Introduction
Glioma is one of the most common primary
malignancies of the central nervous system (CNS), accounting for
approximately 40% of all intracranial tumors (1). According to the WHO glioma grading
criteria, gliomas are divided into low-grade (I and II) and
high-grade (III and IV) gliomas (2). High-grade gliomas, including
anaplastic glioma and pleomorphic glioblastoma, exhibit an
aggressive clinical behavior with a poor prognosis, compared with
low-grade gliomas. A comprehensive treatment strategy, including a
combination of surgery, radiotherapy and chemotherapy is considered
optimal in glioma management (3).
Although major advances have been made in glioma treatment over the
past few decades, the overall survival rate of patients with glioma
remains poor and the results of treatment aimed at the prolonged
disease-free survival of patients with glioma remain disappointing
(4). Since the malignant behavior
of glioma cells can be attributed to their inherent genetic
abnormalities, it is essential to better understand the underlying
molecular mechanisms in order to develop new and effective
treatment strategies for this fatal disease.
Temozolomide (TMZ), a 3-methyl derivative of
mitozolomide that can easily pass through the blood-brain-barrier,
is the commonly used alkylating chemotherapeutic agent in the
treatment of glioblastoma. The results of phase 2 and 3 clinical
trials have confirmed the antitumor activity, with relatively low
toxicity, of TMZ in patietns with glioblastoma. However, even
though several studies have revealed the apoptotic and
anti-proliferative effects of TMZ on glioma cells (5–9), the
mechanisms of action of TMZ in glioma cells remain largely
undefined. Despite it's the succss of TMZ as a chemotherapeutic
agent, gliomas exhibiting resistance to TMZ are common. It has been
indicated that the sensitivity of glioma cells to TMZ depends on
the intracellular level of O6-methylguanine-DNA
methyltransferase (MGMT) repair activity (6).
Members of the sex determining region Y (SRY)-box
protein (SOX) gene family are distinguished by a conservative high
mobility group DNA-binding domain (10). It has been reported that several
SOX genes, including SOX2, SOX4, SOX5, SOX6, SOX10, SOX11, SOX14
and SOX21 are expressed in the central nervous system as
transcription factors, and are also involved in brain tumorigenesis
(11). The SOX9 variant of the SOX
gene family was first found as an important regulator of cartilage
and male gonadal development, the mutations in which lead to
autosomal sex reversal and campomelic dysplasia (12,13).
As an oncogene, the upregulation of SOX9 has been observed in
several types of tumors, such as lung cancer (14,15),
breast cancer (16), colorectal
cancer (17), ovarian cancer
(18) and prostate cancer
(19). Recently, SOX9 was
identified as an oncogene in glioma, the overexpression of which
was found to be closely associated with a poor clinical outcome of
patients with glioma (20).
Despite this reported oncogenic role of SOX9 in glioma, its
mechanisms of action, including the regulated downstream molecular
signaling pathways, have not yet been completely delineated
(20,21). The chemotherapeutic resistance of
glioma to TMZ is not regulated by a single signal pathway or
molecule, but by a complex molecular network. We thus hypothesized
that SOX9 may be an important node in such a molecular network,
participating in the development and progression of glioma, and in
this study, we assessed its effects on the sensitivity of glioma to
TMZ, under in vitro conditions.
Materials and methods
Reagents
TMZ (purity, 99.95%) and U-104 (purity, 99.05%) was
obtained from Selleckchem Co., Ltd. (Houston, TX, USA).
4′,6-Diamidino-2-phenylindole (DAPI) and
3,8-diamino-5-[3-(diethylmethylammonio)propyl]-6-phenyl-phenanthridinium
diiodide (PI) were obtained from Genview Co., Ltd. (Beijing,
China). The following items were purchased from the cited
commercial sources: Anti-carbonic anhydrase IX (ab15086), anti-SOX9
(ab26414), anti-BCL-2 (ab32124) and anti-BAX (ab32503) (both from
Abcam, Cambridge, MA, USA), anti-AKT (#9272), phospho-AKT (Ser473;
#4060), β-actin (8H10D10; #3700) (all from Cell Signaling
Technology, Beverly, MA, USA), goat anti-rabbit IgG (H+L) HRP
(BS13278) and goat anti-mouse IgG (H+L) HRP (BS12478) antibodies
(both from Bioworld Technology (St. Louis Park, MN, USA).
Cell lines
The human glioma cell lines U251 (astrocytoma), U87
(glioblastoma; as regards this cell line, please also see the
Discussion) and 293T cells were obtained from the Chinese Academy
of Sciences Cell Bank, Shanghai, China. All cell lines were
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% fetal bovine serum (FBS) (Gibco, Calrsbad, CA USA), and
incubated at 37°C in a humidified atmosphere containing 5%
CO2. Both cell lines were routinely subcultured twice a
week by trypsinization, following standard procedures.
Construction of stable cell lines
SOX9 expression was knocked down in the glioma cell
lines by shRNA lentivirus infection, using the following target
sequences: negative control (NC), TTCTCCGAACGTGTCACGT; SOX9 KD1,
GCATCCTTCAATTTCTGTATA; and SOX9 KD2, CTCCACCTTCACCTACATGAA.
pGMLV-SC2 (Genomeditech Co., Shanghai, China) was used as the
lentiviral vector. The pGMLV-SC2 negative control and
pGMLV-SC2-SOX9 shRNAs were transfected into 293T cells to produce
the lentivirus, using HG transgene reagent (Genomeditech Co.). The
medium containing the HG transgene reagent was then removed and
replaced with fresh medium after 6 h. The lentivirus was added to
the U251 and U87 cells. Stable cell lines were selected by
culturing the infected U251 and U87 cells in puromycin for 14 days.
The expression of SOX9 was confirmed by reverse
transcription-quantitative PCR (RT-qPCR) and western blot
analysis.
Western blot analysis
Protein analysis was performed using western blot
analysis. The cells were lysed in RIPA + PMSF lysis buffer, and the
resulting total protein concentration was determined using the
Bicinchoninic Acid (BCA) Protein assay kit (Beyotime Biotechnology
Corporation, Shanghai, China). An equal amount of protein samples
were separated by 10% SDS-polyacrylamide gel electrophoresis and
transferred onto polyvinylidene difluoride (PVDF) membranes. After
being blocked with 5% fat-free milk at room temperature for 2 h,
the PVDF membranes were incubated overnight with the primary
antibodies (anhydrase IX, SOX9, BCL-2, AKT and phospho-AKT:
1:1,000, BAX: 1:2,000) at 4°C. Subsequently, the PVDF membranes
were incubated with the secondary antibodies (goat anti-rabbit IgG
(H+L) HRP (BS13278) and goat anti-mouse IgG (H+L) HRP (BS12478)
antibodies: 1:2,000) for 2 h at room temperature. The membranes
were then prepared for enhanced chemiluminescence (ECL), viewed
directly under an enhanced chemiluminescence detection system
(ChemiDocXRS; Bio-Rad, Hercules, CA, USA), and quantified by
densitometry using Image-Pro plus 6.0 (IPP 6.0) software.
RT-qPCR
The mRNA expression of the concerned genes was
assessed by RT-qPCR. The cells were subcultured into 6-well plates
until they were grown to confluence, and then incubated with TMZ
(200 µM) or U-104 (CA9 inhibitor; 100 µM) at
determined concentrations. Post-incubation, total RNA was extracted
from the cells using Total RNA Extraction Reagent (SunShineBio,
Nanjing, China) and the cDNA was synthesized with the TransScript
First-stand cDNA Synthesis Super Mix (TransGen Biotech, Beijing,
China). Subsequently, quantitative PCR was implemented using 20
µl of SsoFast™ EvaGree Supermix (Bio-Rad). PCR reagents were
used according to the manufacturer's instructions. The Bio-Rad iQ5
Real-Time PCR system (Bio-Rad) was used to amplify with the
following scheme: Initial activation step for 10 min at 95°C,
followed by 40 cycles of denaturation for 30 sec at 95°C and 10 sec
at 60°C. Primer sequences used are as follows: GAPDH forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse, 5′-GAAGATGGTGATGGGATTTC-3′;
SOX9 forward, 5′-AGGTGCTCAAAGGCTACGACT-3′ and reverse,
5′-AGATGTGCGTCTGCTCCGTG-3′; BAX forward, 5′-ACACCTGAGCTGACCTTGGA-3′
and reverse, 5′-CCGTGTCCACGTCAGCAATC-3′; BCL-2 forward,
5′-AAGCTGTCACAGAGGGGCTA-3′ and reverse, 5′-GACGGTAGCGACGAGAGAAG-3′;
CA9 forward, 5′-TTTGCCAGAGTTGACGAGGC-3′ and reverse,
5′-GCTCATAGGCACTGTTTTCTTCC-3′. The mRNA level of each gene,
normalized and presented as a ratio to GAPDH, was calculated using
the ΔΔCq method (22).
PI/DAPI staining of glioma cells
The cells in the exponential phase, seeded at
2×104 cells per well in a 48-well plate, were grown to
near confluency, and were then treated with TMZ (200 or 400
µM) or U-104 (100 µM). Apoptosis was detected with a
two-color analysis, DAPI binding and PI uptake, as follows: The
cells were washed in PBS and incubated with 500 µl of DMEM
containing 5 µl DAPI and 5 µl PI, at 37°C temperature
for 20 min. The cells were then observed under an inverted
fluorescence microscope (Nikon Eclipse Ti; Nikon, Tokyo, Japan),
with appropriate filters in the dark. The fluorescence intensity
was excited at 488 nm, and the emission was detected at 530 nm. The
degree of fluorescence was quantifided by the optical density
measurement using Image-Pro Plus 6.0 (IPP 6.0) software.
Microarray and bioinformatics
analyses
Total RNA from the cells, extracted using TRIzol
reagent (Invitrogen), was labeled and hybridized onto the Agilent
One-Color Microarray-Based Gene Expression Analysis platform
(Shanghai Oebiotech Co., Ltd., Shanghai, China). Statistical
analyses were performed using the Genespring GX software (Agilent
Technologies). Changes in gene expression with a 2-fold change were
considered differentially regulated by SOX9. The differentially
expressed genes obtained from the gene chip results were uploaded
into STRING (http://string-db.org/) to analyze the
protein-protein interaction (PPI).
