Introduction
Pancreatic cancer is one of the most lethal solid
tumors with a 5-year survival rate for ~6% (1,2).
Surgical resection remains the most effective treatment (3,4);
however, a significant number of patients that undergo radical
pancreatectomy experience early local recurrence and metastasis
(5,6). We previously reported a subgroup of
patients with pancreatic cancer with a serum signature of
carcinoembryonic antigen (CEA)+/cancer antigen
(CA)125+/CA19-9 ≥1,000 U/ml, who were more likely to
experience metastasis within 6 months following radical resection
(7). Exploring the molecular
mechanisms responsible for this subgroup with a high metastatic
potential may clarify the biological mechanisms responsible for the
high metastatic potential in pancreatic cancer and may improve the
prognosis of patients with this lethal disease.
Receptor-interacting protein kinase 4 (RIPK4) is a
member of the receptor-interacting protein (RIP) kinase family. It
interacts with protein kinase C-δ (PKCδ) and exhibits protein
kinase activity toward autophosphorylation and substrate
phosphorylation (8,9). RIPK4 dysregulation contributes to
tumor occurrence and development in human cancers (10–14);
however, its role in pancreatic cancer remains unclear.
Phosphatidylethanolamine-binding protein 1 (PEBP1), also known as
RAF kinase inhibitory protein (RKIP), is a physiological endogenous
inhibitor of the mitogen-activated protein kinase (MAPK) pathway
(15). It interferes with
RAF1-mediated phosphorylation and MAPK kinase (MEK) activation via
its ability to disrupt the interaction between the two kinases
(16). It has been suggested that
PEBP1 is a suppressor of metastasis in pancreatic cancer, and its
expression is lost in more than half of patients with pancreatic
cancer (17). The loss of PEBP1
expression is significantly associated with pancreatic cancer
metastasis (18), and a poor
overall survival (OS) and disease-free survival (DFS) (19). Although the effect of PEBP1 on MAPK
pathway can be regulated by PKC (20,21),
the association between PEBP1 and RIPK4, which interacts with PKCδ,
remains unclear.
In the present study, we demonstrate that RIPK4 is
highly expressed in the high metastatic potential subgroup of
patients with pancreatic cancer with
CEA+/CA125+/CA19-9 ≥1,000 U/ml. A high RIPK4
expression correlated with a poor prognosis and promoted pancreatic
cancer cell migration and invasion. Mechanistically, these
functions are in part due to its ability to activate RAF1/MEK/ERK
signaling via the regulation of PEBP1 degradation. These results
illuminate the biological mechanisms responsible for the high
metastatic potential of pancreatic cancer and may aid in the
identification of novel therapeutic targets.
Materials and methods
Cell lines and cell culture
The human pancreatic cancer cell lines, PANC-1, MIA
PaCa-2, Capan-1, BxPC-3, SW1990 and CFPAC-1, were purchased from
the American Type Culture Collection (ATCC, Manassas, VA, USA). The
culture medium, subculturing, cryopreservation and culture
conditions were according to standard ATCC methods. Human
pancreatic duct epithelial (HPDE) cells were a kind gift from
Professor Tsao (22). The cells
were grown in DMEM containing 10% fetal bovine serum (FBS) at 37°C
under a humidified 5% CO2 atmosphere. U0126 and MG132
were purchased from Selleck Chemicals (Houston, TX, USA). To
determine whether the expression levels of PEBP1 proteins are
regulated through proteasome-mediated degradation, the cells were
exposed with 10 µM MG132 for 3 h and then harvested for
western blot analysis. In addition, to examine the effect of the
suppression of PEBP1 degradation with MG132 on RAF1/MEK/ERK pathway
activation, the cells were exposed to 1 µM MG132 for 24 h
and then harvested for western blot analysis.
Patients and tissue specimens
The human pancreatic cancer tissues used in this
study were collected from patients diagnosed with pancreatic
adenocarcinoma who underwent radical resection at the Fudan
University Shanghai Cancer Center between 2010 and 2013. The use of
human tissues was approved by the Fudan University Shanghai Cancer
Center Institutional Research Ethics Committee and patient consent
was obtained. The histological grading and pathological annotation
were performed by two independent pathologists at our center. A
total of 79 samples were available for the construction of tissue
microarrays (TMAs) and the evaluation of RIPK4 expression. The
clinicopathological characteristics of the patients are listed in
Table I. The patients were
followed-up until December 2016. In total, 34 cases were used to
examine the association between RIPK4 and PEBP1 expression.
 | Table ICorrelation of clinicopathological
characteristics and with RIPK4 expression in PDAC tissue
samples. |
Table I
Correlation of clinicopathological
characteristics and with RIPK4 expression in PDAC tissue
samples.
| Characteristic | No. | RIPK4 expression
|
|---|
Negative/low
(n=52) | High
(n=27) | P-valuea |
|---|
| Age | | | | |
| ≤60 years | 38 | 26 | 12 | 0.8127 |
| >60 years | 41 | 26 | 15 | |
| Sex | | | | |
| Female | 44 | 25 | 19 | 0.0938 |
| Male | 35 | 27 | 8 | |
| Tumor location | | | | |
| Head | 37 | 25 | 12 | 0.8151 |
| Body and tail | 42 | 27 | 15 | |
| Tumor size | | | | |
| ≤3.0 cm | 32 | 22 | 10 | 0.8096 |
| >3.0 cm | 47 | 30 | 17 | |
| Lymph node
status | | | | |
| Negative | 31 | 23 | 8 | 0.2340 |
| Positive | 48 | 29 | 19 | |
| Tumor
differentiation | | | | |
| Well | 7 | 3 | 4 | 0.4064b |
| Moderate | 47 | 32 | 15 | |
| Poor | 25 | 17 | 8 | |
| TNM stage
(UICC) | | | | |
| IB | 12 | 11 | 1 | 0.0488b |
| IIA | 19 | 14 | 5 | |
| IIB | 48 | 27 | 21 | |
Microarray analysis
Affymetrix Human U133 plus 2.0 (Affymetrix, Santa
Clara, CA, USA) was used to compare differentially expressed genes
between 8 patients with pre-operative serum
CEA+/CA125+/CA19-9 ≥1,000 U/ml
(CEA+, CEA ≥5.2 ng/ml; CA125+, CA125 ≥35
U/ml) and a DFS of ≤6 months, and 8 patients with pre-operative
serum CEA−/CA125−/CA19-9 ≤37 U/ml
(CEA−, CEA <5.2 ng/ml; CA125−, CA125
<35 U/ml) and a DFS of ≥ 24 months. The latter subgroup of
patients with pancreatic cancer had a low metastatic potential and
a better prognosis. Pre-operative levels of such tumor markers were
determined within 1 week prior to resection. The normal upper
limits of serum tumor markers are listed here CA19-9 (37 U/ml), CEA
(5.2 ng/ml) and CA125 (35 U/ml). The Database for Annotation,
Visualization and Integrated Discovery (DAVID) was used to identify
pathways and processes of major biological significance and
importance based on the Gene Ontology (GO) annotation function and
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
function.
