Introduction
Non-small cell lung carcinomas (NSCLCs) comprise
approximately 85% of lung cancers and have an overall 5-year
survival rate of 17.1% (1). The
gold standard for the treatment of lung cancer is surgical
resection, combined with chemotherapy and radiation therapy
(2). Even though some molecular
targeting drugs [such as tyrosine kinase inhibitors (TKIs)] have
been applied to the treatment of patients with NSCLC with epidermal
growth factor receptor (EGFR) mutations (3,4), the
overall survival of patients with this disease remains
discouraging. Therefore, the development of novel therapeutic
strategies is required to improve the clinical outcomes.
Recently, post-operative hyperthermia has been
applied as an important adjuvant therapy to enhance the efficacy of
traditional chemotherapy and/or radiotherapy (5,6). As
past technologies have not been able to deliver heat to tumor sites
effectively and homogeneously without damaging the surrounding
non-tumor tissue, the development of hyperthermia is relatively
outdated in modern medical research. Recent novel techniques (such
as computer modeling and non-invasive thermometry) which directly
apply heat treatment, led us to focus on hyperthermia again.
Numerous clinical data have suggested that microwave (MW) ablation
can improve the oncologic outcomes of patients with hepatocellular
carcinoma (7), colorectal liver
metastases (8) and recurrent
colorectal lung metastases (9). MW
hyperthermia (temperature usually ranges between 42 and 45°C) has
been widely employed in clinical trials of superficial tumors,
including head and neck cancer, or breast cancer recurrences to the
chest wall (10–13). It can also be used effectively in
conjunction with radiation therapy and chemotherapy, and promotes
body's immune response to fight against the target tumor (14,15).
MW therapy is able to kill cancer cells through thermal as well as
non-thermal effects (16).
According to previous studies, MW hyperthermia has been shown to be
a promising alternative non-invasive treatment strategy for various
types of cancer, including lung cancer (17,18).
A better understanding of the molecular mechanisms underlying its
effects may provide further information on clinical hyperthermia
treatment, to a certain extent; however, the precise mechanisms
through which MW hyperthermia affects NSCLC remain
undetermined.
In parallel to clinical research, several aspects of
heat action have been examined in numerous preclinical studies. Due
to the lack of special experimental devices, and the fact that
clinical hyperthermia devices are difficult to be applied in
preclinical studies, the water bath is the common heating system
used in vitro and in vivo in experimental studies
(19,20). When using a water bath to study
hyperthermia, the temperature is the only indicator being
evaluated; the non-thermal effects of MW are not considered.
Additionally, differences in the biological effects induced by
various heat treatments (including a water bath and MW
hyperthermia) under isothermal conditions are not yet clear. In
order to resolve this issue, we developed a special heating
equipment (21) which can provide
MW irradiation under certain temperatures. In this study, using
this system, we investigated the mechanisms underlying the effects
of MW hyperthermia on NSCLC cells in vitro.
Therefore, the aim of this study was to investigate
the effects on NSCLCs induced by two types of heating systems
(water bath and MW hyperthermia) under different temperature
conditions and to explore the potential underlying mechanisms. We
found that MW hyperthermia induced markedly more potent cytotoxic
effects, increased reactive oxygen species (ROS) production,
promoted mitochondrial dysfunction and promoted G2/M
checkpoint arrest, thereby inducing the apoptosis of NSCLCs to a
greater degree than the water bath heating system.
Materials and methods
Cells and cell culture
The human NSCLC cell lines, H460 (EGFR wild-type),
PC-9 (exon 19-deletion EGFR mutant) and H1975 (T790M + L858R EGFR
mutant) and the normal human lung epithelial cell line, BEAS-2B,
were purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). The NSCLC cell lines were maintained in
RPMI-1640 supplemented with 10% fetal bovine serum (FBS) (Gibco,
NY, USA) and 1% penicillin/streptomycin at 37°C in a 5%
CO2 humidified atmosphere. The BEAS-2B cells were
maintained in DMEM supplemented with 5% FBS. Primary astrocyte
cultures were separated from the cerebral cortex of 1-day-old
post-natal Sprague-Dawley rats as previously described (22,23),
with slight modifications. The 1-day-old post-natal rats were
purchased from the Animal Center of the Zhejiang Academy of Medical
Sciences. All the animals were raised at a specific pathogen-free
level (SPF) laboratory at the Animal Center of the Zhejiang Academy
of Medical Sciences. After purchase, the rats were sacrificed
immediately to obtain primary astrocytes. The animals were
anaesthetized with pentobarbital (40 mg/kg, intraperitoneal
injection) and then decapitated. The whole brains were dissected
under sterile conditions. The globulin, striatum, hippocampus and
basal brain tissue were removed; cerebral cortex was collected,
freed from adherent meninges. All experimental procedures were
performed according to the National Institute of Health Guild for
the Care and Use of Laboratory Animals and were in accordance with
the Experimental Animal Welfare Ethics Committee of Zhejiang
Academy of Medical Sciences (2018–045). The tissue was then washed
in phosphate-buffered saline (PBS), cut into small fragments and
digested with trypsin. Cell suspensions were centrifuged at 150 × g
for 10 min and precipitation was re-suspended in Dulbecco's
modified Eagle's medium (DMEM, Life Technologies, Grand Island, NY,
USA) supplemented with 25 mM glucose, 10% FBS, 2 mM glutamine and
1% penicillin/streptomycin. The cells were seeded on
poly-D-lysine-coated flasks, and grown in medium for the first 24
h. The medium was replaced every 3 days. After 12–14 days, the
confluent cultures were shaken overnight to minimized microglial
contamination. The number of glial fibrillary acidic protein
(GFAP)-positive cells in these cultures was >95% (data not
shown).
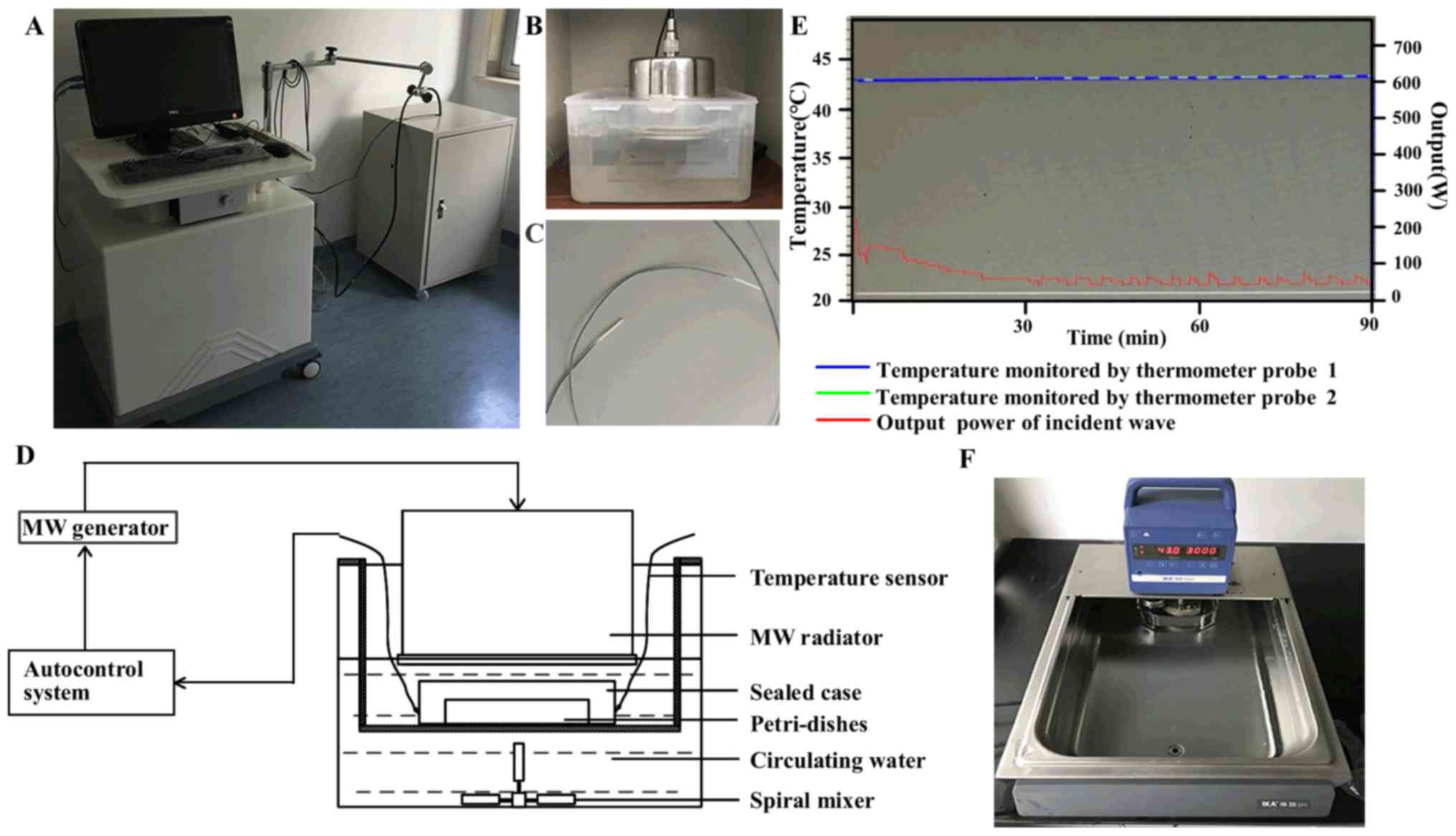
MW hyperthermia system
A novel MW applicator (21) was developed for the experimental
hyperthermia treatment of cancer in vitro, which was
equipped with a 433 MHz MW generator and temperature auto-control
system (Fig. 1). The MW
hyperthermia device consists of an auto-control system (computer),
2 fiber-optic thermometer probes, a MW generator and a MW radiator.
