Introduction
Polyinosinic:polycytidylic acid [Poly(I:C)] is a
synthetic analogue of double-stranded RNA (dsRNA), which is
recognised by pattern recognition receptors, and elicits innate and
adaptive immunity (1). Poly(I:C)
exhibits potential by activating various types of immune cells,
including dendritic cells, T helper (Th)1 cells and Th17 cells
(2). The pathways that initiate
immune activity mainly involve intact intracellular or
extracellular signalling. Intracellularly, dsRNA is recognised by
retinoic acid-inducible gene I protein (RIG-I)-like receptors
[melanoma differentiation-associated protein 5 (MDA5) and RIG-I]
that interact with the interferon (IFN)-β promoter stimulator 1
(IPS-1). Subsequently, IPS-1, which is a mitochondrial signalling
adaptor protein, recruits and phosphorylates IκB kinase ε/tumour
necrosis factor (TNF) receptor-associated factor family
member-associated nuclear factor (NF)-κB activator-binding kinase 1
(TBK1), thus leading to the phosphorylation of interferon
regulatory factor 3 (IRF3). Extracellularly, Toll-like receptors
(TLRs) are the pivotal receptors. Upon Poly(I:C) binding to TLRs,
the TLRs recruit adaptor proteins, including myeloid
differentiation primary response 88, Toll/interlukin-1
receptor-domain-containing adapter-inducing IFN-β and translocating
chain-associated membrane protein, and IRF3 is phosphorylated via
NF-κB signalling followed by the activation of type I IFN
signalling (3,4). A previous study revealed that
Poly(I:C) exerts its antitumour properties in a type I
IFN-dependent or IFN-independent manner in MCF10A, MDAMB-231, IMR32
and HEK293T cells (5).
MDA5 and RIG-I are cytoplasmic sensors of Poly(I:C),
which participate in the Poly(I:C)-induced apoptosis of cancer
cells by eliciting the canonical apoptotic signalling pathway, and
may even boost innate immune responses (6). IRF3 is a downstream molecule of
RIG-I/MDA5, which is usually located in the cytoplasm in its
inactive form, and is a vital transcription factor for type I IFN.
It is activated by phosphorylation at its carboxyl terminus in
response to viral infection, DNA-damaging stress, ultraviolet light
and chemical stimulation. Upon phosphorylation, IRF3 dimerizes and
translocates into the nucleus, where its target genes are
transcribed (7,8). IRF3 exerts dual roles in regulating
the bioactivity of cancer. In previous studies, IRF3 has been
reported to act as an antitumour factor due to its ability to
control cancer cell progression (9,10).
For example, IRF3 inhibits the growth of nasopharyngeal carcinoma
cells, B16 melanoma cells, prostate cancer cells and malignant
glioma cells via regulating the activation of natural killer cells
or type I IFN signalling (9,11-13).
In addition, it has previously been reported that Poly(I:C)-induced
apoptosis of prostate cancer PC3 and DU145 cells is independent of
IRF3 (14). Conversely, the mRNA
and protein expression levels of IRF3 are upregulated in patients
with acute myeloid leukaemia (AML), and IRF3 promotes the
proliferation and survival of AML cells (15). In lung cancer tissues, IRF3 is
aberrantly expressed in either the cytoplasm or nucleus, and its
expression status is not associated with sex, histological grade,
nodal metastasis, pathological stage or recurrence (16). Therefore, the present study aimed
to explore the effects of Poly(I:C) on lung cancer cells and to
reveal the probable role of IRF3 in these effects.
The present study demonstrated that Poly(I:C)
triggered innate immunity via an intracellular signalling pathway,
and induced apoptosis via the extrinsic apoptotic pathway in
non-small cell lung cancer (NSCLC) cells. In addition, the results
indicated that IRF3, activated by MDA5/RIG-I, may regulate the
Poly(I:C)-induced apoptotic pathway. Finally, the results
demonstrated that molecules in the innate immune pathway were
synchronously upregulated; however, this was not accompanied by an
increase in IRF3 phosphorylation, in tumour tissue samples obtained
from patients with NSCLC compared with in matched adjacent
tissues.
Materials and methods
Tissue specimen collection
NSCLC tissues and corresponding adjacent tissues
were obtained from eight patients at Peking University First
Hospital (Beijing, China). Patient characteristics are listed in
Table I. The tissues were
instantly frozen in liquid nitrogen once they were removed from the
patients and were preserved at −80°C. All of the subjects provided
informed consent for their inclusion in the present study prior to
participation. The present study was conducted in accordance with
the Declaration of Helsinki, and was approved by the Ethics
Committee of Peking University First Hospital.
 | Table IClinical information of patients with
non-small cell lung cancer. |
Table I
Clinical information of patients with
non-small cell lung cancer.
| Patients | Sex (M/F) | Age (years) | Pathological
type | Lymphatic
metastasis |
|---|
| #1 | F | 58 | Adenocarcinoma | No |
| #2 | F | 78 | Squamous-cell
carcinoma | Lobar bronchus |
| #3 | M | 59 | Adenocarcinoma | No |
| #4 | M | 60 | Adenocarcinoma | No |
| #5 | F | 80 | Combined small cell
carcinoma and adenocarcinoma | No |
| #6 | F | 53 | Adenocarcinoma | No |
| #7 | M | 57 | Adenocarcinoma | No |
| #8 | F | 57 | Adenocarcinoma | No |
Cell culture and drug pretreatment
The NSCLC cell line A549 was obtained from American
Type Culture Collection (Manassas, VA, USA), and NCI-H1299 cells
were obtained from Peking Union Medical College (Beijing, China).
The A549 cells were cultured with Dulbecco’s modified Eagle’s
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
and the H1299 cells were cultured with Roswell Park Memorial
Institute 1640 medium (Gibco; Thermo Fisher Scientific, Inc.). Both
media were supplemented with 10% foetal bovine serum (Corning
Incorporated, Corning, NY, USA) at 37°C in a humidified incubator
containing 5% CO2.
Drug pretreatment and Poly(I:C)
transfection
Once cell confluence reached 70–80% in the 6-well
plates, the pan-caspase inhibitor Z-VAD-FKM (50 μM;
InvivoGen, San Diego, CA, USA), the caspase-8 inhibitor Z-IETD-FKM
(25 μM; EMD Millipore, Billerica, MA, USA) or the TBK1
inhibitor BX795 (1 μM); (InvivoGen) were used to treat the
cells for 2 h at 37°C. Subsequently, Poly(I:C) (0, 100, 200 and 400
ng/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
transfected into the cells (confluence, 70–80%) for 6 h using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer’s protocol, after
which the cells were cultured for 24 h.
Western blot analysis
The cells/tissues were lysed in
radioimmunoprecipitation assay buffer containing
phenylmethylsulfonyl fluoride and phosphatase inhibitors (Roche
Diagnostics GmbH, Mannheim, Germany) for 5 min. The protein content
in the cell lysates was determined using a bicinchoninic acid
protein assay kit (Thermo Fisher Scientific, Inc.). The lysates,
containing 40 μg protein, were separated by 12% SDS-PAGE, and
proteins were transferred to a polyvinylidene fluoride membrane.
Subsequently, the membrane was blocked with 5% blocking reagent (2
g skimmed milk power dissolved in 40 ml Tris-buffered saline with
1% Tween-20) for 1 h at room temperature. Then it was incubated
with the following primary antibodies (1:1,000 dilutions) overnight
at 4°C: Anti-IRF3 (ab50772; Abcam, Cambridge, UK),
anti-phosphorylated (p)-IRF3 (p-S386) (ab76493; Abcam), anti-MDA5
(ab126630; Abcam), anti-RIG-I (ab180675; Abcam), anti-NAK/TBK1
(ab109735; Abcam), anti-NAK/TBK1 (p-S172) (ab109272; Abcam),
anti-GAPDH (ab9485; Abcam), anti-caspase-3 (622701; BioLegend,
Inc., San Diego, CA, USA), anti-caspase-9 (621901; BioLegend,
Inc.), anti-caspase-8 (645501; BioLegend, Inc.), anti-TNF-related
apoptosis-inducing ligand (TRAIL) antibody (RLT4721; Suzhou Ruiying
Biological Co., Ltd., Suzhou, China) and anti-p-STAT1 (p-Ser727)
antibody (RLP0842; Suzhou Ruiying Biological Co., Ltd.).
