Introduction
Colorectal cancer (CRC) is one of the leading causes
of cancer-associated mortality worldwide. Although the overall
morbidity and mortality rates for CRC have been declining for
decades, they have increased considerably in adults <50 years of
age. In addition, the 5-year relative survival rate for patients
with CRC diagnosed at an advanced stage is just 14%, as not all of
the tumor can be surgically removed (1–3). In
addition to old age and lifestyle factors, genetic changes are also
a cause of CRC (4). In these cases
of CRC, targeted therapy may be effective. Therefore, the
identification of novel tumor markers may contribute to the
diagnosis and treatment of CRC, such as the effect of the clinical
application of carcinoembryonic antigen and carbohydrate antigen
19-9 (5).
The homeobox (HOX) family includes a series of
developmental genes. The encoded homeodomain proteins are important
transcription factors that bind to specific DNA sequences as
monomers or multimers to regulate cell differentiation,
proliferation and apoptosis. At present, 39 human HOX genes have
been identified, which are divided into four clusters: A, B, C and
D. Previous studies have reported that HOX family genes are
involved in the development of human malignancies and are
associated with prognosis (6,7). It
was previously observed that HOXA9, HOXA13 and HOXB7 were
overexpressed in esophageal cancer, and promoted the proliferation
of esophageal cancer cells (8–10).
The expression levels of HOXA1, HOXA10, HOXA13, HOXB7 and HOXC6
were also found to be increased in gastric cancer tissue, and may
promote cell proliferation and metastasis, in addition to being
associated with prognosis (11–17).
In CRC, HOXB7 was also reported to be expressed at a high level,
and interacted with the mitogen-activated protein kinase and
phosphoinositide 3-kinase/AKT pathways to promote tumor cell
proliferation (18).
HOXA6 is a member of the HOX family and encodes a
DNA-binding transcription factor, which may regulate gene
expression, morphogenesis and differentiation. Previous studies
have reported that the HOXA6 gene is expressed at a high level in
several types of malignant tumor, including cerebral glioma and
acute myeloid leukemia, and that it is associated with cell
invasion, proliferation, colony formation and chemosensitivity
(19,20). HOX family members have been
confirmed to be involved in tumor regulation, including in the
occurrence and development of CRC. As HOXA6 is also involved in the
regulation of certain types of malignant tumor, it is reasonable to
suggest that HOXA6 may have a similar effect on tumor regulation in
CRC. Based on this, the aim of the present study was to investigate
the expression of HOXA6 in CRC, and the effect of the expression of
HOXA6 on the proliferation, apoptosis, migration and invasion of
CRC cells.
Materials and methods
Patient specimens
A total of 16 CRC tumor and paired adjacent normal
colorectal tissue samples were collected from the First Affiliated
Hospital of Chongqing Medical University (Chongqing, China) between
September 28, 2017 and November 11, 2017 (Table I). Informed consent was signed by
all patients, and ethics approval was obtained from the Ethics
Committee of the First Affiliated Hospital of Chongqing Medical
University.
 | Table ICharacteristics of the patients with
colorectal cancer. |
Table I
Characteristics of the patients with
colorectal cancer.
| Age (years) | Sex | Diagnosis | Sample collection
date (all in the year 2017) |
|---|
| 74 | Male | Colon carcinoma | September 28 |
| 72 | Male | Rectal carcinoma | September 30 |
| 67 | Male | Rectal carcinoma | October 9 |
| 63 | Female | Rectal carcinoma | October 11 |
| 63 | Male | Colon carcinoma | October 13 |
| 68 | Male | Rectal carcinoma | October 13 |
| 65 | Female | Rectal carcinoma | October 14 |
| 62 | Female | Colon carcinoma | October 17 |
| 66 | Female | Colon carcinoma | October 20 |
| 74 | Male | Colon carcinoma | October 21 |
| 63 | Male | Colon carcinoma | October 25 |
| 80 | Female | Colon carcinoma | October 25 |
| 69 | Male | Colon carcinoma | October 31 |
| 65 | Female | Colon carcinoma | November 6 |
| 49 | Male | Colon carcinoma | November 9 |
| 63 | Male | Colon carcinoma | November 10 |
Cell culture and transfection
The Caco2, HCT116, SW480 and HT-29 human CRC cell
lines were purchased from the American Type Culture Collection
(Manassas, VA, USA) and cultured in RPMI-1640 medium (HyClone/GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (PAN-Biotech GmbH, Aidenbach, Germany) at 37°C
in 5% CO2. Plasmids expressing HOXA6, short hairpin
interfering RNA against HOXA6 (shHOXA6; sh1,
5′-CCTTGTTTCTACCAACAGTCC-3′; sh2, 5′-CCTCGTGTTTCTATTCTGATA-3′; sh3,
5′-GGCGCGCAAATGAGTTCCTAT-3′; sh4, 5′-GACAAGACGTACACCTCACCT-3′)
(21) or a negative control (NC)
sequence (non-targeting shRNA) were purchased from GeneCopeia, Inc.
(Rockville, MD, USA). The CRC cells were transfected using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer’s protocol. For the
selection of stable cell lines, G418 and Puromycin (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) were added to the medium at 48
h following transfection and the cells were maintained under the
aforementioned conditions.
