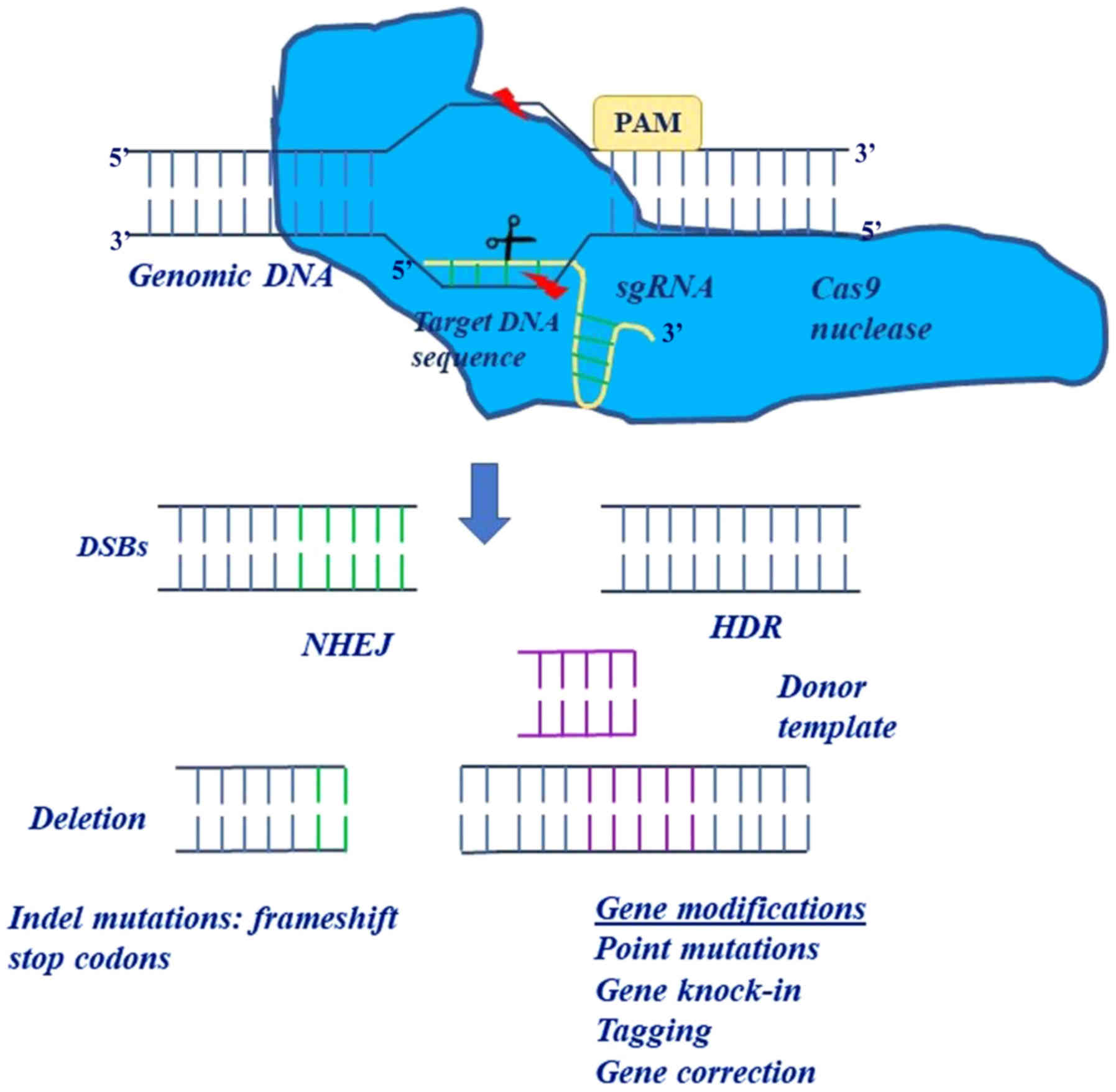
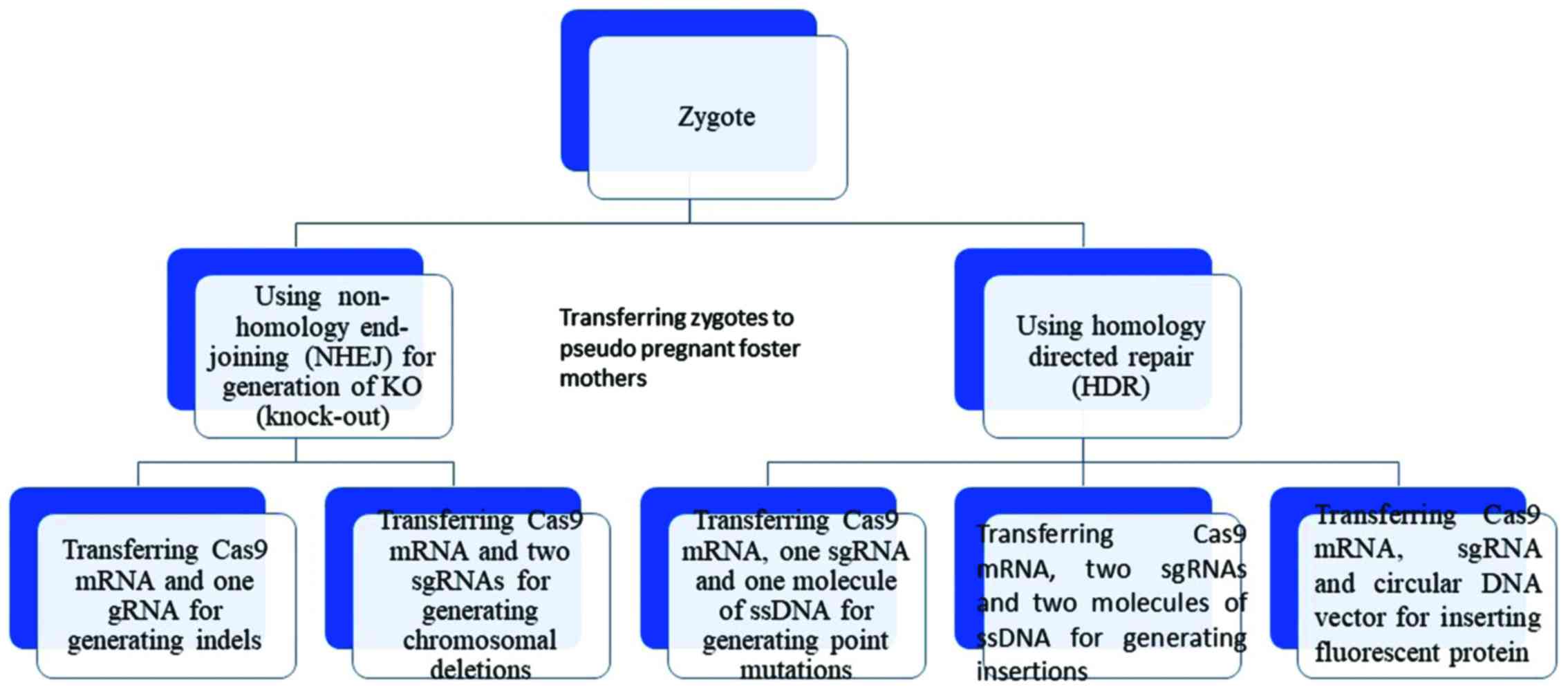
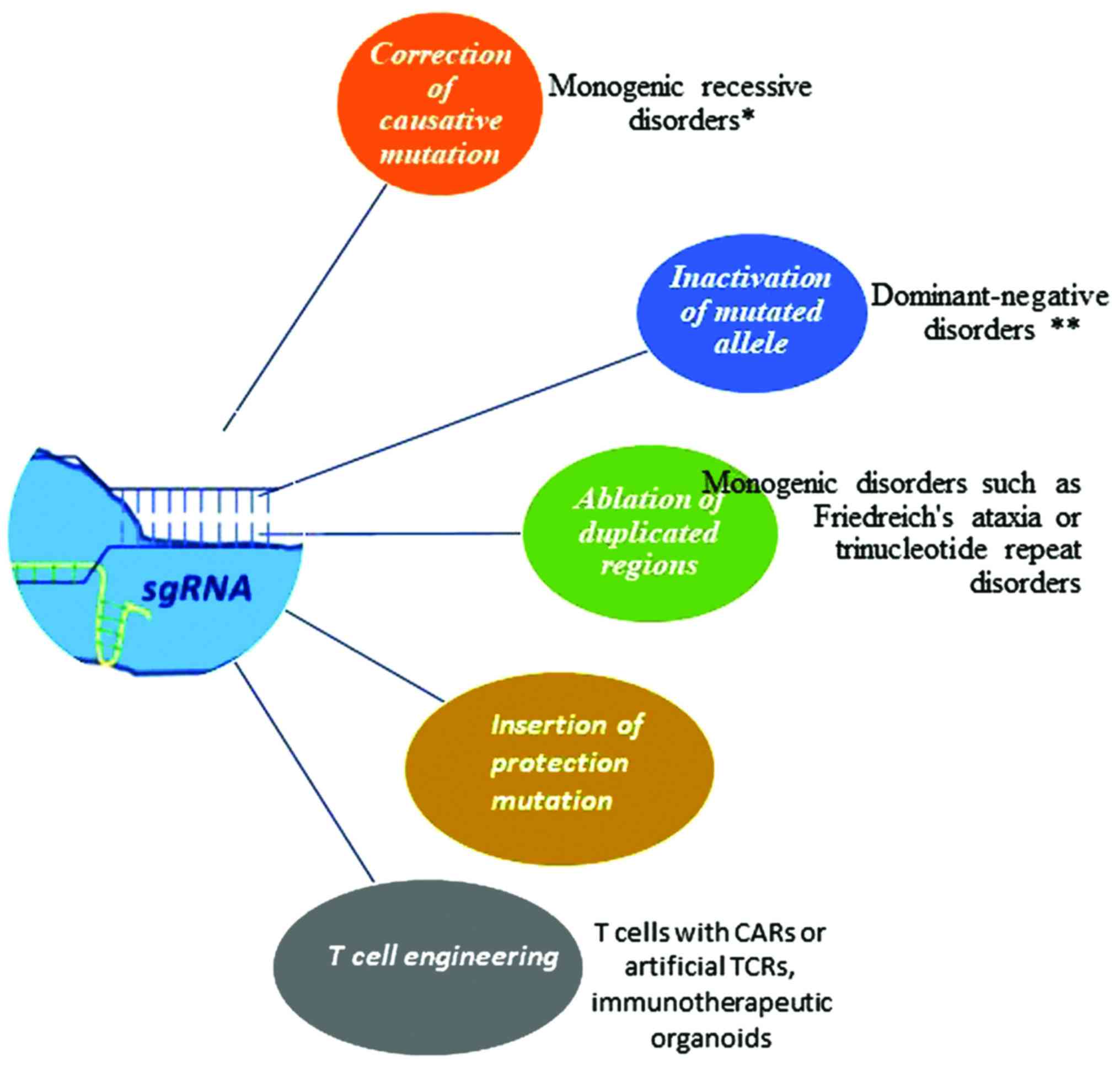
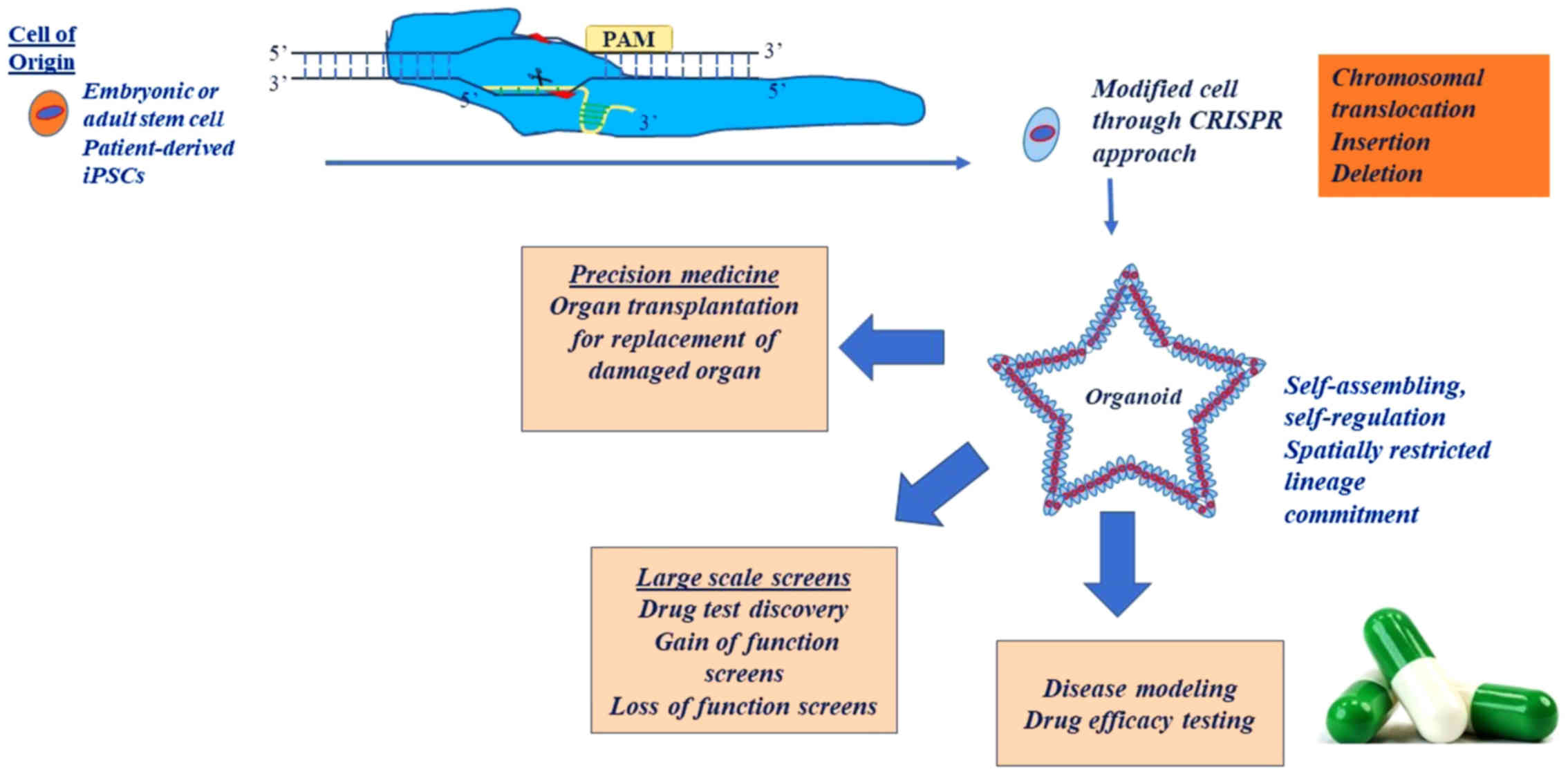
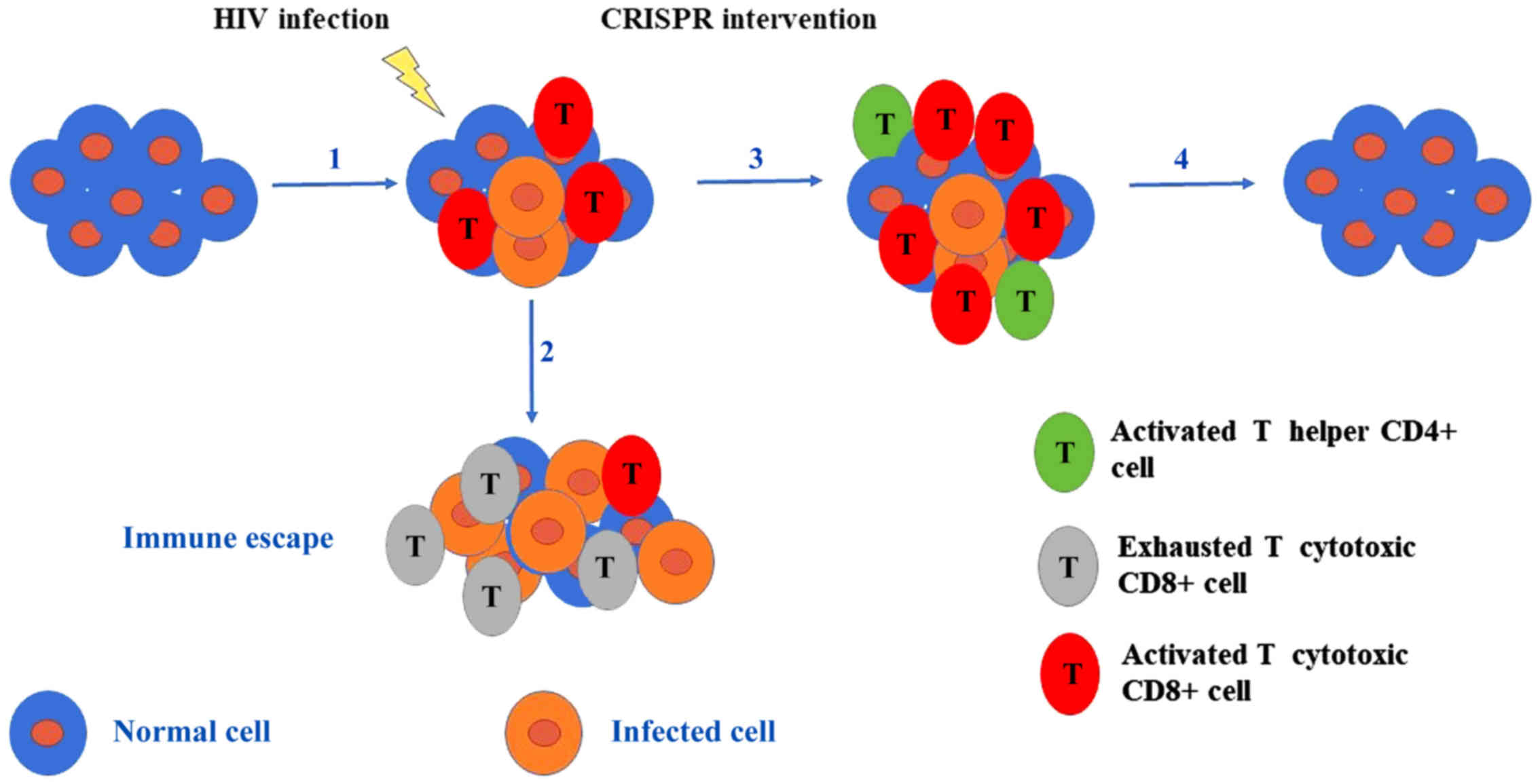
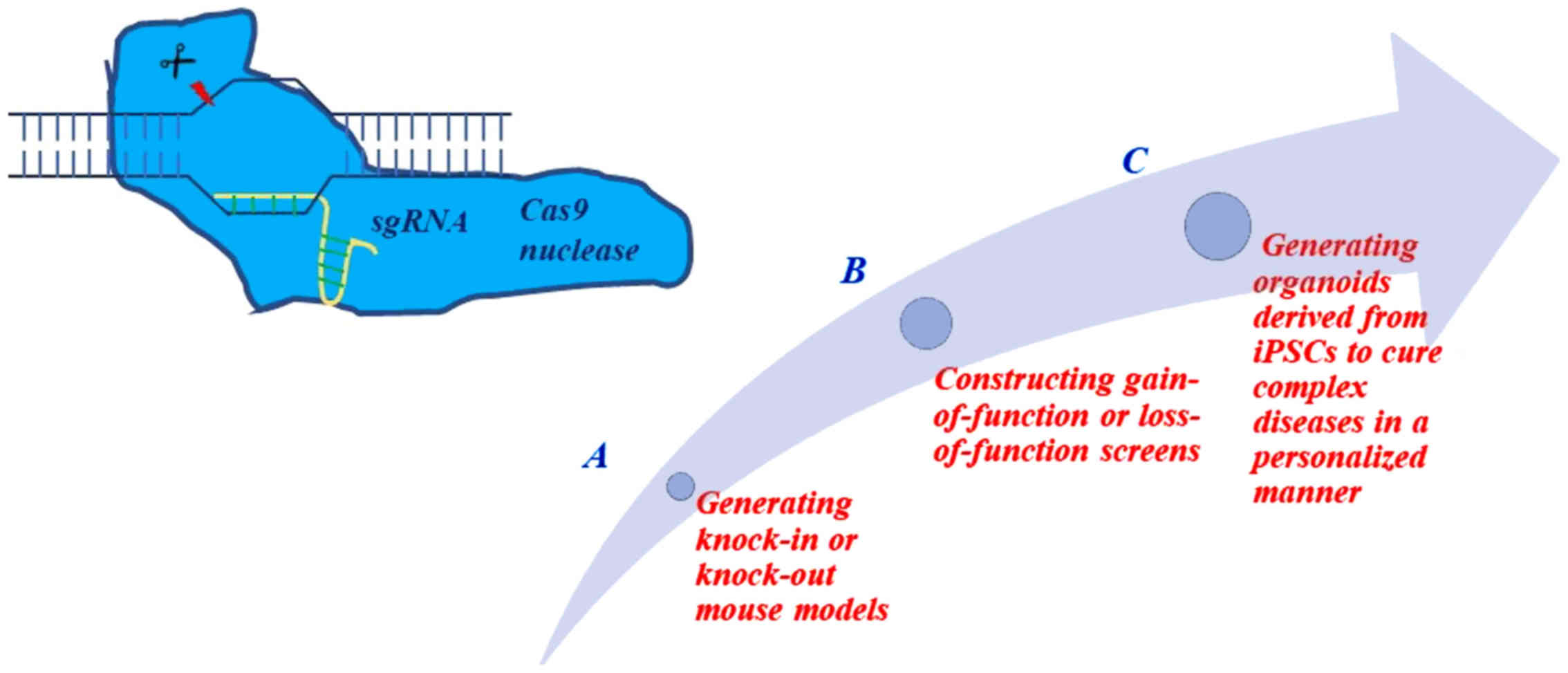
|
1
|
Bedell VM, Wang Y, Campbell JM, Poshusta
TL, Starker CG, Krug RG II, Tan W, Penheiter SG, Ma AC and Leung
AY: In vivo genome editing using a high-efficiency TALEN system.
Nature. 491:114–118. 2012. View Article : Google Scholar
|
|
2
|
Urnov FD, Miller JC, Lee YL, Beausejour
CM, Rock JM, Augustus S, Jamieson AC, Porteus MH, Gregory PD and
Holmes MC: Highly efficient endogenous human gene correction using
designed zinc-finger nucleases. Nature. 435:646–651. 2005.
View Article : Google Scholar
|
|
3
|
Kim EJ, Kang KH and Ju JH: CRISPR-Cas9: A
promising tool for gene editing on induced pluripotent stem cells.
