Introduction
Breast cancer is one of the most commonly diagnosed
cancers in females worldwide (1).
The estimated new cases of female breast cancer and associated
mortalities were 252,710 and 40,610, respectively, in the United
States in 2017 (2) and 268,600 and
69,500, respectively, in China in 2015 (3). Among American women, there were an
estimated 231,840 new cases of invasive breast cancer diagnosed in
2015 (4). The majority of breast
cancer cases are histologically invasive ductal carcinoma (IDC;
also known as infiltrating ductal carcinoma) (5), followed by the invasive lobular
cancer subtype (6). Despite
improvements to various treatment methods, there remains a lack of
effective ways to prevent and cure this malignant disease.
Therefore, identifying specific biomarkers and therapeutic targets
of breast cancer at an early stage is of particular importance.
Collagen type V α1 chain (COL5A1) is a minor
fibrillar collagen which has previously been reported to be
associated with embryonic development, fibrillogenesis and
Ehlers-Danlos syndrome (7). COL5A1
forms a heterotrimer (one α1 and two α2) (8) and usually co-polymerizes with type I
collagen to adjust the diameter of the collagen molecules (9). Overexpression of the COL5A1
gene has been observed in certain pathological conditions,
including inflammation and atherosclerosis (10). However, previous studies have
suggested that COL5A1 may be involved in tumor initiation and
progression in several types of malignant tumor. For example, the
expression of COL5A1 is increased in tongue squamous cell carcinoma
and ovarian cancer, and is associated with certain clinical
characteristics (11,12). Furthermore, COL5A1 has been
identified as a biomarker of human gastric cancer using gene
expression profiling (13) and
RNA-sequencing has revealed that it is associated with the
extracellular matrix (ECM) degradation pathways in papillary
thyroid carcinoma (14). More
recently, comprehensive bioinformatics analysis has revealed that
COL5A1 is one of the key genes between patients with
inflammatory and non-inflammatory breast cancer (15). The collagen family is also a
promising prognostic marker for patients with cancer (11) and contributes to cancer progression
by participating in the ECM-receptor interaction pathway (16). Furthermore, in osteoblasts, COL5A1
has been revealed to be mediated by transforming growth factor-β
(TGF-β) (17), a cytokine that
participates in the invasive progression of breast cancer (18). However, the function of COL5A1 in
IDC of the breast and whether COL5A1 is regulated by TGF-β in IDC
remains unclear.
The present study was performed to detect the
expression of COL5A1 in human IDC compared with its adjacent normal
tissue and fibroadenoma of the breast. The loss-of-function of
COL5A1 and the regulation of COL5A1 protein expression by TGF-β1
were also investigated in breast cancer cells.
Materials and methods
Human subjects and tissue sample
preparation
Informed consent was obtained from patients, and the
present study was approved by the Ethics Committee of Jinshan
Hospital, Fudan University (Shanghai, China). A total of 180
paraffin-embedded breast tissue samples (90 cases of IDC and 90
cases of fibroadenoma) were collected at Jinshan Hospital between
January 2010 and December 2015. The median age of patients was 54
years (range, 33-85 years). No patients underwent chemotherapy and
radiotherapy prior to cytoreductive surgery. The IDC and
fibroadenoma tissues were diagnosed by pathologists.
Immunohistochemical (IHC) staining and
analysis
IHC analysis was performed as previously described
(19). Briefly, the 4%
paraformaldehyde-fixed, paraffin-embedded tissue specimens were
sectioned (4 μm thick), deparaffinized in xylene and
rehydrated in a descending alcohol series. Following blocking for
30 min at room temperature with 10% normal goat serum (Fuzhou
Maixin Biotech Co., Ltd., Fuzhou, China; cat. no. SP KIT-B2), the
sections were incubated with monoclonal rabbit anti-COL5A1 (1:200
dilution; cat. no. SAB4500384, Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 4°C overnight. Following incubation with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary
antibody (1:200 dilution; cat. no. L3012; Signalway Antibody LLC,
College Park, MD, USA) for 1 h at room temperature, the signal was
detected using a DAB kit (Fuzhou Maixin Biotech Co., Ltd.; cat. no.
DAB-0031). Following counterstaining with hematoxylin, a photograph
was taken under a light microscope. The presence of brown color
within a cell was considered to be positive staining. The
immunoreactive staining of COL5A1 in the tissue of a section was
evaluated by two independent pathologists. COL5A1-low and -high
expression in the breast tissue was determined as previously
described (20,21).
Cell culture and TGF-β1
administration
Human breast non-tumorous MCF-12A and cancerous
MCF-7 cells were obtained from the American Type Culture Collection
(Manassas, VA, USA). MCF-12A and MCF-7 cells were cultured in
Dulbecco's modified Eagle's medium/F12 and RPMI-1640 media,
respectively. Following incubation for 24 h in 6-well plates, the
cells were treated with recombinant human TGF-β1 (10 ng/ml
rhTGF-β1; cat. no. 240-B; R&D Systems, Inc., Minneapolis, MN,
USA) at 37°C for 48 h. For the blocking assay, cells were
pretreated with an inhibitor of TGF-β type I receptor kinase (10
μM SB-431542, cat. no. S4317-5MG; Sigma-Aldrich; Merck KGaA)
at 37°C for 30 min, followed by treatment of rhTGF-β1 at 37°C for
48 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA in the tissues and cells was extracted
using TRIzol reagent (cat. no. 9109; Takara Biotechnology Co.,
Ltd., Dalian, China), according to the manufacturer's protocol.
Total RNA (1 μg) was reverse transcribed using a First
Strand cDNA Synthesis kit (cat. no. 04896866001; Roche Diagnostics,
Indianapolis, IN, USA). The primers were synthesized by BioTNT Co.,
Ltd. (Shanghai, China). The sequences of primers were as follows:
human COL5A1 forward, 5′-CTCCCTGCTTTCTTTATCCT-3′ and reverse,
5′-GAGTGTGCTTGGCTATCCTG-3′; human β-actin forward,
5′-AAGGTGACAGCAGTCGGTT-3′ and reverse, 5′-TGTGTGGACTTGGGAGAGG-3′.
PCR amplification was performed on 7300 Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), using a SYBR-Green I Master kit (cat. no. 04707516001; Roche
Diagnostics) with the following steps: 1 cycle of 95°C for 10 min
for denaturation and 40 cycles of 95°C for 5 sec and 60°C for 30
sec for amplification. The 2−ΔΔCq method (22) was used to calculate the relative
amount of COL5A1 normalized to the β-actin control using Sequence
Detection Software v1.4 (Applied Biosystems; Thermo Fisher
Scientific, Inc.).
Western blot analysis
Fresh IDC tissues, adjacent normal tissues and
fibroadenoma were lysed in ice-cold RIPA buffer (cat. no. 89900)
with Pierce™ Protease and Phosphatase Inhibitor Mini Tablets (cat.
no. 88668) (both from Thermo Fisher Scientific, Inc.). Following
dissociation using a homogenizer, the tissue samples were
centrifuged at 800 × g for 5 min at 4°C. The supernatant was
transferred to a new tube and the sample was sonicated for 30 sec,
followed by centrifugation at 20,000 × g for 20 min at 4°C. Protein
concentration was determined using a BCA Protein Assay kit (cat.
no. 23227; Thermo Fisher Scientific, Inc.). MCF-7 and MCF-12A cells
were also lysed in ice-cold RIPA buffer. Total protein (20
μg) was separated on 6% SDS-PAGE and transferred to a PVDF
membrane (cat. no. IPVH00010; EMD Millipore, Billerica, MA, USA).
