Introduction
Prostate cancer is the most common male-related
malignancy worldwide; it affects 1 in 9 men aged 65 years or older
and is the second leading cause of cancer-related mortality in men
(1,2). Therefore, identification of novel
factors that are involved in prostate cancer development and
progression, including tumor cell proliferation, migration and
invasion, may provide useful insights into the effective control of
prostate cancer progression as well as novel diagnostic and
therapeutic approaches. For example, our previous study revealed
that N-Myc downstream-regulated gene 3 (NDRG3) was overexpressed in
prostate cancer tissue specimens and was able to induce
proliferation and migration of prostate cancer cells in
vitro and in a xenograft nude mouse model (3). NDRG3 overexpression was also reported
to increase the expression of C-X-C motif chemokine 5 (CXCL5; also
known as epithelial neutrophil-activating peptide-78) and to
promote tumor cell growth (3–5).
Therefore, further studies on the role of CXCL5 in prostate cancer
may provide valuable information regarding prostate cancer
development and progression.
CXCL5 is a member of the CXC family of chemokines
and is produced following stimulation by other inflammatory
cytokines, such as interleukin (IL)-1 and tumor necrosis factor-α
(6). CXCL5 functions in the cell
through interaction with C-X-C chemokine receptor type 2 (CXCR2) on
the cell surface (7). The CXCL5
protein contains an ELR motif at the amino terminus, which shares
structural homology with IL-8 and, thus, exhibits similar functions
as IL-8, such as regulation of cell proliferation or migration
(5,8,9).
CXCL5 was also reported to serve a role in tumor cell
proliferation, metastasis, neutrophil infiltration and angiogenesis
in tumor lesions (4,5). Previous studies have demonstrated
that CXCL5 is overexpressed in several types of human cancers,
including gastric, pancreatic, liver, and prostate cancers
(4,10–12),
and was associated with poor prognosis (5,10,11,13–15).
In addition, CXCL5 has been considered as a crucial chemokine in
the promotion of human cancer development and progression (4–15).
The present study investigated CXCL5 expression in
normal and cancerous prostate tissues and cell lines, and assessed
the effects of exogenous CXCL5 exposure and lentiviral-mediated
CXCL5 overexpression on the regulation of malignant behaviors of
prostate cancer cells in vitro and in a nude mouse xenograft
model. In addition, the underlying molecular mechanisms of CXCL5
overexpression in prostate cancer cells were investigated to
provide novel insight into the role of CXCL5 in prostate cancer
progression, which may aid in the development of future therapeutic
strategies.
Materials and methods
Cell lines and culture
Prostate cancer cell lines PC-3, DU145 and LNCaP,
the immortalized normal prostate stroma cell line WPMY-1, the
immortalized prostate epithelium cell line RWPE-1 and the 293T
cells used for lentiviral packaging were obtained from American
Type Culture Collection (Manassas, VA, USA). Prostate cancer cell
lines were maintained in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1:100
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.), whereas WPMY-1 and RWPE-1 cell lines were cultured in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS and 1:100
penicillin/streptomycin in a humidified incubator with 5%
CO2 at 37°C. The medium supplemented with FBS and
penicillin/streptomycin was considered as complete medium (CM).
Preparation of CXCL5-lentiviral vector
and stable gene transfection
To overexpress CXCL5 in prostate cells, a
pReceiver-Lv203 CXCL5 expression vector (cat. no. EX-A1113-Lv203)
and a pReceiver-Lv203 empty vector (cat. no. EX-NEG-Lv203) were
constructed by GeneCopoeia Inc. (Rockville, MD, USA), and a
Lenti-Pac HIV Expression Packaging kit (cat. no. HPK-LvTR-40;
GeneCopoeia, Inc. Rockville, MD, USA) was used to produce
CXCL5-carrying lentivirus overexpression vector (CXCL5-OE) and mock
lentivirus empty vector (mock), according to the manufacturer's
instructions. The 293T lenti-viral packaging cells were transfected
using the transfection reagent provided in the Lenti-Pac HIV
Expression Packaging kit, following the manufacturer's protocol.
Briefly, 293T cells (1.5×106 cells) were plated in a
10-mm dish in 10 ml of DMEM supplemented with 10% heat-inactivated
FBS. Following incubation at 37°C for 48 h, DNA-EndoFectin Lenti
complex was directly added to the dish and the cells were incubated
at 37°C for 12 h, after which the culture medium that contains the
DNA-EndoFectin Lenti complex was replaced with fresh DMEM
supplemented with 5% heat-inactivated FBS. A 1/500 volume of the
TiterBoost reagent was added to the culture medium and continued
incubation at 37°C for 48 h. The lentiviral particle-containing
culture medium was collected and centrifuged at room temperature at
1,000 × g for 10 min. Subsequently, PC-3, DU145, or WPMY-1 cells
(2.5×105 cells/well) were incubated in 6-well plates in
1 ml RPMI-1640 or DMEM CM containing lentiviral particle-containing
culture medium at a ratio of 1:1 at 37°C for 48 h. To select the
stable gene-transfected cells, these cell lines were further
cultured and selected by culturing with puromycin (50 ng/ml,
Invitrogen; Thermo Fisher Scientific, Inc.) in RPMI-1640 or DMEM
CM. The stable CXCL5-OE-transfected cells and mock-transfected
cells were isolated and expanded, and CXCL5 expression was
confirmed by ELISA. These isolated cells were used for the
subsequent experiments.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from 2×107 cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
and reverse transcribed into cDNA using the Quant Reverse
Transcriptase (Tiangen Biotech, Beijing, China), both according to
the manufacturers' protocol. cDNA was amplified by PCR to determine
the mRNA expression levels of CXCL5 and its receptor CXCR2, as well
as the expression levels of BAX, NDRG3, ERK, and β-actin. The
primers and PCR thermocycling conditions are provided in Table I and II, respectively. PCR products were
visualized by electrophoresis in 1.2% agarose gels stained with
ethidium bromide. Relative mRNA expression levels were normalized
to β-actin mRNA using Quantity One Software, version 4.2 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The experiments were
repeated in triplicate.
 | Table IPrimer sequences used for
semi-quantitative reverse transcription-polymerase chain
reaction. |
Table I
Primer sequences used for
semi-quantitative reverse transcription-polymerase chain
reaction.
| Gene | Primer sequences
(5′→3′) |
|---|
| β-actin | F:
AGAAAATCTGGCACCACACC |
| R:
CTCCTTAATGTCACGCACGA |
| CXCL5 | F:
GCTACCACTTCCACCTTG |
| R:
CCACTATGAGCCTVVTGT |
| CXCR2 | F:
CAGGAATGTGGCCAAAAAT |
| R:
GGAAACTCCCTCGTGATG |
| BAX | F:
TTGTCGCCCTTTTCTACTTTGCC |
| R:
TCTGAAGATGGGGAGAGGGCAC |
| NDRG3 | F:
GGCGAATTGTCCCCTACCACCAGT |
| R:
CTGCCTCCTGTTCTTACCCACCTA |
| ERK | F:
CTTCTCGCCTCAGTTCGC |
| R:
CTCCTGGATGCTTGTCTGGTAA |
 | Table IIThermocycling conditions of
polymerase chain reaction amplification for each gene. |
Table II
Thermocycling conditions of
polymerase chain reaction amplification for each gene.
| Gene | Denaturation | Annealing | Extension | Cycles |
|---|
| β-actin | 94°C (1 min) | 52.9°C (30
sec) | 72°C (1 min) | 25 |
| CXCL5 | 94°C (1 min) | 64.0°C (30
sec) | 72°C (1 min) | 38 |
| CXCR2 | 94°C (1 min) | 54.0°C (30
sec) | 72°C (1 min) | 35 |
| BAX | 94°C (1 min) | 55.0°C (30
sec) | 72°C (1 min) | 32 |
| NDRG3 | 94°C (1 min) | 61.7°C (30
sec) | 72°C (1 min) | 32 |
| ERK | 94°C (1 min) | 55.6°C (30
sec) | 72°C (1 min) | 30 |
ELISA
Cells (1.5×105 cells/well) were seeded
into 6-well plates with 1 ml cell culture medium containing 0.5%
FBS and incubated at 37°C for 48 h. Cell culture media were
collected and the expression levels of CXCL5 protein were measured
using a Human CXCL5/LIX/ENA-78 PicoKine ELISA kit (cat. no. EK0728;
Wuhan Boster Biological Technology, Ltd., Wuhan, China). Briefly,
96-well ELISA plates were coated with 100 μl of human
recombinant CXCL5 protein from the kit at concentrations of 4,000,
2,000, 1,000, 500, 250, 125, 62.5, 31.2, and 0 pg/ml to create a
standard curve. A total of 100 μl of the media from the
different cell cultures was added to each well and the plates were
incubated at 37°C for 90 min. Subsequently, the plates were washed
with phosphate-buffered saline (PBS) and incubated with 100
μl anti-human CXCL5 antibody working liquid (provided in the
kit) for 1 h at room temperature. The plates were then washed with
PBS again and incubated with ABC working solution at 37°C for 30
min. Following the addition of 100 μl
3,3′,5,5′-tetramethylbenzidine color solution to each well, optical
density was immediately detected at 450 nm using a
spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA) and
the levels of CXCL5 in the media were quantified according to the
standard curve. The experiments were repeated at least three
times.
