Introduction
Gastric cancer (GC) is currently the fourth most
common malignancy in the worldwide scale. It remains the second
leading cause of cancer-related mortality, with a poor prognosis
following diagnosis. Regrettably, a large proportion of patients
with GC are at a late stage when diagnosed due to a lack of
effective screening programs (1–3).
Zinc finger proteins (ZFPs) comprise one of the largest
transcription factor families and are found exclusively in tetrapod
vertebrates (4). The
Krüppel-associated box (KRAB) exists in approximately one-third of
ZFPs (4). KRAB-ZFPs are considered
crucial regulators of diverse cellular progresses, such as cell
differentiation, proliferation, apoptosis and tumorigenesis
(4–6). ZFPs can activate or suppress gene
expression by binding to promoters (7,8). The
ZNF382 gene, a novel zinc finger transcription factor
described previously, is located on chromosome 19q13.12 and
contains only one KRAB domain. It has been shown to be a tumor
suppressor gene (TSG) and is commonly downregulated due to the
hypermethylation of its promoter CpG island in multiple carcinomas,
including GC (4,9). Moreover, ZNF382 can inhibit
activator protein-1 (AP-1) and nuclear factor (NF)-κB signaling and
downregulate multiple oncogenes, including melanogenesis associated
transcription factor (MITF), MYC, cyclin dependent kinase
(CDK)6 and high mobility group AT-hook 2 (HMGA2), and
it can also down-regulate several upstream factors of NF-κB,
including signal transducer and activator of transcription
(STAT)3, STAT5B and inhibitor of DNA binding 1, HLH
protein (ID1) (9).
Epithelial-mesenchymal transition (EMT) is both a
physiological and pathological course, regulating cell phenotype
and function during normal development and tumor development
(including GC) (10–12). Previous studies have verified the
vital role of SNAIL in suppressing E-cadherin expression;
SNAIL downregulates the expression of E-cadherin by
binding to the two E-boxes of the E-cadherin promoter
(13). Reportedly, various
signaling pathways, including NF-κB, Wnt and NOTCH, are involved in
this multi-step event (14,15).
Notably, NOTCH has been identified as a key factor involved in
tumor metastasis (16–18).
As there are limited studies available on
ZNF382, at least to the best of our knowledge, its roles
during EMT and GC are unclear. Thus, in this study, in order to
clarify the role of ZNF382 in GC, the expression level and
the methylation status of its promoter in GC cell lines and paired
gastric tumor tissues were examined. We further examined its
biological function and the potential underlying molecular
mechanisms involved in gastric tumorigenesis.
Materials and methods
Cell culture and tissue samples
Five GC cell lines (AGS, BGC823, MKN28, MKN45 and
SGC7901) were used. The AGS, MKN28 (reported to be a derivative of
the MKN74 GC cell line) (19,20)
and MKN45 cells were acquired from the American Type Culture
Collection (ATCC; Manassas, VA, USA) or provided by Professor Qian
Tao (the Chinese University of Hong Kong, Hong Kong, China). The
BGC823 and SGC7901 cells were purchased from the Cell Resource
Center of Shanghai Institution for Biological Sciences, Chinese
Academy of Sciences (Shanghai, China). The cells were allowed to
grown in RPMI-1640 medium (Gibco-BRL, Karlsruhe, Germany) at
37°C/5% CO2, supplemented with 100 mg/ml streptomycin,
100 U/ml penicillin and 10% fetal bovine serum (FBS; PAA
Laboratories, Linz, Austria). The MKN45 and SGC7901 cell lines
which were transfected with pcDNA3.1-ZNF382-Flag or vector pcDNA3.1
were selected with geneticin (G418). The ectopic expression of
ZNF382 was assayed by RT-PCR and western blot analysis prior to the
other experimental procedures. A total of 5 normal gastric tissues,
138 primary gastric tumor tissues and 64 matched adjacent non-tumor
tissue samples were acquired from the First Affiliated Hospital of
Chongqing Medical University, Chongqing, China (January, 2012 to
November, 2016). Clinical and pathological information was
collected for the majority of the tumor samples. DNA and RNA
extraction for the majority of these tissue samples were performed.
This study was approved by the Ethics Committee of the First
Affiliated Hospital of Chongqing Medical University, and all
patients provided signed informed consent.
DNA and RNA extraction
The QIAamp® DNA Mini kit (Qiagen, Hilden,
Germany) was used for the genomic DNA extraction from the cell
lines and tissues in accordance with the manufacturer's
instructions. TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used for the total RNA
isolation from the cell lines and tissue samples (−80°C for sample
storage).
Semi-quantitative reverse
transcription-PCR (RT-PCR) and quantitative PCR (qPCR)
Briefly, the RNA (1 µg) to 20 µg of
cDNA, the Reverse Transcription system (Promega, Madison, WI, USA)
was used. RT-PCR was carried out as previously described (21). GAPDH was used as an internal
control. RT-PCR was performed (32 cycles for target genes, 23
cycles for GAPDH) with Go-Taq polymerase (Promega). qPCR was
carried out in accordance with the instructions of the ABI 7500
system (Applied Biosystems, Foster City, CA, USA) using
SYBR®-Green qPCR Master Mix (MBI Fermentas, St.
Leon-Rot, Germany). The primers used in this study are listed in
Table I.
 | Table IList of primers used in this
study. |
Table I
List of primers used in this
study.
| PCR | Primer | Sequence
(5′–3′) | Product size
(bp) | PCR cycles | Annealing
temperature (°C) |
|---|
| RT-PCR | ZNF382F |
CCTTACAGGGATCAGTGTCA | 173 | 32 | 58 |
| ZNF382R |
CAACTTGCGGATCATATCAG | | | |
| SOX2F |
AGCAACGGCAGCTACAGCA | 281 | | 58 |
| SOX2R |
TGGGAGGAAGAGGTAACCACAG | | | |
| NANOGF |
ATGAGTGTGGATCCAGCTTG | 190 | | 58 |
| NANOGR |
CCTGAATAAGCAGATCCATGG | | | |
| OCT4F |
AAGGAGAAGCTGGAGCAA | 303 | | 58 |
| OCT4R |
GAGGGTTTCTGCTTTGCAT | | | |
| MMP2F |
TTTGACGGTAAGGACGGACTC | 346 | | 58 |
| MMP2R |
CCTGGAAGCGGAATGGAA | | | |
| MMP11F |
TTCTTCCGAGGCAGGGACTA | 203 | | 58 |
| MMP11R |
AAGCCTTCCAGAGCCTTCAC | | | |
| GAPDHF |
GGAGTCAACGGATTTGGT | 206 | 23 | 58 |
| GAPDHR |
GTGATGGGATTTCCATTGAT | | | |
| RT-qPCR | E-cadF |
TACACTGCCCAGGAGCCAGA | 103 | | 60 |
| E-cadR |
TGGCACCAGTGTCCGGATTA | | | |
| VimentinF |
GACCAGCTAACCAACGACAA | 150 | | 60 |
| VimentinR |
GTCAACATCCTGTCTGAAAGAT | | | |
| SNAIL1F |
CGCGCTCTTTCCTCGTCAG | 181 | | 60 |
| SNAIL1R |
TCCCAGATGAGCATTGGCAG | | | |
| TwistF |
CCACTGAAAGGAAAGGCATC | 122 | | 60 |
| TwistR |
CTATGGTTTTGCAGGCCAGT | | | |
| NOTCH1F |
AGGCATCCTACCCTTTTCTGG | 186 | | 60 |
| NOTCH1R |
GGCTCTGGCAAGTCTCCTACAA | | | |
| NOTCH2F |
AGGCAGGATTTGATGGAGTC | 150 | | |
| NOTCH2R |
TCTCATGGAGGCAGAAGGAT | | | |
| NOTCH3R |
CAGCAAGGCTATGGAACATG | | | |
| NOTCH4F |
CTGCGATAATGCGAGGAAGATA | 144 | | 60 |
| NOTCH4R |
ACGGAGTAAGGCAAGGAGGC | | | |
| HES-1F |
AGATAGCTCGCGGCATTCC | 130 | | 60 |
| HES-1R |
GTACTTCCCCAGCACACTTG | | | |
| JAG1F |
GTGCCGCATCTCACAGCTAT | 167 | | 60 |
| JAG1R |
TGATCTAAGACTGCATCACCA | | | |
| MSP | ZNF382m1 |
GGCGATTAACGGGTCGTTTC | 230 | 40 | 60 |
| ZNF382m2 |
AAAATTTCCAAACCCGACTCG | | | |
| ZNF382U1 |
GTGGTGATTAATGGGTTGTTTT | 233 | | 58 |
| ZNF382U2 |
CAAAATTTCCAAACCCAACTCA | | | |
5-Aza-2′-deoxycytidine (Aza) and
trichostatin A (TSA) treatment
Fresh medium containing 10 mmol/l Aza
(Sigma-Aldrich, Steinheim, Germany) was used for cell culture.
