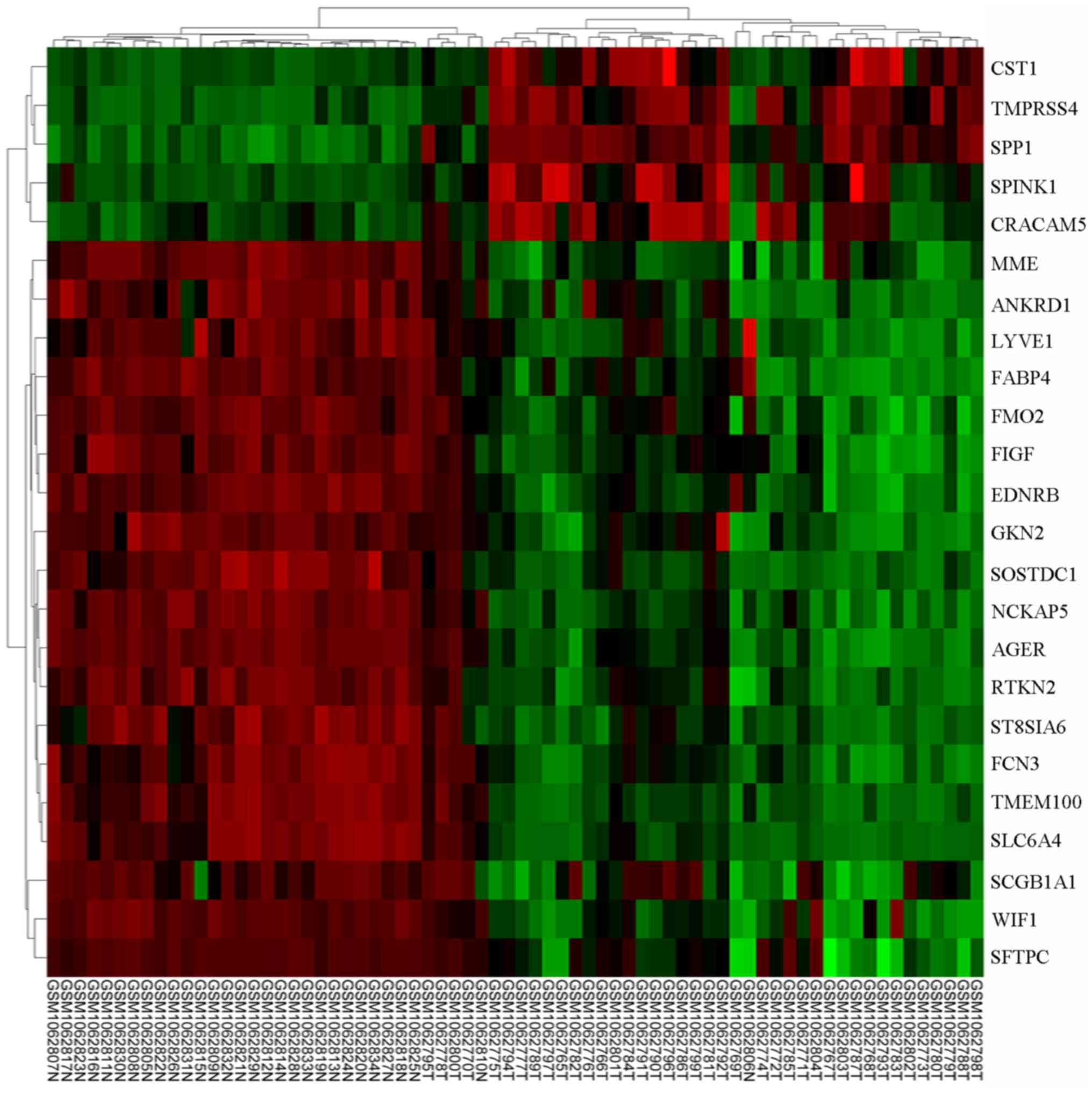
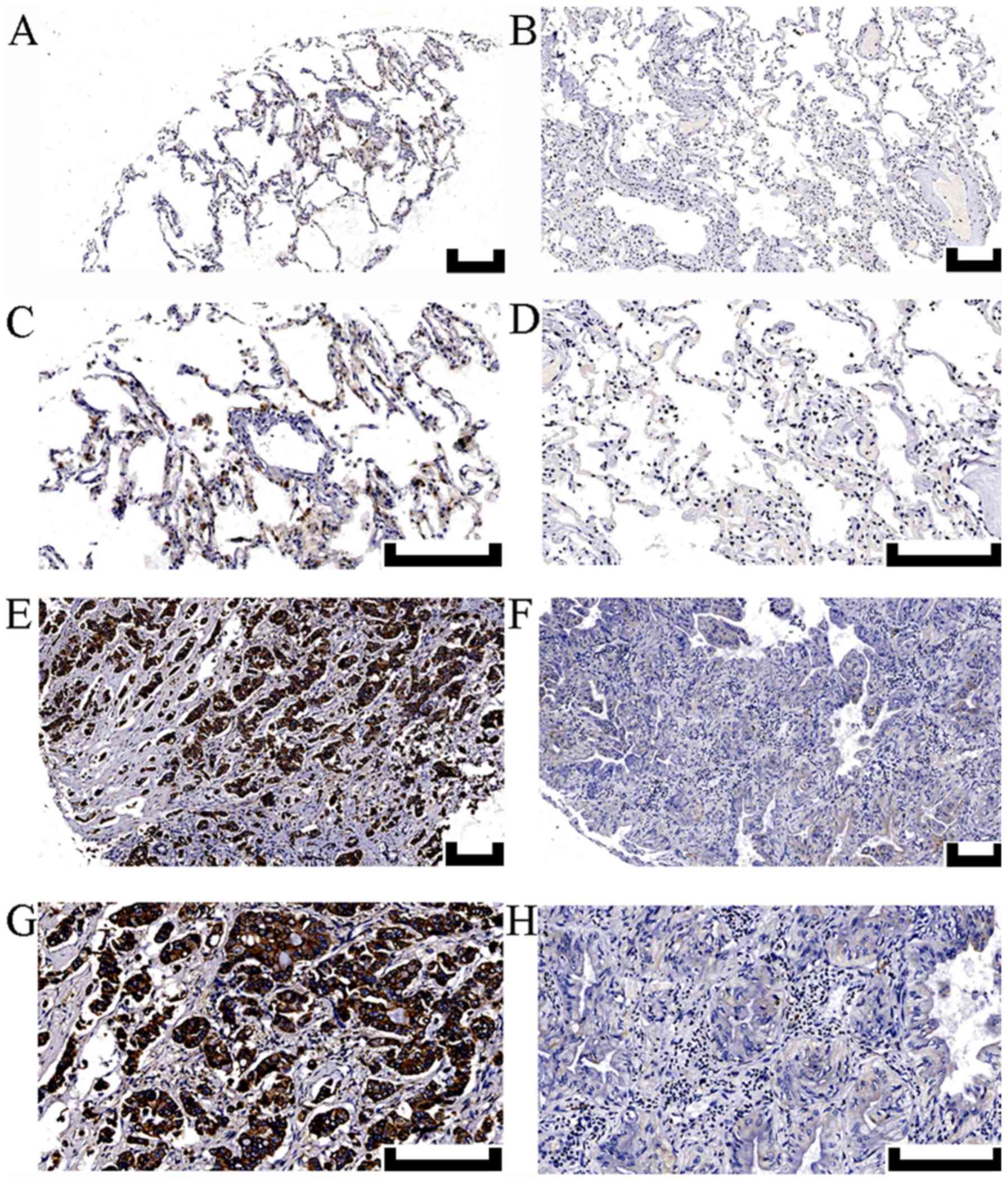
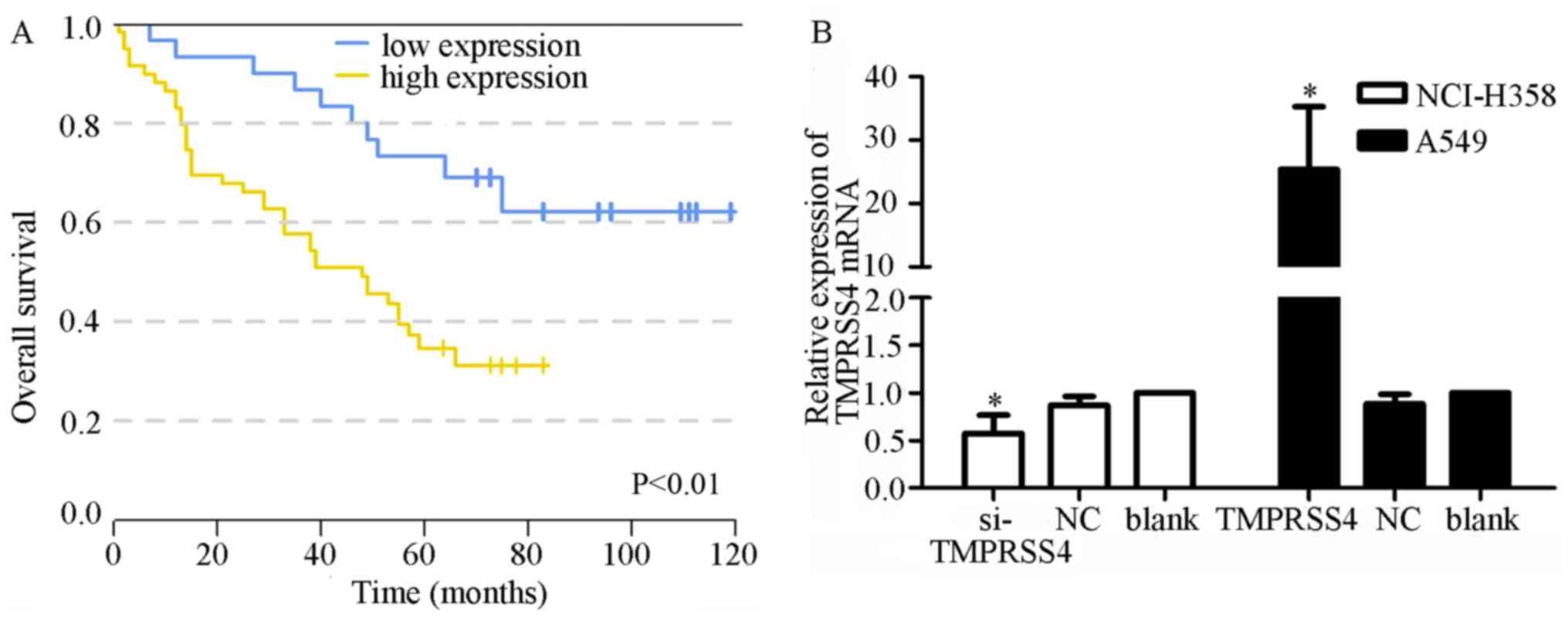
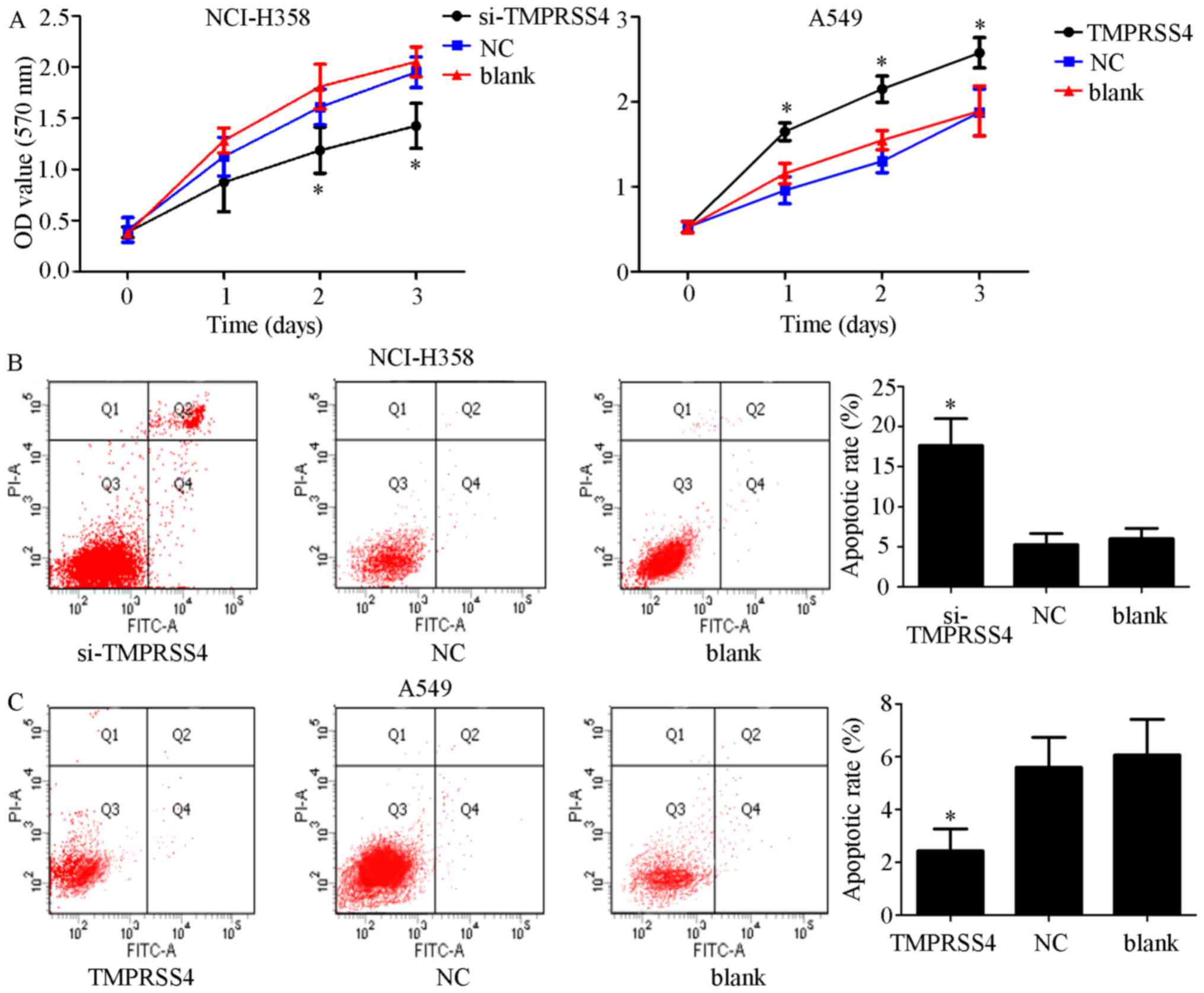
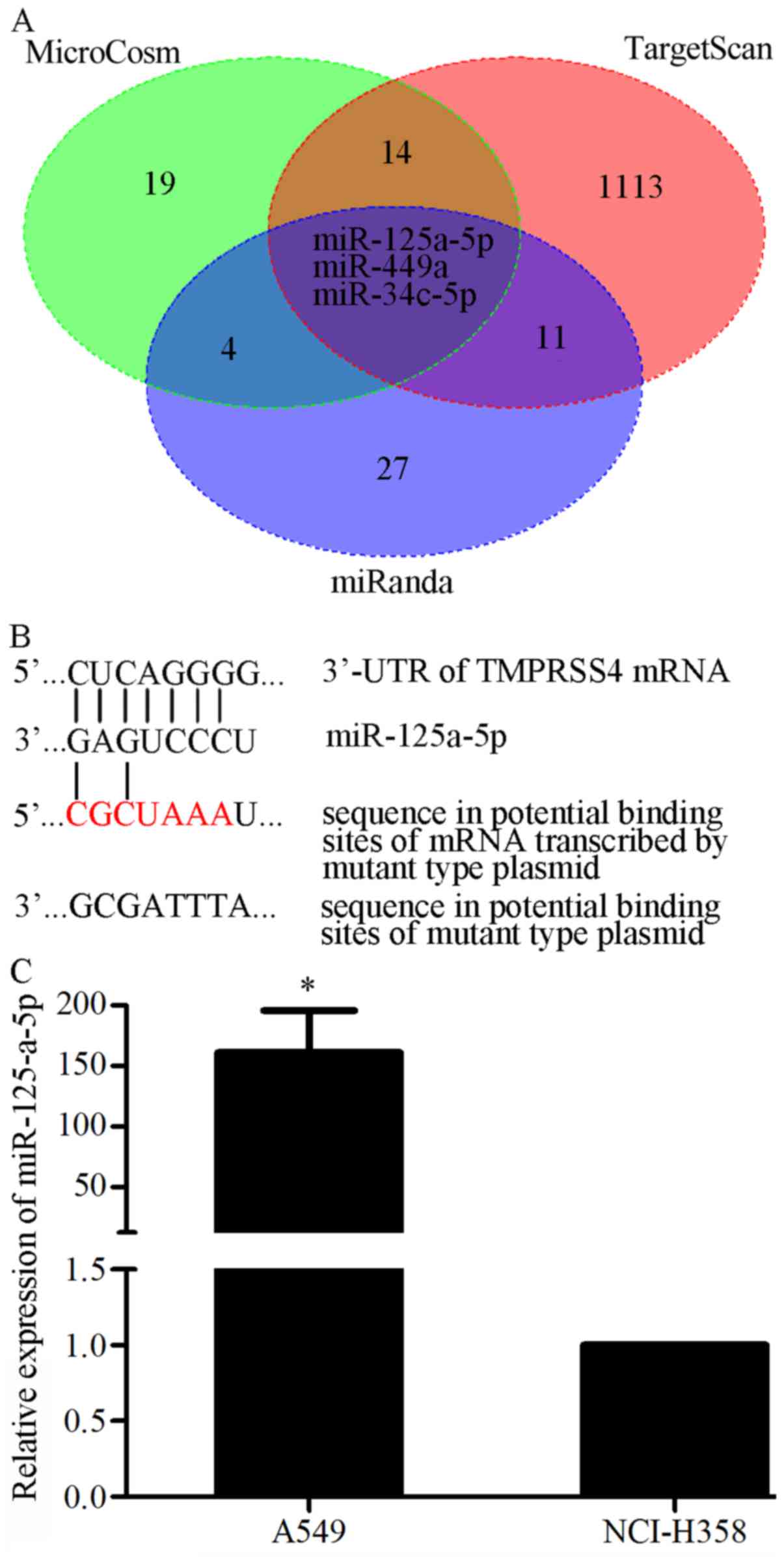
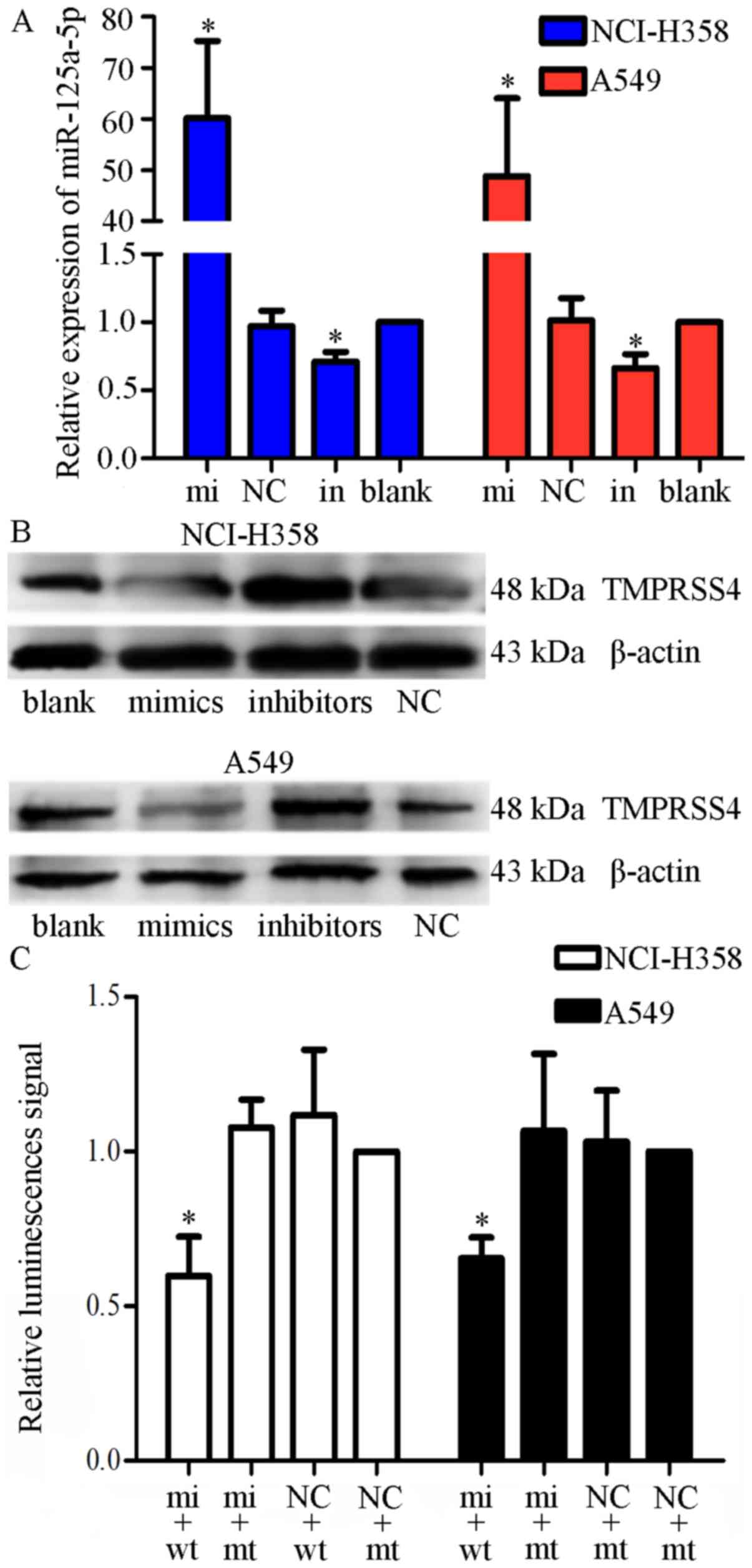
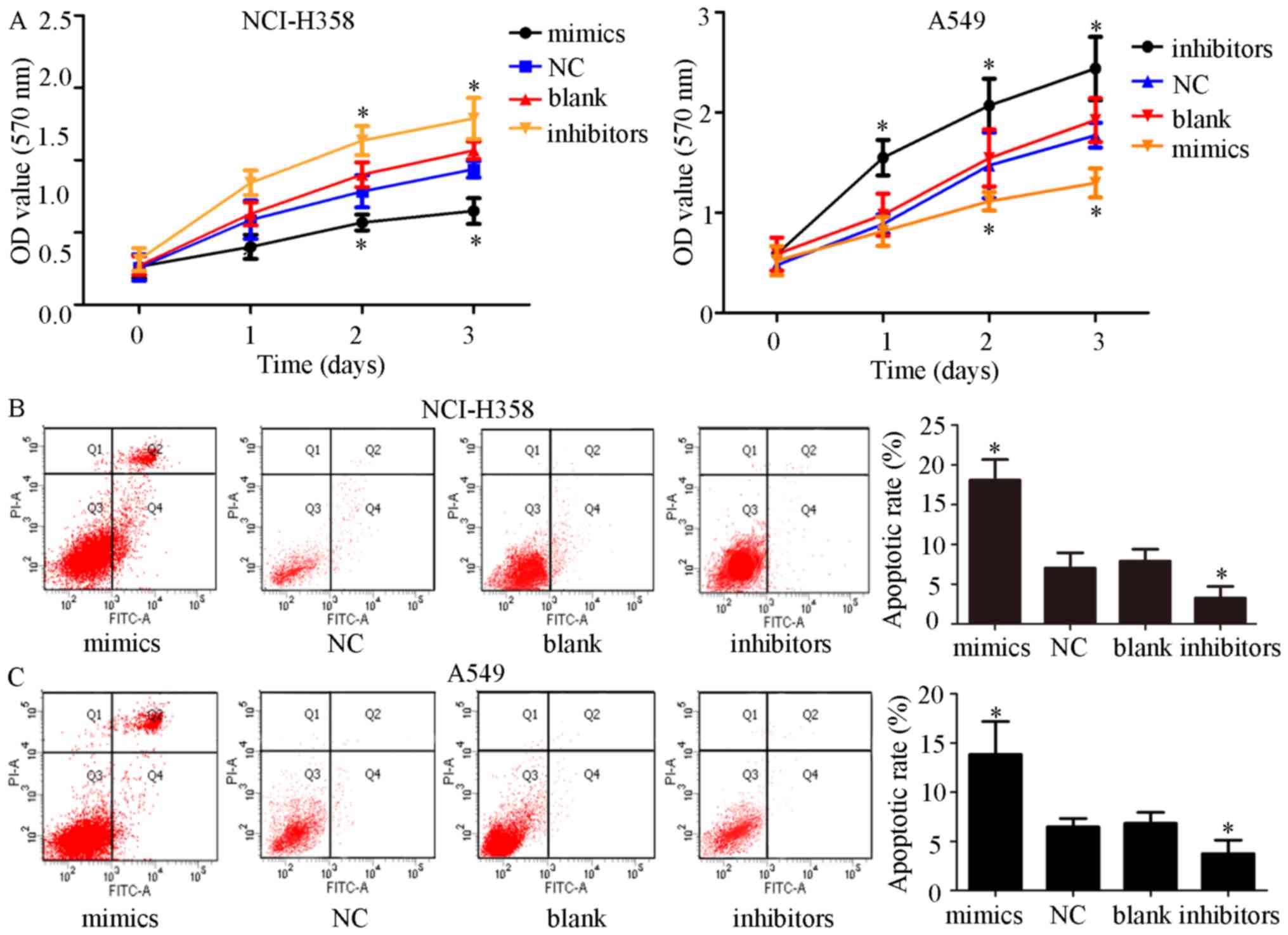
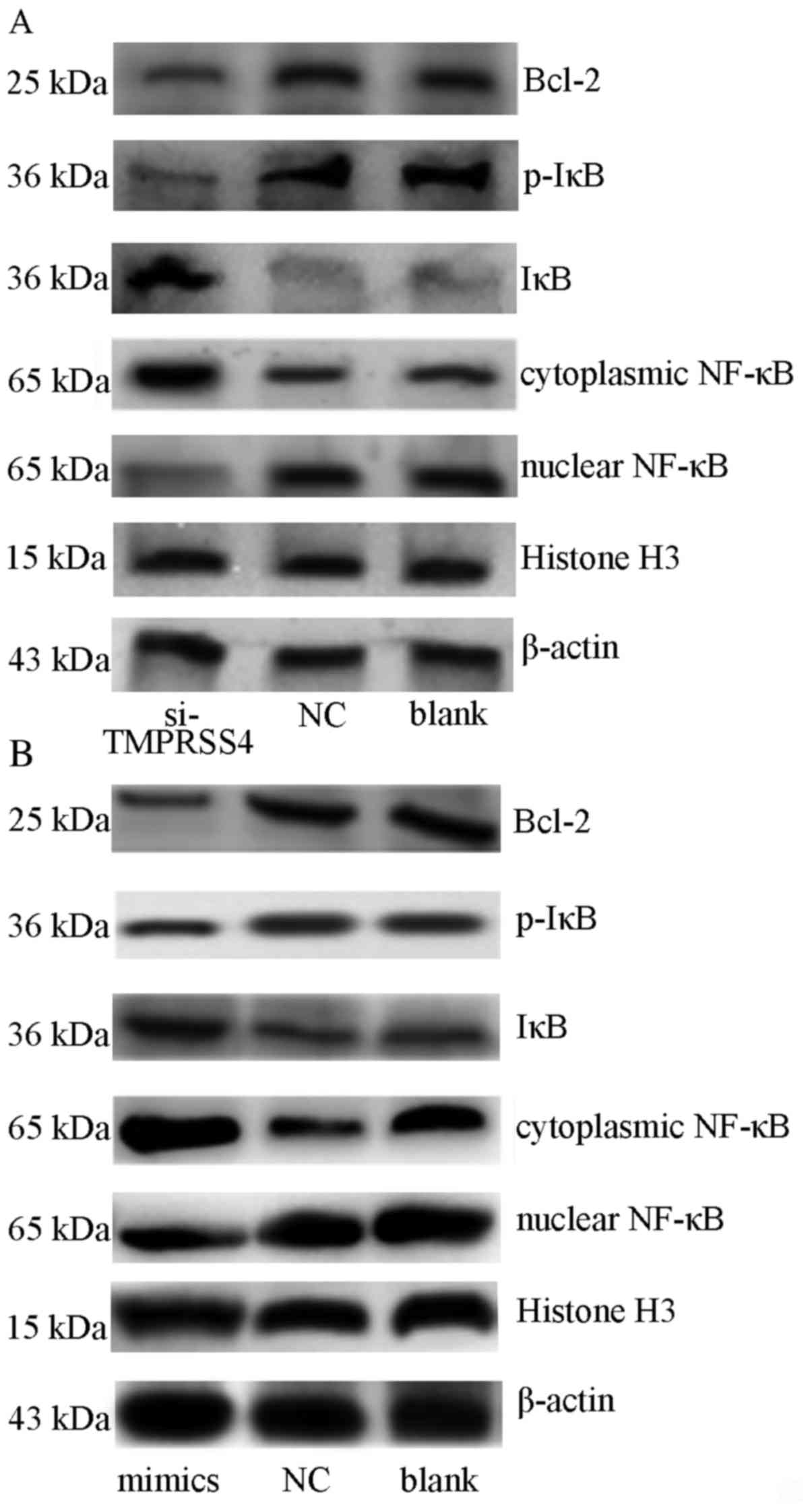
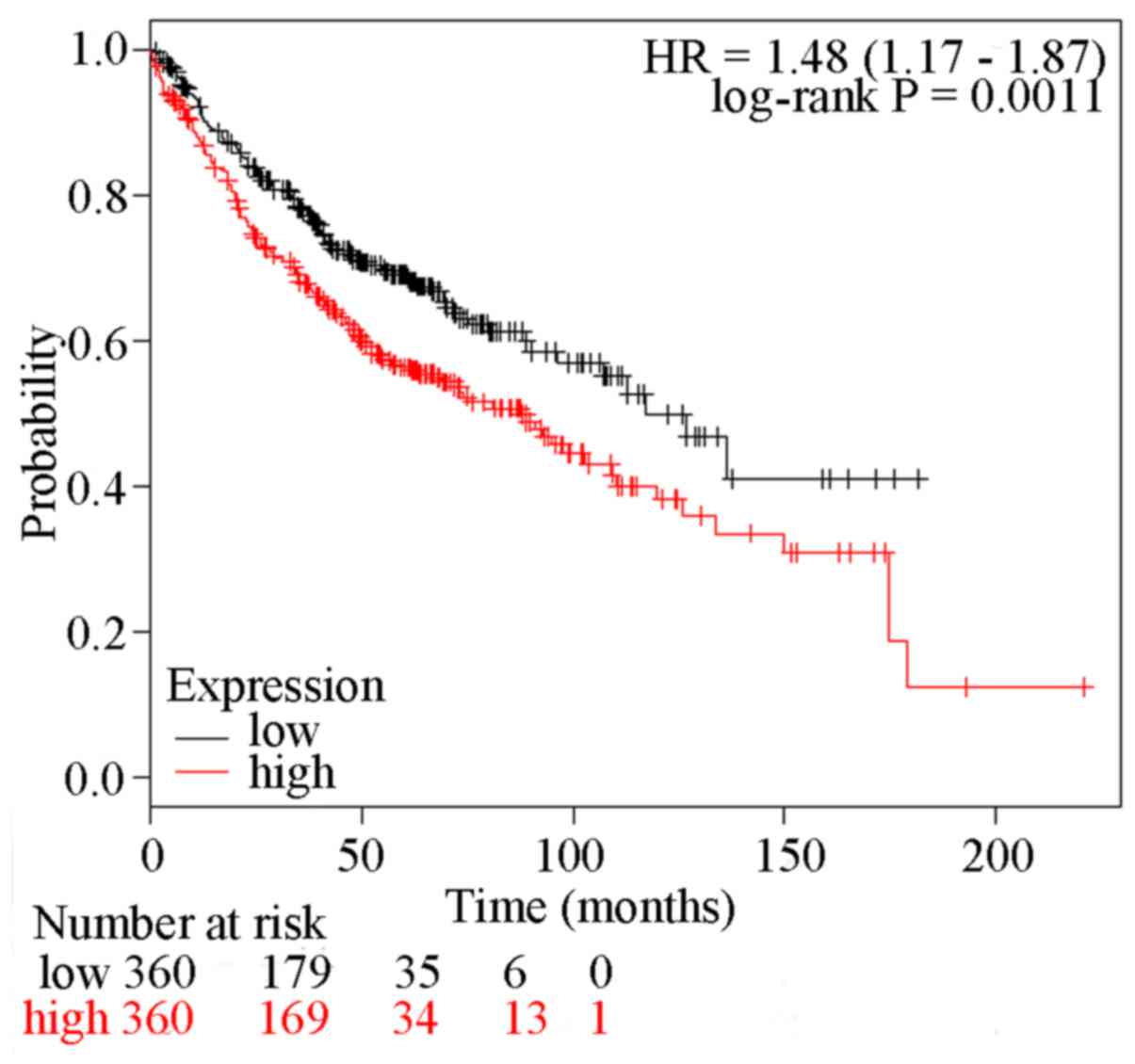
|
1
|
Stahel RA: Adenocarcinoma, a molecular
perspective. Ann Oncol. 18(Suppl 9): ix147–ix149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kadara H, Kabbout M and Wistuba II:
