Introduction
Papillary thyroid cancer (PTC) is the most common
type of thyroid cancer. As epidemiological studies have reported,
the incidence of thyroid cancer has markedly increasing over the
past decades, mainly due to the increasing incidence of PTC
(1). PTC typically has a favorable
prognosis, with an overall 10-year survival rate >90%. However,
the number of patients with refractory PTC has also increased,
which is an obstacle to the effective treatment of PTC (2). Thus, the further understanding of the
molecular mechanisms responsible for the development and
progression of PTC is important in order to improve the prognosis
(3).
Neuregulin 1 (NRG1) is one of the most active
members of the epidermal growth factor (EGF)-like family, located
on chromosome 8p12. NRG1 is a membrane glycoprotein that mediates
cell-cell signaling and plays a critical role in the growth and
development of multiple organ systems. NRG1 is processed
into numerous isoforms by alternative splicing, which allows it to
perform a wide variety of functions (4,5). The
interaction of NRG1 with the dimers of its receptors, including
ErbB2, ErbB3 and ErbB4, results in a number of biological processes
(6). NRG1 has been reported to
interact with several signal pathways, including the
Ras/MAPK/ERK1/2 pathway in Schwann cell development and the PI3K
pathway in melanoma (7,8).
To achieve uncontrolled proliferation, it is
critical for tumor cells to maintain reduction and oxidation
balance, which is also known as redox homeostasis. Reactive oxygen
species (ROS) need to be regulated delicately to a moderate level
so that tumor cells can initiate, proliferate, migrate and invade
in a well-balanced redox homeostasis (9,10).
As byproducts of oxygen metabolism, ROS play critical physiological
roles in biological processes and are important for healthy cell
function (11). However, high ROS
levels are noxious to cancer cells, as DNA damage can be induced by
excessive ROS generation, which eventually leads to apoptosis
(12,13).
Cellular oxidative stress is aggravated when ROS
levels increase, and tumor cells have to confront a severe survival
challenge (12). In order to
survive, tumor cells apply a series of strategies with which to
restore redox homeostasis. For example, tumor cells activate
complex antioxidant pathways to increase the expression of ROS
scavengers, thus neutralizing excessive ROS levels (10). The transcription factor nuclear
factor E2-related factor 2 (NRF2), is a pivotal regulator of a
series of antioxidant enzymes. NRF2 upregulates the expression of
these antioxidant enzymes by binding to the antioxidant response
element (ARE) in their promoter regions (14). In non-stressed cells, NRF2 can be
degraded by the constitutively active Kelch-like ECH-associated
protein 1 (KEAP1)-mediated and cullin-3-dependent
ubiquitin-proteasome pathway, which is disrupted upon oxidative
stress, leading to NRF2 accumulation. NRF2 then translocates to the
nucleus and triggers the antioxidant response (15).
However, to date, and at least to the best of our
knowledge, there have been only a few attempts made at exploring
the role of NRG1 in PTC (16–18).
Thus, in the present study, we aimed to assess the role of NRG1 in
PTC, particularly the association between NRG1 and PTC cell
viability and proliferation. Furthermore, we aimed to elucidate the
underlying mechanisms by exploring the potential role of NRG1 in
the regulation of thyroid cancer-related pathways, including ERK1/2
and redox homeostasis.
Materials and methods
Patients and tissue samples
Following confirmation by pathological diagnosis,
thyroid cancer tissues and corresponding normal thyroid tissues at
least 1 cm away from the tumor were obtained from 196 patients.
These patients were diagnosed and had received a thyroidectomy
between 2003 and 2012 at the Department of Head and Neck Surgery,
Fudan University Shanghai Cancer Center (Shanghai, China). All
tissue samples were fixed in formalin, embedded in paraffin and
sectioned into 5-μm-thick slices. The criteria of the
American Joint Committee on Cancer (8th edition) for thyroid cancer
was used for TNM staging classification (19). This study was approved by the Human
Ethics Committee/Institutional Review Board of Fudan University
Shanghai Cancer Center. All 196 patients signed the written
informed consent. A highly sensitive streptavidin-biotin-peroxidase
detection system was used to perform immunohistochemical staining
with thyroid cancer tissue microarrays derived from the samples of
the 196 patients. To investigate the association between the
expression of NRG1 and that of NRF2, we extracted and analyzed the
data of 490 patients with PTC from The Cancer Genome Atlas (TCGA)
database. NRG1 and NRF2 expression and clinical data of the TCGA
database are available from the link directly: http://www.cbioportal.org/index.do?cancer_study_id=thca_tcga&Z_SCORE_THRESHOLD=2.0&RPPA_SCORE_THRESHOLD=2.0&data_priority=0&case_set_id=thca_tcga_all&gene_list=NRG1%2520NFE2L2&geneset_list=+&tab_index=tab_visualize&Action=Submit&genetic_profile_ids_PROFILE_MUTATION_EXTENDED=thca_tcga_mutations&genetic_profile_ids_PROFILE_COPY_NUMBER_ALTERATION=thca_tcga_gistic.
Patients who fit the following criteria were included: i) Patients
who had PTC as the only malignancy or the first of multiple
malignancies; and ii) patients who received surgical therapy.
Patients who fit the following criteria were excluded: i) Patients
with insufficient data or unknown clinicopathological profiles; ii)
patients with an undetermined histology; and iii) patients with
other types of thyroid cancer (follicular thyroid cancer, medullary
thyroid cancer, anaplastic thyroid cancer, etc.), or secondary
tumors.
Immunohistochemistry (IHC) and IHC
scoring
Briefly, tissue samples obtained from the 196
patients were fixed with formalin (37% formaldehyde dissolved in
water, containing 10% methanol in addition) at room temperature for
24 h. The fixed samples were then embedded in paraffin and
sectioned into 5-μm-thick slices. The slices were mounted on
slides and were then deparaffinized with xylol, hydrated with
gradient ethanol in multiple steps. Citrate buffer (pH 6.0) was
used for antigen retrieval and the slides were heated in this
buffer at 121°C for 15 min. Following the blockage of endogenous
peroxidase by 3% H2O2, the slides were then
blocked by 10% normal goat serum and incubated with primary
antibodies. After being washed with PBS, the slides were developed
using Dako EnVision + Rabbit Polymer (cat. no. K4003) from Dako
(Carpinteria, CA, USA), followed by another thorough washing. The
slides were counterstained with hematoxylin and coverslipped. The
immunohistochemically-stained samples were scored by two
pathologists blinded to the clinical parameters, separately. The
staining intensity was scored as follows: 0 (negative), 1 (weak), 2
(medium) or 3 (strong). The extent of staining was scored as
follows: 0, <5%; 1, 5–25%; 2, 26–50%; 3, 51–75%; and 4, >75%
according to the percentages of the positively stained areas in
relation to the whole tumor area. The immunoreactivity score (IS)
for each sample was generated by multiplying the score for staining
intensity with the score for staining extent. Final staining scores
for samples of <4, 4, 6 and ≥8 were considered to be -, +, ++
and +++, respectively.
