With the development of tumor molecular biology,
progress of the detection and treatment of cancer has led to an
impressive reduction in both mortality and morbidity. However,
cancer still remains one of the most clinically challenging
diseases (1). Today, it is
believed that systemic chemotherapy improves the survival and
quality of life of patients with advanced stage cancer, and
improved the outcome of first-line therapy for advanced and
metastatic cancer have primarily focused on the addition of
targeted agents to platinum-based two-drug regimens (2). Medical studies suggest that
understanding the molecular mechanism of tumors is critical for
improving the diagnosis and treatment. Especially, the level of
certain protein expression is associated with the prognosis and
treatment of malignant tumors (3).
Heat shock protein 90 (Hsp90) accounts for 1–2% of the amounts of
cellular proteins under non-stressed conditions. However, it
contents would go up approximately twice during environmental
stress (4). Hsp90 performs a
series of biological functions via complicated signals regulation
by combining and disaggregation of ATP, and various client proteins
and co-chaperones of Hsp90 are implicated in this process (5). Human Hsp90 includes four isoforms:
Hsp90α and β (cytosolic isoforms), TRAP1 (in mitochondria) and
Grp94 (in endoplasmic reticulum) (6). Hsp90β (Hsp90AB1) is regarded as a
constitutive expression while Hsp90α (Hsp90AA1) as an inducible
expression, it is proved that they have 86% amino acid sequence
identity (7). Hsp90 has been found
as a critical regulator of cell proliferation, development,
mobility and metastasis in malignant tumors, which facilitates
maturation and activation of oncogenic proteins, including many
kinases and transcription regulatory factors (8). Also, Hsp90 exerts anti-apoptotic
activity and affects growth processes tumor cells, and
overexpression of Hsp90 has been obviously associated with drug
resistance and survival time of tumor patients (9). Previous studies show that Hsp90 is
highly expressed in specimens of lung cancer and are associated
with poor post-surgical survival time and lymphatic metastasis of
lung cancer patients (10–13) indicating that upregulation of Hsp90
potentially facilitates proliferation and metastasis of lung
cancer. However, anti-Hsp90 (Hsp90 inhibitors) studies have
demonstrated that downregulation and function disruption of Hsp90
inhibits cell proliferation, motility and metastasis, and induces
apoptosis of lung cancer cells (11,12,14).
Here, we reviewed new findings on the role of Hsp90 in lung cancer,
including the mechanisms and signaling pathways, the pre-clinical
results of Hsp90 inhibition (14).
As a homodimeric protein of ~90 kDa, Hsp90 performs
complicated biological functions reacting with many collaborating
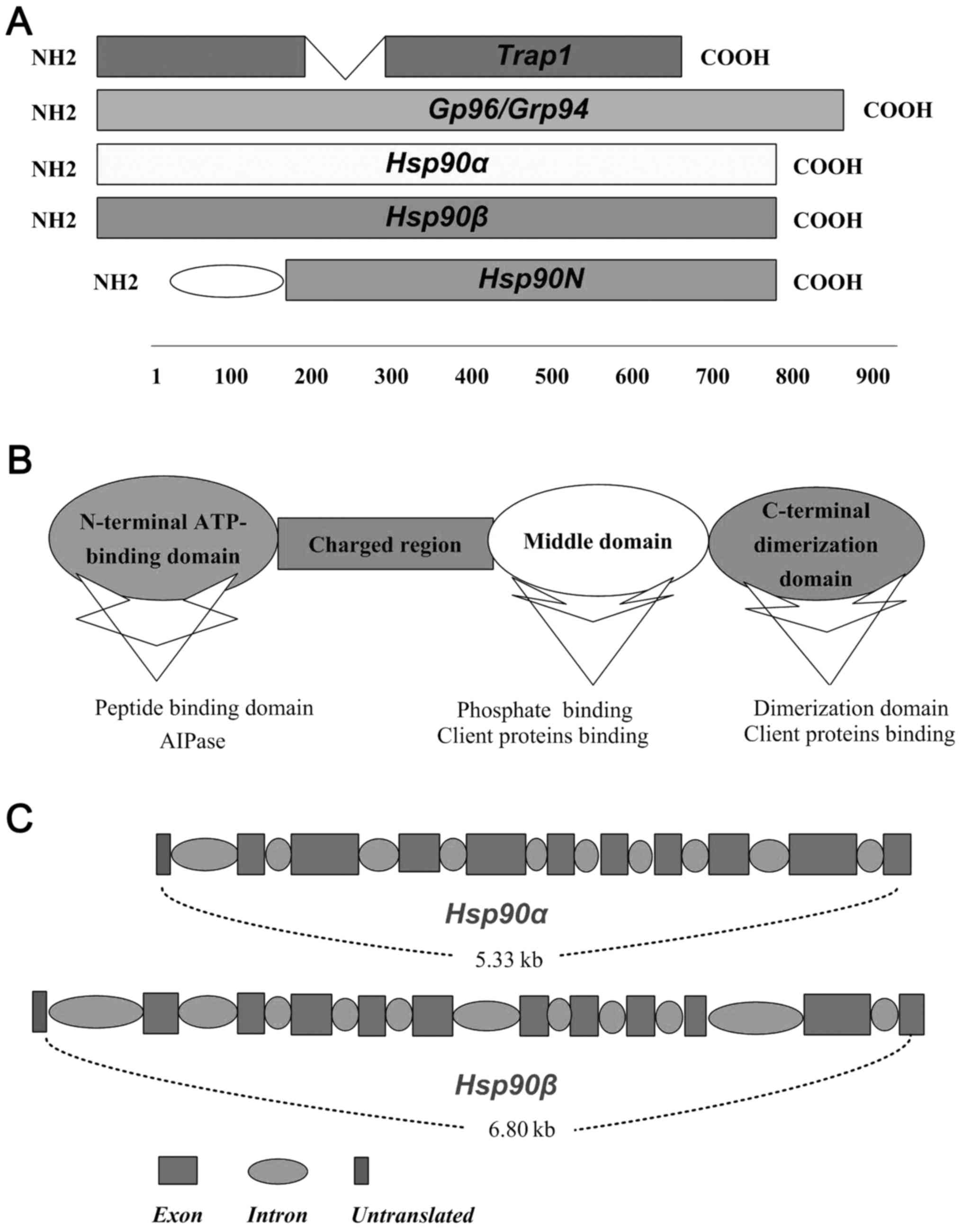
proteins (co-chaperons and clients proteins of Hsp90) (15). As shown in Fig. 1A, Hsp90α, Hsp90β, gp96 and the
TRAP1 are four members of Hsp90 family (7). Different members of Hsp90 family
present the same action pattern but binding to specially appointed
clients proteins, which depends in part on their locations and
distribution within the different cells (16). Each protomer of Hsp90 comprises
three regions, ATP-binding domain (N-domain), middle domain
(M-domain), and C-terminal dimerization domain (C-domain) (17) (Fig.