Western blot analysis
Western blot analysis was carried out as previously
described (23). In brief, the
cells were harvested after being washed with phosphate-buffered
saline (PBS) twice, lysed with RIPA cell lysis buffer for 30 min on
ice, and centrifuged at 12,000 rpm for 15 min at 4°C. The
concentration of total protein was determined using a BCA protein
assay kit. Equal amounts (30 µg/load) of protein samples
were subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) electrophoresis and transferred onto
polyvinylidene fluoride (PVDF) membranes and reacted with primary
antibodies overnight at 4°C. Following incubation with the
secondary antibodies for 1 h at room temperature, the protein bands
were developed with the chemiluminescent reagents and imaged by
ImageQuant™ LAS 4000 (GE Healthcare, Little Chalfont,
Buckinghamshire, UK). Antibodies against the following proteins
were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA):
RIPK4 (sc-377368), RAF1 (sc-7267), MEK1/2 (sc-436), p-MEK1/2
(sc-81503), p-ERK1/2 (sc-136521), RKIP (sc-376925) and p-RKIP
(sc-135779). Antibodies against ERK1/2 (cs-9102) and p-RAF1
(cs-9427) were obtained from Cell Signaling Technology (Danvers,
MA, USA). All these antibodies mentioned above were diluted at
1:1,000. β-actin expression (A2228; Sigma-Aldrich, St. Louis, MO,
USA) was used as a loading control at a dilution of 1:40,000.
Anti-mouse IgG antibody (cs-14709) and anti-rabbit IgG (cs-14708)
which were obtained from Cell Signaling Technology were used as the
secondary antibodies at a dilution of 1:3,000.
Immunohistochemistry
Immunohistochemical staining for RIPK4 (sc-377368)
or PEBP1 (sc-376925) was carried out according to standard
procedures. The percentage of tumor cells with RIPK4 or PEBP1
staining was evaluated (0%, 0; 1-10%, 1; 11-50%, 2; 51-80%, 3 and
81-100%, 4). The staining intensity was evaluated as follows: 0,
negative; 1, weak; 2, moderate; and 3, strong. The numeric values
were multiplied to yield an immunoreactivity score (IRS) ranging
from 0 to 12. The cut-off point for RIPK4 and PEBP1 expression
(high vs. low) was determined using a median IRS score. The
definition for high RIPK4 and PEBP1 was IRS ≥7 (at least moderate
intensity in >50% of tumor cells or at least strong intensity in
>10% of tumor cells). IRS scores were evaluated by two
pathologists. Correlations between RIPK4 and PEBP1 were analyzed
with Pearson's χ2 tests.
Plasmid construction and cell lines
The shRNA oligos targeting human RIPK4
(GCACGATGTATACAGCTTTGC and GGAACCTTCAACCAGCGATCT) were designed and
cloned into the pLKO.1-TRC (Plasmid 10878; Addgene, Cambridge, MA,
USA) cloning vector to generate shRNA constructs. The human RIPK4
coding sequence was cloned into the lentiviral vector
pLENT-EF1α-Puro-CMV to generate RIPK4 expression plasmids. A
pLKO.1-scramble shRNA (Plasmid 1864; Addgene) and a lentiviral
vector pLENT-EF1α-Puro-CMV were used as the negative control. A
lentiviral vector pLV-luci (Cat#VL3612; Inovogen, Beijing, China)
was used to express luciferase in tumor cells. The recombinant
construct was co-transfected into 293T cells together with two
packaging vectors (psPAX2 and pMD2.G). Lentiviral particles were
harvested and filtered to infect pancreatic cancer cell lines
followed by puromycin screening to generate stable cells with RIPK4
overexpression or knockdown. RIPK4 was overexpressed in the Capan-1
(Capan-1-R) and SW1990 (SW1990-R) cells; RIPK4 was knocked down in
the PANC-1 (PANC-1-Rsh1, PANC-1-Rsh2) and MIA PaCa-2 (MIA2-Rsh1,
MIA2-Rsh2) cells. The control cells were Capan-1-vector
(Capan-1-vec), SW1990-vector (SW1990-vec), PANC-1-scramble
(PANC-1-src) and MIA PaCa-2-scramble (MIA2-src).
Transwell cell migration and invasion
assay
The cells (5×104) were seeded into the
upper chamber (24-well insert; Corning, Corning, NY, USA). The
lower chamber was filled with 10% FBS followed by incubation for 48
or 72 h for the migration or invasion assays, respectively. For the
invasion assay, the inserts were previously coated with
extracellular matrix gel (BD Biosciences, San Jose, CA, USA). The
cells on the lower surface were fixed with 4% paraformaldehyde and
stained with 0.05% crystal violet. Three visual fields were
randomly selected, and the numbers of cells were counted under a
microscope (IX71; Olympus, Tokyo, Japan). To examine the effect of
the suppression of PEBP1 degradation by MG132 and blocking
RAF1/MEK/ERK signaling by U0126 on cell migration and invasion, the
cells were exposed with 1 µM MG132 for 24 h and 10 µM
U0126 for 8 h before the cells were trypsinized and seeded onto the
upper chamber of the Transwell, respectively. DMSO was used as the
control.
Establishment of xenograft tumors using
nude mice
A total of 12 BALB/c-nu mice (female, 4-5 weeks of
age; weighing 18-20 g; SLAC Laboratory Animal Co., Ltd., Shanghai,
China) were randomly assigned to 2 groups, and 3×106
SW1990 lucif-erase-tagged pancreatic cancer cells with or without
RIPK4 overexpression in 100 µl PBS were injected into the
spleens of the mice in the 2 groups. At 3 weeks after implantation,
the mice were prepared for IVIS Spectrum In Vivo Imaging
System scanning. After the final imaging, the tumors and tissue
specimens of the mice were surgically dissected after the mice were
sacrificed by cervical dislocation for the observation of
metastasis and follow-up experiments. All animal experiments were
approved by the Institutional Animal Care and Use Committee of
Fudan University.
GEO database analysis
To identify the clinical relevance of increased
RIPK4 expression, we used the Gene Expression Omnibus (GEO)
database (https://www.ncbi.nlm.nih.gov/geo). The expression data
from Mayo Clinic pancreatic tumor and normal samples are available
through GEO (accession no. GSE16515). The experiment consisted of
36 tumor samples and 16 normal samples; a total of 52 samples. The
raw data were downloaded from GEO and affymerix oligo array probe
level data were converted to expression values using R software.
The gene expression level was normalized to the RPKM value.
Network analysis
We used the predictive web interface GeneMANIA
(http://www.genemania.org) to predict interactions
between RIPK4 and the MAPK signaling pathway. The analysis
generated a list of genes with functional similarity based on
currently available proteomics and genomics databases (24).
Quantitative (real-time) PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). The RNA was then reverse
transcribed into cDNA with the ExScript reverse
transcription-polymerase chain reaction (RT-PCR) kit (Takara,
Tokyo, Japan). The expression status of PEBP1 and glyceraldehyde
3-phosphate dehydrogenase (GAPDH) were determined by quantitative
PCR. GAPDH was used as the loading control. The following
oligonucleotide primer was used: PEBP1 forward,
5′-CTCCGATTATGTGGGCTCGG-3′ and reverse, 5′-GGTGGTCTCCAGATCGGTTG-3′;
and GAPDH forward, 5′-CGACCACTTTGTCAAGCTCA-3′ and reverse,
5′-AGGGGAGATTCAGTGTGGTG-3′. All amplifications and detections were
carried out using the Applied Biosystems Prism 7900 system (Applied
Biosystems, Foster City, CA, USA). The assays were performed in
triplicate.
Statistical analysis
The results are expressed as the means ± SD.
