Introduction
Epithelial ovarian cancer (EOC) is the seventh most
common cause of tumor-associated mortality in women and the most
lethal gynecologic malignancy. The American Cancer Society
estimated a total of 22,440 new cases and 14,080 mortalities from
the disease in 2017 (1). The high
fatality rate is predominantly attributed to the late detection and
chemoresistance of EOC. It is a heterogeneous condition composed of
different types of tumors with widely varied clinicopathological
features and behavior. EOC, according to histological criteria, is
divided into five major subtypes: High-grade serous, clear cell,
endometrioid, mucinous and low-grade serous types. These five
subtypes are best considered to be distinct entities, of which
serous type, accounting for 70% of EOC, is the most common
(2,3).
Histological grade has been demonstrated to be an
important prognostic factor for serous ovarian carcinoma in
previous studies (4–7). However, there are several traditional
systems used for grading these tumors with different categories and
number of strata (International Federation of Gynecology and
Obstetrics, World Health Organization and Gynecologic Oncology
Group criteria) (8). In 2002 and
subsequently in 2004, a two-tier system for grading serous ovarian
carcinoma was proposed, according to which tumors are subdivided
into high-grade and low-grade (4,9).
Although a large number of studies report that high-grade serous
carcinoma (HGSC) and low-grade serous carcinoma (LGSC) share the
same origin from fallopian tubal epithelia, they are completely
distinct types of gynecologic tumor, rather than different grades
of the same neoplasm, and there are drastic clinicopathological and
molecular differences between the two (10–13).
HGSC is usually susceptible to platinum-based chemotherapy; however
5- and 10-year survival rates for advanced staged carcinoma are ~25
and 0%, respectively. LGSC progresses slowly with a 5-year survival
rate of 85% and a 10-year survival rate of 50%. It is usually
resistant to standard chemotherapy. Thus, optimal cytoreductive
surgery is more critical for the treatment of LGSC (14). Accurate differential diagnosis may
help to reduce unnecessary morbidity and optimize therapeutic
effectiveness.
Primary cytoreductive surgery has been considered
the standard therapeutic strategy for serous ovarian cancer in many
centers, however neoadjuvant chemotherapy followed by interval
debulking surgery is achieving more and more attention recently. A
clinical trial conducted by Vergote et al (15) among patients with stage IIIC and IV
ovarian cancers demonstrated that there was no difference in
survival rates between patients undergoing primary cytoreductive
surgery and those receiving neoadjuvant chemotherapy followed by
interval debulking surgery. With respect to morbidity and mortality
risk associated with the extent of surgery, neoadjuvant
chemotherapy is preferable. Thus, neoadjuvant chemotherapy is
quickly becoming the superior approach for advanced stage ovarian
carcinoma. Unlike LGSC, HGSC is comparatively more responsive to
chemotherapy. In addition, clinical trials specifically targeting
the crucial molecular alterations of LGSC, such as CI-1040
targeting the mitogen-activated protein kinase pathway, have been
launched and are on-going (16).
Thus, a precise histopathology-based differential diagnosis of HGSC
and LGSC is crucial for decision making by gynecological
oncologists prior to any treatment.
The two-tier system for grading serous ovarian
carcinoma is based primarily on nuclear atypia, with the mitotic
rate used as a secondary criteria. Malpica et al (17) demonstrated that the system is easy
to follow and is reproducible. In most epithelial ovarian tumors,
the nuclear atypia of LGSC and HGSC corresponds to grade 1 and
grade 3, respectively, in a three-grade system. However, nuclear
features of certain tumors (~4% of serous carcinomas) are
intermediary between LGSC and HGSC (18). Thus, distinguishing these tumors of
intermediate grade remains a challenge. Furthermore, subjective
judgments on the criteria of nuclear atypia and mitotic rate from
individual pathologists may also pose another problem for
differential diagnosis of HGSC and LGSC. Thus, it is necessary to
explore and establish objective criteria to assist differential
diagnosis. In recent years, a number of researchers have begun to
concentrate on distinguishing HGSC from LGSC through various
methods, such as immunohisto-chemical markers (19). Although these methods do improve
the accuracy of diagnosis to a certain extent, there have been no
satisfactory results obtained as of yet. Thus, the purpose of the
current study was to investigate useful markers in order to raise
the efficacy in distinguishing HGSC from LGSC.
Materials and methods
Tissue samples for RNA-seq analysis
Flash-frozen primary serous ovarian tumor samples
(n=12) were collected from Qilu Hospital of Shandong University
(Jinan, China) from patients that did not undergo preoperative
chemotherapy: 6 ovarian high-grade serous cancers and 6 ovarian
low-grade serous cancers. In the present study, patients with
primary ovarian serous carcinoma were selected for investigation.
Patients with other malignancies or those that had undergone
preoperative chemotherapy were excluded. The ages of selected
patients with 43–63 years. The samples were collected with the
approval of the Ethics Committee at Qilu Hospital of Shandong
University between January 2014 and December 2015, and with the
signed informed consent from all patients. All tumors were
diagnosed according to the two-tier system of MD Anderson Cancer
Center criteria (4). Frozen
sections (5 μm) were cut, fixed onto slides, and immediately
stored at −20°C. The remaining samples were immediately stored at
liquid nitrogen for subsequent arrays. The slides were then stained
with hematoxylin (0.2%, 5 min) and eosin (1%, 10 min) at room
temperature for reviewing and ensuring accurate diagnosis by two
gynecological pathologists. Tumor samples containing >70% of
invasive cancer were used for sequencing analysis.
RNA extraction and RNA-seq analysis
Total RNA was isolated from cancer samples using
TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer’s protocol. Prior to mRNA sequencing,
the quality of all RNA samples were detected by agarose gel (1%)
electrophoresis and Agilent 2100 bioanalyzer (Agilent Technologies,
Inc., Santa Clara, CA, USA). Following mRNA sequencing using the
Illumina HiSeq X Ten platform, TopHat2 (ccb.jhu.edu/software/tophat) was used to align
paired-end clean reads to the reference genome (20). One HGSC sample was excluded because
of lower proportion of total reads mapped (<80%). Then, HTSeq
v0.9.1 (pypi.python.org/pypi/HTSeq) was applied to count the
read numbers mapped of each gene. Subsequently, reads per kilobase
of exon model per million mapped reads (RPKM) of each gene was
calculated based on the length of the gene and reads count mapped
to this gene. RPKM takes sequencing depth and gene length into
consideration and is currently the most common method for
estimating gene expression levels (21).
Differential expression analysis and
clustering
Differential expression analysis between HGSC and
LGSC was performed using the DESeq R package (version 3.4.2;
r-project.org/). DESeq provides statistical
routines for testing differential expression by the use of negative
binonial distribution. P<0.05 in DESeq was considered to
indicate a statistically significant difference. To identify the
correlation of different samples, differentially expressed genes
were clustered between HGSC and LGSC using unsupervised
hierarchical clustering method with the function of heatmap.2 in R
(version 3.4.2). Manhattan distance metric with average linkage was
used for clustering. To allow for log adjustment, genes with 0 RPKM
were assigned a value of 0.01. We also performed a Gene Ontology
analysis (geneontology.org) on all the
differential genes between HGSC and LGSC.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Ovarian HGSC (n=36) and LGSC (n=15) were used for
validation of differentially expressed genes between them in mRNA
sequencing. They were obtained from Qilu Hospital of Shandong
University between January 2011 and June 2016. The ages of selected
patients are range from 41–76 years. The inclusion and exclusion
criteria of selected patients were the same as described above.
Tumor samples containing >70% of invasive cancer were selected
for research. Based on the above results and the literature, a
number of molecules were selected for further validation and
investigation [lysosomal associated membrane protein 3 (LAMP3), EGF
like domain multiple 6 (EGFL6), cyclin-dependent kinase inhibitor
1A (CDKN1A), anterior gradient 3, protein disulphide isomerase
family member (AGR3), glutathione S-transferase μ1 (GSTM1),
tectonic family member 1 (TCTN1), spermatogenesis associated 18
(SPATA18)]. Of them, LAMP, EGFL6, CDKN1A and AGR3 exhibited
significant differences between HGSC and LGSC, and were selected in
the current study to demonstrate the results. Oligonucleotide
primers (Sangon Biotech Co., Ltd., Shanghai, China) designed for
each gene were as follows: LAMP3 forward, 5′-GCGTCCCTGGCCGTAATTT-3′
and reverse, 5′-TGCTTGCTTAGCTGGTTGCT-3′; EGFL6 forward,
5′-GTCTGTGAAGCTACATGCGAA-3′ and reverse, 5′-CATGGCCGGGGTTTCATTC-3′;
CDKN1A forward, 5′-CGATGGAACTTCGACTTTGTCA-3′ and reverse,
5′-GCACAAGGGTACAAGACAGTG-3′; AGR3 forward,
5′-ATCACCTGATGGGCAATATGTG-3′ and reverse,
5′-GAGTATCTTCCAGCTATGTCAGC-3′; β-actin forward,
5′-TCATGAAGTGTGACGTGGACATC-3′ and reverse,
5′-CAGGAGGAGCAATGATCTTGATCT-3′. β-actin was used as the endogenous
control.
