Introduction
Pancreatic cancer is a lethal malignant neoplasm,
and its morbidity and mortality rates have not noticeably
decreased, despite advances in pancreatic cancer treatment
strategies. Patients suffering from early-stage pancreatic cancer
can be treated by surgery, with a relatively positive outcome.
However, the majority of patients are diagnosed at an advanced
stage of pancreatic cancer. Recently, clinical trials examining
chemotherapy to cure pancreatic cancer have been undertaken to
identify a more effective method with which to prolong the life
expectancy of patients; however, the long-term curative effects and
better choices of different chemotherapeutic combinations remain
uncertain (1–3). Moreover, early metastasis is another
non-negligible cause of a poor outcome for patients with pancreatic
cancer (4). Thus, elucidating the
mechanisms responsible for metastasis is probably an important
strategy for the treatment of pancreatic cancer.
Wiskott-Aldrich syndrome protein family
verprolin-homologous protein 3 (WAVE3) is in the WASP/WAVE family
of actin cytoskeleton remodeling proteins. Some researchers have
demonstrated that WAVE3 is closely related to cell cytokinesis,
motility and proliferation (5).
WAVE3 is overexpressed in certain types of cancer, including
ovarian cancer (6), breast cancer
(7), prostate cancer (8), hepatocellular carcinoma (9), gastric cancer (10), and colorectal cancer (11). High expression levels of WAVE3 are
associated with stronger capabilities for migration and invasion in
some cancer types, and researchers have discovered that WAVE3
promotes the epithelial-mesenchymal transition (EMT) process to
enhance the metastatic potential of certain types of cancer
(10,12). Moreover, WAVE3 has also been shown
to be associated with cell survival in certain types of cancer
(6,8,10–12).
The mechanisms through which WAVE3 influences the biological
properties of certain types of cancer have been examined in a few
studies. For example, WAVE3 has been shown to affect matrix
metalloproteinases (MMPs), mitogen-activated protein kinase (MAPK)
and Snail (6,8,10,11,13).
Thus, WAVE3 enhances the proliferative, migratory and invasive
abilities of cells in certain types of cancer, and similarities and
differences exist in the underlying mechanisms. However, whether
WAVE3 affects pancreatic cancer and the mechanisms through which it
affects the biological characteristics of pancreatic cancer have
not yet been determined.
Phosphatidylinositol 3-kinase (PI3K) and protein
kinase B (PBK/AKT) are the key proteins in the AKT pathway. This
pathway is regulated by multiple mechanisms and is related to a
range of diseases, particularly cancer, and pancreatic cancer is no
exception. Activated AKT is involved in the proliferative, cycle,
growth, survival (also known as anti-apoptosis), migratory and
invasive abilities of cells. Phosphoinositide-dependent kinase
(PDK1) partially activates AKT via the phosphorylation of T308, and
the phosphorylation of S473 by phosphoinositide-dependent kinase 2
(PDK2) is needed for full activation (14,15).
In this study, we focused on determining the effects
of WAVE3 on the biological behavior of pancreatic cancer and aimed
to elucidate the underlying mechanisms of the effect of WAVE3 on
pancreatic cancer. The findings of this study may aid in the
development of novel treatment strategies targeting WAVE3 and/or
the AKT pathway for pancreatic cancer.
Materials and methods
Patients and samples
A cumulative total of 87 pairs of pancreatic cancer
tissues and pancreatic cancer-adjacent non-cancerous samples were
collected from patients that underwent radical surgery at the First
Affiliated Hospital of Sun Yat-Sen University (Guangzhou, China)
from January, 2014 to December, 2015. The patients with pancreatic
cancer who suffered from other types of cancer or who received
chemotherapy and/or radiotherapy prior to surgery were excluded.
Tumor-node-metastasis staging was assessed according to the Cancer
Staging Manual (7th edition) of the American Joint Committee on
Cancer (16). The tumor grade was
detected by pathological sectioning. Overall survival was defined
as the period from the date of surgery to the date of death (or the
last follow-up). We obtained follow-up survival information by
telephone from 79 of the 87 pancreatic cancer patients following
surgery. Die to the lack of contact, we were not able to follow-up
the remaining 8 patients. The study was approved by the Institute
Research Ethics Committee of the First Affiliated Hospital at Sun
Yat-Sen University. As this study is a retrospective study, the
patient consent was allowed to be waived by the ethics
committee.
Immunohistochemistry
The paraffin-embedded tissue sections were first
baked in an oven at 60°C for 2 h before being immediately
deparaffinized with xylene and rehydrated with diminishing
concentrations of alcohol. The endogenous peroxidase activity in
the tissues was then blocked with 3% H2O2 at
room temperature for 15 min. Antigen retrieval was performed at a
high temperature in saline sodium citrate (pH 6.0) for 15 min.
After slow cooling, the tissue sections were stained with an
anti-WAVE3 antibody (1:200, 2806S; Cell Signaling Technology,
Danvers, MA, USA) at 4°C overnight. After washing with
phosphate-buffered saline (PBS), the sections were incubated with
HRP-labeled goat anti-rabbit IgG for 15 min at room temperature
(Beyotime, Shanghai, China), stained with the DAB+ Staining Kit
(Gene Tech, Shanghai, China) and counterstained with hematoxylin.
Two experienced pathologists independently scored the magnitude of
the staining according to the staining intensity and the area that
was positive for staining. The score of the positive staining
intensity was defined according to the following standards
(17): Zero points was assigned to
no positive staining, one point was assigned to a light yellow
color (weak staining), two points were assigned to a yellow-brown
color (moderate staining), and three points were assigned to a
brown color (strong staining). For the area of positive staining,
tumor cells without positive staining were awarded zero points,
those with 30% positive staining were awarded one point, those with
30–60% positive staining were awarded two points, and those with
>60% positive staining were awarded three points. Multiplying
the staining intensity score by the score for the positively
stained area provided the final immunohistochemistry staining
score. The results could then be distinguished by a score of 0, 1,
2, 3, 4, 6 or 9. The median of the scores was statistically
analyzed (approximately 4.3), which was equal to 4. Thus, the
cut-off value was 4. Finally, an immunohistochemistry staining
score ≤4 was defined as a tissue with a low WAVE3 expression, while
scores >4 were defined as having a high WAVE3 expression.
Cells and cell culture
Five pancreatic cancer cell lines (Panc-1, BxPC-3,
HPAF-II, SW-1990 and CFPAC-1) and a non-neoplastic human pancreatic
ductal epithelial cell line (hTERT-HPNE) were cultured for use in
the following experiments. The Panc-1 cells were cultured from an
adenocarcinoma at the head of the pancreas, which has been
demonstrated to have a better migratory and invasive ability. The
CFPAC-1 cells were obtained from a liver metastasis that has been
demonstrated to be well-differentiated (18). All the pancreatic cancer cell lines
were purchased from the Chinese Academy of Sciences (Beijing,
China). The hTERT-HPNE cells were obtained from the prestigious
Professor Huang Peng at the Sun Yat-Sen University Cancer Center.
The Panc-1 and hTERT-HPNE cells were maintained in DMEM
supplemented with 10% FBS (Gibco/Thermo Fisher Scientific, Waltham,
MA, USA), 100 U/ml penicillin and streptomycin; the CFPAC-1 cells
were maintained in Iscove’s modified Dulbecco’s medium IMDM
supplemented with 10% FBS, 100 U/ml penicillin and streptomycin;
the BxPC-3 cells were maintained in RPMI-1640 supplemented with 10%
FBS, 100 U/ml penicillin and streptomycin; the HPAF-II cells were
maintained in Eagle’s minimal essential medium (EMEM) supplemented
with 10% FBS, 100 U/ml penicillin and streptomycin; and the SW-1990
cells were maintained in L-15 supplemented with 10% FBS, 100 U/ml
penicillin and streptomycin. All the cell lines were cultured at
37°C in a humidified incubator with 5% CO2 and used for
experiments during the logarithmic growth phase.
Cell transfection
The cells were cultivated in 6-well plates and
transfected with WAVE3 siRNA or a negative control siRNA using
Lipofectamine® 3000 Transfection reagent (Invitrogen,
Carlsbad, CA, USA) following the manufacturer’s instructions.
Briefly, the cells were cultured in an incubator at 37°C, and after
24 h, the cellular proteins were extracted or certain functional
experiments were performed (Transwell assays, plate clone formation
assay, CCK-8 test, flow cytometry and co-immunoprecipitation).
Three candidate WAVE3 siRNA sequences were assessed for the highest
interference efficiency by western blot analysis. The WAVE3 siRNA
sequences were WAVE3-1 (GGU UUC AAA GAA CAG CAU UTT), WAVE3-2 (GCA
AAC AUG CUG AAG ACA UTT), and WAVE3-3 (GCU CUG CCU GAA GGG AUU
ATT). The negative control siRNA sequence was sense: UUC UCC GAA
CGU GUC ACG UTT. The above-mentioned siRNA sequences were designed
by Shanghai GenePharma Co., Ltd. (Shanghai, China).
Western blot analysis
The cells were lysed with a radioimmunoprecipitation
assay (RIPA) lysis buffer and PMSF (both from Cell Signaling
Technology) for 30 min using a RIPA to PMSF volume ratio of 100:1.