The MW radiator is connected to a MW generator via a flexible cable
and enclosed in a circulating water bath used as a protection
against overheating of the cells. During MW hyperthermia treatment,
the temperature of the cultured cells was measured with a
temperature sensor under the dishes, and the temperature of
surrounding circulating water was monitored via another sensor
probe. Compared with a common thermistor thermometer, these
thermometer probes used in our device can avoid the effects of
electromagnetic waves, such as MW and allow for the measurement of
temperatures within ±0.2°C of accuracy. The temperature was
automatically controlled by a decrease/increase in the power output
to maintain the temperature at the set value. Cell culture dishes
or plates are positioned in the sealed box under the MW radiator.
The MW heating system in vitro model is schematically
illustrated in Fig. 1. Following
initial irradiation, the temperatures and MW output reached a
plateau with an output value of approximately 50 W, and the maximum
output value of MW was set at 200 W.
Hyperthermia treatment
The cells were seeded in culture plates prior to
treatment. For MW hyperthermia treatment, the cell culture dishes
or plates were exposed to MW irradiation at the indicated
temperatures for the indicated periods of time. For water bath
treatments, the cell culture dishes or plates were immersed in a
circulating water bath (IKA group, Staufen, Germany) at 43°C for 60
min or 90 min (Fig. 1F). For
recovery following hyperthermia treatment, the cells were placed in
an incubator at 37°C until further analysis.
Cell viability assay (cell counting kit-8
assay)
The inhibitory effects on tumor cell viability
observed following treatment with the MW hyperthermia device or
water bath were determined by CCK-8 assay. The NSCLC cells, H460,
PC-9 and H1975, were seeded in 96-well plates at 1×104
cells/well. The cells were exposed to different temperatures
(moderate hyperthermia) for 60 or 90 min. In the negative control
group, the cells were incubated at 37°C in a CO2
incubator instead of MW irradiation or the water bath, and then
incubated in a CO2 incubator. After 6, 12 or 24 h of
treatment, 10 µl of CCK-8 solution (MedChem Express,
Princeton, NJ, USA) were added to each well, and the cells were
cultured for a further 1–3 h at 37°C. Cell viability in each group
was measured at 450 nm using a Multiskan Spectrum spectrophotometer
(Thermo Fisher Scientific, Rockford, IL, USA). The cells were also
pre-treated with or without the caspase-3 inhibitor, Ac-DEVD-CHO
(Selleckchem, Houston, TX, USA), for 3 h prior to MW hyperthermia
(43°C for 90 min) for 24 h. Cell viability in each group was then
measured.
Apoptosis detection by flow
cytometry
For each cell line, 2×105 cells/well were
seeded in a 6-well plate. Following hyper-thermia treatment with MW
or the water bath system, the cells were harvested and then stained
with the Annexin V-FITC Apoptosis kit (BD Biosciences, Franklin
Lakes, NJ, USA) according to the manufacturer's instructions and
analyzed using a flow cytometer (BD Biosciences). Annexin
V+/PI− cells were considered early apoptotic
and Annexin V+/PI+ cells were considered late
apoptotic. The cells were also pre-treated with or without
Ac-DEVD-CHO (50 µM) for 3 h prior to MW hyperthermia (43°C
for 90 min) for 24 h.
Caspase-3 activity assay
Caspase-3 activity was determined using a Caspase-3
Activity Assay kit (Beyotime Institute of Biotechnology, Shanghai,
China) following the manufacturer's instructions. Briefly, the
cells were treated with heat using a water bath or MW hyperthermia
device and collected. The cells were washed with PBS twice. Lysis
buffer was added by incubation on ice for 20 min. Cell lysates were
centrifuged at 12,000 × g for 15 min at 4°C, and the protein
concentration of the supernatants was determined using the Bradford
protein assay kit (Beyotime Institute of Biotechnology). This was
followed by incubation at 37°C for 1–2 h and the addition of
Ac-DEVD-pNA (10 µl) and mixing with the cell lysates. The
activity of caspase-3 was then quantified at 405 nm with a
Multiskan Spectrum spectrophotometer (Thermo Fisher Scientific).
The cells were also pre-treated with or without Ac-DEVD-CHO (50
µM) for 3 h prior to MW hyperthermia (43°C for 90 min) for
24 h.
Measurement of intracellular ROS
generation
The detection of intracellular ROS generation
following treatment was performed using the fluorescence probe,
2′,7′- dichlorodihydrofluorescein diacetate (DCFH-DA, Beyotime
Institute of Biotechnology). The cells were seeded in a 35-mm
culture plate (Thermo Fisher Scientific) at a density of
5×105 cells/ml in a volume of 1 ml. The cells were
treated with 43°C (moderate hyperthermia) with MW or a water bath
for 90 min. After 6 h of treatment, the cells were pre-loaded with
10 µM DCFH-DA in FBS-free RPMI-1640 medium for 20 min. After
washing 3 times for 10 min with PBS, the cells were mounted under a
fluorescenc microscope (Olympus BX61; Olympus, Tokyo, Japan) at an
excitation of 488 nm and emission of 525 nm. Using the Image-Pro
Plus program, the mean fluorescence intensity of the images was
assessed and normalized to obtain relative ratios that were
compared between the experimental groups.
Detection of mitochondrial membrane
potential (MMP)
MMP was determined using the fluorescent probe, JC-1
(Beyotime Institute of Biotechnology), according to the
instructions of the manufacturer. JC-1 is a cationic dye. Under
normal conditions, the mitochondrial membrane exhibits red
fluorescence; when MMP is depolarized, red fluorescence turns into
green fluorescence. Briefly, following treatment, the cells were
incubated with 5 µg/ml JC-1 staining solution for 20 min.
After washing 2 times with PBS, the cells were observed under a
confocal fluorescence microscope (Leica Microsystems AG, Mannheim,
Germany). At least 10 visual fields in each were analyzed. All
experiments were repeated at least 3 times.
Cell cycle analyses by flow
cytometry
In order to obtain the distribution of cells in
different phases of the cell cycle, the cells were fixed with 70%
ice cold ethanol, stored overnight at −20°C, and subsequently
stained using the cycle test plus DNA reagent kit according to the
manufacturer's instructions (BD Biosciences). The samples were
finally analyzed using a flow cytometer (BD Biosciences).
Western blot analysis
Following the different treatments, total protein
was extracted using RIPA lysing buffer. Lysates were centrifuged at
15,000 × g for 10 min at 4°C. Protein was quantified using a BCA
protein kit (Thermo Fisher Scientific). Equal amounts (40
µg) of tissue lysates were separated by 10% SDS-PAGE and
transferred onto PVDF membranes (Bio-Rad, Hercules, CA, USA). The
PVDF membranes were blocked with 5% non-fat milk at room
temperature for 1–2 h and then incubated with specific primary
antibodies at 4°C overnight. The following antibodies were used:
anti-ataxia telangiectasia mutated (ATM; sc-377293, 1:500, Santa
Cruz Biotechnology, Santa Cruz, CA, USA), anti-phosphorylated
(p-)ATM (13050S, 1:1,000, Cell Signaling Technology, Danvers, MA,
USA), anti-p-checkpoint kinase 2 (Chk2; 2197S, 1:1,000, Cell
Signaling Technology), anti-p21 (ab109520, 1:1,000, Abcam,
Cambridge, MA, USA), anti-cdc 25c (4688S, 1:1,000, Cell Signaling
Technology), anti-cyclin B1 (12231S, 1:1,000, Cell Signaling
Technology), anti-cdc 2 (28439S, 1:1,000, Cell Signaling
Technology), anti-β-actin (sc-47778, 1:1,000, Santa Cruz
Biotechnology). After washing with TBST, the membranes were
incubated with secondary antibodies (anti-rabbit IgG, sc-2357,
1:5,000 and anti-mouse IgG, sc-2005, 1:5,000 from Santa Cruz
Biotechnology) at room temperature for a further 2 h. The protein
bands were visualized using the ECL system (Beyotime Institute of
Biotechnology). Images were captured using the Odyssey infrared
imaging system (LI-COR Biosciences, Lincoln, NE, USA). All
experiments were repeated at least 3 times.
Statistical analysis
All analyses represented at least in triplicate
experiments in vitro. Data are presented as the means ± SEM.