Pulmonary adenocarcinoma: A renewed entity in 2011. Respirology.
17:50–65. 2012. View Article : Google Scholar
|
|
3
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar
|
|
4
|
de Aberasturi AL and Calvo A: TMPRSS4: An
emerging potential therapeutic target in cancer. Br J Cancer.
112:4–8. 2015. View Article : Google Scholar :
|
|
5
|
Bhasin MK, Ndebele K, Bucur O, Yee EU, Otu
HH, Plati J, Bullock A, Gu X, Castan E, Zhang P, et al:
Meta-analysis of transcriptome data identifies a novel 5-gene
pancreatic adenocarcinoma classifier. Oncotarget. 7:23263–23281.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Larzabal L, Nguewa PA, Pio R, Blanco D,
Sanchez B, Rodríguez MJ, Pajares MJ, Catena R, Montuenga LM and
Calvo A: Overexpression of TMPRSS4 in non-small cell lung cancer is
associated with poor prognosis in patients with squamous histology.
Br J Cancer. 105:1608–1614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Villalba M, Diaz-Lagares A, Redrado M, de
Aberasturi AL, Segura V, Bodegas ME, Pajares MJ, Pio R, Freire J,
Gomez-Roman J, et al: Epigenetic alterations leading to TMPRSS4