Reverse transcription-polymerase chain
reaction (RT-PCR) and reverse transcription-quantitative PCR
(RT-qPCR) analyses
Total RNA was extracted from tissue samples and CRC
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RNA was reverse transcribed into cDNA using the GoScript™
Reverse Transcription system (Promega Corp., Madison, WI, USA). All
PCR primers were purchased from Genscript (Nanjing, China): HOXA6
forward, 5′-TACACGCGCTACCAGACAC-3′ and reverse,
5′-GCGTGGAATTGATGAGCTTGTTT-3′ (product length, 178 bp); β-actin
forward, 5′-TGGAACGGTGAAGGTGACAG-3′ and reverse,
5′-AACAACGCATCTCATATTTGGAA-3′ (product length, 125 bp). RT-qPCR was
performed with GoTaq qPCR Master mix (Promega Corporation)
(nuclease-free water: 3.6 μl; upstream primer: 0.2
μl; downstream primer: 0.2 μl; 2X GoTaq®
qPCR Master Mix: 5 μl; cDNA: 1 μl. Thermocycling
steps: 95°C-10 min; 95°C-15 sec; 60°C-1 min, 40 cycles) and the
data were analyzed using the 2−ΔΔCq method (22). RT-PCR was performed with GoTaq
polymerase (Promega Corporation) according to the manufacturer’s
protocol (2X GoTaq Green Master Mix: 5 μl; upstream primer:
0.6 μl; downstream primer: 0.6 μl; cDNA: 2 μl;
nuclease-free water: 1.8 μl. Thermocycling steps: 95°C-2
min; 95°C-30 sec, 55°C-30 sec, 72°C-30 sec; 72°C-3 min. HOXA6: 32
cycles, β-actin: 23 cycles.). The PCR products were analyzed with
2% agarose gel electrophoresis, and subjected to densitometric
analysis with a gel imaging system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Western blot analysis
Proteins were extracted from the cells at 72 h
post-transfection using RIPA lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). The protein concentration was
determined using a BCA kit (Beyotime Institute of Biotechnology).
Subsequent to 10% SDS-PAGE separation (40 μg), the proteins
were transferred onto a PVDF membrane. The membrane was blocked in
5% skim milk at room temperature for 1–2 h, and then incubated at
4°C overnight with the primary antibodies. Following incubation
with appropriate horseradish peroxidase-conjugated anti-rabbit
secondary antibodies (#7074, dilution: 1:3,000) (Cell Signaling
Technology, Inc., Danvers, MA, USA) for 2 h at room temperature,
the membranes were visualized with an ECL kit (Beyotime Institute
of Biotechnology). The grayscale value of images was analyzed using
Fusion software (Fusion FX, Vilber lourmat). The primary antibodies
for western blot analysis included rabbit anti-HOXA6 (YT2212,
1:800) and anti-β-actin (A283, 1:1,000) from ImmunoWay
Biotechnology Company (Plano, TX, USA), and rabbit anti-B-cell
lymphoma-2 (Bcl-2, #3498, 1:3,000), anti-Bcl-2-associated X protein
(Bax, #5023, 1:3,000), anti-cleaved-caspase-3 (#9662, 1:1,000),
anti-poly (ADP-ribose) polymerase (PARP, #9532, 1:3,000),
anti-N-cadherin (#13116, 1:3,000), anti-E-cadherin (#3195, 1:3,000)
and anti-Vimentin (#5741, 1:3,000) from Cell Signaling Technology,
Inc.
Cell proliferation assay
Cell proliferation was assessed using a cell
counting kit-8 (CCK-8) assay. The stably transfected cells,
including the Caco2-HOXA6, Caco2-NC, HT-29-shHOXA6 and HT-29-NC
cells, were seeded in a 96-well plate at a density of
4×103 cells/well, and cultured overnight. CCK-8 reagent
was then added into the wells and the absorbance at 450 nm was
detected following culture for 24, 48 and 72 h.
For the colony formation assay, the
stably transfected cells were seeded in a 6-well plate at a density
of 500 cells/well
Following culture for 2 weeks, the clones were fixed
with paraformaldehyde and stained with gentian violet. The numbers
of clones containing >50 cells were counted under an inverted
phase contrast microscope (Leica, Wetzlar, Germany).
A 5-ethynyl-2′-deoxyuridine (EdU) assay was also
performed to measure cell proliferation ability. An EdU kit
(RiboBio Co., Ltd., Guangzhou, China) was used according to the
manufacturer’s protocol. Images were captured with a fluorescence
microscope (Olympus Corporation, Tokyo, Japan).
Cell migration and invasion
Cell migration and invasion were measured using
Transwell assays. To determine the rate of cell migration, 200
μl of RPMI-1640 medium containing 5×104 cells was
added into the upper chamber, and 700 μl RPMI-1640
containing 10% FBS was added into the lower chamber. Following
incubation for 48 h, the migrated cells were fixed and stained with
crystal violet for 15 min. Subsequently, the cells were viewed and
counted under an inverted phase contrast microscope in five fields
of view. The invasion ability was measured with Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA). The Matrigel was diluted
with RPMI-1640 and added to the upper chamber (100 μl/well).
Following solidification of the Matrigel, 100 μl of
RPMI-1640 containing 1×105 cells was added into the
upper chamber; the remaining steps were the same as for the
migration assay.
Cell migration was also detected using a
wound-healing assay. The stably transfected cells were seeded in a
6-well plate. At confluence, the cell monolayer was scratched,
washed with PBS, and maintained in serum-free RPMI-1640. Following
incubation for 0, 24 and 48 h, images were captured using an
inverted microscope.
Cell apoptosis
Flow cytometry was used to detect the rate of
apoptosis of Caco2 and HT-29 cells. At 48 h post-transfection, the
cells were harvested with EDTA-free trypsin and suspended in PBS
buffer. The cells were stained with an Annexin V-FITC/PI kit (BD
Biosciences) according to the manufacturer’s protocol, and
immediately analyzed by flow cytometry.