Statistical analysis
All data are expressed as the means ± SD. Data
analysis was performed using the Student's t-test or one-way ANOVA,
followed by Newman-Keuls test. The differences were considered
statistically significant at P<0.05.
Results
Lentivirus mediated SOX9 knockdown
reduces CA9 expression in glioma cells
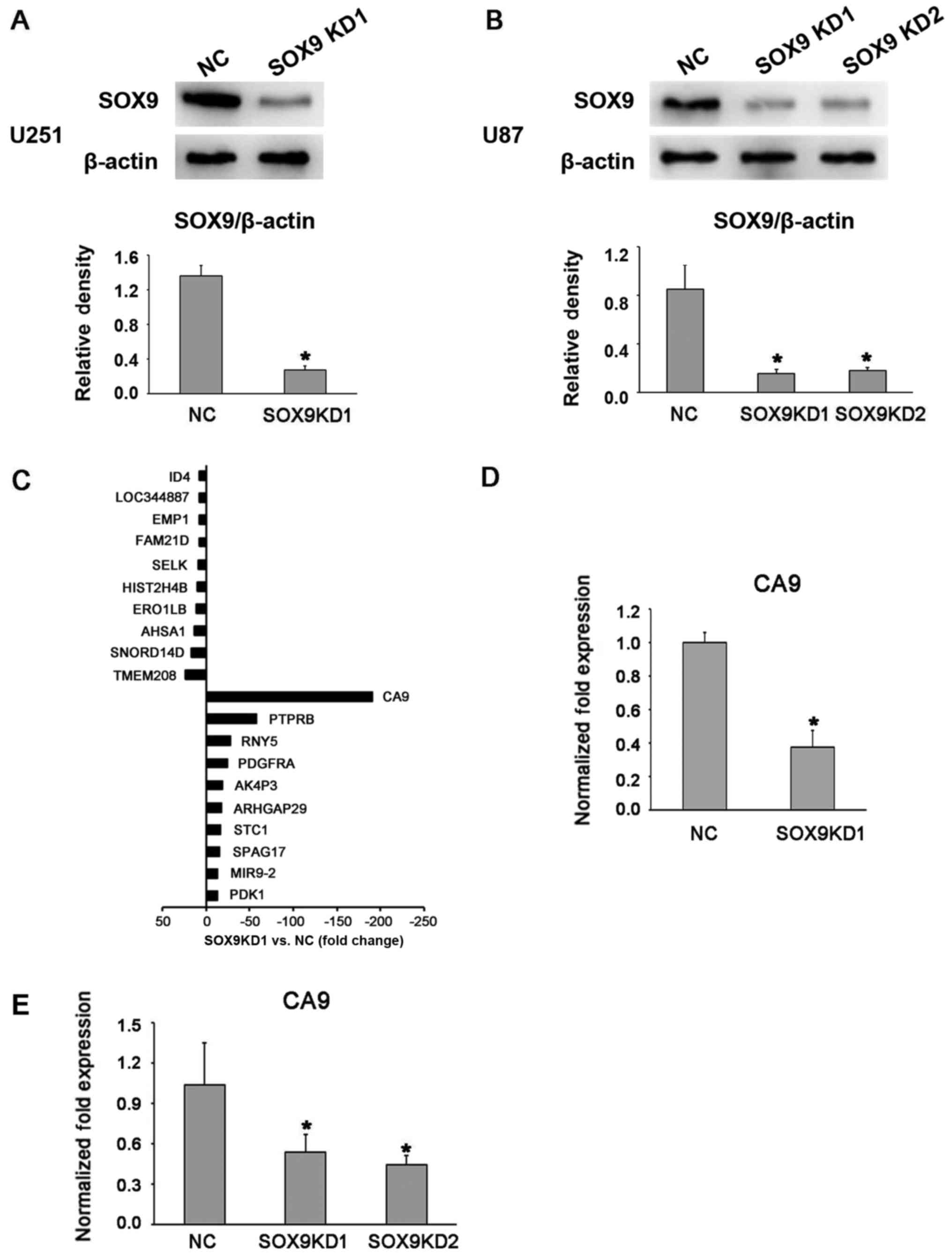
SOX9 expression in the U87 and U251 glioma cell
lines was effectively knocked down through lentivirus infection
with shRNA (Fig. 1A and B), and
stable cell lines were established through puromycin-based
selection. Both shRNA sequences, referred to as SOX9KD1 and
SOX9KD2, were found to be equally effective at knocking down SOX9
in the U87 cell line. Thus SOX9KD1 sequence alone was used in the
assessments of U251 cell line. The microarray-based gene expression
analysis was performed to analyze the SOX9 downstream target genes
in these cells, with the negative control shRNA-infected cells
serving as the control. The results revealed that, in response to
SOX9 downregulation, the CA9 gene not only topped the list of the
top 10 downregulated genes, but also topped the list of all the
SOX9-mediated differentially regulated genes (Table I and Fig. 1C). In order to further validate the
results, we assessed the mRNA expression of CA9 in the infected U87
and U251 cells, by RT-qPCR, which revealed a significant inhibition
of CA9 expression after the silencing of SOX9 (Fig. 1D and E).
 | Table IList of the top 10 downregulated
genes and the top 10 upregulated genes following SOX9
knockdown. |
Table I
List of the top 10 downregulated
genes and the top 10 upregulated genes following SOX9
knockdown.
| Probe set ID | [(SOX9-1) vs. (NC)
fold change ≥1.5] | Regulation
[(SOX9-1) vs. (NC)] | Gene symbol |
|---|
|
TC09000191.hg.1 | −190.23251 | Down | CA9 |
|
TC12001718.hg.1 | −57.696537 | Down | PTPRB |
|
TC12001420.hg.1 | −27.711525 | Down | RNY5 |
|
TC04002931.hg.1 | −24.433125 | Down | PDGFRA |
|
TC12001366.hg.1 | −19.318275 | Down | AK4P3 |
|
TC01002884.hg.1 | −18.039902 | Down | ARHGAP29 |
|
TC08001062.hg.1 | −16.57504 | Down | STC1 |
|
TC01003040.hg.1 | −15.059714 | Down | SPAG17 |
|
TC05001577.hg.1 | −13.410678 | Down | MIR9-2 |
|
TC02001031.hg.1 | −13.375226 | Down | PDK1 |
|
TC16000535.hg.1 | 23.395475 | Up | TMEM208 |
|
TC11002392.hg.1 | 16.466776 | Up | SNORD14D |
|
TC14000491.hg.1 | 12.988753 | Up | AHSA1 |
|
TC01004004.hg.1 | 10.898638 | Up | ERO1LB |
|
TC01001173.hg.1 | 9.65654 | Up | HIST2H4B |
|
TC03001474.hg.1 | 8.374862 | Up | SELK |
|
TC10000331.hg.1 | 7.95951 | Up | FAM21D |
|
TC12000189.hg.1 | 7.489741 | Up | EMP1 |
|
TC03001014.hg.1 | 7.4074697 | Up | LOC344887 |
|
TC03002706.hg.1 | 7.384494 | Up | ID4 |
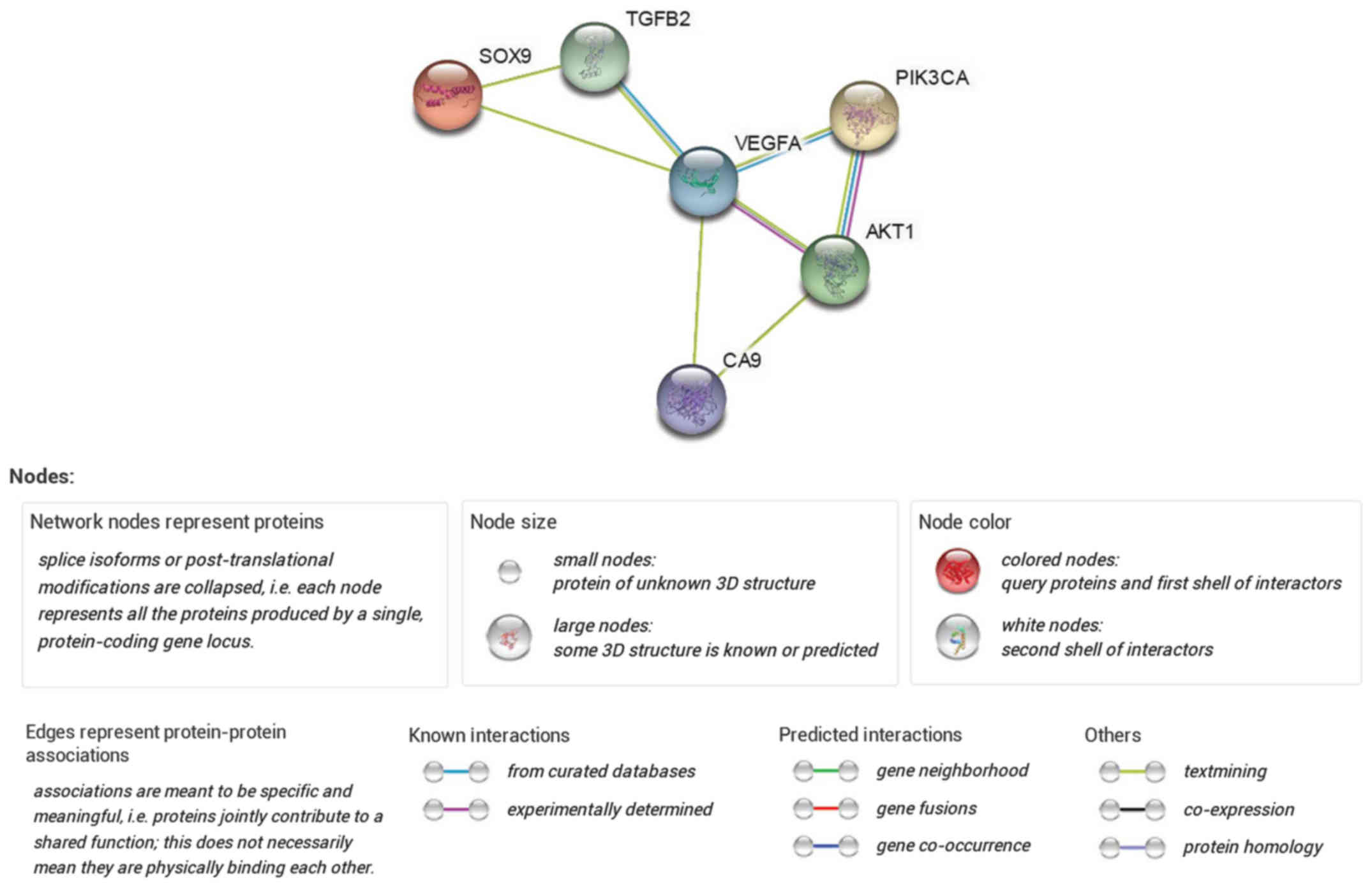
Furthermore, PPI networks were constructed with the
differentially expressed genes obtained from the gene chip results
(Fig. 2). In the network, SOX9 was
found strongly associated with CA9, and together they constructed a
combined network of downstream survival and apoptotic signals.