Korean J Intern Med (Korean Assoc Intern Med). 32:42–61. 2017.
|
|
4
|
Cong L, Ran FA, Cox D, Lin S, Barretto R,
Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al: Multiplex
genome engineering using CRISPR/Cas systems. Science. 339:819–823.
2013. View Article : Google Scholar
|
|
5
|
Wu X, Scott DA, Kriz AJ, Chiu AC, Hsu PD,
Dadon DB, Cheng AW, Trevino AE, Konermann S, Chen S, et al:
Genome-wide binding of the CRISPR endonuclease Cas9 in mammalian
cells. Nat Biotechnol. 32:670–676. 2014. View Article : Google Scholar
|
|
6
|
Doudna JA and Charpentier E: Genome
editing. The new frontier of genome engineering with CRISPR-Cas9.
Science. 346:12580962014. View Article : Google Scholar
|
|
7
|
Wiedenheft B, Sternberg SH and Doudna JA:
RNA-guided genetic silencing systems in bacteria and archaea.
Nature. 482:331–338. 2012. View Article : Google Scholar
|
|
8
|
Mali P, Yang L, Esvelt KM, Aach J, Guell
M, DiCarlo JE, Norville JE and Church GM: RNA-guided human genome
engineering via Cas9. Science. 339:823–826. 2013. View Article : Google Scholar
|
|
9
|
Wright AV, Nuñez JK and Doudna JA: Biology
and applications of CRISPR systems: Harnessing nature' s toolbox
for genome engineering. Cell. 164:29–44. 2016. View Article : Google Scholar
|
|
10
|
van der Oost J, Westra ER, Jackson RN and
Wiedenheft B: Unravelling the structural and mechanistic basis of
CRISPR-Cas systems. Nat Rev Microbiol. 12:479–492. 2014. View Article : Google Scholar
|
|
11
|
Mougiakos I, Bosma EF, de Vos WM, van
Kranenburg R and van der Oost J: Next generation prokaryotic
engineering: The CRISPR-Cas Toolkit. Trends Biotechnol. 34:575–587.
2016. View Article : Google Scholar
|
|
12
|
Bolotin A, Quinquis B, Sorokin A and
Ehrlich SD: Clustered regularly interspaced short palindrome
repeats (CRISPRs) have spacers of extrachromosomal origin.
Microbiology. 151:2551–2561. 2005. View Article : Google Scholar
|
|
13
|
Westra ER, Semenova E, Datsenko KA,
Jackson RN, Wiedenheft B, Severinov K and Brouns SJ: Type I-E
CRISPR-cas systems discriminate target from non-target DNA through
base pairing-independent PAM recognition. PLoS Genet.
9:e10037422013. View Article : Google Scholar
|
|
14
|
Jinek M, Chylinski K, Fonfara I, Hauer M,
Doudna JA and Charpentier E: A programmable dual-RNA-guided DNA
endonuclease in adaptive bacterial immunity. Science. 337:816–821.
2012. View Article : Google Scholar
|
|
15
|
Larson MH, Gilbert LA, Wang X, Lim WA,
Weissman JS and Qi LS: CRISPR interference (CRISPRi) for
sequence-specific control of gene expression. Nat Protoc.
8:2180–2196. 2013. View Article : Google Scholar
|
|
16
|
Mojica FJ, Díez-Villaseñor C,
García-Martínez J and Almendros C: Short motif sequences determine
the targets of the prokaryotic CRISPR defence system. Microbiology.
155:733–740. 2009. View Article : Google Scholar
|
|
17
|
Mei Y, Wang Y, Chen H, Sun ZS and Ju XD:
Recent progress in CRISPR/Cas9 technology. J Genet Genomics.
43:63–75. 2016. View Article : Google Scholar
|
|
18
|
Ran FA, Hsu PD, Wright J, Agarwala V,
Scott DA and Zhang F: Genome engineering using the CRISPR-Cas9
system. Nat Protoc. 8:2281–2308. 2013. View Article : Google Scholar
|
|
19
|
Drost J and Clevers H: Who is in the
Driver' s Seat: Tracing cancer genes using CRISPR-barcoding. Mol
Cell. 63:352–354. 2016. View Article : Google Scholar
|
|
20
|
Lin S, Staahl BT, Alla RK and Doudna JA:
Enhanced homology-directed human genome engineering by controlled
timing of CRISPR/Cas9 delivery. eLife. 3:e047662014. View Article : Google Scholar
|
|
21
|
Ghezraoui H, Piganeau M, Renouf B, Renaud
JB, Sallmyr A, Ruis B, Oh S, Tomkinson AE, Hendrickson EA,
Giovannangeli C, et al: Chromosomal translocations in human cells
are generated by canonical nonhomologous end-joining. Mol Cell.
55:829–842. 2014. View Article : Google Scholar
|
|
22
|
Roukos V and Misteli T: The biogenesis of
chromosome translocations. Nat Cell Biol. 16:293–300. 2014.
View Article : Google Scholar
|
|
23
|
Komor AC, Kim YB, Packer MS, Zuris JA and
Liu DR: Programmable editing of a target base in genomic DNA
without double-stranded DNA cleavage. Nature. 533:420–424. 2016.
View Article : Google Scholar
|
|
24
|
Suzuki K, Tsunekawa Y, Hernandez-Benitez
R, Wu J, Zhu J, Kim EJ, Hatanaka F, Yamamoto M, Araoka T, Li Z, et
al: In vivo genome editing via CRISPR/Cas9 mediated
homology-independent targeted integration. Nature. 540:144–149.
2016. View Article : Google Scholar
|
|
25
|
Nishimasu H, Ran FA, Hsu PD, Konermann S,
Shehata SI, Dohmae N, Ishitani R, Zhang F and Nureki O: Crystal
structure of Cas9 in complex with guide RNA and target DNA. Cell.
156:935–949. 2014. View Article : Google Scholar
|
|
26
|
Christian M, Cermak T, Doyle EL, Schmidt
C, Zhang F, Hummel A, Bogdanove AJ and Voytas DF: Targeting DNA
double-strand breaks with TAL effector nucleases. Genetics.
186:757–761. 2010. View Article : Google Scholar
|
|
27
|
Kabadi AM, Ousterout DG, Hilton IB and
Gersbach CA: Multiplex CRISPR/Cas9-based genome engineering from a
single lentiviral vector. Nucleic Acids Res. 42:e1472014.
View Article : Google Scholar
|
|
28
|
Sadikovic B, Al-Romaih K, Squire JA and
Zielenska M: Cause and consequences of genetic and epigenetic
alterations in human cancer. Curr Genomics. 9:394–408. 2008.
View Article : Google Scholar
|
|
29
|
Baliou S, Adamaki M, Kyriakopoulos AM,
Spandidos DA, Panayiotidis M, Christodoulou I and Zoumpourlis V:
Role of the CRISPR system in controlling gene transcription and
monitoring cell fate (Review). Mol Med Rep. 17:1421–1427. 2018.
|
|
30
|
Swiech L, Heidenreich M, Banerjee A, Habib
N, Li Y, Trombetta J, Sur M and Zhang F: In vivo interrogation of
gene function in the mammalian brain using CRISPR-Cas9. Nat
Biotechnol. 33:102–106. 2015. View Article : Google Scholar
|
|
31
|
Yang H, Wang H, Shivalila CS, Cheng AW,
Shi L and Jaenisch R: One-step generation of mice carrying reporter
and conditional alleles by CRISPR/Cas-mediated genome engineering.
Cell. 154:1370–1379. 2013. View Article : Google Scholar
|
|
32
|
Torres R, Martin MC, Garcia A, Cigudosa
JC, Ramirez JC and Rodriguez-Perales S: Engineering human
tumour-associated chromosomal translocations with the RNA-guided
CRISPR-Cas9 system. Nat Commun. 5:39642014. View Article : Google Scholar
|
|
33
|
Canver MC, Bauer DE, Dass A, Yien YY,
Chung J, Masuda T, Maeda T, Paw BH and Orkin SH: Characterization
of genomic deletion efficiency mediated by clustered regularly
interspaced short palindromic repeats (CRISPR)/Cas9 nuclease system
in mammalian cells. J Biol Chem. 292:25562017. View Article : Google Scholar
|
|
34
|
Lupiáñez DG, Kraft K, Heinrich V, Krawitz
P, Brancati F, Klopocki E, Horn D, Kayserili H, Opitz JM, Laxova R,
et al: Disruptions of topological chromatin domains cause
pathogenic rewiring of gene-enhancer interactions. Cell.
161:1012–1025. 2015. View Article : Google Scholar
|
|
35
|
Choi PS and Meyerson M: Targeted genomic
rearrangements using CRISPR/Cas technology. Nat Commun. 5:37282014.
View Article : Google Scholar
|
|
36
|
Cho SW, Kim S, Kim Y, Kweon J, Kim HS, Bae
S and Kim JS: Analysis of off-target effects of CRISPR/Cas-derived
RNA-guided endonucleases and nickases. Genome Res. 24:132–141.
2014. View Article : Google Scholar
|
|
37
|
Wang H, Yang H, Shivalila CS, Dawlaty MM,
Cheng AW, Zhang F and Jaenisch R: One-step generation of mice
carrying mutations in multiple genes by CRISPR/Cas-mediated genome
engineering. Cell. 153:910–918. 2013. View Article : Google Scholar
|
|
38
|
Yang L, Guell M, Byrne S, Yang JL, De Los
Angeles A, Mali P, Aach J, Kim-Kiselak C, Briggs AW, Rios X, et al:
Optimization of scarless human stem cell genome editing. Nucleic
Acids Res. 41:9049–9061. 2013. View Article : Google Scholar
|
|
39
|
Yin H, Xue W, Chen S, Bogorad RL,
Benedetti E, Grompe M, Koteliansky V, Sharp PA, Jacks T and
Anderson DG: Genome editing with Cas9 in adult mice corrects a
disease mutation and phenotype. Nat Biotechnol. 32:551–553. 2014.
View Article : Google Scholar
|
|
40
|
Schumann K, Lin S, Boyer E, Simeonov DR,
Subramaniam M, Gate RE, Haliburton GE, Ye CJ, Bluestone JA, Doudna
JA, et al: Generation of knock-in primary human T cells using Cas9
ribonucleoproteins. Proc Natl Acad Sci USA. 112:10437–10442. 2015.
View Article : Google Scholar
|
|
41
|
Renaud JB, Boix C, Charpentier M, De Cian
A, Cochennec J, Duvernois-Berthet E, Perrouault L, Tesson L,
Edouard J, Thinard R, et al: Improved genome editing efficiency and
flexibility using modified oligonucleotides with TALEN and
CRISPR-Cas9 nucleases. Cell Rep. 14:2263–2272. 2016. View Article : Google Scholar
|
|
42
|
Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech
L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, Jovanovic M, et
al: CRISPR-Cas9 knockin mice for genome editing and cancer
modeling. Cell. 159:440–455. 2014. View Article : Google Scholar
|
|
43
|
Kaulich M, Lee YJ, Lönn P, Springer AD,
Meade BR and Dowdy SF: Efficient CRISPR-rAAV engineering of
endogenous genes to study protein function by allele-specific RNAi.