Apoptosis was also measured using a terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
kit (Roche Applied Science, Indianapolis, IN, USA), according to
the manufacturer’s protocol. Following TUNEL staining, the cells
were stained with DAPI for 5 min and washed with PBS. A
fluorescence microscope (Leica) was used to capture images.
Statistical analysis
Each experiment was independently repeated three
times. Statistical analysis was performed with SPSS version 22 (IBM
SPSS, Armonk, NY, USA). All data are presented as the mean ±
standard deviation and were analyzed using Student’s t-test (two
groups) or two-way analysis of variance [for the results of CCK-8
assay (Fig. 2A) and wound-healing
assay (Fig. 4B)] followed by
Bonferroni’s test. A value of P<0.05 was considered to indicate
a statistically significant difference.
Results
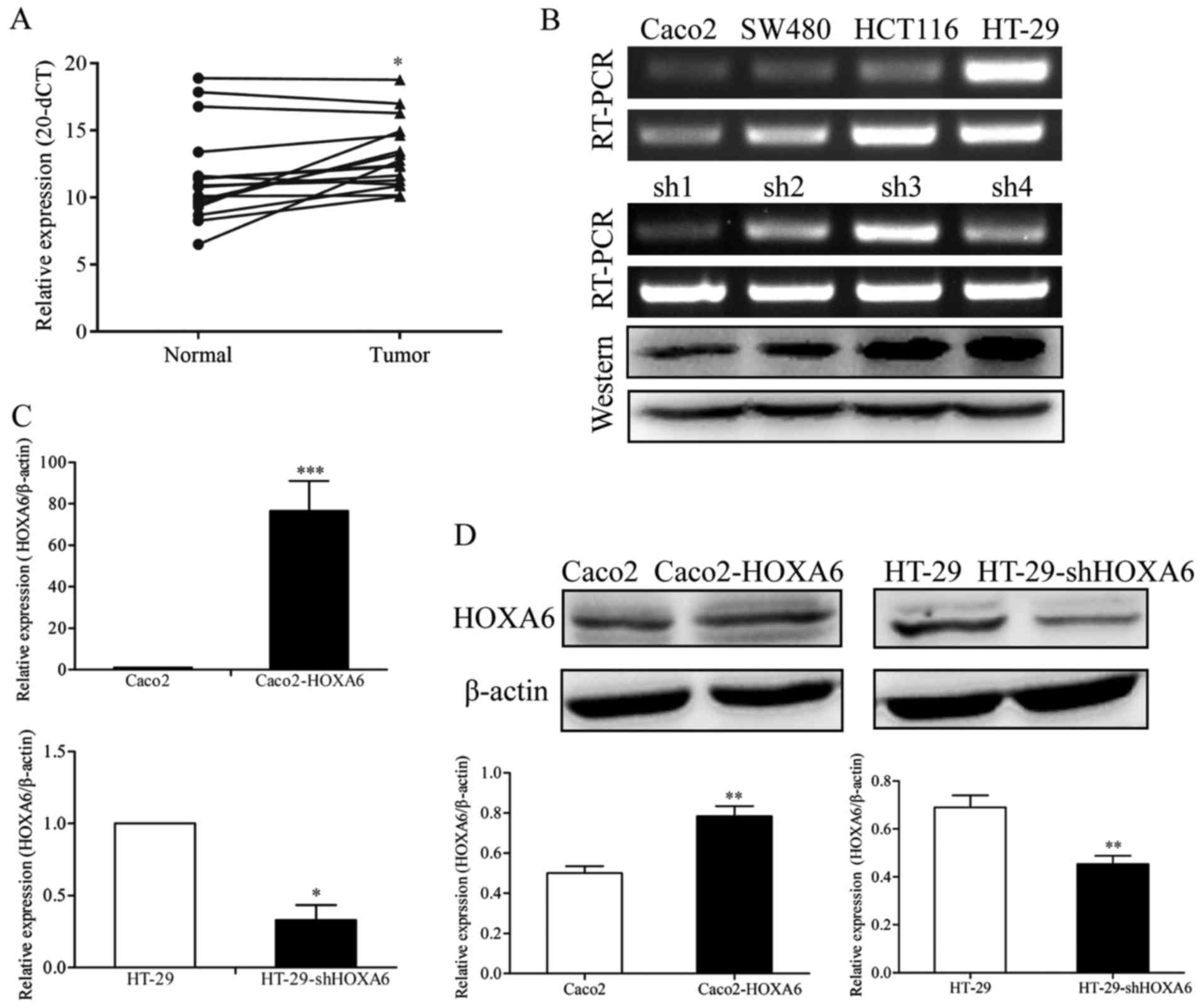
Expression of HOXA6 in human CRC tissues
and cell lines
The detection of the mRNA expression of HOXA6 in CRC
and normal colorectal tissue samples was performed by RT-qPCR
analysis. The results showed that the mRNA levels of HOXA6 in the
CRC tissues were significantly upregulated, compared with those in
the paired normal colorectal tissue samples (P=0.0103) (Fig. 1A). Subsequently, the expression
levels of HOXA6 in four CRC cell lines (Caco2, SW480, HCT116 and
HT-29) were detected by RT-PCR analysis. The mRNA expression level
of HOXA6 was highest in the HT-29 cells and lowest in Caco2 cells
(Fig. 1B). Therefore, these two
cell lines were selected for the subsequent experiments.