The student t-test was used for single-group comparisons, and
one-way ANOVA followed by Tukey's post hoc test was used for
multiple comparisons. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
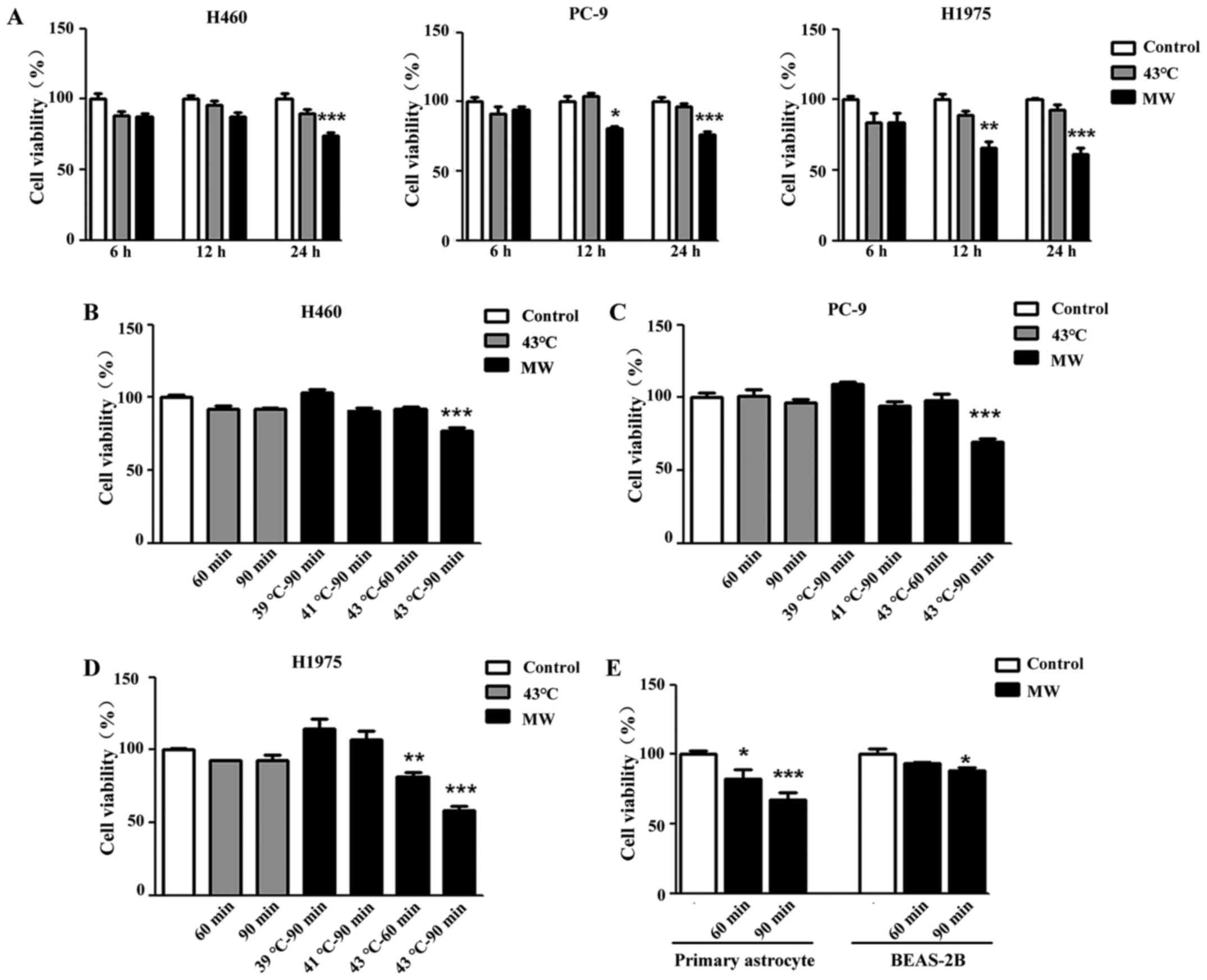
MW hyperthermia treatment inhibits tumor
cell viability
To examine the inhibitory effect of hyperthermia on
tumor cell growth, CCK-8 assay was used to evaluate cell viability.
All 3 different NSCLC cells (H460, PC-9 and H1975) were treated
with MW hyperthermia or a water bath, while the control cell
monolayers were maintained at 37°C. Following 90 min of heat
treatment and a subsequent 6 h of incubation, heat treatment using
a water bath or MW did not affect cell viability, while 12 h of
incubation attenuated the viability of the PC-9 and H1975 cells
treated with MW hyperthermia (Fig.
2A). After of 24 h pf incubation, cell viability was decreased
significantly by MW hyperthermia treatment in all 3 cell lines
(Fig. 2A). As shown in Fig. 2B–D, the mean value of percentage
cell viability based upon the control (100%) was 77.35±1.89% (MW at
43°C for 90 min, P<0.001) in the H460 cell line, 69.75±1.82% (MW
at 43°C for 90 min, P<0.001) in the PC-9 cell line, and
81.84±2.93% (MW at 43°C for 60 min, P<0.01) and 57.88±3.42% (MW
at 43°C for 90 min, P<0.001) in the H1975 cell line. Compared
with the control group, no significant differences were observed in
the 3 cell lines treated with the water bath. Furthermore, we
examined the side-effects of MW hyperthermia on some normal cells
(murine primary astrocyte and human lung epithelial cells,
BEAS-B2). In primary astrocytes, cell viability decreased as the
irradiation time and temperature increased (MW at 43°C for 90 min,
P<0.001). As for the normal lung epithelial cells, the BEAS-2B
cells were the least sensitive to MW irradiation in the experiments
shown in Fig. 2E. MW hyperthermia
did not affect the viability of the BEAS-2B cells treated with 43°C
mild hyperthermia for 60 min, although it induced 11.85% cell death
with 43°C MW hyperthermia for 90 min (P<0.05).
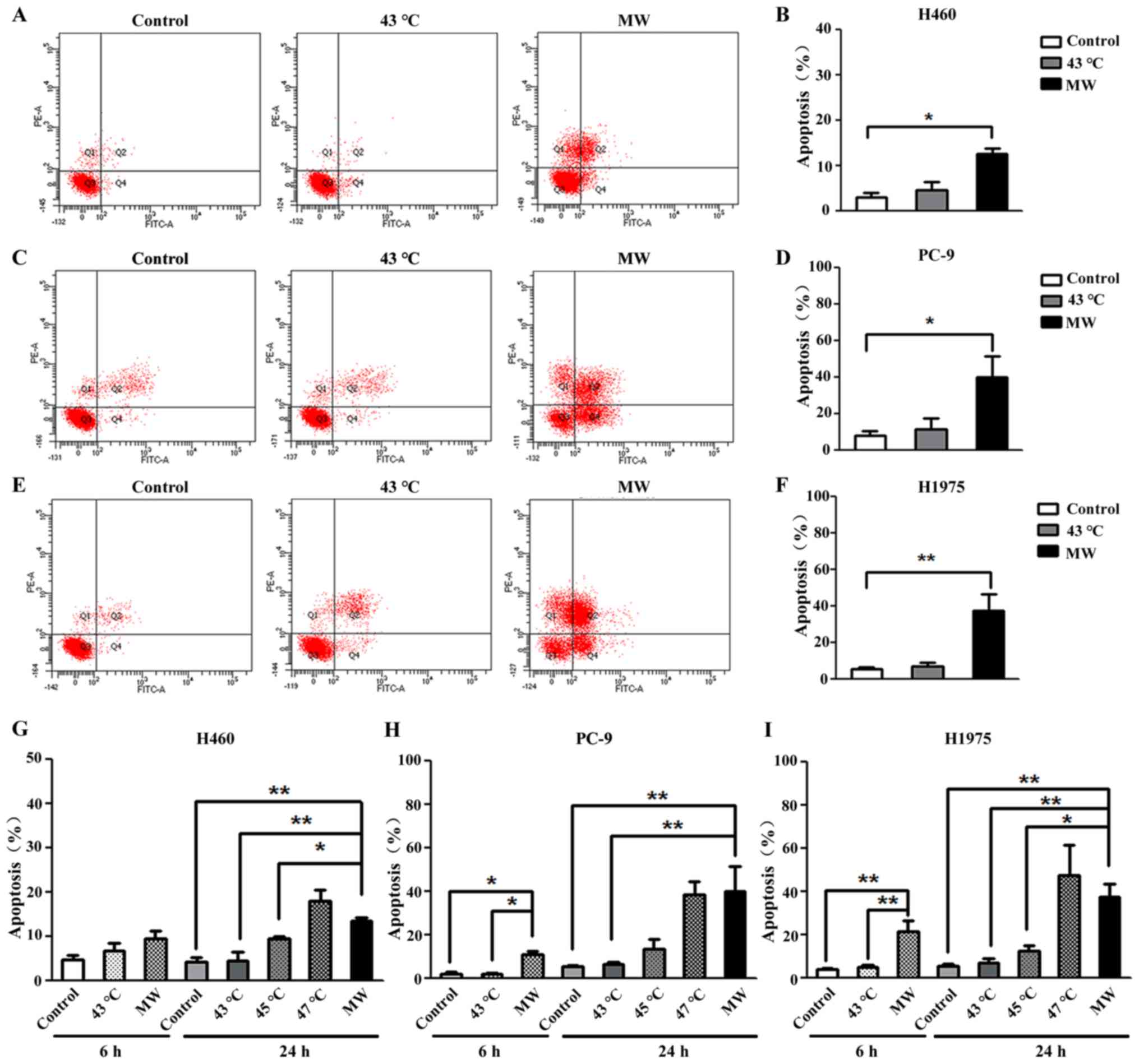
MW hyperthermia treatment induces
caspase-3-dependent apoptosis
In order to determine the type of cell death induced
by MW hyperthermia, the effects of heat treatment on apop-tosis
were examined. Annexin V-positive and PI-negative cells represent
early apoptotic cells, and double-positive cells are defined as
late apoptotic cells. For the H460 cells, the total number of
apoptotic cells (including early apoptotic and late apoptotic
cells) increased significantly (3.89-fold compared to the control)
following treatment with MW at 43°C for 90 min (Fig. 3A and B). For the PC-9 cells, a
5.56-fold increase in the total number of apoptotic cells was
observed following treatment with MW at 43°C for 90 min (Fig. 3C and D). For the H1975 cells, an
8-fold increase in the total number of apoptotic cells was observed
following treatment with MW at 43°C for 90 min (Fig. 3E and F). The mean value of
percentage apoptosis of the cells treated with MW hyperthermia was
12.45±1.20%, 40.00±11.22% and 37.70±9.17% in the H460, PC-9 and
H1975 cells, respectively. No significant differences were observed
between the control groups and water bath-treated groups in all 3
NSCLC cell lines. These results suggested that the enhanced the
inhibitory effects on cancer cell survival were associated with
cell apoptosis in the cells treated with MW hyperthermia.