Each RNA sample (<1 μg) was reverse
transcribed using the Reverse Transcription system (Takara Bio,
Inc., Otsu, Japan) in 20 μl reaction system. Following 37°C
incubation for 15 min, the reaction system was incubated in 85°C
for 5 sec and then stored at −20°C. RT-qPCR was performed using
SYBR-Green qPCR master mix (Takara Bio, Inc.), with the ROX as
internal reference dye. PCR was conducted using the ABI PRISM
7900HT System (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The qPCR cycling conditions were as follows: Predegeneration at
95°C for 30 sec, then dena-turation at 95°C for 5 sec and annealing
at 60°C for 34 sec, the last two steps were repeated for a total of
40 cycles. All experiments were performed in triplicate for target
and reference genes. Statistical analysis was conducted using
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Western blot analysis
HGSC (n=8) and seven LGSC (n=7) samples were
included for this analysis. They were obtained from Qilu Hospital
of Shandong University from January 2010 to December 2014. The ages
of selected patients range from 43–76 years. The inclusion and
exclusion criteria of selected patients were as described above.
All samples were cut into pieces before they were lysed on ice with
radioimmunoprecipitation assay buffer (Biocolor Ltd.,
Carrickfergus, UK) and protease inhibitors. The protein
concentration was determined using a bicinchoninic acid assay kit
(Beyotime Institute of Biotechnology, Haimen, China). Protein
samples (30 μg) were separated using 12% (separating gel)
and 6% (stacking gel) SDS-polyacrylamide gel and then
electrotransferred onto poly-vinylidene difluoride membrane (EMD
Millipore, Billerica, MA, USA). Following blocking with 5% non-fat
milk at room temperature for 1 h, the membranes were incubated
overnight at 4°C with the mouse anti-human monoclonal antibody
against AGR3 (1:1,000; cat. no. ab82400; Abcam, Cambridge, UK),
mouse anti-human monoclonal antibody against tumor protein 53
(TP53; 1:1,000; cat. no. M7001; Dako; Agilent Technologies, Inc.)
and mouse anti-human monoclonal antibody against β-actin (1:2,000;
cat. no. A2228; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Subsequently, the membranes were incubated with specific
horseradish peroxidase-labeled secondary antibodies to mouse IgG
(1:5,000; cat. no. 074-1806; Kirkegaard & Perry Laboratories
Inc., Gaithersburg, MD, USA) at room temperature for 1 h. Signal
was detected with enhanced chemiluminescence using Western Lighting
Plus ECL (PerkinElmer, Inc., Waltham, MA, USA) by ImageQuant LAS
4000 (GE Healthcare Life Sciences, Little Chalfont, UK).
Immunohistochemistry (IHC) analysis
Protein expression of AGR3 and TP53 was detected in
samples of HGSC (n=145) and LGSC (n=30). Cases were reviewed by two
gynecological pathologists, and a diagnosis of high and low-grade
serous ovarian carcinomas was established according to the two-tier
system of MD Anderson Cancer Center criteria. The clinicopathologic
characteristics of all samples are shown in Table I. Formalin-fixed (10% formalin
fixation for 24–48 h at room temperature) and paraffin-embedded
tissues were sectioned at 4 μm and incubated at 70°C for 30
min. Tissue sections were dewaxed at room temperatures as follows:
xylene I (100%) for 15 min, xylene II (100%) for 15 min, and then a
graded series of ethanol (100, 95, 80 and 70%) for 5 min each. To
uncover the antigens sufficiently, the sections were submerged for
antigenic retrieval in citrate buffer (for AGR3, pH 6.0) and EDTA
buffer (for TP53, pH 9.0) at 96–99°C for 15 min, then cooled to
room temperature. The activity of endogenous peroxidases was
inhibited with 3% hydrogen peroxide at 37°C for 15 min.
Subsequently, the non-specific binding sites were blocked with goat
serum from the IHC reagent kit (cat. no. SP9000; OriGene
Technologies, Inc., Beijing, China). The mouse anti-human
monoclonal antibody for AGR3 (cat. no. ab82400; Abcam) was applied
at a dilution of 1:4,000 and mouse anti-human monoclonal antibody
for TP53 (cat. no. ZM0408; OriGene Technologies, Inc.) at the
working concentration. Then, the slides were stored in a moist
chamber at room temperature for 2 h. Following washing of the
sections (PBS, 3 times for 3 min), they were incubated with the
secondary antibody from IHC reagent kit (OriGene Technologies,
Inc.) at 37°C for 20 min, and then the sections were washed again
as above. Sections were stained with diaminobenzidine for 1.5 min
at room temperature and counterstained with hematoxylin (0.2%) for
2 min at room temperature. Negative controls were made by omitting
the primary antibody. A light microscope (Nikon Corporation, Tokyo,
Japan) was used to visualize the stained tissue sections.
 | Table IMain clinicopathological
characteristics of patients enrolled in the immunohistochemistry
study. |
Table I
Main clinicopathological
characteristics of patients enrolled in the immunohistochemistry
study.
| Characteristic | HGSC (n=145) | LGSC (n=30) |
|---|
| Age at
diagnosis | | |
| Mean ± SD | 55.43±9.74 | 50.20±11.48 |
| Median
(range) | 56 (35-78) | 49 (24-77) |
| FIGO stage (n) | | |
| I+II | 30 | 13 |
| III+IV | 115 | 17 |
| CA125 (n) | | |
| <200 U/ml | 20 | 14 |
| ≥200 U/ml | 125 | 16 |
AGR3 was scored as positive or negative and TP53 was
scored as aberrant expression or wild-type pattern. The
subcel-lular localization of AGR3 on staining was predominantly
found to be membranous, whereas for TP53 it was nuclear. More than
or equal to 20% positively stained in epithelial cells were defined
as positive for AGR3. While aberrant expression of TP53 was defined
as 0 (negative or occasional cells positive) or ≥75% of cells
staining, according to clinical practice and previous studies
(22), and also because of
evidence that these TP53-staining patterns correlated with the
mutational status of TP53 (23,24).
Thus, absence and overexpression of TP53 were considered as
aberrant expression, indicating various types of TP53 mutation. The
usefulness of TP53 and AGR3 to distinguish HGSC and LGSC was
evaluated with a χ2 test. Then, the TP53/AGR3
differential diagnostic performance was quantified with sensitivity
and specificity calculations with morphological classification as
the gold standard. Sensitivity, also termed the true positive rate,
is defined as the proportion of positives that are correctly
identified as such. Specificity, also termed the true negative
rate, is defined as the proportion of negatives that are correctly
identified as such. In order to evaluate the reliability of the
diagnostic test, positive and negative predictive value were
calculated. The positive and negative predictive values (PPV and
NPV, respectively) are defined as the proportions of positive and
negative results in diagnostic tests that are true positive and
true negative results, respectively. Receiver operating
characteristic (ROC) analysis was performed to compare diagnostic
effectiveness of TP53 and AGR3. Then, unsupervised clustering
analysis of TP53 and AGR3 IHC scores was performed using heatmap.2
function in R (version 3.4.2). Based on clustering results,
combination of TP53 and AGR3 data were tabulated to distinguish
HGSC and LGSC. All statistical analyses were performed using SPSS
22.0 (IBM Corp., Armonk, NY, USA).