Verification of the knockdown efficiency
of the shHOXA6 plasmid
To analyze the knockdown efficiency of four
interference plasmids targeting HOXA6 (shRNA1/2/3/4-HOXA6), the
expression of HOXA6 in transfected cells was examined by RT-PCR and
western blot analyses. HOXA6 was expressed the least in the cells
transfected with shRNA1-HOXA6, as confirmed by RT-PCR and western
blot analyses (Fig. 1B). Based on
these results, shRNA1-HOXA6 was selected as the most efficient
interference plasmid for the remaining experiments.
Verification of the expression of HOXA6
in transfected cells
The Caco2 cells were transfected with plasmids
expressing HOXA6 or an NC and HT-29 cells were transfected with
shHOXA6 or NC plasmids. The expression of HOXA6 was detected by
RT-qPCR and western blot analyses. The results showed that the mRNA
and protein expression levels were significantly upregulated in the
Caco2-HOXA6 cells (P=0.0008 and 0.0012) and suppressed in the
HT-29-shHOXA6 cells (P=0.0225 and 0.0026) (Fig. 1C and D).
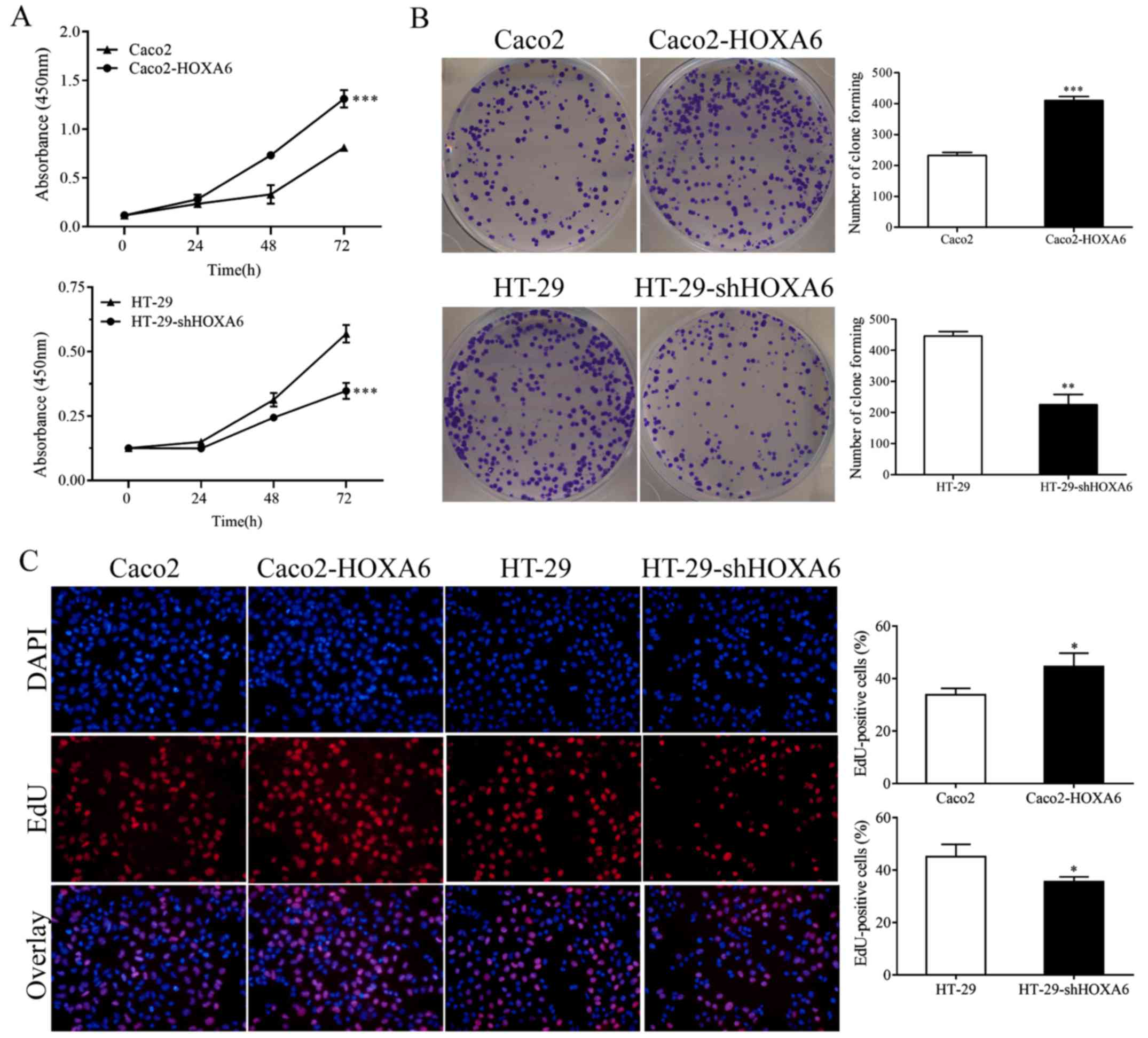
HOXA6 enhances the proliferation rate of
CRC cells
To examine the effect of HOXA6 on CRC cell
proliferation, CCK-8, colony formation and EdU assays were
performed. The CCK-8 assay demonstrated that the Caco2-HOXA6 cells
grew significantly faster than the Caco2-NC cells (P<0.0001),
whereas the growth rate of the HT-29-shHOXA6 cells was
significantly lower, compared with that of the HT-29-NC cells
(P<0.0001) (Fig. 2A). In
addition, the colony formation assay demonstrated that the
Caco2-HOXA6 cells formed more colonies than the Caco2-NC cells
(P<0.0001), whereas the HT-29-shHOXA6 cells formed fewer
colonies than the HT-29-NC cells (P=0.0004) (Fig. 2B). The EdU assay demonstrated that
the upregulated expression of HOXA6 promoted cell proliferation
(P=0.0301), whereas the downregulation of HOXA6 suppressed cell
proliferation (P=0.0310) (Fig.