Increasing the temperature of the water bath proportionally
increased the percentages of apoptotic cells, which were
4.54±1.87%, 9.30±0.70%, 17.83±2.62% in the H460 cells (Fig. 3G), 6.33±1.24%, 13.26±4.71% and
38.30±5.89% in the PC-9 cells (Fig.
3H), and 7.06±1.83%, 12.10±2.95% and 47.43±13.87% in the H1975
cells (Fig. 3I) at 43, 45 and
47°C, respectively.
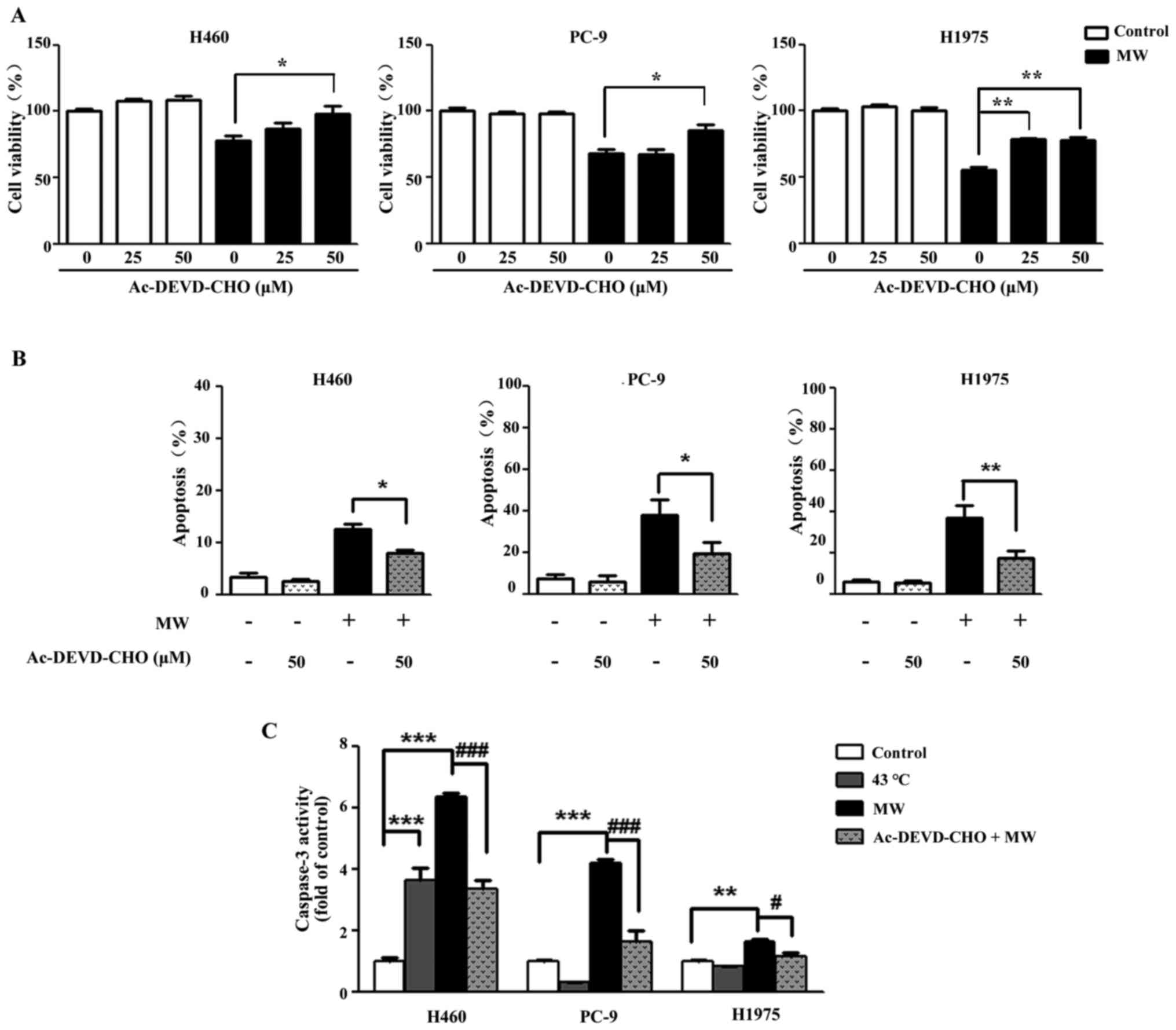
To examine whether caspase-3 activation is involved
in the apoptosis triggered by MW hyperthermia, the cells were
treated with Ac-DEVD-CHO, a caspase-3 specific inhibitor for 3 h
prior to MW hyperthermia. Following MW treatment in the presence or
absence of Ac-DEVD-CHO, cell viability was examined at 24 h. As
shown in Figure 4A, Ac-DEVD-CHO
markedly attenuated MW hyperthermia-induced cell death compared
with the cells treated with MW alone. Furthermore, the apoptosis
induced by MW was partially reversed by Ac-DEVD-CHO (Fig. 4B). Compared with each control
group, the level of caspase-3 activation was markedly increased in
the MW hyperthermia group (8.52-fold in the H460 cells, 4.14-fold
in the PC-9 cells and 1.64-fold in the H1975 cells). Furthermore,
we found that Ac-DEVD-CHO significantly attenuated the increase in
caspase-3 production compared with the cells treated with MW
hyperthermia alone for 24 h (Fig.
4C). These results indicated that caspase-3 was involved in the
apoptosis induced by MW hyperthermia.