Nucleic Acids Res. 43:e452015. View Article : Google Scholar
|
|
44
|
Li Y, Park AI, Mou H, Colpan C, Bizhanova
A, Akama-Garren E, Joshi N, Hendrickson EA, Feldser D, Yin H, et
al: A versatile reporter system for CRISPR-mediated chromosomal
rearrangements. Genome Biol. 16:1112015. View Article : Google Scholar
|
|
45
|
Xiao A, Wang Z, Hu Y, Wu Y, Luo Z, Yang Z,
Zu Y, Li W, Huang P, Tong X, et al: Chromosomal deletions and
inversions mediated by TALENs and CRISPR/Cas in zebrafish. Nucleic
Acids Res. 41:e1412013. View Article : Google Scholar
|
|
46
|
Heyer J, Kwong LN, Lowe SW and Chin L:
Non-germline genetically engineered mouse models for translational
cancer research. Nat Rev Cancer. 10:470–480. 2010. View Article : Google Scholar
|
|
47
|
Xue W, Chen S, Yin H, Tammela T,
Papagiannakopoulos T, Joshi NS, Cai W, Yang G, Bronson R and
Crowley DG: et al CRISPR-mediated direct mutation of cancer genes
in the mouse liver. Nature. 514:380–384. 2014. View Article : Google Scholar
|
|
48
|
Zuckermann M, Hovestadt V, Knobbe-Thomsen
CB, Zapatka M, Northcott PA, Schramm K, Belic J, Jones DT, Tschida
B, Moriarity B, et al: Somatic CRISPR/Cas9-mediated tumour
suppressor disruption enables versatile brain tumour modelling. Nat
Commun. 6:73912015. View Article : Google Scholar
|
|
49
|
Doerks T, Copley RR, Schultz J, Ponting CP
and Bork P: Systematic identification of novel protein domain
families associated with nuclear functions. Genome Res. 12:47–56.
2002. View Article : Google Scholar
|
|
50
|
Guerra C, Mijimolle N, Dhawahir A, Dubus
P, Barradas M, Serrano M, Campuzano V and Barbacid M: Tumor
induction by an endogenous K-ras oncogene is highly dependent on
cellular context. Cancer Cell. 4:111–120. 2003. View Article : Google Scholar
|
|
51
|
Findlay GM, Boyle EA, Hause RJ, Klein JC
and Shendure J: Saturation editing of genomic regions by multiplex
homology-directed repair. Nature. 513:120–123. 2014. View Article : Google Scholar
|
|
52
|
Hacisuleyman E, Goff LA, Trapnell C,
Williams A, Henao-Mejia J, Sun L, McClanahan P, Hendrickson DG,
Sauvageau M, Kelley DR, et al: Topological organization of
multichromosomal regions by the long intergenic noncoding RNA
Firre. Nat Struct Mol Biol. 21:198–206. 2014. View Article : Google Scholar
|
|
53
|
Krishnaswamy JK, Singh A, Gowthaman U, Wu
R, Gorrepati P, Sales Nascimento M, Gallman A, Liu D, Rhebergen AM,
Calabro S, et al: Coincidental loss of DOCK8 function in
NLRP10-deficient and C3H/HeJ mice results in defective dendritic
cell migration. Proc Natl Acad Sci USA. 112:3056–3061. 2015.
View Article : Google Scholar
|
|
54
|
Billon P, Bryant EE, Joseph SA, Nambiar
TS, Hayward SB, Rothstein R and Ciccia A: CRISPR-mediated base
editing enables efficient disruption of eukaryotic genes through
induction of STOP codons. Moll Cell. 67:1068–79.e4. 2017.
View Article : Google Scholar
|
|
55
|
Blasco RB, Karaca E, Ambrogio C, Cheong
TC, Karayol E, Minero VG, Voena C and Chiarle R: Simple and rapid
in vivo generation of chromosomal rearrangements using CRISPR/Cas9
technology. Cell Rep. 9:1219–1227. 2014. View Article : Google Scholar
|
|
56
|
Maddalo D, Manchado E, Concepcion CP,
Bonetti C, Vidigal JA, Han YC, Ogrodowski P, Crippa A, Rekhtman N,
de Stanchina E, et al: In vivo engineering of oncogenic chromosomal
rearrangements with the CRISPR/Cas9 system. Nature. 516:423–427.
2014. View Article : Google Scholar
|
|
57
|
Nishio M, Kim DW, Wu YL, Nakagawa K,
Solomon BJ, Shaw AT, Hashigaki S, Ohki E, Usari T, Paolini J, et
al: Crizotinib versus chemotherapy in Asian patients with advanced
ALK-positive non-small cell lung cancer. Cancer Res Treat. Jul
6–2017.Epub ahead of print. View Article : Google Scholar
|
|
58
|
Ding Q, Strong A, Patel KM, Ng SL, Gosis
BS, Regan SN, Cowan CA, Rader DJ and Musunuru K: Permanent
alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circ
Res. 115:488–492. 2014. View Article : Google Scholar
|
|
59
|
Yang DG, Chung YC, Lai YK, Lai CW, Liu HJ
and Hu YC: Corrigendum to 'Avian Influenza Virus Hemagglutinin
Display on Baculovirus Envelope: Cytoplasmic Domain Affects Virus
Properties and Vaccine Potential'. Mol Ther. 15:17362007.
View Article : Google Scholar
|
|
60
|
Cancer Genome Atlas N; Cancer Genome Atlas
Network: Comprehensive molecular characterization of human colon
and rectal cancer. Nature. 487:330–337. 2012. View Article : Google Scholar
|
|
61
|
Sánchez-Rivera FJ, Papagiannakopoulos T,
Romero R, Tammela T, Bauer MR, Bhutkar A, Joshi NS, Subbaraj L,
Bronson RT, Xue W, et al: Rapid modelling of cooperating genetic
events in cancer through somatic genome editing. Nature.
516:428–431. 2014. View Article : Google Scholar
|
|
62
|
Chiou SH, Winters IP, Wang J, Naranjo S,
Dudgeon C, Tamburini FB, Brady JJ, Yang D, Grüner BM, Chuang CH, et
al: Pancreatic cancer modeling using retrograde viral vector
delivery and in vivo CRISPR/Cas9-mediated somatic genome editing.
Genes Dev. 29:1576–1585. 2015. View Article : Google Scholar
|
|
63
|
Annunziato S, Kas SM, Nethe M, Yücel H,
Del Bravo J, Pritchard C, Bin Ali R, van Gerwen B, Siteur B, Drenth
AP, et al: Modeling invasive lobular breast carcinoma by
CRISPR/Cas9-mediated somatic genome editing of the mammary gland.
Genes Dev. 30:1470–1480. 2016. View Article : Google Scholar
|
|
64
|
Oh B, Hwang S, McLaughlin J, Solter D and
Knowles BB: Timely translation during the mouse oocyte-to-embryo
transition. Development. 127:3795–3803. 2000.
|
|
65
|
Flemr M and Bühler M: Single-step
generation of conditional knockout mouse embryonic stem cells. Cell
Rep. 12:709–716. 2015. View Article : Google Scholar
|
|
66
|
Malina A, Mills JR, Cencic R, Yan Y,
Fraser J, Schippers LM, Paquet M, Dostie J and Pelletier J:
Repurposing CRISPR/Cas9 for in situ functional assays. Genes Dev.
27:2602–2614. 2013. View Article : Google Scholar
|
|
67
|
Heckl D, Kowalczyk MS, Yudovich D,
Belizaire R, Puram RV, McConkey ME, Thielke A, Aster JC, Regev A
and Ebert BL: Generation of mouse models of myeloid malignancy with
combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat
Biotechnol. 32:941–946. 2014. View Article : Google Scholar
|
|
68
|
Chen C, Liu Y, Rappaport AR, Kitzing T,
Schultz N, Zhao Z, Shroff AS, Dickins RA, Vakoc CR, Bradner JE, et
al: MLL3 is a haploinsufficient 7q tumor suppressor in acute
myeloid leukemia. Cancer Cell. 25:652–665. 2014. View Article : Google Scholar
|
|
69
|
Zhong C, Yin Q, Xie Z, Bai M, Dong R, Tang
W, Xing YH, Zhang H, Yang S, Chen LL, et al: CRISPR-Cas9-mediated
genetic screening in mice with haploid embryonic stem cells
carrying a Guide RNA Library. Cell Stem Cell. 17:221–232. 2015.
View Article : Google Scholar
|
|
70
|
Park CY, Kim DH, Son JS, Sung JJ, Lee J,
Bae S, Kim JH, Kim DW and Kim JS: Functional correction of large
factor VIII gene chromosomal inversions in hemophilia a
patient-derived iPSCs using CRISPR-Cas9. Cell Stem Cell.
17:213–220. 2015. View Article : Google Scholar
|
|
71
|
Huang L, Holtzinger A, Jagan I, BeGora M,
Lohse I, Ngai N, Nostro C, Wang R, Muthuswamy LB, Crawford HC, et
al: Ductal pancreatic cancer modeling and drug screening using
human pluripotent stem cell- and patient-derived tumor organoids.
Nat Med. 21:1364–1371. 2015. View Article : Google Scholar
|
|
72
|
Wang J, Exline CM, DeClercq JJ, Llewellyn
GN, Hayward SB, Li PW, Shivak DA, Surosky RT, Gregory PD, Holmes
MC, et al: Homology-driven genome editing in hematopoietic stem and
progenitor cells using ZFN mRNA and AAV6 donors. Nat Biotechnol.
33:1256–1263. 2015. View Article : Google Scholar
|
|
73
|
Hoban MD, Cost GJ, Mendel MC, Romero Z,
Kaufman ML, Joglekar AV, Ho M, Lumaquin D, Gray D, Lill GR, et al:
Correction of the sickle cell disease mutation in human
hematopoietic stem/progenitor cells. Blood. 125:2597–2604. 2015.
View Article : Google Scholar
|
|
74
|
Xie F, Ye L, Chang JC, Beyer AI, Wang J,
Muench MO and Kan YW: Seamless gene correction of β-thalassemia
mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac.
Genome Res. 24:1526–1533. 2014. View Article : Google Scholar
|
|
75
|
Roberts SA, Dong B, Firrman JA, Moore AR,
Sang N and Xiao W: Engineering factor Viii for hemophilia gene
therapy. J Genet Syndr Gene Ther. 1:12011.
|
|
76
|
Stanek LM, Sardi SP, Mastis B, Richards
AR, Treleaven CM, Taksir T, Misra K, Cheng SH and Shihabuddin LS:
Silencing mutant huntingtin by adeno-associated virus-mediated RNA
interference ameliorates disease manifestations in the YAC128 mouse
model of Huntington' s disease. Hum Gene Ther. 25:461–474. 2014.
View Article : Google Scholar
|
|
77
|
Shin JW, Kim KH, Chao MJ, Atwal RS, Gillis
T, MacDonald ME, Gusella JF and Lee JM: Permanent inactivation of
Huntington's disease mutation by personalized allele-specific
CRISPR/Cas9. Hum Mol Genet. 25:4566–4576. 2016.
|
|
78
|
Monteys AM, Ebanks SA, Keiser MS and
Davidson BL: CRISPR/Cas9 editing of the mutant huntingtin allele in
vitro and in vivo. Mol Ther. 25:12–23. 2017. View Article : Google Scholar
|
|
79
|
Xu X, Tay Y, Sim B, Yoon SI, Huang Y, Ooi
J, Utami KH, Ziaei A, Ng B, Radulescu C, et al: Reversal of
Phenotypic Abnormalities by CRISPR/Cas9-Mediated Gene Correction in
Huntington Disease Patient-Derived Induced Pluripotent Stem Cells.