2C). These results indicated that the expression of HOXA6
enhanced the proliferative capacity of the CRC cells.
HOXA6 inhibits apoptosis in CRC
cells
The effect of HOXA6 on CRC cell apoptosis was
evaluated with flow cytometry and a TUNEL assay. The results of the
two assays revealed that the rate of apoptosis of the Caco2-HOXA6
cells was inhibited, compared with that of the Caco2-NC cells
(P<0.05), whereas the rate of apoptosis of the HT-29-shRNA cells
was significantly higher, compared with that of the HT-29-NC cells
(P<0.01) (Fig. 3A and B).
Western blot analysis was performed to detect the protein
expression levels of PARP, Bcl-2, Bax and caspase-3. The protein
expression levels of cleaved PARP, Bax and cleaved caspase-3 were
inhibited in the Caco2-HOXA6 cells and increased in the HT-29-shRNA
cells, whereas the expression of Bcl-2 was increased in the
Caco2-HOXA6 cells and suppressed in the HT-29-shHOXA6 cells,
compared with levels in the NC cells (Fig. 3C). These results demonstrated that
HOXA6 inhibited the apoptosis of the CRC cells.
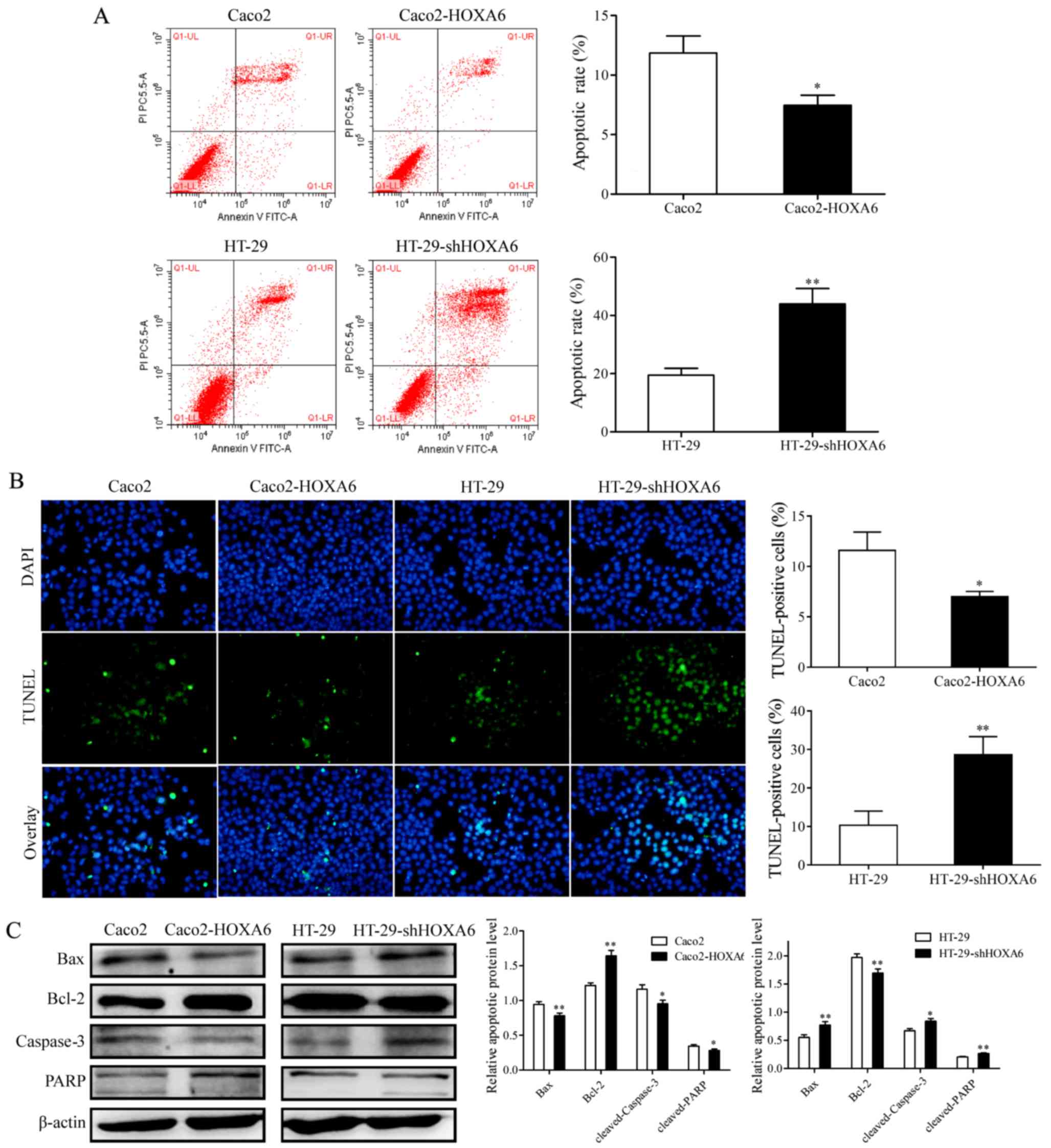 | Figure 3Rate of apoptosis in transfected
cells, as detected by flow cytometry and TUNEL assays, and the
expression of apoptosis-related proteins. (A) Results of flow
cytometry for the rate of apoptosis in the transfected cells. (B)
Results of the TUNEL assay, which also measured the rate of
apoptosis (×200 magnification). Cells stained with both TUNEL and
DAPI were considered as apoptotic cells. (C) Western blot analysis
to detect the protein expression levels of Bax, Bcl-2, caspase-3
and PARP. Three independent experiments were performed for each
