Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer in men and the second most commonly diagnosed
cancer in women worldwide (1).
According to cancer statistics in China, the incidence and
mortality rates of CRC continue to increase (2). Tumor stage is the most important
prognostic factor in CRC. For example, the 5-year survival rate of
patients diagnosed with CRC in the USA in 2001–2007 was 90.1% for
patients with CRC at the localized stage, but 11.7% for patients
with distant tumor spread (3).
However, only 40% of patients diagnosed with CRC were detected at
the localized stage (4).
Therefore, to improve the early diagnosis of patients with CRC,
understanding the pathogenesis of this disease is important.
The tripartite motif (TRIM) protein family comprises
proteins consisting of three domains, a RING finger protein domain,
a B-box type I domain, and a B-box type II domain, followed by a
coiled-coil region, and a highly variable C-terminal region
(5). To date, >70 TRIM
genes have been recognized in the human genome (6–8). As
a member of the TRIM family, TRIM27 (also known as RFP) inherited
the basic structure of this family. TRIM27 was first
identified as a gene involved in the generation of the RET
transforming gene activated by DNA rearrangement (9–11).
In the majority of human tissues, TRIM27 has been reported
to be detectable (12). The role
of TRIM27 in cancer has received increased attention. It has been
reported that TRIM27 can act as an oncogene in ovarian
cancer, endometrial cancer, breast cancer and lung cancer (13–16).
In addition, TRIM27 is important in promoting anticancer drug
resistance in specific tumors (17,18).
These studies suggested that TRIM27 may have an oncogenic role in
various types of tumor. In CRC, TRIM27 has been reported to be
upregulated and can predict chemotherapy resistance (18,19).
However, the exact role of TRIM27 in the progression of CRC remains
to be fully elucidated.
Epithelial-mesenchymal transition (EMT) is a
developmental process that promotes invasion and metastasis in
various types of tumor (20).
During EMT, E-cadherin and N-cadherin are the most commonly
detected epithelial and mesenchymal markers, respectively.
Vimentin, as a major member of the intermediate filament, is
expressed in almost all mesenchymal cells, and is important in
maintaining cell integrity and resisting external injury (21). The multi-step process of EMT
involves multiple regulatory mechanisms, including the activation
of phosphorylated AKT serine/threonine kinase (p-AKT) (22). However, until now, the association
between TRIM27 and EMT in CRC has not been investigated.
The aim of the present study was to analyze the
expression of TRIM27 in CRC tissues and adjacent normal tissues.
The study also aimed to further investigate the biological role of
TRIM27 in CRC cells in vivo and in vitro via the
inhibition and overexpression of TRIM27. Finally, the potential
mechanism underlying the effects of TRIM27 on the progression of
CRC was examined.
Materials and methods
Patients and tissue specimens
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Nanjing Medical
University (Nanjing, China; Ethics no. 2010-SR-091.A1). In total,
80 pairs of human CRC tissues and adjacent normal tissues were
collected from patients with CRC, who had signed an informed
consent form, between 2010 and 2012 at the First Affiliated
Hospital of Nanjing Medical University. All the patients were aged
between 27 and 88 years old (average, 61.6 years old), and none of
the patients had a history of radiotherapy or chemotherapy prior to
surgery. All samples were immediately preserved in liquid nitrogen
within 5 min following resection and then placed at −70°C for
long-term preservation. The tumor-node-metastasis stage was
determined based on the National Comprehensive Cancer Network
(23).
CRC cell lines and culture
conditions
All CRC cell lines (LoVo, HCT116, SW480, DLD-1 and
HT29) and normal epithelial colon cells (NCM460) were purchased
from the American Type Culture Collection (Manassas, VA, USA). All
cell lines were cultured in Dulbecco’s modified Eagle’s medium
(DMEM) supplemented with 10% fetal bovine serum (both from Winsent,
Inc., St. Bruno, QC, Canada), 100 U/ml of penicillin and 100 µg/ml
of streptomycin in a humid incubator (stabilized at 5%
CO2 and 37°C)
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR analysis)
Total RNA was extracted from CRC tissues and cells
using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer’s protocol.
The cDNA was produced from the RNA by performing reverse
transcription using a PrimeScript RT reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China). The RT-qPCR experiment was
performed in a 20 µl volume consisting of 2 µl cDNA, 1.2 µl
primers, 6.8 µl dH2O and 10 µl SYBR, using a SYBR-Green
PCR kit (Roche Diagnostics, Indianapolis, IN, USA). The final
reaction was performed in a StepOnePlus Real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and comprised:
Hot-start DNA polymerase activation to 95°C for 10 min; 40 cycles
of 95°C for 15 sec and 60°C for 1 min; followed by one cycle of
melt curve analysis at 95°C for 15 sec, 60°C for 1 min, and 95°C
for 15 sec. The specific primers were as follows: TRIM27, forward,
5′-AGCCCATGATGCTCGACTG-3′ and reverse, 5′-GGGCACGACACGTTAGTCT-3′;
GAPDH, forward, 5′-AGAAGGCTG GGGCTCATTTG-3′ and reverse,
5′-AGGGGCCATCCACAGTCTTC-3′. TRIM27 expression was normalized to
GAPDH and relative expression levels were calculated using the
2−ΔΔCq method (24).
Immunohistochemistry (IHC)
To detect the level of TRIM27 in CRC, 50 CRC tissues
and 25 adjacent normal tissues were evaluated using IHC. The
formalin-fixed and paraffin-embedded tumor and normal tissue
sections (4-µm) were deparaffinized in xylene and rehydrated in
different concentrations of alcohol and distilled water, followed
by microwave antigen retrieval. The sections were washed in
phosphate-buffered saline (PBS) three times and were placed in 3%
H2O2 for 20 min in the dark, followed by
three washes with PBS. The tissues were then soaked in 5% BSA
(Servicebio, Wuhan, China) for 30 min. Finally, the tissues were
reacted with anti-TRIM27 antibodies (diluted 1:1,000, polyclonal,
rabbit, cat. no. ab78393; Abcam, Cambridge, MA, USA) overnight at
4°C, followed by incubation with anti-rabbit antibodies
(horseradish peroxidase-tagged, diluted 1:1,000, polyclonal, cat.
no. ab6721; Abcam) at room temperature for 50 min, and with the
color agent diaminobenzidine. The nuclei were counterstained with
hematoxylin, and the sections were dehydrated using different
grades of ethyl alcohol and xylene. The sections were then viewed
under an inverted microscope (NIKON ECLIPSE TI-SR; Nikon
Corporation, Tokyo, Japan). Based on the staining intensity, the
level of TRIM27 was graded as 0 (no staining), 1 (+), 2 (++), and 3
(+++). According to the proportion of TRIM27-positive cells, the
scores were as follows: 0 (negative), 1 (<30%), 2 (31–60%), 3
(>60%). The total score was calculated as the intensity score
plus the positive rate score. Scores ≥4 were regarded as a high
level of TRIM27, and scores <4 were regarded as a low level of
TRIM27.
Knockdown and overexpression of
TRIM27
Small interference RNA (siRNA) targeting
TRIM27 and a negative control sequence (NC) were designed by
GenePharma Corporation (Shanghai, China). The target sequences are
shown in Table I (13). The TRIM27 inhibitor
lentivirus [short hairpin (sh)TRIM27] in vivo experiment was
designed based on the sequence of siRNA918, with the specific
sequence: 5′-GCAGCTGATATCACTCCTTA-3′. For the overexpression of
TRIM27, a plasmid expressing TRIM27 was designed by
GeneCopoeia, Inc. (Rockville, ME, USA). The above siRNAs and
plasmid were transfected into cells using Lipofectamine®
3000, according to the manufacturer’s protocol (Invitrogen; Thermo
Fisher Scientific, Inc.).
 | Table ISequences of small interference RNAs
designed for TRIM27 knockdown. |
Table I
Sequences of small interference RNAs
designed for TRIM27 knockdown.
| Name | Sequence |
|---|
|
TRIM27-homo-1251 | F:
5′-GCAGUCAGAUAUGGAGAAATT-3′ |
| R:
5′-UUUCUCCAUAUCUGACUGCTT-3′ |
|
TRIM27-homo-1578 | F:
5′-GGCAGUGUCUUUGUGGUAUTT-3′ |
| R:
5′-AUACCACAAAGACACUGCCTT-3′ |
|
TRIM27-homo-918 | F:
5′-GCAGCUGUAUCACUCCUUATT-3′ |
| R:
5′-UAAGGAGUGAUACAGCUGCTT-3′ |
| GAPDH-negative
control | F:
5′-UUCUCCGAACGUGUCACGUTT-3′ |
| R:
5′-ACGUGACACGUUCGGAGAATT-3′ |
| GAPDH-positive
control | F:
5′-GUAUGACAACAGCCUCAAGTT-3′ |
| R:
5′-CUUGAGGCUGUUGUCAUACTT-3′ |
Cell viability assay
To investigate the effect of TRIM27 on the
proliferation of CRC cells, 2×103 cells per well were
cultured in 96-well plates with each well containing 100 µl of
medium. Cell viability was detected using a Cell Counting Kit-8
(CCK-8; Dojindo Molecular Technologies, Inc., Kummamoto, Japan)
assay according to the manufacturer’s protocol. After 24, 48, 72
and 96 h, 10 µl of CCK-8 assay reagent was added to each well mixed
with 90 µl of serum-free medium. The absorbance was measured 2 h
later using a microplate reader at a test wavelength of 450 nm and
a reference wavelength of 630 nm.
