Introduction
Gallbladder carcinoma (GBC) is one of the most
common malignancies of the biliary tract, ranked as the 5th most
common type of gastrointestinal cancer (1,2). GBC
is associated with an aggressive behavior and metastasizes to
regional lymph nodes during the early stages of the disease
(3). The majority of patients with
GBC are diagnosed at the late stages of the disease and the
recurrence and chemoresistance rates are very high (4). Over the past decade, the 5-year
survival of patients with GBC remains at a low rate of
approximately 5% and the mean survival rate ranges from 13.2 to 19
months (5,6), mainly due to the potent growth
properties and early metastastic capacity of the disease. Molecular
biological studies on GBC have aimed to shed light on the
pathogenesis of the disease in an attempt to prolong the survival
time of patients with GBC, by elucidating the underling mechanisms
and identifying potential therapeutic targets (7). However, further studies are warranted
to fully understand the mechanism responsible for the disease and
to identify novel therapeutic targets.
Liver kinase B1 (LKB1) plays important roles in cell
polarity, trafficking and metabolism (8). It is involved in maintaining cell
metabolism and energy homeostasis (9). A recent study by Yamada and Bastie
demonstrated that LKB1 exerted a growth-suppressive effect on cells
by activating AMPK and AMPK-related kinases (10), thus playing a vital role in
maintaining cell polarity, thereby inhibiting inappropriate
expansion of tumor cells (11).
LKB1 germline mutations have been detected in patients with
Peutz-Jeghers syndrome, a disease characterized by the accumulation
of non-cancerous gastrointestinal polyps and an increased risk of
cancer (12). Additionally, LKB1
somatic mutations occur more frequently in human lung, cervical and
breast cancers (13). However, the
regulatory role of LKB1 in GBC remains unknown.
The majority of neoplasms are composed of
heterogeneous populations, including a distinct subset of cancer
stem cells (CSCs) (14). These
cells possess the properties of self-renewal, differentiation
potential, resistance to chemotherapy and a high tumorigenicity
(15). Previous studies have
demonstrated that GBC CSCs can be identified by CD44, CD133, EpCAM
and other biomarkers (16–18). Numerous stemness-associated
transcription factors and signaling pathways have been reported to
be involved in GBC CSC maintenance and propagation (19). Accumulating evidence indicates that
chemoresistance and recurrence in patients with GBC are closely
associated with the existence of GBC CSCs (20–22).
Therefore, a more effective therapeutic strategy may be developed
if the molecular mechanisms underlying CSC regulation are
elucidated (23). However, the
detailed regulatory mechanisms responsible for GBC CSC generation
and expansion remain far from being fully understood.
In the present study, we found that LKB1 expression
was abnormally reduced in >80% of GBC tissues, and that the
downregulation of LKB1 mRNA expression was associated with the poor
prognosis of patients with GBC. In addition, we found that LKB1
suppressed GBC the proliferation, metastasis and expansion of GBC
CSCs through the JAK/signal transducer and activator of
transcription 3 (STAT3) cascade.
Materials and methods
Patients and samples
A total of 157 tissue samples were randomly
retrieved from patients with GBC who underwent curative resection
at the Third Affiliated Hospital of PLA Second Military Medical
University [also known as Eastern Hepatobiliary Surgery Hospital
(Shanghai, China)]. In total, 40 tissue samples from patients with
GBC were used for RT-PCR assay and the clinicopathological
characteristics of the patients with GBC are shown in Table I. In addition, 12 tissues from
patients with GBC were used for western blot analysis; and 2 tissue
samples were used for immunohistochemical analysis. A total of 105
tissue samples were also used for clinical prognosis analysis and
the clinicopathological characteristics of these patients are shown
in Table II. Overall survival
(OS) was defined as the time interval from the date of surgery to
the date of death. Cumulative recurrence was defined as the time
interval from surgery to the evidence of recurrence. Patient
informed consent was also obtained and the procedure of human
sample collection was approved by the Ethics Committee of Eastern
Hepatobiliary Surgery Hospital.
 | Table IClinicopathological data of patients
in cohort 1. |
Table I
Clinicopathological data of patients
in cohort 1.
| Variables | No. |
|---|
| Sex | |
| Male | 15 |
| Female | 25 |
| Age (years) | |
| Medium | 52 |
| Range | 29–75 |
| Tumor size
(cm) | |
| ≤3 | 12 |
| >3 | 28 |
| Local invasion | |
| Yes | 14 |
| No | 26 |
| Lymph-node
metastasis | |
| Yes | 13 |
| No | 27 |
| Distant
metastasis | |
| Yes | 13 |
| No | 27 |
| TNM stage | |
| I–II | 19 |
| III–IV | 21 |
 | Table IIClinicopathological data of patients
in cohort 2. |
Table II
Clinicopathological data of patients
in cohort 2.
| Variables | No. |
|---|
| Sex | |
| Male | 40 |
| Female | 65 |
| Age (year) | |
| Medium | 55 |
| Range | 30-74 |
| Tumor size
(cm) | |
| ≤3 | 38 |
| >3 | 67 |
| Local invasion | |
| Yes | 33 |
| No | 72 |
| Lymph-node
metastasis | |
| Yes | 36 |
| No | 69 |
| Distant
metastasis | |
| Yes | 36 |
| No | 69 |
| TNM stage | |
| I–II | 51 |
| III–IV | 54 |
Cell lines and cell culture
The GBC cell lines, SGC-996 and GBC-SD, were
purchased from the Chinese Academy of Sciences, Shanghai, China.
The GBC cells were cultured in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% fetal bovine serum (FBS) and 2 mM
L-glutamine, and 25 µg/ml of gentamicin and maintained at
37°C in a 5% CO2 incubator. All these reagents were
purchased from Shanghai Basalmedia Technologies Co., Ltd.
(Shanghai, China). The cultured cells were digested with 0.5%
trypsin and moved to a new 6-well plate twice a week.
The lentiviral vector expressing LKB1 and its
control virus were produced as previously described (24). The lentiviral vectors were
purchased from Shanghai GenePharma (Shanghai, China). The
lentiviral vectors were mixed with PolyJet (Polyplus, New York, NY,
USA), and then added to the cells. The GBC-SD or SGC-996 cells were
infected with LKB1 or its control virus and the stable infectants
were screened by puromycin.
The SGC-996 or GBC-SD LKB1 and their control cells
were treated with 10 µM AZD-1480 (Cat. no. S2162; Selleck.
cn) or left untreated, and were then subjected to CCK8 assay (96
h), Transwell assay (24 h) or spheroid formation assay (7
days).
Cell proliferation assays
For cell proliferation analysis, the GBC-SD or
SGC-996 LKB1 and their control cells were seeded in 96-well plates
(3×103 cells/well). ATP activity was measured using a
Cell Counting kit-8 at the indicated time-points. The procedure was
as follows: The cell suspension (100 µl/well) was inoculated
in a 96-well plate, and the plate was pre-incubated in a humidified
incubator at 37°C for 1 h. This was followed by the addition of 10
µl of the CCK-8 solution to each well of the plate, and
incubation of the plate for 1 h in the incubator. Finally, the
absorbance was measured at 450 nm using a microplate reader
(Synergy H1; BioTek Instruments, Inc., Winooski, VT, USA).
For cell EdU immunofluorescence staining, the GBC
cells were seeded into 96-well plates and examined using the EdU
kit (RiboBio, Guangzhou, China). The results were quantified with a
Zeiss axiophot photomicroscope (Carl Zeiss, Jena, Germany) and
Image-Pro plus 6.0 software.
Colony formation assay
For colony formation assay, the GBC-SD or SGC-996
LKB1 and their control cells were seeded in 12-well plates
(3×103 cells/well). The cells were incubated at 37°C for
7 days and then fixed with with 10% neutral formalin for >4 h.