assay. *P<0.05 and **P<0.01. TUNEL,
terminal deoxynucleotidyl transferase dUTP nick end labeling;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-assocated X protein; PARP,
poly(ADP-ribose) polymerase; sh, short hairpin RNA; HOXA6, homeobox
A6. |
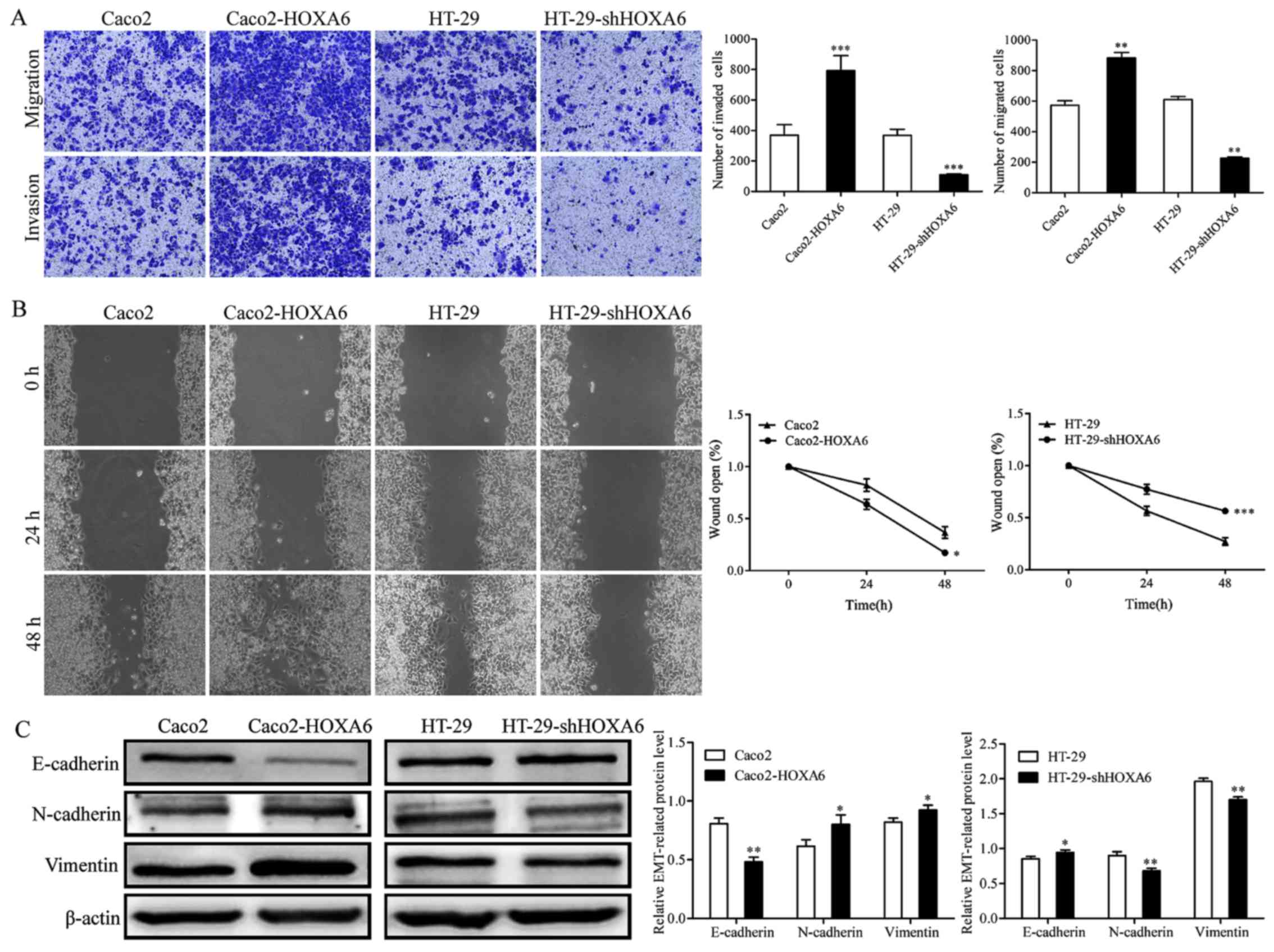
Expression of HOXA6 enhances CRC cell
migration and invasion
Transwell and wound-healing assays were performed to
analyze the effect of HOXA6 on CRC cell migration and invasion. The
results of the Transwell assay showed that the numbers of migrated
and invaded Caco2-HOXA6 cells were significantly increased, whereas
the numbers of migrated and invaded HT-29-shHOXA6 cells were
suppressed, compared with those in the NC (Fig. 4A). The wound-healing assay
demonstrated that the Caco2-HOXA6 cells migrated at a faster rate
than the Caco2-NC cells (P=0.0186), whereas the HT-29-shHOXA6 cells
migrated at a slower rate than the HT-29-NC cells (P=0.0003)
(Fig. 4B). The results of the
western blot analysis showed that the expression levels of
N-cadherin and Vimentin increased, whereas the expression of
E-cadherin decreased in the Caco2-HOXA6 cells, with the opposite
pattern observed in the HT-29-shHOXA6 cells (Fig. 4C). These results confirmed that
HOXA6 enhanced the migration and invasion of the CRC cells.
Discussion
The occurrence and development of tumors are
associated with several factors, including behavioral and
environmental factors. The occurrence of tumors is the result of
the accumulation of mutations in a number of genes over an extended
period of time. The identification of affected genes may provide a
basis for the early diagnosis and treatment of cancer.