Two-tailed unpaired Student's t-tests, one-way analysis of
variance, or Pearson's χ2 tests were used to evaluate
the data. Survival curves were plotted using the Kaplan-Meier
method and compared by the log-rank test. All statistical analyses
were performed using SPSS 19.0 software (IBM Corp., Armonk, NY,
USA). Differences were considered significant at P<0.05.
Results
RIPK4 expression is upregulated in
pancreatic cancer and is associated with a poor outcome following
surgery and a high metastatic potential
A cDNA microarray was used to perform the
contrastive analysis of the gene expression profiles between 8
patients with pre-operative serum
CEA+/CA125+/CA19-9 ≥1,000 U/ml, DFS of ≤6
months, and 8 patients with pre-operative serum
CEA−/CA125−/CA19-9 ≤37 U/ml, a DFS of ≥24
months. Over 100 genes had at least a 1.5-fold difference in
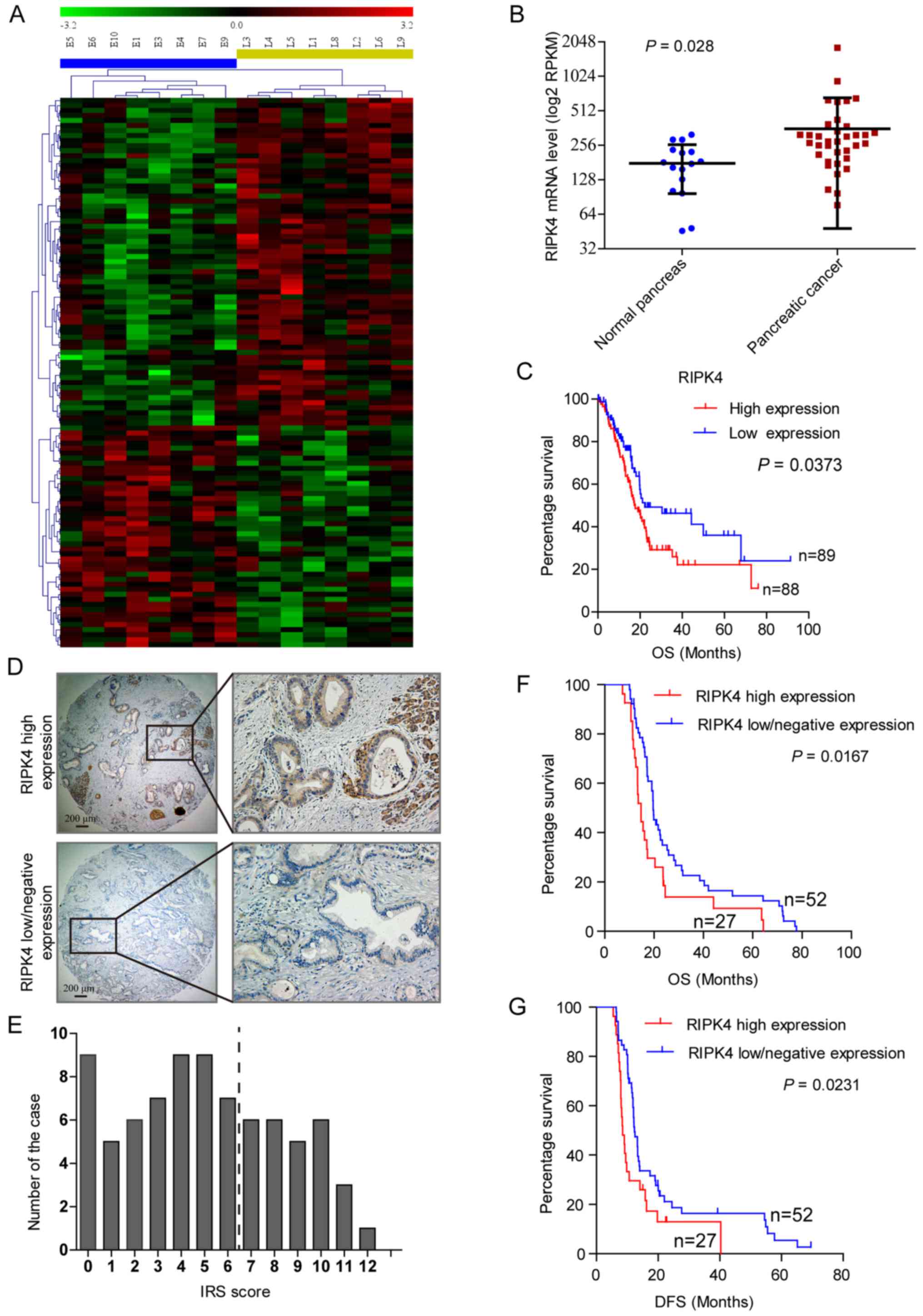
expression between the 2 groups (P<0.05) (Fig. 1A), and 17 genes were differentially
expressed with at least a 2-fold change in expression (P<0.05)
(Table II). The RIPK4 mRNA
levels were upregulated by 2.2-fold. To determine the clinical
relevance of the increased expression of RIPK4, we first analyzed
the RIPK4 mRNA levels in 52 clinical specimens (GSE16515),
including 36 pancreatic cancer tissue specimens and 16 normal
pancreatic tissue specimens, which are available through the Gene
Expression Omnibus (GEO) database. The RIPK4 mRNA level (normalized
to the RPKM value) was higher in the pancreatic cancer tissues than
in the normal pancreatic tissues (P<0.05) (Fig. 1B). A survival analyses using the
clinical and follow-up TCGA pancreatic cancer data revealed that
the patients with a high RIPK4 expression had a shorter OS than
those with low RIPK4 levels (17.7 vs. 22.2 months, P<0.05)
(Fig. 1C).
 | Table IIDifferentially expressed genes in
human pancreatic cancer samples
(CEA+/CA125+/CA19-9 ≥1,000 U/ml, DFS of ≤6
months vs. CEA−/CA125−/CA19-9 ≤37 U/ml, DFS
of ≥24 months). |
Table II
Differentially expressed genes in
human pancreatic cancer samples
(CEA+/CA125+/CA19-9 ≥1,000 U/ml, DFS of ≤6
months vs. CEA−/CA125−/CA19-9 ≤37 U/ml, DFS
of ≥24 months).
| Probe_Set_ID | Gene_symbol | P-value | Fold changea |
|---|
| Upregulated
gene | 205922_at | VNN2 | 0.03327158 | 2.761510733 |
| 206336_at | CXCL6 | 0.030429421 | 2.682334096 |
| 213150_at | HOXA10 | 0.015566485 | 2.648941024 |
| 204006_s_at |
FCGR3A/FCGR3B | 0.032846977 | 2.459635693 |
| 1569344_a_at | – | 0.034275931 | 2.421933712 |
| 226926_at | DMKN | 0.019513244 | 2.310987677 |
| 221215_s_at | RIPK4 | 0.036130949 | 2.231740768 |
| 211504_x_at | ROCK2 | 0.020972521 | 2.132222303 |
| 222830_at | GRHL1 | 0.029119833 | 2.122296769 |
| Downregulated
gene | 213268_at | CAMTA1 | 0.003757686 | 0.495911419 |
| 209465_x_at | PTN | 0.01256999 | 0.484851788 |
| 203896_s_at | PLCB4 | 0.011971674 | 0.479533229 |
| 235645_at | ESCO1 | 0.012775165 | 0.472722222 |
| 244700_at | SEC61B | 0.008219772 | 0.466590487 |
| 243790_at | ZNF585A | 0.033921682 | 0.46249514 |
| 211737_x_at | PTN | 0.025599908 | 0.448126885 |
| 241803_s_at | – | 0.029068249 | 0.382177815 |
Immunohistochemistry was performed to detect RIPK4
protein expression in a TMA of 79 pancreatic cancer tissues. RIPK4
localized to the cell membrane and cytoplasm and was detectable in
70 (89%) cases. Representative samples are shown in Fig. 1D. In total, 52 (66%) cases were
defined as having a negative/low expression, and 27 (34%) had a
high expression (Fig. 1D and E).