Cell culture
Two human papillary thyroid cancer cell lines, K1
and TPC1, were purchased from the University of Colorado 125 Cancer
Center Cell Bank and were cultured in RMPI-1640 medium containing
10% FBS (Invitrogen, Carlsbad, CA, USA). 293T cells were purchased
from the Type Culture Collection Cell Bank, Chinese Academy of
Sciences and were cultured in Dulbecco’s modified Eagle’s medium
containing 10% FBS (Invitrogen). All cells were maintained at 37°C
with 5% CO2 in proper humidity. It should be noted that
the K1 cells are considered to be a mixed thyroid gland papillary
carcinoma type, as it has been reported that the K1 cells are
contaminated by GLAG-66, which is also derived from thyroid gland
papillary carcinoma (20,21).
Antibodies
NRG1 antibody (10527-1-AP), NRF2 antibody
(16396-1-AP), GAPDH (60004-1-Ig) and ERK1/2 antibody (16443-1-AP)
were purchased from Proteintech Group, Inc. (Chicago, IL, USA)
β-actin antibody (sc-47778) was obtained from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). The secondary antibodies,
anti-mouse (SA00001-1) antibody conjugated with horseradish
peroxidase (HRP) and anti-rabbit (SA00001-2) antibody conjugated
with HRP, were purchased from Proteintech Group, Inc. All
antibodies were diluted as per the manufacturer’s instructions when
they were applied for specific experiments. NRG1 antibody and NRF2
antibody were diluted at a ratio of 1:50 for IHC and were diluted
1:1,000 for western blot analysis. GAPDH antibody and ERK1/2
antibody were diluted at a ratio of 1:1,000 for western blot
analysis. β-actin antibody was diluted 1:500 for western blot
analysis.
Plasmids
The pLKO.1-TRC cloning vector (Addgene plasmid
10878) was used to generate shRNA constructs against NRG1. The
21-bp target sequences against NRG1 were CGTGGAATCAAACGAGATCAT and
CCACAGAAGGAGCAAATACTT respectively. These two constructs were
afterwards used to produce lentiviruses and establish stable the
cell lines named as ‘shNRG1-1’ and ‘shNRG1-2’, respectively. For
lentivirus production, the packaging plasmid psPAX2 and the
envelope plasmid pMD2.G were kind gifts from Dr Yi Qin, Pancreatic
Cancer Institute, Fudan University.
Lentivirus production and stable cell
line selection
To produce lentiviral particles, the shRNA
constructs were co-transfected with the psPAX2 and pMD2.G plasmids
at a ratio of 4:3:1 into the 293T cells using Lipofectamine™ 2000
Transfection Reagent (11668027; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Lentiviral particles were harvested after 48 h
of transfection. After being infected by the lentiviral particles,
the K1 and TPC1 cells were selected by puromycin, respectively to
obtain cell lines that stably expressed shRNA.
Flow cytometric analysis of ROS
generation and intracellular glutathione (GSH) activity assay
An oxidant-sensitive fluorescent probe (DCFH-DA) was
used to detect intracellular ROS levels (Sigma-Aldrich, St. Louis,
MO, USA). Briefly, the cells were washed twice by
phosphate-buffered saline (PBS), and were then stained with DCFH-DA
(10 μmol/l) at 37°C for 20 min as per the manufacturer’s
instructions. DCFH-DA was deacetylated by intracellular
non-specific esterase, and was then oxidized by ROS to generate the
fluorescent compound, 2,7-dichlorofluorescein (DCF). The DCF
fluorescence intensity was detected using a FACScan flow cytometer
(BD Biosciences, San Jose, CA, USA). Intracellular GSH activity
that reflects the oxidative status of cells was determined using
the GSH/GSSG Ratio Detection Assay kit (ab138881; Abcam, Cambridge,
MA, USA).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
TRIzol reagent (10296010; Thermo Fisher Scientific,
Inc.) was used to extract total RNA from the cells as per the
manufacturer’s instructions. cDNA reverse transcription was
achieved using the PrimeScript™ RT Master Mix (RR036A; Takara Bio,
Inc., Beijing, China). Relative quantitative (real-time) PCR was
performed using SYBR® Premix Ex Taq™ II (Tli RNaseH
Plus) (RR820Q; Takara Bio, Inc.) to determine the mRNA expression
levels of candidate genes normalized to β-actin, using the ABI
7900HT Real-Time PCR system (Applied Biosystems, Waltham, MA, USA)
by the standard protocol. The thermocycling conditions are as
follows: step 1, 50°C for 2 min; step 2, 95°C for 5 min (step 1 and
step 2 are the hold stage); step 3, 95°C for 10 sec; step 4, 60°C
for 1 min (recycle step 3 and step 4 to a total of 40 cycles,
making the PCR stage); step 5, 95°C for 15 sec; step 6, 60°C for 1
min; step 7, 95°C for 15 sec (step 5 to step 7 are the melt curve
stage). All reactions were conducted in triplicate. Relative
quantification (ΔΔCq) was carried out as previously described
(22). The primers used for PCR
were as follows: NRG1 forward, 5′-CTAACATAGGAGAGTTAGGTGGC-3′
and reverse, 5′CTGTGGGCCAGTTAAACCTCTT-3′; ME1 forward,
5′-CCTCACTACTGCTGAGGTTATAGC-3′ and reverse,
5′-CGGTTCAGGATAAACTGTGGCTG-3′; TXNRD1 forward,
5′-GCAATCCAGGCAGGAAGATTGCT-3′ and reverse,
5′-CTCTTGACGGAATCGTCCATTCC-3′; GCLC forward,
5′-GTGGTACTGCTCACCAGAGTG-3′ and reverse,
5′-AGCTCCGTGCTGTTCTGGGCCTT-3′; GCLM forward,
5′-ATCTTGCCTCCTGCTGTGTGATGC-3′ and reverse,
5′-CAATGACCGAATACCGCAGTAGCC-3′; HMOX1 forward,
5′-GCTCTGGAAGGAGCAAAATCACACC-3′ and reverse,
5′-TATGACCCTTGGGAAACAAAG TCTGG-3′; NQO1 forward,
5′-AAGCCCAGACCAACTTCT-3′ and reverse, 5′-GCGTTTCTTCCATCCTTC-3′;
GAPDH forward, 5′-GAACGGGAAGCTCACTGG-3′ and reverse,
5′-GCCTGCTTCACCACCTTCT-3′; and β-actin forward,
5′-CACCATTGGCAATGAGCGGTTC-3′ and reverse,
5′-AGGTCTTTGCGGATGTCCACGT-3′.
Western blot analysis
Cell lysates were obtained from 1×106
cultured cells with a mixture of RIPA protein extraction reagent,
protease inhibitor and phosphatase inhibitor (11836153001 and
4906845001; Roche, Shanghai, China). The protein concentration was
determined by bicinchoninic acid assay (BCA). Equal amounts (50
μg) of total protein lysate were separated by 5 to 10%
SDS-PAGE and then transferred onto PVDF membranes. The membranes
were then blocked in 5% non-fat milk at room temperature for 1 h.
