of
BAD, and provoked a strong inhibition of the expression of
BCL-2. Thus, physiological concentrations of melatonin
potentiated the cytotoxic effects of low concentrations of
docetaxel, which may allow the therapeutic concentrations of
anticancer drugs to be reduced.
In breast cancer patients, docetaxel is administered
in doses oscillating from 75–200 mg/m2 every 3 weeks,
roughly equivalent to concentrations of 1 μM in breast
cancer cells in culture medium. For this reason, we then examined
the changes in gene expression profiles when the MCF‑7 cells were
treated with docetaxel (1 μM), and found that 8 genes were
upregulated and 36 were downregulated by at least 1.5‑fold. When
docetaxel and melatonin were simultaneously added to the culture
medium, 15 genes were upregulated and 29 downregulated by at least
1.5‑fold. We further analyzed the expression of 9 genes involved in
cell cycle progression (TP53 and CDKN1A) angiogenesis
(CDH13), apoptosis (BAX, BAD and
BCL-2), and gene transcription (GATA3, MUC1
and c-MYC). The genome guardian p53 maintains genome
stability (46), and the
expression of p53 and p21WAF1 is reduced in locally advanced breast
cancer patients receiving neoadjuvant docetaxel plus epirubicin
(47). The induction of p53 and
p21WAF1 by melatonin has been suggested as part of the mechanism
through which melatonin inhibits the proliferation of breast cancer
cells (48). In the present study,
docetaxel decreased the expression of TP53, CDKN1A,
two key regulators of cell cycle. An intact p53 predicts
sensitivity to breast cancer therapy and higher levels of p21
indicate a more indolent type of breast cancer (49). In our hands, docetaxel also
decreased the expression of CDH13, the loss of which has
been shown to be associated with tumor malignancy, invasiveness and
metastasis (50). Of note,
melatonin counteracted the negative effect of docetaxel, increasing
the expression of these 3 genes, considered as tumor suppressors
with a key role in cell cycle control and tumor progression.
BAX and BAD are pro-apoptotic and BCL-2 is an
anti-apoptotic factor classified as an oncogene (51). BAD neutralizes anti-apoptotic BCL-2
members, which in turn, modulate the intrinsic apoptotic pathway by
binding and neutralizing other proteins that act as mitochondrial
permeabilizers, such as the pro-apoptotic protein, BAX (52). BAX expression is upregulated
by p53 and BAX is involved in p53-mediated apoptosis. BAD
(BCL-2-associated death promoter) is a pro-apoptotic member of the
BCL-2 gene family involved in the initiation of apoptosis.
BAD forms heterodimers with anti-apoptotic proteins (such as BCL-2)
inactivating them, allowing for BAX-triggered apoptosis (51). Docetaxel has been reported to both
stimulate and inhibit the apoptosis of human melanoma (53), whereas in MCF-7 cells, nanomolar
concentrations of docetaxel have been shown to induce apoptosis
likely through the phosphorylation of BCL-2 (54). In this study, we found that
treatment with docetaxel (1 μM) significantly increased the
expression of BAX and BAD, and decreased the levels
of BCL-2. Importantly, melatonin potentiated the stimulatory
effects of docetaxel on the expression of the pro-apoptotic genes,
BAX and BAD, and further inhibited the expression of
the anti-apoptotic gene, BCL-2. Our results strongly suggest
that melatonin potentiates the pro-apoptotic effects of docetaxel
in breast cancer cells by modulating the expression of the
BAX, BAD and BCL-2 genes.
GATA3 is a transcription factor differentially
expressed in breast cancer (55).
GATA3 mediates the transcriptional upregulation of MUC1
(56). MUC1 expression has been
found in plasma cells of patients with lymph node metastasis or
micro-metastasis (57).
c-MYC is a classical oncogene that is mutated, translocated
or overexpressed in many types of tumors and is upregulated by
estradiol in MCF-7 cells, whereas melatonin abolishes this effect
almost completely (58).
Melatonin, in combination with arsenic trioxide, has been shown to
stimulate apoptosis by increasing p53 protein levels, increasing
the ratio of BAX/BCL-2 and suppressing survivin-mediated
c-MYC and hTERT expression (44). In this study, we found that
docetaxel induced the expression of GATA3, MUC1 and
c-MYC, whereas melatonin counteracted the stimulatory
effects of this chemotherapeutic agent. Again, melatonin seemed to
exert a protective effect, since the inhibition of the expression
of MUC1, GATA3 and the proto-oncogene, c-MYC,
may well correlate with both lower levels of the expression of
factors involved in cellular growth and a less aggressive invasion
phenotype.
