Introduction
Breast cancer is the most common cause of
cancer-associated mortality in women and ~1.7 million cases of
breast cancer are diagnosed worldwide each year (1). Out of all breast cancer cases, 15–20%
are characterized as triple-negative, which is negative for the
expression of estrogen and progesterone receptors and does not
exhibit amplification of the HER2/Neu gene (2,3).
Hormonal or trastuzumab-based therapies are generally ineffective
at treating triple-negative breast cancer (TNBC); therefore,
taxane, cisplatinum derivatives and bevacizumab, as well as
anthracycline-based chemotherapies used alone or in combination
with surgery and/or radiotherapy, remain the standard methods of
treating TNBC (2,4). The overall survival rate of patients
with TNBC is poor compared with those that have other subtypes of
breast cancer (2); therefore,
novel chemotherapeutic agents to treat this subtype of breast
cancer are required.
DNA topoisomerases are highly specialized nuclear
enzymes. By breaking and rejoining DNA strands, they overcome the
DNA supercoiling that occurs during DNA replication, transcription
and repair (5,6). Human DNA topoisomerases are molecular
targets of several classes of antineoplastic agents, including
camptothecins, anthracyclines and epipodophyllotoxins, which are
used to treat various types of cancer, including breast, lung,
prostate and hematological cancer (7,8).
Fluoroquinolones are a class of synthetic
antibiotics that interact with topoisomerase II-DNA complexes and
inhibit helix rejoining, resulting in the formation of
double-stranded DNA breaks (9).
Fluoroquinolones, including ciprofloxacin, enrofloxacin,
moxifloxacin and gatifloxacin exhibit activity not only against
bacterial topoisomerase II (DNA gyrase) and topoisomerase IV, but
also against eukaryotic topoisomerase IIα, the analogue of DNA
gyrase. Therefore, certain members of these antibiotics exhibit
marked cytotoxicity in various mammalian cancer cell lines
(10–15). However, it should be noted that
certain treatments for cancer may not distinguish between tumor and
non-tumor cells in patients and may therefore induce undesirable
side effects, such as genotoxicity (16).
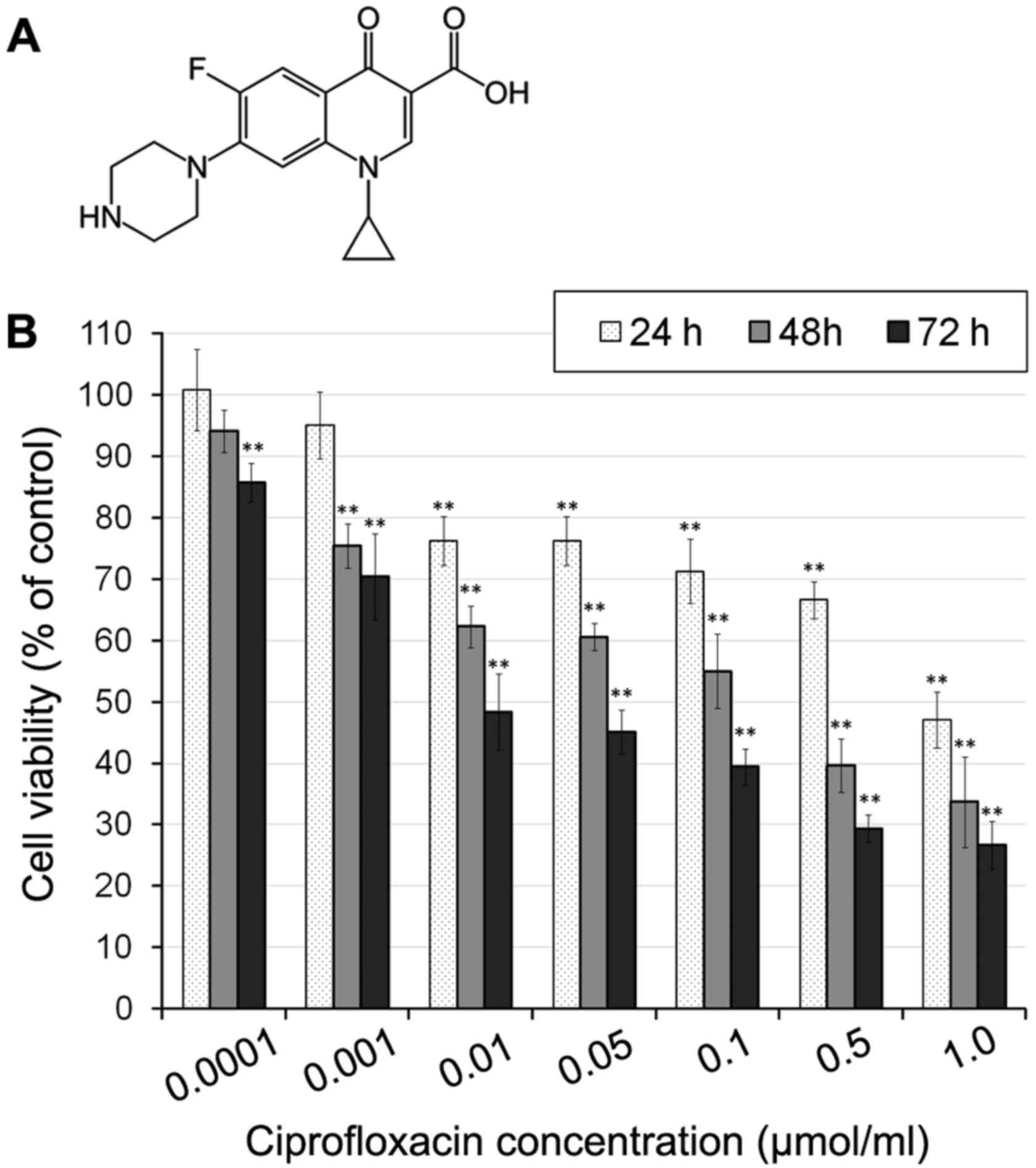
Ciprofloxacin (Fig.
1A) is a well tolerated second-generation fluoroquinolone
antibiotic used to treat patients with community and
hospital-acquired infections (16,17).
It has been suggested that ciprofloxacin is less susceptible to
efflux-mediated resistance compared with conventional topoisomerase
II chemotherapeutics, including doxorubicin and etoposide (18).
It is estimated that DNA topoisomerase IIα
(TOP2A) is frequently co-amplified with the human epidermal
growth factor receptor (HER2) oncogene in 40–50% of breast
cancer cases (19). This may mean
that the clinical response to topoisomerase II inhibitor-based
chemotherapy is more marked in patients with HER2-positive
breast cancer. However, TOP2A amplification may also occur
in HER2-negative breast cancer (20).
The overproduction of reactive oxygen species (ROS)
and depletion of cellular antioxidants, including reduced
glutathione (GSH), stimulate the induction of cancer cell apoptosis
(21). Hence, strategies aiming at
disrupting redox homeostasis may be used to chemosensitize tumors
and may represent a novel method of treating cancer (22).
The overproduction of ROS mediates the signal
transduction of apoptosis; increased levels of ROS may induce
oxidative stress, loss of cell function and cellular apoptosis. ROS
are also able induce lipid peroxidation and the cross-linking of
thiol groups in proteins (21).
Furthermore, tumors may be sensitized to chemotherapy and other
antitumor treatments by inhibiting antioxidant defenses, such as
NADPH or GSH, via metabolic suppression. Therefore, methods of
regulating redox signaling in tumor cells may represent promising
novel methods of treating cancer (22).
The overexpression of p53 deregulates regulation of
the cell cycle, DNA synthesis and cell apoptosis. Stimulation of
the p53 pathway is one of the mechanisms by which anticancer drugs
induce the apoptosis of cancer cells (23). Several p53 downstream target gene
products, such as the pro-apoptotic Bax protein, are able to
mediate apoptosis; when these products are expressed in concert,
apoptosis is induced (24).
Mitochondrial dysfunction mediated by members of the Bcl-2 family,
including anti-apoptotic Bcl-2 and pro-apoptotic Bax, is a well
known early event of apoptosis (25). p53 may also inhibit Bcl-2
expression and trigger mitochondrial apoptotic signaling by
increasing levels of ROS (25,26).
The MDA-MB-231 cell line, is negative for the
expression of the estrogen and progesterone receptors and
HER2/Neu amplification and is one of the most
commonly studied TNCB cell lines (27).
Studies have demonstrated that ciprofloxacin induces
time- and dose-dependent growth inhibition, apoptosis and
modulation of the cell cycle in human colorectal (11), pancreatic (13) and human prostate (14) cancer cell lines. However, to the
best of our knowledge, there have been no studies investigating the
cytotoxic effect of ciprofloxacin in a TNBC cell line. Therefore,
the present study investigated the impact of ciprofloxacin on the
viability, redox balance and apoptosis of MDA-MB-231 breast cancer
cells.