Stem Cell Reports. 8:619–633. 2017. View Article : Google Scholar
|
|
80
|
Chung CY, Khurana V, Auluck PK, Tardiff
DF, Mazzulli JR, Soldner F, Baru V, Lou Y, Freyzon Y, Cho S, et al:
Identification and rescue of α-synuclein toxicity in Parkinson
patient-derived neurons. Science. 342:983–987. 2013. View Article : Google Scholar
|
|
81
|
Long C, McAnally JR, Shelton JM, Mireault
AA, Bassel-Duby R and Olson EN: Prevention of muscular dystrophy in
mice by CRISPR/Cas9-mediated editing of germline DNA. Science.
345:1184–1188. 2014. View Article : Google Scholar
|
|
82
|
Schwank G, Koo BK, Sasselli V, Dekkers JF,
Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK,
et al: Functional repair of CFTR by CRISPR/Cas9 in intestinal stem
cell organoids of cystic fibrosis patients. Cell Stem Cell.
13:653–658. 2013. View Article : Google Scholar
|
|
83
|
Firth AL, Menon T, Parker GS, Qualls SJ,
Lewis BM, Ke E, Dargitz CT, Wright R, Khanna A, Gage FH, et al:
Functional gene correction for cystic fibrosis in lung epithelial
cells generated from patient iPSCs. Cell Rep. 12:1385–1390. 2015.
View Article : Google Scholar
|
|
84
|
Wu Y, Liang D, Wang Y, Bai M, Tang W, Bao
S, Yan Z, Li D and Li J: Correction of a genetic disease in mouse
via use of CRISPR-Cas9. Cell Stem Cell. 13:659–662. 2013.
View Article : Google Scholar
|
|
85
|
Wu Y, Zhou H, Fan X, Zhang Y, Zhang M,
Wang Y, Xie Z, Bai M, Yin Q, Liang D, et al: Correction of a
genetic disease by CRISPR-Cas9-mediated gene editing in mouse
spermatogonial stem cells. Cell Res. 25:67–79. 2015. View Article : Google Scholar
|
|
86
|
Xie N, Gong H, Suhl JA, Chopra P, Wang T
and Warren ST: Reactivation of FMR1 by CRISPR/Cas9-mediated
deletion of the expanded CGG-repeat of the Fragile X chromosome.
PLoS One. 11:e01654992016. View Article : Google Scholar
|
|
87
|
Horii T, Tamura D, Morita S, Kimura M and
Hatada I: Generation of an ICF syndrome model by efficient genome
editing of human induced pluripotent stem cells using the CRISPR
system. Int J Mol Sci. 14:19774–19781. 2013. View Article : Google Scholar
|
|
88
|
Chang CW, Lai YS, Westin E,
Khodadadi-Jamayran A, Pawlik KM, Lamb LS Jr, Goldman FD and Townes
TM: Modeling human severe combined immunodeficiency and correction
by CRISPR/Cas9-enhanced gene targeting. Cell Rep. 12:1668–1677.
2015. View Article : Google Scholar
|
|
89
|
Flynn R, Grundmann A, Renz P, Hanseler W,
James WS, Cowley SA and Moore MD: CRISPR-mediated genotypic and
phenotypic correction of a chronic granulomatous disease mutation
in human iPS cells. Exp Hematol. 43:838–848.e3. 2015. View Article : Google Scholar
|
|
90
|
Osborn MJ, Belanto JJ, Tolar J and Voytas
DF: Gene editing and its application for hematological diseases.
Int J Hematol. 104:18–28. 2016. View Article : Google Scholar
|
|
91
|
Shinkuma S, Guo Z and Christiano AM:
Site-specific genome editing for correction of induced pluripotent
stem cells derived from dominant dystrophic epidermolysis bullosa.
Proc Natl Acad Sci USA. 113:5676–5681. 2016. View Article : Google Scholar
|
|
92
|
Bassuk AG, Zheng A, Li Y, Tsang SH and
Mahajan VB: Precision Medicine: Genetic Repair of Retinitis
Pigmentosa in Patient-Derived Stem Cells. Sci Rep. 6:199692016.
View Article : Google Scholar
|
|
93
|
Ruan GX, Barry E, Yu D, Lukason M, Cheng
SH and Scaria A: CRISPR/Cas9-mediated genome editing as a
therapeutic approach for leber congenital amaurosis 10. Mol Ther.
25:331–341. 2017. View Article : Google Scholar
|
|
94
|
Mandegar MA, Huebsch N, Frolov EB, Shin E,
Truong A, Olvera MP, Chan AH, Miyaoka Y, Holmes K, Spencer CI, et
al: CRISPR Interference Efficiently Induces Specific and Reversible
Gene Silencing in Human iPSCs. Cell Stem Cell. 18:541–553. 2016.
View Article : Google Scholar
|
|
95
|
Couzin-Frankel J: Breakthrough of the year
2013. Cancer immunotherapy Science. 342:1432–1433. 2013.
|
|
96
|
Guye P, Ebrahimkhani MR, Kipniss N,
Velazquez JJ, Schoenfeld E, Kiani S, Griffith LG and Weiss R:
Genetically engineering self-organization of human pluripotent stem
cells into a liver bud-like tissue using Gata6. Nat Commun.
7:102432016. View Article : Google Scholar
|
|
97
|
Sharma SV, Haber DA and Settleman J: Cell
line-based platforms to evaluate the therapeutic efficacy of
candidate anticancer agents. Nat Rev Cancer. 10:241–253. 2010.
View Article : Google Scholar
|
|
98
|
Lutsenko S: Introduction to the minireview
series on modern technologies for in-cell biochemistry. J Biol
Chem. 291:3757–3758. 2016. View Article : Google Scholar
|
|
99
|
Sato T, Vries RG, Snippert HJ, van de
Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters
PJ, et al: Single Lgr5 stem cells build crypt-villus structures in
vitro without a mesenchymal niche. Nature. 459:262–265. 2009.
View Article : Google Scholar
|
|
100
|
Clevers H: Modeling development and
disease with organoids. Cell. 165:1586–1597. 2016. View Article : Google Scholar
|
|
101
|
Lancaster MA and Knoblich JA: Generation
of cerebral organoids from human pluripotent stem cells. Nat
Protoc. 9:2329–2340. 2014. View Article : Google Scholar
|
|
102
|
Sayin VI and Papagiannakopoulos T:
Application of CRISPR-mediated genome engineering in cancer
research. Cancer Lett. 387:10–17. 2017. View Article : Google Scholar
|
|
103
|
Boj SF, Hwang CI, Baker LA, Chio II, Engle
DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, et al:
Organoid models of human and mouse ductal pancreatic cancer. Cell.
160:324–338. 2015. View Article : Google Scholar
|
|
104
|
Nantasanti S, Spee B, Kruitwagen HS, Chen
C, Geijsen N, Oosterhoff LA, van Wolferen ME, Pelaez N, Fieten H,
Wubbolts RW, et al: Disease modeling and gene therapy of copper
storage disease in canine hepatic organoids. Stem Cell Rep.
5:895–907. 2015. View Article : Google Scholar
|
|
105
|
Walsh AJ, Cook RS, Sanders ME, Arteaga CL
and Skala MC: Drug response in organoids generated from frozen
primary tumor tissues. Sci Rep. 6:188892016. View Article : Google Scholar
|
|
106
|
van de Wetering M, Francies HE, Francis
JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J,
Taylor-Weiner A, Kester L, et al: Prospective derivation of a
living organoid biobank of colorectal cancer patients. Cell.
161:933–945. 2015. View Article : Google Scholar
|
|
107
|
Huch M, Gehart H, van Boxtel R, Hamer K,
Blokzijl F, Verstegen MM, Ellis E, van Wenum M, Fuchs SA, de Ligt
J, et al: Long-term culture of genome-stable bipotent stem cells
from adult human liver. Cell. 160:299–312. 2015. View Article : Google Scholar
|
|
108
|
Barker N, Huch M, Kujala P, van de
Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H,
van den Born M, et al: Lgr5(+ve) stem cells drive self-renewal in
the stomach and build long-lived gastric units in vitro. Cell Stem
Cell. 6:25–36. 2010. View Article : Google Scholar
|
|
109
|
Huch M, Bonfanti P, Boj SF, Sato T,
Loomans CJ, van de Wetering M, Sojoodi M, Li VS, Schuijers J,
Gracanin A, et al: Unlimited in vitro expansion of adult bi-potent
pancreas progenitors through the Lgr5/R-spondin axis. EMBO J.
32:2708–2721. 2013. View Article : Google Scholar
|
|
110
|
Huch M, Dorrell C, Boj SF, van Es JH, Li
VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, et
al: In vitro expansion of single Lgr5+ liver stem cells induced by
Wnt-driven regeneration. Nature. 494:247–250. 2013. View Article : Google Scholar
|
|
111
|
Gao D, Vela I, Sboner A, Iaquinta PJ,
Karthaus WR, Gopalan A, Dowling C, Wanjala JN, Undvall EA, Arora
VK, et al: Organoid cultures derived from patients with advanced
prostate cancer. Cell. 159:176–187. 2014. View Article : Google Scholar
|
|
112
|
Karthaus WR, Iaquinta PJ, Drost J,
Gracanin A, van Boxtel R, Wongvipat J, Dowling CM, Gao D, Begthel
H, Sachs N, et al: Identification of multipotent luminal progenitor
cells in human prostate organoid cultures. Cell. 159:163–175. 2014.
View Article : Google Scholar
|
|
113
|
Heo I and Clevers H: Expanding intestinal
stem cells in culture. Cell Res. 25:995–996. 2015. View Article : Google Scholar
|
|
114
|
Cooks T, Pateras IS, Tarcic O, Solomon H,
Schetter AJ, Wilder S, Lozano G, Pikarsky E, Forshew T, Rosenfeld
N, et al: Mutant p53 prolongs NF-κB activation and promotes chronic
inflammation and inflammation-associated colorectal cancer. Cancer
Cell. 23:634–646. 2013. View Article : Google Scholar
|
|
115
|
Kazanjian A and Shroyer NF: NOTCH
signaling and ATOH1 in colorectal cancers. Curr Colorectal Cancer
Rep. 7:121–127. 2011. View Article : Google Scholar
|
|
116
|
Fujii M, Shimokawa M, Date S, Takano A,
Matano M, Nanki K, Ohta Y, Toshimitsu K, Nakazato Y, Kawasaki K, et
al: A colorectal tumor organoid library demonstrates progressive
loss of niche factor requirements during tumorigenesis. Cell Stem
Cell. 18:827–838. 2016. View Article : Google Scholar
|
|
117
|
Drost J, van Jaarsveld RH, Ponsioen B,
Zimberlin C, van Boxtel R, Buijs A, Sachs N, Overmeer RM, Offerhaus
GJ, Begthel H, et al: Sequential cancer mutations in cultured human
intestinal stem cells. Nature. 521:43–47. 2015. View Article : Google Scholar
|
|
118
|
Bargou R, Leo E, Zugmaier G, Klinger M,
Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, et
al: Tumor regression in cancer patients by very low doses of a T
cell-engaging antibody. Science. 321:974–977. 2008. View Article : Google Scholar
|
|
119
|
Chames P and Baty D: Bispecific antibodies
for cancer therapy: The light at the end of the tunnel. MAbs.