Subsequently, the membranes were incubated with goat anti-mouse
immunoglobulin G (IgG)-horseradish peroxidase (HRP) (sc-2005;
1:5,000 dilution; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
and goat anti-rabbit IgG-HRP (sc2004; 1:5,000 dilution; Santa Cruz
Biotechnology, Inc.) secondary antibodies for 2 h at room
temperature. The blots were visualised using the Luminata Forte
Western HRP Substrate (EMD Millipore).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells or tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA (2 μg) was reverse transcribed to cDNA
using a first-strand cDNA synthesis kit (Invitrogen; Thermo Fisher
Scientific, Inc.), which was conducted according to the
manufacturer’s protocol. Subsequently, relative gene expression was
determined by qPCR using the SYBR kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and ABI 7500 real-time PCR detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
amplification conditions were as follows: An initial denaturation
step at 95°C for 10 min, followed by 40 cycles of denaturation at
95°C for 15 sec and annealing/extending at 60°C for 1 min. The
following primer sequences were used: IRF3 forward,
5′-TCAGGAGTTGGGGACTTTTC and reverse, 5′-GAATGTCTTCCTGGGTATCAG; and
β-actin forward, 5′-ATATCGCCGCGCTCGTCGTC and reverse,
5′-CATGCCCACCATCACGCC CTG-3′. The MDA5 and RIG-1 primer sequences
used are noted in previous studies (17,18),
as are the sequences for C-X-C motif chemokine ligand 10 (CXCL-10)
and IFN-β (19), and TRAIL
(5). β-actin was used as an
internal reference. The relative mRNA expression levels were
normalised to β-actin mRNA. The results of the cell samples were
calculated as 2−ΔΔCq (20), whereas the 2−ΔCq method
was used to analyse the tissue samples.
ELISA
After the indicated treatments, the supernatants
were collected and an ELISA analysis was performed to detect the
expression levels of human CXCL-10 using an ELISA kit (555046; BD
Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer’s protocol.
Apoptosis analysis
An equal number of A549 cells
(1.0×105/ml) were seeded into a 6-well plate. Following
transfection of A549 cells with Poly(I:C) for 24 h, with or without
caspase inhibitor pretreatment, the cells were harvested
(1.0×106). Subsequently, the apoptotic cells were
stained using the Annexin V-fluorescein isothiocyanate/propidium
iodide Cell Apoptosis Detection kit (Beijing Transgen Biotech Co.,
Ltd., Beijing, China) according to the manufacturer’s protocol.
Each sample was assessed by fluorescence-activated cell sorting
using a flow cytometer (BD Biosciences) and the data were analyzed
using FlowJo v10 software (FlowJo LLC, Ashland, OR, USA).
Lentivirus short hairpin (sh)RNA
infection
The lentivirus shRNA pLent-U6-shIRF3-GFP-Puro and
the empty vector pLent-U6-GFP-Puro were purchased from Vigene
Biosciences, Inc. (Jinan, China). The sequence of IRF3 shRNA was as
follows:
5′-CCGGCCCTTCATTGTAGATCTGATTCTCGAGAATCAGATCTACAATGAAGGGTTTTT-3′.
A549 cells were infected with the lentiviruses once cell confluence
reached 70-80%. A total of 48 h postinfection, a low concentration
of puromycin (5 mg/ml) was used to select lentivirus-infected
cells. After 14 days of screening, the stably infected cells were
obtained.
Plasmid construction
IRF3 cDNA was obtained from peripheral blood cells
collected from a healthy human volunteer, and PCR was performed
with primers tagged with restriction endonuclease sequences
(XhoI and AgeI; Thermo Fisher Scientific, Inc.).
Subsequently, the PCR products, or the vector plasmid pEGFP-N1
(Clontech Laboratories, Inc., Mountainview, CA, USA), were
incubated with XhoI or AgeI for 1.5 h and were
ligated with T4 ligase for 1.5 h (Thermo Fisher Scientific, Inc.)
at room temperature. Following ligation, the mix was transformed
into competent Escherichia coli (Beijing Transgen Biotech
Co., Ltd.) cells by heat shock treatment (42°C for 90 sec in a
water bath). Finally, the transformed bacteria were cultured with
Lennox L Broth Base (Invitrogen; Thermo Fisher Scientific, Inc.)
containing kanamycin (30 μg/ml; Gibco; Thermo Fisher
Scientific, Inc.) and the recombinant plasmid IRF3-pEGFP-N1 was
identified by PCR using the IRF3 primer.
Plasmid and small interfering (si)RNA
transfection
Once cell confluence reached 80–90%, the expression
plasmid IRF3-pEGFP-N1 and the empty vector were transfected into
A549 cells, whereas the IRF3-pEnter with His-tag (Vigene
Biosciences, Inc.) and the empty vector were transfected into H1299
cells using Lipofectamine® 3000. The culture medium was
changed after 6 h, and IRF3 expression was assessed after 48 h of
transfection. For siRNA transfection, the RIG-I- and MDA5-specific
siRNAs, and the scrambled sequence were prepared (Suzhou
GenePharma, LLC, Suzhou, China). The sequences used were as
follows: Scrambled, 5′-CAUAGCGUCCUUGAUCACAUU-3′; RIG-I,
5′-AACGAUUCCAUCACUAUCCAUdTdT-3′ (21); MDA5, 5′-GGUGUAAGAGAGCUACUAAtt-3′
(22). siRNA transfection was
performed using Lipofectamine® 3000 once cell confluence
reached 70–80%, and the concentration of siRNAs used was 50 nM.
Subsequently, cells were analysed after 36 h of transfection.
Statistical analysis
Statistical analyses were performed using SPSS 20
(IBM Corporation, Armonk, NY, USA). The data were presented as the
means ± standard error of the mean. Semi-quantification of western
blotting was analysed using ImageJ2 software (National Institutes
of Health, Bethesda, MD, USA). For comparisons between two groups,
Student’s t-test was used, whereas comparisons among multiple
groups were made using univariate analysis of variance, followed by
least significant difference post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Three independent experiments were conducted.
Results
Poly(I:C) induces apoptosis via the
extrinsic apoptotic pathway in A549 cells
It was hypothesised that Poly(I:C) may induce
apoptosis of NSCLC cells via the canonical apoptotic pathway. The
present study demonstrated that the number of apoptotic A549 cells
increased in a concentration-dependent manner following
transfection with Poly(I:C) for 24 h (Fig. 1A and B). The mRNA expression levels
of TRAIL were significantly increased (data not shown), and the
protein expression levels of TRAIL, and cleaved caspase-3 and -8,
were markedly increased. Conversely, cleaved caspase-9 expression
was slightly increased post-transfection (Fig. 1C). To further determine the role of
caspases in Poly(I:C)-induced apoptosis of A549 cells, the cells
were treated with the pancaspase inhibitor Z-VAD-FMK and the
caspase-8 inhibitor Z-IETD-FMK for 2 h prior to transfection with
100 ng/ml Poly(I:C) for 24 h. As expected, apoptosis was
significantly inhibited (Fig. 1D and
E) and cleaved caspase-8 expression was decreased following
Z-VAD-FMK pretreatment (Fig. 1F).
These findings indicated that Poly(I:C) may induce apoptosis of
A549 cells via activation of an extrinsic apoptotic pathway.