For p-AKT 308 and p-AKT 473, the cells were also lysed with
Phosphatase Inhibitor Cocktail I (MedChemExpress, Monmouth
Junction, NJ, USA). A BCA Protein Assay kit (Thermo Scientific
Pierce, Rockford, IL, USA) was used to measure the total protein
concentrations. Aliquots (40 µg) of total cellular proteins
were resolved by SDS-PAGE (5–12%), electrotransferred onto PVDF
membranes and then blocked with 5% skim milk (w/v) at room
temperature for 1 h. The membranes were then incubated with primary
antibodies against WAVE3 (1:1,000, 2806S), PI3K (1:1,000, 4249S),
AKT (1:1,000, 4691S), p-AKT 308 (1:1,000, 13038S), p-AKT 473
(1:1,000, 4060S) (all from Cell Signaling Technology), PDK2
(1:1,000, ab68164; Abcam, Cambridge, MA, USA), E-Cadherin (1:1,000,
3195S), N-Cadherin (1:1,000, 13116S), Slug (1:500, 9585S), Snail
(1:500, 3879S), Vimentin (1:1,000, 5741S), zincfinger Ebox binding
homeobox 1 (ZEB1; 1:1,000, 3396S), p53 (1:500, 2527S), Bcl-2
(1:500, 4223S) (all from Cell Signaling Technology), cyclin D1
(1:20,000, ab134175; Abcam) and GAPDH (1:6,000, G8795;
Sigma-Aldrich), which served as an internal control on an orbital
shaker at 4°C overnight, and the secondary antibodies (HRP-labeled
goat-anti-mouse (1:1,000, 14709S) and HRP-labeled goat-anti-rabbit
(1:1,000, 14708S) (both from Cell Signaling Technology) were added,
and the mixtures were incubated for 1 h at room temperature. The
protein-antibody complexes were then detected by chemiluminescence
(Pierce ECL Western Blotting Substrate; Thermo Scientific
Pierce).
Cell growth curve
The cell lines seeded in medium with 10% FBS were
added to 96-well plates at a density of 2×103 per well.
Each cell line consisted of 6 replicate wells per plate and 4
plates. Following incubation at 37°C for 24, 48, 72 and 96 h, the
cell viability in each well was determined using Cell Counting kit
(CCK)-8 (Beyotime). The absorbance was then recorded at 450 nm with
a 96-well plate reader (Thermo Fisher Scientific).
Plate clone-forming assay
The cells were cultured with medium containing 10%
FBS in 6-well plates at a density of 500 cells per well at 37°C in
5% CO2. After 14 days, the cell clones were washed with
PBS, fixed in 4% formaldehyde for 15 min, and stained with crystal
violet. The number of stained cell clones with diameters larger
than 1 mm were counted and analyzed as previously described.
Cell apoptosis and cell cycle
analysis
According to the instructions supplied with the eGFP
Annexin V and PI Apoptosis kit (GeneCopoeia, Inc., Rockville, MD,
USA), eGFP Annexin V and PI double staining and flow cytometry were
conducted to analyze cell apoptosis. The pancreatic cancer cell
lines (Panc-1 and CFPAC-1) were plated in 6-well plates
(1×106 cells/well) and harvested after a 24 h of
incubation. The cells were washed with PBS at pH 7.4 and
centrifuged at 110 × g for 5 min, 3 times. Single-cell suspensions
were then resuspended in 500 µl of binding buffer containing
5 µl of eGFP Annexin V and 2 µl of PI in the dark for
15 min. Cell apoptosis was analyzed by flow cytometry using FlowJo
7.6 (Tree Star, Inc., Ashland, OR, USA) software.
The cells were collected as described
above
After washing 3 times with PBS, the cells were
resuspended in cold PBS, fixed in 70% ethanol, and stored at 4°C
for 30 min. The cells were centrifuged at 110 × g for 5 min,
resuspended in 500 µl of PI (50 µg/ml) and then
incubated on ice in the dark for 15 min. Cell cycle analysis was
conducted by flow cytometry and analyzed using ModFit LT 4.1
(Verity Software House, Topsham, ME, USA) software.
Wound healing assays
The cells were cultivated in 6-well plates at a
density of 1×106 cells per well. The cells were cultured
in medium containing 10% FBS for 24 h at 37°C and 5%
CO2. A scratch wound was then created by introducing a
vertical line on the cell surface with a 200-µl plastic
pipette tip. Cell debris was removed by washing 3 times with PBS,
followed by the addition of medium containing 1% FBS and mitomycin
C (MedChemExpress). A region with a defined area within the scratch
was imaged using a microscope equipped with a digital camera at one
(0 h), 24 and 48 h. The length of the scratch was measured using
Image-Pro Plus 6.0. The migration rate was calculated as follows:
(% at 0 h) = (D0−DT)/D0 ×100% (D0, distance measured at 0 h; DT,
distance measured at 24 or 48 h).
Transwell migration and invasion
assays
The tumor cell migration and invasion potentials
were determined using Transwell® chambers (8 µm;
Corning, Corning, NY, USA, BioCoat Control Cell Culture Inserts and
BioCoat Matrigel Invasion Chambers). Three independent experiments
were conducted under the same conditions. The upper Boyden
chambers, containing serum-free medium, were seeded with
2×105 cells (for migration), while 5×105
cells were seeded in the upper membranes with serum-free medium
(for invasion). Subsequently, 600 µl of medium containing
20% FBS was added to the lower chamber. The cells were cultured in
a 37°C incubator with 5% CO2. Twenty-four hours later,
the cells were washed 3 times with PBS and fixed in 4% formaldehyde
for 10 min. Non-invading cells in the upper chamber (for migration)
or on the upper membrane (for invasion) were removed carefully with
a cotton swab, and the cells were then washed again 3 times with
PBS. The cells that adhered to the lower surface of the upper
chamber (for migration) or the upper membrane (for invasion) were
stained with 0.1% crystal violet. Ten randomly selected fields of
fixed cells at ×200 magnification were captured using the
microscope equipped with a digital camera, and the numbers of cells
that were stained purple were counted. The numbers of cells in the
different groups were statistically analyzed as previously
described.
Statistical analysis
All statistical analyses were conducted using SPSS
for Windows, version 20.0 (SPSS, Inc., Chicago, IL, USA). The
significance of the results obtained for the different groups was
analyzed by the Student’s t-test and one-way analysis of variance
(ANOVA) followed by appropriate post hoc tests (Fishers’ Least
Significant Difference). Differences between categorical variables
were compared using the Chi square test, while when the number of
variables was <5, Fisher’s exact test was used to detect the
difference. The survival curve was calculated using the
Kaplan-Meier method and analyzed with the log-rank test using
GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). In all
the statistical analyses, statistical significance was set as
P<0.05.
Results
Association between WAVE3 expression and
the clinicopathological characteristics of patients with pancreatic
cancer
To determine the role of WAVE3 protein expression in
the development of pancreatic cancer, the association between the
clinicopathological characteristics of the patients with pancreatic
cancer and WAVE3 protein expression was analyzed. The data
presented in Table I demonstrate
that a high expression level of WAVE3 was associated with lymphatic
metastasis (P<0.05), poorly differentiated tumors (P<0.05)
and high pre-operative CA19-9 levels (P<0.05). There were no
obvious associations between WAVE3 protein expression and sex
(P=0.2535), age (P=0.2555), distant metastasis (P=0.2775),
pre-operative CA125 (P=0.5564) and CEA (P=0.7736) levels, smoking
history (P=0.4193), history of alcohol consumption (P=0.5747) or
clinical stage (P=0.7469), while in clinical stage II, there was no
evidence that single tumors with vascular invasion (T2a) or
multiple tumors with or without vascular invasion (T2b) were
related to WAVE3 expression (P=0.2833).
 | Table IAssociation of WAVE3 expression with
the clinicopathological characteristics of the patients with
pancreatic cancer. |
Table I
Association of WAVE3 expression with
the clinicopathological characteristics of the patients with
pancreatic cancer.
