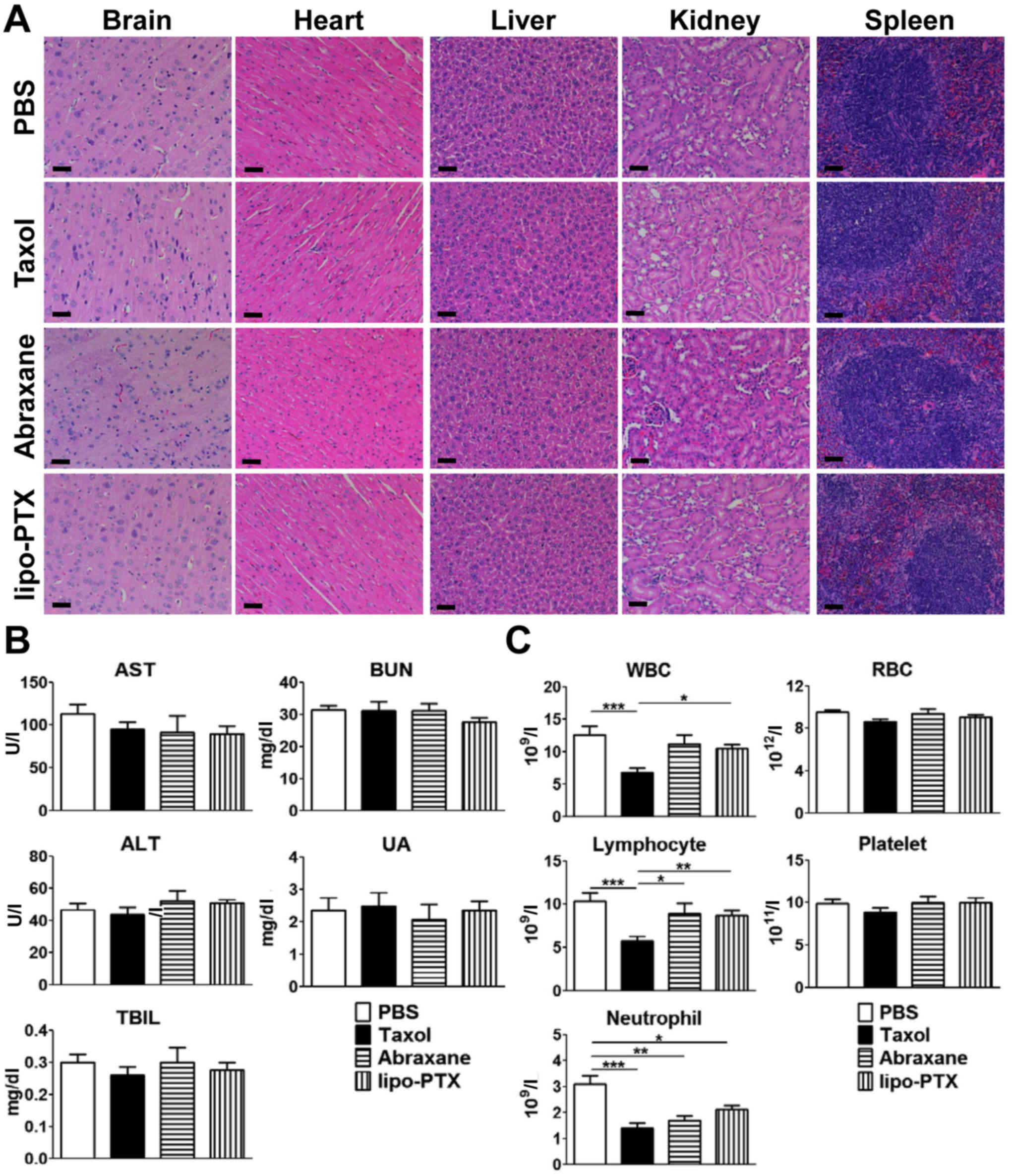
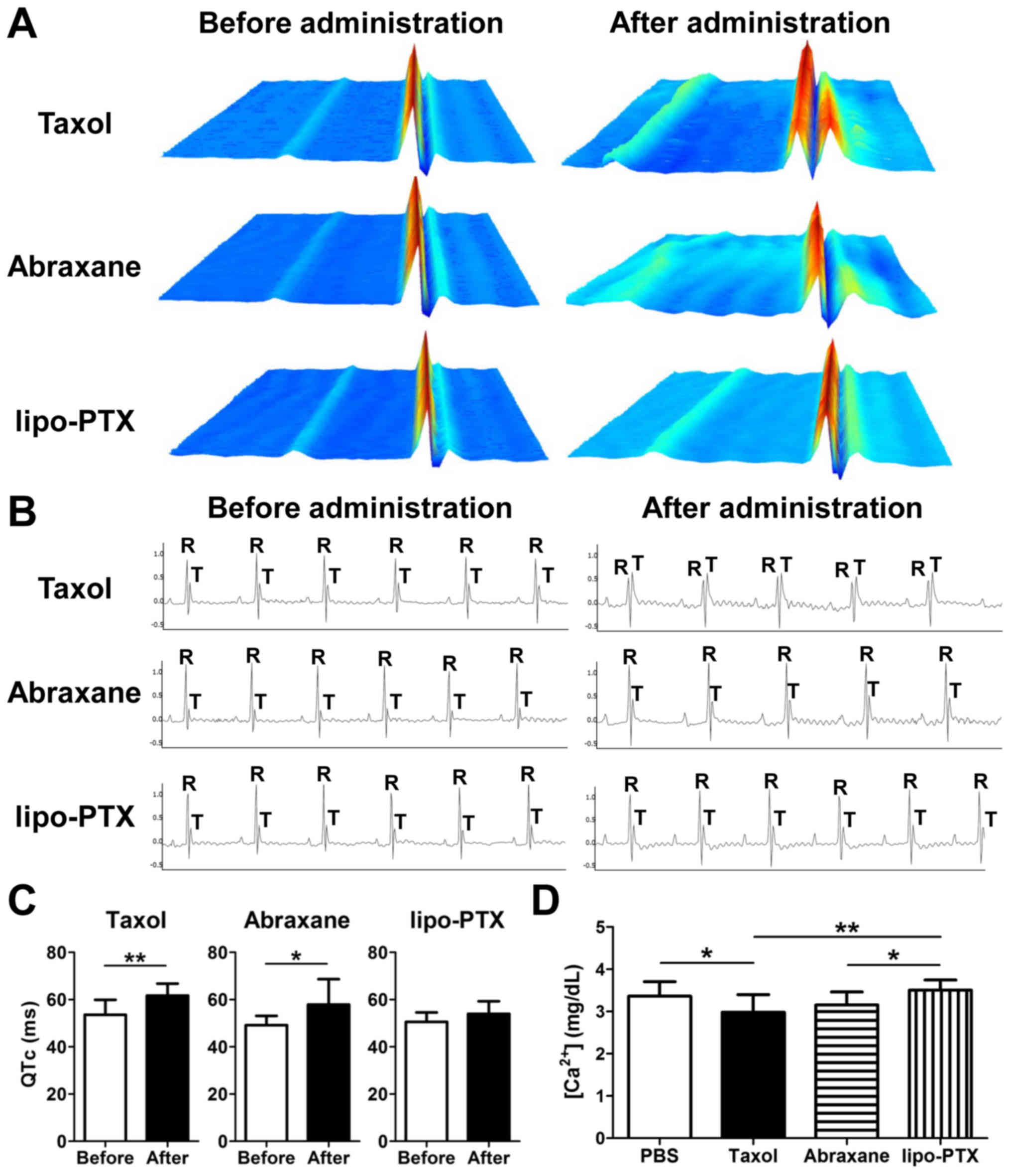
|
1
|
Kampan NC, Madondo MT, McNally OM, Quinn M
and Plebanski M: Paclitaxel and its evolving role in the management
of ovarian cancer. BioMed Res Int. 2015.413076:2015.
|
|
2
|
Rowinsky EK and Donehower RC: Paclitaxel
(taxol). N Engl J Med. 332:1004–1014. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Javeed A, Ashraf M, Riaz A, Ghafoor A,
Afzal S and Mukhtar MM: Paclitaxel and immune system. Eur J Pharm
Sci. 38:283–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hadzic T, Aykin-Burns N, Zhu Y, Coleman
MC, Leick K, Jacobson GM and Spitz DR: Paclitaxel combined with
inhibitors of glucose and hydroperoxide metabolism enhances breast
cancer cell killing via H2O2-mediated
oxidative stress. Free Radic Biol Med. 48:1024–1033. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alexandre J, Hu Y, Lu W, Pelicano H and
Huang P: Novel action of paclitaxel against cancer cells: Bystander
effect mediated by reactive oxygen species. Cancer Res.