The cells were dyed with crystal violet (Beyotime, Haimen, China).
The cells were photographed under a microscope (Olympus, Tokyo,
Japan).
Cell migration assay
For cell migration experiments, 2×105
cells were seeded into the upper chamber of a polycarbonate
Transwell in serum-free DMEM. The lower chamber was supplemented
with DMEM containing 20% FBS as chemoattractant. The cells were
incubating for 24 h and the chamber was fixed with 10% neutral
formalin for >4 h. The cells were dyed with crystal violet
(Beyotime). The cells were then counted under a microscope
(Olympus) and the cell number is expressed as the average number of
the cells in each field.
Cell invasion assay
For cell invasion experiments, 2×105
cells were seeded into the upper chamber of a Matrigel-coated
Boyden chamber in serum-free DMEM. The lower chamber was
supplemented with DMEM containing 20% FBS as a chemoattractant. The
cells were incubating for 48 h and the chamber was fixed with 10%
neutral formalin for >4 h. The cells were dyed with crystal
violet (Beyotime). The cells were counted under a microscope
(Olympus) and the cell number is expressed as the average number of
the cells in each field.
Flow cytometric analysis
The GBC cells were incubated with the primary
anti-CD44 (Cat. no. 15675-1-AP; Proteintech, Rosemont, IL, USA) or
anti-CD133 (Cat. no. 18470-1-AP; Proteintech) for 30 min at room
temperature. The cells were then subjected to flow cytometry using
a MoFlo XDP cell sorter from Beckman Coulter (Indianapolis, IN,
USA) according to the manufacturer's instructions.
The GBC-SD LKB1 or SGC-996 LKB1 and their control
cells were incubated with the primary anti-CD44 (Cat. no.
15675-1-AP; Proteintech) or anti-CD133 (Cat. no. 18470-1-AP;
Proteintech) for 30 min at room temperature. Flow cytometric
analysis was performed using a MoFlo XDP cell sorter from Beckman
Coulter according to the manufacturer's instructions.
Spheroid formation assay
The GBC cells were cultured in a 6-well or 96-well
ultra-low attachment culture plate for 1 week, and the total number
of spheres was counted under a microscope (Olympus).
Limiting dilution assay
Various numbers (64, 32, 16, 8, 4, 2 cells per well)
of GBC-SD or SGC-996 LKB1 and their control cells were seeded into
96-well ultra-low attachment culture plates for 1 week, and the
number of spheres was counted under a microscope (Olympus). CSC
proportions were analyzed using Poisson distribution statistics and
the L-Calc Version 1.1 software program (StemCell Technologies,
Inc., Vancouver, Canada) as previously described (25).
Reverse transcription-quantitative
PCR
RNA was extracted from the cells and tissues using
TRIzol reagent (15596-018; Invitrogen, Carlsbad, CA, USA). Total
cDNA was synthesized using the ThermoScrip™ RT-PCR system
(11146-057; Invitrogen). The total mRNA expression in the cells was
measured by RT-PCR using the ABI PRISM 7300 Real-Time PCR system
(Applied Biosystems, Foster City, CA, USA). The primers were
purchased from Invitrogen/Thermo Fisher Scientific (Shanghai,
China) and the primers sequences are presented in Table III. The PCR cycling conditions
were as follows: 95°C degeneration for 10 min; 95°C modification
for 30 sec, 60°C annealing for 30 sec, 72°C extension for 40 sec,
total of 40 cycles, 72°C terminal extension for 10 min.
 | Table IIISequences of PCR primers used in this
study. |
Table III
Sequences of PCR primers used in this
study.
| LKB1 (human) | F (5′- 3′)
TTGGGACCCCAGAGAAAACC |
| R (5′- 3′)
CGATGGCGTTTCTCGTGTTT |
| ABCG2 (human) | F (5′- 3′)
CGCACAGAGCAAAGCCATTT |
| R (5′- 3′)
GCAAGGGGCTAGAAGAAGGG |
| MDR-1) (human) | F (5′- 3′)
CCTAGGAGTACTCACTTCAGGA |
| R (5′- 3′)
CCAATCAGCCTCACCACAGA |
| CD34 (human) | F (5′- 3′)
AGGAGAAAGGCTGGGCGAAG |
| R (5′- 3′)
GTTGTCTTGCTGAATGGCCG |
| OCT4) (human) | F (5′- 3′)
CTTGCTGCAGAAGTGGGTGGAGGAA |
| R (5′- 3′)
CTGCAGTGTGGGTTTCGGGCA |
| β-actin)
(human) | F (5′- 3′)
GGCCCAGAATGCAGTTCGCCTT |
| R (5′- 3′)
AATGGCACCCTGCTCACGCA |
Western blot analysis
The tissue samples from the patients were lysed with
cell lysis buffer (Beyotime) followed by supersonic splitting. The
total protein was quantified using the BCA Protein Quantification
kit. A total of 20 µg of protein were subjected to sodium
dodecyl sulfate polyacrylamide gel electrophoresis and then
transferred onto nitrocellulose membranes. The membranes were
blocked with 10% non-fat milk and incubated with the primary
antibodies (LKB1, p-STAT3, STAT3, p-AKT, p-MEK, E-cadherin and
GAPDH) overnight. The protein band, specifically bound to the
primary antibody, was detected using an IRDye 800CW-conjugated
secondary antibody and LI-COR imaging system (LI-COR Biosciences,
Lincoln, NE, USA).
The cells were also lysed using cell lysis buffer,
and western blot analysis was then carried as described above. The
total protein was quantified using the BCA Protein Quantification
kit. A total of 20 µg protein of cell extracts were
subjected to western blot analysis with one of the antibodies
against LKB1, STAT3, p-AKT, p-MEK, E-cadherin and GAPDH (Abcam,
Cambridge, MA, USA). The protein band, specifically bound to the
primary antibody, was detected using an IRDye 800CW-conjugated
secondary antibody and LI-COR imaging system (LI-COR Biosciences).
The details of the antibodies used in this study are shown in
Table IV.
 | Table IVPrimary antibodies used in this
study. |
Table IV
Primary antibodies used in this
study.
| Human antigens | Antibody | Catalogue no. | Manufacturer | Dilution |
|---|
| LKB1 | Mouse
polyclonal | ab15095 | Abcam, Cambridge,
MA, USA | 1:500 for western
blot analysis |
| LKB1 | Rabbit
polyclonal | 10746-1-AP | Proteintech, Wuhan,
China | 1:50 for IHC |
| p-STAT3 | Rabbit
polyclonal | ab76315 | Abcam, Cambridge,
MA, USA | 1:500 for western
blot analysis |
| STAT3 | Rabbit
polyclonal | ab119352 | Abcam, Cambridge,
MA, USA | 1:500 for western
blot analysis |
| p-AKT | Rabbit
polyclonal | ab38449 | Abcam, Cambridge,
MA, USA | 1:500 for western
blot analysis |
| p-MEK | Rabbit
polyclonal | ab96379 | Abcam, Cambridge,
MA, USA | 1:500 for western
blot analysis |
| E-cadherin | Mouse
polyclonal | ab1416 | Abcam, Cambridge,
MA, USA | 1:500 for western
blot analysis |
| GAPDH | Mouse
monoclonal | ab8245 | Abcam, Cambridge,
MA, USA | 1:1000 for western
blot analysis |
| CD44 | Rabbit
monoclonal | 15675-1-AP | Proteintech, Wuhan,
China | 1:10 for FACS |
| CD133 | Rabbit
monoclonal | 18470-1-AP | Proteintech, Wuhan,
China | 1:10 for FACS |
Luciferase reporter assay
The GBC cells transfected with the pGL-STAT3-luc and
pRL-TK-Renilla-luc plasmids (GenePharma). Luciferase
activity was measured using a Synergy 2 Multidetection Microplate
Reader (BioTek Instruments, Inc.). Data were normalized for
transfection efficiency by dividing the firefly luciferase activity
by Renilla luciferase activity.