In conclusion, our results point once more to
melatonin as a useful molecule with a potential to be considered as
an adjuvant in breast cancer therapy. Since the nocturnal increase
in plasma melatonin is much lower in patients with
estrogen-positive breast cancer than in healthy women (59), the administration of melatonin at
physiological doses may compensate this deficit of melatonin in
these patients. We report herein that the concomitant use of
melatonin and docetaxel sensitizes human breast cancer cells to the
chemotherapeutic agent. When docetaxel is used at low
concentrations, combinations of melatonin plus docetaxel have a
synergistic effect in arresting cell proliferation and inducing the
apoptosis in MCF-7 cells. Melatonin also modulates changes in gene
expression induced by docetaxel at higher concentrations
(equivalent to the docetaxel dose in chemotherapy protocols). Our
results suggest that patients receiving docetaxel as part of their
chemotherapy treatment may benefit in the next future with a
co‑treatment with melatonin as an adjuvant agent, allowing for a
reduction in the dose of the chemotherapeutic agent administered,
which may result in better tolerance and less adverse effects. The
efficacy of melatonin when administered together with chemotherapy
has been tested in several trials; however, to date, at least to
the best of our knowledge, it has never been tested in combination
with docetaxel. Our results indicate that it may be worthy to
perform randomized, double blind, placebo-controlled trials in the
near future in breast cancer patients to clarify the efficacy of
this association.
Acknowledgments
The present study was supported by grants from the
Spanish Science Technology and Innovation Ministry (grant no.
SAF2016–77103-P) and the Research Institute Valdecilla (grant no.
APG/12).
References
|
1
|
Cos S, Martínez-Campa C, Mediavilla MD and
Sánchez-Barceló EJ: Melatonin modulates aromatase activity in MCF-7
human breast cancer cells. J Pineal Res. 38:136–142. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cos S, González A, Martínez-Campa C,
Mediavilla MD, Alonso-González C and Sánchez-Barceló EJ: Melatonin
as a selective estrogen enzyme modulator. Curr Cancer Drug Targets.
8:691–702. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cos S, González A, Güezmes A, Mediavilla
MD, Martínez-Campa C, Alonso-González C and Sánchez-Barceló EJ:
Melatonin inhibits the growth of DMBA-induced mammary tumors by
decreasing the local biosynthesis of estrogens through the
modulation of aromatase activity. Int J Cancer. 118:274–278. 2006.
View Article : Google Scholar
|
|
4
|
Rato AG, Pedrero JG, Martínez MA, del Río
B, Lazo PS and Ramos S: Melatonin blocks the activation of estrogen
receptor for DNA binding. FASEB J. 13:857–868. 1999.PubMed/NCBI
|
|
5
|
Bouhoute A and Leclercq G: Calmodulin
decreases the estrogen binding capacity of the estrogen receptor.
Biochem Biophys Res Commun. 227:651–657. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
del Río B, García Pedrero JM,
Martínez-Campa C, Zuazua P, Lazo PS and Ramos S: Melatonin, an
endogenous‑specific inhibitor of estrogen receptor alpha via
calmodulin. J Biol Chem. 279:38294–38302. 2004. View Article : Google Scholar
|
|
7
|
Aronica SM, Kraus WL and Katzenellenbogen
BS: Estrogen action via the cAMP signaling pathway: Stimulation of
adenylate cyclase and cAMP-regulated gene transcription. Proc Natl
Acad Sci USA. 91:8517–8521. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Godson C and Reppert SM: The Mel1a
melatonin receptor is coupled to parallel signal transduction
pathways. Endocrinology. 138:397–404. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cos S and Blask DE: Melatonin modulates
growth factor activity in MCF-7 human breast cancer cells. J Pineal
Res. 17:25–32. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Molis TM, Spriggs LL, Jupiter Y and Hill
SM: Melatonin modulation of estrogen-regulated proteins, growth
factors, and proto-oncogenes in human breast cancer. J Pineal Res.