Plate colony formation assay
The cells (500 per well) were cultured in 6-well
plates to investigate the effect of TRIM27 on the efficiency of
colony formation in CRC cells. After 7 days, each well was washed
with PBS three times at room temperature. The cells were then fixed
in each well using ethyl alcohol for 30 sec and stained for 20 min
using crystal violet dye. Following washing with PBS, colonies (≥50
cells/colony) in each well were manually counted and images were
captured using a digital camera (Canon DS126211; Canon, Tokyo,
Japan).
Wound-healing assay
The CRC cells (4×105) were seeded in
6-well plates and cultured until they reached a density of 90%. A
200-µl micropipette tip was then used make a wound in the cells and
the medium was replaced with serum-free medium. Electron microscopy
was used to observe the shape of wound at 0 and 24 h.
Transwell assay
Cell migration and invasion were assayed in a
24-well plate with polycarbonate sterile chambers (8-µm filters; BD
Biosciences, Franklin Lakes, NJ, USA) with or without Matrigel
coating. The CRC cells (2×104) were cultured with 100 µl
of serum-free DMEM in the upper chamber and 600 µl of DMEM + 10%
serum in the lower chamber. After 24 h, the lower chamber was
washed twice with PBS and crystal violet dye was added to the lower
chamber incubated for 20 min. The lower chamber was washed with PBS
three times, following which a cotton bud was used to remove cells
and medium from the upper chambers. The migrated or invaded cells
in the lower chambers were observed under an electron
microscope.
Cell apoptosis analysis
Following 48 h of the siRNA or plasmid transfection,
the cells were collected and washed with cold PBS. The cells were
then mixed with an Annexin V-FITC Apoptosis Detection Kit I (BD
Biosciences) in flow cytometry tubes, following the manufacturer’s
protocol. The number of apoptotic cells was analyzed using flow
cytometry (BD Biosciences).
Cell cycle analysis
At 48 h following siRNA or plasmid transfection, the
transfected CRC cells were collected in PBS. Following washing
twice with PBS, the cells were fixed with 75% ethyl alcohol at 4°C
overnight. The following day, the separated ethyl alcohol was
removed, and the cells were fixed with 500 µl of propidium iodide
(PI) staining solution and incubated for 30 min in the dark at room
temperature. The analysis of the cell cycle was performed using
fluorescence-activated cell sorting with a FACSCalibur flow
cytometer with CellQuest software (version 3.0; BD
Biosciences).
Western blot analysis
Protein was extracted from the CRC cells using a
Radioimmunoprecipitation Assay kit (Beyotime Institute of
Biotechnology, Shanghai, China) according to the manufacturer’s
protocol. The protein concentration, which determined the quantity
loaded for SDS-PAGE, was determined using the Bicinchoninic Acid
Protein Assay kit (Beyotime Institute of Biotechnology). The
proteins (40 µg) were separated using 10% SDS-PAGE in running
buffer and transferred onto polyvinylidene fluoride membranes (EMD
Millipore, Bedford, MA, USA) in transfer buffer. The membranes were
then blocked in 5% non-fat milk for >2 h and incubated with
specific primary antibodies at 4°C overnight. Following washing
with TBST three times (10 min each time), the membranes were
incubated with secondary antibodies (anti-rabbit or anti-mouse) at
room temperature for 2 h. The immunoreactive protein bands were
visualized using ECL Plus (EMD Millipore) with a bio-imaging
system. The specific primary and secondary antibodies were as
follows: TRIM27 (diluted 1:1,000, polyclonal, rabbit, cat. no.
ab78393), E-cadherin (diluted 1:500, monoclonal, mouse, cat. no.
ab1416), N-cadherin (diluted 1:1,000, poly-clonal, rabbit, cat. no.
ab18203), vimentin (diluted 1:2,000, monoclonal, rabbit, cat. no.
ab92547), AKT (diluted 1:500, polyclonal, rabbit, cat. no. ab8805),
p-AKT (diluted 1:500, polyclonal, rabbit, cat. no. ab38449) (all
from Abcam), anti-rabbit secondary antibodies (diluted 1:5,000,
polyclonal, cat. no. GAB007), anti-mouse secondary antibodies
(diluted 1:5,000, polyclonal, cat. no. GAM007) (both from Hangzhou
Multi Sciences Biotech Co., Ltd., Hangzhou, China). GAPDH (diluted
1:5,000, monoclonal, mouse; Abcam) was used as an internal
control.
Tumor xenograft in a nude mouse
model
The animal experiment was approved by Animal Ethics
Committee of Nanjing Medical University. A total of 20 male mice
(age, 3–4 weeks; weight, 13–15 g) were purchased from the Animal
Center of Nanjing Medical University after signing the
animal-raising agreement. The mice were maintained under the
following conditions: Room temperature, 20–26°C and 12-h light/dark
cycle. The mice received 5 g food and 100 ml water per 100 g body
weight per day. To detect whether TRIM27 can affect tumor growth in
mice, 2×106 of differently treated LoVo cells mixed with
200 µl of PBS were subcutaneously injected into the anesthetized
mice. Each mouse was randomly injected with cells treated with
shTRIM27 or NC in their right and left armpits. At 4 weeks
post-injection, all mice were sacrificed and the tumor tissues were
surgically removed. To further investigate the role of TRIM27 in
tumor metastasis in vivo, 2×106 LoVo cells
suspended in 200 µl of PBS were injected into the mouse tail vein.
The liver tissues were removed after 7 weeks and stained with
hematoxylin and eosin.
Statistical analysis
The results are expressed as the mean ± standard
deviation. The mRNA expression was analyzed using unpaired t-tests.
IHC and the clinical features were analyzed using Pearson
χ2 tests. One-way analysis of variance and the
least-significant difference post hoc test were the main
statistical methods used to compare datasets containing multiple
groups. Cumulative survival analysis was assessed using the
Kaplan-Meier method. Independent prognostic factors were identified
using univariate and multivariate Cox proportional hazard
regression models. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS (version 15.0; SPSS, Inc., Chicago, IL, USA)
and GraphPad Prism5 (GraphPad Software, Inc., La Jolla, CA,
USA).