|
Characteristics | WAVE3 expression
| P-valuea |
|---|
| High | Low |
|---|
| Sex | | | 0.2535 |
| Male | 31 | 24 | |
| Female | 22 | 10 | |
| Ageb | | | 0.2555 |
| ≥62 | 30 | 15 | |
| <62 | 23 | 19 | |
| Lymphatic
metastasis | | | 0.0004 |
| Negative | 27 | 30 | |
| Positive | 26 | 4 | |
| Distant
metastasis | | | 0.2775 |
| Negative | 50 | 34 | |
| Positive | 3 | 0 | |
| Clinical stage | | | 0.7469 |
| I | 9 | 9 | |
| II | 40 | 23 | 0.2833 |
| T2a | 17 | 13 | |
| T2b | 23 | 10 | |
| III | 3 | 2 | |
| IV | 1 | 0 | |
|
Differentiation | | | <0.0001 |
| Poor | 25 | 2 | |
| Moderate | 28 | 25 | |
| Well | 0 | 7 | |
| Pre-surgery level
of CA19-9c | | | <0.0001 |
| High | 49 | 16 | |
| Low | 4 | 18 | |
| Pre-surgery level
of CA125d | | | 0.5564 |
| High | 9 | 4 | |
| Low | 44 | 30 | |
| Pre-surgery level
of CEAe | | | 0.7736 |
| High | 10 | 5 | |
| Low | 43 | 29 | |
| Smoking
history | | | 0.4193 |
| Yes | 15 | 7 | |
| No | 38 | 27 | |
| History of alcohol
consumption | | | 0.5747 |
| Yes | 12 | 6 | |
| No | 41 | 28 | |
Varying expression levels of WAVE3 in
pancreatic cancer tissues and adjacent non-cancerous tissues
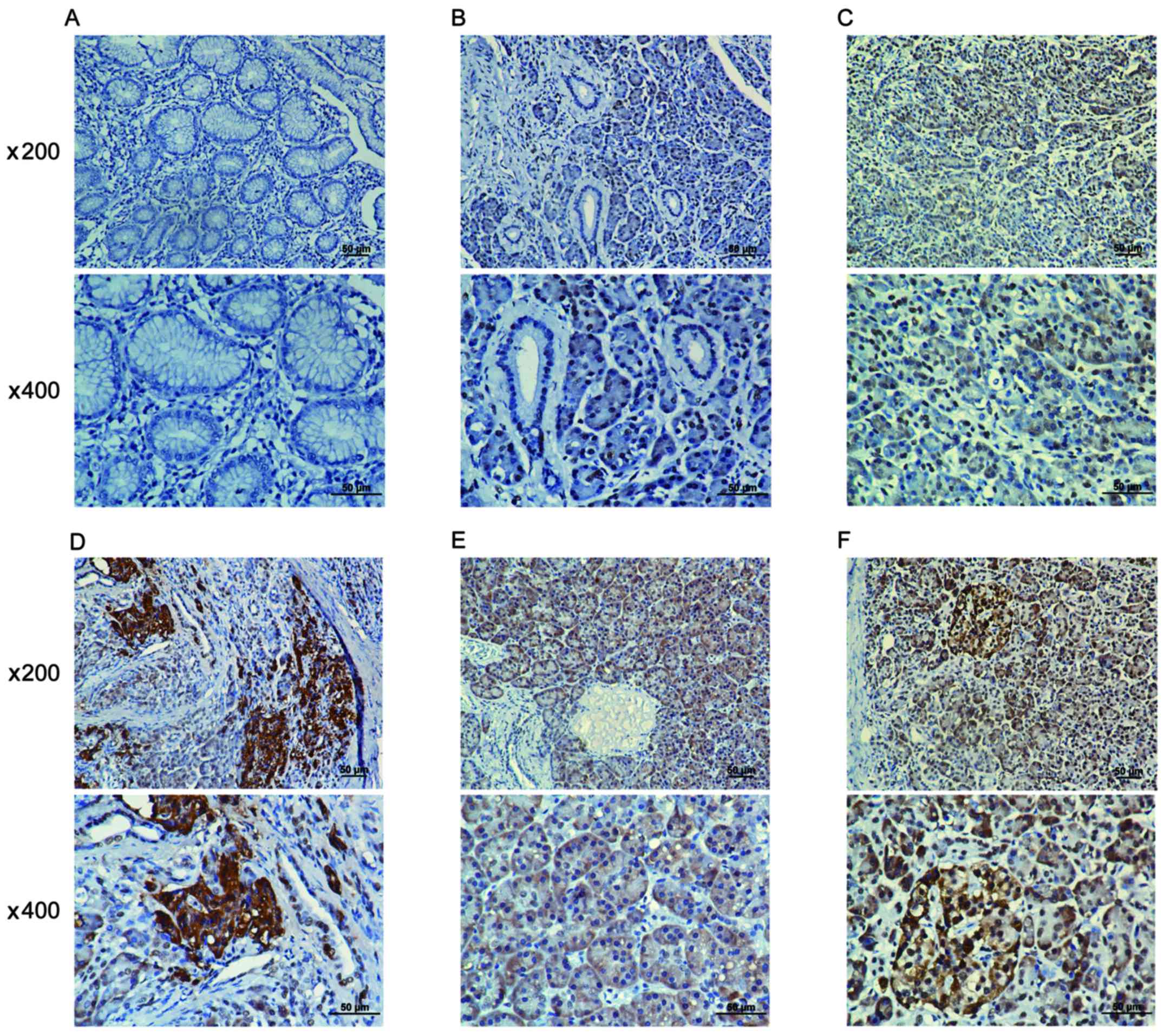
A positive WAVE3 protein expression was obseved in
the cytoplasm of pancreatic cancer cell tissues (Fig. 1B–F), while in the adjacent
non-cancerous tissues, a low or scarce WAVE3 protein expression was
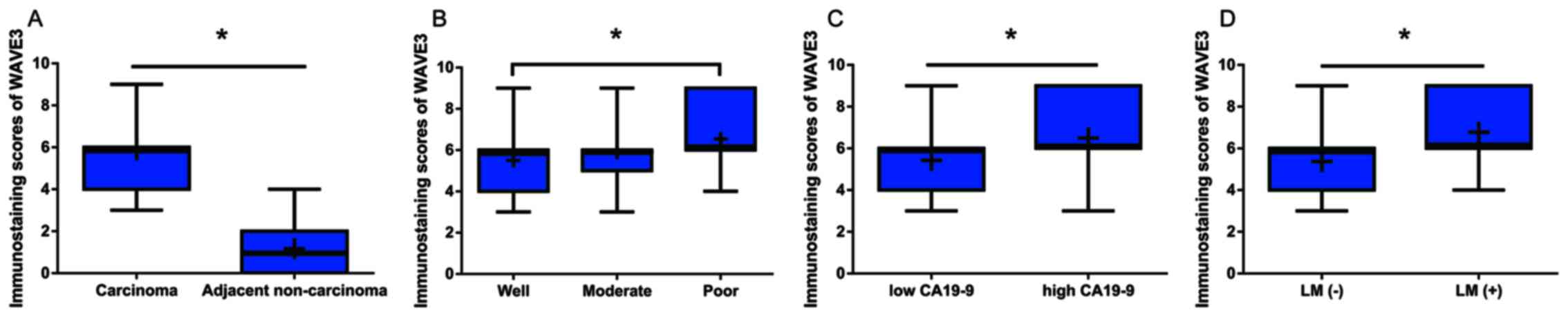
detected (Fig. 1A). Moreover, the
immunostaining scores for WAVE3 protein expression in the
pancreatic cancer tissues were significantly higher than those in
the non-cancerous tissues (Fig.
2A). Among the pancreatic cancer cases, the levels of WAVE3
expression were lower in the well-differentiated adenocarcinomas
than in the poorly differentiated adenocarcinomas (Fig. 2B). Furthermore, the WAVE3 protein
levels were prominently higher in the pancreatic cancer cases with
higher pre-operative CA19-9 levels and lymphatic metastasis than in
those without these characteristics (Fig. 2C and D).
The prognostic value of WAVE3 expression
in patients with pancreatic cancer
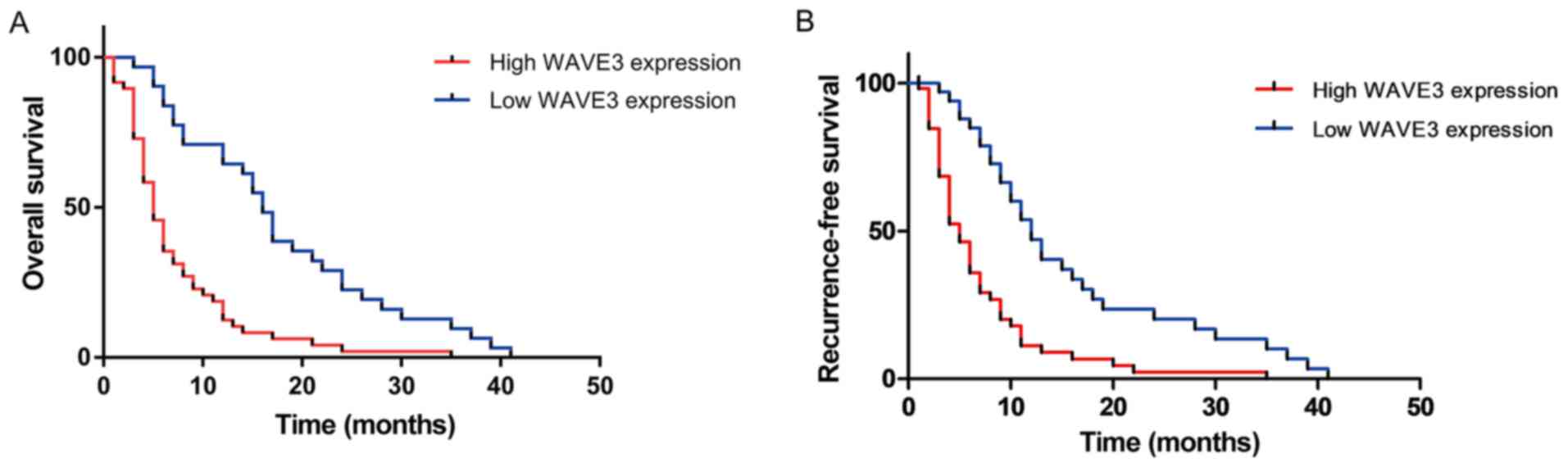
To elucidate the prognostic value of WAVE3 in
patients pancreatic cancer, the associations of WAVE3 expression
with survival time and recurrence-free survival time were detected
in 79 of the 87 pancreatic cancer patients. As shown in Fig. 3A, a prominent difference in the
overall survival between the low WAVE3 expression group (median
survival time, 16.0 months) and the high WAVE3 expression group
(median survival time, 5.0 months; P<0.05) was observed. As
shown in Fig. 3B, a significant
difference in recurrence-free survival between the low WAVE3
expression group (median recurrence free survival time, 12.0
months) and the high WAVE3 expression group (median recurrence free
survival time, 5.0 months; P<0.05).