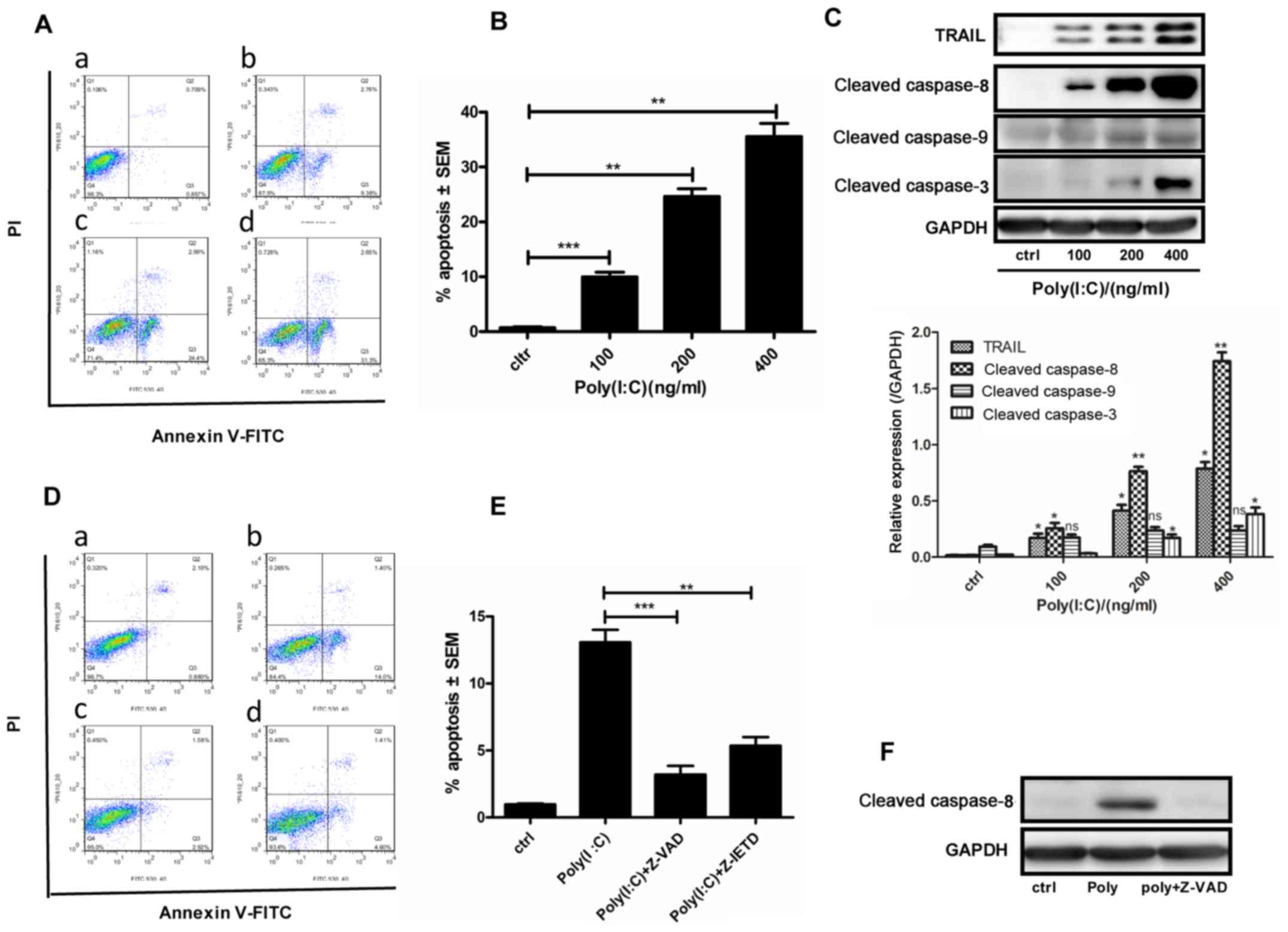 | Figure 1Poly(I:C) induces apoptosis of A549
cells via the extrinsic apoptotic pathway. (A–D) A549 cells were
transfected with 0, 100, 200 or 400 ng/ml Poly(I:C) for 24 h; the
control group was treated with Lipofectamine® 3000
alone. (A) Flow cytometry was used to detect apoptotic cells. (B)
Histogram of the percentage of apoptotic cells in panel (A). (C)
Western blotting was used to examine the protein expression levels
of TRAIL, cleaved caspase-8, -9 and -3. (D) Flow cytometry was used
to detect apoptosis of cells pretreated with the pan-caspase
inhibitor Z-VAD or the caspase-8 inhibitor Z-IETD for 2 h prior to
100 ng/ml Poly(I:C) transfection for 24 h. (E) Histogram of the
percentage of apoptotic cells in panel (E). (F) Cleaved caspase-8
expression was detected by western blotting in A549 cells
pretreated with the pan-caspase inhibitor Z-VAD.
*P<0.02; **P<0.005;
***P<0.001. FITC, fluorescein isothiocyanate; ns, not
significant; PI, propidium iodide; Poly(I:C),
polyinosinic:polycytidylic acid; SEM, standard error of the mean;
TRAIL, tumour necrosis factor-related apoptosis-inducing
ligand. |
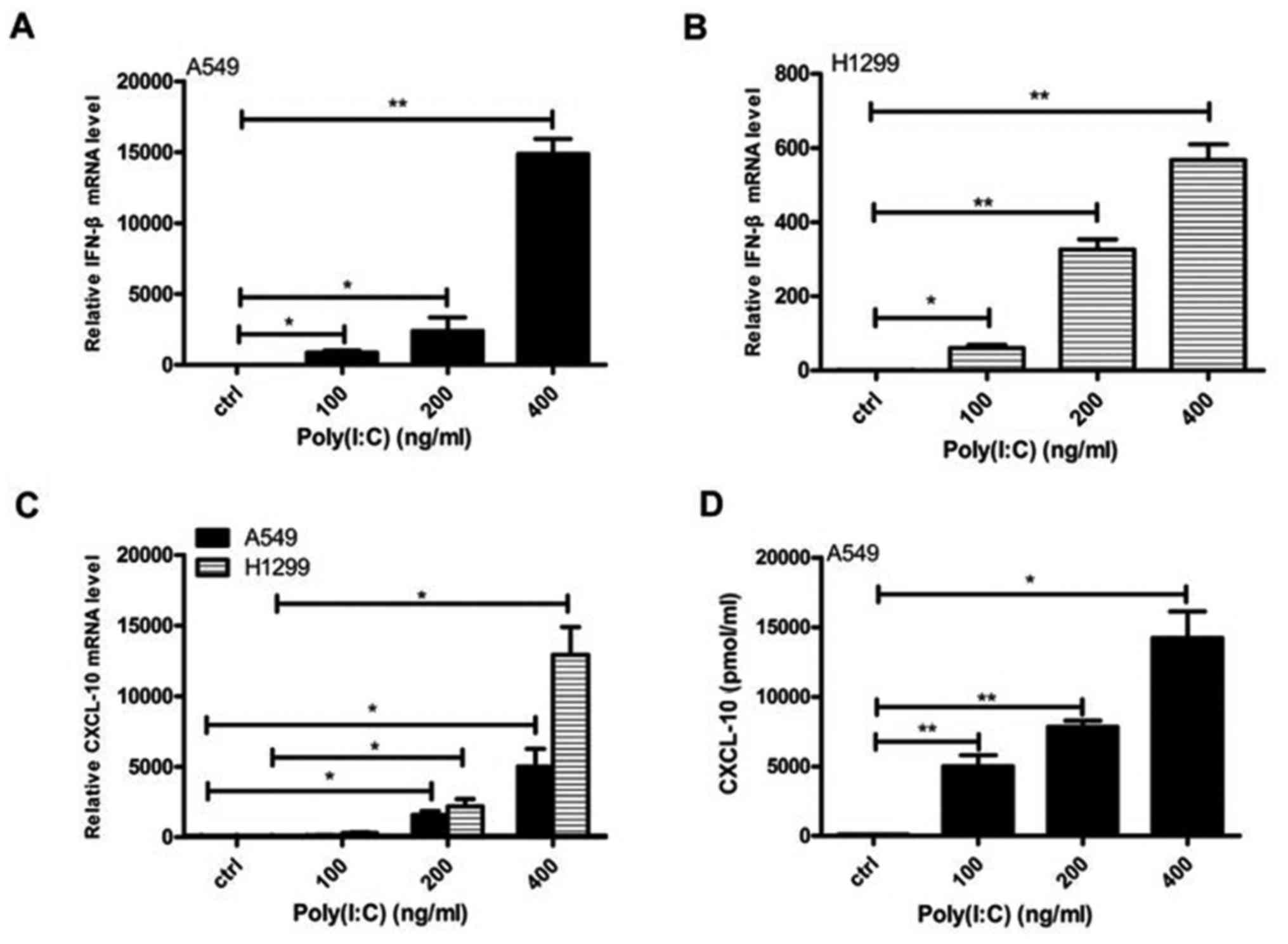
Poly(I:C) transfection activates innate
immunity in NSCLC cells
The present study transfected Poly(I:C) into the
NSCLC cell lines A549 and H1299. Subsequently, the mRNA expression
levels of IFN-β and CXCL-10 were increased in both cell lines, and
the secretion of CXCL-10 was increased in A549 cells in a
concentration-dependent manner (Fig.