Results
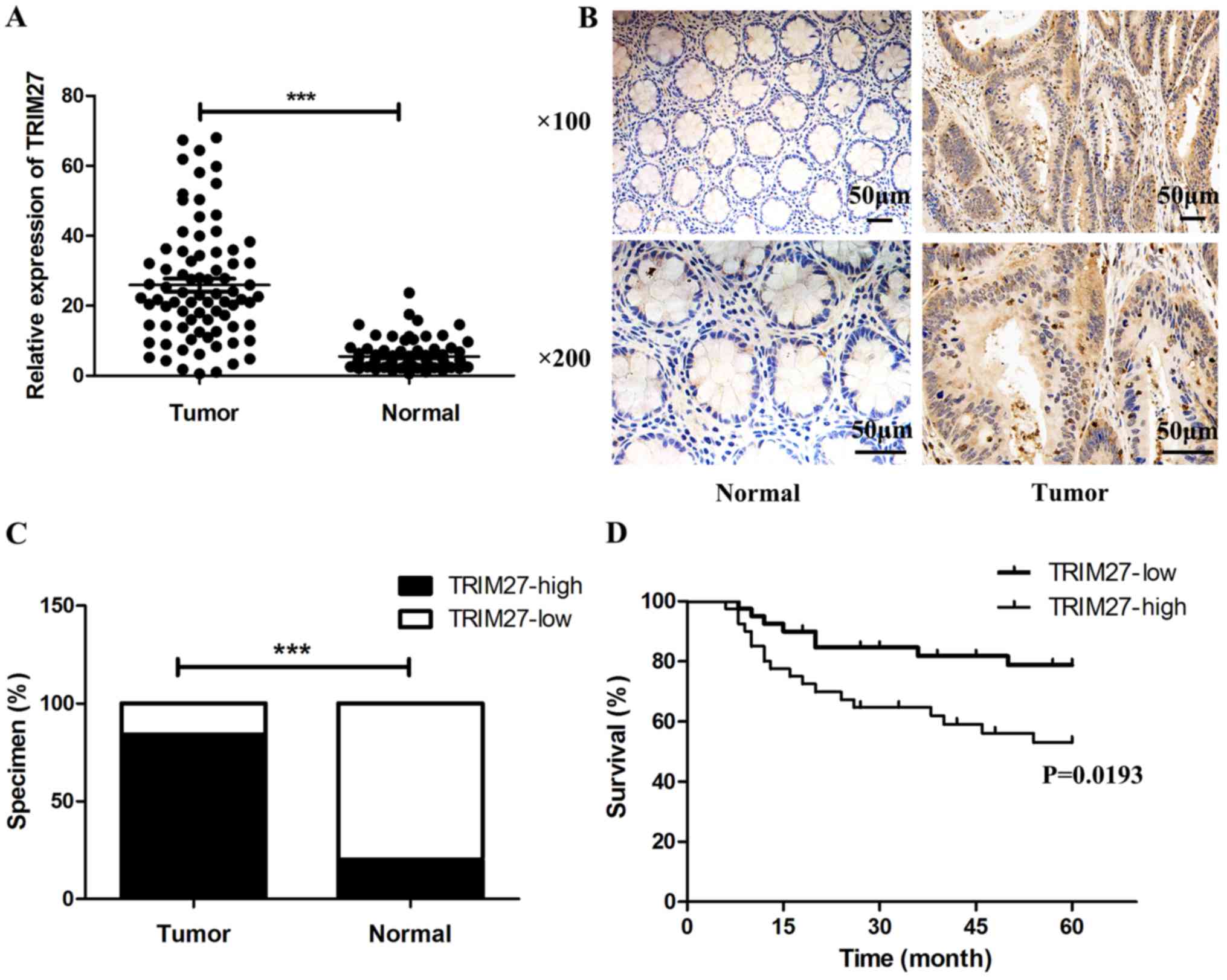
TRIM27 is upregulated in CRC tissues and
correlated with clinicopathological features
To reveal the role of TRIM27 in the progression of
CRC, the mRNA expression of TRIM27 was first detected in 80
pairs of CRC tissues and adjacent normal tissues using RT-qPCR
analysis. It was found that the expression of TRIM27 was
significantly upregulated in CRC tissues (Fig. 1A). Then we detected the presence of
TRIM27 using IHC in 50 CRC tissues and 25 adjacent normal tissues.
IHC also showed that the protein level of TRIM27 was significantly
upregulated in CRC tissues (Fig. 1B
and C; Table II). The
patients were next divided into two groups based on the average
TRIM27 levels, and the association between levels of TRIM27 and the
clinicopathological features was analyzed. It was found that a
higher expression of TRIM27 was associated with deeper invasion
(P=0.005), increased lymph node metastasis (P=0.014), more advanced
tumor stage (P=0.004), and increased liver metastasis (P=0.043)
(Table III). However, no
significant association was found for sex, age, CEA, or location.
Kaplan-Meier curves were used to analyze the effect of TRIM27 on
overall survival (OS) in patients with CRC. The median follow-up
time was 60 months, and the result showed that higher mRNA
expression of TRIM27 predicted poorer prognosis in patients
diagnosed with CRC (Fig. 1D).
Multivariate analysis further suggested that TRIM27 was an
independent prognostic factor for CRC (Table IV).
 | Table IIAnalysis of the expression of
tripartite motif-containing 27 in colorectal cancer tissues and
adjacent normal tissues by immunohistochemistry. |
Table II
Analysis of the expression of
tripartite motif-containing 27 in colorectal cancer tissues and
adjacent normal tissues by immunohistochemistry.
| Specimen | Total score | Number (%) | P-value |
|---|
| Carcinoma | 0–3 | 8 (10.7) | <0.001 |
| 4–6 | 42 (56.0) | |
| Normal | 0–3 | 20 (26.7) | |
| 4–6 | 5 (6.6) | |
 | Table IIImRNA expression of TRIM27 in
colorectal carcinoma and adjacent normal tissues. |
Table III
mRNA expression of TRIM27 in
colorectal carcinoma and adjacent normal tissues.
| Clinical
feature | n (%) | TRIM27
expression
| P-value |
|---|
| High, n (%) | Low, n (%) |
|---|
| Sex | | 40 | 40 | |
| Male | 52 (65.0) | 25 (31.3) | 27 (33.8) | 0.639 |
| Female | 28 (35.0) | 15 (18.7) | 13 (16.2) | |
| Age (years) | | | | |
| >60 | 36 (45.0) | 17 (21.3) | 19 (23.8) | 0.653 |
| ≤60 | 44 (55.0) | 23 (28.7) | 21 (26.2) | |
| Depth of
invasion | | | | |
| T1/T2 | 21 (26.3) | 5 (6.3) | 16 (20.0) | 0.005b |
| T3/T4 | 59 (73.7) | 35 (43.7) | 24 (30.0) | |
| Lymph node
metastasis | | | | |
| Absent | 39 (48.8) | 14 (17.5) | 25 (31.3) | 0.014a |
| Present | 41 (51.2) | 26 (32.5) | 15 (18.7) | |
| Tumor stage | | | | |
| I/II | 37 (46.3) | 12 (15.0) | 25 (31.2) | 0.004b |
| III/IV | 43 (53.7) | 28 (35.0) | 15 (18.8) | |
| Liver
metastasis | | | | |
| Yes | 10 (12.5) | 8 (10.0) | 2 (2.5) | 0.043a |
| No | 70 (87.5) | 32 (40.0) | 38 (47.5) | |
| Location | | | | |
| Rectum | 35 (43.8) | 19 (23.8) | 16 (20.0) | 0.499 |
| Colon | 45 (56.2) | 21 (26.2) | 24 (30.0) | |
| CEA (ng/ml) | | | | |
| <4.7 | 31 (38.8) | 13 (16.2) | 18 (22.5) | 0.250 |
| >4.7 | 49 (61.2) | 27 (33.8) | 22 (27.5) | |
 | Table IVUnivariate and multivariate analysis
of the association of prognosis with clinicopathologic parameters
and expression of TRIM27 in colorectal cancer. |
Table IV
Univariate and multivariate analysis
of the association of prognosis with clinicopathologic parameters
and expression of TRIM27 in colorectal cancer.
| Variable | Univariable
analysis | Multivariable
analysis |
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex (male vs.
female) | 0.83
(0.38–1.82) | 0.642 | – | NA |
| Age (>60 vs. ≤60
years) | 0.92
(0.42–1.99) | 0.823 | – | NA |
| Depth of invasion
(T3/T4 vs. T1/T2) | 1.50
(1.04–2.16) | 0.029a | 2.48
(0.86–7.15) | 0.093 |
| Lymph node
metastasis (present vs. absent) | 3.18
(1.23–8.22) | 0.017a | 2.56
(1.04–6.28) | 0.040a |
| Liver metastasis
(present vs. absent) | 3.28
(1.28–8.41) | 0.013a | 2.95
(1.19–7.31) | 0.020a |
| Location (rectum
vs. colon) | 1.31
(0.61–2.82) | 0.495 | – | NA |
| CEA (>4.7 vs.