1B). Constructively, the 25-kDa N-terminal of Hsp90 is
relatively conserved, which is linked with 55 kDa C-terminal
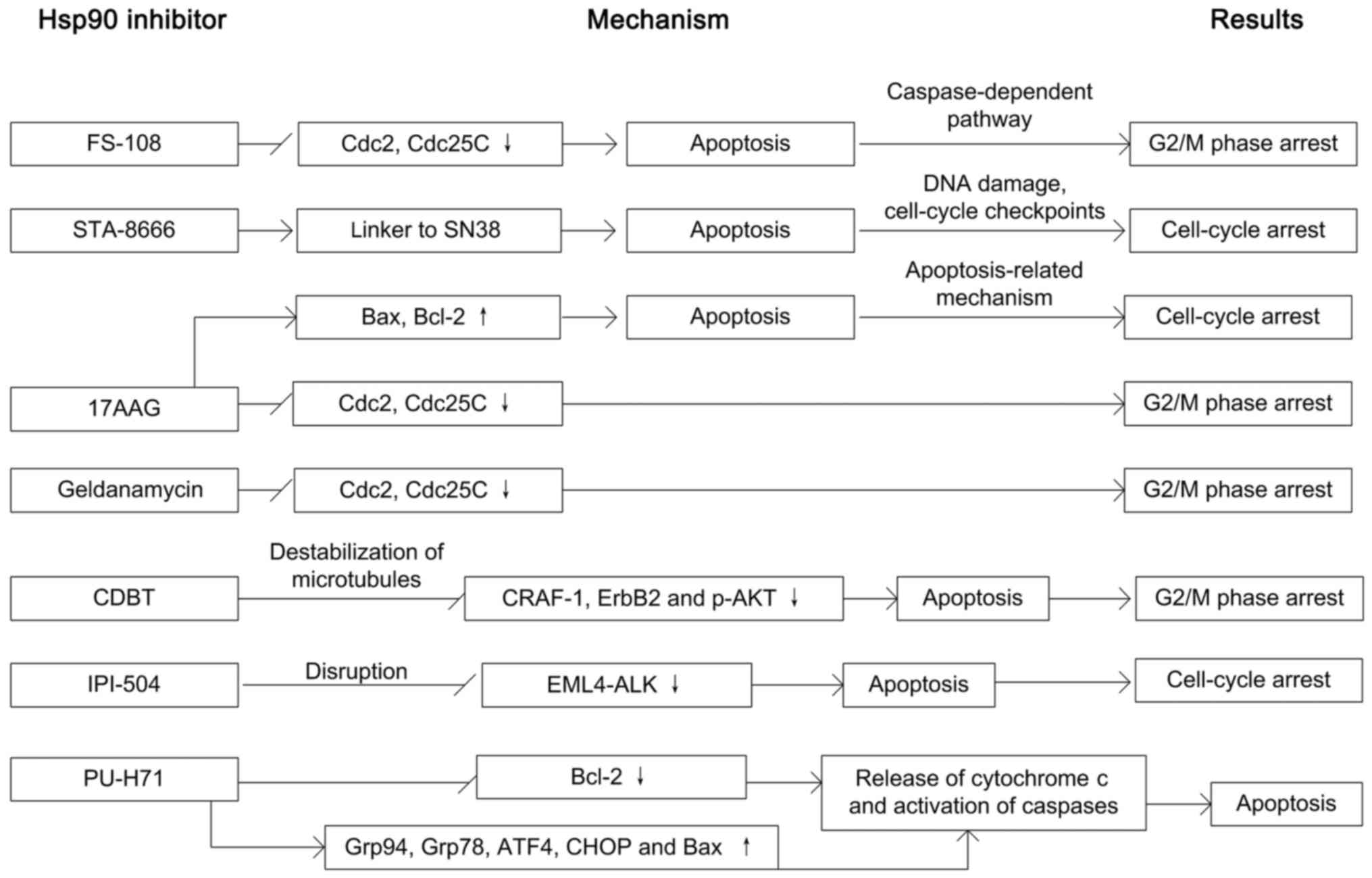
domains by a charged linker region and middle domain (16) (Fig.
1B). The middle domain of Hsp90 is ~35 kDa, has been
investigated as a binding site for client proteins and nuclear
localization signal, which is implicated in recognising of
collaborating proteins and adjusting the activation molecular
chaperones (18) (Fig. 1B). Hsp90 exerts relevant functions
via binding and hydrolysis of ATP like a molecular clamp, which
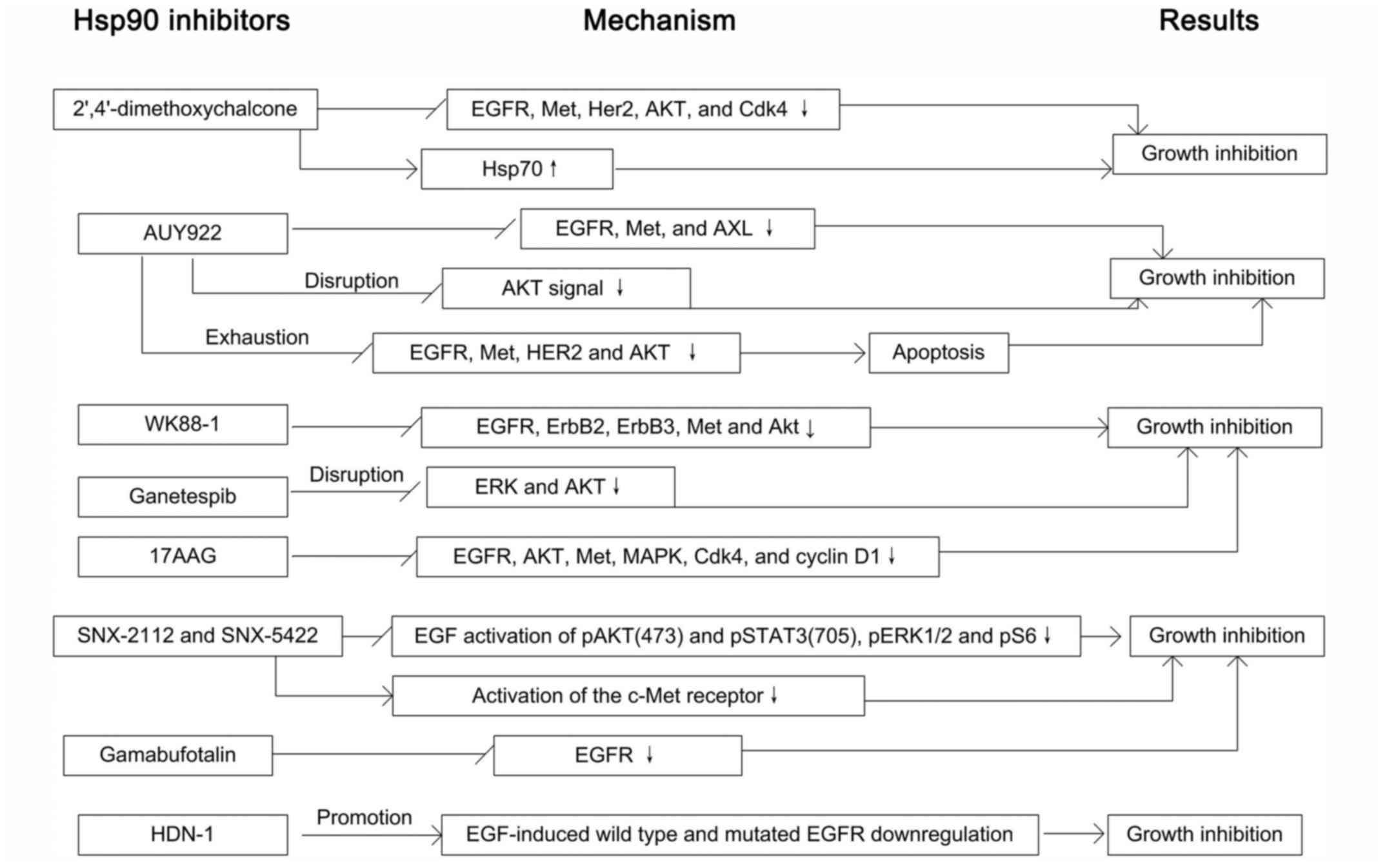
facilitates the combining and dissociation of its client proteins
(19) (Fig. 1B). The important two members of
Hsp90, Hsp90α and Hsp90β, exist as a result of the duplication of
the original gene and share 86% homology (20). In humans, the chromosome 14q32.33
encodes the Hsp90α, while Hsp90β is located at 6p21. Hsp90-coding
genes include intron sequences and the second exon is the region of
translational initiation of both Hsp90α and Hsp90β (7) (Fig.
1C).