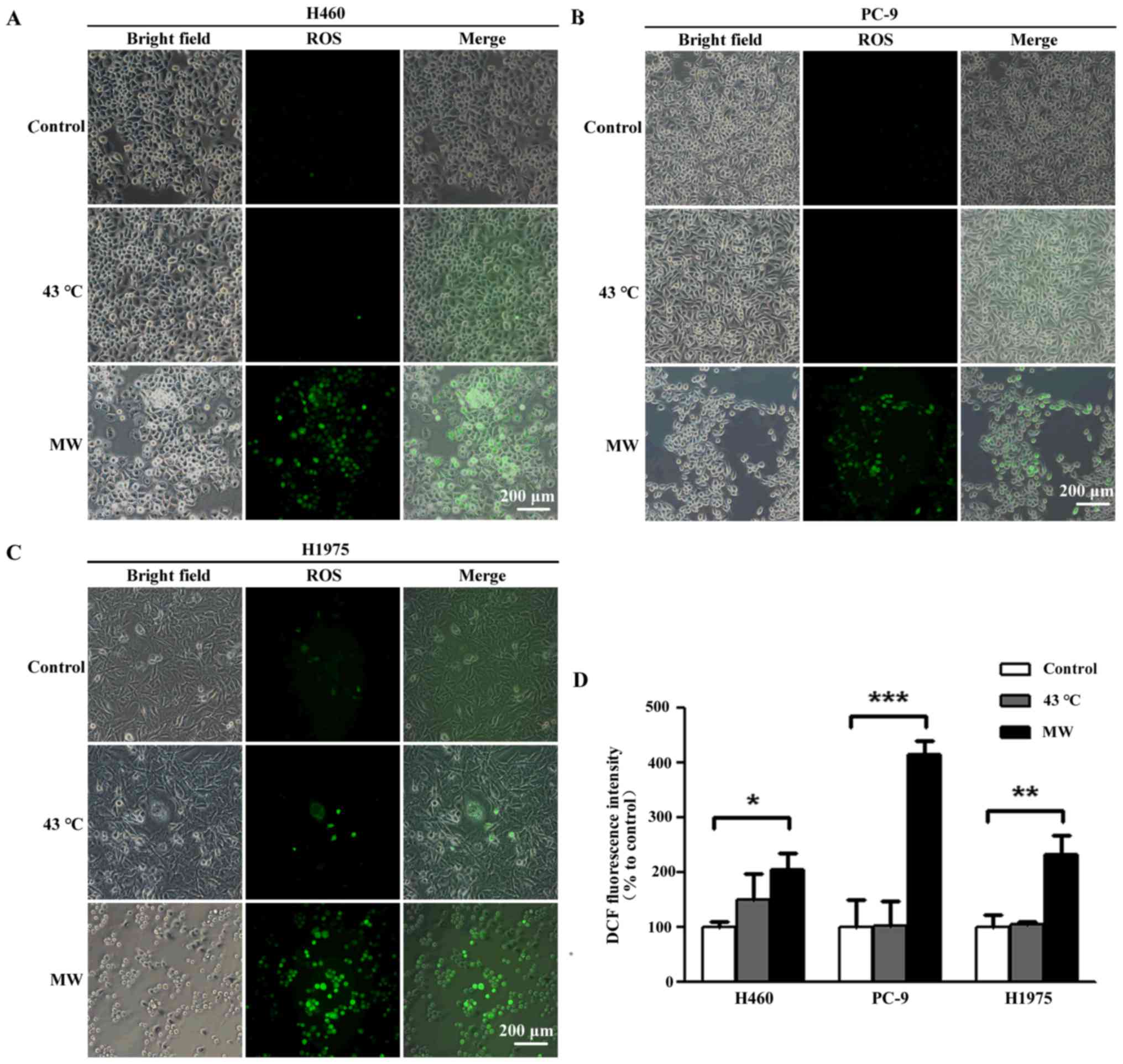
MW hyperthermia treatment increases ROS
levels
To investigate whether oxidative stress contributes
to the apoptosis induced by MW hyperthermia, we measured
intracellular ROS production using a DCFDA fluorescence probe, an
indicator of total cellular ROS. ROS production increased 6 h
following MW hyperthermia (2.07-fold in the H460 cells, 2.31-fold
in the H1975 cells and 4.41-fold in the PC-9 cells; Fig. 5), while heat-treatment using a
water bath induced a slight, yet insignificant, increase in ROS
levels (1.49-fold in the H460 cells, 1.04-fold in the H1975 cells
and 1.01-fold in the PC-9 cells), compared to each control group.
Taken together, our results demonstrated that oxidative stress
contributed to the apoptosis of NSCLC cells induced by MW
hyperthermia.
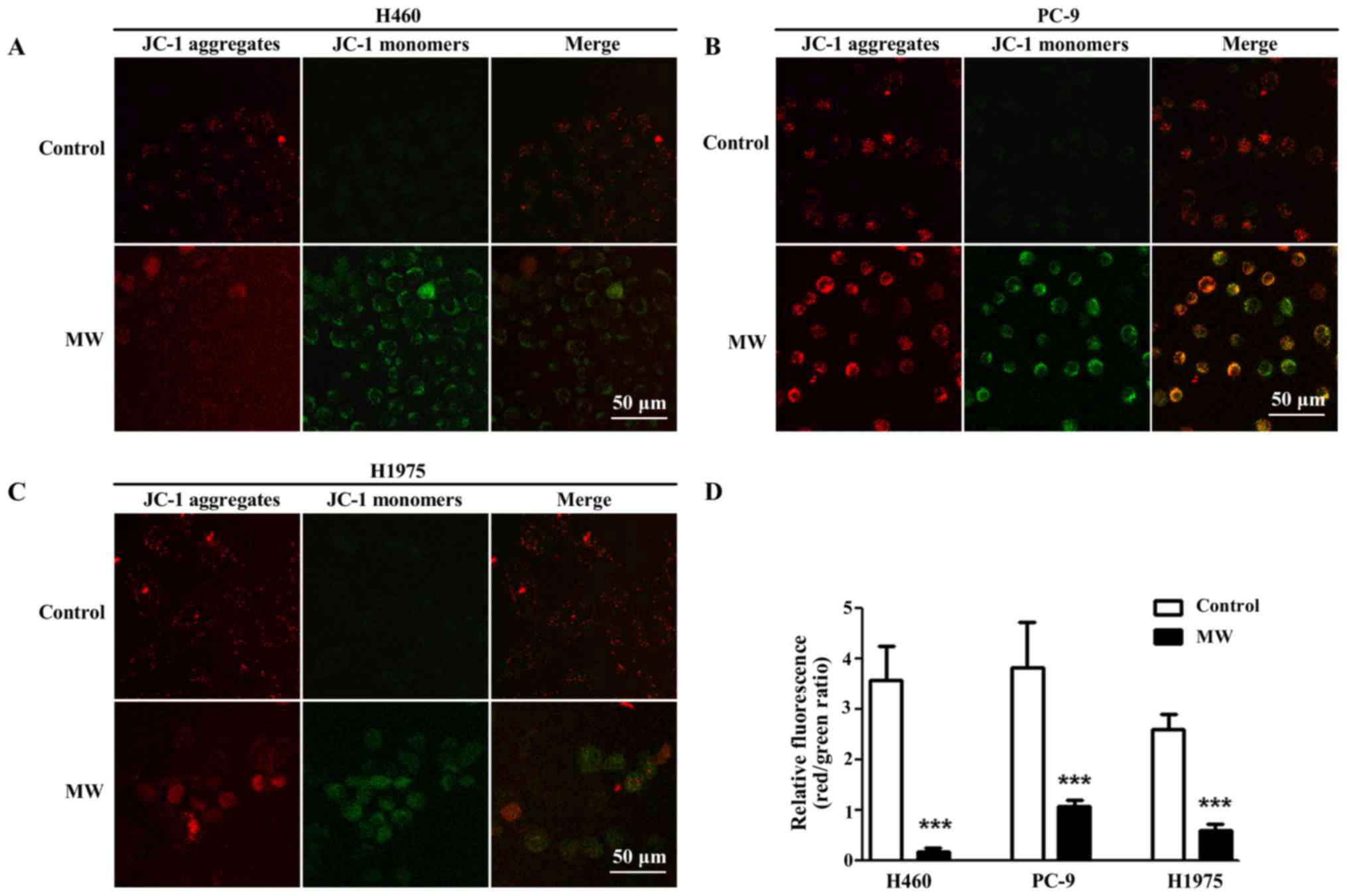
MW hyperthermia treatment decreases MMP
in NSCLC cells
To determine whether the change in MMP was induced
by MW hyperthermia, JC-1 staining was performed. JC-1 is an ideal
fluorescent probe commonly used to detect MMP. In normal healthy
cells, JC-1 accumulates in the mitochondria with red fluorescence,
while in apoptotic cells, it depolarizes MMP, and JC-1 is diffused
in the cytosol with green fluorescence. Thus, the transition from
red fluorescence to green fluorescence of JC-1 staining suggests
mitochondrial depolarization and apoptosis. We found that MMP was
decreased in the NSCLC cells following treatment with MW
hyperthermia (Fig. 6).