Following blocking with 5% non-fat milk for 1 h at room
temperature, the membrane was incubated with a primary antibodies
at 4°C overnight and subsequently incubated with HRP-conjugated
goat anti-rabbit or anti-mouse IgG (1:5,000 dilution; cat. nos.
L3012 and L3032; Signalway Antibody LLC) for 1 h at room
temperature. The following primary antibodies were used: Rabbit
anti-COL5A1 (1:600 dilution; cat. no. SAB4500384; Sigma-Aldrich;
Merck KGaA), mouse anti-Smad2 and rabbit anti-phosphorylated-Smad2
(1:2,000 dilution; cat. nos. 3103 and 3108; Cell Signaling
Technology, Inc., Danvers, MA, USA), and rabbit anti-β-actin
(1:5,000 dilution; cat. no. 66009-1-Ig; Wuhan Sanying
Biotechnology, Wuhan, China). Signals were measured using the
Tanon-4500 Gel Imaging System (Tanon Science and Technology Co.,
Ltd., Shanghai, China) following the addition of Immobilon™ Western
Chemiluminescent HRP Substrate (cat. no. WBKLS0100; EMD Millipore)
and analyzed using GIS ID Analysis Software v4.1.5 (Tanon Science
and Technology Co., Ltd.).
Small interfering (si)RNA
transfection
A total of 2×105 cells/well were plated
into 6-well plates for 24 h, and were then transfected with 50
nM/well human COL5A1-siRNA (COL5A1-siR) or scramble, nonspecific
control-siRNA (C-siR). The sequences of human COL5A1-siR were
5′-GGGAUUCCUUCAAGGUUUATT-3′ (sense) and 5′-UAAACCUUGAAGGAAUCCCTT-3′
(antisense). The sequences of C-siR were 5′-UUCUCCGAACG
UGUCACGUTT-3′ (sense) and 5′-ACGUGACACGUUCGGAGAATT-3′ (antisense).
GAPDH was used as a positive control with the sequences of
5′-UGACCUCAACUACAUGGUUTT-3′ (sense) and 5′-AACCAUGUAGUUGAGGUCATT-3′
(anti-sense). All siRNAs were purchased from Shanghai GenePharma
Co., Ltd., (Shanghai, China). Transfection was performed using
X-tremeGENE siRNA Transfection Reagent (cat. no. 4476093001; Roche
Diagnostics).
Cell viability assay
Cells were seeded in a 96-well plate at a density of
3,000/well for 24 h, and then transfected with 0.5 μg/well
human COL5A1 siRNA (COL5A1-siR) or control scramble siRNA (C-siR)
and incubated for 24 to 48 h. Cell viability was measured using the
WST-1 kit (cat. no. W201; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) according to the manufacturer's protocol. The
optical density of each well was read at 450 nm using a plate
reader (BioTek Instruments, Inc., Winooski, VT, USA). The
experiment was repeated at least three times.
Wound healing assay
Following COL5A1-siR transfection, a scraping wound
was made in the cell culture using a 1 ml pipette tip. Following
removal of the detached cells and cell debris by washing with
culture medium, the attached cells were incubated in medium
containing 10% fetal bovine serum (FBS; cat. no. 35-010-CV; Corning
Life Sciences, Tewksbury, MA, USA) for 48 h. Photos were taken
using an inverted microscope and the width of each gap was analyzed
using MATLAB software version R2015b (The MathWorks, Inc., Natick,
MA, USA).
Transwell invasion assay
Transwell chambers (cat. no. 3422; Corning Life
Sciences) were inserted into 24-well plates with the addition of
0.1 ml of pre-warmed and diluted Matrigel Matrix (cat. no. 356234;
Corning Life Sciences). Following gel formation for 1 h and
hydration for 2 h at 37°C, the transfected cells (104
cells/well) were seeded into the upper chamber with serum-free
medium. In the lower chamber, 0.5 ml culture medium with 10% FBS
was added. Following cell culture at 37°C for 24 h, media in the
upper and lower chambers were aspirated. Non-invaded cells on the
inner surface of the membrane were removed with a cotton swab and
the invaded cells on the outer surface of the membrane were fixed
with 4% paraformaldehyde for 10 min and stained with crystal violet
for 30 min at room temperature. The cells were observed under an
inverted microscope and photographed.
Bioinformatics analysis
ONCOMINE (www.oncomine.org) was used to confirm the reliability
of the comparison of COL5A1 expression between normal breast
tissues and IDC tissues. The survival rate was obtained from The
Cancer Genome Atlas (TCGA, http://cancergenome.nih.gov/) database, which provides
abundant gene expression data of human cancers through
RNA-sequencing analysis.
Statistical analysis
All data were analyzed using Stata 11 software
(StataCorp LLC, College Station, TX, USA). The Kaplan-Meier method
was used to analyze survival data and the log-rank test was used
for single-factor analysis. For multi-group comparisons, one-way
analysis of variance was used followed by Bonferroni post hoc test.
For comparisons between two groups, the associations between COL5A1
protein expression and histological type or clinicopathological
characteristics, a χ2 test was applied. Results are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
COL5A1 is overexpressed in human breast
cancer
Positive staining of COL5A1 protein in ductal
epithelial cells of breast tissue derived from patients with
malignant IDC was observed by IHC (Fig. 1A). The positive rate of high COL5A1
protein expression was significantly increased in IDC tissues
(malignant) compared with adjacent normal tissues (normal) and
fibroadenoma tissues (benign; P<0.05) (Fig. 1B). Overexpression of COL5A1 at the
mRNA and protein levels was further confirmed in IDC tissues by
RT-qPCR (Fig. 1C) and western blot
analysis (Fig. 1D and E).