The discovery and structural characterization of the
ATP-binding site in the N-terminal domain of HSP90, made it
possible to determine the degree to which the ATPase activity of
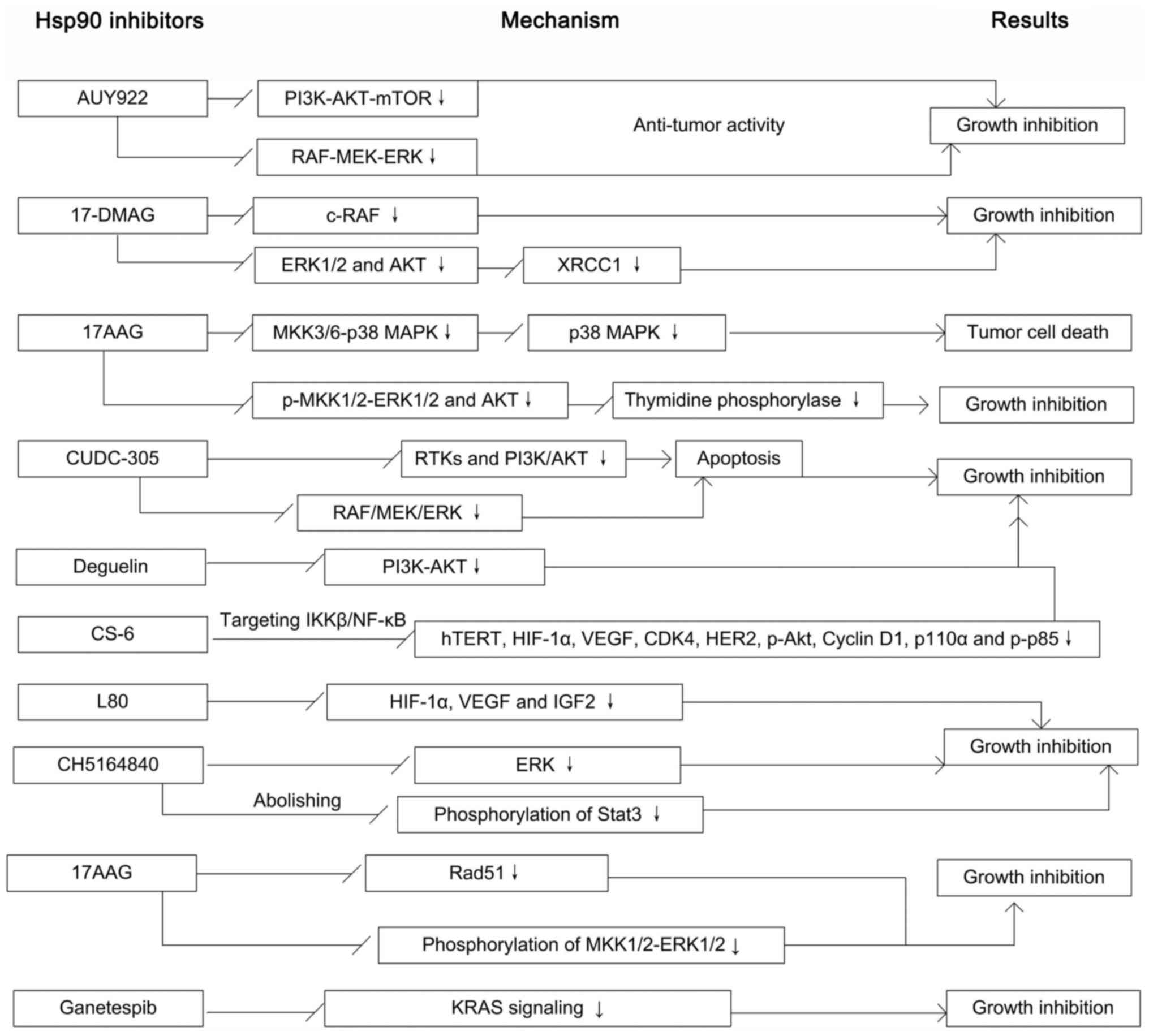
HSP90 contributed to the essential biological functions of HSP90 as
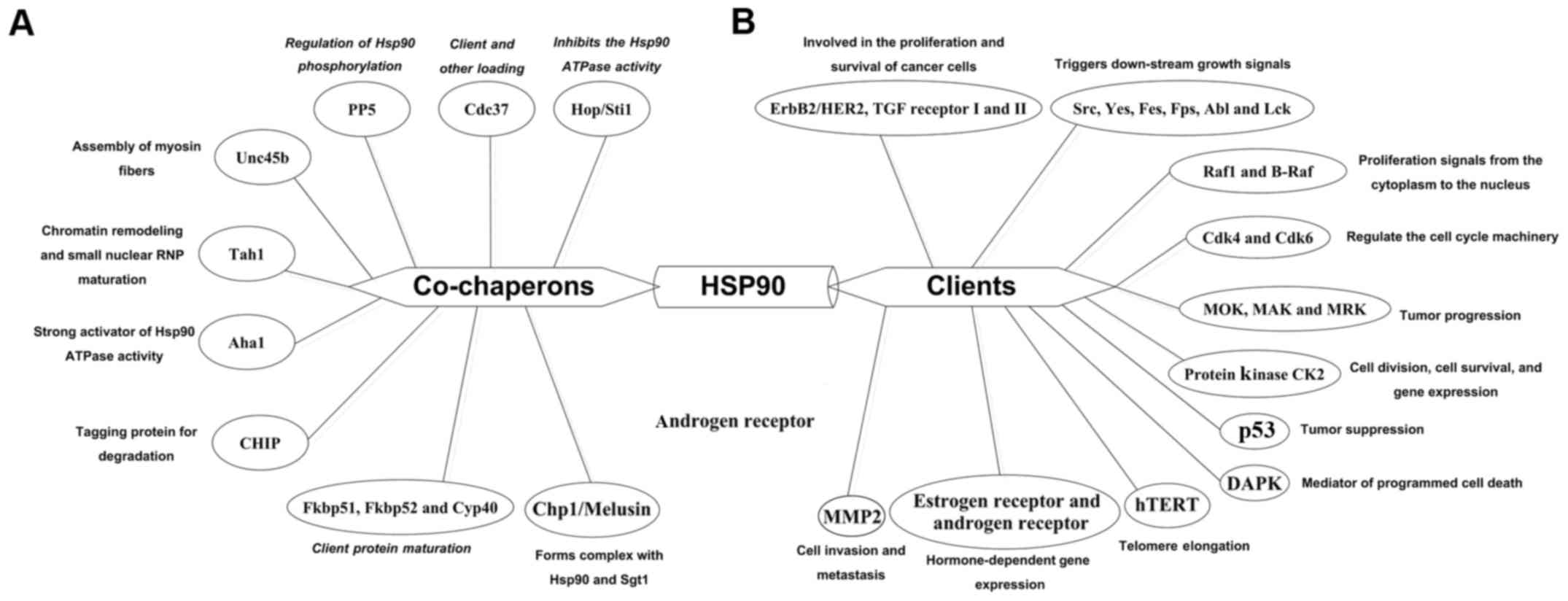
a molecular chaperone. There are more than 20 different proteins
that regulate the activity and function of Hsp90, which are known
as co-chaperones (Fig. 2A). Of
them, Hop/Sti1 inhibits the activity of Hsp90 ATPase and recruits
steroid hormone receptor to Hsp90 (21,22).
Cdc37 helps the loading of other co-chaperones of Hsp90 (18), p23/Sba1 inhibits the ATPase
activity of Hsp90 and also promotes the maturation of client
proteins (22,23). PP5 stabilises the status of Hsp90
phosphorylation promoting the efficient processing of client
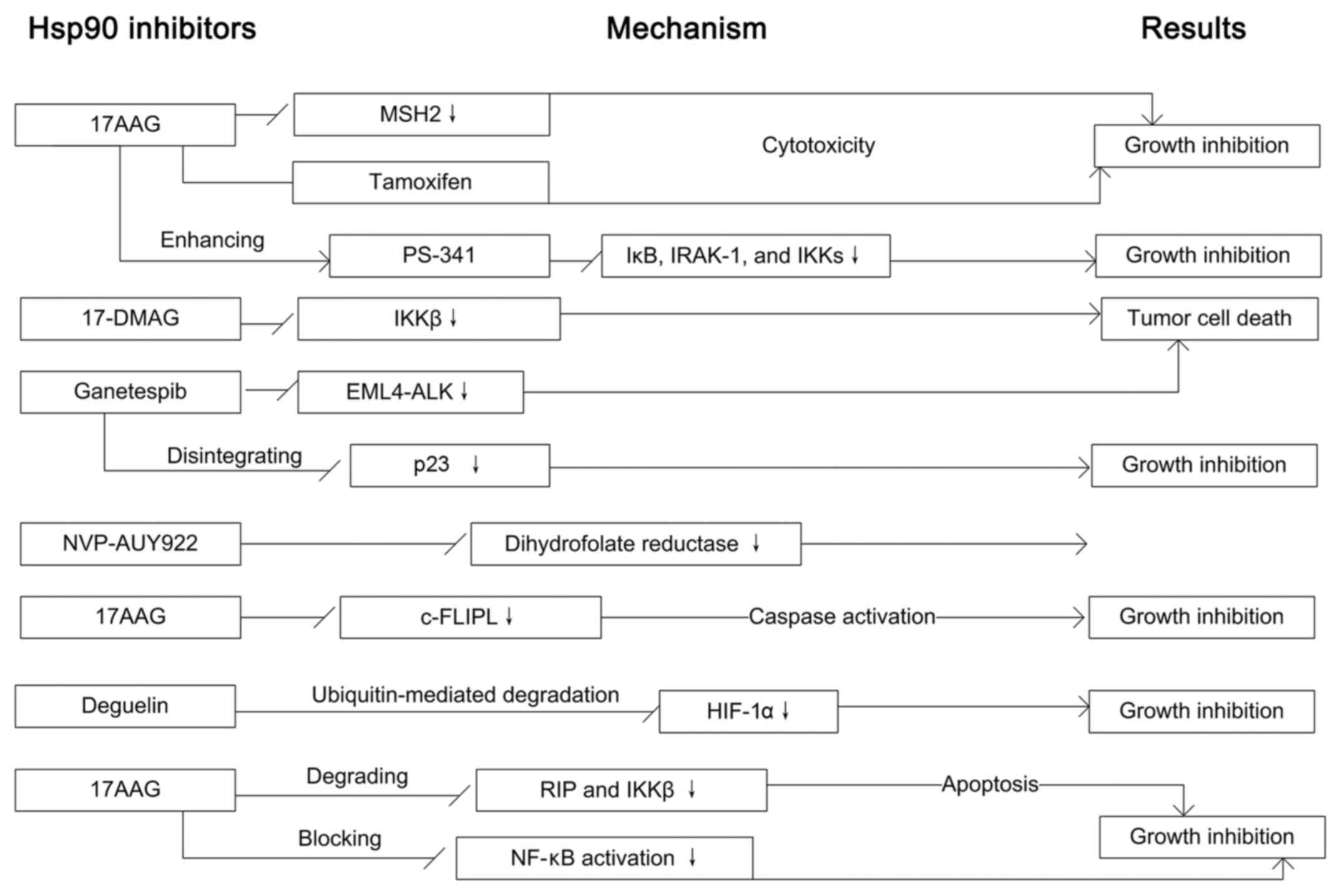
proteins (24,25). Human peptidyl prolyl
cis/trans isomerases, Fkbp51, Fkbp52 and Cyp40
improve the client protein maturation of Hsp90 (26). unc45b forms a stable complex with
Hsp90 and selectively combines the myosin motor domain, and
promotes motor domain folding (27). Together with the Tah1 cofactor,
Hsp90 stabilize Pih1/nop17 and increases the chromatin remodeling
and small nuclear ribonucleoprotein maturation (28). Aha1 induces Hsp90 rearrangements
that speeds up the conformational cycle, which defines a controlled
progression through distinct intermediates (29). Co-chaperones of Hsp90 are also
involved in other physiological processes, such as
mitochondrial/chloroplast protein import, nuclear migration and
melanoma progression (30).