Protein extraction and western
blotting
Total protein was extracted from cells
(3×106) or 20 mg of xenografted tissues using Pierce
Lysis Buffer (Thermo Fisher Scientific, Inc.) on ice for 30 min and
centrifuged at 14,000 × g for 15 min at 4°C. Protein concentrations
in the supernatant were determined using the Bicinchoninic Acid
Protein Assay kit (Wuhan Boster Biological Technology, Ltd.). A
total of 30 μg of each sample was separated by 12% SDS-PAGE
and transferred onto poly-vinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked in 5%
skimmed milk in PBS for 2 h at room temperature and incubated
overnight at 4°C with the following primary antibodies: Rabbit
polyclonal anti-BAX (1:1,000; cat. no. TA890001; OriGene
Technologies, Inc., Beijing, China), goat polyclonal anti-NDRG3
(1:200; cat. no. sc-19470; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), rabbit polyclonal anti-extracellular signal-regulated
kinase 1/2 (ERK1/2; 1:1,000; cat. no. AF0155; Affinity Biosciences,
Inc., Cincinnati, OH, USA), rabbit polyclonal anti-CXCR2 (1:500;
cat. no. sc-30008; Santa Cruz Biotechnology, Inc.), rabbit
polyclonal anti-IL-18 (1:500; cat. no. sc-7954; Santa Cruz
Biotechnology, Inc.), rabbit polyclonal anti-Bcl-2 (1:500; cat. no.
sc-492; Santa Cruz Biotechnology, Inc.), rabbit polyclonal
anti-caspase-3 (1:500; cat. no. sc-7148; Santa Cruz Biotechnology,
Inc.), and mouse monoclonal anti-β-actin (1:1,000; cat. no.
TA811000; OriGene Technologies, Inc.). The membranes were
subsequently washed with PBS containing 0.05% Tween-20 and
incubated with the horseradish peroxidase (HRP)-conjugated goat
anti-rabbit, rabbit anti-goat or goat anti-mouse secondary
antibodies (1:10,000; cat. nos. BA1054, BA1060, and BA1050,
respectively; Wuhan Boster Biological Technology, Ltd.) at 37°C for
30 min. Immunoreactive protein bands were detected using the Pierce
Enhanced Chemiluminescence solution (Thermo Fisher Scientific,
Inc.) and the intensity of each target band was normalized to
β-actin and quantified using ImageJ software (version 1.45s;
National Institutes of Health, Bethesda, MD, USA). Each experiment
was repeated at least three times.
Tissue samples and immunohistochemical
analysis
Prostate tissue samples were collected from patients
(age, 56–72 years) with prostate cancer (n=13), with benign
prostate hyperplasia (BPH; n=3), with cystic dilatation prostate
(n=3), and with normal prostate (n=3). Of the 13 patients with
prostate cancer, 1 had Gleason score 4 tumor, 2 had Gleason score
5, 1 had Gleason score 6, 2 had Gleason score 7, 3 had Gleason
score 8, 3 had Gleason score 9, 1 had Gleason score 10, and of the
13 cases, 7 had metastatic tumors. All patients had undergone
surgical treatment at The Affiliated Hospital of Jiamusi University
(Jiamusi, China) between June 2013 and June 2015. This study was
approved by the Ethics Committee of Jiamusi University (approval
no. JMSU-195) and written informed consent was provided by all
patients prior to enrolment.
A tissue microarray (TMA) comprising 50 prostate
cancer, 20 BPH, and 10 normal prostate tissue samples was purchased
from Alenabio (Xi'an, China). Among the 50 cancer tissues, patients
were aged between 20 and 87 years and had Gleason scores between 4
and 10 (4 with Gleason score 4; 7 with Gleason score 5; 9 with
Gleason score 6; 8 with Gleason score 7; 2 with Gleason score 8; 9
with Gleason score 9; 11 with Gleason score 10) and of the 50
cases, 19 with metastatic tumors).
Tissue samples were fixed in 10% buffered formalin
at room temperature for 24 h, embedded in paraffin and sectioned
into 5 μm-thick sections. These and the TMA (5 μm)
sections were deparaffinized in xylene at room temperature and
rehydrated in a descending ethanol series. The sections were
subjected to antigen retrieval in a high-pressure cooker with 0.01
M citric acid buffer for 3 min, and endogenous peroxidase activity
was quenched by incubating the sections in 3%
H2O2 in PBS for 30 min at room temperature.
The sections were blocked with 20% normal goat serum (cat. no.
AR0009; Wuhan Boster Biological Technology, Ltd.) at 37°C for 1 h,
followed by incubation with a mouse monoclonal anti-CXCL5 antibody
(1:50; cat. no. sc-73931; Santa Cruz Biotechnology, Inc.) at 4°C
overnight. Following this incubation, the sections were washed with
PBS and incubated with a HRP-conjugated secondary goat anti-mouse
immunoglobulin G (1:200; cat. no. BA1050; Wuhan Boster Biological
Technology, Ltd.) for 30 min at the room temperature. Subsequently,
the sections were washed with PBS and incubated with the
Avidin-Biotin Enzyme Complex (Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA) for 30 min at room temperature, followed by
incubation with 3,3′-diaminobenzidine solution at the room
temperature for 5 min for colorimetric determination of protein
expression, and then counterstained with hematoxylin at room
temperature for 15 sec. For quantification, Image-Pro Plus software
(version 6.0; Media Cybernetics, Inc., Rockville, MD, USA) was used
to determine the average optical density and relative area of total
CXCL5 expression in each tissue section within three random fields
at ×400 magnification under an Olympus BX-60 Epifluorescence
microscope (Olympus Corporation, Tokyo, Japan).
Cell treatments and proliferation
assay
To determine the effects of exogenous, autocrine and
paracrine CXCL5 expression on the proliferation of prostate cancer
cells, the following three experimental treatments were conducted:
i) Exogenous assay, in which PC-3 and DU145 cells were seeded
(2×103 cells/well) into 96-well plates and incubated in
CM overnight at 37°C in a 5% CO2 incubator; following
removal of the culture media, 100 μl of CM containing
different concentrations of recombinant CXCL5 (0, 10, 20, 40 or 60
ng/ml; PeproTech, Inc.) was added to each well; ii) autocrine
assay, in which the untransfected parental, mock-transfected, and
CXCL5-OE-transfected PC-3 and DU145 cells were seeded
(2×103 cells/well) into 96-well plates and incubated in
100 μl of CM; and iii) paracrine assay, in which parental,
mock-transfected and CXCL5-OE-transfected WPMY-1 cells were seeded
(5×105 cells/well) into 100 mm cell culture dishes and
incubated in CM at 37°C for 48 h. The WPMY-1 CM was collected and
subsequently added to PC-3 and DU145 cell cultures (containing
2×103 cells/well in 96-well plates) at different
dilutions (20, 50, 80 or 100%). The three groups of cells were
incubated at 37°C in a 5% CO2 incubator for 72 h without
any subsequent medium change. Cell proliferation was then
determined by using the Cell Counting Kit-8 (CCK-8; Boster
Biotechnology), following the manufacturer's protocol. Briefly, 10
μl CCK-8 reagent was added into each well and cells were
incubated at 37°C for 1 h. The optical density was measured at 450
nm using a spectrophotometer (BioTek Instruments, Inc.). Each assay
was repeated at least thrice.
Colony formation assay
PC-3 and DU145 cells were seeded (100 cells/well)
into 24-well plates and incubated with CM containing 60 ng/ml (for
PC-3 cells) or 20 ng/ml (for DU145 cells) CXCL5 for 12 days
(according to pre-test experiments; data not shown) at 37°C with 5%
CO2 until the colonies were visible. The aforementioned
CXCL5-containing medium was replaced every 3 days. The colonies
were stained with 0.01% crystal violet solution at room temperature
for 15 min and counted under an Olympus BX-60 Epifluorescence
microscope BX-60 (Olympus Corporation).
Transwell migration assay
The Transwell migration assay was performed using
24-well chambers with polycarbonate membrane filters (pore size, 8
μm; Corning, Inc., Corning, NY, USA), which was modified
from our previously described protocol (16). The effects of exogenous, autocrine,
and paracrine CXCL5 exposure on the migratory capcity of prostate
cancer cells was determined as follows: i) Exogenous assay, in
which PC-3 cells (2×104 cells/well) or DU145 cells
(5×103 cells/well) were seeded into the upper Transwell
chamber with serum-free RPMI-1640 medium; 600 μl RPMI-1640
medium with 10% FBS and various concentrations of CXCL5 (0, 10, 20,
40, or 60 ng/ml) was added into the lower chamber; ii) Autocrine
assay, in which parental, mock-transfected, and
CXCL5-OE-transfected PC-3 cells (2×104 cells/well) or
DU145 cells (5×103 cells/well) were seeded into the
upper chamber with serum-free RPMI-1640 medium; 600 μl
RPMI-1640 medium with 10% FBS was added into the lower chamber; and
iii) Paracrine assay, in which PC-3 cells (2×104
cells/well) or DU145 cells (5×103 cells/well) were
seeded into the upper Transwell chamber containing serum-free
RPMI-1640 medium; 600 μl of the conditional medium prepared
by dissolving different ratios of culture media from parental,
mock-transfected, and CXCL5-OE-transfected WPMY-1 cells (20, 50, 80
or 100%) in DMEM containing 10% FBS was added into the lower
chamber. In addition, a co-culture system was used in the paracrine
assay, in which PC-3 cells (2×104 cells/well) or DU145
cells (5×103 cells/well) were seeded into the upper
chamber containing RPMI-1640 medium, with parental,
mock-transfected, or CXCL5-OE-transfected WPMY-1 cells
(3×104 cells/well) were cultured in the lower chamber
containing 600 μl of DMEM containing 10% FBS. For all
experimental groups, cells were incubated at 37°C in a 5%
CO2 incubator for 48 h; subsequently, the upper surfaces
of the membranes were scraped with a cotton swab to remove the
non-migrating cells, and the filters were washed with PBS, fixed
and stained with 95% ethanol containing 0.5% crystal violet
solution at room temperature for 30 min. The filters were then
washed with PBS and the migrating cells were counted in five random
fields using a bright field microscope at ×50 magnification.