HOXA6, a member of the HOX family, has been reported
to be involved in the regulation of certain types of malignancy.
The present study investigated the effect of the expression of
HOXA6 on CRC cell proliferation, apoptosis, migration and invasion,
and examined the expression of associated proteins. Initially, the
RT-qPCR results demonstrated that HOXA6 was upregulated in CRC
samples compared with normal tissues. Subsequently, by upregulating
and downregulating the expression of HOXA6 with plasmid
transfection, it was found that the upregulated expression of HOXA6
promoted proliferation, migration and invasion, and inhibited
apoptosis, whereas the downregulated expression of HOXA6 had the
opposite effects. Finally, the expression levels of associated
proteins were detected. The upregulated expression of HOXA6
increased the expression levels of cleaved PARP, cleaved caspase-3,
Bax, N-cadherin and Vimentin, but decreased the expression levels
of Bcl-2 and E-cadherin. The downregulated expression of HOXA6
induced the opposite results. These results suggested that HOXA6
promoted proliferation and metastasis, and inhibited apoptosis in
CRC.
Apoptosis is the process of programmed cell death,
which eliminates unnecessary or unhealthy cells from the body.
Cancer cells avoid apoptosis, and gain an advantage in survival and
proliferation by promoting anti-apoptotic mechanisms and
downregulating pro-apoptotic programs (23). Apoptosis is regulated by the Bcl-2
and caspase families, particularly caspase-3. The activation of
caspase-3 is necessary for efficient apoptosis (24). The balance between pro-apoptotic
and anti-apoptotic Bcl-2 family proteins is also significant in
apoptosis. Previous studies have identified that the Bcl-2/Bax
ratio was increased in tumor tissues, causing a reduced rate of
apoptosis (25,26). PARP is cleaved by caspase-3 during
apoptosis, which means that PARP cleavage is an important indicator
of apoptosis and caspase-3 activation (27,28).
This is consistent with the experimental results of the present
study described above.
Tumor metastasis is the main cause of the poor
prognosis of CRC. Following the determination of changes in
migration and invasion, the expression of epithelial-mesenchymal
transition (EMT)-related proteins was detected. It has been
demonstrated that EMT initiates the isolation of cancer cells from
primary carcinomas, which subsequently migrate and spread further
(29,30). Its core features are a decreased
expression of E-cadherin and increased expression of Vimentin. The
experimental results in the present study confirmed that an
increase in the expression of HOXA6 promoted EMT.
The findings of the present study suggested that
HOXA6 was associated with the proliferation, apoptosis, migration
and invasion of CRC cells, and further elucidated the potential
mechanisms by which HOXA6 inhibited apoptosis and promoted
migration and invasion. However, there were number of shortcomings
in the present study and the potential mechanism underlying the
regulation of cell proliferation requires further investigation.
The number of clinical samples was insufficient, and additional
samples are required to verify the expression of HOXA6 in
colorectal tumors. There was also a lack of relevant clinical
information regarding the study participants. In addition, HOXA6,
as a transcription factor, mediates its biological effect by
regulating the expression of downstream genes, which may provide a
direction for further investigations.
In conclusion, the expression of HOXA6 was
demonstrated to be associated with the proliferation, apoptosis,
migration and invasion of CRC cells through experiments involving
HOXA6 knockdown or transfection. These results suggest that HOXA6
is involved in the regulation of CRC, which may inform the
development of strategies for the diagnosis and treatment of
CRC.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
CCK-8
|
cell counting kit-8
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
EMT
|
epithelial-mesenchymal transition
|
Acknowledgments
The authors would like to thank Dr Tingxiu Xiang and
the Chongqing Key Laboratory of Molecular Oncology and Epigenetics
for providing the equipment, materials and technical supports.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
SW and FW made substantial contributions to the
study conception, design, acquisition of data, analysis and
interpretation of the data and were involved in the drafting of the
manuscript. ZJ was involved in revising the manuscript critically
for important intellectual content. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was signed by all patients, and
ethics approval was obtained from the Ethics Committee of the First
Affiliated Hospital of Chongqing Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RG, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar
|
|
4
|
Wu S, Wu F and Jiang Z: Identification of
hub genes, key miRNAs and potential molecular mechanisms of
colorectal cancer. Oncol Rep. 38:2043–2050. 2017. View Article : Google Scholar
|
|
5
|
Mourtzikou A, Stamouli M, Kroupis C,
Christodoulou S, Skondra M, Kastania A, Pectasides D, Athanasas G
and Dimas C: Evaluation of carcinoembryonic antigen (CEA),
epidermal growth factor receptor (EGFR), epithelial cell adhesion
molecule EpCAM (GA733-2), and carbohydrate antigen 19-9 (CA 19-9)
levels in colorectal cancer patients and correlation with
clinicopathological characteristics. Clin Lab. 58:441–448.
2012.
|
|
6
|
Shah N and Sukumar S: The Hox genes and
their roles in oncogenesis. Nat Rev Cancer. 10:361–371. 2010.
View Article : Google Scholar
|
|
7
|
Bhatlekar S, Fields JZ and Boman BM: HOX
genes and their role in the development of human cancers. J Mol Med
(Berl). 92:811–823. 2014. View Article : Google Scholar
|
|
8
|
Takahashi O, Hamada J, Abe M, Hata S,
Asano T, Takahashi Y, Tada M, Miyamoto M, Kondo S and Moriuchi T:
Dysregulated expression of HOX and ParaHOX genes in human
esophageal squamous cell carcinoma. Oncol Rep. 17:753–760.