<4.7) | 0.68
(0.32–1.42) | 0.303 | – | NA |
| TRIM27 expression
(high vs. low) | 2.63
(1.14–6.05) | 0.023a | 2.52
(1.02–6.25) | 0.046a |
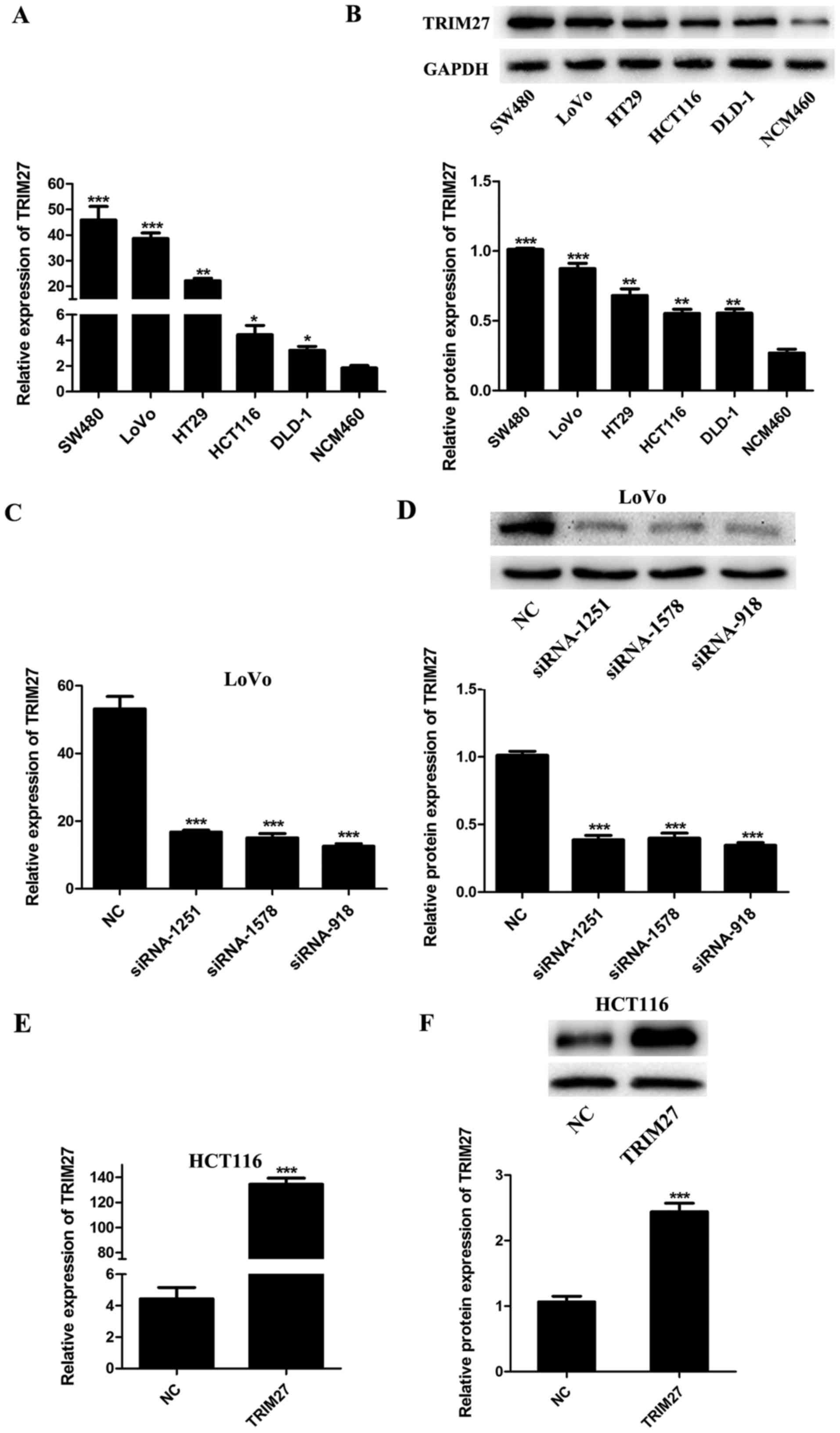
Expression of TRIM27 is upregulated in
CRC cells and can be regulated by siRNAs and plasmids
To evaluate the expression level of TRIM27 in CRC
cells, five CRC cell lines (SW480, LoVo, HT29, HCT116 and DLD-1)
and the NCM460 normal colon epithelial cell line were detected by
RT-qPCR and western blot analyses. It was found that the mRNA and
protein levels of TRIM27 were upregulated in the CRC cell lines
compared with those in the NCM460 cells (Fig. 2A and B). To knock down or
overexpress TRIM27, the LoVo and HCT116 cells were
transfected with a TRIM27 inhibitor and TRIM27-overexpressing
plasmid, respectively. According to the results of RT-qPCR and
western blot analysis, the expression of TRIM27 was significantly
inhibited by siRNA-918, siRNA-1251 and siRNA-1578 in LoVo cells
(Fig. 2C and D), and was
overexpressed by trans-fection with the TRIM27-overexpressing
plasmid in HCT116 cells (Fig. 2E and
F). To further investigate the role of TRIM27 in CRC, the
TRIM27-overexpressing plasmid and siRNA-918 were selected to
perform further in vitro experiments.
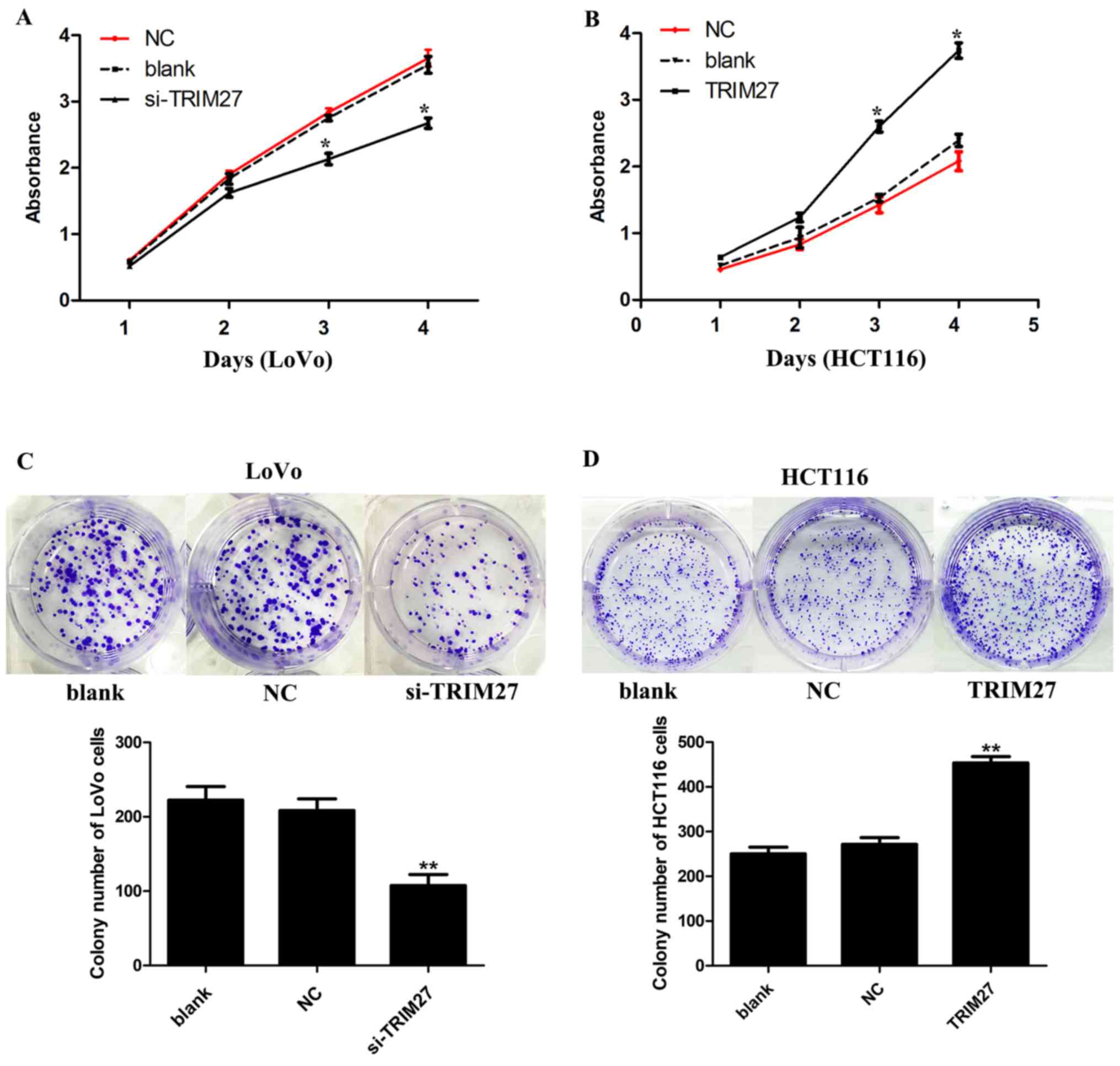
TRIM27 significantly increases CRC cell
proliferation and colony formation in LoVo and HCT116 cells
CCK-8 and colony formation assays were used to
detect the effect of TRIM27 on the proliferation of CRC cells. It
was found that the knockdown of TRIM27 significantly
inhibited cell proliferation in LoVo cells (Fig. 3A). By contrast, cell proliferation
was promoted in HCT116 cells overexpressing TRIM27 (Fig. 3B). Consistently, in the colony
formation assay, it was found that LoVo cells transfected with the
siRNA showed reduced formation of colonies (Fig. 3C), whereas HCT116 cells transfected
with the TRIM27-overexpressing plasmid showed the opposite result
(Fig. 3D).