Statistical analysis
GraphPad Prism (GraphPad Software, Inc. La Jolla,
CA, USA) was used for all statistical analyses. Statistical
analysis was carried out using a t-test or the Bonferroni Multiple
Comparisons test. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
LKB1 expression is downregulated in human
GBC tissues and predicts the poor prognosis of patients with
GBC
To explore the function of LKB1 in GBC progression,
we examined the mRNA and protein expression of LKB1 in GBC tissues.
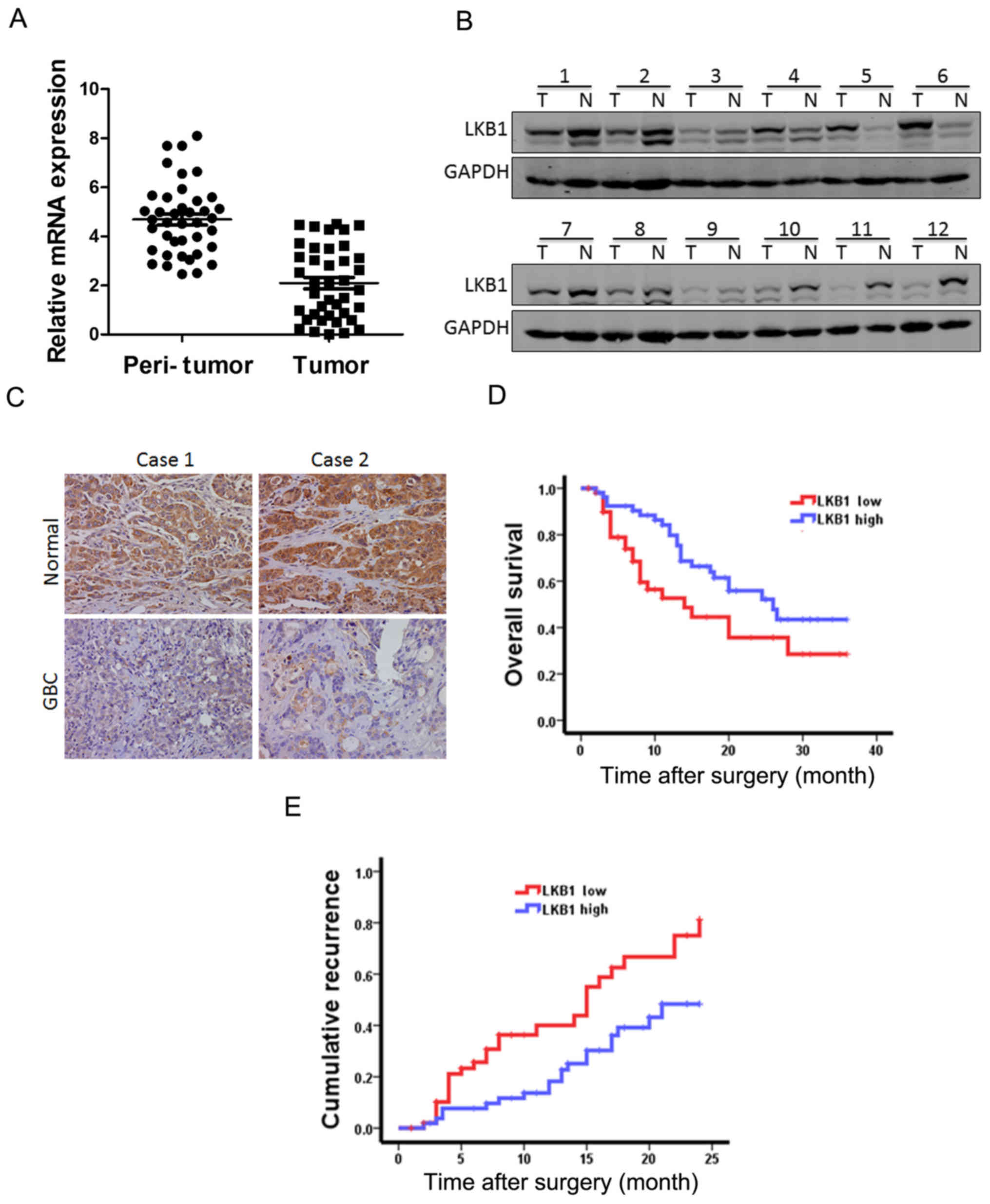
As shown in Fig. 1A, LKB1 mRNA
expression was markedly decreased in the tumor tissue, namely in
82.5% of the GBC cases (33/40) compared with the paired
non-tumorous tissues, with higher LKB1 transcripts observed in
17.5% of the patients. Moreover, the results of western blot
analysis revealed the downregulation of LKB1 expression in the
tumor tissues and the upregulation in the surrounding normal
tissues (Fig. 1B). Furthermore,
immumohistochemical staining revealed that the expression of LKB1
was decreased in the patients with GBC (Fig. 1C).
According to the LKB1 mRNA expression levels which
were above or below the median score, 105 patients with GBC were
classified into the 'high-LKB1' expression group (n=52) and the
'low-LKB1' expression group (n=53). The survival time in the
'low-LKB1' expression group was shorter than that in the
'high-LKB1' expression group (Fig. 1D
and E). Taken together, LKB1 could be a valuable prognostic
factor for GBC patients.
Overexpression of LKB1 suppresses GBC
cell proliferation
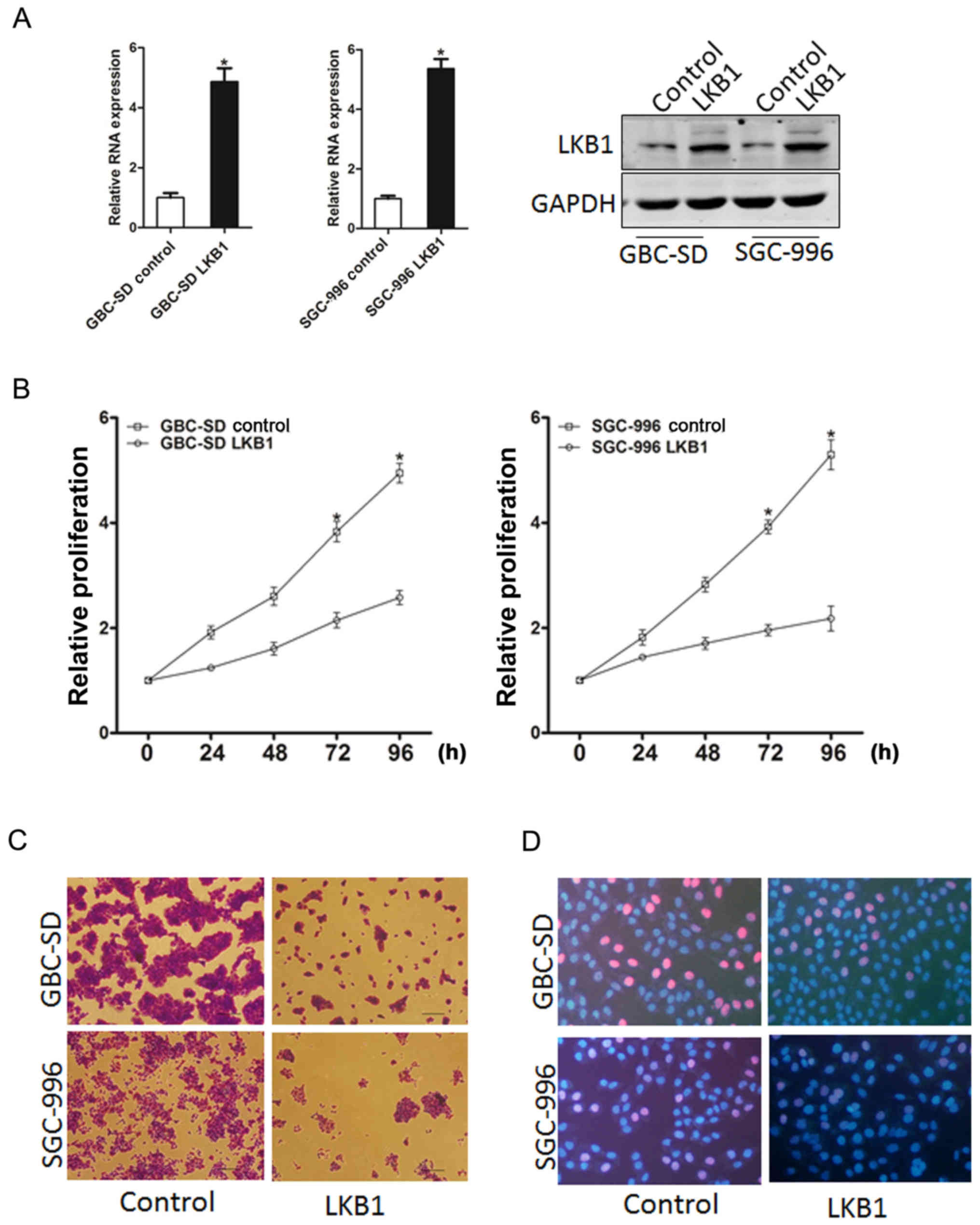
In order to elucidate the role of LKB1 in GBC cell
growth, GBC-SD and SGC-996 cells were infected with LKB1
overexpression lentivirus. The stable infectants were examined by
RT-qPCR and western blot analysis (Fig. 2A). As was expected, LKB1
overexpression suppressed the markedly proliferation of the GBC
cells (Fig. 2B). Moreover, GBC
cells stably overexpressing LKB1 formed smaller and fewer colonies
(Fig. 2C). Consistently,
5-ethynyl-2′-deoxyuridine (EdU) staining confirmed that the ectopic
expression of LKB1 suppressed cell proliferation (Fig. 2D). Taken together, the
above-mentioned results demonstrated that LKB1 suppressed GBC cell
growth.