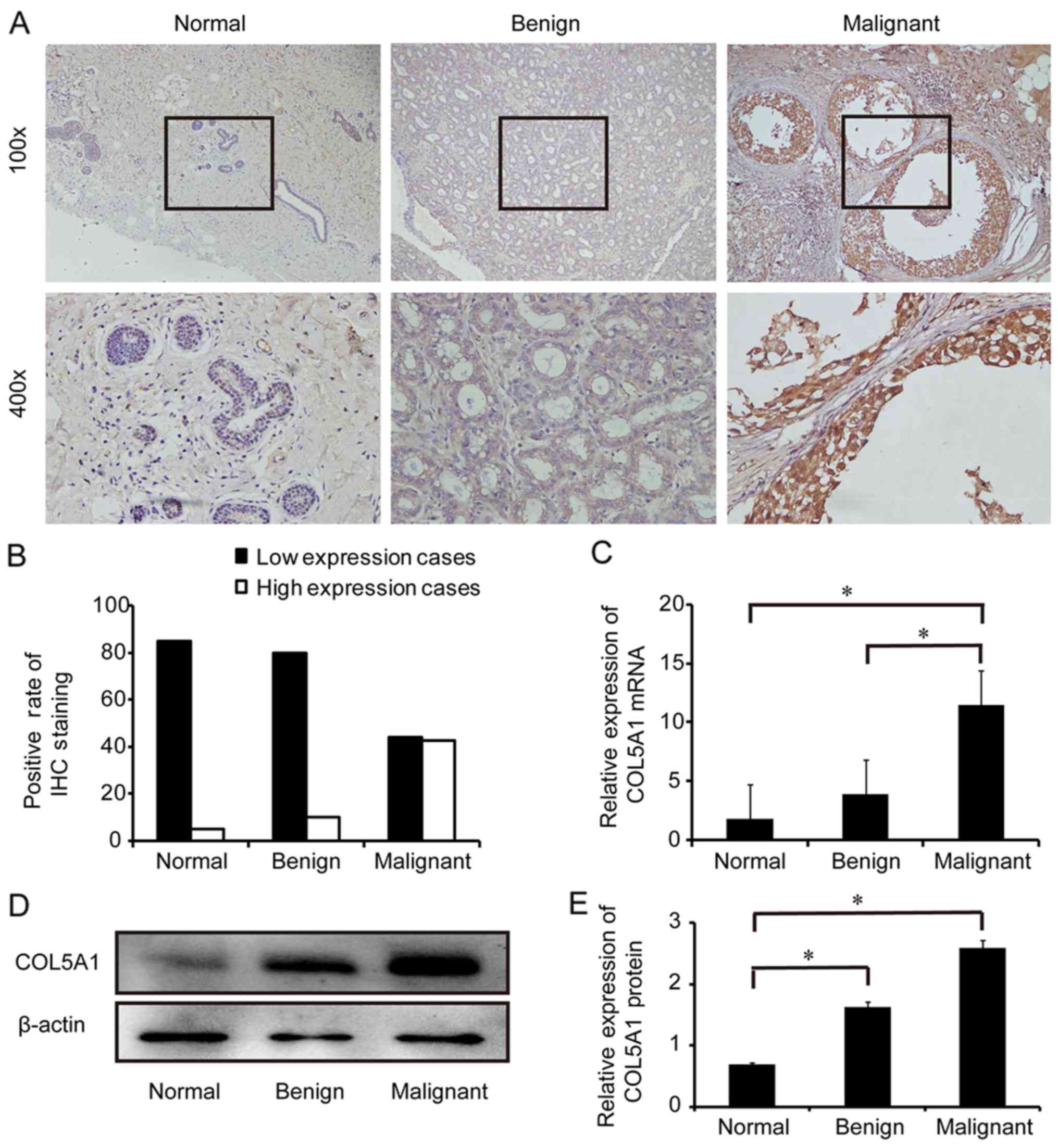 | Figure 1Expression of COL5A1 in breast
tissues. (A) Expression of COL5A1 protein in adjacent normal
tissues (Normal), fibroadenomas (Benign) and invasive ductal
carcinomas (Malignant) was detected by IHC using a specific
antibody against COL5A1. The bottom panel shows amplified images
from the square area of the upper panel. Original magnification,
×100 and ×400. (B) Quantitative analysis of the positive rate of
COL5A1 protein expression in normal, benign, and malignant tissues
(each group: n=90 cases). (C) Reverse transcription-quantitative
polymerase chain reaction analysis of COL5A1 mRNA expression in
normal, benign, and malignant tissues (n=3 each). (D) Western blot
analysis of COL5A1 protein expression in normal, benign, and
malignant tissues. Representative images are presented. (E)
Histogram with the semi-quantification of the gels in D (n=3 each).
*P<0.05, with comparisons indicated by lines. COL5A1,
collagen type V α1 chain; IHC, immunohistochemistry. |
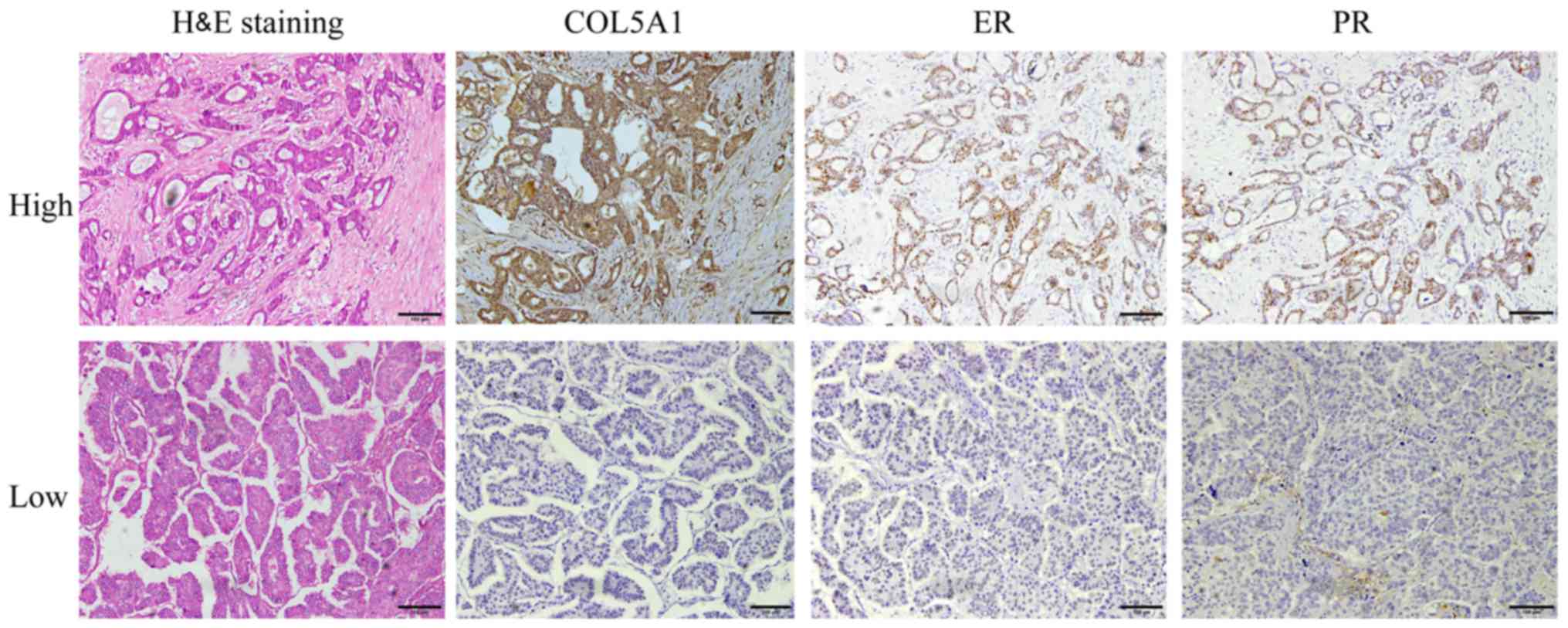
COL5A1 expression is associated with the
expression of ER and PR in patients with breast cancer
Next, our group examined whether the expression of
COL5A1 protein was associated with the clinicopathological features
of patients with IDC. The expression of COL5A1 protein was not
significantly associated with the majority of clinicopathological
features, including age (≥60 vs. <60), lymph node metastasis
(yes vs. no), tumor size (≤2.0 vs. >2 cm), histological grades
and clinical stages (all P>0.05) (Table I). However, through comparisons of
COL5A1 protein expression with breast cancer-associated markers,
expression of COL5A1 protein was revealed to be associated with the
expression of ER and PR (P<0.05) (Fig. 2 and Table I), but was not associated with the
expression of Her2 and Ki-67, a proliferation marker (Table I).
 | Table IAssociations between COL5A1 and
clinicopathological characteristics of patients with invasive
ductal carcinoma (n=90). |
Table I
Associations between COL5A1 and
clinicopathological characteristics of patients with invasive
ductal carcinoma (n=90).