The client proteins of Hsp90 have been found to be
related to a wide aspects of physiological procedures including the
regulation of cell cycle, communication of cell signals and
regulation of cell transcription and post-transcriptional
adjustment (Fig. 2B). Hsp90 plays
a critical role in the function and stability of ErbB2/HER2 by
binding to IGF-I and TGF receptor I and II (18,31,32).
The members of Src family, Yes, Fes, Fps, Abl and Lck, have been
shown to be related to the exertion of Hsp90 function, which
activate the cascade reaction of downstream molecules (18,33,34).
Two proteins of MAPK pathway, ERK1 and ERK2 regulate the growth of
cells by phosphorylating many kinds of substrates within the
nucleus. In addition, Hsp90 correlates with the structure and
function of Raf-1 and B-Raf by interacting with Cdc37 (18,35,36).
Clearly, the activity of CK2 depends upon the appearance of Hsp90
(12,37), estrogen and androgen receptors,
require the assistance of Hsp90 to enable the steroid hormone
ligand to bind (16). Inhibiting
Hsp90 results in the proteolysis of hTERT, which affects the
function of hTERT (38,39). Downregulation of Hsp90 reduces the
expression of MMP9 and protects the MMP2 from degradation in
malignant cells (40,41). Also, p53 interacts with Hsp90 in a
relatively folded state, which may be chalked up to the
destabilization by Hsp90 (42,43).
Some Hsp90 client proteins, including RAF, ErbB2, EGFR, MAK and
hTERT, have been found to interact with Hsp90 and play important
roles in the development of lung cancer (44,45).
Currently, there are still some limitations on
investigating the expression of Hsp90 in lung cancer. First, we
still lack large number of samples and multiple center research.
There is no high quality research concerning the expression of
Hsp90 in lung tissues, blood, BALF, and malignant pleural effussion
(MPE). Second, previous studies do not focus on Hsp90 gene
mutations and abnormal copy. Because a great deal of studies on
inhibitors of Hsp90 that fight against lung cancer show that not
all lung cancer respond with definite efficacy to inhibitors of
Hsp90.
From 2000 to now, investigating the relation between
Hsp90 and lung cancer has become a very active field, especially on
the efficacy of Hsp90 inhibitors that fight against lung cancer. It
seems that different Hsp90 inhibitors affect different signaling
pathways via specific signal molecules in development of lung
cancer.
The combined treatment of Hsp90 inhibitors and
conventional photon radiation has shown more effective tumor growth
inhibition than radiation alone, and a number of Hsp90 inhibitors
are also known to sensitize cancer cells to radiation. Hsp90
inhibitor ganetespib sensitizes NSCLC cells to radiation (91) via potentiating the effect of
radiotherapy and eliminating radioresistant residual cells
(49). A purine-scaffold Hsp90
inhibitor, PU-H71, promotes the sensitivity of the lung cancer
cells to radiation by inhibiting the repair of DSBs (92). 17-AAG and 17DMAG (Hsp90 inhibitors)
have been reported to be potent radio-sensitisers, achieving
radiation enhancement ratios ranging from 2.3 to 2.7 (93). Co-treatment of 17-DMAG with
radiation has a synergistic antitumor activity in NSCLC cells,
which involves in inhibition of DNA repair and correlates with the
BER and ATM-regulated pathways (94). Combination of irradiation and
17-AAG displays an additive effect on cell growth inhibition by
downregulating the expressions of Cdc25C and Cdc2 (51). Hsp90 inhibitor NVP-AUY922 results
in radiosensitization, which is accompanied by DNA repair effect,
cell cycle progression and abrogation of homologous recombination
(95). Co-treatment of NVP-AUY922
and 17-AAG leads to upregulation of HIF-1α and thus shows a
promotion of radiosensitivity (96). Especially, celastrol disrupts the
ATP-binding activity of Hsp90, and thus reinforces the
radiation-induced cell killing by decreasing levels of EGFR, ErbB2
and survivin and increasing p53 expression (97). Hsp90 inhibitor deguelin suppresses
radioresistant lung cancer cells and combined treatment of
radiation with deguelin cuts down the viability and vascularization
of radioresistant cells by blocking the HIF-1α/Hsp90 interaction
and HIF-1α expression (98).
The potential anticancer activity of some Hsp90
inhibitors for treating lung cancer has been proven in preclinical
in vitro and in vivo models. For instance, recently a
clinical trial evaluated the activity and safety of Hsp90 inhibitor
ganetespib in combination with docetaxel in advanced NSCLC, which
showed that combination of ganetespib significantly prolongs the
PFS and OS and the combination treatment of ganetespib and
docetaxel does not have obvious additional side effects (109). NSCLC patients in a previous study
were divided into three groups: cohort A (EGFR mutants), B (KRAS
mutants), or C (with out mutations of both). Patients of the three
groups were all administered ganetespib of 200 mg/m2 via
intravenous infusion (1/week; rest for a week after 3 weeks), until
disease progression. The results showed that ganetespib monotherapy
presents a manageable side effect profile as well as clinical
activity in heavily pretreated patients with advanced NSCLCs,
particularly in patients with tumors harboring ALK gene
rearrangement (110). Another
prospective, non-randomized, multicenter, phase II study of IPI-504
monotherapy has shown that IPI-504 has certain clinical activity in
patients with NSCLC, particularly among patients with ALK
rearrangements (111). However,
the clinical research on the Hsp90 inhibitor for treating lung
cancer is only at the beginning, it seems that these studies did
not produce an impressive breakthrough as we expected. Because
Hsp90 conduces to the maturation and stability many mutated or
overexpressed oncogenic proteins, targeting Hsp90 has been
considered as an effective anticancer therapy. Although the
WCLC2016, GALAXY-2 study showed that the addition of ganetespib as
a rescue treatment on the basis of docetaxel did not improve
efficacy. The combination application of Hsp90 inhibitors and other
traditional chemotherapy is still greatly worthy of further
exploration. However, the clinical research of Hsp90-dependent
molecular targeted therapy (Hsp90 inhibitors) is an area where we
have really fallen far behind. We must acknowledge that the main
reason of leading to the embarrassing situation correlates with
medical ethics and strict approval system of new drugs in all
countries; after all, we are faced with humans. However, clinical
research and application is the only way and the ultimate goal.