After 3 days, the cells were treated with 100 nmol/l TSA
(Sigma-Aldrich) for the following 24 h. Cells were then collected
for RNA extraction (22).
DNA bisulfite treatment and
methylation-specific PCR (MSP)
DNA bisulfite modifications and MSPs were carried
out in accordance with previously described methods (23,24).
The primers used for MSP are listed in Table I. AmpliTaq®-Gold DNA
Polymerase (Applied Biosystems) was used, and 40 amplification
cycles were performed in both the methylated and unmethylated
tissue samples. Products were detected on 2% (w/v) agarose gels
with 100 bp DNA markers (MBI Fermentas, Vilnius, Lithuania).
ZNF382 overexpression in GC cell
lines
Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used in accordance with the instructions for
the transfection of MKN45 and SGC7901 cells with 4 µg
pcDNA3.1-ZNF382-Flag or pcDNA3.1 plasmids (provided by Professor
Qian Tao at the Chinese University of Hong Kong) in non-serum
medium; 6 h later, the medium was replaced with fresh non-selective
growth medium for 48 h. The transfected cells were then cultured
with selective medium containing G418 (10 µl/ml) for 14 days
and the cultures were maintained with 5 µl/ml G418. Total
RNA was extracted from the cells following transfection and
digested with TURBO™ DNase (Ambion, Austin, TX, USA). RT-PCR and
western blot analysis were carried out to confirm the stable
overexpression of ZNF382.
Immunohistochemical staining (IHC)
assay
The gastric cancer tissue samples (n=55) were
formalin-fixed and paraffin-embedded. These tissue samples included
55 GC tissues and 29 matched tumor adjacent tissues.
Immunohistochemical staining was then performed as previously
described (22,25). The sections were incubated with a
rabbit monoclonal antibody (HPA049259, anti-ZNF382 antibody, 1:50
dilution; Sigma-Aldrich, St. Louis, MO, USA) at 4°C overnight. The
following day, samples were rinsed with phosphate-buffered saline
(PBS), incubated with rabbit secondary antibody (SP-9001, 1:100
dilution; ZSGB-BIO, Beijing, China) at 37°C for 30 min, stained
with diaminobenzidine for 33 sec, and counterstained with
hematoxylin for 5 sec to visualize the nuclei. Each section was
assessed and scored by two independent pathologists who were
blinded to the origin of all tissues. The widely accepted German
semi-quantitative scoring criteria was used for scoring, and the
staining index was determined by multiplying the score for staining
intensity with the score for staining extent, as previously
described (26). Clinical data
included age, sex, histological type, differentiation grade and TNM
stage. Clinical follow-up data for 55 patients were censored for
the analysis.
Colony formation assay
Stable MKN45 and SGC7901 cells were planted in a
6-well plate at 100 cells/well for 14 days, and the medium was
refreshed every 2–3 days. The cells were fixed in 4%
paraformaldehyde for 30 min, and after staining with Gentian Violet
(ICM Pharma, Singapore, Singapore) for 20 min, surviving colonies
with >50 cells/colony were counted using ImageJ (V.1.8.0)
software (National Institutes of Health, Bethesda, MD, USA) for the
analysis. The experiments were repeated 3 times.
Cell viability
CCK-8 assay was performed according to the
manufacturer's instructions (Beyotime, Shanghai, China). Stable
MKN45 and SGC7901 cells were seeded in 96-well plates
(2×103 cells/well) and cultured for 24, 48 or 72 h, and
the medium in each well was then replaced with 100 ml RPMI-1640
(10% FBS) containing 10 ml CCK-8 solution and incubated at 37°C for
2 h. The absorbance at 450 nm was measured using a microplate
reader (Multiskan MK3; Thermo Fisher Scientific, former Fermentas,
Schwerte, Germany) at 24, 48 and 72 h. All experiments were
assessed in triplicate.
Flow cytometric analysis
To investigate the cell cycle status, the cells were
collected and centrifuged (200 × g for 5 min), rinsed twice with
PBS and fixed with 70% ethanol at 4°C overnight. The following day,
the cells were treated with 50 mg/l propidium iodide (PI)
(Beyotime) for 30 min in the condition of 4°C in the dark. For
apoptosis analyses, the cells were washed, collected, resuspended
in PBS, stained with Annexin V-FITC (BD Pharmingen, San, Jose, CA,
USA) and PI, and analyzed using a flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA).
Wound-healing assay
The cells were cultured in 6-well plates. Using a
sterile tip to scratched a straight linear wound when the cultured
cells were confluent. After rinsing with PBS, the cells were
incubated with fresh growth medium. Images of the cells were
captured with a 10× objective lens (Nikon, Tokyo, Japan) at 0, 24
and 48 h after wounding for the SGC7901 cells and at 0, 12 and 24 h
for the MKN45 cells. The experiments were performed in
triplicate.
Migration and invasion assay
The migratory and invasive abilities of the GC cells
were also investigated using Transwell chambers (8 µm pore
size; Corning, New York, NY, USA) with or without a Matrigel (BD
Biosciences) barrier added to the top chamber. The MKN45 and
SGC7901 cells transfected with the ZNF382 overexpression
vector or the control vector were collected, washed twice in
non-serum medium, and seeded into the upper Transwell chamber.
Approximately 800 µl of medium with 10% FBS was added to the
lower chambers. Following incubation at 37°C and 5% CO2
for 24 h, the cells were fixed in 4% paraformaldehyde for 30 min
and stained with crystal violet (DC079; Genview, Beijing, China)
for 20 min at room temperature. Cells on the upper side of the
chamber were wiped off with a cotton bud. Cells from 6 random
fields were captured and counted under a microscope (×100
magnification, CTR4000; Leica, Wetzlar, Germany).
Immunofluorescence staining
The MKN45 and SGC7901 cells were cultured in 24-well
plates with glass coverslips in the wells and transiently
transfected with pcDNA3.1-Flag-ZNF382. Forty-eight hours later, the
cells were fixed in 4% paraformaldehyde for 30 min, then
permeabilized in 0.1% Triton X-100 for 5 min, followed by blocking
with 1% bovine serum albumin (BSA) in PBS for 1 h. The cells were
then incubated with primary antibodies against ZNF382 (HPA049259,
1:200 dilution; Sigma-Aldrich) and E-cadherin (sc-8426, 1:200
dilution) or Vimentin (sc-6260, 1:200 dilution) (both from Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C overnight,
then incubated with Alexa Fluor® 594-conjugated
(#A-11032, 1:200 dilution; Invitrogen; Thermo Fisher Scientific,
Inc.) or FITC-conjugated (#111-585-003, 1:200 dilution; Jackson
ImmunoResearch, West Grove, PA, USA) secondary antibody against
rabbit or mouse IgG. 4′,6-Diamidino-2-phenylindole (DAPI) was used
for nuclei counterstaining. Images were captured using a confocal
laser scanning microscope (×200 and ×400 magnification).