SOX9 knockdown enhances the
chemosensitivity of glioma cells to TMZ
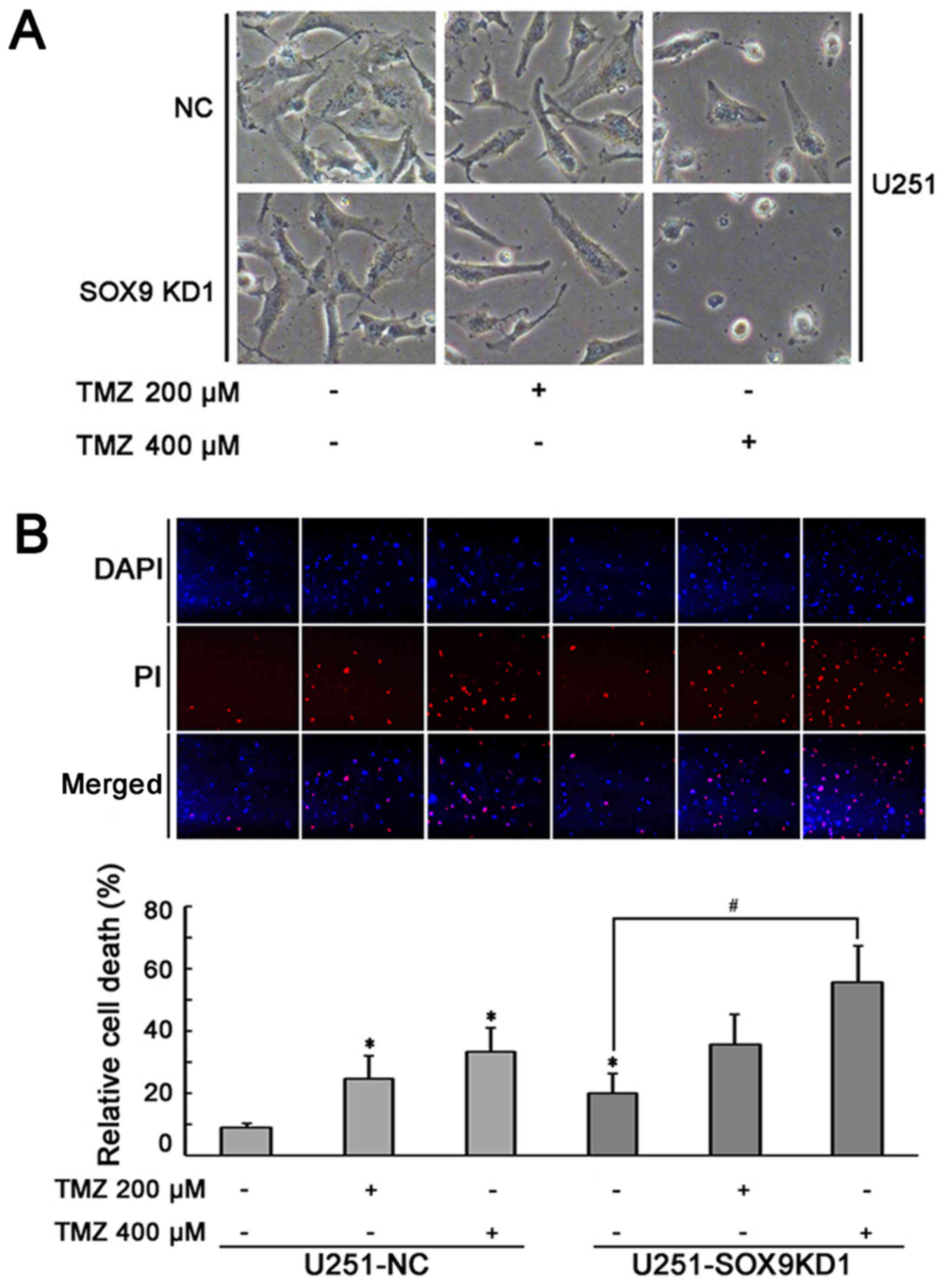
The role of the SOX9 gene in the sensitivity of U87
and U251 cells to TMZ was examined by incubating the U87 and U251
cells in which SOX9 was knocked down (SOX9KD), and the negative
control shRNA-infected cells (NC), with various concentrations of
TMZ (200 and 400 µM) for 24 h. Under a light microscope, the
number of U251 and U87 cells per field of vision appeared lower in
the SOX9KD group, compared to the negative control shRNA-infected
glioma cells (Fig. 3A and C),
revealing the importance of SOX9 in glioma. In the presence of TMZ,
both cell lines exhibited morphological changes (rounding of the
cells) and a more obvious growth arrest, which was directly
proportional to the TMZ concentration used (Fig. 3A and C). This effect was markedly
enhanced in the SOX9KD group compared with the negative control
cells. PI staining based on the two-color analysis technique, under
a fluorescent microscope, revealed an increased death of glioma
cells in response to SOX9 downregulation, compared to the negative
control cells. Furthermore, a significant increase in cellular
apoptosis was evident in response to TMZ treatment, in both cell
lines, which was significantly increased by SOX9 downregulation
(Fig. 3B and D). The response was
directly proportional to the concentration of TMZ used. These
results indicate the involvement of SOX9 in the sensitivity of
glioma cells to TMZ.
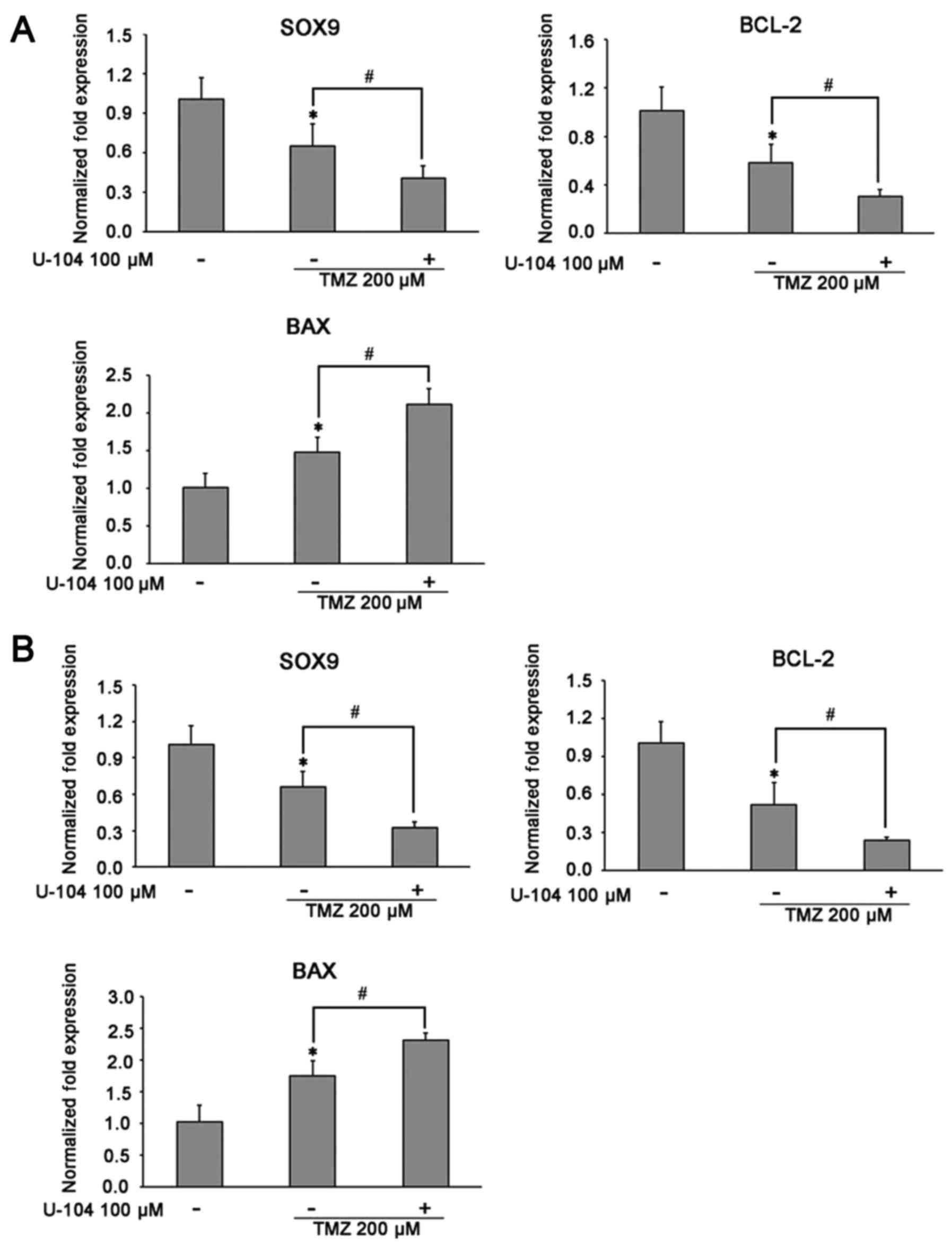
