Introduction
Colorectal cancer (CRC) is among the most common
types of cancer and the third leading cause of cancer-associated
mortality worldwide. Therefore, it is an important topic among
oncologists and cancer biologists (1–3).
Although the mortality rate of CRC has decreased significantly with
early detection and surgery and chemotherapy treatments, recurrence
and drug resistance have become more common (4). Therefore, there is an urgent
requirement to develop novel anticancer drugs and/or therapeutic
strategies for the treatment of colorectal cancer.
Clove, the dried bud of Syzygium aromaticum,
is a traditional medicinal herb widely used in Asian countries.
Clove has been indicated to possess various biological properties
including anti-infammatory (5,6),
antivira (7), antibacterial
(8), antioxidant (8,9) and
antitumor (8,10,11)
activities. Our previous study demonstrated that the active
fraction of clove (AFC) was effective against various types of
cancer cell, including lung, breast, liver, pancreatic, ovarian and
cervical cancer (12). Two
components of AFC have been identified as demonstrating
cytotoxicity against various types of cancer cells: Oleanonic acid
(OA) and eugenol (12). It was
also demonstrated that OA was able to induce apoptosis of cancer
cells via the mitochondrial pathway (13). Combination of OA and fluorouracil
(5-FU) treatments synergistically potentiated the cytotoxicity of
5-FU against human pancreatic cancer Pan-28 cells (13). AFC was more effective than a single
isolated component of OA or eugenol against human colon cancer
HT-29 xenografts in vivo (12). However, the mechanistic action of
AFC remains unclear. Therefore, the present study had the
interesting aim of elucidating the mechanism of action of AFC.
Apoptosis (programmed cell death) is a highly
regulated and controlled process and serves a crucial role in the
development and treatment of cancer (14–16).
Apoptosis occurs through two major molecular pathways, the
intrinsic (mitochondrial) and the extrinsic (death
receptor-mediated) pathways (16,17).
The intrinsic pathway involves the mitochondria and mainly affects
the Bcl-2 and caspase families (17). The extrinsic pathways involves
death signals, including TNF-α with TNF receptor 1 (TNFR1) and
activates caspase-8 to cleave procaspase-3 into its active form
(16). Apoptosis is an important
anticancer mechanism and numerous anticancer drugs execute their
anticancer activity via induction of apoptosis (18).
Autophagy is a survival-promoting pathway and serves
a complicated role in cell development, growth and tumorigenesis to
regulate inhibition of cancer cell proliferation or promotion of
cancer cell survival (19–22). Numerous anticancer agents display
antitumor activity via autophagy of cancer cells (23–25).
The phosphoinositide 3-kinase/Akt/mechanistic target of rapamycin
signaling pathway is a major signal transduction cascade involved
in cell proliferation, survival and metabolism, and it serves an
important role in the development and therapy of colorectal cancer
(26,27). Upregulation of PI3K expression
results in the inhibition of apoptosis in colon cancer SW480 cells
(28). The PI3K/Akt/mTOR signaling
pathway is also an important therapeutic target and a previous
study demonstrated that the dual PI3K/mTOR inhibitor, NVP-BEZ235,
was effective against colorectal cancer cells in vitro and
in vivo (29). The
PI3K/Akt/mTOR signaling pathway is also involved in the autophagic
process, and activation of the pathway attenuates autophagy in
cancer cells. A recent study also demonstrated that OA, one of the
main components of AFC, is capable of inducing protective autophagy
in cancer cells via the PI3K/Akt/mTOR signaling pathway (30). However, the effect of AFC on
autophagy remains unclear. In the present study, AFC-induced
autophagic effects were evaluated in human colorectal cancer
HCT-116 cells by morphological observation, flow cytometry and
western blotting. The results revealed that AFC induced apoptosis
via the PI3K/Akt/mTOR-mediated autophagic pathway in colorectal
cancer HCT-116 cells.
Materials and methods
Reagents and antibodies
Rapamycin and 3-methyladenine (3-MA) were purchased
from Medchem Express Co., Ltd. (Shanghai, China). Baflomycin A1
(BA) was purchased from Beijing Hua MEIKO Biotechnology Co., Ltd.
(Beijing, China). The Annexin V-FITC apoptosis detection kit was
purchased from BD Biosciences (San Diego, CA, USA). Cell culture
media, DMEM, RPMI-1640, and McCoy's5A were purchased from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cell Counting
kit-8 (CCK-8), Hoechst 33258, Ad-mCherry-GFP-LC3B, dimethyl
sulfoxide (DMSO), fetal bovine serum (FBS), penicillin,
streptomycin, monoclonal antibodies of β-actin (cat. no. AA128-1),
horseradish peroxidase (HRP)-labeled goat anti-rat IgG(H+L)
(#A0216) and HRP-labeled goat anti-rabbit IgG(H+L) (cat. no. A0208)
were purchased from Beyotime Institute of Biotechnology (Shanghai,
China). LY294002, the caspase-3 (cat. no. ab32351), caspase-9 (cat.
no. ab32539) and Poly(ADP-ribose) polymerase (PARP; cat. no.
ab191217) antibodies were purchased from Abcam (Cambridge, UK).
LC3B (cat. no. 3868), Beclin-1 (cat. no. 3495), PI3K (cat. no.
4292), p-PI3K at Tyr458 (cat. no. 4228), Akt (cat. no. 9272), p-Akt
at Ser473 (cat. no. 9271), mTOR (cat. no. 2972) and p-mTOR at
Ser2448 (cat. no. 2971) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Insulin-like growth factor-I
(IGF-I) was purchased from R&D Systems, Inc. (Minneapolis, MN,
USA).
Cell culture
The human colorectal carcinoma cell lines, HCT-116,
SW620, HCT8, HT29 and LoVo were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA). Cells were grown in
RPMI-1640, DMEM or McCoy's5A (Gibco; Thermo Fisher Scientific,
Inc.) culture medium containing 10% heat-inactivated FBS, 100 U/ml
penicillin and 100 mg/ml streptomycin at 37°C in 5% CO2.
Cells in the logarithmic phase were routinely renewed with fresh
medium every 2–3 days.
Extraction, isolation and
characterization of individual compounds with cytotoxic
activity
Air-dried, powdered clove was purchased from Qingdao
Company of Traditional Chinese Medicine (Qingdao, Shandong, China).
The isolation and extraction of clove was performed as previously
described (31). Briefly, 10.0 kg
powdered clove was hydrated with 95% ethanol at room temperature
for 72 h. Subsequent to filtration, the solution was concentrated
to generate an ethanol extract and the extract was further
extracted with ethyl acetate at 60°C for 2 h, and the supernant was
concentrated using a vacuum rotary evaporator (Yamato Sci., Tokyo,
Japan). The ethyl acetate extract of clove (EAEC) was fractionated
using silica gel column chromatography (CC; 200 and 400 mesh) and
Sephadex LH-20 CC. The resulting fraction was defined as active
fraction from clove (AFC). The purity was characterized by the
Department of Chemistry and Molecular Engineering at Qingdao
University (Qingdao) using 1H- and 13C-NMR
analysis, confirming that ACF contained three main compounds,
including eugenol (32.32%) and oleanonic Acid (23.60). AFC
underwent a comprehensive residue screen for 172 pesticides by the
Pacific Agricultural Laboratory (Portland, OR, USA) and no
pesticides were detected. Similarly, AFC was analyzed for heavy
metal content by Avomeen Analytical Services (Ann Arbor, MI, USA)
and no heavy metal was detected.