Nude mouse tumor cell xenograft
assay
Male nude mice (nu/nu; age, 5 weeks; weight 13–16 g;
n=7) were purchased from Charles River Laboratories International,
Inc. (Beijing, China). The mice were housed in cages under
laminar-flow HEPA-filtered hoods at 25°C with a 12-h light/dark
cycle and were provide with sterile rodent chow and sterile water
ad libitum. Mock and CXCL5-overexpressing PC-3 cells were
cultured and harvested at the exponential growth phase, washed with
PBS, and resuspended in serum-free RPMI-1640 medium. The trypan
blue exclusion assay was used to ensure that cell viability was
>99% prior to injection. PC-3 cells (1×106 cells in
100 μl culture medium) were subcutaneously injected into
male athymic mice; each mouse was injected with
CXCL5-overexpressing cells into one side of the flank, and the mock
control cells were injected into the other side for simultaneous
comparison. The animals were sacrificed with CO2 at week
8 post-injetion and the tumor cell xenografts were harvested for
analysis and comparison. The experiments were repeated three
times.
Statistical analysis
All data are presented as the mean ± standard
deviation. Comparisons between two groups were analyzed using
Student's t-test, and comparison among multiple groups was analyzed
by analysis of variance followed by Student-Newman-Keuls post hoc
test or by Kruskal-Wallis test with a Nemenyi test pairwise
comparison post hoc test. All statistical analyses were performed
using SPSS software version 19.0 (IBM Corp., Armonk, NY, USA).
P<0.05 was considered statistically significant.
Results
Upregulation of CXCL5 in vitro and ex
vivo
The expression levels of CXCL5 mRNA were examined by
RT-PCR in the prostate cancer cell lines PC-3, DU145 and LNCaP, in
the normal prostate stroma cell line WPMY-1, and in the normal
prostate epithelium cell line RWPE-1. The results demonstrated that
CXCL5 mRNA expression was high in PC-3, DU145, WPMY-1, and RWPE-1
cells and expression was low in LNCaP cells (Fig. 1A). ELISA was used to determine
whether CXCL5 protein was produced and secreted by these cell
lines, and the results revealed that each of these cell lines
secreted CXCL5 protein (Fig. 1B),
which suggested that CXCL5 may function through the autocrine and
paracrine systems in prostate cells. In addition, a significantly
increased level of CXCL5 secretion was observed in
CXCL5-overexpressing PC-3, DU145, and WPMY-1 cells compared with
their respective parental and mock controls (Fig. 1B), which may lay the foundation for
subsequent autocrine and paracrine assays. Our previous study
demonstrated a high expression level of CXCR2 protein in DU145,
LNCaP, and RWPE-1 cells and low CXCR2 expression in PC-3 and WPMY-1
cells (16), which suggested that
CXCL5 and CXCR2 expression may lack specificity in prostate
cells.
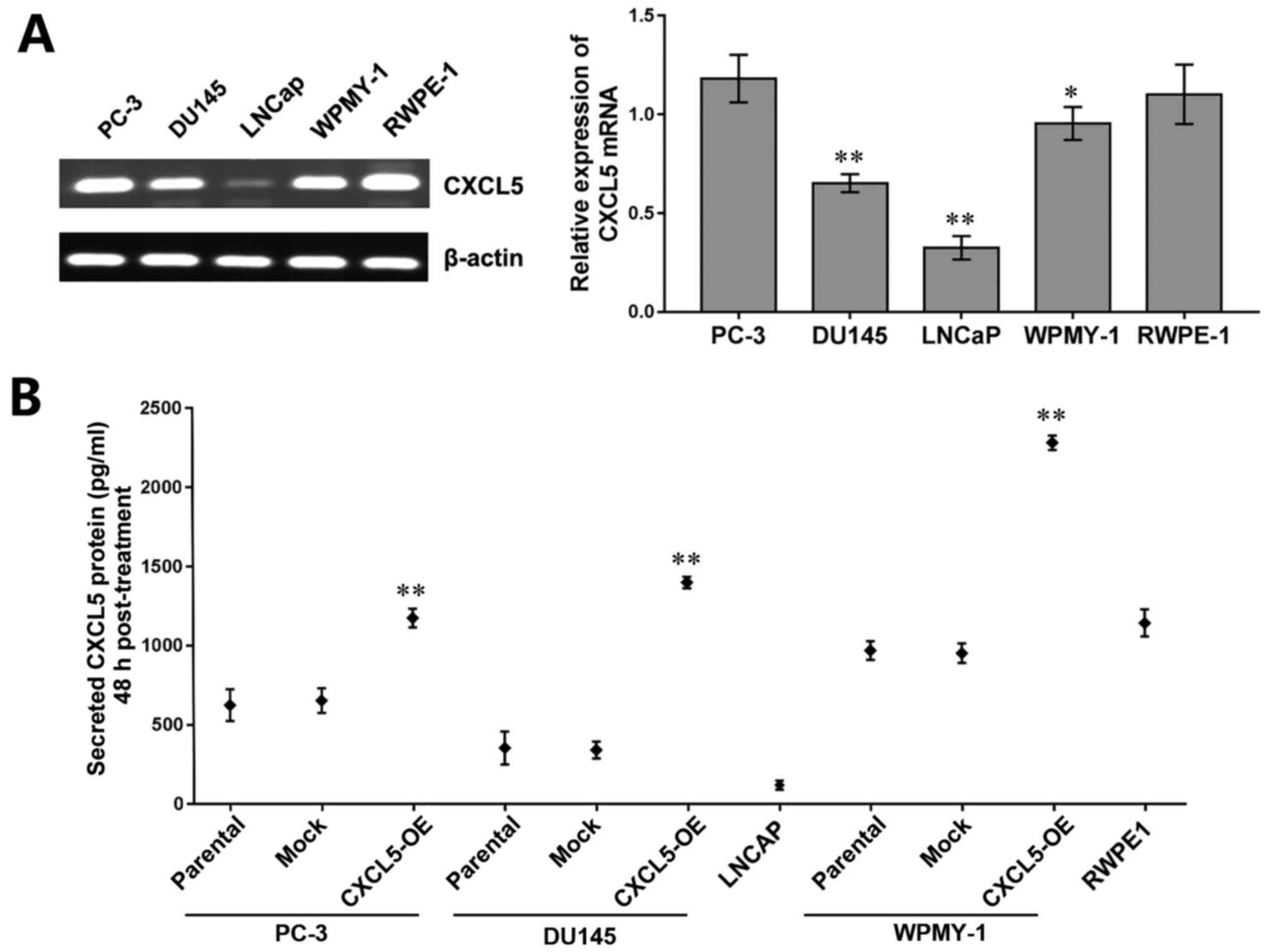 | Figure 1CXCL5 expression in prostate cancer
cells. (A) Reverse transcription-polymerase chain reaction results
and semi-quantitative analysis of CXCL5 mRNA expression levels in
PC-3, DU145, LNCaP, WPMY-1, and RWPE-1 cell lines; β-actin was used
to normalize the data; *P<0.05 and
**P<0.01 vs. RWPE-1 cells. (B) ELISA analysis of the
concentrations of secreted CXCL5 proteins 48 h post-treatment in
untransfected parental, mock-transfected, and CXCL5-OE-transfected
PC-3, DU145 and WPMY-1 cells, as well as LNCaP and RWPE-1 cells.
**P<0.01 vs. respective parental or mock cells.
CXCL5, C-X-C motif chemokine 5; CXCL5-OE,
lentiviral-CXCL5-overexpression vector-transfected cells; mock,
lentiviral empty vector-transfected cells. |
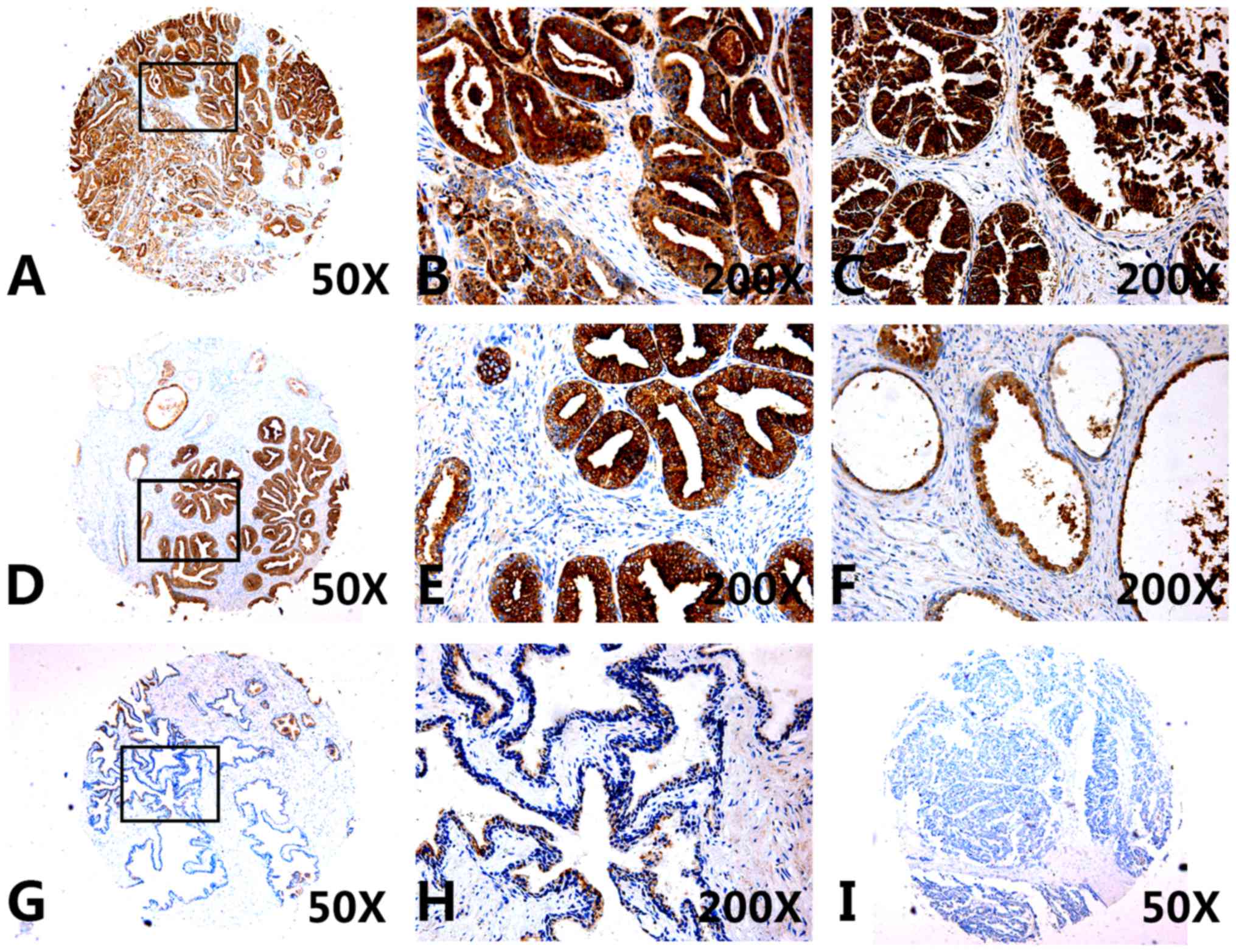
Furthermore, CXCL5 protein expression levels were
also examined in tissue specimens from patients with prostate
cancer, BPH or cystic dilatation prostate, as well as in normal
prostate tissue. CXCL5 expression was significantly higher in
prostate cancer tissues (Fig.