18:93–103. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cos S, Blask DE, Lemus‑Wilson A and Hill
AB: Effects of melatonin on the cell cycle kinetics and
'estrogen-rescue' of MCF-7 human breast cancer cells in culture. J
Pineal Res. 10:36–42. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alonso-González C, González A,
Martínez-Campa C, Menéndez-Menéndez J, Gómez-Arozamena J,
García-Vidal A and Cos S: Melatonin enhancement of the
radiosensitivity of human breast cancer cells is associated with
the modulation of proteins involved in estrogen biosynthesis.
Cancer Lett. 370:145–152. 2016. View Article : Google Scholar
|
|
13
|
Leon-Blanco MM, Guerrero JM, Reiter RJ,
Calvo JR and Pozo D: Melatonin inhibits telomerase activity in the
MCF-7 tumor cell line both in vivo and in vitro. J Pineal Res.
35:204–211. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martínez-Campa CM, Alonso-González C,
Mediavilla MD, Cos S, González A and Sánchez-Barceló EJ: Melatonin
down-regulates hTERT expression induced by either natural estrogens
(17beta-estradiol) or metalloestrogens (cadmium) in MCF-7 human
breast cancer cells. Cancer Lett. 268:272–277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cos S, Fernández R, Güézmes A and
Sánchez-Barceló EJ: Influence of melatonin on invasive and
metastatic properties of MCF-7 human breast cancer cells. Cancer
Res. 58:4383–4390. 1998.PubMed/NCBI
|
|
16
|
González A, Alvarez-García V,
Martínez-Campa C, Alonso-González C and Cos S: Melatonin promotes
differentiation of 3T3‑L1 fibroblasts. J Pineal Res. 52:12–20.
2012. View Article : Google Scholar
|
|
17
|
Alvarez-García V, González A,
Alonso-González C, Martínez-Campa C and Cos S: Melatonin interferes
in the desmoplastic reaction in breast cancer by regulating
cytokine production. J Pineal Res. 52:282–290. 2012. View Article : Google Scholar
|
|
18
|
Alvarez-García V, González A,
Martínez-Campa C, Alonso-González C and Cos S: Melatonin modulates
aromatase activity and expression in endothelial cells. Oncol Rep.
29:2058–2064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lissoni P, Barni S, Mandalà M, Ardizzoia
A, Paolorossi F, Vaghi M, Longarini R, Malugani F and Tancini G:
Decreased toxicity and increased efficacy of cancer chemotherapy
using the pineal hormone melatonin in metastatic solid tumour
patients with poor clinical status. Eur J Cancer. 35:1688–1692.
1999. View Article : Google Scholar
|
|
20
|
Lissoni P, Chilelli M, Villa S, Cerizza L
and Tancini G: Five years survival in metastatic non-small cell
lung cancer patients treated with chemotherapy alone or
chemotherapy and melatonin: A randomized trial. J Pineal Res.
35:12–15. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sookprasert A, Johns NP, Phunmanee A,
Pongthai P, Cheawchanwattana A, Johns J, Konsil J, Plaimee P,
Porasuphatana S and Jitpimolmard S: Melatonin in patients with
cancer receiving chemotherapy: A randomized, double-blind,
placebo-controlled trial. Anticancer Res. 34:7327–7337.
2014.PubMed/NCBI
|
|
22
|
Seely D, Wu P, Fritz H, Kennedy DA, Tsui
T, Seely AJ and Mills E: Melatonin as adjuvant cancer care with and
without chemotherapy: A systematic review and meta-analysis of
randomized trials. Integr Cancer Ther. 11:293–303. 2012. View Article : Google Scholar
|
|
23
|
Wang YM, Jin BZ, Ai F, Duan CH, Lu YZ,
Dong TF and Fu QL: The efficacy and safety of melatonin in
concurrent chemotherapy or radiotherapy for solid tumors: A
meta-analysis of randomized controlled trials. Cancer Chemother
Pharmacol. 69:1213–1220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reiter RJ, Rosales-Corral SA, Tan DX,
Acuña-Castroviejo D, Qin L, Yang SF and Xu K: Melatonin, a full
service anti-cancer agent: Inhibition of initiation, progression
and metastasis. Int J Mol Sci. 18:E8432017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martínez-Campa C, Menéndez-Menéndez J,
Alonso-González C, González A, Álvarez‑García V and Cos S: What is
known about melatonin, chemotherapy and altered gene expression in
breast cancer (Review). Oncol Lett. 13:2003–2014. 2017.
|
|
26
|
Xiang S, Dauchy RT, Hauch A, Mao L, Yuan
L, Wren MA, Belancio VP, Mondal D, Frasch T, Blask DE, et al:
Doxorubicin resistance in breast cancer is driven by light at
night-induced disruption of the circadian melatonin signal. J
Pineal Res. 59:60–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Granzotto M, Rapozzi V, Decorti G and
Giraldi T: Effects of melatonin on doxorubicin cytotoxicity in
sensitive and pleiotropically resistant tumor cells. J Pineal Res.