High expression of WAVE3 in pancreatic
cancer cell lines
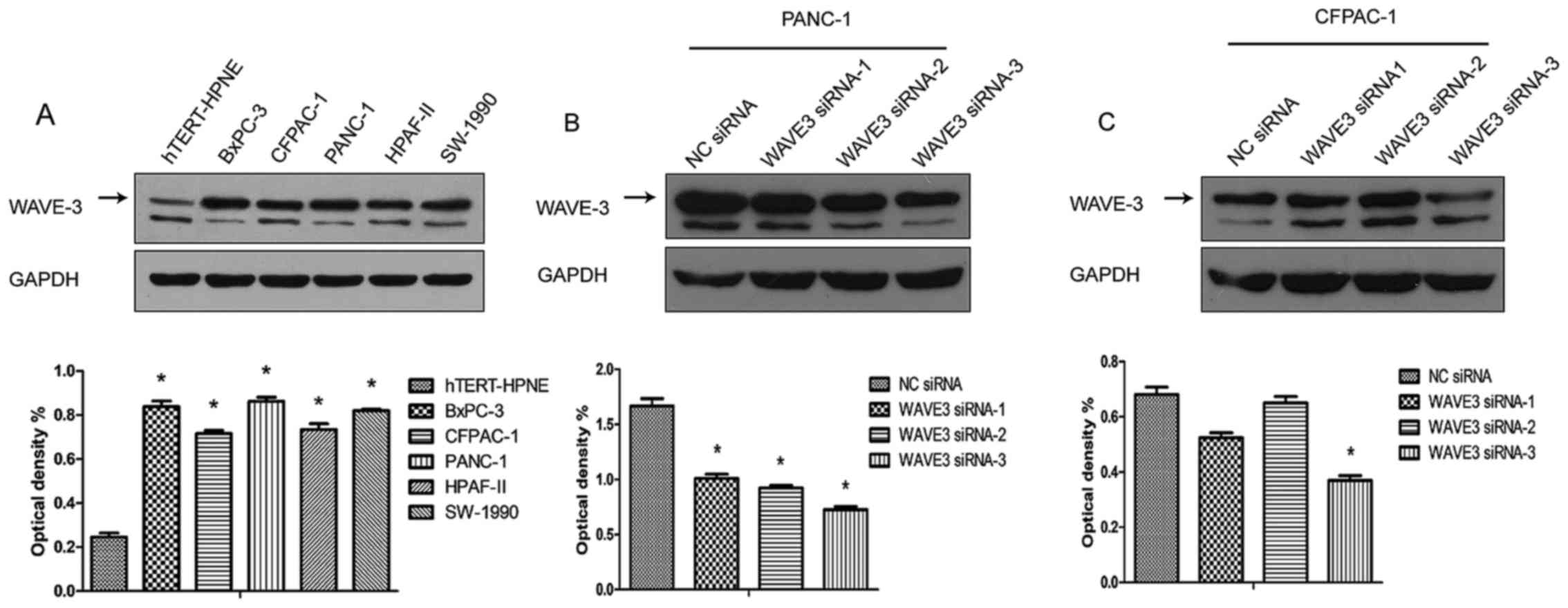
The pancreatic cancer cell lines, BxPC-3, CFPAC-1,
PANC-1, HPAF-II and SW-1990, and a non-neoplastic human pancreatic
ductal epithelial cell line (hTERT-HPNE) were cultured, and the
protein expression of WAVE3 was detected in different cells types
by western blot analysis. The results demonstrated that the WAVE3
expression levels were much higher in the pancreatic cancer cell
lines than in the non-neoplastic human pancreatic ductal epithelial
cell line (hTERT-HPNE). In addition, the different pancreatic
cancer cell lines presented differential levels of WAVE3 protein
expression. A previous study demonstrated that the PANC-1 cells
exhibited better migratory and invasive abilities, while the
CFPAC-1 cells were shown to be well-differentiated (18). In this study, PANC-1 expressed
relatively higher WAVE3 protein levels, while the CFPAC-1 cells
presented lower WAVE3 protein levels (Fig. 4A). Thus, the PANC-1 and CFPAC-1
were selected for use in the following experiments. The protein
expression of WAVE3 was knocked down by WAVE3 siRNA in both the
PANC-1 and CFPAC-1 cell lines. Three different sequences of WAVE3
siRNA (Si-1, Si-2 and Si-3) were used to knock down its expression.
The results demonstrated that Si-3 was the most effective siRNA.
The expression level of WAVE3 was decreased in both the PANC-1 and
CFPAC-1 cell lines following WAVE3 siRNA interference (Fig. 4B and C).
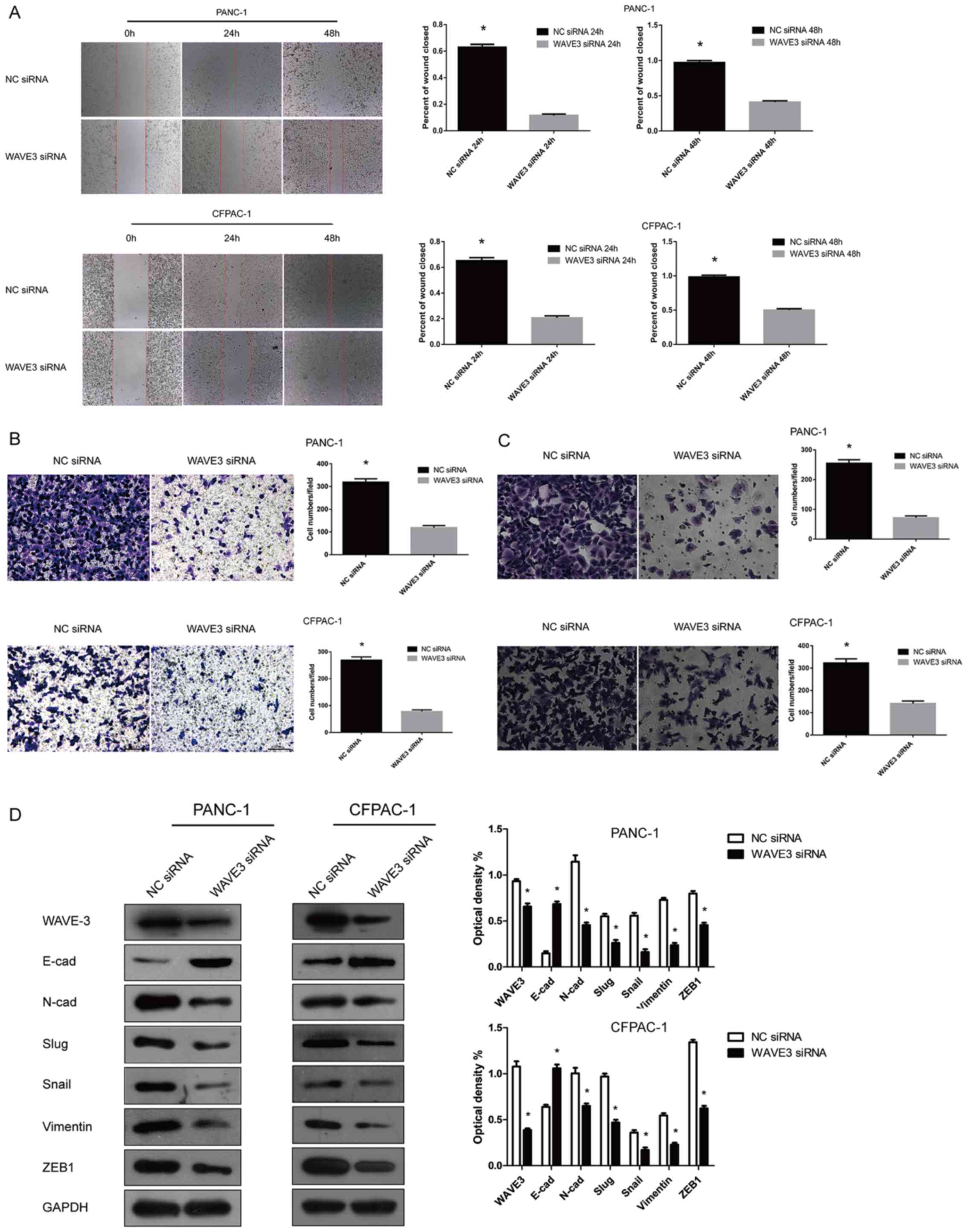
Knockdown of WAVE3 inhibits the migratory
and invasive potentials of pancreatic cancer cells
The expression of WAVE3 in the PANC-1 and CFPAC-1
cell lines was knocked down by transfection with WAVE3 siRNA
(Si-3). Wound healing and Transwell (migration and invasion) assays
were then conducted to determine the invasive and metastastic
potentials of the pancreatic cancer cells with or without
transfection. Following RNA interference, the PANC-1 and CFPAC-1
cell lines displayed weakened cell migratory and invasive
capabilities compared with the control group (P<0.05) (Fig. 5A–C). We also observed that the
expression levels of certain EMT-related proteins were altered
after the knockdown of the expression of WAVE3 in the PANC-1 and
CFPAC-1 cell lines. As indicated, the expression levels of certain
EMT-related proteins, such as Slug, Snail, Vimentin, ZEB1 and
N-Cadherin, were predominantly decreased, while E-cadherin protein
expression was markedly increased (Fig. 5D). Thus, the knockdown of WAVE3 led
to the acquisition of an epithelial-like phenotype from a
neural-like phenotype in the pancreatic cancer cell lines (19). Overall, the knockdown of WAVE3
negatively influenced the migratory and invasive capabilities of
the PANC-1 and CFPAC-1 cell lines.