Drug preparation and treatment
AFC was diluted with cell culture medium to final
concentrations of 25, 50, 100, 200 and 400 µg/ml for the
treatment of HCT-116 cells for 24, 48 or 72 h. The PI3K inhibitor,
LY294002, and the autophagylysosomal inhibitor, BA, were dissolved
in DMSO and diluted with culture medium. The final concentration of
DMSO in the test solutions was <0.1%. This concentration of DMSO
did not cause any adverse responses in cells. 3-MA was dissolved in
heated sterile double distilled water to achieve a 100 mM stock
solution, and was then diluted with culture medium for a final
concentration of 2 mM. IGF-I (a PI3K activator) was reconstituted
to 10 µg/ml in sterile PBS, and diluted with culture medium
for a final concentration of 50 ng/ml. HCT-116 cells were
pretreated with 10 µM LY294002, 1 nM BA, 2 mM 3-MA or 50
ng/ml IGF-I for 1 h, then further treated with 100 µg/ml AFC
for 48 h. Cells were treated with fresh medium without serum as a
vehicle control.
Cell viability assay
Cell viability was determined by CCK-8 assay as
previously described (12).
Briefly, cells at 80–90% confluency were seeded in 96-well plates
at a density of 5×103 cells/well. After a 24-h
incubation 25, 50, 100, 200 or 400 µg/ml AFC, or vehicle
control, was added to the wells. After 24, 48 or 72 h 10% CCK-8
reagent (10 µl/well) was added and incubated for 1 h at
37°C. The absorbance value (optical density; OD) was measured at a
wavelength of 450 nm with a SpectraMAX M3 microplate
spectrophotometer (Molecular Devices Corporation, Sunnyvale, CA,
USA) and the cell viability ratio was calculated using the
following formula: Cell viability rate = (OD of the experimental
group/OD of the control group) ×100%. The half maximal inhibitory
concentration (IC50) values were determined using a
nonlinear best fit method by GraphPad Prism 6 (GraphPad Software,
Inc., La Jolla, CA, USA). All experiments were performed 3 times in
triplicate.
GFP-LC3 transfection
HCT-116 cells were seeded onto coverslips in a
24-well plate at a density of 5×104 cells/well and were
transfected with Ad-mCherry-GFP-LC3B. Following 24 h of culture,
the cells were treated with AFC (100 µg/ml) or medium
(control) for 48 h then fixed with 4% polyoxymethylene and observed
with an EVOS™ FL Imaging system (AMF4300; Thermo Fisher Scientific
Inc.). Autophagic cells presenting ≥5 mRFP-GFP-LC3 dots were
counted. All experiments were performed 3 times in triplicate.
Apoptotic assay of HCT-116 cells by
Hoechst 33258 staining
Morphological assessment of apoptotic cells was
analyzed using Hoechst 33258 staining as previously described
(31). Briefly, HCT-116 cells were
seeded in a 24-well plate at a density of 5×104
cells/well. Following culture for 24 h, the cells were treated with
25, 50 or 100 µg/ml AFC or the same volume of vehicle
control. Following incubation for another 48 h, the cells were
fixed with 4% paraformaldehyde for 10 min at room temperature, and
the medium was removed and washed with PBS for 15 min. The cells
were treated with 10 µg/ml Hoechst 33258 staining at room
temperature for 10 min, and washed with PBS for 15 min in the dark
to reduce background. The morphology of the cells was visualized
and photographed under a DMR fluorescence microscope at ×400
magnification (Leica Microsystems GmbH, Wetzlar, Germany) using
fluorescence excitation at 340 nm. The apoptotic index was
calculated using the following formula: Apoptotic index = apoptotic
cell number/total cell number ×100%. A minimum of 4 fields of view
of each well, containing ≥500 cells, were required to calculate the
rate of apoptosis. All experiments were performed 3 times in
triplicate.
Observation of autophagosomes by
transmission electron microscopy
HCT-116 cells were seeded in a 24-well plate at a
density of 5×104 cells/well. Following 24 h of culture,
the cells were treated with 25, 50 and 100 µg/ml AFC or the
equivalent volume of medium (vehicle control) for 48 h. The cells
were collected by trypsinization and washed twice with PBS, then
fixed in 2.5% glutaraldehyde for 90 min and post-fixed in 1% osmium
tetraoxide for 30 min at room temperature. Subsequent to 3 washes
with PBS, the cells were progressively dehydrated in an ascending
alcohol series (50, 70, 95 and 100%), and embedded in Epon resin.
The ultrathin sections were contrasted with uranyl acetate and lead
citrate for electron microscopy observation. The ultrastructure of
the cells was then examined under a transmission electron
microscope (JEM-1230; JEOL, Ltd., Tokyo, Japan). All experiments
were performed 3 times in triplicate.
Apoptotic assay of HCT-116 cells with
Annexin V-FITC/PI and flow cytometry
AFC-induced apoptosis in HCT-116 cells was also
evaluated by flow cytometry with Annexin V-FITC apoptosis detection
kit (cat. no. 556547BD Pharmingen; BD Biosciences), according to
the manufacturer's protocol. Briefly, HCT-116 cells at 80–90%
confluency were seeded in 6-well plates at a density of
4×105 cells/well and cultured for 24 h. Then, the cells
were treated with 100 µg/ml AFC or vehicle control for 48 h.
After treatment, the cells were harvested by cryogenic
centrifugation at 4°C, 1,500 × g for 5 min and washed twice with
4°C PBS. The cells were resuspended in 1× binding buffer at a
concentration of 1×106 cells/ml. A total of 100
µl of the solution (1×105 cells) was transferred
to a 5-ml culture tube, and 5 µl Annexin V-FITC and 5
µl PI were successively added to the cells and incubated at
room temperature in the dark for 15 min. The quantity of stained
cells was analyzed using a flow cytometer (FACSCalibur; BD
Biosciences, Franklin Lakes, NJ, USA). All experiments were
performed 3 times in triplicate.
Western blotting analysis
HCT-116 cells were seeded at a density of
4×105 cells/well in 6-well plates, and the culture
medium (2 ml) was replaced after 24 h in culture, with 25, 50 or
100 µl/ml AFC, or vehicle control for 24 h prior to
harvesting. The cells were lysed on ice with
radioimmunoprecipitation assay buffer [0.5% NP-40, 50 mM Tris-HCl,
120 mM NaCl, 1 mM EDTA, 0.1 mM Na3VO4, 1 mM
NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1 µg/ml
leupeptin, pH 7.5] for 30 min in the presence of PhosSTOP (Roche
Molecular Systems, Inc., Basel, Switzerland) with PMSF and then
centrifuged at 9600 × g for 20 min at 4°C. The supernatant was
collected and stored in aliquots at −80°C until analysis. Protein
concentrations were determined using BCA protein assay kit
(Beyotime Institution of Biotechnology, Shanghai, China) and
equalized prior to loading. Equal amounts of protein (40 µg)
were separated by 15% SDS-PAGE and blotted onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). Following
blocking with 5% non-fat dry milk or bovine serum albumin in 1×
Tris-buffered saline with Tween (TBST; 20 mM Tris-HCl, 150 mM NaCl
and 0.05% Tween-20) for 1 h at room temperature, the membranes were
incubated with primary antibodies at a dilution of 1:1,000
(caspase-9, PARP, LC3B, Beclin-1, PI3K, p-PI3K at Tyr458, Akt,
p-Akt at Ser473, mTOR, p-mTOR at Ser2448) and 1;5,000 (β-actin and
caspase-3) at 4°C overnight. Then, the membranes were incubated
secondary antibodies at a dilution of 1:2,000 at room temperature
for 1 h. β-actin was used as a loading control. Following 3 washes
in TBST, the membranes were developed by incubation with ECL
Western detection reagents (Thermo Fisher Scientific Inc.). The
specific protein bands were visualized using a chemiluminescence
reagent (EMD Millipore) and imaged using a VersaDoc imaging system
(Bio-Rad Laboratories, Hercules, CA, USA). All experiments were
performed three times in triplicate. The relative protein
expression was quantified by Image J software (National Institutes
of Health, Bethesda, ML, USA). For each sample, the grayscale value
of each band was normalized to that of corresponding β-actin and
the ratio of LC3-II/I was calculated using the normalized value of
LC3-II/LC3-I.