2A–E; Table III), compared
with compared with expression in the non-cancerous tissue
specimens, including cystic dilatation prostate tissues (Fig. 2F), normal prostate tissues
(Fig. 2G–H), and BPH tissues
(Fig. 2I). The average optical
density and relative area data indicated that the levels of CXCL5
protein expression in both non-metastatic cancer tissues (non-METs)
and METs were significantly higher compared with non-prostate
cancer tissues (non-PCa), which indicated that the increased
expression of CXCL5 mayb be associated with prostate cancer
development and progression (Table
III). However, CXCL5 expression was not associated with Gleason
scores or age of the patients (data not shown).
 | Table IIILevels of CXCL5 protein expression in
human prostate tissues as determined by immunohistochemistry. |
Table III
Levels of CXCL5 protein expression in
human prostate tissues as determined by immunohistochemistry.
| Tissue | n | CXCL5 expression
|
|---|
| Average optical
density | Relative area |
|---|
| Non-PCaa | 39 | 0.303±0.018 |
8,4487.05±7,1405.03 |
| Non-METs | 37 | 0.329±0.049b |
133,185.24±74,874.95b |
| METs | 26 | 0.322±0.026b |
159,082.46±67,890.32b |
Effects of exogenous CXCL5 exposure on
tumor cell proliferation, colony formation, and migration
Owing to the tendency of LNCaP cells to form
colonies in culture instead of a cell monolayer, these cells were
not used in subsequent experiments. Tumor cell proliferation,
colony formation and migratory ability were assessed in PC-3 and
DU145 cell lines following treatment with various concentrations of
exogenous recombinant CXCL5 protein (0, 10, 20, 40 and 60 ng/ml). A
significant increase in proliferation was observed following
treatment with 60 ng/ml (PC-3 cells) or with 10 and 20 ng/ml (DU145
cells) of exogenous CXCL5 (Fig.
3A), although DU145 cells exposed to higher concentrations of
exogenous CXCL5 (for example, 60 ng/ml) had a significant decrease
in proliferation compared with untreated control cells. As the 60
and 10–20 ng/ml doses of exogenous CXCL5 were indicated as the
optimal concentrations for regulation of PC-3 and DU145 cell
proliferation, respectively, 60 ng/ml was used for PC-3 and 20
ng/ml for DU145 cells in the subsequent colony formation assays;
the treated PC-3 and DU145 cells exhibited a notable increase in
colony formation (Fig. 3B and
Table IV).
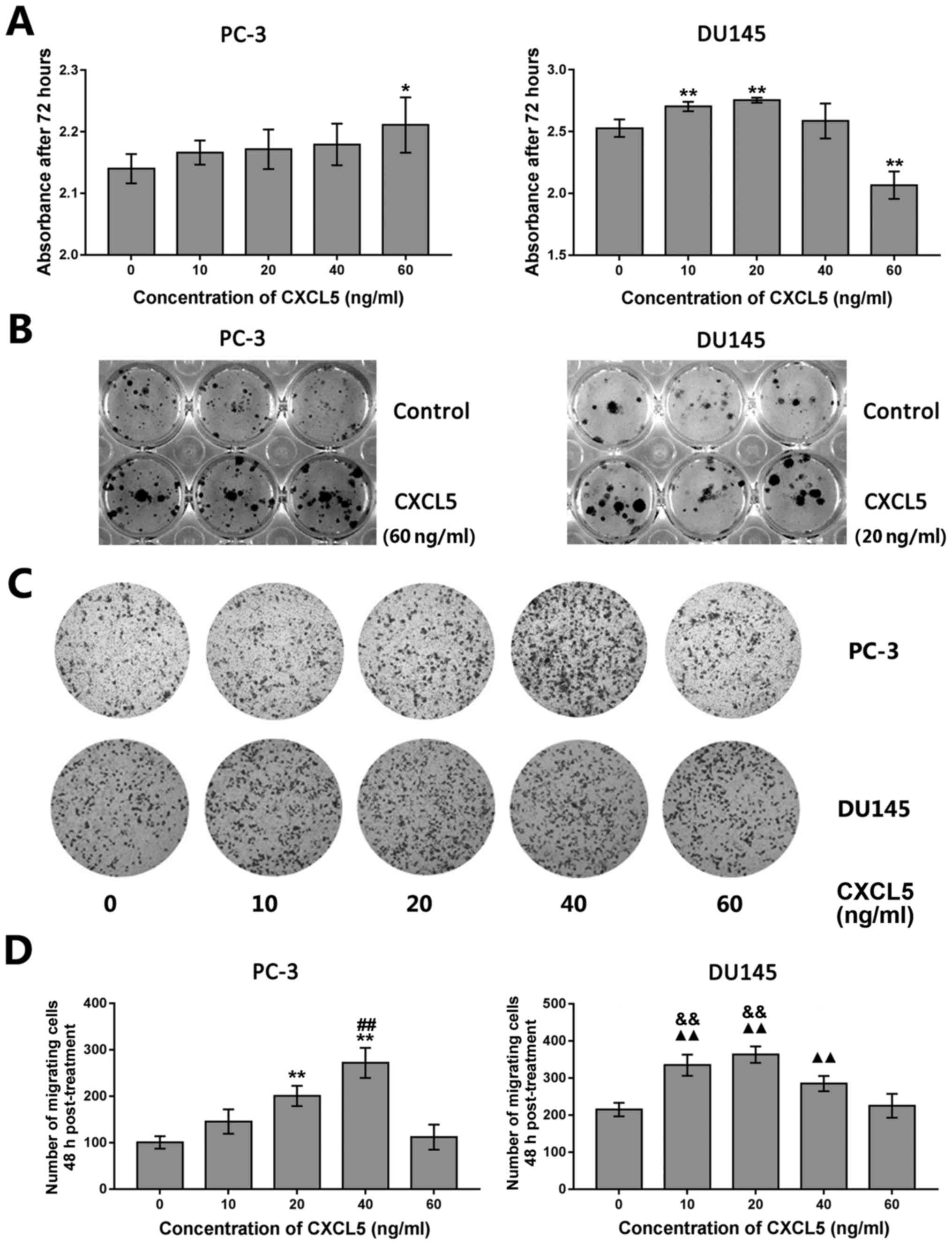 | Figure 3Effects of exogenous CXCL5 protein
exposure on prostate cancer cell proliferation, colony formation,
and migration in vitro. (A) PC-3 and DU145 cells were
treated with various concentrations of recombinant CXCL5 (0, 10,
20, 40 and 60 ng/ml) for 72 h and proliferation was assessed using
the CCK-8 assay. *P<0.05 and **P<0.01
vs. untreated (0 ng/ml) control cells. (B) PC-3 and DU145 cells
were treated with 60 ng/ml or 20 ng/ml recombinant CXCL5,
respectively, for 12 days and colony formation was examined. (C)
Transwell migration assay for PC-3 and DU145 cells treated with
various concentrations of CXCL5 (0, 10, 20, 40, and 60 ng/ml) for
48 h. (D) Results from statistical analysis of Transwell tumor cell
migration assay from (C). **P<0.01 vs. 0, 10 or 60
ng/ml; ##P<0.01 vs. 20 ng/ml; ▲▲P<0.01
vs. 0 or 60 ng/ml; &&P<0.01 vs. 40 ng/ml.
CCK-8, Cell Counting Kit-8; CXCL5, C-X-C motif chemokine 5. |
 | Table IVEffects of exogenous CXCL5 expression
on colony formation of prostate cancer cells. |
Table IV
Effects of exogenous CXCL5 expression
on colony formation of prostate cancer cells.
| Dose of CXCL5
(ng/ml) | PC-3 | DU145 |
|---|
| 0 | 35.50±6.80 | 13.63±6.00 |
| 20 | ND | 32.63±5.42a |
| 60 | 66.38±9.41a | ND |
Transwell migration assays were conducted on PC-3
and DU145 cells following treatment with various concentrations of
exogenous CXCL5 (0, 10, 20, 40 and 60 ng/ml). The results
demonstrated that CXCL5 at a dose of 20 or 40 ng/ml for PC-3 cells
or 10, 20 and 40 ng/ml for DU145 cells significantly promoted tumor
cell migration capacity compared with respective cells treated with
0 ng/ml of CXCL5 (Fig. 3C and
D).