67:3512–3517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adams JD, Flora KP, Goldspiel BR, Wilson
JW, Arbuck SG and Finley R: Taxol: A history of pharmaceutical
development and current pharmaceutical concerns. J Natl Cancer Inst
Monogr. 15:141–147. 1993.
|
|
7
|
Goldspiel BR: Clinical overview of the
taxanes. Pharmacotherapy. 17:110S–125S. 1997.PubMed/NCBI
|
|
8
|
Gelderblom H, Verweij J, Nooter K and
Sparreboom A: Cremophor EL: The drawbacks and advantages of vehicle
selection for drug formulation. Eur J Cancer. 37:1590–1598. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szebeni J, Alving CR, Savay S, Barenholz
Y, Priev A, Danino D and Talmon Y: Formation of
complement-activating particles in aqueous solutions of Taxol:
Possible role in hypersensitivity reactions. Int Immunopharmacol.
1:721–735. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
U.S. Department of Health and Human
Services: TAXOL® (paclitaxel) injection label.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf.
|
|
11
|
Weiss RB, Donehower RC, Wiernik PH, Ohnuma
T, Gralla RJ, Trump DL, Baker JR Jr, Van Echo DA, Von Hoff DD and
Leyland-Jones B: Hypersensitivity reactions from taxol. J Clin
Oncol. 8:1263–1268. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sparreboom A, van Tellingen O, Nooijen WJ
and Beijnen JH: Nonlinear pharmacokinetics of paclitaxel in mice
results from the pharmaceutical vehicle Cremophor EL. Cancer Res.
56:2112–2115. 1996.PubMed/NCBI
|
|
13
|
Sparreboom A, van Zuylen L, Brouwer E,
Loos WJ, de Bruijn P, Gelderblom H, Pillay M, Nooter K, Stoter G
and Verweij J: Cremophor EL-mediated alteration of paclitaxel
distribution in human blood: Clinical pharmacokinetic implications.
Cancer Res. 59:1454–1457. 1999.PubMed/NCBI
|
|
14
|
Surapaneni MS, Das SK and Das NG:
Designing Paclitaxel drug delivery systems aimed at improved
patient outcomes: Current status and challenges. ISRN Pharmacol.
2012.623139:2012.
|
|
15
|
Meng Z, Lv Q, Lu J, Yao H, Lv X, Jiang F,
Lu A and Zhang G: Prodrug strategies for Paclitaxel. Int J Mol Sci.
17:7962016. View Article : Google Scholar :
|
|
16
|
Yardley DA: nab-Paclitaxel mechanisms of
action and delivery. J Control Release. 170:365–372. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ibrahim NK, Desai N, Legha S, Soon-Shiong
P, Theriault RL, Rivera E, Esmaeli B, Ring SE, Bedikian A,
Hortobagyi GN, et al: Phase I and pharmacokinetic study of ABI-007,
a Cremophor-free, protein-stabilized, nanoparticle formulation of
paclitaxel. Clin Cancer Res. 8:1038–1044. 2002.PubMed/NCBI
|
|
18
|
Gradishar WJ, Tjulandin S, Davidson N,
Shaw H, Desai N, Bhar P, Hawkins M and O'Shaughnessy J: Phase III
trial of nanoparticle albumin-bound paclitaxel compared with
polyethylated castor oil-based paclitaxel in women with breast
cancer. J Clin Oncol. 23:7794–7803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
US Department of Health and Human
Services: ABRAXANE® label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021660s041lbl.pdf.
|
|
20
|
eHealthMe: Abraxane and myocardial
infarction. https://www.ehealthme.com/ds/abraxane/myocardial-infarction/.
|
|
21
|
Feng L and Mumper RJ: A critical review of
lipid-based nanoparticles for taxane delivery. Cancer Lett.
334:157–175. 2013. View Article : Google Scholar
|
|
22
|
Hong SS, Choi JY, Kim JO, Lee MK, Kim SH
and Lim SJ: Development of paclitaxel-loaded liposomal nanocarrier
stabilized by triglyceride incorporation. Int J Nanomedicine.