Materials and methods
Reagents
Ciprofloxacin hydrochloride was obtained from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Dulbecco's modified
Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin,
streptomycin, amphotericin B and trypsin/EDTA were all purchased
from Cytogen GmbH (Wetzlar, Germany). WST-1 was purchased from
Roche Diagnostics GmbH (Mannheim, Germany). Solution 3 (used in the
fixed cell-cycle-DAPI assay), consisting of DAPI (1 µg/ml)
and Triton X-100 (0.1%) in PBS; solution 7 (used in the
mitochondrial potential assay), consisting of JC-1 (200
µg/ml) in dimethyl sulfoxide (DMSO); solution 8 (used in the
mitochondrial potential assay) consisting of DAPI (1 µg/ml)
in PBS; solution 5 (used to measure intracellular GSH levels)
consisting of VitaBright-48™ (VB-48™; 400 µg/ml), propidium
iodide (PI; 500 µg/ml) and acridine orange (AO; 1.2
µg/ml) in DMSO; solution 15 consisting of Hoechst 33342 (500
µg/ml); and solution 16 consisting of PI (500 µg/ml)
were all purchased from ChemoMetec (Allerod, Denmark). Annexin
V-CF488A conjugate and Annexin V binding buffer were obtained from
Biotium, Inc. (Fremont, CA, USA).
MDA-MB-231 cell culture
The human epithelial metastatic breast cancer cell
line MDA-MB-231 was purchased from the ATCC (ATCC®
HTB-26™; Manassas, VA, USA). Cells were cultured in DMEM
supplemented with 10% FBS, penicillin (10,000 U/ml), streptomycin
(10 mg/ml) and amphotericin B (0.25 mg/ml) at 37°C in 5%
CO2.
Cell viability assay
The viability of MDA-MB-231 cells was evaluated
using a WST-1-based microplate colorimetric assay following a
previously described protocol (28). Briefly, 2,500 cells/well were
pre-incubated in DMEM for 24 h. Subsequently, the medium was
replaced with different concentrations of ciprofloxacin (0.0001,
0.001, 0.01, 0.05, 0.1, 0.5 and 1.0 µmol/ml) and cells were
incubated with the drug for 24, 48 or 72 h. WST-1 was added 3 h
prior to the end of the incubation periods. The absorbance of
samples was measured at 440 nm with a reference wavelength of 650
nm using a microplate reader. The viability of MDA-MB-231 cells
that were not treated with ciprofloxacin (controls) were normalized
to 100% for each assay and the results of the experiments were
expressed as the percentage of the controls. The assay was
performed in three independent experiments in triplicate.
Assessment of intracellular GSH
levels
GSH levels in MDA-MB-231 cells were measured using
the NucleoCounter® NC-3000™ (ChemoMetec) fluorescence
image cytometer following a previously reported protocol (28). MDA-MB-231 cells were seeded in T-75
flasks (2×106 cells/flask) and pretreated in DMEM for 24
h. Subsequently, the medium was replaced with various
concentrations of ciprofloxacin (0.01, 0.1 and 1.0 µmol/ml)
and cells were incubated with the drug for a further 24 h. Samples
were treated with solution 5 according the manufacturer's protocol
and analysis was conducted using NucleoView NC-3000 software,
version 1.4 (ChemoMetec). The assay was performed in three
independent experiments in triplicate.
Annexin V assay
During apoptosis, the presence of phosphatidylserine
on the exterior surface of the plasma membrane may be detected
using Annexin V-fluorescein isothiocyanate (Annexin V-FITC). This
assay is combined with the analysis of exclusion of plasma membrane
integrity (PI probe). MDA-MB-231 cells were seeded in T-75 flasks
at a density of 2×106 cells/flask for 24 h. Cells were
then treated with various concentrations of ciprofloxacin (0.01,
0.1 and 1.0 µmol/ml). Following 24 h incubation, cells were
harvested by trypsinization and counted using the NucleoCounter
image cytometer. A total of 3.0×105 cells were suspended
in 100 µl Annexin V binding buffer. For each sample, 2
µl Annexin V-CF 488A conjugate and 2 µl solution 15
(Hoechst 33342, final concentration 10 µg/ml) were added and
samples were incubated at 37°C for 15 min using a heating block.
Stained cells were then centrifuged at 400 × g at room temperature
for 5 min and washed twice with Annexin V binding buffer. Cell
pellets were then resuspended in 100 µl Annexin V binding
buffer supplemented with solution 16 (10 µg/ml PI) and
analyzed immediately using the fluorescence image cytometer.
Scatter-plots were used to determine the proportion of healthy
cells (Annexin V-negative/PI-negative cells), early apoptotic cells
(Annexin V-positive/PI-negative cells) and late apoptotic cells
(Annexin V-positive/PI-positive cells). NucleoView NC-3000
software, version 1.4. was used for data analysis. The assay was
performed in three independent experiments in triplicate.
Mitochondrial membrane potential
assay
The mitochondrial transmembrane potential was
measured using the NucleoCounter NC-3000 fluorescence image
cytometer following a previously described protocol (28). MDA-MB-231 cells were seeded in T-75
flasks (2×106 cells/flask) and pretreated in DMEM for 24
h. Subsequently, the medium was replaced with different
concentrations of ciprofloxacin (0.01, 0.1 and 1.0 µmol/ml)
and cells were incubated for 24 and 48 h. Samples were stained with
solutions 7 at 37°C for 15 min. At the end of analysis, cell
pellets were resuspended in 250 µl solution 8 and analyzed
immediately using NucleoView NC-3000 software, version 1.4
(ChemoMetec). The assay was performed in three independent
experiments in triplicate.
Quantitative determination of p53, Bax
and Bcl-2 proteins
The expression of p53, Bax and Bcl-2 in cell lysates
following treatment with different concentrations of ciprofloxacin
(0.01, 0.1 and 1.0 µmol/ml) for 24 h were measured using
human p53 (Biovendor, Brno, Czech Republic), human Bax (Enzo
Biochem, New York, NY, USA) and human Bcl-2 (Biovendor)
enzyme-linked immunosorbent assay (ELISA) kits (cat. nos. RAF082R,
ADI-900-138 and RAF005R, respectively), following the
manufacturer's protocol. The expression of p53, Bax and Bcl-2 were
normalized to total protein content and expressed as percentages of
the controls (lysates of cells that were not treated with
ciprofloxacin). Total protein concentration in each lysate was
determined using the Pierce BCA Protein assay kit (cat. no. 23225;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), with bovine
serum albumin as a standard. The assay was performed in three
independent experiments in triplicate.
Fixed cell cycle-DAPI assay
Cell cycle analysis of MDA-MB-231 cells was
performed using the NucleoCounter NC-3000 fluorescence image
cytometer following a previously described protocol (28). In brief, MDA-MB-231 cells were
seeded in T-75 flasks (2×106 cells/flask) and pretreated
in growth medium for 24 h. The medium was then replaced with
different concentrations of ciprofloxacin (0.01, 0.1 and 1.0
µmol/ml) and cells were incubated with the drug for a
further 24 h. Cells were fixed with 70% cold-ethanol at 4°C for 24
h, stained with solution 3 containing DAPI for 5 min at 37°C and
analyzed using the NucleoView NC-3000 software, version 1.4. The
assay was performed in three independent experiments in
triplicate.
Statistical analysis
For all experiments, at least three separate
experiments (n=3) were performed in triplicate and the results are
presented as the mean ± standard error of the mean. Statistical
analysis was performed using GraphPad Prism 6.01 (GraphPad
Software, Inc., La Jolla, CA, USA). Differences among groups were
assessed using one-way analysis of variance followed by Dunnett's
test. P<0.05 was determined to indicate a significant
difference.
Results
Ciprofloxacine reduces MDA-MB-231 cell
viability
To investigate the effect of ciprofloxacin on the
viability of human MDA-MB-231 breast cancer cells, a WST-1 assay
was performed. The results demonstrated that ciprofloxacin
significantly decreased cell viability in a dose- and
time-dependent manner (Fig. 1).
Incubation with 0.01–1 µmol/ml ciprofloxacin for 24 h
significantly decreased MDA-MB-231 cell viability by between 24±3
and 53±4%, compared with the control (Fig. 1B). The cytotoxic effects of
ciprofloxacin increased following an increase in the incubation
period to 48 h, when the viability of MDA-MB-231 cells treated with
1.0 µmol/ml ciprofloxacin decreased to 33±6% compared with
the control. Following 72 h incubation, a significant decrease in
cell viability was observed following incubation with all
concentrations of ciprofloxacin (Fig.