promoter hypomethylation and protein overexpression predict poor
prognosis in squamous lung cancer patients. Oncotarget.
7:22752–22769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu SL, Chen HY, Chang GC, Chen CY, Chen
HW, Singh S, Cheng CL, Yu CJ, Lee YC, Chen HS, et al: MicroRNA
signature predicts survival and relapse in lung cancer. Cancer
Cell. 13:48–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tong Z, Liu N, Lin L, Guo X, Yang D and
Zhang Q: miR-125a-5p inhibits cell proliferation and induces
apoptosis in colon cancer via targeting BCL2, BCL2L12 and MCL1.
Biomed Pharmacother. 75:129–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia CW, Sun Y, Zhang TT, Lu ZH and Chen J:
Effects of miR-125a-5p on cell proliferation, apoptosis and cell
cycle of pancreatic cancer cells. Zhongguo Yi Xue Ke Xue Yuan Xue
Bao. 38:415–421. 2016.PubMed/NCBI
|
|
11
|
Fu Y and Cao F: MicroRNA-125a-5p regulates
cancer cell proliferation and migration through NAIF1 in prostate
carcinoma. OncoTargets Ther. 8:3827–3835. 2015. View Article : Google Scholar
|
|
12
|
Zhong L, Sun S, Shi J, Cao F, Han X and