2). Furthermore, MDA5/RIG-I expression was upregulated, and
their downstream molecules, TBK1 and IRF3, were activated (p-TBK1,
p-IRF3) (data not shown). However, these alterations were not
observed when Poly(I:C) was directly added to the medium (data not
shown).
MDA5/RIG-I/IRF3 axis is associated with
the effects of Poly(I:C) transfection on NSCLC cell lines
When the NSCLC A549 and H1299 cell lines were
pretreated with BX795 for 2 h, and were then transfected with
Poly(I:C) for 24 h, p-IRF3 was inhibited, whereas no changes in
total IFR3 were detected (Fig.
3A). In addition, poly(I:C)-induced increases in IFN-β and
CXCL-10 mRNA expression in both cell lines, and CXCL-10 secretion
in A549 cells, were abrogated by BX795 (Fig. 3B–D). Furthermore, IRF3 was stably
knocked down by shRNA in A549 cells, which inhibited the increase
in p-IFR3 levels and CXCL-10 secretion following Poly(I:C)
transfection (Fig. 3E and F).
Furthermore, both cell lines were transfected with Poly(I:C) for 24
h following transfection with RIG-I and MDA5 siRNAs. Notably, the
expression levels of p-IRF3 were decreased (Fig. 3G), which was accompanied by a
decrease in IFN-β and CXCL-10 mRNA expression, and CXCL-10
secretion in A549 cells (Fig. 3F, H
and I). These results indicated that responses to Poly(I:C)
transfection were regulated by the MDA5/RIG-I/IRF3 axis in NSCLC
cells.
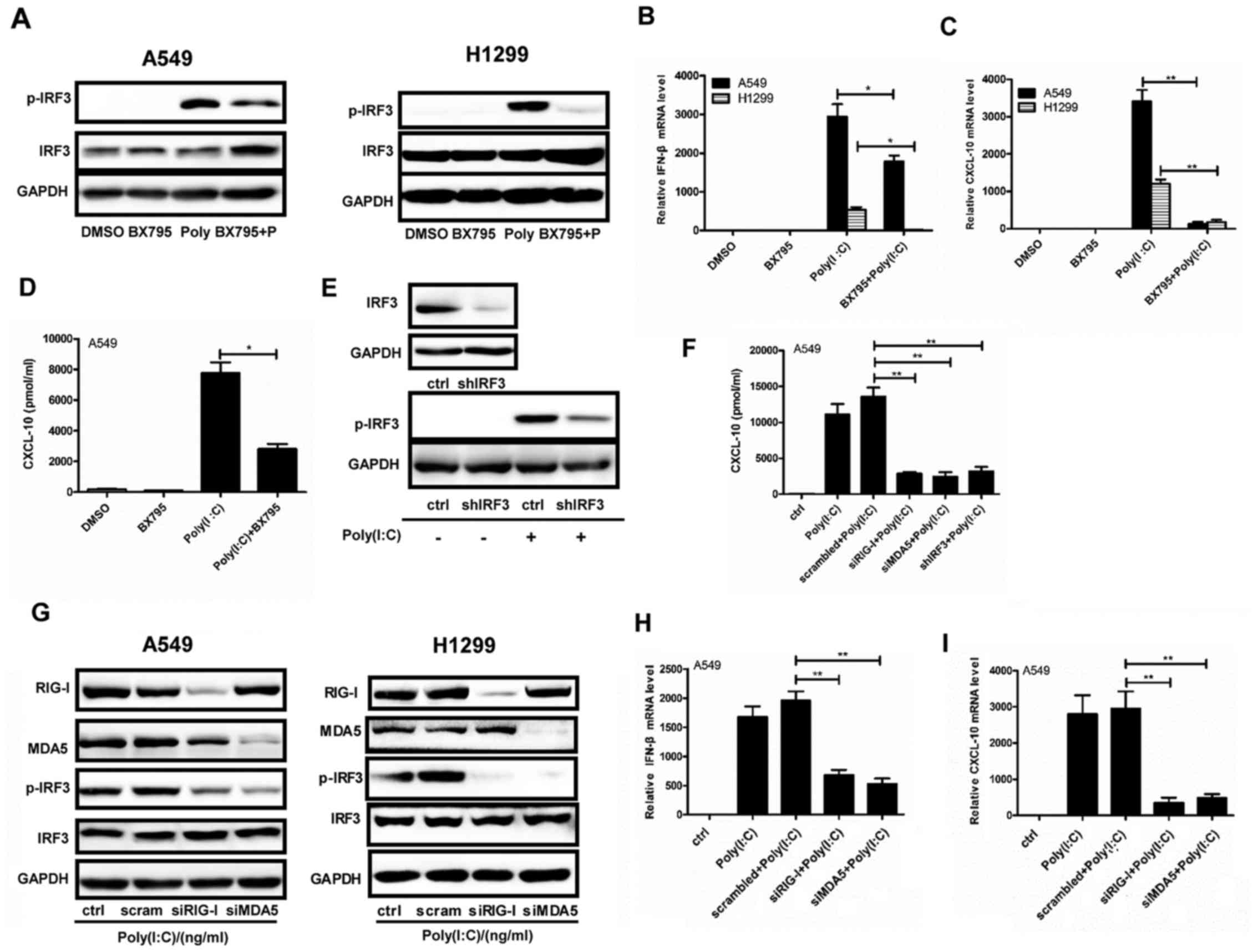 | Figure 3MDA5/RIG-I/IRF3 axis mediates the
responses of NSCLC cell lines to Poly(I:C) transfection. (A–D)
Poly(I:C) (200 ng/ml) was transfected into A549 and H1299 cells for
24 h following 2 h pretreatment with BX795. DMSO was used as a
control. (A) Protein expression levels of IRF3 and p-IRF3 were
examined by western blotting. (B and C) IFN-β and CXCL-10
expression was quantified by qPCR. (D) CXCL-10 secretion was
determined in A549 cell supernatants by ELISA. (E) IRF3 was stably
downregulated by infection with a lentivirus containing shIFR3, the
protein expression levels of p-IRF3 were detected by western
blotting after Poly(I:C) transfection for 24 h. (F–I) siRNAs
against RIG-I or MDA5 were transfected into cells for 36 h prior to
Poly(I:C) transfection (200 ng/ml) for 24 h. Scrambled sequences
were used as a negative control. (F) CXCL-10 was detected by ELISA
in A549 cells with RIG-I or MDA5 or IRF3 depletion after Poly(I:C)
transfection for 24 h. (G) Western blotting was used to examine the
protein expression levels of RIG-I, MDA5, IRF3 and p-IRF3. (H and
I) IFN-β and CXCL-10 mRNA expression was assessed by qPCR in A549
cells with RIG-I or MDA5 depletion following Poly(I:C) transfection
for 24 h. *P<0.05; **P<0.005. CXCL-10,
C-X-C motif chemokine ligand 10; DMSO, dimethyl sulfoxide; IFN-β,
interferon-β; IRF3, interferon regulatory factor 3; MDA5, melanoma
differentiation-associated protein 5; p-IRF3, phosphorylated-IRF3;
Poly(I:C), polyinosinic:polycytidylic acid; qPCR, quantitative
polymerase chain reaction; RIG-I, retinoic acid-inducible gene I
protein; sh, short hairpin RNA; si, small interfering RNA. |
IRF3 mediates apoptosis of NSCLC cells
via STAT1 phosphorylation
The present study initially treated NSCLC cells with
BX795 or shRNA to downregulate IRF3; the results demonstrated that
inhibition of IRF3 suppressed Poly(I:C)-induced TRAIL, and cleaved
caspase-3 and -8 expression, and decreased p-STAT1 (Fig. 4A and B). Conversely, IRF3
overexpression in NSCLC cells induced upregulation of TRAIL,
cleaved caspase-3 and -8, and p-STAT1 (Fig. 5). Furthermore, knockdown of MDA5 or
RIG-1 reduced TRAIL expression in both cell lines (data not shown).