Inhibitory effect of TMZ on glioma cells:
Its mechanisms of action
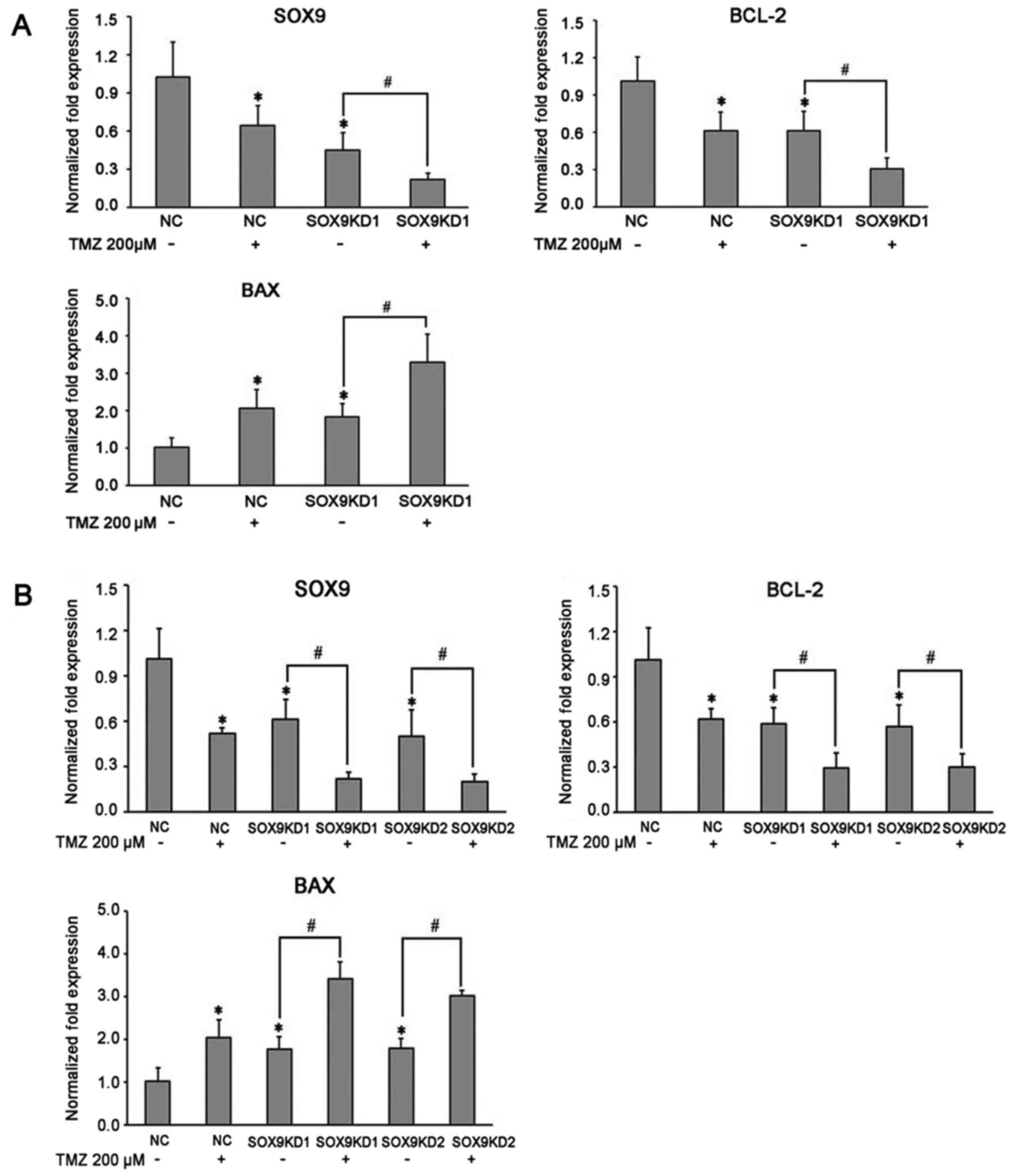
The mechanisms underlying the inhibitory effects of
TMZ on U87 and U251 glioma cell growth wereevaluated by assessing
the related survival molecules in the TMZ-treated glioma cells by
western blot analysis and RT-qPCR. After exposing the glioma cells
to TMZ (200 µM) for 16 h, the expression of SOX9 was found
to be significantly downregulated in both the U87 and U251 cells
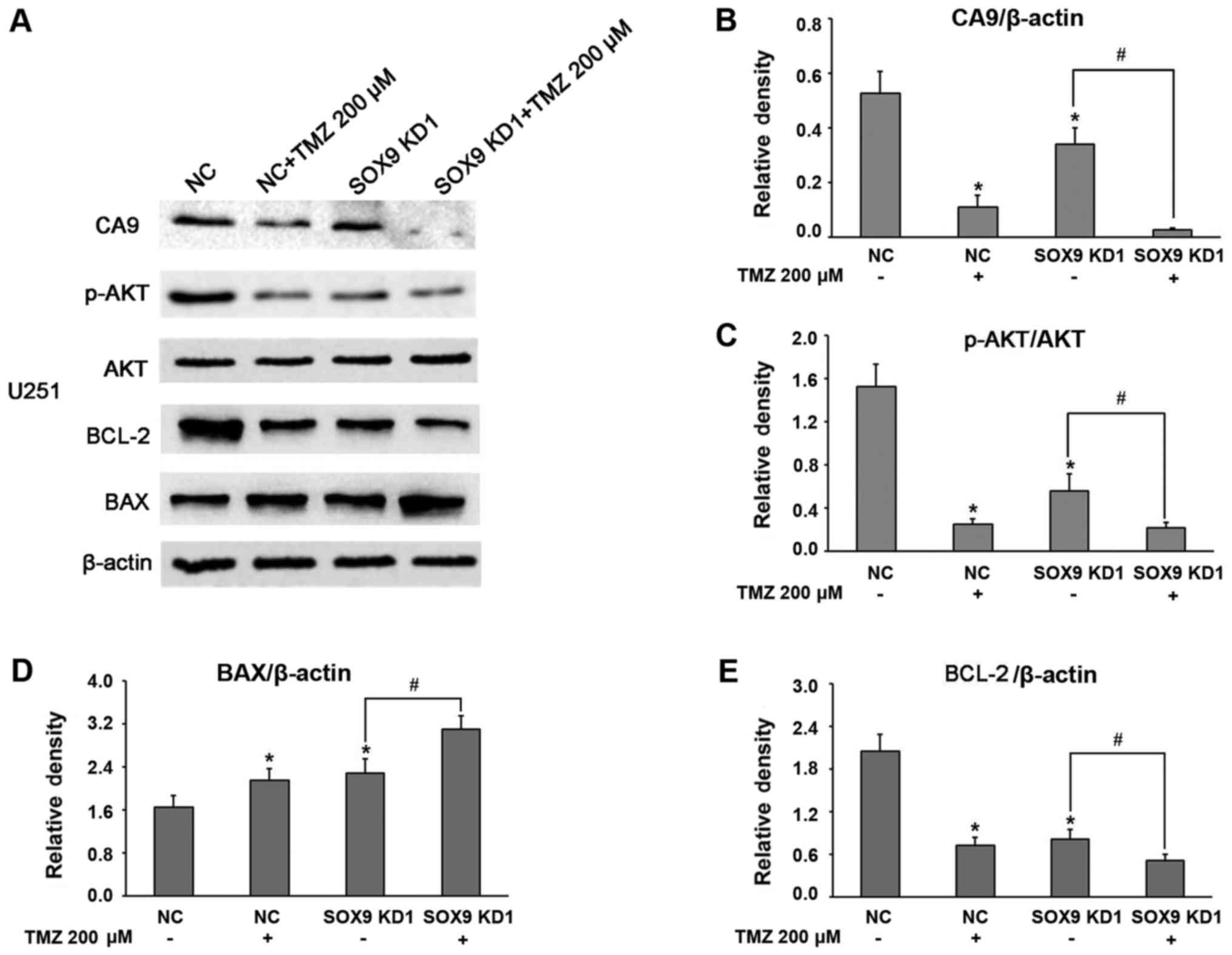
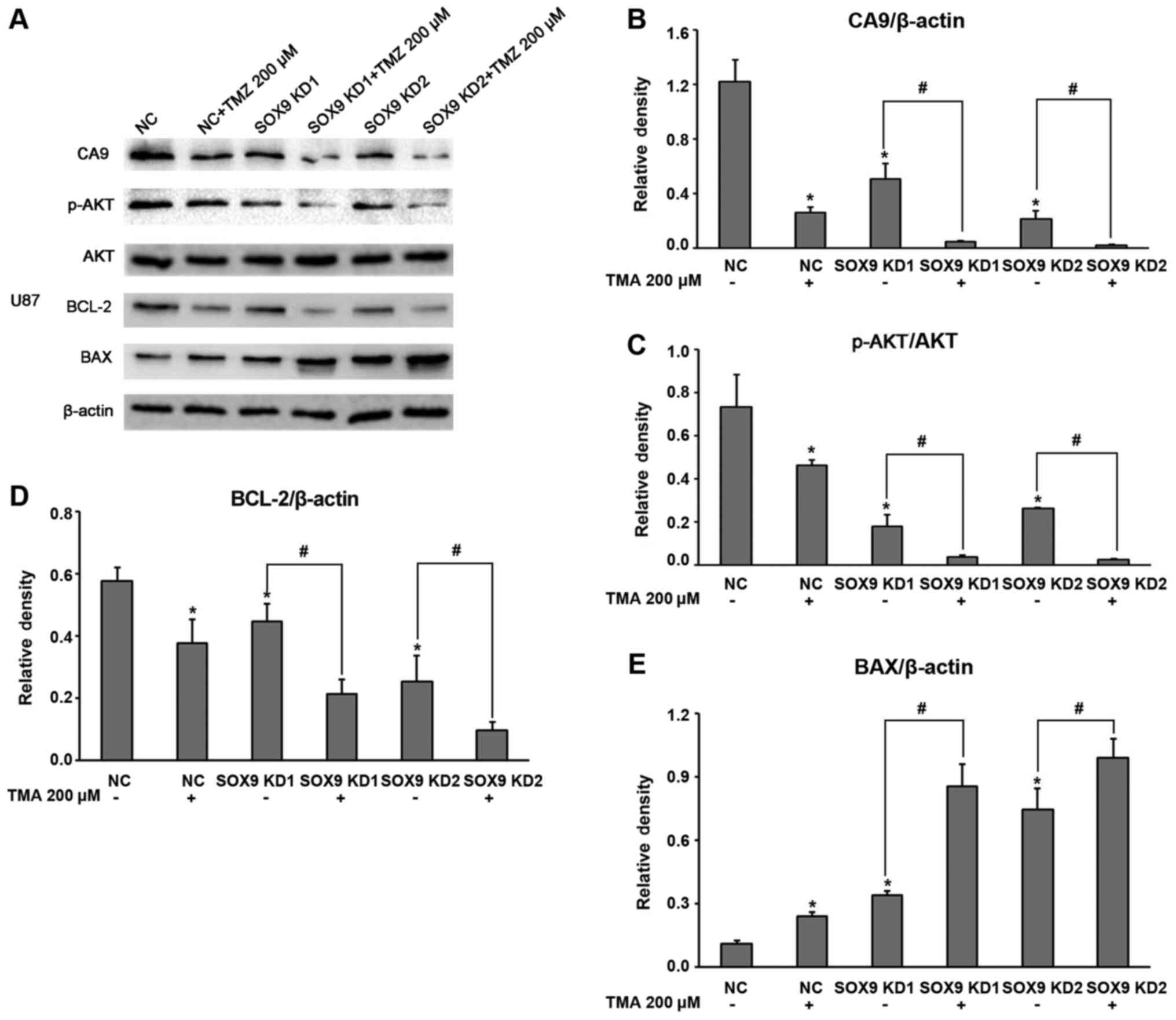
(Fig. 4A and B). Of note, TMZ was
equally efficient in further decreasing SOX9 expression in the
glioma cells in which SOX9 was silenced (SOX9KD group) (Fig. 4A and B). Considering the
association between SOX9 and CA9, we also examined the effect of
TMZ on CA9 expression in glioma cells. The results revealed that
TMZ significantly downregulated CA9 expression in the glioma cells,
with the effect being more significant in the cells in the SOX9KD
group (Figs. 5A and B, and
6A and B). These results
demonstrated the inhibitory effects of TMZ on SOX9 and CA9 protein
expression in glioma cells. Moreover, TMZ also significantly
decreased Akt phosphorylation, in cells in both the negative
control and SOX9KD groups, although the change in the expression of
Akt remained insignificant (Figs. 5A
and C, and 6A and C). Based on
these findings, we predicted that TMZ may interact with SOX9 and
vary the downstream signaling pathway by the direct or indirect