1B). The exposure of cells to 0.0001–1.0 µmol/ml
ciprofloxacin resulted in a decrease of cell viability of between
15±3 and 74±4%, compared with the control.
The strongest decrease in cell viability
following incubation for 24, 48 and 72 h was observed following
treatment with 1.0 µmol/ml ciprofloxacin
Furthermore, for MDA-MB-231 cells treated with
ciprofloxacin for 24, 48 and 72 h, the concentrations of
ciprofloxacin that decreased cell viability by 50% were 0.83, 0.14
and 0.03 µmol/ml, respectively.
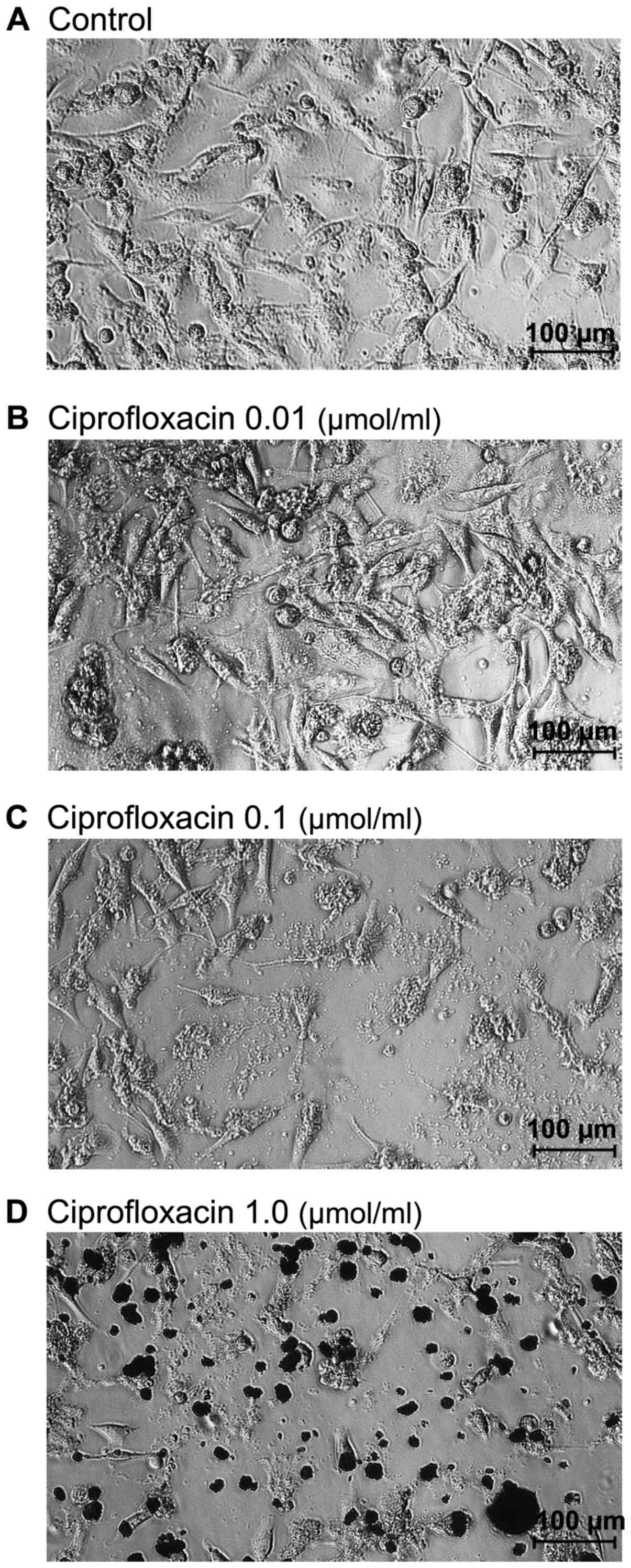
Ciprofloxacin induces morphological
changes in MDA-MB-231 cells
Morphological changes of MDA-mB-231 cells were
determined using a light-inverted microscope at a magnification of
×100. As presented in Fig. 2A,
untreated MDA-MB-231 cells grew adherently in culture flask and had
a regular shape and size. Treatment with the lowest concentration
of ciprofloxacin (0.01 µmol/ml) did not affect the
morphology of MDA-MB-231 cells (Fig.
2B). By contrast, cells treated with higher concentrations of
ciprofloxacin (0.1 and 1.0 µmol/ml) for 24 h lost their
shape, became round and start to detach from the flask (Fig. 2C and D). A decrease in the number
of cells, as well as a loss in cell-cell contact was also
observed.
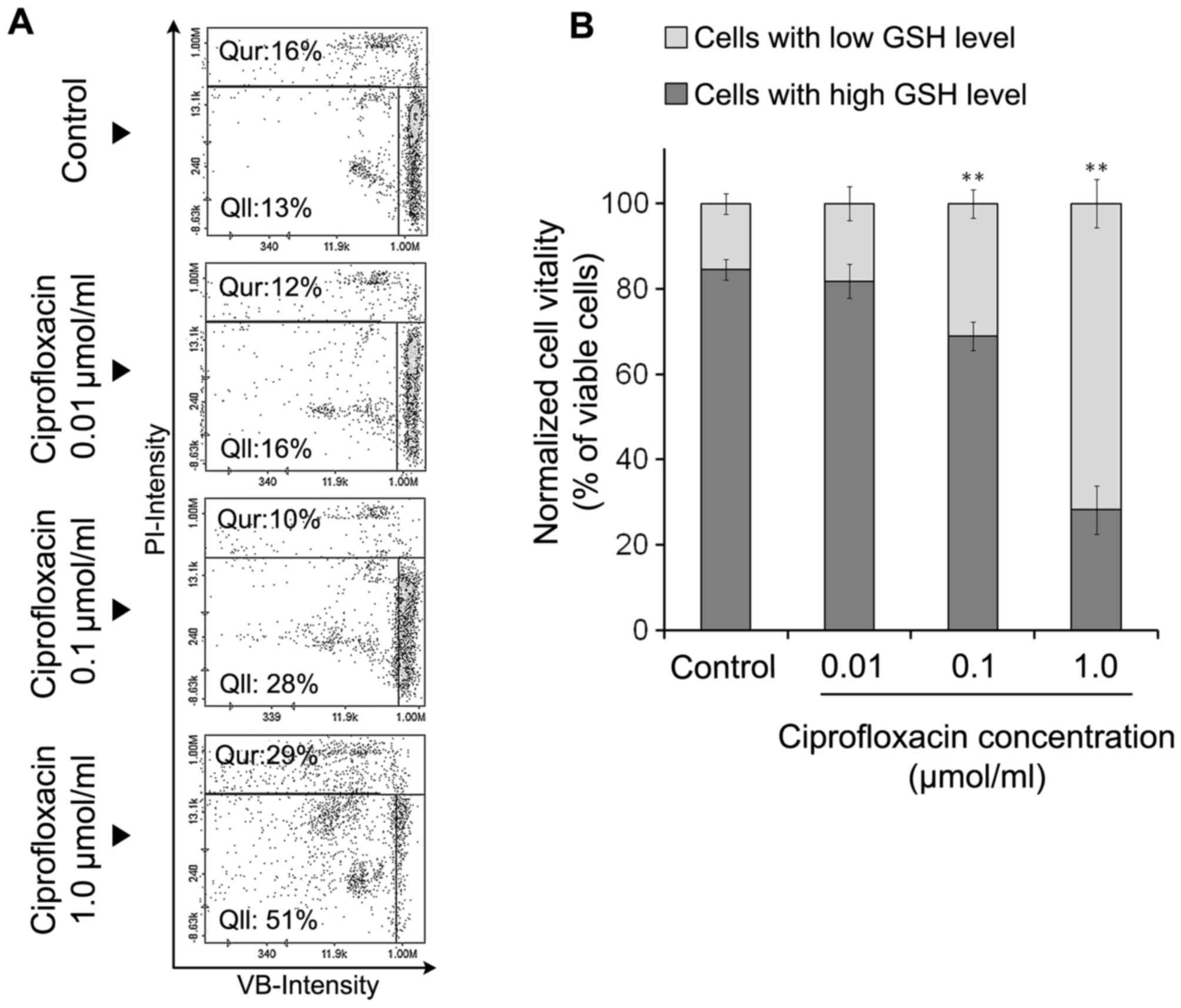
The effect of ciprofloxacin on cellular
GSH levels
There is a strong correlation between cellular GSH
depletion and the progression of apoptosis (22). In the present study, MDA-MB-231
cells were stained with three different reagents: A stain for all
nucleated cells (AO), a stain staining dead cells alone (PI) and
VB-48™, a stain that stained all viable cells in an
intensity-dependent manner dependent on GSH levels. The results
indicated that ciprofloxacin decreased GSH levels in MDA-MB-231
cells (Fig. 3A and B). Following
the exposure of MDA-MB-231 cells to 0.01, 0.1 and 1.0
µmol/ml ciprofloxacin for 24 h, the percentage of
PI-negative/VB-48™-negative cells exhibiting low levels of GSH
increased from 13% (control) to 16±2, 28±4 and 51±5%, respectively.