Overexpression of LKB1 suppresses GBC
cells migration and invasion in vitro
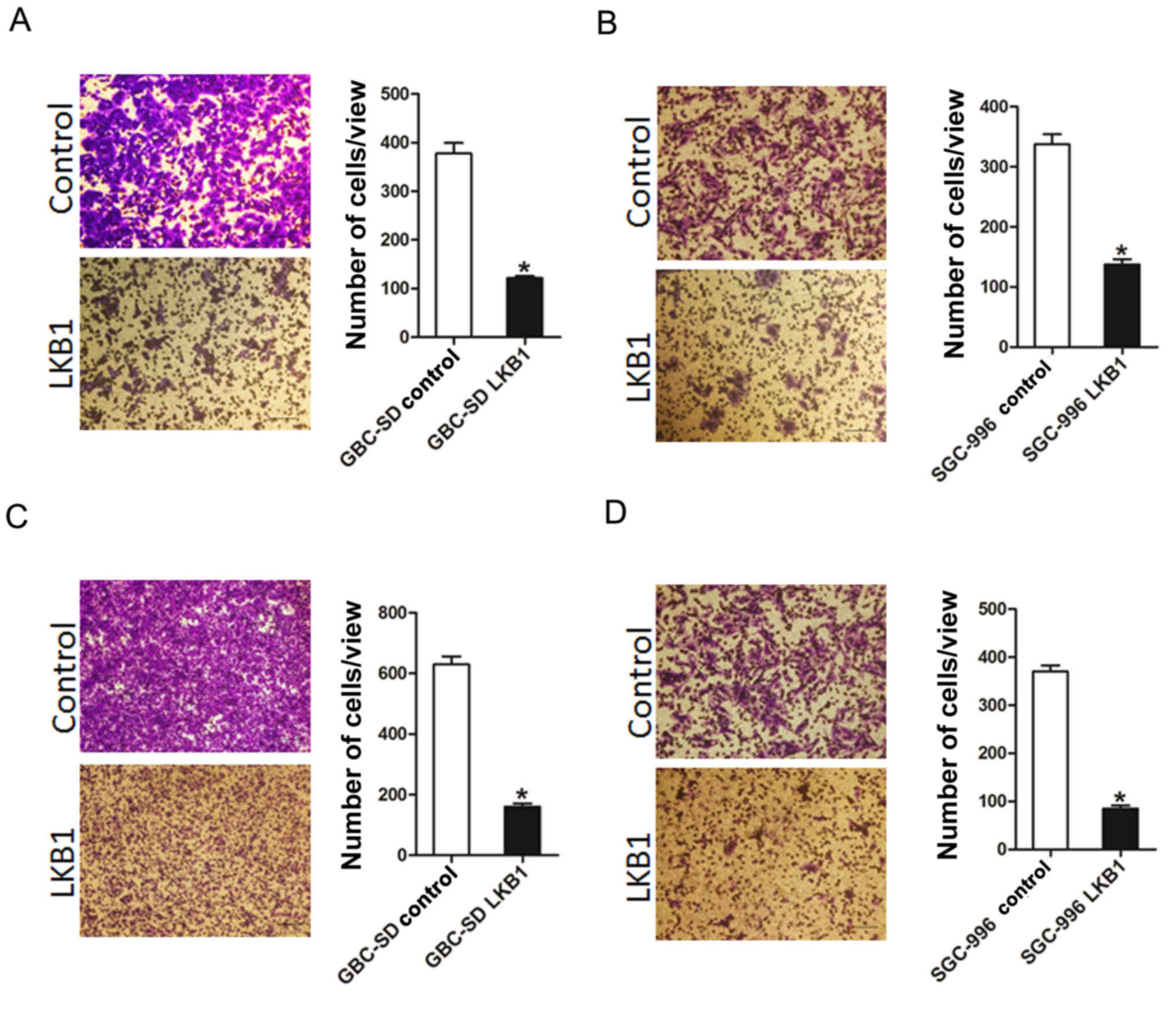
To further elucidate the effects of LKB1 on GBC cell
metastasis, Transwell assay was performed, which demonstrated that
the migratory ability of the GBC cells was attenuated following the
ectopic overexpression of LKB1 (Fig.
3A and B). In addition, the invasive ability of the GBC cells
was impaired following the overexpression of LKB1 (Fig. 3C and D). Collectively, our results
demonstrated that LKB1 disrupted the metastatic potential of GBC
cells.