effect of SOX9 on CA9. By employing RT-qPCR, we further examined
the effects of TMZ on the apoptosis regulatory proteins, BCL-2/BAX,
in glioma cells. The results revealed that TMZ effectively
inhibited BCL-2 mRNA expression, while upregulating BAX expression
in both cell lines, with an enhanced effect observed in the cells
in which SOX9 was knocked down (Figs.
4A and B, 5D and E, and
6D and E). These results indicate
the possible involvement of SOX9 in the chemosensitivity of glioma
cells to TMZ.
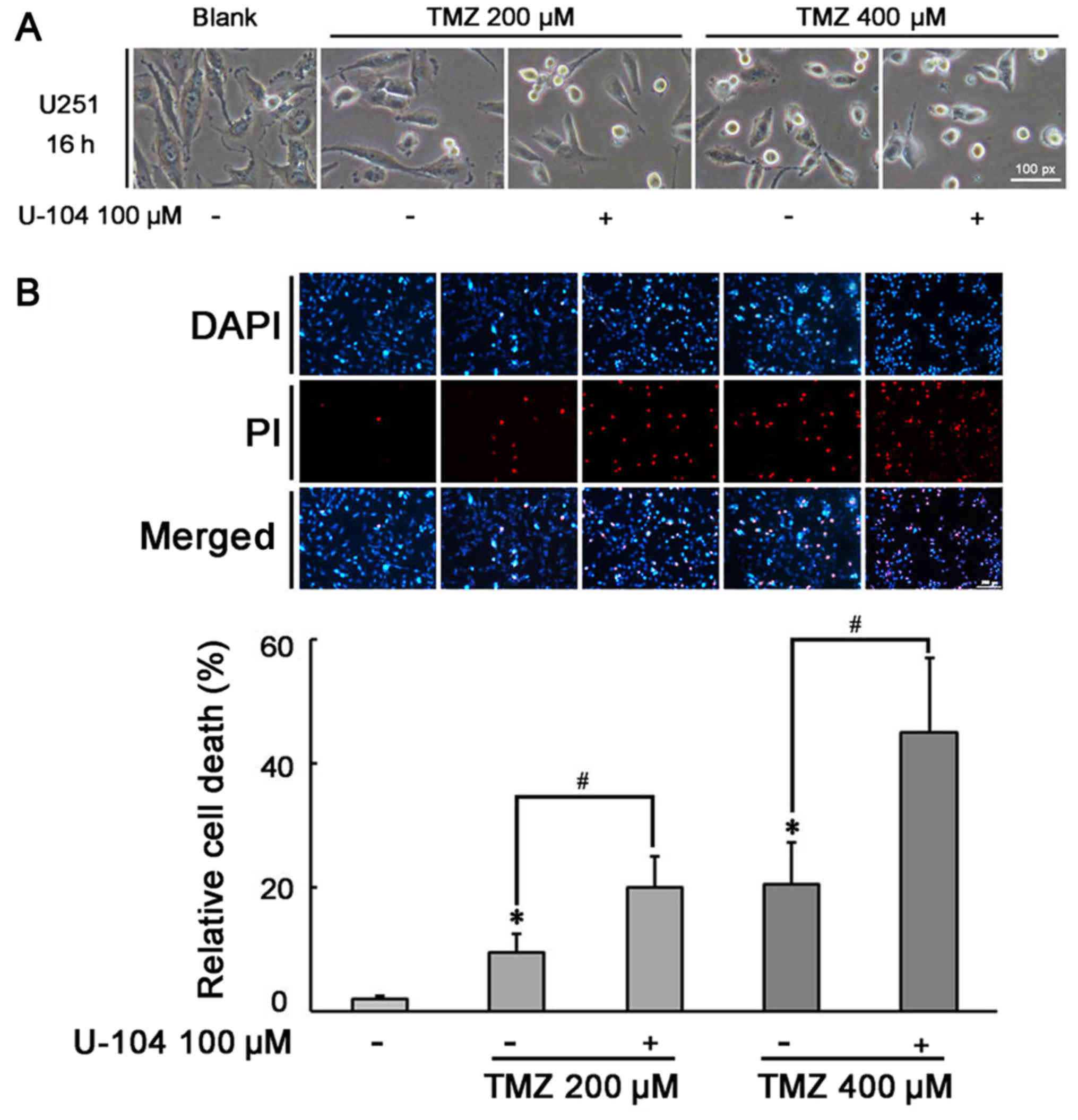
CA9 inhibitor enhances the pro-apoptotic
effects of TMZ on U87 and U251 cells
In view of the above-mentioned results, we
hypothesized that CA9 expression may also be involved in cellular
resistance to TMZ. To examine this hypothesis, we treated the
glioma cells with TMZ (200 and 400 µM) in the presence of
CA9 inhibitor (U-104; 100 µM) for 24 h. Under a light
microscope, fewer cells per field of vision, with an increased
number of cells with an altered morphology, as well cell bodies
that had become smaller and rounded as a result of cytoplasmic
retraction, were observed in the U-104 + TMZ group, compared to the
TMZ alone group (Fig. 7A and C).
Consistently, PI staining revealed a significant increase in the
number of apoptotic cells in the U-104 + TMZ group compared to the
TMZ alone group (Fig. 7B and D).
The effect was directly proportional to the concentration of TMZ
used. These results suggest that CA9 may also be involved in the
chemosensitivity of glioma cells to TMZ.