31:206–213. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Woo SM, Min KJ and Kwon TK:
Melatonin‑mediated Bim up-regulation and cyclooxygenase-2 (COX-2)
down-regulation enhances tunicamycin-induced apoptosis in
MDA-MB-231 cells. J Pineal Res. 58:310–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yun M, Kim EO, Lee D, Kim JH, Kim J, Lee
H, Lee J and Kim SH: Melatonin sensitizes H1975 non-small-cell lung
cancer cells harboring a T790M-targeted epidermal growth factor
receptor mutation to the tyrosine kinase inhibitor gefitinib. Cell
Physiol Biochem. 34:865–872. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Plaimee P, Weerapreeyakul N, Barusrux S
and Johns NP: Melatonin potentiates cisplatin-induced apoptosis and
cell cycle arrest in human lung adenocarcinoma cells. Cell Prolif.
48:67–77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pariente R, Pariente JA, Rodríguez AB and
Espino J: Melatonin sensitizes human cervical cancer HeLa cells to
cisplatin-induced cytotoxicity and apoptosis: Effects on oxidative
stress and DNA fragmentation. J Pineal Res. 60:55–64. 2016.
View Article : Google Scholar
|
|
32
|
Uguz AC, Cig B, Espino J, Bejarano I,
Naziroglu M, Rodríguez AB and Pariente JA: Melatonin potentiates
chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic
tumor cells. J Pineal Res. 53:91–98. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma C, Li LX, Zhang Y, Xiang C, Ma T, Ma ZQ
and Zhang ZP: Protective and sensitive effects of melatonin
combined with adriamycin on ER+ (estrogen receptor)
breast cancer. Eur J Gynaecol Oncol. 36:197–202. 2015.
|
|
34
|
Koşar PA, Nazıroğlu M, Övey IS and Çiğ B:
Synergic effects of doxorubicin and melatonin on apoptosis and
mitochondrial oxidative stress in MCF-7 breast cancer cells:
Involvement of TRPV1 channels. J Membr Biol. 249:129–140. 2016.
View Article : Google Scholar
|
|
35
|
Rodríguez-García A, Mayo JC, Hevia D,
Quirós-González I, Navarro M and Sainz RM: Phenotypic changes
caused by melatonin increased sensitivity of prostate cancer cells
to cytokine-induced apoptosis. J Pineal Res. 54:33–45. 2013.
View Article : Google Scholar
|
|
36
|
Lee SE, Kim SJ, Youn JP, Hwang SY, Park CS
and Park YS: MicroRNA and gene expression analysis of
melatonin-exposed human breast cancer cell lines indicating
involvement of the anticancer effect. J Pineal Res. 51:345–352.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
39
|
Iwao-Koizumi K, Matoba R, Ueno N, Kim SJ,
Ando A, Miyoshi Y, Maeda E, Noguchi S and Kato K: Prediction of
docetaxel response in human breast cancer by gene expression
profiling. J Clin Oncol. 23:422–431. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lumachi F, Chiara GB, Foltran L and Basso
SM: Proteomics as a guide for personalized adjuvant chemotherapy in
patients with early breast cancer. Cancer Genomics Proteomics.
12:385–390. 2015.PubMed/NCBI
|
|
41
|
Kiefer T, Ram PT, Yuan L and Hill SM:
Melatonin inhibits estrogen receptor transactivation and cAMP
levels in breast cancer cells. Breast Cancer Res Treat. 71:37–45.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
González A, Cos S, Martínez-Campa C,
Alonso-González C, Sánchez-Mateos S, Mediavilla MD and
Sánchez-Barcelo EJ: Selective estrogen enzyme modulator actions of
melatonin in human breast cancer cells. J Pineal Res. 45:86–92.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vriend J and Reiter RJ: Breast cancer
cells: Modulation by melatonin and the ubiquitin-proteasome system
- a review. Mol Cell Endocrinol. 417:1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nooshinfar E, Bashash D, Safaroghli-Azar
A, Bayati S, Rezaei-Tavirani M, Ghaffari SH and Akbari ME:
Melatonin promotes ATO-induced apoptosis in MCF-7 cells: Proposing
novel therapeutic potential for breast cancer. Biomed Pharmacother.