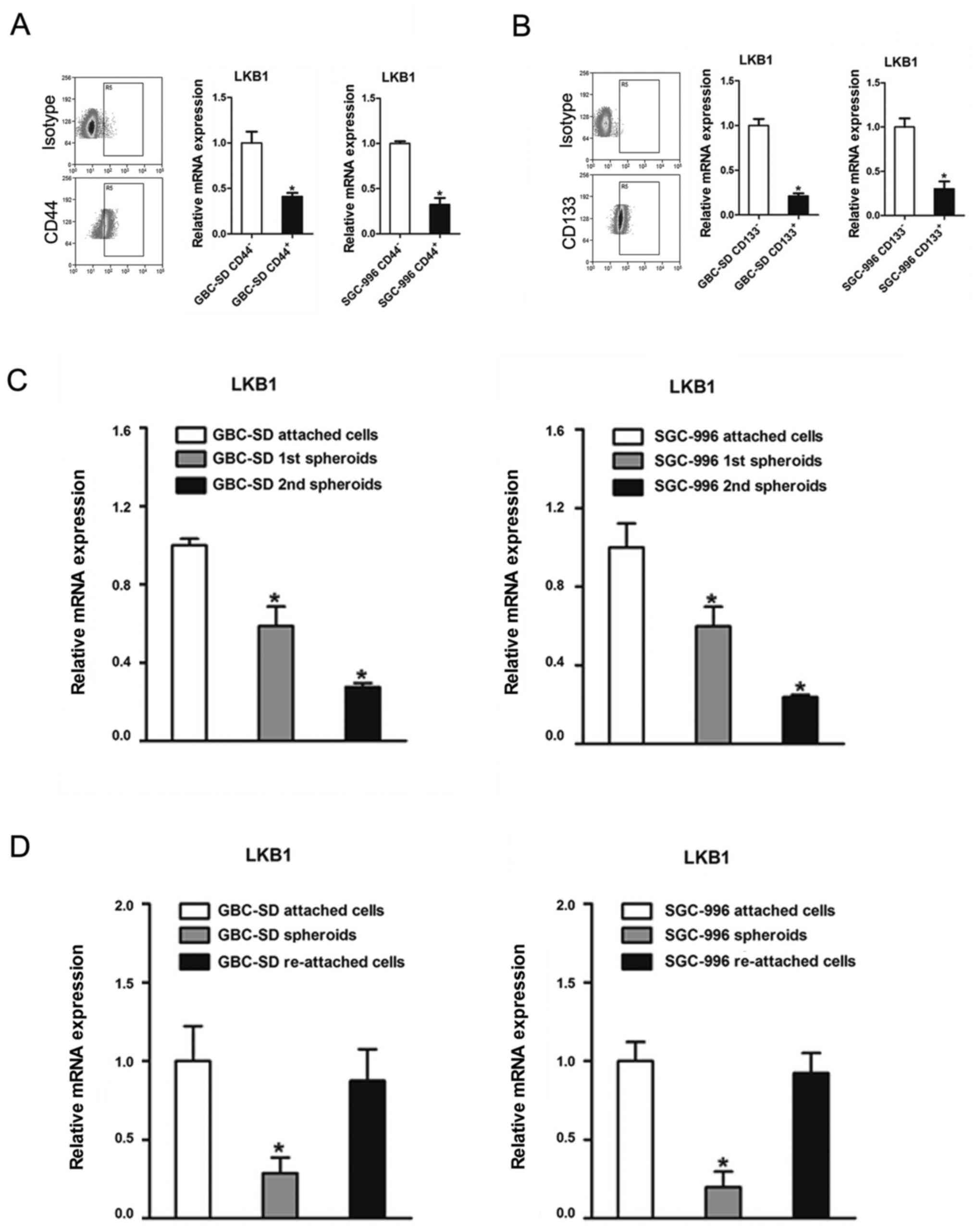
Downregulation of LKB1 in GBC CSCs
It has been reported that LKB1 is downregulated in
breast stem-like cells (26).
However, whether LKB1 regulates the maintenance of GBC CSCs
remained unknown. In this study, we found that cluster of
differentiation (CD)44-positive (CD44+) or
CD133-positive (CD133+) GBC cells, which are considered
as GBC CSCs, exhibited a decreased expression of LKB1 compared with
the CD44− or CD133− cells (Fig. 4A and B). In serial passages of
GBC-SD or SGC-996 spheroids, the expression of LKB1 was also found
to be gradually decreased (Fig.
4C). Of note, LKB1 expression was partially recovered when the
spheroids were re-seeded in the attached plates (Fig. 4D).
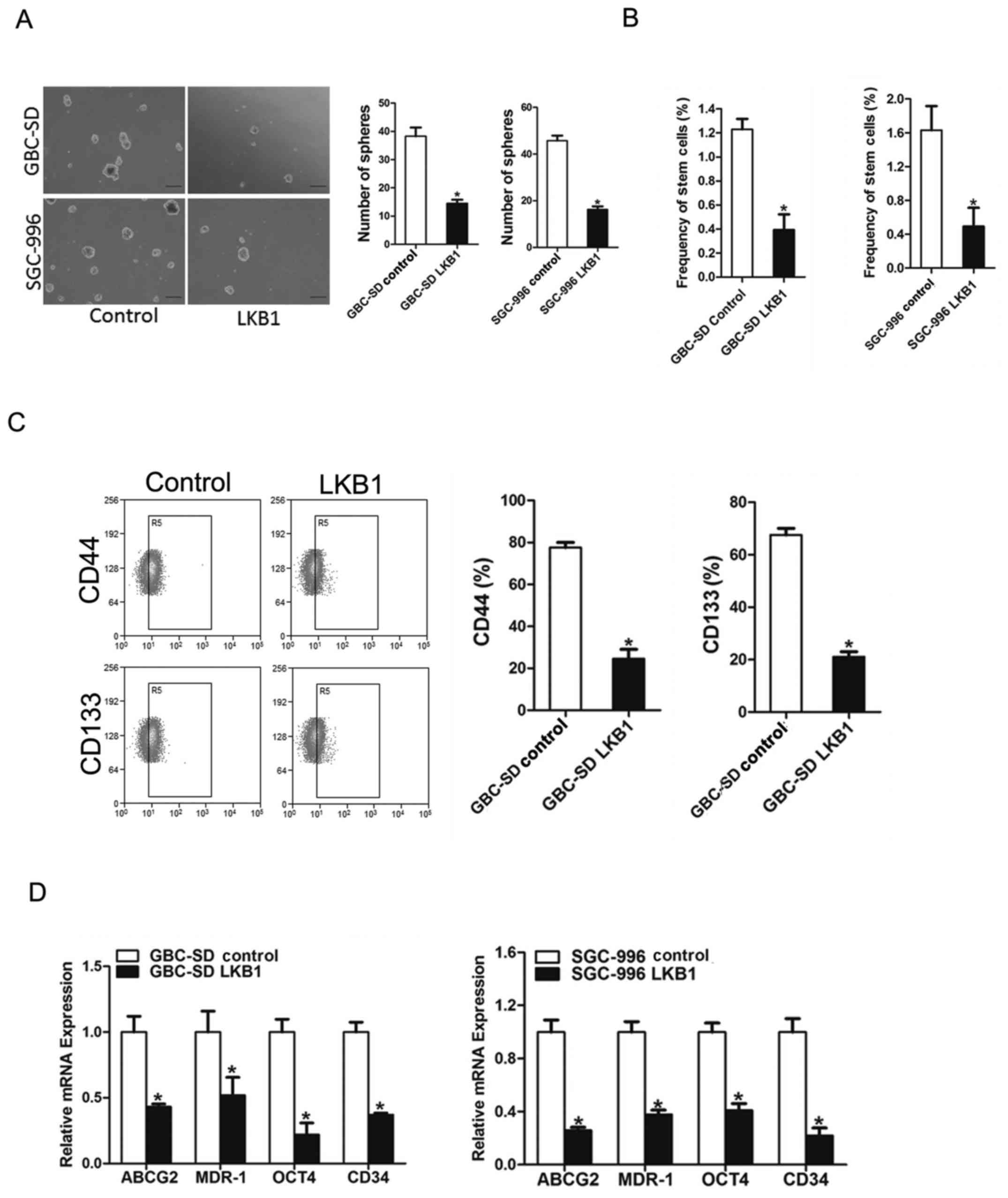
LKB1 represses GBC CSCs expansion
To explore the significance of LKB1 in GBC CSCs,
LKB1 stable overexpressing transfectants of GBC cells were used. As
expected, fewer spheroids were formed in the GBC cells
overexpressing LKB1 as compared with the control cells (Fig. 5A). Consistently, an in vitro
limiting dilution assay illustrated that LKB1 overexpression was
markedly decreased in the CSC population in the GBC cells (Fig. 5B). Moreover, the expression levels
of LKB1 (Fig. 5C and D), GBC
stemness-associated transcription factors and CSC markers were also
suppressed in the spheroids overexpressing which further supported
that LKB1 could suppress GBC CSCs expansion.