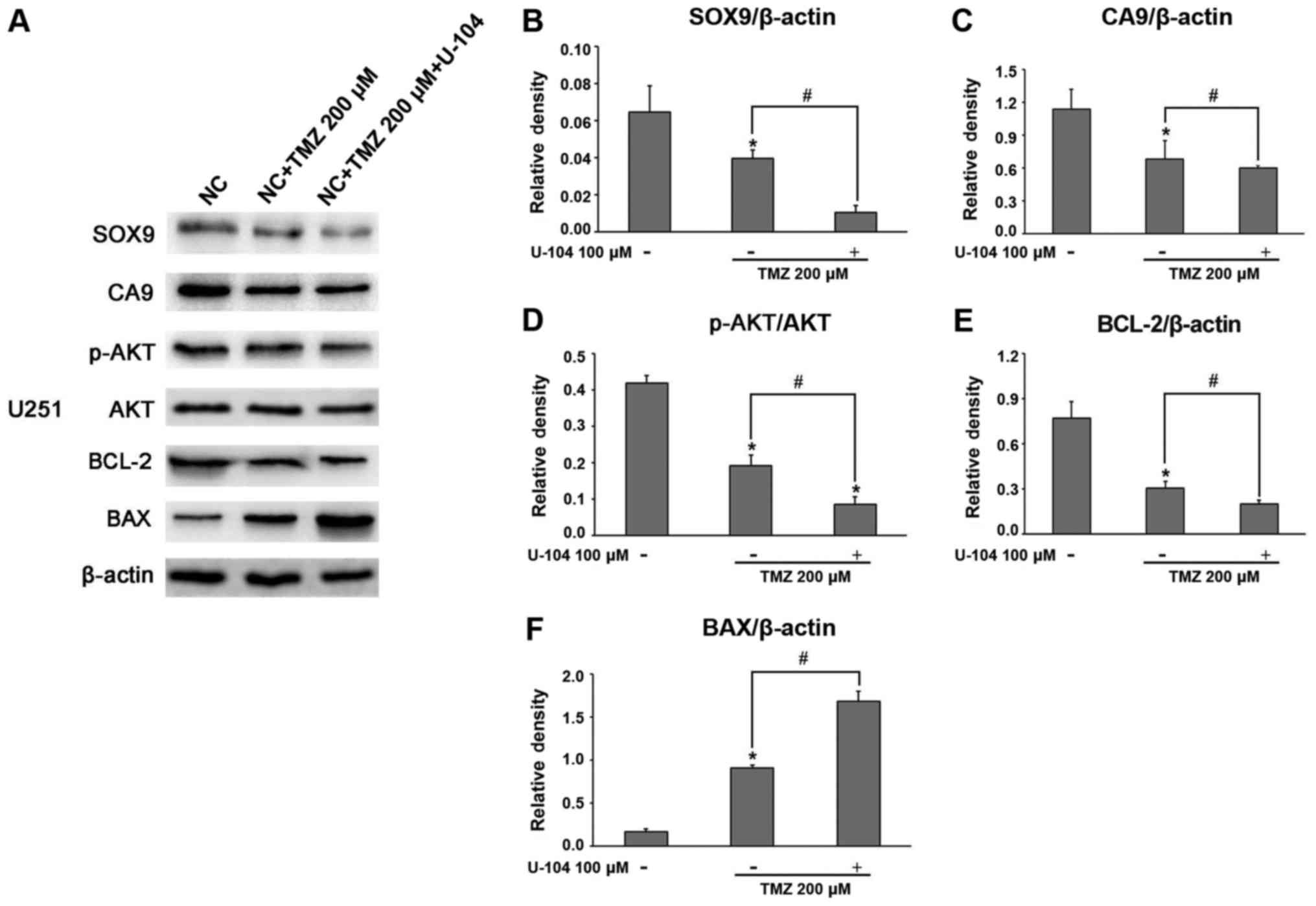
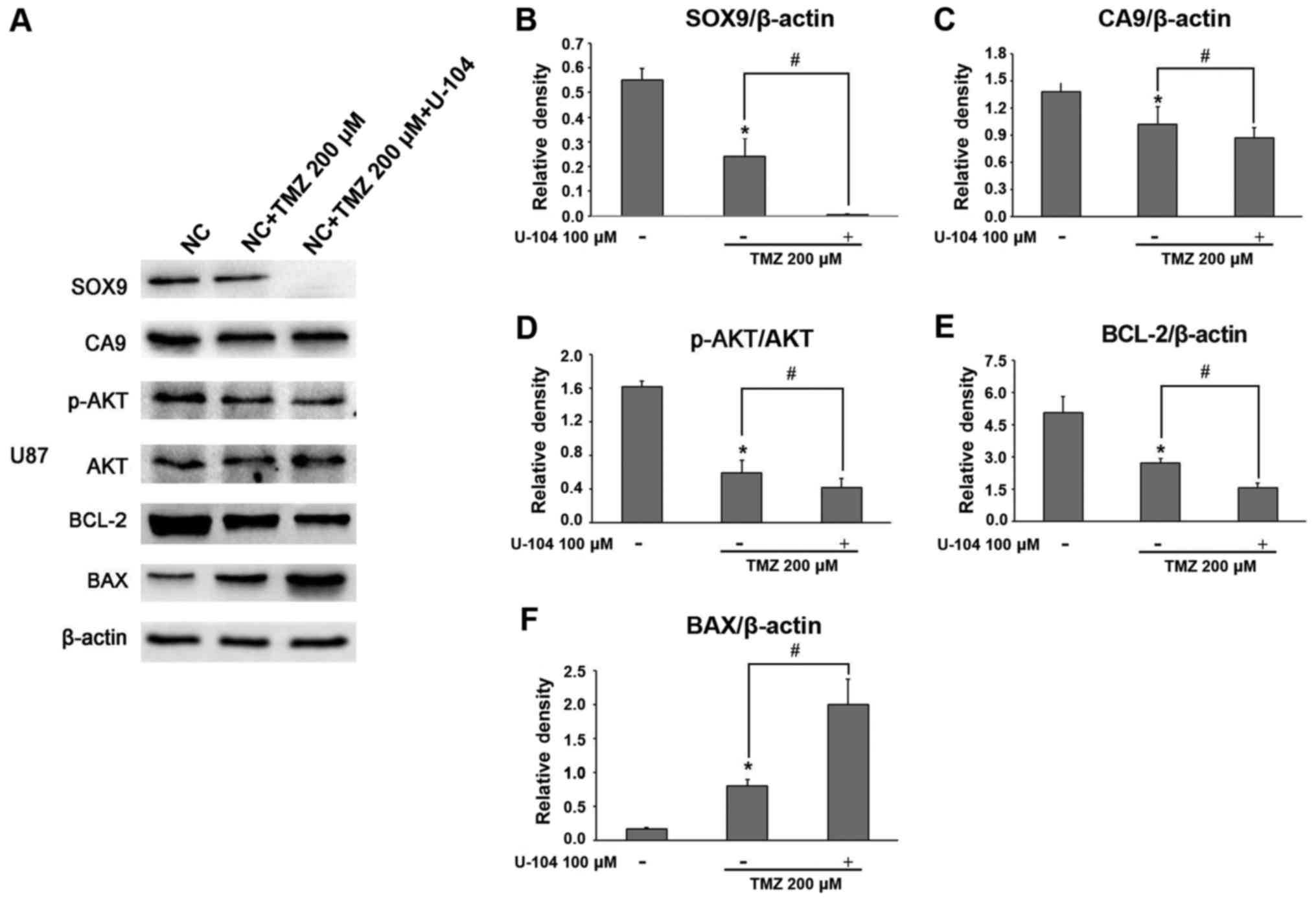
Synergistic effect of TMZ and CA9
inhibitor (U-104) on glioma cell apoptosis: Mechanisms of
action
To examine the role of CA9 inhibition in
TMZ-mediated glioma cell apoptosis, the associated survival pathway
genes were assessed. The results revealed that, synchronous with
the decrease in CA9 expression, the TMZ + U-104 combination
substantially decreased SOX9 expression in the glioma cells,
compared to the TMZ alone group (Figs.
8A–C, 9A–C, and 10A and B). Furthermore, TMZ + U-104
markedly suppressed the phosphorylation of Akt (Figs. 8D and 9D), while also significantly altering the
BCL-2 and BAX expression levels in the glioma cells (Figs. 8E and F, 9E and F, and 10A and B).
Discussion
It has previously been demonstrated that SOX9 plays
a key role in the regulation of cellular proliferation and
senescence, and in the self-renewal of cancer stem cells, thus
supporting tumor development in various types of cancer, including
brain tumors, colorectal cancer and prostate cancer (17,23,24).
Notably, the inhibition of SOX9 expression has been shown to
decrease tumor cell proliferation (25) and to suppress the self-renewal
ability of the cells (26),
subsequently resulting in tumor regression. Several studies have
witnessed the increased expression of SOX9 in glioblastoma
(20), the decreased expression of
which is linked to a better prognosis by inducing cell cycle arrest
in glioma cells (20,27). The fndings of this study also
demonstrated that SOX9 inhibition induced the apoptosis of U251 and
U87 glioma cells. It has been reported that SOX9 can be regulated
by multiple signaling pathways, including EGFR (28), Notch (29), Wnt (30) and hypoxia (31). Moreover, SOX9 can in turn regulate
several pathways, such as Wnt (32), BMI1 (17), PI3K/Akt (21,33).
Due to the important role of these signaling pathways in
maintaining the stemness of glioma stem cells and the modulation of
their chemoresistance, we hypothesized that SOX9 may also play an
important role in the regulation of drug resistance in glioma.
TMZ is currently the most efficient chemotherapy for
GBM. Indeed, TMZ combined-radiotherapy treatment has been shown to
extend the overall survival rate of patients from approximately 3
to 12% at 4 years compared to treatment with radiotherapy alone
(34). TMZ inhibits glioma cell
proliferation and migration, and promotes apoptosis (35). The expression levels of SOX9, CA9
and p-AKT are closely related to the proliferation, migration and
apoptosis of various tumors (36–38).
In this study, we found that TMZ alone can effectively inhibited
the expression of SOX9, CA9 and p-AKT (Figs. 8A–D and 9A–D). It has been demonstrated that TMZ
enhances the effects of radiotherapy in a randomized phase III
study (34). However, the damage
to glioma cells induced by TMZ can be repaired by
O6-methylguanine-DNA methyltransferase (MGMT), thus
inducing treatment resistance, while the methylation of the MGMT
promoter leads to an increase in TMZ sensitivity (39). Of note, the U251 and U87 glioma
cell lines used in our experiments exhibited MGMT promoter
hypermethylation status (40). The
findings of this study revealed that the glioma cells exhibited
high levels of SOX9 expression, and were less sensitive to TMZ
treatment compared to the cells in which SOX9 was silenced. This
increase in sensitivity was significant, demonstrating the key role
of SOX9 in TMZ sensitivity. Given that SOX9 can be postulated to be
one of the key term responsible for resistance to current GBM
chemotherapy. Therefore, targeting the activity of SOX9 may provide
a novel and promising therapeutic strategy for glioma.
A previous in vitro study examined the role
of SOX9 in the apoptosis of human glioma cells (20). However, the mechanism of
SOX9-mediated TMZ-induced cell death has not yet been adequately
understood. As is known, apoptosis is regulated by several protein
families, including the upstream BCL-2 family (e.g., the
anti-apoptotic BCL-2 and pro-apoptotic BAX) and the downstream
caspase family (e.g., caspase-3) (41,42).