Statistical analysis
HTSeq (v0.9.1; open-source software available from
www-huber.embl.de/HTSeq or from the
Python Package Index at pypi.python.org/pypi/HTSeq) was used to analyze
RNA-seq data. Differential expression analysis between HGSC and
LGSC was performed using the DESeq R package (version 3.4.2;
open-source available from www.r-project.org/). Data achieved from RT-qPCR are
presented as the mean ± standard deviation and analyzed through
t-test using GraphPad Prism (version 5.01; GraphPad Software, Inc.,
La Jolla, CA, USA) software. χ2 test was performed for
IHC analysis using SPSS 19.0 software (IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
HGSC and LGSC are two distinct diseases
with different gene expression profiles
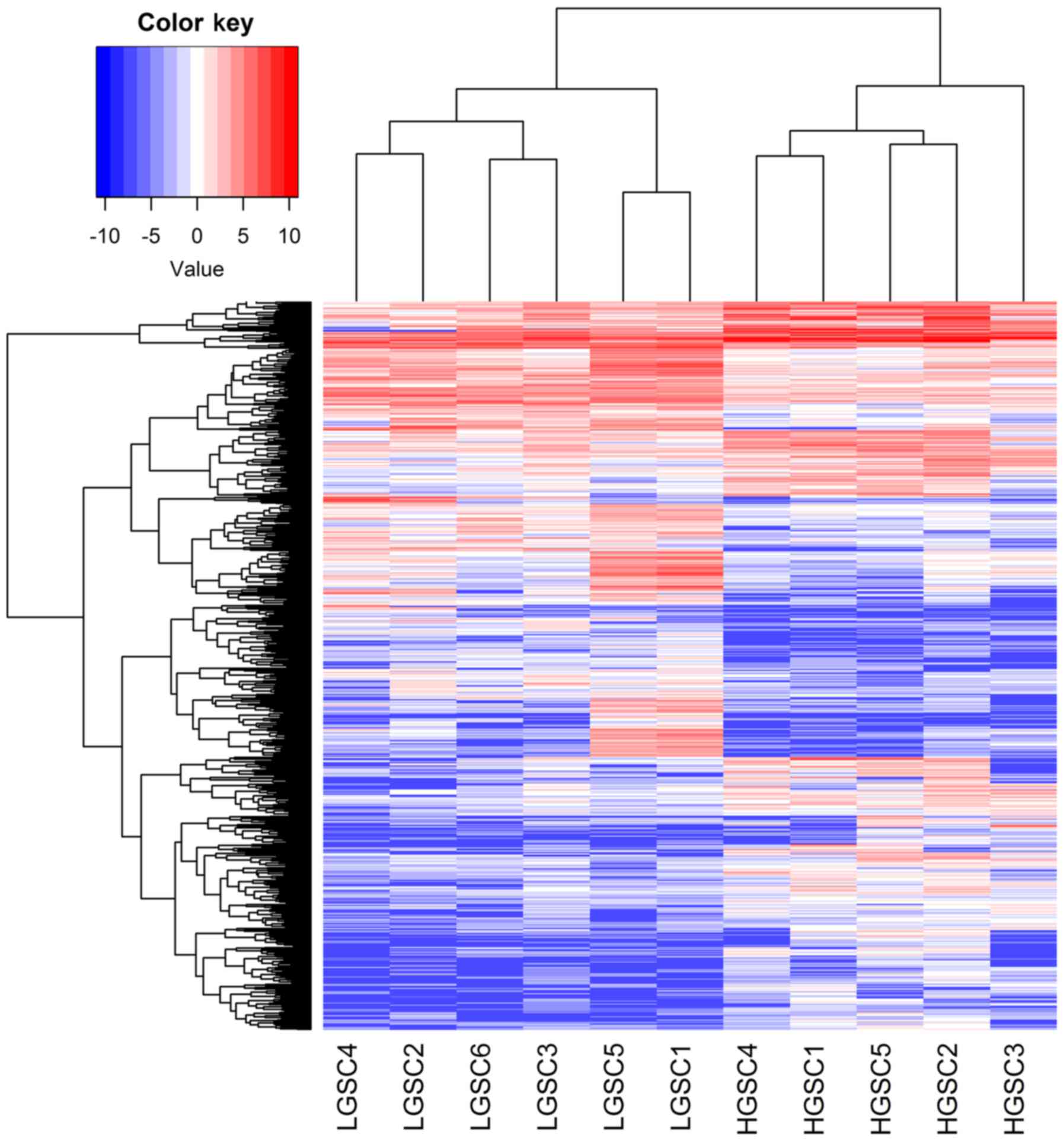
Comparing HGSC and LGSC using the DESeq R package,
699 differentially expressed genes (>2-fold difference) were
identified, 367 of which were upregulated in HGSC. Unsupervised
hierarchical cluster analyses on differentially expressed genes
identified by mRNA sequencing between HGSC and LGSC demonstrated
that HGSC clearly segregated from LGSC (Fig. 1). A number of differentially
expressed genes between them were in accordance with previous
studies (14). According to the
Gene Ontology analysis, significant groups of differential genes
between HGSC and LGSC associated with specific functional
processes, including ‘pattern specification process’, ‘DNA
metabolic process’ and ‘nucleic acid metabolic process’.
To validate the results and to further investigate
novel differential diagnostic markers, certain differential genes
were analyzed by RT-qPCR. As shown in Fig. 2, LAMP3 and EGFL6 were upregulated
in HGSC, while CDKN1A and AGR3 were significantly downregulated
compared with LGSC. These results, in accordance with mRNA
sequencing results, indicate that gene expression profile in HGSC
is different from that in LGSC. Among these genes, AGR3, which
exhibited the greatest difference between HGSC and LGSC was
selected for further analysis.
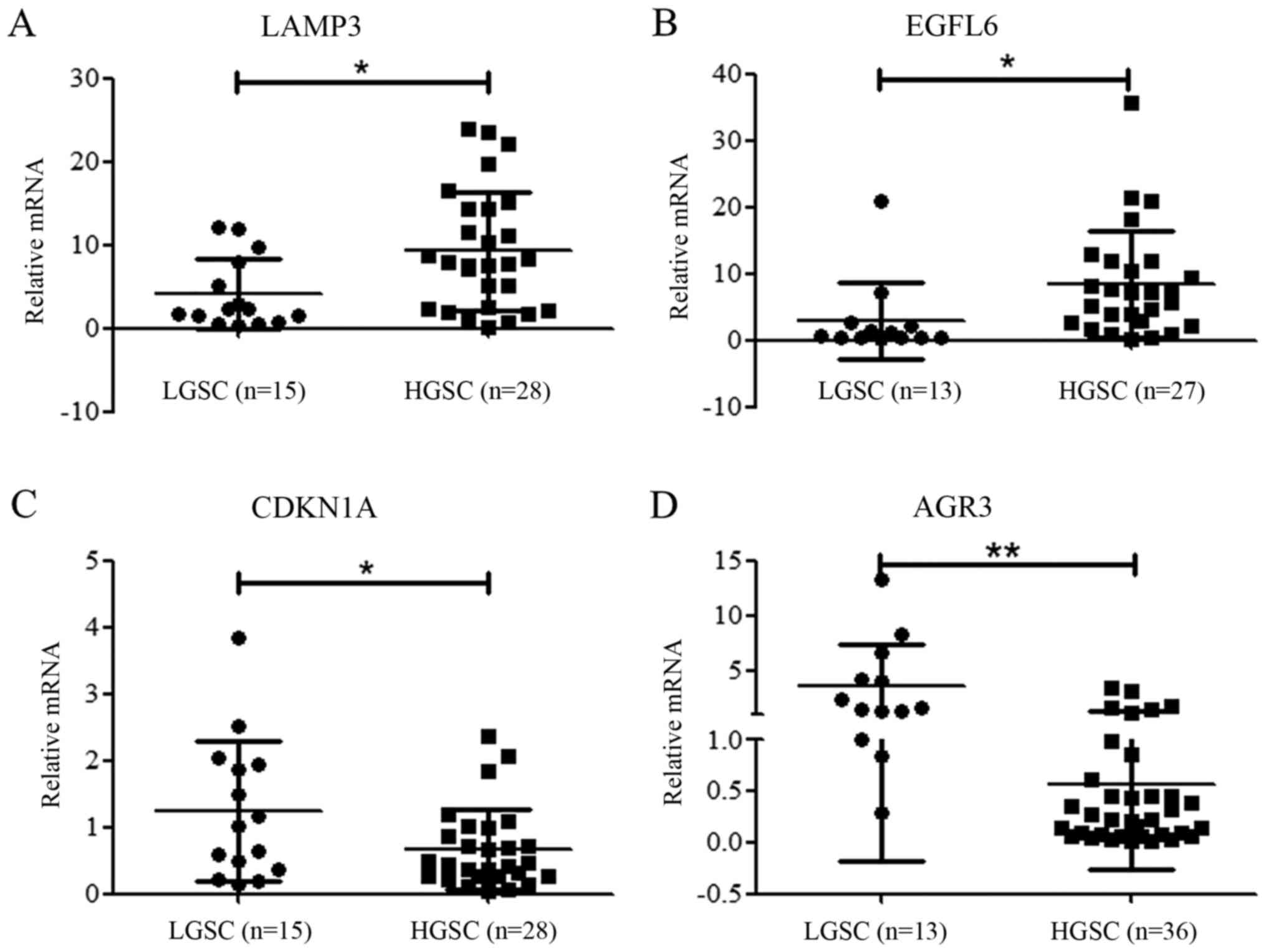 | Figure 2Expression of LAMP3, EGFL6, CDKN1A and
AGR3 in HGSC and LGSC. RT-qPCR confirmed a trend toward increased
expression of (A) LAMP3 and (B) EGFL6 in HGSC samples compared with
LGSC (P=0.0137 and P=0.0365 respectively). RT-qPCR confirmed the
decreased expression of (C) CDKN1A and (D) AGR3 in HGSC samples
compared with LGSC (P=0.0285 and P<0.001 respectively).