Survival analyses revealed that a high RIPK4 expression was
associated with a decreased OS (14.7 vs. 19.6 months, P<0.05)
and DFS (8.5 vs. 12.3 months, P<0.05) (Fig. 1F and G). These findings suggest
that a higher RIPK4 expression is associated with a poor outcome
and tumor metastasis in patients with pancreatic cancer.
RIPK4 promotes pancreatic cancer cell
migration and invasion in vitro and in vivo
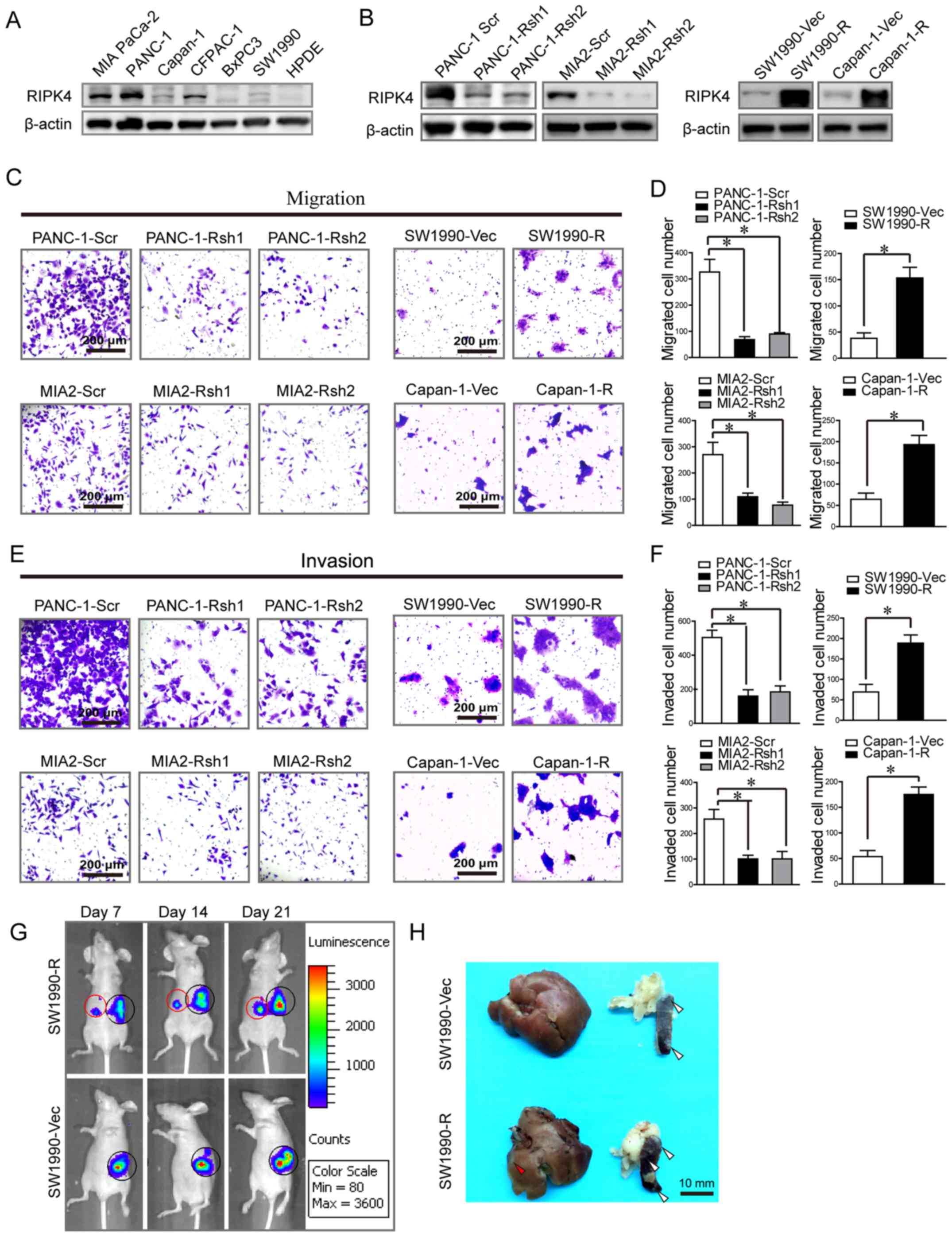
Western blot analysis was used to determine the
RIPK4 protein levels in several pancreatic cancer cell lines. The
results revealed a high expression in the MIA PaCa-2, PANC-1 and
CFPAC-1 cells; a low expression in the Capan-1, BxPC-3 and SW1990
cells; and barely detectable levels in HDPE cells (Fig. 2A). RIPK4 was then overexpressed in
the Capan-1 and SW1990 cells, and knocked down in the PANC-1 and
MIA PaCa-2 cells (Fig. 2B).
Transwell cell migration/invasion assays revealed that RIPK4
knockdown with shRNA oligos markedly decreased the number of PANC-1
and MIA PaCa-2 migrating/invading cells [PANC-1 migrating cells:
327±83 vs. 68±20, P<0.05; 327±83 vs. 89±10, P<0.05; PANC-1
invading cells: 504±74 vs. 160±66, P<0.05; 504±74 vs. 185±60,
P<0.05; and MIA PaCa-2 migrating cells: 270±72 vs. 109±24,
P<0.05; 270±72 vs. 77±20, P<0.05; MIA PaCa-2 invading cells:
257±65 vs. 100±25, P<0.05; 257±65 vs. 100±51, P<0.05
(PANC-1-Scr vs. PANC-1-Rsh1 and PANC-1-Scr vs. PANC-1-Rsh2,
respectively and MIA2-Scr vs. MIA2-Rsh1 and MIA2-Scr vs. MIA2-Rsh2,
respectively) (Fig. 2C–F)].
Consistent with these findings, RIPK4 overexpression markedly
increased the number of migrating/invading Capan-1 and SW1990 cells
[Capan-1 migrating cells: 64±25 vs. 193±37, P<0.05; Capan-1
invading cells: 54±20 vs. 175±25, P<0.05; and SW1990 migrating
cells: 38±18 vs. 153±35, P<0.05; SW1990 invading cells: 69±32
vs. 189±34, P<0.05 (Capan-1-Vec vs. Capan-1-R, respectively and
SW1990-Vec vs. SW1990-R) (Fig.
2C–F)].
We then injected luciferase-tagged SW1990 cells
overexpressing RIPK4 into the spleens of nude mice. At week 3,
increased liver metastasis was observed in the RIPK4 overexpression
group (SW1990-R) compared to the control group (SW1990-Vec). The
liver metastasis rate was 83% (5 of 6) in the RIPK4 overexpression
group compared with 17% (1 of 6) in the control group (Fig. 2G and H). The loss- and
gain-of-function assays performed in vitro and in
vivo suggested that RIPK4 promoted pancreatic cancer cell
migration.