|
Characteristics | COL5A1 expression
| χ2 | P-value |
|---|
| Low (n=46) | High (n=44) |
|---|
| Age, years | | | 1.6601 | 0.198 |
| <60 | 35 | 28 | | |
| ≥60 | 11 | 16 | | |
| LN metastasis | | | 2.1583 | 0.142 |
| Yes | 32 | 24 | | |
| No | 14 | 20 | | |
| Tumor size, cm | | | 0.0004 | 0.985 |
| ≤2.0 | 21 | 20 | | |
| >2.0 | 25 | 24 | | |
| Histological
grade | | | 0.6771 | 0.071 |
| 1 | 4 | 2 | | |
| 2 | 29 | 28 | | |
| 3 | 13 | 14 | | |
| Clinical stage | | | 3.0605 | 0.382 |
| I | 15 | 14 | | |
| II | 30 | 28 | | |
| III | 0 | 2 | | |
| IV | 1 | 0 | | |
| ER expression | | | 5.5178 | 0.019a |
| High | 11 | 25 | | |
| Low | 25 | 19 | | |
| PR expression | | | 5.4049 | 0.020a |
| High | 18 | 28 | | |
| Low | 28 | 26 | | |
| Her2
expression | | | 0.0389 | 0.844 |
| High | 25 | 23 | | |
| Low | 21 | 21 | | |
| Ki-67
expression | | | 1.1552 | 0.561 |
| 0–25% | 31 | 34 | | |
| 26–50% | 13 | 9 | | |
| 51–75% | 2 | 1 | | |
| 76–100% | 0 | 0 | | |
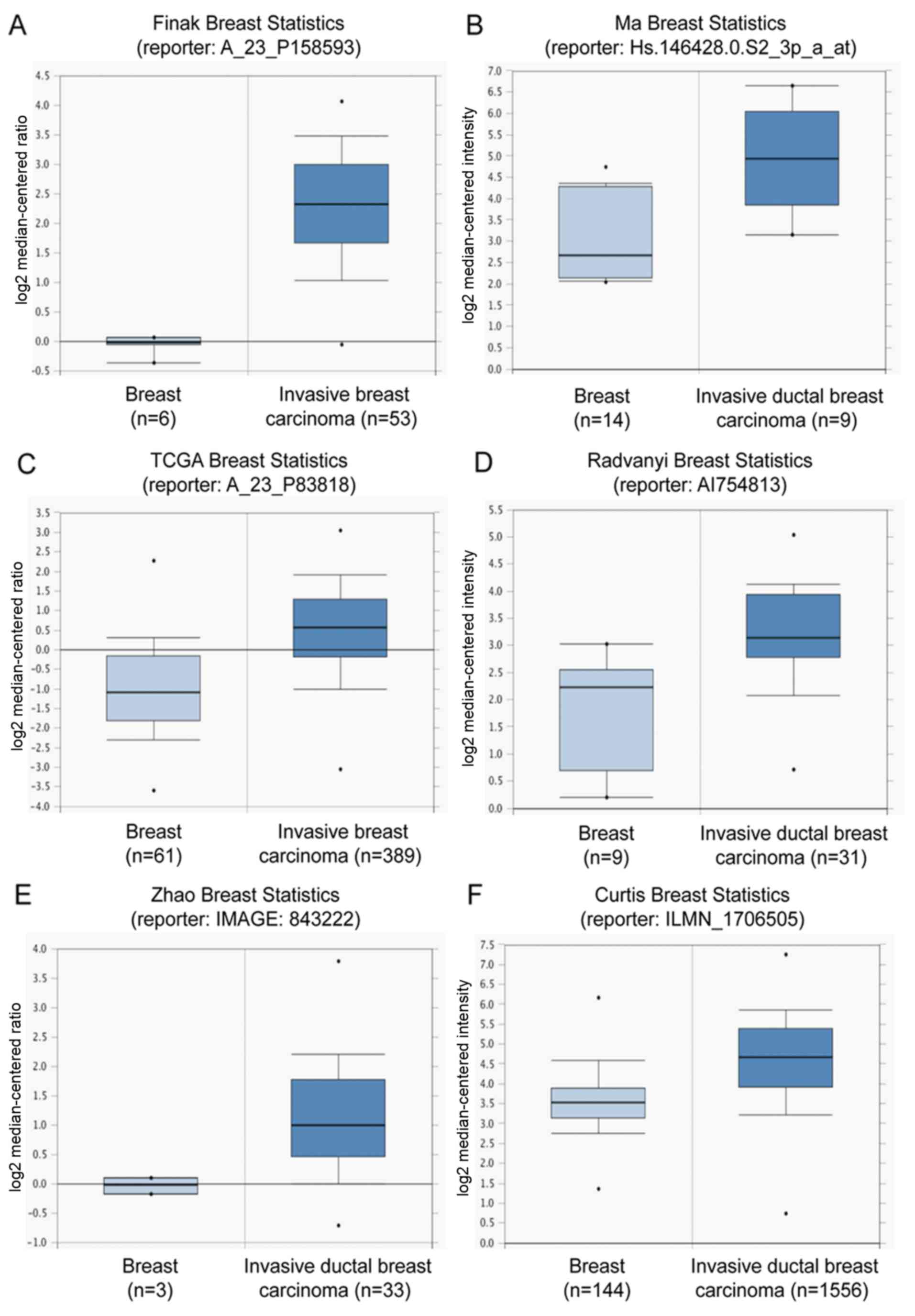
Using bioinformatics analysis of the Oncomine
database (www.oncomine.org), these results were
confirmed to be consistent with data from public databases. A high
level of COL5A1 mRNA expression was observed in invasive breast
carcinomas compared with normal breast tissues in the microarray
data-sets from different groups with a fold change >2 (Fig. 3). There were fold changes of 5.043
(P<0.001; Fig. 3A) in the
datasets of Finak (23), 3.585
(P<0.0001; Fig. 3B) in the
datasets of Ma (24), 2.84
(P<0.0001; Fig. 3C) in the
datasets of TCGA (http://tcga-data.nci.nih.gov/tcga/), 2.703 (P=0.001;
Fig. 3D) in the datasets of
Radvanyi (25), 2.178
(P<0.0001; Fig. 3E) in the
datasets of Zhao (26), and 2.005
(P<0.0001; Fig. 3F) in the
datasets of Curtis (27),
respectively. These data indicated that COL5A1 may be involved in
IDC development.
Kaplan-Meier Plotter datasets were analyzed
(http://kmplot.com/analysis/) (28), and the survival plots revealed that
high expression of COL5A1 mRNA was associated with distant
metastasis free survival in patients with breast cancer (Fig. 3G), but was not associated with
overall survival (OS), relapse-free survival or progression-free
survival.