Only this way, can we bring benefits to lung cancer patients from
Hsp90 inhibitors.
The clinical benefit of current anticancer regimens
for lung cancer therapy is still limited due to moderate efficacy,
drug resistance, and early recurrence. Therefore, the development
of effective new anticancer drugs for first-line therapy and for
optimal second-line treatment is necessary. Study on the molecular
mechanism of Hsp90 in lung cancer has made some progress as well as
greatly promoting the development of Hsp90-dependent molecular
targeted drugs. However, there are some shortcomings in current
research. So far, no large sample numbers and multiple centers on
the expression of Hsp90 in lung cancer are reported, including in
lung tissues, blood, bronchoalveolar lavage fluid and malignant
pleural effussion. In addition, no studies keep a watchful eye on
gene mutations and copy abnormality of Hsp90, which may be
correlated with the efficacy of Hsp90 inhibitors such as EGFR
mutations. Importantly, although clinical studies to evaluate the
activity and safety of Hsp90 inhibitors in treating lung cancer
patients has been reported occasionally, it seems that quality and
scale of these studies could not support the evidence of clinical
application of Hsp90 inhibitors. To assess whether Hsp90 inhibitor
proves to be a successful therapeutic strategy in treating lung
cancer patients, we still need to do further research, such as
construction of drug delivery vehicles, design of clinical research
and evaluation of side effects. Future research should focus on
assessing the activity and safety of Hsp90 inhibitors in clinical
lung cancer patients and establish the most effective Hsp90
inhibitors for treating lung cancer.
Research shows that Hsp90 is highly expressed in
lung cancer and that upregulation of Hsp90 potentially facilitates
proliferation and metastasis of lung cancer. However, anti-Hsp90
(Hsp90 inhibitors) studies have demonstrated that downregulation
and function disruption of Hsp90 inhibits cell proliferation,
motility and metastasis, and induces apoptosis of lung cancer
cells. However, there is an urgent need for a comprehensive
assessment of Hsp90 protein expression and gene abnormality in
large cohorts of lung cancer. In addition, high quality clinical
research on Hsp90 inhibitors are also needed for evaluating the
efficacy and safety in clinical recommendation. Actually, we still
know relatively little as to how the Hsp90 regulates tumorigenesis
of lung cancer at the molecular level, thus improved understanding
of the molecular mechanisms and signaling pathways correlated with
Hsp90 present an interesting challenge, and a future important
direction.
This study was supported by grants from the
Scientific Research Plan projects of Shaanxi Province Education
Department (no. 17JK0661).
|
1
|
Biaoxue R, Xiguang C, Hua L and Shuanying
Y: Stathmin-dependent molecular targeting therapy for malignant
tumor: The latest 5 years' discoveries and developments. J Transl
Med. 14:2792016. View Article : Google Scholar
|
|
2
|
Biao-xue R, Xi-guang C, Shuan-ying Y, Wei
L and Zong-juan M: EphA2-dependent molecular targeting therapy for
malignant tumors. Curr Cancer Drug Targets. 11:1082–1097. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Biaoxue R, Hua L, Wenlong G and Shuanying
Y: Overexpression of stathmin promotes metastasis and growth of
malignant solid tumors: A systemic review and meta-analysis.
Oncotarget. 7:78994–79007. 2016.PubMed/NCBI
|
|
4
|
Tatokoro M, Koga F, Yoshida S and Kihara
K: Heat shock protein 90 targeting therapy: State of the art and
future perspective. EXCLI J. 14:48–58. 2015.PubMed/NCBI
|
|
5
|
Chehab M, Caza T, Skotnicki K, Landas S,
Bratslavsky G, Mollapour M and Bourboulia D: Targeting Hsp90 in
urothelial carcinoma. Oncotarget. 6:8454–8473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hall JA, Forsberg LK and Blagg BS:
Alternative approaches to Hsp90 modulation for the treatment of
cancer. Future Med Chem. 6:1587–1605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prodromou C: Mechanisms of Hsp90
regulation. Biochem J. 473:2439–2452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khurana N and Bhattacharyya S: Hsp90, the
concertmaster: Tuning transcription. Front Oncol. 5:1002015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lianos GD, Alexiou GA, Mangano A, Mangano
A, Rausei S, Boni L, Dionigi G and Roukos DH: The role of heat
shock proteins in cancer. Cancer Lett. 360:114–118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biaoxue R, Xiling J, Shuanying Y, Wei Z,
Xiguang C, Jinsui W and Min Z: Upregulation of Hsp90-beta and
annexin A1 correlates with poor survival and lymphatic metastasis
in lung cancer patients. J Exp Clin Cancer Res. 31:702012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SH, Ji JH, Park KT, Lee JH, Kang KW,
Park JH, Hwang SW, Lee EH, Cho YJ, Jeong YY, et al: High-level
expression of Hsp90β is associated with poor survival in resectable
non-small-cell lung cancer patients. Histopathology. 67:509–519.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyata Y and Yahara I: The 90-kDa heat
shock protein, HSP90, binds and protects casein kinase II from
self-aggregation and enhances its kinase activity. J Biol Chem.
267:7042–7047. 1992.PubMed/NCBI
|
|
13
|
Rong B, Zhao C, Liu H, Ming Z, Cai X, Gao
W and Yang S: Identification and verification of Hsp90-beta as a
potential serum biomarker for lung cancer. Am J Cancer Res.
4:874–885. 2014.PubMed/NCBI
|
|
14
|
Pillai RN and Ramalingam SS: Heat shock
protein 90 inhibitors in non-small-cell lung cancer. Curr Opin
Oncol. 26:159–164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pennisi R, Ascenzi P and Di Masi A: Hsp90:
A new player in DNA repair? Biomolecules. 5:2589–2618. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maloney A and Workman P: HSP90 as a new
therapeutic target for cancer therapy: The story unfolds. Expert
Opin Biol Ther. 2:3–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J and Buchner J: Structure, function
and regulation of the hsp90 machinery. Biomed J. 36:106–117. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Verma S, Goyal S, Jamal S, Singh A and
Grover A: Hsp90: Friends, clients and natural foes. Biochimie.
127:227–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Fok JH and Davies FE: Heat shock
proteins in multiple myeloma. Oncotarget. 5:1132–1148. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
García R, Merino D, Gómez JM, Nistal JF,
Hurlé MA, Cortajarena AL and Villar AV: Extracellular heat shock
protein 90 binding to TGFβ receptor I participates in TGFβ-mediated
collagen production in myocardial fibroblasts. Cell Signal.
28:1563–1579. 2016. View Article : Google Scholar
|
|
21
|
Prodromou C, Siligardi G, O'Brien R,
Woolfson DN, Regan L, Panaretou B, Ladbury JE, Piper PW and Pearl
LH: Regulation of Hsp90 ATPase activity by tetratricopeptide repeat
(TPR)-domain co-chaperones. EMBO J. 18:754–762. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Richter K, Muschler P, Hainzl O, Reinstein
J and Buchner J: Sti1 is a non-competitive inhibitor of the Hsp90
ATPase Binding prevents the N-terminal dimerization reaction during
the ATPase cycle. J Biol Chem. 278:10328–10333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Obermann WM, Sondermann H, Russo AA,
Pavletich NP and Hartl FU: In vivo function of Hsp90 is dependent
on ATP binding and ATP hydrolysis. J Cell Biol. 143:901–910. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Silverstein AM, Galigniana MD, Chen MS,
Owens-Grillo JK, Chinkers M and Pratt WB: Protein phosphatase 5 is
a major component of glucocorticoid receptor hsp90 complexes with
properties of an FK506-binding immunophilin. J Biol Chem.
272:16224–16230. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wandinger SK, Suhre MH, Wegele H and
Buchner J: The phosphatase Ppt1 is a dedicated regulator of the
molecular chaperone Hsp90. EMBO J. 25:367–376. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pirkl F and Buchner J: Functional analysis
of the Hsp90-associated human peptidyl prolyl cis/trans isomerases
FKBP51, FKBP52 and Cyp40. J Mol Biol. 308:795–806. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Srikakulam R, Liu L and Winkelmann DA:
Unc45b forms a cytosolic complex with Hsp90 and targets the
unfolded myosin motor domain. PLoS One. 3:e21372008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao R, Kakihara Y, Gribun A, Huen J, Yang
G, Khanna M, Costanzo M, Brost RL, Boone C, Hughes TR, et al:
Molecular chaperone Hsp90 stabilizes Pih1/Nop17 to maintain R2TP
complex activity that regulates snoRNA accumulation. J Cell Biol.