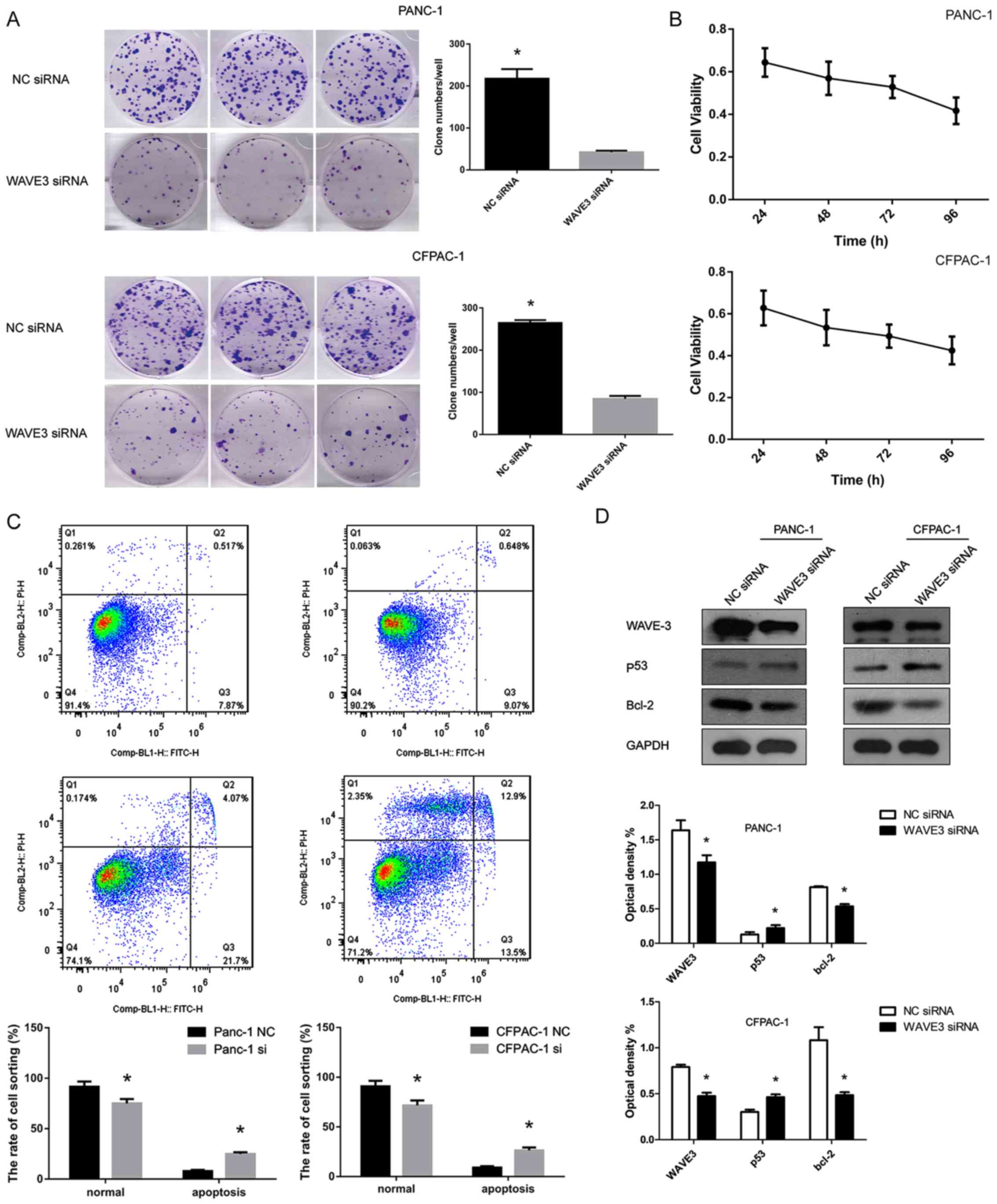
Suppression of WAVE3 negatively affects
the survival of pancreatic cancer cells
The cell growth curve was generated over 4
consecutive days using the CCK-8 assay. Moreover, plate clone
formation assays were conducted to detect the cell survival
abilities of the PANC-1 and CFPAC-1 cell lines. WAVE3 played a
significant role in cell growth. Following WAVE3 siRNA interference
(Si-3), the PANC-1 and CFPAC-1 cell lines exhibited a decrease in
cell viability in the CCK-8 assays and poorer cell growth than the
control cells in the plate clone formation assays (P<0.05)
(Fig. 6A and B). Flow cytometry
demonstrated that the decreased WAVE3 protein expression led to an
increased rate of apoptosis of the pancreatic cancer cell lines
(P<0.05) (Fig. 6C). Western
blot analysis revealed that the protein level of p53 increased
following the knockdown of WAVE3 in the PANC-1 and CFPAC-1 cell
lines, while the expression of its downstream protein, Bcl-2, which
is inhibited by p53, was decreased in comparison to that in the
control group (Fig. 6D).
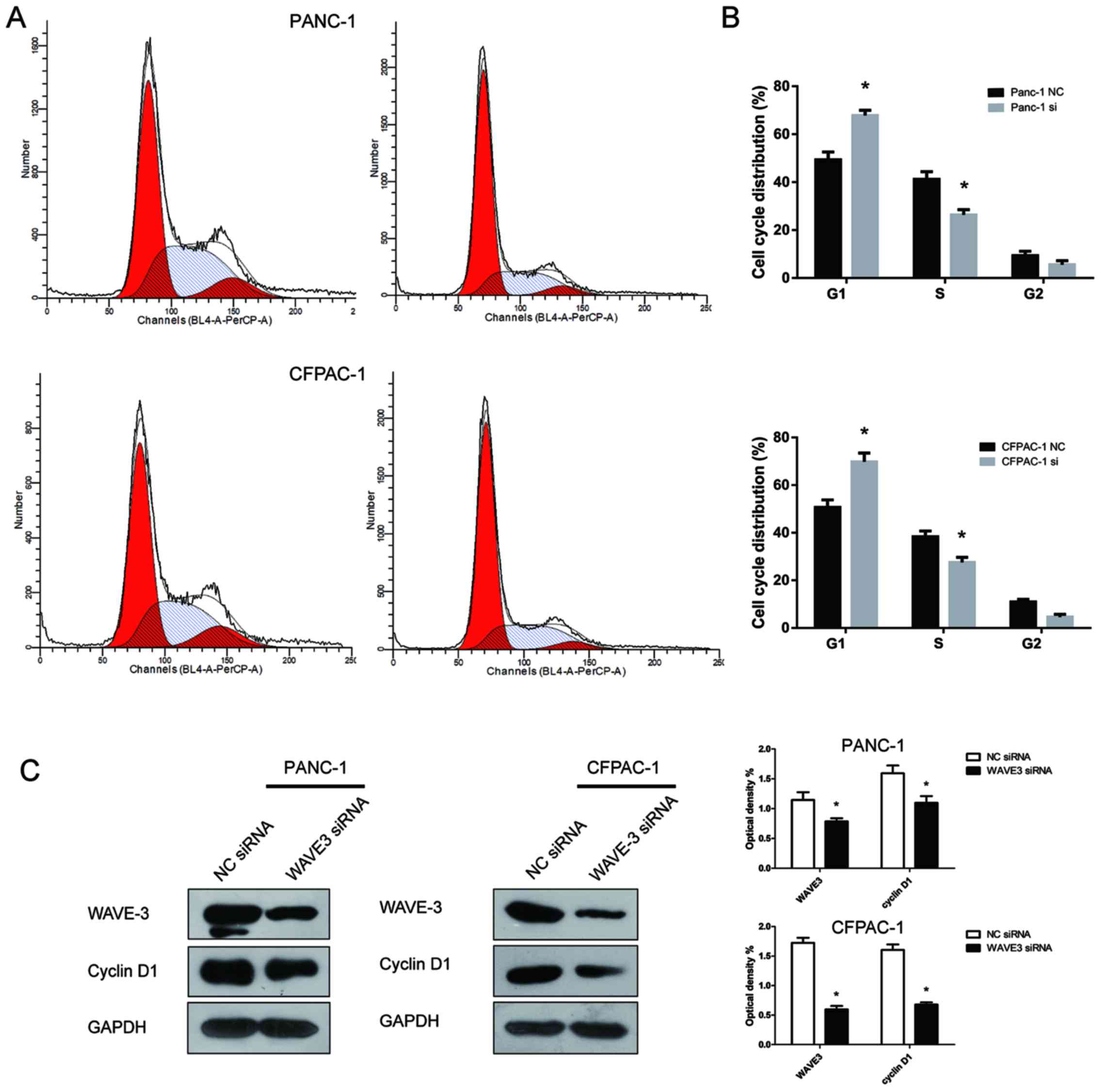
WAVE3 affects the cell cycle of
pancreatic cancer cells
The role of WAVE3 expression in the pancreatic
cancer cell cycle was assessed by flow cytometry. Compared with the
control groups, the fraction of cells in the G1 phase increased
following the knockdown of WAVE3, while the fraction in S phase
decreased (P<0.05) (Fig. 7A and
B). Thus, the knockdown of WAVE3 induced cell cycle arrest in
the G1 phase. To explore the molecular mechanisms involved in the
induction of G1 phase caused by WAVE3 knockdown, western blot
analysis was conducted to assess the protein expression of cyclin
D1, a key cell cycle-regulated protein that influences the G1 phase
transition to S phase. The cyclin D1 protein level decreased after
the knockdown of WAVE3 (Fig. 7C).