*P<0.05, **P<0.01. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; HGSC,
high-grade serous carcinoma; LGSC, low-grade serous carcinoma;
LAMP3, lysosomal associated membrane protein 3; EGFL6, EGF like
domain multiple 6; CDKN1A, cyclin-dependent kinase inhibitor 1A;
AGR3, anterior gradient homolog 3. |
Significant differential expression of
TP53 and AGR3 protein in HGSC and LGSC
Previous researches have confirmed the use of TP53
in the differential diagnosis of HGSC and LGSC. On this basis, the
diagnostic utility of AGR3 alone and combined with TP53 were
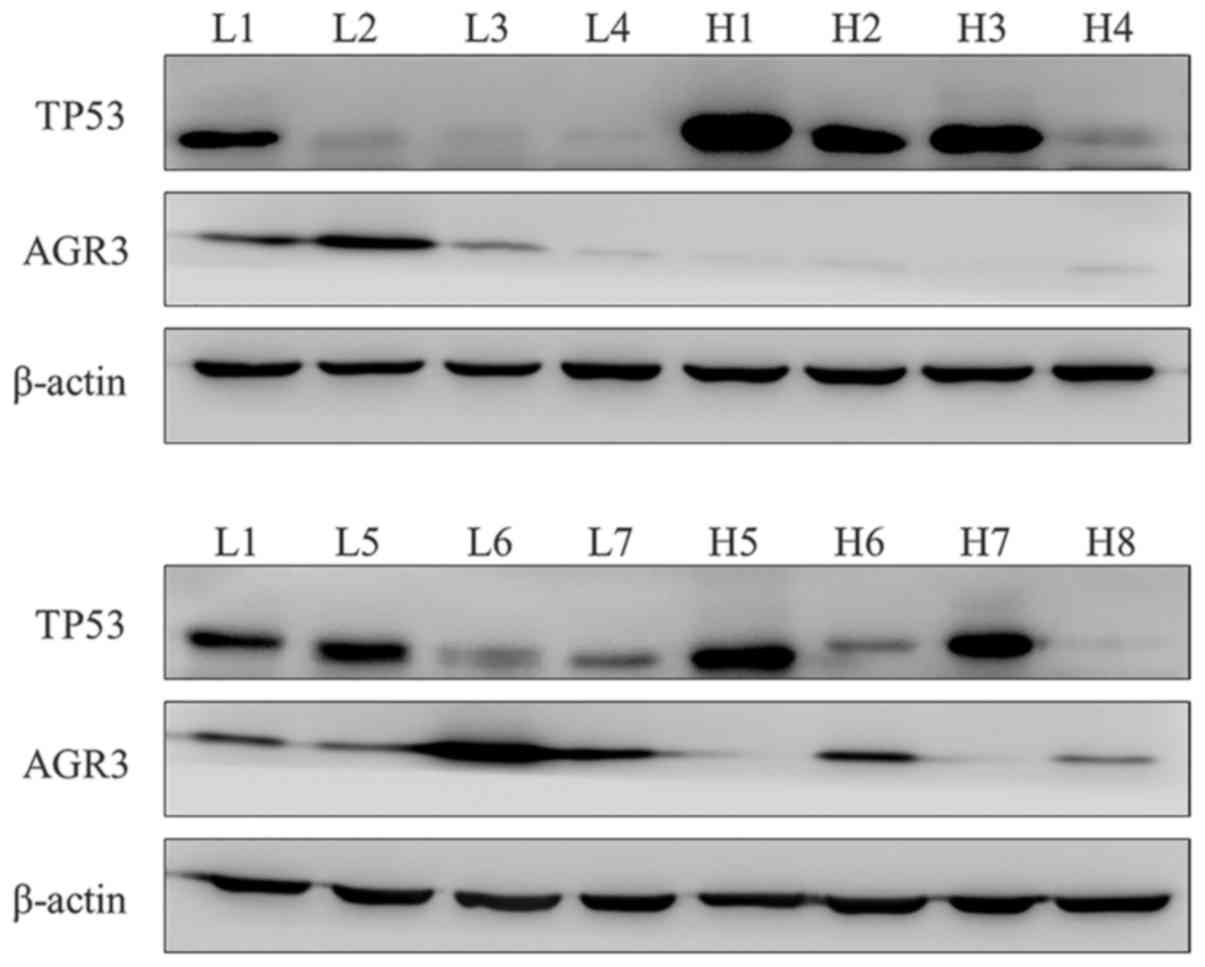
investigated in the current study. The protein expression of TP53
and AGR3 in HGSC (n=8) and LGSC (n=7) were examined through western
blot analysis. TP53 exhibited overexpression or absence in 6 of 8
HGSC compared with LGSC, as expected. Increased expression of AGR3
was observed in LGSC compared with HGSC samples (Fig. 3). Taken together, these findings
suggest that the differential expression of TP53 and AGR3 between
HGSC and LGSC may serve as markers for differential diagnosis.
TP53 and AGR3 were useful to distinguish
HGSC from LGSC
In order to assess the diagnostic performance of
TP53 and AGR3, immunohistochemical staining was performed on 145
HGSC and 30 LGSC samples to analyze sensitivity and specificity of
these markers in diagnosis. The subcellular localization of TP53
staining was predominantly nuclear. Additionally, the percentage of
positive cells, rather than immu-nointensity, was used for
evaluation of TP53 in the majority of previous studies (22,25).
With respect to AGR3, its subcellular localization was
predominantly membranous. There was no different immunointensity
between two samples with sharp distinction in positive cell
percentage. Thus, the percentage of positive cells was used for
evaluation of TP53 and AGR3, as in previous studies (25,26).
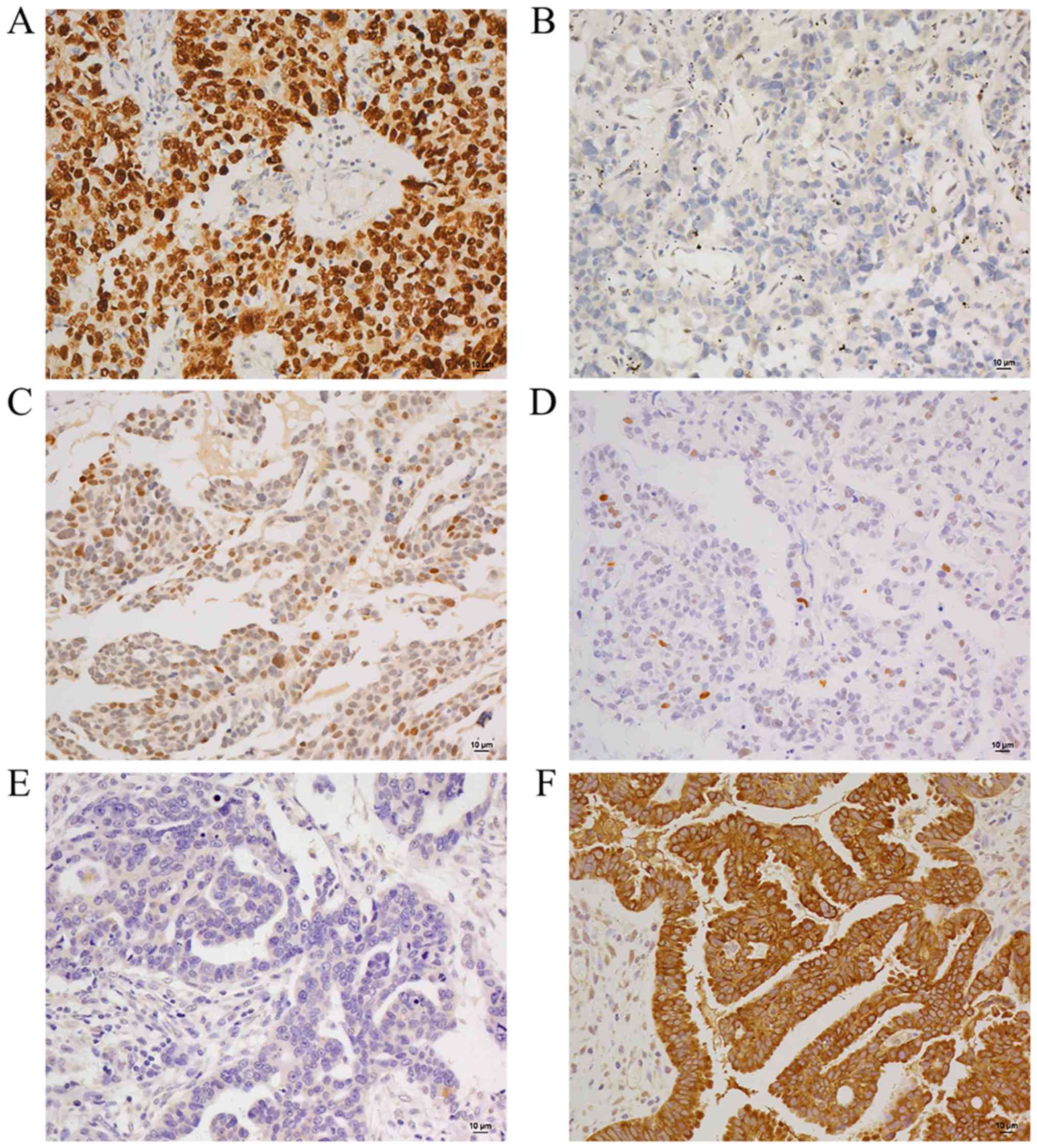
Representative staining features of TP53 and AGR3 in HGSC and LGSC
are shown in Fig. 4. TP53 was
scored as absent (negative or occasional positive cells), wild-type
pattern (0–75%) or overexpression (≥75%) and AGR3 was scored as
either negative (<20%) or positive (≥20%). The staining results
for TP53 and AGR3 across the available cohorts were shown in
Tables II and III. TP53 and AGR3 were efficient in
distinguishing HGSC from LGSC (P<0.001 for both). To test the
diagnostic reliability of TP53 and AGR3, positive and negative
predictive values were also calculated in the same samples again.