180:563–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hessling M, Richter K and Buchner J:
Dissection of the ATP-induced conformational cycle of the molecular
chaperone Hsp90. Nat Struct Mol Biol. 16:287–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li J, Soroka J and Buchner J: The Hsp90
chaperone machinery: Conformational dynamics and regulation by
co-chaperones. Biochim Biophys Acta. 1823:624–635. 2012. View Article : Google Scholar
|
|
31
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu W, Mimnaugh E, Rosser MF, Nicchitta C,
Marcu M, Yarden Y and Neckers L: Sensitivity of mature Erbb2 to
geldanamycin is conferred by its kinase domain and is mediated by
the chaperone protein Hsp90. J Biol Chem. 276:3702–3708. 2001.
View Article : Google Scholar
|
|
33
|
Schwartzberg PL: The many faces of Src:
Multiple functions of a prototypical tyrosine kinase. Oncogene.
17:1463–1468. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Perdew GH, Wiegand H, Vanden Heuvel JP,
Mitchell C and Singh SSA: A 50 kilodalton protein associated with
raf and pp60(v-src) protein kinases is a mammalian homolog of the
cell cycle control protein cdc37. Biochemistry. 36:3600–3607. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stancato LF, Chow YH, Owens-Grillo JK, Yem
AW, Deibel MR Jr, Jove R and Pratt WB: The native v-Raf hsp90 p50
heterocomplex contains a novel immunophilin of the FK506 binding
class. J Biol Chem. 269:22157–22161. 1994.PubMed/NCBI
|
|
37
|
Miyata Y and Yahara I: Interaction between
casein kinase II and the 90-kDa stress protein, HSP90.
Biochemistry. 34:8123–8129. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Holt SE, Aisner DL, Baur J, Tesmer VM, DY
M, Ouellette M, Trager JB, Morin GB, Toft DO, Shay JW, et al:
Functional requirement of p23 and Hsp90 in telomerase complexes.
Genes Dev. 13:817–826. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
DeZwaan DC, Toogun OA, Echtenkamp FJ and
Freeman BC: The Hsp82 molecular chaperone promotes a switch between
unextendable and extendable telomere states. Nat Struct Mol Biol.
16:711–716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sarkar S, Dutta D, Samanta SK,
Bhattacharya K, Pal BC, Li J, Datta K and Mandal C and Mandal C:
Oxidative inhibition of Hsp90 disrupts the super-chaperone complex
and attenuates pancreatic adenocarcinoma in vitro and in vivo. Int
J Cancer. 132:695–706. 2013. View Article : Google Scholar
|
|
41
|
Song X, Wang X, Zhuo W, Shi H, Feng D, Sun
Y, Liang Y, Fu Y, Zhou D and Luo Y: The regulatory mechanism of
extracellular Hsp90{alpha} on matrix metalloproteinase-2 processing
and tumor angiogenesis. J Biol Chem. 285:40039–40049. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Walerych D, Gutkowska M, Klejman MP,
Wawrzynow B, Tracz Z, Wiech M, Zylicz M and Zylicz A: ATP binding
to Hsp90 is sufficient for effective chaperoning of p53 protein. J
Biol Chem. 285:32020–32028. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park SJ, Borin BN, Martinez-Yamout MA and
Dyson HJ: The client protein p53 adopts a molten globule-like state
in the presence of Hsp90. Nat Struct Mol Biol. 18:537–541. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Q, Zhai S, Li L, Li X, Zhou H, Liu
A, Su G, Mu Q, Du Y and Yan B: Anti-tumor selectivity of a novel
tubulin and HSP90 dual-targeting inhibitor in non-small cell lung
cancer models. Biochem Pharmacol. 86:351–360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang L, Yu Z, Wang Y, Wang X, Zhang L,
Wang C, Yue Q, Wang X, Deng S, Huo X, et al: Quantitative
proteomics reveals molecular mechanism of gamabufotalin and its
potential inhibition on Hsp90 in lung cancer. Oncotarget.
7:76551–76564. 2016.PubMed/NCBI
|
|
46
|
Su JM, Hsu YY, Lin P and Chang H: Nuclear
accumulation of heat-shock protein 90 is associated with poor
survival and metastasis in patients with non-small cell lung
cancer. Anticancer Res. 36:2197–2203. 2016.PubMed/NCBI
|
|
47
|
Gallegos Ruiz MI, Floor K, Roepman P,
Rodriguez JA, Meijer GA, Mooi WJ, Jassem E, Niklinski J, Muley T,
van Zandwijk N, et al: Integration of gene dosage and gene
expression in non-small cell lung cancer, identification of HSP90
as potential target. PLoS One. 3:e00017222008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu W, Wu Y, Wang L, Gao L, Wang Y, Liu X,
Zhang K, Song J, Wang H, Bayer TA, et al: Protein signature for
non-small cell lung cancer prognosis. Am J Cancer Res. 4:256–269.
2014.PubMed/NCBI
|
|
49
|
Gomez-Casal R, Epperly MW, Wang H, Proia
DA, Greenberger JS and levina V: Radioresistant human lung
adenocarcinoma cells that survived multiple fractions of ionizing
radiation are sensitive to HSP90 inhibition. Oncotarget.
6:44306–44322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu T, Wang X and Zhang L: The correlation
between the up-regulation of Hsp90 and drug resistance to cisplatin
in lung cancer cell line. Zhongguo Fei Ai Za Zhi. 14:472–477.
2011.In Chinese. PubMed/NCBI
|
|
51
|
Senju M, Sueoka N, Sato A, Iwanaga K,
Sakao Y, Tomimitsu S, Tominaga M, Irie K, Hayashi S and Sueoka E:
Hsp90 inhibitors cause G2/M arrest associated with the reduction of
Cdc25C and Cdc2 in lung cancer cell lines. J Cancer Res Clin Oncol.
132:150–158. 2006. View Article : Google Scholar
|
|
52
|
Wu Y, Huang B, Liu Q and Liu Y: Heat shock
protein 90-β over-expression is associated with poor survival in
stage I lung adenocarcinoma patients. Int J Clin Exp Pathol.
8:8252–8259. 2015.
|
|
53
|
Wang M, Feng L, Li P, Han N, Gao Y and
Xiao T: Hsp90AB1 protein is overexpressed in non-small cell lung
cancer tissues and associated with poor prognosis in lung
adenocarcinoma patients. Zhongguo Fei Ai Za Zhi. 19:64–69. 2016.In
Chinese. PubMed/NCBI
|
|
54
|
Shi Y, Liu X, Lou J, Han X, Zhang L, Wang
Q, Li B, Dong M and Zhang Y: Plasma levels of heat shock protein 90
alpha associated with lung cancer development and treatment
responses. Clin Cancer Res. 20:6016–6022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang YQ, Shen AJ, Sun JY, Wang X, Liu HC,
Zhang MM, Chen DQ, Xiong B, Shen JK, Geng MY, et al: Targeting
Hsp90 with FS-108 circumvents gefitinib resistance in EGFR mutant
non-small cell lung cancer cells. Acta Pharmacol Sin. 37:1587–1596.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gaponova AV, Nikonova AS, Deneka AY, Kopp
MC, Kudinov AE, Skobeleva N, Khazak V, Ogawa LS, Cai KQ, Duncan KE,
et al: A novel HSP90 inhibitor-drug conjugate to Sn38 is highly
effective in small cell lung cancer. Clin Cancer Res. 22:5120–5129.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Niu B, Lin J and Feng T: Effects of 17-AAG
on the proliferation and apoptosis of human lung cancer A549 and
H446 cells. Zhonghua Jie He He Hu Xi Za Zhi. 38:267–272. 2015.In
Chinese. PubMed/NCBI
|
|
58
|
Hirakawa H, Fujisawa H, Masaoka A, Noguchi
M, Hirayama R, Takahashi M, Fujimori A and Okayasu R: The
combination of Hsp90 inhibitor 17AAG and heavy-ion irradiation
provides effective tumor control in human lung cancer cells. Cancer
Med. 4:426–436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang Q, Zhai S, Li L, Li X, Jiang C,
Zhang C and Yan B: P-glycoprotein-evading anti-tumor activity of a
novel tubulin and HSP90 dual inhibitor in a non-small-cell lung
cancer model. J Pharmacol Sci. 126:66–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Normant E, Paez G, West KA, Lim AR, Slocum
KL, Tunkey C, McDougall J, Wylie AA, Robison K, Caliri K, et al:
The Hsp90 inhibitor IPI-504 rapidly lowers EML4-ALK levels and
induces tumor regression in ALK-driven NSCLC models. Oncogene.