Following this treatment, the membranes were probed with primary
antibodies against NRG1, ERK1/2, NRF2, β-actin and GAPDH at 4°C
overnight. Following incubation in a solution of goat anti-rabbit
or anti-mouse IgG at room temperature for 1 h, the membranes were
washed using TBST and then treated with enhanced chemiluminescence
reagents (Thermo Fisher Scientific, Inc.).
Cell proliferation assays
The Cell Counting kit-8 (CCK-8; CK04; Dojindo
Laboratories, Shanghai, China) assay and plate clone formation
assay were used to evaluate cell viability and the cell
proliferative capability. Briefly, 1,000 cells were seeded in 1
well of a 96-well plate for detection for 5 days. At the same time
each day, the cell culture medium was changed and CCK-8 reagents
were added at a ratio to medium of 1:10. Following 2 h of
incubation, the optical density at 450 nm of each well was measured
using a Synergy™ H4 Hybrid Multi-Mode Microplate Reader (BioTek,
Winooski, VT, USA) and transformed into cell numbers accordingly.
For clone formation assay, 500 cells were plated into a well of a
6-well plate and cultured in a cell incubator for 14 days with
regular medium changes. The cells were then washed with PBS and
fixed with 4% paraformaldehyde (P1110; Solarbio Biotechnology,
Shanghai, China) at room temperature for 30 min. Then cells were
stained with crystal violet staining solution (0.5%) (60506ES60;
Yeasen Biotechnology, Shanghai, China) at room temperature for 30
min and then washed with PBS 3 times. The plate was dried at room
temperature and images were aqcuired using a NEM-AL10 device
developed by Honor, Huawei (Guangdong, China). Clone numbers were
counted by Image J software and analyzed.
Statistical analysis
All statistical analyses were conducted using the
SPSS software program (version 22.0; IBM Corp., Armonk, NY, USA).
Pearson’s χ2 test was conducted to assess the
association between NRG1 expression and patient outcomes (Table I) and between NRG1 expression and
NRF2 expression in thyroid cancer samples (Table II). A value of P<0.05 was
considered to indicate a statistically significant difference.
Pearson’s correlation coefficient test was used to assess the
correlation between the expression of NRG1 and the expression of
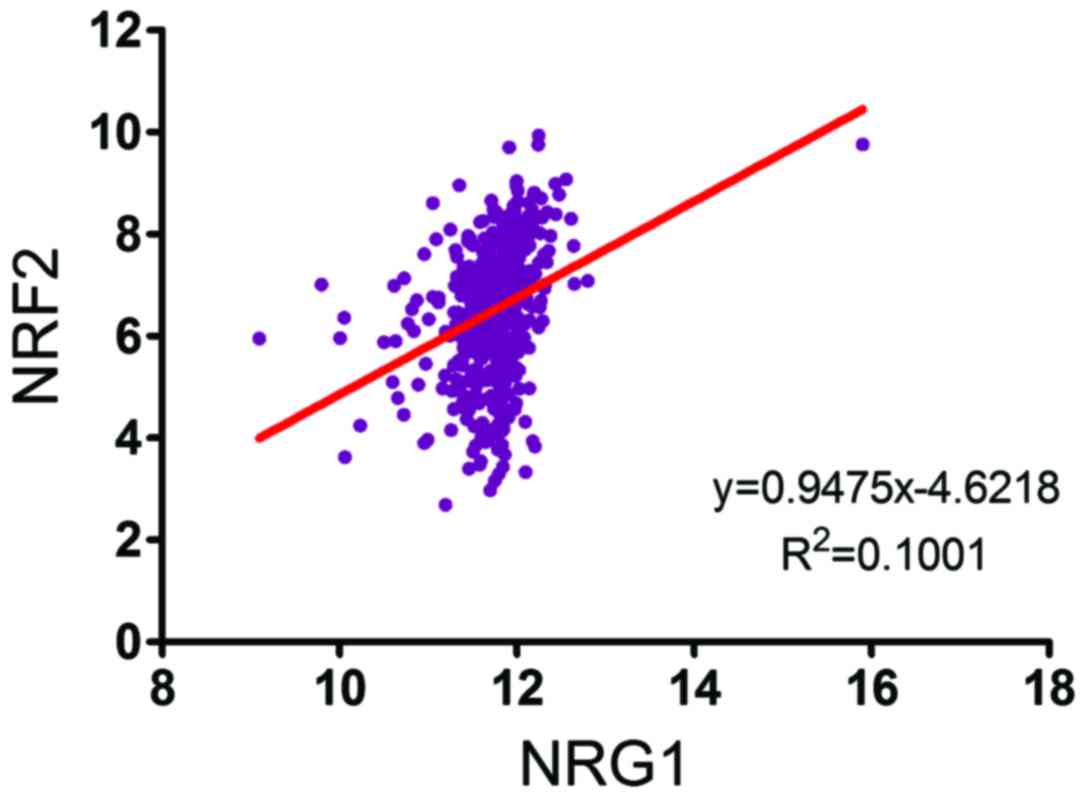
NRF2 (Fig. 5). Values of P<0.05
and R2>0.1 were considered to indicate statistically significant
differences. One-way ANOVA with Dunnett’s Multiple Comparison test
was used to determine the statistical significance and P-values in
Figs. 2Figure 3–4 and a value of P<0.05 was considered
to indicate a statistically significant difference.
 | Table IAssociation between NRG1 and lymph
node metastasis in patients with thyroid cancer according to
immunohistochemical analysis and patient outcomes. |
Table I
Association between NRG1 and lymph
node metastasis in patients with thyroid cancer according to
immunohistochemical analysis and patient outcomes.
| Tumor tissue | Regional lymph node
metastasisa
| Recurrence (local
or distant)b
|
|---|
| Negative | Positive | Negative | Positive |
|---|
| NRG1 | | | | |
| − | 43 (22.0) | 24 (12.2) | 66 (33.7) | 1 (0.5) |
| + | 21 (10.7) | 36 (18.4) | 49 (25) | 8 (4.1) |
| ++ | 23 (11.7) | 49 (25.0) | 60 (30.6) | 12 (6.1) |
 | Table IIPositive association between NRG1 and
NRF2 in PTC tissues according to immunohistochemical analysis. |
Table II
Positive association between NRG1 and
NRF2 in PTC tissues according to immunohistochemical analysis.
| Tumor tissue | NRF2
|
|---|
| − | + | ++ |
|---|
| NRG1 | | | |
| − | 36 (18.3) | 19 (9.7) | 12 (6.1) |
| + | 11 (5.6) | 30 (15.3) | 16 (8.2) |
| ++ | 9 (4.6) | 26 (13.3) | 37 (18.9) |
Results
NRG1 is a potential prognostic marker of
thyroid cancer
The clinicopathological characteristics of the
patients have been provided in a previous study by our group
(23). IHC staining and scoring
was used to detect the protein levels of NRG1 in the PTC samples.