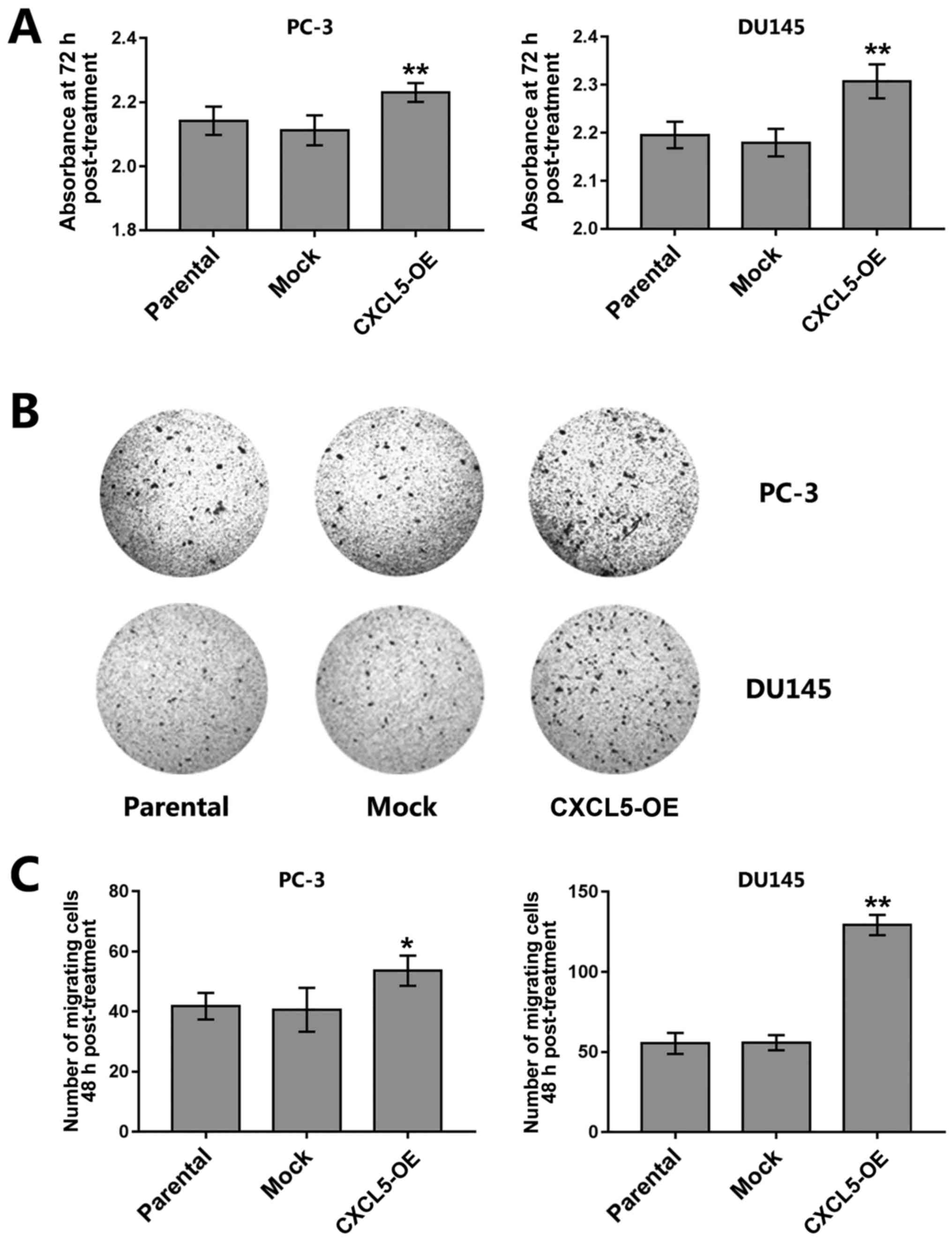
Autocrine/paracrine effects of CXCL5
overexpression on regulation of prostate cancer cell malignant
phenotypes
Results from CCK-8 assays demonstrated that the
proliferation of CXCL5-overexpressing PC-3 and DU145 cells was
increased compared with that of parental or mock control cells
(Fig. 4A). CXCL5 overexpression
also significantly increased the migratory capacity of PC-3 and
DU145 cells compared with that of parental and mock cells (Fig. 4B and C).
Furthermore, a stable CXCL5-overexpressing WPMY-1
prostate stromal cell line was established and various
concentrations (20, 50, 80 and 100%) of the conditional medium from
parental, mock or CXCL5-overexpressing WPMY-1 cells was
subsequently prepared and added to the PC-3 and DU145 cell
cultures. PC-3 cells cultured with 100% and DU145 cells cultured
with either 80 or 100% CXCL5-overexpressing WPMY-1 conditioned
medium exhibited a significant increase in tumor cell proliferation
compared with the proliferation of cells cultured with similar
concentrations of conditional medium from parental or mock WPMY-1
cells (Fig. 5A); no significant
differences in tumor cell proliferation were identified for cells
culutred with 20, 50 or 80% of the WPMY-1 CM. Similar phenomena
were also observed in prostate cancer cell migration assays; for
example, PC-3 and DU145 cells cultured with 100%
CXCL5-overexpressing WPMY-1 conditional medium exhibited a
significant increase in migration capacity compared with cells
cultured with similar concentrations of conditioned medium from
parental or mock WPMY-1 cells (Fig. 5B
and C). In addition, PC-3 and DU145 cells that were co-cultured
with CXCL5-overexpressing WPMY-1 cells exhibited an increase in
number of migrating cells compared with the number of migrating
tumor cells co-cultured with parental or mock WPMY-1 cells
(Fig. 5D and E).
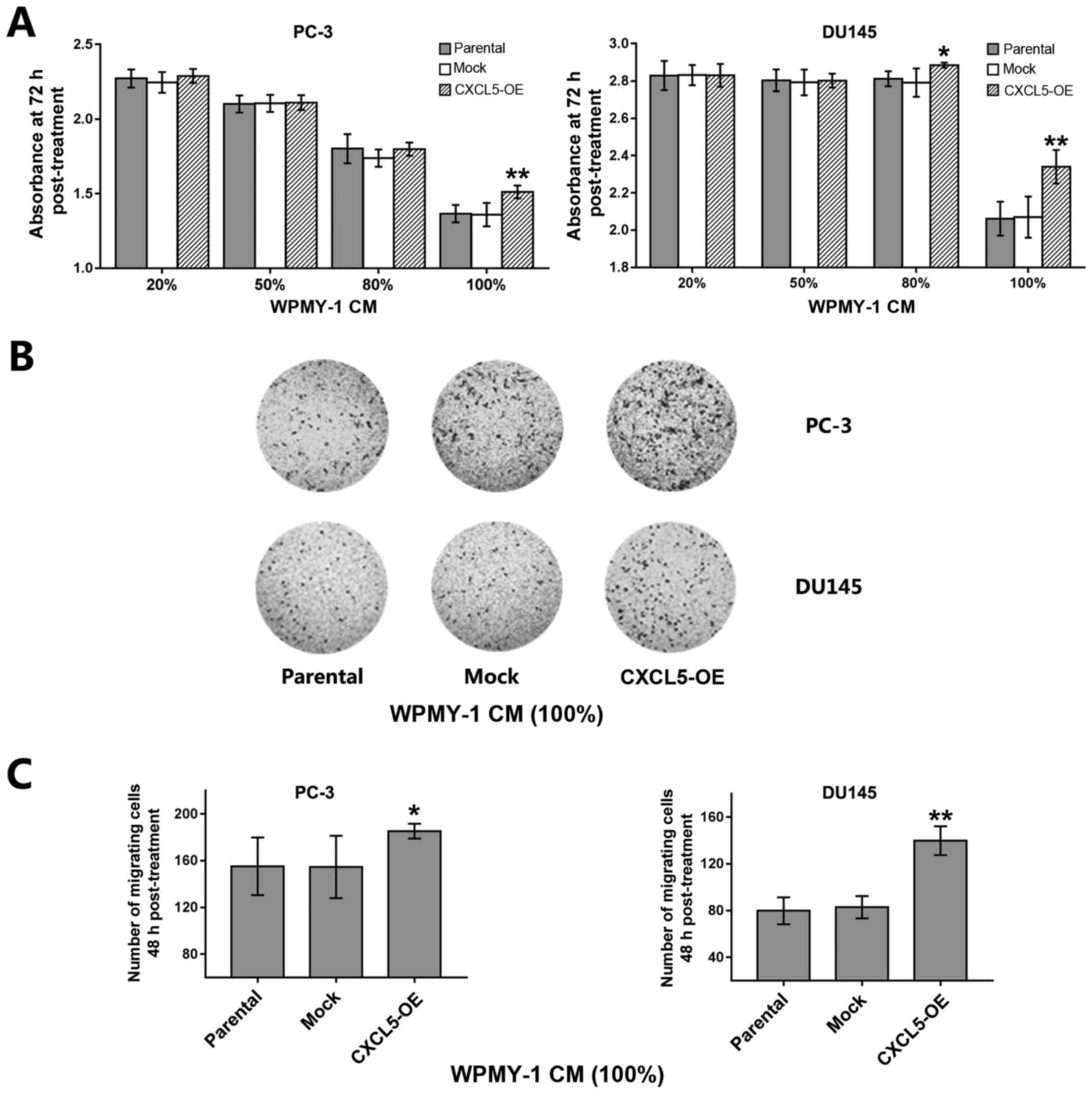 | Figure 5Effects of CXCL5-overexpressing WPMY
cells on prostate cancer cell malignant potential are paracrine in
fashion. (A) PC-3 and DU145 cells were cultured for 72 h in CM (20,
50, 80 or 100%) obtained from untransfected parental,
mock-transfected, and CXCL5-OE-transfected WPMY cells and
proliferation was analyzed by CCK-8 assay. (B) Representative
images of Transwell migration assay for PC-3 and DU145 cells
incubated with 100% parental, mock or CXCL5-OE WPMY-1 CM. (C)
Analysis of migratory ability in PC-3 and DU145 cells from (B). (D)
Representative images of Transwell migration assay for PC-3 and
DU145 cells that were co-cultured with parental, mock, or CXCL5-OE
WPMY cells for 48 h. (E) Analysis of cell migratory ability of PC-3
and DU145 cells from (D). *P<0.05 and
**P<0.01 vs. parental or mock. CCK-8, Cell Counting
Kit-8; CM, conditioned medium; CXCL5, C-X-C motif chemokine 5;
CXCL5-OE, lentiviral-CXCL5-overexpression vector-transfected cells;
mock, lentiviral empty vector-transfected cells. |
Effects of CXCL5 overexpression on BAX,
NDRG3, ERK, and CXCR2 expression in prostate cancer cells
To explore the underlying gene expression of the
CXCL5-mediated prostate cancer cell malignat pheotypes, the mRNA
and protein expression levels of known tumor-related genes were
examined. The results demonstrated that the expression levels of
NDRG3, ERK1/2, and CXCR2 mRNA and protein were significantly
increased, whereas the expression levels of BAX mRNA and protein
were significantly reduced in CXCL5-overexpressing PC-3 cells
compared with those of parental and mock control cells (Fig. 6A–D).