Thus, the G1 phase arrest induced by the knockdown of WAVE3 in
pancreatic cancer cell lines was mediated via the downregulation of
cyclin D1.
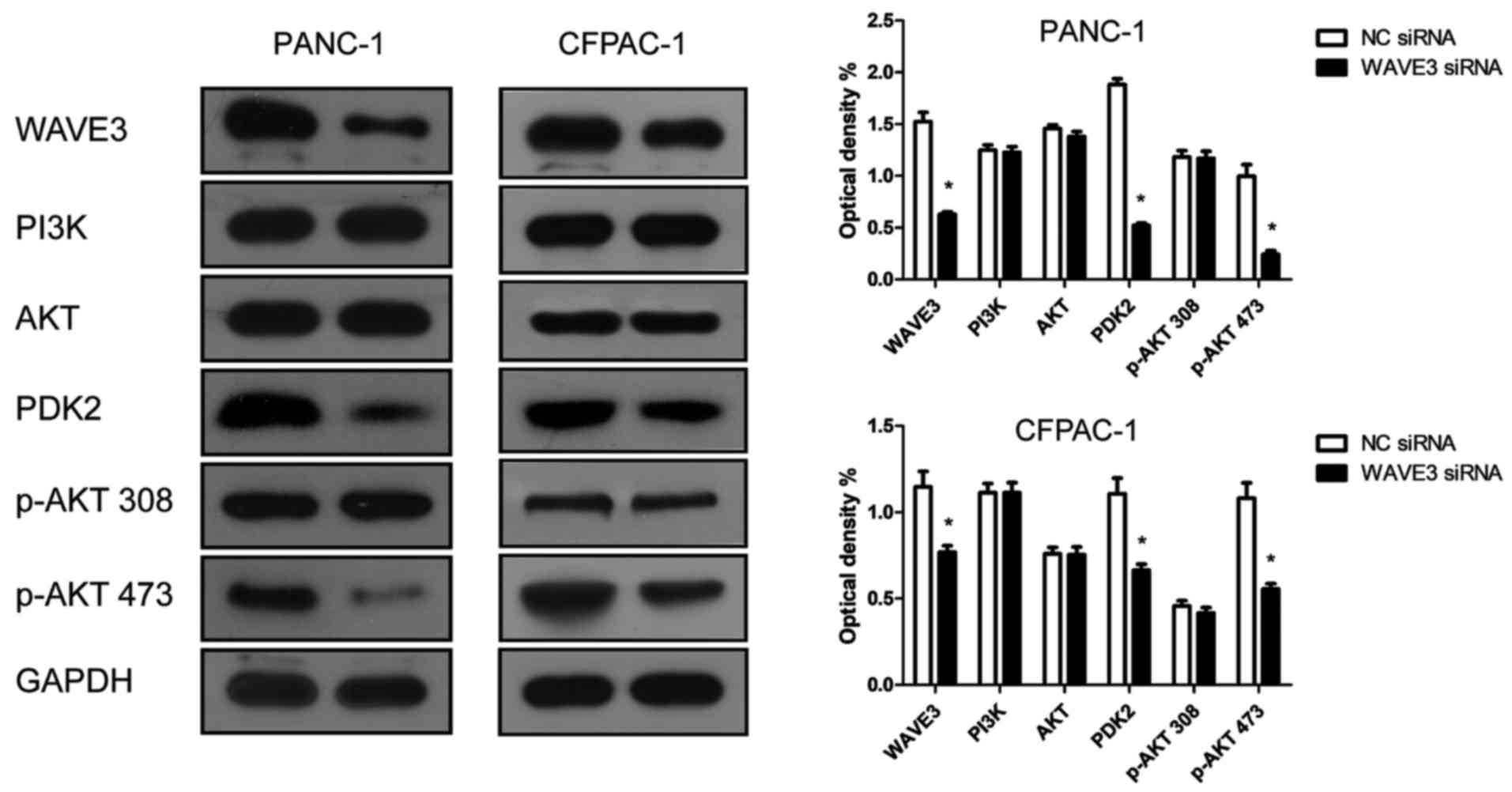
WAVE3 affects the biological behaviors of
pancreatic cancer cells via the AKT pathway
Western blot analysis was conducted to determine
whether WAVE3 affects the AKT pathway. Following the knockdown of
WAVE3 by siRNA (Si-3), the protein expression of PDK2 and p-AKT 473
was decreased compared with that in the control groups in both the
PANC-1 and CFPAC-1 cell lines (Fig.
8). Therefore, the knockdown of WAVE3 suppressed the expression
of PDK2, thus inhibiting the activation of p-AKT 473 and altering
downstream protein expression levels. On the whole, our data
indicate that WAVE3 affects the survival, invasion, migration and
the cell cycle of pancreatic cancer cell lines (PANC-1 and CFPAC-1)
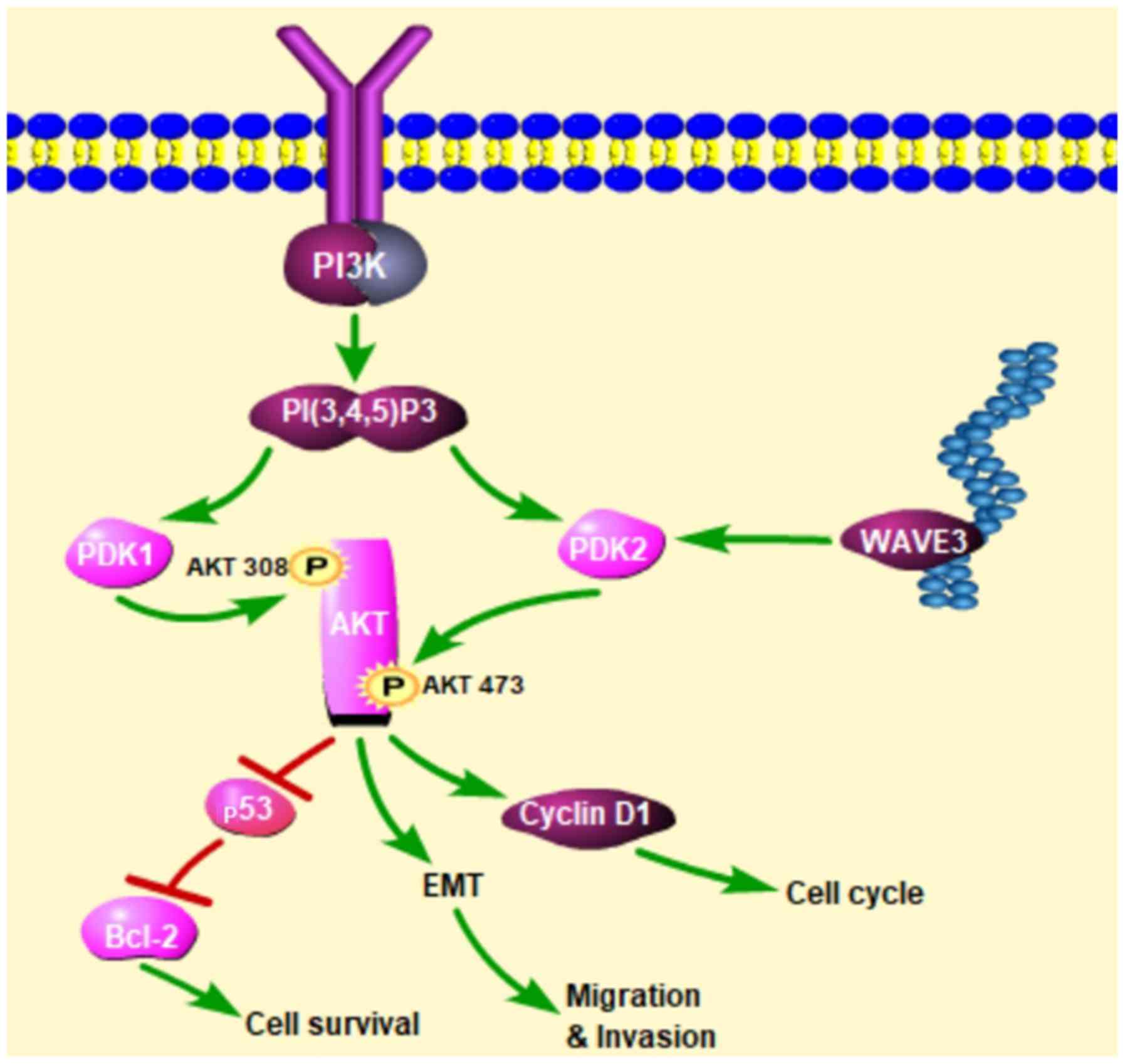
via the AKT pathway (Fig. 9).