Western blot analysis
This assay was conducted as previously described
(27). Protein extraction reagent
(Thermo Fisher Scientific, Inc.), containing phenylmethanesulfonyl
fluoride, protease inhibitor and phosphatase inhibitor cocktail
(Sigma-Aldrich), was used for cell lysis (all proteins were
extracted from cells). Following disruption using the Ultrasonic
Cell Disruptor (Ningbo Scientz Biotechnology Co., Ltd., Ningbo,
China), the cell suspensions were centrifuged (200 × g for 4°C),
and the supernatant was collected. To determine the concentration
of proteins, a BCA protein kit (Thermo Fisher Scientific, Inc.) was
used. Sodium dodecyl sulphate/polyacrylamide gel electrophoresis
was conducted for the separation of a total of 40 mg protein
lysate. Proteins were then transferred onto a polyvinylidene
fluoride membrane (Bio-Rad, Hercules, CA, USA). Membranes were then
incubated with blocking buffer (PBS with 5% non-fat milk and 0.1%
Tween-20) for 1 h at room temperature. Several primary antibodies
were used: ZNF382 (HPA049259, 1:1,000 dilution; Sigma-Aldrich),
E-cadherin (sc-8426, 1:1,000 dilution), Vimentin (sc-6260, 1:1,000
dilution) (both from Santa Cruz Biotechnology, Inc.), SNAIL1
(ab135708, 1:1,000 dilution; Abcam, Cambridge, UK), NOTCH1
(sc-376403, 1:1,000 dilution), NOTCH3 (sc-515825, 1:1,000
dilution), HES-1 (sc-166378, 1:1,000 dilution) and SOX2 (sc-365823,
1:1,000 dilution); GAPDH (sc-47724, 1:1,000 dilution) (all from
Santa Cruz Biotechnology, Inc.) was used as a control. Anti-rabbit
IgG (#7074, 1:3,000 dilution) and anti-mouse IgG (#7076, 1:3,000
dilution) (both from Cell Signaling Technology, Danvers, MA, USA)
horseradish peroxidase conjugate secondary antibodies were used.
The membranes were visualized using the enhanced chemiluminescence
(ECL) detection kit (Amersham Pharmacia Biotech, Piscataway, NJ,
USA).
Statistical analysis
All data were analyzed with the use of SPSS
software, version 19.0 (SPSS Inc., Chicago, IL, USA). The
Chi-square (also termed χ2) test was used for the
analysis of the results of immunohistochemistry. The Student's
t-tests, Chi-square test and Fisher's exact test were used for the
comparison of the methylation status and clinicopathological
characteristics of the patients with GC. For all assays, a value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
ZNF382 expression is downregulated in
both GC cell lines and primary GC tissues
Semi-quantitative RT-PCR was carried out to examine
the expression of ZNF382 in several gastric tumor cell lines
and 5 normal gastric tissues. ZNF382 expression was
significantly suppressed in 3 of the 5 GC cell lines, and
ZNF382 was faintly expressed in the AGS and MKN28 cell
lines. By contrast, ZNF382 was strongly expressed in the 5
normal gastric tissues (Fig. 2A).
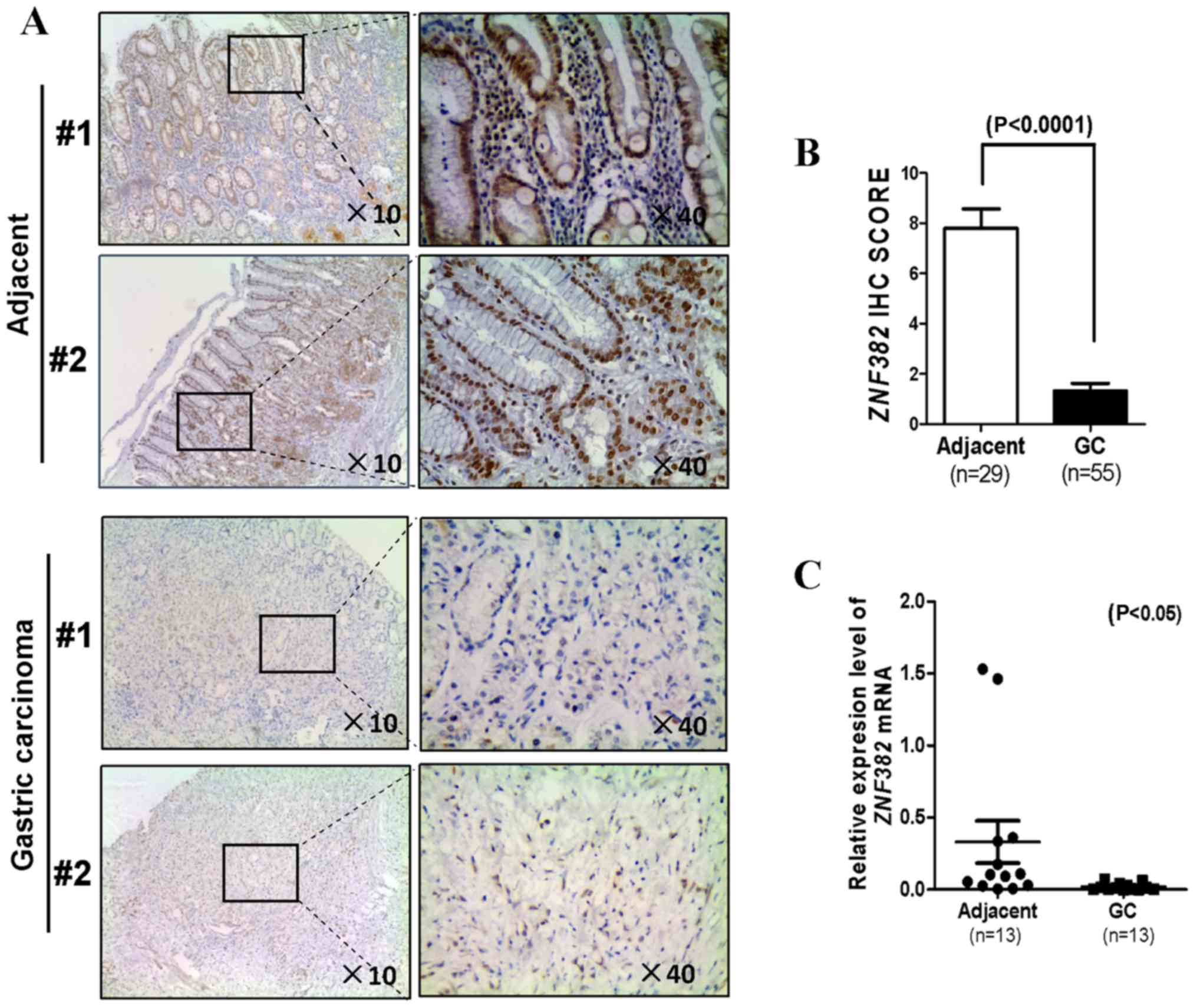
ZNF382 expression in the GC tissue samples and paired tumor
adjacent samples was then assayed by qPCR and immunohistochemistry.
A total of 55 GC tissues and 29 matched adjacent non-tumor tissues
were collected to determine the ZNF382 protein levels by
immunohistochemistry. We found that ZNF382 was located
predominantly in the nucleus (Fig.