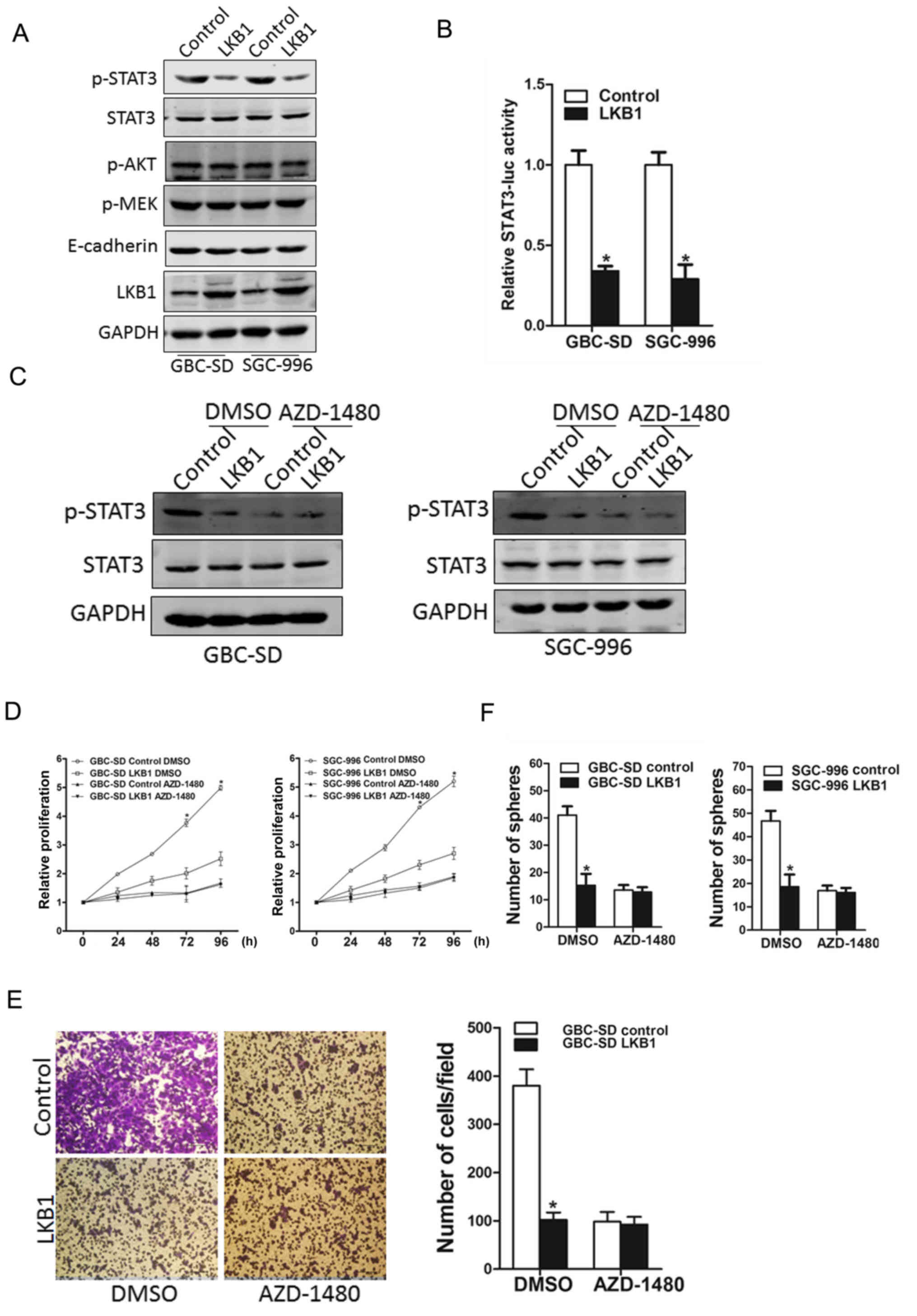
LKB1 inhibits GBC cell progression via
the JAK/STAT3 cascade
Several signaling pathways, including JAK/STAT3,
PI3K/Akt, MEK/ERK and EMT have been reported to play a role in the
proliferation of GBC cells (2–2). In
this study, our data demonstrated that PI3K/Akt and MEK/ERK
pathways, and EMT were not affected by LKB1 overexpression, while
the phosphorylation of the STAT3 molecule was evidently activated
in both the SGC-996 and GBC-SD LKB1 control cells (Fig. 6A). STAT3 reporter assay further
confirmed the effects of LKB1 on STAT3 activation (Fig. 6B). In addition, the use of the
JAK2/STAT3 inhibitor, AZD-1480, led to the distinct inactivation of
the phosphorylation of STAT3 in the LKB1-overexpressig GBC cells
and the control cells (Fig. 6C).
More importantly, the inhibitor, AZD-1480, diminished the distinct
differences in the growth capacity between the LKB1-overexpressing
GBC cells and the control cells (Fig.
6D), and also eliminated the differences in the metastatic
ability between the LKB1-overexpressing GBC cells and the control
cells (Fig. 6E). The use of
AZD-1480 also abolished the distinct differences in the
self-renewal ability between the LKB1-overexpressing GBC cells and
the control cells (Fig. 6F),
suggesting that LKB1 suppressed the GBC cell growth ability by
inhibiting JAK2/STAT3 signaling.
Discussion
GBC is the most common malignant tumors of the
biliary system. The majority of patients with GBC are diagnosed at
a late stage or are affected by distant metastasis, and thus, the
prognosis is poor (33).
Post-operative recurrence in patients with GBC remains very high,
and treatments involving radiotherapy or chemotherapy are only
effective for a few patients (34). However, the underlying molecular
mechanisms responsible for the development of GBC remain elusive.
It is therefore necessary to obtain a better understanding of the
molecular mechanisms behind the pathogenesis of GBC in order to
identifying GBC-specific targets for therapeutic intervention. In
this study, to the best of our knowledge, we demonstrate for the
first time that a decreased expression of LKB1 is associated with
the prognosis of patients with GBC, suggesting that LKB1 may thus
prove to be a valuable prognostic marker for patients with GBC.
It has been reported that LKB1 functions as a tumor
suppressor gene in the initiation and progression of human cancers
(35), and may prove to be a novel
marker for the diagnosis and treatment of cancers. However, the
potential role of LKB1 in GBC has not been reported to date, at
least to the best of our knowledge. In this study, LKB1 was found
to be downregulated in GBC tissues and to be associated with the
prognosis of patients with GBC. We also found that the ectopic
overexpression of the LKB1 gene by lentivirus transfection
inhibited GBC cell proliferation and metastasis in
vitro.
The presence of CSCs in solid tumors has been
confirmed, and these cells have the ability of self-renewal and
differentiation, a high tumorigenic potential, and resistance to
chemotherapeutics (36). The
existence of GBC CSCs is considered to be the origin of the
chemoresistance and recurrence of patients with GBC (22). Thus, it is mandatory that the
molecular mechanisms underlying GBC CSC regulation are
investigated, so as to develop novel therapeutic strategies
targeting CSCs. It has been reported that LKB1 regulates the
quiescence and metabolic homeostasis of hematopoietic stem cells
(13). However, whether LKB1 is
involved in GBC CSC expansion remains to be fully elucidated. The
data from the present study indicated that LKB1 was downregulated
in the CD44- and CD133-positive GBC cells (CD44 and CD133 are
markers of CSCs). Moreover, LKB1 overexpression in the GBC cells
suppressed the self-renewal capacity of the GBC CSCs, and
downregulated the levels of stemness-associated transcription
factors and CSC markers.