Discussion
Pancreatic cancer is considered a malignant tumor
and is associated with high mortality rates even with improved
treatment methods (20).
Metastasis is the non-negligible cause of a poor prognosis of
patients with pancreatic cancer. A series of studies have
demonstrated the involvement of EMT in the progression of
pancreatic cancer metastasis (21–23).
Thus, exploring the mechanisms of EMT in pancreatic cancer is a
very important research strategy for the treatment of pancreatic
cancer.
The majority of research investigating WAVE3 has
demonstrated its overexpression in each cancer type studied, such
as ovarian cancer, breast cancer, prostate cancer, hepatocellular
carcinoma, gastric cancer and colorectal cancer (6–11).
As mentioned previously, some studies have shown that high
expression levels of WAVE3 lead to enhanced migratory and invasive
capabilities via EMT in certain types of cancer (10,12).
In addition, researchers have suggested that WAVE3 is linked to
cell survival in certain types of cancer (6,8,10–12).
However, the function and molecular mechanisms of WAVE3 in EMT and
its association with cell survival remain unclear due to the
limited number of studies.
In the present study, the protein expression levels
of WAVE3 were significantly higher in pancreatic cancer tissues
than in non-cancerous tissues. Furthermore, the clinical data of
our retrospective study demonstrated that the overexpression of
WAVE3 was associated with lymphatic metastasis (P<0.05), poorly
differentiated tumors (P<0.05) and high pre-operative CA19-9
levels (P<0.05) and that the high expression of WAVE3 indicated
a poor outcome of patients with pancreatic cancer. In vitro,
the WAVE3 expression levels were also markedly higher in the
pancreatic cancer cell lines compared with those in a
non-neoplastic human pancreatic ductal epithelial cell line.
Additionally, a comparison of the different pancreatic cancer cell
lines suggested that the WAVE3 expression levels may be associated
with cell differentiation and mobility. Thus, we selected PANC-1
(better migration and invasion abilities and higher expression of
the WAVE3 protein) and CFPAC-1 (well-differentiated and a lower
WAVE3 expression) to conduct the following experiments. Our
analysis revealed that the cell migratory and invasive capacities
were inhibited following the knockdown of WAVE3. Western blot
analysis demonstrated that the knockdown of WAVE3 decreased the
expression of certain EMT-related proteins, such as Slug, Snail,
Vimentin, ZEB1 and N-Cadherin, and upregulated E-cadherin
expression. Thus, the pancreatic cancer cell lines acquired an
epithelial-like phenotype from a neural-like phenotype, decreasing
their migratory and invasive potentials, following WAVE3 knockdown.
WAVE3 suppression decreased the proliferation and increased the
apoptosis of the pancreatic cancer cell lines. Flow cytometry
revealed that the knockdown of WAVE3 induced cell cycle arrest in
the G1 phase. Taken together, the suppression of WAVE3 decreased
the proliferation, migration and invasion abilities and increased
the apoptosis of the pancreatic cancer cell lines analyzed.
A number of studies have shown that Snail inhibits
the expression of E-cadherin, which is closely related to cancer
invasion and metastasis via EMT (24,25).
The enhancement of AKT activity with Snail stabilization is
essential for EMT and, subsequently, improves the invasive and
metastatic potential of tumors (26,27).
High-intensity p-AKT expression is related to poor outcomes of
patients with pancreatic ductal adenocarcinoma (28). The inhibition of the AKT pathway in
pancreatic cancer cell lines (PANC-1 and HPAF) shows a promising
effect by reducing cell growth and promoting apoptosis (29). Taken together, from the
above-mentioned findings, we hypothesized that WAVE3 influences the
proliferation, migration and invasion of pancreatic cancer cell
lines via the AKT pathway.
To further confirm this hypothesis, western blot
analysis was conducted to determine whether WAVE3 affects the AKT
pathway to alter the biological behaviors of pancreatic cancer cell
lines. The knockdown of WAVE3 decreased the expression of PDK2 and
then negatively inhibited the phosphorylation of its downstream
molecule, AKT, at the Ser473 site. As a result, the expression of
downstream proteins in the AKT pathway was affected, accordingly
affectin cell survival, invasion and migration as well as the cell
cycle (Fig. 9). The p53 and Bcl-2
proteins downstream of the AKT pathway are known to affect cell
survival, and another downstream protein, cyclin D1, influences the
cell cycle (15,30). Therefore, western blot analysis was
conducted to assess whether the expression levels of p53, Bcl-2 and
cyclin D1 were altered following the knockdown of WAVE3. The p53
protein level was increased following the knockdown of WAVE3, while
the expression of its downstream protein, Bcl-2, which is inhibited
by p53, was decreased. Moreover, the protein level of cyclin D1 was
decreased following the knockdown of WAVE3. This result was
consistent with the phenomenon that the knockdown of WAVE3 induced
cell cycle arrest in the G1 phase. Taken together, WAVE3 influences
the AKT pathway to modify the biological behaviors of pancreatic
cancer cell lines.
Previous studies have focused on gene mutations in
pancreatic cancer, and certain genes have been shown to be related
to pancreatic cancer, including KRAS, ATM (31), TP53, BRAF (32), SMAD4 and CDKN2A (33). The molecular mechanisms of the
mutated genes listed above were discovered in several studies
(31–33); however, the corresponding novel
agents targeting these mutated genes have not been identified. In
this study, WAVE3 was regarded as a potential target for the
treatment of pancreatic cancer as we discovered that it influenced
the AKT pathway and then significantly affected the biological
behaviors of pancreatic cancer cell lines. In this study, the
knockdown of WAVE3 reversed the malignant behaviors of pancreatic
cell lines. Therefore, novel agents targeted toward WAVE3 and/or
the AKT pathway are worth exploring for the treatment of pancreatic
cancer.
In conclusion, in this study, we demonstrated that
the expression levels of WAVE3 in pancreatic cancer tissues were
prominently higher than those in the non-cancerous tissues. Our
clinical data revealed that the overexpression of WAVE3 was
associated with lymphatic metastasis, poorly differentiated tumors
and high pre-operative levels of CA19-9. Moreover, a high
expression of WAVE3 was associated with a poor prognosis of
patients with pancreatic cancer. In the cell culture experiments,
the WAVE3 expression levels were markedly higher in the pancreatic
cancer cell lines than in a non-neoplastic human pancreatic ductal
epithelial cell line. Furthermore, the suppression of WAVE3
inhibited the cell proliferative, migratory and invasive
capabilities and promoted the apoptosis of the pancreatic cancer
cell lines. The mechanism underlying this phenomenon was WAVE3
knockdown inhibiting PDK2 expression, which negatively affected the
phosphorylation of it downstream molecule, AKT, at the Ser473 site.
Therapies targeted toward WAVE3 and/or the AKT pathway may be worth
exploring for the treatment of pancreatic cancer.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
CZ proposed the study. SH and CH performed the
research. WC and YL collected and analyzed the data. XY, JL, LL and
QW provided the clinical specimens and clinical data. AW offered
the technical assistance. All authors contributed to the
conception, design and interpretation of the study and to further
drafts. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Institute Research
Ethics Committee of the First Affiliated Hospital at Sun Yat-Sen
University. As this study is a retrospective study, the patient
consent was allowed to be waived by the ethics committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Professor Anxun Wang
(Department of Oral and Maxillofacial Surgery, the First Affiliated
Hospital of Sun Yat-Sen University, Guangzhou, China) for providing
technical assistance and Professor Peng Huang (Sun Yat-Sen
University Cancer Center, Guangzhou, China) for kindly providing
the non-neoplastic human pancreatic ductal epithelial cell line
(hTERT-HPNE).