1A). The majority of the tumor tissue samples (51/55) had a
lower level of ZNF382, while the adjacent non-tumor samples (21/29)
exhibited a higher ZNF382 level (P<0.0001) (Fig. 1B). The mRNA expression level of
ZNF382 in 13 additional GC samples was markedly decreased in
comparison with the paired tumor adjacent tissues (P<0.05)
(Fig. 1C). These findings
indicated that ZNF382 expression was downregulated in both
GC cells and primary GC tissues. No association was observed
between the ZNF382 expression level and the clinicopathological
characteristics of the patients with GC (Table II).
 | Table IIAssociation between the
clinicopathological characteristics of the patients with gastric
cancer and ZNF382 expression. |
Table II
Association between the
clinicopathological characteristics of the patients with gastric
cancer and ZNF382 expression.
| Parameter | No. | ZNF382
expression
| P-value |
|---|
| None | Low | Moderate | High |
|---|
| Sex | | | | | | 0.283 |
| Female | 15 | 5 | 10 | 0 | 0 | |
| Male | 40 | 20 | 16 | 2 | 2 | |
| Age (years) | | | | | | 0.504 |
| ≤60 | 28 | 13 | 14 | 1 | 0 | |
| >60 | 27 | 13 | 11 | 1 | 2 | |
| Tumor size
(cm) | | | | | | 0.668 |
| ≤3 | 12 | 6 | 5 | 1 | 0 | |
| >3 | 43 | 20 | 20 | 1 | 2 | |
| Metastasis | | | | | | 0.731 |
| None | 37 | 17 | 17 | 2 | 1 | |
| Yes | 18 | 9 | 8 | 0 | 1 | |
| Grade | | | | | | 0.793 |
| G2 | 14 | 6 | 7 | 1 | 0 | |
| G3 | 41 | 20 | 17 | 2 | 2 | |
| T Stage | | | | | | 0.714 |
| Ta-T2 | 8 | 3 | 5 | 0 | 0 | |
| T3-T4 | 47 | 24 | 20 | 2 | 1 | |
ZNF382 downregulation in GC cell lines by
promoter CpG methylation
We then determined whether promoter CpG methylation
is the primary cause for the downregulation of ZNF382 in GC
cell lines. The ZNF382 promoter methylation status in 4 GC
cell lines was detected with the use of MSP. The hypermethylation
of the ZNF382 promoter was observed in 3 of the 4 gastric
tumor cell lines (Fig. 2A). To
examine whether ZNF382 suppression was due directly to
promoter methylation, we treated the MKN45 and SGC7901 cells with
Aza and TSA, and then performed RT-PCR. The restoration of
ZNF382 expression was observed following treatment with Aza
and TSA (Fig. 2B).
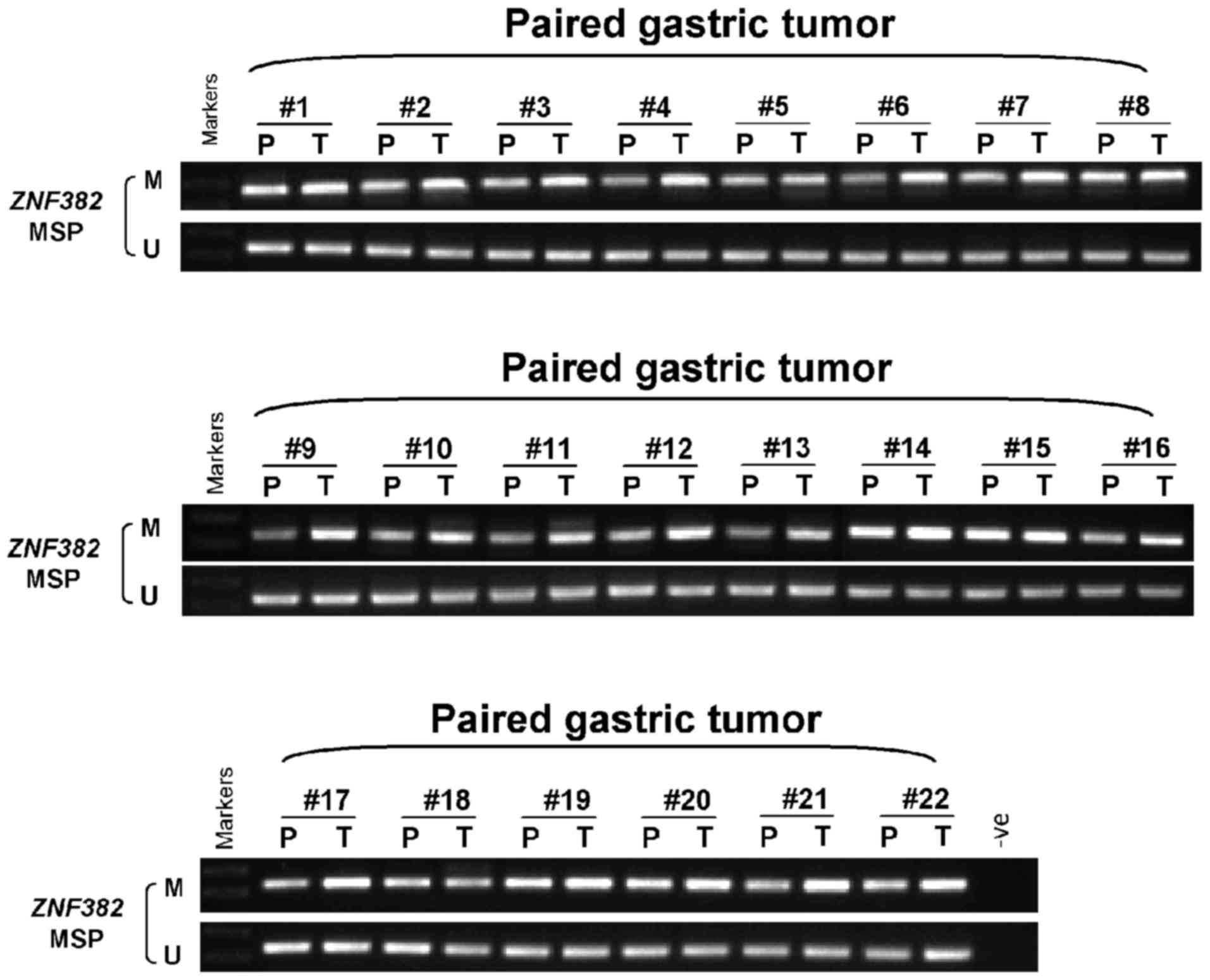
We further assayed the methylation of the
ZNF382 promoter in 70 GC tissues, as well as 22 matched
adjacent non-tumor gastric tissues. The results revealed that
ZNF382 was methylated in 100% (70/70) of primary GC tissues
(Figs. 2C and 3), and the methylation level was
significantly higher in the majority of the GC tissues compared
with the adjacent non-tumor tissues (Fig. 3). However, we failed to identify
any inter-relation between ZNF382 promoter methylation and
the patient clinicopathological characteristics (data not
shown).
ZNF382 inhibits colony formation and
proliferation, and induces cell cycle arrest and the apoptosis of
GC cell lines
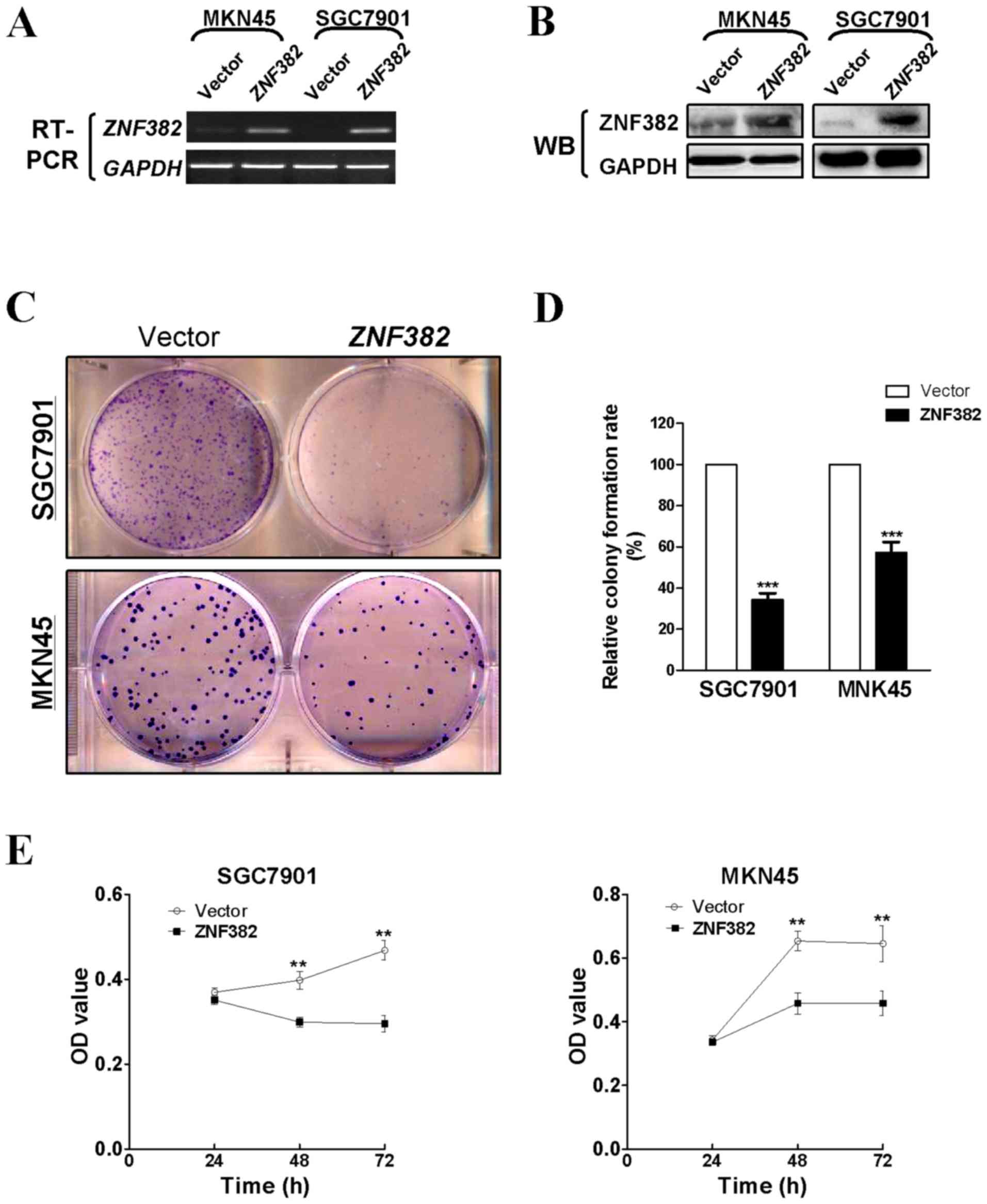
Several methods were used to determine the function
of ZNF382 in GC cells. To investigate whether ZNF382 affects
cell growth in GC, colony formation and CCK8 assays were carried
out using stably transfected MKN45 and SGC7901 cells. ZNF382
expression in the cell lines was verified by RT-PCR and western
blot analysis (Fig. 4A and B). The
ectopic expression of ZNF382 markedly reduced the ability of
the GC cells to form colonies compared with the controls
(P<0.001) (Fig. 4C and D). Cell
viability also decreased markedly at 48 and 72 h (P<0.01)
(Fig. 4E).