Statistical analysis
All experiments were repeated ≥3 times. Data were
analyzed using SPSS 19.0 statistical software (IBM Corporation,
Armonk, NY, USA) and expressed as the mean ± standard deviation.
One-way analysis of variance and Tukey's test were used to compare
the means among groups. P<0.05 was considered to indicate a
statistically significant difference, and highly significant
differences were indicated by P<0.01 and P<0.001.
Results
AFC inhibits the proliferation of
colorectal cancer cells
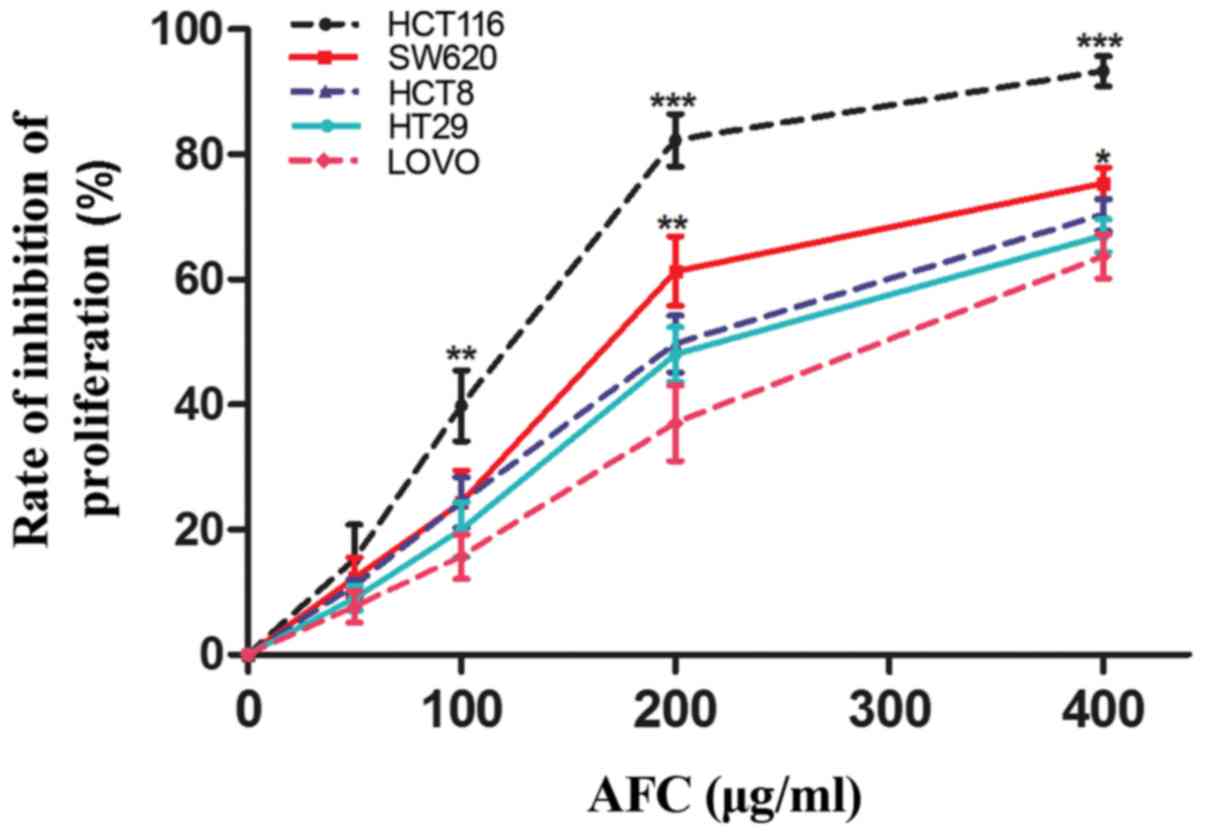
We initially investigated the effects of AFC on
various types of human colorectal cancer cells using a CCK-8 assay.
The results demonstrated that AFC was able to significantly inhibit
proliferation of HCT116, HT-29, SW620, HCT8 and LoVo cells
(Fig. 1; Table I). Compared with other cells,
HCT116 cells were the most sensitive to AFC. Therefore, HCT116
cells were selected for use in subsequent experiments to
investigate the potential mechanisms of the effect AFC in human
colorectal cancer cells.
 | Table IIC50 of active fraction of
clove in terms of proliferation of various cell lines. |
Table I
IC50 of active fraction of
clove in terms of proliferation of various cell lines.
| Cell line | Tumor type | IC50
µg/ml (mean ± standard deviation) |
|---|
| HCT116 | Human colorectal
cancer cell | 113.5±7.83 |
| SW620 | Human colorectal
cancer | 174.9±9.52 |
| HCT8 | Human colorectal
cancer | 211.7±7.29 |
| HT29 | Human colorectal
cancer | 232.3±15.42 |
| LoVo | Human colorectal
cancer | 280.9±12.61 |
AFC inhibits cell viability and induces
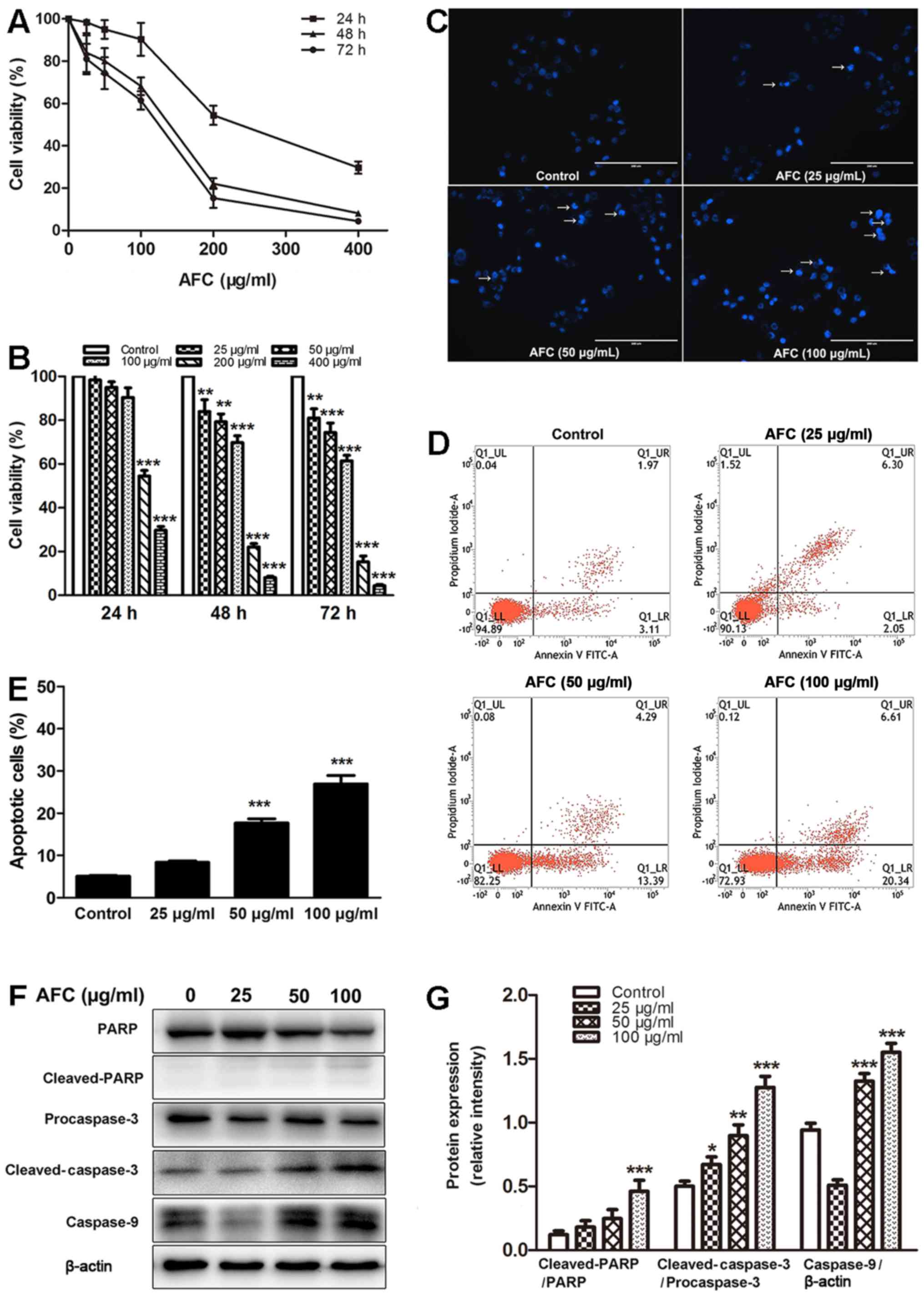
apoptosis of HCT-116 cells
The effects of AFC on cell viability of human
colorectal cancer HCT-116 cells was investigated by CCK-8 assay.