RIPK4 promotes pancreatic cancer cell
migration and invasion via the RAF1/MEK/ERK pathway
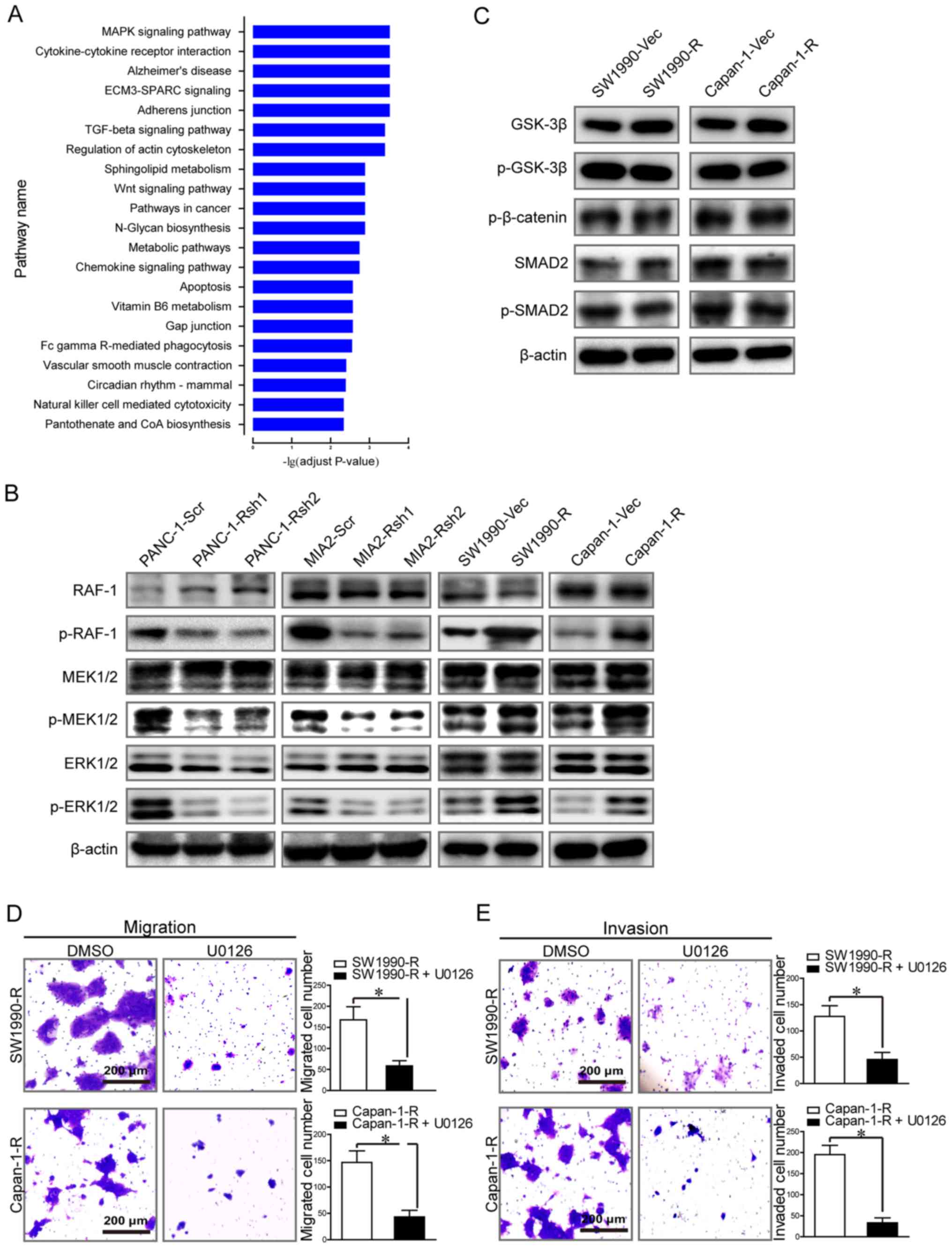
We performed a KEGG pathway enrichment analysis of
differentially expressed genes (at least a 1.5-fold change,
P<0.05) between the two subgroups. These genes were mainly
enriched in functional pathways that are critical in cancer, such
as the MAPK, TGF-β and Wnt signaling pathways (adjusted P<0.001)
(Fig. 3A). The results of western
blot analysis revealed that RIPK4 knockdown induced RAF1, MEK1/2
and ERK1/2 inactivation in the PANC-1 and MIAPaCa-2 cells, as
indicated by reduced phosphorylation. Consistently, RIPK4
overexpression enhanced RAF1, MEK1/2 and ERK1/2 phosphorylation in
the Capan-1 and SW1990 cells (Fig.
3B). However, the levels of total Smad2 and phospho-Smad2 in
the TGF-β signaling pathway and GSK-3β, phospho-GSK-3β, and
phospho-β-catenin in the Wnt signaling pathway remained unaltered,
irrespective of RIPK4 expression (Fig.
3C). These thus data indicate that RIPK4 may be associated with
the activation of RAF1/MEK/ERK signaling. We therefore wished to
determine whether the blocking of the activation of RAF1/MEK/ERK
signaling can alter the impact of RIPK4 on tumor metastasis.
Treatment of RIPK4-overexpressing cells with U0126 (a highly
selective inhibitor of MEK1/2) significantly reduced cell migration
and invasion in vitro. Transwell assays revealed that U0126
decreased the number of migrating/invading Capan-1 and SW1990
RIPK4-overexpressing cells [Capan-1 migrating cells: 147±38 vs.
43±22, P<0.05; Capan-1 invading cells: 195±38 vs. 33±20,
P<0.05; and SW1990 migrating cells: 168±54 vs. 59±21, P<0.05;
SW1990 invading cells: 128±36 vs. 46±23, P<0.05 (SW1990-R vs.
SW1990-R + U0126 and Capan-1-R vs. Capan-1-R + U012) (Fig. 3D and E)]. Collectively, these data
demonstrate that RIPK4 promotes pancreatic cancer cell migration
and invasion via the RAF1/MEK/ERK pathway.