Treatment of MDA-MB-231 cells with the highest concentration of
ciprofloxacin (1.0 µmol/ml) for 24 h increased the
percentage of PI-positive cells (dead cells) from 16±2% (control)
to 29±3%. The exposure of cells to lower concentrations of
ciprofloxacin (0.01 and 0.1 µmol/ml) had no significant
effect on the percentage of PI positive cells compared with the
control (Fig. 3A). According to
the quantitative analysis of image cytometry data (Fig. 3B), 24-h incubation of MDA-MB-231
cells with the drug in concentration of 1.0 µmol/ml resulted
in a dramatic increase in the ratio of viable cells with low GSH
levels to viable cells with high GSH levels.
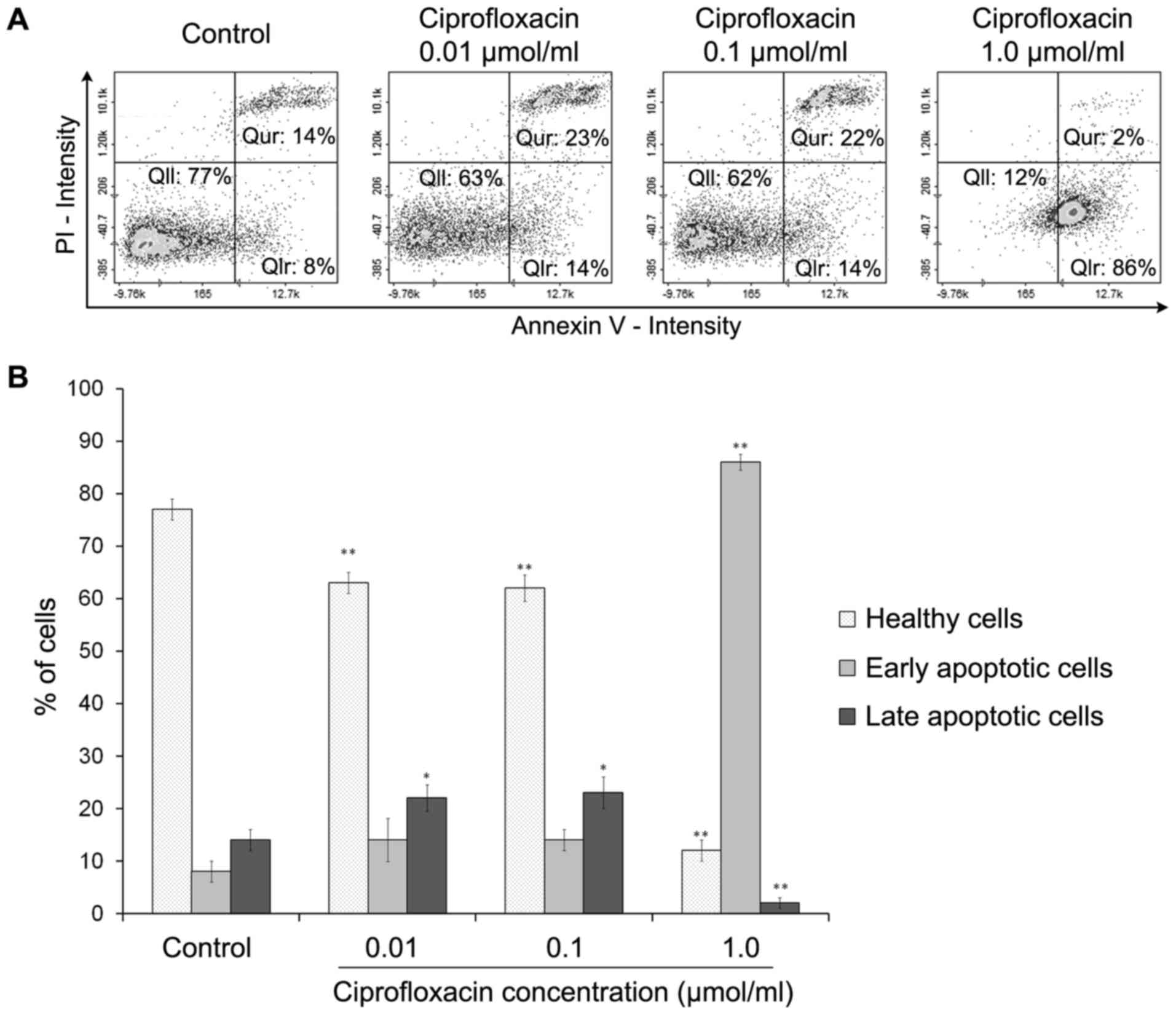
Ciprofloxacin induces the apoptosis of
MDA-MB-231 cells
Cell apoptosis was estimated using an Annexin V
assay. Phosphatidylserine, which is normally located in the inner
leaflet of the plasma membrane, is exported to the outer plasma
membrane leaflet during apoptosis. Annexins are a group of cellular
proteins that bind to phospholipids, such as phosphatidylserine
(26). By conjugating a
fluorescent label to Annexin V it is possible to identify and
quantify apoptotic cells. Annexin V also binds to
phosphatidylserine in late apoptotic cells, but as the membrane
integrity of these cells is lost, they may be distinguished from
early apoptotic cells using PI. The exposure of MDA-MB-231 cells to
ciprofloxacin induced apoptosis (Fig.
4). Following treatment of cells with 0.01 and 0.1
µmol/ml ciprofloxacin for 24 h, the proportion of early
apoptotic (Annexin V-positive/PI-negative) cells increased from
8±2% (control) to 14±3%. The increase in apoptosis was significant
following the exposure of cells to the highest concentration of
ciprofloxacin (1.0 µmol/ml); the percentage of early
apoptotic cells increased from 8±2% (control) to 86±4%. The
treatment of MDA-MB-231 cells with 0.01 and 0.1 µmol/ml
ciprofloxacin significantly increased the percentages of late
apoptotic (Annexin V-positive/PI-positive) cells by ~9±2% compared
with the controls. However, treatment with 1.0 µmol/ml
ciprofloxacin significantly decreased the proportion of late
apoptotic cells compared with the control (Fig. 4B).
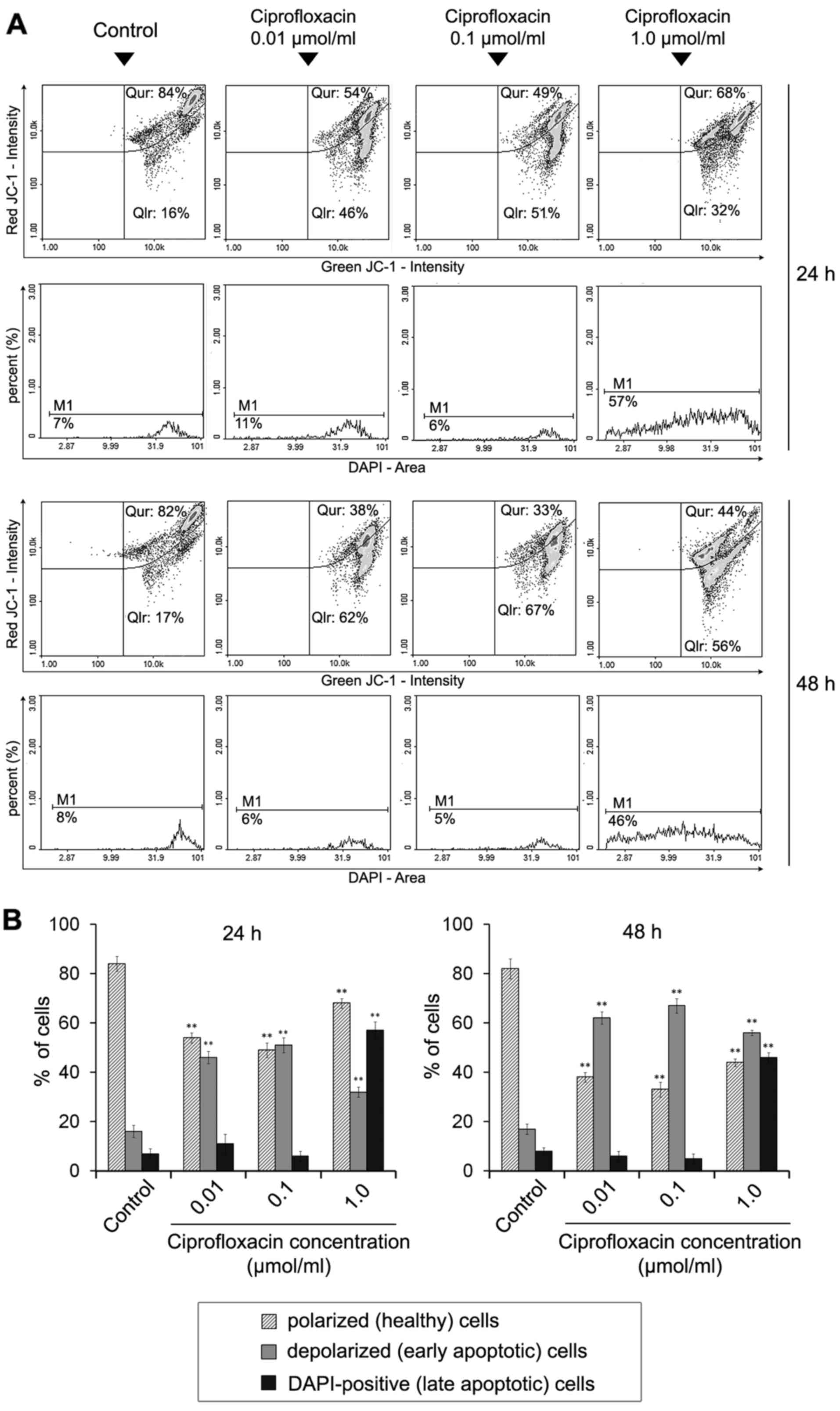
The effect of ciprofloxacin on the
mitochondrial membrane potential in MDA-MB-231 cells
Dysregulation of the mitochondrial potential is an
event that occurs early on in apoptosis (26). To detect apoptosis-associated
alterations in the mitochondrial membrane in ciprofloxacin-treated
breast cancer cells, staining with the lipophilic cationic dyes
JC-1 and DAPI were performed, followed by image cytometric
analysis. In polarized (healthy) cells, the negative charge
established by the intact mitochondrial membrane potential
facilitates the accumulation of JC-1 in the mitochondrial matrix,
whereas in depolarized (early-apoptotic) cells, JC-1 localizes to
the cytosol in its monomeric form (26). Cellular JC-1 aggregates and
monomers were detected as red and green fluorescence, respectively.