30:2581–2586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gallerne C, Prola A and Lemaire C: Hsp90
inhibition by PU-H71 induces apoptosis through endoplasmic
reticulum stress and mitochondrial pathway in cancer cells and
overcomes the resistance conferred by Bcl-2. Biochim Biophys Acta.
1833:1356–1366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Seo YH: Discovery of
2′,4′-dimethoxychalcone as a Hsp90 inhibitor and its effect on
iressa-resistant non-small cell lung cancer (NSCLC). Arch Pharm
Res. 38:1783–1788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Choi YJ, Kim SY, So KS, Baek IJ, Kim WS,
Choi SH, Lee JC, Bivona TG, Rho JK and Choi CM: AUY922 effectively
overcomes MET- and AXL-mediated resistance to EGFR-TKI in lung
cancer cells. PLoS One. 10:e01198322015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ueno T, Tsukuda K, Toyooka S, Ando M,
Takaoka M, Soh J, Asano H, Maki Y, Muraoka T, Tanaka N, et al:
Strong anti-tumor effect of NVP-AUY922, a novel Hsp90 inhibitor, on
non-small cell lung cancer. Lung Cancer. 76:26–31. 2012. View Article : Google Scholar
|
|
65
|
Jang WJ, Jung SK, Kang JS, Jeong JW, Bae
MK, Joo SH, Park GH, Kundu JK, Hong YS and Jeong CH: Anti-tumor
activity of WK88-1, a novel geldanamycin derivative, in
gefitinib-resistant non-small cell lung cancers with Met
amplification. Cancer Sci. 105:1245–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Smith DL, Acquaviva J, Sequeira M, Jimenez
JP, Zhang C, Sang J, Bates RC and Proia DA: The HSP90 inhibitor
ganetespib potentiates the antitumor activity of EGFR tyrosine
kinase inhibition in mutant and wild-type non-small cell lung
cancer. Target Oncol. 10:235–245. 2015. View Article : Google Scholar :
|
|
67
|
Koizumi H, Yamada T, Takeuchi S, Nakagawa
T, Kita K, Nakamura T, Matsumoto K, Suda K, Mitsudomi T and Yano S:
Hsp90 inhibition overcomes HGF-triggering resistance to EGFR-TKIs
in EGFR-mutant lung cancer by decreasing client protein expression
and angiogenesis. J Thorac Oncol. 7:1078–1085. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kobayashi N, Toyooka S, Soh J, Yamamoto H,
Dote H, Kawasaki K, Otani H, Kubo T, Jida M, Ueno T, et al: The
anti-proliferative effect of heat shock protein 90 inhibitor,
17-DMAG, on non-small-cell lung cancers being resistant to EGFR
tyrosine kinase inhibitor. Lung Cancer. 75:161–166. 2012.
View Article : Google Scholar
|
|
69
|
Rice JW, Veal JM, Barabasz A, Foley B,
Fadden P, Scott A, Huang K, Steed P and Hall S: Targeting of
multiple signaling pathways by the Hsp90 inhibitor SnX-2112 in EGFR
resistance models as a single agent or in combination with
erlotinib. Oncol Res. 18:229–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Song X, Zhao Z, Qi X, Tang S, Wang Q, Zhu
T, Gu Q, Liu M and Li J: Identification of
epipolythiodioxopiperazines HDN-1 and chaetocin as novel inhibitor
of heat shock protein 90. Oncotarget. 6:5263–5274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Park KS, Oh B, Lee MH, Nam KY, Jin HR,
Yang H, Choi J, Kim SW and Lee DH: The HSP90 inhibitor, NVP-AUY922,
sensitizes KRAS-mutant non-small cell lung cancer with intrinsic
resistance to MEK inhibitor, trametinib. Cancer Lett. 372:75–81.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Takeuchi S, Fukuda K, Arai S, Nanjo S,
Kita K, Yamada T, Hara E, Nishihara H, Uehara H and Yano S:
Organ-specific efficacy of HSP90 inhibitor in multiple-organ
metastasis model of chemorefractory small cell lung cancer. Int J
Cancer. 138:1281–1289. 2016. View Article : Google Scholar
|
|
73
|
Tung CL, Jian YJ, Syu JJ, Wang TJ, Chang
PY, Chen CY, Jian YT and Lin YW: Down-regulation of ERK1/2 and
AKT-mediated X-ray repair cross-complement group 1 protein (XRCC1)
expression by Hsp90 inhibition enhances the gefitinib-induced
cytotoxicity in human lung cancer cells. Exp Cell Res. 334:126–135.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tung CL, Chiu HC, Jian YJ, Jian YT, Chen
CY, Syu JJ, Wo TY, Huang YJ, Tseng SC and Lin YW: Down-regulation
of MSH2 expression by an Hsp90 inhibitor enhances
pemetrexed-induced cytotoxicity in human non-small-cell lung cancer
cells. Exp Cell Res. 322:345–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Weng SH, Tseng SC, Huang YC, Chen HJ and
Lin YW: Inhibition of thymidine phosphorylase expression by using
an HSP90 inhibitor potentiates the cytotoxic effect of cisplatin in
non-small-cell lung cancer cells. Biochem Pharmacol. 84:126–136.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bao R, Lai CJ, Wang DG, Qu H, Yin L,
Zifcak B, Tao X, Wang J, Atoyan R, Samson M, et al: Targeting heat
shock protein 90 with CUDC-305 overcomes erlotinib resistance in
non-small cell lung cancer. Mol Cancer Ther. 8:3296–3306. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kim WY, Chang DJ, Hennessy B, Kang HJ, Yoo
J, Han SH, Kim YS, Park HJ, Seo SY, Mills G, et al: A novel
derivative of the natural agent deguelin for cancer chemoprevention
and therapy. Cancer Prev Res (Phila). 1:577–587. 2008. View Article : Google Scholar
|
|
78
|
Lee SC, Min HY, Choi H, Kim HS, Kim KC,
Park SJ, Seong MA, Seo JH, Park HJ, Suh YG, et al: Synthesis and
evaluation of a novel deguelin derivative, L80, which disrupts ATP
binding to the C-terminal domain of heat shock protein 90. Mol
Pharmacol. 88:245–255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ono N, Yamazaki T, Tsukaguchi T, Fujii T,
Sakata K, Suda A, Tsukuda T, Mio T, Ishii N, Kondoh O, et al:
Enhanced antitumor activity of erlotinib in combination with the
Hsp90 inhibitor CH5164840 against non-small-cell lung cancer.
Cancer Sci. 104:1346–1352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ko JC, Chen HJ, Huang YC, Tseng SC, Weng
SH, Wo TY, Huang YJ, Chiu HC, Tsai MS, Chiou RY, et al: HSP90
inhibition induces cytotoxicity via down-regulation of Rad51
expression and DNA repair capacity in non-small cell lung cancer
cells. Regul Toxicol Pharmacol. 64:415–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Acquaviva J, Smith DL, Sang J, Friedland
JC, He S, Sequeira M, Zhang C, Wada Y and Proia DA: Targeting
KRAS-mutant non-small cell lung cancer with the Hsp90 inhibitor
ganetespib. Mol Cancer Ther. 11:2633–2643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ko JC, Chiu HC, Syu JJ, Chen CY, Jian YT,
Huang YJ, Wo TY, Jian YJ, Chang PY, Wang TJ, et al: Down-regulation
of MSH2 expression by Hsp90 inhibition enhances cytotoxicity
affected by tamoxifen in human lung cancer cells. Biochem Biophys
Res Commun. 456:506–512. 2015. View Article : Google Scholar
|
|
83
|
Lee KH, Jang AH and Yoo CG:
17-allylamino-17-demethoxygel-danamycin and the enhancement of
PS-341-induced lung cancer cell death by blocking the NF-kappaB and
PI3K/Akt pathways. Am J Respir Cell Mol Biol. 53:412–421. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Qu Z, Dong H, Xu X, Feng W and Yi X:
Combined effects of 17-DMAG and TNF on cells through a mechanism
related to the NF-kappaB pathway. Diagn Pathol. 8:702013.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sang J, Acquaviva J, Friedland JC, Smith
DL, Sequeira M, Zhang C, Jiang Q, Xue L, Lovly CM, Jimenez JP, et
al: Targeted inhibition of the molecular chaperone Hsp90 overcomes
ALK inhibitor resistance in non-small cell lung cancer. Cancer
Discov. 3:430–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Garon EB, Finn RS, Hamidi H, Dering J,
Pitts S, Kamranpour N, Desai AJ, Hosmer W, Ide S, Avsar E, et al:
The HSP90 inhibitor NVP-AUY922 potently inhibits non-small cell
lung cancer growth. Mol Cancer Ther. 12:890–900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang Q, Sun W, Hao X, Li T, Su L and Liu
X: Down-regulation of cellular FLICE-inhibitory protein (Long Form)
contributes to apoptosis induced by Hsp90 inhibition in human lung
cancer cells. Cancer Cell Int. 12:542012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Shimamura T, Perera SA, Foley KP, Sang J,
Rodig SJ, Inoue T, Chen L, Li D, Carretero J, Li YC, et al:
Ganetespib (STA-9090), a nongeldanamycin HSP90 inhibitor, has
potent antitumor activity in in vitro and in vivo models of
non-small cell lung cancer. Clin Cancer Res. 18:4973–4985. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Oh SH, Woo JK, Yazici YD, Myers JN, Kim
WY, Jin Q, Hong SS, Park HJ, Suh YG, Kim KW, et al: Structural
basis for depletion of heat shock protein 90 client proteins by
deguelin. J Natl Cancer Inst. 99:949–961. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wang X, Ju W, Renouard J, Aden J, Belinsky
SA and Lin Y: 17-allyl-amino-17-demethoxygeldanamycin
synergistically potentiates tumor necrosis factor-induced lung
cancer cell death by blocking the nuclear factor-kappaB pathway.