Chen Z: MicroRNA-125a-5p plays a role as a tumor suppressor in lung
carcinoma cells by directly targeting STAT3. Tumour Biol.
39:1010428317697579. 2017. View Article : Google Scholar
|
|
13
|
Zhu WY, Luo B, An JY, He JY, Chen DD, Xu
LY, Huang YY, Liu XG, Le HB and Zhang YK: Differential expression
of miR-125a-5p and let-7e predicts the progression and prognosis of
non-small cell lung cancer. Cancer Invest. 32:394–401. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kabbout M, Garcia MM, Fujimoto J, Liu DD,
Woods D, Chow CW, Mendoza G, Momin AA, James BP, Solis L, et al:
ETS2 mediated tumor suppressive function and MET oncogene
inhibition in human non-small cell lung cancer. Clin Cancer Res.
19:3383–3395. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding W, Hu W, Yang H, Ying T and Tian Y:
Prognostic correlation between MTA2 expression level and colorectal
cancer. Int J Clin Exp Pathol. 8:7173–7180. 2015.PubMed/NCBI
|
|
16
|
Chen T, Yang M, Yu Z, Tang S, Wang C, Zhu
X, Guo J, Li N, Zhang W, Hou J, et al: Small GTPase RBJ mediates
nuclear entrapment of MEK1/MEK2 in tumor progression. Cancer Cell.