The results demonstrated that AFC inhibited the viability of
HCT-116 cells in a dose- and time-dependent manner (Fig. 2A and B). Next, the effect of AFC on
the induction of apoptosis of HCT-116 cells was investigated by
Hoechst 33258 staining, which is commonly used to detect cell
apoptosis by observation of chromatin condensation under a
fluorescence microscope (31). As
demonstrated in Fig. 2C, following
treatment with AFC (25, 50 and 100 µg/ml) for 48 h, the
cells exhibited typical apoptotic morphological features, including
chromatin condensation, nuclear shrinkage and the formation of
apoptotic bodies. Annexin V is a sensitive method for detection of
early apoptosis of cancer cells by using fluorescein (FITC) as
fluorescent probe (32).
Therefore, the rate of apoptosis of HCT-116 cells was investigated
by flow cytometric analysis using Annexin V-FITC/PI double
staining. The results demonstrated that the percentage of apoptotic
cells increased when HCT-116 cells were treated with AFC compared
with control (Fig. 2D and E). To
study the underlying mechanism of AFC-induced apoptosis, the
cleavage of PARP, caspase-3, and caspase-9 was analyzed by western
blotting. As demonstrated in Fig. 2F
and G, AFC cleaved PARP, pro-caspase-3 and pro-caspase-9 into
their active forms. These data indicate that AFC inhibits cell
viability through inducing apoptosis of HCT-116 cells.
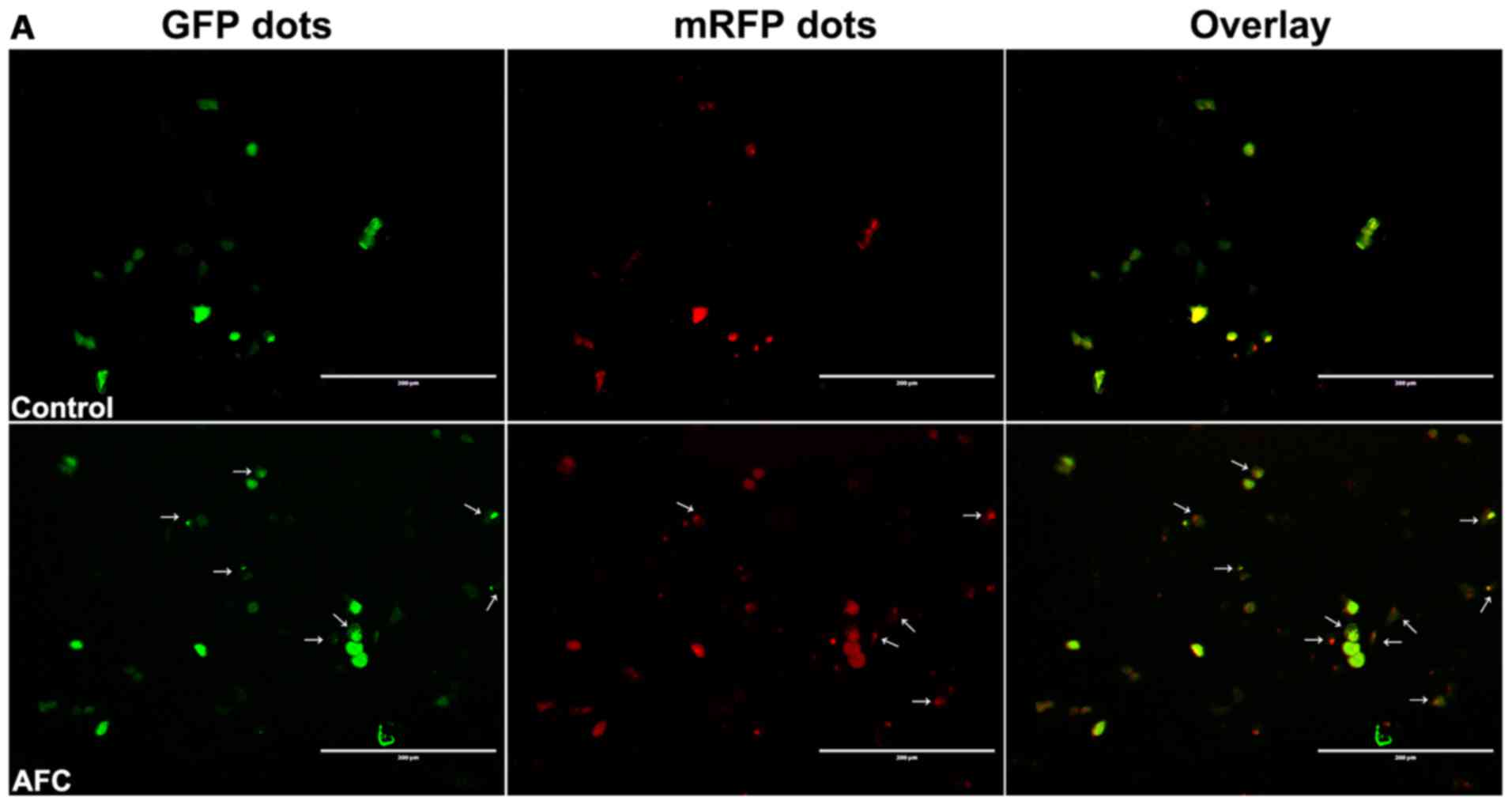
AFC induces autophagy in HCT-116
colorectal cancer cells
To study AFC-induced autophagy, the autophagic flow
of LC3-puncta was firstly determined. HCT-116 cells were
transfected with Ad-mCherry-GFP-LC3B and GFP-LC3 puncta were
observed under a fluorescence microscope. As indicated by Fig. 3A, fluorescence of GFP-LC3 puncta
was frequently observed in HCT-116 cells treated with AFC, whereas
the cells treated with vehicle control exhibited a relatively
homogeneous LC3 expression pattern. In addition, a large number of
autophagic bodies and autophagylysosomes were observed in HCT-116
cells treated with AFC (Fig. 3B)
using transmission electron microscopy.
It has been well established that the
microtubule-associated protein 1A/1B-light chain 3 (LC3) is a
central protein in the autophagy pathway and closely associated
with autophagosome appearance. Thus, it serves as a reliable marker
to monitor autophagy (33).