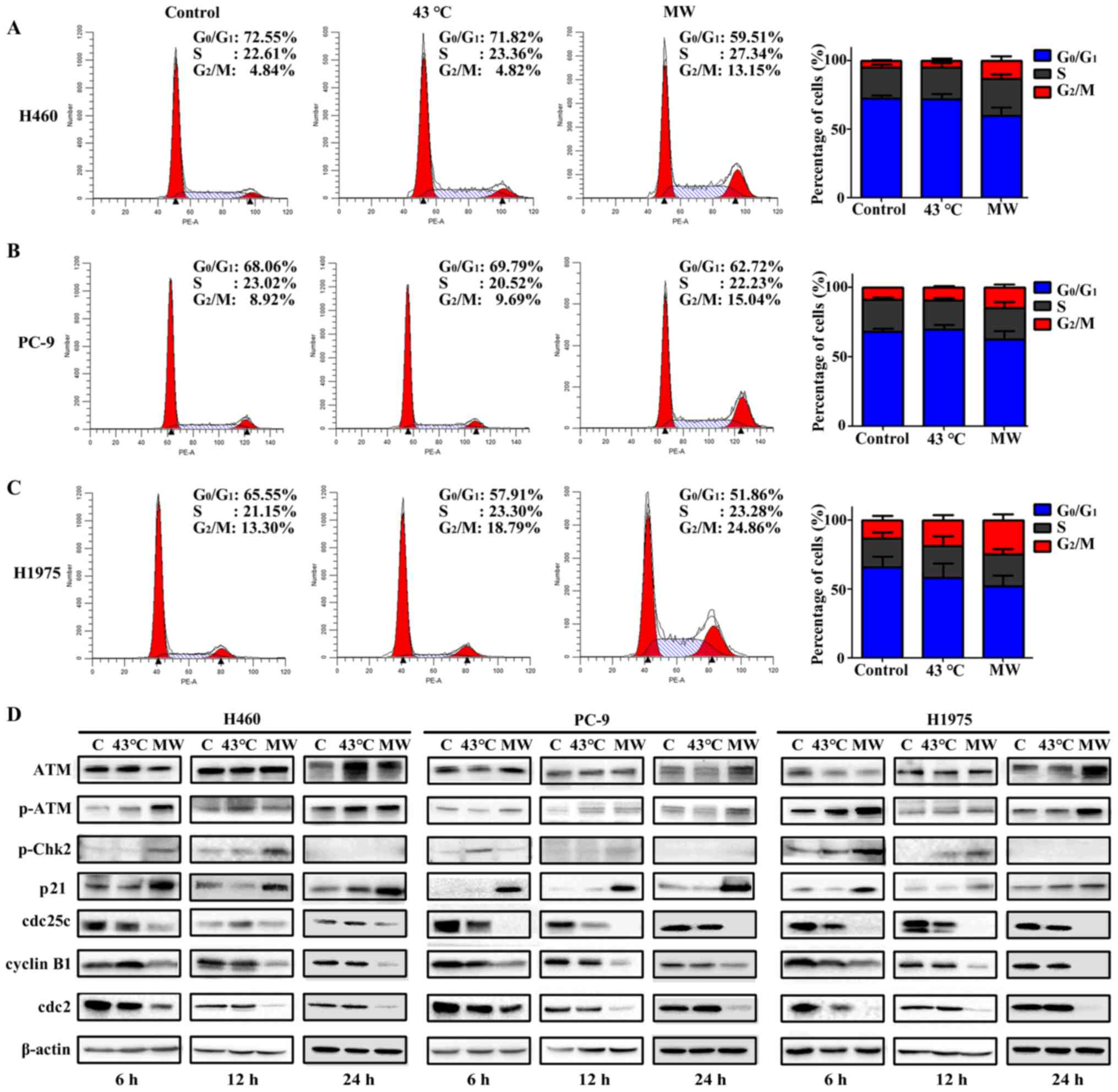
MW hyperthermia treatment induces
G2/M arrest
The cells were treated with MW hyperthermia or a
water bath, and the cell cycle distributions were analyzed by flow
cytometry. As shown in Fig. 7A–C,
the water bath treatment had no significant effect on cell cycle
distribution, while MW hyperthermia treatment markedly induced
G2/M phase arrest, indicated by the was significant
increase in the G2/M phase cell population compared with
that of the other groups. Compared with the control group, the
proportion of cells in the G2/M phase increased with MW
hyperthermia treatment (2.72-fold for the H460 cells, 1.88-fold for
the PC-9 cells and 2.10-fold for the H1975 cells; Fig. 7A–C).
To examine the effects of MW hyperthermia on the
cell cycle in more detail, the cell cycle regulator proteins for
cell transiting through the G2/M phase were examined by
western blot analysis. In all cell lines, ATM protein expression
was not altered considerably, while the phosphorylation of ATM was
increased in the MW hyperthermia group at 6 and 12 h, when compared
with the control group. p-Chk2 protein expression was increased in
the MW group at 6 and 12 h, but was not detected at 24 h. We
speculated that Chk2 phosphorylation was detected transiently
following MW exposure, which in turn rapidly activated downstream
signaling pathways. In the PC-9 cells, Chk2 phosphorylation was
increased slightly in the cells treated with the water bath at 6 h,
compared with the control group. The expression of p21 was markedly
increased in the MW hyperthermia group at 6, 12 and 24 h in all
cell lines. The expression levels of cdc25c, cdc2 and cyclin B1 in
the MW hyperthermia group were markedly decreased at 6, 12 and 24 h
in the various NSCLC lines. The protein expression levels of
regulators of the G2/M checkpoint were not markedly
altered with water bath treatment (Fig. 7D). Our results indicated that the
ATM signaling pathway may play a critical role in G2/M
arrest induced by MW hyperthermia.
Discussion
In this study, we examined the effects of MW
hyperthermia (433 MHz) on different NSCLC cells in vitro
using a self-developed device. Under the isothermal conditions
(43°C for 90 min), MW hyperthermia inhibited cell survival and
increased the cell apoptotic rates to a greater extent than water
bath treatment in vitro, and induced G2/M cell
cycle arrest. The mechanisms involved may be related to the
activation of the ATM pathway.
Over the past few decades, scientists have developed
a large number of equipment and techniques for use in clinical
hyperthermia (24). Previous
studies have indicated the positive effects of hyperthermia
treatments in clinical trials (25); however, the potential underlying
mechanisms remain unclear. The goal of thermal biological research
is to disclose the mechanisms responsible for the thermal effects,
in order to further reveal the mechanisms of thermal
radiosensitization and chemosensitization. In preclinical research,
water baths are the most common heating system used to investigate
hyperthermia, due to the lack of special thermal therapy
instruments. It is difficult to explain the antitumor mechanisms of
hyperthermia in the clinical setting. Water baths exert thermal
effects alone, while MW hyperthermia therapies also exert
non-thermal effects on cancer cells. A specifically designed device
is needed that will mimic the clinical treatment on heating cancer
cells in vitro. Electromagnetic heating devices operating
from 8–915 MHz have been proposed for the hyperthermia treatment of
cancer (24,26). To mimic the clinical conditions, we
developed a novel MW applicator for the experimental hyperthermia
treatment of cancer in vitro, which was equipped with a 433
MHz (ISM frequency in Europe: a frequency allocated for industry,
science and medicine) (27) MW
generator (Fig. 1). Using this
novel MW system, we found that the viability of the H460, PC-9 and
H1975 cells was decreased by MW hyperthermia, but not by the water
bath at 43°C. These results indicated that the thermal-tolerant
cells could be killed by MW hyperthermia. Caspase-3 was found to be
involved in MW hyperthermia-induced apoptosis. Our results are
consistent with those of another study in that MW hyperthermia
induced cell apoptosis (28). The
efficacy of hyperthermia depends on the treatment temperature and
duration in cancer cells (29).
Furthermore, we found that increasing the temperature of the water
bath resulted in similar apoptotic rates to MW hyperthermia at 43°C
(Fig. 3G–I). Pawlik et al
reported that heat-treated cells at 43.5 and 45°C exhibited marked
apoptotic phenomena (30). This
indicated that the degree of temperature was one of the essential
parameters in inducing apoptosis, but most importantly, MW may play
a key role in the antitumor effects. Our results indirectly
confirmed that MW hyperthermia exerted non-thermal effects on
cancer cell death. Asano et al reported that normothermic MW
irradiation induced cell death via heat-independent apoptosis
(31). The potential mechanisms
underlying these non-thermal effects of MW warrant further
investigation. These results indicated that our novel MW device may
be applied to preclinical studies in thermal biology, which is
difficult to achieve when employing typically used water bath
device. Moreover, we found that long-term exposure to MW (90 min)
may have some side-effects on primary astrocytes and normal lung
epithelial cells, BEAS-2B cells (Fig.
2E). Our results indicated that local treatment with MW
hyperthermia in the clinical setting may be beneficial for
eliminating the damage to normal surrounding tissue. On the whole,
our results provide give a clear explanation of the distinct
cell-killing effect of MW hyperthermia and the intrinsic molecular
mechanisms using this novel device.
Hyperthermia may enhance the production of
intracellular (ROS) (32,33). Hyperthermia-induced oxidative
stress is crucial in the initiation of apoptotic cell death
(28,34). Therefore, we speculated that ROS
may play a role in cell apoptosis induced by MW hyperthermia. The
results revealed that the accumulation of ROS was observed in the
MW-treated NSCLC cells. It has been recognized that ROS can damage
a variety of cellular components, leading to DNA damage,
mitochondrial dysfunction and apoptosis. The excessive accumulation
of ROS can open the mitochondrial permeability transition pore,
releasing pro-apoptotic proteins, and finally activating the
caspase cascade and inducing apoptosis (35). Furthermore, we found that MMP was
depolarized in all NSCLC cell lines following treatment with MW
hyperthermia. It is well known that depolarized MMP is an indicator
of mitochondrial dysfunction during apoptosis (36), and it is an early event that
coincides with caspase activation. The level of caspase-3
activation was markedly increased in the MW hyperthermia group
(8.52-fold in the H460 cells, 4.14-fold in the PC-9 cells and
1.64-fold in the H1975 cells; Fig.
4C). The caspase-3 specific inhibitor, Ac-DEVD-CHO,
significantly attenuated the increase in caspase-3 activity
compared with MW hyperthermia alone. Our data suggested that ROS
accumulation and MMP depolarization contributed to the MW
hyperthermia-induced caspase-3-dependent apoptosis of human NSCLC
cells.
There is accumulating evidence to indicate that
hyper-thermia inhibits cell growth via inducing apoptosis and/or
cell cycle checkpoint activation in tumor cells (37,38).