Accumulating evidence has illustrated that the
JAK/STAT3 pathway plays a pivotal role in tumor activation,
although the detailed underlying mechanisms have yet to be
determined (37–39). Activated STAT3 modulates the
function of numerous substrates, including cell proliferation and
apoptosis (40). There is ample
evidence to indicate that the STAT3 pathway is uncontrolled in GBC
(28,41). It was found in our study that LKB1
plays a negative role in GBC cells and inhibits GBC cell growth and
metastasis by inactivating STAT3 signaling. In addition, these
effects were attenuated by the JAK2 inhibitor, AZD-1480. Mouse
embryos with STAT3-deficiency have also been shown to be unable to
develop beyond embryonic day 7, when gastrulation begins (42). STAT3 activation has been shown to
be involved in the early developmental stage of embryonic stem
cells (43). It has been reported
that IL-6 promotes CSC expansion via STAT3 activation in numerous
tumors, including breast, colon, lung, prostate cancers as well as
GBC (27,44–46).
The data of the present study demonstrated that LKB1 suppressed GBC
CSC expansion via the inactivation of JAK/STAT3 pathway.
Furthermore, these effects were abolished by JAK2 inhibitor,
AZD-1480.
In conclusion, in this study, at least to the best
of our knowledge, we demonstrate for the first time that LKB1 is
downregulated in GBC tissues, and its ectopic overexpression
suppresses the growth, metastasis and self-renewal ability of GBC
cells. Moreover, LKB1 suppressed GBC cell progression via
dysregulating JAK/STAT3 signaling in vitro. The findings of
the present study not only shed new light on the mechanisms
responsible for GBC progression, but also suggest that LKB1 may be
a novel prognostic marker and a potential therapeutic target
against GBC and GBC CSCs.
Acknowledgments
Not applicable.
References
|
1
|
Zhu AX, Hong TS, Hezel AF and Kooby DA:
Current management of gallbladder carcinoma. Oncologist.
15:168–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wistuba II and Gazdar AF: Gallbladder
cancer: Lessons from a rare tumour. Nat Rev Cancer. 4:695–706.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lazcano-Ponce EC, Miquel JF, Muñoz N,
Herrero R, Ferrecio C, Wistuba II, Alonso de Ruiz P, Aristi Urista
G and Nervi F: Epidemiology and molecular pathology of gallbladder
cancer. CA Cancer J Clin. 51:349–364. 2001. View Article : Google Scholar
|
|
4
|
Batra Y, Pal S, Dutta U, Desai P, Garg PK,
Makharia G, Ahuja V, Pande GK, Sahni P, Chattopadhyay TK, et al:
Gallbladder cancer in India: A dismal picture. J Gastroenterol
Hepatol. 20:309–314. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu XS, Shi LB, Li ML, Ding Q, Weng H, Wu
WG, Cao Y, Bao RF, Shu YJ, Ding QC, et al: Evaluation of two
inflammation-based prognostic scores in patients with resectable
gallbladder carcinoma. Ann Surg Oncol. 21:449–457. 2014. View Article : Google Scholar
|
|
6
|
Butte JM, Matsuo K, Gönen M, D'Angelica
MI, Waugh E, Allen PJ, Fong Y, DeMatteo RP, Blumgart L, Endo I, et
al: Gallbladder cancer: Differences in presentation, surgical
treatment, and survival in patients treated at centers in three
countries. J Am Coll Surg. 212:50–61. 2011. View Article : Google Scholar
|
|
7
|
Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z,
Liu C, Shen B, Wang XA, Wu W, et al: Whole-exome and targeted gene
sequencing of gallbladder carcinoma identifies recurrent mutations
in the ErbB pathway. Nat Genet. 46:872–876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McInnes KJ, Brown KA, Hunger NI and
Simpson ER: Regulation of LKB1 expression by sex hormones in
adipocytes. Int J Obes. 36:982–985. 2012. View Article : Google Scholar
|
|
9
|
Mans LA, Querol Cano L, van Pelt J,
Giardoglou P, Keune WJ and Haramis AG: The tumor suppressor LKB1
regulates starvation-induced autophagy under systemic metabolic
stress. Sci Rep. 7:73272017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamada E and Bastie CC: Disruption of Fyn
SH3 domain interaction with a proline-rich motif in liver kinase B1
results in activation of AMP-activated protein kinase. PLoS One.
9:e896042014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng X, Chi J, Zhi J, Zhang H, Yue D,
Zhao J, Li D, Li Y, Gao M and Guo J: Aurora-A-mediated
phosphorylation of LKB1 compromises LKB1/AMPK signaling axis to
facilitate NSCLC growth and migration. Oncogene. 37:502–511. 2018.
View Article : Google Scholar
|
|
12
|
Jenne DE, Reimann H, Nezu J, Friedel W,
Loff S, Jeschke R, Müller O, Back W and Zimmer M: Peutz-Jeghers
syndrome is caused by mutations in a novel serine threonine kinase.
Nat Genet. 18:38–43. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Fillmore Brainson C, Koyama S,
Redig AJ, Chen T, Li S, Gupta M, Garcia-de-Alba C, Paschini M,
Herter-Sprie GS, et al: Lkb1 inactivation drives lung cancer
lineage switching governed by Polycomb Repressive Complex 2. Nat
Commun. 8:149222017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh AK, Arya RK, Maheshwari S, Singh A,
Meena S, Pandey P, Dormond O and Datta D: Tumor heterogeneity and
cancer stem cell paradigm: Updates in concept, controversies and
clinical relevance. Int J Cancer. 136:1991–2000. 2015. View Article : Google Scholar
|
|
15
|
Kreso A and Dick JE: Evolution of the
cancer stem cell model. Cell Stem Cell. 14:275–291. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma KL, Yadav A, Gupta A, Tulsayan S,
Kumar V, Misra S, Kumar A and Mittal B: Association of genetic
variants of cancer stem cell gene CD44 haplotypes with gallbladder
cancer susceptibility in North Indian population. Tumour Biol.
35:2583–2589. 2014. View Article : Google Scholar
|
|
17
|
Yin S, Li J, Hu C, Chen X, Yao M, Yan M,
Jiang G, Ge C, Xie H, Wan D, et al: CD133 positive hepatocellular
carcinoma cells possess high capacity for tumorigenicity. Int J
Cancer. 120:144–450. 2007. View Article : Google Scholar
|
|
18
|
Yamashita T, Ji J, Budhu A, Forgues M,
Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, et al:
EpCAM-positive hepatocellular carcinoma cells are tumor-initiating
cells with stem/progenitor cell features. Gastroenterology.
136:101–024. 2009. View Article : Google Scholar
|
|
19
|
Jin K, Xiang Y, Tang J, Wu G, Li J, Xiao
H, Li C, Chen Y and Zhao J: miR-34 is associated with poor
prognosis of patients with gallbladder cancer through regulating
telomere length in tumor stem cells. Tumour Biol. 35:150–510. 2014.
View Article : Google Scholar
|
|
20
|
Yadav A, Gupta A, Rastogi N, Agrawal S,
Kumar A, Kumar V and Mittal B: Association of cancer stem cell
markers genetic variants with gallbladder cancer susceptibility,
prognosis, and survival. Tumour Biol. 37:183–844. 2016.
|
|
21
|
Wang X: Identification of cancer stem
cells in gallbladder carcinoma: A platform for the discovery of
novel therapeutic targets. Cancer Biol Ther. 10:119–193. 2010.
View Article : Google Scholar
|
|
22
|
Shi C, Tian R, Wang M, Wang X, Jiang J,
Zhang Z, Li X, He Z, Gong W and Qin R: CD44+
CD133+ population exhibits cancer stem cell-like
characteristics in human gallbladder carcinoma. Cancer Biol Ther.