Beclin-1 was the first gene identified to induce autophagy
(34). Therefore, the effect of
AFC on the expression of LC3 and Beclin-1 was next investigated. As
demonstrated by Fig. 3C–E, the
expression levels of LC3-II and Beclin-1 in HCT-116 cells were
significantly increased by AFC in a dose- and time-dependent
manner. These data indicated that AFC treatment not only results in
apoptosis, but also induces autophagy in HCT-116 cells.
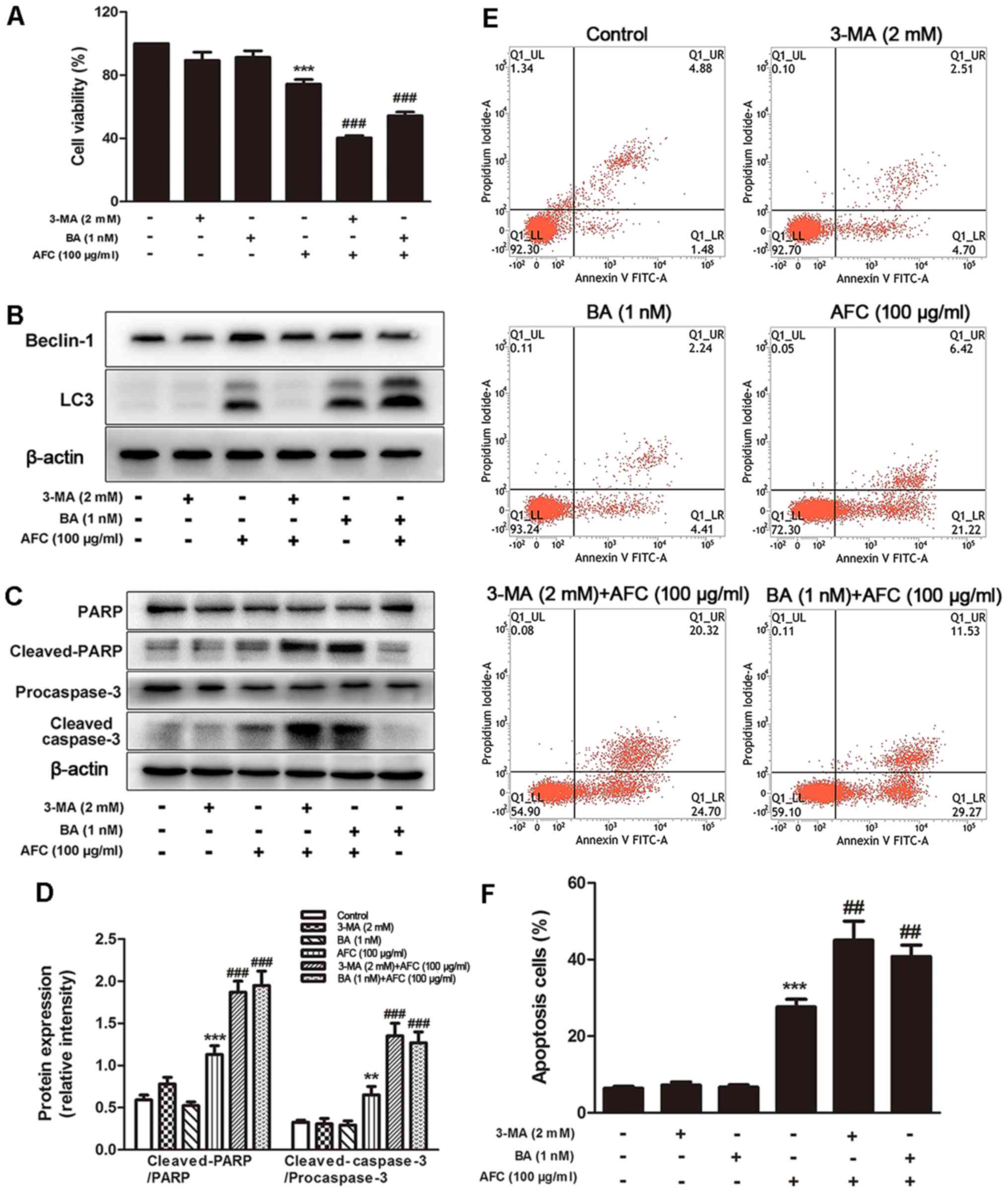
Inhibition of autophagy enhances
AFC-induced apoptosis in HCT-116 cells
It has been documented that autophagy could
facilitate cell survival in adverse microenvironments and that
inhibition of autophagy leads to increased cytotoxicity and
induction of apoptosis (35). The
effect of inhibition of autophagy on apoptosis induced by AFC in
HCT-116 cells was investigated using 2 inhibitors, 3-MA and BA.
3-MA suppresses class III phosphatidylinositol 3-kinase (PI3K),
essential for the initiation of the early stages of autophagy
(36), while BA, an inhibitor of
the vacuolar-type ATPase, inhibits the fusion of autophagosomes
with lysosomes, preventing autophagic degradation (37). As demonstrated in Fig. 4A, cell viability was significantly
decreased in HCT-116 cells treated with the combination of AFC (100
µg/ml) and 3-MA (2 mM) or BA (1 nM). The resulting cell
viability was 40.33±2.52 and 54.34±4.04%, respectively, compared
with 73.25±5.13% for cells treated with AFC alone. This result
indicated that the inhibitory effect of AFC on HCT-116 cell
proliferation was enhanced by autophagic inhibitors of 3-MA and
BA.
Recent studies suggest that increased LC3 expression
could reflect either increased autophagosome formation, due to
increased autophagic activity, or reduced autophagosome turnover
(33). Therefore, the effects of
AFC on LC3 expression in the presence of 3-MA or BA were also
studied. As demonstrated in Fig.
4B, 2 mM 3-MA decreased the
expression of LC3, but 1 nM BA increased the level of LC3
expression. The opposite effects of 3-MA and BA on LC3 are
associated with blocking autophagy at different stages: 3-MA
inhibits autophagosome formation, whereas BA prevents degradation
of LC3 in autophagolysosomes and in turn increases the LC3
expression level (38).
Additionally, 3-MA significantly decreased the LC3 expression
induced by AFC, whereas BA increased the LC3 expression induced by
AFC treatment (Fig. 4B). These
data suggested that 3-MA and BA inhibited autophagy induced by AFC
at different stages.
Furthermore, the effect of AFC on the expression of
apoptotic genes in the presence of 3-MA or BA was determined. The
expression of cleaved-PARP and cleaved-caspase-3 were increased
significantly with combined treatment of AFC and 3-MA or BA
compared with either AFC, 3-MA or BA alone in HCT-116 cells
(P<0.05; Fig. 4C and D).
Similar effects were evident in the Annexin V-FITC/PI double
staining assay, in which combined treatment of AFC and 3-MA or BA
increased apoptosis compared with AFC, 3-MA or BA alone in HCT-116
cells (Fig. 4E and F). These data
suggest that autophagy induced by AFC exerted a suppressive effect
on the apoptotic pathways of HCT-116 cells.