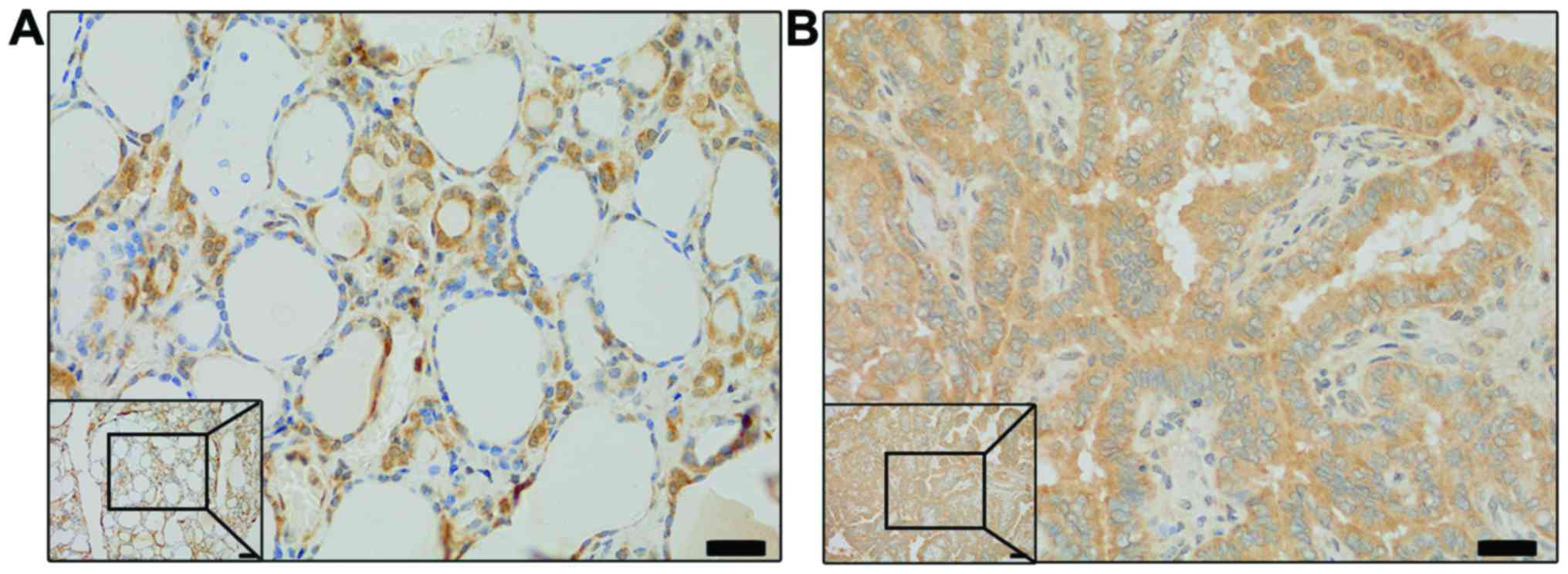
The NRG1 level was higher in the PTC samples than that in the
normal tissues (Fig. 1). In order
to examine the role of NRG1 in the lymph node metastasis (LNM) of
PTC, we examined its level in a PTC tissue microarray composed of
196 patient samples. Further analysis demonstrated that NRG1 was a
positive indicator of PTC LNM at diagnosis (Table I).
NRG1 regulates the viability and
proliferation of thyroid cancer
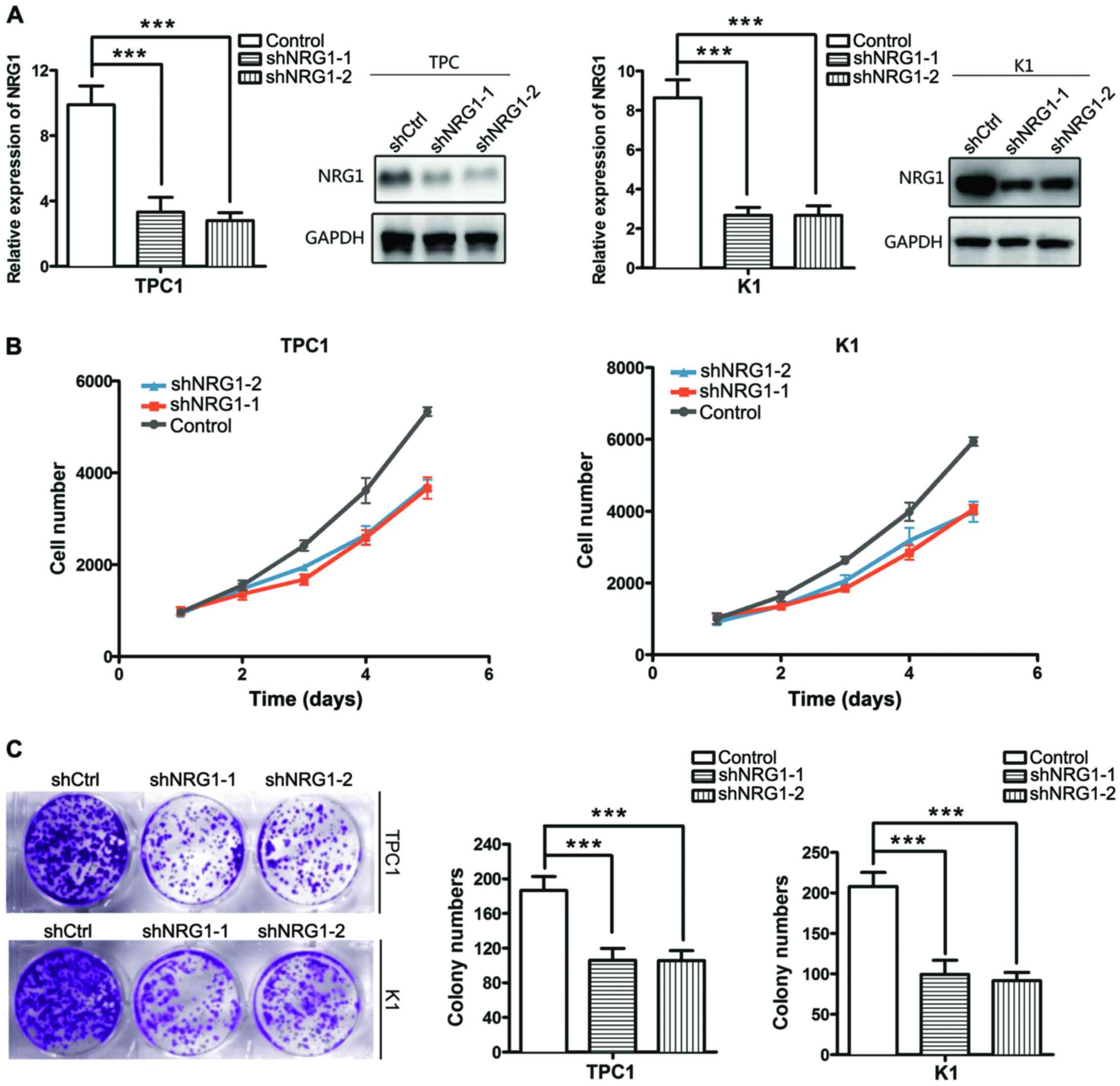
To determine the biological role of NRG1 in thyroid
cancer, we generated PTC cells K1 and TPC1) in which NRG1 was
knocked down using shRNA (two shRNA constructs effectively
decreased NRG1 expression. The knockdown efficiency was detected by
RT-qPCR and western blot analysis after 48 h of transfection
(Fig. 2A). To ascertain the
contribution of NRG1 to the proliferation of PTC cells, a CCK-8
cell proliferation assay was conducted. Cell viability was
significantly decreased when NRG1 was knocked down (Fig. 2B). Plate clone formation assay was
also performed to detect the effect of NRG1 on thyroid cancer cell
proliferation. The colony numbers of TPC1 and K1 cells were
significantly decreased when NRG1 expression was silenced (Fig. 2C).