These results suggested that Poly(I:C) activated the apoptotic
pathway in NSCLC cells via the MDA5/RIG-I/IRF3 pathway. In
addition, STAT1 phosphorylation may also engage with IRF3-mediated
apoptosis of NSCLC cells.
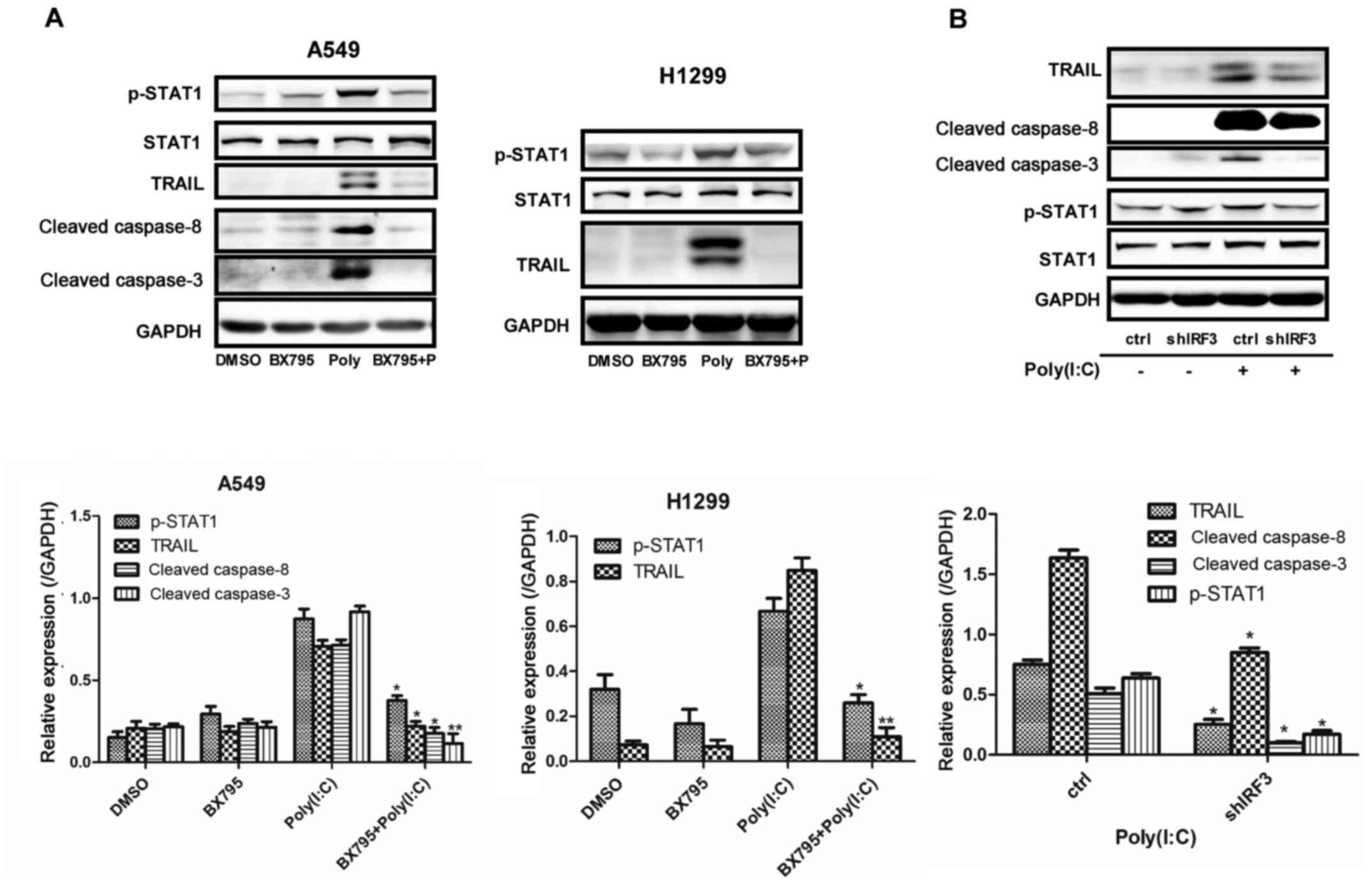 | Figure 4Apoptosis of non-small cell lung
cancer cells was inhibited by the suppression of IRF3 activity. (A)
Expression levels of p-STAT1, TRAIL, and cleaved caspase-3 and -8,
were detected by western blotting in A549 and H1299 cells
pretreated with BX795 for 2 h prior to Poly(I:C) transfection for
24 h. *P<0.01; **P<0.005 vs. Poly(I:C)
group. (B) In A549 cells with stable downregulation of IRF3,
western blotting was conducted to detect TRAIL, p-STAT1, and
cleaved caspase-3 and -8 expression, following Poly(I:C)
transfection for 24 h. *P<0.01;
**P<0.005 vs. ctrl group. IRF3, interferon regulatory
factor 3; p-IRF3, phosphorylated-IRF3; Poly(I:C),
polyinosinic:polycytidylic acid; p-STAT1, phosphorylated signal
transducer and activator of transcription 1; sh, short hairpin RNA;
TRAIL, tumour necrosis factor-related apoptosis-inducing
ligand. |
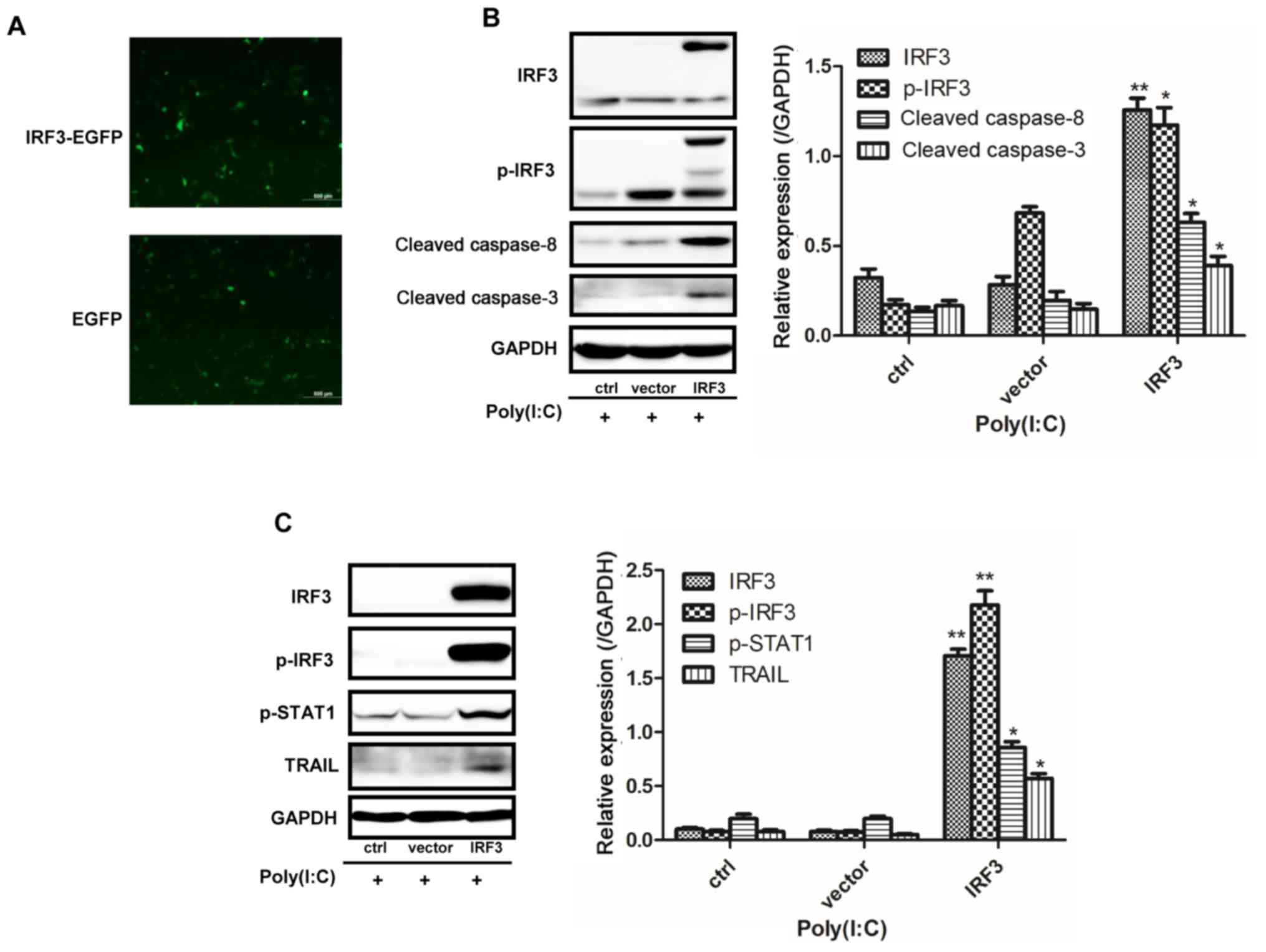 | Figure 5IRF3 overexpression promotes the
apoptosis of NSCLC cells. (A) Successful infection with the
p-EGFP-N1 plasmid in A549 cells. (B) A549 cells overexpressed IRF3
following infection with an IRF3-EGFP plasmid, and exhibited
increased expression of p-IRF3, cleaved caspase-3 and -8 following