AFC inhibits the activation of the
PI3K/Akt/mTOR signaling pathway in HCT-116 cells
It has been well documented that the PI3K/Akt/mTOR
signaling pathway serves a key role in regulating both apoptosis
and autophagy through divergent pathways (39). Therefore, the effect of AFC on the
PI3K/Akt/mTOR signaling pathway in HCT-116 cells was investigated
by western blotting. Treatment with AFC inhibited PI3K
phosphorylation, decreased the levels of phospho-Akt, and
downregulated phospho-mTOR in a dose-dependent manner (Fig. 5A). The ratios of p-Akt/Akt and
p-mTOR/mTOR were significantly decreased following AFC treatment
(Fig. 5B). LY294002 is a
well-characterized inhibitor of PI3K (40), and the effect of combined AFC (100
µg/ml) and LY294002 (10 µM) on the expression of
p-Akt/Akt and p-mTOR/mTOR on HCT-116 cells was investigated by
western blotting. The results revealed that the combination of AFC
and LY294002 treatment was more effective in decreasing of the
ratios of p-Akt/Akt (P<0.001) and p-mTOR/mTOR (P<0.05)
compared with AFC or LY294002 alone (Fig. 5C and D). Insulin-like growth
factor-I (IGF-I), a PI3K activator, is capable of upregulating PI3K
expression, as well as its downstream targets, Akt and mTOR
(41). IGF-I treatment (50 ng/ml)
significantly increased the phosphorylation of Akt and mTOR
(Fig. 5E and F). When cells were
pretreated with IGF-I (50 ng/ml), the effect of AFC was
significantly attenuated. Furthermore, significant differences in
the expression of the LC3-II/LC3-I, cleaved-PARP/PARP and
cleaved-caspase-3/procaspase-3 ratios were observed between AFC
treatment alone and the combination of AFC and IGF-I (Fig. 5G and H). These data indicate that
the effect of AFC on induction of apoptosis and autophagy is
associated with inhibition of the PI3K/Akt/mTOR signaling
pathway.
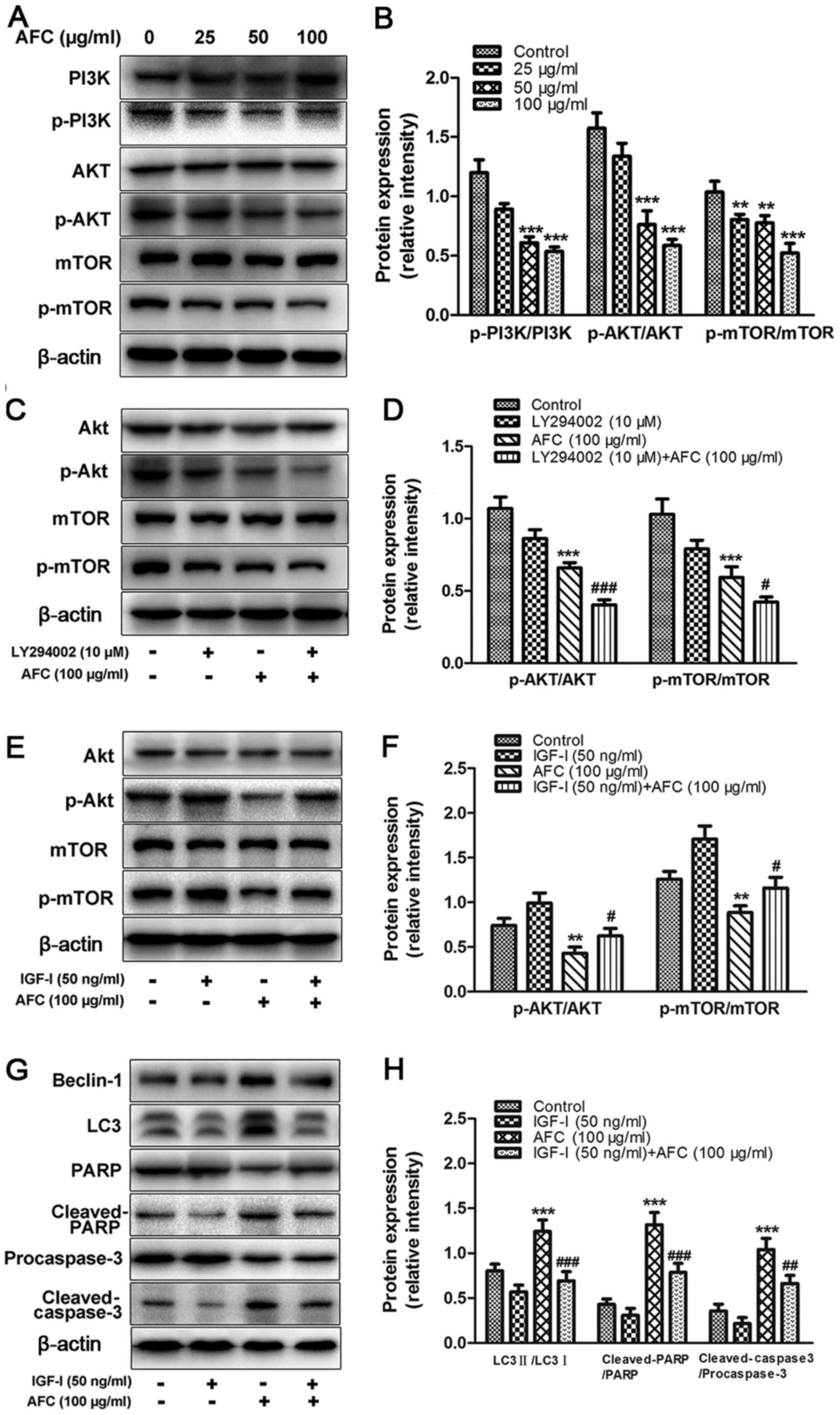 | Figure 5AFC inhibits activation of the
PI3K/Akt/mTOR signaling pathway. (A) The protein expression levels
of phospho-PI3K, phospho-Akt and phospho-mTOR in HCT-116 cells
following treatment with 25, 50 or 100 µg/ml AFC for 48 h.
(B) The intensities of bands were quantified by densitometric
analysis. (C) The protein expression levels of phospho-Akt and
phosphomTOR in HCT-116 cells following treatment with 100
µg/ml AFC with or without 10 µM LY294002 for 48 h.
(D) Quantification of the western blotting results. (E) The
expression levels of phospho-Akt and phospho-mTOR in HCT-116 cells
following treatment with 100 µg/ml AFC with or without 50
ng/ml IGF-I for 48 h. (F) Quantification of the western blotting
results. (G) The expression levels of LC3, Beclin-1, caspase-3 and
PARP protein in HCT-116 cells following treatment with 100
µg/ml AFC for 48 h with or without IGF-I pretreatment (50
ng/ml) for 1 h. (H) Quantification of the western blotting results,
using actin as a loading control. All data are expressed as the
mean ± standard deviation from 3 independent experiments in
triplicate. **P<0.01 and ***P<0.001 vs.
vehicle control; #P<0.05, ##P<0.01 and
###P<0.001 vs. AFC alone. AFC, active fraction of
clove; PI3K, phosphoinositide 3-kinase; mTOR, mechanistic target of
rapamycin; IGF-I, Insulin-like growth factor-I; p-,
phosphorylated. |
Discussion
In the present study, the antitumor effect of AFC
was investigated in human colorectal cancer cells. It was
demonstrated that AFC inhibited cell proliferation and apoptosis
and induced autophagy in a concentration- and time-dependent manner
(Figs. 2Figure 3–4). The effect of AFC on induction of
apoptosis and autophagy was demonstrated to occur via inhibition of
the PI3K/Akt/mTOR signaling pathway (Fig. 5). Furthermore, autophagy induced by
AFC was demonstrated to suppress apoptotic pathways, and inhibition
of autophagy by autophagic inhibitors, 3-MA and BA, enhanced the
effects of AFC on cytotoxicity and apoptosis of HCT-116 cells
(Fig. 4).
In our previous study, two active components of AFC,
OA and Eugenol, were identified (12). OA has been demonstrated to exhibit
anticancer efficacy against various types of human cancer cells
(12,42,43).