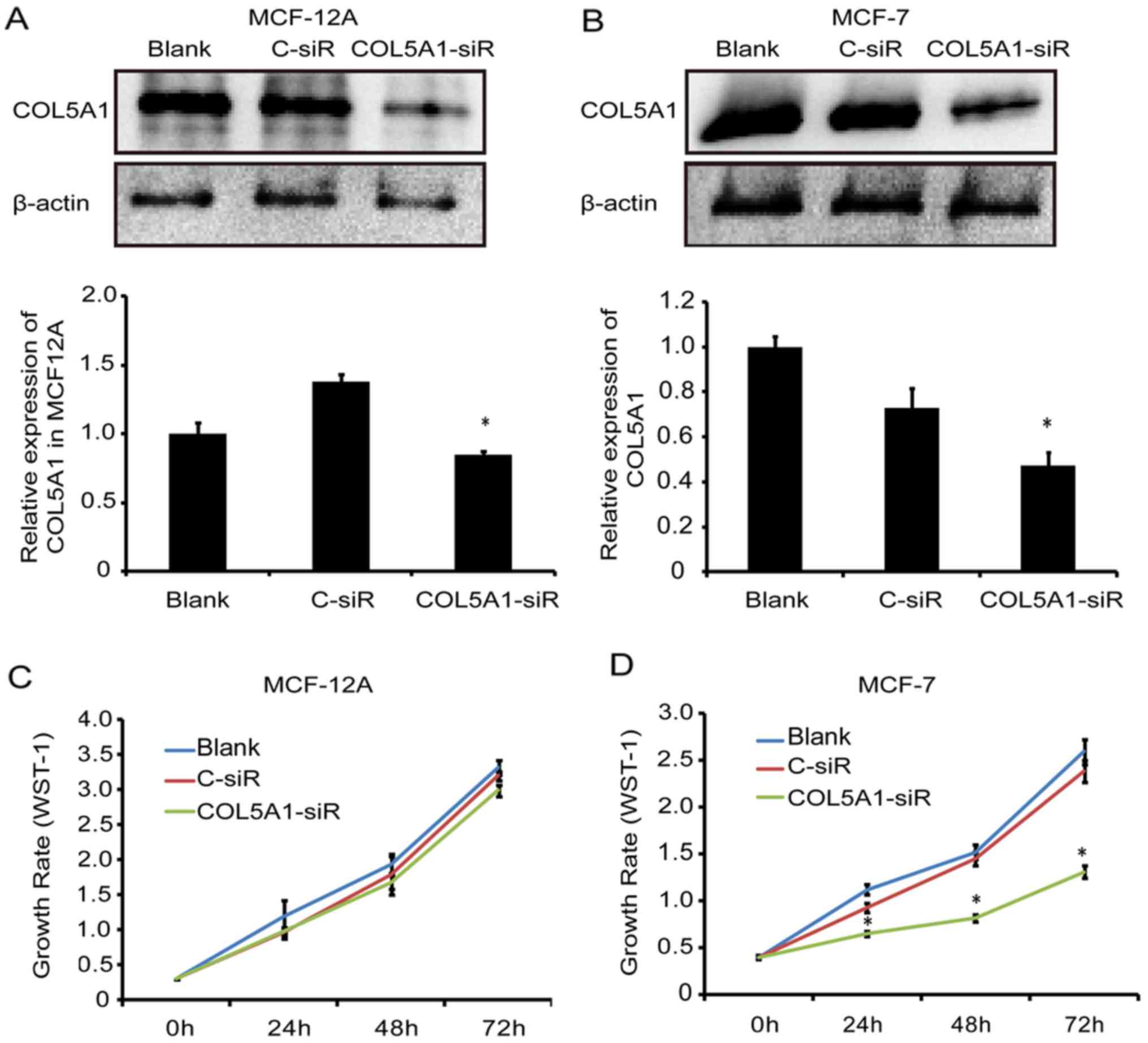
Knockdown of COL5A1 inhibits breast cell
viability, migration, and invasion
To determine the effect of COL5A1 on the biological
functions of breast cells, a loss-of-function approach was applied.
Following transfection with a specific siRNA for 24 h, a
significant decrease of COL5A1 protein was observed in MCF-12A and
MCF-7 cells (P<0.05; Fig. 4A and
B). Cell viability was significantly decreased in MCF-7 cells
(P<0.05) but not in MCF-12A cells (P>0.05) following
COL5A1-siRNA transfection for 24, 48 and 72 h (Fig. 4C and D).
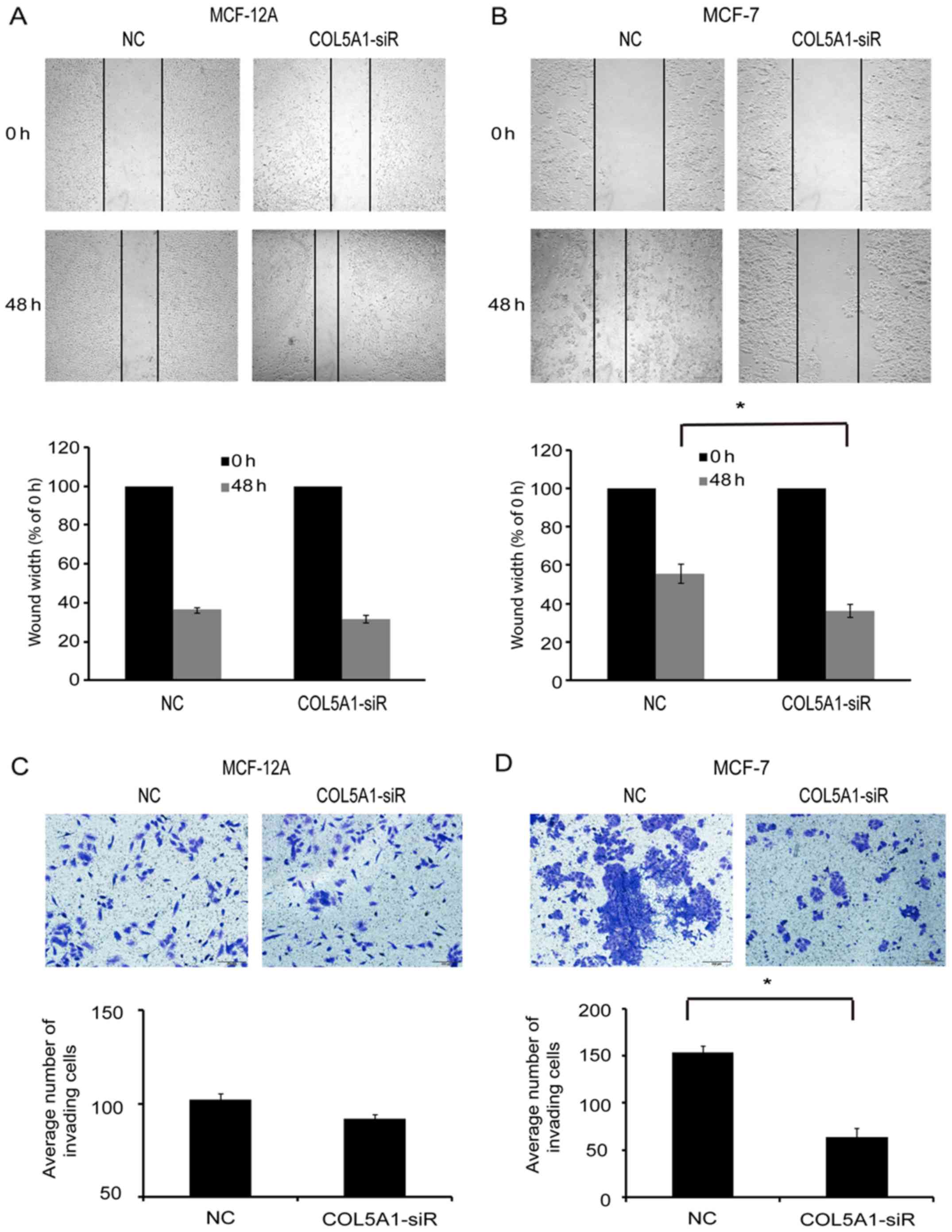
Next, the migration of cells was examined following
COL5A1 knockdown. The wound healing assay revealed that there was
no difference in the width of the wound between
COL5A1-siRNA-transfected and control-siRNA (NC)-transfected MCF-12A
cells (P>0.05; Fig. 5A).
However, wound width was significantly decreased in MCF-7 cells
following COL5A1-siRNA transfection compared with NC at 48 h
(P<0.05; Fig. 5B). Furthermore,
knockdown of COL5A1 did not affect MCF-12A cell invasion (Fig. 5C) but significantly decreased the
number of invading MCF-7 cells in the Transwell assay (Fig. 5D). These data indicated that
COL5A1-siRNA influenced breast cancer MCF-7 cell viability,
migration, and invasion, but this did not occur in the non-tumorous
cell line MCF-12A.