Poly(I:C) transfection. (C) H1299 cells overexpressed IRF3
following infection with an IRF3-pEnter with His-tag plasmid, and
exhibited increased expression of p-IRF3, p-STAT3 and TRAIL.
*P<0.02; **P<0.005 vs. vector group.
EGFP, enhanced green fluorescent protein; IRF3, interferon
regulatory factor 3; p-IRF3, phosphorylated-IRF3; Poly(I:C),
polyinosinic:polycytidylic acid; p-STAT1, phosphorylated signal
transducer and activator of transcription 1; TRAIL, tumour necrosis
factor-related apoptosis-inducing ligand. |
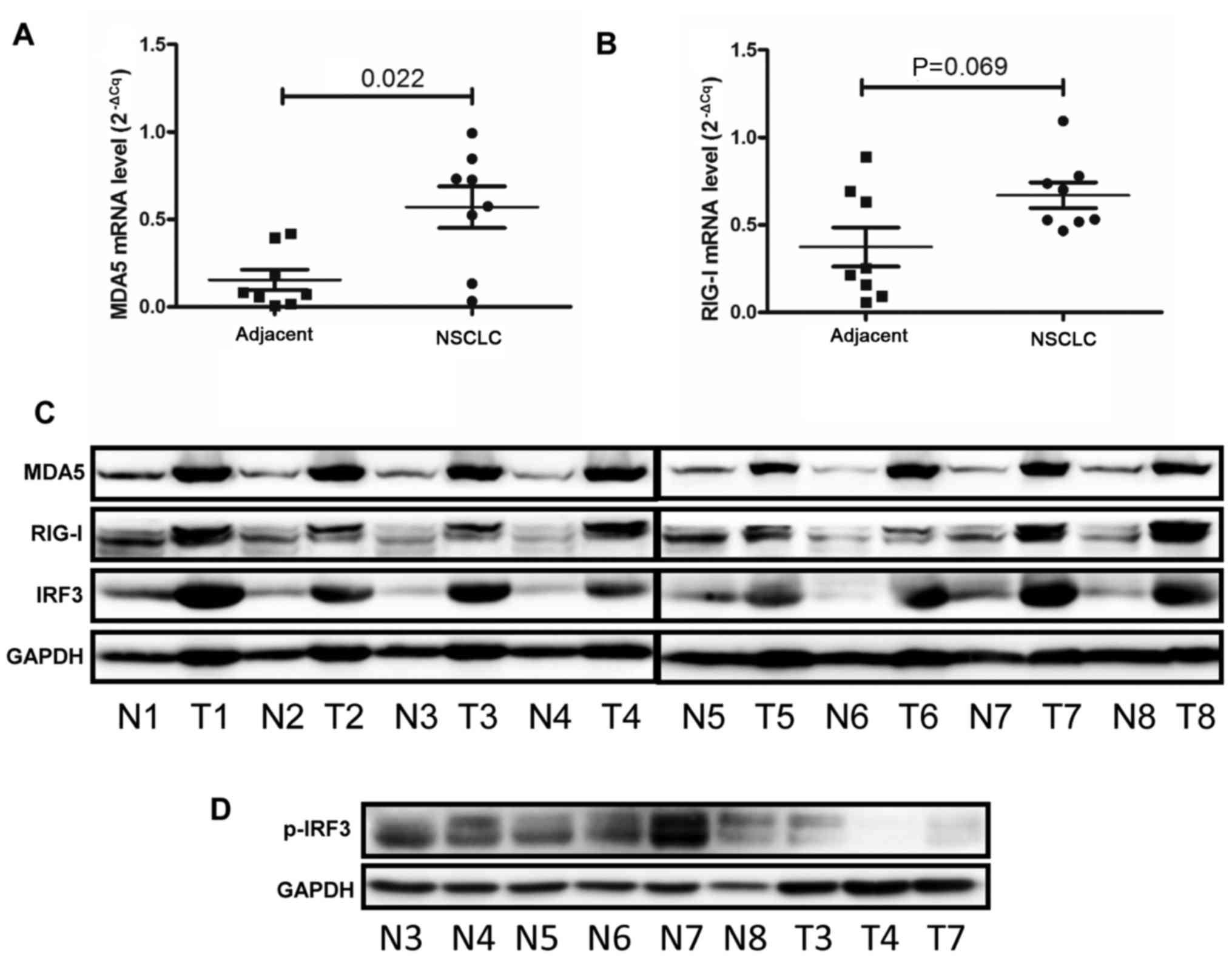
Activation of IRF3 is inhibited in NSCLC
surgical samples
The present study collected surgical NSCLC samples
from eight patients. The results of qPCR revealed that the mRNA
expression levels of MDA5 were increased by ~3-fold in NSCLC
tissues compared with in adjacent normal tissues (Fig. 6A). Conversely, RIG-1 mRNA
expression exhibited no marked alterations in NSCLC tissues
compared with in adjacent normal tissues (Fig. 6B). Western blotting demonstrated
that MDA5, RIG-1 and IRF3 were increased in tumour tissues
(Fig. 6C). However, p-IRF3 was
decreased in the three tumour tissues compared with in the six
normal samples (Fig. 6D). These
results suggested that innate immunity may be impaired in
NSCLC.
Discussion
Poly(I:C) is a synthetic analogue of dsRNA, which
may be used as a vaccine adjuvant to boost innate and adaptive
immunity (6,23,24).