25:682–696. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Aberasturi AL, Redrado M, Villalba M,
Larzabal L, Pajares MJ, Garcia J, Evans SR, Garcia-Ros D, Bodegas
ME, Lopez L, et al: TMPRSS4 induces cancer stem cell-like
properties in lung cancer cells and correlates with ALDH expression
in NSCLC patients. Cancer Lett. 370:165–176. 2016. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Hamamoto J, Soejima K, Naoki K, Yasuda H,
Hayashi Y, Yoda S, Nakayama S, Satomi R, Terai H, Ikemura S, et al:
Methylation-induced downregulation of TFPI-2 causes TMPRSS4
overexpression and contributes to oncogenesis in a subset of
non-small-cell lung carcinoma. Cancer Sci. 106:34–42. 2015.
View Article : Google Scholar :
|
|
20
|
Nguyen TH, Weber W, Havari E, Connors T,
Bagley RG, McLaren R, Nambiar PR, Madden SL, Teicher BA, Roberts B,
et al: Expression of TMPRSS4 in non-small cell lung cancer and its
modulation by hypoxia. Int J Oncol. 41:829–838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin J, Shen X, Chen L, Bao LW and Zhu LM:
TMPRSS4 promotes invasiveness of human gastric cancer cells through
activation of NF-kappaB/MMP-9 signaling. Biomed Pharmacother.
77:30–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng D, Kong H and Li Y: TMPRSS4 as a
poor prognostic factor for triple-negative breast cancer. Int J Mol
Sci. 14:14659–14668. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang A, Zhou H, Zhao H, Quan Y, Feng B
and Zheng M: High expression level of TMPRSS4 predicts adverse
outcomes of colorectal cancer patients. Med Oncol. 30:7122013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang A, Zhou H, Zhao H, Quan Y, Feng B
and Zheng M: TMPRSS4 correlates with colorectal cancer pathological
stage and regulates cell proliferation and self-renewal ability.
Cancer Biol Ther. 15:297–304. 2014. View Article : Google Scholar :
|
|
25
|
Li T, Zeng ZC, Wang L, Qiu SJ, Zhou JW,
Zhi XT, Yu HH and Tang ZY: Radiation enhances long-term metastasis
potential of residual hepatocellular carcinoma in nude mice through
TMPRSS4-induced epithelial-mesenchymal transition. Cancer Gene
Ther. 18:617–626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim S, Kang HY, Nam EH, Choi MS, Zhao XF,
Hong CS, Lee JW, Lee JH and Park YK: TMPRSS4 induces invasion and
epithelial-mesenchymal transition through upregulation of integrin
alpha5 and its signaling pathways. Carcinogenesis. 31:597–606.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guan H, Liang W, Liu J, Wei G, Li H, Xiu
L, Xiao H and Li Y: Transmembrane protease serine 4 promotes
thyroid cancer proliferation via CREB phosphorylation. Thyroid.
25:85–94. 2015. View Article : Google Scholar :
|
|
28
|
Chikaishi Y, Uramoto H, Koyanagi Y, Yamada
S, Yano S and Tanaka F: TMPRSS4 expression as a marker of
recurrence in patients with lung cancer. Anticancer Res.
36:121–127. 2016.PubMed/NCBI
|
|
29
|
Larzabal L, El-Nikhely N, Redrado M,
Seeger W, Savai R and Calvo A: Differential effects of drugs
targeting cancer stem cell (CSC) and non-CSC populations on lung
primary tumors and metastasis. PLoS One. 8:e797982013. View Article : Google Scholar :
|
|
30
|
Larzabal L, de Aberasturi AL, Redrado M,
Rueda P, Rodriguez MJ, Bodegas ME, Montuenga LM and Calvo A:
TMPRSS4 regulates levels of integrin α5 in NSCLC through miR-205
activity to promote metastasis. Br J Cancer. 110:764–774. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsieh TH, Hsu CY, Tsai CF, Long CY, Chai
CY, Hou MF, Lee JN, Wu DC, Wang SC and Tsai EM: miR-125a-5p is a
prognostic biomarker that targets HDAC4 to suppress breast
tumorigenesis. Oncotarget. 6:494–509. 2015. View Article : Google Scholar :
|
|
32
|
Hashiguchi Y, Nishida N, Mimori K, Sudo T,
Tanaka F, Shibata K, Ishii H, Mochizuki H, Hase K, Doki Y, et al:
Down-regulation of miR-125a-3p in human gastric cancer and its
clinicopathological significance. Int J Oncol. 40:1477–1482.
2012.PubMed/NCBI
|
|
33
|
Qin X, Wan Y, Wang S and Xue M:
MicroRNA-125a-5p modulates human cervical carcinoma proliferation
and migration by targeting ABL2. Drug Des Devel Ther. 10:71–79.
2015.
|
|
34
|
Xia JT, Chen LZ, Jian WH, Wang KB, Yang
YZ, He WL, He YL, Chen D and Li W: MicroRNA-362 induces cell
proliferation and apoptosis resistance in gastric cancer by
activation of NF-κB signaling. J Transl Med. 12:332014. View Article : Google Scholar
|
|
35
|
Correction for Jeon et al: Correction for
Jeon et al. A set of NF-κB-regulated microRNAs induces acquired
TRAIL resistance in Lung cancer. Proc Natl Acad Sci USA.
114:E25422017. View Article : Google Scholar
|