NRG1 regulates the ERK1/2 pathway and the
expression of NRF2
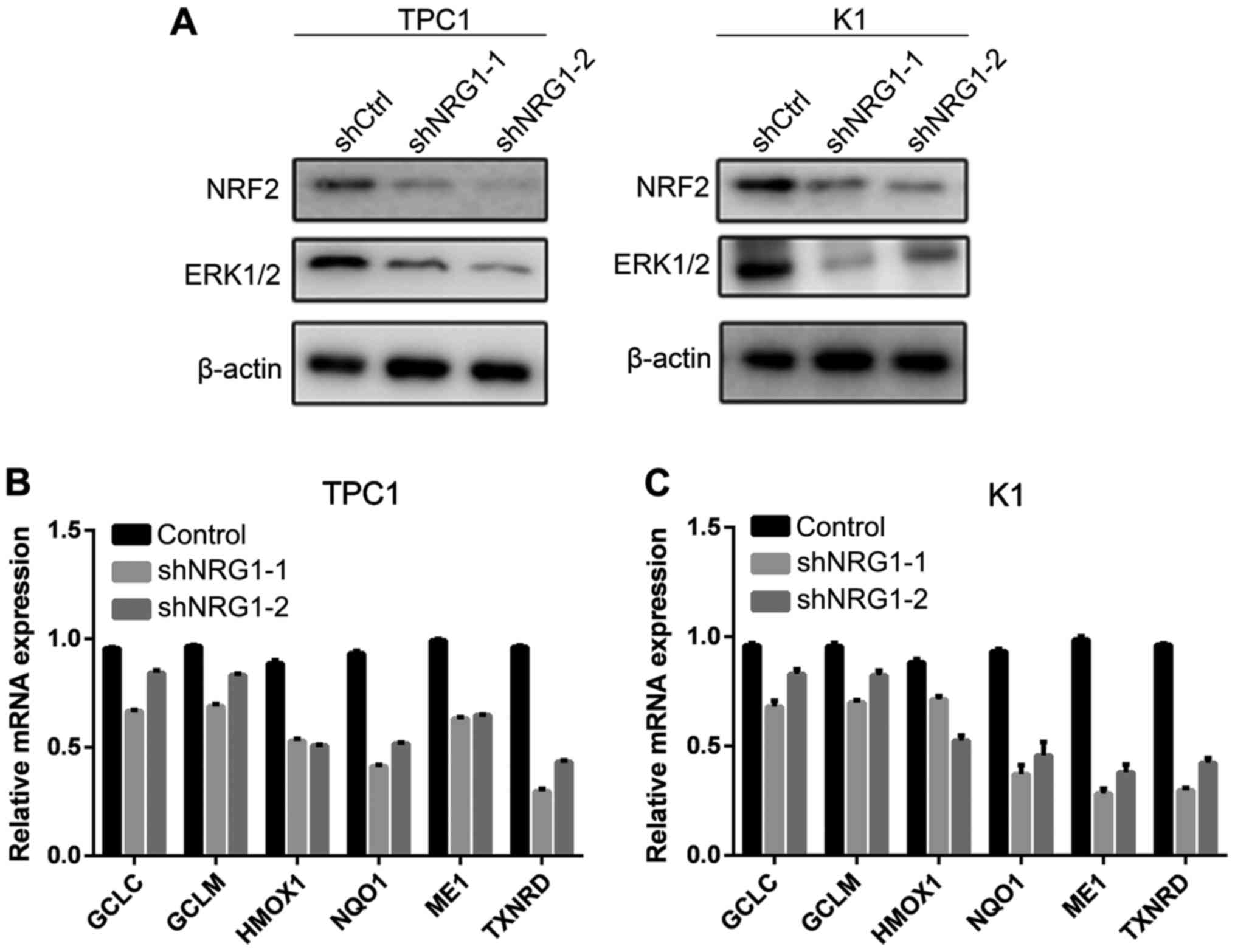
To explore the potential mechanisms through which
NRG1 regulates the viability and proliferation of thyroid cancer
cells, we further examined the association between NRG1 and other
thyroid cancer-related pathways. The results revealed that both the
expression of ERK1/2 and NRF2 decreased when NRG1 was knocked down
(Fig. 3A).
NRG1 is essential for the transcription
of NRF2 target genes
We further detected the downstream targets of the
NRF2/ARE pathway to explore the biological effects of NGR1. The
results of RT-qPCR revealed that NRG1 silencing decreased the
expression of ARE-driven genes, including GCLC, GCLM,
HMOX1, NQO1, ME1 and TXNRD (Fig. 3B and C). These results suggest that
NRG1 is an upstream transcriptional regulator of antioxidant
enzymes, whose regulatory function is dependent on the NRF2/ARE
pathway.