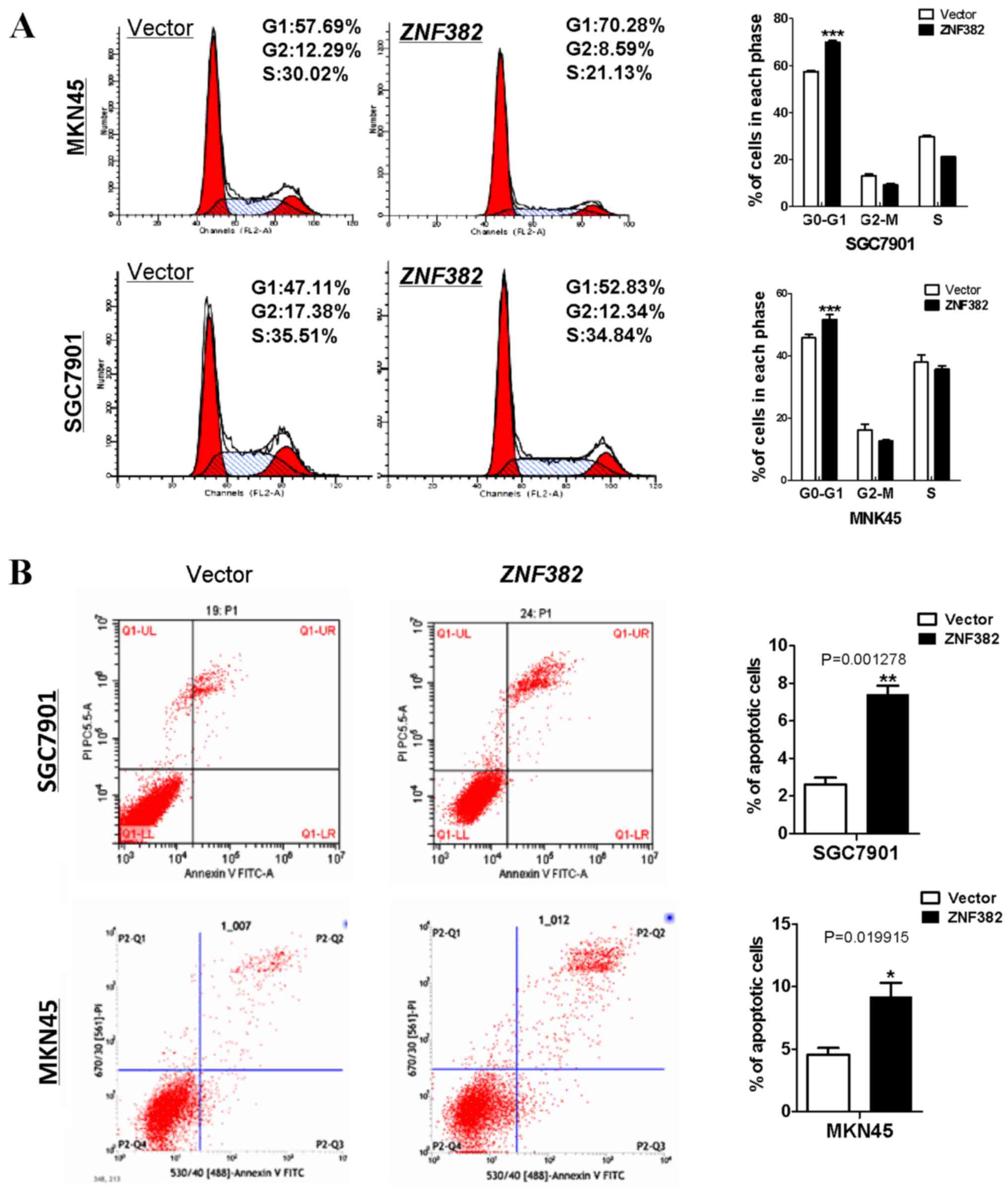
In addition, flow cytometry was used to determine
whether ZNF382 affects the cell cycle and apoptosis of GC
cells. It was found that a greater number of
ZNF382-expressing cells had accumulated in the G0/G1 phase
of the cell cycle compared with the controls (P<0.001) (Fig. 5A). Subsequently, Annexin V-FITC/PI
staining assay was performed to assess the rate of apoptosis. We
found that ZNF382 exerted a pro-apoptotic effect on these
two GC cell lines (P<0.05 and P<0.01) (Fig. 5B), suggesting that ZNF382 acts as a
potential tumor suppressor in GC.