Aberrant TP53 staining (0 or ≥75% positive staining) was detected
in 127/145 (87.6%) of HGSC and in 4/30 (13.3%) of LGSC samples. The
positive staining of AGR3 in HGSC and LGSC was 15/145 (11.3%) and
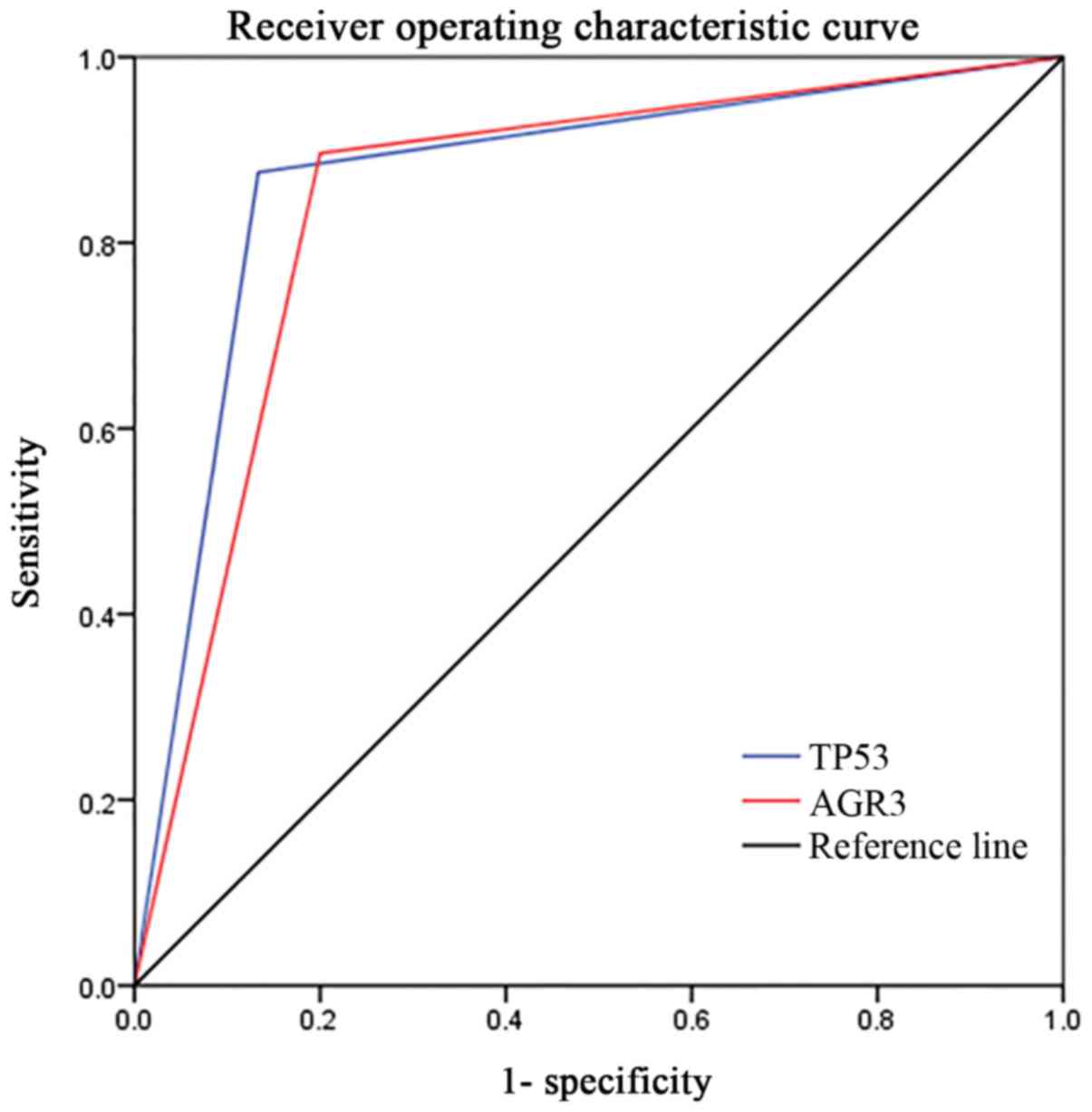
24/30 (80.0%) respectively. ROC analysis produced an area under the
curve of 0.871 for TP53, and 0.848 for AGR3 (Fig. 5). Thus, AGR3 was also a useful
marker with respect to differential diagnosis. PPV and NPV were
96.9 and 59.1% for TP53, and 61.5 and 95.6% for AGR3, which reminds
us to combine the two markers for an overall analysis.
 | Table IITest performance of TP53 for
diagnosis of HGSC. |
Table II
Test performance of TP53 for
diagnosis of HGSC.
| TP53
expression | HGSC | LGSC | P-value |
|---|
| Abnormal | 127 | 4 | <0.001 |
| Normal | 18 | 26 | |
| Total | 145 | 30 | |
 | Table IIITest performance of AGR3 for
diagnosis of LGSC. |
Table III
Test performance of AGR3 for
diagnosis of LGSC.