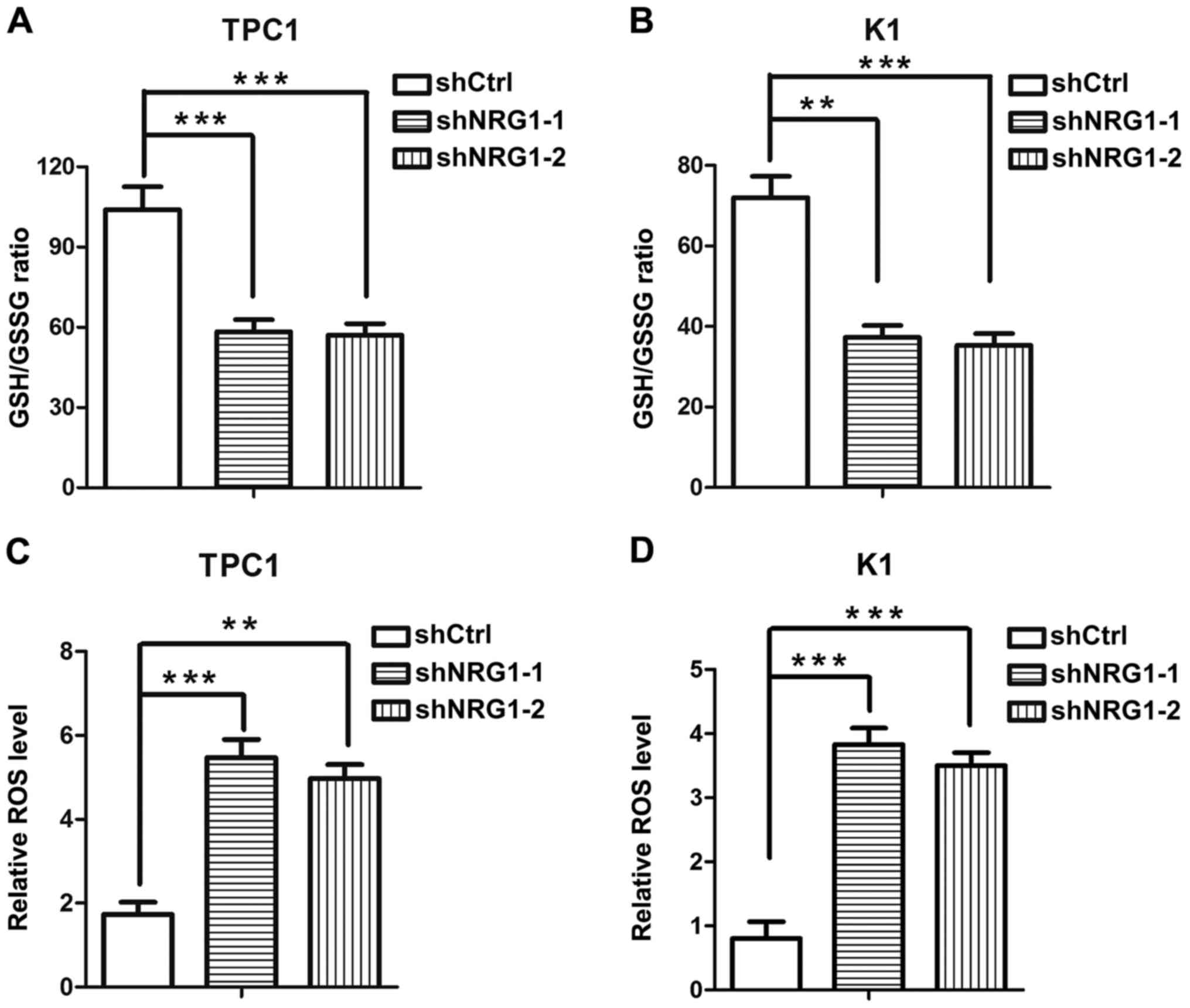
NRG1 modulates the redox status of
thyroid cancer cells
Subsequently, we attempted to explore the
contribution of NRG1 to redox homeostasis. We found that when NRG1
was knocked down, the GSH/GSSG ratios in the PTC cells were
significantly decreased, suggesting that NRG1 maintains the
reductive status of cells (Fig. 4A and
B). The intracellular ROS levels were examined to examine the
effect of NRG1 on the oxidative stress level of PTC cells directly.
Consistent with the changes observed in the GSH/GSSG ratio levels,
the knockdown of NRG1 significantly elevated the intracellular ROS
levels of the PTC cells, indicating that PTC cells in which NRG1
expression is knocked down are subjected to more severe oxidative
stress (Fig. 4C and D). These
results demonstrate that NRG1 plays an important regulatory role in
the redox balance of PTC cells.
NRG1 expression is positively associated
with NRF2 expression in PTC samples
The association between NRG1 and NRF2 was analyzed
by IHC staining of PTC tissues from 196 patients treated at the
Department of Head and Neck Surgery, Fudan University Shanghai
Cancer Center and by the analysis of data from 490 patient data
extracted from TCGA database. In the 196 PTC samples, the NRG2
level was high, in parallel with the high level of NRG1 in the PTC
samples. Statistical analysis indicated a positive association
between NRG1 and NRG2 in the PTC tissue microarray (Table II). Linear regression analysis of
the expression of NRG1 and NRF2 of 490 patients with PTC derived
from the TCGA database revealed a similar pattern (Fig. 5). These results suggest a positive
correlation between the expression of NRG1 and NRF2, implying the
role of NRG1 in redox homeostasis clinically.
Mechanisms through which NRG1 regulates
redox homeostasis in PTC cells
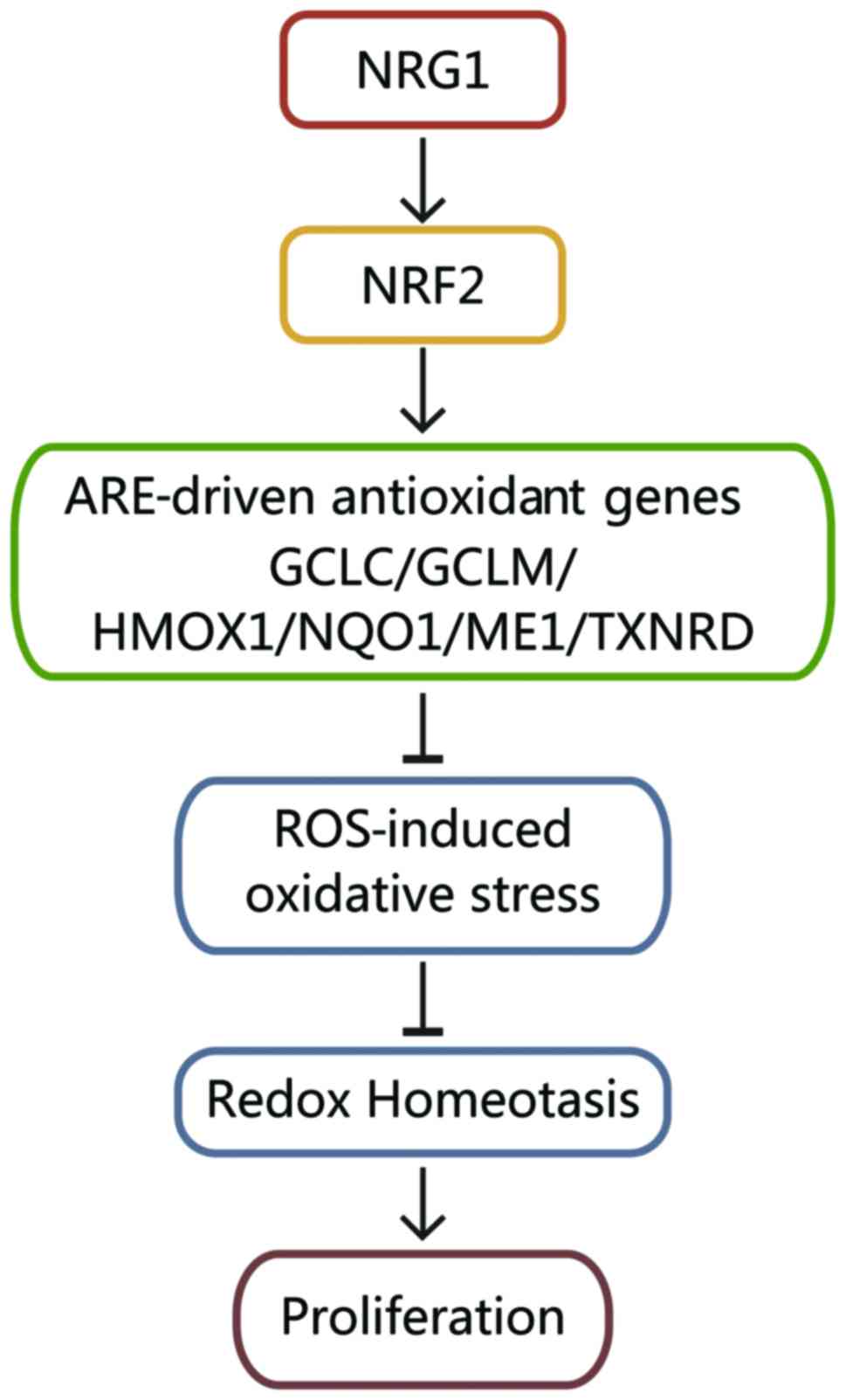
According to the results mentioned above, we
explored the mechanisms through which NRG1 regulates redox
homeostasis in PTC cells. NRG1 positively regulates NRF2, which
upregulates ARE-related antioxidant genes (GCLC, GCLM, HMOX1,
NQO1, ME1 and TXNRD). Through these mechanisms, NRG1
reduces ROS-induced oxidative stress and maintains the moderate
redox homeostasis that is essential for the proliferation of
papillary thyroid cancer cells (Fig.