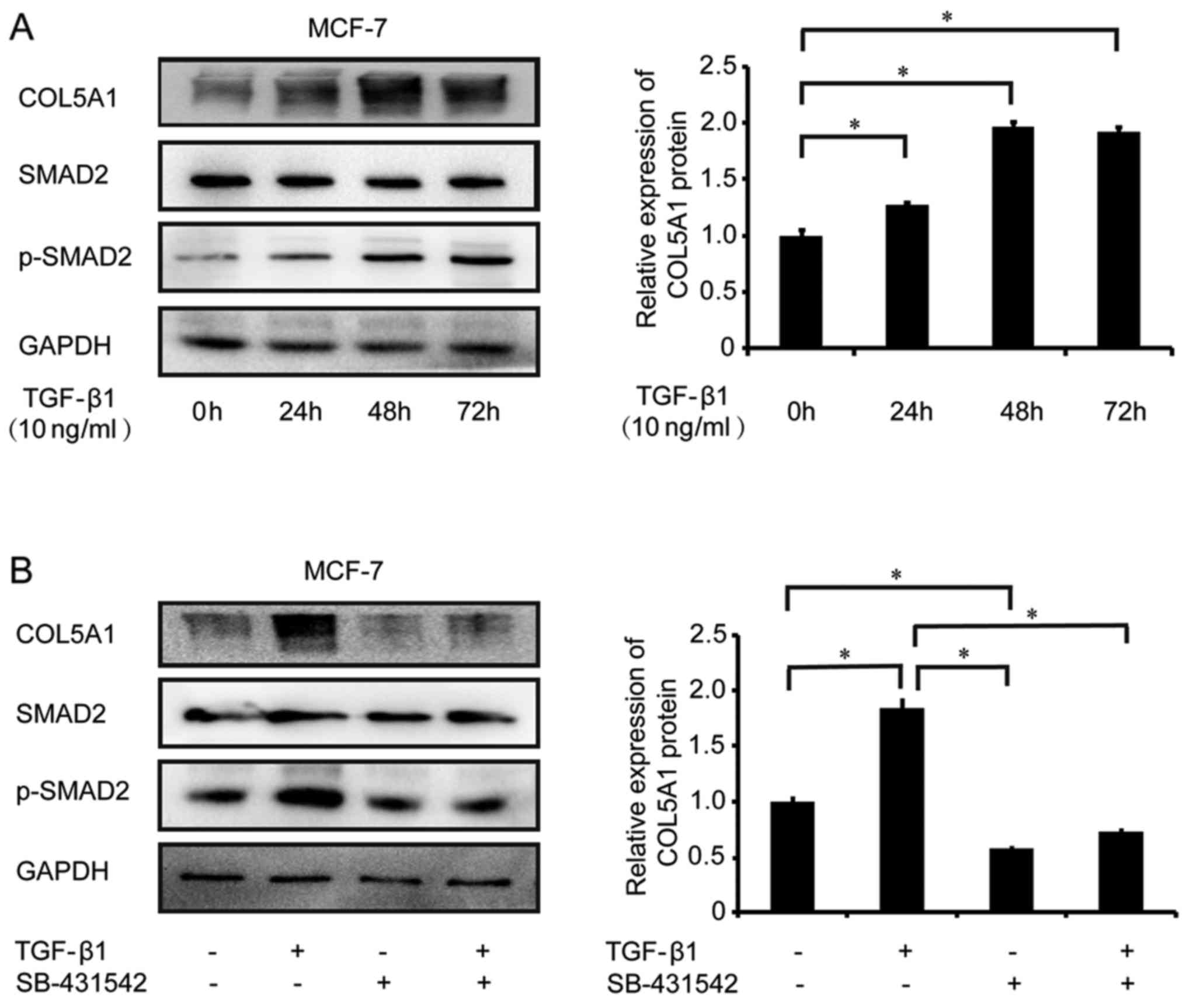
COL5A1 expression is regulated by the
TGF-β signaling pathway in breast cancer cells
As the TGF-β signaling pathway is involved in breast
tumorigenesis (29), our group
subsequently investigated whether the TGF-β signaling pathway
regulates COL5A1 expression. Using western blot analysis, COL5A1
protein expression was revealed to be significantly increased
following treatment with 10 ng/ml TGF-β1 in MCF-7 cells at 24, 48
and 72 h (P<0.05; Fig. 6A). An
increase in Smad2 phosphorylation, which is a TGF-β signaling
transducer protein activated following TGF-β1 treatment, was also
observed in TGF-β1-treated MCF-7 cells, suggesting that TGF-β
signaling exists in these cells (Fig.
6A). TGF-β1-mediated COL5A1 expression was blocked by its type
I receptor inhibitor SB-431542 (10 nM; Fig. 6B), indicating that COL5A1 is
regulated by the TGF-β signaling pathway.
Discussion
The present study demonstrated the overexpression of
COL5A1 in human IDC. Collagen is one of the main components of the
ECM and functions in cell adhesion, migration, differentiation and
tissue regeneration, and at least 28 types of collagen have been
identified (30). Type V collagen
is a minor fibrillar collagen and has three subtypes: COL5A1,
COL5A2 and COL5A3. Despite it at a low level in certain tissues,
COL5A1 has been implicated in biological and pathophysiological
processes and serves an important function in ECM regulation and
assembly (9).
Previous studies have revealed differential
expression of COL5A1 in several types of cancer, including serous
ovarian cancer, gastric cancer, meningioma, tongue squamous cell
carcinoma and invasive bladder transition cell carcinoma (11-13,31,32).
The present study focused on IDC, the most common histological type
of breast cancer. The results clearly demonstrated that COL5A1 was
overexpressed in malignant tumor IDCs compared with adjacent normal
tissues and benign tumor fibroadenomas, suggesting that COL5A1 may
have biological functions in the development of breast cancer.
Bioinformatics analyses of the microarray data from the public
datasets confirmed the observation that COL5A1 was overexpressed in
malignant breast tumors, and may be associated with metastasis.
Although no correlation was observed between COL5A1 expression and
OS in patients with breast cancer from the Kaplan-Meier Plotter
database, an association between COL5A1 expression and poor OS of
patients with ovarian cancer has been reported (12). In order to investigate whether the
expression of COL5A1 is associated with OS, a large cohort study in
patients with breast cancer is required in the future.
The present study demonstrated that increased COL5A1
expression in IDC was associated with estrogen receptor (ER) and
progesterone receptor (PR) expression, indicating that COL5A1, like
Her3 and Ki-67 (33,34), may serve a prognostic function in
patients with IDC. Estrogen is a major driver of breast tumor cell
growth (35) and ERα+
breast cancer is the leading cause of breast cancer-associated
mortality (36). ER and PR, along
with matrix metalloproteinase-2, have a prognostic value in
patients with breast cancer (37).
Indeed, the ER and PR status of human breast cancer represent
important prognostic and predictive markers for human breast cancer
(38,39). COL5A1 may also be a prognostic
marker and an important contributor to tumor cell behavior as
regulated by these hormone pathways. However, further studies are
required in the future.
COL5A1 has a mutual interaction with other subtypes
of collagen (9). Abnormal
expression of COL5A1 is observed in a variety of diseases. Natural
genetic mutation of the COL5A1 gene leads to defects in its
expression and results in a congenital connective tissue dysplasia
known as Ehlers-Danlos syndrome (40). In zebrafish embryos, COL5A1 is
expressed in the spinal cord, somite, interstitial, cranial neural
crest, and head cartilage (41).