Abbreviations:
|
WAVE3
|
Wiskott-Aldrich syndrome protein
family verprolin-homologous protein 3
|
|
MMPs
|
matrix metalloproteinases
|
|
MAPK
|
mitogen-activated protein kinase
|
|
IHC
|
immunohistochemistry
|
|
CCK-8
|
cell counting kit-8
|
|
PBK/AKT
|
protein kinase B
|
|
PDK2
|
pyruvate dehydrogenase kinase isoform
2
|
|
EMT
|
epithelial-mesenchymal transition
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
PDK1
|
phosphoinositide-dependent kinase
|
|
PBS
|
phosphate-buffered saline
|
|
ANOVA
|
one-way analysis of variance
|
|
ZEB1
|
zincfinger Ebox binding homeobox 1
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar
|
|
2
|
Wang-Gillam A, Li CP, Bodoky G, Dean A,
Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, et
al NAPOLI-1 Study Group: Nanoliposomal irinotecan with fluorouracil
and folinic acid in metastatic pancreatic cancer after previous
gemcitabine-based therapy (NAPOLI-1): A global, randomised,
open-label, phase 3 trial. Lancet. 387:545–557. 2016. View Article : Google Scholar
|
|
3
|
Deplanque G and Demartines N: Pancreatic
cancer: Are more chemotherapy and surgery needed. Lancet.
389:985–986. 2017. View Article : Google Scholar
|
|
4
|
Kamisawa T, Isawa T, Koike M, Tsuruta K
and Okamoto A: Hematogenous metastases of pancreatic ductal
carcinoma. Pancreas. 11:345–349. 1995. View Article : Google Scholar
|
|
5
|
Sossey-Alaoui K: Surfing the big WAVE:
Insights into the role of WAVE3 as a driving force in cancer
progression and metastasis. Semin Cell Dev Biol. 24:287–297.
2013.
|
|
6
|
Lu J, Wang SL, Wang YC, Wu YN, Yu X, Zhao
WZ and Wang JH: High WAVE3 expression correlates with
proliferation, migration and invasion in human ovarian cancer.
Oncotarget. 8:41189–41201. 2017.
|
|
7
|
Davuluri G, Schiemann WP, Plow EF and
Sossey-Alaoui K: Loss of WAVE3 sensitizes triple-negative breast
cancers to chemotherapeutics by inhibiting the STAT-HIF-1α-mediated
angiogenesis. JAK-STAT. 3:e10092762015. View Article : Google Scholar
|
|
8
|
Moazzam M, Ye L, Sun PH, Kynaston H and
Jiang WG: Knockdown of WAVE3 impairs HGF induced migration and
invasion of prostate cancer cells. Cancer Cell Int. 15:512015.
View Article : Google Scholar
|
|
9
|
Ji Y, Li B, Zhu Z, Guo X, He W, Fan Z and
Zhang W: Overexpression of WAVE3 promotes tumor invasiveness and
confers an unfavorable prognosis in human hepatocellular carcinoma.
Biomed Pharmacother. 69:409–415. 2015. View Article : Google Scholar
|
|
10
|
Yue Z, Feng W, Xiangke L, Liuxing W,
Qingxia F and Jianbo G: WAVE3 promotes epithelial-mesenchymal
transition of gastric cancer through upregulation of Snail. Cancer
Gene Ther. 21:499–506. 2014. View Article : Google Scholar
|
|
11
|
Zhang Y, Guan XY, Dong B, Zhao M, Wu JH,
Tian XY and Hao CY: Expression of MMP-9 and WAVE3 in colorectal
cancer and its relationship to clinicopathological features. J
Cancer Res Clin Oncol. 138:2035–2044. 2012. View Article : Google Scholar
|
|
12
|
Taylor MA, Davuluri G, Parvani JG,
Schiemann BJ, Wendt MK, Plow EF, Schiemann WP and Sossey-Alaoui K:
Upregulated WAVE3 expression is essential for TGF-β-mediated EMT
and metastasis of triple-negative breast cancer cells. Breast
Cancer Res Treat. 142:341–353. 2013. View Article : Google Scholar
|
|
13
|
Sossey-Alaoui K, Ranalli TA, Li X, Bakin
AV and Cowell JK: WAVE3 promotes cell motility and invasion through
the regulation of MMP-1, MMP-3, and MMP-9 expression. Exp Cell Res.
308:135–145. 2005. View Article : Google Scholar
|
|
14
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar
|
|
15
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar
|
|
16
|
Edge SB; American Joint Committee on
Cancer; ACS: AJCC Cancer Staging Manual. 7th. Springer; New York:
2009
|
|
17
|
Chang B, Li S, He Q, Liu Z, Zhao L, Zhao T
and Wang A: Deregulation of Bmi-1 is associated with enhanced
migration, invasion and poor prognosis in salivary adenoid cystic
carcinoma. Biochim Biophys Acta. 1840.3285–3291. 2014.
|
|
18
|
Deer EL, González-Hernández J, Coursen JD,
Shea JE, Ngatia J, Scaife CL, Firpo MA and Mulvihill SJ: Phenotype
and genotype of pancreatic cancer cell lines. Pancreas. 39:425–435.
2010. View Article : Google Scholar
|
|
19
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar
|
|
20
|
Middleton G, Palmer DH, Greenhalf W,
Ghaneh P, Jackson R, Cox T, Evans A, Shaw VE, Wadsley J and Valle
JW: Vandetanib plus gemcitabine versus placebo plus gemcitabine in
locally advanced or metastatic pancreatic carcinoma (ViP): A
prospective, randomised, double-blind, multicentre phase 2 trial.
Lancet Oncol. 18:486–499. 2017. View Article : Google Scholar
|
|
21
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK and
Vonderheide RH: EMT and dissemination precede pancreatic tumor
formation. Cell. 148:349–361. 2012. View Article : Google Scholar
|
|
22
|
Hao K, Tian XD, Qin CF, Xie XH and Yang
YM: Hedgehog signaling pathway regulates human pancreatic cancer
cell proliferation and metastasis. Oncol Rep. 29:1124–1132. 2013.
View Article : Google Scholar
|
|
23
|
Bo H, Zhang S, Gao L, Chen Y, Zhang J,
Chang X and Zhu M: Upregulation of Wnt5a promotes
epithelial-to-mesenchymal transition and metastasis of pancreatic
cancer cells. BMC Cancer. 13:4962013. View Article : Google Scholar
|
|
24
|
Yang MH, Chen CL, Chau GY, Chiou SH, Su
CW, Chou TY, Peng WL and Wu JC: Comprehensive analysis of the
independent effect of twist and snail in promoting metastasis of
hepatocellular carcinoma. Hepatology. 50:1464–1474. 2009.
View Article : Google Scholar
|
|
25
|
Oda H, Tsukita S and Takeichi M: Dynamic
behavior of the cadherin-based cell-cell adhesion system during
Drosophila gastrulation. Dev Biol. 203:435–450. 1998. View Article : Google Scholar
|
|
26
|
Liu L, Dai Y, Chen J, Zeng T, Li Y, Chen
L, Zhu YH, Li J, Li Y and Ma S: Maelstrom promotes hepatocellular
carcinoma metastasis by inducing epithelial-mesenchymal transition
by way of Akt/GSK-3β/Snail signaling. Hepatology. 59:531–543. 2014.
View Article : Google Scholar
|
|
27
|
Liu A, Shao C, Jin G, Liu R, Hao J, Song
B, Ouyang L and Hu X: miR-208-induced epithelial to mesenchymal
transition of pancreatic cancer cells promotes cell metastasis and
invasion. Cell Biochem Biophys. 69:341–346. 2014. View Article : Google Scholar
|
|
28
|
Yamamoto S, Tomita Y, Hoshida Y, Morooka
T, Nagano H, Dono K, Umeshita K, Sakon M, Ishikawa O and Ohigashi
H: Prognostic significance of activated Akt expression in
pancreatic ductal adenocarcinoma. Clin Cancer Res. 10:2846–2850.
2004. View Article : Google Scholar
|
|
29
|
Stoll V, Calleja V, Vassaux G, Downward J
and Lemoine NR: Dominant negative inhibitors of signalling through
the phos-phoinositol 3-kinase pathway for gene therapy of
pancreatic cancer. Gut. 54:109–116. 2005. View Article : Google Scholar
|
|
30
|
Tang F, Wang Y, Hemmings BA, Rüegg C and
Xue G: PKB/Akt-dependent regulation of inflammation in cancer.
Semin Cancer Biol. 48:62–69. 2018. View Article : Google Scholar
|
|
31
|
Biankin AV, Waddell N, Kassahn KS, Gingras
MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J,
et al Australian Pancreatic Cancer Genome Initiative: Pancreatic
cancer genomes reveal aberrations in axon guidance pathway genes.
Nature. 491:399–405. 2012. View Article : Google Scholar
|
|
32
|
Raphael BJ, Hruban RH, Aguirre AJ, Moffitt
RA, Yeh JJ, Stewart C, Robertson AG, Cherniack AD, Gupta M, Getz G,
et al Cancer Genome Atlas Research Network: Integrated Genomic
Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell.
32:185–203.e13. 2017. View Article : Google Scholar
|
|
33
|
Waddell N, Pajic M, Patch AM, Chang DK,
Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, et al
Australian Pancreatic Cancer Genome Initiative: Whole genomes
redefine the mutational landscape of pancreatic cancer. Nature.
518:495–501. 2015. View Article : Google Scholar
|