6).
Discussion
PTC is the most common thyroid cancer. PTC typically
has a favorable prognosis, with an overall 10-year survival rate
>90%. As epidemiological studies have reported, the incidence of
thyroid cancer, particularly that of PTC, has markedly increased
over the past decades worldwide (1,24,25).
In addition, the number of the patients with refractory thyroid
cancer has also increased. In order to improve the prognosis of
patients with PTC, it is of utmost importance to elucidate the
biological mechanisms underlying the initiation and progression of
PTC.
Cells can only live with intracellular homeostases
to conduct their biological progresses, such as pH homeostasis,
temperature homeostasis and redox homeostasis. In order to maintain
intracellular homeostasis, cells manage to react rapidly to
perturbations of redox homeostasis through a series of redox
balancing mechanisms (26). ROS
produced by oxygen metabolism are one of the perturbations to redox
homeostasis. Excessive ROS productoin and ROS-mediated cell damage
give rise oxidative stress in cells. Thus, cells under oxidative
stress employ the antioxidant mechanism to survive and proliferate,
and the NRF2/ARE signaling pathway plays a pivotal role in this
process (27).
NRG1, a membrane glycoprotein, mediates cell-cell
signaling and is essential for the development and growth of
multiple organ systems. NRG1 can be processed into numerous
isoforms, which allows it to perform a wide range of functions.
NRG1 has been reported to interact with a number of important
signaling pathways, including the ErbB pathway, Ras/MAPK/ERK1/2
pathway and PI3K pathway (4,5,7,8).
According to the microarray analysis of the human
genome chip in this study, NRG1 expression was upregulated in the
PTC tissue samples compared with corresponding non-tumor thyroid
tissue samples obtained at the Fudan University Shanghai Cancer
Center. It was also revealed that NRG1 regulates the expression of
NRF2 in PTC cells and in clinical tissue samples.
Downstream targets of the NRF2/ARE pathway are
various antioxidant enzymes, whose expression levels protect cancer
cells from oxidative stress (28).
The expression levels of several antioxidant enzymes were examined
in this study, including GCLC, GCLM, HMOX1, NQO1, ME1 and TXNRD.
ME1 is a nicotinamide adenine dinucleotide phosphate
(NADP)-dependent malic enzyme producing NADPH that can be used for
antioxidation (29). TXNRD1, one
of pyridine nucleotide oxidoreductases, also balances redox
homeostais (30). Both GCLC and
GCLM are biosynthetic enzymes of GSH, which participates in
antioxidant reactions and redox homeostasis maintenance (31). HMOX1, acting as an essential enzyme
in heme catabolism, can counteract inflammation by upregulating
interleukin (IL)-10 and IL-1 receptor agonist IL-1 receptor agonist
(IL-1RA) expression (32). NQO1
encodes NAD(P)H dehydrogenase, which can protect cellular membranes
from peroxidative injury (33).
In this study, we demonstrated that NRG1 may be a
regulator of redox homeostasis, by promoting the transcription of
these target antioxidant enzymes via NRF2. Due to the upregulation
of these antioxidant enzymes by NRF2, intracellular ROS levels are
regulated to moderate levels, creating a homeostasis that is
essential for the survival, proliferation, migration and invasion
for PTC cells.
In conclusion, in this study, we demonstrated that
NRG1 activates the antioxidant pathway by upregulating the
expression of antioxidant enzymes through NRF2, thus modulating
redox homeostasis in PTC (Fig. 6).
However, to clarify the exact mechanisms through which NRG1
activates the antioxidant response, further studies are warranted.
The role of NRG1 in ROS detoxification suggests the potential use
of NRG1 as a therapeutic target in the treatment of PTC.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (no. 81702649 to NQ, nos.
81572622 and 81272934 to QHJ, nos. 81472498 and 81772851 to YLW and
no. 81502317 to WJW) and from the Shanghai Rising-Star Program (no.
15QA1401100 to YLW).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors’ contributions
TTZ, NQ, GHS, YXZ and RLS were involved in the
conception and design of the study. LZ, TTZ and YW were involved in
the acquisition of the clinical data. NQ, LZ, WJW, YLW and QHJ
conducted the experiments. YJW and XMM analyzed and interpreted the
data. TTZ, NQ, LZ and RLS wrote the manuscript. All authors have
read and approved the manuscript.
Ethics approval and consent to
participate
This study was approved by the Human Ethics
Committee/Institutional Review Board of Fudan University Shanghai
Cancer Center. All 196 patients signed the written informed
consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Lim H, Devesa SS, Sosa JA, Check D and
Kitahara CM: Trends in thyroid cancer incidence and mortality in
the United States, 1974–2013. JAMA. 317:1338–1348. 2017. View Article : Google Scholar
|
|
2
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM and
Schlumberger M: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar
|
|
3
|
Acosta-Ortega J, Montalbán-Romero S,
García-Solano J, Sánchez- Sánchez C and Pérez-Guillermo M:
Simultaneous medullary carcinoma of the thyroid gland and Hodgkin’s
lymphoma in bilateral lymph nodes of the neck: A potential pitfall
in fine-needle aspiration cytology. Diagn Cytopathol. 31:255–258.