10:118–190. 2010.
|
|
23
|
Shi CJ, Gao J, Wang M, Wang X, Tian R, Zhu
F, Shen M and Qin RY: CD133(+) gallbladder carcinoma cells exhibit
self-renewal ability and tumorigenicity. World J Gastroenterol.
17:296–971. 2011. View Article : Google Scholar
|
|
24
|
Han T, Xiang DM, Sun W, Liu N, Sun HL, Wen
W, Shen WF, Wang RY, Chen C, Wang X, et al: PTPN11/Shp2
overexpression enhances liver cancer progression and predicts poor
prognosis of patients. J Hepatol. 63:65–60. 2015. View Article : Google Scholar
|
|
25
|
Roccograndi L, Binder ZA, Zhang L, Aceto
N, Zhang Z, Bentires-Alj M, Nakano I, Dahmane N and O'Rourke DM:
SHP2 regulates proliferation and tumorigenicity of glioma stem
cells. J Neurooncol. 135:48–96. 2017. View Article : Google Scholar
|
|
26
|
Sengupta S, Nagalingam A, Muniraj N,
Bonner MY, Mistriotis P, Afthinos A, Kuppusamy P, Lanoue D, Cho S,
Korangath P, et al: Activation of tumor suppressor LKB1 by honokiol
abrogates cancer stem-like phenotype in breast cancer via
inhibition of oncogenic Stat3. Oncogene. 36:570–721. 2017.
View Article : Google Scholar
|
|
27
|
Enyu L, Na W, Chuanzong Z, Ben W, Xiaojuan
W, Yan W, Zequn L, Jianguo H, Jiayong W, Benjia L, et al: The
clinical significance and underlying correlation of pStat-3 and
integrin αvβ6 expression in gallbladder cancer. Oncotarget.
8:1946–9477. 2017. View Article : Google Scholar
|
|
28
|
Kong X, Ma MZ, Zhang Y, Weng MZ, Gong W,
Guo LQ, Zhang JX, Wang GD, Su Q, Quan ZW, et al: Differentiation
therapy: Sesamin as an effective agent in targeting cancer
stem-like side population cells of human gallbladder carcinoma. BMC
Complement Altern Med. 14:2542014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin YP, Hu YP, Wu XS, Wu YS, Ye YY, Li HF,
Liu YC, Jiang L, Liu FT, Zhang YJ, et al: miR-14-p targeting of
ITGA6 suppresses tumour growth and angiogenesis by downregulating
PLGF expression via the PI3K/AKT pathway in gallbladder carcinoma.
Cell Death Dis. 9:1822018. View Article : Google Scholar
|
|
30
|
Mohri D, Ijichi H, Miyabayashi K,
Takahashi R, Kudo Y, Sasaki T, Asaoka Y, Tanaka Y, Ikenoue T,
Tateishi K, et al: A potent therapeutics for gallbladder cancer by
combinatorial inhibition of the MAPK and mTOR signaling networks. J
Gastroenterol. 51:711–721. 2016. View Article : Google Scholar
|
|
31
|
Wang SH, Wu XC, Zhang MD, Weng MZ, Zhou D
and Quan ZW: Upregulation of H19 indicates a poor prognosis in
gallbladder carcinoma and promotes epithelial-mesenchymal
transition. Am J Cancer Res. 6:15–26. 2015.
|
|
32
|
Xiong L, Wen Y, Miao X and Yang Z: NT5E
and FcGBP as key regulators of TGF-1-induced epithelial-mesenchymal
transition (EMT) are associated with tumor progression and survival
of patients with gallbladder cancer. Cell Tissue Res. 355:365–374.
2014. View Article : Google Scholar
|
|
33
|
Zhao S, Cao Y, Liu SB, Wang XA, Bao RF,
Shu YJ, Hu YP, Zhang YJ, Jiang L, Zhang F, et al: The E545K
mutation of PIK3CA promotes gallbladder carcinoma progression
through enhanced binding to EGFR. J Exp Clin Cancer Res. 35:972016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Caldow Pilgrim CH, Groeschl RT, Quebbeman
EJ and Gamblin TC: Recent advances in systemic therapies and
radiotherapy for gallbladder cancer. Surg Oncol. 22:61–67. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Trapp EK, Majunke L, Zill B, Sommer H,
Andergassen U, Koch J, Harbeck N, Mahner S, Friedl TWP, Janni W, et
al: LKB1 pro-oncogenic activity triggers cell survival in
circulating tumor cells. Mol Oncol. 11:1508–1526. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang S, Balch C, Chan MW, Lai HC, Matei
D, Schilder JM, Yan PS, Huang TH and Nephew KP: Identification and
characterization of ovarian cancer-initiating cells from primary
human tumors. Cancer Res. 68:4311–4320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Sun W, Shen W, Xia M, Chen C,
Xiang D, Ning B, Cui X, Li H, Li X, et al: Long non-coding RNA DILC
regulates liver cancer stem cells via IL-6/STAT3 axis. J Hepatol.
64:1283–1294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hill DG, Yu L, Gao H, Balic JJ, West A,
Oshima H, McLeod L, Oshima M, Gallimore A, D'Costa K, et al:
Hyperactive gp130/STAT3-driven gastric tumourigenesis promotes
submucosal tertiary lymphoid structure development. Int J Cancer.
Feb 8–2018.Epub ahead of print. View Article : Google Scholar
|
|
39
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jin G, Yang Y, Liu K, Zhao J, Chen X, Liu
H, Bai R, Li X, Jiang Y, Zhang X, et al: Combination curcumin and
(−)-epigallocatechin-3-gallate inhibits colorectal carcinoma
microenvironment-induced angiogenesis by JAK/STAT3/IL-8 pathway.
Oncogenesis. 6:e3842017. View Article : Google Scholar
|
|
41
|
Wang W, Zhan M, Li Q, Chen W, Chu H, Huang
Q, Hou Z, Man M and Wang J: FXR agonists enhance the sensitivity of
biliary tract cancer cells to cisplatin via SHP dependent
inhibition of Bcl-xL expression. Oncotarget. 7:34617–34629.
2016.PubMed/NCBI
|
|
42
|
Takeda K, Noguchi K, Shi W, Tanaka T,
Matsumoto M, Yoshida N, Kishimoto T and Akira S: Targeted
disruption of the mouse Stat3 gene leads to early embryonic
lethality. Proc Natl Acad Sci USA. 94:3801–3804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He N, Chen X, Wang D, Xu K, Wu L, Liu Y,
Tao H, Zhao Q, Cao X, Li Y, et al: VE-cadherin regulates the
self-renewal of mouse embryonic stem cells via LIF/Stat3 signaling
pathway. Biomaterials. 158:34–43. 2018. View Article : Google Scholar
|
|
44
|
Schroeder A, Herrmann A, Cherryholmes G,
Kowolik C, Buettner R, Pal S, Yu H, Müller-Newen G and Jove R: Loss
of androgen receptor expression promotes a stem-like cell phenotype
in prostate cancer through STAT3 signaling. Cancer Res.
74:1227–1237. 2014. View Article : Google Scholar
|
|
45
|
Korkaya H, Kim GI, Davis A, Malik F, Henry
NL, Ithimakin S, Quraishi AA, Tawakkol N, D'Angelo R, Paulson AK,
et al: Activation of an IL6 inflammatory loop mediates trastuzumab
resistance in HER2+ breast cancer by expanding the
cancer stem cell population. Mol Cell. 47:570–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jinushi M, Chiba S, Yoshiyama H, Masutomi
K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A and Tahara H:
Tumor-associated macrophages regulate tumorigenicity and anticancer
drug responses of cancer stem/initiating cells. Proc Natl Acad Sci
USA. 108:12425–12430. 2011. View Article : Google Scholar : PubMed/NCBI
|