A decrease in the red/green fluorescence intensity ratio indicated
the induction of apoptosis and mitochondrial depolarization.
Late-apoptotic cells were detected as blue fluorescent
(DAPI-positive) cells (Fig. 5A).
Following image cytometric analysis (Fig. 5B), the percentages of mitochondrial
membrane depolarized cells following treatment with 0.01, 0.1 and
1.0 µmol/ml ciprofloxacin for 24 h was significantly
increased and determined to be 46±4, 51±5 and 32±3%, respectively,
compared with 16±2% in control cells. The effect was markedly
increased following an increase in the incubation time to 48 h; the
proportions of mitochondrial membrane depolarized cells following
treatment with 0.01, 0.1 and 1.0 µmol/ml ciprofloxacin were
62±4, 67±2 and 56±4%, while the value determined for the control
was 17±2%. The results indicate that ciprofloxacin increases the
proportion of membrane-depolarized cells in a dose- and
time-dependent manner.
A significant increase in blue DAPI fluorescence was
observed following exposure of MDA-MB-231 cells to 1.0
µmol/ml ciprofloxacin compared with the control, indicating
the induction of late apoptosis. The percentage of late apoptotic
(DAPI-positive) cells were 57±3 and 46±2% following 24 and 48 h
incubation, respectively. The values determined for the controls
were 7±1 and 8±2%, respectively.
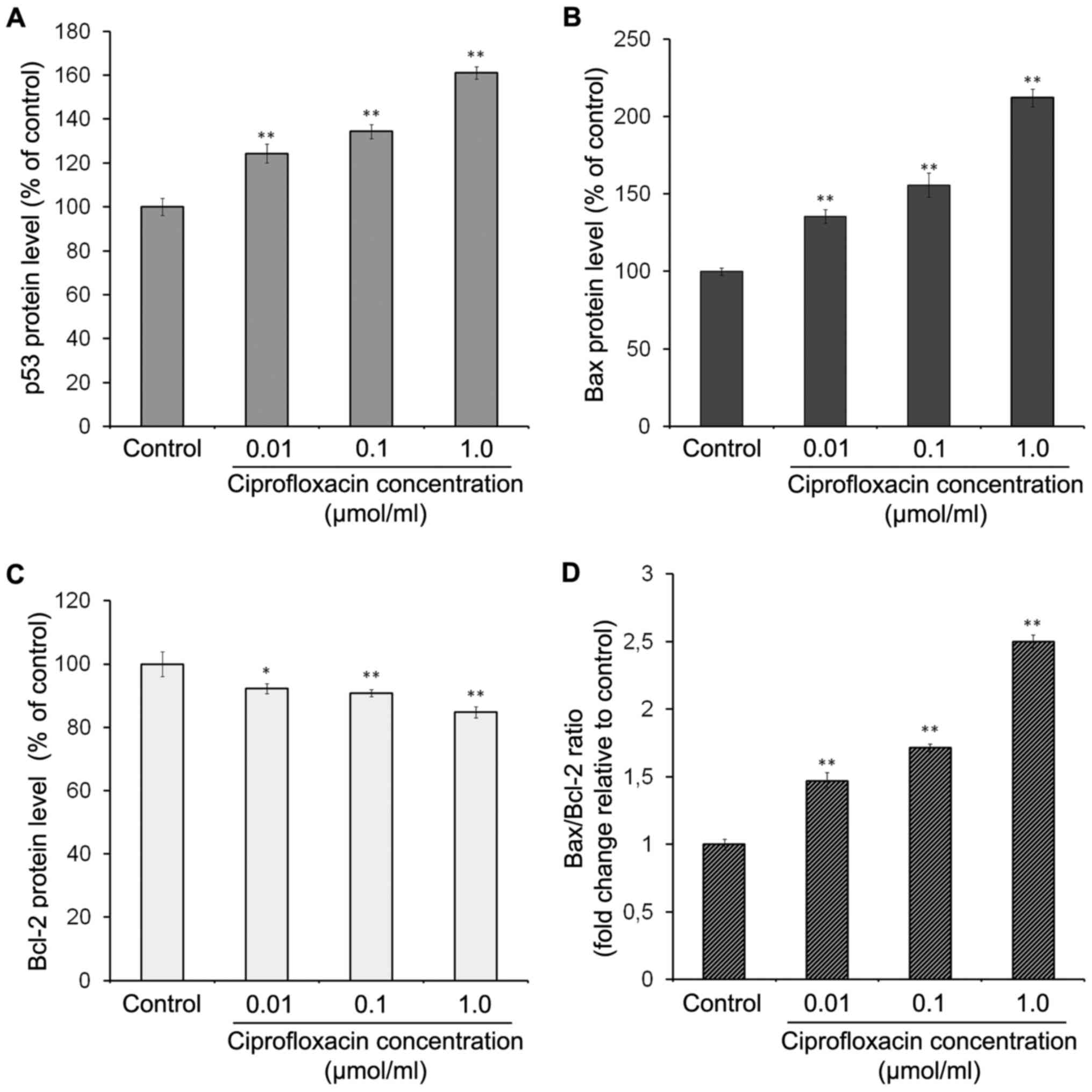
The effect of ciprofloxacin on the
expression of apoptotic proteins in MDA-MB-231 cells
To characterize the signaling pathways involved in
ciprofloxacin-induced apoptosis, the expression of p53, Bax and
Bcl-2 proteins was measured. p53 is a tumor suppressor protein,
which can induce apoptosis in response to various stress signals,
including irradiation, DNA damage and chemotherapeutic agents
(29). The results of ELISA
demonstrated that ciprofloxacin significantly enhanced the
expression of p53 in a concentration-dependent manner (Fig. 6A). Following exposure of MDA-MB-231
cells to 0.01, 0.1 and 1.0 µmol/ml ciprofloxacin for 24 h,
the expression of p53 increased by 24±3, 34±2 and 61±5%,
respectively, compared with the controls.
The Bcl-2 protein family serves an
important role in mitochondria-dependent apoptosis and regulates
the mitochondrial membrane potential
Bax proteins are pro-apoptotic and Bcl-2 proteins
are anti-apoptotic (26,29). The results of ELISA demonstrated
that ciprofloxacin significantly enhanced Bax expression in a
concentration-dependent manner (Fig.