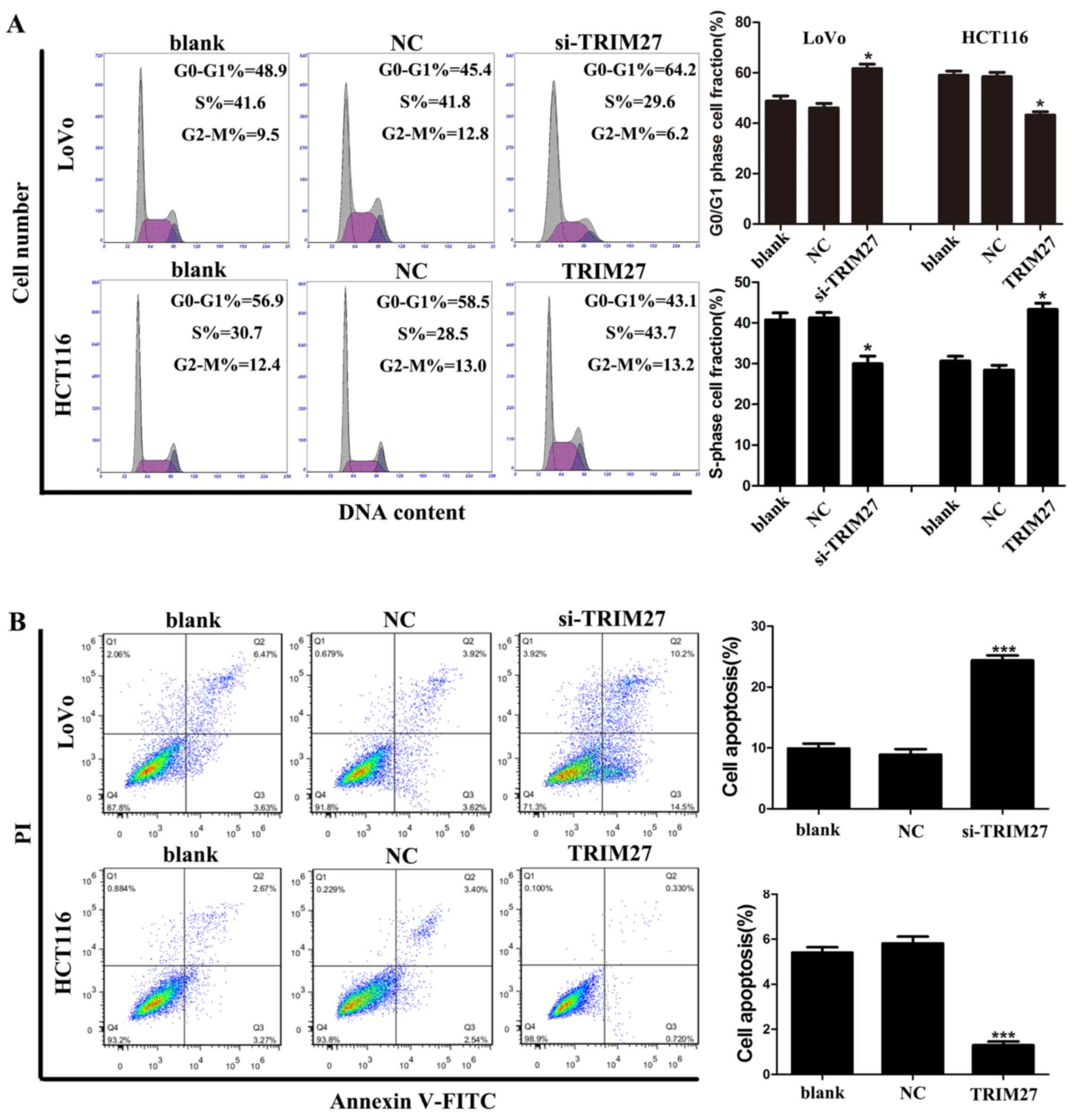
TRIM27 promotes apoptosis resistance and
cell cycle G0-G1/S transition in CRC cells
Flow cytometry was used to investigate whether
TRIM27 regulates apoptosis and the cell cycle of CRC cells. It was
found that the LoVo cells transfected with the siRNA showed a
significant increase in the percentage of cells in the G0-G1 phase
and a reduced percentage of cells in the S phase, whereas HCT116
cells transfected with the TRIM27-overexpressing plasmid showed the
reverse result (Fig. 4A).
Similarly, LoVo cells transfected with the siRNA showed a
significant increase in the percentage of apoptotic cells, whereas
HCT116 cells transfected with the TRIM27-overexpressing plasmid
showed fewer apoptotic cells (Fig.
4B). Taken together, these results suggested that TRIM27
promoted the proliferation of CRC cells by promoting apoptosis
resistance and cell cycle transition in the G0-G1/S phase.
Regulation of TRIM27 influences the
invasion and metastasis of CRC cells in vitro
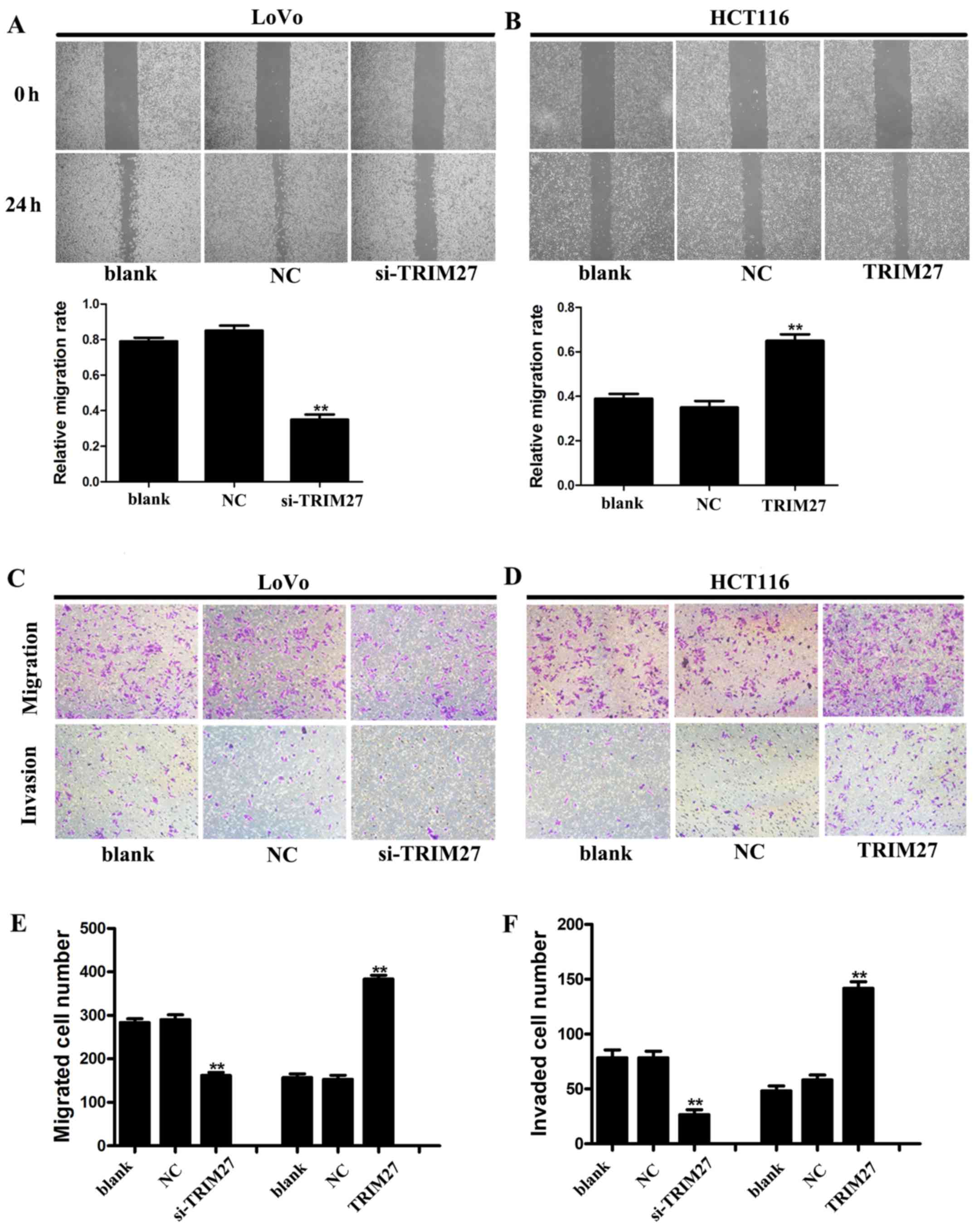
Wound-healing and Transwell assays were used to
detect the metastasis and invasiveness in LoVo and HCT116 cells. In
the wound-healing assay, it was found that the wound area was wider
in LoVo cells transfected with siRNA (Fig. 5A), but narrower in HCT116 cells
transfected with the TRIM27-overexpressing plasmid (Fig. 5B). In the Transwell assay, the
numbers of cells that traversed the Transwell and Matrigel were
significantly reduced by TRIM27 knockdown in the LoVo cells,
whereas the overexpression of TRIM27 in HCT116 cells increased the
number of cells that traversed the Transwell and Matrigel (Fig. 5C–F). These results suggested that
TRIM27 promoted the invasion and metastasis of CRC cells.