Knockout of COL5A1 causes severe dysfunction in the synthesis of
collagen fibers (42,43). Notably, the present study
demonstrated that knockdown of COL5A1 resulted in a decrease of the
viability, migration, and invasion of breast cancer MCF-7 cells,
but not of non-tumorous MCF-12A cells. As the expression of COL5A1
was relatively increased in MCF-7 cells compared with MCF-12A
cells, an increase in its expression in cancerous cells may cause
the change of cellular characteristics. Inhibiting collagen V has
been demonstrated to lower the rate of growth, migration and
invasion in 8701-BC breast cancer cells (44). These data indicate that COL5A1 is a
cancer-associated molecule and may be a potential target for breast
cancer treatment.
Despite the finding that COL5A1 was overexpressed in
IDC, the mechanism underlying the regulation remains unclear. The
TGF-β signaling pathway serves a key function in the regulation of
ECM, including collagens (45),
and mediates the epithelial-mesenchymal transition (46). Therefore, TGF-β may be involved in
the regulation of COL5A1 in breast cancer cells. The present study
demonstrated that COL5A1 protein expression was increased following
TGF-β1 stimulation, and this increase was blocked in the presence
of an inhibitor of TGF-β type I receptor. These data indicated that
COL5A1 was regulated by the TGF-β signaling pathway in breast
cancer cells, similar to the result observed in osteoblasts
(17).
In conclusion, COL5A1 is overexpressed in IDC and
regulated by TGF-β1. An increase of COL5A1 reflects the breast
tumor progression. COL5A1 may serve as a novel biomarker and become
a therapeutic target for the treatment of human breast IDC.
Acknowledgments
Not applicable.
Abbreviations:
|
COL5A1
|
collagen type V α1 chain
|
|
ECM
|
extracellular matrix
|
|
ER
|
estrogen receptor
|
|
Her2
|
human epidermal growth factor
receptor-2
|
|
IHC
|
immunohistochemistry
|
|
IDC
|
invasive ductal carcinoma
|
|
PR
|
progesterone receptor
|
|
TGF-β
|
transforming growth factor-β
|
Notes
[1]
Funding
The present study was supported by grants from the
Shanghai Municipal Commission of Health and Family Planning (grant
no. 201640287), the National Natural Science Foundation of China
(grant no. 81272880), the Natural Science Foundation of Shanghai
(grant no. 17ZR1404100), the Science and Technology Commission of
Shanghai Municipality (grant no. 124119b1300) and the Science and
Technology Commission of Jinshan District (grant no. 2015-3-2).
[2] Availability
of data and materials
All data generated or analyzed during this study are
included in this published article.
[3] Authors'
contributions
WR conducted experiments, and performed data
analysis, figure generation and manuscript writing. YZ contributed
to the collection of clinical samples and pathological diagnosis.
LZ, QL, JZ performed part of the experiments and bioinformatics
analyses. GX contributed to the experimental design, data analysis,
figure generation and manuscript writing. All authors read and
approved the final manuscript.
[4] Ethics
approval and consent to participate
The present study was approved by the Ethics
Committee of Jinshan Hospital, Fudan University (no. E-2017-11-01;
Shanghai, China).
[5] Consent for
publication
Informed consent was obtained from all patients.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DeSantis CE, Fedewa SA, Goding Sauer A,
Kramer JL, Smith RA and Jemal A: Breast cancer statistics, 2015:
Convergence of incidence rates between black and white women. CA
Cancer J Clin. 66:31–42. 2016. View Article : Google Scholar
|
|
5
|
Muñoz-Díaz MM, Fernández-Aceñero MJ,
Salvadores P and Schneider J: Utility of a simplified molecular
classification of tumors for predicting survival of patients with
invasive ductal breast carcinoma. Anticancer Res. 29:4727–4730.
2009.PubMed/NCBI
|
|
6
|
Chen Z, Yang J, Li S, Lv M, Shen Y, Wang
B, Li P, Yi M, Zhao X, Zhang L, et al: Invasive lobular carcinoma
of the breast: A special histological type compared with invasive
ductal carcinoma. PLoS One. 12:e01823972017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roulet M, Ruggiero F, Karsenty G and
LeGuellec D: A comprehensive study of the spatial and temporal
expression of the col5a1 gene in mouse embryos: A clue for
understanding collagen V function in developing connective tissues.
Cell Tissue Res. 327:323–332. 2007. View Article : Google Scholar
|
|
8
|
Fichard A, Tillet E, Delacoux F, Garrone R
and Ruggiero F: Human recombinant alpha1(V) collagen chain.
Homotrimeric assembly and subsequent processing. J Biol Chem.
272:30083–30087. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wenstrup RJ, Florer JB, Brunskill EW, Bell
SM, Chervoneva I and Birk DE: Type V collagen controls the
initiation of collagen fibril assembly. J Biol Chem.
279:53331–53337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borradaile NM and Pickering JG: Polyploidy
impairs human aortic endothelial cell function and is prevented by
nicotinamide phosphoribosyltransferase. Am J Physiol Cell Physiol.
298:C66–C74. 2010. View Article : Google Scholar
|
|
11
|
Suresh A, Vannan M, Kumaran D, Gümüs ZH,
Sivadas P, Murugaian EE, Kekatpure V, Iyer S, Thangaraj K and
Kuriakose MA: Resistance/response molecular signature for oral
tongue squamous cell carcinoma. Dis Markers. 32:51–64. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheon DJ, Tong Y, Sim MS, Dering J, Berel
D, Cui X, Lester J, Beach JA, Tighiouart M, Walts AE, et al: A
collagen-remodeling gene signature regulated by TGF-β signaling is
associated with metastasis and poor survival in serous ovarian
cancer. Clin Cancer Res. 20:711–723. 2014. View Article : Google Scholar
|
|
13
|
Sun H: Identification of key genes
associated with gastric cancer based on DNA microarray data. Oncol
Lett. 11:525–530. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu J, Zhang W, Xia Q, Liu F, Li L, Zhao
S, Gao X, Zang C, Ge R and Sun Y: RNA sequencing identifies crucial
genes in papillary thyroid carcinoma (PTC) progression. Exp Mol
Pathol. 100:151–159. 2016. View Article : Google Scholar
|
|
15
|
Chai F, Liang Y, Zhang F, Wang M, Zhong L
and Jiang J: Systematically identify key genes in inflammatory and
noninflammatory breast cancer. Gene. 575:600–614. 2016. View Article : Google Scholar
|
|
16
|
Huang Y, Tao Y, Li X, Chang S, Jiang B, Li
F and Wang ZM: Bioinformatics analysis of key genes and latent
pathway interactions based on the anaplastic thyroid carcinoma gene
expression profile. Oncol Lett. 13:167–176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kahai S, Vary CP, Gao Y and Seth A:
Collagen, type V, alpha1 (COL5A1) is regulated by TGF-beta in
osteoblasts. Matrix Biol. 23:445–455. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lo PK, Zhang Y, Yao Y, Wolfson B, Yu J,
Han SY, Duru N and Zhou Q: Tumor-associated myoepithelial cells
promote the invasive progression of ductal carcinomain situthrough
activation of TGFβ signaling. J Biol Chem. 292:11466–11484. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou D, Zhang L, Sun W, Guan W, Lin Q, Ren
W, Zhang J and Xu G: Cytidine monophosphate kinase is inhibited by
the TGF-β signalling pathway through the upregulation of
miR-130b-3p in human epithelial ovarian cancer. Cell Signal.