A vast array of genes are not only induced but also repressed by
dsRNA. The inducible genes include interferon-stimulated genes,
apoptosis-associated genes, cytokines and growth factor genes, and
genes associated with metabolism and biosynthesis, RNA synthesis,
protein synthesis and degradation, cytoskeletal components,
transporters and the extracellular matrix. The repressed genes
include those involved in metabolism, cell cycle regulation and
cell adhesion (25). In the
present study, the results indicated that the MDA5/RIG-I innate
immunity pathway was activated and that type I IFN was induced when
Poly(I:C) was transfected into NSCLC cells, which indicated that
intracellular Poly(I:C) elicited innate immune responses by
activating the MDA5/RIG-I pathway (24). However, when Poly(I:C) was added to
the medium directly, it did not induce the innate immune response
in NSCLC cells, which may result from a deficiency of TLR3
expression, which is the mainstay sensor for extracellular
Poly(I:C) (3). However, the exact
mechanism requires further investigation. The TBK1 inhibitor BX795
inhibits phosphoinositide-dependent kinase-1 and TBK1 and,
subsequently blocks phosphorylation of IRF3 (26). Treatment with BX795 or knockdown of
IRF3 prior to Poly(I:C) transfection led to the inhibition of
innate immunity. These findings suggested that the
MDA5/RIG-I/TBK1/IRF3 signalling pathway was initiated by
intracellular Poly(I:C), and was intact in NSCLC cells, as in other
cancer cells (14,27,28).
Previous studies have revealed that Poly(I:C)
induces apoptosis of prostate cancer cells, pancreatic cancer cells
and other cells (27,28). Furthermore, the canonical apoptotic
pathways, including the caspase-9-dependent intrinsic pathway and
the caspase-8-dependent extrinsic pathway, engage Poly(I:C)-induced
apoptosis to some degree (14,27).
In the present study, Poly(I:C)-induced apoptosis of A549 cells
mainly via the extrinsic pathway, as determined by the slight
change in activated caspase-9 expression, and this was associated
with an upregulation of TRAIL. TRAIL belongs to the TNF family and
induces apoptosis by binding death receptor (DR)4 or DR5, in order
to activate the extrinsic apoptotic pathway (29,30).
Furthermore, IRF3 serves a crucial role in TLR3-mediated apoptosis
of androgen-sensitive prostate cancer LNCaP cells (12), whereas it is not implicated in
androgen-resistant prostate cancer cells (14). In the present study, downregulation
of IRF3 in NSCLC cells resulted in a decline in the expression of
apoptosis-related proteins, and vice versa. A previous study
revealed that IRF3 binds and transactivates the TRAIL promoter in
the nucleus where the IRF3 response element is located (31). Therefore, active IRF3 may shuttle
into the nucleus of NSCLC cells and directly target TRAIL to
activate extrinsic apoptosis. However, another study elucidated the
concept of RIG-I-induced IRF3-mediated apoptosis, revealing that
IRF3 in the cytoplasm interacts with B-cell lymphoma 2-associated X
protein and causes activation of caspase-9 and -3 (24), which mainly activate intrinsic
apoptosis. Therefore, in the present study, it is likely that the
results are consistent with the first mechanism. Notably, the
present study demonstrated that variation of STAT1 phosphorylation
was in accordance with the alterations in the apoptotic pathway.
This finding suggested that activation of STAT1 by type I IFN may
affect DNA damage and adaptive immunity (32), and may modulate the expression of
FAS, FAS ligand and casapse-1, and the function of p53 to
accelerate apoptosis (33). In
addition, STAT1 suppresses the cell cycle by inducing p53, NF-κB
p65, cyclin A, cyclin D1, cyclin E, F-box and WD repeat domain
containing 7, Hes family BHLH transcription factor 1 and
cyclin-dependent kinase 2 expression. Conversely, the Janus kinase
(JAK)2/STAT1 pathway participates in the upregulation of programmed
death-ligand 1 and may serve a pivotal role in inducing tumour
immune escape (34). Generally,
IRF3 is indeed involved in Poly(I:C)-induced apoptosis of NSCLC
cells; however, the exact mechanism is ambiguous, and whether the
JAK/STAT1 pathway orchestrates IRF3-mediated apoptosis in NSCLC
remains to be determined.
In human tissue samples, the present study
demonstrated that MDA5, RIG-I and IRF3 were increased in NSCLC
samples, thus indicating that the innate immune pathway was intact
in NSCLC. Notably, p-IRF3 expression was decreased, which was
inconsistent with the increase of IRF3, in three samples. These
findings suggested that MDA5 and RIG-I may be positively correlated
with the overall survival of patients with NSCLC, similar to in
patients with hepatocellular carcinoma (35). However, IRF3 may also be favourable
to the survival of cancer cells via producing various inflammatory
factors into the tumour microenvironment (36). In patients with herpes simplex
encephalitis, a G-A mutation at the 854 base-pair region in exon 6
prevents IRF3 from forming homodimers and being phosphorylated at
S286, which disrupts its transcriptional activity and the innate
immune response (37). Whether the
same mutation occurs in patients with NSCLC is currently
unknown.
In conclusion, the present study revealed that the
innate immune pathway was intact in NSCLC cells, and IRF3 was
involved in regulating the apoptotic pathway. However, in NSCLC
tissue samples, the innate immunity pathway may be disrupted. These
findings may provide novel insights into the role of IRF3 in innate
immunity and apoptosis in NSCLC. In addition, IRF3 may be
considered a target to induce apoptosis in NSCLC therapy.
Acknowledgments
The authors would like to thank Professor Dingfang
Bu for his expert technical guidance.
Abbreviations:
|
MDA5
|
melanoma differentiation-associated
protein 5
|
|
RIG-I
|
retinoic acid-inducible gene I
protein
|
|
IRF3
|
interferon regulatory factor 3
|
|
IPS-1
|
interferon-β promoter stimulator 1
|
|
TBK1
|
IκB kinase ε/tumour necrosis factor
receptor-associated factor family member-associated nuclear
factor-κB activator-binding kinase 1
|
|
TLRs
|
Toll-like receptors
|
Notes
[1]
Funding
The present study was supported by the Beijing
Natural Science Foundation (grant no. 7151011).
[2] Availability
of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors’
contributions
XL conceived and designed the experiments; LY
performed the experiments, analysed the data and wrote the paper;
DS ZL and ZZ helped collect the tissue specimens; QH, YW and XC
conducted statistical analysis.
[4] Ethics
approval and consent to participate
The present study was approved by the Ethics
Committee of Peking University First Hospital (Beijing, China). All
of the subjects gave their informed consent for inclusion before
they participated in the study. The peripheral blood cells were
collected from a healthy volunteer and informed consent was
obtained as well.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Jin B, Cheng LF, Wu K, Yu XH and Yeo AE:
Application of dsRNA in cancer immunotherapy: Current status and
future trends. Anticancer Agents Med Chem. 14:241–255. 2014.
View Article : Google Scholar
|
|
2
|
Jin B, Sun T, Yu XH, Liu CQ, Yang YX, Lu
P, Fu SF, Qiu HB and Yeo AE: Immunomodulatory effects of dsRNA and
its potential as vaccine adjuvant. J Biomed Biotechnol.
2010:6904382010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cui J, Chen Y, Wang HY and Wang RF:
Mechanisms and pathways of innate immune activation and regulation
in health and cancer. Hum Vaccin Immunother. 10:3270–3285. 2014.
View Article : Google Scholar
|
|
4
|
Kawai T and Akira S: Signaling to
NF-kappaB by Toll-like receptors. Trends Mol Med. 13:460–469. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar S, Ingle H, Mishra S, Mahla RS,
Kumar A, Kawai T, Akira S, Takaoka A, Raut AA and Kumar H: IPS-1
differentially induces TRAIL, BCL2, BIRC3 and PRKCE in type I
interferons-dependent and -independent anticancer activity. Cell
Death Dis. 6:e17582015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao X, Ai M, Guo Y, Zhou X, Wang L, Li X
and Yao C: Poly I:C-induced tumor cell apoptosis mediated by
pattern-recognition receptors. Cancer Biother Radiopharm.