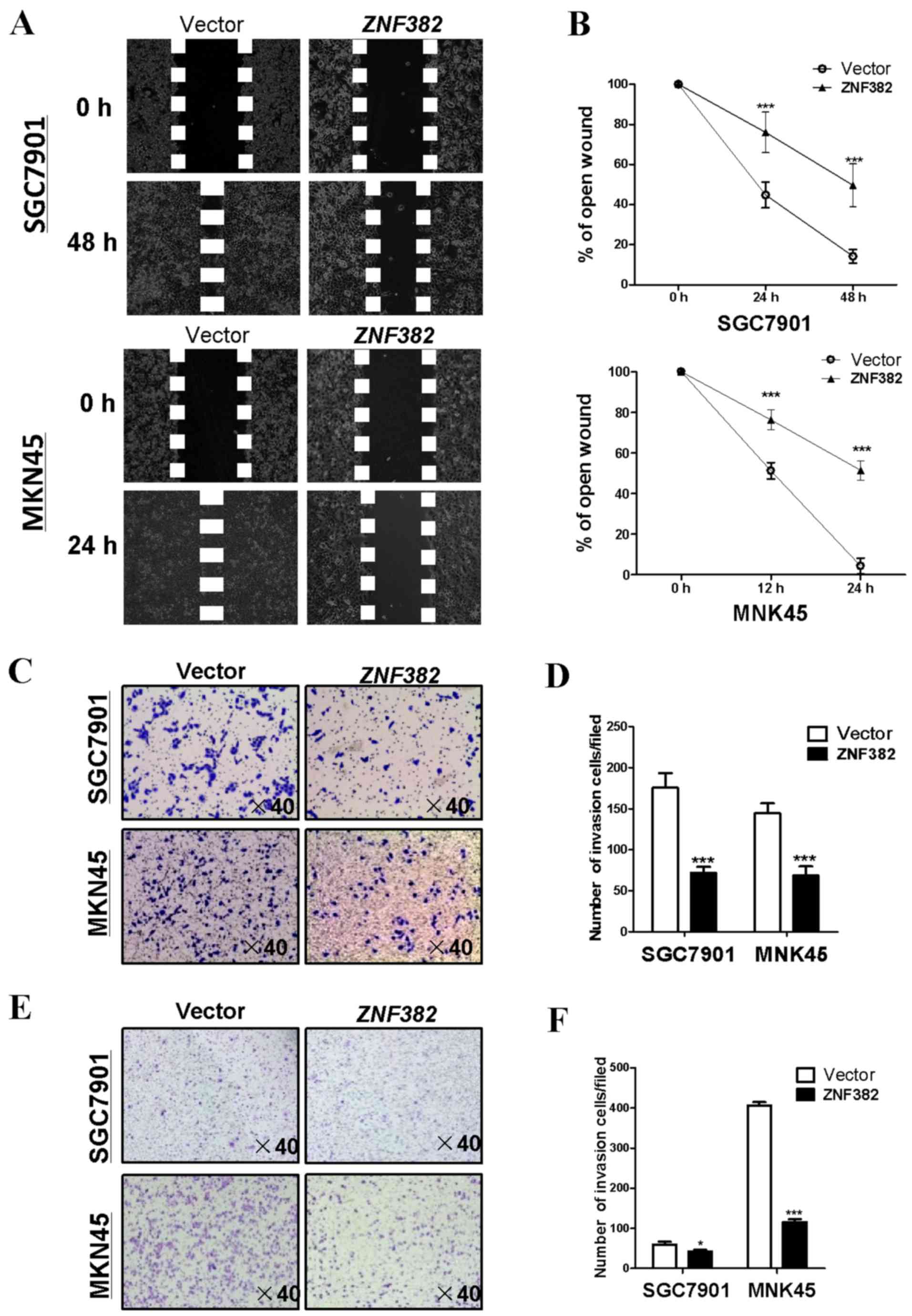
ZNF382 suppresses cell migration and
invasion in gastric tumor cells
The effects of ZNF382 on the migration and invasion
of GC cells were investigated using wound-healing and Transwell
assays. The results of wound-healing assay revealed that
ZNF382-expressing SGC7901 cells were less able to migrate
along the edges of wounds at 24 and 48 h compared with the
controls, while the same phenomenon was observed in the MKN45 cells
at 12 and 24 h (P<0.001) (Fig. 6A
and B). Furthermore, the results of Transwell assay illustrated
that the number of migrated cells was markedly decreased in the
ZNF382-transfected cells compared with the controls
(P<0.001) (Fig. 6C and D). In
the Transwell assay, which included a Matrigel barrier,
ZNF382 overexpression was associated with the inhibition of
GC cell invasion through the Matrigel before traversing the
Transwell chamber membrane (P<0.05, P<0.001) (Fig. 6E and F), indicating that
ZNF382 inhibits the migration and invasion of GC cells.
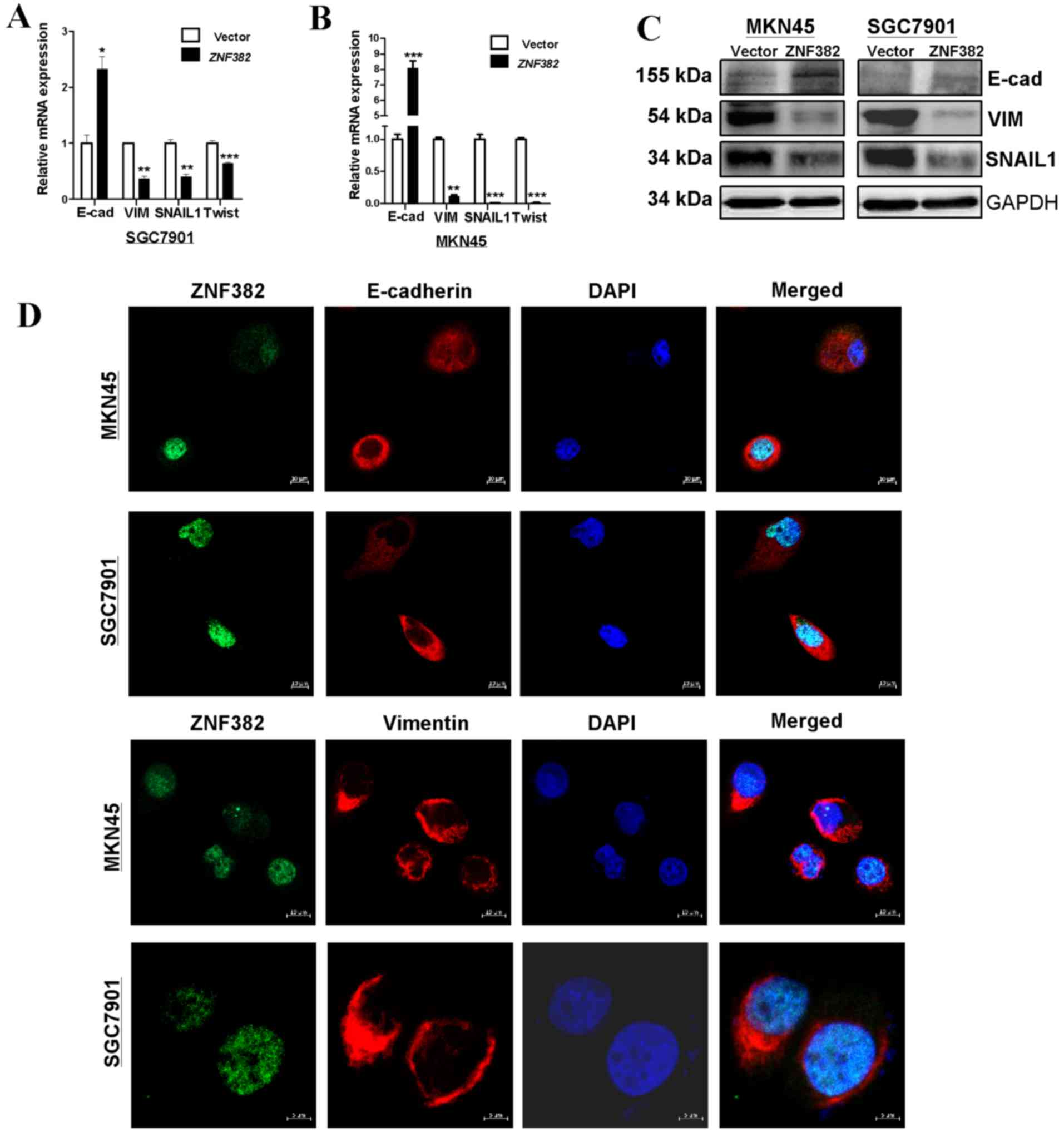
ZNF382 can reverse EMT through NOTCH
signaling in GC cells
We then examined whether ZNF382 can affect EMT in GC
cells. The results indicated that ectopic ZNF382 expression
reversed EMT to mesenchymal-to-epithelial transition in both cell
lines examined (MKN45 and SGC7901). The results of western blot
analysis and qPCR confirmed that E-cadherin expression was
increased in the cells transfected with ZNF382, and the
expression of SNAIL1, Twist and Vimentin was decreased (Fig. 7A–C). Moreover, immunofluorescence
revealed increased staining for E-cadherin and decreased staining
for Vimentin in the ZNF382-expressing cells (Fig. 7D), indicating that ZNF382
suppressed EMT in GC cells.