1:539–547. 2009. View Article : Google Scholar
|
|
120
|
Stagg J, Lejeune L, Paquin A and Galipeau
J: Marrow stromal cells for interleukin-2 delivery in cancer
immunotherapy. Hum Gene Ther. 15:597–608. 2004. View Article : Google Scholar
|
|
121
|
Compte M, Nuñez-Prado N, Sanz L and
Alvarez-Vallina L: Immunotherapeutic organoids: A new approach to
cancer treatment. Biomatter. 3:e238972013. View Article : Google Scholar
|
|
122
|
Fuller MK, Faulk DM, Sundaram N, Shroyer
NF, Henning SJ and Helmrath MA: Intestinal crypts reproducibly
expand in culture. J Surg Res. 178:48–54. 2012. View Article : Google Scholar
|
|
123
|
Grün D, Lyubimova A, Kester L, Wiebrands
K, Basak O, Sasaki N, Clevers H and van Oudenaarden A: Single-cell
messenger RNA sequencing reveals rare intestinal cell types.
Nature. 525:251–255. 2015. View Article : Google Scholar
|
|
124
|
Onuma K, Ochiai M, Orihashi K, Takahashi
M, Imai T, Nakagama H and Hippo Y: Genetic reconstitution of
tumori-genesis in primary intestinal cells. Proc Natl Acad Sci USA.
110:11127–11132. 2013. View Article : Google Scholar
|
|
125
|
Vazin T and Schaffer DV: Engineering
strategies to emulate the stem cell niche. Trends Biotechnol.
28:117–124. 2010. View Article : Google Scholar
|
|
126
|
Lancaster MA, Renner M, Martin CA, Wenzel
D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP and
Knoblich JA: Cerebral organoids model human brain development and
microcephaly. Nature. 501:373–379. 2013. View Article : Google Scholar
|
|
127
|
Murphy SV and Atala A: 3D bioprinting of
tissues and organs. Nat Biotechnol. 32:773–785. 2014. View Article : Google Scholar
|
|
128
|
Lindemans CA, Calafiore M, Mertelsmann AM,
O'Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM,
Lawrence G, et al: Interleukin-22 promotes
intestinal-stem-cell-mediated epithelial regeneration. Nature.
528:560–564. 2015. View Article : Google Scholar
|
|
129
|
Wilson SS, Tocchi A, Holly MK, Parks WC
and Smith JG: A small intestinal organoid model of non-invasive
enteric pathogen-epithelial cell interactions. Mucosal Immunol.
8:352–361. 2015. View Article : Google Scholar
|
|
130
|
Wu K, House L, Liu W and Cho WC:
Personalized targeted therapy for lung cancer. Int J Mol Sci.
13:11471–11496. 2012. View Article : Google Scholar
|
|
131
|
Tang H and Shrager JB: CRISPR/Cas-mediated
genome editing to treat EGFR-mutant lung cancer: A personalized
molecular surgical therapy. EMBO Mol Med. 8:83–85. 2016. View Article : Google Scholar
|
|
132
|
Cancer Genome Atlas Research Network;
Electronic address edsc, Cancer Genome Atlas Research N:
Comprehensive and Integrated Genomic Characterization of Adult Soft
Tissue Sarcomas. Cell. 171:950–65.e28. 2017. View Article : Google Scholar
|
|
133
|
Barretina J, Caponigro G, Stransky N,
Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV,
Sonkin D, et al: The cancer cell line encyclopedia enables
predictive modelling of anticancer drug sensitivity. Nature.
483:603–607. 2012. View Article : Google Scholar
|
|
134
|
Consortium EP; ENCODE Project Consortium:
An integrated encyclopedia of DNA elements in the human genome.
Nature. 489:57–74. 2012. View Article : Google Scholar
|
|
135
|
Shalem O, Sanjana NE, Hartenian E, Shi X,
Scott DA, Mikkelson T, Heckl D, Ebert BL, Root DE, Doench JG, et
al: Genome-scale CRISPR-Cas9 knockout screening in human cells.
Science. 343:84–87. 2014. View Article : Google Scholar
|
|
136
|
Wang T, Wei JJ, Sabatini DM and Lander ES:
Genetic screens in human cells using the CRISPR-Cas9 system.
Science. 343:80–84. 2014. View Article : Google Scholar
|
|
137
|
Koike-Yusa H, Li Y, Tan EP,
Velasco-Herrera MC and Yusa K: Genome-wide recessive genetic
screening in mammalian cells with a lentiviral CRISPR-guide RNA
library. Nat Biotechnol. 32:267–273. 2014. View Article : Google Scholar
|
|
138
|
Sanjana NE: Genome-scale CRISPR pooled
screens. Anal Biochem. 532:95–99. 2017. View Article : Google Scholar
|
|
139
|
Fellmann C, Gowen BG, Lin PC, Doudna JA
and Corn JE: Cornerstones of CRISPR-Cas in drug discovery and
therapy. Nat Rev Drug Discov. 16:89–100. 2017. View Article : Google Scholar
|
|
140
|
Wang T, Birsoy K, Hughes NW, Krupczak KM,
Post Y, Wei JJ, Lander ES and Sabatini DM: Identification and
characterization of essential genes in the human genome. Science.
350:1096–1101. 2015. View Article : Google Scholar
|
|
141
|
González F, Zhu Z, Shi ZD, Lelli K, Verma
N, Li QV and Huangfu D: An iCRISPR platform for rapid,
multiplexable, and inducible genome editing in human pluripotent
stem cells. Cell Stem Cell. 15:215–226. 2014. View Article : Google Scholar
|
|
142
|
Shi J, Wang E, Milazzo JP, Wang Z, Kinney
JB and Vakoc CR: Discovery of cancer drug targets by CRISPR-Cas9
screening of protein domains. Nat Biotechnol. 33:661–667. 2015.
View Article : Google Scholar
|
|
143
|
Qi LS, Larson MH, Gilbert LA, Doudna JA,
Weissman JS, Arkin AP and Lim WA: Repurposing CRISPR as an
RNA-guided platform for sequence-specific control of gene
expression. Cell. 152:1173–1183. 2013. View Article : Google Scholar
|
|
144
|
Gilbert LA, Larson MH, Morsut L, Liu Z,
Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH,
Doudna JA, et al: CRISPR-mediated modular RNA-guided regulation of
transcription in eukaryotes. Cell. 154:442–451. 2013. View Article : Google Scholar
|
|
145
|
Gilbert LA, Horlbeck MA, Adamson B,
Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh
HL, Bassik MC, et al: Genome-Scale CRISPR-Mediated Control of Gene
Repression and Activation. Cell. 159:647–661. 2014. View Article : Google Scholar
|
|
146
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar
|
|
147
|
Konermann S, Brigham MD, Trevino AE, Joung
J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS,
Nishimasu H, et al: Genome-scale transcriptional activation by an
engineered CRISPR-Cas9 complex. Nature. 517:583–588. 2015.
View Article : Google Scholar
|
|
148
|
Cheng AW, Wang H, Yang H, Shi L, Katz Y,
Theunissen TW, Rangarajan S, Shivalila CS, Dadon DB and Jaenisch R:
Multiplexed activation of endogenous genes by CRISPR-on, an
RNA-guided transcriptional activator system. Cell Res.
23:1163–1171. 2013. View Article : Google Scholar
|
|
149
|
Rathe SK, Moriarity BS, Stoltenberg CB,
Kurata M, Aumann NK, Rahrmann EP, Bailey NJ, Melrose EG, Beckmann
DA, Liska CR, et al: Using RNA-seq and targeted nucleases to
identify mechanisms of drug resistance in acute myeloid leukemia.
Sci Rep. 4:60482014. View Article : Google Scholar
|
|
150
|
Tzelepis K, Koike-Yusa H, De Braekeleer E,
Li Y, Metzakopian E, Dovey OM, Mupo A, Grinkevich V, Li M, Mazan M,
et al: A CRISPR Dropout Screen Identifies Genetic Vulnerabilities
and Therapeutic Targets in Acute Myeloid Leukemia. Cell Rep.
17:1193–1205. 2016. View Article : Google Scholar
|
|
151
|
Ruiz S, Mayor-Ruiz C, Lafarga V, Murga M,
Vega-Sendino M, Ortega S and Fernandez-Capetillo O: A Genome-wide
CRISPR Screen Identifies CDC25A as a Determinant of Sensitivity to
ATR Inhibitors. Mol Cell. 62:307–313. 2016. View Article : Google Scholar
|
|
152
|
Wanzel M, Vischedyk JB, Gittler MP, Gremke
N, Seiz JR, Hefter M, Noack M, Savai R, Mernberger M, Charles JP,
et al: CRISPR-Cas9-based target validation for p53-reactivating
model compounds. Nat Chem Biol. 12:22–28. 2016. View Article : Google Scholar
|
|
153
|
Rath O and Kozielski F: Kinesins and
cancer. Nat Rev Cancer. 12:527–539. 2012. View Article : Google Scholar
|
|
154
|
Chen C, Siegel D, Gutierrez M, Jacoby M
and Hofmeister CC: Safety and efficacy of selinexor in relapsed or
refractory multiple myeloma and Waldenstrom macroglobulinemia.
Blood. 131:855–863. 2018. View Article : Google Scholar
|
|
155
|
Steinhart Z, Pavlovic Z, Chandrashekhar M,
Hart T, Wang X, Zhang X, Robitaille M, Brown KR, Jaksani S,
Overmeer R, et al: Genome-wide CRISPR screens reveal a Wnt-FZD5
signaling circuit as a druggable vulnerability of RNF43-mutant
pancreatic tumors. Nat Med. 23:60–68. 2017. View Article : Google Scholar
|
|
156
|
Zhu S, Li W, Liu J, Chen CH, Liao Q, Xu P,
Xu H, Xiao T, Cao Z, Peng J, et al: Genome-scale deletion screening
of human long non-coding RNAs using a paired-guide RNA CRISPR-Cas9
library. Nat Biotechnol. 34:1279–1286. 2016. View Article : Google Scholar
|
|
157
|
Jaitin DA, Weiner A, Yofe I, Lara-Astiaso
D, Keren-Shaul H, David E, Salame TM, Tanay A, van Oudenaarden A
and Amit I: Dissecting immune circuits by linking CRISPR-pooled
screens with single-cell RNA-Seq. Cell. 167:1883–1896.e15. 2016.
View Article : Google Scholar
|
|
158
|
Hinrichs CS and Rosenberg SA: Exploiting
the curative potential of adoptive T-cell therapy for cancer.
Immunol Rev. 257:56–71. 2014. View Article : Google Scholar
|
|
159
|
Jensen MC and Riddell SR: Designing
chimeric antigen receptors to effectively and safely target tumors.
Curr Opin Immunol. 33:9–15. 2015. View Article : Google Scholar
|
|
160
|
Eyquem J, Mansilla-Soto J, Giavridis T,
van der Stegen SJ, Hamieh M, Cunanan KM, Odak A, Gönen M and
Sadelain M: Targeting a CAR to the TRAC locus with CRISPR/Cas9
enhances tumour rejection. Nature. 543:113–117. 2017. View Article : Google Scholar
|
|
161
|
Cyranoski D: Chinese scientists to pioneer
first human CRISPR trial. Nature. 535:476–477. 2016. View Article : Google Scholar
|
|
162
|
Topalian SL, Drake CG and Pardoll DM:
Immune checkpoint blockade: A common denominator approach to cancer
therapy. Cancer Cell. 27:450–461. 2015. View Article : Google Scholar
|
|
163
|
Yang Y, Kelly P, Shaffer AL III, Schmitz
R, Yoo HM, Liu X, Huang DW, Webster D, Young RM, Nakagawa M, et al:
Targeting non-proteolytic protein ubiquitination for the treatment
of diffuse large B cell lymphoma. Cancer Cell. 29:494–507. 2016.