11:4465–4477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng Q, Yu MZ, Wang JC, Hou WJ, Gao LY, Ma
XF, Pei XW, Niu YJ, Liu XY, Qiu C, et al: Synergistic inhibition of
breast cancer by co-delivery of VEGF siRNA and paclitaxel via
vapreotide-modified core-shell nanoparticles. Biomaterials.
35:5028–5038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Immordino ML, Brusa P, Arpicco S, Stella
B, Dosio F and Cattel L: Preparation, characterization,
cytotoxicity and pharma-cokinetics of liposomes containing
docetaxel. J Control Release. 91:417–429. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Crosasso P, Ceruti M, Brusa P, Arpicco S,
Dosio F and Cattel L: Preparation, characterization and properties
of sterically stabilized paclitaxel-containing liposomes. J Control
Release. 63:19–30. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. The National Academies Press; Washington, DC: 2011
|
|
27
|
Gregoriadis G: Drug entrapment in
liposomes. FEBS Lett. 36:292–296. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Allen TM and Chonn A: Large unilamellar
liposomes with low uptake into the reticuloendothelial system. FEBS
Lett. 223:42–46. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee TY, Lin CT, Kuo SY, Chang DK and Wu
HC: Peptide-mediated targeting to tumor blood vessels of lung
cancer for drug delivery. Cancer Res. 67:10958–10965. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu CH, Kuo YH, Hong RL and Wu HC:
α-Enolase-binding peptide enhances drug delivery efficiency and
therapeutic efficacy against colorectal cancer. Sci Transl Med.
7:290ra912015. View Article : Google Scholar
|
|
31
|
Bartlett GR: Phosphorus assay in column
chromatography. J Biol Chem. 234:466–468. 1959.PubMed/NCBI
|
|
32
|
Fiandaca MS, Berger MS and Bankiewicz KS:
The use of convection-enhanced delivery with liposomal toxins in
neuroon-cology. Toxins (Basel). 3:369–397. 2011. View Article : Google Scholar
|
|
33
|
Hillaireau H and Couvreur P: Nanocarriers'
entry into the cell: Relevance to drug delivery. Cell Mol Life Sci.
66:2873–2896. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eiseman JL, Eddington ND, Leslie J,
MacAuley C, Sentz DL, Zuhowski M, Kujawa JM, Young D and Egorin MJ:
Plasma pharmacokinetics and tissue distribution of paclitaxel in
CD2F1 mice. Cancer Chemother Pharmacol. 34:465–471. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Howarth FC, Calaghan SC, Boyett MR and
White E: Effect of the microtubule polymerizing agent taxol on
contraction, Ca2+ transient and L-type Ca2+
current in rat ventricular myocytes. J Physiol. 516:409–419. 1999.
View Article : Google Scholar
|
|
36
|
Zhang K, Heidrich FM, DeGray B, Boehmerle
W and Ehrlich BE: Paclitaxel accelerates spontaneous calcium
oscillations in cardiomyocytes by interacting with NCS-1 and the
InsP3R. J Mol Cell Cardiol. 49:829–835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boehmerle W, Splittgerber U, Lazarus MB,
McKenzie KM, Johnston DG, Austin DJ and Ehrlich BE: Paclitaxel
induces calcium oscillations via an inositol 1,4,5-trisphosphate
receptor and neuronal calcium sensor 1-dependent mechanism. Proc
Natl Acad Sci USA. 103:18356–18361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boehmerle W, Zhang K, Sivula M, Heidrich
FM, Lee Y, Jordt SE and Ehrlich BE: Chronic exposure to paclitaxel
diminishes phosphoinositide signaling by calpain-mediated neuronal
calcium sensor-1 degradation. Proc Natl Acad Sci USA.
104:11103–11108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Drummond DC, Meyer O, Hong K, Kirpotin DB
and Papahadjopoulos D: Optimizing liposomes for delivery of
chemotherapeutic agents to solid tumors. Pharmacol Rev. 51:691–743.
1999.PubMed/NCBI
|
|
40
|
McIntosh TJ: The effect of cholesterol on
the structure of phosphatidylcholine bilayers. Biochim Biophys
Acta. 513:43–58. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Deniz A, Sade A, Severcan F, Keskin D,
Tezcaner A and Banerjee S: Celecoxib-loaded liposomes: Effect of
cholesterol on encapsulation and in vitro release characteristics.