83:456–465. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Proietti S, Cucina A, Dobrowolny G,
D'Anselmi F, Dinicola S, Masiello MG, Pasqualato A, Palombo A,
Morini V, Reiter RJ, et al: Melatonin down-regulates MDM2 gene
expression and enhances p53 acetylation in MCF-7 cells. J Pineal
Res. 57:120–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Berger C, Qian Y and Chen X: The
p53-estrogen receptor loop in cancer. Curr Mol Med. 13:1229–1240.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tiezzi DG, Andrade JM, Ribeiro-Silva A,
Zola FE, Marana HR and Tiezzi MG: HER-2, p53, p21 and hormonal
receptors proteins expression as predictive factors of response and
prognosis in locally advanced breast cancer treated with
neoadjuvant docetaxel plus epirubicin combination. BMC Cancer.
7:36–46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mediavilla MD, Cos S and Sánchez-Barceló
EJ: Melatonin increases p53 and p21WAF1 expression in MCF‑7 human
breast cancer cells in vitro. Life Sci. 65:415–420. 1999.
View Article : Google Scholar
|
|
49
|
Elledge RM and Allred DC: Prognostic and
predictive value of p53 and p21 in breast cancer. Breast Cancer Res
Treat. 52:79–98. 1998. View Article : Google Scholar
|
|
50
|
Moelans CB, Verschuur-Maes AH and van
Diest PJ: Frequent promoter hypermethylation of BRCA2, CDH13, MSH6,
PAX5, PAX6 and WT1 in ductal carcinoma in situ and invasive breast
cancer. J Pathol. 225:222–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tudor G, Aguilera A, Halverson DO, Laing
ND and Sausville EA: Susceptibility to drug-induced apoptosis
correlates with differential modulation of Bad, Bcl-2 and Bcl-xL
protein levels. Cell Death Differ. 7:574–586. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Correia C, Lee SH, Meng XW, Vincelette ND,
Knorr KL, Ding H, Nowakowski GS, Dai H and Kaufmann SH: Emerging
understanding of Bcl-2 biology: Implications for neoplastic
progression and treatment. Biochim Biophys Acta. 1853:1658–1671.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mhaidat NM, Zhang XD, Jiang CC and Hersey
P: Docetaxel-induced apoptosis of human melanoma is mediated by
activation of c-Jun NH2-terminal kinase and inhibited by the
mitogen-activated protein kinase extracellular signal-regulated
kinase 1/2 pathway. Clin Cancer Res. 13:1308–1314. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Berchem GJ, Bosseler M, Mine N and
Avalosse B: Nanomolar range docetaxel treatment sensitizes MCF-7
cells to chemotherapy induced apoptosis, induces G2M arrest and
phosphorylates bcl-2. Anticancer Res. 19A:535–540. 1999.
|
|
55
|
Zang H, Li N, Pan Y and Hao J:
Identification of upstream transcription factors (TFs) for
expression signature genes in breast cancer. Gynecol Endocrinol.
33:193–198. 2017. View Article : Google Scholar
|
|
56
|
Abba MC, Nunez MI, Colussi AG, Croce MV,
Segal-Eiras A and Aldaz CM: GATA3 protein as a MUC1 transcriptional
regulator in breast cancer cells. Breast Cancer Res. 8:R642006.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Puttipanyalears C, Kitkumthorn N,
Buranapraditkun S, Keelawat S and Mutirangura A: Breast cancer
upregulating genes in stromal cells by LINE-1 hypermethylation and
micro-metastatic detection. Epigenomics. 8:475–486. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Girgert R, Hanf V, Emons G and Gründker C:
Membrane-bound melatonin receptor MT1 down-regulates estrogen
responsive genes in breast cancer cells. J Pineal Res. 47:23–31.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tamarkin L, Danforth D, Lichter A, DeMoss
E, Cohen M, Chabner B and Lippman M: Decreased nocturnal plasma
melatonin peak in patients with estrogen receptor positive breast
cancer. Science. 216:1003–1005. 1982. View Article : Google Scholar : PubMed/NCBI
|