Previous studies have demonstrated that treatment with TMZ alters
the expression of pro-apoptotic BAX and anti-apoptotic BCL-2, which
are involved in the mitochondrial pathway of apoptosis (43,44).
It has been demonstrated that the knockdown of SOX9 suppresses cell
growth in vitro by affecting the level of apoptosis-related
molecules, including the expression of BCL-2 and BAX (45). Thus, in this study, we examined the
levels of Bax and Bcl-2 protein expression in U251 and U87 cells in
which SOX9 was knocked down following treatmet with TMZ. The
results demonstrated a significant increase in BAX expression and a
decrease in BCL-2 expression, in the cells in which SOX9 was
knocked down compared to the normal glioma cells, in response to
TMZ treatment. This indicated an efficient and promoting effect of
SOX9 knockdown on the TMZ-mediated regulation of the BAX/BCL-2
ratio.
In the present study, we found that genomes
regulating cell proliferation and cell cycle progression, such as
AHSA1 (46), EMP1 (47), ID4 (48) and PDGFRA (49) (shown in Table I), were markedly altered in the
cells in which SOX9 was knocked down. The results also revealed
that the downregulation of SOX9 suppressed the growth of glioma
cells through the CA9-AKT pathway. Thus, a close association was
established between SOX9 and CA9, which was also demonstrated by a
PPI network. It has been shown that this regulation occurs at the
post-transcriptional level and that there is a feedback loop
between the two. The proteoglycan domain of CA9 has previously been
linked to negative regulation of cell adhesion through the
modulation of β-catenin and E-cadherin interactions (50), whereas the intracellular domain has
been shown to contribute to AKT activation (51). CA9 knockdown has shown to reduce
the growth rate of tumor xenografts, as reported in two recent
studies (37,52). In this study, we identified that
SOX9 and CA9 expression were linked together in glioma cells and
that the oncogenic activity of SOX9 is at least partially mediated
by the latter. Moreover, the decreased levels of CA9 observed in
the U251 and U87 cells in which SOX9 was knocked down suggest that
SOX9 may be involved in the regulation of glioma.
In an effort to identify the molecules that may
efficiently inhibit the expression of SOX9 (direct or indirectly),
we found that, in the U251 and U87 glioma cells, the CA9 inhibitor
significantly decreased SOX9 expression along with the subsequent
cascade of AKT signaling, demonstrating that the pharmacological
silencing of SOX9 is feasible using inhibitors of these signaling
pathways. Furthermore, a significant alteration in the
apoptosis-associated molecules, namely BCL-2 downregulation and BAX
upregulation, was also observed in these glioma cells in which CA9
was inhibited. It is vital to note that PI3K pathways are
aberrantly active in a high percentage of glioblastomas (53), which may indicate that their action
may be modulated through CA9 and SOX9.
An increasing amount of evidence indicates that
combining drugs with chemotherapeutic agents is a more effective
therapeutic option in cancer. The findings of the present study
identified that the concomitant treatment with a CA9 inhibitor
(U-104) and TMZ led to a further decrease in AKT phosphorylation,
the downregulation of BCL-2 and upregulation of BAX levels, all of
which were significant enough to exert a greater cytotoxic effect
on the glioma cells, compared to the cells treated with TMZ alone.
This suggests that the addition of U-104 to TMZ treatment may
potentially enhance the efficacy of TMZ therapy against human
glioma.
Currently, there is little information about the
association between SOX9 and CA9 in the regulation of glioma drug
resistance. Of note, the results of this study confirm the
association between them. The results of this study, together with
prior evidence, demonstrate that the assessment of the activity of
SOX9/CA9 may be a valuable prognostic and predictive marker in
glioma.
Although there are many controversies about the U87
MG cell line, we ensure that the aforementioned misidentification
issue of the U87MG cell line from ATCC is unlikely to affect the
outcomes of our study. The analysis by Allen et al indicated
that the U87MG ATCC cell line is likely to be derived from another
patient with glioma (54). That is
the reason why it differs from the U87 cell line established by
Uppsala University. It has been reported that the U87 MG ATCC line
is of central nervous system origin, although its source is unknown
(54). Recently, the U87MG cell
line from ATCC has still been used in studies on glioma (55–57).
In future studies, we will replace the U87MG cell line with other
classical cell lines that have been validated in order to further
refine our experimental results.
Acknowledgments
Not applicable.
Funding
This study was supported by grants from the National
Natural Scientifc Foundation of China to YZ (no. 81272419), the
National Natural Scientifc Foundation of China to YT (no.
81572983), the Social Development of Technology Research Projects
in Shaanxi Province to PZ (no. 2015SF027), the Social Development
of Technology Research Projects in Shaanxi Province to HL (no.
2016SF191), and the Beijing Key Laboratory of Brain Major Diseases
Open Project to YT (2015).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YZ, YT, WJ and YC conceived and designed the
majority of the experiments. XX, PZ, XW, HY and HL developed the
methodology. XX and ZW performed the majority of the experiments.
XX and NL performed the analysis and interpretation of the data.
XX, ZW and NL wrote, reviewed and revised the manuscript. YZ and YT
supported study supervision. All authors have read and approved the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dunn GP, Rinne ML, Wykosky J, Genovese G,
Quayle SN, Dunn IF, Agarwalla PK, Chheda MG, Campos B, Wang A, et
al: Emerging insights into the molecular and cellular basis of
glioblastoma. Genes Dev. 26:756–784. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Radner H, Blümcke I, Reifenberger G and
Wiestler OD: The new WHO classification of tumors of the nervous
system 2000. Pathology and genetics. Pathologe. 23:260–283. 2002.In
German. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qi J, Yang H, Wang X and Tu Y: The
progress in molecular biomarkers of gliomas. Cancer Transl Med.
2:125–129. 2016. View Article : Google Scholar
|
|
4
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Athanassiou H, Synodinou M, Maragoudakis
E, Paraskevaidis M, Verigos C, Misailidou D, Antonadou D, Saris G,
Beroukas K and Karageorgis P: Randomized phase II study of
temozolomide and radiotherapy compared with radiotherapy alone in
newly diagnosed glioblastoma multiforme. J Clin Oncol.
23:2372–2377. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagane M, Kobayashi K, Ohnishi A, Shimizu
S and Shiokawa Y: Prognostic significance of O6-methylguanine-DNA
methyltransferase protein expression in patients with recurrent
glioblastoma treated with temozolomide. Jpn J Clin Oncol.
37:897–906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Groves MD, Puduvalli VK, Hess KR, Jaeckle
KA, Peterson P, Yung WK and Levin VA: Phase II trial of
temozolomide plus the matrix metalloproteinase inhibitor,
marimastat, in recurrent and progressive glioblastoma multiforme. J
Clin Oncol. 20:1383–1388. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bektas M, Johnson SP, Poe WE, Bigner DD
and Friedman HS: A sphingosine kinase inhibitor induces cell death
in temozolomide resistant glioblastoma cells. Cancer Chemother
Pharmacol. 64:1053–1058. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bocangel DB, Finkelstein S, Schold SC,
Bhakat KK, Mitra S and Kokkinakis DM: Multifaceted resistance of
gliomas to temozolomide. Clin Cancer Res. 8:2725–2734.
2002.PubMed/NCBI
|
|
10
|
Gubbay J, Collignon J, Koopman P, Capel B,
Economou A, Münsterberg A, Vivian N, Goodfellow P and Lovell-Badge
R: A gene mapping to the sex-determining region of the mouse Y
chromosome is a member of a novel family of embryonically expressed
genes. Nature. 346:245–250. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
de la Rocha AM, Sampron N, Alonso MM and
Matheu A: Role of SOX family of transcription factors in central
nervous system tumors. Am J Cancer Res. 4:312–324. 2014.PubMed/NCBI
|
|
12
|
Wagner T, Wirth J, Meyer J, Zabel B, Held
M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, et al:
Autosomal sex reversal and campomelic dysplasia are caused by
mutations in and around the SRY-related gene SOX9. Cell.
79:1111–1120. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Foster JW, Dominguez-Steglich MA, Guioli
S, Kwok C, Weller PA, Stevanović M, Weissenbach J, Mansour S, Young
ID, Goodfellow PN, et al: Campomelic dysplasia and autosomal sex
reversal caused by mutations in an SRY-related gene. Nature.
372:525–530. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang SS, Fang WT, Hou YH, Huang SF, Yen
BL, Chang JL, Li SM, Liu HP, Liu YL, Huang CT, et al: Upregulation
of SOX9 in lung adenocarcinoma and its involvement in the
regulation of cell growth and tumorigenicity. Clin Cancer Res.
16:4363–4373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Capaccione KM, Hong X, Morgan KM, Liu W,
Bishop JM, Liu L, Markert E, Deen M, Minerowicz C, Bertino JR, et
al: Sox9 mediates Notch1-induced mesenchymal features in lung
adenocarcinoma. Oncotarget. 5:3636–3650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chakravarty G, Rider B and Mondal D:
Cytoplasmic compartmentalization of SOX9 abrogates the growth
arrest response of breast cancer cells that can be rescued by
trichostatin A treatment. Cancer Biol Ther. 11:71–83. 2011.
View Article : Google Scholar
|
|
17
|
Matheu A, Collado M, Wise C, Manterola L,
Cekaite L, Tye AJ, Canamero M, Bujanda L, Schedl A, Cheah KS, et
al: Oncogenicity of the developmental transcription factor Sox9.
Cancer Res. 72:1301–1315. 2012. View Article : Google Scholar :
|
|
18
|
Raspaglio G, Petrillo M, Martinelli E, Li
Puma DD, Mariani M, De Donato M, Filippetti F, Mozzetti S, Prislei
S, Zannoni GF, et al: Sox9 and Hif-2α regulate TUBB3 gene
expression and affect ovarian cancer aggressiveness. Gene.