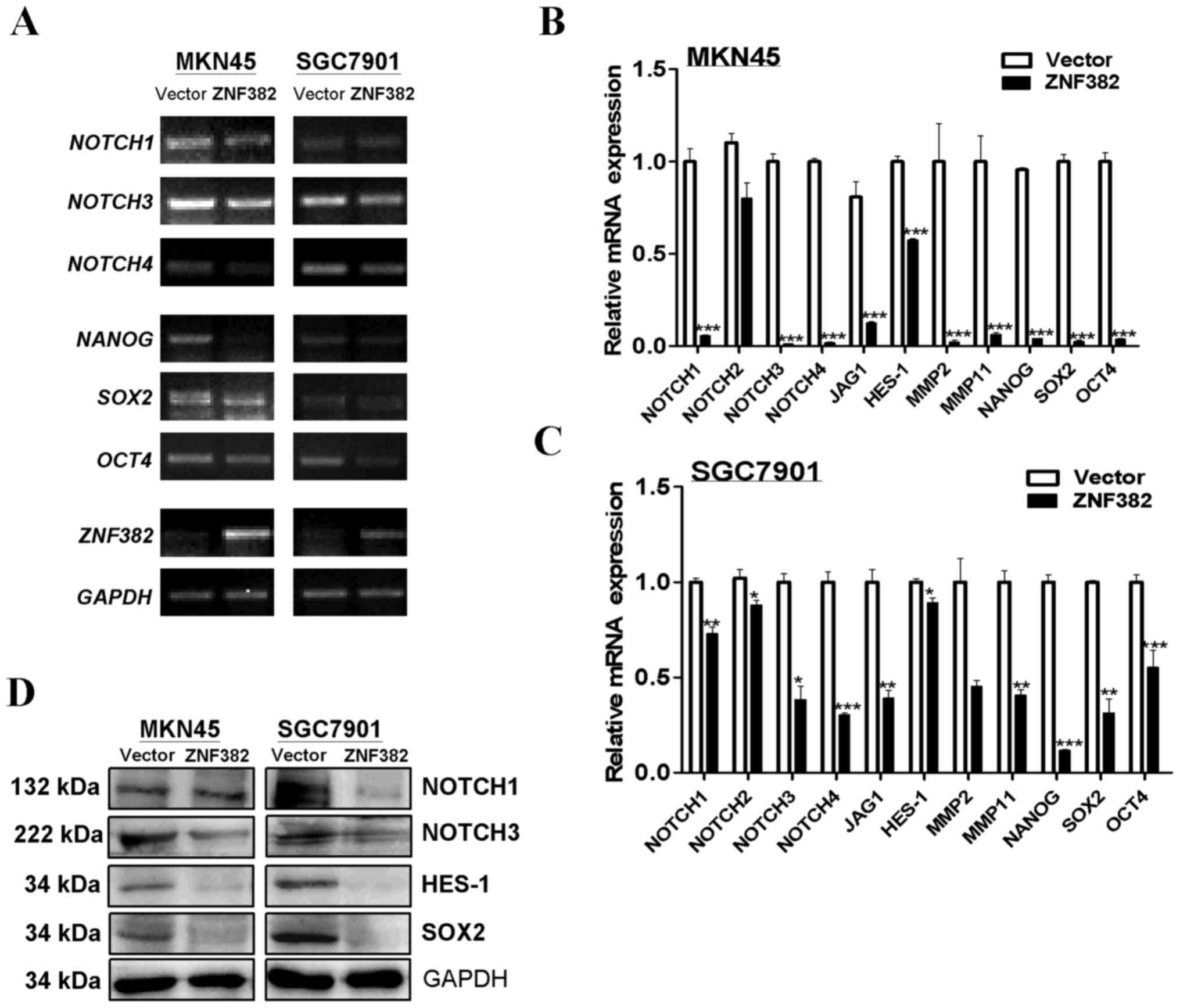
Recent studies have illustrated that NOTCH signaling
plays a role in promoting EMT in multiple carcinoma types (14,16–18).
In this study, we thus examined whether ZNF382 is related to
this pathway. The results of RT-PCR and qPCR revealed that the
ectopic expression of ZNF382 downregulated the important
receptor and ligand markers of the NOTCH signaling pathway (e.g.,
NOTCH1, NOTCH2, NOTCH3, NOTCH4 and
JAG1) in the MKN45 and SGC7901 cells (Fig. 8A–C). The results were verified by
western blot analysis, revealing that the NOTCH signaling
downstream target, HES-1, was also downregulated in
ZNF382-expressing cells (Fig. 8D).
Thus, these findings suggest that ZNF382 reverses the EMT
process by antagonizing NOTCH signaling, although this requires
further investigation.
Finally, cells that have stem-like properties are
tightly connected with EMT in tumor cells. Thus, we investigated
whether ZNF382 suppresses stemness in GC cells. Several markers of
cell stemness, such as NANOG, octamer-binding transcription
factor 4 (OCT4) and SOX2, were downregulated in
ZNF382-expressing cells (Fig.
8A–C). These results were confirmed by western blot analysis
(Fig. 8D), illustrating that
ZNF382 suppresses both EMT and stemness in GC cells.
Discussion
Previous research has revealed that ZNF382 is
commonly silenced by the methylation of its promoter, and that
ZNF382 exists in multiple carcinoma types, including
colorectal, nasopharyngeal, gastric and breast carcinomas as a
tumor suppressor (9). However, as
a novel member of the KRAB-ZFP family, little is known about its
role in GC. Thus, it is worth elucidating the direct association
between ZNF382 and GC. In this study, we observed that
ZNF382 expression was decreased in several GC cell lines and
GC tissues. We also noted that ZNF382 expression was decreased in
the AGS and MKN28 cells, while it was silent in the BGC823, MKN45
and SGC7901 cells. MSP and demethylation treatment revealed that
the downregulation of ZNF382 in the GC cell lines and GC
tumors was a result of promoter methylation. We then examined the
tumor-repressive function of ZNF382 in the MKN45 and SGC7901
cells. The ectopic expression of ZNF382 in these two cell
lines markedly repressed clonogenicity, suppressed cell
proliferation, restrained migration and invasion, and induced
apoptosis; these data illustrate that ZNF382 functions as a
tumor suppressor in GC cells.
Moreover, ZNF382 binds to target promoters
and acts as a transcriptional repressor. Therefore, investigating
the target genes affected by ZNF382 may prove to be pivotal
for revealing the underlying mechanisms of its suppressive effect.
As such, RT-PCR and qPCR assays were carried out to screen the
downstream target genes of ZNF382. Our results revealed that the
ectopic expression of ZNF382 significantly reversed EMT to a
mesenchymal-to-epithelial transition in both the MKN45 and SGC7901
cells, evidenced by the increased expression of the epithelial
marker, E-cadherin, and the decreased expression of the mesenchymal
markers, Vimentin, SNAIL1 and Twist. These findings indicate that
ZNF382 may serve as a transcriptional repressor, reversing EMT in
GC cells.
NOTCH is bound by its ligands, which is followed by
the cleavage and release of the NOTCH intracellular domain (NICD).
NICD regulates downstream target genes by translocating to the
nucleus and binding specific transcriptional regulators (12,28).
As recently reported, the NOTCH signaling pathway promotes EMT in
multiple carcinoma types, including GC (12,14).
However, it remains unclear as to whether ZNF382 is associated with
the NOTCH signaling pathway in GC. RT-PCR and qPCR assays revealed
that the ectopic expression of ZNF382 downregulated the expression
of NOTCH1, NOTCH2, NOTCH3, NOTCH4,
HES-1 and JAG1, as well as that of several stem cell
markers (OCT4, SOX2 and NANOG). Some of these
results were confirmed by western blot analysis, which indicated
that ZNF382 overexpression downregulates NOTCH1, NOTCH3 and
its downstream target, HES-1, in the MKN45 and SGC7901 cells. Thus,
we hypothesized that ZNF382 may reverse EMT by antagonizing
NOTCH signaling; however, further investigations are required to
determine the exact mechanisms through which ZNF382 regulates EMT
via NOTCH signaling. Moreover, further studies such as sphere
forming assay are warranted to determine whether ZNF382 suppresses
stemness properties.