6B). Following treatment of MDA-MB-231 cells with 0.01, 0.1 and
1.0 µmol/ml ciprofloxacin for 24 h, the expression of Bax
increased by 35±4, 56±3 and 112±6%, respectively, compared with the
control (Fig. 6B). By contrast,
the same concentrations of ciprofloxacin suppressed the expression
of Bcl-2 by 8±1, 10±2 and 15±2%, respectively, compared with the
control (Fig. 6C). Consequently,
the Bax/Bcl-2 ratio significantly increased following treatment
with ciprofloxacin in a concentration-dependent manner (Fig. 6D).
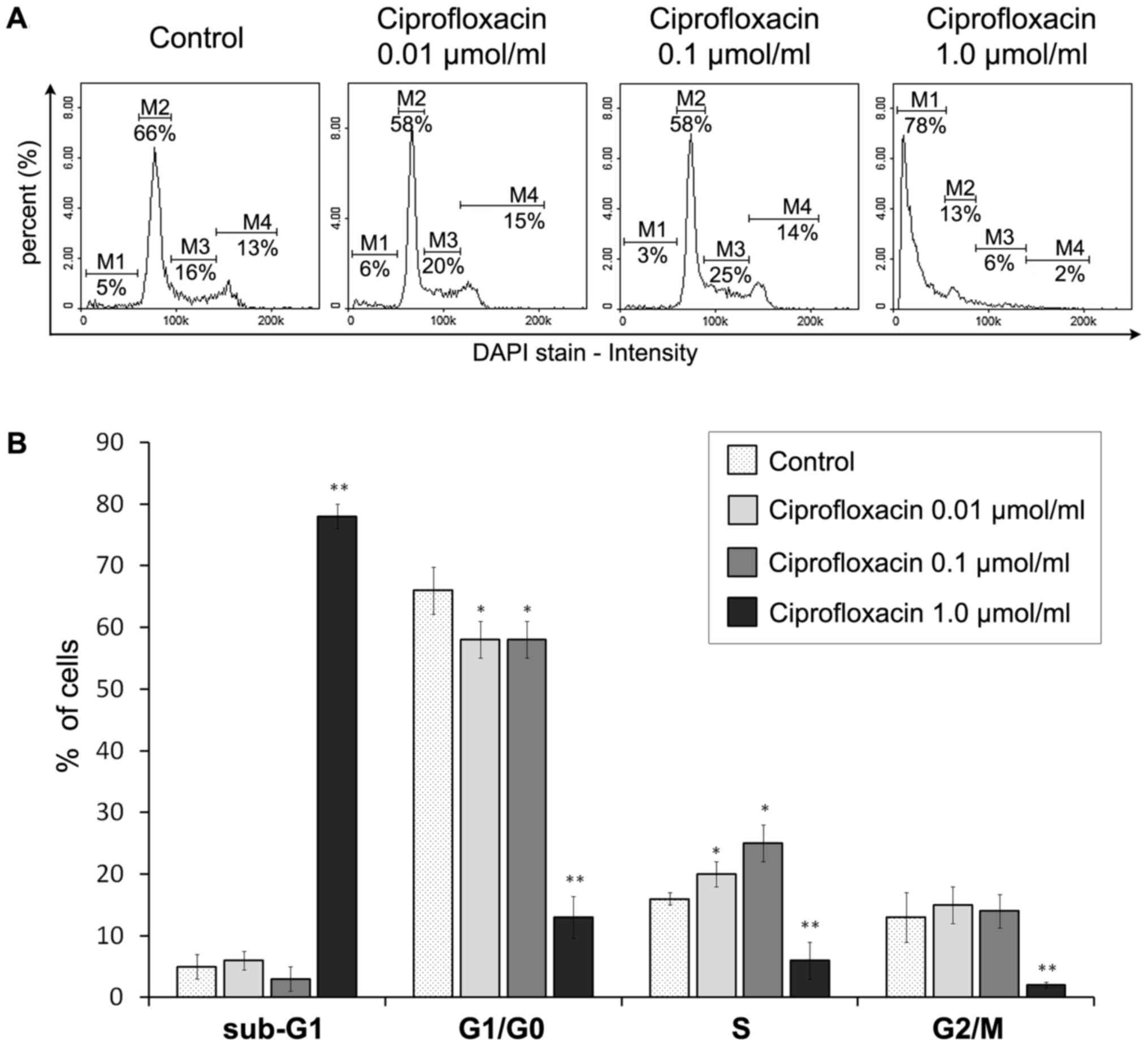
Ciprofloxacin mediates S-phase arrest and
DNA fragmentation in MDA-MB-231 cells
The impact of ciprofloxacin on the MDA-MB-231 breast
cancer cell cycle was assessed using a fluorescence image
cytometer. Based on the measurement of DNA content in individual
cells from the cell population, the proportion of cells occupying
the four main phases of the cell cycle was estimated (Fig. 7). Treatment with 0.01 and 0.1
µmol/ml ciprofloxacin for 24 h significantly increased the
proportion of cells in the S-phase from 16±2% in the control to
20±2 and 25±3%, respectively (Fig.
7B). This indicates that ciprofloxacin induces S-phase arrest
in MDA-MB-231 cells. Furthermore, ciprofloxacin induced DNA
fragmentation, a late event in the apoptosis pathway. This
phenomenon was only identified following treatment of cells with
1.0 µmol/ml ciprofloxacin, where the proportion of cells in
the sub-G1 phase (having less than one DNA equivalent)
significantly increased from 5±1 to 78±3%. Furthermore, there was a
significant decrease in the proportion of cells in the
G1/G0 and G2/M phases, from 66±2
to 13±3% and from 13±2 to 2±1%, respectively.
Discussion
Breast cancer is the most common malignancy and the
second most common cause of cancer-associated mortality among women
worldwide (30). Current methods
of treating breast cancer, such as chemotherapy, often cause the
development of high systemic toxicity and drug resistance, leading
to the therapeutic failure (3).
Fluoroquinolones are broad-spectrum synthetic
antibiotics widely used to treat various infections (16). Certain members of these antibiotics
exhibit antitumor activity in vitro in a number of different
cancer cell lines (12,13,15,31)
and also in vivo (32).
This antitumor activity was linked to the inhibition of the
eukaryotic analogue of DNA gyrase, topoisomerase IIα activity. To
the best of our knowledge, the current study was the first to
determine the impact of ciprofloxacin on cell viability, GSH
levels, the apoptosis pathway and cell cycle distribution in human
triple-negative MDA-MB-231 breast cancer cells.
Ciprofloxacin decreased the viability of MDA-MB-231
breast cancer cells in a time- and concentration-dependent manner.
Following 24 h incubation with 0.01–1.0 µmol/ml
ciprofloxacin, the viability of MDA-MB-231 decreased by 47%
compared with the control. The cytotoxic effect intensified with
the duration of incubation: 1.0 µmol/ml ciprofloxacin
decreased the viability of MDA-MB-231 cells by 67 and 74% compared
with the control following incubation for 48 and 72 h,
respectively. Microscopic analysis indicated that MDA-MB-231 cells
became rounded and lost their cell-cell contact following treatment
with ciprofloxacin. Characteristic morphological features of
apoptosis, including cell shrinkage, were also observed.
The cytotoxic effects of ciprofloxacin in A549 human
non-small cell lung cancer, C6 rat glioblastoma and B16 mouse
melanoma cell lines were demonstrated by Kloskowski et al
(31). The authors demonstrated
that ciprofloxacin at concentrations of 0.4 and 0.3 µmol/ml
(in A549 cells), 16.2 and 2.9 µmol/ml (in C6 cells), and 1.1
and 0.2 µmol/ml (in B16 cells) caused a 50% decrease in cell
viability following 24 and 48 h incubation, respectively. The
results of the present study detected a 50% decrease in the
viability of MDA-MB-231 cells following 24 and 48 h incubation with
0.83 µmol/ml and 0.14 µmol/ml ciprofloxacin,
respectively. This indicates that ciprofloxacin induces more
cytotoxicity in TNBC MDA-MB-231 cells than in A549, C6 and B16
cells.
Previous studies have demonstrated that
fluoroquinolones, including lomefloxacin (33), norfloxacin and moxifloxacin
(34) and sparfloxacin (35) may alter the activity of the
cellular antioxidant enzymes superoxide dismutase, catalase and GSH
peroxidase. It has therefore been hypothesized that
fluoroquinolones may induce cellular oxidative stress by triggering
the generation of ROS, particularly the superoxide radical anion
and hydrogen peroxide.
GSH, the most abundant cellular thiol and the
primary determinant of cellular redox homeostasis, is an important
mediator in apoptotic pathways (36,37).
Elevated GSH levels may disrupt apoptosis either by facilitating
DNA repair or by buffering oxidative stress. Therefore, GSH
depletion increases the sensitivity of cells to apoptosis induced
by various chemical agents and radiation (38,39).
The present study examined whether the cytotoxic response of
MDA-MB-231 cells following ciprofloxacin treatment may be
associated with decreases in intracellular GSH levels.