Our previous study indicated that treatment of human pancreatic
pan-28 cancer cells with OA induced apoptosis via a
mitochondrially-mediated apoptotic pathway (13). It was also demonstrated that OA is
able to induce protective autophagy in multiple types of cancer
cells (30). In the present study,
it was indicated that AFC-induced autophagy decreased its effect on
induction of apoptosis in human HCT-116 cancer cells. Eugenol has
also been demonstrated to possess moderate antitumor activity
(44,45), and to trigger apoptosis of breast
cancer cells through E2F1/survivin down-regulation (46). The combination of myricetin and
methyl Eugenol enhanced the anticancer activity of cisplatin
against HeLa cervical cancer cells (47). However, whether a synergistic
effect of anticancer activity exists of OA and Eugenol remains
unclear and requires further investigation. AFC may contain other
anticancer components. Studies are on-going in our laboratory to
address the complicated mechanism of the anticancer activity of
clove.
Clove is traditionally believed in Chinese medicine
to aid gastrointestinal function and to alleviate pain, and
historically used to treat nausea, gastric spasm and sore throat
(48). In a previous study it was
demonstrated that oral administration of aqueous clove (100
µl/mouse/day for 21 weeks) decreased the incidence of tumor
development by >50% in a mouse model of benzo[a]pyrene
(BP)-induced lung carcinogenesis, and that the chemo-preventive
effect of clove may be due to inhibition of anti-apoptotic gene
expression, including that of Bcl-2, VEGFA and CD44 (10). Our previous study also revealed
that clove extracts were capable of inducing apoptosis via
mitochondrial pathways in a number of cancer cell lines (12). Considering its low toxicity and its
effectiveness, AFC has potential for development as a novel
anticancer agent.
In the present study, AFC treatment was demonstrated
to enhance autophagy in HCT-116 cells in a dose- and time-dependent
manner. Autophagy has been indicated to be induced by anticancer
drugs during the induction of apoptosis. Therefore, inducing
autophagy-associated cell death of cancer cells may be useful in
cancer treatment (49–52). An extract from the tuber of
Amorphaphallus was reported to suppress the growth of
proliferation of SGC-7901 and AGS cancer cells by induction of
apoptosis and autophagy (53).
Fenugreek extract also displayed anticancer effects through
induction of autophagy and autophagy-associated death in human T
lymphoma jurkat cells (54). The
present study suggests that AFC extracts may be used in combination
with classical chemotherapeutic agents to achieve an optimized
outcome in the treatment of cancer.
In conclusion, the data from the present study
indicate that AFC was able to induce typical apoptosis and
autophagy in human colorectal cancer HCT-116 cells. Furthermore,
the autophagy inhibitors, 3-MA and BA, potentiated the
pro-apoptotic activity of AFC in HCT-116 cells. AFC also inhibited
the phosphorylation of members of the PI3K/Akt/mTOR signaling
pathway. These data may provide scientific rationale for improving
the existing understanding of the anticancer mechanism of clove and
to further develop AFC as a promising novel anticancer agent used
alone or in combination with other chemotherapeutic agents for the
treatment of colorectal cancer.
Acknowledgments
Not applicable.
References
|
1
|
Brouquet A and Nordlinger B: Metastatic
colorectal cancer outcome and fatty liver disease. Nat Rev
Gastroenterol Hepatol. 10:266–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuipers EJ, Rösch T and Bretthauer M:
Colorectal cancer screening - optimizing current strategies and new
directions. Nat Rev Clin Oncol. 10:130–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schmoll HJ and Stein A: Colorectal cancer
in 2013: Towards improved drugs, combinations and patient
selection. Nat Rev Clin Oncol. 11:79–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanko Y, Mohammed A, Okasha MA, Umar AH
and Magaji RA: Anti-nociceptive and anti-inflammatory activities of
ethanol extract of Syzygium aromaticum flower bud in Wistar rats
and mice. Afr J Tradit Complement Altern Med. 5:209–212.
2008.PubMed/NCBI
|
|
6
|
Han X and Parker TL: Anti-inflammatory
activity of clove (Eugenia caryophyllata) essential oil in human
dermal fibroblasts. Pharm Biol. 55:1619–1622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aboubakr HA, Nauertz A, Luong NT, Agrawal
S, El-Sohaimy SA, Youssef MM and Goyal SM: In vitro antiviral
activity of clove and ginger aqueous extracts against Feline
calicivirus, a surrogate for human norovirus. J Food Prot.
79:1001–1012. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abd El Azim MHM, El-Mesallamy AMD,
El-Gerby M and Awad A: Anti-tumor, antioxidant and antimicrobial
and the phenolic constituents of clove flower Buds
(Syzygiumaromaticum). J Microb Biochem Technol. 10:s8–s007.
2014.
|
|
9
|
Gülçin I, Şat G, Beydemir S, Elmastaş M
and Küfrevioǧlu I: Comparison of antioxidant activity of clove
(Eugenia caryophylata Thunb.) buds and lavender (Lavandulastoechas
L.). Food Chem. 87:393–400. 2004. View Article : Google Scholar
|
|
10
|
Banerjee S, Panda CK and Das S: Clove
(Syzygium aromaticum L.), a potential chemopreventive agent for
lung cancer. Carcinogenesis. 27:1645–1654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kubatka P, Uramova S, Kello M, Kajo K,
Kruzliak P, Mojzis J, Vybohova D, Adamkov M, Jasek K, Lasabova Z,
et al: Antineoplastic effects of clove buds (Syzygium aromaticum
L.) in the model of breast carcinoma. J Cell Mol Med. 21:2837–2851.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Schmitz JC, Wei J, Cao S, Beumer
JH, Strychor S, Cheng L, Liu M, Wang C, Wu N, et al: Clove extract
inhibits tumor growth and promotes cell cycle arrest and apoptosis.
Oncol Res. 21:247–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei J, Liu M, Liu H, Wang H, Wang F, Zhang
Y, Han L and Lin X: Oleanolic acid arrests cell cycle and induces
apoptosis via ROS-mediated mitochondrial depolarization and
lysosomal membrane permeabilization in human pancreatic cancer
cells. J Appl Toxicol. 33:756–765. 2013. View Article : Google Scholar
|
|
14
|
Ameisen JC: On the origin, evolution, and
nature of programmed cell death: A timeline of four billion years.
Cell Death Differ. 9:367–393. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gerl R and Vaux DL: Apoptosis in the
development and treatment of cancer. Carcinogenesis. 26:263–270.
2005. View Article : Google Scholar
|
|
16
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Putcha GV, Harris CA, Moulder KL, Easton
RM, Thompson CB and Johnson EM Jr: Intrinsic and extrinsic pathway
signaling during neuronal apoptosis: Lessons from the analysis of
mutant mice. J Cell Biol. 157:441–453. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016. View Article : Google Scholar
|
|
19
|
Apel A, Zentgraf H, Büchler MW and Herr I:
Autophagy-A double-edged sword in oncology. Int J Cancer.
125:991–995. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J, Zhang Y, Qu J, Xu L, Hou K, Zhang
J, Qu X and Liu Y: β-Elemene-induced autophagy protects human
gastric cancer cells from undergoing apoptosis. BMC Cancer.
11:1832011. View Article : Google Scholar
|
|
21
|
White E: The role for autophagy in cancer.
J Clin Invest. 125:42–46. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kimmelman AC and White E: Autophagy and
tumor metabolism. Cell Metab. 25:1037–1043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zarzynska JM: The importance of autophagy
regulation in breast cancer development and treatment. BioMed Res
Int. 2014:7103452014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li JP, Yang YX, Liu QL, Pan ST, He ZX,
Zhang X, Yang T, Chen XW, Wang D, Qiu JX, et al: The
investigational Aurora kinase A inhibitor alisertib (MLN8237)
induces cell cycle G2/M arrest, apoptosis, and autophagy via p38
MAPK and Akt/mTOR signaling pathways in human breast cancer cells.
Drug Des Devel Ther. 9:1627–1652. 2015.PubMed/NCBI
|
|
25
|
Nagelkerke A, Bussink J, Geurts-Moespot A,
Sweep FC and Span PN: Therapeutic targeting of autophagy in cancer.