2007.
|
|
9
|
Lv J, Cao XF, Ji L, Zhu B, Wang DD, Tao L
and Li SQ: Association of β-catenin, Wnt1, Smad4, Hoxa9, and Bmi-1
with the prognosis of esophageal squamous cell carcinoma. Med
Oncol. 29:151–160. 2012. View Article : Google Scholar
|
|
10
|
Gu ZD, Shen LY, Wang H, Chen XM, Li Y,
Ning T and Chen KN: HOXA13 promotes cancer cell growth and predicts
poor survival of patients with esophageal squamous cell carcinoma.
Cancer Res. 69:4969–4973. 2009. View Article : Google Scholar
|
|
11
|
Han Y, Lu S, Wen YG, Yu FD, Zhu XW, Qiu
GQ, Tang HM, Peng ZH and Zhou CZ: Overexpression of HOXA10 promotes
gastric cancer cells proliferation and HOXA10(+)/CD44(+) is
potential prognostic biomarker for gastric cancer. Eur J Cell Biol.
94:642–652. 2015. View Article : Google Scholar
|
|
12
|
Sentani K, Oue N, Naito Y, Sakamoto N,
Anami K, Oo HZ, Uraoka N, Aoyagi K, Sasaki H and Yasui W:
Upregulation of HOXA10 in gastric cancer with the intestinal mucin
phenotype: Reduction during tumor progression and favorable
prognosis. Carcinogenesis. 33:1081–1088. 2012. View Article : Google Scholar
|
|
13
|
He YX, Song XH, Zhao ZY and Zhao H: HOXA13
upregulation in gastric cancer is associated with enhanced cancer
cell invasion and epithelial-to-mesenchymal transition. Eur Rev Med
Pharmacol Sci. 21:258–265. 2017.
|
|
14
|
He X, Liu Z, Xia Y, Xu J, Lv G, Wang L, Ma
T, Jiang L, Mou Y, Jiang X, et al: HOXB7 overexpression promotes
cell proliferation and correlates with poor prognosis in gastric
cancer patients by inducing expression of both AKT and MARKs.
Oncotarget. 8:1247–1261. 2017.
|
|
15
|
Joo MK, Park JJ, Yoo HS, Lee BJ, Chun HJ,
Lee SW and Bak YT: The roles of HOXB7 in promoting migration,
invasion, and anti-apoptosis in gastric cancer. J Gastroenterol
Hepatol. 31:1717–1726. 2016. View Article : Google Scholar
|
|
16
|
Chen SW, Zhang Q, Xu ZF, Wang HP, Shi Y,
Xu F, Zhang WJ, Wang P and Li Y: HOXC6 promotes gastric cancer cell
invasion by upregulating the expression of MMP9. Mol Med Rep.
14:3261–3268. 2016. View Article : Google Scholar
|
|
17
|
Zhang Q, Jin XS, Yang ZY, Wei M, Liu BY
and Gu QL: Upregulated Hoxc6 expression is associated with poor
survival in gastric cancer patients. Neoplasma. 60:439–445. 2013.
View Article : Google Scholar
|
|
18
|
Liao WT, Jiang D, Yuan J, Cui YM, Shi XW,
Chen CM, Bian XW, Deng YJ and Ding YQ: HOXB7 as a prognostic factor
and mediator of colorectal cancer progression. Clin Cancer Res.
17:3569–3578. 2011. View Article : Google Scholar
|
|
19
|
Guo YB, Shao YM, Chen J, Xu SB, Zhang XD,
Wang MR and Liu HY: Effect of overexpression ofHOXgenes on its
invasive tendency in cerebral glioma. Oncol Lett. 11:75–80. 2016.
View Article : Google Scholar
|
|
20
|
Dickson GJ, Liberante FG, Kettyle LM,
O’Hagan KA, Finnegan DP, Bullinger L, Geerts D, McMullin MF, Lappin
TR, Mills KI, et al: HOXA/PBX3 knockdown impairs growth and
sensitizes cytogenetically normal acute myeloid leukemia cells to
chemotherapy. Haematologica. 98:1216–1225. 2013. View Article : Google Scholar
|
|
21
|
Cheng TL and Chang WT: Construction of
simple and efficient DNA vector-based short hairpin RNA expression
systems for specific gene silencing in mammalian cells. Methods Mol
Biol. 408:223–241. 2007. View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Fernald K and Kurokawa M: Evading
apoptosis in cancer. Trends Cell Biol. 23:620–633. 2013. View Article : Google Scholar
|
|
24
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar
|
|
25
|
Zeren T, Inan S, Vatansever HS and Sayhan
S: Significance of apoptosis related proteins on malignant
transformation of ovarian tumors: A comparison between Bcl-2/Bax
ratio and p53 immunoreactivity. Acta Histochem. 116:1251–1258.
2014. View Article : Google Scholar
|
|
26
|
Samarghandian S, Nezhad MA and Mohammadi
G: Role of caspases, Bax and Bcl-2 in chrysin-induced apoptosis in
the A549 human lung adenocarcinoma epithelial cells. Anticancer
Agents Med Chem. 14:901–909. 2014. View Article : Google Scholar
|
|
27
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly(ADP-ribose)
polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP
mutant increases rates of apoptosis in transfected cells. J Biol
Chem. 274:22932–22940. 1999. View Article : Google Scholar
|
|
28
|
Mullen P: PARP cleavage as a means of
assessing apoptosis. Methods Mol Med. 88:171–181. 2004.
|
|
29
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: Emt: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar
|
|
30
|
Heerboth S, Housman G, Leary M, Longacre
M, Byler S, Lapinska K, Willbanks A and Sarkar S: EMT and tumor
metastasis. Clin Transl Med. 4:62015. View Article : Google Scholar
|