Ciprofloxacin at concentrations 0.1 and 1.0 µmol/ml caused
2- and 4-fold decreases in the proportion of cells exhibiting
reduced GSH levels. These results suggest that ciprofloxacin may
trigger apoptosis in MDA-MB-231 cells by decreasing intracellular
thiol levels.
In the present study, Annexin V/PI double staining
was performed to determined whether ciprofloxacin induces apoptosis
in MDA-MB-231 breast cancer cells. The proportion of apoptotic
cells, including early (Annexin V-positive/PI-negative) and late
(Annexin V-positive/PI-positive) apoptotic cells, was increased
following the exposure of cells to high concentrations of
ciprofloxacin. A 4-fold increase in the proportion of apoptotic
cells occurred following treatment with 1.0 µmol/ml
ciprofloxacin.
To the best of our knowledge, there have been no
previous studies investigating the effect of fluoroquinolones on
human TNBC cells. It is hypothesized that fluoroquinolones inhibit
bacterial type II topoisomerase (DNA gyrase), however there is also
evidence to suggest that they may affect the viability of cells,
including cancer cells (12,13,33–35).
The anticancer activity of topoisomerase inhibitors may potentially
occur via the inhibition of mitochondrial DNA synthesis, which
subsequently induces mitochondrial injury, disorders in the
respiratory chain and depletion of intracellular ATP stores. Energy
depletion favors apoptosis, as it may induce cell cycle arrest in
the S- and/or G2/M phases (26).
The present study investigated the
mitochondria-associated events that occur during apoptosis in
MDA-MB-231 cells following treatment with ciprofloxacin. It was
demonstrated that ciprofloxacin induces apoptosis in breast cancer
cells due to mitochondrial membrane breakdown. Treatment with 0.01,
0.1 and 1.0 µmol/ml ciprofloxacin for 24 h increased the
proportion of depolarized/apoptotic cells by 30, 35 and 16%,
respectively, compared with the control. This effect was more
pronounced when the cells were treated with the drug for 48 h; the
proportion of depolarized/apoptotic cells reached 50%. Furthermore,
the proportion of late apoptotic cells increased following
treatment with ciprofloxacin, reaching a peak following treatment
with 1.0 µmol/ml ciprofloxacin for 24 h. Similar results
were obtained by Herold et al (11), where the cytotoxic response of
human colorectal carcinoma cells to ciprofloxacin treatment was
mediated by the mitochondrial apoptosis pathway.
It has been reported that one of the key responses
of drug-induced DNA damage is the expression of p53 that leads to
induction of apoptosis via the intrinsic mitochondrial pathway
(26,29). The results demonstrated that
ciprofloxacin induces apoptosis in MDA-MB-231 cells and that this
was accompanied by the upregulation of p53 expression. This
suggests that the activation of p53 pathway may be involved in the
apoptosis of MDA-MB-231 cells following treatment with
ciprofloxacin. It was also observed that ciprofloxacin mediates the
upregulation of Bax and downregulation of Bcl-2 expression, thus
inducing apoptosis. Therefore, ciprofloxacin may stimulate the
opening of the mitochondrial permeability transition pores via the
Bax/Bcl-2-dependent pathway.
It has been demonstrated that cell cycle regulation
is a method of regulating cell growth (26). Therefore, anticancer therapies may
be used to block the cancer cell cycle. In the present study,
fluorescence image cytometer analysis revealed that lower
concentrations of ciprofloxacin (0.01 and 0.1 µmol/ml)
induced S-phase cell cycle arrest in MDA-MB-231 cells, suggesting
that this occurs via topoisomerase II inhibition. It has been
demonstrated by Kloskowski et al (31) that in human non-small lung cancer
cells, ciprofloxacin induces cell cycle arrest at the
G2/M checkpoint. Therefore, different molecular
mechanisms of drug action may dominate depending on the cell type
and origin. Furthermore, different molecular pathways may be
activated by various fluoroquinolone derivatives in the same cell
line (40).
The results of the Annexin V assay indicated that
ciprofloxacin increased the proportion of apoptotic cells. To
determine whether this increase was also triggered by
apoptosis-associated DNA fragmentation, the induction of DNA
fragmentation in MDA-MB-231 cells following exposure to
ciprofloxacin was investigated. The highest concentration of
ciprofloxacin (1.0 mM) induced oligonucleosomal DNA fragmentation
(the presence of sub-G1 fraction), strongly suggesting
that apoptosis was induced via the p53-dependent pathway. These
results are consistent with the results of our recent study, which
revealed that ciprofloxacin mediates the induction of S-phase
arrest and apoptosis in COLO829 melanoma cells (41). They are also consistent with the
results of study by Yadav et al (13), which identified the ability of
ciprofloxacin to induce DNA fragmentation and S-phase arrest in
human pancreatic cancer cells.
Serum concentrations of ciprofloxacin in humans
following two oral doses of 750 mg are ~10-fold lower (42) than the concentrations that have a
significant cytotoxic and pro-apoptotic effect on the MDA-MB-231
cell line. However, concentrations of ciprofloxacin in the targeted
tissues may exceed those in the serum. Indeed, the concentration of
ciprofloxacin following oral administration is up to 7 times higher
in the lung tissue than in the serum (43). Furthermore, according to the
results of our previous study, ciprofloxacin forms complexes with
melanin and therefore this drug may accumulate in tissues
containing high levels of melanin (44). Melanin biopolymers are present not
only in the basal layer of the epidermis, but also in the outer
parts of the breast, including the nipple and areola, hair
follicles, uveal tract of the eye, the inner ear and central
nervous system (45). In most
cases of breast cancer in which the dermo-epidermal junction is
breached, the accumulation of melanin within breast tumors may
occur due to colonization by melanocytes, which are the cells
responsible for melanin synthesis (46). Therefore, it is possible that
ciprofloxacin concentrations in breast cancer cells may be
significantly higher than in the serum and therefore a cytotoxic
response, as well as the induction of apoptosis in the presence of
this drug, may occur.
In conclusion, to the best of our knowledge, the
present study is the first to indicate that ciprofloxacin induces
concentration- and time-dependent decreases in human MDA-MB-231
breast cancer cell viability, induces apoptosis via the
p53/Bax/Bcl-2 signaling pathway and induces S-phase cell cycle
arrest. This suggests a mechanism of eukaryotic topoisomerase
poisoning. The results of the present study provide important
molecular data concerning the cellular cascade, which may explain
the cytotoxic effects of ciprofloxacin on human TMBC cells and may
provide a novel insight into the therapeutic properties of
ciprofloxacin. Further in vivo studies are required to
determine the potential use of ciprofloxacin to treat TNBC.
Acknowledgments
Not applicable
Notes
[1]
Funding
The present study was supported by the Medical
University of Silesia Grant no. KNW-2-007/N/7/K.
[2] Availability
of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
[3] Author
contributions
AB conceived, designed, performed the experiments
and wrote the paper; JR, performed the experiments, DW, ZR and MR
performed the experiments and analyzed the data; EB conceived and
designed the experiments. All authors have read and approved the
final manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-An update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar
|
|
2
|
Yao H, He G, Yan S, Chen C, Song L, Rosol
TJ and Deng X: Triple-negative breast cancer: Is there a treatment
on the horizon? Oncotarget. 8:1913–1924. 2017.
|
|
3
|
Anders C and Carey LA: Understanding and
treating triple-negative breast cancer. Oncology (Williston Park).
22:1233–1240. 12432008.
|
|
4
|
Wahba HA and El-Hadaad HA: Current
approaches in treatment of triple-negative breast cancer. Cancer
Biol Med. 12:106–116. 2015.PubMed/NCBI
|
|
5
|
Wang JC: Cellular roles of DNA
topoisomerases: A molecular perspective. Nat Rev Mol Cell Biol.
3:430–440. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tse-Dinh YC: Exploring DNA topoisomerases
as targets of novel therapeutic agents in the treatment of
infectious diseases. Infect Disord Drug Targets. 7:3–9. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaur P, Kaur V and Kaur S: DNA
Topoisomerase II: promising target for anticancer drugs.
Multi-Targeted Approach to Treatment of Cancer. Springer; pp.
323–338. 2015
|
|
8
|
Cowell IG and Austin CA: Mechanism of
generation of therapy related leukemia in response to
anti-topoisomerase II agents. Int J Environ Res Public Health.
9:2075–2091. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aldred KJ, Kerns RJ and Osheroff N:
Mechanism of quinolone action and resistance. Biochemistry.