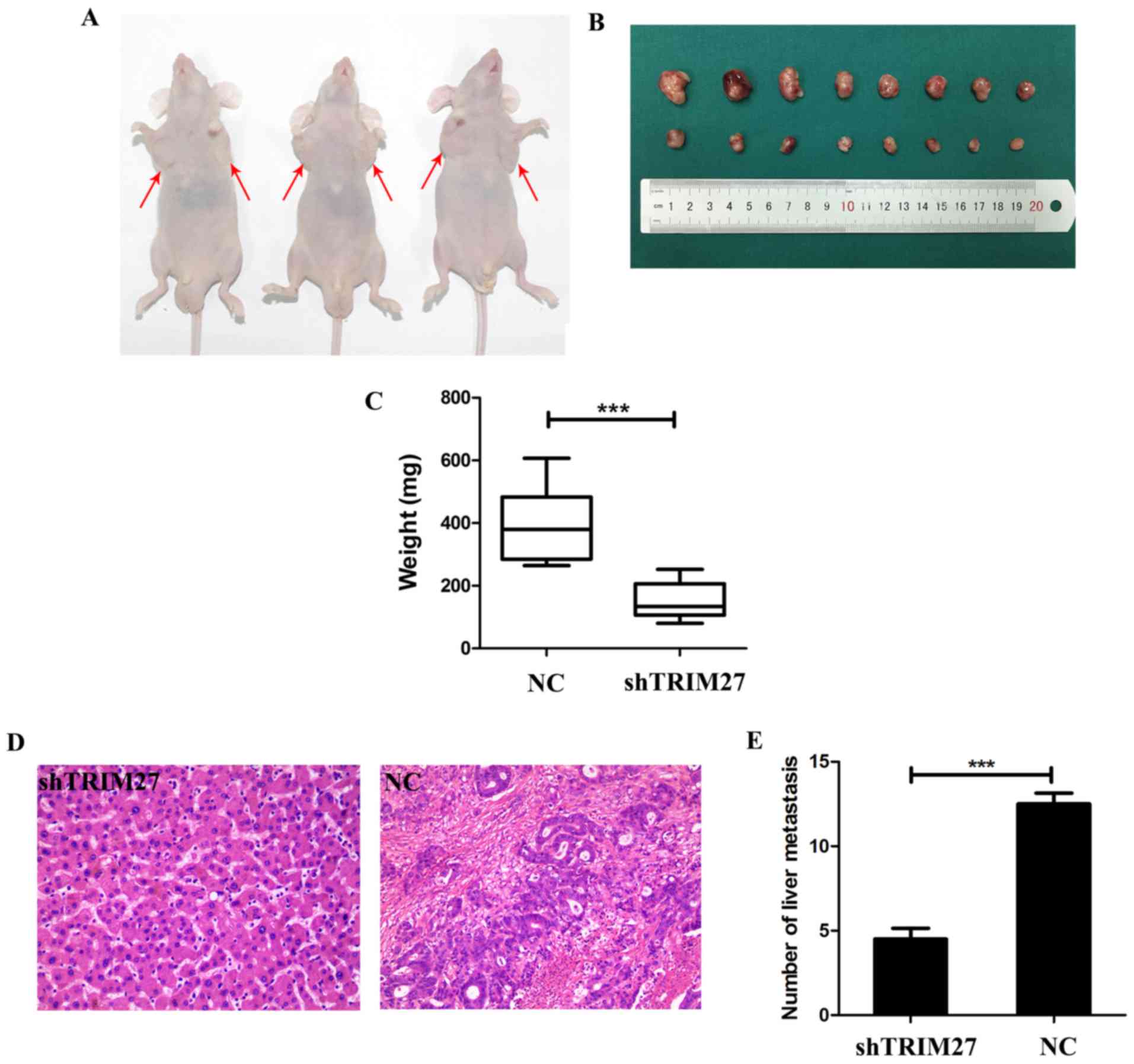
Downregulation of TRIM27 significantly
suppresses tumor proliferation and metastasis in nude mice
To determine whether TRIM27 affects tumor growth
in vivo, tumor xenografting was performed in nude mice. At 4
weeks post-implantation, the tumor size (Fig. 6A and B) and weight (Fig. 6C) was significantly decreased in
the group treated with shTRIM27 compared with the untreated
control. To further investigate whether TRIM27 affects tumor
metastasis in vivo, a tail vein metastatic assay was
performed in nude mice using LoVo cells transfected with shTRIM27.
At 7 weeks post-injection, the results of hematoxylin and eosin
staining showed that the knockdown of TRIM27 correlated
closely with reduced liver metastasis (Fig. 6D and E). These in vivo
results further confirmed that TRIM27 promoted proliferation and
metastasis in CRC.
TRIM27 promotes the process of EMT and
activation of p-AKT in CRC cells
To further detect whether TRIM27 was associated with
the EMT process, the levels of EMT-associated proteins in LoVo and
HCT116 cells were evaluated using western blot analysis. Following
TRIM27 knockdown, it was found that the level of the
epithelial marker E-cadherin increased, and the level of the
mesenchymal marker N-cadherin decreased. The opposite effect was
observed in HCT116 cells transfected with the TRIM27-overexpressing
plasmid (Fig. 7). Further
investigation revealed that the knockdown of TRIM27
decreased the level of vimentin in LoVo cells; however, the level
of vimentin increased in HCT116 cells following the overexpression
of TRIM27 (Fig. 7). These results
suggested that TRIM27 promoted EMT in CRC cells. Additionally, the
levels of p-AKT and AKT in LoVo and HCT116 cells were detected. It
was found that the level of p-AKT increased when TRIM27 was
overexpressed, but decreased when TRIM27 was knocked down.
However, no significant changes occurred in the expression of AKT,
suggesting that TRIM27 promoted the activation of p-AKT (Fig. 7).