2004. View
Article : Google Scholar
|
|
4
|
Falls DL: Neuregulins: Functions, forms,
and signaling strategies. Exp Cell Res. 284:14–30. 2003. View Article : Google Scholar
|
|
5
|
Stefansson H, Steinthorsdottir V,
Thorgeirsson TE, Gulcher JR and Stefansson K: Neuregulin 1 and
schizophrenia. Ann Med. 36:62–71. 2004. View Article : Google Scholar
|
|
6
|
Wadugu B and Kühn B: The role of
neuregulin/ErbB2/ErbB4 signaling in the heart with special focus on
effects on cardiomyocyte proliferation. Am J Physiol Heart Circ
Physiol. 302:H2139–H2147. 2012. View Article : Google Scholar
|
|
7
|
Sheean ME, McShane E, Cheret C, Walcher J,
Müller T, Wulf-Goldenberg A, Hoelper S, Garratt AN, Krüger M and
Rajewsky K: Activation of MAPK overrides the termination of myelin
growth and replaces Nrg1/ErbB3 signals during Schwann cell
development and myelination. Genes Dev. 28:290–303. 2014.
View Article : Google Scholar
|
|
8
|
Cheng H, Terai M, Kageyama K, Ozaki S,
McCue PA, Sato T and Aplin AE: Paracrine effect of NRG1 and HGF
drives resistance to MEK inhibitors in metastatic uveal melanoma.
Cancer Res. 75:2737–2748. 2015. View Article : Google Scholar
|
|
9
|
Vyas S, Zaganjor E and Haigis MC:
Mitochondria and cancer. Cell. 166:555–566. 2016. View Article : Google Scholar
|
|
10
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View
Article : Google Scholar
|
|
11
|
Sena LA and Chandel NS: Physiological
roles of mitochondrial reactive oxygen species. Mol Cell.
48:158–167. 2012. View Article : Google Scholar
|
|
12
|
Favaro E, Bensaad K, Chong MG, Tennant DA,
Ferguson DJ, Snell C, Steers G, Turley H, Li JL and Günther UL:
Glucose utilization via glycogen phosphorylase sustains
proliferation and prevents premature senescence in cancer cells.
Cell Metab. 16:751–764. 2012. View Article : Google Scholar
|
|
13
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE and Wong M: Association of
reactive oxygen species levels and radioresistance in cancer stem
cells. Nature. 458:780–783. 2009. View Article : Google Scholar
|
|
14
|
Li W and Kong AN: Molecular mechanisms of
Nrf2-mediated antioxidant response. Mol Carcinog. 48:91–104. 2009.
View Article : Google Scholar
|
|
15
|
Taguchi K, Motohashi H and Yamamoto M:
Molecular mechanisms of the Keap1-Nrf2 pathway in stress response
and cancer evolution. Genes Cells. 16:123–140. 2011. View Article : Google Scholar
|
|
16
|
He H, Li W, Liyanarachchi S, Wang Y, Yu L,
Genutis LK, Maharry S, Phay JE, Shen R, Brock P and de la Chapelle
A: The role of NRG1 in the predisposition to papillary thyroid
carcinoma. J Clin Endocrinol Metab. Nov 7–2017.Epub ahead of
print.
|
|
17
|
Jendrzejewski J, Liyanarachchi S, Nagy R,
Senter L, Wakely PE, Thomas A, Nabhan F, He H, Li W and Sworczak K:
Papillary thyroid carcinoma: Association between germline DNA
variant markers and clinical parameters. Thyroid. 26:1276–1284.
2016. View Article : Google Scholar
|
|
18
|
Rogounovitch TI, Bychkov A, Takahashi M,
Mitsutake N, Nakashima M, Nikitski AV, Hayashi T, Hirokawa M,
Ishigaki K and Shigematsu K: The common genetic variant rs944289 on
chromosome 14q13.3 associates with risk of both malignant and
benign thyroid tumors in the Japanese population. Thyroid.
25:333–340. 2015. View Article : Google Scholar
|
|
19
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th. Springer;
Chicago, IL: 2017, View Article : Google Scholar
|
|
20
|
Capes-Davis A, Theodosopoulos G, Atkin I,
Drexler HG, Kohara A, MacLeod RA, Masters JR, Nakamura Y, Reid YA,
Reddel RR and Freshney RI: Check your cultures! A list of
cross-contaminated or misidentified cell lines. Int J Cancer.
127:1–8. 2010. View Article : Google Scholar
|
|
21
|
Ribeiro FR, Meireles AM, Rocha AS and
Teixeira MR: Conventional and molecular cytogenetics of human
non-medullary thyroid carcinoma: Characterization of eight cell
line models and review of the literature on clinical samples. BMC
Cancer. 8:3712008. View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Shi RL, Qu N, Liao T, Wang YL, Wang Y, Sun
GH and Ji QH: Expression, clinical significance and mechanism of
Slit2 in papillary thyroid cancer. Int J Oncol. 48:2055–2062. 2016.
View Article : Google Scholar
|
|
24
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar
|
|
25
|
La Vecchia C, Malvezzi M, Bosetti C,
Garavello W, Bertuccio P, Levi F and Negri E: Thyroid cancer
mortality and incidence: A global overview. Int J Cancer.
136:2187–2195. 2015. View Article : Google Scholar
|
|
26
|
Jaramillo MC and Zhang DD: The emerging
role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev.
27:2179–2191. 2013. View Article : Google Scholar
|
|
27
|
Hayes AJ, Skouras C, Haugk B and Charnley
RM: Keap1-Nrf2 signalling in pancreatic cancer. Int J Biochem Cell
Biol. 65:288–299. 2015. View Article : Google Scholar
|
|
28
|
Zhou S, Ye W, Shao Q, Zhang M and Liang J:
Nrf2 is a potential therapeutic target in radioresistance in human
cancer. Crit Rev Oncol Hematol. 88:706–715. 2013. View Article : Google Scholar
|
|
29
|
Jiang P, Du W, Mancuso A, Wellen KE and
Yang X: Reciprocal regulation of 53 and malic enzymes modulates
metabolism and senescence. Nature. 493:689–693. 2013. View Article : Google Scholar
|
|
30
|
Dai B, Yoo SY, Bartholomeusz G, Graham RA,
Majidi M, Yan S, Meng J, Ji L, Coombes K and Minna JD:
KEAP1-dependent synthetic lethality induced by AKT and TXNRD1
inhibitors in lung cancer. Cancer Res. 73:5532–5543. 2013.
View Article : Google Scholar
|
|
31
|
Lien EC, Lyssiotis CA, Juvekar A, Hu H,
Asara JM, Cantley LC and Toker A: Glutathione biosynthesis is a
metabolic vulnerability in PI(3)K/Akt-driven breast cancer. Nat
Cell Biol. 18:572–578. 2016. View
Article : Google Scholar
|
|
32
|
Piantadosi CA, Withers CM, Bartz RR,
MacGarvey NC, Fu P, Sweeney TE, Welty-Wolf KE and Suliman HB: Heme
oxygenase-1 couples activation of mitochondrial biogenesis to
anti-inflammatory cytokine expression. J Biol Chem.
286:16374–16385. 2011. View Article : Google Scholar
|
|
33
|
Ross D and Siegel D: NAD(P)H:quinone
oxidoreductase 1 (NQO1, DT-diaphorase), functions and
pharmacogenetics. Methods Enzymol. 382:115–144. 2004. View Article : Google Scholar
|