53:1565–1574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seo K, Holt R, Jung YS, Rodriguez CO Jr,
Chen X and Rebhun RB: Fluoroquinolone-mediated inhibition of cell
growth, S-G2/M cell cycle arrest, and apoptosis in canine
osteosarcoma cell lines. PLoS One. 7:e429602012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herold C, Ocker M, Ganslmayer M, Gerauer
H, Hahn EG and Schuppan D: Ciprofloxacin induces apoptosis and
inhibits proliferation of human colorectal carcinoma cells. Br J
Cancer. 86:443–448. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yadav V, Sultana S, Yadav J and Saini N:
Gatifloxacin induces S and G2-phase cell cycle arrest in pancreatic
cancer cells via p21/p27/p53. PLoS One. 7:e477962012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yadav V, Varshney P, Sultana S, Yadav J
and Saini N: Moxifloxacin and ciprofloxacin induces S-phase arrest
and augments apoptotic effects of cisplatin in human pancreatic
cancer cells via ERK activation. BMC Cancer. 15:5812015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aranha O, Grignon R, Fernandes N,
McDonnell TJ, Wood DP Jr and Sarkar FH: Suppression of human
prostate cancer cell growth by ciprofloxacin is associated with
cell cycle arrest and apoptosis. Int J Oncol. 22:787–794.
2003.PubMed/NCBI
|
|
15
|
Aranha O, Wood DP Jr and Sarkar FH:
Ciprofloxacin mediated cell growth inhibition, S/G2-M cell cycle
arrest, and apoptosis in a human transitional cell carcinoma of the
bladder cell line. Clin Cancer Res. 6:891–900. 2000.PubMed/NCBI
|
|
16
|
Oliphant CM and Green GM: Quinolones: A
comprehensive review. Am Fam Physician. 65:455–464. 2002.PubMed/NCBI
|
|
17
|
Talla V and Veerareddy P: Oxidative stress
induced by fluoroquinolones on treatment for complicated urinary
tract infections in Indian patients. J Young Pharm. 3:304–309.
2011. View Article : Google Scholar
|
|
18
|
Bisacchi GS and Hale MR: A 'Double-Edged'
scaffold: Antitumor power within the antibacterial quinolone. Curr
Med Chem. 23:520–577. 2016. View Article : Google Scholar :
|
|
19
|
Arriola E, Marchio C, Tan DS, Drury SC,
Lambros MB, Natrajan R, Rodriguez-Pinilla SM, Mackay A, Tamber N,
Fenwick K, et al: Genomic analysis of the HER2/TOP2A amplicon in
breast cancer and breast cancer cell lines. Lab Invest. 88:491–503.
2008. View Article : Google Scholar
|
|
20
|
Żaczek AJ, Markiewicz A, Seroczyńska B,
Skokowski J, Jaśkiewicz J, Pieńkowski T, Olszewski WP, Szade J,
Rhone P, Welnicka-Jaskiewicz M, et al: Prognostic significance of
TOP2A gene dosage in HER-2-negative breast cancer. Oncologist.
17:1246–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shyur LF, Lee SH, Chang ST, Lo CP, Kuo YH
and Wang SY: Taiwanin A inhibits MCF-7 cancer cell activity through
induction of oxidative stress, upregulation of DNA damage
checkpoint kinases, and activation of p53 and FasL/Fas signaling
pathways. Phytomedicine. 18:16–24. 2010. View Article : Google Scholar
|
|
22
|
Panieri E and Santoro MM: ROS homeostasis
and metabolism: A dangerous liason in cancer cells. Cell Death Dis.
7:e22532016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blaydes JP, Craig AL, Wallace M, Ball HM,
Traynor NJ, Gibbs NK and Hupp TR: Synergistic activation of
p53-dependent transcription by two cooperating damage recognition
pathways. Oncogene. 19:3829–3839. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Burns TF and El-Deiry WS: The p53 pathway
and apoptosis. J Cell Physiol. 181:231–239. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pietenpol JA and Stewart ZA: Cell cycle
checkpoint signaling: Cell cycle arrest versus apoptosis.
Toxicology. 181–182:475–481. 2002. View Article : Google Scholar
|
|
26
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chavez KJ, Garimella SV and Lipkowitz S:
Triple negative breast cancer cell lines: One tool in the search
for better treatment of triple negative breast cancer. Breast Dis.
32:35–48. 2010. View Article : Google Scholar
|
|
28
|
Beberok A, Wrześniok D, Szlachta M, Rok J,
Rzepka Z, Respondek M and Buszman E: Lomefloxacin induces oxidative
stress and apoptosis in COLO829 melanoma cells. Int J Mol Sci.
18:E21942017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bai L and Wang S: Targeting apoptosis
pathways for new cancer therapeutics. Annu Rev Med. 65:139–155.
2014. View Article : Google Scholar
|
|
30
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
31
|
Kloskowski T, Gurtowska N, Olkowska J,
Nowak JM, Adamowicz J, Tworkiewicz J, Dębski R, Grzanka A and Drewa
T: Ciprofloxacin is a potential topoisomerase II inhibitor for the
treatment of NSCLC. Int J Oncol. 41:1943–1949. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thadepalli H, Salem F, Chuah SK and
Gollapudi S: Antitumor activity of trovafloxacin in an animal
model. In Vivo. 19:269–276. 2005.PubMed/NCBI
|
|
33
|
Beberok A, Buszman E, Otręba M and
Wrześniok D: Impact of lomefloxacin on antioxidant enzymes activity
in normal melanocytes HEMa-LP. Curr Issues Pharm Med Sci.
25:426–429. 2012. View Article : Google Scholar
|
|
34
|
Beberok A, Wrześniok D, Otręba M, Miliński
M, Rok J and Buszman E: Effect of norfloxacin and moxifloxacin on
melanin synthesis and antioxidant enzymes activity in normal human
melanocytes. Mol Cell Biochem. 401:107–114. 2015. View Article : Google Scholar :
|
|
35
|
Beberok A, Wrześniok D, Otręba M and
Buszman E: Impact of sparfloxacin on melanogenesis and antioxidant
defense system in normal human melanocytes HEMa-LP - An in vitro
study. Pharmacol Rep. 67:38–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hall AG: Review: The role of glutathione
in the regulation of apoptosis. Eur J Clin Invest. 29:238–245.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mirkovic N, Voehringer DW, Story MD,
McConkey DJ, McDonnell TJ and Meyn RE: Resistance to
radiation-induced apoptosis in Bcl-2-expressing cells is reversed
by depleting cellular thiols. Oncogene. 15:1461–1470. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dai J, Weinberg RS, Waxman S and Jing Y:
Malignant cells can be sensitized to undergo growth inhibition and
apoptosis by arsenic trioxide through modulation of the glutathione
redox system. Blood. 93:268–277. 1999.
|
|
40
|
Blau H, Klein K, Shalit I, Halperin D and
Fabian I: Moxifloxacin but not ciprofloxacin or azithromycin
selectively inhibits IL-8, IL-6, ERK1/2, JNK, and NF-kappaB
activation in a cystic fibrosis epithelial cell line. Am J Physiol
Lung Cell Mol Physiol. 292:L343–L352. 2007. View Article : Google Scholar
|
|
41
|
Beberok A, Wrześniok D, Minecka A, Rok J,
Delijewski M, Rzepka Z, Respondek M and Buszman E:
Ciprofloxacin-mediated induction of S-phase cell cycle arrest and
apoptosis in COLO829 melanoma cells. Pharmacol Rep. 70:6–13. 2017.
View Article : Google Scholar
|
|
42
|
Shah A, Lettieri J, Kaiser L, Echols R and
Heller AH: Comparative pharmacokinetics and safety of ciprofloxacin
400 mg i.v. thrice daily versus 750 mg po twice daily. J Antimicrob
Chemother. 33:795–801. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rohwedder R, Bergan T, Caruso E,
Thorsteinsson SB, Della Torre H and Scholl H: Penetration of
ciprofloxacin and metabolites into human lung, bronchial and
pleural tissue after 250 and 500 mg oral ciprofloxacin.
Chemotherapy. 37:229–238. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Beberok A, Buszman E, Wrześniok D, Otręba
M and Trzcionka J: Interaction between ciprofloxacin and melanin:
The effect on proliferation and melanization in melanocytes. Eur J
Pharmacol. 669:32–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
d'Ischia M, Wakamatsu K, Cicoira F, Di
Mauro E, Garcia-Borron JC, Commo S, Galván I, Ghanem G, Kenzo K,
Meredith P, et al: Melanins and melanogenesis: From pigment cells
to human health and technological applications. Pigment Cell
Melanoma Res. 28:520–544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wyatt AJ, Agero ALC, Delgado R, Busam KJ
and Marghoob AA: Cutaneous metastatic breast carcinoma with
melanocyte colonization: A clinical and dermoscopic mimic of
malignant melanoma. Dermatol Surg. 32:949–954. 2006.PubMed/NCBI
|