View Article : Google Scholar
|
|
164
|
DeWitt MA, Magis W, Bray L, Wang T, Berman
JR, Urbinati F, Heo SJ, Mitros T, Muñoz DP, Boffelli D, et al:
Selection-free genome editing of the sickle mutation in human adult
hemato-poietic stem/progenitor cells. Sci Transl Med.
8:360ra1342016. View Article : Google Scholar
|
|
165
|
Zhen S, Hua L, Liu YH, Gao LC, Fu J, Wan
DY, Dong LH, Song HF and Gao X: Harnessing the clustered regularly
inter-spaced short palindromic repeat (CRISPR)/CRISPR-associated
Cas9 system to disrupt the hepatitis B virus. Gene Ther.
22:404–412. 2015. View Article : Google Scholar
|
|
166
|
Dong C, Qu L, Wang H, Wei L, Dong Y and
Xiong S: Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease
efficiently inhibits viral replication. Antiviral Res. 118:110–117.
2015. View Article : Google Scholar
|
|
167
|
Price AA, Sampson TR, Ratner HK, Grakoui A
and Weiss DS: Cas9-mediated targeting of viral RNA in eukaryotic
cells. Proc Natl Acad Sci USA. 112:6164–6169. 2015. View Article : Google Scholar
|
|
168
|
Wang J and Quake SR: RNA-guided
endonuclease provides a therapeutic strategy to cure latent
herpesviridae infection. Proc Natl Acad Sci USA. 111:13157–13162.
2014. View Article : Google Scholar
|
|
169
|
Zhen S, Hua L, Takahashi Y, Narita S, Liu
YH and Li Y: In vitro and in vivo growth suppression of human
papillomavirus 16-positive cervical cancer cells by CRISPR/Cas9.
Biochem Biophys Res Commun. 450:1422–1426. 2014. View Article : Google Scholar
|
|
170
|
Leen AM, Myers GD, Sili U, Huls MH, Weiss
H, Leung KS, Carrum G, Krance RA, Chang CC, Molldrem JJ, et al:
Monoculture-derived T lymphocytes specific for multiple viruses
expand and produce clinically relevant effects in immunocompromised
individuals. Nat Med. 12:1160–1166. 2006. View Article : Google Scholar
|
|
171
|
Saglio F, Hanley PJ and Bollard CM: The
time is now: Moving toward virus-specific T cells after allogeneic
hematopoietic stem cell transplantation as the standard of care.
Cytotherapy. 16:149–159. 2014. View Article : Google Scholar
|
|
172
|
Lieberman J, Skolnik PR, Parkerson GR III,
Fabry JA, Landry B, Bethel J and Kagan J: Safety of autologous, ex
vivo-expanded human immunodeficiency virus (HIV)-specific cytotoxic
T-lymphocyte infusion in HIV-infected patients. Blood.
90:2196–2206. 1997.
|
|
173
|
Brodie SJ, Lewinsohn DA, Patterson BK,
Jiyamapa D, Krieger J, Corey L, Greenberg PD and Riddell SR: In
vivo migration and function of transferred HIV-1-specific cytotoxic
T cells. Nat Med. 5:34–41. 1999. View
Article : Google Scholar
|
|
174
|
Tan R, Xu X, Ogg GS, Hansasuta P, Dong T,
Rostron T, Luzzi G, Conlon CP, Screaton GR, McMichael AJ, et al:
Rapid death of adoptively transferred T cells in acquired
immunodeficiency syndrome. Blood. 93:1506–1510. 1999.
|
|
175
|
Lam S, Sung J, Cruz C, Castillo-Caro P,
Ngo M, Garrido C, Kuruc J, Archin N, Rooney C, Margolis D, et al:
Broadly-specific cytotoxic T cells targeting multiple HIV antigens
are expanded from HIV+ patients: implications for immunotherapy.
Mol Ther. 23:387–395. 2015. View Article : Google Scholar
|
|
176
|
Patel S, Lam S, Cruz CR, Wright K, Cochran
C, Ambinder RF and Bollard CM: Functionally active HIV-specific T
cells that target Gag and Nef can be expanded from virus-naive
donors and target a range of viral epitopes: Implications for a
cure strategy after allogeneic hematopoietic stem cell
transplantation. Biol Blood Marrow Transplant. 22:536–541. 2016.
View Article : Google Scholar
|
|
177
|
Sung JA, Lam S, Garrido C, Archin N,
Rooney CM, Bollard CM and Margolis DM: Expanded cytotoxic T-cell
lymphocytes target the latent HIV reservoir. J Infect Dis.
212:258–263. 2015. View Article : Google Scholar
|
|
178
|
Colovos C, Villena-Vargas J and Adusumilli
PS: Safety and stability of retrovirally transduced chimeric
antigen receptor T cells. Immunotherapy. 4:899–902. 2012.
View Article : Google Scholar
|
|
179
|
Scholler J, Brady TL, Binder-Scholl G,
Hwang WT, Plesa G, Hege KM, Vogel AN, Kalos M, Riley JL, Deeks SG,
et al: Decade-long safety and function of retroviral-modified
chimeric antigen receptor T cells. Sci Transl Med. 4:132ra532012.
View Article : Google Scholar
|
|
180
|
Joseph A, Zheng JH, Follenzi A, Dilorenzo
T, Sango K, Hyman J, Chen K, Piechocka-Trocha A, Brander C,
Hooijberg E, et al: Lentiviral vectors encoding human
immunodeficiency virus type 1 (HIV-1)-specific T-cell receptor
genes efficiently convert peripheral blood CD8 T lymphocytes into
cytotoxic T lymphocytes with potent in vitro and in vivo
HIV-1-specific inhibitory activity. J Virol. 82:3078–3089. 2008.
View Article : Google Scholar
|
|
181
|
Mitsuyasu RT, Anton PA, Deeks SG, Scadden
DT, Connick E, Downs MT, Bakker A, Roberts MR, June CH, Jalali S,
et al: Prolonged survival and tissue trafficking following adoptive
transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T
cells in human immunodeficiency virus-infected subjects. Blood.
96:785–793. 2000.
|
|
182
|
Deeks SG, Wagner B, Anton PA, Mitsuyasu
RT, Scadden DT, Huang C, Macken C, Richman DD, Christopherson C,
June CH, et al: A phase II randomized study of HIV-specific T-cell
gene therapy in subjects with undetectable plasma viremia on
combination antiretroviral therapy. Mol Ther. 5:788–797. 2002.
View Article : Google Scholar
|
|
183
|
Koretzky GA: Multiple roles of CD4 and CD8
in T cell activation. J Immunol. 185:2643–2644. 2010. View Article : Google Scholar
|
|
184
|
Hütter G and Ganepola S: Eradication of
HIV by transplantation of CCR5-deficient hematopoietic stem cells.
Sci World J. 11:1068–1076. 2011. View Article : Google Scholar
|
|
185
|
Bitinaite J, Wah DA, Aggarwal AK and
Schildkraut I: FokI dimerization is required for DNA cleavage. Proc
Natl Acad Sci USA. 95:10570–10575. 1998. View Article : Google Scholar
|
|
186
|
Yuan J, Wang J, Crain K, Fearns C, Kim KA,
Hua KL, Gregory PD, Holmes MC and Torbett BE: Zinc-finger nuclease
editing of human cxcr4 promotes HIV-1 CD4(+) T cell resistance and
enrichment. Mol Ther. 20:849–859. 2002. View Article : Google Scholar
|
|
187
|
Tebas P, Stein D, Tang WW, Frank I, Wang
SQ, Lee G, Spratt SK, Surosky RT, Giedlin MA, Nichol G, et al: Gene
editing of CCR5 in autologous CD4 T cells of persons infected with
HIV. N Engl J Med. 370:901–910. 2014. View Article : Google Scholar
|
|
188
|
Pattanayak V, Ramirez CL, Joung JK and Liu
DR: Revealing off-target cleavage specificities of zinc-finger
nucleases by in vitro selection. Nat Methods. 8:765–770. 2011.
View Article : Google Scholar
|
|
189
|
Hou P, Chen S, Wang S, Yu X, Chen Y, Jiang
M, Zhuang K, Ho W, Hou W, Huang J, et al: Genome editing of CXCR4
by CRISPR/cas9 confers cells resistant to HIV-1 infection. Sci Rep.
5:155772015. View Article : Google Scholar
|
|
190
|
Kaminski R, Chen Y, Fischer T, Tedaldi E,
Napoli A, Zhang Y, Karn J, Hu W and Khalili K: Elimination of HIV-1
genomes from human T-lymphoid cells by CRISPR/Cas9 gene editing.
Sci Rep. 6:225552016. View Article : Google Scholar
|
|
191
|
Berger C, Jensen MC, Lansdorp PM, Gough M,
Elliott C and Riddell SR: Adoptive transfer of effector
CD8+ T cells derived from central memory cells
establishes persistent T cell memory in primates. J Clin Invest.
118:294–305. 2008. View Article : Google Scholar
|
|
192
|
Chapuis AG, Casper C, Kuntz S, Zhu J,
Tjernlund A, Diem K, Turtle CJ, Cigal ML, Velez R, Riddell S, et
al: HIV-specific CD8+ T cells from HIV+ individuals receiving HAART
can be expanded ex vivo to augment systemic and mucosal immunity in
vivo. Blood. 117:5391–5402. 2011. View Article : Google Scholar
|
|
193
|
Hashimoto M and Takemoto T:
Electroporation enables the efficient mRNA delivery into the mouse
zygotes and facilitates CRISPR/Cas9-based genome editing. Sci Rep.
5:113152015. View Article : Google Scholar
|
|
194
|
Chu VT, Weber T, Graf R, Sommermann T,
Petsch K, Sack U, Volchkov P, Rajewsky K and Kühn R: Efficient
generation of Rosa26 knock-in mice using CRISPR/Cas9 in C57BL/6
zygotes. BMC Biotechnol. 16:42016. View Article : Google Scholar
|
|
195
|
Cottle RN, Lee CM, Archer D and Bao G:
Controlled delivery of β-globin-targeting TALENs and CRISPR/Cas9
into mammalian cells for genome editing using microinjection. Sci
Rep. 5:160312015. View Article : Google Scholar
|
|
196
|
Oude Blenke E, Evers MJ, Mastrobattista E
and van der Oost J: CRISPR-Cas9 gene editing: Delivery aspects and
therapeutic potential. J Control Release. 244:139–148. 2016.
View Article : Google Scholar
|
|
197
|
Han X, Liu Z, Jo MC, Zhang K, Li Y, Zeng
Z, Li N, Zu Y and Qin L: CRISPR-Cas9 delivery to hard-to-transfect
cells via membrane deformation. Sci Adv. 1:e15004542015. View Article : Google Scholar
|
|
198
|
D'Astolfo DS, Pagliero RJ, Pras A,
Karthaus WR, Clevers H, Prasad V, Lebbink RJ, Rehmann H and Geijsen
N: Efficient intracellular delivery of native proteins. Cell.
161:674–690. 2015. View Article : Google Scholar
|
|
199
|
Ha JS, Lee JS, Jeong J, Kim H, Byun J, Kim
SA, Lee HJ, Chung HS, Lee JB and Ahn DR: Poly-sgRNA/siRNA
ribonucleoprotein nanoparticles for targeted gene disruption. J
Control Release. 250:27–35. 2017. View Article : Google Scholar
|
|
200
|
Sun W, Ji W, Hall JM, Hu Q, Wang C, Beisel
CL and Gu Z: Self-assembled DNA nanoclews for the efficient
delivery of CRISPR-Cas9 for genome editing. Angew Chem Int Ed Engl.