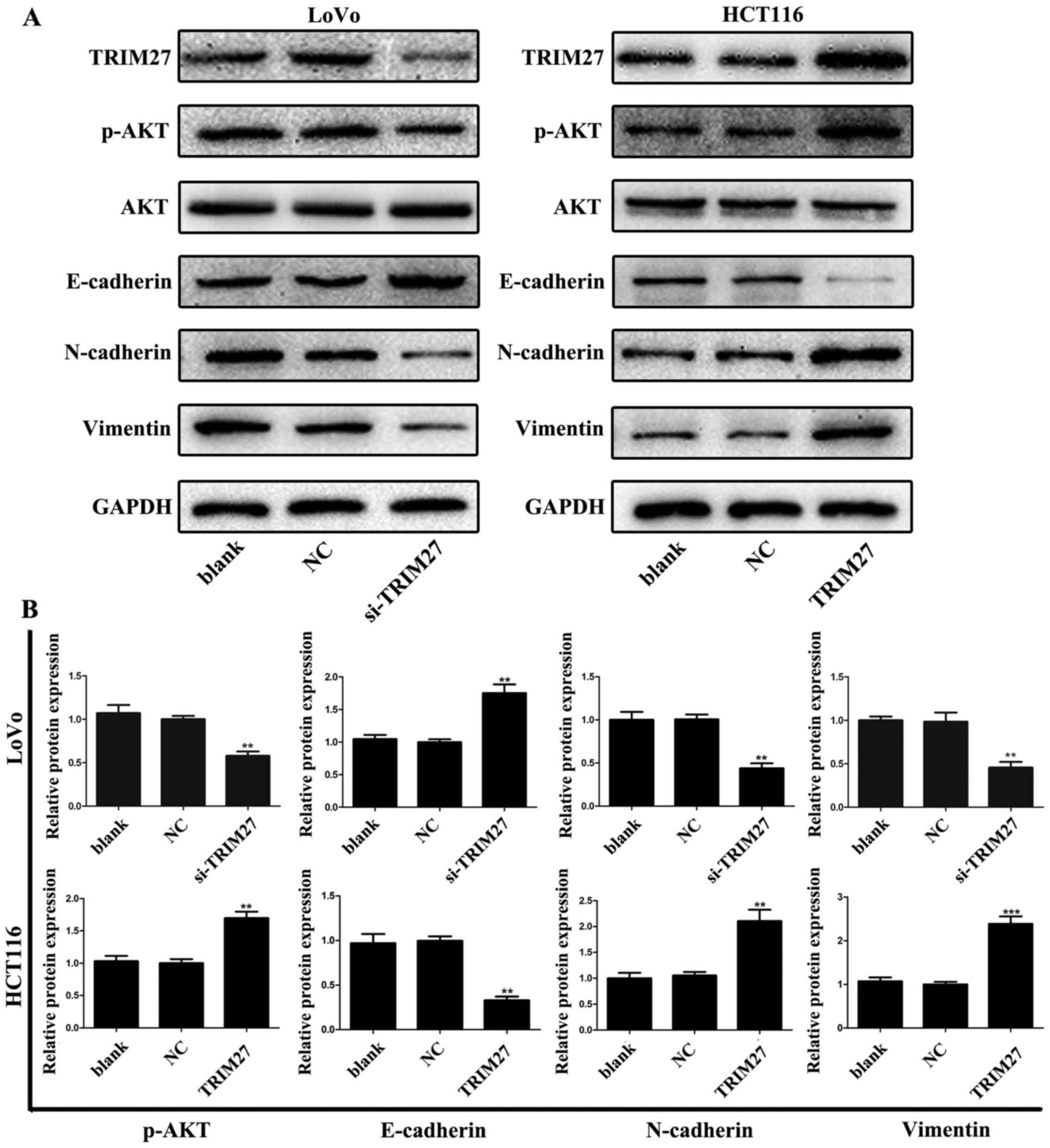 | Figure 7TRIM27 promotes the activation of
p-AKT and the epiethlium-mesenchymal transition process in
colorectal cancer cells. (A) Expression levels of p-AKT, AKT,
E-cadherin, N-cadherin and vimentin in LoVo and HCT116 cells
following TRIM27 knockdown and overexpression were detected using
western blot analysis. (B) Relative protein levels of p-AKT,
E-cadherin, N-cadherin and vimentin were quantified in LoVo and
HCT116 cells. Data are presented as the mean ± standard deviation
from three independent experiments; **P<0.01 and
***P<0.001, compared with controls. TRIM27,
tripartite motif-containing 27; si-TRIM27, small interference RNA
target TRIM27; p-, phosphorylated; NC, negative control
sequence. |
Discussion
The TRIM protein family has been recognized to
comprise regulatory proteins in a variety of tumors, some of which
have been reported to be associated with CRC (25–28).
TRIM27, which belongs to the TRIM family, has been reported to be
involved in various tumor processes. For example, in ovarian
cancer, Ma et al showed that TRIM27 knockdown induced
cell cycle arrest and apoptosis by upregulating the expression of
p-p38 and downregulating the level of p-AKT (13). In endometrial cancer, Tsukamoto
et al and Tezel et al revealed that TRIM27
knockdown significantly impaired cancer cell migration and
invasion, with concomitant decreases in levels of integrin b1 and
a2 (14,29). In lung cancer, Iwakoshi et
al found that TRIM27 was involved in mutated epidermal growth
factor receptor (EGFR) signaling and can be a prognostic factor for
lung cancer with EGFR mutations (16). All of the above studies suggested
that TRIM27 acts as an oncogene in facilitating tumor
proliferation and metastasis in various tumors.
The first step of the present study involved
determining whether TRIM27 was upregulated in CRC tissues, with
further analysis to determine whether it was associated with
patient prognosis. The results suggested that TRIM27 was
significantly upregulated in CRC tissues, and a higher expression
level of TRIM27 predicted a poorer prognosis, which was in
accordance with the conclusion of Kato et al and
Zoumpoulidou et al (18,19).
The present study further showed that a higher expression level of
TRIM27 predicted deeper invasion, increased lymph node metastasis,
higher tumor stage, and increased distant metastases in CRC
tissues. This suggested that TRIM27 is an oncogene in CRC.
To test this hypothesis, the expression of TRIM27 was detected in
CRC cells, and the results showed that TRIM27 was upregulated in
CRC cells. Further investigation revealed that TRIM27 significantly
promoted cell proliferation in vivo and in vitro,
possibly by reducing cell apoptosis and promoting cell cycle G1/S
transition. In addition, the results of wound-healing and Transwell
assays showed that TRIM27 promoted invasion and metastasis, which
was further supported by the results of tail vein metastasis assays
in mice. Therefore, it was concluded that TRIM27 has an oncogenic
role by promoting the proliferation, invasion, and metastasis of
CRC cells.
EMT is a transformation in which epithelial cells
break down cell-cell and cell-extracellular matrix connections
(30). This process is considered
to be closely associated with tumor invasion and metastasis in
various types of cancer, and the overall process is always
accompanied by decreased expression of epithelial markers and
increased expression of mesenchymal markers (31,32).
Although an association between EMT and TRIM27 has not been
reported until now, other TRIM family members, including TRIM14,
TRIM16 and TRIM59 have been reported to regulate EMT (28,33,34).
Therefore, the present study assessed a series of proteins
associated with EMT using western blot analysis. The results
suggested that TRIM27 significantly promoted EMT, which verified
the above hypothesis. The activation of AKT has been reported to
transduce signals to regulate multiple biological processes,
including cellular proliferation, survival, growth, angiogenesis,
migration and EMT, in various types of cancer (35). In addition, Ma et al
revealed that TRIM27 knockdown inhibited the expression of
p-AKT in ovarian cancer (13).
This suggested that TRIM27 may also be associated with the
expression of p-AKT in CRC, and the results of the present study
supported this. Therefore, it was hypothesized that TRIM27-mediated
EMT may be promoted by the activation of p-AKT. However, the exact
association between TRIM27, EMT, and p-AKT in promoting the
proliferation, invasion and metastasis of CRC remains to be
elucidated. Further in-depth investigations are required, with a
focus on evaluating the role of TRIM27 in CRC.
In conclusion, the present study demonstrated that
TRIM27 was significantly upregulated in CRC tissues, which
indicated the TRIM27 acts as an oncogenic protein, and its
expression level predicts poor prognosis in patients with CRC.
Furthermore, TRIM27 promoted proliferation, invasion and
metastasis, possibly by promoting EMT and the activation of p-AKT
in CRC cells. Therefore, TRIM27 represents a potential prognostic
and therapeutic target in CRC. Further investigations are required
to determine the detailed mechanisms underlying the effects of
TRIM27 in CRC.
Funding
This study was supported in part by the Jiangsu Key
Medical Discipline (General Surgery) (grant no. ZDXKA2016005).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
YS, ZF, YZ and YF conceived and designed the study.