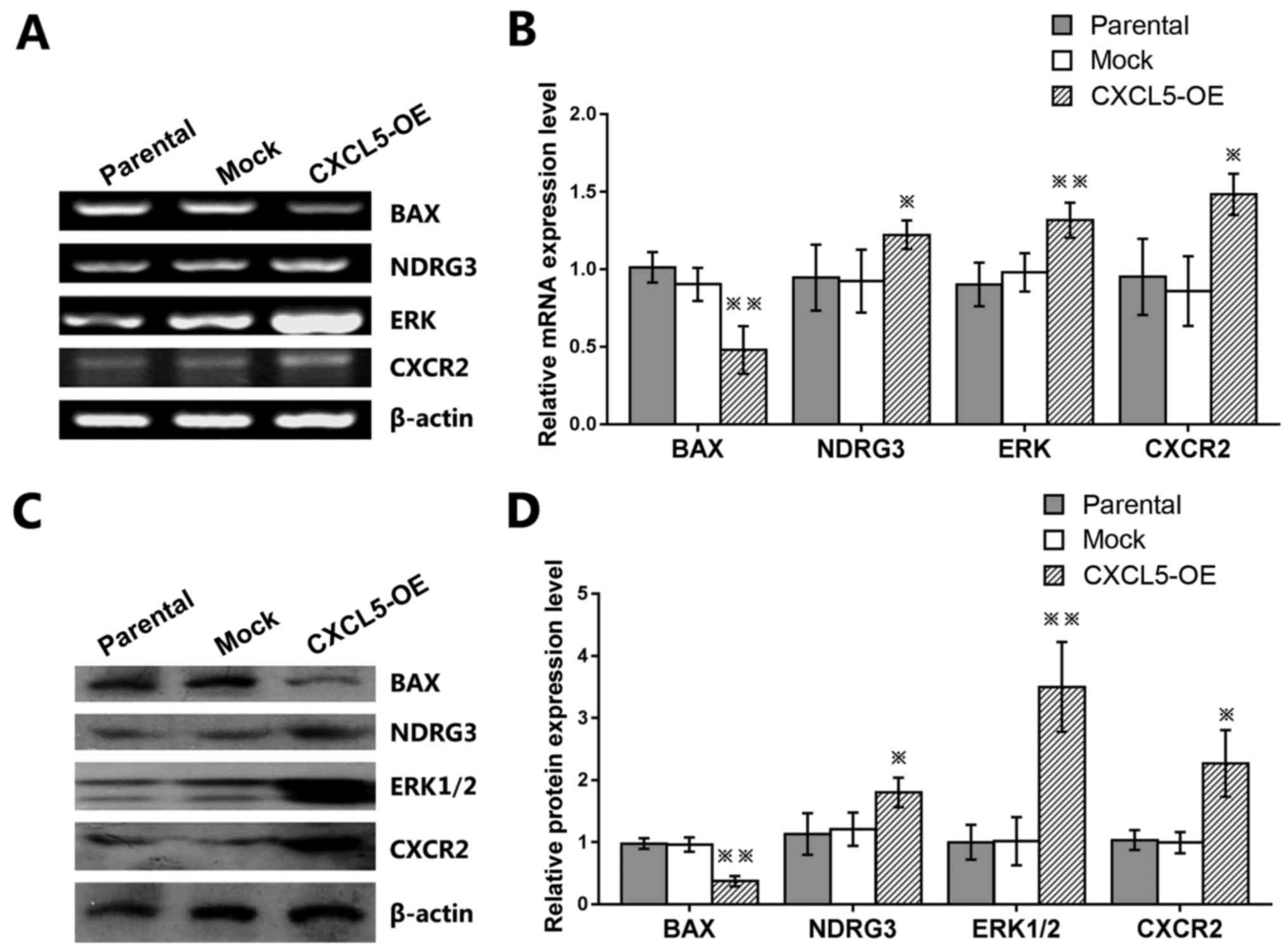 | Figure 6Effects of CXCL5 gene expression in
PC-3 prostate cancer cells. Untransfected parental,
mock-transfected, or CXCL5-OE-transfected PC-3 cells were used to
determine the effects of CXCL5 on the (A and B) mRNA and (C and D)
protein expression levels of BAX, NDRG3, ERK, and CXCR2.
*P<0.05 and **P<0.01 vs. parental or
mock. CXCL5, C-X-C motif chemokine 5; ERK, extracellular
signal-regulated kinase; NDRG3, N-Myc downstream-regulated gene 3.
CXCR2, C-X-C chemokine receptor type 2; CXCL5-OE,
lentiviral-CXCL5-overexpression vector-transfected cells; mock,
lentiviral empty vector-transfected cells. |
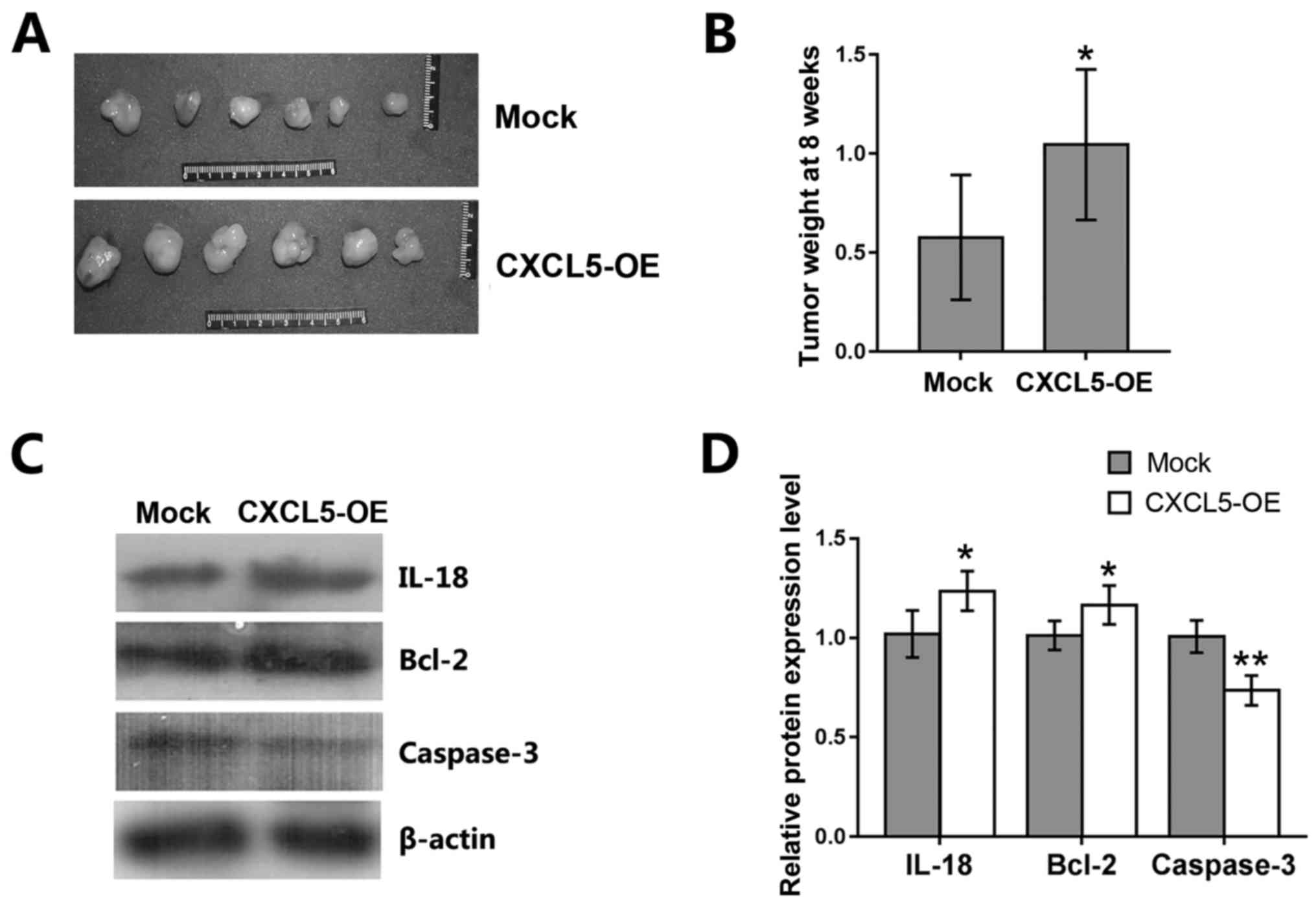
Effects of CXCL5 overexpression on
prostate cancer cells in vivo
To assess the in vivo effects of CXCL5
overexpression on prostate cancer cells, a nude mouse xenograft
assay was performed by subcutaneous injection of
CXCL5-overexpressing and mock-treated PC-3 cells into nude mice.