35:197–207. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Gui L, Zhang Y, Zhang J, Shi J and
Xu G: Cystatin B is a progression marker of human epithelial
ovarian tumors mediated by the TGF-β signaling pathway. Int J
Oncol. 44:1099–1106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu Y, Guo M, Zhang L, Xu T, Wang L and Xu
G: Biomarker triplet NAMPT/VEGF/HER2 as a de novo detection panel
for the diagnosis and prognosis of human breast cancer. Oncol Rep.
35:454–462. 2016. View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Finak G, Bertos N, Pepin F, Sadekova S,
Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu
A, et al: Stromal gene expression predicts clinical outcome in
breast cancer. Nat Med. 14:518–527. 2008. View Article : Google Scholar
|
|
24
|
Ma XJ, Dahiya S, Richardson E, Erlander M
and Sgroi DC: Gene expression profiling of the tumor
microenvironment during breast cancer progression. Breast Cancer
Res. 11:R72009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Radvanyi L, Singh-Sandhu D, Gallichan S,
Lovitt C, Pedyczak A, Mallo G, Gish K, Kwok K, Hanna W, Zubovits J,
et al: The gene associated with trichorhinophalangeal syndrome in
humans is overexpressed in breast cancer. Proc Natl Acad Sci USA.
102:11005–11010. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao H, Langerød A, Ji Y, Nowels KW,
Nesland JM, Tibshirani R, Bukholm IK, Kåresen R, Botstein D,
Børresen-Dale AL, et al: Different gene expression patterns in
invasive lobular and ductal carcinomas of the breast. Mol Biol
Cell. 15:2523–2536. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Curtis C, Shah SP, Chin SF, Turashvili G,
Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, et
al METABRIC Group: The genomic and transcriptomic architecture of
2,000 breast tumours reveals novel subgroups. Nature. 486:346–352.
2012.PubMed/NCBI
|
|
28
|
Györffy B, Lanczky A, Eklund AC, Denkert
C, Budczies J, Li Q and Szallasi Z: An online survival analysis
tool to rapidly assess the effect of 22,277 genes on breast cancer
prognosis using microarray data of 1,809 patients. Breast Cancer
Res Treat. 123:725–731. 2010. View Article : Google Scholar
|
|
29
|
Moore-Smith L and Pasche B: TGFBR1
signaling and breast cancer. J Mammary Gland Biol Neoplasia.
16:89–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mienaltowski MJ and Birk DE: Structure,
physiology, and biochemistry of collagens. Adv Exp Med Biol.
802:5–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kliese N, Gobrecht P, Pachow D, Andrae N,
Wilisch-Neumann A, Kirches E, Riek-Burchardt M, Angenstein F,
Reifenberger G, Riemenschneider MJ, et al: miRNA-145 is
downregulated in atypical and anaplastic meningiomas and negatively
regulates motility and proliferation of meningioma cells. Oncogene.
32:4712–4720. 2013. View Article : Google Scholar
|
|
32
|
Ewald JA, Downs TM, Cetnar JP and Ricke
WA: Expression microarray meta-analysis identifies genes associated
with Ras/MAPK and related pathways in progression of
muscle-invasive bladder transition cell carcinoma. PLoS One.
8:e554142013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hammoda GE, El-Hefnawy SM, Abdou AG and
Abdallah RA: Human epidermal growth factor receptor-3 mRNA
expression as a prognostic marker for invasive duct carcinoma not
otherwise specified. J Clin Diagn Res. 11:XC01–XC05.
2017.PubMed/NCBI
|
|
34
|
Engels CC, Fontein DB, Kuppen PJ, de
Kruijf EM, Smit VT, Nortier JW, Liefers GJ, van de Velde CJ and
Bastiaannet E: Immunological subtypes in breast cancer are
prognostic for invasive ductal but not for invasive lobular breast
carcinoma. Br J Cancer. 111:532–538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim SH, Hwang KA and Choi KC: Treatment
with kaempferol suppresses breast cancer cell growth caused by
estrogen and triclosan in cellular and xenograft breast cancer
models. J Nutr Biochem. 28:70–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barcus CE, Keely PJ, Eliceiri KW and
Schuler LA: Prolactin signaling through focal adhesion complexes is
amplified by stiff extracellular matrices in breast cancer cells.
Oncotarget. 7:48093–48106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ramos EA, Silva CT, Manica GC, Pereira IT,
Klassen LM, Ribeiro EM, Cavalli IJ, Braun-Prado K, Lima RS, Urban
CA, et al: Worse prognosis in breast cancer patients can be
predicted by immunohistochemical analysis of positive MMP-2 and
negative estrogen and progesterone receptors. Rev Assoc Med Bras
(1992). 62:774–781. 2016. View Article : Google Scholar
|
|
38
|
Sharangpani GM, Joshi AS, Porter K,
Deshpande AS, Keyhani S, Naik GA, Gholap AS and Barsky SH:
Semi-automated imaging system to quantitate estrogen and
progesterone receptor immunoreactivity in human breast cancer. J
Microsc. 226:244–255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamamoto-Ibusuki M, Yamamoto Y, Yamamoto
S, Fujiwara S, Fu P, Honda Y, Iyama K and Iwase H: Comparison of
prognostic values between combined immunohistochemical score of
estrogen receptor, progesterone receptor, human epidermal growth
factor receptor 2, Ki-67 and the corresponding gene expression
score in breast cancer. Mod Pathol. 26:79–86. 2013. View Article : Google Scholar
|
|
40
|
Monroe GR, Harakalova M, van der Crabben
SN, Majoor-Krakauer D, Bertoli-Avella AM, Moll FL, Oranen BI,
Dooijes D, Vink A, Knoers NV, et al: Familial Ehlers-Danlos
syndrome with lethal arterial events caused by a mutation in
COL5A1. Am J Med Genet A. 167:1196–1203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fang M, Adams JS, McMahan BL, Brown RJ and
Oxford JT: The expression patterns of minor fibrillar collagens
during development in zebrafish. Gene Expr Patterns. 10:315–322.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun M, Connizzo BK, Adams SM, Freedman BR,
Wenstrup RJ, Soslowsky LJ and Birk DE: Targeted deletion of
collagen V in tendons and ligaments results in a classic
Ehlers-Danlos syndrome joint phenotype. Am J Pathol. 185:1436–1447.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun M, Chen S, Adams SM, Florer JB, Liu H,
Kao WW, Wenstrup RJ and Birk DE: Collagen V is a dominant regulator
of collagen fibrillogenesis: Dysfunctional regulation of structure
and function in a corneal-stroma-specific Col5a1-null mouse model.
J Cell Sci. 124:4096–4105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Luparello C, Sheterline P, Pucci-Minafra I
and Minafra S: A comparison of spreading and motility behaviour of
8701-BC breast carcinoma cells on type I, I-trimer and type V
collagen substrata. Evidence for a permissive effect of type
I-trimer collagen on cell locomotion. J Cell Sci. 100:179–185.
1991.PubMed/NCBI
|
|
45
|
Rubiś P, Wiśniowska-Smiałek S, Wypasek E,
Rudnicka-Sosin L, Hlawaty M, Leśniak-Sobelga A, Kostkiewicz M and
Podolec P: 12-month patterns of serum markers of collagen
synthesis, transforming growth factor and connective tissue growth
factor are similar in new-onset and chronic dilated cardiomyopathy
in patients both with and without cardiac fibrosis. Cytokine.
96:217–227. 2017. View Article : Google Scholar
|
|
46
|
Margetts PJ, Bonniaud P, Liu L, Hoff CM,
Holmes CJ, West-Mays JA and Kelly MM: Transient overexpression of
TGF-{beta}1 induces epithelial mesenchymal transition in the rodent
peritoneum. J Am Soc Nephrol. 16:425–436. 2005. View Article : Google Scholar
|