Cell cycle checkpoints are important for regulating cell growth. In
the present study, the results indicated that MW hyperthermia was
found to arrest the cell cycle of NSCLC cells at the
G2/M phase. Furthermore, we focused on investigating the
potential molecular mechanisms of MW hyperthermia-induced
G2/M checkpoint arrest. It is well known that cell cycle
progression requires the precise expression and activation of
several cyclins proteins and cyclin-dependent kinases (39). The ATM plays a key role in the
activation of cell cycle checkpoints (40). The ATM pathway responds not only to
DNA double-strand breaks, but also to damage induced by various
types of stress (41–43). p21 is an important member of the
family of cyclin kinase inhibitors, mediated G2/M cell
cycle arrest (44). When DNA
damage occurs, activated ATM induces Chk2 phosphorylation and p21
activation, consequently regulating cdc25c. Chk2 can induce
G2/M cell cycle arrest by decreasing the protein
expression of cdc25c (45). It is
well known that in the G2/M checkpoint, cdc2 and cyclin
B1 are the important regulators, and the activated cyclin B1/cdc 2
complex can trigger the transition from the G2 to the M
phase (46,47). In the present study, we found that
MW hyperthermia activated ATM and Chk2, further regulating the
protein expression levels of p21, cdc25c, cyclin B1 and cdc2. Our
results indicated that the mechanisms responsible for the effects
of MW hyperthermia on G2/M phase arrest may be related
to the regulation of the ATM-Chk2/p21-cdc25c signaling pathway.
Of note, we found that MW hyperthermia treatment
induced a significantly higher proportion of apoptotic NSCLC cells
with EGFR mutations (40.00±11.22% in the PC-9 cells with exon19
deletion in the EGFR gene, 37.70±9.17% in the H1975 cells with
L858R/T790M double mutations in the EGFR gene) than in the H460
cells with wild-type EGFR (12.45±1.20%) (Fig. 3B, D and F). Thus, we hypothesized
that the sensitivity of the cells to MW may differ among cells with
different EGFR gene types. It has been reported that EGFR
mutation-positive NSCLC is associated with the regression of
patient tumors to intrapleural perfusion with hyperthermic
chemotherapy complete treatment in clinical trials, and that the
mechanisms involved may be related to the decreased protein level
of EFGR (48). Our results provide
experimental evidence to support the thesis that EGFR
mutation-positive NSCLC cells may be more sensitive towards
hyperthermia. These results indicate that MW hyperthermia may be
one of the most promising adjuvant therapies against NSCLC tumors
with mutations in the EGFR gene.
In conclusion, in this study, we report for the
first time, at least to the best of our knowledge, that the
exposure of NSCLC cells to a specially designed MW device (433 MHz)
increases ROS production, mitochondrial dysfunction, promotes
caspase-3-dependent cell apoptosis and induces G2/M cell
cycle arrest by regulating the ATM signaling pathway. Heat
treatment under a water bath did not have the same effect. Our
results demonstrated that the MW hyperthermia device specially
designed by our group may be a good tool for hyperthermia research
in vitro.
Acknowledgments
The authors would like to thank the Experimental
Animal laboratory of the Zhejiang Academy of Medical Sciences for
assisting with the culture of astrocytes.
Funding
This study was supported by grants from the National
Natural Science Foundation (81602555), Zhejiang Medical and Health
Technology project of Zhejiang (2018247705). The funders had no
role in the study design, data collection and analysis, decision to
publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YYZ, SLM and SRZ designed the experiments; SLM and
SRZ were involved in project administration; YYZ, SLM and SRZ wrote
and edited the manuscript; YYZ, QW, ZBW, JJZ, LCZ, and YY performed
experiments and analyzed the data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed according
to the National Institute of Health Guild for the Care and Use of
Laboratory Animals and were in accordance with the Experimental
Animal Welfare Ethics Committee of Zhejiang Academy of Medical
Sciences (2018-045).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al International Association for the
Study of Lung Cancer Staging and Prognostic Factors Committee,
Advisory Boards, and Participating Institutions; International
Association for the Study of Lung Cancer Staging and Prognostic
Factors Committee Advisory Boards and Participating Institutions:
The IASLC Lung Cancer Staging Project: Proposals for Revision of
the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the
TNM Classification for Lung Cancer. J Thorac Oncol. 11:39–51. 2016.
View Article : Google Scholar
|
|
2
|
Torre LA, Siegel RL and Jemal A: Lung
cancer statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar
|
|
3
|
Cross DA, Ashton SE, Ghiorghiu S, Eberlein
C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ,
et al: AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated
resistance to EGFR inhibitors in lung cancer. Cancer Discov.
4:1046–1061. 2014. View Article : Google Scholar
|
|
4
|
Wu YL, Sequist LV, Tan EH, Geater SL,
Orlov S, Zhang L, Lee KH, Tsai CM, Kato T, Barrios CH, et al:
Afatinib as first-line treatment of older patients with EGFR
mutation-positive non-small-cell lung cancer: Subgroup analyses of
the LUX-lung 3, LUX-lung 6, and LUX-lung 7 trials. Clin Lung
Cancer. S1525-7304(18): 30051–2. 2018.
|
|
5
|
Issels RD, Lindner LH, Verweij J, Wust P,
Reichardt P, Schem BC, Abdel-Rahman S, Daugaard S, Salat C,
Wendtner CM, et al European Organisation for Research and Treatment
of Cancer Soft Tissue and Bone Sarcoma Group (EORTC-STBSG);
European Society for Hyperthermic Oncology (ESHO): Neo-adjuvant
chemotherapy alone or with regional hyperthermia for localised
high-risk soft-tissue sarcoma: A randomised phase 3 multicentre
study. Lancet Oncol. 11:561–570. 2010. View Article : Google Scholar
|
|
6
|
Datta NR, Rogers S, Ordóñez SG, Puric E
and Bodis S: Hyperthermia and radiotherapy in the management of
head and neck cancers: A systematic review and meta-analysis. Int J
Hyperthermia. 32:31–40. 2016. View Article : Google Scholar
|
|
7
|
Han JB, Kong FW, Ding H, Zhang YF, Liu JM,
Wei Q, Hu L, Zhao L, Xu CJ and Yi YX: Hepatectomy combined with
microwave ablation of the spleen for treatment of hepatocellular
carcinoma complicated with splenomegaly: A retrospective study. Mol
Clin Oncol. 6:204–208. 2017. View Article : Google Scholar
|
|
8
|
Poulou LS, Thanos L, Ziakas PD, Merikas E,
Achimastos A, Gennatas C and Syrigos KN: Thermal ablation may
improve outcomes in patients with colorectal liver metastasis: A
case-control study. J BUON. 22:673–678. 2017.
|
|
9
|
Bäcklund M and Freedman J: Microwave
ablation and immune activation in the treatment of recurrent
colorectal lung metastases: A case report. Case Rep Oncol.
10:383–387. 2017. View Article : Google Scholar
|
|
10
|
Sherar MD, Liu FF, Newcombe DJ, Cooper B,
Levin W, Taylor WB and Hunt JW: Beam shaping for microwave
waveguide hyperthermia applicators. Int J Radiat Oncol Biol Phys.
25:849–857. 1993. View Article : Google Scholar
|
|
11
|
Vernon CC, Hand JW, Field SB, Machin D,
Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD,
González González D, et al International Collaborative Hyperthermia
Group: Radiotherapy with or without hyperthermia in the treatment
of superficial localized breast cancer: Results from five
randomized controlled trials. Int J Radiat Oncol Biol Phys.
35:731–744. 1996. View Article : Google Scholar
|
|
12
|
Kouloulias V, Triantopoulou S, Vrouvas J,
Gennatas K, Ouzounoglou N, Kouvaris J, Karaiskos P, Aggelakis P,
Antypas C, Zygogianni A, et al: Combined chemoradiotherapy with
local microwave hyperthermia for treatment of T3N0 laryngeal
carcinoma: A retrospective study with long-term follow-up. Acta
Otorhinolaryngol Ital. 34:167–173. 2014.
|
|
13
|
Kouloulias V, Triantopoulou S,
Efstathopoulos E, Platoni K, Kouvaris J, Uzunoglou N, Antypas C,
Karaiskos P, Aggelakis P, Vrouvas J, et al: Microwave hyperthermia
in conjunction with radiotherapy in superficial tumours:
Correlation of thermal parameters with tumour regression. West
Indian Med J. 62:752–757. 2013.
|
|
14
|
Agostinelli E, Belli F, Dalla Vedova L,
Marra M, Crateri P and Arancia G: Hyperthermia enhances
cytotoxicity of amine oxidase and spermine on drug-resistant LoVo
colon adenocarcinoma cells. Int J Oncol. 28:1543–1553. 2006.
|
|
15
|
Li L, Wang W, Pan H, Ma G, Shi X, Xie H,
Liu X, Ding Q, Zhou W and Wang S: Microwave ablation combined with
OK-432 induces Th1-type response and specific antitumor immunity in
a murine model of breast cancer. J Transl Med. 15:232017.
View Article : Google Scholar
|
|
16
|
Asano M, Sakaguchi M, Tanaka S, Kashimura
K, Mitani T, Kawase M, Matsumura H, Yamaguchi T, Fujita Y and
Tabuse K: Effects of normothermic conditioned microwave irradiation
on cultured cells using an irradiation system with semiconductor
oscillator and thermo-regulatory applicator. Sci Rep. 7:412442017.