In conclusion, we found that promoter methylation is
a key mechanism contributing to the downregulation of ZNF382
in GC cells. We further confirmed that ZNF382 is a
functional TSG in GC by inducing cell apoptosis and suppressing
tumor cell growth and metastasis and may be considered as a tumor
marker for GC.
Acknowledgments
The authors would like to thank Professor Qian Tao
(the Chinese University of Hong Kong, Hong Kong, China) for
providing some cell lines, primers and plasmids.
References
|
1
|
Riquelme I, Letelier P, Riffo-Campos AL,
Brebi P and Roa JC: Emerging role of miRNAs in the drug resistance
of gastric cancer. Int J Mol Sci. 17:4242016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Catalano V, Labianca R, Beretta GD, Gatta
G, de Braud F and Van Cutsem E: Gastric cancer. Crit Rev Oncol
Hematol. 71:127–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Urrutia R: KRAB-containing zinc-finger
repressor proteins. Genome Biol. 4:2312003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gebelein B, Fernandez-Zapico M, Imoto M
and Urrutia R: KRAB-independent suppression of neoplastic cell
growth by the novel zinc finger transcription factor KS1. J Clin
Invest. 102:1911–1919. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knight RD and Shimeld SM: Identification
of conserved C2H2 zinc-finger gene families in the Bilateria.
Genome Biol. 2:RESEARCH0016. 2001.PubMed/NCBI
|
|
7
|
Cowger JJ, Zhao Q, Isovic M and Torchia J:
Biochemical characterization of the zinc-finger protein 217
transcriptional repressor complex: Identification of a ZNF217
consensus recognition sequence. Oncogene. 26:3378–3386. 2007.
View Article : Google Scholar
|
|
8
|
Huntley S, Baggott DM, Hamilton AT,
Tran-Gyamfi M, Yang S, Kim J, Gordon L, Branscomb E and Stubbs L: A
comprehensive catalog of human KRAB-associated zinc finger genes:
Insights into the evolutionary history of a large family of
transcriptional repressors. Genome Res. 16:669–677. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng Y, Geng H, Cheng SH, Liang P, Bai Y,
Li J, Srivastava G, Ng MH, Fukagawa T, Wu X, et al: KRAB zinc
finger protein ZNF382 is a proapoptotic tumor suppressor that
represses multiple oncogenes and is commonly silenced in multiple
carcinomas. Cancer Res. 70:6516–6526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan Y, Wang YF, Su HF, Fang N, Zou C, Li
WF and Fei ZH: Decreased expression of the long noncoding RNA
LINC00261 indicate poor prognosis in gastric cancer and suppress
gastric cancer metastasis by affecting the epithelial–mesenchymal
transition. J Hematol Oncol. 9:572016. View Article : Google Scholar
|
|
12
|
Zang M, Zhang B, Zhang Y, Li J, Su L, Zhu
Z, Gu Q, Liu B and Yan M: CEACAM6 promotes gastric cancer invasion
and metastasis by inducing epithelial-mesenchymal transition via
PI3K/AKT signaling pathway. PLoS One. 9:e1129082014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimokawa M, Haraguchi M, Kobayashi W,
Higashi Y, Matsushita S, Kawai K, Kanekura T and Ozawa M: The
transcription factor Snail expressed in cutaneous squamous cell
carcinoma induces epithelial-mesenchymal transition and
down-regulates COX-2. Biochem Biophys Res Commun. 430:1078–1082.
2013. View Article : Google Scholar
|
|
14
|
Colas E, Pedrola N, Devis L, Ertekin T,
Campoy I, Martínez E, Llauradó M, Rigau M, Olivan M, Garcia M, et
al: The EMT signaling pathways in endometrial carcinoma. Clin
Transl Oncol. 14:715–720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen W, Zhang H, Wang J, Cao G, Dong Z, Su
H, Zhou X and Zhang S: Lentiviral-mediated gene silencing of
Notch-4 inhibits in vitro proliferation and perineural invasion of
ACC-M cells. Oncol Rep. 29:1797–1804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fender AW, Nutter JM, Fitzgerald TL,
Bertrand FE and Sigounas G: Notch-1 promotes stemness and
epithelial to mesenchymal transition in colorectal cancer. J Cell
Biochem. 116:2517–2527. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kostina AS, Uspensky VE, Irtyuga OB,
Ignatieva EV, Freylikhman O, Gavriliuk ND, Moiseeva OM, Zhuk S,
Tomilin A, Kostareva AA, et al: Notch-dependent EMT is attenuated
in patients with aortic aneurysm and bicuspid aortic valve. Biochim
Biophys Acta. 1862:733–740. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan X, Wu H, Han N, Xu H, Chu Q, Yu S,
Chen Y and Wu K: Notch signaling and EMT in non-small cell lung
cancer: Biological significance and therapeutic application. J
Hematol Oncol. 7:872014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Xu M, Zhang X, Chu F and Zhou T:
MAPK/c-Jun signaling pathway contributes to the upregulation of the
anti-apoptotic proteins Bcl-2 and Bcl-xL induced by Epstein-Barr
virus-encoded BARF1 in gastric carcinoma cells. Oncol Lett.
15:7537–7544. 2018.PubMed/NCBI
|
|
20
|
Capes-Davis A, Theodosopoulos G, Atkin I,
Drexler HG, Kohara A, MacLeod RA, Masters JR, Nakamura Y, Reid YA,
Reddel RR, et al: Check your cultures! A list of cross-contaminated
or misidentified cell lines. Int J Cancer. 127:1–8. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ying J, Li H, Seng TJ, Langford C,
Srivastava G, Tsao SW, Putti T, Murray P, Chan AT and Tao Q:
Functional epigenetics identifies a protocadherin PCDH10 as a
candidate tumor suppressor for nasopharyngeal, esophageal and
multiple other carcinomas with frequent methylation. Oncogene.
25:1070–1080. 2006. View Article : Google Scholar
|
|
22
|
Xiang T, Li L, Yin X, Yuan C, Tan C, Su X,
Xiong L, Putti TC, Oberst M, Kelly K, et al: The ubiquitin
peptidase UCHL1 induces G0/G1 cell cycle arrest and apoptosis
through stabilizing p53 and is frequently silenced in breast
cancer. PLoS One. 7:e297832012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tao Q, Huang H, Geiman TM, Lim CY, Fu L,
Qiu GH and Robertson KD: Defective de novo methylation of viral and
cellular DNA sequences in ICF syndrome cells. Hum Mol Genet.
11:2091–2102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tao Q, Swinnen LJ, Yang J, Srivastava G,
Robertson KD and Ambinder RF: Methylation status of the
Epstein-Barr virus major latent promoter C in iatrogenic B cell
lymphoproliferative disease. Application of PCR-based analysis. Am
J Pathol. 155:619–625. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mu H, Wang N, Zhao L, Li S, Li Q, Chen L,
Luo X, Qiu Z, Li L, Ren G, et al: Methylation of PLCD1 and
adenovirus-mediated PLCD1 overexpression elicits a gene therapy
effect on human breast cancer. Exp Cell Res. 332:179–189. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan X, Zhou T, Tai YH, Wang C, Zhao J, Cao
Y, Chen Y, Zhang PJ, Yu M, Zhen C, et al: Elevated expression of
CUEDC2 protein confers endocrine resistance in breast cancer. Nat
Med. 17:708–714. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yin X, Xiang T, Li L, Su X, Shu X, Luo X,
Huang J, Yuan Y, Peng W, Oberst M, et al: DACT1, an antagonist to
Wnt/β-catenin signaling, suppresses tumor cell growth and is
frequently silenced in breast cancer. Breast Cancer Res.
15:R232013. View Article : Google Scholar
|
|
28
|
Güngör C, Zander H, Effenberger KE,
Vashist YK, Kalinina T, Izbicki JR, Yekebas E and Bockhorn M: Notch
signaling activated by replication stress-induced expression of
midkine drives epithelial-mesenchymal transition and
chemoresistance in pancreatic cancer. Cancer Res. 71:5009–5019.
2011. View Article : Google Scholar : PubMed/NCBI
|