Eight weeks following tumor cell injection, tumor xenografts were
resected from the mice; weight of tumor cell xenografts was
significantly higher in mice implanted with CXCL5-overexpressing
PC-3 cells compared with those implanted with mock PC-3 cells
(Fig. 7A and B). Western blot
analysis of the tumor xenografts revealed that the levels of IL-18
and Bcl-2 proteins were increased, whereas caspase-3 levels were
decreased in CXCL5-overexpressing PC-3 xenografts compared with
mock PC-3 xenografts (Fig. 7C and
D).
Discussion
Prostate cancer is the most frequently diagnosed
malignancy in men in the United States, accounting for >35% of
all male cancers diagnosed (17).
Thus, research on the molecular mechanisms of prostate cancer
development and progression may identify novel strategies to
effectively control prostate cancer. To this end, chemokines, their
receptors, and cancer-related inflammation serve important roles in
cancer developemnt, including prostate cancer (18–20),
and cancer initiation (21). Human
cancer development may be initialized through crosstalk between
tumor cells and the adjacent microenvironment, where a number of
chemokines are continuously secreted by other cancer cells and/or
stromal cells (22–24). Many types of cancer cells are able
to manipulate the microenvironment to optimize growth and
metastatic advantages, whereas auto-crine/paracrine signaling leads
to communication of tumor cells with the neighboring non-tumor
cells (25–27). Thus, chemokines and their receptors
appear to serve vital roles in formation of peri- and intratumoral
infiltrations (23,28). Such intercellular crosstalk between
tumor and neighboring cells through chemokines in the extracellular
environment may serve crucial roles in the establishment of
mesenchymal status and tumor progression and metastasis (8,22,29).
In this regard, the present study assessed the
effects of CXCL5 on prostate cancer cells. CXCL5 mRNA was
demonstrated to be highly expressed in prostate cancer cells and
tissues, in normal prostate epithelial cells, and in prostate
stromal cells, and exogenous delivery of CXCL5 protein resulted in
increased proliferation and migration of prostate cancer cells. A
previous study revealed that proliferation of prostate cancer LNCaP
and 22Rv1 cells was significantly induced in response to very low
levels of exogenous CXCL5 protein, whereas higher levels of
exogenous CXCL5 reduced tumor cell growth (4). Although different prostate cancer
cell lines were used, data from the present study demonstrated a
similar phenomenon in which low doses of CXCL5 induced tumor cell
proliferation, whereas higher doses of CXCL5 inhibited tumor cell
proliferation. Taken together, these data suggested that a high
level of ligands may reduce the affinity of the receptors to their
ligands. Results from the present study demonstrated that 10–20
ng/ml doses of exogenous CXCL5 were the most optimal concentrations
for regulation of DU145 cell proliferation and migration; however,
the data also indicated that treatment of PC3 cells with a higher
concentration of exogenous CXCL5 resulted in an increase in tumor
cell proliferation, whereas lower concentrations contributed to
tumor cell migration. Indeed, cell proliferation and migration are
two phenotypic traits of all tumor cells, and although they may be
related, their mechanisms usually differ. Therefore, the present
study hypothesized that different concentrations of exogenous CXCL5
may promote PC3 cell proliferation and migration through different
signaling pathways, but further investigations are needed to
confirm this.
RT-PCR and ELISA data from the present study
demonstrated that CXCL5 is expressed in prostate cancer cells,
normal prostate epithelium, and stromal cells, and it is
acknowledged that various types of cells in the microenvironment
continuously secrete abundant chemokines to promote cancer
development through their ability to serve as autocrine or
paracrine signals with crosstalk between tumor cells and
neighboring cells (22–24); therefore, whether CXCL5 was able to
modify prostate cancer cell malignant behaviors was examined by
focusing on its role in autocrine/paracrine mechanisms. The data
indicated that CXCL5 overexpression in PC-3 or DU145 cells, culture
of these cells in conditional medium from CXCL5-overexpressing WPMY
cells, or co-culture of these cells with CXCL5-overexpressing WPMY
cells all induced prostate cancer cell malignant phenotypes in an
autocrine/paracrine fashion in vitro. Moreover, nude mouse
xenograft assays demonstrated that CXCL5 overexpression promoted
prostate cancer cell xenograft formation and growth in nude mice,
which further confirmed our in vitro data supporting CXCL5
induction of prostate cancer cell proliferation. However, future
research is needed to assess whether knockdown of CXCL5 expression
can inhibit tumor formation and growth. Although a previous study
indicated that exogenous CXCL5 promotes malignant behavior of
prostate cancer cells (4), the
present study demonstrated the autocrine and paracrine effects of
CXCL5 on the malignant behavior of prostate cancer in
CXCL5-overexpressing prostate adenocarcinoma cells and stromal
cells, which may secrete CXCL5 protein in the tumor
microenvironment. The novelty of this study is in the examination
of the source of CXCL5, the CXCL5-producing cells, in the tumor
microenvironment.
In addition, CXCL5 overexpression was able to
regulate the expression of CXCL5-related signaling pathway genes in
prostate cancer cells, such as BAX, NDRG3, ERK1/2, CXCR2, Bcl-2,
IL-18, and caspase-3 proteins. The interferon γ-inducing factor
IL-18 not only enhances cytokine production by T and natural killer
cells, but also induces their proliferation and cytolytic activity
(30,31). Previous studies reported that
engineering tumor cells to produce IL-18 resulted in reduced
tumorigenicity and that systemic administration of recombinant
IL-18 led to notable tumor inhibition (32,33).
Another study reported that leptin-induced IL-18 expression was
regulated via nuclear factor (NF)-κB/NF-κB1 signaling in
tumor-associated macrophages, whereas it was regulated through
phosphoinositide 3-kinase (PI3K)/RAC-α serine/threonine-protein
kinase (AKT) signaling in breast cancer cells, which led to tumor
invasion and metastasis (34).
Furthermore, the ERK signaling cascade serves a crucial role in
development, progression, metastasis, and angiogenesis of various
types of human cancers (35–38).
In addition, BAX is a pro-apoptotic factor and caspase-3 is an
apoptosis executor, whereas Bcl-2 is an anti-apoptotic protein that
promotes cell survival (39–42).
These proteins in the BAX/Bcl-2/caspase-3 signaling pathway
interact with each other to regulate apoptosis in various normal
and tumor cells (39–42). The present study data on CXCL5
regulation of these gene expressions indicated that the expression
of these proteins may be important in mediating CXCL5 activity in
prostate cancer progression. However, further studies are needed to
determine the importance of each of these proteins in prostate
cancer. In addition, our previous study revealed that NDRG3
overexpression significantly increased CXCL5 expression in prostate
cancer cells (3), and the present
study data demonstrated that CXCL5 overexpression was able to
induce NDRG3 expression in PC-3 prostate cancer cells, which
suggested a potential positive-feedback loop of CXCL5 and NDRG3 to
promote prostate cancer progression. Future studies will
investigate the underlying gene regulations involved in the
regulation of CXCL5 in prostate cancer development and
progression.
Acknowledgments
The authors would like to thank the Research
Institute for Geriatric Tumor and Life Sciences Experimental
Centre, Jiamusi University (Jiamusi, China) for assistance with
some of the experiments.
Funding
This study was supported in part by grants from The
National Natural Science Foundation of China (grant no. 81272854),
The Natural Science Foundation of Heilongjiang Province (grant no.
D200966), The Science and Innovation Team Foundation of Department
of Education in Heilongjiang Province (grant no. cxtd-2016-03), and
The Postgraduate Science and Innovation Foundation of Jiamusi
University (grant no. YM2016_006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ and YL performed cell culture and cell
experiments. SG, JL, and XJ conducted animal experiments. YQ, ML,
and DJ collected tissue samples and drafted the manuscript. MS, HY
and WS performed reverse transcription-polymerase chain reaction,
ELISA and western blot analyses. JW and SW performed
immunohistochemistry. HS performed all statistical analyses. WW and
PZ conceived and designed the study. All authors have read and
approved the final version of this manuscript.
Ethics approval and consent to
participate
The Medical Ethics committee at Jiamusi University
(Jiamusi, China) approved all procedures performed in the present
study involving animals and human participants (approval no.
JMSU-195), which were in accordance with ethical standards, and all
patients provided written informed consent prior to participation
in this study.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no conflict of
interest with regard to this work.
References
|
1
|
Catalona WJ: Prostate Cancer Screening.
Med Clin North Am. 102:199–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
International Agency for Research on
Cancer: World Cancer Report. 2014, http://www.who.int/cancer/publications/WRC_2014/en/.