| AGR3
expression | HGSC | LGSC | Total | P-value |
|---|
| Positive | 15 | 24 | 39 | <0.001 |
| Negative | 130 | 6 | 136 | |
| Total | 145 | 30 | 175 | |
In order to evaluate the diagnostic performance of a
combination of TP53 and AGR3 staining, a heat map was produced by
unsupervised hierarchical cluster analysis in R. With two markers
(TP53 and AGR3, each with two outcomes), four combinations/cluster
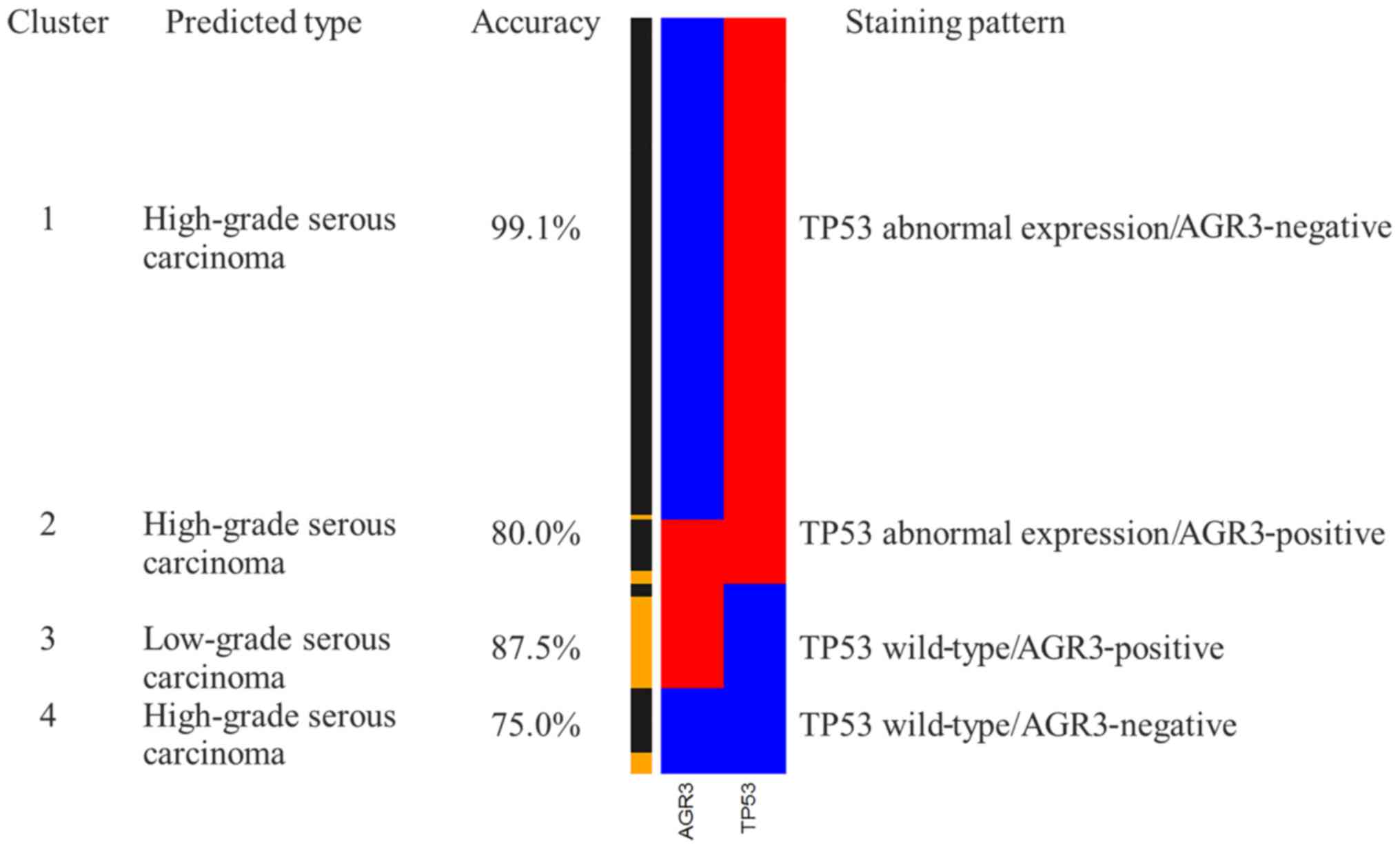
groups were derived (Fig. 6).
Three of these four cluster groups were associated with HGSC.
Cluster 1 (TP53 aberrant expression and AGR3-negative), accounting
for 66.3% of the cases, 99.1% were diagnosed as HGSC by the gold
standard morphology. Cluster 2 (TP53 aberrant expression and
AGR3-positive), which formed the smallest group with 8.6% of the
cases, was associated with the diagnosis of HGSC. Cluster 4 (TP53
wild-type staining and AGR3-negative) was also most likely
associated with HGSC. However, a substantial proportion of LGSC was
found in cluster 3 (TP53 wild-type staining and AGR3-positive).
Given that only cluster 3 is predictive for LGSC,
the performance of cluster 3 was analyzed for use in diagnosis of
LGSC with sensitivity and specificity shown in Table IV. The sensitivity of cluster 3 to
predict LGSC is 70.0% (21/30) with a specificity of 97.9%
(142/145). In order to test the predictive performance for clinical
diagnosis, PPV and NPV were calculated using the current samples.
PPV and NPV of cluster 3 were 87.5 and 94.0%, respectively. In
addition, the accuracy of differential diagnosis was 93.1%
(163/175). These results suggest that a combination of TP53 and
AGR3 may be highly effective in distinguishing HGSC from LGSC.
 | Table IVTest performance of cluster 3 for
diagnosis of LGSC. |
Table IV
Test performance of cluster 3 for
diagnosis of LGSC.
| Cluster | HGSC | LGSC | P-value |
|---|
| 3 |
3 | 21 | <0.001 |
| 1,2,4 | 142 | 9 | |
| Total | 145 | 30 | |
Discussion
HGSCs and LGSCs exhibit distinct characteristics,
and possess diverse pathogenesis and prognosis. HGSC is highly
aggressive, grows rapidly and almost always presents at an advanced
stage. It directly develops from serous tubal intra-epithelial
carcinoma (27). According to The
Cancer Genome Atlas ovarian cancer study, TP53 mutation occurs in
96% of HGSCs, while mutations of KRAS proto-oncogene, GTPase
(KRAS), B-Raf proto-oncogene, serine/threonine kinase (BRAF) or
(erb-b2 receptor tyrosine kinase 2) ERBB2 are infrequent (28). LGSC accounts for a smaller
proportion of all ovarian serous carcinomas. LGSC generally
exhibits indolent biological behavior and is usually confined to
the ovary at presentation. It has been hypothesized to develop in a
slow step-wise manner from a serous cystadenoma or adenofibroma
(29–31). LGSC is relatively stable and
expresses normal levels of TP53, but also is characterized by
mutations of the KRAS, BRAF or ERBB2 genes (8). Furthermore, HGSC and LGSC respond
differentially to similar therapeutic protocols, so it is crucial
to distinguish HGSC from LGSC prior to the initiation of treatment.
The final diagnosis relies on histopathological features, however
clinical behavior, ancillary examinations, molecular biology
characteristics and especially immunohistochemical markers are all
useful in differentiating HGSC from LGSC. Currently, a two-tier
system is widely used to subdivide ovarian serous carcinoma into
HGSC and LGSC. Excellent inter- and intra-observer reproducibility
of the two-tier system for grading ovarian serous carcinoma has
been demonstrated. However, certain difficulties remain in
differential diagnosis of HGSC and LGSC. On one hand, certain
tumors with intermediate characteristics cannot be categorized
accurately even with the application of the two-tier grading
scheme, especially in local hospitals. On the other hand, current
clinical practice can only rely on limited samples from
paracentesis or biopsies of pelvic/peritoneal implants prior to the
commencement of neoadjuvant chemotherapy or targeted therapy. Under
such conditions, a number of markers (including P16, Ki67 and TP53)
are investigated for differential diagnosis of ovarian serous
carcinoma (22,32). In a previous study, the expression
of P16 was analyzed in HGSC and LGSC. The cases were
semi-quantitatively scored as 0 (negative or occasional positive
cells), 1+ (<10% cells positive), 2+ (10–25% cells positive), 3+
(26–50% cells positive), 4+ (51–75% cells positive) or 5+ (>75%
cells positive). In the previous study, 5+ staining (>75%
positive cells) was observed in 27% of LGSC and in 83% of HGSC
(32). A diffusely positive
staining pattern (5+ staining) may result into an erroneous
diagnosis because many metastatic ovarian tumors arising from
uterine serous carcinomas also exhibit the same staining pattern
for P16. Similarly, the Ki67 proliferation index and the expression
of TP53 are both higher in HGSC compared with LGSC. In a study by
O’Neill et al (22), the
proliferation index was significantly different between these two
tumor types (23% for low-grade and 55% for high-grade). TP53 5+
staining (>75% positive cells) was observed in 18% of LGSC and
64% of HGSC (22). Additionally,
another study reported that aberrant expression of TP53 (completely
absent or ≥60% of cells stained) occurred in 89% of HGSC and 6% of
LGSC (25). These studies indicate
that HGSC is characterized by diffuse expression of TP53, while the
expression of TP53 is much lower in LGSC. To the best of our
knowledge, molecular alteration of TP53 is a defining feature of
HGSC and TP53 is also currently one of the best available choices
for differential diagnosis (33).
However, TP53 staining does not perfectly differentiate HGSC from
LGSC on the basis of morphology. Thus, we intend to identify novel
and effective markers to be used in consolidation with TP53 in
order to distinguish HGSC from LGSC efficiently on the basis of
conventional histopathology.
In total, 699 differentially expressed genes were
identified comparing HGSC and LGSC using DESeq R package and
dendrograms were produced by unsupervised hierarchical clustering
using heatmap.2 function in ‘gplots’ R-package. HGSC was separated
from LGSC completely. The results support the hypothesis of two
distinct pathways of HGSC and LGSC yielding two different disease
entities. This is in accordance with the different clinical and
molecular presentations of the two. In agreement with the
sequencing data, HGSC expressed higher levels of LAMP3 and EGFL6
mRNA compared with LGSC, as determined by RT-qPCR (P<0.05).