PEBP1 mediates the interaction between
RIPK4 and the RAF1/MEK/ERK pathway
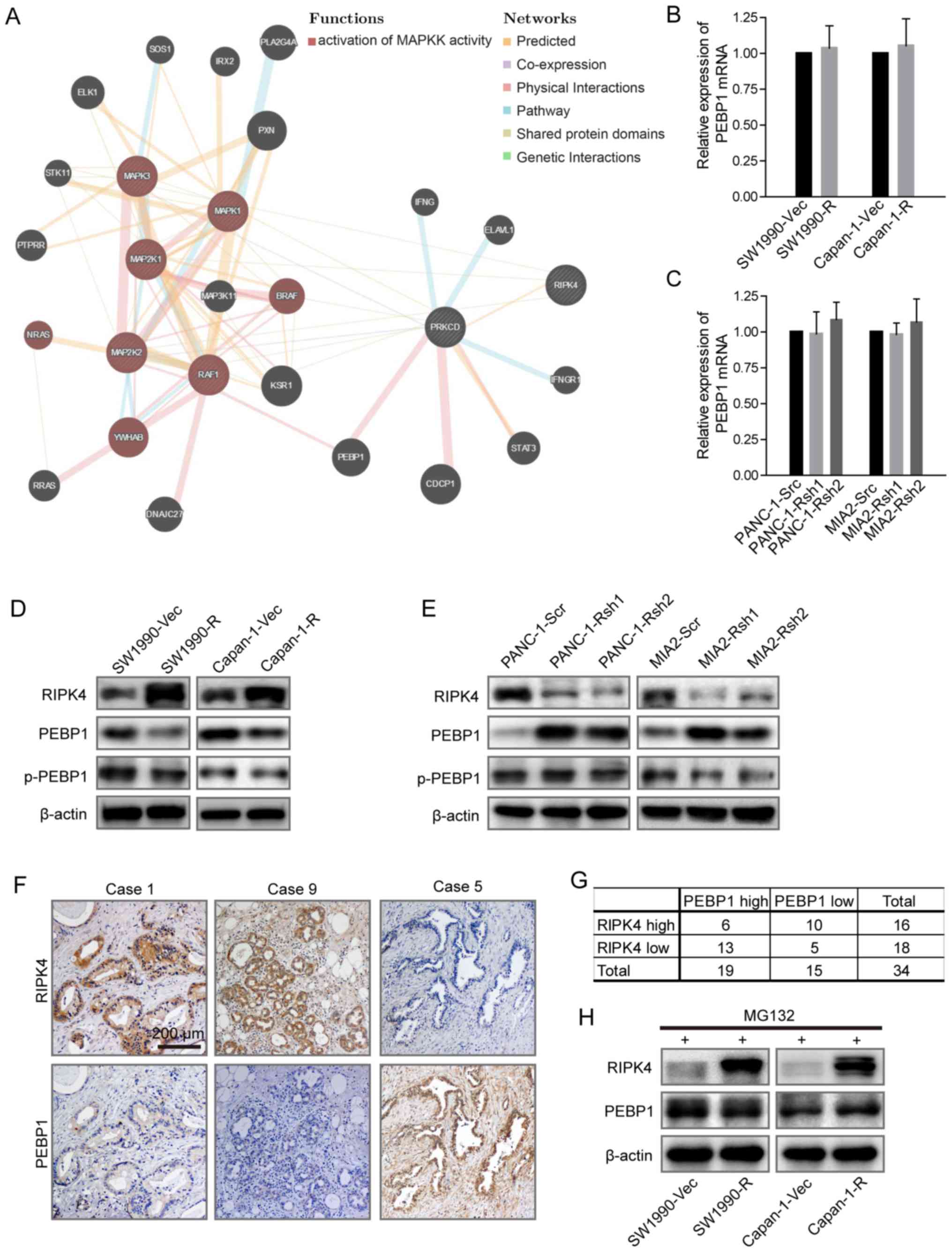
We performed a protein interaction network analysis
to elucidate the mechanisms through which RIPK4 induces
RAF1/MEK/ERK signaling. The network predicted that PEBP1 played a
critical role in the interaction between RIPK4 and the activation
of RAF1/MEK/ERK signaling (Fig.
4A). We examined PEBP1 expression by RT-PCR and western blot
analysis in the cells in which RIPK4 was overexpressed or knock
down. The results revealed that the PEBP1 mRNA levels were not
significantly altered (Fig. 4B and
C). Of note, the PEBP1 protein level inversely correlated with
RIPK4 expression; however, PEBP1 phosphorylation was not markedly
affected by RIPK4 expression (Fig. 4D
and E). Consistent with this finding, a negative correlation
between RIPK4 and PEBP1 was also observed in 34 pancreatic cancer
patient tissues (P=0.0418 with Pearson's χ2 test)
(Fig. 4F and G). These results
suggest that RIPK4 regulates PEBP1 expression at the
post-translational level. We then treated RIPK4-overexpressing
cells with MG132 (an inhibitor of the 26S proteasome) to examine
whether PEBP1 is regulated through classic protein degradation. The
inhibition of proteolysis by MG132 almost eliminated the change in
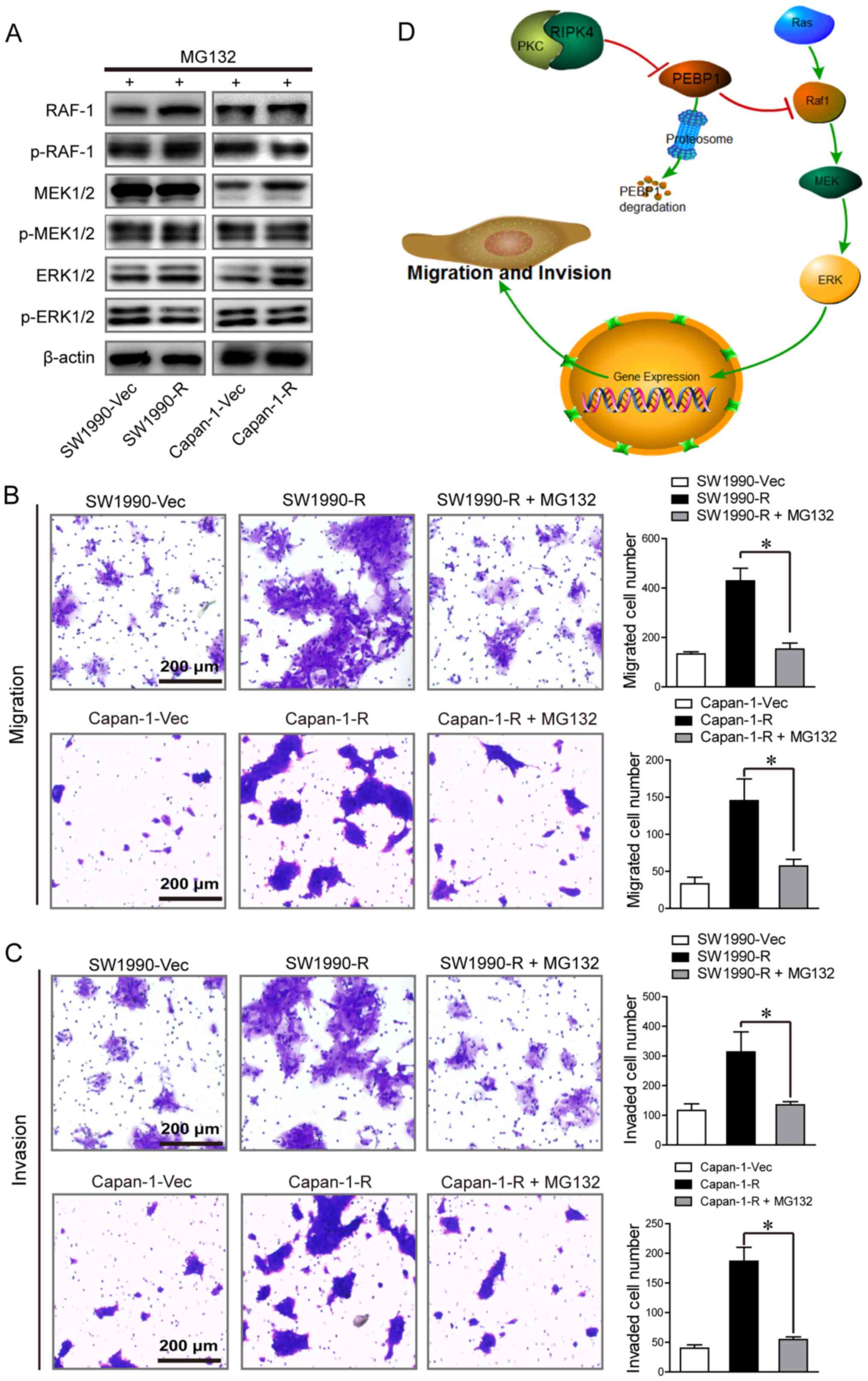
the PEBP1 protein level induced by RIPK4 overexpression (Fig. 4H). Collectively, these findings
suggest that the negative interplay between RIPK4 and BEBP1 is
largely regulated through proteasome-mediated protein
degradation.