27:530–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clément JF, Bibeau-Poirier A, Gravel SP,
Grandvaux N, Bonneil E, Thibault P, Meloche S and Servant MJ:
Phosphorylation of IRF-3 on Ser 339 generates a hyperactive form of
IRF-3 through regulation of dimerization and CBP association. J
Virol. 82:3984–3996. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Escalante CR, Nistal-Villán E, Shen L,
García-Sastre A and Aggarwal AK: Structure of IRF-3 bound to the
PRDIII-I regulatory element of the human interferon-beta enhancer.
Mol Cell. 26:703–716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tarassishin L and Lee SC: Interferon
regulatory factor 3 alters glioma inflammatory and invasive
properties. J Neurooncol. 113:185–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Romieu-Mourez R, Solis M, Nardin A, Goubau
D, Baron-Bodo V, Lin R, Massie B, Salcedo M and Hiscott J: Distinct
roles for IFN regulatory factor (IRF)-3 and IRF-7 in the activation
of antitumor properties of human macrophages. Cancer Res.
66:10576–10585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duan Y, Li Z, Cheng S, Chen Y, Zhang L, He
J, Liao Q, Yang L, Gong Z and Sun LQ: Nasopharyngeal carcinoma
progression is mediated by EBER-triggered inflammation via the
RIG-I pathway. Cancer Lett. 361:67–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gambara G, Desideri M, Stoppacciaro A,
Padula F, De Cesaris P, Starace D, Tubaro A, Del Bufalo D,
Filippini A, Ziparo E, et al: TLR3 engagement induces
IRF-3-dependent apoptosis in androgen-sensitive prostate cancer
cells and inhibits tumour growth in vivo. J Cell Mol Med.
19:327–339. 2015. View Article : Google Scholar :
|
|
13
|
Moore TC, Kumm PM, Brown DM and Petro TM:
Interferon response factor 3 is crucial to poly-I:C induced NK cell
activity and control of B16 melanoma growth. Cancer Lett.
346:122–128. 2014. View Article : Google Scholar :
|
|
14
|
Palchetti S, Starace D, De Cesaris P,
Filippini A, Ziparo E and Riccioli A: Transfected poly(I:C)
activates different dsRNA receptors, leading to apoptosis or
immunoadjuvant response in androgen-independent prostate cancer
cells. J Biol Chem. 290:5470–5483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian WL, Jiang ZX, Wang F, Guo R, Tang P,
Huang YM and Sun L: IRF3 is involved in human acute myeloid
leukemia through regulating the expression of miR-155. Biochem
Biophys Res Commun. 478:1130–1135. 2016. View Article : Google Scholar
|
|
16
|
Tokunaga T, Naruke Y, Shigematsu S, Kohno
T, Yasui K, Ma Y, Chua KJ, Katayama I, Nakamura T, Hishikawa Y, et
al: Aberrant expression of interferon regulatory factor 3 in human
lung cancer. Biochem Biophys Res Commun. 397:202–207. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tatsuta T, Imaizumi T, Shimoyama T, Sawaya
M, Kunikazu T, Matsumiya T, Yoshida H, Satoh K and Fukuda S:
Expression of melanoma differentiation associated gene 5 is
increased in human gastric mucosa infected with Helicobacter
pylori. J Clin Pathol. 65:839–843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Le Goffic R, Pothlichet J, Vitour D,
Fujita T, Meurs E, Chignard M and Si-Tahar M: Cutting Edge:
Influenza A virus activates TLR3-dependent inflammatory and
RIG-I-dependent antiviral responses in human lung epithelial cells.
J Immunol. 178:3368–3372. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo R, Li Y, Ning J, Sun D, Lin L and Liu
X: HnRNP A1/A2 and SF2/ASF regulate alternative splicing of
interferon regulatory factor-3 and affect immunomodulatory
functions in human non-small cell lung cancer cells. PLoS One.
8:e627292013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Yoboua F, Martel A, Duval A, Mukawera E
and Grandvaux N: Respiratory syncytial virus-mediated NF-kappa B
p65 phosphorylation at serine 536 is dependent on RIG-I, TRAF6, and
IKK beta. J Virol. 84:7267–7277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parsons KS, Hsu AC and Wark PA: TLR3 and
MDA5 signalling, although not expression, is impaired in asthmatic
epithelial cells in response to rhinovirus infection. Clin Exp
Allergy. 44:91–101. 2014. View Article : Google Scholar
|
|
23
|
Cheng YS and Xu F: Anticancer function of
polyinosinic-polycytidylic acid. Cancer Biol Ther. 10:1219–1223.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chattopadhyay S and Sen GC:
dsRNA-activation of TLR3 and RLR signaling: Gene
induction-dependent and independent effects. J Interferon Cytokine
Res. 34:427–436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geiss G, Jin G, Guo J, Bumgarner R, Katze
MG and Sen GC: A comprehensive view of regulation of gene
expression by double-stranded RNA-mediated cell signaling. J Biol
Chem. 276:30178–30182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clark K, Plater L, Peggie M and Cohen P:
Use of the pharmaco-logical inhibitor BX795 to study the regulation
and physiological roles of TBK1 and IkappaB kinase epsilon: A
distinct upstream kinase mediates Ser-172 phosphorylation and
activation. J Biol Chem. 284:14136–14146. 2009. View Article : Google Scholar :
|
|
27
|
Duewell P, Beller E, Kirchleitner SV,
Adunka T, Bourhis H, Siveke J, Mayr D, Kobold S, Endres S and
Schnurr M: Targeted activation of melanoma
differentiation-associated protein 5 (MDA5) for immunotherapy of
pancreatic carcinoma. OncoImmunology. 4:e10296982015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kübler K, tho Pesch C, Gehrke N, Riemann
S, Dassler J, Coch C, Landsberg J, Wimmenauer V, Pölcher M,
Rudlowski C, et al: Immunogenic cell death of human ovarian cancer
cells induced by cytosolic poly(I:C) leads to myeloid cell
maturation and activates NK cells. Eur J Immunol. 41:3028–3039.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bernardo AR, Cosgaya JM, Aranda A and
Jiménez-Lara AM: Synergy between RA and TLR3 promotes type I
IFN-dependent apoptosis through upregulation of TRAIL pathway in
breast cancer cells. Cell Death Dis. 4:e4792013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kiraz Y, Adan A, Kartal Yandim M and Baran
Y: Major apoptotic mechanisms and genes involved in apoptosis.
Tumour Biol. 37:8471–8486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kirshner JR, Karpova AY, Kops M and Howley
PM: Identification of TRAIL as an interferon regulatory factor 3
transcriptional target. J Virol. 79:9320–9324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheon H, Borden EC and Stark GR:
Interferons and their stimulated genes in the tumor
microenvironment. Semin Oncol. 41:156–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stephanou A and Latchman DS: STAT-1: A
novel regulator of apoptosis. Int J Exp Pathol. 84:239–244. 2003.
View Article : Google Scholar
|
|
34
|
Doi T, Ishikawa T, Okayama T, Oka K,
Mizushima K, Yasuda T, Sakamoto N, Katada K, Kamada K, Uchiyama K,
et al: The JAK/STAT pathway is involved in the upregulation of
PD-L1 expression in pancreatic cancer cell lines. Oncol Rep.
37:1545–1554. 2017. View Article : Google Scholar
|
|
35
|
No authors listed: RIG-I is a tumor
suppressor and biomarker of IFN-alpha efficacy in HCC. Cancer
Discov. 4:OF122014. View Article : Google Scholar
|
|
36
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Andersen LL, Mørk N, Reinert LS,
Kofod-Olsen E, Narita R, Jørgensen SE, Skipper KA, Höning K, Gad
HH, Østergaard L, et al: Functional IRF3 deficiency in a patient
with herpes simplex encephalitis. J Exp Med. 212:1371–1379. 2015.
View Article : Google Scholar : PubMed/NCBI
|