While the mRNA levels of CDKN1A and AGR3 were higher in LGSC
(P<0.05). AGR3, which exhibited the greatest variation out of
the four molecules between HGSC and LGSC, was selected for further
analysis. TP53 was included in the current study based on its
diagnostic utility. Along with mRNA levels, AGR3 protein expression
was also higher in LGSC, as demonstrated using western blot
analysis. The findings suggested that AGR3 may be used as an
efficient marker and may be beneficial in differential diagnosis
between HGSC and LGSC. However, the comparison of TP53 expression
between HGSC and LGSC using western blotting was solely
circumstantial evidence. Regrettably, mutation data on HGSC and
LGSC was not analyzed. Furthermore, overexpression of TP53
demonstrated by western blotting cannot rule in or rule out the
role of TP53 mutations.
In the current study, aberrant expression of TP53 (0
or ≥75% positive) exhibited a sensitivity 87.6% and specificity
86.7% for HGSC. Overexpression and absence of TP53 in HGSC
indicates missense and null mutations, respectively. Although, it
is generally acknowledged that vast majority of HGSCs are
characterized by TP53 mutations and numerous studies have reported
that TP53 staining patterns were distinctly different between HGSC
and LGSC (25,34). However, based on a study by Singer
et al (35),
immunohistochemistry does not accurately predict mutation status.
Furthermore, TP53 staining does not perfectly differentiate HGSC
from LGSC. Therefore, it is necessary to seek additional markers to
improve the current diagnostic efficacy.
The differential diagnostic value of AGR3 for HGSC
and LGSC were investigated. To the best of our knowledge, there are
only limited reports of AGR3 expression in normal and cancer
tissue. The current study confirmed that the expression of AGR3 was
markedly different between HGSC and LGSC. Following analysis of the
expression with varied cutoff values, 20% AGR3-positive cells was
determined to be the best cut-off to distinguish HGSC from LGSC.
Therefore, 20% or more positively stained epithelial cells was
defined as AGR3-positive. Positive expression of AGR3 has a
sensitivity of 80.0% and a specificity of 89.7% for LGSC. Although
there is some overlap in the extent of staining in HGSC vs. LGSC,
these findings indicate that a morphologically problematic serous
ovarian carcinoma with negative expression of AGR3 is unlikely to
be LGSC. ROC analysis produced a similar area under the curve for
AGR3 compared to TP53, suggesting that AGR3 is comparably as
important as TP53 for differential diagnosis of HGSC and LGSC. PPV
and NPV of AGR3 staining for LGSC were 61.5 and 95.6%,
respectively. The low PPV may be due to the finite number of LGSC
samples. In accordance, the expression of AGR3 was also reported to
be significantly higher in LGSC than HGSC by western blot analysis
in a previous study by King et al (26). However, the previous study could
not distinguish HGSC from LGSC using AGR3 IHC, which was likely
attributable to the different anti-AGR3 antibody used and different
cutoff values in the two studies. In the current study, 20% was the
best cutoff to efficiently distinguish HGSC from LGSC. The results
support the association of AGR3 with the differentiation of serous
ovarian cancer. In conclusion, AGR3 is a useful marker for
differential diagnosis of HGSC and LGSC based on the results of the
present study. Subsequently, statistical validation was performed
for combined application of AGR3 and TP53 in the differential
diagnosis of HGSC and LGSC. The strength of the study is depicted
by the PPV (87.5%) and NPV (94.0%). Furthermore, the accuracy of
diagnosis increased from 87.4% to 93.1% by including AGR3. Thus, a
panel of two antibodies is more effective and it improves the
accuracy of the diagnosis. IHC staining with a wild-type TP53
pattern and AGR3-positive expression produced a specificity of
97.9% and a sensitivity of 70.0% for LGSC. The low sensitivity may
be attributed to a proportion of samples with negative expression
of AGR3 and aberrant expression of TP53 in LGSC. The outcomes
ascertained from the current study provide compelling evidence for
the use of these two markers for the differential diagnosis of HGSC
and LGSC.
In summary, these results suggest that a combination
of TP53 and AGR3 staining is superior to TP53 alone for
differential diagnosis of HGSC and LGSC on the basis of morphology.
Furthermore, it may improve accuracy of diagnosis and be beneficial
for the clinical management, optimizing therapeutic regimen and
thus lowering the overall risk of mortality. On this basis, the two
markers may be useful to test additional ovarian carcinomas of
‘uncertain’ subtype. However, there is no statistical data at
present because of limitation in the number of rare cases. Future
prospective clinical studies should be conducted on a larger cohort
of various ovarian carcinoma subtypes to validate the clinical
utility of the model.
Glossary
Abbreviations
Abbreviations:
|
HGSC
|
high-grade serous carcinoma
|
|
LGSC
|
low-grade serous carcinoma
|
|
AGR3
|
anterior gradient homolog 3
|
|
ROC
|
receiver operating characteristic
|
|
EOC
|
epithelial ovarian cancer
|
Acknowledgments
Not applicable.
Funding
This study was supported by National Natural Science
Foundation of China (grant nos. 81572554 and 81272857), the
National Clinical Research Center for Gynecological Oncology (grant
no. 2015BAI13B05) and the Natural Science Foundation of Shandong
Province (grant nos. ZR2014HM070 and ZR2015HM079).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
BK designed and supervised the research. NL provided
technical support and revised the paper. CQ was a major contributor
in collecting tissue samples and writing the manuscript. YW
performed statistical analysis. XW reviewed the sections used for
further study and interpreted all data from immunohistochemistry.
QZ collected and analyzed RNA-seq experiments. YL carried out
RT-qPCR analysis. YX performed western blotting. CJ and HB
conducted all immunohistochem-istry experiments. WZ reviewed and
confirmed the sections used for further study. XY directed the
research. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Samples were collected with the approval of the
Ethics Committee at Qilu Hospital of Shandong University (Jinan,
China), and with the signed informed consent from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar
|
|
2
|
Prat J: Ovarian carcinomas: Five distinct
diseases with different origins, genetic alterations, and
clinicopathological features. Virchows Archiv. 460:237–249. 2012.
View Article : Google Scholar
|
|
3
|
Köbel M, Kalloger SE, Boyd N, McKinney S,
Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, et al:
Ovarian carcinoma subtypes are different diseases: Implications for
biomarker studies. PLoS Med. 5:e2322008. View Article : Google Scholar
|
|
4
|
Malpica A, Deavers MT, Lu K, Bodurka DC,
Atkinson EN, Gershenson DM and Silva EG: Grading ovarian serous
carcinoma using a two-tier system. Am J Surg Pathol. 28:496–504.
2004. View Article : Google Scholar
|
|
5
|
Bertelsen K, Holund B and Andersen E:
Reproducibility and prognostic value of histologic type and grade
in early epithelial ovarian cancer. Int J Gynecol Cancer. 3:72–79.
1993. View Article : Google Scholar
|
|
6
|
Shimizu Y, Kamoi S, Amada S, Hasumi K,
Akiyama F and Silverberg SG: Toward the development of a universal
grading system for ovarian epithelial carcinoma. I. Prognostic
significance of histopathologic features - problems involved in the
architectural grading system. Gynecol Oncol. 70:2–12. 1998.
View Article : Google Scholar
|
|
7
|
Silverberg SG: Histopathologic grading of
ovarian carcinoma: A review and proposal. Int J Gynecol Pathol.
19:7–15. 2000. View Article : Google Scholar
|
|
8
|
Vang R, Shih IeM and Kurman RJ: Ovarian
low-grade and high-grade serous carcinoma: Pathogenesis,
clinicopathologic and molecular biologic features, and diagnostic
problems. Adv Anat Pathol. 16:267–282. 2009. View Article : Google Scholar
|
|
9
|
Malpica A, Deavers MT, Lu K, Liu J,
Atkinson EN, Gershenson DM and Silva EG: Grading ovarian serous
carcinomas using a two-tier system. Mod Pathol. 15:202a–203a.
2002.
|
|
10
|
Tone AA, Begley H, Sharma M, Murphy J,
Rosen B, Brown TJ and Shaw PA: Gene expression profiles of luteal
phase fallopian tube epithelium from BRCA mutation carriers
resemble high-grade serous carcinoma. Clin Cancer Res.