View Article : Google Scholar
|
|
17
|
Zagar TM, Oleson JR, Vujaskovic Z,
Dewhirst MW, Craciunescu OI, Blackwell KL, Prosnitz LR and Jones
EL: Hyperthermia combined with radiation therapy for superficial
breast cancer and chest wall recurrence: A review of the randomised
data. Int J Hyperthermia. 26:612–617. 2010. View Article : Google Scholar
|
|
18
|
Mallory M, Gogineni E, Jones GC, Greer L
and Simone CB II: Therapeutic hyperthermia: The old, the new, and
the upcoming. Crit Rev Oncol Hematol. 97:56–64. 2016. View Article : Google Scholar
|
|
19
|
Lee H, Park HJ, Park CS, Oh ET, Choi BH,
Williams B, Lee CK and Song CW: Response of breast cancer cells and
cancer stem cells to metformin and hyperthermia alone or combined.
PLoS One. 9:e879792014. View Article : Google Scholar
|
|
20
|
Gao F, Ye Y, Zhang Y and Yang J: Water
bath hyperthermia reduces stemness of colon cancer cells. Clin
Biochem. 46:1747–1750. 2013. View Article : Google Scholar
|
|
21
|
Wu ZB, Ma SL, Zhu J, Long JP, Li XD, Yao
XY, Lu HQ, Zhang YM, Chen SM and Jing SS: An experimental device
for heating tumor cell. CN Patent 2015205415415. Filed July 23,
2015; issued December 2, 2015.
|
|
22
|
Wang R, Zhang X, Zhang J, Fan Y, Shen Y,
Hu W and Chen Z: Oxygen-glucose deprivation induced glial scar-like
change in astrocytes. PLoS One. 7:e375742012. View Article : Google Scholar
|
|
23
|
Levison SW and McCarthy KD: Astroglia in
culture. Nerve Cell Culture. G Banker and K Goslin: MIT Press;
Cambridge, MA: pp. 309–336. 1991
|
|
24
|
Stauffer PR: Evolving technology for
thermal therapy of cancer. Int J Hyperthermia. 21:731–744. 2005.
View Article : Google Scholar
|
|
25
|
van Leeuwen CM, Oei AL, Chin KWTK, Crezee
J, Bel A, Westermann AM, Buist MR, Franken NAP, Stalpers LJA and
Kok HP: A short time interval between radiotherapy and hyperthermia
reduces in-field recurrence and mortality in women with advanced
cervical cancer. Radiat Oncol. 12:752017. View Article : Google Scholar
|
|
26
|
Rietveld PJ, van Putten WL, van der Zee J
and van Rhoon GC: Comparison of the clinical effectiveness of the
433 MHz Lucite cone applicator with that of a conventional
waveguide applicator in applications of superficial hyperthermia.
Int J Radiat Oncol Biol Phys. 43:681–687. 1999. View Article : Google Scholar
|
|
27
|
Paulides MM, Bakker JF, Chavannes N and
Van Rhoon GC: A patch antenna design for application in a
phased-array head and neck hyperthermia applicator. IEEE Trans
Biomed Eng. 54:2057–2063. 2007. View Article : Google Scholar
|
|
28
|
Xing F, Zhan Q, He Y, Cui J, He S and Wang
G: 1800MHz microwave induces p53 and p53-mediated caspase-3
activation leading to cell apoptosis in vitro. PLoS One.
11:e01639352016. View Article : Google Scholar
|
|
29
|
Zhang JF, Yan XM, Lan B, Lei YR, Li XH,
Gao S, Guo YF and Guo F: Molecular mechanisms of synergistic
induction of apoptosis by the combination therapy with hyperthermia
and cisplatin in prostate cancer cells. Biochem Biophys Res Commun.
479:159–165. 2016. View Article : Google Scholar
|
|
30
|
Pawlik A, Nowak JM, Grzanka D, Gackowska
L, Michalkiewicz J and Grzanka A: Hyperthermia induces cytoskeletal
alterations and mitotic catastrophe in p53-deficient H1299 lung
cancer cells. Acta Histochem. 115:8–15. 2013. View Article : Google Scholar
|
|
31
|
Asano M, Tanaka S, Sakaguchi M, Matsumura
H, Yamaguchi T, Fujita Y and Tabuse K: Normothermic microwave
irradiation induces death of HL-60 cells through heat-independent
apoptosis. Sci Rep. 7:114062017. View Article : Google Scholar
|
|
32
|
Wang Z, Cai F, Chen X, Luo M, Hu L and Lu
Y: The role of mitochondria-derived reactive oxygen species in
hyperthermia-induced platelet apoptosis. PLoS One. 8:e750442013.
View Article : Google Scholar
|
|
33
|
Katschinski DM, Boos K, Schindler SG and
Fandrey J: Pivotal role of reactive oxygen species as intracellular
mediators of hyperthermia-induced apoptosis. J Biol Chem.
275:21094–21098. 2000. View Article : Google Scholar
|
|
34
|
Hou CH, Lin FL, Hou SM and Liu JF:
Hyperthermia induces apoptosis through endoplasmic reticulum and
reactive oxygen species in human osteosarcoma cells. Int J Mol Sci.
15:17380–17395. 2014. View Article : Google Scholar
|
|
35
|
Lee H, Kim S, Choi BH, Park MT, Lee J,
Jeong SY, Choi EK, Lim BU, Kim C and Park HJ: Hyperthermia improves
therapeutic efficacy of doxorubicin carried by mesoporous silica
nanocontainers in human lung cancer cells. Int J Hyperthermia.
27:698–707. 2011. View Article : Google Scholar
|
|
36
|
Gu Y, Chen T, Fu S, Sun X, Wang L, Wang J,
Lu Y, Ding S, Ruan G, Teng L, et al: Perioperative dynamics and
significance of amino acid profiles in patients with cancer. J
Transl Med. 13:352015. View Article : Google Scholar
|
|
37
|
Ahmed K, Tabuchi Y and Kondo T:
Hyperthermia: An effective strategy to induce apoptosis in cancer
cells. Apoptosis. 20:1411–1419. 2015. View Article : Google Scholar
|
|
38
|
Furusawa Y, Iizumi T, Fujiwara Y, Zhao QL,
Tabuchi Y, Nomura T and Kondo T: Inhibition of checkpoint kinase 1
abrogates G2/M checkpoint activation and promotes
apoptosis under heat stress. Apoptosis. 17:102–112. 2012.
View Article : Google Scholar
|
|
39
|
Roskoski R Jr: Cyclin-dependent protein
kinase inhibitors including palbociclib as anticancer drugs.
Pharmacol Res. 107:249–275. 2016. View Article : Google Scholar
|
|
40
|
Kurz EU and Lees-Miller SP: DNA
damage-induced activation of ATM and ATM-dependent signaling
pathways. DNA Repair (Amst). 3:889–900. 2004. View Article : Google Scholar
|
|
41
|
Shaltiel IA, Krenning L, Bruinsma W and
Medema RH: The same, only different - DNA damage checkpoints and
their reversal throughout the cell cycle. J Cell Sci. 128:607–620.
2015. View Article : Google Scholar
|
|
42
|
Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K
and Linn S: Molecular mechanisms of mammalian DNA repair and the
DNA damage checkpoints. Annu Rev Biochem. 73:39–85. 2004.
View Article : Google Scholar
|
|
43
|
Lavin MF and Kozlov S: ATM activation and
DNA damage response. Cell Cycle. 6:931–942. 2007. View Article : Google Scholar
|
|
44
|
Beecken WD, Ringel EM, Babica J, Oppermann
E, Jonas D and Blaheta RA: Plasmin-clipped beta(2)-glycoprotein-I
inhibits endothelial cell growth by down-regulating cyclin A, B and
D1 and up-regulating p21 and p27. Cancer Lett. 296:160–167. 2010.
View Article : Google Scholar
|
|
45
|
Yin H, Jiang M, Peng X, Cui H, Zhou Y, He
M, Zuo Z, Ouyang P, Fan J and Fang J: The molecular mechanism of
G2M cell cycle arrest induced by AFB1 in the jejunum.
Oncotarget. 7:35592–35606. 2016.
|
|
46
|
Wang Z, Fan M, Candas D, Zhang TQ, Qin L,
Eldridge A, Wachsmann-Hogiu S, Ahmed KM, Chromy BA, Nantajit D, et
al: Cyclin B1/Cdk1 coordinates mitochondrial respiration for
cell-cycle G2/M progression. Dev Cell. 29:217–232. 2014. View Article : Google Scholar
|
|
47
|
Cheng YM, Tsai CC and Hsu YC:
Sulforaphane, a dietary isothiocyanate, induces G(2)/M arrest in
cervical cancer cells through cyclin B1 downregulation and
GADD45beta/CDC2 association. Int J Mol Sci. 17:15302016. View Article : Google Scholar
|
|
48
|
Zhang H, Zhan C, Ke J, Xue Z, Zhang A, Xu
K, Shen Z, Yu L and Chen L: EGFR kinase domain mutation positive
lung cancers are sensitive to intrapleural perfusion with
hyperthermic chemotherapy (IPHC) complete treatment. Oncotarget.
7:3367–3378. 2016.
|