542:173–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomsen MK, Ambroisine L, Wynn S, Cheah
KS, Foster CS, Fisher G, Berney DM, Møller H, Reuter VE, Scardino
P, et al Transatlantic Prostate Group: SOX9 elevation in the
prostate promotes proliferation and cooperates with PTEN loss to
drive tumor formation. Cancer Res. 70:979–987. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, He S, Yuan J, Mao X, Cao Y, Zong
J, Tu Y and Zhang Y: Oncogenic role of SOX9 expression in human
malignant glioma. Med Oncol. 29:3484–3490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Swartling FJ, Ferletta M, Kastemar M,
Weiss WA and Westermark B: Cyclic GMP-dependent protein kinase II
inhibits cell proliferation, Sox9 expression and Akt
phosphorylation in human glioma cell lines. Oncogene. 28:3121–3131.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
23
|
Swartling FJ, Savov V, Persson AI, Chen J,
Hackett CS, Northcott PA, Grimmer MR, Lau J, Chesler L, Perry A, et
al: Distinct neural stem cell populations give rise to disparate
brain tumors in response to N-MYC. Cancer Cell. 21:601–613. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang G, Lunardi A, Zhang J, Chen Z, Ala U,
Webster KA, Tay Y, Gonzalez-Billalabeitia E, Egia A, Shaffer DR, et
al: Zbtb7a suppresses prostate cancer through repression of a
Sox9-dependent pathway for cellular senescence bypass and tumor
invasion. Nat Genet. 45:739–746. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang YJ, Xu F, Zhang YJ, Li HB, Han JC
and Li L: miR-206 inhibits non small cell lung cancer cell
proliferation and invasion by targeting SOX9. Int J Clin Exp Med.
8:9107–9113. 2015.PubMed/NCBI
|
|
26
|
Larsimont JC, Youssef KK, Sánchez-Danés A,
Sukumaran V, Defrance M, Delatte B, Liagre M, Baatsen P, Marine JC,
Lippens S, et al: Sox9 controls self-renewal of oncogene targeted
cells and links tumor initiation and invasion. Cell Stem Cell.
17:60–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao J, Zhang JY, Li YH and Ren F:
Decreased expression of SOX9 indicates a better prognosis and
inhibits the growth of glioma cells by inducing cell cycle arrest.
Int J Clin Exp Pathol. 8:10130–10138. 2015.PubMed/NCBI
|
|
28
|
Ling S, Chang X, Schultz L, Lee TK, Chaux
A, Marchionni L, Netto GJ, Sidransky D and Berman DM: An
EGFR-ERK-SOX9 signaling cascade links urothelial development and
regeneration to cancer. Cancer Res. 71:3812–3821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haller R, Schwanbeck R, Martini S, Bernoth
K, Kramer J, Just U and Rohwedel J: Notch1 signaling regulates
chondrogenic lineage determination through Sox9 activation. Cell
Death Differ. 19:461–469. 2012. View Article : Google Scholar :
|
|
30
|
Blache P, van de Wetering M, Duluc I,
Domon C, Berta P, Freund JN, Clevers H and Jay P: SOX9 is an
intestine crypt transcription factor, is regulated by the Wnt
pathway, and represses the CDX2 and MUC2 genes. J Cell Biol.
166:37–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lafont JE, Talma S, Hopfgarten C and
Murphy CL: Hypoxia promotes the differentiated human articular
chondrocyte phenotype through SOX9-dependent and -independent
pathways. J Biol Chem. 283:4778–4786. 2008. View Article : Google Scholar
|
|
32
|
Bastide P, Darido C, Pannequin J, Kist R,
Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande
F, et al: Sox9 regulates cell proliferation and is required for
Paneth cell differentiation in the intestinal epithelium. J Cell
Biol. 178:635–648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ikegami D, Akiyama H, Suzuki A, Nakamura
T, Nakano T, Yoshikawa H and Tsumaki N: SOX9 sustains chondrocyte
survival and hypertrophy in part through Pik3ca-Akt pathways.
Development. 138:1507–1519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Linz U: Commentary on effects of
radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 2009.10:459–466. View Article : Google Scholar
Cancer. 116:1844–1846. 2010. View Article : Google Scholar
|
|
35
|
Yu XZ, Han GK, Yang WT, Li ZY and Jin F:
Effect of temozolomide on cell proliferation and migration ability
of glioma TJ905 cells. J Xinxiang Medical University. 32:111–114.
2015.
|
|
36
|
Liu N, Zhang L, Wang Z, Cheng Y, Zhang P,
Wang X, Wen W, Yang H, Liu H, Jin W, et al: MicroRNA-101 inhibits
proliferation, migration and invasion of human glioblastoma by
targeting SOX9. Oncotarget. 8:19244–19254. 2017.
|
|
37
|
Lou Y, McDonald PC, Oloumi A, Chia S,
Ostlund C, Ahmadi A, Kyle A, Auf dem Keller U, Leung S, Huntsman D,
et al: Targeting tumor hypoxia: Suppression of breast tumor growth
and metastasis by novel carbonic anhydrase IX inhibitors. Cancer
Res. 71:3364–3376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song L, Li D, Gu Y, Wen ZM, Jie J, Zhao D
and Peng LP: MicroRNA-126 targeting PIK3R2 inhibits NSCLC A549 cell
proliferation, migration, and invasion by regulation of
PTEN/PI3K/AKT pathway. Clin Lung Cancer. 17:e65–e75. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gaspar N, Marshall L, Perryman L, Bax DA,
Little SE, Viana-Pereira M, Sharp SY, Vassal G, Pearson AD, Reis
RM, et al: MGMT-independent temozolomide resistance in pediatric
glioblastoma cells associated with a PI3-kinase-mediated HOX/stem
cell gene signature. Cancer Res. 70:9243–9252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pyko IV, Nakada M, Sabit H, Teng L,
Furuyama N, Hayashi Y, Kawakami K, Minamoto T, Fedulau AS and
Hamada J: Glycogen synthase kinase 3β inhibition sensitizes human
glioblastoma cells to temozolomide by affecting O6-methylguanine
DNA methyltransferase promoter methylation via c-Myc signaling.
Carcinogenesis. 34:2206–2217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jarskog LF, Selinger ES, Lieberman JA and
Gilmore JH: Apoptotic proteins in the temporal cortex in
schizophrenia: High Bax/Bcl-2 ratio without caspase-3 activation.
Am J Psychiatry. 161:109–115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kobayashi T, Masumoto J, Tada T, Nomiyama
T, Hongo K and Nakayama J: Prognostic significance of the
immunohistochemical staining of cleaved caspase-3, an activated
form of caspase-3, in gliomas. Clin Cancer Res. 13:3868–3874. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ma J, Murphy M, O'Dwyer PJ, Berman E, Reed
K and Gallo JM: Biochemical changes associated with a
multidrug-resistant phenotype of a human glioma cell line with
temozolomide-acquired resistance. Biochem Pharmacol. 63:1219–1228.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Das A, Banik NL, Patel SJ and Ray SK:
Dexamethasone protected human glioblastoma U87MG cells from
temozolomide induced apoptosis by maintaining Bax:Bcl-2 ratio and
preventing proteolytic activities. Mol Cancer. 3:362004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stoeckl S, Goettl C, Grifka J and Graessel
S: In vitro gene knockdown of SOX9 in rat MSC affects Bcl2
expression. Eur J Cell. 89(Suppl 1): S142010.
|
|
46
|
Shao J, Wang L, Zhong C, Qi R and Li Y:
AHSA1 regulates proliferation, apoptosis, migration, and invasion
of osteosarcoma. Biomed Pharmacother. 77:45–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun GG, Wang YD, Cui DW, Cheng YJ and Hu
WN: EMP1 regulates caspase-9 and VEGFC expression and suppresses
prostate cancer cell proliferation and invasion. Tumour Biol.
35:3455–3462. 2014. View Article : Google Scholar
|
|
48
|
Jason CP, Chaudhary J and Evans A:
Abstract LB-6: bHLH transcription factor Id4 plays a regulatory
role in cell cycle control, apoptosis, and senescence in
androgen-insensitive prostate cancer. Cancer Res. 70(Suppl):
321–327. 2010. View Article : Google Scholar
|
|
49
|
Chen D, Zuo D, Luan C, Liu M, Na M, Ran L,
Sun Y, Persson A, Englund E, Salford LG, et al: Glioma cell
proliferation controlled by ERK activity-dependent surface
expression of PDGFRA. PLoS One. 9:e872812014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Svastová E, Zilka N, Zat'ovicová M,
Gibadulinová A, Ciampor F, Pastorek J and Pastoreková S: Carbonic
anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via
interaction with β-catenin. Exp Cell Res. 290:332–345. 2003.
View Article : Google Scholar
|
|
51
|
Dorai T, Sawczuk IS, Pastorek J, Wiernik
PH and Dutcher JP: The role of carbonic anhydrase IX overexpression
in kidney cancer. Eur J Cancer. 41:2935–2947. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ebos JM, Lee CR and Kerbel RS: Tumor and
host-mediated pathways of resistance and disease progression in
response to antiangiogenic therapy. Clin Cancer Res. 15:5020–5025.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
McLendon R, Friedman A, Bigner D, et al
Cancer Genome Atlas Research Network: Comprehensive genomic
characterization defines human glioblastoma genes and core
pathways. Nature. 455:1061–1068. 2008. View Article : Google Scholar
|
|
54
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8:354re32016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Franco DG, Moretti IF and Marie SK:
Abstract 1498: Melatonin inhibits mitochondrial transcription
factor A expression in glioblastoma U87MG cell culture inducing an
anti-tumorigenic effect. Cancer Res. 77(Supplement): 14982017.
View Article : Google Scholar
|
|
56
|
Lan YL, Wang X, Xing JS, Yu ZL, Lou JC, Ma
XC and Zhang B: Anti-cancer effects of dopamine in human glioma:
Involvement of mitochondrial apoptotic and anti-inflammatory
pathways. Oncotarget. 8:88488–88500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Han MZ, Xu R, Xu YY, Zhang X, Ni SL, Huang
B, Chen AJ, Wei YZ, Wang S, Li WJ, et al: TAGLN2 is a candidate
prognostic biomarker promoting tumorigenesis in human gliomas. J
Exp Clin Cancer Res. 36:1552017. View Article : Google Scholar : PubMed/NCBI
|