14:4067–4078. 2008. View Article : Google Scholar
|
|
11
|
Marquez RT, Baggerly KA, Patterson AP, Liu
J, Broaddus R, Frumovitz M, Atkinson EN, Smith DI, Hartmann L,
Fishman D, et al: Patterns of gene expression in different
histotypes of epithelial ovarian cancer correlate with those in
normal fallopian tube, endometrium, and colon. Clin Cancer Res.
11:6116–6126. 2005. View Article : Google Scholar
|
|
12
|
Li J, Abushahin N, Pang S, Xiang L,
Chambers SK, Fadare O, Kong B and Zheng W: Tubal origin of
‘ovarian’ low-grade serous carcinoma. Mod Pathol. 24:1488–1499.
2011. View Article : Google Scholar
|
|
13
|
Qiu C, Lu N, Wang X, Zhang Q, Yuan C, Yan
S, Dongol S, Li Y, Sun X, Sun C, et al: Gene expression profiles of
ovarian low-grade serous carcinoma resemble those of fallopian tube
epithelium. Gynecol Oncol. 147:634–641. 2017. View Article : Google Scholar
|
|
14
|
May T, Shoni M, Crum CP, Xian W,
Vathipadiekal V, Birrer M, Rosen B, Tone A and Murphy KJ: Low-grade
and high-grade serous Mullerian carcinoma: Review and analysis of
publicly available gene expression profiles. Gynecol Oncol.
128:488–492. 2013. View Article : Google Scholar
|
|
15
|
Vergote I, Tropé CG, Amant F, Kristensen
GB, Ehlen T, Johnson N, Verheijen RHM, van der Burg MEL, Lacave AJ,
Panici PB, et al European Organization for Research and Treatment
of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group:
Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV
ovarian cancer. N Engl J Med. 363:943–953. 2010. View Article : Google Scholar
|
|
16
|
Pohl G, Ho CL, Kurman RJ, Bristow R, Wang
TL and Shih IeM: Inactivation of the mitogen-activated protein
kinase pathway as a potential target-based therapy in ovarian
serous tumors with KRAS or BRAF mutations. Cancer Res.
65:1994–2000. 2005. View Article : Google Scholar
|
|
17
|
Malpica A, Deavers MT, Tornos C, Kurman
RJ, Soslow R, Seidman JD, Munsell MF, Gaertner E, Frishberg D and
Silva EG: Interobserver and intraobserver variability of a two-tier
system for grading ovarian serous carcinoma. Am J Surg Pathol.
31:1168–1174. 2007. View Article : Google Scholar
|
|
18
|
Ayhan A, Kurman RJ, Yemelyanova A, Vang R,
Logani S, Seidman JD and Shih IeM: Defining the cut point between
low-grade and high-grade ovarian serous carcinomas: A
clinico-pathologic and molecular genetic analysis. Am J Surg
Pathol. 33:1220–1224. 2009. View Article : Google Scholar
|
|
19
|
Kalloger SE, Köbel M, Leung S, Mehl E, Gao
D, Marcon KM, Chow C, Clarke BA, Huntsman DG and Gilks CB:
Calculator for ovarian carcinoma subtype prediction. Mod Pathol.
24:512–521. 2011. View Article : Google Scholar
|
|
20
|
Kim D, Pertea G, Trapnell C, Pimentel H,
Kelley R and Salzberg SL: TopHat2: Accurate alignment of
transcriptomes in the presence of insertions, deletions and gene
fusions. Genome Biol. 14:R362013. View Article : Google Scholar
|
|
21
|
Mortazavi A, Williams BA, McCue K,
Schaeffer L and Wold B: Mapping and quantifying mammalian
transcriptomes by RNA-Seq. Nat Methods. 5:621–628. 2008. View Article : Google Scholar
|
|
22
|
O’Neill CJ, Deavers MT, Malpica A, Foster
H and McCluggage WG: An immunohistochemical comparison between
low-grade and high-grade ovarian serous carcinomas: Significantly
higher expression of p53, MIB1, BCL2, HER-2/neu, and C-KIT in
high-grade neoplasms. Am J Surg Pathol. 29:1034–1041. 2005.
|
|
23
|
Yemelyanova A, Vang R, Kshirsagar M, Lu D,
Marks MA, Shih IeM and Kurman RJ: Immunohistochemical staining
patterns of p53 can serve as a surrogate marker for TP53 mutations
in ovarian carcinoma: An immunohistochemical and nucleotide
sequencing analysis. Mod Pathol. 24:1248–1253. 2011. View Article : Google Scholar
|
|
24
|
Cole AJ, Dwight T, Gill AJ, Dickson KA,
Zhu Y, Clarkson A, Gard GB, Maidens J, Valmadre S, Clifton-Bligh R,
et al: Assessing mutant p53 in primary high-grade serous ovarian
cancer using immunohistochemistry and massively parallel
sequencing. Sci Rep. 6:261912016. View Article : Google Scholar
|
|
25
|
Altman AD, Nelson GS, Ghatage P, McIntyre
JB, Capper D, Chu P, Nation JG, Karnezis AN, Han G, Kalloger SE, et
al: The diagnostic utility of TP53 and CDKN2A to distinguish
ovarian high-grade serous carcinoma from low-grade serous ovarian
tumors. Mod Pathol. 26:1255–1263. 2013. View Article : Google Scholar
|
|
26
|
King ER, Tung CS, Tsang YTM, Zu Z, Lok
GTM, Deavers MT, Malpica A, Wolf JK, Lu KH, Birrer MJ, et al: The
anterior gradient homolog 3 (AGR3) gene is associated with
differentiation and survival in ovarian cancer. Am J Surg Pathol.
35:904–912. 2011. View Article : Google Scholar
|
|
27
|
Vang R, Shih IeM and Kurman RJ: Fallopian
tube precursors of ovarian low- and high-grade serous neoplasms.
Histopathology. 62:44–58. 2013. View Article : Google Scholar
|
|
28
|
Bell D, Berchuck A, Birrer M, Chien J,
Cramer DW, Dao F, Dhir R, DiSaia P, Gabra H, Glenn P, et al Cancer
Genome Atlas Research Network: Integrated genomic analyses of
ovarian carcinoma. Nature. 474:609–615. 2011. View Article : Google Scholar
|
|
29
|
Smith Sehdev AE, Sehdev PS and Kurman RJ:
Noninvasive and invasive micropapillary (low-grade) serous
carcinoma of the ovary: A clinicopathologic analysis of 135 cases.
Am J Surg Pathol. 27:725–736. 2003. View Article : Google Scholar
|
|
30
|
Bell DA, Longacre TA, Prat J, Kohn EC,
Soslow RA, Ellenson LH, Malpica A, Stoler MH and Kurman RJ: Serous
borderline (low malignant potential, atypical proliferative)
ovarian tumors: Workshop perspectives. Hum Pathol. 35:934–948.
2004. View Article : Google Scholar
|
|
31
|
Longacre TA, McKenney JK, Tazelaar HD,
Kempson RL and Hendrickson MR: Ovarian serous tumors of low
malignant potential (borderline tumors): Outcome-based study of 276
patients with long-term (> or =5-year) follow-up. Am J Surg
Pathol. 29:707–723. 2005. View Article : Google Scholar
|
|
32
|
O’Neill CJ, McBride HA, Connolly LE,
Deavers MT, Malpica A and McCluggage WG: High-grade ovarian serous
carcinoma exhibits significantly higher p16 expression than
low-grade serous carcinoma and serous borderline tumour.
Histopathology. 50:773–779. 2007. View Article : Google Scholar
|
|
33
|
Vang R, Levine DA, Soslow RA, Zaloudek C,
Shih IeM and Kurman RJ: Molecular alterations of TP53 are a
defining feature of ovarian high-grade serous carcinoma: A rereview
of cases lacking TP53 mutations in the Cancer Genome Atlas Ovarian
Study. Int J Gynecol Pathol. 35:48–55. 2016. View Article : Google Scholar
|
|
34
|
Sundov D, Caric A, Mrklic I, Gugic D,
Capkun V, Hofman ID, Mise BP and Tomic S: P53, MAPK, topoisomerase
II alpha and Ki67 immunohistochemical expression and KRAS/BRAF
mutation in ovarian serous carcinomas. Diagn Pathol. 8:212013.
View Article : Google Scholar
|
|
35
|
Singer G, Stöhr R, Cope L, Dehari R,
Hartmann A, Cao DF, Wang TL, Kurman RJ and Shih IeM: Patterns of
p53 mutations separate ovarian serous borderline tumors and low-
and high-grade carcinomas and provide support for a new model of
ovarian carcinogenesis: A mutational analysis with
immunohistochemical correlation. Am J Surg Pathol. 29:218–224.
2005. View Article : Google Scholar
|