Accessed April 17, 2017.
|
|
3
|
Wang W, Li Y, Li Y, Hong A, Wang J, Lin B
and Li R: NDRG3 is an androgen regulated and prostate enriched gene
that promotes in vitro and in vivo prostate cancer cell growth. Int
J Cancer. 124:521–530. 2009. View Article : Google Scholar
|
|
4
|
Begley LA, Kasina S, Mehra R, Adsule S,
Admon AJ, Lonigro RJ, Chinnaiyan AM and Macoska JA: CXCL5 promotes
prostate cancer progression. Neoplasia. 10:244–254. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawamura M, Toiyama Y, Tanaka K, Saigusa
S, Okugawa Y, Hiro J, Uchida K, Mohri Y, Inoue Y and Kusunoki M:
CXCL5, a promoter of cell proliferation, migration and invasion, is
a novel serum prognostic marker in patients with colorectal cancer.
Eur J Cancer. 48:2244–2251. 2012. View Article : Google Scholar
|
|
6
|
Chang MS, McNinch J, Basu R and Simonet S:
Cloning and characterization of the human neutrophil-activating
peptide (ENA-78) gene. J Biol Chem. 269:25277–25282.
1994.PubMed/NCBI
|
|
7
|
Persson T, Monsef N, Andersson P, Bjartell
A, Malm J, Calafat J and Egesten A: Expression of the
neutrophil-activating CXC chemokine ENA-78/CXCL5 by human
eosinophils. Clin Exp Allergy. 33:531–537. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Long X, Ye Y, Zhang L, Liu P, Yu W, Wei F,
Ren X and Yu J: IL-8, a novel messenger to cross-link inflammation
and tumor EMT via autocrine and paracrine pathways (Review). Int J
Oncol. 48:5–12. 2016. View Article : Google Scholar
|
|
9
|
Xu X, Huang P, Yang B, Wang X and Xia J:
Roles of CXCL5 on migration and invasion of liver cancer cells. J
Transl Med. 12:1932014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li A, King J, Moro A, Sugi MD, Dawson DW,
Kaplan J, Li G, Lu X, Strieter RM, Burdick M, et al: Overexpression
of CXCL5 is associated with poor survival in patients with
pancreatic cancer. Am J Pathol. 178:1340–1349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park JY, Park KH, Bang S, Kim MH, Lee J-E,
Gang J, Koh SS and Song SY: CXCL5 overexpression is associated with
late stage gastric cancer. J Cancer Res Clin Oncol. 133:835–840.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH,
Wang Z, Huang XW, Fan J and Zhou J: Overexpression of CXCL5
mediates neutrophil infiltration and indicates poor prognosis for
hepatocellular carcinoma. Hepatology. 56:2242–2254. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuo PL, Huang MS, Hung JY, Chou SH, Chiang
SY, Huang YF, Yang C-J, Tsai M-J, Chang W-A and Hsu Y-L:
Synergistic effect of lung tumor-associated dendritic cell-derived
HB-EGF and CXCL5 on cancer progression. Int J Cancer. 135:96–108.
2014. View Article : Google Scholar
|
|
14
|
Speetjens FM, Kuppen PJK, Sandel MH, Menon
AG, Burg D, van de Velde CJH, Tollenaar RAEM, de Bont HJGM and
Nagelkerke JF: Disrupted expression of CXCL5 in colorectal cancer
is associated with rapid tumor formation in rats and poor prognosis
in patients. Clin Cancer Res. 14:2276–2284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu X, Qiao Y, Liu W, Wang W, Shen H, Lu
Y, Hao G, Zheng J and Tian Y: CXCL5 is a potential diagnostic and
prognostic marker for bladder cancer patients. Tumour Biol.
37:4569–4577. 2016. View Article : Google Scholar
|
|
16
|
Gui SL, Teng LC, Wang SQ, Liu S, Lin YL,
Zhao XL, Liu L, Sui H-Y, Yang Y, Liang L-C, et al: Overexpression
of CXCL3 can enhance the oncogenic potential of prostate cancer.
Int Urol Nephrol. 48:701–709. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mantovani A: Cancer: Inflaming metastasis.
Nature. 457:36–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mantovani A, Savino B, Locati M, Zammataro
L, Allavena P and Bonecchi R: The chemokine system in cancer
biology and therapy. Cytokine Growth Factor Rev. 21:27–39. 2010.
View Article : Google Scholar
|
|
20
|
Vindrieux D, Escobar P and Lazennec G:
Emerging roles of chemokines in prostate cancer. Endocr Relat
Cancer. 16:663–673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Verbeke H, Geboes K, Van Damme J and
Struyf S: The role of CXC chemokines in the transition of chronic
inflammation to esophageal and gastric cancer. Biochim Biophys
Acta. 1825:117–129. 2012.
|
|
22
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Douglas MR, Morrison KE, Salmon M and
Buckley CD: Why does inflammation persist: A dominant role for the
stromal microenvironment? Expert Rev Mol Med. 4:1–18. 2002.
View Article : Google Scholar
|
|
24
|
Hembruff SL and Cheng N: Chemokine
signaling in cancer: Implications on the tumor microenvironment and
therapeutic targeting. Cancer Ther. 7A:254–267. 2009.
|
|
25
|
Amagai Y, Tanaka A, Matsuda A, Jung K,
Ohmori K and Matsuda H: Stem cell factor contributes to
tumorigenesis of mast cells via an autocrine/paracrine mechanism. J
Leukoc Biol. 93:245–250. 2013. View Article : Google Scholar
|
|
26
|
Huang CK, Yang CY, Jeng YM, Chen CL, Wu
HH, Chang YC, Ma C, Kuo WH, Chang KJ, Shew JY, et al:
Autocrine/paracrine mechanism of interleukin-17B receptor promotes
breast tumorigenesis through NF-κB-mediated antiapoptotic pathway.
Oncogene. 33:2968–2977. 2014. View Article : Google Scholar
|
|
27
|
Lee JL, Lin CT, Chueh LL and Chang CJ:
Autocrine/paracrine secreted Frizzled-related protein 2 induces
cellular resistance to apoptosis: A possible mechanism of mammary
tumorigenesis. J Biol Chem. 279:14602–14609. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rollins BJ: Inflammatory chemokines in
cancer growth and progression. Eur J Cancer. 42:760–767. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Strieter RM: Chemokines: Not just
leukocyte chemoattractants in the promotion of cancer. Nat Immunol.
2:285–286. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tough DF, Borrow P and Sprent J: Induction
of bystander T cell proliferation by viruses and type I interferon
in vivo. Science. 272:1947–1950. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsutsui H, Nakanishi K, Matsui K,
Higashino K, Okamura H, Miyazawa Y and Kaneda K: IFN-gamma-inducing
factor up-regulates Fas ligand-mediated cytotoxic activity of
murine natural killer cell clones. J Immunol. 157:3967–3973.
1996.PubMed/NCBI
|
|
32
|
Hashimoto W, Osaki T, Okamura H, Robbins
PD, Kurimoto M, Nagata S, Lotze MT and Tahara H: Differential
antitumor effects of administration of recombinant IL-18 or
recombinant IL-12 are mediated primarily by Fas-Fas ligand- and
perforin-induced tumor apoptosis, respectively. J Immunol.
163:583–589. 1999.
|
|
33
|
Micallef MJ, Tanimoto T, Kohno K, Ikeda M
and Kurimoto M: Interleukin 18 induces the sequential activation of
natural killer cells and cytotoxic T lymphocytes to protect
syngeneic mice from transplantation with Meth A sarcoma. Cancer
Res. 57:4557–4563. 1997.PubMed/NCBI
|
|
34
|
Li K, Wei L, Huang Y, Wu Y, Su M, Pang X,
Wang N, Ji F, Zhong C and Chen T: Leptin promotes breast cancer
cell migration and invasion via IL-18 expression and secretion. Int
J Oncol. 48:2479–2487. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bian Y, Yu Y, Wang S and Li L:
Up-regulation of fatty acid synthase induced by EGFR/ERK activation
promotes tumor growth in pancreatic cancer. Biochem Biophys Res
Commun. 463:612–617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hamaoka Y, Negishi M and Katoh H: EphA2 is
a key effector of the MEK/ERK/RSK pathway regulating glioblastoma
cell proliferation. Cell Signal. 28:937–945. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu L, Cao Y, Chen C, Zhang X, McNabola A,
Wilkie D, Wilhelm S, Lynch M and Carter C: Sorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer
Res. 66:11851–11858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Uekita T, Fujii S, Miyazawa Y, Iwakawa R,
Narisawa-Saito M, Nakashima K, Tsuta K, Tsuda H, Kiyono T, Yokota
J, et al: Oncogenic Ras/ERK signaling activates CDCP1 to promote
tumor invasion and metastasis. Mol Cancer Res. 12:1449–1459. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Floros KV, Thomadaki H, Florou D, Talieri
M and Scorilas A: Alterations in mRNA expression of
apoptosis-related genes BCL2, BAX, FAS, caspase-3, and the novel
member BCL2L12 after treatment of human leukemic cell line HL60
with the anti-neoplastic agent etoposide. Ann N Y Acad Sci.
1090:89–97. 2006. View Article : Google Scholar
|
|
40
|
Hajiahmadi S, Panjehpour M, Aghaei M and
Shabani M: Activation of A2b adenosine receptor regulates ovarian
cancer cell growth: Involvement of Bax/Bcl-2 and caspase-3. Biochem
Cell Biol. 93:321–329. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sharifi AM, Hoda FE and Noor AM: Studying
the effect of LPS on cytotoxicity and apoptosis in PC12 neuronal
cells: Role of Bax, Bcl-2, and caspase-3 protein expression.
Toxicol Mech Methods. 20:316–320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zeng J, Chen S, Li N, Chen L, Su J, Niu G,
Zhu S and Liang Y: Sasanquasaponin from Camellia oleifera Abel.
induces apoptosis via Bcl-2, Bax and caspase-3 activation in HepG2
cells. Mol Med Rep. 12:1997–2002. 2015. View Article : Google Scholar : PubMed/NCBI
|