Cancer Res. 66:1089–1095. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wang Y, Liu H, Diao L, Potter A, Zhang J,
Qiao Y, Wang J, Proia DA, Tailor R, Komaki R, et al: Hsp90
inhibitor ganetespib sensitizes non-small cell lung cancer to
radiation but has variable effects with chemoradiation. Clin Cancer
Res. 22:5876–5886. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Segawa T, Fujii Y, Tanaka A, Bando S,
Okayasu R, Ohnishi K and Kubota N: Radiosensitization of human lung
cancer cells by the novel purine-scaffold Hsp90 inhibitor, PU-H71.
Int J Mol Med. 33:559–564. 2014. View Article : Google Scholar
|
|
93
|
Camphausen K and Tofilon PJ: Inhibition of
Hsp90: A multitarget approach to radiosensitization. Clin Cancer
Res. 13:4326–4330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Koll TT, Feis SS, Wright MH, Teniola MM,
Richardson MM, Robles AI, Bradsher J, Capala J and Varticovski L:
HSP90 inhibitor, DMAG, synergizes with radiation of lung cancer
cells by interfering with base excision and ATM-mediated DNA
repair. Mol Cancer Ther. 7:1985–1992. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Laszlo A, Thotala D and Hallahan DE:
Membrane phospholipids, EML4-ALK, and Hsp90 as novel targets in
lung cancer treatment. Cancer J. 19:238–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Schilling D, Bayer C, Li W, Molls M,
Vaupel P and Multhoff G: Radiosensitization of normoxic and hypoxic
h1339 lung tumor cells by heat shock protein 90 inhibition is
independent of hypoxia inducible factor-1α. PLoS One. 7:e311102012.
View Article : Google Scholar
|
|
97
|
Lee JH, Choi KJ, Seo WD, Jang SY, Kim M,
Lee BW, Kim JY, Kang S, Park KH, Lee YS, et al: Enhancement of
radiation sensitivity in lung cancer cells by celastrol is mediated
by inhibition of Hsp90. Int J Mol Med. 27:441–446. 2011.PubMed/NCBI
|
|
98
|
Kim WY, Oh SH, Woo JK, Hong WK and Lee HY:
Targeting heat shock protein 90 overrides the resistance of lung
cancer cells by blocking radiation-induced stabilization of
hypoxia-inducible factor-1alpha. Cancer Res. 69:1624–1632. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Beck TN, Korobeynikov VA, Kudinov AE,
Georgopoulos R, Solanki NR, Andrews-Hoke M, Kistner TM, Pépin DPK,
Nicolas E, et al: Anti-Müllerian hormone signaling regulates
epithelial plasticity and chemoresistance in lung cancer. Cell Rep.
16:657–671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Proia DA, Sang J, He S, Smith DL, Sequeira
M, Zhang C, Liu Y, Ye S, Zhou D, Blackman RK, et al: Synergistic
activity of the Hsp90 inhibitor ganetespib with taxanes in
non-small cell lung cancer models. Invest New Drugs. 30:2201–2209.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tsai MS, Weng SH, Chen HJ, Chiu YF, Huang
YC, Tseng SC, Kuo YH and Lin YW: Inhibition of p38 MAPK-dependent
excision repair cross-complementing 1 expression decreases the DNA
repair capacity to sensitize lung cancer cells to etoposide. Mol
Cancer Ther. 11:561–571. 2012. View Article : Google Scholar
|
|
102
|
Hashida S, Yamamoto H, Shien K, Ohtsuka T,
Suzawa K, Maki Y, Furukawa M, Soh J, Asano H, Tsukuda K, et al:
Hsp90 inhibitor NVP-AUY922 enhances the radiation sensitivity of
lung cancer cell lines with acquired resistance to EGFR-tyrosine
kinase inhibitors. Oncol Rep. 33:1499–1504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sawai A, Chandarlapaty S, Greulich H,
Gonen M, Ye Q, Arteaga CL, Sellers W, Rosen N and Solit DB:
Inhibition of Hsp90 down-regulates mutant epidermal growth factor
receptor (EGFR) expression and sensitizes EGFR mutant tumors to
paclitaxel. Cancer Res. 68:589–596. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lee SC, Min HY, Choi H, Bae SY, Park KH,
Hyun SY, Lee HJ, Moon J, Park SH, Kim JY, et al: Deguelin analogue
SH-1242 inhibits Hsp90 activity and exerts potent anticancer
efficacy with limited neurotoxicity. Cancer Res. 76:686–699. 2016.
View Article : Google Scholar
|
|
105
|
Yang YC, Cheng TY, Huang SM, Su CY, Yang
PW, Lee JM, Chen CK, Hsiao M, Hua KT and Kuo ML: Cytosolic PKM2
stabilizes mutant EGFR protein expression through regulating
Hsp90-EGFR association. Oncogene. 35:3387–3398. 2016. View Article : Google Scholar
|
|
106
|
Chen Z, Akbay E, Mikse O, Tupper T, Cheng
K, Wang Y, Tan X, Altabef A, Woo SA, Chen L, et al: Co-clinical
trials demonstrate superiority of crizotinib to chemotherapy in
ALK-rearranged non-small cell lung cancer and predict strategies to
overcome resistance. Clin Cancer Res. 20:1204–1211. 2014.
View Article : Google Scholar :
|
|
107
|
Tanimoto A, Yamada T, Nanjo S, Takeuchi S,
Ebi H, Kita K, Matsumoto K and Yano S: Receptor ligand-triggered
resistance to alectinib and its circumvention by Hsp90 inhibition
in EML4-ALK lung cancer cells. Oncotarget. 5:4920–4928. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Rolfo C, Passiglia F, Castiglia M, Raez
LE, Germonpre P, Gil-Bazo I, Zwaenepoel K, De Wilde A, Bronte G,
Russo A, et al: ALK and crizotinib: After the honeymoon…what else?
Resistance mechanisms and new therapies to overcome it. Transl Lung
Cancer Res. 3:250–261. 2014.
|
|
109
|
Ramalingam S, Goss G, Rosell R,
Schmid-Bindert G, Zaric B, Andric Z, Bondarenko I, Komov D, Ceric
T, Khuri F, et al: A randomized phase II study of ganetespib, a
heat shock protein 90 inhibitor, in combination with docetaxel in
second-line therapy of advanced non-small cell lung cancer
(GALAXY-1). Ann Oncol. 26:1741–1748. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Socinski MA, Goldman J, El-Hariry I,
Koczywas M, Vukovic V, Horn L, Paschold E, Salgia R, West H,
Sequist LV, et al: A multi-center phase II study of ganetespib
monotherapy in patients with genotypically defined advanced
non-small cell lung cancer. Clin Cancer Res. 19:3068–3077. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Sequist LV, Gettinger S, Senzer NN,
Martins RG, Jänne PA, Lilenbaum R, Gray JE, Iafrate AJ, Katayama R,
Hafeez N, et al: Activity of IPI-504, a novel heat-shock protein 90
inhibitor, in patients with molecularly defined non-small-cell lung
cancer. J Clin Oncol. 28:4953–4960. 2010. View Article : Google Scholar : PubMed/NCBI
|