Biosci Rep. 30:365–373. 2010. View Article : Google Scholar
|
|
42
|
Sharma A, Mayhew E, Bolcsak L, Cavanaugh
C, Harmon P, Janoff A and Bernacki RJ: Activity of paclitaxel
liposome formulations against human ovarian tumor xenografts. Int J
Cancer. 71:103–107. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu Y, Ran R, Chen J, Kuang Q, Tang J, Mei
L, Zhang Q, Gao H, Zhang Z and He Q: Paclitaxel loaded liposomes
decorated with a multifunctional tandem peptide for glioma
targeting. Biomaterials. 35:4835–4847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li XY, Zhao Y, Sun MG, Shi JF, Ju RJ,
Zhang CX, Li XT, Zhao WY, Mu LM, Zeng F, et al: Multifunctional
liposomes loaded with paclitaxel and artemether for treatment of
invasive brain glioma. Biomaterials. 35:5591–5604. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ruttala HB and Ko YT: Liposomal
co-delivery of curcumin and albumin/paclitaxel nanoparticle for
enhanced synergistic antitumor efficacy. Colloids Surf B
Biointerfaces. 128:419–426. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Barbosa MV, Monteiro LO, Carneiro G,
Malagutti AR, Vilela JM, Andrade MS, Oliveira MC, Carvalho-Junior
AD and Leite EA: Experimental design of a liposomal lipid system: A
potential strategy for paclitaxel-based breast cancer treatment.
Colloids Surf B Biointerfaces. 136:553–561. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Assanhou AG, Li W, Zhang L, Xue L, Kong L,
Sun H, Mo R and Zhang C: Reversal of multidrug resistance by
co-delivery of paclitaxel and lonidamine using a TPGS and
hyaluronic acid dual-functionalized liposome for cancer treatment.
Biomaterials. 73:284–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schmitt-Sody M, Strieth S, Krasnici S,
Sauer B, Schulze B, Teifel M, Michaelis U, Naujoks K and Dellian M:
Neovascular targeting therapy: Paclitaxel encapsulated in cationic
liposomes improves antitumoral efficacy. Clin Cancer Res.
9:2335–2341. 2003.PubMed/NCBI
|
|
49
|
Strieth S, Eichhorn ME, Werner A, Sauer B,
Teifel M, Michaelis U, Berghaus A and Dellian M: Paclitaxel
encapsulated in cationic liposomes increases tumor microvessel
leakiness and improves therapeutic efficacy in combination with
Cisplatin. Clin Cancer Res. 14:4603–4611. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fetterly GJ, Grasela TH, Sherman JW, Dul
JL, Grahn A, Lecomte D, Fiedler-Kelly J, Damjanov N, Fishman M,
Kane MP, et al: Pharmacokinetic/pharmacodynamic modeling and
simulation of neutropenia during phase I development of
liposome-entrapped paclitaxel. Clin Cancer Res. 14:5856–5863. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Slingerland M, Guchelaar HJ, Rosing H,
Scheulen ME, van Warmerdam LJ, Beijnen JH and Gelderblom H:
Bioequivalence of Liposome-Entrapped Paclitaxel Easy-To-Use
(LEP-ETU) formulation and paclitaxel in polyethoxylated castor oil:
A randomized, two-period crossover study in patients with advanced
cancer. Clin Ther. 35:1946–1954. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yeh ET and Bickford CL: Cardiovascular
complications of cancer therapy: Incidence, pathogenesis,
diagnosis, and management. J Am Coll Cardiol. 53:2231–2247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gardner ER, Dahut W and Figg WD:
Quantitative determination of total and unbound paclitaxel in human
plasma following Abraxane treatment. J Chromatogr B Analyt Technol
Biomed Life Sci. 862:213–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sparreboom A, Scripture CD, Trieu V,
Williams PJ, De T, Yang A, Beals B, Figg WD, Hawkins M and Desai N:
Comparative preclinical and clinical pharmacokinetics of a
cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and
paclitaxel formulated in Cremophor (Taxol). Clin Cancer Res.
11:4136–4143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Desai N, Trieu V, Yao Z, Louie L, Ci S,
Yang A, Tao C, De T, Beals B, Dykes D, et al: Increased antitumor
activity, intratumor paclitaxel concentrations, and endothelial
cell transport of cremophor-free, albumin-bound paclitaxel,
ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res.
12:1317–1324. 2006. View Article : Google Scholar : PubMed/NCBI
|