Part II: Pharmacological modulation of treatment-induced autophagy.
Semin Cancer Biol. 31:99–105. 2015. View Article : Google Scholar
|
|
26
|
Shimizu T, Tolcher AW, Papadopoulos KP,
Beeram M, Rasco DW, Smith LS, Gunn S, Smetzer L, Mays TA, Kaiser B,
et al: The clinical effect of the dual-targeting strategy involving
PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced
cancer. Clin Cancer Res. 18:2316–2325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Porta C, Paglino C and Mosca A: Targeting
PI3K/Akt/mTOR signaling in cancer. Front Oncol. 4:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhirnov OP and Klenk HD: Control of
apoptosis in influenza virus-infected cells by up-regulation of Akt
and p53 signaling. Apoptosis. 12:1419–1432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roper J, Richardson MP, Wang WV, Richard
LG, Chen W, Coffee EM, Sinnamon MJ, Lee L, Chen PC, Bronson RT, et
al: The dual PI3K/mTOR inhibitor NVP-BEZ235 induces tumor
regression in a genetically engineered mouse model of PIK3CA
wild-type colorectal cancer. PLoS One. 6:e251322011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J, Zheng L, Zhong J, Wu N, Liu G and
Lin X: Oleanolic acid induces protective autophagy in cancer cells
through the JNK and mTOR pathways. Oncol Rep. 32:567–572. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang S, Zhao Q, Xiang H, Liu M, Zhang Q,
Xue W, Song B and Yang S: Antiproliferative activity and
apoptosis-inducing mechanism of constituents from Toona sinensis on
human cancer cells. Cancer Cell Int. 13:122013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wlodkowic D, Telford W, Skommer J and
Darzynkiewicz Z: Apoptosis and beyond: Cytometry in studies of
programmed cell death. Methods Cell Biol. 103:55–98. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, et al: Guidelines for the use
and interpretation of assays for monitoring autophagy (3rd
edition). Autophagy. 12:1–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang XH, Jackson S, Seaman M, Brown K,
Kempkes B, Hibshoosh H and Levine B: Induction of autophagy and
inhibition of tumorigenesis by beclin 1. Nature. 402:672–676. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu ZX, Liang J, Haridas V, Gaikwad A,
Connolly FP, Mills GB and Gutterman JU: A plant triterpenoid,
avicin D, induces autophagy by activation of AMP-activated protein
kinase. Cell Death Differ. 14:1948–1957. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu YT, Tan HL, Shui G, Bauvy C, Huang Q,
Wenk MR, Ong CN, Codogno P and Shen HM: Dual role of
3-methyladenine in modulation of autophagy via different temporal
patterns of inhibition on class I and III phosphoinositide
3-kinase. J Biol Chem. 285:10850–10861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Klionsky DJ, Baehrecke EH, Brumell JH, Chu
CT, Codogno P, Cuervo AM, et al: A comprehensive glossary of
autophagy-related molecules and processes (2nd edition). Autophagy.
7:1273–1294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Periyasamy-Thandavan S, Jiang M, Wei Q,
Smith R, Yin XM and Dong Z: Autophagy is cytoprotective during
cisplatin injury of renal proximal tubular cells. Kidney Int.
74:631–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shao X, Lai D, Zhang L and Xu H: Induction
of autophagy and apoptosis via PI3K/AKT/TOR pathways by
azadirachtin A in spodopteralitura cells. Sci Rep. 6:54822016.
View Article : Google Scholar
|
|
40
|
Cheng Y, Diao D, Zhang H, Guo Q, Wu X,
Song Y and Dang C: High glucose-induced resistance to
5-fluorouracil in pancreatic cancer cells alleviated by
2-deoxy-D-glucose. Biomed Rep. 2:188–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Povsic TJ, Kohout TA and Lefkowitz RJ:
β-arrestin1 mediates insulin-like growth factor 1 (IGF-1)
activation of phosphati-dylinositol 3-kinase (PI3K) and
anti-apoptosis. J Biol Chem. 278:51334–51339. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li H, He N, Li X, Zhou L, Zhao M, Jiang H
and Zhang X: Oleanolic acid inhibits proliferation and induces
apoptosis in NB4 cells by targeting PML/RARα. Oncol Lett.
6:885–890. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shanmugam MK, Dai X, Kumar AP, Tan BK,
Sethi G and Bishayee A: Oleanolic acid and its synthetic
derivatives for the prevention and therapy of cancer: Preclinical
and clinical evidence. Cancer Lett. 346:206–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ghosh R, Nadiminty N, Fitzpatrick JE,
Alworth WL, Slaga TJ and Kumar AP: Eugenol causes melanoma growth
suppression through inhibition of E2F1 transcriptional activity. J
Biol Chem. 280:5812–5819. 2005. View Article : Google Scholar
|
|
45
|
Slamenová D, Horváthová E, Wsólová L,
Sramková M and Navarová J: Investigation of anti-oxidative,
cytotoxic, DNA-damaging and DNA-protective effects of plant
volatiles eugenol and borneol in human-derived HepG2, Caco-2 and
VH10 cell lines. Mutat Res. 677:46–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Al-Sharif I, Remmal A and Aboussekhra A:
Eugenol triggers apoptosis in breast cancer cells through
E2F1/survivin down-regulation. BMC Cancer. 13:6002013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yi JL, Shi S, Shen YL, Wang L, Chen HY,
Zhu J and Ding Y: Myricetin and methyl eugenol combination enhances
the anticancer activity, cell cycle arrest and apoptosis induction
of cis-platin against HeLa cervical cancer cell lines. Int J Clin
Exp Pathol. 8:1116–1127. 2015.PubMed/NCBI
|
|
48
|
Pourgholami MH, Kamalinejad M, Javadi M,
Majzoob S and Sayyah M: Evaluation of the anticonvulsant activity
of the essential oil of Eugenia caryophyllata in male mice. J
Ethnopharmacol. 64:167–171. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Amaravadi RK, Yu D, Lum JJ, Bui T,
Christophorou MA, Evan GI, Thomas-Tikhonenko A and Thompson CB:
Autophagy inhibition enhances therapy-induced apoptosis in a
Myc-induced model of lymphoma. J Clin Invest. 117:326–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chresta CM, Davies BR, Hickson I, Harding
T, Cosulich S, Critchlow SE, Vincent JP, Ellston R, Jones D, Sini
P, et al: AZD8055 is a potent, selective, and orally bioavailable
ATP-competitive mammalian target of rapamycin kinase inhibitor with
in vitro and in vivo antitumor activity. Cancer Res. 70:288–298.
2010. View Article : Google Scholar
|
|
51
|
Li X and Fan Z: The epidermal growth
factor receptor antibody cetuximab induces autophagy in cancer
cells by downregulating HIF-1alpha and Bcl-2 and activating the
beclin 1/hVps34 complex. Cancer Res. 70:5942–5952. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sasaki K, Tsuno NH, Sunami E, Tsurita G,
Kawai K, Okaji Y, Nishikawa T, Shuno Y, Hongo K, Hiyoshi M, et al:
Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on
colon cancer cells. BMC Cancer. 10:3702010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen X, Yuan LQ, Li LJ, Lv Y, Chen PF and
Pan L: Suppression of gastric cancer by extract from the tuber of
amorphophallus konjac via induction of apoptosis and autophagy.
Oncol Rep. 38:1051–1058. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Al-Daghri NM, Alokail MS, Alkharfy KM,
Mohammed AK, Abd-Alrahman SH, Yakout SM, Amer OE and Krishnaswamy
S: Fenugreek extract as an inducer of cellular death via autophagy
in human T lymphoma Jurkat cells. BMC Complement Altern Med.
12:2022012. View Article : Google Scholar : PubMed/NCBI
|