54:12029–12033. 2015. View Article : Google Scholar
|
|
201
|
Ramakrishna S, Kwaku Dad AB, Beloor J,
Gopalappa R, Lee SK and Kim H: Gene disruption by cell-penetrating
peptide-mediated delivery of Cas9 protein and guide RNA. Genome
Res. 24:1020–1027. 2014. View Article : Google Scholar
|
|
202
|
Chew WL, Tabebordbar M, Cheng JK, Mali P,
Wu EY, Ng AH, Zhu K, Wagers AJ and Church GM: A multifunctional
AAV-CRISPR-Cas9 and its host response. Nat Methods. 13:868–874.
2016. View Article : Google Scholar
|
|
203
|
Long C, Amoasii L, Mireault AA, McAnally
JR, Li H, Sanchez-Ortiz E, Bhattacharyya S, Shelton JM, Bassel-Duby
R and Olson EN: Postnatal genome editing partially restores
dystrophin expression in a mouse model of muscular dystrophy.
Science. 351:400–403. 2016. View Article : Google Scholar
|
|
204
|
Nelson CE, Hakim CH, Ousterout DG, Thakore
PI, Moreb EA, Castellanos Rivera RM, Madhavan S, Pan X, Ran FA, Yan
WX, et al: In vivo genome editing improves muscle function in a
mouse model of Duchenne muscular dystrophy. Science. 351:403–407.
2016. View Article : Google Scholar
|
|
205
|
Gomez EJ, Gerhardt K, Judd J, Tabor JJ and
Suh J: Light-Activated Nuclear translocation of adeno-associated
virus nanoparticles using phytochrome B for enhanced, tunable, and
spatially programmable gene delivery. ACS Nano. 10:225–237. 2016.
View Article : Google Scholar
|
|
206
|
Ran FA, Cong L, Yan WX, Scott DA,
Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS, et
al: In vivo genome editing using Staphylococcus aureus Cas9.
Nature. 520:186–191. 2015. View Article : Google Scholar
|
|
207
|
Zuris JA, Thompson DB, Shu Y, Guilinger
JP, Bessen JL, Hu JH, Maeder ML, Joung JK, Chen ZY and Liu DR:
Cationic lipid-mediated delivery of proteins enables efficient
protein-based genome editing in vitro and in vivo. Nat Biotechnol.
33:73–80. 2015. View Article : Google Scholar
|
|
208
|
Wang M, Zuris JA, Meng F, Rees H, Sun S,
Deng P, Han Y, Gao X, Pouli D, Wu Q, et al: Efficient delivery of
genome-editing proteins using bioreducible lipid nanoparticles.
Proc Natl Acad Sci USA. 113:2868–2873. 2016. View Article : Google Scholar
|
|
209
|
Gabriel R, Lombardo A, Arens A, Miller JC,
Genovese P, Kaeppel C, Nowrouzi A, Bartholomae CC, Wang J, Friedman
G, et al: An unbiased genome-wide analysis of zinc-finger nuclease
specificity. Nat Biotechnol. 29:816–823. 2011. View Article : Google Scholar
|
|
210
|
Chiarle R, Zhang Y, Frock RL, Lewis SM,
Molinie B, Ho YJ, Myers DR, Choi VW, Compagno M, Malkin DJ, et al:
Genome-wide translocation sequencing reveals mechanisms of
chromosome breaks and rearrangements in B cells. Cell. 147:107–119.
2011. View Article : Google Scholar
|
|
211
|
Hsu PD, Scott DA, Weinstein JA, Ran FA,
Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, et al: DNA
targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol.
31:827–832. 2013. View Article : Google Scholar
|
|
212
|
Heigwer F, Kerr G and Boutros M: E-CRISP:
Fast CRISPR target site identification. Nat Methods. 11:122–123.
2014. View Article : Google Scholar
|
|
213
|
Crosetto N, Mitra A, Silva MJ, Bienko M,
Dojer N, Wang Q, Karaca E, Chiarle R, Skrzypczak M, Ginalski K, et
al: Nucleotide-resolution DNA double-strand break mapping by
next-generation sequencing. Nat Methods. 10:361–365. 2013.
View Article : Google Scholar
|
|
214
|
Kim D, Bae S, Park J, Kim E, Kim S, Yu HR,
Hwang J, Kim JI and Kim JS: Digenome-seq: Genome-wide profiling of
CRISPR-Cas9 off-target effects in human cells. Nat Methods.
12:237–243. 2015. View Article : Google Scholar
|
|
215
|
Tsai SQ, Zheng Z, Nguyen NT, Liebers M,
Topkar VV, Thapar V, Wyvekens N, Khayter C, Iafrate AJ, Le LP, et
al: GUIDE-seq enables genome-wide profiling of off-target cleavage
by CRISPR-Cas nucleases. Nat Biotechnol. 33:187–197. 2015.
View Article : Google Scholar
|
|
216
|
Fu Y, Sander JD, Reyon D, Cascio VM and
Joung JK: Improving CRISPR-Cas nuclease specificity using truncated
guide RNAs. Nat Biotechnol. 32:279–284. 2014. View Article : Google Scholar
|
|
217
|
Kim D, Kim S, Kim S, Park J and Kim JS:
Genome-wide target specificities of CRISPR-Cas9 nucleases revealed
by multiplex Digenome-seq. Genome Res. 26:406–415. 2016. View Article : Google Scholar
|
|
218
|
Frock RL, Hu J, Meyers RM, Ho YJ, Kii E
and Alt FW: Genome-wide detection of DNA double-stranded breaks
induced by engineered nucleases. Nat Biotechnol. 33:179–186. 2015.
View Article : Google Scholar
|
|
219
|
Jinek M, Jiang F, Taylor DW, Sternberg SH,
Kaya E, Ma E, Anders C, Hauer M, Zhou K, Lin S, et al: Structures
of Cas9 endonucleases reveal RNA-mediated conformational
activation. Science. 343:12479972014. View Article : Google Scholar
|
|
220
|
Ran FA, Hsu PD, Lin C-Y, Gootenberg JS,
Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, et
al: Double nicking by RNA-guided CRISPR Cas9 for enhanced genome
editing specificity. Cell. 154:1380–1389. 2013. View Article : Google Scholar
|
|
221
|
McCaffrey J, Sibert J, Zhang B, Zhang Y,
Hu W, Riethman H and Xiao M: CRISPR-CAS9 D10A nickase
target-specific fluorescent labeling of double strand DNA for whole
genome mapping and structural variation analysis. Nucleic Acids
Res. 44. e112016. View Article : Google Scholar
|
|
222
|
Tsai SQ, Wyvekens N, Khayter C, Foden JA,
Thapar V, Reyon D, Goodwin MJ, Aryee MJ and Joung JK: Dimeric
CRISPR RNA-guided FokI nucleases for highly specific genome
editing. Nat Biotechnol. 32:569–576. 2014. View Article : Google Scholar
|
|
223
|
Wyvekens N, Topkar VV, Khayter C, Joung JK
and Tsai SQ: Dimeric CRISPR RNA-guided FokI-dCas9 nucleases
directed by truncated gRNAs for highly specific genome editing. Hum
Gene Ther. 26:425–431. 2015. View Article : Google Scholar
|
|
224
|
Kleinstiver BP, Pattanayak V, Prew MS,
Tsai SQ, Nguyen NT, Zheng Z and Joung JK: High-fidelity CRISPR-Cas9
nucleases with no detectable genome-wide off-target effects.
Nature. 529:490–495. 2016. View Article : Google Scholar
|
|
225
|
Hou Z, Zhang Y, Propson NE, Howden SE, Chu
LF, Sontheimer EJ and Thomson JA: Efficient genome engineering in
human pluripotent stem cells using Cas9 fro Neisseria meningitidis.
Proc Natl Acad Sci USA. 110:15644–15649. 2013. View Article : Google Scholar
|
|
226
|
Walsh RM and Hochedlinger K: A variant
CRISPR-Cas9 system adds versatility to genome engineering. Proc
Natl Acad Sci USA. 110:15514–15515. 2013. View Article : Google Scholar
|
|
227
|
Raza U, Saatci Ö, Uhlmann S, Ansari SA,
Eyüpoğlu E, Yurdusev E, Mutlu M, Ersan PG, Altundağ MK, Zhang JD,
et al: The miR-644a/CTBP1/p53 axis suppresses drug resistance by
simultaneous inhibition of cell survival and epithelial-mesenchymal
transition in breast cancer. Oncotarget. 7:49859–49877. 2016.
View Article : Google Scholar
|
|
228
|
Singh R, Gupta SC, Peng WX, Zhou N,
Pochampally R, Atfi A, Watabe K, Lu Z and Mo YY: Regulation of
alternative splicing of Bcl-x by BC200 contributes to breast cancer
pathogenesis. Cell Death Dis. 7:e22622016. View Article : Google Scholar
|
|
229
|
Engel BJ, Bowser JL, Broaddus RR and
Carson DD: MUC1 stimulates EGFR expression and function in
endometrial cancer. Oncotarget. 7:32796–32809. 2016. View Article : Google Scholar
|
|
230
|
Zhang X, Choi PS, Francis JM, Imielinski
M, Watanabe H, Cherniack AD and Meyerson M: Identification of
focally amplified lineage-specific super-enhancers in human
epithelial cancers. Nat Genet. 48:176–182. 2016. View Article : Google Scholar
|
|
231
|
Kavlashvili T, Jia Y, Dai D, Meng X, Thiel
KW, Leslie KK and Yang S: Inverse relationship between progesterone
receptor and Myc in endometrial cancer. PLoS One. 11:e01489122016.
View Article : Google Scholar
|
|
232
|
Kawamura N, Nimura K, Nagano H, Yamaguchi
S, Nonomura N and Kaneda Y: CRISPR/Cas9-mediated gene knockout of
NANOG and NANOGP8 decreases the malignant potential of prostate
cancer cells. Oncotarget. 6:22361–22374. 2015. View Article : Google Scholar
|
|
233
|
Mazur PK, Herner A, Mello SS, Wirth M,
Hausmann S, Sánchez-Rivera FJ, Lofgren SM, Kuschma T, Hahn SA,
Vangala D, et al: Combined inhibition of BET family proteins and
histone deacet-ylases as a potential epigenetics-based therapy for
pancreatic ductal adenocarcinoma. Nat Med. 21:1163–1171. 2015.
View Article : Google Scholar
|
|
234
|
Zhang Z, Christin JR, Wang C, Ge K, Oktay
MH and Guo W: Mammary-stem-cell-based somatic mouse models reveal
breast cancer drivers causing cell fate dysregulation. Cell Rep.
16:3146–3156. 2016. View Article : Google Scholar
|
|
235
|
Walton J, Blagih J, Ennis D, Leung E,
Dowson S, Farquharson M, Tookman LA, Orange C, Athineos D, Mason S,
et al: CRISPR/Cas9-mediated Trp53 and Brca2 knockout to generate
improved murine models of ovarian high-grade serous carcinoma.
Cancer Res. 76:6118–6129. 2016. View Article : Google Scholar
|
|
236
|
Liang P, Xu Y, Zhang X, Ding C, Huang R,
Zhang Z, Lv J, Xie X, Chen Y, Li Y, et al: CRISPR/Cas9-mediated
gene editing in human tripronuclear zygotes. Protein Cell.
6:363–372. 2015. View Article : Google Scholar
|