Suppression of PEBP1 degradation
attenuates RIPK4-induced RAF1/MEK/ERK pathway activation and
pancreatic tumor cell migration and invasion
We then examined the effect of PEBP1 on
RIPK4-mediated activation of RAF1/MEK/ERK signaling and tumor cell
migration and invasion. The results of western blot analysis
revealed that the phosphorylation of RAF1, MEK1/2 and ERK1/2
induced by RIPK4 overexpression was almost fully prevented by the
suppression of PEBP1 degradation with MG132 (Fig. 5A). Furthermore, RIPK4
overexpression-mediated cell migration and invasion were
significantly abolished by the suppression of PEBP1 degradation in
the Capan-1 and SW1990 cells [Capan-1 migrating cells: 144±39 vs.
59±13, P<0.05; Capan-1 invading cells: 181±32 vs. 53±6,
P<0.05; and SW1990 migrating cells: 432±56 vs. 150±27,
P<0.05; SW1990 invading cells: 313±66 vs. 132±8, P<0.05
(Capan-1-R vs. Capan-1-R + MG132 and SW1990-R vs. SW1990-R + MG132)
(Fig. 5B and C)]. These results
indicate that RIPK4 promotes tumor cell metastasis at least partly
by down-regulating PEBP1 expression.
Discussion
We previously identified a unique subgroup of
patients with pancreatic cancer with a pre-operative serum
signature of CEA+/CA125+/CA19-9 ≥1,000 U/ml.
These patients usually have poor surgical outcomes and are more
likely to experience distant metastasis within 6 months after
radical surgery (7). The molecular
mechanisms underlying this aggressive phenotype remain unclear. In
this study, we thus performed a high-throughput gene expression
screen to identify key molecules or signaling pathways involved in
the metastatic potential of the patients with this serum signature.
Patients with pre-operative serum levels of CEA-/CA125-/CA19-9 ≤37
U/ml and a DFS of ≥24 months were included as the control group.
This analysis revealed that RIPK4 was one of the most critical
genes upregulated in the subgroup of patients with pre-operative
serum levels of CEA+/CA125+/CA19-9 ≥1,000
U/ml, and this significantly correlated with the prognosis of
patients with pancreatic cancer. As the role of RIPK4 in cancer has
been reported previously, we mainly examined the function of RIPK4
in pancreatic cancer metastasis.
Our clinical relevance analysis revealed a higher
RIPK4 expression in pancreatic cancer tissues compared to normal
pancreatic tissues. Notably, an increased RIPK4 expression
predicted a poor OS and DFS. Functional assays demonstrated that
RIPK4 promoted pancreatic cancer cell migration and invasion in
vitro and in vivo. KEGG pathway enrichment analysis of
differentially expressed genes indicated that MAPK signaling may
play an important role in the high metastatic potential in this
group of pancreatic cancer patients. To although no studies have
reported that RIPK4 regulates MAPK signaling (at least to the best
of our knowledge), crosstalk between PKC and the MAPK pathway has
been reported (25). PKCδ has been
found to participate in ERK activation (26). Of note, the interaction between
PKCδ and RIPK4 has been observed only for the catalytic domain of
PKCδ, not for the regulatory domain (8). It is therefore reasonable to assume
that RIPK4 is a downstream signaling molecule of PKCδ and may serve
as an intermediary in PKCδ and MAPK pathway activation. This
hypothesis is supported by our findings that RIPK4 expression did
not affect PKC phosphorylation (data not shown). However, RIPK4
obviously regulated the phosphorylation of RAF1, MEK1/2 and ERK1/2.
Blocking the activation of RAF1/MEK/ERK signaling pathway
significantly reduced cell migration and invasion in vitro.
Collectively, these results suggest that RIPK4 promotes pancreatic
cancer cell migration and invasion via the RAF1/MEK/ERK
pathway.
A protein interaction network analysis predicted
that PEBP1 plays a critical role in mediating the interaction
between RIPK4 and RAF1/MEK/ERK activation. PEBP1 is a physiological
endogenous inhibitor of the RAF1/MEK/ERK pathway that can inhibit
pancreatic cancer cell proliferation, migration and invasion
(27); however, its association
with RIPK4 remains unclear. PEBP1 can act as a signaling switch
between the PKC and RAF1/MEK/ERK signaling cascades (20). The PKC-mediated phosphorylation of
PEBP1 on serine 153 abrogates its ability to bind to RAF1 and
inhibit downstream MAPK signaling (21). Only classic and atypical, but not
novel PKC isoforms phosphorylate PEBP1 at this residue (26,28).
This suggests that PKCδ may be ineffective, and that the regulatory
effects of PKCδ on RAF1/MEK/ERK signaling may not be due to PEBP1
phosphorylation. Similarly, RIPK4 may also not regulate PEBP1
phosphorylation. This is suggested by our result showing that RIPK4
did not affect PEBP1 phosphorylation at serine 153. We did find a
negative correlation between RIPK4 and PEBP1 expression in tissues
from patients with pancreatic cancer, and RIPK4 reduced PEBP1
expression by inducing proteasome-mediated degradation. This result
is consistent with a report that the steady state of total PEBP1
protein expression is regulated through proteasome-mediated
degradation (21). Of note,
non-phosphorylated PEBP1 is more likely to be degraded by
proteasome-mediated degradation (21,29).
Therefore, as a protein kinase that interacts with PKCδ, RIPK4 may
activate RAF1/MEK/ERK signaling by inducing proteasome-mediated
PEBP1 degradation rather than regulating PEBP1 phosphorylation.
In conclusion, this study reveals an elevated RIPK4
expression in a unique subgroup of patients with pancreatic cancer
with levels of CEA+/CA125+/CA19-9 ≥1,000
U/ml. RIPK4 overexpression markedly promoted pancreatic cancer cell
migration and invasion via RAF1/MEK/ERK activation. RIPK4 may
stimulate RAF1/MEK/ERK signaling by inducing proteasome-mediated
PEBP1 degradation. The direct mechanisms underlying RIPK4-induced
proteasome-mediated PEBP1 degradation remains unclear. Future
studies are warranted to determine whether other genes identified
in the microarray are associated with the high metastatic potential
of pancreatic cancer.
Acknowledgments
The authors would like to thank Huan-Yu Xia and Ying
Yang for their assistance in collecting the patient data.
Notes
[1]
Funding
This study was supported by grants from the National
Science Foundation for Distinguished Young Scholars of China (no.
81625016) and the National Natural Science Foundation of China
(nos. 81472670, 81402397 and 81402398). The funding agencies had no
role in the study design, data collection and analyses, decision to
publish, or preparation of this manuscript.
[2] Authors'
contributions
ZHQ, HXX and LL were responsible for the study
design, original article drafting and editing, data acquisition and
data analysis. WQW and WJ were responsible for article revision.
HLG was responsible for data analysis. SRZ and JZX were responsible
for data acquisition and patient follow-up. CTW were responsible
for data interpretation and methodology. SL was responsible for
patient follow-up. QXN was responsible for supervision. XJY was
responsible for funding acquisition and supervision.