YZ, DJ, SW and WQ performed the experiments and acquired data. YF
and CZ carried out the patient follow-up. DJ, QW, BJ and ZZ
analyzed and interpreted the data. YZ, BJ and CZ performed the
statistical analysis. YZ and YF drafted and edited the manuscript.
All authors have given final approval of the version to be
published.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Nanjing Medical
University (ethics no. 2010-SR-091.A1).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Hao Fan and Dr
Yuanguangyan Zhang (the First Affiliated Hospital of Nanjing
Medical University, Jiangsu, China) for providing language and
technology support.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar
|
|
3
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar
|
|
4
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar
|
|
5
|
Micale L, Chaignat E, Fusco C, Reymond A
and Merla G: The tripartite motif: Structure and function. Adv Exp
Med Biol. 770:11–25. 2012. View Article : Google Scholar
|
|
6
|
Miyamoto K, Nakamura N, Kashiwagi M, Honda
S, Kato A, Hasegawa S, Takei Y and Hirose S: RING finger, B-box,
and coiled-coil (RBCC) protein expression in branchial epithelial
cells of Japanese eel, Anguilla japonica. Eur J Biochem.
269:6152–6161. 2002. View Article : Google Scholar
|
|
7
|
Meroni G and Diez-Roux G: TRIM/RBCC, a
novel class of ‘single protein RING finger’ E3 ubiquitin ligases.
BioEssays. 27:1147–1157. 2005. View Article : Google Scholar
|
|
8
|
Nisole S, Stoye JP and Saïb A: TRIM family
proteins: Retroviral restriction and antiviral defence. Nat Rev
Microbiol. 3:799–808. 2005. View Article : Google Scholar
|
|
9
|
Takahashi M, Ritz J and Cooper GM:
Activation of a novel human transforming gene, ret, by DNA
rearrangement. Cell. 42:581–588. 1985. View Article : Google Scholar
|
|
10
|
Takahashi M and Cooper GM: ret
transforming gene encodes a fusion protein homologous to tyrosine
kinases. Mol Cell Biol. 7:1378–1385. 1987. View Article : Google Scholar
|
|
11
|
Takahashi M, Inaguma Y, Hiai H and Hirose
F: Developmentally regulated expression of a human
‘finger’-containing gene encoded by the 5′ half of the ret
transforming gene. Mol Cell Biol. 8:1853–1856. 1988. View Article : Google Scholar
|
|
12
|
Fagerberg L, Hallström BM, Oksvold P,
Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S,
Danielsson A and Edlund K: Analysis of the human tissue-specific
expression by genome-wide integration of transcriptomics and
antibody-based proteomics. Mol Cell Proteomics. 13:397–406. 2014.
View Article : Google Scholar
|
|
13
|
Ma Y, Wei Z, Bast RC Jr, Wang Z, Li Y, Gao
M, Liu Y, Wang X, Guo C, Zhang L, et al: Downregulation of TRIM27
expression inhibits the proliferation of ovarian cancer cells in
vitro and in vivo. Lab Invest. 96:37–48. 2016. View Article : Google Scholar
|
|
14
|
Tsukamoto H, Kato T, Enomoto A, Nakamura
N, Shimono Y, Jijiwa M, Asai N, Murakumo Y, Shibata K and Kikkawa
F: Expression of Ret finger protein correlates with outcomes in
endometrial cancer. Cancer Sci. 100:1895–1901. 2009. View Article : Google Scholar
|
|
15
|
Tezel GG, Uner A, Yildiz I, Guler G and
Takahashi M: RET finger protein expression in invasive breast
carcinoma: Relationship between RFP and ErbB2 expression. Pathol
Res Pract. 205:403–408. 2009. View Article : Google Scholar
|
|
16
|
Iwakoshi A, Murakumo Y, Kato T, Kitamura
A, Mii S, Saito S, Yatabe Y and Takahashi M: RET finger protein
expression is associated with prognosis in lung cancer with
epidermal growth factor receptor mutations. Pathol Int. 62:324–330.
2012. View Article : Google Scholar
|
|
17
|
Horio M, Kato T, Mii S, Enomoto A, Asai M,
Asai N, Murakumo Y, Shibata K, Kikkawa F and Takahashi M:
Expression of RET finger protein predicts chemoresistance in
epithelial ovarian cancer. Cancer Med. 1:218–229. 2012. View Article : Google Scholar
|
|
18
|
Kato T, Shimono Y, Hasegawa M, Jijiwa M,
Enomoto A, Asai N, Murakumo Y and Takahashi M: Characterization of
the HDAC1 complex that regulates the sensitivity of cancer cells to
oxidative stress. Cancer Res. 69:3597–3604. 2009. View Article : Google Scholar
|
|
19
|
Zoumpoulidou G, Broceño C, Li H, Bird D,
Thomas G and Mittnacht S: Role of the tripartite motif protein 27
in cancer development. J Natl Cancer Inst. 104:941–952. 2012.
View Article : Google Scholar
|
|
20
|
Li L and Li W: Epithelial-mesenchymal
transition in human cancer: Comprehensive reprogramming of
metabolism, epigenetics, and differentiation. Pharmacol Ther.
150:33–46. 2015. View Article : Google Scholar
|
|
21
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar
|
|
22
|
Xu W, Yang Z and Lu N: A new role for the
PI3K/Akt signaling pathway in the epithelial-mesenchymal
transition. Cell Adhes Migr. 9:317–324. 2015. View Article : Google Scholar
|
|
23
|
Benson AB III, Venook AP, Bekaii-Saab T,
Chan E, Chen YJ, Cooper HS, Engstrom PF, Enzinger PC, Fenton MJ,
Fuchs CS, et al: National Comprehensive Cancer Network: Colon
cancer, version 3.2014. J Natl Compr Canc Netw. 12:1028–1059. 2014.
View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Xu W, Xu B, Yao Y, Yu X, Cao H, Zhang J,
Liu J and Sheng H: RNA interference against TRIM29 inhibits
migration and invasion of colorectal cancer cells. Oncol Rep.
36:1411–1418. 2016. View Article : Google Scholar
|
|
26
|
Tan Z, Liu X, Yu E, Wang H, Tang L, Wang H
and Fu C: Lentivirus-mediated RNA interference of tripartite motif
68 inhibits the proliferation of colorectal cancer cell lines
SW1116 and HCT116 in vitro. Oncol Lett. 13:2649–2655. 2017.
View Article : Google Scholar
|
|
27
|
Lee OH, Lee J, Lee KH, Woo YM, Kang JH,
Yoon HG, Bae SK, Songyang Z, Oh SH and Choi Y: Role of the focal
adhesion protein TRIM15 in colon cancer development. Biochim
Biophys Acta. 1853.409–421. 2015.
|
|
28
|
Sun Y, Ji B, Feng Y, Zhang Y, Ji D, Zhu C,
Wang S, Zhang C, Zhang D and Sun Y: TRIM59 facilitates the
proliferation of colorectal cancer and promotes metastasis via the
PI3K/AKT pathway. Oncol Rep. 38:43–52. 2017. View Article : Google Scholar
|
|
29
|
Tezel GG, Ordulu Z, Hımmetoğlu C and
Usubütün A: The selective expression of ret finger protein in
endometrial cancer: Can RFP be a marker of serous carcinomas. Turk
Patoloji Derg. 28:213–219. 2012.
|
|
30
|
Radisky DC and LaBarge MA:
Epithelial-mesenchymal transition and the stem cell phenotype. Cell
Stem Cell. 2:511–512. 2008. View Article : Google Scholar
|
|
31
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar
|
|
32
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar
|
|
33
|
Guo Xu G, Xu Y, Wang D, Shen Y, Wang Y, Lv
F, Song Y, Jiang F and Zhang DY: TRIM14 regulates cell
proliferation and invasion in osteosarcoma via promotion of the AKT
signaling pathway. Sci Rep. 7:424112017. View Article : Google Scholar
|
|
34
|
Tan H, Qi J, Chu G and Liu Z: Tripartite
motif 16 inhibits the migration and invasion in ovarian cancer
cells. Oncol Res. 25:551–558. 2017. View Article : Google Scholar
|
|
35
|
Suman S, Kurisetty V, Das TP, Vadodkar A,
Ramos G, Lakshmanaswamy R and Damodaran C: Activation of AKT
signaling promotes epithelial-mesenchymal transition and tumor
growth in colorectal cancer cells. Mol Carcinog. 53(Suppl 1):
E151–E160. 2014. View Article : Google Scholar
|