[3] Availability
of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
[4] Ethics
approval and consent to participate
The use of human tissues was approved by the Fudan
University Shanghai Cancer Center Institutional Research Ethics
Committee and patient consent was obtained.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Long J, Luo GP, Xiao ZW, Liu ZQ, Guo M,
Liu L, Liu C, Xu J, Gao YT, Zheng Y, et al: Cancer statistics:
Current diagnosis and treatment of pancreatic cancer in Shanghai,
China. Cancer Lett. 346:273–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koay EJ, Amer AM, Baio FE, Ondari AO and
Fleming JB: Toward stratification of patients with pancreatic
cancer: Past lessons from traditional approaches and future
applications with physical biomarkers. Cancer Lett. 381:237–243.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hackert T, Ulrich A and Büchler MW:
Borderline resectable pancreatic cancer. Cancer Lett. 375:231–237.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartwig W, Werner J, Jäger D, Debus J and
Büchler MW: Improvement of surgical results for pancreatic cancer.
Lancet Oncol. 14:e476-e4852013. View Article : Google Scholar
|
|
6
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu L, Xu H, Wang W, Wu C, Chen Y, Yang J,
Cen P, Xu J, Liu C, Long J, et al: A preoperative serum signature
of CEA+/CA125+/CA19-9 ≥ 1000 U/ml indicates poor outcome to
pancreatectomy for pancreatic cancer. Int J Cancer. 136:2216–2227.
2015. View Article : Google Scholar
|
|
8
|
Bhr C, Rohwer A, Stempka L, Rincke G,
Marks F and Gschwendt M: DIK, a novel protein kinase that interacts
with protein kinase Cdelta. Cloning, characterization, and gene
analysis. J Biol Chem. 275:36350–36357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang D, Lin J and Han J:
Receptor-interacting protein (RIP) kinase family. Cell Mol Immunol.
7:243–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Azizmohammadi S, Azizmohammadi S, Safari
A, Kaghazian M, Sadrkhanlo M, Behnod V and Seifoleslami M:
High-level expression of RIPK4 and EZH2 contributes to lymph node
metastasis and predicts favorable prognosis in patients with
cervical cancer. Oncol Res. 25:495–501. 2017. View Article : Google Scholar
|
|
11
|
Liu DQ, Li FF, Zhang JB, Zhou TJ, Xue WQ,
Zheng XH, Chen YB, Liao XY, Zhang L, Zhang SD, et al: Increased
RIPK4 expression is associated with progression and poor prognosis
in cervical squamous cell carcinoma patients. Sci Rep. 5:119552015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang X, McGann JC, Liu BY, Hannoush RN,
Lill JR, Pham V, Newton K, Kakunda M, Liu J, Yu C, et al:
Phosphorylation of Dishevelled by protein kinase RIPK4 regulates
Wnt signaling. Science. 339:1441–1445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee P, Jiang S, Li Y, Yue J, Gou X, Chen
SY, Zhao Y, Schober M, Tan M and Wu X: Phosphorylation of Pkp1 by
RIPK4 regulates epidermal differentiation and skin tumorigenesis.
EMBO J. 36:1963–1980. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Zhu W, Zhou Y, Xu W and Wang H:
RIPK4 is down-regulated in poorly differentiated tongue cancer and
is associated with migration/invasion and cisplatin-induced
apoptosis. Int J Biol Markers. 29:e150-e1592014. View Article : Google Scholar
|
|
15
|
Yeung K, Janosch P, McFerran B, Rose DW,
Mischak H, Sedivy JM and Kolch W: Mechanism of suppression of the
Raf/MEK/extracellular signal-regulated kinase pathway by the raf
kinase inhibitor protein. Mol Cell Biol. 20:3079–3085. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeung K, Seitz T, Li S, Janosch P,
McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, et
al: Suppression of Raf-1 kinase activity and MAP kinase signalling
by RKIP. Nature. 401:173–177. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song SP, Zhang SB, Li ZH, Zhou YS, Li B,
Bian ZW, Liao QD and Zhang YD: Reduced expression of Raf kinase
inhibitor protein correlates with poor prognosis in pancreatic
cancer. Clin Transl Oncol. 14:848–852. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karamitopoulou E, Zlobec I, Gloor B,
Kondi-Pafiti A, Lugli A and Perren A: Loss of Raf-1 kinase
inhibitor protein (RKIP) is strongly associated with high-grade
tumor budding and correlates with an aggressive phenotype in
pancreatic ductal adenocarcinoma (PDAC). J Transl Med. 11:3112013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HS, Kim GY, Lim SJ and Kim YW: Loss of
Raf-1 kinase inhibitory protein in pancreatic ductal
adenocarcinoma. Pathology. 42:655–660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hellmann J, Rommelspacher H and Wernicke
C: Long-term ethanol exposure impairs neuronal differentiation of
human neuroblastoma cells involving neurotrophin-mediated
intracellular signaling and in particular protein kinase C. Alcohol
Clin Exp Res. 33:538–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moen EL, Wen S, Anwar T, Cross-Knorr S,
Brilliant K, Birnbaum F, Rahaman S, Sedivy JM, Moss SF and
Chatterjee D: Regulation of RKIP function by Helicobacter pylori in
gastric cancer. PLoS One. 7:e378192012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furukawa T, Duguid WP, Rosenberg L,
Viallet J, Galloway DA and Tsao MS: Long-term culture and
immortalization of epithelial cells from normal adult human
pancreatic ducts trans-fected by the E6E7 gene of human papilloma
virus 16. Am J Pathol. 148:1763–1770. 1996.PubMed/NCBI
|
|
23
|
Qi Z, Liu M, Liu Y, Zhang M and Yang G:
Tetramethoxychalcone, a chalcone derivative, suppresses
proliferation, blocks cell cycle progression, and induces apoptosis
of human ovarian cancer cells. PLoS One. 9:e1062062014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Warde-Farley D, Donaldson SL, Comes O,
Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT,
et al: The GeneMANIA prediction server: Biological network
integration for gene prioritization and predicting gene function.
Nucleic Acids Res. 38(Suppl 2): W214–W220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maines MD: Biliverdin reductase: PKC
interaction at the crosstalk of MAPK and PI3K signaling pathways.
Antioxid Redox Signal. 9:2187–2195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mugami S, Dobkin-Bekman M, Rahamim-Ben
Navi L and Naor Z: Differential roles of PKC isoforms (PKCs) in
GnRH stimulation of MAPK phosphorylation in gonadotrope derived
cells. Mol Cell Endocrinol. Apr 6–2017.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dai H, Chen H, Liu W, You Y, Tan J, Yang
A, Lai X and Bie P: Effects of Raf kinase inhibitor protein
expression on pancreatic cancer cell growth and motility: An in
vivo and in vitro study. J Cancer Res Clin Oncol. 142:2107–2117.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Corbit KC, Trakul N, Eves EM, Diaz B,
Marshall M and Rosner MR: Activation of Raf-1 signaling by protein
kinase C through a mechanism involving Raf kinase inhibitory
protein. J Biol Chem. 278:13061–13068. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baritaki S, Yeung K, Palladino M, Berenson
J and Bonavida B: Pivotal roles of snail inhibition and RKIP
induction by the proteasome inhibitor NPI-0052 in tumor cell
chemoimmunosensitization. Cancer Res. 69:8376–8385. 2009.
View Article : Google Scholar : PubMed/NCBI
|